Abstract
The mammalian E2A, HEB, and E2-2 genes encode a unique class of basic helix-loop-helix (bHLH) transcription factors that are evolutionarily conserved and essential for embryonic and postnatal development. While the structural and functional similarities among the gene products are well demonstrated, it is not clear why deletion of E2A, but not HEB or E2-2, leads to a complete arrest in B-lymphocyte development. To understand the molecular basis of the functional specificity between E2A and HEB/E2-2 in mammalian development, we generated and tested a panel of E2A knockin mutations including subtle mutations in the E12 and E47 exons and substitution of both E12 and E47 exons with a human HEB cDNA. We find that the alternatively spliced E12 and E47 bHLH proteins of the E2A gene play similar and additive roles in supporting B lymphopoiesis. Further, we find that HEB driven by the endogenous E2A promoter can functionally replace E2A in supporting B-cell commitment and differentiation toward completion. Finally, the postnatal lethality associated with E2A disruption is fully rescued by the addition of HEB. This study suggests that the functional divergence among E12, E47, and HEB in different cell types is partially defined by the context of gene expression.
B lymphocytes in mammals are derived from hematopoietic stem cells (HSC) present in the liver during fetal development and bone marrow in adult life. The same HSC also generate T lymphocytes, erythrocytes, macrophages, and other cell types in blood and the lymphoid organs. Once committed to the B-cell lineage, the HSC follow a stepwise differentiation pathway to become B lymphocytes, which subsequently participate in diverse humoral immune responses. It is not entirely known how and when B-lineage cells are first specified from HSC and how B lymphopoiesis is maintained throughout life.
B-lineage development can be divided roughly into three discrete stages: progenitor (pro-B), precursor (pre-B), and mature B-cell stages. Rearrangements of immunoglobulin (Ig) genes initiate at the pro-B stage and proceed to completion at the pre-B stage. Pre-B cells expressing functional but nonself-reactive B-cell receptors are selected for survival and expansion to become mature B cells (18). In addition, B cells at various stages of development express lineage-specific markers, such as B220, CD43, and CD19 surface antigens (9, 19). These lineage markers, combined with the status of Ig rearrangements and expression, establish road signs of normal and abnormal progression of B lymphopoiesis (9).
Regulation at the transcriptional level is crucial in each step of B-cell development. Indeed, recent studies have shown that E2A, EBF, Pax5, Ikaros, and several other transcription factors play key roles in the pro- and pre-B stages of development (2, 14, 29, 31, 35). Disruption of genes encoding each one of these transcription factors arrests B-cell development at either the pro- or pre-B-cell stage. Since deletion of E2A blocks B-cell development prior to the initiation of Ig gene rearrangement, E2A seems to play an extremely early role. Furthermore, E2A is the only gene that has been shown to be capable of triggering Ig gene D-J rearrangement in non-B cells, making E2A a key regulatory component for B-lineage commitment (6, 23).
E2A is a founding member of the basic-helix-loop-helix (bHLH) gene family, which is defined by the conserved bHLH structure, a protein domain involved in DNA binding and protein dimerization. E2A encodes two bHLH proteins, E12 and E47, which contain different bHLH domains resulting from alternative splicing (15, 27). E12 and E47, together with the gene products of the E2-2 and HEB genes (10, 11), comprise a unique subfamily of bHLH proteins, commonly known as the E-protein family. These E proteins are ubiquitously, although not evenly, expressed and are capable of forming heterodimers with a variety of bHLH proteins, including tissue-specific bHLH proteins such as MyoD (13, 16) and broadly expressed inhibitory proteins such as Id1 (4). A large body of evidence indicates that E2A proteins participate in tissue-specific regulation through heterodimer formation with the tissue-specific bHLH proteins (13, 16). This activity of E2A can be inhibited by Id proteins that dimerize with E2A and form a non-DNA binding heterodimer (4, 32). Thus, E2A plays a central role in tissue-specific gene regulation by mediating positive and negative signals. However, gene knockout studies have shown that this generic function of E2A may not be provided by E2A alone, since most tissue types and organs can develop in the absence of the E2A gene (2, 34, 35).
The function of E2A in B-cell development is thought to be mediated by E2A protein homodimers. Studies have demonstrated that E47 is capable of forming homodimers in the physiological conditions of B cells but not other cell types (5, 25). E47 homodimerization is regulated posttranslationally through at least two possible means: a disulfide bond can covalently link two monomers and prevent dimer dissociation (5), and hypophosphorylation at the region upstream of the basic domain may enhance homodimer formation (25). Therefore, it has been generally believed that the initiation and maintenance of E47 homodimers in B cells underlies the specificity of E2A function in B-cell development. E12, on the other hand, does not form homodimers because of the inhibitory effect of an acidic region adjacent to the bHLH domain (27). Nonetheless, a recent study has suggested that E12 may collaborate with E47 in supporting B-lineage commitment (3). The molecular basis of this cooperation between E12 and E47 is not known.
The function of E2-2 and HEB in B-cell development has also been investigated through targeted mutation in mice (36). Mice lacking either E2-2 or HEB, in contrast to the E2A mutation, are still able to generate substantial amounts of B-lineage cells, suggesting that neither E2-2 nor HEB is essential to B-cell commitment and maturation. However, the number of pro-B cells produced in E2-2 or HEB mutant mice is slightly reduced and can be reduced further in the compound-heterozygous background of E2A and E2-2 or E2A and HEB (36). Based on these observations, we have proposed that E2-2 and HEB can modulate, but may not replace, E2A activity, and the interaction between E2A and E2-2 or HEB is due to titration of the Id proteins, the common dimerization partners of all E proteins (28, 36).
To further explore the functional specificity of individual E proteins in B-cell development, we generated and analyzed a panel of E2A mutations. First, we used gene targeting to convert the endogenous E47 into a dominant negative form by introducing a point mutation into the basic region of the bHLH domain of E47. This mutation, together with a previously generated E12 knockout allele, demonstrated that either E12 or E47 is sufficient to support a limited, but definitive, B-lineage commitment. However, a sustained and optimal B-cell production requires both E12 and E47. Second, we used a knockin approach to replace the mouse E2A gene with a human HEB cDNA. When the HEB cDNA was tested on the E2A-null background, we found that two copies, but not one, of human HEB can support B-cell development. This study demonstrates that E47, E12, and HEB have similar structural features required for B-cell development. Further, the results suggest that B-cell commitment and maturation are regulated by a threshold level of E proteins.
MATERIALS AND METHODS
Construction of E47bm and E2Aheb alleles.
The E47bm (basic-region mutation) targeting construct was built on a 9-kb KpnI-BamHI fragment of the E2A genomic DNA isolated from the 129/sv strain. A 2-kb subclone covering the E47 exon was used in site-directed mutagenesis (Amersham Sculptor kit) for introduction of the E47 basic-region mutation. The mutation was verified by sequencing before subcloning into the targeting construct. A phosphoglycerate kinase (PGK)-neomycin phosphoryltransferase (Neo) cassette was inserted into the unique XbaI site located downstream of the E2A gene. A (PGK)-thymidine kinase (TK) cassette was placed outside the targeting module and used as a negative selection marker to eliminate the nonhomologous recombination events.
