Abstract
Following ingestion of fruits, vegetables and derived products, (poly)phenols that are not absorbed in the upper gastrointestinal tract pass to the colon, where they undergo microbiota-mediated ring fission resulting in the production of a diversity of low molecular weight phenolic catabolites, which appear in the circulatory system and are excreted in urine along with their phase II metabolites. There is increasing interest in these catabolites because of their potential bioactivity and their use as biomarkers of (poly)phenol intake. Investigating the fate of dietary (poly)phenolics in the colon has become confounded as a result of the recent realisation that many of the phenolics appearing in biofluids can also be derived from the aromatic amino acids, l-phenylalanine and l-tyrosine, and to a lesser extent catecholamines, in reactions that can be catalysed by both colonic microbiota and endogenous mammalian enzymes. The available evidence, albeit currently rather limited, indicates that substantial amounts of phenolic catabolites originate from phenylalanine and tyrosine, while somewhat smaller quantities are produced from dietary (poly)phenols. This review outlines information on this topic and assesses procedures that can be used to help distinguish between phenolics originating from dietary (poly)phenols, the two aromatic amino acids and catecholamines.
Keywords: Bioactive phenolic catabolites, Dietary (poly)phenolics, Microbiota-mediated catabolism, Endogenous metabolism, Aromatic amino acids, Catecholamines
1. Introduction
Dietary (poly)phenols, including C6–C3–C6 flavonoids, are found principally as conjugates linked to sugars including glucose and rutinose. Conjugates of C6–C3 cinnamic acids include quinic and tartaric acid esters. Only relatively small amounts of (poly)phenol conjugates are absorbed without undergoing structural modification. In most instances, after ingestion the attached conjugating moiety is removed in the proximal gastrointestinal (GI) tract by the action of lactase phlorizin hydrolase in the brush border of the small intestine epithelial cells and/or cytosolic β-glucosidase within the epithelial cells. The released aglycones are modified in epithelial/hepatic cells and appear in the bloodstream as phase II sulfate, glucuronide and/or methylated metabolites [1]. Ingested (poly)phenols not absorbed in the upper GI tract pass into the colon where they are subjected to the action of the resident microbiota which catalyse cleavage of the conjugating moiety [[2], [3], [4], [5], [6], [7], [8]]. The released aglycones are subjected to further microbiota-mediated catabolism, including ring fission, which yields a complex mixture of low molecular weight phenolics. These catabolites are absorbed, a portion as phase II metabolites, with what remains being voided in feces [1,9,10].
There is particular interest in colon-derived phenolic catabolites as biomarkers of (poly)phenol intake [[11], [12], [13], [14]]. However, attention is also being focused on their potential involvement in the protective effects of diets rich in fruits and vegetables against the development of non-communicable chronic conditions including coronary heart disease, inflammation, cancer and reduced cognitive function [[15], [16], [17], [18], [19], [20], [22], [23], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]].
Although largely ignored in recent years, with one notable exception [38], there is compelling evidence that the majority of phenolic catabolites detected in plasma and urine are not unique products of dietary (poly)phenols as they can also originate via a diversity of routes from the aromatic amino acids l-phenylalanine 1 and l-tyrosine 2, and to a lesser degree catecholamines such as dopamine 3 [39]. This review focusses the origins of low molecular weight phenolics, summarizes studies on the metabolism of, phenylalanine, tyrosine and dopamine, and evaluates protocols that can be used to help distinguish their metabolites from those derived from dietary (poly)phenols. The terminology and synonyms used for phenolic catabolites over the years are confusing and as a consequence the nomenclature proposed by Kay et al. [40] is used throughout this article.
2. Evolution of techniques for the analysis of phenolic acids and related compounds
In the 1960s,GC-MS with ∼2.0 mm i. d. rather than capillary GC columns, came into use to for the analysis of phenolics after derivatization to form methyl esters and/or trimethylsilyl ethers. Their phase II metabolites could not be analysed directly as even after derivatization they lacked the volatility necessary to elute from GC columns. In the early 2000's it became possible to analyse phenolics and their phase II conjugates by HPLC-MS without a prerequisite derivatization step. However, at the time the sensitivity of MS detectors was poor and many reference compounds were lacking and as a consequence relatively few phenolics were detected [2,41,42]. Current instrumentation detection limits and selectivity have improved greatly and UHPLC-HR-MS and UHPLC-QQQ-MS instruments are now the analytical platforms of choice for identification and quantification, respectively. As a consequence the number of phenolics that can be monitored has increased markedly in recent years. For instance, Pereira-Caro et al. [43] identified 65 phenolic compounds in urine collected after ingestion of orange juice, while Carregosa et al. [44] reported that in human intervention studies using diets rich in (poly)phenols, 137 low molecular weight phenolics have been detected in plasma.
The large expanding number of phenolics detected in biofluids presents its own analytical problems even with advanced UHPLC-HR-MS and UHPLC-QQQ-MS. Two recent reviews discuss in depth potential pitfalls in the characterization and quantification of (poly)phenol metabolites, and colonic catabolites appearing in biofluids, and how they can be avoided [45,46].
3. The fate of dietary (poly)phenols in the colon
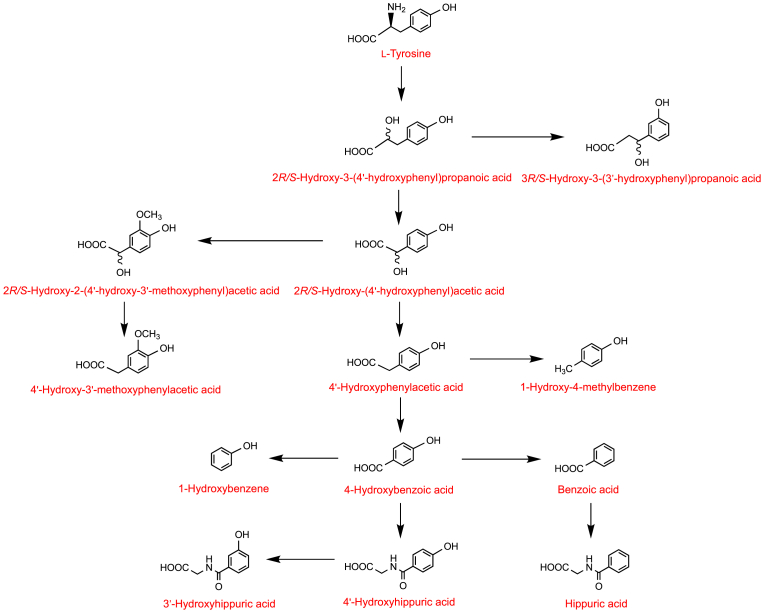
Although there are undoubtedly variations on this theme, most notably with flavan-3-ols (see Section 8.1), on entering the colon most C6–C3–C6 flavonoids are subjected to microbiota-mediated O1–C2 and C4–C10 ring fission (Fig. 1) The released A-ring generates phenolic and aliphatic compounds. The cleaved B-ring-forms phenolic acids with a three-carbon side chain, namely phenylpropanoic acids [1]. Cinnamic acids may be absorbed per se or after hydrogenation to phenylpropanoic acids [47]. Arguably, most metabolism of the C6–C3 phenylpropanoic acids involves mammalian enzymes with a β-oxidation catalysing the removal of two carbons from the side chain yielding C6–C1 benzoic acids. In addition a minor route involving, an α-oxidation or a microbiota-catalysed reaction converts the C6–C3 phenylpropanoids to C6–C2 phenylacetic acids which can also be converted to benzoic acids. Benzoic acids, as well as being catabolized yielding phenol derivatives, such as 1,2-dihydroxybenzene (4, aka catechol), are metabolized to hippuric acids via a glycination step that is catalysed by hepatic enzymes [1,48] (Fig. 1). Elaborations of the basic pathway include the formation of catabolites with a hydroxylated side chain, such 2R/S-hydroxy-3-(4′-hydroxyphenyl)propanoic acid 5, 3R/S-hydroxy-3-(3′-hydroxyphenyl)propanoic acid 6 and 2R/S-hydroxy-2-(4′-hydroxyphenyl)acetic acid 7 [39], and the formation of methoxy, glucuronide, and sulfate phase II metabolites [1]. There is considerable inter-person variation in the relative yields of the numerous phenolics and their phase II metabolites [49,50].
Fig. 1.
Proposed outline of the pathways for the metabolism of flavonoids and cinnamic acids in the lower gastrointestinal tract after cleavage of the conjugating moiety. Red arrows indicate microbiota-mediated conversions and the blue arrows reactions catalysed by mammalian enzymes. Fine arrows are potential minor routes. Additions to the pathways include the formation of methoxy, glucuronide, and sulfate phase II metabolites. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Many phenolic catabolites are common colonic degradation products of a number of different (poly)phenols others, however, have more restricted origins and their presence in biofluids is associated exclusively with the intake of specific (poly)phenols [9]. For example, potentially bioactive C6–C5 phenyl-γ-valerolactones [26,35,49], such as 4R/S-5-(4′-hydroxyphenyl)-γ-valerolactone-3′-sulfate 8, and phenylvaleric acids including 4R/S-hydroxy-5-(3′-hydroxyphenyl)valeric acid-4′-glucuronide 9, as noted in Section 8.1, are the products of microbiota-catalysed O1–C2 ring fission of (−)-epicatechin 10, as well as related flavan-3-ol monomers and procyanidins. The C6–C5 catabolites of (−)-epicatechin have a peak plasma concentration time (Tmax) of ca. 6–7 h and an apparent elimination half-life (AT1/2) of ca. 6 h. They persist in the circulatory system much longer than C6–C3–C6 metabolites, such as (−)-epicatechin-3′-glucuronide 11, which originate from the small intestine and typically have respective Tmax and AT1/2 times of ca. 1 h and 2 h (Fig. 2) [51,52]. Although C6–C5 valeric acids are also microbiota catabolites of cereal grain alkyl-resorcinols [53] they differ from the flavan-3-ol phenylvaleric acids as they lack a side chain hydroxyl group.
Fig. 2.
Plasma pharmacokinetic profiles of (−)-epicatechin-3′-glucuronide and 4R/S-5-(4′-hydroxyphenyl)-γ-valerolactone-3′-sulfate following the ingestion of [2–14C1](−)-epicatechin [51,52], and urolithins derived from ellagitannins after the consumption of raspberries [60].
Another example is the urolithins, catabolites of ellagitannins such as sanguiin H-6 12, which is found in raspberries, and punicalagin 13 and punicalin 14 which occur in pomegranates [[54], [55], [56], [57]]. Ellagitannins are absorbed poorly, if at all, in the upper GI tract and on reaching the colon undergo microbiota-mediated conversion to urolithins, but first they are hydrolysed by tannin-hydrolase and the released hexa-hydroxydiphenic acid undergoes spontaneous lactonization to form ellagic acid 15 [58]. Lactone-ring cleavage and decarboxylation of ellagic acid yields a pentahydroxy-urolithin, 3,4,8,9,10-pentahydroxy-urolithin (16, aka urolithin M5). Dehydroxylation reactions then occur generating a series of tetrahydroxy-trihydroxy-, dihydroxy- and finally a mono-hydroxy-urolithin [58]. The urolithins form phase II conjugates and urinary excretion starts ca. 16 h post-ellagitannin ingestion and can continue for a further 4 days [57,59]. They are also long-lived in the circulatory system compared to other phenolic catabolites, including phenyl-γ-valerolactones, first appearing in the circulatory system ca. 8 h after ellagitannin intake (Fig. 2) [60] and attaining peak plasma concentrations (Cmax) of up to 5 μmol/L [58,59].
