Abstract
Onchocerciasis treatment and control relies mainly on the use of ivermectin which has high activity against the microfilarial stage of Onchocerca volvulus but limited activity against the long-lived, tissue dwelling adult nematodes. As this neglected tropical disease has now been targeted for elimination, there is an urgent need for new drugs to combat these parasites, ideally with macrofilaricidal activity. In this study, we have examined the anti-Onchocerca activity of a range of existing FDA-approved drugs with a view to repurposing, which can lead to rapid and relatively inexpensive development. From the Pharmakon-1600 library, 106 drugs were selected and tested against O. gutturosa adult male parasites using a concentration of 1.25 × 10−5 M in an in vitro 5-day standard assay to assess motility and viability (using MTT/formazan colorimetry). The findings revealed that 44 drugs produced marginal/moderate activity (50–99% motility and/or MTT reductions) including cefuroxime sodium, methenamine, primaquine phosphate and rivastigmine tartrate, while 23 drugs produced good activity (100% motility reductions and significant MTT reductions), including atovaquone, isradipine, losartan, rifaximin, cefaclor and pyrantel pamoate. Although this study represents only a first step, some of the identified hits indicate there are potential anti-Onchocerca drug candidates worthy of further investigation.
Keywords: onchocerciasis, drug discovery, anthelmintics, O. gutturosa, motility and MTT inhibition, FDA-approved drugs
1. Introduction
Onchocerciasis (river blindness) is caused by the tissue-dwelling filarial worm Onchocerca volvulus. The infection is transmitted between humans, by the bite of the blackfly vector of the Simulium genus. Circulating microfilariae (mf) accumulate in the skin but in high-intensity infections, the mf can also enter the tissues of the eye. Death of the mf causes the pathologies of pruritus, skin atrophy, skin de-pigmentation, papular rash, eye lesions and blindness in humans [1]. Related to high morbidity is reduced work productivity, which can then lead to social stigmatisation; this poverty promoting nematode infection has been included within the group of neglected tropical diseases (NTDs) [2].
Without an available vaccine, current preventive chemotherapy and elimination of onchocerciasis rely on mass drug administration (MDA) programs (large scale distribution without diagnosis and supervision of health-care staff) that distribute ivermectin (Mectizan®, Merck, Rahway, NJ, USA) either on an annual or biannual basis [2]. This drug, belonging to the family of macrocyclic lactones, targets only the mf stage of the parasite by killing mf and exerting embryostatic effects on the adult female worm; that is, mf release from the uterus is temporarily suppressed [3]. Both of these mechanisms have the effect of reducing the skin mf by up to 99% two months after treatment [4]. In order to have an impact on filarial transmission reduction, treatment needs to be administered for the duration of the reproductive life span of the long-lived (up to 15 years [5]), tissue-dwelling adult worms, so program success is constrained by the absence of drugs with macrofilaricidal activity. There are now concerns of developing ivermectin resistance in Onchocerca parasites, as has already been reported in nematodes of veterinary importance [6,7,8], and construed in human onchocerciasis [9]. A study in endemic communities exposed to frequent rounds of treatment in Ghana demonstrated that although ivermectin retained microfilarial activity, sub-optimal responses to treatment could be due to the development of resistance by O. volvulus, resulting in a decreased effect on the inhibition of mf release [9]. Similarly, in Cameroon, studies on communities given multiple rounds of ivermectin therapy compared to those that were ivermectin naive indicated that continuous exposure to this drug had a reduced embryostatic effect on the female adult worms [10]. The findings from these studies highlight the possible emerging problems of ivermectin resistance and the urgent need for alternative methods of treatment. Recent studies demonstrated that the closely related drug moxidectin (registered for use in humans by the United States Food and Drug Administration (US FDA) in 2018) may offer improved treatment over ivermectin, although no macrofilaricidal effect has been observed so far [11,12].
The Pharmakon 1600 Collection, a US FDA-approved library, consists of 1600 drugs that have reached clinical evaluation and demonstrated biological activity against known targets. By screening a selection of this drug set, the identification of any potential antifilarial candidates could be rapidly repurposed and prove useful in onchocerciasis elimination programs, as many of the drugs represented in the library are available on the market. Humans are the only viable hosts of O. volvulus and there are no laboratory models that support the complete life cycle of this parasite; as such, drug discovery for onchocerciasis has to rely in part, on the use of surrogate parasites and animal hosts. Several in vitro and in vivo standard operating protocols for testing drugs against the adult stage of Onchocerca have been developed and optimized [13] and references therein. Using a World Health Organisation approved 5-day motility/MTT-based assay with the cattle filarial nematode Onchocerca gutturosa, in this study we have assessed the activity of 106 selected drugs with a range of biological activities (see Table 1).
Table 1.
Selected Pharmakon-1600 drugs (106) for in vitro evaluation against O. gutturosa adult male parasites. Bioactivity was categorised by MicroSource Discovery Systems, Inc. (Gaylordsville, CT, USA).