A separate and more generic targeting vector was made for introducing any cDNA sequence into the E2A locus. This construct contains, from 5′ to 3′, a 6-kb E2A homologous sequence, the Neo coding sequence fused in frame with E2A, a unique cloning site for introduction of foreign cDNA, and a short 3′ homologous sequence to facilitate PCR detection of recombination events. A full-length human HEB cDNA (11) was inserted between the Neo marker and the 3′ homologous sequence. Translation of the HEB cDNA was provided by the encephalomyocarditis virus internal ribosomal entry site (IRES) added in front of the HEB cDNA (12). Transcription of the HEB cDNA was provided by the endogenous E2A promoter when the construct was integrated into the E2A locus.
Gene targeting was performed in an embryonic stem (ES) cell line (a gift from A. Imamoto and P. Soriano) derived from a 129/sv mouse. Clones carrying targeting events were identified by PCR and subsequently confirmed by Southern analysis. Chimeric mice were derived by injecting ES cells into blastocysts harvested from C57BL/6 mice. Germ line transmission was tested by crossing chimeric males with C57BL/6 females, and the resulting heterozygous mice were maintained thereafter on the mixed background of 129/sv and C57BL/6. All mice were maintained in a specific-pathogen-free environment throughout the study.
PCR genotyping.
DNA used in genotyping was prepared from toes cut at approximately 10 days of age. Toe tissue was digested at 50°C for more than 3 h in 0.1 ml of lysis buffer containing 100 mM NaCl, 10 mM Tris (pH 8.0), 25 mM EDTA, 0.5% sodium dodecyl sulfate, and freshly added proteinase K (0.1 mg/ml). DNA was purified by extraction with phenol once and chloroform once and addition of 2 volumes of 95% ethanol. After a wash with 70% ethanol, the DNA pellet was resuspended in 0.5 ml of Tris-EDTA buffer. PCR was performed by adding 1 μl of DNA into 15 μl of the reaction cocktail, which consisted of 1× PCR buffer, 10% dimethyl sulfoxide, 1 mM deoxynucleoside triphosphate, 0.1 μM each primer, and Taq polymerase; 10× PCR buffer contained 166 mM ammonium sulfate, 670 mM Tris (pH 8.8), 67 mM MgCl2, 50 mM 2-mercaptoethanol, and 67 μM EDTA. PCR was performed with an MJ thermocycler using the following program: step 1, 93°C for 1.5 min; step 2, 93°C for 0.5 min; step 3, 57°C for 0.5 min; step 4, 65°C for 3 min; repetition of steps 2 to 4 40 times. For each allele, three PCR primers were used for simultaneous amplification of both mutant and wild-type alleles. Sequences of PCR primers used in genotyping and the results expected from use of each primer set are shown in Tables 1 and 2.
TABLE 1.
PCR primer sequences
| Name | Sequence | Location |
|---|---|---|
| YZ-104 | 5′-ATG TGT GGT GGC CCA CAC TTG T-3′ | E2A antisense |
| YZ-177 | 5′-TTG TGG ACA TTT TCT AGG CAG-3′ | E2A sense |
| YZ-164 | 5′-AAG AAC GAG GCC TTC CGT GTC-3′ | E2A sense |
| E19 | 5′-CCG AGC TCC TTA AAG GCC TCA-3′ | E12 antisense |
| E21 | 5′-CAG TAC AGA TGA GGT GCT GTC C-3′ | E47 sense |
| YZ-24 | 5′-GTT GTG CCC AGT CAT AGC CG-3′ | Neo antisense |
| YZ-29 | 5′-TCG CAG CGC ATC GCC TTC TA-3′ | Neo sense |
TABLE 2.
PCR primers and expected PCR results
| Allele | Primer set
|
Size (kb) of products
|
|||
|---|---|---|---|---|---|
| Common | Mutant | Wild type | Mutant | Wild type | |
| E2Ako | YZ-104 | YZ-29 | YZ-164 | 2.0 | 1.7 |
| E2Agal | YZ-177 | YZ-24 | E19 | 0.9 | 1.1 |
| E2Aheb | YZ-177 | YZ-24 | E19 | 0.9 | 1.1 |
| E12ko | YZ-177 | YZ-24 | E19 | 1.2 | 1.1 |
| E47bm | YZ-104 | YZ-29 | YZ-164 | 2.0 | 1.7 |
Western and RT-PCR analyses.
Western analysis was carried out by first separating nuclear extracts on a sodium dodecyl sulfate–10% polyacrylamide gel and then blotting the proteins to a nitrocellulose membrane. Anti-E2A polyclonal sera (provided by T. Kadesch), anti-E2A monoclonal antibody (Yae; Santa Cruz Biotechnology), and anti-HEB polyclonal sera (Santa Cruz) were used to detect relevant E proteins. Horseradish peroxidase-conjugated secondary antibodies were used in an enhanced chemiluminescence (ECL) reaction (Amersham). Reverse transcription (RT)-PCR was carried out essentially as described (20). Briefly, 0.1 μg of cytoplasmic RNA was used in each RT reaction, and 1/10 of the reaction mixture was used for each PCR. PCR was carried out for 22 cycles with elongation factor 1 alpha (EF1α) primers and 28 cycles with all of the E2A primers.
Flow cytometry.
Bone marrow, spleen, and fetal liver cells were isolated from various age group mice and used immediately for fluorescence-activated cell sorting (FACS) analysis. Cell suspensions were stained with a combination of a fluorescein isothiocyanate (FITC)-conjugated antibody and a phycoerythrin (PE)-conjugated antibody plus 7-amino-actiomycin D (7AAD). Live cells were analyzed on a FACScan (Becton Dickinson) for simultaneous detection and recording of FITC, PE, and 7AAD signals. Data were processed by using the CellQuest program (Becton Dickinson). 7AAD is a DNA dye that stains permeable or dead cells (Molecular Probes). The use of 7AAD was crucial for eliminating nonspecific staining of antibodies to dead cells. Anti-mouse CD19 antibody was provided by S. Sato and T. F. Tedder (21). All other antibodies were purchased from Sigma, Jackson Immunology, and Pharmingen.
Irradiation and stem cell reconstitution.
C57BL/6 mice congenic for the Ly5A allotype marker were purchased from Jackson Laboratories. Host mice at 8 to 12 weeks of age were irradiated with 1,100 rads 1 day before stem cell transfusion and maintained in sterile bedding and antibiotics thereafter. Donor cells were prepared from either frozen stocks or freshly isolated fetal liver or bone marrow cells. From 0.1 × 106 to 0.5 × 106 total fetal liver or 0.5 × 105 to 2 × 105 bone marrow cells were delivered to the host in 0.2 ml of phosphate-buffered saline through tail vein injection. For each donor type, two to five recipients were used in the test. Mice were bled at 1 month and sacrificed at 2 months after irradiation for FACS analysis.
RESULTS
E2A alleles used in this study.
Table 3 lists the E2A alleles used in this study. E2Ako and E2Agal are two independent alleles with both E12 and E47 exons deleted from the gene. The E2Ako allele (also known as E2AΔbhlh) has been thoroughly characterized and reported in an earlier study (35). The phenotype of E2Agal is essentially the same as E2Ako except that the former expresses a β-galactosidase marker driven by the endogenous E2A promoter. In this report, both alleles are used interchangeably as E2A-null controls. The E12ko allele has been generated and analyzed at the ES cell level in a previous study (34). Essentially, the Neo coding sequence was inserted into the E12 exon to produce a E2A-Neo fusion protein and cause a disruption of E12 (34). The mouse strain carrying this mutation was generated and characterized in this study. E47bm and E2Aheb are newly created alleles and are fully described in this report.
