Abstract
This study delves into the critical issue of water pollution caused by the presence of metal oxides, synthetic dyes, and dissolved organic matter, shedding light on their potential ramifications for both the environment and human health. Metal oxides, ubiquitous in industrial processes and consumer products, are known to leach into water bodies, posing a significant threat to aquatic ecosystems. Additionally, synthetic dyes, extensively used in various industries, can persist in water systems and exhibit complex chemical behavior. This review provides a comprehensive examination of the toxicity associated with metal oxides, synthetic dyes, and dissolved organic matter in water systems. We delve into the sources and environmental fate of these contaminants, highlighting their prevalence in natural water bodies and wastewater effluents. The study highlights the multifaceted impacts of them on human health and aquatic ecosystems, encompassing effects on microbial communities, aquatic flora and fauna, and the overall ecological balance. The novelty of this review lies in its unique presentation, focusing on the toxicity of metal oxides, dyes, and dissolved organic matter. This approach aims to facilitate the accessibility of results for readers, providing a streamlined and clear understanding of the reported findings.
Keywords: toxicity, metal oxides, dissolved organic matter, dyes, water, aquatic lives, environments, human health
1. Introduction
Water is a vital resource for sustaining life, and its quality is paramount for the well-being of ecosystems and human populations. However, in recent decades, increasing industrialization, urbanization, and agricultural practices have led to the release of various pollutants into water bodies, posing significant threats to aquatic ecosystems and human health [1]. Among these pollutants, metal oxides, dyes, and dissolved organic matter have emerged as notable concerns due to their diverse sources and potential toxicity [2].
Metal oxides, arising from both natural geological processes and human activities, impact water bodies through pathways like runoff, industrial discharges, and atmospheric deposition [3]. While well-known hazardous elements such as lead, mercury, cadmium, chromium, and arsenic pose risks, the spectrum extends to diverse metal oxides from 3d, 4d, and 5d transition metal series [4]. In the 3d series, iron oxides (Fe2O3, Fe3O4), copper oxides (CuO, Cu2O), and manganese oxides (MnO2) contribute to water pollution via natural weathering and anthropogenic activities like industrial emissions and urban runoff [5]. Additional contributors like nickel oxide (NiO), zinc oxide (ZnO), selenium oxides, and titanium oxide (TiO2) further emphasize the complexity of metal oxide pollution [6,7]. Expanding to the 4d and 5d transition metals, ruthenium oxides (RuO2) [8], rhodium oxides (Rh2O3), tantalum oxides (Ta2O5), and tungsten oxides (WO3) introduce diverse chemical characteristics from industrial processes, raising concerns about their ecological impacts [9]. Accumulation of these metal oxides in aquatic ecosystems disrupts environmental balance, impacting fish, plankton, and benthic organisms [10]. Bioaccumulation raises concerns about transference through the food chain, posing risks to human health [11]. This interplay underscores the need to understand metal oxides’ sources, behavior, and toxicological impacts on aquatic ecosystems [12]. A multifaceted approach is crucial for developing effective strategies to monitor, mitigate, and prevent the deleterious effects of these contaminants on water quality and human health.
Dyes, commonly used in industries like textiles, leather, and paper, contribute to water pollution by discharging effluents containing unutilized dyes [13]. The vibrant and diverse colors associated with dyes may be visually appealing, but their environmental impact is a cause for concern [14]. Many dyes utilized across diverse industries exhibit a concerning characteristic—persistence and resistance to degradation [15]. These pose substantial challenges for wastewater treatment plants and significantly heighten the risk of bioaccumulation in aquatic organisms [16]. Among the myriad of persistent dyes, several notable examples underline the severity of this issue [17]. Reactive dyes, known for their chemical ability to bond with fibers, exemplify persistence in the textile industry [18].
Similarly, direct dyes employed in the dyeing of cellulose fibers include notorious persisters like congo Red [19], direct blue 1, direct black 38, and direct blue 95 [20]. Disperse dyes, favored for synthetic fibers like polyester, introduce compounds such as disperse yellow 3 [21], disperse yellow 7 [22], disperse red 1 [23], and disperse orange 1 [24], demonstrating resistance to degradation. The azo dyes category, marked by azo bonds, boasts examples like orange II, sudan I, and acid red 88—known for their stability and persistence [25]. Vat dyes, commonly used in cotton dyeing [26,27], feature resilient options like indigo, benzanthrone, and anthraquinone [28,29]. Triarylmethane dyes, including malachite green and crystal violet, exhibit persistence, particularly in aquaculture applications [30]. The persistence of these dyes in wastewater streams necessitates advanced treatment methods, as traditional approaches often struggle to degrade these compounds effectively [31]. This persistence challenges the efficiency of wastewater treatment and raises the bioaccumulation specter in aquatic organisms. The consequences of this resistance to degradation extend beyond environmental concerns, encompassing potential ecological imbalances and posing risks to human health [32].
