Abstract
Background: Due to low adherence to HPV vaccination programs, the European region struggles with vaccination rates lower than 30% among the targeted population. The present report investigated various socio-demographic and psychological factors influencing European parents’ hesitancy towards vaccinating their children. Methods: As of September 2023, four databases were searched. After applying inclusion criteria, all articles comparing psychological and socio-demographic factors in parents who vaccinate or do not vaccinate their children were included. Results: Twenty-five primary publications met the criteria, totaling 385,460 responders, of which 311,803 want to or have already vaccinated their child, and 73,555 do not wish to do so. Immigrant and employment status, religion, age of parents and the child’s gender seemed to influence their decision to vaccinate their child. Previous experience with vaccines, perceived safety and efficacy and the mother’s previous abnormal CCS results also impacted their decision. The caregivers’ education, gender, civil status, number of children, family history of neoplasia or mother’s CCS attendance did not influence their hesitancy to vaccine. Conclusion: Multiple demographic, social, economic and psychological barriers involved in the parents’ hesitancy to vaccinate their children against the HPV virus were highlighted. Specific at-risk categories that need to be targeted with information, education and vaccination campaigns were identified.
Keywords: HPV vaccination, hesitancy, Europe, parents, cervical cancer prevention, socio-demographic, psychological factors
1. Introduction
Among female neoplasia cases worldwide, cervical cancer is the pathology currently holding second place concerning its incidence and mortality, despite widely available preventive measures [1,2]. The World Health Organization (WHO) recently proposed the Cervical Cancer Elimination Initiative (also called the 90–70–90 rule), a strategy that aims to fully vaccinate 90% of girls by the age of 15 years old, screen 70% of women with high-performance tests (such as HPV testing or typing) by the age of 35 and again by 45 years old and treat 90% of women with precancerous lesions or invasive cancer by 2030 [3]. Despite this ambitious initiative, from 2021, official cervical cancer screening recommendations were available in only 69% of all countries worldwide, of which 78% were still using cytology as a primary test. Only 35% of them used HPV testing (usually a Polymerase Chain Reaction–PCR test) as a standard, alone or in combination with classic cytology [4].
Meanwhile, the HPV vaccine was approved by the FDA in 2006, initially covering four HPV strains [5]. Currently, Gardasil 9 offers protection against nine strains: 6, 11, 16, 18, 31, 33, 45, 52 and 58 [6]. Despite tremendous efforts to implement vaccination programs worldwide, more than 17 years after its initial approval, only 55% of the WHO Member States introduced the HPV vaccine as a primary prophylaxis measure, and only 33 out of those 107 countries have gender-neutral programs, vaccinating girls and boys [7]. In Europe, 77% of countries have introduced the HPV vaccination [7], the majority with the aid of a National Immunization Program in 2007, shortly after EMA approved the product. Some countries only adhered later, such as Croatia in 2016 [8]. However, different implementation strategies were used, resulting in high heterogeneity regarding vaccine availability. For example, the costs could be partially or fully reimbursed, covered by the National Health Authorities, while other countries offer the vaccine only if fully paid for by the recipients. Moreover, the recommended age for vaccinating children (ranging from 9 to 15 years old) varies significantly between countries regarding the policy of reimbursement and availability through the public health system; some reimburse the vaccine regardless of the ages of the recipients, while only a few offer gender-neutral programs [8]. As of 2022, in the European Region, full vaccination coverage (three doses of HPV vaccine) in females was around 29%, while in males, the percentage was less than half, at around 14%. In total, 38% of females received the first dose, while only 18% of males initiated the vaccination scheme [9]. Most programs targeted young boys and girls, while some countries offered catch-up alternatives; parental consent was required.
This poor coverage of HPV vaccination highlights the discrepancies among European countries regarding vaccination implementation and parents’ (or caretakers’) hesitancy toward initiating or continuing the scheme. Vaccination hesitancy was defined by the World Health Organization (WHO) as the delay in acceptance or refusal despite available access to vaccines [10]. Multiple reasons can influence the decision to vaccinate oneself or one’s child, varying from personal beliefs, insufficient education, low-quality information or a lack of information, safety concerns, a lack of confidence or demanding access to vaccination programs [10,11,12,13]. Therefore, this systematic review and meta-analysis aimed to identify other psychological, social, demographic and economic factors (such as concerns related to the early debut of sexual activity, previous childhood vaccinations, parents’ history of gynecological pathologies, gender of the children, perceived susceptibility and perceived risk, level of education, marital status, income and many more) that could influence European parents’ (or caregivers’) intention to vaccinate their children against HPV.
2. Materials and Methods
2.1. Research Protocol and Information Sources
A systematic review and meta-analysis were performed to identify psychological and social factors influencing parents’ (or caretakers’) intention to vaccinate their children against HPV. This report followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). There was no separate review protocol, and this study was not preregistered. No Ethical Committee approval or informed consent was required since the data were collected from previous publications.
2.2. Search Strategy
A search was performed on 13 September 2023 in four databases: PubMed (US National Library of Medicine, Bethesda, MD, USA), EMBASE (Elsevier, Amsterdam, The Netherlands), Scopus (Elsevier), and Web of Science (Philadelphia, PA, USA). A predefined search formula was used to identify relevant studies: ((hpv vaccin*) OR (human papillomavirus vaccin*) OR (human papilloma virus vaccin*)) AND ((knowledge, attitudes, practice) OR (barriers) OR (facilitators) OR (decision) OR (inten*)) AND ((parent*) OR (caregive*) OR (child*)). No additional filters or limits were used.
2.3. Eligibility Criteria
Inclusion criteria (formulated to adhere to PICO strategy):
Population: Parents and caregivers from European countries (EU-27) and the United Kingdom;
Intervention: All studies evaluating psychological, demographic and social factors;
Comparison: Vaccinated/intent to vaccinate compared with non-vaccinated/no intent to vaccinate their children;
Outcome: Vaccination status/intention to vaccinate;
Study type: Self-report quantitative studies, cross-sectional studies and non-experimental studies.
Exclusion Criteria
Duplicates, other reviews and meta-analyses, other study designs (qualitative studies, intervention studies correspondence, conference proceedings, books and chapters, validation or development of scale/questionnaire) and studies that assess other topics were excluded. Studies in languages other than English and studies with no available full text were also excluded.
2.4. Selection Process
Two reviewers independently screened the studies; if discrepancies were found, a third reviewer solved the issue. No automation tools were used.
2.5. Data Collection Process
Two independent reviewers collected data from individual studies, and relevant information was extracted.
2.6. Risk of Bias Assessment and Quality Assessment
Funnel plots were used to evaluate the risk of bias. The Newcastle–Ottawa Quality Assessment Scale (NOS), adapted for cross-sectional studies, was used to assess the quality of the included studies [14]. Two reviewers evaluated each study.
2.7. Synthesis of Methods and Results
The articles were screened by title and abstract. Articles that met the eligibility criteria (comparison groups, evaluating psychological and social characteristics) were included in the full-text assessment. Eligible articles were included in the systematic review assessing parents (or caretakers) with daughters or sons (regardless of the number of children) who were vaccinated, non-vaccinated or intended to vaccinate. Relevant data were extracted from the included articles: age, gender, educational level, civil status, employment, religion, the immigrant status of parents (or caretakers), number of children, mothers’ CCS attendance and Pap Smear results, family history of any cancer, each child’s gender, previous history of childhood vaccination, perceived efficacy and safety of the HPV vaccine, risks behaviors, HPV knowledge and awareness, costs of the HPV vaccine, perceived consequences, risks and susceptibility decision-making process and barriers toward HPV vaccination.
Combinable data were considered when results were presented either as an already calculated effect of measure (Odds Ratio—OR; Relative Risk—RR; Risk Difference—RD) or numbers were provided regarding the number of events in each group (presence vs. absence of a factor) and the total population number, so that the OR could be calculated. In that instance, OR = (a/c)/b/d), where a = the number of events in the exposed group; b = the number of controls in the exposed group; c = the number of events in the unexposed group; and d = the number of controls in the unexposed group. When other effects of measures were presented (RR or RD), appropriate transformations to OR were performed. If the results were not combinable, a simple systematic review was provided. Odds Ratio (OR) was used as a primary parameter with a Confidence Interval (CI) of 95%. I2 was used to evaluate heterogeneity, and the threshold were set according to Cochrane’s recommendations as follows [15]: 0% to 40%—the heterogeneity might not be important; 30% to 60%—may represent moderate heterogeneity; 50% to 90%—may represent substantial heterogeneity; 75% to 100%—considerable heterogeneity. Fixed or Random Effects models were applied accordingly based on the differences between smaller and larger studies or causes of heterogeneity and bias. As when there is no heterogeneity, a random-effects or fixed-effects model will provide results similar to identical ones, a classical fixed-effects model was applied. Data analysis was performed in Review Manager 5.4 (RevMan). Regarding additional analyses, subgroup analyses were planned if feasible (type of included studies, quality assessment scoring, child’s gender or age, gender of parent), while sensitivity analyses were depicted by excluding one study at a time from the meta-analysis. A good sensitivity was considered to exist when the results remained in the same CI.