TABLE 3.
Summary of E2A mutant alleles
| Allele | Mutation of E2A | Expression | Reference |
|---|---|---|---|
| E2Ako | Deletion E12 and E47 bHLH exons | Null | 35 |
| E2Agal | Deletion E12 and E47 bHLH exons | β-Galacto- sidase | 34 |
| E12ko | Disruption of E12 bHLH domain | E47 | 34 |
| E47bm | Mutation in E47 basic region | E47bm, E12 | This report |
| E2Aheb | Deletion E12 and E47 bHLH exons | HEB | This report |
Disruption of the DNA binding domain of E47.
We used a dominant negative strategy to investigate the function of E47 and its potential dimerization partner(s). In principle, a point mutation in the E47 basic region disrupts the DNA binding but not the protein dimerization activities of E47. Such a mutation not only abolishes the function of E47 homodimer but can inhibit the function of putative E47 heterodimers and thus may help reveal the identity and function of E47 dimerization partner(s).
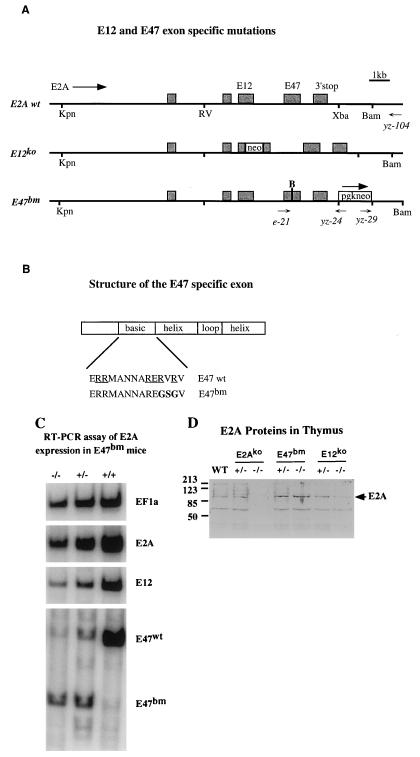
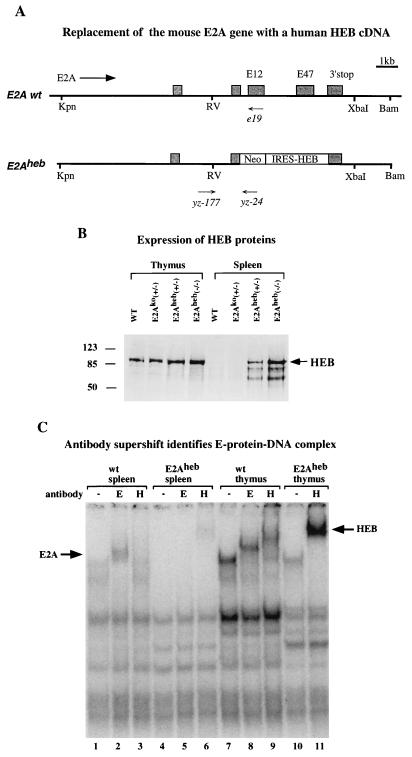
The mutation of E47 was introduced into the E2A locus by gene targeting (Fig. 1A). This change replaced two conserved arginines with glycines in the protein and added a new BamHI site in the DNA. Mutation at the identical positions has been shown previously to be a true dominant negative mutation in gel shift assays (30). In addition to the mutation in the basic region, the gene targeting construct also contained PGK-Neo as a positive selection marker and PGK-TK as a negative selection marker. Integration of PGK-Neo into the E2A locus was determined by PCR analysis with primers YZ-29 and YZ-104, which specifically detects homologous recombination at the 3′ region. The positive clones were subsequently analyzed by a second round of PCR using primers E21 and YZ-24. An integration of the basic region mutation was scored by restriction enzyme digestion of the PCR products, which should contain a BamHI site if the E47 basic mutation is retained after recombination. The frequency of gene targeting after the double selection was approximately 30%. About half of the targeted clones carried the basic mutation. This mutant allele was named E47bm. The E47bm mouse strain used in the subsequent experiments was derived from two independently targeted clones and bred in the 129/sv and C57BL/6 mixed background.
FIG. 1.
(A) The 3′ portion of the mouse E2A genomic DNA (from KpnI to BamHI) was used in the gene targeting experiment. The exons contained within this piece of genomic DNA are shown as shaded boxes. The direction of the E2A gene, positions of E12 and E47 exons, and the 3′ polyadenylation site of the E2A gene are shown. The structures of E12ko and E47bm alleles are shown according to the scale of the wild type (wt). The E12ko allele was generated by insertion of a promoterless neo gene into the E12 exon (34). The E47bm allele was generated by cointegration of a point mutation and a selection marker into the E2A gene. “B” above the E47 exon of the E47bm allele indicates the position of the basic-region mutation and the addition of a BamHI site. A PGK-Neo cassette running in the same direction as the E2A gene is inserted into the unique XbaI site downstream of the E2A gene. The names, positions, and directions of PCR primers used in the experiment are shown below the constructs. RV, EcoRV. (B) Detailed structure of the E47-specific exon. Sequences of the basic region of E47 wild-type and E47bm alleles are shown. Underlines indicate amino acid residues that are conserved in the bHLH gene family, and boldface indicates the changes made in E47bm. (C) RT-PCR analysis of thymocyte RNA prepared from E47bm homozygous, E47bm heterozygous, and wild-type animals. For each reverse-transcribed RNA sample, four sets of PCRs were performed with primers specific to (i) EF1α gene, (ii) E2A exons common to both E12 and E47, (iii) the E12-specific exon, and (iv) the E47-specific exon. To differentiate E47 wild-type and the E47bm alleles, PCR product generated with E47 primers was digested with BamHI, which is present in the E47bm but not the wild-type allele (in lanes E47bm +/− and E47bm −/−). Results are representative of three repeated experiments with multiple individuals from each genotype. (D) Western analysis of E2A proteins in the thymus isolated from the wild-type, E2Ako, E47bm, and E12ko mice. Genotypes of the samples are indicated at the top. Size markers in kilodaltons are shown at the left, and positions of the full-length E2A proteins are indicated at the right.
To determine whether the expression of E2A is affected by the PGK-Neo marker inserted downstream of the E2A gene, RT-PCR was used to evaluate the RNA levels of E12 and E47 in E47bm mice (Fig. 1C). The newly introduced BamHI site served as an allelic marker to distinguish the mutant allele and the wild-type allele in E47bm heterozygous mice. Because of a severe impairment of B-cell development in E47bm mice, thymocyte RNA were used in this assay. Four sets of PCR primers, including EF1α as an internal control, were used for each sample. This semiquantitative RT-PCR assay showed that the expression levels of E2A in E47bm homozygous mice was reduced to about 60% of the wild-type level (comparing E2A with EF1α), and the ratio of E47/E12 splicing was increased approximately 80% (comparing E47bm with E12). Although the cause of the change in splicing is still under investigation, the analysis clearly demonstrates the presence of the E12 transcript in the E47bm mice.