Dissolved organic matter (DOM), a diverse blend of organic compounds which passes through a 0.45 μm filter, is pivotal in shaping water quality within aquatic ecosystems [33,34]. While a natural component, human-driven activities such as deforestation, agriculture, and urbanization amplify its presence in water bodies [35]. The spectrum of DOM includes various components such as humic acids, derived from the decomposition of organic matter, microplastics, fulvic acids, tannins, amino acids, and plant-derived sugars [36,37]. These compounds not only contribute to the color of water but also engage in intricate interactions with metal oxides [38]. The formation of complexes between DOM and metal oxides can lead to alterations in toxicity, impacting the overall health of aquatic ecosystems [39]. Humic and fulvic acids, for instance, are known for forming complexes with metals, influencing their bioavailability [40]. Recognizing the diversity of DOM components is crucial for understanding the nuanced dynamics of these interactions, emphasizing the need for sustainable practices to manage and mitigate the repercussions on water quality and ecological balance [41].
While previous studies have often examined these pollutants in isolation, understanding their synergistic effects is essential for comprehensively assessing water quality. Metal oxides, arising from both natural processes and human activities, can interact with synthetic dyes and DOM, forming complex chemical reactions with potential ecological consequences [39]. In addition, the coexistence of metal oxides, synthetic dyes, and dissolved organic matter in irrigation water engenders a complex web of interactions with profound implications for soil properties. Metal oxides, originating from natural processes and anthropogenic activities, introduce variations in soil pH and influence the solubility and precipitation of other pollutants [42]. Synthetic dyes, known for their persistence, can alter the soil’s chemical composition, affecting nutrient availability and microbial activity [43]. Concurrently, dissolved organic matter, a complex mixture of organic compounds, interacts with metal oxides, potentially forming complexes that impact soil structure and nutrient cycling [44,45]. The interplay of these pollutants in irrigation water may trigger intricate reactions, influencing the overall soil environment. Understanding the implications of metal oxides, dyes, and dissolved organic matter in water is crucial for comprehending the complex interplay between contaminants and their effects on the environment and human health [46,47]. This review aims to explore the current knowledge regarding these pollutants’ sources, distribution, and toxicological impacts, shedding light on the potential risks they pose to aquatic ecosystems and human health. By showcasing the toxicity aspects of these contaminants, the review enhances the usability of the information, making it more readily applicable for those seeking insights into the environmental and health impacts of metal oxides, synthetic dyes, and dissolved organic matter. The elucidation of the ecological disruptions caused by metal oxides, coupled with the persistent challenges of synthetic dyes and the variable toxicity of dissolved organic matter, underscores the intricate dynamics influencing both environmental integrity and human health. The unique focus on showcasing the toxicity aspects enhances the practical usability of the reported findings, making them more accessible for researchers.
2. Source of Metal Oxides and Toxicity
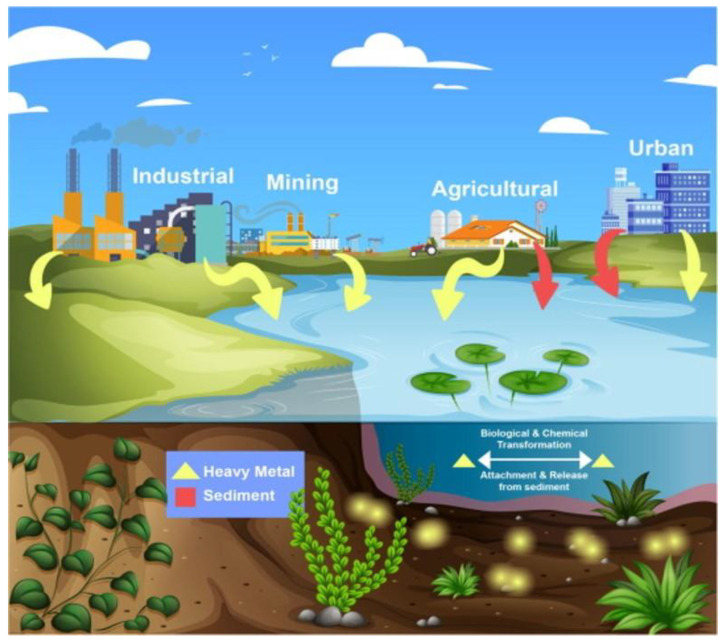
Metal oxide contaminants infiltrate the environment through various pathways, showcasing the intricate interplay of natural and anthropogenic processes. Erosion of rocks and minerals contributes to the release of metal ions, while industrial activities discharge metal oxides into waterways [36]. Agricultural runoff carries metal-based fertilizers into water bodies, impacting aquatic ecosystems. Urban runoff from corroded structures adds to water pollution. Mining operations disturb soil, releasing metal oxides into rivers. Natural disasters mobilize metal oxides, affecting water quality. Atmospheric deposition introduces metals into ecosystems, and improper waste disposal in landfills perpetuates metal persistence. Biological processes contribute to metal accumulation, and intentional use of metal-based materials in applications like adsorbents and nanoparticles introduces these contaminants. Recognizing these diverse sources is crucial for implementing effective measures to safeguard water quality and environmental well-being. A summary of the sources (Figure 1) and effects of toxic metal oxides on human health (Figure 2) and aquatic life is presented in Table 1.