There is a large inter-individual variability in the levels of urolithins, and this has been associated with varying colonic microbiota compositions. The variability is not only quantitative with low vs. high producers [58], but also qualitative with more subjects excreting 3,8-dihydroxy- than 3-hydroxy-urolithin derivatives [57,58], leading to the concept of metabolic phenotypes [57,61,62]. There is evidence that obese subjects producing 3-hydroxyurolithin 17 (aka urolithin B) and 3,9-dihydroxy-urolithin 18 (aka isourolithin A) are at increased risk of cardiovascular disease while a 3,8-dihydroxy-urolithin (19, aka urolithin A) phenotype provides protection [63]. A comprehensive evaluation of the metabolism and bioactivity of urolithins and the involvement of the gut microbiota has been published by Garcia-Villalba et al. [64].
Isoflavones and lignans can be added to the aforementioned flavan-3-ols and procyanidins and ellagitannins as classes of (poly)phenols that give rise to unique colon-derived catabolites. Isoflavones are converted to S-equol 20, desmethylangolensins, such as O-desmethylangolensin 21, and 2-(phenyl)propanoic acid 22 [65], while lignans yield enterodiol 23 and enterolactone 24 [66].
4. Feeding protocols for the investigation of colonic catabolites of dietary (poly)phenols
A frequently used protocol in human clinical bioavailability studies with test meals or beverages has involved collecting urine over a 12 h overnight fasting period after adherence to a low (poly)phenol diet for at least 36 h. Participants then ingest the test food/beverage after which plasma and urine are collected at intervals over a 24 h or 48 h period during which time they continue the restricted low (poly)phenol diet. The collected fluids are analysed by UHPLC-HR-MS and/or UHPLC-QQQ-MS.
Cumulative washout in urine indicates the percentage recovery of ingested (poly)phenols as metabolites and catabolites. In view of the presence of background low molecular weight phenolics, the quantity of phenolics in 12 h pre-feed baseline urine is used on a per-hour basis to subtract from the 0–24/0-48 h post-feed levels, thus minimising the risk of over-estimating phenolics produced from the ingested supplement.
Typical data obtained with this approach in a bioavailability study by Ludwig and co-workers [60], in which raspberries containing of 292 μmol of anthocyanins, principally cyanidin-3-O-sophoroside 25, were ingested by 9 subjects, are presented in Table 1. Excretion of anthocyanins was a mere 0.007% of intake, however, significant increases in 14 phenolic acids were noted with a substantial amount of hippuric acid 26 dominating both baseline phenolics and those excreted post-raspberry consumption. There were marked person to person variations with both pre- and post-supplement excretion of hippuric acid with amounts excreted by some subjects being substantially higher than the 292 μmol of ingested anthocyanins. The large quantity of hippuric acid in baseline urine demonstrates that it originates from sources other than the ingested anthocyanins. Excretion of phenolics, without taking hippuric acid into account, was equivalent to 15% of the anthocyanin intake (Table 1).
Table 1.
Increased urinary excretion of phenolic acids 0–48 h after acute intake of 300 g of blended raspberries containing 292 μmol of anthocyanins (99.2% cyanidin-based)[60] a.
| Phenolic acids | Baselineb | Total (0–48 h)c |
|---|---|---|
| Cinnamic acids | ||
| 4′-Hydroxycinnamic acid-3′-sulfate | – | 1.1 ± 0.1 |
| 4′-Hydroxy-3′-methoxycinnamic acid | 1.8 ± 0.3 | 5.9 ± 2.1 |
| 3′-Methoxycinnamic acid-4′-sulfate | 1.6 ± 0.2 | 6.5 ± 2.6 |
| 3′-Methoxycinnamic acid-4′-glucuronide | 0.7 ± 0.1 | 1.8 ± 0.5 |
| 4′-Methoxycinnamic acid-3′-sulfate | – | 0.5 ± 0.2 |
| 4′-Methoxycinnamic acid-3′-glucuronide | 0.3 ± 0.1 | 1.0 ± 0.4 |
| Total cinnamic acids | 4.4 ± 0.4 | 16.8 ± 3.6 |
| Phenylpropanoic acids | ||
| 3-(4′-Hydroxypheny)propanoic acid-3′-sulfate | – | 1.1 ± 0.3 |
| Total phenylpropanoids | – | 1.1 ± 0.3 |
| Phenylacetic acids | ||
| 3′,4′-Dihydroxyphenylacetic acid | 0.4 ± 0.2 | 2.9 ± 1.3 |
| 4′-Hydroxy-3′-methoxyphenylacetic acid | – | 0.2 ± 0.2 |
| Total phenylacetic acids | 0.4 ± 0.2 | 3.1 ± 1.7 |
| Benzoic acids | ||
| 3-Hydroxybenzoic acid-4-sulfate | 0.1 ± 0.0 | 0.2 ± 0.2 |
| 4-Hydroxybenzoic acid-3-sulfate | 0.1 ± 0.0 | 0.3 ± 0.1 |
| 4-Hydroxybenzoic acid | – | 6.4 ± 4.8 |
| Total benzoic acids | 0.2 ± 0.0 | 6.9 ± 5.0 |
| Hippuric acids | ||
| 4′-Hydroxyhippuric acid | 7.0 ± 2.1 | 16.1 ± 1.9 |
| Hippuric acid | 294 ± 47 | 239 ± 55 |
| Total phenolics excluding hippuric acid | 12.0 ± 2.3 | 44.0 ± 8.1 (15%)d |
Data expressed in μmol as mean values ± SE (n = 9).
Subjects were on a 0–36 h a low (poly)phenol diet, after which they fasted for 12 h and baseline urine excreted during this period was collected prior to the ingestion of raspberries.
Phenolic acids collected 0–48 h following raspberry intake after subtraction on an excretion per hour basis, of baseline excretion values. All listed phenolics exhibited significantly higher excretion above baseline values (p < 0.05).
The amount excreted as a percentage of the 292 μmol of ingested anthocyanins.
The time of appearance of phenolics in the circulatory system indicates whether absorption has occurred in the upper or lower GI tract, or both, as illustrated by the Ludwig et al. raspberry study [60]. Fig. 3 illustrates the plasma profiles of 3′-methoxycinnamic acid-4′-sulfate 27, 3′,4′-dihydroxyphenylacetic acid 28 and hippuric acid 26 obtained after the ingestion of raspberries. The cinnamic acid has a Tmax of 1 h characteristic of metabolism and absorption in the small intestine while the longer 6 h Tmax of 3′4′-dihydroxyphenylacetic acid indicates it is a product of catabolism in the lower GI tract. In keeping with the urine data, the major plasma phenolic was hippuric acid with a plasma concentration of ca. 2000 nmol/L which changed little following raspberry consumption. This along with its high baseline level of urinary excretion indicates that hippuric acid originates principally from compounds other than the ingested anthocyanins and estimating what would appear to be the relatively small amounts derived from the raspberry anthocyanins is not feasible. The most obvious source would be benzoic acid but it was not detected in the raspberries. Similarly high levels of hippuric acid have been obtained in other bioavailability studies [39,[67], [68], [69], [70]].
Fig. 3.
Plasma pharmacokinetic profiles of 3′-methoxycinnamic acid-4′-sulfate, 3′,4′-dihydroxyphenylacetic acid and hippuric acid following the ingestion of 300 g of raspberry purée [60].
Arguably, the best strategy to minimise the risk of over-inflating the yield of metabolites associated with the test meal/beverage is to extend the washout period until the excretion of these metabolites has become asymptotic. Washout of metabolites sequestered in tissues and/or bound to serum albumin has rarely been studied explicitly but metabolites from this source may still be detected in urine after two days on a low (poly)phenol diet, and as a consequence require a longer washout period. This is almost certainly the case with metabolites that originate from ellagitannins, and possibly proanthocyanidins, and thearubigins, and even some simple flavonoids, which are capable of binding strongly to the gut mucosa and thus expanding the period over which absorption and excretion occurs [71]. Washout periods may also be extended by matrix effects such as the presence of fibre with the ingested supplement [72]. Ideally they should be longer than 48 h. However, there are practical limitations as to how long volunteers are willing to follow a low (poly)phenol diet, although arguably less than two days is likely to compromise the results by reducing the yield, and possibly distorting the metabolite profile. Moreover, it precludes access to washout data of value in exploring, among other things, tissue accumulation which is otherwise little studied.
Fecal samples have been analysed only rarely in bioavailability investigations [51,73,74] despite the information obtained being very relevant to determining the fraction of phenolic catabolites that is not absorbed and are potentially bioactive in the colon. However, anaerobic ex vivo incubation of (poly)phenols and related compounds with gut bacteria and fecal material have been widely used and has provided key information on microbiota-mediated catabolism [34,38,42,68,70,[75], [76], [77], [78], [79], [80], [81]], but not the accompanying in vivo phase II metabolism conversions that are catalysed by mammalian enzymes [43,47,51,67,69,82]. Ex vivo incubations with specific substrates, especially those labelled with 13C or 2H, can provide valuable information. With unlabelled substrates, the use of phenylalanine- and tyrosine-free media, and blank incubations, are able to preclude inputs from protein degradation products that can complicate matters as discussed in Section 6.
A further approach to define the role of the gut microbiota is to perform feeding studies with healthy volunteers and healthy ileostomists who have had their colon removed surgically. The plasma, urine, and feces of healthy volunteers with an intact colon include metabolites originating from the colonic microbiota, but these are not present in samples provided by the ileostomists because their colon has been removed [[2], [3], [4], [5], [6], [7], [8],82,83]. Some metabolites such as the free cinnamic acids may be absorbed in the stomach or the small intestine by both groups, while the portion, often substantial, released from unabsorbed conjugates by the colonic microbiota will not be produced by the ileostomists [5]. Analysis of their ileal effluent will reveal these untransformed substrates plus any metabolites excreted in bile and/or effluxed from the upper GI tract enterocytes. These substrates and metabolites cannot otherwise be discriminated and quantified because they are further transformed by the colonic microbiota and may be absorbed and recycled in volunteers with an intact colon.
5. Phenolic compounds derived from amino acids and catecholamines
Phenylalanine 1 is an essential amino acid as it is not synthesized de novo in animals and must be provided by the diet. It occurs in many animal and plant-derived foods including beef, pork, lamb, venison, poultry, tuna, cottage cheese, milk, lentils, soy beans, peanuts and fruits [84]. In the body it can undergo hepatic 4′-hydroxylation to form tyrosine 2. Both aromatic amino acids are incorporated into proteins, and released during protein breakdown when there is no entry of the amino acids into the body from dietary sources [85].
Some of the background phenolics found in plasma and urine, especially hippuric acid as noted in Section 5, are not derived exclusively from dietary (poly)phenols. There are reports that they are also catabolites of the aromatic amino acids phenylalanine, tyrosine, and, to a lesser degree, as they occur in much smaller amounts, catecholamines including dopamine 3. Compelling evidence on the involvement of these compounds can be found in papers from the 1960s and 1970s which until recently have escaped notice [39].
In 1961 Grümer reported that [14C]phenylalanine 1 administered intravenously to phenylketonuric subjects, where conversion to tyrosine is blocked, was converted to radiolabelled hippuric acid 26 which was detected in urine collected for a 6 h period with a 4.7% yield [86]. This indicates that the conversion of phenylalanine to hippuric acid was probably hepatic in origin and had by-passed the gut microbiota.
In 1972 and 1975, Curtius and colleagues reported on the metabolism of deuterated phenylalanine and tyrosine ingested orally by healthy subjects, participants with hyperphenylalaninemia and phenylketonuria, and mentally retarded patients [87,88]. These studies used GC-MS to identify labelled metabolites in urine which allowed metabolic pathways to be inferred but provided little quantitative information, and none on phase-II conjugates. Potential pathways of the metabolism of phenylalanine and tyrosine are illustrated, respectively, in Fig. 4, Fig. 5. Hippuric acid 26 and 4′-hydroxyphenylacetic acid 29 were the main metabolites of [2H2]phenylalanine which was also converted to benzoic acid 30 and 4′-hydroxyhippuric acid 31.