Western analysis of thymus extracts showed that the expression level of total E2A proteins in the E47bm homozygous animals is approximately the same as in wild-type controls (Fig. 1D). Although separate anti-E2A antibodies have been used to verify this result, we cannot yet distinguish E12 and E47 with the reagents available.
E47 is not essential for survival and reproduction.
Previous studies on the E2A knockout mice have shown that deletion of the E2A gene resulted in a high frequency of postnatal death and deficiency in B-lineage formation (2, 35). Subsequent studies also show that the surviving E2Ako females are sterile, while males are fertile (data not shown). In contrast to the E2Ako mice, the survival rates of E47bm mice are indistinguishable from those of their wild-type or heterozygous littermates, and both males and females are fertile. This result indicates that the DNA binding activity of E47 is not essential for postnatal survival and reproduction. Furthermore, E47bm may play a dominant role in keeping these mice alive and fertile, perhaps by keeping Id levels in check (33). Although the dominant negative effect of E47bm cannot be completely understood before a true E47-null allele is available (experiment is under way), the improved viability and fertility already makes the E47bm mice a valuable strain (see below).
Mice without normal E47 proteins are able to produce B cells in neonatal but not adult life.
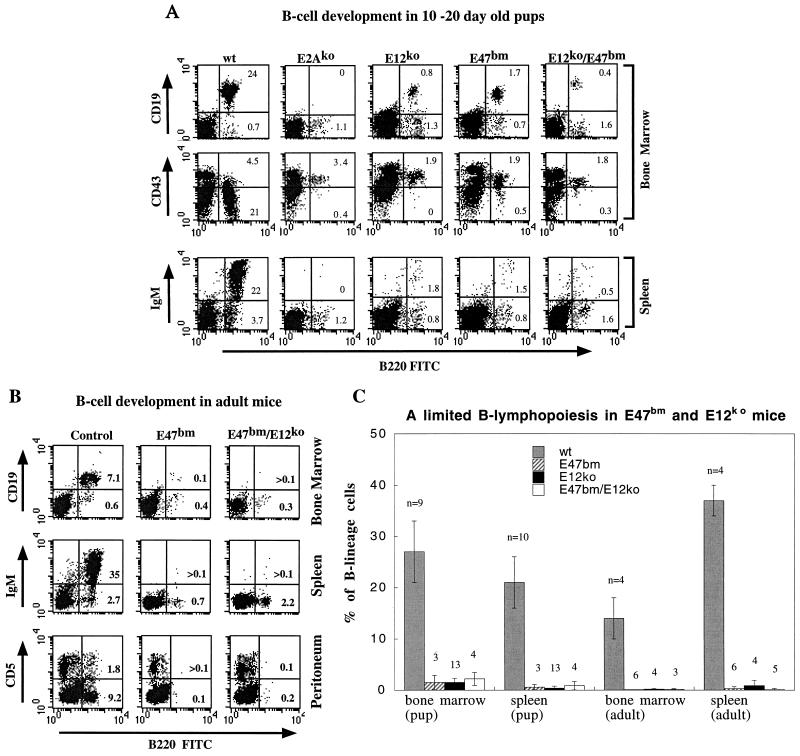
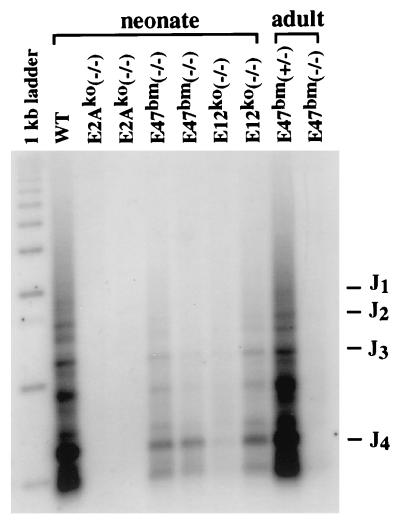
The earliest committed B-lineage cells are characterized by surface expression of B220, CD43, and CD19 antigens (9, 19). As cells mature from pro-B to pre-B cells, both B220 and CD19 are gradually upregulated, whereas CD43 is downregulated. Mature B cells in the bone marrow and spleen express, in addition to B220 and CD19, surface IgM. When these markers are used to analyze E47bm neonates, we find a 10-fold reduction of pro- and pre-B-cell numbers in the bone marrow and more than a 20-fold reduction of B-cell numbers in the spleen (Fig. 2A and C). B cells found in E47bm bone marrow are further verified by PCR analysis of the Ig heavy-chain locus, which detects substantial DH-JH rearrangement (Fig. 3). No B cells are detected by either flow cytometry or PCR analysis of DH-JH rearrangements in the same age group of E2A-null mice. This result indicates that in the absence of normal E47 protein, E12 is sufficient to support a limited but definitive B-cell commitment at the neonatal stage.
FIG. 2.
(A) Three-channel FACS analysis of live bone marrow (top two panels) and spleen B cells (bottom panel) prepared from 10- to 20-day-old pups carrying various E2A mutations. The genotype of each animal is indicated above each vertical panel, which is composed of three FACS analyses of the same animal. B220-FITC and 7AAD were included in all the stainings. In addition, CD19-PE or CD43-PE was used in the bone marrow samples, and IgM-PE was used in the spleen samples. The variation in the levels of CD19-PE staining is due to batch difference in the antibody used. To enrich lymphocytes and to exclude dead cells in the analysis, size gate and 7AAD gate were used for all the dot plots displayed. The relative percentages of pro-B, pre-B, and mature B cells in total live cells are given in each plot. FACS analysis of B cells in adult mice. Wild-type control, E47bm homozygous and E47bm/E12ko transheterozygous mice were analyzed with B220 and 7AAD plus CD19 for bone marrow, IgM for spleen, and CD5 for peritoneum samples. Presentation of data is as described for panel A. (C) Average B-cell numbers as a percentage of total nucleated bone marrow or spleen populations. Both sample size and standard deviation are shown with each data bar. Four genotype groups of animals, wild-type (wt), E47bm homozygous, E12ko homozygous, and E47bm/E12ko transheterozygous, are included. Pup is equivalent to 10 to 20 days old; adult is more than 2 months old.
FIG. 3.
PCR analysis of Ig heavy-chain gene D-J rearrangements. Genomic DNA was purified from bone marrow of either 10- to 20-day-old neonates or 2-month-old adults. PCR was carried out with a J4-specific primer (24) and a D-segment-specific primer that hybridizes to the upstream sequence of most D segments (8). PCR products representing specific D-J rearrangements were revealed by a radiolabeled oligonucleotide that hybridizes to the internal side of the J4 primer. The running positions of DNA size markers and J segments are indicated on the left and right side, respectively. WT, wild type.
This limited B lymphopoiesis in E47bm mice, however, is observed only during the first few weeks of postnatal life. Analysis of 2- to 4-month-old-mice failed to detect B cells in bone marrow, spleen, and peritoneum (Fig. 2B and C). Although less than 1% of B220+ CD19− cells were detected in bone marrow, PCR analysis did not show any DH-JH rearrangement in these cells, indicating that these B220+ CD19− cells had not yet committed to the B-cell lineage (Fig. 3). Recent studies by several groups have shown that bone marrow B220+ CD19− cells contain progenitors of B-lymphoid, natural killer, and other undetermined cell types (19).
Mice without E12 have limited capacity to generate B cells.
