Abstract
Background
Acute gout flares cause significant pain and disability and it is important to provide quick and effective pain relief. Traditional options for managing acute flares include colchicine, non‐steroidal anti‐inflammatory drugs (NSAIDs) and glucocorticoids.
Objectives
To assess the benefits and harms of interleukin‐1 inhibitors (anakinra, canakinumab, rilonacept) in acute gout.
Search methods
We searched The Cochrane Library, MEDLINE and EMBASE on 19 June 2013. We applied no date or language restrictions. We performed a handsearch of the abstracts from the European League Against Rheumatism (EULAR) (2009 to 2012) and American College of Rheumatology (ACR) (2009 to 2011) conferences and of the references of all included trials. We also screened the Clinical Trials Registry Platform of the World Health Organization and Clinical Trials Registry Platform of the US National Institutes of Health.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐randomised clinical trials (controlled clinical trials (CCTs)) assessing an interleukin‐1 inhibitor (anakinra, canakinumab or rilonacept) against placebo or another active treatment (colchicine, paracetamol, NSAIDs, glucocorticoids (systemic or intra‐articular), adrenocorticotropin hormone, a different interleukin‐1 blocking agent or a combination of any of the above) in adults with acute gout.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed the risk of bias and extracted the data. If appropriate, we pooled data in a meta‐analysis. We assessed the quality of the evidence using the GRADE approach.
Main results
We included four studies (806 participants) in the review. The studies had an unclear risk of selection bias and low risk of performance and attrition biases. One study each had an unclear risk of detection and selection bias.
Three studies (654 participants) compared subcutaneous canakinumab compared with intramuscular triamcinolone acetonide 40 mg in the treatment of acute gout flares of no more than five days' duration. Doses of canakinumab were varied (10 to 150 mg), but most people (255/368) were treated with canakinumab 150 mg. None of the studies provided data on participant‐reported pain relief of 30% or greater. Moderate‐quality evidence indicated that canakinumab 150 mg was probably superior to triamcinolone acetonide 40 mg in terms of pain relief, resolution of joint swelling and in achieving a good treatment response at 72 hours following treatment, but was probably associated with an increased risk of adverse events.
Mean pain (0‐ to 100‐mm visual analogue scale (VAS), where 0 mm was no pain) was 36 mm after triamcinolone acetonide treatment; pain was further reduced by a mean of 11 mm with canakinumab treatment (mean difference (MD) ‐10.6 mm, 95% confidence interval (CI) ‐15.2 to ‐5.9). Forty‐four per cent of participants treated with canakinumab had resolution of joint swelling at 72 hours compared with 32% of participants treated with triamcinolone (risk ratio (RR) 1.39, 95% CI 1.11 to 1.74, number needed to treat for an addition beneficial outcome (NNTB) 9); 65% of participants treated with canakinumab assessed their response to treatment as good or excellent compare with 47% of participants treated with triamcinolone acetonide (RR 1.37, 95% CI 1.16 to 1.61, NNTB 6). Function or health‐related quality of life were not measured. In both groups, 0.7% of participants withdrew from treatment (RR 1.1, 95% CI 0.2 to 7.2); there was one death and one alteration of laboratory results in each of the treatment groups. Adverse events were more frequent in participants receiving canakinumab (61%) compared with triamcinolone acetonide (51%; RR 1.2, 95% CI 1.1 to 1.4, number needed to treat for an addition harmful outcome (NNTH) 10).
Low‐quality evidence from one study (152 participants with an acute gout flare of no more than 48 hours' duration and affecting fewer than four joints) comparing rilonacept 320 mg with indomethacin (50 mg three times a day for three days followed by 25 mg three times a day for up to nine days) indicated that indomethacin may improve pain more than rilonacept at 24 to 72 hours, and there may be no evidence of a difference in withdrawal rates or adverse events. The mean change (improvement) in pain from baseline with indomethacin was 4.3 points (measured on a 0 to 10 numerical rating scale, where 0 was no pain); pain was improved by a mean of only 2.5 points with rilonacept (MD 2.52, 95% CI 0.29 to 4.75, 25% less improvement in absolute pain with rilonacept). Inflammation, function health‐related quality of life and participant global assessment of treatment success were not measured. Rates of study withdrawals due to adverse events were low in both groups: 1/75 (1%) participants in the rilonacept group compared with 2/76 (3%) participants in the indomethacin group (RR 0.5, 95% CI 0.05 to 5.5). Adverse events were reported in 27/75 (36%) participants in the rilonacept group and 23/76 (30%) in the indomethacin group (RR 1.2, 95% CI 0.8 to 1.9).
Authors' conclusions
Moderate‐quality evidence indicated that compared with a single suboptimal 40‐mg dose of intramuscular injection of triamcinolone acetonide, a single subcutaneous dose of 150 mg of canakinumab probably results in better pain relief, joint swelling and participant‐assessed global assessment of treatment response in people with an acute gout flare but is probably associated with an increased risk of adverse events. The cost of canakinumab is over 5000 times higher than triamcinolone acetonide; however, there are no data on the cost‐effectiveness of this approach. We found no studies comparing canakinumab with more commonly used first‐line therapies for acute gout flares such as NSAIDs or colchicine. Low‐quality evidence indicated that compared with maximum doses of indomethacin (50 mg three times a day), 320 mg of rilonacept may provide less pain relief with a similar rate of adverse events.
Keywords: Adult; Humans; Acute Disease; Antibodies, Monoclonal; Antibodies, Monoclonal/administration & dosage; Antibodies, Monoclonal, Humanized; Gout; Gout/drug therapy; Injections, Intramuscular; Injections, Subcutaneous; Interleukin‐1; Interleukin‐1/antagonists & inhibitors; Randomized Controlled Trials as Topic; Recombinant Fusion Proteins; Recombinant Fusion Proteins/administration & dosage; Triamcinolone; Triamcinolone/administration & dosage
Plain language summary
Interleukin‐1 inhibitors for acute gout
Background: what is an acute gout flare and what are interleukin‐1 inhibitors?
Gout results from the deposition of crystals of uric acid in and around joints. The main treatments for gout are drugs that lower uric acid blood levels and resolve the crystal deposits. Acute gout flares result in significant pain and disability and treatment aims at reducing the pain and resolving the arthritis quickly.
Interleukin‐1 inhibitors (canakinumab, rilonacept and anakinra) modify the immune system and reduce inflammation. They can be injected in a single dose or repeated doses.
Study characteristics
This summary of a Cochrane review presents what we know from research about the effect of interleukin‐1 inhibitors for treating acute gout flares. After searching for all relevant studies to 19 June 2013, we included four studies (806 participants, mean age 52 years, 92% men). Three studies assessed the use of a single injection of canakinumab (10 to 150 mg) compared with a single suboptimal 40 mg intramuscular injection of triamcinolone acetonide (steroid injection) and one study assessed the use of a single subcutaneous injection of rilonacept 320 mg compared with maximum doses of oral indomethacin (50 mg three times a day for three days) (a non‐steroidal anti‐inflammatory drug (NSAID)) in people with acute gout.
Key results: what happens to people with an acute gout flare who are injected with canakinumab compared with triamcinolone acetonide
Pain on a scale of 0 to 100 points (lower scores mean reduced pain)
‐ People who took canakinumab rated their pain to be 11 points lower at three days compared with people who had an injection of triamcinolone acetonide.
‐ People who had a subcutaneous injection of canakinumab rated their pain to be 25 on a scale of 0 to 100.
‐ People who had an injection of triamcinolone acetonide rated their pain to be 36 on a scale of 0 to 100.
Swelling of the joint
‐ 12 more people out of 100 reported no swelling in their joint three days after treatment with canakinumab compared with people who had an injection of triamcinolone acetonide.
‐ 44 people out of 100 who had canakinumab reported no swelling.
‐ 32 people out of 100 who had triamcinolone reported no swelling.
Participant assessment of good or excellent treatment response
‐ 17 more people out of 100 rated their treatment as good or excellent after three days' treatment with canakinumab compared with people who had an injection of triamcinolone.
‐ 64 people out of 100 who had canakinumab reported a good or excellent treatment response.
‐ 47 people out of 100 who had triamcinolone reported a good or excellent treatment response.
Withdrawal from the study due to side effects
Participant withdrawal due to side effects
‐ 1 out of 100 people withdrew due to side effects after treatment with canakinumab or injection of triamcinolone.
Side effects
‐ 10 more people out of 100 had a side effect after treatment with canakinumab compared with people who had an injection of triamcinolone.
‐ 61 people out of 100 who had canakinumab had a side effect.
‐ 51 people out of 100 who had triamcinolone had a side effect.
Quality of the evidence
We found moderate‐quality evidence that, compared with a single intramuscular injection of the steroid, triamcinolone acetonide, canakinumab injection probably improves pain, swelling and the number of good or excellent treatment responses, but probably results in more side effects. Possible side effects included back pain, headache, high blood pressure, joint pain and a rise in the liver enzyme gamma‐glutamyl transpeptidase. Function or quality of life were not measured. Further research may change the estimates.
There was low‐quality evidence from one study that rilonacept injection may improve pain less than indomethacin and may not be associated with an increase in side effects. Inflammation, disability, quality of life and participant assessment of treatment success were not measured. Further research is likely to change these estimates.
We found no studies comparing canakinumab with more commonly used therapies for acute gout flares such as NSAIDs or colchicine.
Summary of findings
Summary of findings for the main comparison. Canakinumab versus triamcinolone acetonide.
| Canakinumab versus triamcinolone acetonide | ||||||
| Participant or population: acute gout Settings: hospital Intervention: canakinumab versus triamcinolone acetonide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Triamcinolone acetonide | Canakinumab | |||||
| Pain: at 72 hours (canakinumab 150 mg) 100‐mm VAS (0 = no pain, 100 = unbearable pain) | Mean pain: at 72 hours in the control groups was 35.73 mm | Mean pain: at 72 hours in the intervention groups was 10.57 lower (15.24 to 5.9 lower) | ‐ | 443 (2 studies) | ⊕⊕⊕⊝ moderate1 | Absolute improvement: 11% lower with canakinumab (15% to 6% lower). Relative % change: 14% more improvement with canakinumab (8% to 21% more improvement)2 NNTB 7 (95% CI 5 to 12)4 |
|
Inflammation: participants with no swelling at 72 hours (canakinumab 150 mg) Assessed by the investigator as no swelling, mild/palpable, moderate/visible, severe/bulging beyond the joint margins |
315 per 1000 | 438 per 1000 (350 to 548) | RR 1.39 (1.11 to 1.74) | 523 (3 studies) | ⊕⊕⊕⊝ moderate1 | Absolute improvement: 12% more events with canakinumab (4% to 20% more). Relative % change: 39% improvement with canakinumab (11% to 74% worse). NNTB 9 (95% CI 5 to 29)4 |
| Functional disability ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Health‐related quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
|
Participant global assessment of response to treatment: good or excellent at 72 hours (canakinumab 150 mg) Assessed by the participant on a 5‐point Likert scale (excellent, good, acceptable, slight or poor) |
470 per 1000 | 643 per 1000 (545 to 756) | RR 1.37 (1.16 to 1.61) | 478 (3 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference: 18% more events with canakinumab (7% to 29% more). Relative % change: 37% improvement with canakinumab (16% to 61% improvement). NNTB 6 (95% CI 4 to 14) |
| Study withdrawals due to AE (all canakinumab doses) | 7 per 1000 | 7 per 1000 (1 to 51) | RR 1.07 (0.16 to 7.23) | 654 (3 studies) | ⊕⊕⊝⊝ low1,3 | Absolute risk difference: 0% more events with canakinumab (2% more to 1% less). Relative % change: 7% worse with canakinumab (84% improvement to 623% worse). NNTH = n/a |
| Participants with at least 1 AE (all canakinumab doses) | 507 per 1000 | 608 per 1000 (532 to 705) | RR 1.2 (1.05 to 1.39) | 654 (3 studies) | ⊕⊕⊕⊝ moderate1 | Absolute risk difference: 10% more events with canakinumab (1% to 18% more). Relative % change: 20% worse with canakinumab (5% to 39% worse). NNTH = 10 (95% CI 6 to 40) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse events; CI: confidence interval; NNTB: number needed to treat to for an additional beneficial outcome benefit; NNTH: number needed to treat to for an additional harmful outcome benefit; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of selection bias.