| Bioactivity | Drug |
|---|---|
| Antibacterial | Carbadox; Cefaclor; Cefamandole nafate; Cefoperazone; Cefoxitin sodium; Cefsulodin sodium; Ceftibuten; Cefuroxime sodium; Chlorhexidine dihydrochloride; Chloroxylenol; Demeclocycline hydrochloride; Doxycycline hydrochloride; Furazolidone; Gramicidin; Lasalocid sodium; Merbromin; Methacycline hydrochloride; Methenamine; Minocycline hydrochloride; Nitrofurantoin; Nitroxoline; Ofloxacin; Oxytetracycline; Rifampcin; Rifaximin; Sulfaquinoxaline sodium; Teicoplanin |
| Anticancer | Azaserine; Bleomycin; Daunorubicin; Doxorubicin; Epirubicin hydrochloride; Isotretinon; Lomustine; Mitoxantrone hydrochloride; Tretinoin |
| Antihypertensive/ vasodilator |
Dipyridamole; Guanethidine; Losartan; Nicardipine hydrochloride; Nicotinyl alcohol tartrate; Nifedipine |
| Anti-infective | Benzethonium chloride; Broxyquinoline; Dequalinium chloride; Methylbenzethonium chloride; Nitrofurazone; Oxyquinoline hemisulfate; Phenylethyl alcohol; Resorcinol monoacetate |
| Anti-inflammatory/ antihistamine |
Dexamethasone acetate; Doxylamine succinate; Meloxicam sodium; Prednisolone tebutate; Sulfasalazine |
| Antiparasitic | Amitraz; Atovaquone; Candicidin; Clorsulon; Diethylcarbamazine citrate; Flubendazole; Hexetidine; Homidium bromide; Iodoquinol; Levamisole hydrochloride; Moxidectin; Primaquine phosphate; Pyrantel pamoate; |
| Antiviral | Oseltamivir phosphate; Valganciclovir hydrochloride |
| Neurological | Acepromazine maleate; Almotriptan; Ampyzine sulfate; Apomorphine hydrochloride; Armodafinil; Bupropion; Chlorpromazine; Danazol; Desipramine hydrochloride; Dopamine hydrochloride; Isradipine (also antihypertensive/vasodilator); Methsuximide; Methylphenidate hydrochloride; Olanzapine; Oxidopamine hydrochloride; Penfluridol; Rivastigmine tartrate; Selegiline hydrochloride; Zaleplon |
| Various | Alendronate sodium; Anisindione; Ascorbyl palmitate; Bromhexine hydrochloride; Butacaine; β-Carotene; Clopidogrel sulfate; Dienestrol; Dioxybenzone; Docusate sodium; Fluorescein; Mangafodipir trisodium; Methylergonovine maleate; Propoxycaine hydrochloride; Riboflavin; Sennoside A; Tetrahydrozoline hydrochloride |
2. Materials and Methods
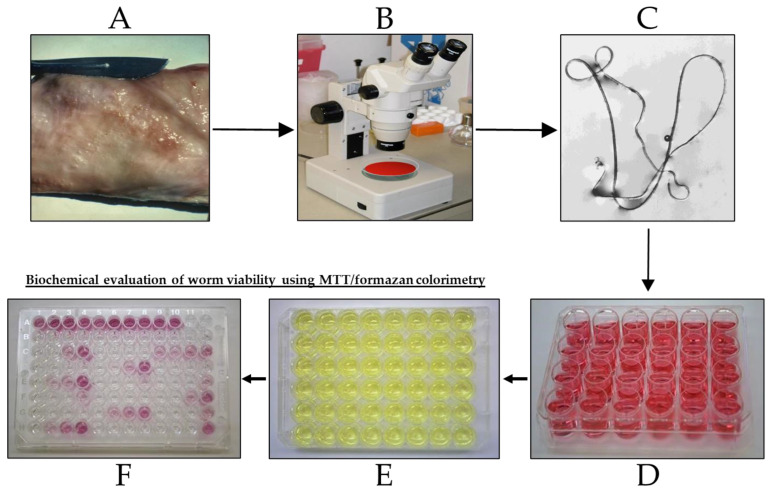
A workflow diagram for the experimental procedure used in this study is depicted in Figure 1.
Figure 1.
Workflow diagram for the Onchocerca gutturosa adult worm in vitro 5-day motility/MTT assay. (A) Nuchal ligament connective tissue from naturally infected cattle; (B) Tissue in culture medium placed under a dissecting microscope (40× magnification) to isolate worms using fine forceps; (C) Isolated worm floating on tissue culture medium, worm length approximately 2 cm; (D) Worms were maintained individually in each well of a 24-well plate containing 2 mL culture medium, at 37 °C under an atmosphere of 5% CO2 in air; this was replaced after 24 h with culture medium containing the test drug at a final concentration of 1.25 × 10−5 M. Worm motility was determined microscopically every 24 h up to 120 h; (E) Worm viability was then assessed by the transfer of each worm to the well of a 48-well plate containing 0.5 mg/mL MTT, incubation at 37 °C for 30 min; (F) Each worm was transferred to a well in a 96-well plate containing 200 µL dimethylsulfoxide to solubilize the formazan. After 1 h at 23–26 °C, the plate was gently agitated and formazan formation measured using absorbance (490 nm) on an ELISA reader (Dynatech, Willenhall, UK).
2.1. Parasites—Onchocerca gutturosa
Adult male worms were obtained postmortem from naturally infected, freshly slaughtered cattle in Gambia, West Africa. The material used in this study was purchased from local butchers by the West Africa Livestock Innovation Centre (WALIC), Banjul, Gambia. The adult male worms were dissected from the cattle nuchal ligament connective tissue and transferred to each well of a sterile 24-well (2 mL) plate (Fisher Scientific, Loughborough, UK), and maintained for at least 24 h in culture before use. The culture medium used was Minimum Essential Medium (MEM) with Earl’s Salts and L-Glutamine (Life Technologies Ltd., Loughborough, UK) supplemented with 10% heat inactivated new-born calf serum (Life Technologies Ltd., UK) and 200 units/mL penicillin, 200 µg/mL streptomycin and 0.5 µg/mL amphotericin B (Life Technologies Ltd., UK). Only normally active worms were used for the test and all assays were conducted at 37 °C under an atmosphere of 5% CO2 in air.