Figure 1.
Diagrammatic illustration of toxic metals in the environment [48]. Copyright 2022, reproduced with permission from Elsevier B.V.
Table 1.
Sources and effects of toxic metal oxides on human health and aquatic life.
| Metal Oxides | Source | Toxicity in Human Health | Toxicity in Aquatic Life | Refs. |
|---|---|---|---|---|
| Lead oxide (PbO) | Industrial processes, mining | Neurological effects, developmental issues | Adverse effects on fish and invertebrates | [47] |
| Mercury oxide (HgO) | Coal combustion, mining | Neurological damage, kidney damage | Bioaccumulation in fish, toxic to invertebrates | [49] |
| Cadmium oxide (CdO) | Smelting, battery production | Kidney damage, bone effects | Toxic to aquatic organisms, disrupts ecosystems | [50] |
| Arsenic oxide (As2O3) | Mining, agricultural runoff | Skin lesions, cancer risk | Toxic to fish, invertebrates, and plants | [51,52] |
| Nickel oxide (NiO) | Metal refining, combustion | Respiratory issues, carcinogenic, infertility | Toxic to aquatic invertebrates | [53] |
| Zinc oxide (ZnO) | Mining, industrial processes | Gastrointestinal issues | Adverse effects on fish and aquatic plants | [54] |
| Selenium oxides | Mining, agricultural runoff | Selenosis, liver damage | Toxic to fish and aquatic invertebrates | [55] |
| Titanium oxide (TiO2) | Paints, sunscreens, industrial use | Limited health risks (in nanoparticle form) | Low toxicity, but environmental concerns | [56] |
| Copper oxide (CuO) | Mining, agricultural pesticides | Gastrointestinal issues, liver damage | Toxic to fish, invertebrates, and aquatic organism | [57] |
| Chromium oxide (Cr2O3) | Metal plating, leather tanning | Respiratory issues, carcinogenic | Toxic to aquatic organisms, bio accumulative | [58] |
| Vanadium pentoxide (V2O5) | Metal smelting, fuel additives | Respiratory and cardiovascular issues, cytotoxic | Toxic to fish, invertebrates, and aquatic plants | [59] |
| Manganese oxides (MnO2) | Mining, industrial processes | Neurological effects, respiratory issues | Adverse effects on fish and aquatic invertebrates | [60] |
| Ruthenium oxides (RuO2) | Anthropogenic activities, metal refining, electronics | Respiratory irritation and, in extreme cases, lung damage | Potential ecotoxicological effects | [60] |
| Rhodium oxides (Rh2O3) | Precious metal refining, automotive catalysis, and electronics manufacturing | Irritate the respiratory tract, and, like many metal oxides | Ecotoxicological impacts in high concentration | [61] |
| Tantalum oxides (Ta2O5) | Electronics, capacitors, metallurgical operations | Teratogenic, respiratory irritation, and reproductive toxicity | Limited information is available on the specific effects of tantalum oxides on aquatic life | [62,63] |
| Tungsten oxides (WO3) | Metal manufacturing, alloys | Cytotoxic, respiratory irritation, and genotoxic | Eco-toxicological effects of tungsten oxides are not extensively studied | [64] |
Additionally, the toxicity findings for several metal oxides vary, and the available information on some compounds is limited. Palladium oxide (PdO) is relatively inert, and while specific toxicity data are limited [65], respiratory and skin irritation precautions are advised. Iridium oxides (IrO2) have limited toxicity data [66], but as with many metal oxides, careful handling is necessary to prevent respiratory hazards. Osmium tetroxide (OsO4) is highly toxic, causing severe respiratory and skin irritation, eye damage, and its use is confined to controlled laboratory settings [67]. Platinum oxides (PtO2) have limited specific toxicity information, but occupational exposure should be managed to prevent potential cytotoxic issues [68]. Hafnium oxides (HfO2) have limited information on cytotoxicity [69], and precautions are recommended during handling. Rhenium oxides (ReO3) and niobium pentoxide (Nb2O5) have limited toxicity data, with rhenium and niobium considered to have low toxicity [70,71]. Comprehensive studies are needed to fully understand exposure to these metal oxides’ potential health and environmental implications. Occupational safety measures, including proper handling and exposure prevention, remain crucial in industrial settings.