Fig. 4.
Potential pathways for the metabolism of deuterated phenylalanine following oral intake. The arrows usually represent more than one metabolic step, sometimes many more. Based on data of Curtius et al. [87,88].
Fig. 5.
Potential pathways for the metabolism of deuterated tyrosine following oral intake. The arrows usually represent more than one metabolic step, sometimes many more. Based on data of Curtius et al. [87,88].
When subjects were given the antibiotic neomycin for 3 days pre-feed to inhibit the gut microbiota, ingested 2H2-tyrosine was no longer converted to benzoic acid and hippuric acid although deuterated 4-hydroxybenzoic acid 32 and 4′-hydroxyhippuric acid 31 were still detected in urine [88]. This finding suggests that in the upper GI tract a portion of the ingested [2H2]tyrosine is absorbed into the circulatory system and undergoes hepatic side chain shortening, resulting in the formation of 4-hydroxybenzoic acid and conversion to 4′-hydroxyhippuric acid. Tyrosine remaining in the GI tract passes to the colon where the microbiota catalyse aliphatic side chain shortening and 4′-dehydroxylation with the resultant benzoic acid 30 being absorbed and converted to hippuric acid 26. As a consequence, pre-feed treatment with neomycin inhibited the gut microbiota and therefore blocked hippuric acid production but not that of 4′-hydroxyhippuric acid [86]
Deuterated tyrosine 2, 2R/S-hydroxy-3-(4′-hydroxyphenyl)propanoic acid 5, 3-(4′-hydroxyphenyl)propanoic acid 33, 4′-hydroxyphenylacetic acid 29 and 4-hydroxybenzoic acid 32 were all used as substrates in anaerobic incubations with fecal material [89]. The resultant conversions are presented in the pathways illustrated in Fig. 6. It is of note in Fig. 6 that in some instances 4′-hydroxylated C6–C3 phenolics were converted by the microbiota to 3′-hydroxylated products, such as the transformation of 3-(4′-hydroxyphenyl)propanoic acid 33 to 3-(3′-hydroxyphenyl)propanoic acid 34. This is based on an NIH-shift of the aliphatic side chain, a conversion in which certain anaerobic species move the three carbon side chain of tyrosine and/or the associated 4′-metabolites around the ring such that the 4′-hydroxyl becomes a 3′-hydroxyl [90].
Fig. 6.
Proposed catabolism of deuterated tyrosine and related phenolics following fecal incubations. Routes from (A) [2H2]tyrosine, (B) [2H2]2-hydroxy-3-(4′-hydroxyphenyl)propanoic acid, (C) [2H2]3-(4′-hydroxyphenyl)propanoic acid, (D) [2H4]4′-hydroxyphenylacetic acid and (E) [2H2]4-hydroxybenzoic acid. The arrows usually represent more than one metabolic step, sometimes many more. Based on data of Curtius et al. [89].
The third aromatic amino acid tryptophan 35, is a precursor for serotonin 36 synthesis and, in normal conditions, its degradation in mammals involves hepatic conversions to picolinic acid 37, quinolinic acid 38, and a number of indole derivatives, while a branch in the brain proceeds to kynurenine 39. One of the products of kynurenine is 1,2-dihydroxybenzene 4 which is degraded to water and carbon dioxide [91]. This may not be relevant to circulating levels of 1,2-dihydroxybenzene, and in any case the amount of the benzene derivatives produced via this route are likely to be extremely small compared to that originating from phenylalanine, tyrosine and dietary (poly)phenols.
Catecholamine synthesis in vivo begins with dietary phenylalanine and/or tyrosine being metabolized to 3′,4′,dihydroxyphenylalanine (40, l-DOPA) that is decarboxylated yielding dopamine 3 [[92], [93], [94]]. In turn, dopamine is converted to norepinephrine and epinephrine (aka adrenaline) (see Fig. 7) in adrenomedullary chromaffin cells. Norepinephrine is also produced by sympathetic nerve endings. Only a small fraction of catecholamines is released from storage vesicles of sympathetic nerves and enters the circulation. Dietary sources of dopamine, and other catecholamines, undergo sulfation in the GI tract before entering the bloodstream and, as a consequence, almost all circulating forms are sulfate derivatives. Extraneural metabolism of dopamine by catechol-O-methyltransferase and monoamine oxidase leads to the formation of 3′-methoxytryptamine, 3′,4′-dihydroxyphenylacetic acid and 4′-hydroxy-3′-methoxyphenylacetic acid as illustrated in Fig. 7 [94]. 3′,4′-Dihydroxyphenylacetic acid 28 is a biomarker of catecholamine-secreting tumors. However, false positive results can occur [95,96] as the dihydroxyphenylacetic acid is also a colonic catabolite of the dietary flavonol quercetin 41 which as glycoside conjugates occurs widely in fruits and vegetables, and is found in onions in especially high concentrations [56]. However, the contribution of dopamine to the phenolic acid catabolite pool, like that of tryptophan, is likely to be very small compared to that of phenylalanine and tyrosine.
Fig. 7.
Synthesis and catabolism of dopamine and related catecholamines. After Olguín et al. [94].
6. Phenolics excreted in urine by subjects on a low (poly)phenol diet
An investigation involving participants with a full GI tract and ileostomists has provided insights into the origins of at least some of the “background” phenolics in feeding studies with test meals or beverages. Urine collected from the two groups of subjects over a 12 h fasting period, after prior adherence to a low (poly)phenol diet for 36 h, was subjected to detailed analysis by HPLC-HR-MS [39]. Over 70 phenolics were detected and quantified. Many were present in sub-μmol amounts excreted in statistically similar amounts by both groups. Total excretion of the different categories of phenolics are summarised in Table 2 along with information on key individual phenolics which were excreted in μmol amounts by subjects with a full GI tract and, in some but not all instances, lower quantities by ileostomists.
Table 2.
Urinary excretion of selected phenolics by participants with (n = 8) and without a colon (n = 10), who were on a low (poly)phenol diet for 0–36 h, after which they fasted for 12 h. Urine collected in the 36–48 h period of the study was analysed by UHPLC-HR-MS [39] a.
| Phenolics | With colon | Without colon | ANOVA p value |
|---|---|---|---|
| Cinnamic acids (13) | |||
| Total cinnamic acids | 3.8 ± 0.4 | 4.8 ± 0.4 | ns |
| Phenylpropanoic acids (14) | |||
| 3-(3′-Methoxyphenyl)propanoic acid-4′-sulfate | 2.4 ± 0.2 | 0.33 ± 0.03 | * |
| 3-(Phenyl)propanoic acid-4′-sulfate | 1.2 ± 0.2 | 0.04 ± 0.00 | * |
| Total phenylpropanoic acids | 23.5 ± 2.2 | 21.5 ± 3.2 | ns |
| Phenylhydracrylic acids (3) | |||
| 3R/S-Hydroxy-3-(3′-hydroxy-4′-methoxyphenyl)propanoic acid | 4.9 ± 0.3 | 4.8 ± 0.3 | ns |
| 3R/S-Hydroxy-3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid | 3.1 ± 0.3 | 0.6 ± 0.05 | * |
| 3R/S-Hydroxy-3-(3′-hydroxyphenyl)propanoic acid | 13.4 ± 1.1 | 6.1 ± 0.3 | * |
| Total phenylhydracrylic acids | 21.4 ± 1.7 | 11.5 ± 0.7 | * |
| Phenylacetic acids (10) | |||
| 3′4′-Dihydroxyphenylacetic acid | 10.5 ± 0.9 | 12.6 ± 0.9 | ns |
| 3′-Methoxyphenylacetic acid-4′-sulfate | 1.3 ± 0.08 | 1.2 ± 0.1 | ns |
| 4′-Methoxyphenylacetic acid-3′-sulfate | 2.6 ± 0.4 | 2.8 ± 0.5 | ns |
| Total phenylacetic acids | 19.0 ± 1.8 | 23.6 ± 1.9 | ns |
| Mandelic acids (2) | |||
| 2R/S-Hydroxy-2-(4′-hydroxyphenyl)acetic acid | 13.4 ± 1.2 | 10.8 ± 0.4 | ns |
| 2R/S-Hydroxy-2-(4′-Hydroxy-3′-methoxyphenyl)acetic acid | 19.3 ± 1.3 | 17.8 ± 1.0 | ns |
| Total mandelic acids | 32.7 ± 2.5 | 28.6 ± 1.4 | ns |
| Benzoic acids and aldehydes (16) | |||
| 3,4-Dihydroxybenzoic acid | 4.3 ± 0.6 | 0.65 ± 0.06 | * |
| Benzoic acid-sulfate-1 | 6.7 ± 0.8 | 0.80 ± 0.06 | * |
| Total benzoic acids and aldehydes | 25.4 ± 3.3 | 18.7 ± 1.7 | ns |
| Benzene catabolites (15) | |||
| 1-Hydroxybenzene-2-sulfate | 14.9 ± 1.1 | 5.1 ± 0.3 | * |
| Methoxybenzene-sulfate-2 | 22.2 ± 2.1 | 1.5 ± 0.1 | * |
| Hydroxy-methoxybenzene-sulfate-1 | 15.3 ± 0.8 | 4.5 ± 0.7 | * |
| Hydroxy-methoxybenzene-sulfate −2 | 4.2 ± 0.3 | 1.4 ± 0.2 | * |
| Total hydroxybenzenes | 80.6 ± 7.5 | 33.2 ± 3.3 | * |
| Hippuric acids (4) | |||
| Hippuric acid | 366 ± 28 | 238 ± 13 | ns |
| 3′-Hydroxyhippuric acid | 17.3 ± 2.3 | 0.90 ± 0.04 | * |
| 4′-Hydroxyhippuric acid | 19.9 ± 1.4 | 12.3 ± 0.5 | ns |
| Total hippuric acids | 403 ± 32 | 252 ± 14 | ns |
| Total phenolics | 609 ± 51 | 394 ± 27 | * |
Asterisks denote a statistically significant difference among two groups of subjects.
*p value < 0.05; ns – not statistically significant; n.d. not detected.
Figures in italicized parentheses are the total number of phenolics detected in each category including those excreted in sub-μmol amounts that were not statistically different. The total quantities of phenolics excreted in each phenolic category are presented in italics.
Data expressed as mean values in μmol ± SE.
Hippuric acid 26 was by far the major aromatic detected in the study with 366 ± 28 μmol for participants with a colon and 238 ± 13 μmol excreted by the ileostomists. The two levels of excretion are not statistically different because of large differences in the amounts excreted by individual subjects. Hippuric acid dominated accounting on average for 60% of the total excreted phenolics for both volunteer categories. This implies substantial production of hippuric acid, from surplus phenylalanine and/or tyrosine, and to a lesser degree because of their lower levels, from catecholamines, via endogenous routes independent of the colonic microbiota. Potential intermediates in these conversions, such as benzoic acid 30, were present in much lower levels, presumably as a result of being rapidly turned over and converted to hippuric acid. It is of note, however, that there were similar levels of excretion by volunteers with an intact colon and ileostomists of 2R/S-hydroxy-2-(4′-hydroxyphenyl)acetic acid 7 (13.4 μmol vs 10.8 μmol), 2R/S-hydroxy-2-(4′-hydroxy-3′-methoxyphenyl)acetic acid 42 (19.3 μmol vs 17.8 μmol), and 3′,4′-dihydroxyphenylacetic acid 28 (10.5 μmol vs 12.6 μmol) [39], all known metabolites of tyrosine 2 [88,89].