2 Control group baseline mean (standard deviation) pain, 0‐ to 100‐mm VAS, 73.6 mm (12.6 mm). 3 Low number of events.
4 Note: NNTB or NNTH = n/a when result was not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (Cochrane Musculoskeletal Group editorial office), assuming a minimal clinically important difference in pain on 15 mm (0‐ to 100‐mm VAS).
Summary of findings 2. Rilonacept versus indomethacin for acute gout.
| Rilonacept versus indomethacin for acute gout | ||||||
| Participant or population: acute gout Settings: hospital Intervention: rilonacept versus indomethacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Indomethacin | Rilonacept | |||||
| Pain ‐ change from baseline to 24 to 72 hours Assessed on numerical rating scale (0 = no pain; 10 = extreme pain) | Mean pain reduction from baseline at 24 to 72 hours in the control groups was 4.33 | Mean pain change from baseline in the intervention groups was
2.52 lower (0.29 lower to 4.75 lower) |
‐ | 148 (1 study) | ⊕⊕⊝⊝ low1 | Absolute risk difference: 25% less improvement in pain with rilonacept (3% to 48% less). Relative % change: 37% less improvement with rilonacept (4% to 70% less) NNTB with indomethacin 6 (95% CI 3 to 70)2 |
| Inflammation ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Functional disability ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Health‐related quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Participant global assessment of treatment success ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Adverse events ‐ study withdrawals due to adverse events | 26 per 1000 | 13 per 1000 (1 to 144) | RR 0.51 (0.05 to 5.47) | 151 (1 study) | ⊕⊕⊝⊝ low1 | Absolute risk difference: 1% less events with rilonacept (6% less to 3% more). Relative % change: 49% improvement with rilonacept (95% improvement to 447% worsening). NNTH = n/a |
| Adverse events ‐ participants with at least 1 adverse event | 303 per 1000 | 360 per 1000 (227 to 569) | RR 1.19 (0.75 to 1.88) | 151 (1 study) | ⊕⊕⊝⊝ low1 | Absolute risk difference: 6% more events with rilonacept (9% less to 21% more). Relative % change: 19% worsening with rilonacept (25% improvement to 88% worsening). NNTH = n/a |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Small population size and thus potential imprecision; risk of selection and detection bias.
2 Note: NNTH = n/a when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (Cochrane Musculoskeletal Group editorial office), assuming a minimal clinically important difference in pain on 1.5 points (0 to 10 scale).
Background
Description of the condition
Gout is a prevalent disease that results from monosodium urate (MSU) crystal deposition in and around joints. It affects up to 1% to 2% of adults and is the most common inflammatory arthritis in men (Smith 2010). Elevation of serum uric acid (sUA) levels ‐ hyperuricaemia ‐ is a prerequisite for gout, although not all people with hyperuricaemia develop gout (Campion 1987). As sUA rises, the saturation threshold is exceeded and salts (MSU crystals) can form and deposit; these deposits can cause acute and persistent inflammation.
Gout usually presents as repeated episodes of acute arthritis in peripheral joints separated by asymptomatic intercritical periods. These acute arthritis episodes ‐ also known as flares ‐ occur most frequently in the first metatarsophalangeal joint but other frequently affected joints include knee, ankle and tarsus. Less commonly, gout can cause acute inflammation in tendons or superficial bursae. Flares tend to resolve spontaneously in under one week, especially in gout of short duration. If left untreated, MSU crystal deposits will increase, as will symptoms: polyarticular flares become common, symptoms persist between attacks and tophi can become apparent in soft tissues and joints. Although the prevalence of tophaceous gout varies among populations, in one study, tophi were detected in three‐quarters of the people with untreated gout of 20 years or more duration (Gutman 1973). Chronic crystal deposits can produce bone erosions and joint damage (Dalbeth 2009). Data have also been accumulating in support of the association between chronic gout and both the metabolic syndrome and cardiovascular morbidity and mortality (Choi 2007).
The main goal in gout therapy is lowering sUA, which allows urate deposits to disappear slowly (Pascual 2007). Current available treatments for urate lowering include allopurinol, febuxostat and, in some countries, uricosuric agents such as benzbromarone or probenecid.
However, acute flares cause significant pain and disability and it is also important to provide quick and effective pain relief in people with an acute gouty arthritis. Traditional options for managing acute flares include the use of colchicine, non‐steroidal anti‐inflammatory drugs (NSAIDs), glucocorticoids and adrenocorticotropin hormone. Adrenocorticotropin hormone is produced by the pituitary gland and its principal effect is the increased production and release of glucocorticoids. The choice of agent, dose and route is guided mainly by co‐morbidities (Neogi 2011). These same drugs, albeit at different doses, are often used as prophylaxis of acute flares; this is especially useful when initiating urate‐lowering therapy (ULT).
Description of the intervention
Interleukin‐1 inhibitors have been appraised for the treatment of different inflammatory conditions. Currently three agents are available: anakinra, canakinumab and rilonacept. Anakinra is a human interleukin‐1 receptor antagonist licensed in some countries for use in rheumatoid arthritis in people who are non‐responsive to methotrexate (approved dose 100 mg/day subcutaneously). Canakinumab is a fully human monoclonal anti‐interleukin‐1β antibody approved in some countries for use in the treatment of cryopyrin‐associated syndromes (approved dose in adults 320‐mg loading dose followed by 160 mg once‐weekly subcutaneously). Cryopyrin‐associated syndromes are a spectrum of autoinflammatory syndromes including familial cold autoinflammatory syndrome, Muckle‐Wells syndrome (a periodic fever associated with recurrent hives and deafness) and neonatal‐onset multisystem inflammatory disease. They share many clinical features and are associated with mutations in the gene encoding cryopyrin, an essential protein in the production of active interleukin‐1. In 2011, The Food and Drug Administration (FDA) in the US rejected the approval of canakinumab for the treatment of gout flares due to safety concerns (FDA 2011). Rilonacept is a fusion protein that contains the extracellular portions of type I interleukin‐1 receptor and interleukin‐1 receptor accessory protein; it is used for life‐threatening cryopyrin‐associated syndromes (150 mg subcutaneously every eight weeks in adults).
The optimal dose of these therapies in gout is still under debate and will possibly vary depending on the individual inflammatory burden or the aim of the therapy (treatment of refractory acute flare versus prevention of acute flares).
Several potential harms deriving from interleukin‐1 inhibitors must be noted. As with most drugs administered subcutaneously, there is a possibility of injection site reactions. The blocking of the inflammatory cascade may be associated with an increased overall risk of infections and serious infections. The risk of malignancies is currently unknown but a potential risk cannot be excluded. Finally, the risk of severe hypersensitivity reactions, not uncommon with injectable proteins, must be taken into consideration.
How the intervention might work
Interleukin‐1 is a cytokine ‐ a small molecule used extensively in intercellular communication. There is evidence that interleukin‐1 plays a pivotal role in inflammation induced by MSU crystals. MSU crystals have shown in vitro ability to activate the NALP3 inflammasome (also known as cryopyrin); this is a key step in the production of active interleukin‐1 (Martinon 2006). Moreover, in an animal in vivo model of crystal‐induced peritonitis, impaired neutrophil influx was detected in inflammasome‐deficient mice and mice deficient in the interleukin‐1β receptor (Martinon 2006), highlighting the key role of interleukin‐1 in MSU crystal‐induced inflammation.
In humans with acute gouty arthritis, the blockade of interleukin‐1 might block the inflammatory cascade and, therefore, treat gout‐related flares. Given their mode of action, interleukin‐1 blockade is not expected to have a significant effect on sUA levels.
Why it is important to do this review
The goal of this review was to summarise systematically all current data on the benefit and safety of interleukin‐1 inhibitors in treating inflammation associated with acute gout flares. This will provide clinicians with useful information in order to optimise treatment of gout‐related acute inflammation. Policy‐makers also need to keep abreast with the current literature in terms of benefit and safety of interleukin‐1 inhibitors and this review will provide an up‐to‐date summary of available evidence.
To our knowledge, this is the first systematic review on interleukin‐1 inhibitors for the treatment of acute gout.
Objectives
To assess the benefit and safety of interleukin‐1 inhibitors (anakinra, canakinumab, rilonacept) in acute gout.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) or quasi‐randomised controlled trial (controlled clinical trial (CCTs)) comparing interleukin‐1 inhibitors with another therapy (active or placebo) in people with acute gout for inclusion. We excluded studies published in languages other than English if they did not have an English abstract.
We only included trials that were published as full articles or were available as a full trial report.
Types of participants
We considered trials that included adults (aged 18 years or older) with acute gout for inclusion if they had an episode of acute joint inflammation ‐ often referred to as flare ‐ and had a diagnosis of gout as described by the study authors. Common ways of diagnosing gout include identification of MSU crystals in synovial fluid, several classification and diagnostic criteria, or expert opinion. The American College of Rheumatology (ACR) preliminary criteria for the classification of the acute arthritis of primary gout remain the most frequently used criteria for gout diagnosis in clinical trials (Wallace 1977). According to these criteria, a person can be classified as having gout if MSU crystals are identified in a synovial fluid sample or in a tophus aspirate, or if any six out of the 12 clinical, radiographic and laboratory individual items are fulfilled.
Types of interventions
All trials evaluating interleukin‐1 inhibitors (anakinra, canakinumab, rilonacept) were eligible for inclusion. We included trials if they evaluated interleukin‐1 inhibitors alone or in combination with other pharmacological or non‐pharmacological treatments. We included all dosages of interleukin‐1 inhibitors.
Comparators could be:
placebo;
colchicine;
paracetamol;
NSAIDs, including traditional agents and cyclo‐oxygenase‐2 inhibitors (coxibs);
glucocorticoids (systemic or intra‐articular);
adrenocorticotropin hormone;
a different interleukin‐1 blocking agent;
combination therapy (combinations of any of the above).
Types of outcome measures
OMERACT (Outcome Measures in Rheumatology) is an international network interested in outcome measures across the spectrum of rheumatology intervention studies. The OMERACT 9 conference defined a recommended core and discretionary set of domains for outcome measures in clinical studies of acute and chronic gout (Schumacher 2009). For acute gout, core domains included pain, joint swelling, joint tenderness, participant global assessment and activity limitations. Safety outcomes were not included in the final core domains, even though they were proposed in the preliminary set (Schumacher 2005).
Intense pain is the hallmark of an acute gout attack; it has also been a consistent outcome measure in clinical trials involving acute gouty arthritis, although the instruments and time intervals used to measure pain are varied (Grainger 2009). It is recognised that interpreting the meaning of average changes in continuous measures of pain (e.g. mean change on a 100‐mm visual analogue scale (VAS)), is hampered where participants report either very good or very poor pain relief (Moore 2010). For trials of interventions for chronic pain, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has recommended that dichotomous pain outcomes (the proportion of participants improved by 30% or greater and 50% or greater) be reported (Dworkin 2008), although no recommendations have yet been published for acute pain. Therefore, we elected to include a dichotomous pain outcome measure (the proportion of participants reporting 30% or greater pain relief) as the primary benefit measure in this review. However, as most trials of interventions for acute gout report continuous measures, we also included mean change in pain score as a secondary benefit measure.