Parallel to the analysis of E47bm mutation, we also tested an E12-specific mutation, which had been generated and analyzed previously at the ES cell level (34). This mutant allele was made by inserting the Neo coding region into the bHLH domain of the E12-specific exon. This mutation was introduced recently into mice through blastocyst injection, and the resulting mice were subsequently bred into 129/sv and C57BL/6 backgrounds. The expression of E47 in the E12ko homozygous background was verified by RT-PCR analysis (34) and by Western analysis in this study (Fig. 1D). In contrast to mice with the E47bm mutation, the survival rate for E12ko mice was lower than normal but significantly higher than that of E2A knockout mice (data not shown). With limited breeding, E12ko males were found to be normal in reproduction, while females were sterile.
At the neonatal stage, E12ko mice showed limited capacity to produce B cells. Similar to the phenotype of E47bm mice, a small number of B cells were found in the bone marrow and spleens of neonatal E12ko mice. The bone marrow B cells were mostly B220+ CD19+ CD43+ and DH-JH-rearranged pro-B cells, and the splenic B cells were B220+ IgM+ mature B cells (Fig. 2A and C; Fig. 3). This result indicates that in the absence of E12, E47 alone is capable of supporting B-cell commitment. However, since the number of B cells produced in E12ko mice is less than 10% of the wild-type level, the function of E47 must be limited. A noted difference between E12ko and E47bm mice is that small numbers of B cells are frequently, but not always, found in the spleens of adult E12ko mice (Fig. 2C). Although the number of B cells is about 1 to 5% of the wild-type level, they can produce a substantial amount of serum Igs (data not shown).
E47bm/E12ko transheterozygous mice are phenotypically similar to the E47bm or E12ko homozygous mice.
It was not clear why E47bm and E12ko mice had only limited capacity to produce B cells. One possible interpretation is that the levels of functional E12 or E47 proteins in E47bm or E12ko mice are reduced below a threshold level which is required for maintaining normal B lymphopoiesis. RT-PCR analysis clearly showed a small but detectable reduction of E12 and E47 messages in E47bm and E12ko mice, respectively. In the E47bm mice, the level of functional E12 proteins may be reduced even further by a dominant negative effect from the E47bm allele, but we cannot say if this suboptimal gene expression is sufficient to account for the impairment of B lymphopoiesis in these mice.
Alternatively, the lack of sustained B lymphopoiesis in E47bm and E12ko mice may be due to the disruption of synergy between the two E proteins, a possibility suggested by Bain et al. (3). To evaluate this scenario, we analyzed the E47bm/E12ko transheterozygous mice. The transheterozygous mice were born and lived to adulthood without any superficial difference from the control littermates. Although both E12 and E47 messages are present in these transheterozygous mice (data not shown), their capacity to produce B-lineage cells is about the same as that of homozygous E12ko or E47bm mice (Fig. 2A and C); i.e., small numbers of B-lineage cells are detected in neonates but not in adults. This result indicates that coexpression of wild-type E47 and E12 proteins in the same animal is sufficient to initiate B lymphopoiesis in the neonates but not sufficient to maintain B lymphopoiesis in the adult. Given the potential dominant negative effect from the E47bm allele, we cannot say how much of the functional E12 and E47 proteins are available to support B lymphopoiesis in the E47bm/E12ko mice. However, the phenotypic similarity among E47bm homozygous, E12ko homozygous, and E47bm/E12ko transheterozygous mice indicates an underlying common defect in these mice.
Stem cell transfusion confirms a B-cell-specific role for E12 and E47.
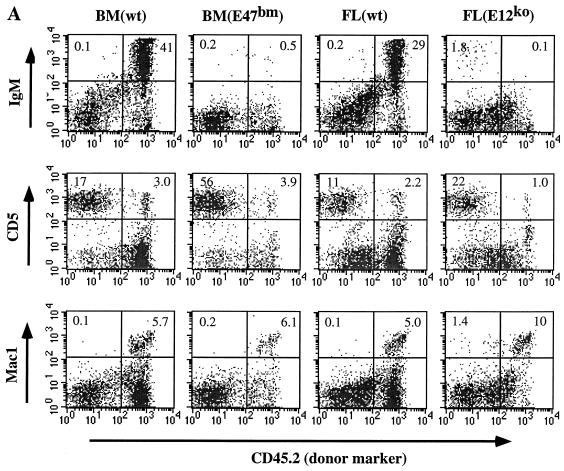
E12ko and E47bm mice have a limited capacity to generate B-lineage cells. This suppression of B lymphopoiesis could be due to an intrinsic defect within the B-cell lineage, a nonsupportive stromal environment, or both. To test this possibility, we performed a stem cell transfusion test. HSC from either the fetal livers of E12ko mice or the bone marrow of E47bm neonates were used as donors to complement lethally irradiated wild-type hosts. As shown in Fig. 4A, HSC from both E12ko and E47bm mice are capable of providing radioprotection and giving rise to T-lymphoid, myeloid cells but not B cells. Meanwhile, a normal number of B cells can be generated when wild-type fetal liver or bone marrow is used as the donor. This result confirms the transfusion test with the E2A-null cells as donors (36) and indicates that disruption of either E12 or E47 specifically limits the potential of hematopoietic stem cells to give rise to B-lineage cells.
FIG. 4.
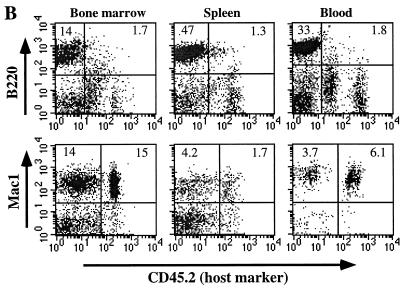
(A) FACS analysis of spleen samples from stem cell transfusion experiments. Sources of donor stem cells are indicated at the top (BM, bone marrow; wt, wild type; FL, fetal liver). Each vertical panel represents separate FACS analysis for B cells (IgM), T cells (CD5), and myeloid cells (Mac1) from the same splenic sample. CD45.2 (Pharmingen) is an allotype marker that is present on the donor cells and absent on host cells. To eliminate nonspecific staining and dead cells, 7AAD was also included in all the staining reactions. Percentages of cells given in the quadrants are calculated based on total live cells rather than the size-gated population. Data are representative of two to five independent transfusion tests for each donor type. (B) FACS analysis of an E47bm mouse (representative of three mice) reconstituted with the wild-type HSC. Cells from bone marrow, spleen, and blood were analyzed with a B-cell marker (B220) and a myeloid cell marker (Mac1). Donor cells were derived from wild-type C57BL/6Ly5A mice and were negative for CD45.2, whereas host cells were positive for the CD45.2 marker. Gating and data presentation are as in panel A.
Adoptive transfer in the reverse direction has been impossible in the past because of the lethality of E2A knockout. The E47bm mice generated in this study provided excellent hosts for transfusion tests. We now know that wild-type HSC can provide complete radioprotection and give rise to B-lineage cells in the lethally irradiated E47bm hosts (Fig. 4B), demonstrating a permissive stromal environment for B lymphopoiesis in E47bm mice. Therefore, the lack of B lymphopoiesis in adult E47bm mice is almost exclusively due to a B-lineage autonomous defect.
Replacement of E2A with a human HEB cDNA.