Eleven phenolics were excreted in significantly larger amounts by subjects with a colon than by ileostomists (Table 2). Among the compounds in this category were two phenylhydracrylic acids, two benzoic acid derivatives, four sulfo-benzene conjugates and 3′-hydroxyhippuric acid 43. The sulfo-benzene metabolites were the largest group of compounds in this category. These phenolics are likely to be produced principally from tyrosine and phenylalanine in the low (poly)phenol diet which will also contain proteins that are broken down in the small intestine by the action of chymotrypsin, trypsin and releasing further quantities of the two aromatic amino acids.
It is evident that the metabolic pathways associated with phenylalanine, tyrosine and catecholamines are complex and overlap with the pathways originating from most dietary (poly)phenols and to disentangle them is often difficult/impossible. It is, for instance, not possible, absolutely, to determine the origin of many of the “background” phenolics in Table 2. For example, as noted above, phenolics of tyrosine/catecholamine metabolism, such as 3′,4′-dihydroxyphenylacetic acid 28 and 4′-hydroxy-3′-methoxyphenylacetic acid (44, aka homovanillic acid) [96] are also colonic catabolites of quercetin 41 [2].
Yet further complexities arise with the microbiota-mediated aliphatic side chain shift of certain 3′- and 4′-hydroxy C6–C3 metabolites [90]. This overlaps with 3′-hydroxyphenyl catabolites produced by the gut microbiota 4′-dehydroxylation of 3′,4′-dihydroxy C6–C3 substrates which frequently dominate phenolics derived from dietary (poly)phenols. Clearly, excluding these shared metabolites will under-estimate the true yield from the test meal/beverage (poly)phenols, but including them will lead to an over-estimate of the yield. However, if such a metabolite is bioactive and has some potentially beneficial effect does it matter whether it is derived from amino acid or the catecholamines rather than dietary (poly)phenols?
In most instances it is difficult/impossible to determine to what extent bioactive phenolics are derived from dietary (poly)phenols or alternative sources. However, plasma pharmacokinetic profiles can be of assistance. For instance, the profile of hippuric acid 26 in Fig. 3 is in keeping with most of the glycinated benzoic acid being derived from phenylalanine and tyrosine released from breakdown of endogenous proteins. Information on plasma profiles obtained with ileostomists could also be of value. In addition, more clarity will become apparent when, as discussed in Section 4, the ingested dietary supplement contains (poly)phenols that yield metabolites that have never been reported in studies of amino acid or catecholamine metabolism. These are of particular interest because any physiological effect associated with them can be more readily traced back to the test meal/beverage.
7. (Poly)phenol bioavailability studies using isotopically-labelled substrates
The use of isotopically-labelled (poly)phenol substrates in bioavailability feeding studies is the gold standard to discriminate between phenolics derived from (poly)phenolics in the colon and unlabelled products in biofluids originating from surplus amino acids, catecholamines and other sources. However, this approach has rarely been used. There are only two human (poly)phenol feeding studies reported between 2012 and 2016 that have employed such methodology, one with 14C- labelled (−)-epicatechin 10 [51,52], and the other with 13C-labelled cyanidin-3-O-glucoside 45 [74,97]. In part, this is likely to be because of the difficulties in obtaining appropriately labelled substrates, that have been approved for human consumption. Obtaining ethical permission for investigations with radiolabeled substrates is not straight forward and when it is obtained rigorous conditions are applied under which the feeding studies can be carried out, and this is reflected in the incumbent associated costs. For instance, after the intake of 14C-labelled (−)-epicatechin individual participants were only allowed to be discharged from the research facility after 6–9 days, when radioactivity excreted in urine and voided feces over 24 h was <1% of the administered dose [51].
7.1. [2–14C1](−)-Epicatechin
A feeding study reported by Ottaviani et al. [51] utilized radiolabelled (−)-epicatechin. Eight male subjects ingested 300 μCi (270 μmol) of [2–14C1](−)-epicatechin ([2–14C]EC) after which radioactivity in blood, urine and feces, collected at regular intervals over a period of up to 8 days, were initially monitored using liquid scintillation counting. This revealed an almost total recovery of radioactivity in 0–48 h urine (82 ± 5%) and feces (12 ± 3%) indicating minimal long term tissue deposition of the compounds derived from the ingested flavan-3-ol monomer. Urine collected from participants post-48 h contained negligible amounts of radioactivity demonstrating almost complete 0–48 h washout of the [2–14C]EC and its derived products. Radioactivity in feces was voided in a more irregular manner and over a longer 5-day period without a concomitant appearance of radioactivity in plasma or urine, arguably as a consequence of the 14C-labelled catabolites being bound to fecal material in the colon. This clearly shows that washout metabolites from tissues via urine did not extend beyond 48 h. However, it cannot be assumed that also applies when (poly)phenols are ingested along with more complex food matrices.
HPLC-MS-MS detected 12 glucuronide, sulfate and methylated metabolites, referred to as structurally related (−)-epicatechin metabolites (SREMs), in plasma [51,52]. After attaining an overall Cmax of 1223 nmol/L with a Tmax of ca. 1.0 h after intake of [2–14C]EC), the SREMs declined rapidly with an apparent elimination half-life (AT1/2) of 1.9 h and, in almost all instances, had disappeared from the circulatory system within 8 h. The Tmax is indicative of absorption in the upper GI tract (see Fig. 2). A series of microbiota-derived C6–C5 ring fission catabolites (5C-RFCs), with a summed Cmax of 588 nmol/L appeared in plasma with Tmax of ca. 6 h. They were present in the circulatory systems for longer than the SREMs having an AT1/2 of 5.7 h. The main 5C-RFC was 4R/S-5-(4′-hydroxyphenyl)-γ-valerolactone-3′-sulfate 8 (see Fig. 2) along with lower concentrations of other valerolactones and phenylvaleric acids such as 4R/S-hydroxy-5-(3′-hydroxyphenyl)valeric acid-4′-glucuronide 9.
Using HPLC-MS-MS in conjunction with an on-line radioactivity detector [96] enabled 14C-SREMs and 5C-RFMs, along with 14C-labelled hippuric acids and low molecular weight phenolic acid catabolites, to be monitored in 0–48 h urine and feces. Urinary metabolites consisted of SREMs (20%), 5C-RFCs (42%), two and three carbon side chain-RFCs (7%) and hippuric acid (13%) together with 3′-hydroxyhippuric acid 43 (8%) along with a number of minor, unidentified radiolabelled products [51,52]. Hence, 70% of the ingested radiolabelled (−)-epicatechin was converted to microbiota-derived catabolites. The radioactivity in 0–48 h feces was associated predominantly with 4R/S-hydroxy-5-(phenyl)valeric acids and a smaller amount of 3-(3′-hydroxyphenyl)propanoic acid 34.
Proposed pathways, via which [2–14C]EC entering the colon is subject to a microbiota-mediated catabolism, a well as step involving human enzymes, that lead to benzoic acids which are subjected to hepatic glycination and converted to hippuric acids, are summarised in Fig. 8. The high recovery of radioactivity originating from the ingested [2–14C]EC demonstrates that benzoic acids are metabolized primarily to hippuric acids, with very limited, if any conversion to C6–C0 benzene derivatives by the colonic microbiota, as this would have result in the loss of the14C label with removal of the carboxyl group.
Fig. 8.
Proposed routes for the fate of [2–14C](−)-epicatechin passing from the small to the large intestine. Red arrows indicate microbiota-mediated conversions, and blue arrows steps catalysed by mammalian enzymes in colonocytes and/or hepatocytes. Asterisks indicate proposed intermediates that do not accumulate in detectable quantities. Detected in plasma (P), urine (U) and feces (F). The red circles indicates the position of 14C-label. Fine arrows are potential minor routes. Based on the data of Ottaviani et al. [51] and Borges et al. [52]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
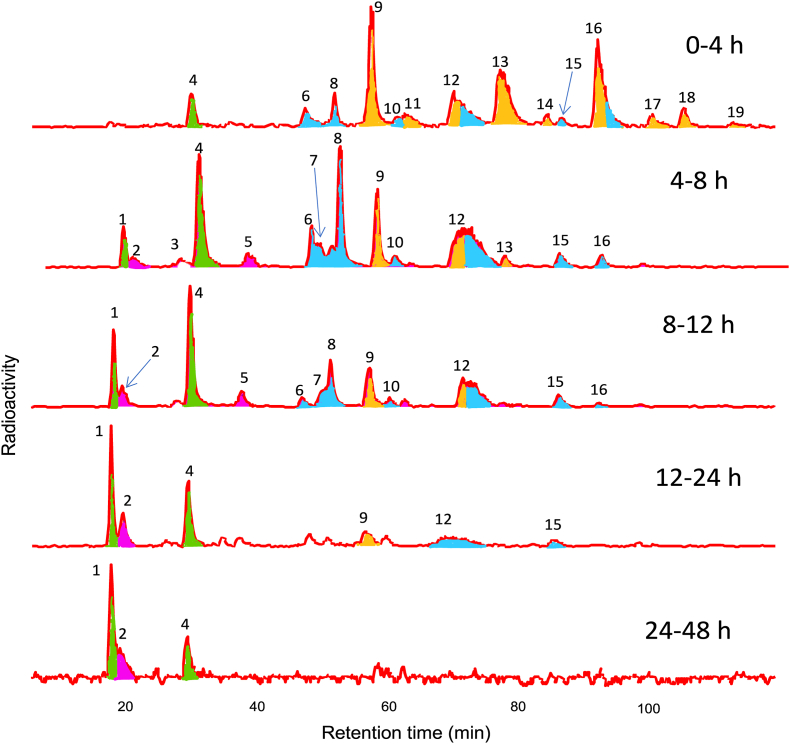
The use of [2–14C]EC had a specific activity of 14.5 μCi μmol−1 was invaluable in monitoring the overall levels of radioactivity in body fluids after supplementation, and in following the diversity of metabolites and catabolites in urine as shown in the HPLC radioactivity traces illustrated in Fig. 9. However, it was not possible to distinguish the 14C molecular ion from the much bigger 12C ion by MS because of the low intensity signal of the 14C+2 ion. This was due to i) only one of the fifteen EC carbons being labelled with 14C and ii) with a specific activity of 14.5 μCi μmol−1, the C-2 position contained both 12C- and 14C molecules with 12C predominating, comprising >90% of the mixture. As a consequence MS identifications of metabolites in radioactive HPLC peaks were based on co-chromatography with unlabelled 12C standards with the level of radioactivity in peaks facilitating accurate quantification. A further limitation is that because of the low intermittent rate of decay of the 14C label, HPLC radioactivity detectors require at least a ca. 10 s time constant to achieve useable signal/noise ratios [98]. This results in peak tailing which impacts adversely on chromatographic resolution, necessitating the use of a long shallow HPLC mobile phase gradient, such as that used in Fig. 9. Of necessity this precludes the use of rapid UHPLC. However, employing extensively labelled 13C instead of 14C-substrates, enables 13C ions to be readily distinguished from 12C fragments by MS, sacrificing the need for a detector time constant, and thus facilitating the use of rapid analysis UHPLC.
Fig. 9.