Major outcomes
Pain: participant reported pain relief of 30% or greater. If this was not reported, mean change in pain score on VAS, numerical rating scale or Likert scale.
Inflammation (joint swelling, erythema, tenderness): if an individual trial reported more than one measure, we extracted only one according to the following hierarchy: swelling, erythema, tenderness. Where applicable, we extracted data both in an index joint and as the total number of inflamed joints.
Function: as assessed by the Health Assessment Questionnaire (HAQ) or by any other method. We considered that disability and activity limitations were comparable concepts to function.
Health‐related quality of life (HRQoL): as reported by generic questionnaires (such as the 36‐item Short Form (SF‐36)) or by disease‐specific questionnaires (such as the Gout Assessment Questionnaire (GAQ) or Gout Impact Scale (GIS)).
Participant global assessment of treatment success.
Number of withdrawals due to adverse events (AE).
Number and type of AEs and serious adverse events (SAE).
We extracted outcomes at all time points measured in the included trials. We planned to pool available data into short‐term (up to two weeks), medium‐term (two to six weeks) and long‐term (more than six weeks) outcomes, if data were available.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases:
The Cochrane Library including the Cochrane Central Register of Controlled Trials (CENTRAL) (to 19 June 2013) (Appendix 1);
MEDLINE via PubMed (1946 to 19 June 2013) (Appendix 2);
EMBASE via Embase.com (1974 to 19 June 2013) (Appendix 3).
We did not limit the search by language, year of publication or type of publication.
Searching other resources
We performed a manual search of the reference lists of included trials.
We searched the abstracts of the two major international rheumatology scientific meetings ‐ the European League Against Rheumatism (EULAR) and ACR for the years 2010 and 2011. We also searched EULAR abstracts for the 2012 meeting.
For trials published after 1 July 2005, we screened the Clinical Trials Registry Platform of the World Health Organization (apps.who.int/trialsearch) and Clinical Trials Registry Platform of the US National Institutes of Health (clinicaltrials.gov).
Data collection and analysis
We used EndNoteX5 software to manage records retrieved from electronic databases. We tracked results from handsearches on a Microsoft Excel spreadsheet. We used a data extraction sheet in Microsoft Word in order to record the retrieved information from each individual trial.
Selection of studies
Two review authors (FS, MW) independently reviewed the retrieved records to identify trials that fulfilled inclusion criteria and kept a record of reasons for exclusion. We resolved disagreements by consensus. If no agreement was met, a third review author (LC) acted as arbiter. We retrieved the full text of all possibly relevant articles. We translated studies into English when necessary.
Data extraction and management
Two review authors (FS, MW) independently extracted data from the trials using pre‐set forms, including study design, characteristics of the study population, treatment dosage, outcomes and timing of the outcomes. We resolved differences in data extraction by referring back to the original articles and reaching a consensus. An arbiter (LC) helped resolve differences if needed. If necessary, we contacted the authors of the studies to obtain additional information.
We entered the collected data into Review Manager 5 (RevMan 2011).
Assessment of risk of bias in included studies
Two review authors (FS, MW) independently assessed risk of bias for all included studies using a 'Risk of bias' table and The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011a). This included assessing the bias in each of the following domains: random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; completeness of outcome data; selective reporting and other sources of bias (baseline imbalance, source of funding). We graded each of these domains as 'high risk' of bias, 'low risk' of bias or 'unclear risk' of bias (either lack of adequate information or uncertainty over the potential for bias). We resolved disagreements by consensus; if a consensus could not be reached, a third review author (LC) acted as arbiter.
Measures of treatment effect
We analysed the results of the studies using Review Manager 5 (RevMan 2011). We only performed a meta‐analysis if the data of the studies were sufficiently clinically and statistically homogeneous.
We presented the results of dichotomous data as risk ratios (RR) with the corresponding 95% confidence intervals (95% CI). An RR greater than 1.0 indicated a beneficial effect of interleukin‐1‐blocking therapy.
We presented the results of continuous data as mean differences (MD) between the intervention and comparator groups with the corresponding 95% CIs. When different scales were used to measure the same conceptual domain, we calculated standardised mean differences (SMD) with the corresponding 95% CIs instead. For the calculation of SMD, we divided the MD by the standard deviation, resulting in a unit‐less measure of treatment effect. SMDs larger than zero indicated a beneficial effect of the interleukin‐1‐blocking agents. As described by Cohen (Cohen 1988), an SMD of 0.2 indicates a small beneficial effect, 0.5 a medium effect and 0.8 a large effect in favour of interleukin‐1 blockade. We translated SMDs back into MDs on a scale of 0 to 10 in order to facilitate appraisal by clinicians. We planned to back‐translate SMD to a typical scale (e.g. 0 to 10 for pain) by multiplying the SMD by a typical among‐person standard deviation (e.g. the standard deviation of the control group at baseline from the most representative trial) (Schünemann 2011b).
We treated the results of count data (such as the number of gout flares) as continuous outcome data if events were common and as rate ratios if they were rare events.
For studies containing more than two intervention groups, making multiple pair‐wise comparisons between all possible pairs of intervention groups possible, we included the same group of participants only once in the meta‐analysis.
Unit of analysis issues
We assessed whether each study evaluated participants or acute flares as a unit of analysis. In the event that cross‐over trials had been identified in which the reporting of continuous outcome data precluded paired analysis, we would not have included these data in a meta‐analysis in order to avoid unit of analysis error. Where carry‐over effects were thought to have existed and where sufficient data existed, we would have included only data from the first period in the analysis.
We extracted data from all time points and combined them into short term (up to two weeks), medium term (two to six weeks) and long term (greater than six weeks) outcomes, though this depended on the feasibility of doing so from the available data. If more than one time point was reported within the short‐term subgroup and given the natural history of acute gout flares that tend to be self limiting within the first seven to 14 days, we extracted the time point closer to 72 hours. In the medium‐ or long‐term subgroup, when more than one time point was reported, we extracted the later time point only within each subgroup.
Dealing with missing data
When data were missing or incomplete, we sought further information from the study authors.
In cases where individual data were missing from the reported results and no further information was forthcoming from the study authors, we assumed the missing values to have a poor outcome. For dichotomous variables that measure AEs, we calculated the withdrawal rate using the number of participants that received treatment as the denominator (worst‐case analysis). For dichotomous outcomes that measure benefits, we performed the worst‐case analysis using the number of randomised individuals as the denominator. For continuous variables, we calculated the MD or the SMD based on the number of participants analysed at the time point. If the number of participants analysed was not available, we used the number of randomised participants in each group at baseline.
Where possible, we calculated missing standard deviations from other statistics such as standard errors, CIs or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). If we could not calculate standard deviations, we imputed them from other studies in the meta‐analysis (Higgins 2011c).
Assessment of heterogeneity
We assessed clinical and statistical heterogeneity between studies.
First, we assessed studies for clinical homogeneity with respect to study participants, intervention groups, outcome measures and timing of outcome.
For studies judged as clinically similar, we assessed statistical heterogeneity using the I2 statistic (Deeks 2011). We used the following approximate thresholds for the interpretation of the I2 statistic: 0% to 40% heterogeneity might not be important, 30% to 60% represents moderate heterogeneity, 50% to 90% represents substantial heterogeneity and greater than 75% represents considerable heterogeneity. In cases of considerable heterogeneity, we explored the data further, including subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
To assess the potential for reporting bias, we determined whether the protocol of the RCT was published before recruitment of participants began. For trials published after 1 July 2005, we screened the Clinical Trials Registry Platform of the World Health Organization (apps.who.int/trialsearch) and Clinical Trials Registry Platform of the US National Institutes of Health (clinicaltrials.gov). We evaluated if selective reporting of outcomes was present.
We planned to compare the fixed‐effect estimate against the random‐effects model. In the event of the possible presence of small sample bias in the published literature (i.e. in which the intervention effect was more beneficial in smaller studies), the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate (Sterne 2011).
If applicable, we could have explored reporting bias using a funnel plot if 10 or more studies are available; however, we included only four studies in this review.
Data synthesis
If we considered studies sufficiently homogeneous, we pooled data in a meta‐analysis using a random‐effects model irrespective of the I2 statistic results.
We performed analysis using Review Manager 5 (RevMan 2011), and produced forest plots for all analyses.
'Summary of findings' table
We produced a 'Summary of findings' table using GRADEpro software. This table provided key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the outcomes (short‐ and long‐term outcomes for pain, total number of withdrawals due to adverse effects, function and quality of life), as recommended by The Cochrane Collaboration (Schünemann 2011a).
The 'Summary of findings' table included an overall grading of the evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011b).
In addition to the absolute and relative magnitude of effect provided in the 'Summary of findings' table, for dichotomous outcomes, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) from the control group event rate (unless the population event rate was known) and the RR using the Visual Rx NNT calculator (Cates 2004).
For continuous outcomes, we calculated the NNTB and NNTH using the Wells calculator software available at the Cochrane Musculoskeletal Group (CMSG) editorial office. We determined the minimal clinically important difference (MCID) for each outcome for input into the calculator. We assumed an MCID of 1.5 points on a 0‐ to 10‐point VAS scale for pain and 10 points on a 0‐ to 100‐point scale for function.
In the 'Summary of findings' table, we presented data from the following outcomes:
pain (note: as none of the included trials reported pain as proportion improved by 30% or more as pre‐specified as our main benefit outcome, we included pain as a continuous measure in the 'Summary of findings' table);
inflammation: joint swelling;
function;
HRQoL;
participant global assessment of treatment success;
study withdrawals due to AEs;
proportion of participants with AEs.
Subgroup analysis and investigation of heterogeneity
We anticipated possible differences in response to treatment according to participant age and gender. Age may be associated with co‐morbidities and possibly with a greater chance of AEs (Busquets 2011).
Gout affects mostly men. Reports indicate that gout in women might have different epidemiological and clinical characteristics than gout in men (Harrold 2006).
Thus, we planned the following subgroup analyses:
participant's age (65 years or older versus under 65 years),
participant's gender (men versus women).
We had planned to extract the primary outcome by subgroups from each trial (i.e. pain in people under 65 years of age, and pain in people aged 65 years or older; and similarly, extract outcome by gender in a separate analysis), and informally compare the magnitudes of effect to assess possible differences in response to treatment between the two age groups (and the two sexes). However, as anticipated, the trials did not report the outcomes by subgroups, which precluded the planned subgroup analyses.
Sensitivity analysis
We had planned sensitivity analyses to explore the impact of any bias attributable to inadequate or unclear allocation concealment and outcome assessor blinding. However, we deemed all of the included trials to be at unclear risk of bias for allocation concealment and all blinded outcome assessment; therefore, we performed no sensitivity analyses.
Results
Description of studies
Results of the search
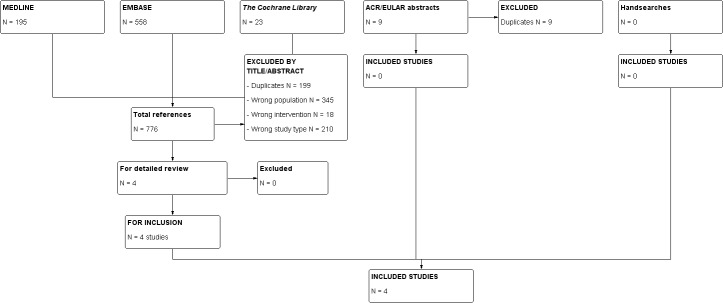
Figure 1 shows the details of the retrieved references and those that met the criteria for inclusion.