2.2. Origin of Drugs Tested
The drugs of the Pharmakon 1600 Collection were supplied by MicroSource Discovery Systems Inc. (Gaylordsville, CT, USA) as 10 mM DMSO stock solutions in microtubes in a 96-well plate format and stored at −20 °C. The positive control drug, Immiticide® (melarsomine dihydrochloride, Merial, Duluth, GA, USA) was supplied as a dry solid, known amounts of this drug were solubilized in 1 mL DMSO.
2.3. In Vitro Drug Activity against O. gutturosa Adult Worms as Described by Townson et al. [13]
The selected Pharmakon drugs with the positive control Immiticide® were screened using a final concentration of 1.25 × 10−5 M. At this concentration, the drugs were not toxic to mammalian cells (LLMCK2 monkey kidney cells, evaluated by microscopy). Two trials were performed using different numbers of worms (according to availability) and drugs. Trial 1: Four worms per group were used for the test and positive control drugs, and eight worms for the untreated control group. Only 100 of the selected 106 drugs were tested due to an insufficient number of available worms. Trial 2: This was performed using three separate assays; 23 drugs were tested, of which 17 were retested from Trial 1 (confirmation tests), and the 6 remaining drugs that could not be tested in Trial 1; two worms per group were used for the test and positive control drugs and six worms for the untreated control group. Worm viability was assessed using the following parameters: measurement of mean worm motility scores on a scale of 0 (immotile) to 10 (maximum) every 24 h, terminating at 120 h, using an Olympus inverted microscope. Biochemical evaluation of worm viability using MTT/formazan colorimetry was carried out after the last motility reading (120 h), outlined in Figure 1. Inhibition of formazan formation is correlated with worm damage or death (viability). The results of the test drugs were compared to the untreated controls. The results were calculated as motility reduction (%) and MTT reduction (%) compared to the untreated controls (Microsoft Office Excel, 2010) and designated: good activity, 100% motility and/or MTT reduction; moderate/marginal activity, 50–99% motility and/or MTT reduction; inactive, <50% motility reduction and MTT reduction. The test drug is considered active when the motility and/or MTT reductions of ≥50% is observed by comparison to the untreated controls. Statistical analysis was performed on only the Trial 1 data, using the two-tailed t test for the comparison between the motility and optical density means of the test drug and untreated control (Microsoft Office Excel, 2010) with a significance level of p < 0.05.
3. Results
Of the 106 selected drugs tested, 39 were inactive (<50% motility reduction and MTT reduction), 44 showed marginal/moderate activity (50–99% motility and/or MTT reduction) and 23 showed good activity (100% motility and/or MTT reduction) after 120 h drug exposure.
The results for the 44 drugs that showed moderate or marginal activity in Trial 1 are shown in Table 2. For the majority of these drugs, the MTT reductions correlated with the motility reductions, indicating that the worms did not only have reduced motility but were permanently damaged. Examination of the data shows that for 13 drugs with the greatest motility reductions of >80%, the MTT reduction was ≥70% (p-values < 0.001), with the highest number of hits in the antibacterial (cefuroxime sodium, demeclocycline hydrochloride, methenamine), antiparasitic (amitraz, primaquine phosphate) and neurological (armodafinil, chlorpromazine, dopamine hydrochloride, rivastigmine tartrate) bioactivity groups.
Table 2.
Results of the 44 identified drugs with moderate or marginal activity after 120 h drug exposure. Mot Redn—motility reduction; MTT Redn—MTT reduction.
| Drug | Mot Red | MTT Red | ||
|---|---|---|---|---|
| % | p-Value | % | p-Value | |
| IMMITICIDE (positive control) | 100.00 | <0.0001 | 91.09 | <0.0001 |
| Acepromazine Maleate | 53.57 | <0.0001 | 57.67 | <0.001 |
| Amitraz | 89.29 | <0.0001 | 77.23 | <0.0001 |
| Ampyzine Sulfate | 78.57 | <0.0001 | 76.98 | <0.0001 |
| Apomorphine Hydrochloride | 78.57 | <0.0001 | 76.49 | <0.0001 |
| Armodafinil | 82.14 | <0.0001 | 84.65 | <0.0001 |
| Ascorbyl Palmitate | 57.14 | <0.0001 | 67.08 | <0.001 |
| Bleomycin | 53.57 | <0.0001 | 53.22 | <0.01 |
| Bromhexine Hydrochloride | 57.14 | <0.0001 | 56.93 | <0.01 |
| Broxyquinoline | 82.14 | <0.0001 | 82.18 | <0.0001 |