Gradient elution reversed phase HPLC with an on-line radioactivity monitor. Analysis of urine collected 0–4, 4–8, 8–12, 12–24 and 24–48 h after the ingestion of [2–14C](−)-epicatechin by one subject. Peak identifications: (1) 3′-hydroxyhippuric acid, (2) 3-(3′,4′-dihydroxyphenyl)propionic acid, (3) unknown, 4) hippuric acid, 5) a hydroxyphenylacetic acid-sulfate, 6) a 4R/S-5-(phenyl)-γ-valerolactone-sulfo-glucuronide, (7) 4R/S-hydroxy-5-(3′-hydroxyphenyl)valeric acid-4′-sulfate, (8) 4R/S-hydroxy-5-(4′-hydroxyphenyl)valeric acid-3′-sulfate, (9) (−)-epictechin-3′-glucuronide, (10) a 4R/S-hydroxy-5-(phenyl)valeric acid-sulfate, (11) (−)-epictechin-5-sulfate, (12) an (−)-epicatechin-sulfo-glucuronide, and 4R/S-5-(4′-hydroxyphenyl)-γ-valerolactone-3′-sulfate, (13) (−)-epictechin-3′-sulfate, (14) 3′-methoxy-(−)-epicatechin-4′-sulfate, (15) unknown, (16) 3′-methoxy-(−)-epicatechin-5-sulfate and 4R/S-5-(3′-methoxyphenyl)-γ-valerolactone-4′-sulfate, (17–19) methoxy-(−)-epicatechin-sulfates. Peak colors: orange – structurally-related epicatechin metabolites, blue – C6–C5 catabolites, red – C6–C2/3 catabolites, green – hippuric acids. Based on data of Ottaviani et al. [51] and Borges et al. [52]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
7.2. [6,8,10,3′,5′-13C5]Cyanidin-3-O-glucoside
A13C-labelled anthocyanin, namely [6,8,10,3′,5′-13C5]cyanidin-3-O-glucoside, was synthesized by Zhang et al. [99]. The anthocyanin had a 99% 13C enrichment at each of the labelled positions, and as three 13C molecules were incorporated into the A-ring and two into the B-ring (Fig. 10) it was possible with MS detection to ascertain whether catabolites were derived from the A- or B-ring of cyanidin.
Fig. 10.
Structure and 13C labelling of [6,8,10,3′,5′-13C5 ]cyanidin-3-O-glucoside [99]. The red circles indicates the positions of 13C-label. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In a bioavailability study reported by Czank et al. [74] and de Ferrars et al. [97], after an overnight fast, 8 participants each ingested 0.5 g (1114 μmol) of the 13C5-anthocyanin. Blood was collected 0, 0.5, 1, 2, 4 6, 24 and 48 h after intake and urine and feces after 0–6, 6–24 and 24–48 h. Labelled CO2 was measured in breath collected after 0, 1, 2,4, 6, 24 and 48 h. Measuring overall levels of recovered 13C was not as straight forward as monitoring 14C with liquid scintillating counting [51,52], and necessitated the use of isotopic ratio-mass spectrometry as described by Morrison et al. [[100], [101]]. This showed mean recoveries of 13C to be 5.4 ± 0.7% in urine, 6.9 ± 1.6% in breath and 32 ± 6% in feces which gives a relative bioavailability of ≥12.3 ± 1.4% calculated from the combined elimination in urine and breath. The metabolite profile of the 13C-anthocyanin is, therefore, very different to that of [2–14C]EC where there was an 82% recovery of the 14C-label in urine and 12% in feces (51,52).
An array of 13C-labelled catabolites was detected in serum, urine and feces after intake of the [13C]cyanidin-3-O-glucoside. These are summarised in Table 3, Table 4 where Kay et al. nomenclature [40] replaces synonyms used by de Ferrars et al. [97]. In addition, estimates of compounds in urine and feces that were originally given in μg have been converted to μmol values. All but three of the >20 catabolites detected were 13C2 rather than 13C3-labelled compounds indicating that they originated from the B-ring of cyanidin-3-O-glucoside.
Table 3.
Recovery of13C-labelled anthocyanins and phenolic catabolites in plasma and urine collected 0–48 h after the ingestion of 1114 μmol13C5-cyanidin-3-O-glucoside by 8 subjects. Figures in italicized parentheses indicate the number of volunteers in which individual compounds were detected. Based on the data of de Ferrars et al. [97].
| 13C-Labelled anthocyanins and phenolic catabolites | Plasma |
Urine |
||
|---|---|---|---|---|
| Tmax (h) | Cmax (nmol/L) | Peak excretion (h) | Total 0–48 h recovery (μmol) | |
| 13C5-Anthocyanins | ||||
| Cyanidin-3-O-glucoside | 1.8 | 141 ± 70 (5) | 1–2 | 0.24 ± 0.11 (7) |
| Cyanidin-glucuronidea | – | – | 1–2 | 0.07 ± 0.03 (5) |
| Peonidin-3-O-glucoside | – | – | 1–2 | 0.05 ± 0.02 (7) |
| Methoxycyanidin-glucuronideb | – | – | 1–2 | 0.004 ± 0.002 (4) |
| Methoxycyanidin-3-O-glucoside-glucuronide | – | – | 1–2 | 0.11 ± 0.05 (5) |
| Total anthocyanins | 0.47 | |||
| 13C2-cinnamic and phenylacetic acids | ||||
| 4′-Hydroxy-3′-methoxycinnamic acid | 8.2 | 827 ± 371 (7) | 24–48 | 4.1 ± 1.5 (8) |
| 4′-Hydroxyphenylacetic acid | – | – | 4–5 | 0.33 ± 0.13 (3) |
| 3′,4′-Dihydroxyphenylacetic acid | – | – | 24–48 | 0.18 (1) |
| Total | 4.6 | |||
| 13C2-benzoic acids and benzaldehydes | ||||
| 3,4-Dihydroxybenzoic acid | 3.3 | 146 ± 74 (8) | 1–2 | 0.46 ± 0.13 (8) |
| 3/4-Hydroxybenzoic acids | – | – | 1–2 | 0.09 ± 0.03 (5) |
| 4-Hydroxy-3-methoxybenzoic acid | 12.5 | 1845 ± 838 (2) | 1–2 | 5.6 ± 2.1 (4) |
| 3-Hydroxy-4-methoxybenzoic acid | 2.0 | 195 (1) | 1–2 | 0.47 ± 0.32 (4) |
| 3,4-Dihydroxy-methoxybenzoic acid | 8.4 | 12 ± 5 (8) | 3–4 | 0.11 ± 0.04 (8) |
| Benzoic acid-4-glucuronide | 10.9 | 74 ± 20 (7) | 4–5 | 0.17 ± 0.06 (7) |
| 3-Hydroxybenzoic acid-4-glucuronide | 3.8 | 68 ± 61 (8) | 1–2 | 0.30 ± 0.12 (8) |
| 4-Hydroxybenzoic acid-3-glucuronide | 2.7 | 11 ± 3 (5) | 1–2 | 1.8 ± 0.1 (8) |
| 3-Hydroxybenzoic acid-4-sulfate | 11.4 | 157 ± 116 (8) | 1–2 | 2.1 ± 0.8 (8) |
| 4-Hydroxybenzoic acid-3-sulfate | 1–2 | 1.4 ± 0.4 (8) | ||
| 3-Methoxybenzoic acid-4-glucuronide | 4.8 | 24 ± 4 (8) | 4–5 | 1.8 ± 0.3 (8) |
| 3-Methoxybenzoic acid-4-sulfate | 30 | 430 ± 299 (4) | 3–4 | 1.8 ± 0.4 (7) |
| 4-Methoxybenzoic acid-3-glucuronide | 4.3 | 35 ± 5 (8) | 5–6 | 1.5 ± 0.3 (8) |
| 4-Methoxybenzoic acid-3-sulfate | – | – | 3–4 | 0.74 ± 0.37 (5) |
| 4-Hydroxybenzaldehyde | 5.6 | 667 ± 653 (7) | 5–6 | 0.08 ± 0.07 (2) |
| 3,4-Dihydroxybenzaldehyde | – | – | 0–1 | 0.05 ± 0.02 (6) |
| Total benzoic acid and benzaldehydes | 18.5 | |||
| 13C2-hippuric acid | ||||
| Hippuric acid | 15.7 | 1962 ± 1389 (8) | 6–24 | 13.3 ± 1.2 (8) |
| Total | 13.3 (1.2% recovery) | |||
| 13C3-Catabolites | ||||
| 4′-Hydroxy-3′-methoxycinnamic acid | 13.3 | 87 ± 38 (6) | 0–1 | 1.1 ± 0.9 (8) |
| 2-Hydroxy-4-methoxybenzoic acid | – | – | 3–4 | 0.38 (1) |
| 2,4,6-Trihydroxybenzaldehyde | 2.8 | 582 ± 536 (4) | 6–24 | 0.42 ± 0.13 (8) |
| Total | – | – | – | 1.5 |
| Total recovery of anthocyanins and phenolic catabolites | – | – | – | 38.4 μmol, 3.4% of intake |
Two isomers.
Three isomers, – not detect/not estimated.
Table 4.
Recovery of13C-labelled anthocyanins and phenolic catabolites in feces collected 0–48 h after the ingestion of 1114 μmol13C5-cyanidin-3-O-glucoside by 8 subjects. Figures in italicized parentheses indicate the number of volunteers in which individual compounds were detected. Based on data of de Ferrars et al. [97].
| 13C-Labelled anthocyanins and phenolic catabolites | Total recovery (μmol) |
|---|---|
| 13C5-Anthocyanins | |
| Cyanidin-3-O-glucoside | 0.82 (1) |
| Total | 0.82 |
| 13C2-cinnamic and phenylacetic acids | |
| 3′,4′,-Dihydroxycinnamic acid | 2.1 (2) |
| 4′-Hydroxy-3′-methoxycinnamic acid | 19.5 (6) |
| 3′,4′-Dihydroxyphenylacetic acid | 0.14 (5) |
| 4′-Hydroxyphenylacetic acid | 0.41 (1) |
| Total | 22.2 |
| 13C2-Benzoic acids and benzaldehydes | |
| 3,4-Dihydroxybenzoic acid | 3.7 (7) |
| 2,3-Dihydroxybenzoic acid | 2.8 (2) |
| Hydroxybenzoic acid | 0.12 (3) |
| 2-Hydroxy-4-methoxybenzoic acid | 1.6 (2) |
| 4-Hydroxy-3-methoxybenzoic acid | 0.61 (3) |
| 3-Hydroxy-4-methoxybenzoic acid | 0.12 (1) |
| 3,4-Dihydroxy-methoxybenzoic acid | 1.4 (6) |
| Dimethoxybenzoic acid | 0.19 (1) |
| Benzoic acid-4-glucuronide | 0.07 (8) |
| 3-Hydroxybenzoic acid-4-glucuronide | 0.11 (1) |
| 4-Hydroxybenzoic acid-3-glucuronide | 0.22 (3) |
| 3-Hydroxybenzoic acid-4-sulfate | 0.11 (3) |
| 4-Hydroxybenzoic acid-3-sulfate | 0.18 (4) |
| 3-Methoxybenzoic acid-4-glucuronide | 0.01 (3) |
| 3-Methoxybenzoic acid-4-sulfate | 0.04 (3) |
| 4-Methoxybenzoic acid-3-glucuronide | 0.04 (3) |
| 4-Methoxybenzoic acid-3-sulfate | 0.71 (2) |
| 3,4-Dihydroxybenzaldehyde | 0.06 (6) |
| 4-Hydroxybenzaldehyde | 0.01 (2) |
| 4-Methoxybenzaldehyde | 0.15 (1) |
| Total | 12.2 |
| 13C2-Hippuric acid | |
| Hippuric acid | 0.22 (3) |
| Total | 0.22 |
| 13C3-Catabolites | |
| 4′-Hydroxy-3′-methoxycinnamic acid | 3.6 (5) |
| 2,4,6-Trihydroxybenzaldehyde | 0.70 (8) |
| Total | 4.3 |
| Total recovery of anthocyanins and phenolic catabolites | 39.7 μmol, 3.5% of intake |
While there were some similarities, many more urinary phenolics were detected in the [13C5]cyanidin-3-O-glucoside study than in the raspberry cyanidin-3-O-sophoroside feed by Ludwig et al. [60]. However, a number of 13C-catabolites were detected in relatively small quantities in samples from ≤4 of the 8 subjects who participated in the study (Table 3, Table 4). Furthermore, there were much more marked volunteer-to-volunteer variations in the 13C-labelled study than in the study in which raspberries were ingested [60]. There were also quantitative and qualitative differences in the catabolic profiles, such as the more complex excretion of 13C2-benzoic acid conjugates. These differences could be a consequence of the ingestion of the 1114 μmol bolus of [13C5]cyanidin-3-O-glucoside compared with the 292 μmol of anthocyanins that were ingested in a raspberry matrix, Arguably, the high 13C dose exaggerated both the inherent instability of the cyanidin moiety [102] and volunteer differences in the capacity of their colonic microbiota to catabolise the anthocyanin.