1.
Study flowchart.
Of the 776 references identified by the search, we considered four references of potential interest. All four RCTs were finally included in the review (Schlesinger 2012a; Schlesinger 2012b; So 2010a; Terkeltaub 2013). All included trials were registered prior to the start of recruitment (ClinicalTrials.gov registration numbers: Schlesinger 2012a NCT01029652; Schlesinger 2012b NCT01080131; So 2010a NCT00798369; Terkeltaub 2013 NCT00855920).
Included studies
Details of the included studies are provided in the Characteristics of included studies table. We included four studies (806 participants, mean age 52 years, 92% men). Three trials assessed the use of a single injection of canakinumab at varying doses (10 to 150 mg) compared with a single suboptimal intramuscular injection of triamcinolone acetonide 40 mg in people with acute gout of not more than five days' duration (Schlesinger 2012a; Schlesinger 2012b; So 2010a), and one trial assessed the use of a single subcutaneous injection of rilonacept 320 mg compared with maximum doses of oral indomethacin (50 mg three times a day for three days) in people with acute gout of no more than 48 hours' duration (Terkeltaub 2013).
Of the three RCTs evaluating the use of canakinumab versus triamcinolone acetonide in people with acute gouty arthritis, one trial presented their outcomes in two different papers (Schlesinger 2011; So 2010b), while the other two RCTs were presented together in a single paper (Schlesinger 2012a;Schlesinger 2012b). A wide range of doses of canakinumab were assessed in So 2010a, while in Schlesinger 2012a and Schlesinger 2012b, the canakinumab dose was always a single 150‐mg subcutaneous injection (to be repeated if a new gout flare occurred). Therefore, most of the available evidence for canakinumab is for a 150‐mg dose.
So 2010a was an RCT comparing the benefit and safety of different doses of canakinumab (administered as a single subcutaneous injection) and a placebo intramuscular injection to triamcinolone acetonide (40 mg) in 200 participants with an acute gout flare of no more than five days of duration. Of the 200 participants, 57 received placebo and 143 received canakinumab (28 participants received canakinumab 10 mg, 29 received canakinumab 25 mg, 29 received canakinumab 50 mg, 29 received canakinumab 90 mg and 28 received canakinumab 150 mg). To be included, participants had to be refractory or intolerant to NSAIDs or colchicine (or both) therapy or have contraindications to their use, and have moderate‐severe pain (defined as a baseline pain score 50 or greater as measured on a 100‐mm VAS ranging from no pain (0 mm) to unbearable pain (100 mm)). Participants were mostly men (93%) and had a mean age of 51.9 years. The study was multinational with 89 participating centres in 11 different countries. Most acute gout flares involved only one joint: 63% monoarticular, 28% oligoarticular and 9% polyarticular. Participants were treated with a single dose and benefit and safety outcomes were evaluated at scheduled visits at 72 hours, seven days, four weeks and eight weeks post dose. Pain was also assessed at six, 12, 24 and 48 hours and four, five and six days post dose.
Schlesinger 2012a and Schlesinger 2012b assessed the use of canakinumab (150 mg subcutaneously) versus triamcinolone acetonide (40 mg intramuscular injection) administered during a gout flare. Both treatment groups also received a complementary intramuscular or subcutaneous placebo injection. Participants could receive repeat injections of the same baseline study drug (in the same dosage) if they developed a new flare as long as the period between consecutive drug administration was at least 14 days and the injection was given within the first five days of the initiation of the new flare. Four hundred and fifty‐six participants were enrolled, 230 in Schlesinger 2012a (115 participants received canakinumab 150 mg and 115 participants received triamcinolone) and 226 in Schlesinger 2012b (112 participants received canakinumab 150 mg and 114 participants received triamcinolone). Sixty (26.7%) participants in the canakinumab group and 89 (38.9%) participants in the triamcinolone group received multiple doses of the medication and 335 participants continued into the 12‐week extensions. The double‐blind was continued into these 12‐week extensions, so we extracted safety data for this review for the whole of the 24 weeks. Inclusion required the presence of an acute gout flare of no more than five days' duration, and being refractory to, intolerant of or having a contraindication to NSAIDs or colchicine (or both) therapy, and a baseline pain score 50 or greater as measured on a 100‐mm VAS. Both studies were multicentre; the β‐RELIEVED study was carried out in 16 countries in Europe, America, Asia and Australia (see Schlesinger 2012a), while β‐RELIVED II study was conducted mainly in the US (see Schlesinger 2012b). Participants were typically gouty, predominantly men (91%), with a mean age of 53.0 years and with predominantly monoarticular flares (53%). Benefit and safety outcomes were evaluated at 24 hours, 72 hours, seven days, four weeks, eight weeks, 12 weeks, 16 weeks, 20 weeks and 24 weeks post dose. Pain was also assessed at six, 12 and 48 hours and four, five and six days post treatment.
One single RCT evaluated the use of rilonacept against an NSAID in people with an acute gout flare (Terkeltaub 2013). Participants were randomised into one of three arms: 1. rilonacept monotherapy: rilonacept 320 mg subcutaneously at baseline (with oral placebo), 2. indomethacin monotherapy: indomethacin orally 50 mg three times daily for three days and then 25 mg three times daily for up to nine days (plus placebo subcutaneously at baseline) or 3. combination therapy: rilonacept (320 mg subcutaneously at baseline) (with oral placebo) plus indomethacin orally 50 mg three times daily for three days and then 25 mg three times daily for up to nine days (plus placebo subcutaneously at baseline). The study enrolled 225 participants: 75 received rilonacept monotherapy, 76 received indomethacin monotherapy and 74 received combination therapy. Inclusion required a history of relief of a previous gout flare with NSAIDs, an acute gout flare of no more than 48 hours' duration and a baseline pain score of 3 or greater in a 5‐point Likert scale, together with swelling and tenderness in the index joint. Participants were mostly men (94%) with a mean age of 50.3 years. Flares of more than three joints were excluded; no data were available on the proportion of monoarticular flares. Benefit outcomes were evaluated at 24, 48 and 72 hours from baseline. Pain was also assessed using an electronic diary at four, eight, 12 and 24 hours and then daily until the flare ended. Safety was assessed on‐site on days four, eight and a final safety assessment on day 31.
Excluded studies
We excluded no studies after full‐text review.
Risk of bias in included studies
Overall, the four studies had an unclear risk of selection bias and were at low risk of performance and attrition biases. One study each may have been susceptible to detection and selection bias (see Characteristics of included studies table and Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were described as randomised, but the methods of randomisation and allocation concealment were not described in any of the included trials, resulting in an unclear risk of selection bias.
Blinding
We judged all three canakinumab trials to be at low risk of performance and detection bias. All participants and over 80% of investigators were blinded to study medication in So 2010a. Most outcomes in So 2010a were either participant‐reported outcomes (e.g. pain, participant global assessment of response to treatment) or objective measures (e.g., C‐reactive protein) with the exception of physician assessment of response to treatment. In the other two canakinumab trials (Schlesinger 2012a; Schlesinger 2012b), the study personnel who administered the study medication were unblinded but did not participate in outcome assessments. All participants, investigators other than those who administered the study medication and assessors were blinded to treatment allocation.
In the single rilonacept trial, Terkeltaub 2013 described the study as double‐blind and double‐dummy but did not specify who was blinded; benefit assessment included only pain as assessed by the participant, so there was a possibility of detection bias.
Incomplete outcome data
Risk of attrition bias was low in all included trials. All randomised participants were accounted for and withdrawal rates were between 4% and 15%, with similar withdrawal rates between groups in all studies.
Selective reporting
In So 2010a, the pain results were only reported using the VAS scores and no data on the Likert scale score were provided. In addition, not all pre‐specified time points for the pain outcomes were provided (six, 12, 24 and 48 hours, four, five and six days), so there was a potential risk of bias due to selective reporting of results.
In both Schlesinger 2012a and Schlesinger 2012b, secondary outcomes were not reported in the available paper. It was stated that they will be reported elsewhere at a further time point. However, results for some of the secondary outcomes were available at the ClinicalTrials.gov web page, thus, the risk of selective reporting is low for these studies.
Terkeltaub 2013 only pre‐specified and reported on one outcome (pain), thus, there was a low risk of selection bias. No assessment of other core OMERACT domains such as joint inflammation, HRQoL, participant global assessment or function were pre‐specified or provided.
Other potential sources of bias
It was unclear if all studies were subject to other bias. In So 2010a, there was an imbalance in baseline pain intensity between groups in the VAS score (but not the Likert scale) with mean scores in the canakinumab 150 mg group being the lowest. However, a lower VAS score can potentially underestimate the benefit of canakinumab; therefore, we considered the risk of bias to be low. Novartis Pharma AG, who participated in the study design, data gathering, data analysis and decision to publish, funded all three canakinumab studies (Schlesinger 2012a; Schlesinger 2012b; So 2010a).
Regeneron Pharmaceutics Inc., who participated in the study design, data analysis and writing the manuscript, funded the single rilonacept study (Terkeltaub 2013).
Effects of interventions
Subcutaneous canakinumab (in various doses) versus intramuscular triamcinolone acetonide 40 mg
All three canakinumab trials were clinically homogeneous (Schlesinger 2012a; Schlesinger 2012b; So 2010a). Inclusion and exclusion criteria were similar, the resultant participant population was comparable and the control used was triamcinolone acetonide 40 mg (single injection). Therefore, data from all three trials could be pooled (28 participants with canakinumab 10 mg, 29 participants with canakinumab 25 mg, 29 participants with canakinumab 50 mg, 29 participants with canakinumab 90 mg, 255 participants with canakinumab 150 mg, 286 participants with triamcinolone acetonide).
Pain
None of the included studies provided data on the proportion of participants with an improvement in pain of 30% or greater. Pooling data from two trials (443 participants) canakinumab 150 mg provided greater pain relief at 72 hours on the 100‐mm VAS (at 72 hours: canakinumab 25.1; triamcinolone acetonide 35.73; MD ‐10.6, 95% CI ‐15.2 to ‐5.9) (Analysis 1.1; Figure 3). Data on the mean VAS score at 72 hours was unavailable from the third study. Lower doses of canakinumab did not result in superior pain relief of canakinumab at 72 hours as measured by per cent change from baseline compared with triamcinolone acetonide (data not shown).
1.1. Analysis.

Comparison 1 Canakinumab versus triamcinolone acetonide (TA), Outcome 1 Pain: 100‐mm visual analogue scale at 72 hours.
3.

Forest plot of comparison: 1 Canakinumab 150 mg subcutaneously versus triamcinolone acetonide (TA) 40 mg intramuscularly, outcome: 1.1 Pain: 100‐mm visual analogue scale at 72 hours.
Inflammation
Pooling data from three trials (523 participants), participants receiving canakinumab were more likely to present with complete absence of swelling at 72 hours (107/248 (43%) participants in the canakinumab 150 mg group and 87/276 (32%) participants in the triamcinolone acetonide group; RR 1.4, 95% CI 1.1 to 1.7) (Analysis 1.2). No data on the effect of lower canakinumab doses on inflammation were available (only data on the canakinumab 150 mg dose were presented in the published papers).
1.2. Analysis.

Comparison 1 Canakinumab versus triamcinolone acetonide (TA), Outcome 2 Inflammation: participants with no swelling at 72 hours.
Function
No trial provided data for function.
Health‐related quality of life
No trial provided data for HRQoL.