3. Results
3.1. Study Selection
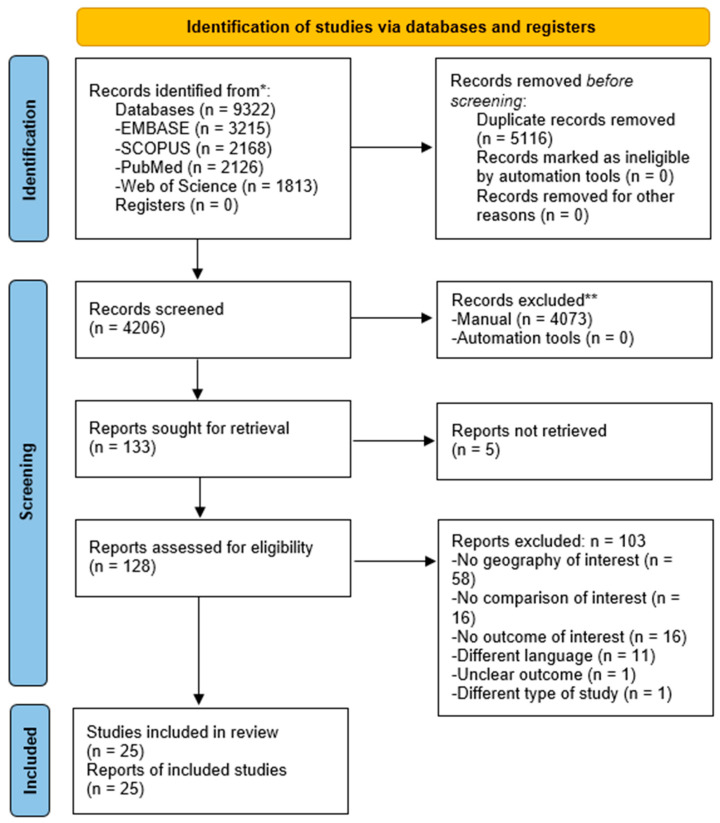
A systematic search was performed in four databases on 13 September 2023, resulting in 9322 articles; after removing the duplicates, 4206 publications were screened by title and abstract. In total, 133 were assessed for full-text, and 25 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] were included in the systematic review and meta-analysis (Figure 1).
Figure 1.
The PRISMA flow chart shows the process involved in searching and filtering the primary publications. * Number of records identified from each database or register searched. ** If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Overall, 25 primary publications were included in the systematic review, totaling 385,460 responders (parents or caregivers), of which 311,803 want to or already have vaccinated their child (boy or girl), and 73,555 did not vaccinate or do not intend to vaccinate their child (Table 1). The quality assessment of the included studies can be consulted in Table 2, and a graphical representation can be consulted in Supplementary Material S2 (Chart S1).
Table 1.
Characteristics of the included studies.
| Study | Country | Respondents | Parent’s Age (in years) | Gender of Children | Vaccination Status | Children’s Age (in years) | Recruitment Site | Data Collection Period | Sample Size | Number of Vaccinated Children | Number of Non-Vaccinated Children |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baumann et al. (2019) [29] | Denmark | Mothers | <20–>40 (at parturition) | Female | Vaccinated vs. non-vaccinated | 13 | Danish Civil Registration System (CRS) | 2015–2016 | 3558 | 2899 | 659 |
| Bedford et al. (2021) [35] | United Kingdom | Parents | Not specified | Female | Initiated vs. non-initiated | 14 | Millennium Cohort Study (MCS) | 2014–2016 | 5654 | 5265 | 399 |
| Brabin et al. (2006) [16] | United Kingdom | Parents | ≤30–>45 | Not specified | Consenting vs. non-consenting | 11–12 | Manchester City Council’s Education Department | 2005 | 317 | 239 | 78 |
| Dahlström et al. (2009) [17] | Sweden | Mothers and fathers | <41–>45 | Female and male | Intention vs. non-intention | 12–15 | Swedish Population Register | 2007 | 13,946 | 10,537 | 3303 |
| David et al. (2022) [30] | France | Parents | 32–58 | Female | Vaccinated vs. non-vaccinated | 9–19 | Hospital Femme-Mère Enfant and Necker-Enfants-Malades | 2020–2021 | 71 | 28 | 43 |
| Dib et al. (2022) [37] | France | Mothers | 43.5 (mean) | Female | Vaccinated vs. non-vaccinated | 11–14 | Not specified | Not specified | 1102 | 425 | 677 |
| Domínguez-Riscart et al. (2023) [40] | Spain | Caregivers (mostly mothers) | 44.1 (mean) | Trans-gender | Intention vs. non-intention | 9–16 | Hospital Puerta del Mar and Universitario Reina Sofia | 2022 | 65 | 14 | 51 |
| Donders et al. (2007) [18] | Belgium | Mothers | 35.8 (mean) | Female and male | Intention vs. non-intention | Not specified | Hospital Hart | 2007 | 309 | 285 | 23 |
| Feiring et al. (2015) [31] | Norway | Mothers and fathers | ≤25–>35 (at parturition) | Female | Initiated vs. non-initiated | Not specified | The Norwegian Central Population Registry | 2013 | 84,139 | 65,843 | 18,296 |
| Ganczak et al. (2018) [26] | Poland | Mothers and fathers | 29–67 | Not specified | Intention vs. non-intention | 13–16 | High School | 2013–2014 | 450 | 383 | 67 |
| Gefenaite et al. (2012) [32] | Netherlands | Parents | 35–55 | Female | Vaccinated vs. non-vaccinated | 13 | Institute for Public Health and the Environment | 2009 | 469 | 307 | 162 |
| Grandahl et al. (2016) [22] | Sweden | Parents | 43.2 (mean) | Children | Consenting vs. declining | 11–12 | Swedish National Population Register | Not specified | 200 | 186 | 14 |
| Haesebaert et al. (2014) [19] | France | Mothers | 18–65 | Female | Favorable vs. uncertain/opposed | 14–18 | General Practitioner | 2008 | 91 | 55 | 36 |
| Hansen et al. (2015) [20] | Norway | Mothers and fathers | <35–≥50 | Female | Initiated vs. non-initiated | 12–13 | Norwegian Immunisation Registry (SYSVAK) | 2013 | 90,540 | 70,870 | 19,670 |
| Lenselink (2007) [23] | Netherlands | Mothers and fathers | 42.2 (mean) | Female and male | Intention vs. non-intention | 10–12 | School | Not specified | 356 | 313 | 32 |
| Mari et al. (2022) [24] | Italy | Parents | 44.5 (mean) | Male | Intention vs. non-intention | 6–18 | Buzzi Hospital | 2021–2022 | 339 | 251 | 88 |
| Marlow et al. (2007) [38] | United Kingdom | Mothers | 41.1 (mean) | Female | Consenting vs. non-consenting | 8–14 | School | 2006 | 684 | 513 | 171 |
| Pelucchi et al. (2010) [21] | Italy | Mothers and fathers | <40–≥50 | Female and male | Consenting vs. non-consenting | 10- 13 | School | 2008 | 2144 | 1438 | 706 |
| Rousset-Jablonski (2021) [33] | France | Accompanying adults (parents) | 32–58 | Female | Vaccinated vs. non-vaccinated | 9–17 | Pediatric or pediatric and adult Cystic Fibrosis centers | 2018–2019 | 104 | 34 | 76 |
| Schreiber et al. (2015) [34] | Denmark | Mothers | Not specified | Female | Initiated vs. non-initiated | Not specified | Danish Civil Registration System | Not specified | 65,926 | 61,162 | 4764 |
| Sobierajski et al. (2023) [27] | Poland | Parents | <30–>49 | Children | Vaccinated/intention vs. non-vaccinated/non-intention | 9–15 | Central Statistical Office’s data | 2022 | 360 | 233 | 127 |
| Spencer et al. (2013) [36] | United Kingdom | Mothers | 25–65 | Female | Initiation vs. non-initiation | 12–15 | National Health Authority Information System | Not specified | 112,451 | 89,088 | 23,363 |
| Sypien & Zielonka (2022) [28] | Poland | Parents | ≤34–>34 | Female and male | Intention vs. non-intention | Not specified | University Children’s Hospital | 2021 | 302 | 95 | 207 |
| Waller et al. (2019) [25] | United Kingdom | Parents | 40.5 (mean) | Female and male | Decided vs. non-decided/undecided | 9–12 | School | 2019 | 1156 | 718 | 438 |
| Woodhall et al. (2007) [39] | Finland | Parents | ≤40–≥51 | Female and male | Accepting vs. declining | 15 (mean) | Tampere city | 2005 | 727 | 622 | 105 |
Table 2.
NOS scoring for the included studies. The Newcastle–Ottawa Scale (NOS), adapted for cross-sectional studies, was used.
| Study | Selection (Out of 5 *) | Comparability (Out of 2 *) | Outcome (Out of 3 *) | TOTAL (Out of 10 *) |
|---|---|---|---|---|
| Baumann et al. (2019) [29] | 3 | 2 | 2 | 7 |
| Bedford et al. (2021) [35] | 3 | 2 | 2 | 7 |
| Brabin et al. (2006) [16] | 3 | 2 | 2 | 7 |
| Dahlström et al. (2009) [17] | 3 | 2 | 2 | 7 |
| David et al. (2022) [30] | 1 | 2 | 2 | 5 |
| Dib et al. (2022) [37] | 2 | 2 | 2 | 6 |
| Domínguez-Riscart et al. (2023) [40] | 1 | 2 | 2 | 5 |
| Donders et al. (2007) [18] | 1 | 2 | 2 | 5 |
| Feiring et al. (2015) [31] | 4 | 2 | 3 | 9 |
| Ganczak et al. (2018) [26] | 1 | 2 | 2 | 5 |
| Gefenaite et al. (2012) [32] | 3 | 2 | 3 | 8 |
| Grandahl et al. (2016) [22] | 1 | 2 | 2 | 5 |
| Haesebaert et al. (2014) [19] | 1 | 2 | 2 | 5 |
| Hansen et al. (2015) [20] | 3 | 2 | 3 | 8 |
| Lenselink (2007) [23] | 1 | 2 | 2 | 5 |
| Mari et al. (2022) [24] | 1 | 2 | 2 | 5 |
| Marlow et al. (2007) [38] | 1 | 2 | 2 | 5 |
| Pelucchi et al. (2010) [21] | 1 | 2 | 2 | 5 |
| Rousset-Jablonski (2021) [33] | 1 | 2 | 2 | 5 |
| Schreiber et al. (2015) [34] | 3 | 2 | 3 | 8 |
| Sobierajski et al. (2023) [27] | 3 | 2 | 2 | 7 |
| Spencer et al. (2013) [36] | 3 | 2 | 3 | 8 |
| Sypien & Zielonka (2022) [28] | 1 | 2 | 2 | 5 |
| Waller et al. (2019) [25] | 2 | 2 | 2 | 6 |
| Woodhall et al. (2007) [39] | 2 | 2 | 2 | 6 |
* Total score.