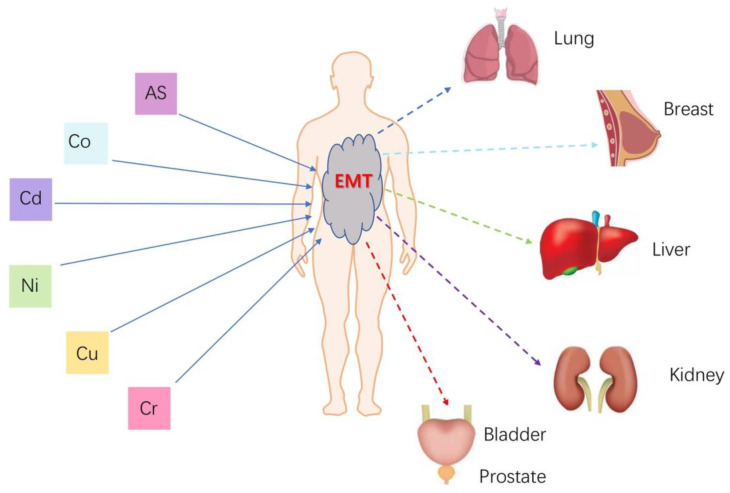
Figure 2.
Metal ion toxicity in humans [72]. Copyright 2022, reproduced with permission from Springer Nature.
The metal oxides listed (Table 1) exhibit common adverse effects on human health and aquatic life. Neurological impacts, respiratory issues, and carcinogenicity are recurring concerns for human health, emphasizing the potential health risks associated with exposure. In aquatic ecosystems, these metal oxides threaten fish, invertebrates, and plants, disrupting the balance of ecosystems. Bioaccumulation, respiratory irritation, and ecological toxicity are shared consequences, highlighting the need for comprehensive management strategies to mitigate these contaminants’ impact on human and environmental well-being.
3. Source of Dyes and Toxicity
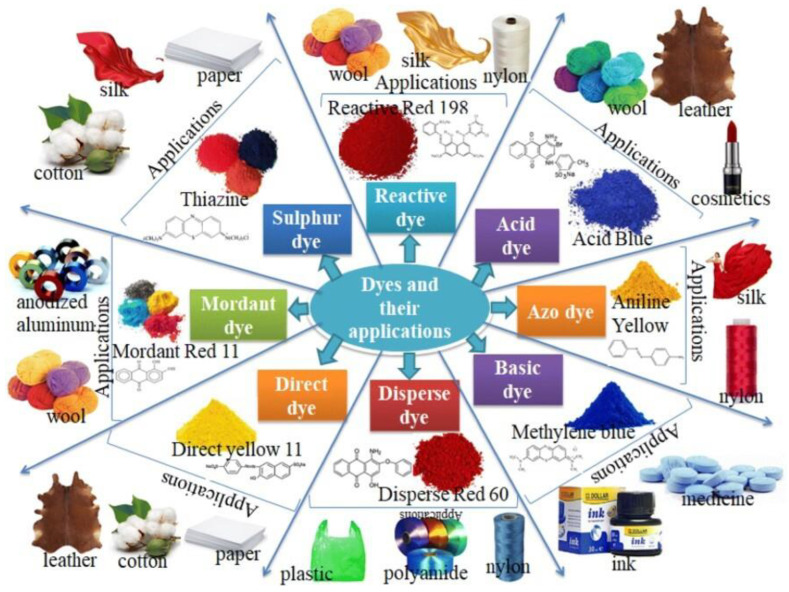
Synthetic dyes encompass diverse types, each designed for specific applications [73]. Acid dyes, known for vibrant hues, find purpose in coloring wool and silk and staining bacteria and yeast [74,75]. Basic dyes with cationic properties excel in dyeing synthetic fibers like acrylic [76]. Disperse dyes are the go-to for coloring polyester and acetate textiles. Reactive dyes form durable bonds with fibers, making them a staple in textile dyeing. Vat dyes, prized for colorfastness, are ideal for dyeing cotton. Direct dyes are versatile, easy to apply, and suitable for natural fibers like cotton and wool. Sulfur dyes, cost-effective and deep in color, are favored for dyeing cotton. Metal complex dyes offer intense colors and resistance to fading in applications such as textiles and inks. Azo dyes [77], a diverse category, find use in textiles, plastics, and printing inks for their vivid hues [78]. Solvent dyes bring vibrant coloration to non-polar solvents like plastics and waxes. This diversity underscores these dyes’ specialized roles in various industries, contributing to the vibrant spectrum of colored products in our daily lives (Figure 3).
Figure 3.
Exploring various dye varieties and their diverse industrial applications [79]. Copyright 2023, reproduced with permission from the Royal Society of Chemistry (RSC); RSC Publishing; Cold Spring Harbor Laboratory Press.