Participant‐reported global assessment of treatment success
Based upon data from three trials (478 participants), participants receiving canakinumab 150 mg were more likely to assess their response to therapy as good or excellent at 72 hours compared with participants receiving triamcinolone acetonide (161/248 (65%) participants in the canakinumab group and 108/230 (47%) participants in the triamcinolone acetonide group; RR 1.4, 95% CI 1.2 to 1.6) (Analysis 1.3). One single study (158 participants) provided data on lower doses of canakinumab compared with triamcinolone and there were no significant between‐group differences in this outcome.
1.3. Analysis.

Comparison 1 Canakinumab versus triamcinolone acetonide (TA), Outcome 3 Participant global assessment of response to treatment: good or excellent at 72 hours.
Study withdrawals due to adverse events
Pooling data from three trials (654 participants), canakinumab conferred no increased risk of study withdrawal due to AE compared with triamcinolone acetonide at 12 weeks. Two participants withdrew from each group (2/368 (0.5%) in the canakinumab group and 2/286 (0.7%) in the triamcinolone acetonide group; RR 1.07, 95% CI 0.16 to 7.23) (Analysis 1.4). These withdrawals were due to one death and one alteration of laboratory results in each of the treatment groups.
1.4. Analysis.

Comparison 1 Canakinumab versus triamcinolone acetonide (TA), Outcome 4 Adverse events (all canakinumab doses).
Adverse events and serious adverse events
We pooled data from three trials (654 participants). We pooled all canakinumab doses for these safety analyses. A slight increase in the risk of AE and SAE was observed in the canakinumab versus the triamcinolone acetonide group (Analysis 1.4; Figure 4). Two hundred and eight out of 368 (57%) participants receiving canakinumab had at least one AE compared with 145/286 (51%) participants receiving triamcinolone acetonide (RR 1.2, 95% CI 1.1 to 1.4). Regarding SAE, 21/368 (6%) participants in the canakinumab group had an SAE compared with 8/286 (3%) participants in the triamcinolone acetonide group (RR 2.3, CI 1.0 to 5.2). Common AEs included back pain, headache, hypertension, arthralgia and gamma‐glutamyl transferase increase. SAE in the canakinumab groups were of diverse nature; of note, four serious infections occurred in the canakinumab group (jaw abscess, limb abscess, pneumonia and gastroenteritis) and none in the triamcinolone acetonide group. One death was observed per drug class (one from intracranial haemorrhage in the canakinumab group and one from pulmonary embolism in the triamcinolone acetonide group); investigators considered both deaths to be unrelated to the study drugs.
4.

Forest plot of comparison: 1 Canakinumab versus triamcinolone acetonide (TA), outcome: 1.4 Adverse events (all canakinumab doses).
Subcutaneous rilonacept 320 mg versus oral indomethacin
Pain
One study did not provide data on the proportion of participants who improved by 30% or more with respect to pain over time (Terkeltaub 2013). Indomethacin provided greater pain relief from baseline to 24 to 72 hours than rilonacept as measured by a 0 to 10 numerical rating scale (‐1.81 in the rilonacept group and ‐4.33 in the indomethacin group; MD 2.52, 95% CI 0.29 to 4.75) (Analysis 2.1; Figure 5).
2.1. Analysis.

Comparison 2 Rilonacept versus indomethacin, Outcome 1 Pain.
5.

Forest plot of comparison: 2 Rilonacept versus indomethacin, outcome: 2.1 Change in pain.
Inflammation
No data were provided for inflammation.
Function
No data were provided for function.
Health‐related quality of life
No data were provided for this outcome.
Participant‐reported global assessment of treatment success
No data were provided for participant global assessment of treatment success.
Study withdrawals due to adverse events
There was no difference in study withdrawals due to AE (1/75 (1%) participants in the rilonacept group and 2/76 (3%) participant in the indomethacin group; RR 0.51, 95% CI 0.05 to 5.47) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Rilonacept versus indomethacin, Outcome 2 Adverse events.
Adverse events and serious adverse events
There was no increased risk of AEs with rilonacept compared with indomethacin (27/75 (36%) participants had at least one AE in the rilonacept group and 23/76 (30%) participants in the indomethacin group; RR 1.2, 95% CI 0.8 to 1.9) (Analysis 2.2). No SAEs or deaths were observed in either of the monotherapy groups.
Subcutaneous rilonacept 320 mg plus oral indomethacin versus oral indomethacin alone
Pain
The study did not provide data on the proportion of participants who improved by 30% or more with respect to pain over time. Rilonacept plus indomethacin did not provide greater pain relief from baseline to 24 to 72 hours compared with indomethacin alone as measured by a 0 to 10 numerical rating scale (‐3.87 in the rilonacept plus indomethacin group and ‐4.33 in the indomethacin alone group; MD 0.46, 95% CI ‐2.30 to 3.22). (Analysis 3.1).
3.1. Analysis.

Comparison 3 Rilonacept plus indomethacin versus indomethacin, Outcome 1 Pain.
Inflammation
No data were provided for inflammation.
Function
No data were provided for function.
Health‐related quality of life
No data were provided for HRQoL.
Participant‐reported global assessment of treatment success
No data were provided for participant‐reported global assessment of treatment success.
Study withdrawals due to adverse events
There was no difference in study withdrawals due to AE (2/74 (3%) participants in the rilonacept plus indomethacin group and 2/76 (3%) participants in the indomethacin alone group; RR 1.03, 95% CI 0.15 to 7.10) (Analysis 3.2).
3.2. Analysis.

Comparison 3 Rilonacept plus indomethacin versus indomethacin, Outcome 2 Adverse events.
Adverse events and serious adverse events
There was no increased risk of AEs by using rilonacept in addition to indomethacin compared with indomethacin alone (34/74 (46%) participants had at least one AE in the rilonacept group and 23/76 (30%) participants had at least one AE in the indomethacin group; RR 1.52, 95% CI 1.00 to 2.31) (Analysis 3.2). In the rilonacept plus indomethacin group, three participants had an SAE (hypertensive cardiomyopathy, myocardial infarction, ulcerative colitis, tubulointerstitial nephritis, pyoderma gangrenosum) and one participant died due to a hypertensive cardiomyopathy. No deaths or SAE were observed in the indomethacin alone group (RR 7.19, 95% CI 0.38 to 136.77).
Discussion
Summary of main results
In this systematic literature review, we retrieved four trials including 879 participants with acute gout that involved treatment with an interleukin‐1 inhibitor.
Canakinumab versus triamcinolone
Based upon two trials (443 participants), there was moderate‐quality evidence that a single subcutaneous injection of canakinumab 150 mg provided greater pain relief at 72 hours compared with a single intramuscular injection of triamcinolone acetonide 40 mg (100‐mm VAS, MD ‐10.6, 95% CI ‐15.2 to ‐5.9) (Table 1). Based upon three trials (523 participants), there was moderate‐quality evidence that a greater proportion of people have complete resolution of joint swelling at 72 hours (RR 1.39, 95% CI 1.1 to 1.7). Based upon three trials (478 participants), there was moderate‐quality evidence that a greater proportion of participants assess their response to treatment as good or excellent at 72 hours (RR 1.37, 95 CI 1.2 to 1.6). There were no data about its comparative effect on function or HRQoL.
Based upon three trials (654 participants), there was moderate‐quality evidence that there were no between‐group differences in study withdrawals due to AEs (RR 1.1, 95% CI 0.2 to 7.2). However, there was moderate‐quality evidence that canakinumab 150 mg results in a slightly greater proportion of participants that experience at least one AE (RR 1.2, 95% CI 1.1 to 1.4)
Rilonacept versus indomethacin
Based upon a single trial comparing rilonacept with indomethacin (152 participants), there was low‐quality evidence that rilonacept may provide less pain relief than indomethacin at 24 to 72 hours (0‐10 numerical rating scale, MD 2.5, 95% CI 0.3 to 4.8) with no difference in the rate of study withdrawals due to AEs (RR 0.5, 95% CI 0.05 to 5.5) or of AEs (RR 1.2, 95% CI 0.8 to 1.9) (Table 2). There were no data on the effect of rilonacept on functional disability, HRQoL, inflammation or participant‐reported response to treatment.
Based upon a single trial comparing rilonacept plus indomethacin with indomethacin (150 participants), there was low‐quality evidence that rilonacept plus indomethacin does not provide greater pain relief than indomethacin alone at 24 to 72 hours (0‐10 numerical rating scale, MD 0.5, 95% CI ‐2.3 to 3.2) with no difference in the rate of study withdrawals due to AEs (RR 1.0, 95% CI 0.2 to 7.1) or in the rate of AEs (RR 1.5, 95% CI 1.0 to 2.3). There were no data on the effect of rilonacept plus indomethacin on functional disability, HRQoL, inflammation or participant‐reported response to treatment.
Overall completeness and applicability of evidence
Demographic data of the participants in these studies seem representative of the average gout population: that is, men (92%), aged in their 50's (mean 52.2 years) who commonly present with monoarticular flares (56%). Only 29% to 55% of the study participants were treated with ULTs when enrolled despite a high sUA level and the presence of previous flares. Although outside current recommendations, this reflects the reality in many clinical settings, where many people are never prescribed or do not adhere to ULT (Perez‐Ruiz 2011). In addition, in the canakinumab trials, people were excluded if they were morbidly obese or if they had significant co‐morbidities (e.g. uncontrolled diabetes, uncontrolled hypertension, congestive heart failure, thyroid disease) (Schlesinger 2012a; Schlesinger 2012b; So 2010a). Therefore, many people frequently encountered in gout clinics may not have been eligible for trial participation. The single rilonacept study did not exclude people with significant co‐morbidities, resulting in a population with a greater external validity (Terkeltaub 2013).
In the canakinumab studies, participants were included with a flare of up to five days' duration (Schlesinger 2012a; Schlesinger 2012b; So 2010a). The aim during gout flares is to treat people as early as possible (many times with a "pills in the pocket" approach (Richette 2010)) and part of patient education in gout is helping them detect the early symptoms of a flare so that adequate medication can be taken early. Clinical experience suggests that 'early' flares might respond differently to fully established or refractory flares. In Schlesinger 2012a and Schlesinger 2012b, approximately one‐fifth of the flares had a duration of four or five days, which may not reflect treatment initiation in routine care. In the rilonacept study, participants were included and received their medication within 48 hours of the beginning of the flare, also resulting in a higher applicability to real‐life situations.
Concerns have also been raised about the chosen comparator in the canakinumab trials. The two therapies that are most frequently used in the treatment of acute gout attacks are colchicine and NSAIDs. While the most recent ACR recommendations also include systemic glucocorticoids as a possible a first‐line therapy, they specified a single 60‐mg dose of intramuscular triamcinolone acetonide followed by oral prednisone or prednisolone of an alternative regimen although no consensus could be reached regarding the use of intramuscular triamcinolone as monotherapy (Khanna 2012). It is possible that the lower dosage of triamcinolone acetonide used in the canakinumab studies could have biased the trial results in favour of canakinumab.
No cost‐effectiveness data were available; however, the cost of a single dose of canakinumab 150 mg is far greater than the costs of a dose of triamcinolone 40 mg (e.g. in the UK around EUR 11,900 versus EUR 2) (NICE 2013). Given the cost difference and the increase in AEs, limiting the use of canakinumab to a selected population such as people refractory to, with contraindications to or intolerant of current mainstream therapies, might be a potential strategy. This is the approach chosen by the European Medicine Agency (EMA) while the FDA has rejected the license for canakinumab for acute gout flares given safety concerns.
We found no evidence regarding canakinumab compared with colchicine or NSAIDs, rilonacept compared with colchicine or glucocorticoids or other interleukin‐1 inhibitors such as anakinra.