Analyses of E47bm and E12ko mice suggest that the B-cell-specific function of E2A has at least two features: it requires a structural domain shared by both E12 and E47, and it demands a minimal dosage of functional E2A proteins. To further explore the molecular basis of these phenomena, we compared the functions of E2A proteins and HEB protein, which is encoded by a separate E-protein gene. The sequence similarity at the amino acid level between HEB and E2A is 82% (for E47) and 89% (for E12) within the bHLH domain and is significantly lower outside the bHLH domain (11). We had shown previously by gene disruption that HEB is not essential for B-cell commitment and differentiation. However, mice compound-heterozygous for E2A and HEB disruption have reduced capacity for producing pro-B cells in fetal liver, suggesting the involvement of HEB in B lymphopoiesis.
To allow a direct comparison of the function of E2A and HEB in the context of B-cell development, we introduced an HEB cDNA into the E2A locus through gene targeting. A full-length human HEB cDNA driven by an IRES (see Materials and Methods) was used to replace the E12 and E47 exons. Selection of targeting events was achieved by adding the neo gene in frame with the E2A coding sequence (Fig. 5A). Gene-targeting events with this HEB insertional construct were obtained at a frequency of 65% after G418 selection, demonstrating the selection efficiency of the construct. The validity of this targeting strategy for expression of an inserted cDNA under the endogenous E2A promoter has been demonstrated by the construction and analysis of the E2Agal allele, in which inactivation of the E2A gene is concomitant with the expression of a β-galactosidase marker by the endogenous E2A promoter (34, 35). We have since shown that this E2Agal allele drives β-galactosidase expression in a broad pattern throughout embryogenesis (data not shown).
FIG. 5.
(A) Gene targeting construct for generating the E2Aheb allele. The Neo marker was introduced in the E2A gene as a fusion protein. IRES-driven human HEB cDNA was inserted downstream of the Neo cassette but in front of the E2A translation termination site. Both E12 and E47 exons are completely deleted from the targeting construct. Primers used for PCR genotyping the mutant and wild-type (wt) alleles are indicated (see Materials and Methods). Designations are as in Fig. 1A. (B) Western blot analysis of thymus (lanes 1 to 4) and spleen (lanes 5 to 8) nuclear extracts from 10-day-old neonatal mice of indicated genotypes. Wild-type, E2Ako heterozygote, E2Aheb heterozygote, and E2Aheb homozygote samples are included in the assay as indicated. The anti-HEB polyclonal sera used in this assay (Santa Cruz) cross-react with both human and mouse HEB proteins. Size markers in kilodaltons and the HEB band are indicated on the left and right, respectively. (C) Gel shift analysis of E-protein complex formation, using μE5 radiolabeled probe. μE5 probe (25) was incubated with nuclear extracts from splenocytes (lanes 1 to 6) or thymocytes (lanes 7 to 11) derived from wild-type (lanes 1, 2, 3, 7, 8, and 9) or E2Aheb (lanes 4, 5, 6, 10, and 11) animals. Anti-E2A antibody (Yae) was added in lanes 2, 5, and 8, and anti-HEB antibody (Santa Cruz) was added in lanes 3, 6, 9, and 11. The E2A- and HEB-dependent supershifts are indicated by arrows on the left and right sides, respectively. Assay conditions are as described by Sawada and Littman (22).
Mice carrying the newly created gene replacement allele, named E2Aheb, were tested by Western analysis for expression of the human HEB protein. Whole-cell extracts from spleen and thymus were probed with anti-HEB antibodies, which cross-react with both human and mouse HEB proteins. As shown in Fig. 5B, the level of the endogenous HEB proteins is low in the spleen and high in the thymus in wild-type animals. Replacement of the E2A gene with the HEB cDNA leads to a moderate increase of HEB proteins in the thymus and a dramatic increase of HEB proteins in the spleen. Disruption of the E2A proteins was verified by anti-E2A antibodies (data not shown). Because no increase of HEB expression is seen in E2Ako heterozygous mice, we conclude that the increased HEB proteins are derived from the human HEB cDNA rather than from upregulation of the endogenous mouse HEB gene.
A gel shift assay with the Ig μE5 enhancer was used to evaluate the activity of HEB proteins produced from the E2A locus. It has been well documented that μE5 is specifically recognized by E2A-containing protein dimers in B-cell extracts (1). As shown in Fig. 5C, the E2A-μE5 complex can be seen as a supershift with anti-E2A antibody in wild-type splenocytes (lane 2) but not in E2Aheb−/− splenocytes (lane 5). In contrast, an HEB-μE5 complex is seen as a supershift with anti-HEB antibody in the E2Aheb splenocytes (lane 6) but not in the wild-type splenocytes (lane 3), suggesting that the E2A-dependent μE5 binding activity has been replaced by HEB. However, HEB may have a lower affinity for the μE5 site than E2A does in the splenocytes since the HEB-μE5 complex can only be seen as a supershift (lane 6). The activity of HEB proteins was also tested in thymocyte extracts, where a replacement of the HEB-E2A heterodimers (lane 7 to 9) by HEB homodimers (lane 10, 11) was observed in the E2Aheb mice. This result is consistent with an observation made by Sawada and Littman (22) that E proteins in wild-type T cells were heterodimers between E2A and HEB.
A copy number-dependent rescue of B-cell development by E2Aheb.
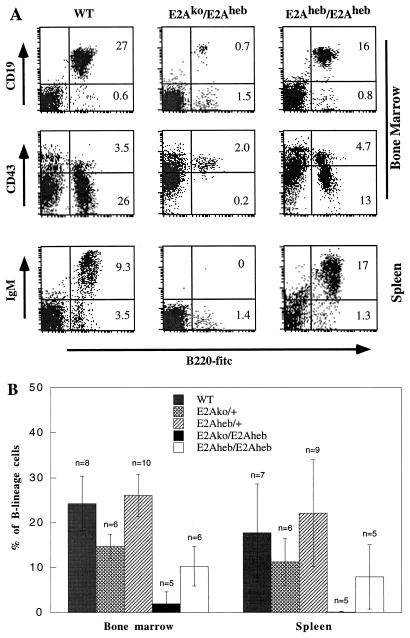
The activity of E2Aheb in B lymphopoiesis was tested by analyzing B-cell contents in the bone marrow and spleens of preweaning-age animals, a stage when B lymphopoiesis is sensitive to the E2A protein dosage. Even in heterozygous animals, a contribution to B-cell development from the E2Aheb allele can be readily seen by comparison of E2Aheb heterozygous mice with E2Ako heterozygous mice. As shown in Fig. 6B, E2Aheb heterozygous mice generate twice as many B cells as E2Ako heterozygous mice do. The number of B cells found in E2Aheb heterozygous mice is no less, and perhaps slightly higher, than the B-cell number in the wild-type controls. We then tested E2Aheb activity on the E2A-null background by crossing E2Aheb heterozygous mice with E2Ako heterozygous mice. Genotyping analysis of 1- to 3-week-old pups showed that the survival rate of transheterozygous offspring is slightly lower than expected (16 wild type, 36 heterozygous, and 11 transheterozygous) but significantly improved from age-matched E2A-null homozygous mice (33). FACS analysis with B-cell lineage markers showed that the E2Aheb/E2Ako mice generate extremely low numbers of pro-B cells (B220+ CD43+ CD19+) in the bone marrow of neonatal mice (Fig. 6). This result indicates that one copy of E2Aheb is insufficient to completely rescue E2A-null defects.