Hence, the primary source of dyes in water arises from untreated industrial effluents discharged into water bodies. Additionally, a non-negligible source of dyes will be domestic washing of clothes. This is a common task at home or in neighborhood laundries and is an activity in which the washing water goes directly into the sewage system. It is worth mentioning this situation and even trying to assess whether the loss of dye, for example, from some denim jeans during successive washing, loses more dye than in their production process. Textile dyeing processes pose a significant environmental threat, with up to 15% of applied dyes escaping wastewater. The extensive use of water in dyeing procedures results in continuous improper discharge. Even after treatment, dye effluents contain high levels of pollutants, including toxic metals, chlorinated compounds, and organic substances. This persistent contamination adversely affects air, soil, plants, and water resources, contributing to severe human health issues [73]. A summary of the sources (Figure 4) and the effects of toxic dyes on human health and aquatic life is presented in Table 2.
Figure 4.
Schematic representation of water pollution originating from industrial effluents [80]. Copyright 2021, reproduced with permission from John Wiley and Sons.
Table 2.
Sources and effects of toxic dyes on human health and aquatic life.
| Synthetic Dye Type | Source | Toxicity in Human Health | Effects on Aquatic Life | Refs. |
|---|---|---|---|---|
| Ionic dyes | Industrial processes, textile dyeing | Skin irritation, respiratory issues | Toxic to fish, invertebrates | [81] |
| Nonionic (disperse): Disperse red 1 Disperse blue 1 |
Textile dyeing, plastics, polyesters | Low acute toxicity, but potential carcinogenic | Adverse effects on aquatic plants | [82] |
| Nonionic (vat): Indanthrene blue rs Vat yellow 2 |
Textile dyeing, printing inks, plastics | Low toxicity, limited health risks | Limited impact on aquatic life | [83] |
| Cationic dyes: Crystal violet, methylene blue, basic blue 9, and malachite green |
Textile dyeing, paper industry | Skin and eye irritation, respiratory issues | Potential toxicity to fish and aquatic plants | [83,84] |
| Anionic (acid): Acid orange 7 Acid red 73 Acid blue 9 Acid red 52 Acid black 1 |
Textile dyeing, leather tanning, paper industry | Skin irritation, respiratory issues | Adverse effects on fish, invertebrates | [85] |
| Anionic (direct): Direct blue 6 Direct blue 86 Direct red 81 Direct yellow 4 Direct black 19 |
Textile dyeing, paper industry, leather tanning | Skin irritation, respiratory issues | Toxic to aquatic invertebrates | [20,86] |
| Anionic (reactive): Reactive blue 19 Reactive red 120 Reactive yellow 145 Reactive black 5 |
Textile dyeing, printing, paper industry | Skin irritation, respiratory issues | Adverse effects on aquatic plants | [87] |
Table 2 summarizes various synthetic dye types and their effects. Ionic dyes cause skin and respiratory issues, impacting fish and invertebrates. Nonionic disperse dyes have low acute toxicity but potential carcinogenic risks, affecting aquatic plants. Nonionic vat dyes exhibit low toxicity with limited impact on aquatic life. Cationic dyes induce skin irritation and respiratory issues, potentially harming fish and aquatic plants. Anionic acid and direct dyes lead to skin irritation and respiratory problems, adversely affecting fish, invertebrates, and aquatic invertebrates. Anionic reactive dyes induce skin irritation and respiratory issues, impacting aquatic plants. This underscores synthetic dyes’ diverse toxic impacts on human health and aquatic ecosystems. Moreover, textile dyes, even at low concentrations, pose environmental hazards due to their non-biodegradable nature. Contaminated water can lead to health issues like skin rashes, headaches, nausea, and, notably, an increased cancer risk. The toxicity of these dyes is categorized into acute and chronic/genotoxic effects, with the latter posing long-term health risks. Reactive dyes, commonly used, are associated with skin irritation and various allergic reactions [80].