7.3. Excretion of 14C-hippuric acid derived from [14C]-(−)-epicatechin and background [12C]-hippuric acid
The 0–12 h urinary excretion of unlabelled 12C-hippuric acid 26 by subjects with a full GI tract over a 12 h fasting period, after prior adherence to a low (poly)phenol diet for 36 h, was 366 ± 28 μmol (Table 2) [39]. Excretion of [14C]hippuric acid over a similar period, after ingestion of 207 μmol [14C](−)-epicatechin, was 13.4 ± 2.9 μmol [52] which corresponds to 7.2% of intake. The size of the endogenous hippuric acid pool was not determined in the 14C-investigation. However, using the 366 ± 28 μmol in Table 2 as a guide, indicates that the combined [12C]- and [14C]-hippuric acid pool was 379 μmol of which the radiolabelled flavan-3-ol metabolite was a minor contributor at 3.5% (Table 5). The vast majority of the hippuric acid, and presumably many of the related phenolic acids, therefore, appear to originate predominantly from other sources with the available evidence pointing principally to phenylalanine and tyrosine. This would also appear to be the case in the [13C5]cyanidin-3-O-glucoside study [74,97] where analysis of 0–48 h urine indicated a 1.2% conversion to [13C2]hippuric acid (Table 5).
Table 5.
Twelve-hour urinary excretion of12C-hippuric acid by subjects on a low (poly)phenol diet [12] compared with excretion of14C-hippuric acid following the ingestion of 207 μmol [14C](−)-epicatechin [51,52]. Subjects in both groups have a full gastrointestinal tract.
| Source | 0–12 h urinary excretion |
|---|---|
| Baseline with colon | 12C-hippuric acid: 366 ± 28 μmol |
| 207 μmol [14C]-(−)-Epicatechin |
14C-hippuric acid:13.4 ± 2.9 μmol (7.2% of intake) Total12/14C-hippuric acid 379 μmol (14C - 3.5%) |
8. Summary
There are number of methods for obtaining information to help distinguish between i) phenolics derived from microbiota-mediated breakdown of dietary (poly)phenols, and ii) the large quantities of phenolics originating from other sources which involve the aromatic amino acids phenylalanine 1 and tyrosine 2, and to a lesser extent catecholamines. Feeding studies using isotopically-labelled substrates remain the gold standard but for a several reasons, principally cost and the difficulties in obtaining ethical permission, they have been used only rarely. Investigations involving the ingestion of plant-derived products containing (poly)phenols, such as ellagitannins, flavan-3-ols, isoflavones, and lignans, have the advantage of being converted by microbiota to phenolic catabolites that have never been reported as products of amino acid metabolism. Nevertheless, investigations involving other (poly)phenols, and designed to at least partially disentangle the production of phenolic catabolites from amino acids and dietary (poly)phenols, are to be encouraged.
Whatever in vitro or in vivo test system is used, UPLC-HR-MS and UHPLC-QQQ-MS are now the analytical methods of choice. Accurate qualitative and quantitative estimates require the use of standards of phenolics and their phase II metabolites. Although there are methods for the synthesis of relevant compounds [103,104], and many are becoming available from commercial sources, because of their number it is all but impossible for investigators to obtain a full complement of the standards that are required. In the circumstances, information should use validated analytical methodology [43,[105], [106], [107]], and the basis of identifications and quantifications should be categorized according to the Metabolic Standards Initiative Metabolite Identification levels of Sumner et al. [108].
Finally, it is becoming apparent that phenylalanine and tyrosine, originating from both turnover of endogenous proteins and dietary sources, can make a substantial contribution to the pools of low molecular weight phenolic catabolites. The potential impact on health of catabolites derived from the various sources of the aromatic amino acids requires further detailed investigation, including updated information on their metabolism and bioactivity. However, any protective effects from this source will be supplemented by phenolic catabolites generated from regular intake of plant-based foods and beverages that are rich in a diversity of (poly)phenols as well as phenylalanine and tyrosine. Clarifying the contributions to the pools of circulating bioactive phenolic catabolites is essential for future, informed dietary health recommendations.
Contributions
M.N.C and A.C. drafted the text, with assistance from P.M. and I.A.L., and monitored edits from the other authors who also provided additional data, helped prepare figures and approved the final text.
Funding
A.C., G.P.-C., G. I.R.G, and T.M.A. were funded by the Distinguished Scientist Fellowship Program (DSFP) of King Saud University, Riyadh, Saudi Arabia. I.A.L. was supported by the Gobierno de Navarra: Grant no. 0011-3947-2021-000034. P.M. received funding from the European Union's Horizon 2020 Research and Innovation Programme: Grant no. 950050; and the National Recovery and Resilience Plan of the Italian Ministry of University and Research and the European Union: Project code PE00000003.
CRediT authorship contribution statement
Michael N. Clifford: Conceptualization, Writing – original draft. Iziar A. Ludwig: Writing – review & editing. Gema Pereira-Caro: Formal analysis, Writing – review & editing. Laila Zeraik: Writing – review & editing. Gina Borges: Conceptualization, Writing – review & editing. Tahani M. Almutairi: Conceptualization. Sara Dobani: Writing – review & editing. Letizia Bresciani: Writing – review & editing. Pedro Mena: Conceptualization, Writing – review & editing. Chris I.R. Gill: Writing – review & editing. Alan Crozier: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103068.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Williamson G., Kay C.D., Crozier A. The bioavailability, transport and bioactivity of dietary flavonoids: a review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018;17:1054–1112. doi: 10.1111/1541-4337.12351. [DOI] [PubMed] [Google Scholar]
- 2.Jaganath I.B., Mullen W., Edwards C.A., Crozier A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006;40:1035–1046. doi: 10.1080/10715760600771400. [DOI] [PubMed] [Google Scholar]
- 3.Borges G., Mullen W., Mullan A., Lean M.E.J., Roberts S.A., et al. Bioavailability of multiple components following acute ingestion of a polyphenol-rich drink. Mol. Nutr. Food Res. 2011;54(Suppl. 2):S268–S277. doi: 10.1002/mnfr.200900611. [DOI] [PubMed] [Google Scholar]
- 4.Stalmach A., Mullen W., Steiling H., Williamson G., et al. Absorption, metabolism, efflux and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010;54:323–334. doi: 10.1002/mnfr.200900194. [DOI] [PubMed] [Google Scholar]
- 5.Stalmach A., Clifford H.M.N., Williamson G., Crozier A. In: Teas, Cocoa and Coffee: Plant Secondary Metabolites and Health. Crozier A., Ashihara H., Tomás-Barbéran F., editors. Blackwell Publishing; Oxford: 2012. Phytochemicals in coffee and the bioavailability of chlorogenic acids; pp. 143–168. ISBN-13: 978-1-4443-3441-8. [Google Scholar]
- 6.Kahle K., Kempf M., Schreiber P., Scheppach W., et al. Intestinal transit and systematic metabolism of apple polyphenols. Eur. J. Nutr. 2011;50:507–522. doi: 10.1007/s00394-010-0157-0. [DOI] [PubMed] [Google Scholar]
- 7.Stalmach A., Edwards C.A., Wightman J., Crozier A. Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol. Nutr. Food Res. 2012;56:497–509. doi: 10.1002/mnfr.201100566. [DOI] [PubMed] [Google Scholar]
- 8.Domínguez-Fernández M., Young P., Yang T., Ludwig I.A., et al. In vivo study of the bioavailability and metabolic profile of (poly) phenols after sous-vide artichoke consumption. Food Chem. 2022;367 doi: 10.1016/j.foodchem.2021.130620. [DOI] [PubMed] [Google Scholar]
- 9.Williamson G., Clifford M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017;139:24–38. doi: 10.1016/j.bcp.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Visvanathan R., Williamson G. Review of factors affecting citrus polyphenol bioavailability and their importance in designing in vitro animal and intervention studies. Compr. Rev. Food Sci. Food Saf. 2022;21:4509–4545. doi: 10.1111/1541-4337.13057. [DOI] [PubMed] [Google Scholar]
- 11.Ottaviani J.I., Britten A., Lucarelli D., Luben R., et al. Biomarker-estimated flavan-3-ol intake is associated with lower blood pressure in cross-sectional analysis in EPIC Norfolk. Sci. Rep. 2020;10:7964. doi: 10.1038/s41598-020-74863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke D D., Rollo M.E., Collins C.E., Wood L., et al. The relationship between dietary polyphenol intakes and urinary polyphenol concentrations in adults prescribed a high vegetable and fruit diet. Nutrients. 2020;12:3431. doi: 10.3390/nu12113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y., Li Y., Ma, X X., Alotaibi W., et al. Comparison between dietary assessment methods and biomarkers in estimating dietary (poly)phenol intake. Food Funct. 2023;14:1369–1386. doi: 10.1039/D2FO02755K. [DOI] [PubMed] [Google Scholar]
- 14.Angelino D., Caffrey A., McNulty H., Gill C.I.R., et al. Association of dietary flavan-3-ol intakes with plasma phenyl-γ-valerolactones: analysis from the TUDA cohort of healthy older adults. Am. J. Clin. Nutr. 2023;118:476–484. doi: 10.1016/j.ajcnut.2023.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy A., Mukamai K.J., Liu L., Franz M., et al. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127:188–196. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., et al. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brickman A.M., Khan U.A., Provenzano F.A., Yeung L.K., et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in old adults. Nat. Neurosci. 2014;17 doi: 10.1038/nn.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Mateos A.M., Vauzour D., Krueger C.G., Shanmuganayagam D., et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch. Toxicol. 2014;88:1803. doi: 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- 19.Sloan R.P., Wall M., Yeung L.-K., Feng T., et al. Insights into the role of diet and dietary flavanols in cognitive ageing: results of a randomised controlled trial. Sci. Rep. 2021;11:3837. doi: 10.1038/s41598-021-83370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sesso H.D., Rist P.M., Aragaki A.K., Rautiainen S., et al. Multivitamins in the prevention of cancer and cardiovascular disease: the COSMOS randomized clinical trial. Am. J. Clin. Nutr. 2022;115:1501–1510. doi: 10.1093/ajcn/nqac05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hidalago M., Martin-Santamaria S., Recio I., Sanchex-Moreno C., de Pascual B., et al. Potential antiflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012;7:295–306. doi: 10.1007/s12263-011-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin H.P., Czank C., Raheem S., Zhang O., et al. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015;59:1095–1106. doi: 10.1002/mnfr.201400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner E.F., Zhang Q., Raheem K.S., O'Hagan D., et al. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J. Nutr. 2016;14:465–473. doi: 10.3945/jn.115.217943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mele L., Carobbio S., Brindani N., Curti C., et al. Phenyl-γ-valerolactones, flavan-3-ol colonic metabolites, protect brown adipocytes from oxidative stress without affecting their differentiation or function. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700074. [DOI] [PubMed] [Google Scholar]