Twenty-eight social, economic, psychological and demographic variables were identified and reported in these publications as influencing the decision to vaccinate. Sixteen factors were presented as combinable data in individual studies and included in subsequent meta-analyses. The rest were evaluated as part of the systematic review and could not be directly compared due to heterogeneity in data-reporting methods. A detailed workflow is available in the Supplementary Material S1—Microsoft Excel file.
3.2. Results of the Systematic Review and Meta-Analysis
3.2.1. Age of Parents
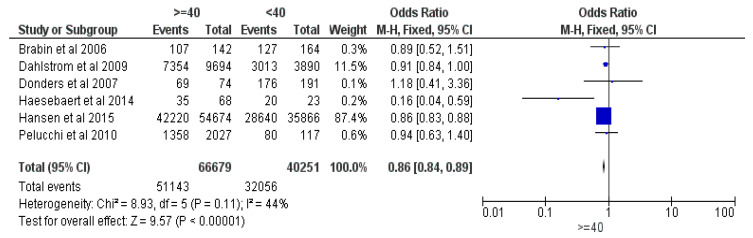
Six studies were included in the meta-analysis of parents’ ages, comparing older (≥40 years old) vs. younger (<40 years old) parents, totaling 106,930 responders, with 83,199 having vaccinated (or intent to vaccinate their) children [16,17,18,19,20,21]. The results revealed an Odds Ratio (OR) of 0.86 in favor of the parents aged less than 40 years old, with a 95% Confidence Interval (CI) between 0.84 and 0.89, at a Z of 9.57 and a p < 0.00001. The Fixed Effects (FE) was deployed due to a relatively small heterogeneity (I2) of 44% (see Figure 2).
Figure 2.
Forrest plot for the age of parent comparison [16,17,18,19,20,21].
3.2.2. Gender of Parent
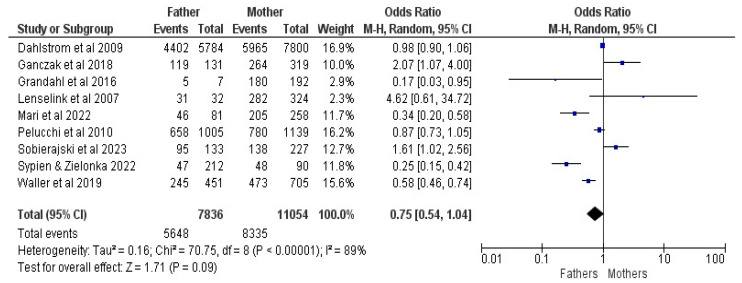
Nine studies were included in the meta-analysis of parents’ gender (comparing fathers and mothers), with 18,890 responders, of whom 13,983 had vaccinated (or an intent to vaccinate their) children [17,21,22,23,24,25,26,27,28]. The results revealed an OR of 0.75, with a 95% CI between 0.54 and 1.04, at a Z of 1.71 and a p = 0.09; Random Effects (RE) were used for an I2 of 89% (See Figure 3).
Figure 3.
Forrest plot for the gender of parent comparison [17,21,22,23,24,25,26,27,28].
3.2.3. Parents Educational Level
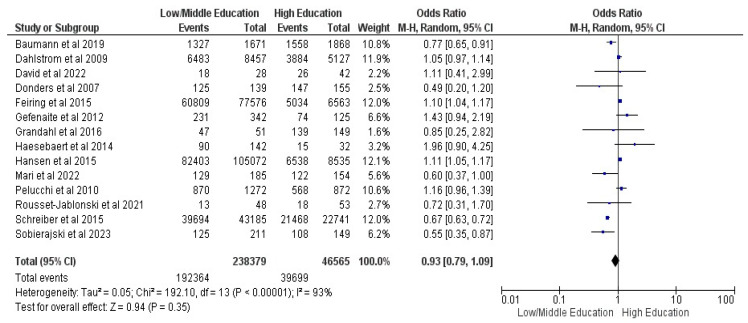
Fourteen studies were included in the parent’s level of education comparison (ISCED 0–4 vs. 5–8), with 284,944 guardians and 232,063 vaccinated children [17,18,19,20,21,22,24,27,29,30,31,32,33,34]. The results showed an OR of 0.93, with a 95% CI between 0.79 and 1.09, at a Z of 0.94 and a p = 0.35, with no statistical significance; Random Effects (RE) were used for an I2 of 93% (see Figure 4).
Figure 4.
Forrest plot for the parent’s educational level comparison [17,18,19,20,21,22,24,27,29,30,31,32,33,34].
3.2.4. Parents Civil Status
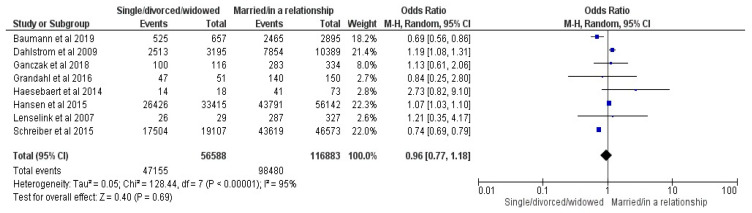
Eight studies were included in the parent’s civil status comparison (single/divorced/widowed vs. married/in a relationship) [17,19,20,22,23,26,29,34]. The results showed an OR of 0.96 with a 95% CI between 0.77 and 1.18, at a Z of 0.4 and a p = 0.69, with no statistical significance; Random Effects (RE) were used for an I2 of 95% (see Figure 5).
Figure 5.
Forrest plot for the parent’s civil status comparison [17,19,20,22,23,26,29,34].
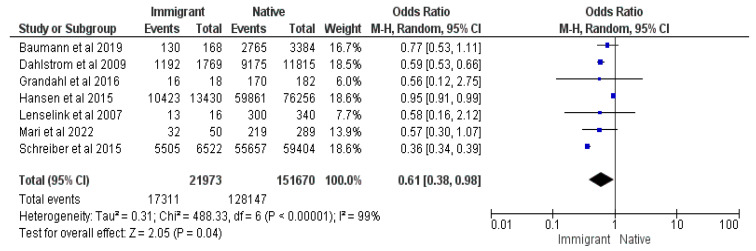
3.2.5. Immigrant Status
Seven studies [17,20,22,23,24,29,34] were included in this meta-analysis, totaling 173,643 responders, of which 145,458 were vaccinated. Odds Ratio (OR) of 0.61, as well as CIs = 0.38 and 0.98, demonstrated statistical significance at a Z value of 2.05 with a p-value = 0.04. Immigrant parents had 39% lower odds of vaccinating their children than native parents in their respective countries (See Figure 6).
Figure 6.
Forrest plot for the immigrant status comparison [17,20,22,23,24,29,34].
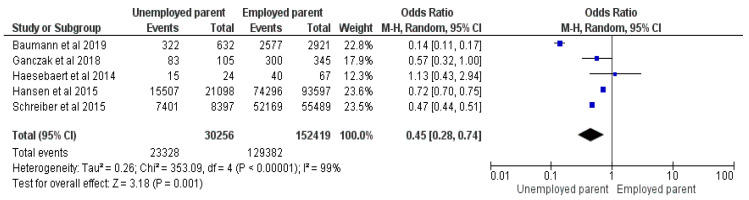
3.2.6. Employment Status of Parent
Five studies were included in the meta-analysis, totaling 182,675 responders with 152,710 vaccinated children, comparing unemployed and employed parents [19,20,26,29,34]. The results exhibit an OR = 0.45, with a 95%CI between 0.28–0.74, at a Z = 3.18, p = 0.001, and I2 = 99%. Random Effects were used due to the high heterogeneity. Unemployed parents had 55% lower odds of vaccinating their children than employed parents (See Figure 7).
Figure 7.
Forrest plot for parent employment status comparison [19,20,26,29,34].
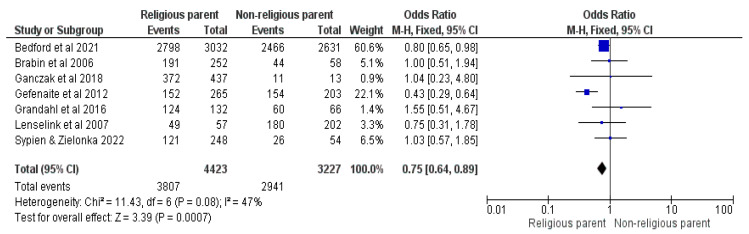
3.2.7. Religion of Parent (Non-Religious vs. Religious)
Seven studies were included in the meta-analysis comparing any religious faith with no faith [16,22,23,26,28,32,35]. The results demonstrate an OR = 0.75, with a 95% CI between 0.64 and 0.89, at a Z = 3.39, with a p = 0.0007 and I2 = 47%; FE were used as a model. Children in religious households had 25% lower odds of getting vaccinated (See Figure 8).
Figure 8.
Forrest plot for the comparison of the religious statuses of the responders [16,22,23,26,28,32,35].
3.2.8. Number of Children
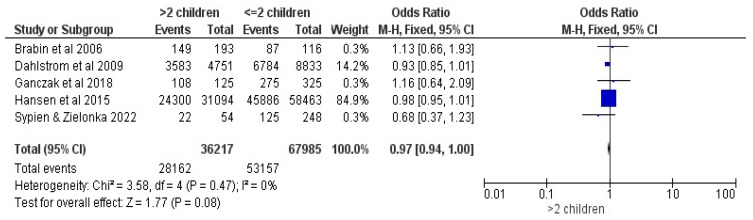
Five studies were included in the comparison regarding the number of children in a household (>two children vs. ≤2) [16,17,20,26,28]. No statistical significance was highlighted, showing an OR of 0.97, with a 95% CI between 0.94 and 1, at a Z = 1.77, with p = 0.08; FE were used at a trivial heterogeneity of I2 = 0% (See Figure 9).