4. Source of Trace Toxic Organic Pollution
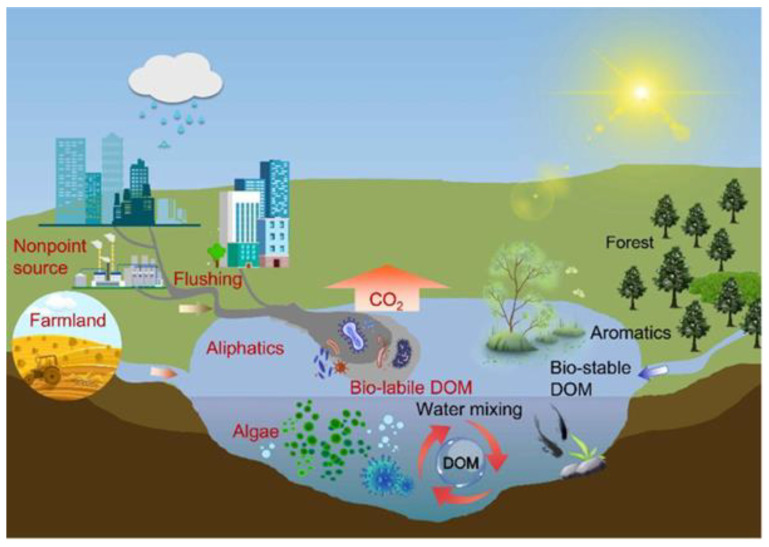
Dissolved organic matter (DOM) or trace toxic organic matters in water originates from diverse natural and human-induced sources [88]. Natural sources include decomposing organic materials such as dead plants, animals, and microorganisms [89]. Soil runoff, facilitated by rainfall or irrigation, carries organic compounds from the land into water bodies [90]. Leaching from plant materials, like leaves and branches, adds to the dissolved organic content influenced by natural ecosystems and human activities like deforestation [91]. Wastewater discharges from domestic or industrial sources introduce organic matter into water, and the decomposition of organic pollutants further contributes to trace toxic organic pollution [92]. Urban runoff, stemming from rainwater washing over impervious surfaces, also contributes to the organic load in water bodies. Aquatic plants and algae release organic compounds through natural processes, and atmospheric deposits, including airborne particles and plant material, can settle into water through precipitation (Figure 5) [88]. Understanding the multifaceted origins of dissolved organic matter is essential for comprehending water quality dynamics and ecosystem health. A summary of the sources (Figure 5) and the effects of trace toxic organic matters on human health and aquatic life is presented in Table 3.
Figure 5.
Schematic illustration of the source of dissolved organic matter [88]. Copyright 2022, reproduced with permission from the American Chemical Society.
Table 3.
Sources and effects of trace toxic organic matters on human health and aquatic life.
| Organic Compound | Source | Toxicity in Human Health | Toxicity in Aquatic Life | Refs. |
|---|---|---|---|---|
| Polycyclic Aromatic Hydrocarbons | Combustion of fossil fuels, industrial processes | Carcinogenic, respiratory, and reproductive issues | Toxic to aquatic bodies | [93,94] |
| Pesticides (e.g., atrazine) | Agricultural runoff, urban runoff | Neurological effects, endocrine disruption | Impacting insects and fish | [95] |
| Benzene | Industrial discharges, urban runoff | Carcinogenic, respiratory, and central nervous system issues | Toxic to aquatic organisms, affecting fish and insects | [96] |
| Chlorinated Compounds | Industrial discharges, atmospheric deposition | Neurological, renal, and developmental issues | Impacting fish and invertebrates | [97] |
| Volatile Organic Compounds | Industrial emissions, vehicle exhaust | Teratogenic, carcinogenic, mutagenic, genetic neurotoxicant and toxicant | Affecting fish and microorganism | [98] |
| Dioxins and Furans | Combustion of waste, forest fires, volcanic eruptions | Respiratory irritation, skin disorders, liver problems, cancer | Fish skin diseases | [99,100] |
| Phthalates | Industrial discharges, plastic leaching, migration, and oxidation | Neurotoxic and genotoxic | Toxic to water bodies animals | [101] |
| Nonylphenol | Industrial discharges, shampoos, detergents | Endocrine disruption, developmental issues | Impacting fish | [102] |
| Endocrine Disruptors | Industrial discharges, wastewater treatment, climate change, cosmetics | Endocrine disruption, reproductive issues, and hormonal imbalance | Affects fish embryonic development | [103] |
| Pharmaceuticals and Personal Care Products | Household wastewater, improper disposal | Variable health effects | Variable effects on aquatic organisms | [104,105] |
| Per- and Polyfluoroalkyl Substances | Industrial processes, firefighting foam, metal coating, wastewater treatment plants | Neurobehavioral toxicity, developmental effects, metabolism abnormalities | Bioaccumulation and toxic effects on aquatic organisms | [106] |
| Cyanobacterial Toxins | Harmful algal blooms, nutrient runoff | Liver damage, potential, carcinogenic effects | Affecting freshwater bodies | [107] |
| Nitrosamines | Agricultural runoff, wastewater treatment, combustion process, domestic source | Carcinogenic potential, potential reproductive issues | Toxic to aquatic and terrestrial organisms | [108,109] |
| Trihalomethanes | Chlorination of drinking water | Carcinogenic potential, reproductive, thyroid hormone endocrine functions issues | Algae toxicity, fragile aquatic ecosystem | [110] |
| Acrylamide | Industrial processes, wastewater treatment plants | Neurotoxic effects, carcinogenic, reprotoxic, and mutagenic | Toxic marine fish | [110] |
| Organophosphate Flame Retardants | Textile manufacturing, electronics production, wood protection | Neurological effects, potential endocrine disruption | Affecting fish reproduction | [111] |
| Polybrominated Diphenyl Ethers | Flame retardants in electronics, textiles | Neurodevelopmental issues, potential carcinogenicity, and immune toxicity | Alters thyroid hormone levels and gene transcription of fish | [112] |