FIG. 6.
(A) FACS analysis of mice carrying one or two copies of E2Aheb. Bone marrow (top two panels) and spleens (bottom panel) from 10-day-old wild-type (WT), E2Ako/E2Aheb transheterozygous, and E2Aheb homozygous mice were analyzed with B-cell markers. Plots were gated on live lymphocytes based on cell size and 7AAD staining. Percentages of cells shown in the quadrants represent the fractions of total live cells analyzed. (B) B-cell contents as a percentage of total live suspension cells for the five genotypes. Percentages of B cells in bone marrow and spleen are calculated based on the analysis of multiple litters with ages ranging from 1 to 3 weeks. Sample size and standard deviation are shown with each data bar. Relatively large standard deviations for most spleen samples are due to a sharp increase of B-cell content in the first 3 weeks of postnatal life.
E2Aheb homozygous mice are recovered with expected frequency (14 +/+, 35 +/−, and 22 −/−) from E2Aheb heterozygous intercross. These E2Aheb homozygous mice are superficially indistinguishable from their wild-type and heterozygous littermates, indicating a general rescue of E2A-null defects by two copies of E2Aheb. Further, interbreeding of E2Aheb homozygous mice has produced healthy offspring. In contrast to the E2Aheb/E2Ako transheterozygous mice, a substantial number of B-lineage cells, including pro- and pre-B cells and mature B cells, were detected in bone marrow and spleen, respectively (Fig. 6). This result indicates a functional replacement of E2A by HEB in both the commitment and maturation processes of B lymphopoiesis. The number of bone marrow B cells in these E2Aheb mice is about 40% of the number in the wild-type littermates, nearly reaching the B-cell number in age-matched E2Ako heterozygous mice (Fig. 6B). Such a log scale increase of B-cell numbers from mice carrying one copy of E2Aheb to mice carrying two copies of E2Aheb strongly argues that a threshold level of E proteins is critical and essential for B-cell commitment.
DISCUSSION
E2A encodes two broadly expressed bHLH proteins that play specific and essential roles in B lymphopoiesis. How functional specificity is achieved by the E2A gene is central to our understanding of E2A-mediated regulatory pathways in B lymphopoiesis. B-cell-specific posttranslational modification of E47 has been postulated as a major event in regulating E2A protein activity, which in principle provides the needed functional specificity. This model suggests that the functional specificity of E2A is mediated by E47 homodimerization, which is detected exclusively in B-lineage cells. Further, the model suggests that the structure of the E47-specific exon provides the functional specificity and excludes any functional significance of E12 and other E proteins in B lymphopoiesis. This model is challenged by a transgenic rescuing test carried out by Bain et al. (3) and is further questioned by two pieces of genetic evidence reported here. First, we have shown that either E12 or E47 alone is capable of supporting limited but definitive B lymphopoiesis. Second, we have shown by gene replacement that HEB can replace the function of E2A in driving B-cell commitment and maturation. Both findings favor the notion that the functional specificity of E2A is largely determined at the transcription level of the E2A gene.
Functional requirement of E47 and E12 throughout B-cell development.
Although E47 or E12 alone is sufficient to support B-cell commitment and differentiation into mature B cells, the entire process is extremely inefficient. Pro-B, pre-B, and mature B cells are generated in both E47bm and E12ko mice but at levels more than 10-fold lower than in their wild-type littermates. In wild-type mice, pro-B cells represent only a small fraction of the total population of B-lineage cells in the bone marrow. In contrast, most B-lineage cells detected in the bone marrow of E47 and E12 knockout mice are B220+ CD43+ CD19+ pro-B cells. The unbalanced ratio of pro-B versus pre-B cells suggests a role for E2A proteins in differentiation from pro-B to pre-B cells. It has been well established that transition from pro-B to pre-B stage is dependent on proper expression and selection of the B-cell receptor genes. Indeed, the Ig heavy-chain and light-chain genes, the terminal deoxynucleotide transferase gene, and surrogate light-chain genes lambda 5 and V-pre-B are among the demonstrated targets of E2A (6, 23, 26). Therefore, E2A proteins, in addition to their unique role in supporting B-lineage commitment, may play a much broader role in later stages of B-cell development.
The combined dosage of E47 and E12 is required for B-cell development.
We have shown that E47bm/E12ko transheterozygous mice have no better capacity to generate B cells than E12ko or E47bm homozygous mice. Therefore, the mere presence of both E12 and E47 proteins is not sufficient to support normal B lymphopoiesis. Comparison with E2Ako heterozygous mice which also carry a single copy of E47 and E12 shows that transheterozygous E47bm/E12ko is composed of an E47bm allele. However, the E2Ako heterozygous mice can produce 50% wild-type levels of pro- and pre-B cells in fetal and neonatal life and near normal levels of B cells in adult life (35). The phenotypic discrepancy between E47bm/E12ko and E2Ako heterozygous mice may be partially due to inadvertent reduction of E12 and E47 expression from the mutated E47bm and E12ko alleles, respectively. Alternatively, the discrepancy may be attributed to a dominant negative effect from the E47bm allele; e.g., E47bm may inhibit the activity of E12 and E47 through competitive dimerization. In either case, the end result is a further reduction of functional E proteins below one-half of the wild-type level in the E47bm/E12ko mice. Perhaps this subtle difference in the level of functional E2A proteins is sufficient to trigger a threshold effect, leading to inefficient B lymphopoiesis in the E47bm/E12ko mice.
Function by context rather than by a unique structure.
The existence of functional specificity among different E-protein genes is supported by gene knockout studies (36). However, it is not clear how functional specificity for each E-protein gene is defined and regulated. Because all three E-protein genes are broadly, although not evenly, expressed in different tissues, transcriptional regulation has been generally discounted as a way to achieve functional specificity. All of the evidence accumulated prior to this study favors the notion that functional specificity of E2A in B-cell development is determined mainly by a unique structure of E2A, in particular the E47 protein (5, 25). HEB and E2-2 can modulate E2A activity in two possible ways: they can interact directly with E12 and E47 through heterodimerization (1, 22) or indirectly affect E2A activity through interactions with the Id proteins (28, 36). Since mice lacking either HEB or E2-2 can still generate a substantial number of B cells (36), these presumed functions of HEB and E2-2 are apparently nonessential. A functional replacement of E2A with HEB, shown in this study, argues that HEB, E2A, and perhaps E2-2 as well share a common protein structure necessary and sufficient to support B-cell commitment and maturation. This finding challenges the existing hypothesis and suggests that the functional specificity of E2A is determined primarily by transcriptional regulation of the E2A gene.
Threshold regulation—from Drosophila to mice.
The high degree of conservation among different E proteins at both structural and functional levels argues that E proteins must play fundamental roles during development. In fact, this conservation of E proteins can be traced back to invertebrates such as Drosophila, in which a single E-protein-equivalent bHLH gene known as daughterless was found. It has been shown that daughterless is involved in regulating differentiation events such as sex determination in a dosage-dependent manner (7, 17). The regulation of differentiation events by daughterless in invertebrates is reminiscent of the dosage-dependent regulation of B-cell development by the E proteins in mammals. In both cases a critical level of bHLH proteins is required to trigger a binary switch leading to irreversible differentiation events, namely, establishment of the female body plan in Drosophila and the B-cell lineage in mice. This structural and functional conservation between the fly daughterless gene and the mouse E2A gene suggests that the threshold mechanism may be more generally used by transcription factors in the regulation of other cell lineages.