Figure 9.
Forrest plot for the comparison of the number of children [16,17,20,26,28].
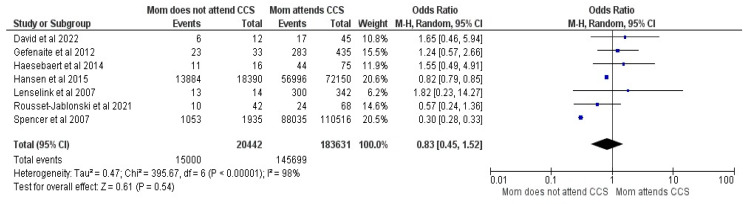
3.2.9. Mothers History of Cervical Cancer Screening (CCS)
Seven studies were included in the meta-analytical comparison between mothers with attendance and non-attendance at CCS and comprised 204,073 responders, of whom 160,699 had vaccinated children [19,20,23,30,32,33,36]. No differences were shown between groups, with OR = 0.83, with a 95% CI between 0.45 and1.52, at a Z value of 0.61, p = 0.54 and a 98% heterogeneity. RE were used due to higher heterogeneity (See Figure 10).
Figure 10.
Forrest plot for the mother’s history of CCS [19,20,23,30,32,33,36].
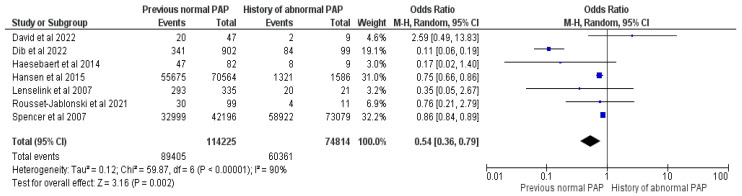
3.2.10. Mother Pap-Smear History
Seven studies summing up 189,039 mothers, of whom 149,766 had vaccinated their children, were included in the meta-analysis comparing mothers’ Pap smear histories (normal vs. abnormal results) [19,20,23,30,33,36,37]. Mothers who had a history of abnormal Pap smear results were 46% more likely to vaccinate their child, evidencing an OR = 0.54, with a 95% CI between 0.36 and 0.79, at a Z = 3.16, with p = 0.002 and I2 = 90%, using RE (See Figure 11).
Figure 11.
Forrest plot for the comparison of the mother’s Pap smear history [19,20,23,30,33,36,37].
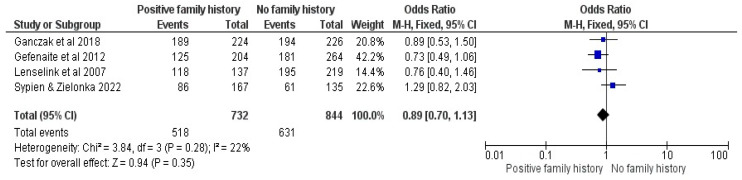
3.2.11. Family History of Cancer
Four studies were included in the family history of cancer comparison [23,26,28,32]. No association was found between a positive family history and the intention to vaccinate children, showing an OR of 0.89, with a 95% CI between 0.70 and 1.13, at a Z = 0.94, p = 0.35 and low I2 = 22% (See Figure 12).
Figure 12.
Forrest plot for the comparison of the family history of cancer [23,26,28,32].
3.2.12. Child Gender (Boys vs. Girls)
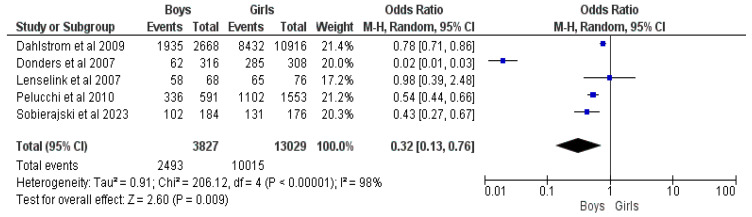
Five studies comprising 16,856 responders with 12,508 vaccinated children were included in the comparison regarding the child’s gender [17,18,21,23,27]. Boys were 68% less likely to be vaccinated by their parents than girls, with an OR = 0.32, a 95% CI = 0.13–0.76, a Z value of 2.60, p = 0.009 and a high I2 = 98% (See Figure 13).
Figure 13.
Forrest plot for the comparison of the child’s gender [17,18,21,23,27].
3.2.13. Previous History of Childhood Vaccination
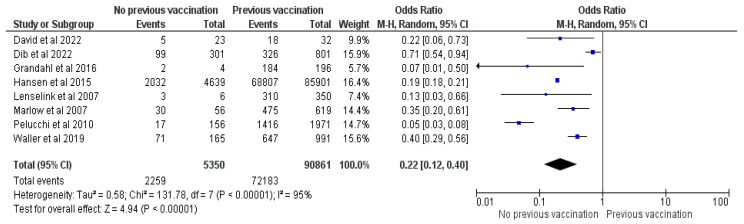
Eight studies [20,21,22,23,25,30,37,38] were included in the meta-analysis containing 96,211 parents and 74,442 vaccinated children. Pooled analysis showed an OR of 0.22, CI = 0.12–0.4, Z = 4.94 and p < 0.00001. Parents who previously refused a vaccine for their child or whose child is not fully vaccinated (with the mandatory schemes) have 88% fewer chances to vaccinate their children against HPV (See Figure 14).
Figure 14.
Forrest plot for the comparison of previous vaccination of child [20,21,22,23,25,30,37,38].
3.2.14. Perceived Efficacy of HPV Vaccination
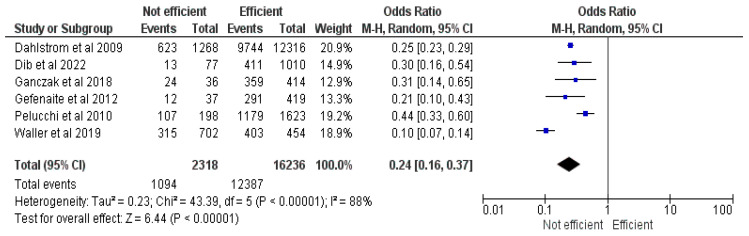
This meta-analysis included six studies [17,21,25,26,32,37] with 18,554 responders, of whom 13,481 had vaccinated their children. The results demonstrate an OR of 0.24, CI = 0.16–0.37, Z = 6.44 and p < 0.00001. Parents who believed the vaccine to be efficient had 76% greater odds of vaccinating their children (See Figure 15).
Figure 15.
Forrest plot for the comparison of perceived efficacy [17,21,25,26,32,37].
3.2.15. Perceived HPV Vaccine Safety
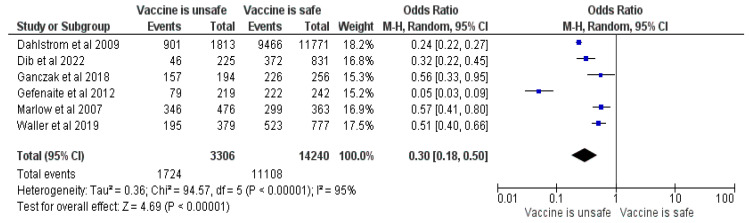
This meta-analysis included six studies [17,25,26,32,37,38] and 17,546 parents, of which 12,832 had vaccinated/intended to vaccinate their children. The results revealed an OR of 0.3, CI = 0.18–0.5, Z = 4.69 and p < 0.00001 (See Figure 16). Responders who thought the vaccine was unsafe had 70% lower odds of vaccinating their child. The main concerns were fear of side effects (long or short term) [16,17,19,23,25,26,28,29,30,32,33,35,37,38,39], too new or a lack of research [19,23,25,30,33,37], fear of needles/injection or pain [35] and a previous bad reaction to vaccination or regret [38].
Figure 16.
Forrest plot for the comparison of perceived safety [17,25,26,32,37,38].
3.2.16. HPV Vaccination Would Encourage Sexual Activity
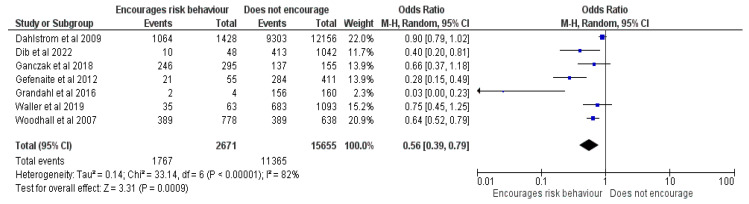
Seven studies [17,22,25,26,32,37,39] totaled 18,326 responders, of whom 13,132 had vaccinated their children and were included in the meta-analysis. The outcome showed an OR of 0.56, CI = 0.39–0.79, Z = 3.31 and p = 0.0009 (See Figure 17). Caregivers who believed that the vaccination would increase the at-risk behavior of their child had lower chances of vaccinating their child. Also, believing that their child had a girlfriend or boyfriend or were sexually active positively influenced the intention to vaccinate, while believing that vaccination would decrease condom usage [21] negatively impacted the intention to vaccinate.
Figure 17.
Forrest plot for the comparison of the perceived increased risk behavior after vaccination [17,22,25,26,32,37,39].
3.2.17. Knowledge Related to HPV
Knowing that the HPV vaccine prevents CC, knowledge related to HPV infection and a positive attitude towards vaccination could influence a parent’s intention to vaccinate their children [21,24,26,27,28,37,38]. In contrast, a lack of or insufficient knowledge about HPV (or the HPV vaccine) could discourage parents from vaccinating their children; other detrimental factors identified were valuing personal knowledge more than experts’ opinions and having a positive attitude toward social media stories or Internet-based information about the vaccine [22,25,28,29,30,32,39]. Five studies found no association between HPV knowledge (regarded as an STI), HPV vaccine knowledge or knowledge related to cervical cancer screening [19,22,26,37,40] and parents’ intention to vaccinate one child.