- 27.Van Rymenant E., Grootaert C., Beerens K., Need P.W P.W., et al. Vasorelaxant activity of twenty-one physiologically relevant (poly)phenolic metabolites on isolated mouse arteries. Food Funct. 2017;8:4331–4335. doi: 10.1039/c7fo01273j. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann H., Rogoll D., Scheppach W., Melcher R., Richling E. Ussing type chamber model to study the intestinal transport and modulation of specific tight-junction genes using a colonic cell line. Mol. Nutr. Food Res. 2009;53:1211–1225. doi: 10.1002/mnfr.200800498. [DOI] [PubMed] [Google Scholar]
- 29.Corral-Jara K.F., Nuthikattu S., Rutledge J., Villablanca A., et al. Structurally-related (–)-epicatechin metabolites and gut microbiota derived metabolites exert genomic modifications via VEGF signaling pathways in brain microvascular endothelial cells under lipotoxic conditions: intergated multi-omic study. J. Proteonomics. 2022;263 doi: 10.1002/mnfr.201700074. [DOI] [PubMed] [Google Scholar]
- 30.Xu X., Luo A., Lu X., Liu M., Wang H., et al. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct.Foods. 2021;87 doi: 10.1016/j.jff.104835. [DOI] [Google Scholar]
- 31.Wang Z.Y., Yin Y., Li D.N., Zhao D.Y., Huang J.Q. Biological activities of p-hydroxycinnamic acids in maintaining gut barrier integrity and function. Foods. 2023;12 doi: 10.3390/foods12132636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Sun X., Zhou F., Xiao S., Zhong L., et al. Protocatechuic acid alleviates dextran-sulfate-sodium-induced ulcerative colitis in mice via the regulation of intestinal flora and ferroptosis. Molecules. 2023;28:3775. doi: 10.3390/molecules28093775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown E.M., Latimer C., Allsopp P., Ternan N.G., McMullan G., et al. In vitro and in vivo models of colorectal cancer: antigenotoxic activity of berries. J. Agric. Food Chem. 2014;62:3852–3866. doi: 10.1021/jf4050759. [DOI] [PubMed] [Google Scholar]
- 34.Dobani S., Latimer C., McDougall G.J., Allwood J.W., et al. Ex-vivo fecal fermentation of human ileal fluid collected after raspberry consumption modifies (poly)phenolics and modulates genoprotective effects in colonic epithelial cells. Redox Biol. 2021;40 doi: 10.1016/j.redox.2021.101862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubert J., Gatto P., Pancher M., Sidarovich V., et al. A screening of native (poly)phenols and gut-related metabolites on 3D HCT116 spheroids reveals gut health benefits of a flavan-3-ol metabolite. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verzelloni E., Pellacani C., Tagliazucchi D., Tagliaferri S., et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011;55(Suppl. 1) doi: 10.1002/mnfr.201000525. S35–S43. [DOI] [PubMed] [Google Scholar]
- 37.Figueira I., Garcia G., Pimpão R.C., Terrasso A.P., Costa I., et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell W., Duncan S.H., Scobbie L., Duncan G., et al. Major phenylpropanoid-derived metabolites in the human gut can arise from the microbial fermentation of protein. Mol. Nutr. Food Res. 2013;57:523–535. doi: 10.1002/mnfr.2012200594. [DOI] [PubMed] [Google Scholar]
- 39.Pereira-Caro G., Cáceres-Jimenez S., Bresciani L., Mena P., et al. Excretion by subjects on a low (poly)phenol diet of phenolic gut microbiota catabolites sequestered in tissues or associated with catecholamines and surplus amino acids. Int. J. Food Sci. Nutr. 2023;74:532–543. doi: 10.1080/09637486.2023.2226369. [DOI] [PubMed] [Google Scholar]
- 40.Kay C.D., Clifford M.N., Mena P., McDougall G.J., et al. Recommendations for standardizing nomenclature for dietary (poly)phenol catabolites. Am. J. Clin. Nutr. 2020;112:1051–1068. doi: 10.1093/ajcn/nqaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aura A.-M., Martin-Lopez P., O'Leary K.A., Williamson G., et al. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005;44:133–142. doi: 10.1007/s00394-004-0502-2. [DOI] [PubMed] [Google Scholar]
- 42.Jaganath I.B., Mullen W., Lean M.E.J., Edwards C.A., et al. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites Free Radic. Biol. Med. 2009;47:1180–1189. doi: 10.1016/j.freeradbiomed.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Pereira-Caro G., Ludwig I.A., Polyviou T., Malkova D., et al. Identification of plasma and urinary metabolites and catabolites derived from orange juice (poly)phenols: analysis by high performance liquid chromatography-high resolution-mass spectrometry. J. Agric. Food Chem. 2016;64:5724–5735. doi: 10.1021/acs.jafc.6b02088. [DOI] [PubMed] [Google Scholar]
- 44.Carregosa D., Pinto C., Ávila-Gálvez M.A., Bastos P., et al. A look beyond dietary (poly)phenols: the low molecular weight phenolic metabolites and their concentrations in human circulation. Compr. Rev. Food Sci. Food Saf. 2022;21:3931–3962. doi: 10.1111/1541-4337.13006. [DOI] [PubMed] [Google Scholar]
- 45.Clifford M.N., Kuhnert N. In: Mol. Nutr. Food Res. 66 Special Issue “Poly)phenol Research: Developments and Future Trends”. Mena P., Crozier A., editors. 2022. LC-MS characterisation and quantification of known and unknown (poly)phenol metabolites - possible pitfalls and their avoidance. [DOI] [PubMed] [Google Scholar]
- 46.Kuhnert N., Clifford M.N. In: Mol. Nutr. Food Res. 66, Special Issue “Poly)phenol Research: Developments and Future Trends”. Mena P., Crozier A., editors. 2022. A practioner's dilemma. Mass spectrometry-based annotations and identification of human plasma and urinary polyphenol metabolites. [DOI] [PubMed] [Google Scholar]
- 47.Stalmach A., Mullen W., Barron D., Uchida K., Yokota T., et al. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine following the ingestion of coffee by humans: identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009;37:1759–1768. doi: 10.1124/dmd.109.028019. [DOI] [PubMed] [Google Scholar]
- 48.Di Pede G., Mena P., Bresciani L., Almutairi T.M., et al. Human colonic catabolism of dietary flavan-3-ol bioactives. Mol. Aspect. Med. 2023;89 doi: 10.1016/j.mam.2022.101107. [DOI] [PubMed] [Google Scholar]
- 49.Mena P., Bresciani L., Brindani N., Ludwig I.A., Pereira-Caro G., et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019;36:714–752. doi: 10.1039/C8NP00062J. [DOI] [PubMed] [Google Scholar]
- 50.Clifford M.N., King L.J., Kerimi A., Pereira-Caro M.G., Williamson G. Metabolism of phenolics in coffee and plant-based foods by canonical pathways: an assessment of the role of fatty acid-β-oxidation to generate biologically-active and -inactive intermediates. Crit. Rev. Food Sci. Nutr. 2022 doi: 10.1080/10408398.2022.2131730. [DOI] [PubMed] [Google Scholar]
- 51.Ottaviani J.I., Borges G., Momma T., Spencer J.P.E., et al. Metabolic fate of [2-14C](–)-epicatechin in humans: wider implications for biomedical assessment of efficacy, safety, and mechanisms of action of polyphenol bioactives. Sci. Rep. 2016;6 doi: 10.1038/srep29034. 101038/srep.29034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borges G., Ottaviani J.I., van der Hooft J.J.J., Schroeter H., Crozier A. Absorption, metabolism, distribution, and excretion of (–)-epicatechin: a review of recent findings. Mol. Aspect. Med. 2018;61:18–30. doi: 10.1016/j.mam.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Landberg R., Aman P., Friberg L.E., Vessby B., et al. Dose response of whole-grain biomarkers: alkylresorcinols in human plasma and their metabolites in urine in relation to intake. Am. J. Clin. Nutr. 2009;89:290–296. doi: 10.3945/ajcn.2008.26709. [DOI] [PubMed] [Google Scholar]
- 54.Mullen W., Yokota T., Lean M.E.J., Crozier A. Analysis of elligitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry. 2003;64:617–624. doi: 10.1016/s0031-9422(03)00281-4. [DOI] [PubMed] [Google Scholar]
- 55.Di Pede G., Favari C., Bresciani L L., Almutairi T., et al. In: Berries and Berry Bioactive Compounds in Promoting Health, Food Chemistry, Function and Analysis, No 1. Klimis-Zacas D., Rodrigues-Mateos A., editors. Royal Society of Chemistry; London: 2022. Occurrence, bioavailability and metabolism of berry (poly)phenols; pp. 41–82. 978-1-83916-216-9. [Google Scholar]
- 56.Crozier A., Yokota T., Jaganath I.B., Marks S., et al. In: Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Crozier A., Clifford M.N., Ashihara H., editors. Blackwell Publishing; Oxford: 2006. Secondary metabolites as dietary components in plant-based foods and beverages; pp. 208–302. -13: 978-1-4051-2509-3. [Google Scholar]
- 57.Garcia-Villalba R., Giménez-Bastida J.A., Ávila-Gálvez M.A., Tomás-Barberán F.A., et al. In: Dietary Polyphenols. Metabolism and Health Effects. Tomás-Barberán F.A., González-Sarrías A., Garcia-Villalba R., editors. Wiley Blackwell; Hoboken, NJ: 2021. Ellagitannins and their gut micorbiota-derived metabolites: urolithins; pp. 319–3364. 978-1-111-956-3716. [Google Scholar]
- 58.Tomás-Barberán F.A., González-Sarrías A., García-Villalba R., Núñez-Sánchez M.A., et al. Urolithins, the rescue of “old” metabolites to understand a “new” concept: metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201500901. [DOI] [PubMed] [Google Scholar]
- 59.Cerdá B., Tomás-Barberán F.A., Espín J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J. Agric. Food Chem. 2005;53:227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 60.Ludwig I.A., Mena P., Calani L., Borges G., et al. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015;89:758–769. doi: 10.1016/j.freeradbiomed.2015.10.400. [DOI] [PubMed] [Google Scholar]
- 61.González-Barrio R., Borges G., Mullen W., Crozier A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010;58:3933–3939. doi: 10.1021/jf100315d. [DOI] [PubMed] [Google Scholar]
- 62.Tomás-Barberán F.A., García-Villalba R., González-Sarrías A., Selma M.V., et al. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014;62:6535–6538. doi: 10.1021/jf5024615. [DOI] [PubMed] [Google Scholar]
- 63.Selma M.V., González-Sarrías A., Salas-Salvadó J., Andrés -Lacueva C., et al. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: comparison between nornoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018;37:897–905. doi: 10.1016/j.clnu.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Villalba R., Giménez-Bastida J.A., Cortés-Martin A., Ávila-Gálvez M.A., et al. Urolithins: a comprehensive update of their metabolism, bioactivity and associated gut microbiota. Mol. Nutr. Food Res. 2022 doi: 10.1002/mnfr.202101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassidy A., Peñalvo J., Hollman P. In: Flavonoids and Related Compounds. Bioavailability and Function. Spencer J.P.E., Crozier A., editors. CRC Press; Boca Raton, FL: 2012. Bioavailability of isoflavones in humans; pp. 109–121. 978-1-4398-4826-5. [Google Scholar]