Quality of the evidence
All trials had an unclear risk of bias. There was unclear risk of selection bias in all studies (no data given on random sequence generation or allocation concealment) and one study had additional concerns on selective reporting bias. All studies were sponsored and supported by the company manufacturing the interleukin‐1 inhibitor being assessed. Although pharmaceutical industry sponsoring is very common, it has been shown that industry‐sponsored drug studies can lead to more favourable results than sponsorship by other sources (Lundh 2012).
There was moderate‐quality evidence that, compared with a single intramuscular injection of the steroid, triamcinolone acetonide, canakinumab injection probably improves pain, swelling and the number of good or excellent treatment responses, but probably results in more side effects. Possible side effects include back pain, headache, hypertension, joint pain and a rise in the liver enzyme gamma‐glutamyl transpeptidase. Function or quality of life were not measured. Further research may change the estimates.
We found no studies comparing canakinumab with more commonly used therapies for acute gout flares such as NSAIDs or colchicine.
There was low‐quality evidence from a single study that rilonacept injection may improve pain less than indomethacin and may not be associated with an increase in AEs. Inflammation, disability, quality of life and participant assessment of treatment success were not measured. Further research is likely to change these estimates.
Potential biases in the review process
We performed a broad search retrieving over 600 hits. We included several different search terms for gout and for currently available interleukin‐1 inhibitors. We believe that the possibility of having missed published relevant studies is low. However, we acknowledge that the lag between the completion of the search and the publication of the review may be a source of bias.
Two review authors extracted all data independently, minimising the risk for data extraction errors. In addition, we searched EULAR and ACR abstracts in an effort to capture completed studies that were still unpublished.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other published systematic reviews on the use of interleukin‐1 inhibitors in acute gout.
The most recent ACR guidelines consider the use of interleukin‐1 inhibitors (anakinra or canakinumab) as off‐label therapies only to be used in people with an inadequate response to one or two conventional therapies (colchicine, NSAIDs, systemic corticosteroids) (Khanna 2012). The guidelines classified evidence for canakinumab as A (supported by more than one RCT or one or more meta‐analyses) and evidence for anakinra as C (consensus opinion, case studies or standard of care). This is consistent with the results of our current review. The panel of the ACR guidelines considered the role of interleukin‐1 inhibitor therapies (anakinra and canakinumab) as uncertain given the lack of RCTs for anakinra and the unclear risk/benefit ratio and lack of FDA approval for canakinumab.
Authors' conclusions
Implications for practice.
Based on the results of this review, in people with an acute gout flare treatment with a single subcutaneous dose of canakinumab 150 mg probably provides greater improvement than treatment with triamcinolone acetonide 40 mg. However, the use of suboptimal doses of glucocorticoid as the comparator might have favoured canakinumab in the existing trials. In addition, canakinumab was associated with a slightly greater risk of AEs (including several serious infections). There were no data available comparing canakinumab with more commonly used first‐line therapies for acute gout flares such as non‐steroidal anti‐inflammatories (NSAIDs) or colchicine. In view of our results and the far greater cost of canakinumab, in countries where canakinumab is licensed for use, it should possibly be reserved for people with a moderate or severe flare have major contraindications or are refractory to other, current mainstream therapies.
Rilonacept may provide less pain relief than maximum doses of indomethacin (50 mg three times a day) with a similar rate of adverse events across groups indicating that current evidence does not support the use of rilonacept for the treatment of acute gout flares. Rilonacept is not licensed for gout in, for example, the EU or US.
Implications for research.
Further randomised controlled trials comparing the effectiveness of canakinumab with commonly used first‐line therapies (such as NSAIDs and colchicine) are needed. In addition, further trials exploring the safety of canakinumab, especially regarding serious infections, should help to define the safety profile of the drug and cost‐effectiveness data should be available before the use of canakinumab can be considered in routine care.
Further randomised controlled trials of rilonacept versus other common first‐line therapies (such as colchicine or glucocorticoids) will help define the effectiveness and safety of rilonacept in acute gout flares. If effectiveness is shown, cost‐effectiveness data should also be available before the use of rilonacept can be considered in routine care.
Planned trials should include participants early in their acute flare (ideally within the first 24 hours as recommended by the American College of Rheumatology (ACR) guidelines (Khanna 2012)).
Trial reporting should include the method of randomisation and treatment allocation concealment; blinding of study participants, study personnel and outcome assessment; follow‐up of all participants who entered the trial and complete reporting of outcomes. Sample sizes should be reported and have adequate power to answer the research question; ideally trials should assess both the benefits and risks of the interventions. To enable comparison and pooling of the results of randomised controlled trials, we suggest that future trials report means with standard deviations for continuous measures and number of events and total numbers analysed for dichotomous measures, and assess outcomes recommended by OMERACT (Outcome Measures in Rheumatology) for studies of acute gout, including pain, joint swelling, joint tenderness, participant global assessment and activity limitations (Schumacher 2009).
What's new
| Date | Event | Description |
|---|---|---|
| 12 September 2011 | Amended | CMSG ID A071 |
Acknowledgements
We are indebted to Louise Falzon, former Trials Search Co‐ordinator, Cochrane Musculoskeletal Group for her help in developing and refining the search strategy and to Renea Johnston, Cochrane Musculoskeletal Group, for assisting with 'Summary of findings' tables.
Appendices
Appendix 1. The Cochrane Library search strategy
1. MeSH descriptor Gout explode all trees
2. gout*:ti,ab
3. tophus:ti,ab
4. tophi:ti,ab
5. tophaceous:ti,ab
6. or/1‐5
7. MeSH descriptor Interleukin‐1 explode all trees
8. Interleukin‐1:ti,ab
9. Il‐1:ti,ab
10. MeSH descriptor Interleukin 1 Receptor Antagonist Protein explode all trees
11. Anakinra:ti,ab
12. Rilonacept:ti,ab
13. Canakinumab:ti,ab
14. Ilaris:ti,ab
15. Kineret:ti,ab
16. Arcalyst:ti,ab
17. "leu?ocyte pyrogen*"data:ti,ab
18. "leukocytic endogenous mediator*":ti,ab
19. "lymphocyte activating factor*":ti,ab
20. or/7‐19
21. 6 and 20
Appendix 2. MEDLINE search strategy
1. gout[MeSH]
2. gout*[ti,ab]
3. tophus[ti,ab]
4. tophi[ti,ab]
5. tophaceous[ti,ab]
6. or/1‐5
7. Interleukin‐1 [MeSH]
8. interleukin‐1[ti,ab]
9. Il‐1[ti,ab]
10. "Interleukin 1 Receptor Antagonist Protein"[Mesh]
11. anakinra[ti,ab]
12. "canakinumab" [Supplementary Concept]
13. canakinumab[ti,ab]
14. "rilonacept" [Supplementary Concept]
15. rilonacept[ti,ab]
16. ilaris[ti,ab]
17. kineret[ti,ab]
18. arcalyst[ti,ab]
19. "leucocyte pyrogen*"[ti,ab]
20. "leukocytic endogenous mediator*"[ti,ab]
21. "lymphocyte activating factor*"[tiab]
22. or/7‐21
20. 6 and 22
Appendix 3. EMBASE search strategy
1. 'gout'/exp
2. gout*:ti,ab
3. tophus:ti,ab
4. tophi:ti,ab
5. tophaceous:ti,ab
6. or/1‐5
7. 'Interleukin 1'/exp
8. 'Interleukin‐1':ti,ab
9. 'Il‐1':ti,ab
10. 'recombinant interleukin 1 receptor blocking agent'/exp
11. Anakinra:ti,ab
12. 'canakinumab'/exp
13. Canakinumab:ti,ab
14. 'rilonacept'/exp
15. Rilonacept:ti,ab
16. Ilaris:ti,ab
17. Kineret:ti,ab
18. Arcalyst:ti,ab
19. leucocyte pyrogen*:ti,ab
20. leukocytic endogenous mediator*:ti,ab
21. lymphocyte activating factor*:ti,ab
22. or/7‐21
23. 6 and 22
Data and analyses
Comparison 1. Canakinumab versus triamcinolone acetonide (TA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: 100‐mm visual analogue scale at 72 hours | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Canakinumab 150 mg | 2 | 443 | Mean Difference (IV, Random, 95% CI) | ‐10.57 [‐15.24, ‐5.90] |
| 2 Inflammation: participants with no swelling at 72 hours | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Canakinumab 150 mg | 3 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.11, 1.74] |
| 3 Participant global assessment of response to treatment: good or excellent at 72 hours | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Canakinumab 10 mg | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.64, 2.16] |
| 3.2 Canakinumab 25 mg | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.62, 2.09] |
| 3.3 Canakinumab 50 mg | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.73, 2.36] |
| 3.4 Canakinumab 90 mg | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.66, 2.19] |
| 3.5 Canakinumab 150 mg | 3 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.16, 1.61] |
| 4 Adverse events (all canakinumab doses) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Study withdrawals due to adverse events | 3 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.16, 7.23] |
| 4.2 Participants with at least 1 adverse event | 3 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.05, 1.39] |
| 4.3 Participants with at least 1 serious adverse event | 3 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.04, 5.15] |
Comparison 2. Rilonacept versus indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Change 24‐72 hours numerical rating scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Study withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Participants with at least 1 adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Participants with at least 1 serious adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Rilonacept plus indomethacin versus indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Change 24‐72 hours numerical rating scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Study withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Participants with at least 1 adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Participants with at least 1 serious adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Schlesinger 2012a.