3.2.18. Recommendation
Receiving the HPV vaccine recommendation from a General Practitioner (GP), a Healthcare Professional (HCP), or a gynecologist [26,28,30,33,37,41] seemed to positively influence the parent’s intention to vaccinate their children [36], as opposed to obtaining the recommendation online. In contrast, parents who were advised against HPV vaccination by relatives, friends or HCPs and those who did not receive any recommendations had lower odds of vaccinating their children [22,28,30,33].
3.2.19. Awareness of HPV
Prior awareness of HPV or receiving education from a GP [17,25,26,41] could positively affect the parents’ intention to vaccinate their children, while being unaware of the HPV vaccine’s existence or purpose negatively influenced their intention to vaccinate [28,33,41].
3.2.20. Need for Information
Receiving information from a GP and Health Authorities or a HCP [24,41] correlated with vaccination status. Caregivers with a higher intention to vaccinate their children [18,25,28,32,37] usually displayed the need for more information to decide, received positive information materials from social media and displayed a higher rate of information searches. One study found no association between vaccination intention and the need for more information regarding HPV, cervical cancer (CC) or HPV vaccination [23]. Searching for information due to mistrust or not receiving guidance from a GP; limited, biased, or unclear information provided by the Government regarding HPV vaccination; a lack of information and feeling the need for more information; or receiving damaging information about vaccination from the media adversely influenced the intention to vaccinate [18,24,28,32,41].
3.2.21. Decision-Making Process and Involvement
Involving the other parent in the decision-making process or thinking that the other parent would want to vaccinate their children positively influenced the decision to vaccinate [25,41]. At the same time, one study found no association between the inclusion of the other parent of the child in the decision-making process and the intention to vaccinate [22]. Believing that the children’s opinion is essential had a positive association with the vaccination intent [23,33]. In contrast, involving their children in the decision-making process or waiting for the children to ask for vaccination negatively influenced parents’ decisions [19,41]. Caregivers believing that their child was incapable of making their own decision regarding vaccination did not seem to influence their decision to vaccinate [32].
3.2.22. Communication about Sexual Life and Vaccination
Parents with difficulties in talking openly with their children, a doctor or any HCP regarding sexual health did not seem to have lesser chances of vaccinating their children [37]. Interestingly, parents who did not address the sexual issues or the HPV vaccine seemed more unlikely to want to vaccinate their children [16,19,37].
3.2.23. HPV Vaccine Cost
The cost of the HPV vaccine correlated with the parents’ decision to vaccinate their children. Having to pay for the vaccine was associated with a lower intention to vaccinate [17,19,28]; reimbursement was mentioned as a solution [28], while one study found no such association [18]. Willingness was higher among girls’ parents than those of boys, even if they had to pay for the vaccine [17].
3.2.24. Perceived Severity and Consequences
Caregivers believing that vaccination assists in preventing a severe disease or health consequences had higher chances of vaccinating their children [19,25]. In contrast, those who believed that HPV infection is not severe were less likely to vaccinate [32]. Not being worried about HPV infection or its consequences did not seem to affect vaccination rates [22,26,32].
3.2.25. Perceived Susceptibility
Feeling that the child would be at risk of contracting STDs or that the child could be infected with HPV had a positive effect on parents’ vaccination intent [16,21,25]; on the contrary, low perceived susceptibility of becoming infected or developing CC [32,39] influenced the intention unfavorably, and one study found no such association [22].
3.2.26. Fear of HPV-Related Disease and Health Concerns
Thinking that vaccination is beneficial or that it can empower parents to protect their children’s health and fear of HPV-related diseases encouraged parents to vaccinate their children [22,28,30,33,38].
3.2.27. Mandatory Vaccination
The non-mandatory character of the HPV vaccine was a paramount factor affecting the parents’ decision to vaccinate, shaping safety concerns and leading to many parents deeming it unnecessary [28,35,37]. In contrast, some parents thought everyone should choose whether or not to vaccinate their children [37].
3.2.28. Trust in the Healthcare System
Parents’ trust in pharmaceutical companies, the government, physicians, pharmacists, researchers and the mainstream media to stop vaccination if serious consequences appeared [23,28,37,38] favorably influenced their intention to vaccinate. A lack of or low trust in the government and the limited information provided, a high number of necessary vaccines, concerns related to fierce side effects and beliefs that pharmaceutical companies influence the government were among the reasons invoked by parents for vaccine refusal [23,32].
3.2.29. Perceived Risks of the Vaccine
Perceiving more risks than benefits regarding the HPV vaccination lowered the parents’ intention to vaccinate their children [22,33,35].
3.2.30. Suitable Age for Vaccination
Perceiving their child to be too young to be vaccinated against HPV negatively influenced the decision to vaccinate [19,30,32,35,37], while two studies found no such association [21,33]. One study found parents’ preference for vaccinating sons younger than 12 [24].
3.2.31. Barriers Related to the HPV Vaccination
The main barriers encountered by parents were the need for many doses (2 or 3), the school not offering the HPV vaccine or their daughter not being enrolled at a school [17,35]. Three studies found no association between locations, the number of necessary doses or the complicated steps involved in getting vaccinated [22,32,37]. Interestingly, one study found parents proposing that the GP prepare the vaccine in the office for same-day vaccination [37].
3.2.32. Gender Neutral Vaccination
Most parents would agree to vaccinate their children if the vaccine was gender-neutral [23,25,32].
3.2.33. Income
Higher-income status was associated with vaccination intent in some studies; however, regarding immigrant parents’ income, lower income was associated with the intention to vaccinate [20,31,34,41]. Lower paternal income and lower income for non-immigrant parents were adversely associated with vaccination intention [20,34].
3.3. Additional Analyses
Sensitivity analyses were conducted for each meta-analysis, excluding one study. For the majority of analyses, robust sensitivity was highlighted. Interestingly, for three comparisons, an excluded study produced an overall OR outside the initial 95%, making the sensitivity of those comparisons inadequate. Therefore, the meta-analyses (the number of children, the mother’s Pap smear history and parents’ age) were performed again without the respective problematic studies. The results stayed the same. Interestingly, for one comparison (mother’s Pap smear history), the heterogeneity dropped significantly from 90% to 43%, although the overall results were unaffected. From the planned subgroup analyses, only one was possible from the planned subgroup analyses based on the quality assessment scoring on the NOS evaluation. Since all the included studies were cross-sectional, the subgroup analyses based on the publication type were impossible. Most articles interviewed mothers or generically described “parents” or caregivers; therefore, comparisons between fathers and mothers were deemed impossible. Regarding children’s gender, not enough data were given to compare males vs. females, with most studies including just females or both genders. At the same time, only one publication included just male children, making the subgroup analysis impossible (due to only one study being included in this category). The heterogeneity presented in the child’s age category significantly affected the possibility of splitting the publication into different age categories. Finally, the NOS scoring data allowed for the split into mediocre and high-quality studies for six comparisons (parents’ education level, civil status, immigration status, employment, the mother’s history of Pap smear results and previous experience with CCS). All other comparisons only included mediocre publications or had just one study with high NOS scoring, making the split into subgroups impossible. The statistically significant effect was only carried over in the high-quality subgroups for the parent’s employment status and the mother’s previous Pap smear results. In contrast, results were insignificant in the low-quality subgroup. For the mother’s previous Pap smear results, the subgroup analysis was performed both with and without Dib et al.’s [37] study, since this publication yielded problematic results in the sensitivity analysis. The results remained the same. Intriguingly, for the parent’s immigration status comparison, the effect only occurred in the low-quality subgroup, while results seemed to be insignificant in the higher-quality publications subgroup. All additional analyses can be consulted in Supplementary Material S2. The original RevMan workflow file can also be consulted in Supplemental Material S3.
4. Discussion
To the best of our knowledge, this is the most comprehensive systematic review and meta-analysis investigating the psychological, social, demographic and economic factors influencing European parents’ decision to vaccinate their children against HPV. The report efficiently identifies social determinants for at-risk categories of parents who would not vaccinate their children: aged more than 40 years old, with immigrant status, unemployment, and religiousness all compounding factors [16,18,19,20,21,22,23,24,26,28,32,34,35,41]. Some studies highlighted that marital status could contribute to hesitancy, while the current meta-analysis showed that civil status had no influence [17,19,20,22,23,26,34,41,42]. Immigrant status was correlated with lower knowledge of HPV and lower intention to vaccinate children against HPV [43,44]. These results emphasise a need for more information with simultaneous social and economic support for at-risk categories. Policies must also consider the discrepancies between European countries regarding the costs of the vaccine, health insurance coverage and out-of-pocket expenses in various regions for these specific at-risk categories to whom general policies might not apply [17,19,28,45,46,47].
A previous history of abnormal Pap smear was correlated with higher HPV vaccine acceptance, while only attending CCS was not associated, suggesting that CCS could create a false belief that screening could be the only method required and would enough protection against CC [19,20,23,30,32,33,36,37]. These results potentially show a need for more knowledge, information and recommendations by HCPs to make an informed decision [18,21,24,25,26,28,30,33,37,38,41,42,48,49,50].
The gender of parents did not influence the decision to vaccinate their child, while the gender of the child showed a higher chance for girls to be vaccinated compared to boys [17,18,21,22,23,24,25,26,27,28]. The systematic review showed a preference for gender-neutral vaccination [23,25,32], possibly highlighting more trust in the efficacy and safety of HPV vaccination while not putting one’s child at risk; in agreement with this, one study highlighted parents of boys advocating for gender-neutral vaccination [51].
The main reasons for refusing the HPV vaccines were fear of side effects and the novelty of the product [17,19,23,25,28,30,32,33,35,37,38,42,45,46,47,50,51,52,53,54,55,56]. In contrast, a previous history of childhood vaccination and the perceived efficacy and safety of the HPV vaccine positively influenced the intention to vaccinate one’s child [21,22,23,25,26,30,32,37,38,47,57], contouring a profile for parents who trust and believe that vaccines are crucial for their child’s health.