| Candicidin | 67.86 | <0.0001 | 72.03 | <0.001 |
| Carbadox | 60.71 | <0.0001 | 66.83 | <0.001 |
| Cefsulodin Sodium | 50.00 | <0.0001 | 21.04 | 0.18 |
| Ceftibuten | 75.00 | <0.0001 | 71.53 | <0.001 |
| Cefuroxime Sodium | 92.86 | <0.0001 | 79.70 | <0.0001 |
| Chlorpromazine | 82.14 | <0.0001 | 79.46 | <0.0001 |
| Clopidogrel Sulfate | 28.57 | <0.01 | 53.47 | <0.01 |
| Demeclocycline Hydrochloride | 82.14 | <0.0001 | 76.24 | <0.001 |
| Dexamethasone Acetate | 78.57 | <0.0001 | 61.14 | <0.001 |
| Dienestrol | 78.57 | <0.0001 | 75.74 | <0.0001 |
| Docusate Sodium | 75.00 | <0.0001 | 66.58 | <0.001 |
| Dopamine Hydrochloride | 82.14 | <0.0001 | 69.55 | <0.001 |
| Doxorubicin | 60.71 | <0.0001 | 64.36 | <0.001 |
| Doxycycline Hydrochloride | 53.57 | <0.0001 | 37.13 | <0.05 |
| Epirubicin Hydrochloride | 75.00 | <0.0001 | 77.48 | <0.0001 |
| Fluorescein | 50.00 | <0.0001 | 45.54 | <0.01 |
| Guanethidine | 78.57 | <0.0001 | 79.95 | <0.0001 |
| Mangafodipir Trisodium | 89.29 | <0.0001 | 76.49 | <0.0001 |
| Methenamine | 92.86 | <0.0001 | 78.47 | <0.0001 |
| Methsuximide | 71.43 | <0.0001 | 66.34 | <0.001 |
| Methylergonovine Maleate | 57.14 | <0.0001 | 55.45 | <0.001 |
| Methylphenidate Hydrochloride | 75.00 | <0.0001 | 88.12 | <0.0001 |
| Minocycline Hydrochloride | 78.57 | <0.0001 | 72.52 | <0.001 |
| Moxidectin | 78.57 | <0.0001 | 71.78 | <0.001 |
| Nicotinyl Alcohol Tartrate | 67.86 | <0.0001 | 70.30 | <0.001 |
| Nifedipine | 92.86 | <0.0001 | 82.92 | <0.0001 |
| Prednisolone Tebutate | 96.43 | <0.0001 | 82.18 | <0.0001 |
| Primaquine Phosphate | 92.86 | <0.0001 | 84.16 | <0.0001 |
| Propoxycaine Hydrochloride | 60.71 | <0.0001 | 60.15 | <0.001 |
| Riboflavin | 60.71 | <0.0001 | 56.19 | <0.01 |
| Rifampin | 57.14 | <0.0001 | 56.44 | <0.01 |
| Rivastigmine Tartrate | 92.86 | <0.0001 | 81.93 | <0.001 |
| Sennoside A | 53.57 | <0.0001 | 52.23 | <0.01 |
| Tetrahydrozoline Hydrochloride | 53.57 | <0.0001 | 53.22 | <0.01 |
| Valganciclovir Hydrochloride | 50.00 | <0.0001 | 53.71 | <0.01 |
The 23 drugs which demonstrated good activity are shown in Table 3 (includes the chemical structures); 17 of these drugs were retests from Trial 1, and 6 drugs were new tests in Trial 2. All of the drugs produced 100% motility reduction and high levels of MTT reduction, and for most drugs there was a good level of concordance between the results of Trial 1 when compared to Trial 2. The highest number of hits was found in the antibacterial (cefaclor, chlorhexidine dihydrochloride, gramicidin, lasalocid sodium, nitrofurantoin, nitrofurazone, nitroxoline, rifaximin), anti-infective (benzethonium chloride, dequalinium chloride, methylbenzethonium chloride, oxyquinoline hemisulfate) and antiparasitic (atovaquone, hexetidine, homidium bromide, iodoquinol, levamisole hydrochloride, pyrantel pamoate) bioactivity groups.
Table 3.
Results of the 23 drugs identified with good activity after 120 h drug exposure. Mot Redn—motility reduction; MTT Redn—MTT reduction; nd—not determined; p-value applies to both motility and MTT reductions.
| Drug (Molecular Weight/Bioactivity) | Molecular Structure | Trial 1 | Trial 2 | |||
|---|---|---|---|---|---|---|
| Mot Redn (%) | MTT Redn (%) | p-Value | Mot Redn (%) | MTT Redn (%) | ||
| IMMITICIDE, positive control (501.34/Anthelmintic) |
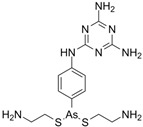
|
100.00 | 91.09 | <0.0001 | 100.00 | Range 77.19–98.88 |
| ATOVAQUONE (366.85/Antimalarial) |
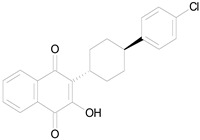
|
100.00 | 90.10 | <0.0001 | 100.00 | 74.42 |
| BENZETHONIUM CHLORIDE (448.09/Antiinfective) |
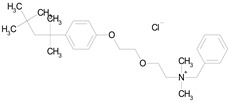
|
100.00 | 88.12 | <0.0001 | 100.00 | 71.91 |
| CHLORHEXIDINE DIHYDROCHLORIDE (578.38/Antibacterial) |
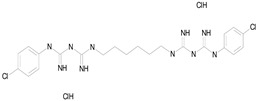
|
100.00 | 90.10 | <0.0001 | 100.00 | 100.00 |
| GRAMICIDIN, gramicidin A shown (1882.34/Antibacterial) |
|
100.00 | 76.24 | <0.0001 | 100.00 | 84.27 |
| IODOQUINOL (396.96/Antiamoebic) |
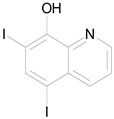
|
100.00 | 80.20 | <0.0001 | 100.00 | 87.64 |
| ISRADIPINE (371.40/Calcium channel blocker) |
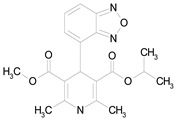
|
100.00 | 87.13 | <0.0001 | 100.00 | 78.95 |
| LASALOCID SODIUM (612.79/Antibacterial) |
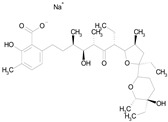
|
100.00 | 89.11 | <0.0001 | 73.33 | 90.70 |
| LEVAMISOLE HYDROCHLORIDE (240.76/Anthelmintic) |
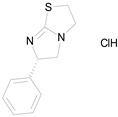
|
100.00 | 92.08 | <0.0001 | 100.00 | 55.81 |