| Methods |
Design: randomised controlled trial Duration: 24 weeks (12 weeks + 12 weeks' extension; both double blind) Blinding: all participants and investigators were blinded. Unblinded study personnel prepared and administered the study medications Sample size: "a sample size of 110 patients per group (per study) was considered to be adequate to produce an overall power for both co‐primary endpoints of approximately 90%..." Analysis: "all efficacy end‐points were analysed using the full analysis set (i.e. all patients as randomised in the core studies who received at least one dose of study drug analysed according to assigned treatment) and all safety assessments were based on the safety set (i.e. all patients who received study drug and had at least one post‐baseline safety assessments, analysed according to received treatment)" Withdrawals: 6 (5%) participants in the canakinumab group: 1 withdrew consent, 3 lost to follow‐up, 2 administrative problems; 10 (8%) participants in the triamcinolone acetonide group: 3 withdrew consent, 1 lost to follow‐up, 1 administrative problem, 1 death, 4 unsatisfactory therapeutic effect |
|
| Participants | 230 participants randomised (115 in canakinumab group; 115 in triamcinolone acetonide); 2 participants in canakinumab group not analysed as they were randomised but received no study medication due to an administrative problem Participant characteristics: Mean age: 54.3 years Sex: men 209/228 Ethnicity: white 189, black 0, Asian 6, other 35 Number of joints involved: monoarticular 102, oligoarticular 87, polyarticular 39 Inclusion criteria: aged 18‐85 years, ACR 1977 preliminary acute gout classification criteria, ≥ 3 self reported flares in the previous 12 months, acute flare for ≤ 5 days, baseline pain intensity ≥ 50 mm on a 0‐100 mm VAS, having contraindications for or intolerance of or unresponsiveness to NSAIDs or colchicine or both, BMI ≤ 45 kg/m2 or less, if taking ULT on a stable dose and regimen for at least 2 weeks prior to randomisation and expected to remain on the same regimen throughout the study Exclusion criteria: use of prohibited medications before screening (corticosteroids, NSAIDs, analgesics, IL‐1 blocker, TNF inhibitor, biological drugs, colchicine); haemodialysis; live vaccination in the 3 months before the start of the study; donation or loss of 400 mL of blood in the 8 weeks before dosing; rheumatoid arthritis, infectious/septic arthritis or other acute inflammatory arthritis; secondary gout; active or recurrent infections or evidence of tuberculosis; risk factors for tuberculosis infection; requirement for antibiotics against latent tuberculosis; history of malignancy of any organ system (except basal cell carcinoma) within the preceding 5 years; significant medical problems including uncontrolled diabetes type 1 or 2, uncontrolled hypertension, congestive heart failure, refractory heart failure, unstable cardiac arrhythmias, unstable symptomatic coronary ischaemia, thyroid disease and any other condition considered by the investigator to immunocompromise the participant; presence of idiopathic thrombocytopenic purpura; long QT syndrome or QTc > 450 mseconds for men or > 470 mseconds for women; history of hypersensitivity to study drugs; pregnant or nursing (lactating) women. Women of childbearing age were required to be using an acceptable method of contraception |
|
| Interventions |
Group 1: canakinumab 150 mg subcutaneous (single dose on day 1 and re‐treatment on each new flare with a minimum period between consecutive administrations of 14 days) plus an intramuscular placebo Group 2: triamcinolone acetonide 40 mg intramuscular (single dose on day 1 and re‐treatment on each new flare with a minimum period between consecutive administrations of 14 days) plus a placebo subcutaneous injection |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Multicentre, multinational (β‐RELIEVED study: Australia, Belgium, Canada, Colombia, Estonia, Germany, Guatemala, Latvia, Lithuania, Mexico, Norway, Poland, Russia, Singapore, Sweden and Ukraine) SAE in the canakinumab group included angina pectoris, arrhythmia, myocardial ischaemia and renal artery occlusion, glaucoma, gastritis and chronic renal failure, spondylolisthesis, spinal cord ischaemia and lumbar spinal stenosis, jaw abscess, increased PSA, hyperglycaemia, device dislocation and pneumonia. SAE in the triamcinolone group included meniscus lesion, vertebrobasilar insufficiency, ischaemic stroke and 2 participants with gouty arthritis Novartis Pharma AG supported the study. Employees of Novartis Pharma AG participated in the design, data gathering, data analysis and data interpretation of the studies. Novartis Pharma also provided aid with medical writing support Trial registered: ClinicalTrials.gov NCT01029652 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Studies are described as random but the method of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | No data provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and investigators were blinded. Unblinded study personnel prepared and administered the study medications but did not participate in outcome assessment. It is unclear what instructions they received regarding participant communication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients and investigators were blinded to the study treatment" Participants and investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 214 of 230 randomised participants completed the study (95% of the canakinumab group and 92% in the triamcinolone acetonide group) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Studies were supported by Novartis Pharma AG. Employees of Novartis Pharma AG participated in the design, data gathering, data analysis and data interpretation of the studies. Novartis Pharma also provided aid with medical writing support |
Schlesinger 2012b.
| Methods |
Design: randomised controlled trial Duration: 24 weeks (12 weeks + 12 weeks' extension; both double‐blind) Blinding: all participants and investigators were blinded. Unblinded study personnel prepared and administered the study medications Sample size: "a sample size of 110 patients per group (per study) was considered to be adequate to produce an overall power for both co‐primary endpoints of approximately 90%..." Analysis: "all efficacy end‐points were analysed using the full analysis set (i.e. all patients as randomised in the core studies who received at least one dose of study drug analysed according to assigned treatment) and all safety assessments were based on the safety set (i.e. all patients who received study drug and had at least one post‐baseline safety assessments, analysed according to received treatment)" Withdrawals: 13 (12%) participants in the canakinumab group: 6 withdrew consent, 5 lost to follow‐up, 1 abnormal laboratory value, 1 death; 11 (10%) participants in the triamcinolone group: 4 withdrew consent, 3 lost to follow‐up, 1 administrative problem, 1 abnormal laboratory value, 2 protocol deviations |
|
| Participants | 226 participants randomised (112 in canakinumab group; 114 in triamcinolone acetonide 114) Participant characteristics: Mean age: 51.6 years Sex: men 205/226 Ethnicity: white 154, black 50, Asian 19, other 3 Number of joints involved: monoarticular 137, oligoarticular 51, polyarticular 38 Inclusion criteria: aged 18‐85 years, ACR 1977 preliminary acute gout classification criteria, ≥ 3 self reported flares in the previous 12 months, acute flare for ≤ 5 days, baseline pain intensity ≥ 50 mm on a 0‐100 mm VAS, having contraindications for or intolerance of or unresponsiveness to NSAIDs or colchicine or both, BMI ≤ 45 kg/m2 or less, if taking ULT on a stable dose and regimen for at least 2 weeks prior to randomisation and expected to remain on the same regimen throughout the study Exclusion criteria: use of prohibited medications before screening (corticosteroids, NSAIDs, analgesics, IL‐1 blocker, TNF inhibitor, biological drugs, colchicine...); haemodialysis; live vaccination in the 3 months before the start of the study; donation or loss of 400 mL of blood in the 8 weeks before dosing; rheumatoid arthritis, infectious/septic arthritis or other acute inflammatory arthritis; secondary gout; active or recurrent infections or evidence of tuberculosis; risk factors for tuberculosis infection; requirement for antibiotics against latent tuberculosis; history of malignancy of any organ system (except basal cell carcinoma) within the preceding 5 years; significant medical problems including uncontrolled diabetes type 1 or 2, uncontrolled hypertension, congestive heart failure, refractory heart failure, unstable cardiac arrhythmias, unstable symptomatic coronary ischaemia, thyroid disease and any other condition considered by the investigator to immunocompromise the participant; presence of idiopathic thrombocytopenic purpura, long QT syndrome or QTc > 450 mseconds for men or > 470 mseconds for women; history of hypersensitivity to study drugs; pregnant or nursing (lactating) women. Women of childbearing age were required to be using an acceptable method of contraception |
|
| Interventions |
Group 1: canakinumab 150 mg subcutaneous (single dose on day 1 and re‐treatment on each new flare with a minimum period between consecutive administrations of 14 days plus an intramuscular placebo Group 2: triamcinolone acetonide 40 mg intramuscular (single dose on day 1 and re‐treatment on each new flare with a minimum period between consecutive administrations of 14 days) plus a placebo subcutaneous injection |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Multicentre, multinational (β‐RELIEVED II study: Canada, Netherlands, Russia, Taiwan, USA) SAE in the canakinumab group included gastroenteritis, convulsion, abscessed limb, atrial fibrillation, pancreatitis, back pain and intervertebral disc protrusion. SAE in the triamcinolone group included diarrhoea, nausea and vomiting and aortic valve incompetence, aortic stenosis, worsening bicuspid aortic valve and cardiomyopathy Novartis Pharma AG supported the study. Employees of Novartis Pharma AG participated in the design, data gathering, data analysis and data interpretation of the studies. Novartis Pharma also provided aided with the medical writing support ClinicalTrials.gov trial identifier: NCT01080131 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Studies were described as random but there were no further data provided |
| Allocation concealment (selection bias) | Unclear risk | No data provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double blind. Participants and investigators were blinded. Unblinded study personnel prepared and administered the study medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients and investigators were blinded to the study treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 202/226 randomised participants completed the study (88% of participants in the canakinumab group and 90% of participants in the triamcinolone group) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Unclear risk | Studies were supported by Novartis Pharma AG. Employees of Novartis Pharma AG participated in the design, data gathering, data analysis and data interpretation of the studies. Novartis Pharma also provided aided with the medical writing support |
So 2010a.
| Methods |
Design: randomised controlled trial Duration: 8 weeks Blinding: single‐blind study (participants) Sample size: "a sample size of 200 patients was considered sufficient to give a half‐width of the 95% confidence interval of the target dose of at most 40mg... " Analysis: not specified if analysis was performed by intention‐to‐treat or per protocol Withdrawals: 1 participant in Group 1 (lost to follow‐up), 1 participant in Group 2 (lost to follow‐up), 2 participants in Group 3 (1 withdrew consent and 1 lost to follow‐up), 1 participant in Group 4 (withdrawal of consent), 1 participant in Group 5 (lost to follow‐up), 3 participants in Group 6 (2 withdrew consent, 1 administrative problem) |
|
| Participants | 200 participants randomised (57 in triamcinolone acetonide group; 143 in canakinumab groups) Participant characteristics: Mean age: 51.9 years Sex: men 186/200 Ethnicity: white 180, black 13, Asian 3, other 4 Number of joints involved: monoarticular 126, oligoarticular 56, polyarticular 18 Inclusion criteria: aged 18‐80 years; history of at least 1 previous flare; ACR 1977 preliminary criteria for gout; acute flare for ≤ 5 days; baseline pain on VAS ≥ 50/100; refractory, intolerant or contraindications to NSAIDs or colchicine or both (based on treating physician assessment); BMI ≤ 40 kg/m2 If on ULT on stable dose regimen (4 weeks before randomisation) and were expected to remain on this regimen throughout the study Exclusion criteria: use of specified medications within specified periods before screening (NSAIDs, corticosteroids, IL‐1 inhibitors, anti‐TNF inhibitors, colchicine...); rheumatoid arthritis; septic or other inflammatory arthritis; severe renal function impairment; drug allergies; idiopathic thrombocytopenic purpura; contraindication to intramuscular injection; donation or blood loss of ≥ 400 mL in the 8 weeks before dosing; live vaccination 3 months before start of study; active or recurrent infection at enrolment; active pulmonary disease; requirement of antibiotics for latent tuberculosis; risk factors for tuberculosis; any surgical, hepatic, haematological, pulmonary, infectious or gastrointestinal condition that compromised the immune system or would place the participant at unacceptable risk if they received immunomodulatory therapy; long QT syndrome or QTc > 450 mseconds for men and > 470 mseconds for women; significant medical problems (i.e. uncontrolled hypertension, uncontrolled diabetes mellitus, thyroid disease, malignancy within the preceding 5 years); women of childbearing age not using an acceptable method of contraception; pregnant or lactating women |
|
| Interventions | Single subcutaneous injection of canakinumab (day 1) at 1 of the following doses: Group 1: 10 mg Group 2: 25 mg Group 3: 50 mg Group 4: 90 mg Group 5: 150 mg plus a saline intramuscular injection OR Group 6: single intramuscular injection of triamcinolone acetonide 40 mg (day 1) plus a placebo subcutaneous injection |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Multicentre, multinational (US, Canada, Argentina, Turkey, Europe) SAE in the canakinumab group included 2 participants with appendicitis, bronchitis and carotid artery stenosis. SAE in the triamcinolone group included a cerebrovascular disorder Novartis Pharma AG supported the study. Employees of Novartis Pharma AG participated in the design, data gathering, data analysis and data interpretation of the studies. Novartis Pharma also provided aided with the medical writing support ClinicalTrials.gov identifier: NCT00798369 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data on method of randomisation provided |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Wherever possible, treatment was administered by an unblinded pharmacist, nurse or physician who was not involved in any of the study assessments, allowing the investigator to be blinded. This was the case for 161/200 patients who thus received double‐blind treatment" Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Wherever possible, treatment was administered by an unblinded pharmacist, nurse or physician who was not involved in any of the study assessments, allowing the investigator to be blinded to treatment. This was the case for 161 of the 200 patients (80.5%) who thus received double‐blind treatment" Most outcomes were directly assessed by the participant, who was blinded. For physician global assessment, 81% of the investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 191/200 participants completed the study; 4 lost to follow‐up in canakinumab group, 2 withdrew consent in each group, 1 withdrawn because of 'administrative problem' in triamcinolone group |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes (pain in Likert scales) or all time points pre‐specified in the protocol at ClinicalTrials.gov were reported |
| Other bias | Unclear risk | Studies were supported by Novartis Pharma AG. Employees of Novartis Pharma AG participated in the design, data gathering, data analysis and data interpretation of the studies. Novartis Pharma also provided aided with the medical writing support |
Terkeltaub 2013.