More reasons for refusing vaccination were the belief that the vaccine would encourage at-risk sexual activities [17,22,25,26,32,37,39,56], believing that one’s child is too young to receive the HPV vaccine while showing a preference for older-age initiation of vaccination [19,30,32,35,37,48]. Other factors were parents’ difficulty discussing with their children the purpose of the HPV vaccine or about sexual life and sexual activity, with parents invoking that these types of discussions could prompt their children to be more curious and encourage risky sexual behavior.
Perceived high susceptibility to contracting the HPV infection, believing one’s child is at risk for severe consequences and perceiving more benefits of HPV vaccination seemed to encourage parents to vaccinate. In contrast, low perceived susceptibility, unproven benefits and more barriers to receiving the HPV vaccine could have the opposite effect [16,19,21,22,25,26,32,33,35,39,42,46,56,57]. In contrast, fear or anxiety regarding a possible HPV infection and anticipating consequences could act as determinants for HPV vaccination.
Interestingly, many of the aforementioned factors influencing vaccine hesitancy were superimposable to those present in various regions around the world such as Africa and Asia [58,59,60,61], although presented data highlighted some worrisome statistics regarding the availability of such products. Moreover, extensive data were published regarding the cost-effectiveness of implementing vaccination programs in these regions [62].
Logistical steps related to the availability of the vaccine, convenience, and the repeated character of the HPV vaccine doses, as well as the lack of school-based vaccination [17,35], have been shown to impede parents from vaccinating their children [45,53] emphasising the efforts necessary to make HPV vaccination readily available with uncomplicated procedures.
Mistrust in HCPs and the health system also discouraged parents from opting for the vaccine. At the same time, the dissimilarities between policies create a lack of sustainable support, all contributing to making parents more hesitant [23,32,51,54]. The non-mandatory character of HPV vaccination [28,35,37] adversely impacted a parent’s decision to vaccinate.
4.1. Limits
This is the most exhaustive investigation linking miscellaneous psychological, social and demographic factors to vaccine hesitancy among European parents. Although a substantial number of studies was initially screened, and the analyses were based on a pool of more than 380,000 responders, some constraints must be acknowledged. Concerning the methodology, the PICO inclusion criteria could be considered a limitation of the present investigation. By confining the publication to the European region, a significant number of studies were excluded from the present analyses (58 publications), potentially spoiling the results. Although further investigations could be undertaken, and exciting comparisons could arise from such calculations, the purpose of the present study was not to investigate other regions. Language criteria (English language only) further restricted the number of included studies. Eleven publications were excluded solely based on this criteria. Given that English is the primary language in only one country included in this study (the United Kingdom, which is also not a part of the European Union anymore), this could be considered a setback contributing to publication bias and heterogeneity. It is also paramount to mention the search formula used to scan all the databases. Although synonyms were employed where possible, and MeSH was used for indexing correct terms, some restrictions can be highlighted, such as the lack of terms concerning fathers. This was mitigated by including words like caregivers, which comprise both genders, but results explicitly show fewer studies investigating fathers alone as responders. Even so, a considerable number of studies were screened from the databases. Database choice can also affect the number and types of articles included. For the present investigation, four noteworthy databases were searched, and although a relatively large number of studies was uncovered (more than 9300 publications), half were duplicates. References included in the most paramount studies in this field were carefully screened, but unfortunately, no other publications were identified through other sources. This issue can also contribute to the nuanced publication bias highlighted in the analyses.
The quality assessment of the included studies was accomplished using a modified NOS scale and highlighted modest-to-good-quality reports, which can be considered another detriment. Cut-off values for the NOS scoring allowed for further subgroup analyses resembling these categories and helped to distinguish results based on the quality of the included publications. This is virtually impossible to mitigate and instead shows the mediocre quality of the studies in this field while highlighting the need for more high-quality research investigating HPV vaccine hesitancy. The lack of RCTs scrutinizing the current matter means there were no available high-quality studies to compare.
Although no considerable publication bias was emphasized (except for the slight warping of the Funnel Plots that can be attributed to the smaller studies exaggerating the effect), some of the heterogeneity was lessened by excluding studies with the help of sensitivity analyses and performing subgroup analyses. Nonetheless, significant heterogeneity remained even after the investigations above and due to data heterogeneity, and no further explorations were possible. We tried explaining the heterogeneity of the included studies (I2 = 0–99%), and perhaps it could be attributed to the population heterogeneity; the geographic discrepancies regarding the HPV vaccination status, incidence, prevalence and mortality; and the types of included studies.
4.2. Future Directions
Noteworthy discrepancies could be indicated throughout European countries regarding the implementation, reimbursement, availability, recommendations and addressability of HPV vaccination programs. Despite tremendous efforts to increase addressability, the European vaccination programs still need help with coverage, which was as low as 30% for females and 14% for males. In comparison, only 77% of European countries have implemented them. These worrisome statistics highlight the need for unified policies at the regional, national and European levels. Such strategies could improve the discrepancies between countries while mitigating the low coverage in precise at-risk categories, such as low-income households or immigrants, through shared funding or universal coverage, regardless of the insurance status of the recipients. The present report identified these distinctive socio-demographic characteristics in households that require intensive awareness and education programs, perhaps regulated at the central level: older caregivers, religious households, unemployed parents and immigrants. More aggressive campaigns must reach these communities, empower these parents to protect their children’s health and safety and improve vaccination coverage.
Detrimental factors, such as complicated procedures, a lack of school-based vaccinations and a lack of invitation-based programs can also be mitigated by improving the delivery system for the vaccine. The present report also highlighted the intricate relationship between the mandatory status of the vaccination and parents’ beliefs, potentially implying a need to implement HPV vaccination as a part of mandatory schemes in a gender-neutral program.
In order to facilitate information and knowledge dissemination among parents, GPs could have a pivotal advocacy role in vaccination campaigns; they are also in direct contact with other at-risk categories, monitoring parents with previous adverse histories regarding vaccinations while being involved in CCS programs. This can be crucial as various interventions can be delivered to mothers that address secondary preventive measures (CCS), highlighting the complementarity of both strategies (primary and secondary prophylaxis). HCPs are also valuable in delivering further recommendations regarding vaccination, as many psychological factors were deemed detrimental to the parent’s intention to vaccinate their children, such as the perceived safety and efficacy and personal misbeliefs regarding risky behaviors after vaccination.
To conclude, unified, simplified, accessible and fair measures must be implemented broadly. At the same time, more aggressive information and education campaigns must be conducted by trusted HCPs, especially in distinct at-risk categories, to improve HPV vaccination addressability and decrease hesitancy among parents. Specific at-risk categories that need to be targeted with information, education and vaccination campaigns were identified.
5. Conclusions
This systematic review and meta-analysis identified crucial determinants that could negatively influence parents’ intention to vaccinate their children: parents older than 40, immigrant status, being unemployed, religious beliefs, previous childhood vaccination refusal, perceiving the vaccine to be unsafe, claiming fear of side effects and believing that children vaccinated against HPV would be encouraged to perform at-risk sexual activities or would result in an early debut. A history of abnormal Pap smear results in mothers and perceiving the HPV vaccine as being effective in preventing diseases would encourage parents to vaccinate their children.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12020127/s1, Supplementary Material S1: Excel Workflow. Supplementary Material S2: Chart S1: NOS scoring for the included publciations; Figure S1: Funnel plot for the age of parent comparison; Table S1: Sensitivity analysis for the age of parent comparison; Figure S2: Forrest plot for the age of parent comparison excluding the Hansen et al. study; Figure S3: Funnel plot for the gender of parent comparison; Table S2: Sensitivity analysis for the gender of parent comparison; Figure S4: Funnel plot for the parent’s educational level comparison; Table S3: Sensitivity analysis for the parent’s educational level comparison; Figure S5: Funnel plot for the parent’s civil status comparison; Table S4: Sensitivity analysis for parent’s civil status comparison; Figure S6: Funnel plot for parent employment status comparison; Table S5: Sensitivity analysis for parent employment status comparison; Figure S7: Funnel plot for the immigrant status comparison; Table S6: Sensitivity analysis for the immigrant status comparison; Figure S8: Funnel plot for the religious status of the responders comparison; Table S7: Sensitivity analysis for the religious status of the responder’s comparison; Figure S9: Funnel plot for the number of children comparison; Table S8: Sensitivity analysis for the number of children comparison; Figure S10: Forrest plot for the number of children comparison excluding the Hansen et al. study; Figure S11: Funnel plot for the mother’s history of CCS; Table S9: Sensitivity analysis for the mother’s history of CCS; Figure S12: Funnel plot for the mother’s Pap history comparison; Table S10: Sensitivity analysis for the mother’s Pap history comparison; Figure S13: Forrest plot for the mother’s Pap history comparison excluding the Dib et al. study; Figure S14: Funnel plot for the family history of cancer comparison; Table S11: Sensitivity analysis for the family history of cancer comparison; Figure S15: Funnel plot for the child’s gender comparison; Table S12: Sensitivity analysis for the child’s gender comparison; Figure S16: Funnel plot for the history of childhood vaccination comparison; Table S13: Sensitivity analysis for History of childhood vaccination for children; Figure S17: Funnel plot for the perceived efficacy comparison; Table S14: Sensitivity analysis for HPV vaccine efficacy comparison; Figure S18: Funnel plot for the perceived safety comparison; Table S15: Sensitivity analysis for perceived safety of the HPV vaccine; Figure S19: Funnel plot for the perceived increased risk behavior after vaccination comparison; Table S16: Sensitivity analysis for the perceived increased risk behavior after vaccination comparison; Figure S20: Forrest plot for the parents’ civil status with subgroups based on the NOS scoring; Figure S21: Forrest plot for the parents’ employment status with subgroups based on the NOS scoring; Figure S22: Forrest plot for the parents’ immigrant status with subgroups based on the NOS scoring; Figure S23: Forrest plot for the mother’s previous CCS attendance with subgroups based on the NOS scoring; Figure S24: Forrest plot for the mother’s previous PAP results with subgroups based on the NOS scoring; Figure S25: Forrest plot for the parents’ education levels with subgroups based on the NOS scoring, Supplementary Material S3: Data Analysis (RevMan file).