| LOSARTAN (422.92/Antihypertensive) |
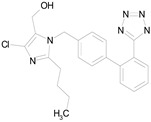
|
100.00 | 88.12 | <0.0001 | 100.00 | 82.56 |
| MELOXICAM SODIUM (373.39/Antiinflammatory) |
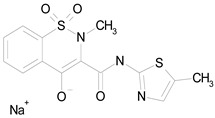
|
100.00 | 89.11 | <0.0001 | 100.00 | 47.67 |
| METHYLBENZETHONIUM CHLORIDE (462.12/Antiinfective) |
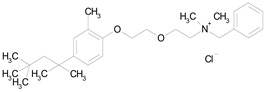
|
100.00 | 91.09 | <0.0001 | 100.00 | 67.44 |
| MITOXANTRONE HYDROCHLORIDE (517.41/Antineoplastic) |
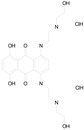
|
100.00 | 91.09 | <0.0001 | 100.00 | 70.93 |
| NITROFURANTOIN (238.16/Antibacterial) |
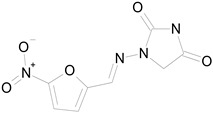
|
100.00 | 75.25 | <0.0001 | 100.00 | 92.70 |
| NITROFURAZONE (198.14/Antibacterial) |
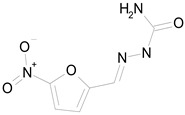
|
100.00 | 85.15 | <0.0001 | 100.00 | 95.51 |
| OXYQUINOLINE HEMISULFATE (243.24/Antiinfective) |
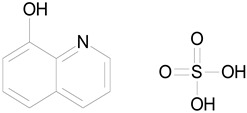
|
100.00 | 86.14 | <0.0001 | 100.00 | 91.57 |
| PYRANTEL PAMOATE (594.69/Anthelmintic) |
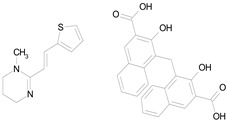
|
100.00 | 85.15 | <0.0001 | 100.00 | 90.45 |
| RIFAXIMIN (785.90/Antibacterial) |
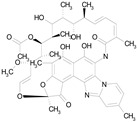
|
100.00 | 91.09 | <0.0001 | 100.00 | 68.60 |
| CEFACLOR (367.81/Antibacterial) |
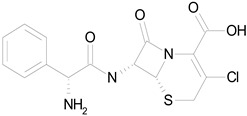
|
nd | nd | nd | 100.00 | 96.51 |
| DEQUALINIUM CHLORIDE (527.59/Antiinfective) |
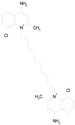
|
nd | nd | nd | 100.00 | 61.40 |
| HEXETIDINE (339.61/Antifungal) |
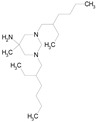
|
nd | nd | nd | 100.00 | 88.60 |
| HOMIDIUM BROMIDE (394.32/Antiprotozoal) |
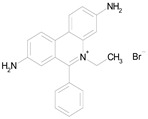
|
nd | nd | nd | 100.00 | 81.58 |
| NITROXOLINE (190.16/Antibacterial) |
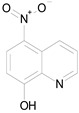
|
nd | nd | nd | 100.00 | 82.46 |
| PENFLURIDOL (523.98/Antipsychotic) |
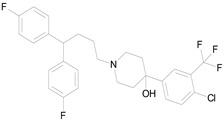
|
nd | nd | nd | 100.00 | 64.04 |
4. Discussion
With the urgent need to identify drugs with potential macrofilaricidal activity against Onchocerca parasites, using the strategy of drug repurposing to identify new drugs for the prompt development of therapeutics for the treatment of filariasis is not a new concept; indeed, the drugs currently in use to treat filarial infections, ivermectin, diethylcarbamazine, moxidectin and doxycycline, have all been repurposed from the veterinary or medical fields [14]. Previous studies have screened libraries and drugs for activity against filarial parasites [15] and the Pharmakon 1600 library itself has also been screened for antischistosomal activity [16]. All human-infecting filarial nematodes, with the exception of L. loa, carry the endosymbiotic Wolbachia bacteria which has been shown to be essential for O. volvulus fertility and viability [17,18]. Studies using drugs from diverse libraries in anti-Wolbachia screens have revealed promising candidates for further development [19]. Rifampicin (also known as rifampin), an antibiotic used for the treatment of tuberculosis, has been developed for testing in clinical trials based on the effect of high dose, long term exposure using in vitro assays against O. gutturosa adult male worms [20] and Wolbachia [21]. In addition, in vivo studies have demonstrated more than 90% Wolbachia depletion using Brugia malayi and Onchocerca ochengi models [22]. To test whether the treatment time for onchocerciasis could be reduced, rifampicin in combination with albendazole is currently being investigated in a clinical trial in Cameroon [23]. Emodepside, a veterinary drug licensed for the oral treatment of gastrointestinal nematodes, exhibited high activity in vitro and in vivo against various filarial parasites [24]. A Phase I clinical trial in healthy humans has been completed [25] and recruitment for a Phase II trial in onchocerciasis patients is currently underway [26]. Similarly, oxfendazole is a drug that is used against intestinal helminths in the veterinary field over several decades. In vitro and in vivo animal studies showed that oxfendazole is also active against filarial nematodes [27,28]. Oxfendazole was tested in Phase 1 clinical trials [29] and will be tested in onchocerciasis, loiasis, mansonellosis and Trichuris trichiura patients in a Phase 2 clinical basket trial [30]. In this study, we aimed to identify selected existing drugs contained within the FDA-approved Pharmakon 1600 library with the potential to be rapidly developed as macrofilaricides against onchocerciasis.