| Polychlorinated Biphenyls | Industrial processes, improper disposal | carcinogenicity, hormone disruption, neurodevelopmental toxicity | Bioaccumulation in fish, dolphins, crabs | [113] |
| Bisphenol A | Plastics, epoxy resins, can linings | Endocrine disruption, potential reproductive issues | Health damage in fish | [114,115] |
| Chlorophenols | Organic synthesis, industrial wastewater, wood industries | Respiratory, dermatological effects, mutagenicity, endocrine-disrupting potency | Affecting fish and bioaccumulation potential in fish tissues | [116] |
| Diethylhexyl Phthalate | Plasticizers in plastics, consumer products | Endocrine disruption, potential reproductive issues | Altered the antioxidant system in the liver, intestine, brain, and gills | [117,118] |
| Glyphosate (Herbicide) | Agricultural runoff, urban runoff | Potential carcinogenicity, endocrine disruption, cytotoxicity | Impact on fish framing | [119,120] |
The toxicity of DOM in human health and aquatic life varies depending on the specific compound. While DOM is a natural component of aquatic ecosystems, human-induced activities contribute significantly to its increased presence [121]. The interactions between DOM and metal oxides, as well as other contaminants, can lead to the formation of toxic complexes, impacting the overall health of aquatic ecosystems [122]. Understanding the sources and toxicological effects of DOM is crucial for effective water quality management and the protection of both human and environmental health. The listed organic compounds (Table 3), sourced from industrial processes and runoff, exhibit diverse toxic effects on human health and aquatic life. Common concerns include carcinogenicity, respiratory issues, neurological effects, and endocrine disruption. The compounds impact fish, invertebrates, and aquatic ecosystems, with varied health consequences across species. The potential for bioaccumulation and disruption of aquatic ecosystems underscores the need for a comprehensive understanding and management of these contaminants.
5. Discussion
This comprehensive review has significantly advanced our comprehension of the intricate dynamics associated with metal oxides, synthetic dyes, and trace toxic organic matter in aquatic ecosystems [123,124]. By delving into the multifaceted challenges posed by these contaminants, the review underscores the urgent need for a holistic approach to water quality management. The elucidation of the ecological disruptions caused by metal oxides, coupled with the persistent challenges of synthetic dyes and the variable toxicity of dissolved organic matter (DOM) or trace toxic organic matter, highlights the complex interplay influencing both environmental integrity and human health. The review’s unique emphasis on showcasing the toxicity aspects enhances the practical usability of the reported findings, rendering them more accessible for researchers, policymakers, and practitioners alike. Moreover, the call for targeted research and the development of innovative water treatment technologies addresses crucial research gaps, paving the way for more effective and sustainable solutions [46]. The integration of these vital components lays the groundwork for making informed decisions in the fields of both environmental and public health.
6. Conclusions
This review illuminates the multifaceted challenges associated with metal oxides, synthetic dyes, and dissolved organic matter or trace toxic organic matter in water ecosystems. The intricate interplay of contaminants, ranging from transition metals to persistent synthetic dyes and complex organic compounds, underscores the critical need for a holistic understanding of their sources and toxicological impacts. The ecological disruption caused by metal oxides, the challenges posed by the persistence of synthetic dyes, and the varied toxicity of trace toxic organic matter necessitate nuanced approaches to water quality management. The review emphasizes the importance of adopting multidisciplinary strategies, encompassing effective monitoring, mitigation measures, and preventive actions, to safeguard environmental integrity and human health. Additionally, the authors propose targeted research to address gaps in understanding contaminant interactions and the development of innovative technologies for water treatment. This paper could emphasize the need for concerted efforts in research, policy development, and practical interventions to ensure the sustainable preservation of water resources.
Author Contributions
H.K.: Conceptualization, writing—original draft preparation, writing—review and editing. C.-W.K.: writing—review and editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Moreover, no animal studies or human participants are involved in this study.