Why three functionally equivalent E-protein genes?
This study also poses an important question: why do mammals need three functionally equivalent E-protein genes? For simplicity, we discuss only the common roles shared by these E proteins, although subtle differences, such as dimerization specificity and affinity to specific DNA sites, clearly exist. It may be argued that a functional diversification at the protein level may not be tolerated if the ancestor E protein plays a fundamental role which has been fixed in evolution. Our study suggests that the function of the E proteins, although fixed, is subject to tissue specific regulation. The transcriptional regulation of the E2A gene in B cells is, perhaps, only one of many tissue-specific regulation events that have occurred during embryonic and tissue development; each event may be regulated by a unique enhancer (or a unique set of enhancers) attached to the gene. Intuitively, there may be a limit for how many tissue-specific enhancers can be added to a single gene. We argue that gene duplication may be a simple way to accommodate the increasing need for tissue-specific regulation of the conserved function provided by the ancestor E-protein gene. This interpretation predicts that as important as transcriptional regulation of the E2A gene is to B-cell development, transcriptional regulation of the HEB and E2-2 genes may be important to other cell types.
ACKNOWLEDGMENTS
We thank Peifeng Cheng for assistance in generating the E47bm mutation, Mike Krangel and Mike Krause for critical reading of the manuscript, and Billie Maciunas for editorial assistance. We are grateful to Duke Cancer Center Flow Cytometry Facility for technical support.
This work was initiated with a fellowship award from the Leukemia Society of America to Y.Z. and supported by a Whitehead Scholarship and an NCI grant (R01CA72433-01) to Y.Z.
REFERENCES
- 1.Bain G, Gruenwald S, Murre C. E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol Cell Biol. 1993;13:3522–3529. doi: 10.1128/mcb.13.6.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain G, Robanus Maandag E C, Izon D J, Amsen D, Kruisbeek A M, Weintraub B C, Krop I, Schlissel M S, Feeney A J, van Roon M, van der Valk M, te Riele H P J, Berns A, Murre C. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 3.Bain G, Robanus Maandag E C, te Riele H P, Feeney A J, Sheehy A, Schlissel M, Shinton S A, Hardy R R, Murre C. Both E12 and E47 allow commitment to the B cell lineage. Immunity. 1997;6:145–154. doi: 10.1016/s1074-7613(00)80421-5. [DOI] [PubMed] [Google Scholar]
- 4.Benezra R, Davis R L, Lockshon D, Turner D L, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 5.Benezra R. An intermolecular disulfide bond stabilizes E2A homodimers and is required for DNA binding at physiological temperatures. Cell. 1994;79:1057–1067. doi: 10.1016/0092-8674(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 6.Choi J K, Shen C P, Radomska H S, Eckhardt L A, Kadesch T. E47 activates the Ig-heavy chain and TdT loci in non-B cells. EMBO J. 1996;15:5014–5021. [PMC free article] [PubMed] [Google Scholar]
- 7.Cline T W. The affairs of daughterless and the promiscuity of developmental regulation. Cell. 1989;59:231–234. doi: 10.1016/0092-8674(89)90280-8. [DOI] [PubMed] [Google Scholar]
- 8.Gu H, Kitamura D, Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991;65:47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- 9.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B-cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henthorn P, Kiledjian M, Kadesch T. Two transcription factors that bind the immunoglobulin enhancer μE5/kE2 motif. Science. 1990;247:467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- 11.Hu J-S, Olson E N, Kingston R E. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992;12:1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang S K, Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990;4:1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- 13.Lassar A B, Murre C, Davis R L, Voronova A, Wright W E, Baltimore D, Kadesch T, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991;66:305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 15.Murre C, McCaw P S, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 16.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Jan Y N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B, Weintraub H, Baltimore D. Interactions between heterodimeric helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 17.Parkhurst S M, Lipshitz H D, Ish-Horowicz D. Achaete-scute feminizing activities and Drosophila sex determination. Development. 1993;117:737–749. doi: 10.1242/dev.117.2.737. [DOI] [PubMed] [Google Scholar]
- 18.Rolink A, Melchers F. B lymphopoiesis in the mouse. Adv Immunol. 1993;53:123–156. doi: 10.1016/s0065-2776(08)60499-x. [DOI] [PubMed] [Google Scholar]
- 19.Rolink A, ten Boekel E, Melchers F, Fearon D T, Krop I, Andersson J. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J Exp Med. 1996;183:187–194. doi: 10.1084/jem.183.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupp R A, Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991;65:927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Ono N, Steeber D A, Pisetsky D S, Tedder T F. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;15:4371–4378. [PubMed] [Google Scholar]
- 22.Sawada S, Littman D R. A heterodimer of HEB and an E12-related protein interacts with the CD4 enhancer and regulates its activity in T-cell lines. Mol Cell Biol. 1993;13:5620–5628. doi: 10.1128/mcb.13.9.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlissel M, Voronova A, Baltimore D. Helix-loop-helix transcription factor E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991;5:1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- 24.Schlissel M S, Corcoran L M, Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991;173:711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen C-P, Kadesch T. B-cell-specific DNA binding by an E47 homodimer. Mol Cell Biol. 1995;15:4518–4524. doi: 10.1128/mcb.15.8.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigvardsson M, O’Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25–36. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- 27.Sun X-H, Baltimore D. An inhibitory domain of E12 transcription factor prevents DNA binding in E12 homodimers but not in E12 heterodimers. Cell. 1991;64:459–470. doi: 10.1016/0092-8674(91)90653-g. [DOI] [PubMed] [Google Scholar]
- 28.Sun X-H. Constitutive expression of the Id1 genes impairs mouse B cell development. Cell. 1994;79:893–900. doi: 10.1016/0092-8674(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 29.Urbanek P, Wang Z-Q, Fetka I, Wagner E, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 30.Voronova A, Baltimore D. Mutations that disrupt DNA binding and dimer formation in the E47 helix-loop-helix protein map to distinct domains. Proc Natl Acad Sci USA. 1990;87:4722–4726. doi: 10.1073/pnas.87.12.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J H, Nichogiannopoulou A, Wu L, Sun L, Sharpe A H, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 32.Wilson R B, Kiledjian M, Shen C P, Benezra R, Zwollo P, Dymecki S M, Desiderio S V, Kadesch T. Repression of immunoglobulin enhancers by the helix-loop-helix protein Id: implications for B lymphoid development. Mol Cell Biol. 1991;11:6185–6191. doi: 10.1128/mcb.11.12.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan W, Young A Z, Soares Y C, Kelley R, Benezra R, Zhuang Y. High incidence of T-cell tumors in E2A-null mice and E2A/Id1 double knockout mice. Mol Cell Biol. 1997;17:7317–7327. doi: 10.1128/mcb.17.12.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang Y, Kim C G, Bartelmez S, Cheng P, Groudine M, Weintraub H. Helix-loop-helix transcription factors E12 and E47 are not essential for skeletal or cardiac myogenesis, erythropoiesis, chondrogenesis, or neurogenesis. Proc Natl Acad Sci USA. 1992;89:12132–12136. doi: 10.1073/pnas.89.24.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang Y, Cheng P F, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three bHLH genes, E2A, E2-2, and HEB. Mol Cell Biol. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]