- 66.Aura A.-M. In: Flavonoids and Related Compounds. Bioavailability and Function. Spencer J.P.E., Crozier A., editors. CRC Press; Boca Raton, FL: 2012. Colon-derived microbial metablites of dietary phenolic compounds; pp. 201–231. 978-1-4398-4826-5. [Google Scholar]
- 67.Pereira-Caro G., Borges G., van der Hooft J., Clifford M.N., et al. Orange juice (poly)phenols are highly bioavailable. Am. J. Clin. Nutr. 2014;100:1385–1391. doi: 10.3945/ajcn.114.090282. [DOI] [PubMed] [Google Scholar]
- 68.Stalmach A., Edwards C.A., Wightman J., Crozier A. Colonic catabolism of dietary (poly)phenolic compounds from Concord grapes. Food Funct. 2013;4:52–62. doi: 10.1039/c2fo30151b. 0.1039/c2fo30151b. [DOI] [PubMed] [Google Scholar]
- 69.Pereira-Caro G., Oliver C.M., Weerakkody R., Singh T., et al. Chronic administration of a microencapsulated probiotic enhances bioavailability of orange juice flavanones in humans. Free Radic. Biol. Med. 2015;84:206–214. doi: 10.1016/j.freeradbiomed.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 70.González-Barrio R., Edwards C.A., Crozier A. Colonic catabolism of ellagitannins, ellagic acid and raspberry anthocyanins: in vivo and in vitro studies. Drug Metab. Dispos. 2011;39:1680–1688. doi: 10.1124/dmd.111.039651. [DOI] [PubMed] [Google Scholar]
- 71.Truchado P., Larrosa M., Garcia-Conesa M.T., Cerda B., et al. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J. Agric. Food Chem. 2012;60:5749–5754. doi: 10.1021/jf203641r. [DOI] [PubMed] [Google Scholar]
- 72.Pereira-Caro G., Almutairi T.M., Cáceres-Jiménez S., Moreno-Rojos J.M., et al. Bioavailability of orange juice (poly)phenols: β-glucan-rich oat bran decreases urinary excretion of flavanone phase II metabolites and enhances excretion of microbiota-derived phenolic catabolites. Free Radic. Biol. Med. 2013;199:34–43. doi: 10.1016/j.freeradbiomed.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 73.Jenner A.M., Rafter J., Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic. Biol. Med. 2005;38:763–772. doi: 10.1016/j.freeradbiomed.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 74.Czank C., Cassidy A., Zhang Q., Morrison D.J., et al. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study. Am. J. Clin. Nutr. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 75.Roowi S., Stalmach A., Mullen W., Lean M.E.J., et al. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010;5:1296–1304. doi: 10.1021/jf9032975. [DOI] [PubMed] [Google Scholar]
- 76.Stoupi S., Williamson G., Drynan J.W., Barron D., et al. A comparison of the in vitro biotransformation of (−)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010;54:747–759. doi: 10.1002/mnfr.200900123. [DOI] [PubMed] [Google Scholar]
- 77.Ludwig I.A., de Peña M., Cid C., Crozier A. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors. 2013;39:623–632. doi: 10.1002/biof.112. [DOI] [PubMed] [Google Scholar]
- 78.Pereira-Caro G., Fernández-Quirós B., Ludwig I.A., Pradas I., et al. Catabolism of citrus flavanones by the probiotics Bifidobacterium longum and Lactobacillus rhamnosus. Eur. J. Nutr. 2018;57:231–242. doi: 10.1007/s00394-016-1312-z. [DOI] [PubMed] [Google Scholar]
- 79.Pereira-Caro G., Moreno-Rojas J.M., Brindani N., Del Rio D., et al. Bioavailability of black tea theaflavins in humans: absorption, metabolisms and colonic catabolism. J. Agric. Food Chem. 2017;65:5365–5374. doi: 10.1021/acs.jafc.7b01707. https://doi.org/10.q021/acs.jafc.7b01707. [DOI] [PubMed] [Google Scholar]
- 80.Diotallevi C., Fontana M., Latimer C., Ternan N.G., et al. Ex vivo fecal fermentatation of human ileal fluid collected after wild strawberry consumption modulates human microbiome community structure and metabolic output and protects against DNA damage in colonic epithelial cells. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.2021004005. [DOI] [PubMed] [Google Scholar]
- 81.Di Pede G., Bresciani L., Brighenti F., Clifford M.N., et al. In: Mol. Nutr. Food Res. 66 Special Issue “Poly)phenol Research: Developments and Future Trends”. Mena P., Crozier A., editors. 2022. In vitro faecal fermentation of monomeric and oligomeric flavan-3-ols: catabolic pathways and stoichiometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stalmach A., Edwards C.A., Wightman J.D., Crozier A. Identification of (poly)phenolic compounds in Concord grape juice and their metabolites in human plasma and urine after juice consumption. J. Agric. Food Chem. 2011;59:9512–9522. doi: 10.1021/jf2015039. [DOI] [PubMed] [Google Scholar]
- 83.Ottaviani J.I., Fong R., Borges G., Kimball J., et al. Flavan-3-ol–methylxanthine interactions: modulation of flavan-3-ol bioavailability in volunteers with a functional colon and an ileostomy. Free Radic. Biol. Med. 2023;196:1–8. doi: 10.1016/j.freeradbiomed.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 84.U.S Department of Agriculture . 2019. Agricultural Research Service, FoodData Central. fdc.nal.usda.gov. [Google Scholar]
- 85.Torres N., Tobón-Cornejo S., Velazquez-Villegs L.A., Noriega L.G., et al. Amino acid catabolism: an overlooked area of metabolism. Nutrients. 2023;15:3378. doi: 10.3390/nu15153378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grümer H.D. Formation of hippuric acid from phenylalanine labelled with carbon-14 in phenylketonuric subjects. Nature. 1961;189:63–64. doi: 10.1038/189063a0. [DOI] [PubMed] [Google Scholar]
- 87.Curtois H.C., Vollmin J.A., Baerlocher K. The use of deuterated phenylalanine for the elucidation of the phenylalanine-tyrosine metabolism. Clin. Chim. Acta. 1972;37:277–285. doi: 10.1016/0009-8981(72)90442-1. [DOI] [PubMed] [Google Scholar]
- 88.Curtius H.C., Redweik U., Steinman B., Leimbacher H., et al. In: Proceedings of the Second International Conference on Stable Isotopes. Klein E.R., Klein P.D., editors. Oak Brook; Illinois: 1975. Use of deuterated tyrosine and phenylalanine in studies of catecholamine and aromatic acid metabolism; pp. 385–391. ark:/67531/metadc1445174. [Google Scholar]
- 89.Curtius H.C., Mettler M., Ettlinger L. Study of the intestinal tyrosine metabolism using stable isotopes and gas chromatography–mass spectrometry. J. Chromatogr. A. 1976;12:569–580. doi: 10.1016/s0021-9673(01)84102-9. [DOI] [PubMed] [Google Scholar]
- 90.Fuchs-Mettler M., Curtius H.C., Ettlinger L. A new rearrangement reaction in tyrosine metabolism. Eur. J. Biochem. 1980;108:527–534. doi: 10.1111/j.1432-1033.1980.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 91.Parthasarathy A., Cross P.J., Dobson R.C.J., Adams L.E., et al. A three-ring circus: metabolism of three proteogenic aromatic amino acids and their role in the health of plants and animals. Front. Mol. Biosci. 2018;5:20. doi: 10.3389/fmolb.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alonso R., Gibson C.J., Wurtman R.J., Agharanya J.C., et al. Elevation of urinary catecholamines and their metabolites following tyrosine administration in humans. Biol. Psychiatr. 1982;17:781–790. PMID: 7115832. [PubMed] [Google Scholar]
- 93.Eisenhofer G., Irwin I.J., Goldstein D.S. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 94.Olguín H.J., Guzmán D.C., Hernández García E., Barragán Mejía G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/9730467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davidson D.F. Elevated urinary dopamine in adults. Ann. Clin. Biochem. 2005;42:200–207. doi: 10.1258/0004563053857851. [DOI] [PubMed] [Google Scholar]
- 96.Combet E., Lean M.E.J., Boyle J.G., Crozier A., et al. Dietary flavonols contribute false-positive elevation of homovanillic acid, a marker of catecholamine-secreting tumours. Clin. Chim. Acta. 2011;412:165–169. doi: 10.1016/j.cca.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 97.De Ferrars R.M., Czank C., Zhang Q., Botting N.P., et al. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014;171 doi: 10.1111/bph.12676. 3268–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reeve D.R., Crozier A. Radioactivity monitor for high performance liquid chromatography. J. Chromatogr. 1977;137:271–281. doi: 10.1016/S0021-9673(00)81350-3. [DOI] [Google Scholar]
- 99.Zhang Q., Botting N.P., Kay C. A gram scale synthesis of a multi 13C-labelled anthocyanin. [6,8,18,3′,5′,13C5]cyanidin-3-glucoside, for use in oral tracer studies in humans. Chem. Commun. 2011;47:10596–10598. doi: 10.1039/c1cc14323a. [DOI] [PubMed] [Google Scholar]
- 100.Morrison D.J., Taylor K., Preston T. Strong anion-exchange liquid chromatography using a Liquiface interface. Rapid Commun. Mass Spectrom. 2010;24:1755–1762. doi: 10.1002/rcm.5139. [DOI] [PubMed] [Google Scholar]
- 101.Morrison D.J., O'Hara J., King R.F., Preston T. Quantitation of plasma 13C-galactose and 13C-glucose during exercise by liquid chromatography/isotopic ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2011;25:2484–2488. doi: 10.1002/rcm.5139. [DOI] [PubMed] [Google Scholar]
- 102.Clifford M.N. Anthocyanins - nature, occurrence and dietary burden. J. Sci. Food Agric. 2000;80:1063–1072. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1063::AID-JSFA605>3.0.CO;2-Q. [DOI] [Google Scholar]
- 103.Barron D., Smarrito-Menozz C., Fumeaux R., Vito F. In: Flavonoids and Related Compounds. Bioavailability and Function. Spencer J.P.E., Crozier A., editors. CRC Press; Boca Raton, FL: 2012. Synthesis of dietary phenolic metabolites and isotopically labelled dietary phenolics; pp. 233–280. 978-1-4398-4826-5. [Google Scholar]
- 104.Zhang Q., Raheem K.S., Botting N.P., Slawin A.M.Z., et al. Flavonoid metabolism: the synthesis of phenolic glucuronides and sulfates as candidate metabolites for bioactivity studies of dietary flavonoids. Tetrahedron. 2012;68:4194–4201. doi: 10.1016/j.tet.2012.03.100. [DOI] [Google Scholar]
- 105.Pereira-Cara G., Ordóñez J.L., Ludwig I.A., Gaillet S., et al. Development and validation of an UHPLC-HRMS protocol for the analysis of flavan-3-ol metabolites and catabolites in urine, plasma and feces of rats with a red wine proanthocyanin extract. Food Chem. 2018;252:49–60. doi: 10.1016/j.foodchem.2018.01.083. 0.1016/j.foodchem.2018.01.083. [DOI] [PubMed] [Google Scholar]
- 106.Cáceres-Jiménez S., Rodríguez-Solana R., Dobani S., Pourshahadi K., et al. UHPLC-HRMS spectrometric analysis: method validation and plasma and urinary metabolites identification of mango pulp intake. J. Agric. Food Chem. 2023;71:11520–11533. doi: 10.1021/acs.jafc.3c03846. [DOI] [PubMed] [Google Scholar]
- 107.Bussy U., Olanrewaju Y., Crozier A., Ottaviani J.I., et al. Development and validation of HPLC-MS2 methodology for the accurate determination of C4−C8 B-type flavanols and procyanidins. Sci. Rep. 2021;11(2012) doi: 10.1038/s41598-021-93993-0. 0.1038/s41598-021-93993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sumner L.W., Amberg A., Barrett D., Beale M.H., et al. Proposed minimum reporting standards for chemical analysis. Chemical analysis working group (CAWG) metabolomics standards initiative (MSI) Metabolomics. 2007;3:211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.