The results of the standard anthelmintics, contained within the antiparasitic bioactivity group, were as expected and demonstrate that screening of diverse libraries using this 5-day motility/MTT in vitro assay is suitable for identifying new drug candidates with activity against Onchocerca parasites. Nevertheless, these types of in vitro assays do not tell us all we need to know about the activity of drugs, since host factors may play an important role. Diethylcarbamazine citrate and flubendazole were inactive in this study and this result corresponds to previous in vitro findings [27,31,32]. However, clinical studies in Mexico demonstrated that flubendazole has high macrofilaricidal activity [33,34] and in vivo laboratory studies confirmed this activity [35]. Recently, the important role of the immune system in supporting the macrofilaricidal efficacy for the related drug oxfendazole was demonstrated in the Litomosoides sigmodontis filarial mouse model [28]. Despite the limitation that some candidates may require an intact immune system for efficacy, several promising candidates were identified. In this study, levamisole hydrochloride displayed good activity (100% motility reduction), the parasites were completely immotile after 5 days of exposure (Table 3), and this result is in accordance with previous studies [31], although the MTT result indicated paralysis rather than worm death. Unsurprisingly, pyrantel pamoate (anthelmintic), which is used for roundworm and pinworm infections, showed good activity against O. gutturosa parasites in this study; this drug is a depolarising neuromuscular blocking agent which causes paralysis of the worms [36]. Treatment of onchocerciasis patients with pyrantel pamoate showed no notable activity against adult worms of O. volvulus [37], possibly due to poor oral uptake or suboptimal dosage/treatment length. Moxidectin showed moderate activity; the motility of the parasites was reduced by 78.6% with a comparable reduction in viability (71.8%) indicating slow killing of the worms in vitro (Table 2). This drug was licensed to treat human onchocerciasis in 2018 [38].
Of the drugs that displayed moderate/marginal activity, cefuroxime sodium, methenamine, primaquine phosphate and rivastigmine tartrate had significant effects on the parasites with motility reductions of >90% and MTT reductions of >70% (p-values < 0.001, Table 2). Oral formulations are available for these drugs and therefore they should be considered “drugs of interest” to be further investigated for use against Onchocerca infections. Cefuroxime sodium and methenamine are used for the treatment of bacterial infections, primaquine phosphate (antimalarial) is used for the treatment of hypnozoites, the dormant form of Plasmodium parasites during malaria tertiana and rivastigmine tartrate has neurological activity in the treatment of dementia associated with Alzheimer’s or Parkinson’s diseases. Rifampin (internationally known as rifampicin) also showed moderate activity (Table 2) and is currently in development for the treatment of onchocerciasis [15]. Such a slow macrofilaricidal efficacy is known for antibiotics that target and eliminate the Wolbachia endosymbionts of filariae, such as doxycycline [15]. The semi-synthetic derivative of rifampin, rifaximin, completely reduced the motility of the parasites (Table 3); however, due to poor absorption, rifaximin is only used to treat gastrointestinal infections.
Drugs belonging to novel classes, with available oral formulations, rendered O. gutturosa male worms completely immotile (good activity, 100% motility reduction) but the parasites were not dead as indicated by the MTT reductions (Table 3); longer exposure to these drugs, or a longer period in culture following exposure to the drug, may result in parasite death. Isradipine (calcium channel blocker) and losartan (angiotensin receptor blocker) are both used to treat hypertension and cause vasodilation by blocking different receptors. Penfluridol with neurological activity is commonly used as an antipsychotic drug. The anticancer drug, mitoxantrone hydrochloride, is used in the treatment of prostate cancer and leukaemia. Of interest is the activity of atovaquone (antimalarial) against the O. gutturosa worms. In addition to its use as prophylaxis and treatment against malaria parasites, it is also used for the treatment of pneumonia caused by fungal infection and some other microbial infections [39]. Several studies have investigated this drug for the treatment of different types of cancers [40]. Also of interest is the antibacterial cefaclor which belongs to the large cephalosporin family of antibiotics; this drug class is structurally related to penicillin and used to treat a wide range of bacterial infections. Cefuroxime sodium, which was moderately/marginally active against the parasites, together with cefaclor, are both second-generation drugs and oral formulations are available for both drugs. We speculate that the active antibacterial drugs tested in this study had a direct effect on worms, with the possibility of an indirect effect by killing Wolbachia in the longer term.
In this study we have taken the first step to identify FDA-approved drugs with potential anti-Onchocerca activity; some of the identified hits should be further investigated for repurposing. Of lower priority are the drugs designed for topical use and those that can only be administered parenterally. Further investigation of the candidate drugs that displayed promising in vitro activity against O. gutturosa adult male parasites was curtailed due to the unexpected, imposed travel and work restrictions with regard to studies in Gambia during the COVID-19 pandemic. Further in vitro trials are required to retest the hits of interest to estimate activity endpoints and EC50 values. This data together with the available pharmacokinetic and toxicity profiles can be used to rapidly inform the development of the drugs for further evaluation against Onchocerca and other filarial species of medical and veterinary importance. In addition, these hits may provide a good starting point to assess related compounds of interest and for the synthesis of new drugs.
Author Contributions
Conceptualization, S.T. (Simon Townson) and I.S.; methodology, S.G., S.T. (Simon Townson) and A.F.; software, S.G.; formal analysis, S.G. and S.T. (Simon Townson); investigation, S.G., S.T. (Simon Townson), A.F., J.S.-K. and J.Z.; data curation, S.G. and A.F.; writing—original draft preparation, S.G. and S.T. (Simon Townson); writing—review and editing, M.P.H., S.G., S.T. (Simon Townson), S.T. (Senyo Tagboto) and I.S.; supervision, S.G., S.T. (Simon Townson) and I.S.; project administration, S.T. (Simon Townson) and I.S.; funding acquisition, S.T. (Simon Townson). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This study was supported by funding from the Drugs for Neglected Diseases initiative, Geneva. M.P.H. is funded under Germany’s Excellence Strategy—EXC2151 390873048. M.P.H. is a member of the German Center for Infection Research (DZIF). M.P.H. received funding from the German Center for Infection Research (TTU 09.701).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brattig N.W., Cheke R.A., Garms R. Onchocerciasis (river blindness)—More than a century of research and control. Acta Trop. 2021;218:105677. doi: 10.1016/j.actatropica.2020.105677. [DOI] [PubMed] [Google Scholar]
- 2.WHO [(accessed on 15 October 2023)]. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1.