Author Contributions
Conceptualization: T.A.-C., D.L.D., A.P. (Aida Puia) and N.-M.J.; methodology, T.A.-C., N.-M.J. and A.P. (Andrei Pașca); software T.A.-C. and A.P. (Andrei Pașca); validation, T.A.-C., D.L.D., A.P. (Aida Puia) and A.P. (Andrei Pașca); formal analysis, T.A.-C., A.P. (Andrei Pașca) and N.-M.J.; investigation, T.A.-C., A.P. (Andrei Pașca) and N.-M.J.; resources, T.A.-C., D.L.D. and A.P. (Aida Puia).; data curation, T.A.-C., A.P. (Andrei Pașca) and N.-M.J., writing—original draft preparation, T.A.-C., writing—review and editing, T.A.-C., D.L.D., A.P. (Aida Puia)., A.P. (Andrei Pașca) and N.-M.J.; visualization T.A.-C. and A.P. (Andrei Pașca); supervision, D.L.D. and A.P. (Aida Puia); project administration, T.A.-C., D.L.D. and A.P. (Aida Puia); funding D.L.D. and A.P. (Aida Puia). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study since the data were collected from previous publications.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors have no conflict of interests to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cancer Today Cancer Today, Top Cancer per Country, Estimated Age-Standardised Incidence Rates. 2020. [(accessed on 5 October 2023)]. Available online: http://gco.iarc.fr/today/home/incidence.
- 2.Cancer Today Cancer Today, Top Cancer per Country, Estimated Age-Standardised Mortality Rate. 2020. [(accessed on 5 October 2023)]. Available online: http://gco.iarc.fr/today/home/mortality.
- 3.WHO Cervical Cancer Elimination Initiative. 2020. [(accessed on 5 October 2023)]. Available online: https://www.who.int/initiatives/cervical-cancer-elimination-initiative.
- 4.Bruni L., Serrano B., Roura E., Alemany L., Cowan M., Herrero R., Poljak M., Murillo R., Broutet N., Riley L.M., et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health. 2022;10:e1115–e1127. doi: 10.1016/S2214-109X(22)00241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singhal P., Marfatia Y.S. Human papillomavirus vaccine. Indian J. Sex. Transm. Dis. AIDS. 2009;30:51. doi: 10.4103/2589-0557.62761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z., Zhang J., Xia N., Zhao Q. Expanded strain coverage for a highly successful public health tool: Prophylactic 9-valent human papillomavirus vaccine. Hum. Vaccines Immunother. 2017;13:2280–2291. doi: 10.1080/21645515.2017.1346755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruni L., Saura-Lázaro A., Montoliu A., Brotons M., Alemany L., Diallo M.S., Afsar O.Z., LaMontagne D.S., Mosina L., Contreras M., et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunisation coverage 2010–2019. Prev. Med. 2021;144:106399. doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 8.Altobelli E., Rapacchietta L., Profeta V.F., Fagnano R. HPV-vaccination and cancer cervical screening in 53 WHO European Countries: An update on prevention programs according to income level. Cancer Med. 2019;8:2524–2534. doi: 10.1002/cam4.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Human Papillomavirus (HPV) Vaccination Coverage. 2022. [(accessed on 5 October 2023)]. Available online: https://immunizationdata.who.int/pages/coverage/hpv.html?CODE=EUR&YEAR=
- 10.Ten Health Issues WHO Will Tackle This Year. [(accessed on 5 October 2023)]. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- 11.Karafillakis E., Simas C., Jarrett C., Verger P., Peretti-Watel P., Dib F., De Angelis S., Takacs J., Ali K.A., Pastore Celentano L., et al. HPV vaccination in a context of public mistrust and uncertainty: A systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum. Vaccines Immunother. 2019;15:1615–1627. doi: 10.1080/21645515.2018.1564436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López N., Garcés-Sánchez M., Panizo M.B., de la Cueva I.S., Artés M.T., Ramos B., Cotarelo M. HPV knowledge and vaccine acceptance among European adolescents and their parents: A systematic literature review. Public Health Rev. 2020;41:10. doi: 10.1186/s40985-020-00126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiboc N.M., Paşca A., Tăut D., Băban A.S. Factors influencing human papillomavirus vaccination uptake in European women and adolescents: A systematic review and meta-analysis. Psychooncology. 2023;33:e6242. doi: 10.1002/pon.6242. [DOI] [PubMed] [Google Scholar]
- 14.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S., Parati G., ESH Working Group on CV Risk in Low Resource Settings Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deeks J.J., Higgins J.P., Altman D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. [(accessed on 15 January 2024)]. Available online: https://training.cochrane.org/handbook/current/chapter-10.
- 16.Brabin L., Roberts S., Farzaneh F., Kitchener H. Future acceptance of adolescent human papillomavirus vaccination: A survey of parental attitudes. Vaccine. 2006;24:3087–3094. doi: 10.1016/j.vaccine.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Dahlström L.A., Tran T.N., Lundholm C., Young C., Sundström K., Sparén P. Attitudes to HPV vaccination among parents of children aged 12-15 years—A population-based survey in Sweden. Int. J. Cancer. 2010;126:500–507. doi: 10.1002/ijc.24712. [DOI] [PubMed] [Google Scholar]
- 18.Donders G.G.G., Gabrovska M., Bellen G., Van Keirsbilck J., Van Den Bosch T., Riphagen I., Verjans M. Knowledge of cervix cancer, human papillomavirus (HPV) and HPV vaccination at the moment of introduction of the vaccine in women in Belgium. Arch. Gynecol. Obstet. 2008;277:291–298. doi: 10.1007/s00404-007-0487-1. [DOI] [PubMed] [Google Scholar]
- 19.Haesebaert J., Lutringer-Magnin D., Kalecinski J., Barone G., Jacquard A.C., Leocmach Y., Régnier V., Vanhems P., Chauvin F., Lasset C. Disparities of Perceptions and Practices Related to Cervical Cancer Prevention and the Acceptability of HPV Vaccination According to Educational Level in a French Cross-Sectional Survey of 18–65 Years Old Women. PLoS ONE. 2014;9:e109320. doi: 10.1371/journal.pone.0109320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen B.T., Campbell S., Burger E., Nygård M. Correlates of HPV vaccine uptake in school-based routine vaccination of preadolescent girls in Norway: A register-based study of 90,000 girls and their parents. Prev. Med. 2015;77:4–10. doi: 10.1016/j.ypmed.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Pelucchi C., Esposito S., Galeone C., Semino M., Sabatini C., Picciolli I., Consolo S., Milani G., Principi N. Knowledge of human papillomavirus infection and its prevention among adolescents and parents in the greater Milan area, Northern Italy. BMC Public Health. 2010;10:378. doi: 10.1186/1471-2458-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grandahl M., Tydén T., Westerling R., Nevéus T., Rosenblad A., Hedin E., Oscarsson M. To Consent or Decline HPV Vaccination: A Pilot Study at the Start of the National School-Based Vaccination Program in Sweden. J. Sch. Health. 2017;87:62–70. doi: 10.1111/josh.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenselink C.H., Gerrits M.M.J.G., Melchers W.J.G., Massuger L.F.A.G., Van Hamont D., Bekkers R.L.M. Parental acceptance of Human Papillomavirus vaccines. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;137:103–107. doi: 10.1016/j.ejogrb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Mari A., Gianolio L., Edefonti V., Khaleghi Hashemian D., Casini F., Bergamaschi F., Sala A., Verduci E., Calcaterra V., Zuccotti G.V., et al. HPV Vaccination in Young Males: A Glimpse of Coverage, Parental Attitude and Need of Additional Information from Lombardy Region, Italy. Int. J. Environ. Res. Public Health. 2022;19:7763. doi: 10.3390/ijerph19137763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waller J., Forster A., Ryan M., Richards R., Bedford H., Marlow L. Decision-making about HPV vaccination in parents of boys and girls: A population-based survey in England and Wales. Vaccine. 2020;38:1040–1047. doi: 10.1016/j.vaccine.2019.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganczak M., Owsianka B., Korzeń M. Factors that Predict Parental Willingness to Have Their Children Vaccinated against HPV in a Country with Low HPV Vaccination Coverage. Int. J. Environ. Res. Public Health. 2018;15:645. doi: 10.3390/ijerph15040645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobierajski T., Małecka I., Augustynowicz E. Feminized vaccine? Parents’ attitudes toward HPV vaccination of adolescents in Poland: A representative study. Hum. Vaccines Immunother. 2023;19:2186105. doi: 10.1080/21645515.2023.2186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sypień P., Zielonka T.M. Knowledge and Awareness of Polish Parents on Vaccination against Human Papillomavirus. Vaccines. 2022;10:1156. doi: 10.3390/vaccines10071156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann A., Andersen B., Østergaard L., Larsen M.B. Sense & Sensibility: Decision-making and sources of information in mothers who decline HPV vaccination of their adolescent daughters. Vaccine X. 2019;2:100020. doi: 10.1016/j.jvacx.2019.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David E., Roy P., Belot A., Quartier P., Bader Meunier B., Aeschlimann F.A., Lega J.-C., Durieu I., Rousset-Jablonski C. Human Papilloma Virus Vaccination in Patients with Rheumatic Diseases in France: A Study of Vaccination Coverage and Drivers of Vaccination. J. Clin. Med. 2022;11:4137. doi: 10.3390/jcm11144137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feiring B., Laake I., Molden T., Cappelen I., Haberg S.E., Magnus P., Steingrímsdóttir Ó.A., Strand B.H., Stålcrantz J., Trogstad L. Do parental education and income matter? A nationwide register-based study on HPV vaccine uptake in the school-based immunisation programme in Norway. BMJ Open. 2015;5:e006422. doi: 10.1136/bmjopen-2014-006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gefenaite G., Smit M., Nijman H.W., Tami A., Drijfhout I.H., Pascal A., Postma M.J., Wolters B.A., van Delden J.J.M., Wilschut J.C., et al. Comparatively low attendance during Human Papillomavirus catch-up vaccination among teenage girls in the Netherlands: Insights from a behavioral survey among parents. BMC Public Health. 