| Methods |
Design: randomised controlled trial Duration: 31 days Blinding: described as double blind Sample size: "a sample size of 75 patients per group was calculated to provide at least 90% power for pair wise comparisons..." Analysis: not specified if analysis was performed as intention‐to‐treat or per protocol Withdrawals: 11 (14.7%) withdrawals in the rilonacept monotherapy group (2 protocol deviations, 1 AE, 2 lack of benefit, 2 request by participant, 2 lost to follow‐up, 2 other reasons); 8 (10.5%) withdrawals in the indomethacin group (2 AE, 2 lack of benefit, 2 request by participant, 2 lost to follow‐up, 1 other reasons); 9 (12.2%) in the combination group (1 AE, 2 lack of benefit, 3 request by participant, 1 death, 1 other reason) |
|
| Participants | 225 participants randomised (75 in the rilonacept monotherapy group, 76 in the indomethacin monotherapy group and 74 in the combination group) Participant characteristics: Mean (SD) age: 50.3 (10.6) years Sex: men 209/222 Ethnicity: white 168, black 41, Asian 9, other 4 Number of joints involved: monoarticular not specified, oligoarticular not specified, polyarticular none Inclusion criteria: aged 18‐70 years, gout diagnosis based on 1977 ACR preliminary criteria for the diagnosis of acute gout, previously demonstrated symptomatic relief with NSAIDs for treatment of acute flares, acute flare of no more than 48 hours of pain onset, pain in index joint ≥ 3 in a 5‐point Likert scale (1 = none, 2 = mild, 3 = moderate, 4 = severe, 5 = extreme), ≥ 1 on a scale of 0 to 3 for assessment of swelling in index joint, ≥ 1 on a scale of 0‐3 for assessment of tenderness in index joint, flare in ≤ 3 joints Exclusion criteria: treatment with short‐acting NSAIDs within 48 hours of randomisation; treatment with other NSAIDs based on duration of action; use of systemic glucocorticoids within 4 weeks; use of colchicine exceeding 0.6 mg twice daily within 7 days; history of NSAID intolerance or absolute contraindication; active or recurrent infections; estimated creatinine clearance < 60 mL/minute; history of bleeding disorders, gastrointestinal bleeding or perforation; poorly controlled hypertension; other cardiovascular risk factors |
|
| Interventions |
Group 1: single injection of rilonacept 320 mg subcutaneously at baseline plus oral placebo 3 times daily for 3 days and then 3 times daily for up to 9 days Group 2: indomethacin orally 50 mg 3 times daily for 3 days and then 25 mg 3 times daily for up to 9 days plus subcutaneous placebo at baseline Group 3: single injection of rilonacept 320 mg subcutaneously at baseline plus indomethacin orally 50 mg 3 times daily for 3 days and then 25 mg 3 times daily for up to 9 days |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
|
|
| Notes | Multicentre, multinational trial Regeneron Pharmaceuticals, Inc. supported the study and funded editorial support in the preparation of the manuscript. Employees of Regeneron Pharmaceuticals, Inc. were study authors and participated in the design, data gathering, data analysis and data interpretation ClinicalTrials.gov identifier: NCT00855920 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no data provided on method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No data provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind; no further specification provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; withdrawal rate was low and similar across groups (10.5‐14.7%) 11 (14.7%) withdrawals in the rilonacept monotherapy group (2 protocol deviations, 1 AE, 2 lack of benefit, 2 request by participant, 2 lost to follow‐up, 2 other reasons); 8 (10.5%) withdrawals in the indomethacin group (2 AE, 2 lack of benefit, 2 request by participant, 2 lost to follow‐up, 1 other reasons); 9 (12.2%) in the combination group (1 AE, 2 lack of benefit, 3 request by subject, 1 death, 1 other reason) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes have been reported |
| Other bias | Unclear risk | This study was funded by Regeneron Pharmaceuticals, Inc. Editorial support in the preparation of this manuscript was funded by Regeneron Pharmaceuticals, Inc. Four authors were employees and stock holders of Regeneron Pharmaceuticals, Inc. Regeneron holds patents related to the content of the manuscript |
ACR: American College of Rheumatology; AE: adverse event; BMI: body mass index; IL: interleukin; NSAID: non‐steroidal anti‐inflammatory drug; PSA: prostate‐specific antigen; SAE: serious adverse event; SD: standard deviation; TNF: tumour necrosis factor; ULT: urate‐lowering therapy; VAS: visual analogue scale.
Differences between protocol and review
Given the time lag between the protocol and the review, EULAR 2012 abstracts were included in the literature search.
The primary and secondary outcomes have been replaced by a list of major outcomes (i.e. those presented in the 'Summary of findings' tables) in the review. This was done to implement GRADE and the use of 'Summary of findings' tables. Other sources of bias have been defined in the methods of the review after the protocol was published, specifically funding source and baseline imbalance.
Contributions of authors
FS wrote the first draft of the review.
MW, MA, LC and RB contributed to the final version of the review by providing comments and suggestions.
All authors approved the final version of the review.
Sources of support
Internal sources
-
Hospital General Universitario de Elda, Elda, Spain.
In‐kind support
-
Rheumatology Unit, Repatriation General Hospital, Daw Park, South Australia and Flinders University of South Australia, Bedford Park, Australia.
In‐kind support
-
Hospital General Universitario de Alicante, Alicante, Spain.
In‐kind support
-
Department of Clinical Epidemiology, Cabrini Hospital, Malvern, Australia.
In‐kind support
-
Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Australia.
In‐kind support
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Schlesinger 2012a {published data only (unpublished sought but not used)}
- NCT01029652. Canakinumab in the treatment of acute gout flares and prevention of new flares in patients unable to use non‐steroidal anti‐inflammatory drugs (NSAIDs) and/or colchicine including a 12 weeks extension and an open‐label 48 weeks extension study (β‐RELIEVED). clinicaltrials.gov/show/NCT01029652 (accessed 24 August 2014).
- Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active‐controlled, double‐blind trials and their initial extensions. Annals of the Rheumatic Diseases 2012;71(11):1839‐48. [DOI] [PubMed] [Google Scholar]
Schlesinger 2012b {published data only (unpublished sought but not used)}
- NCT01080131. Canakinumab in the treatment of acute gout flares and prevention of new flares in patients unable to use non‐steroidal anti‐inflammatory drugs (NSAIDs) and/or colchicines including a 12 week extension and a 1 year open‐label extension study (β‐RELIEVED‐II). clinicaltrials.gov/show/NCT01080131 (accessed 24 August 2014).
- Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active‐controlled, double‐blind trials and their initial extensions. Annals of the Rheumatic Diseases 2012;71(11):1839‐48. [DOI] [PubMed] [Google Scholar]
So 2010a {published data only (unpublished sought but not used)}
- NCT00798369. Targeted dose finding of canakinumab (ACZ885) for management of acute flare in refractory or contraindicated gout patients. clinicaltrials.gov/show/NCT00798369 (accessed 24 August 2014).
- Schlesinger N, Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health‐related quality of life in patients with difficult to treat gout arthritis by suppressing inflammation: results of a randomized, dose‐ranging study. Arthritis Research & Therapy 2011;13(2):R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A, Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult‐to‐treat gouty arthritis: results of a multicenter, phase II, dose‐ranging study. Arthritis & Rheumatism 2010;62(10):3064‐76. [DOI] [PubMed] [Google Scholar]
Terkeltaub 2013 {published data only}
- Terkeltaub RA, Schumacher HR, Carter JD, Baraf HSB, Evans RR, Wang J, et al. Rilonacept in the treatment of acute gouty arthritis: a randomized, controlled clinical trial using indomethacin as the active comparator. Arthritis Research & Therapy 2013;15:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Busquets 2011
- Busquets N, Carmona L, Suris X. Systematic review: safety and efficacy of anti‐TNF in elderly patients. Reumatologia Clinica 2011;7(2):104‐12. [DOI] [PubMed] [Google Scholar]
Campion 1987
- Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. American Journal of Medicine 1987;82(3):421‐6. [DOI] [PubMed] [Google Scholar]
Cates 2004
- Cates C. Dr Chris Cates' EBM web site. Visual Rx version 3. www.nntonline.net/visualrx (accessed 24 August 2014).
Choi 2007
- Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116(8):894‐900. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Dalbeth 2009
- Dalbeth N, Clark B, Gregory K, Gamble G, Sheehan T, Doyle A, et al. Mechanisms of bone erosion in gout: a quantitative analysis using plain radiography and computed tomography. Annals of the Rheumatic Diseases 2009;68(8):1290‐5. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
FDA 2011
- Food, Drug Administration. Center for Drug Evaluation and Research. Summary minutes of the Arthritis Advisory Committee meeting June 21, 2011. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM267388.pdf (accessed 24 August 2014).
Grainger 2009
- Grainger R, Taylor WJ, Dalbeth N, Perez‐Ruiz F, Singh JA, Waltrip RW, et al. Progress in measurement instruments for acute and chronic gout studies. Journal of Rheumatology 2009;36:2346‐55. [DOI] [PubMed] [Google Scholar]
Gutman 1973
- Gutman AB. The past four decades of progress in the knowledge of gout with and assessment of present status. Arthritis & Rheumatism 1973;16:431‐45. [DOI] [PubMed] [Google Scholar]
Harrold 2006
- Harrold LR, Yood RA, Mikuls TR, Andrade SE, Davis J, Fuller J, et al. Sex differences in gout epidemiology: evaluation and treatment. Annals of the Rheumatic Diseases 2006;65(10):1368‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Khanna 2012
- Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis in acute gouty arthritis. Arthritis Care and Research 2012;64(10):1447‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Martinon 2006
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout‐associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237‐41. [DOI] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, WIffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Neogi 2011
- Neogi T. Gout. New England Journal of Medicine 2011;364:443‐52. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Gouty arthritis: canakinumab. www.nice.org.uk/Advice/ESNM23 (accessed 24 August 2014).
Pascual 2007
- Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Annals of the Rheumatic Diseases 2007;66(8):1056‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Ruiz 2011
- Perez‐Ruiz F, Carmona L, García de Yébenes MJ, Pascual E, Miguel E, Ureña I, et al. An audit of the variability of diagnosis and management of gout in the rheumatology setting: the Gout Evaluation and Management Study. Journal of Clinical Rheumatology 2011;17:349‐55. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richette 2010
- Richette P, Bardin T. Colchicine for the treatment of gout. Expert Opinion on Pharmacotherapy 2010;11(17):2933‐8. [DOI] [PubMed] [Google Scholar]
Schlesinger 2011
- Schlesinger N, Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health‐related quality of life in patients with difficult to treat gout arthritis by suppressing inflammation: results of a randomized, dose‐ranging study. Arthritis Research and Therapy 2011;13(2):R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2005
- Schumacher HR, Edwards LN, Perez‐Ruiz F, Becker M, Chen LX, Furst DE, et al. Outcome measures for acute and chronic gout. Journal of Rheumatology 2005;32:2452‐5. [PubMed] [Google Scholar]
Schumacher 2009
- Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. Journal of Rheumatology 2009;36(10):2342‐5. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Smith 2010
- Smith EU, Diaz‐Torne C, Perez‐Ruiz F, March LM. Epidemiology of gout: an update. Best Practice & Research Clinical Rheumatology 2010;24(6):811‐27. [DOI] [PubMed] [Google Scholar]
So 2010b
- So A, Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult‐to‐treat gouty arthritis: results of a multicenter, phase II, dose‐ranging study. Arthritis & Rheumatism 2010;62(10):3064‐76. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Wallace 1977
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis & Rheumatism 1977;20:895‐900. [DOI] [PubMed] [Google Scholar]