- 3.Duke B.O., Zea-Flores G., Muñoz B. The embryogenesis of Onchocerca volvulus over the first year after a single dose of ivermectin. Trop. Med. Parasitol. 1991;42:175–180. [PubMed] [Google Scholar]
- 4.Basáñez M.G., Pion S.D., Boakes E., Filipe J.A., Churcher T.S., Boussinesq M. Effect of single-dose ivermectin on Onchocerca volvulus: A systematic review and meta-analysis. Lancet Infect. Dis. 2008;8:310–322. doi: 10.1016/S1473-3099(08)70099-9. [DOI] [PubMed] [Google Scholar]
- 5.Plaisier A.P., van Oortmarssen G.J., Remme J., Habbema J.D. The reproductive lifespan of Onchocerca volvulus in West African savanna. Acta Trop. 1991;48:271–284. doi: 10.1016/0001-706X(91)90015-C. [DOI] [PubMed] [Google Scholar]
- 6.Geerts S., Gryseels B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin. Microbiol. Rev. 2000;13:207–222. doi: 10.1128/CMR.13.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Samson-Himmelstjerna G., Harder A., Sangster N.C., Coles G.C. Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology. 2005;130:343–347. doi: 10.1017/S0031182004006523. [DOI] [PubMed] [Google Scholar]
- 8.Prichard R.K., Geary T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019;10:69–83. doi: 10.1016/j.ijpddr.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011;5:e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nana-Djeunga H.C., Bourguinat C., Pion S.D., Bopda J., Kengne-Ouafo J.A., Njiokou F., Prichard R.K., Wanji S., Kamgno J., Boussinesq M. Reproductive status of Onchocerca volvulus after ivermectin treatment in an ivermectin-naïve and a frequently treated population from Cameroon. PLoS Negl. Trop. Dis. 2014;8:e2824. doi: 10.1371/journal.pntd.0002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milton P., Hamley J.I.D., Walker M., Basáñez M.G. Moxidectin: An oral treatment for human onchocerciasis. Expert Rev. Anti Infect. Ther. 2020;18:1067–1081. doi: 10.1080/14787210.2020.1792772. [DOI] [PubMed] [Google Scholar]
- 12.Pfarr K.M., Krome A.K., Al-Obaidi I., Batchelor H., Vaillant M., Hoerauf A., Opoku N.O., Kuesel A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 2. Oral anti-infective drugs and drug combinations for off-label use. Parasit. Vectors. 2023;16:394. doi: 10.1186/s13071-023-05909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townson S., Ramirez B., Fakorede F., Mouries M.A., Nwaka S. Challenges in drug discovery for novel antifilarials. Expert Opin. Drug Discov. 2007;2:S63–S73. doi: 10.1517/17460441.2.S1.S63. [DOI] [PubMed] [Google Scholar]
- 14.Tagboto S., Orish V. Drug development for onchocerciasis-the past, the present and the future. Front. Trop. Dis. 2022;3:953061. doi: 10.3389/fitd.2022.953061. [DOI] [Google Scholar]
- 15.Ehrens A., Hoerauf A., Hübner M.P. Current perspective of new anti-Wolbachial and direct-acting macrofilaricidal drugs as treatment strategies for human filariasis. GMS Infect. Dis. 2022;10:Doc02. doi: 10.3205/id000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panic G., Vargas M., Scandale I., Keiser J. Activity Profile of an FDA-Approved Compound Library against Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015;9:e0003962. doi: 10.1371/journal.pntd.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamarozzi F., Halliday A., Gentil K., Hoerauf A., Pearlman E., Taylor M.J. Onchocerciasis: The role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin. Microbiol. Rev. 2011;24:459–468. doi: 10.1128/CMR.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoerauf A. Filariasis: New drugs and new opportunities for lymphatic filariasis and onchocerciasis. Curr. Opin. Infect. Dis. 2008;21:673–681. doi: 10.1097/QCO.0b013e328315cde7. [DOI] [PubMed] [Google Scholar]
- 19.Johnston K.L., Cook D.A.N., Berry N.G., David Hong W., Clare R.H., Goddard M., Ford L., Nixon G.L., O’Neill P.M., Ward S.A., et al. Identification and prioritization of novel anti-Wolbachia chemotypes from screening a 10,000-compound diversity library. Sci. Adv. 2017;3:eaao1551. doi: 10.1126/sciadv.aao1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townson S., Tagboto S., McGarry H.F., Egerton G.L., Taylor M.J. Onchocerca parasites and Wolbachia endosymbionts: Evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 2006;5:4. doi: 10.1186/1475-2883-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenollar F., Maurin M., Raoult D. Wolbachia pipientis growth kinetics and susceptibilities to 13 antibiotics determined by immunofluorescence staining and real-time PCR. Antimicrob. Agents Chemother. 2003;47:1665–1671. doi: 10.1128/AAC.47.5.1665-1671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aljayyoussi G., Tyrer H.E., Ford L., Sjoberg H., Pionnier N., Waterhouse D., Davies J., Gamble J., Metuge H., Cook D.A.N., et al. Short-Course, High-Dose Rifampicin Achieves Wolbachia Depletion Predictive of Curative Outcomes in Preclinical Models of Lymphatic Filariasis and Onchocerciasis. Sci. Rep. 2017;7:210. doi: 10.1038/s41598-017-00322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanji S., Hoerauf A., Klarmann-Schulz U. ISRCTN38954299—The Efficacy of Rifampicin Plus Albendazole against River Blindness (Onchocerciasis) in Cameroon. [(accessed on 5 November 2023)]. Available online: https://www.isrctn.com/ISRCTN38954299.