2012;12:498. doi: 10.1186/1471-2458-12-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rousset-Jablonski C., Haesebaert J., Denis A., Reix P., Llerena C., Perceval M., Touzet S., Durieu I. Human Papilloma Virus Vaccination Among Female Patients Attending French Pediatric Cystic Fibrosis Centers. J. Pediatr. Adolesc. Gynecol. 2021;34:317–323. doi: 10.1016/j.jpag.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Slåttelid Schreiber S.M., Juul K.E., Dehlendorff C., Kjær S.K. Socioeconomic Predictors of Human Papillomavirus Vaccination Among Girls in the Danish Childhood Immunization Program. J. Adolesc. Health. 2015;56:402–407. doi: 10.1016/j.jadohealth.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Bedford H., Firman N., Waller J., Marlow L., Forster A., Dezateux C. Which young women are not being vaccinated against HPV? Cross-sectional analysis of a UK national cohort study. Vaccine. 2021;39:5934–5939. doi: 10.1016/j.vaccine.2021.07.094. [DOI] [PubMed] [Google Scholar]
- 36.Spencer (Nee Pilkington) A.M., Brabin L., Verma A., Roberts S.A. Mothers’ screening histories influence daughters’ vaccination uptake: An analysis of linked cervical screening and human papillomavirus vaccination records in the North West of England. Eur. J. Cancer. 2013;49:1264–1272. doi: 10.1016/j.ejca.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Dib F., Mayaud P., Renaudie C., Launay O., Chauvin P. Determinants of human papillomavirus (HPV) vaccine uptake among girls in France: A population-based telephone survey. Hum. Vaccines Immunother. 2022;18:2083894. doi: 10.1080/21645515.2022.2083894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marlow L.A.V., Waller J., Wardle J. Trust and Experience as Predictors of HPV Vaccine Acceptance. Hum. Vaccines. 2007;3:171–175. doi: 10.4161/hv.3.5.4310. [DOI] [PubMed] [Google Scholar]
- 39.Woodhall S.C., Lehtinen M., Verho T., Huhtala H., Hokkanen M., Kosunen E. Anticipated Acceptance of HPV Vaccination at the Baseline of Implementation: A Survey of Parental and Adolescent Knowledge and Attitudes in Finland. J. Adolesc. Health. 2007;40:466–469. doi: 10.1016/j.jadohealth.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Domínguez-Riscart J., Ariza-Jimenez A.B., Baez-Castillo C., Mateo-Gavira I. Factors associated with knowledge and vaccination intention for human papillomavirus on trans girls by their main caregiver: A cross-sectional study. Front. Immunol. 2023;14:1097449. doi: 10.3389/fimmu.2023.1097449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauman D. Diagnostic methods in pediatric and adolescent gynecology. Endocr. Dev. 2012;22:40–55. doi: 10.1159/000326633. [DOI] [PubMed] [Google Scholar]
- 42.Della Polla G., Pelullo C.P., Napolitano F., Angelillo I.F. HPV vaccine hesitancy among parents in Italy: A cross-sectional study. Hum. Vaccines Immunother. 2020;16:2744–2751. doi: 10.1080/21645515.2020.1744367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anuforo B., McGee-Avila J.K., Toler L., Xu B., Kohler R.E., Manne S., Tsui J. Disparities in HPV vaccine knowledge and adolescent HPV vaccine uptake by parental nativity among diverse multiethnic parents in New Jersey. BMC Public Health. 2022;22:195. doi: 10.1186/s12889-022-12573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Netfa F., Tashani M., Booy R., King C., Rashid H., Skinner S.R. Knowledge, Attitudes and Perceptions of Immigrant Parents Towards Human Papillomavirus (HPV) Vaccination: A Systematic Review. Trop. Med. Infect. Dis. 2020;5:58. doi: 10.3390/tropicalmed5020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen K.H., Santibanez T.A., Stokley S., Lindley M.C., Fisher A., Kim D., Greby S., Srivastav A., Singleton J. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine. 2021;39:2416–2423. doi: 10.1016/j.vaccine.2021.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radisic G., Chapman J., Flight I., Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: A systematic review. Prev. Med. 2017;95:26–37. doi: 10.1016/j.ypmed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Newman P.A., Logie C.H., Lacombe-Duncan A., Baiden P., Tepjan S., Rubincam C., Doukas N., Asey F. Parents’ uptake of human papillomavirus vaccines for their children: A systematic review and meta-analysis of observational studies. BMJ Open. 2018;8:e019206. doi: 10.1136/bmjopen-2017-019206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kornides M.L., McRee A.L., Gilkey M.B. Parents Who Decline HPV Vaccination: Who Later Accepts and Why? Acad. Pediatr. 2018;18((Suppl. 2)):S37–S43. doi: 10.1016/j.acap.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomes J.M., Silva B.M., Santos E.F.d.S., Kelly P.J., Costa A.d.S., Takiuti A.D., de Abreu L.C., Júnior J.M.S., Baracat E.C., Sorpreso I.C.E. Human Papillomavirus (HPV) and the quadrivalent HPV Vaccine among Brazilian adolescents and parents: Factors associated with and divergences in knowledge and acceptance. PLoS ONE. 2020;15:e0241674. doi: 10.1371/journal.pone.0241674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beavis A.L., Meek K., Moran M.B., Fleszar L., Adler S., Rositch A.F. Exploring HPV vaccine hesitant parents’ perspectives on decision-making and motivators for vaccination. Vaccine X. 2022;12:100231. doi: 10.1016/j.jvacx.2022.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Mortensen G., Adam M., Idtaleb L. Parental attitudes towards male human papillomavirus vaccination: A pan-European cross-sectional survey. BMC Public Health. 2015;15:624. doi: 10.1186/s12889-015-1863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rositch A.F., Liu T., Chao C., Moran M., Beavis A.L. Levels of Parental Human Papillomavirus Vaccine Hesitancy and Their Reasons for Not Intending to Vaccinate: Insights From the 2019 National Immunization Survey-Teen. J. Adolesc. Health. 2022;71:39–46. doi: 10.1016/j.jadohealth.2022.01.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonawane K., Zhu Y., Montealegre J.R., Lairson D.R., Bauer C., McGee L.U., Giuliano A.R., A Deshmukh A. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: A nationwide, cross-sectional survey. Lancet Public Health. 2020;5:e484–e492. doi: 10.1016/S2468-2667(20)30139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsui J., Martinez B., Shin M.B., Allee-Munoz A., Rodriguez I., Navarro J., Thomas-Barrios K.R., Kast W.M., Baezconde-Garbanati L. Understanding medical mistrust and HPV vaccine hesitancy among multiethnic parents in Los Angeles. J. Behav. Med. 2023;46:100–115. doi: 10.1007/s10865-022-00283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szilagyi P.G., Albertin C.S., Gurfinkel D., Saville A.W., Vangala S., Rice J.D., Helmkamp L., Zimet G.D., Valderrama R., Breck A., et al. Prevalence characteristics of HPV vaccine hesitancy among parents of adolescents across the, U.S. Vaccine. 2020;38:6027–6037. doi: 10.1016/j.vaccine.2020.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendry M., Lewis R., Clements A., Damery S., Wilkinson C. “HPV? Never heard of it!”: A systematic review of girls’ and parents’ information needs, views and preferences about human papillomavirus vaccination. Vaccine. 2013;31:5152–5167. doi: 10.1016/j.vaccine.2013.08.091. [DOI] [PubMed] [Google Scholar]
- 57.Krawczyk A., Knäuper B., Gilca V., Dubé E., Perez S., Joyal-Desmarais K., Rosberger Z. Parents’ decision-making about the human papillomavirus vaccine for their daughters: I. Quantitative results. Hum. Vaccines Immunother. 2015;11:322–329. doi: 10.1080/21645515.2014.1004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kutz J.-M., Rausche P., Gheit T., Puradiredja D.I., Fusco D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: A systematic review. BMC Public Health. 2023;23:974. doi: 10.1186/s12889-023-15842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong L.P., Wong P.-F., Megat Hashim M.M., Han L., Lin Y., Hu Z., Zhao Q., Zimet G.D. Multidimensional Social and cultural norms influencing HPV vaccine hesitancy in Asia. Hum. Vaccines Immunother. 2020;16:1611–1622. doi: 10.1080/21645515.2020.1756670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dera M., Wondimagegnehu A., Asfaw Z.G. Determinants for hesitancy in human papillomavirus (HPV) vaccine uptake among school girls in Jimma Town, Ethiopia. A mixed approach: Quantitative and qualitative. Reprod. Health. 2023;20:175. doi: 10.1186/s12978-023-01711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ver A.T., Notarte K.I., Velasco J.V., Buac K.M., Nazareno J., Lozañes J.A., Antonio D., Bacorro W. A systematic review of the barriers to implementing human papillomavirus vaccination programs in low- and middle-income countries in the asia-pacific. Asia-Pac. J. Clin. Oncol. 2021;17:530–545. doi: 10.1111/ajco.13513. [DOI] [PubMed] [Google Scholar]
- 62.Ekwunife O.I., O’Mahony J.F., Gerber Grote A., Mosch C., Paeck T., Lhachimi S.K. Challenges in cost-effectiveness analysis modelling of HPV vaccines in low- and middle-income countries: A systematic review and practice recommendations. PharmacoEconomics. 2016;35:65–82. doi: 10.1007/s40273-016-0451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request from the corresponding author.