- 24.Hübner M.P., Townson S., Gokool S., Tagboto S., Maclean M.J., Verocai G.G., Wolstenholme A.J., Frohberger S.J., Hoerauf A., Specht S., et al. Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside. Int. J. Parasitol. Drugs Drug Resist. 2021;17:27–35. doi: 10.1016/j.ijpddr.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillon J.Y., Dennison J., van den Berg F., Delhomme S., Dequatre Cheeseman K., Peña Rossi C., Strub Wourgaft N., Specht S., Pedrique B., Monnot F., et al. Safety, tolerability and pharmacokinetics of emodepside, a potential novel treatment for onchocerciasis (river blindness), in healthy male subjects. Br. J. Clin. Pharmacol. 2021;87:3949–3960. doi: 10.1111/bcp.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DNDi [(accessed on 15 October 2023)]. Available online: https://dndi.org/research-development/portfolio/emodepside/
- 27.Hübner M.P., Martin C., Specht S., Koschel M., Dubben B., Frohberger S.J., Ehrens A., Fendler M., Struever D., Mitre E., et al. Oxfendazole mediates macrofilaricidal efficacy against the filarial nematode Litomosoides sigmodontis in vivo and inhibits Onchocerca spec. motility in vitro. PLoS Negl. Trop. Dis. 2020;14:e0008427. doi: 10.1371/journal.pntd.0008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Risch F., Scheunemann J.F., Reichwald J.J., Lenz B., Ehrens A., Gal J., Fercoq F., Koschel M., Fendler M., Hoerauf A., et al. The efficacy of the benzimidazoles oxfendazole and flubendazole against Litomosoides sigmodontis is dependent on the adaptive and innate immune system. Front. Microbiol. 2023;14:1213143. doi: 10.3389/fmicb.2023.1213143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach T., Galbiati S., Kennedy J.K., Deye G., Nomicos E.Y.H., Codd E.E., Garcia H.H., Horton J., Gilman R.H., Gonzalez A.E., et al. Pharmacokinetics, Safety, and Tolerability of Oxfendazole in Healthy Adults in an Open-Label Phase 1 Multiple Ascending Dose and Food Effect Study. Antimicrob. Agents Chemother. 2020;64:e01018. doi: 10.1128/AAC.01018-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.eWHORM [(accessed on 5 November 2023)]. Available online: https://ewhorm.org/
- 31.Townson S., Connelly C., Dobinson A., Muller R. Drug activity against Onchocerca gutturosa males in vitro: A model for chemotherapeutic research on onchocerciasis. J. Helminthol. 1987;61:271–281. doi: 10.1017/S0022149X00010178. [DOI] [PubMed] [Google Scholar]
- 32.Strote G., Wieland S., Darge K., Comley J.C. In vitro assessment of the activity of anthelmintic compounds on adults of Onchocerca volvulus. Acta Leiden. 1990;59:285–296. [PubMed] [Google Scholar]
- 33.Dominguez-Vazquez A., Taylor H.R., Greene B.M., Ruvalcaba-Macias A.M., Rivas-Alcala A.R., Murphy R.P., Beltran-Hernandez F. Comparison of flubendazole and diethylcarbamazine in treatment of onchocerciasis. Lancet. 1983;1:139–143. doi: 10.1016/S0140-6736(83)92753-8. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie C.D., Geary T.G. Flubendazole: A candidate macrofilaricide for lymphatic filariasis and onchocerciasis field programs. Expert Rev. Anti Infect. Ther. 2011;9:497–501. doi: 10.1586/eri.11.30. [DOI] [PubMed] [Google Scholar]
- 35.Hübner M.P., Ehrens A., Koschel M., Dubben B., Lenz F., Frohberger S.J., Specht S., Quirynen L., Lachau-Durand S., Tekle F., et al. Macrofilaricidal efficacy of single and repeated oral and subcutaneous doses of flubendazole in Litomosoides sigmodontis infected jirds. PLoS Negl. Trop. Dis. 2019;13:e0006320. doi: 10.1371/journal.pntd.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fissiha W., Kinde M.Z. Anthelmintic Resistance and Its Mechanism: A Review. Infect. Drug Resist. 2021;14:5403–5410. doi: 10.2147/IDR.S332378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kale O. Small-scale trials of six drugs against Onchocerca volvulus. Tropenmed. Parasitol. 1978;29:163–167. [PubMed] [Google Scholar]
- 38.Kura K., Milton P., Hamley J.I.D., Walker M., Bakajika D.K., Kanza E.M., Opoku N.O., Howard H., Nigo M.M., Asare S., et al. Can mass drug administration of moxidectin accelerate onchocerciasis elimination in Africa? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023;378:20220277. doi: 10.1098/rstb.2022.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baggish A.L., Hill D.R. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 2002;46:1163–1173. doi: 10.1128/AAC.46.5.1163-1173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng G., Hardy M., Topchyan P., Zander R., Volberding P., Cui W., Kalyanaraman B. Potent inhibition of tumour cell proliferation and immunoregulatory function by mitochondria-targeted atovaquone. Sci. Rep. 2020;10:17872. doi: 10.1038/s41598-020-74808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.