Funding Statement
Basic Science Research Program supported this research through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2019R1I1A3A02059471).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hussain S., Khanam T., Ullah S., Aziz F., Sattar A., Hussain I., Saddique M.A., Maqsood A., Ding C., Wang X., et al. Assessment and Exposure Analysis of Trace Metals in Different Age Groups of the Male Population in Southern Punjab, Pakistan. Toxics. 2023;11:958. doi: 10.3390/toxics11120958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briffa J., Sinagra E., Blundell R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon. 2020;6:e04691. doi: 10.1016/j.heliyon.2020.e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar H., Singh G., Mishra V.K., Singh R.P., Singh P. Chapter 9—Airborne Heavy Metals Deposition and Contamination to Water Resources. In: Shukla S.K., Kumar S., Madhav S., Mishra P.K.B.T.-M., editors. Advances in Environmental Pollution Research. Elsevier; Amsterdam, The Netherlands: 2023. pp. 155–173. [Google Scholar]
- 4.Tolkou A.K., Toubanaki D.K., Kyzas G.Z. Detection of Arsenic, Chromium, Cadmium, Lead, and Mercury in Fish: Effects on the Sustainable and Healthy Development of Aquatic Life and Human Consumers. Sustainability. 2023;15:16242. doi: 10.3390/su152316242. [DOI] [Google Scholar]
- 5.Rainbow P.S. Trace Metals in the Environment and Living Organisms: The British Isles as a Case Study. Cambridge University Press; Cambridge, UK: 2018. [Google Scholar]
- 6.Samim A.R., Singh V.K., Vaseem H. Assessment of Hazardous Impact of Nickel Oxide Nanoparticles on Biochemical and Histological Parameters of Gills and Liver Tissues of Heteropneustes Fossilis. J. Trace Elem. Med. Biol. 2022;74:127059. doi: 10.1016/j.jtemb.2022.127059. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary R.G., Bhusari G.S., Tiple A.D., Rai A.R., Somkuvar S.R., Potbhare A.K., Lambat T.L., Ingle P.P., Abdala A.A. Metal/Metal Oxide Nanoparticles: Toxicity, Applications, and Future Prospects. Curr. Pharm. Des. 2019;25:4013–4029. doi: 10.2174/1381612825666191111091326. [DOI] [PubMed] [Google Scholar]
- 8.Dogra V., Kaur G., Kumar R., Kumar S. Toxicity Profiling of Metallosurfactant Based Ruthenium and Ruthenium Oxide Nanoparticles towards the Eukaryotic Model Organism Saccharomyces Cerevisiae. Chemosphere. 2021;270:128650. doi: 10.1016/j.chemosphere.2020.128650. [DOI] [PubMed] [Google Scholar]
- 9.Egorova K.S., Ananikov V.P. Which Metals Are Green for Catalysis? Comparison of the Toxicities of Ni, Cu, Fe, Pd, Pt, Rh, and Au Salts. Angew. Chem. Int. Ed. 2016;55:12150–12162. doi: 10.1002/anie.201603777. [DOI] [PubMed] [Google Scholar]
- 10.Kahlon S.K., Sharma G., Julka J.M., Kumar A., Sharma S., Stadler F.J. Impact of Heavy Metals and Nanoparticles on Aquatic Biota. Environ. Chem. Lett. 2018;16:919–946. doi: 10.1007/s10311-018-0737-4. [DOI] [Google Scholar]
- 11.Kumar S., Prasad S., Yadav K.K., Shrivastava M., Gupta N., Nagar S., Bach Q.-V., Kamyab H., Khan S.A., Yadav S., et al. Hazardous Heavy Metals Contamination of Vegetables and Food Chain: Role of Sustainable Remediation Approaches—A Review. Environ. Res. 2019;179:108792. doi: 10.1016/j.envres.2019.108792. [DOI] [PubMed] [Google Scholar]
- 12.Bai C., Tang M. Toxicological Study of Metal and Metal Oxide Nanoparticles in Zebrafish. J. Appl. Toxicol. 2020;40:37–63. doi: 10.1002/jat.3910. [DOI] [PubMed] [Google Scholar]
- 13.Yaseen D.A., Scholz M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019;16:1193–1226. doi: 10.1007/s13762-018-2130-z. [DOI] [Google Scholar]
- 14.Houk V.S. The Genotoxicity of Industrial Wastes and Effluents: A Review. Mutat. Res. Genet. Toxicol. 1992;277:91–138. doi: 10.1016/0165-1110(92)90001-P. [DOI] [PubMed] [Google Scholar]
- 15.Góralczyk-Bińkowska A., Długoński A., Bernat P., Długoński J., Jasińska A. Environmental and Molecular Approach to Dye Industry Waste Degradation by the Ascomycete Fungus Nectriella Pironii. Sci. Rep. 2021;11:23829. doi: 10.1038/s41598-021-03446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P., Qanungo K. Challenges in Effluents Treatment Containing Dyes. Adv. Res. Text. Eng. 2022;7:1075. [Google Scholar]
- 17.Lellis B., Fávaro-Polonio C.Z., Pamphile J.A., Polonio J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019;3:275–290. doi: 10.1016/j.biori.2019.09.001. [DOI] [Google Scholar]
- 18.Eletmany M.R., Abdellah I.M., El-Shafei A. Sustainable Cotton Dyeing with Reactive Dyes for Enhanced Color Fastness and Durable Antimicrobial Properties; Proceedings of the NC Global Health Alliance Annual Conference; Raleigh, NC, USA. 1 November 2023. [Google Scholar]
- 19.Siddiqui S.I., Allehyani E.S., Al-Harbi S.A., Hasan Z., Abomuti M.A., Rajor H.K., Oh S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes. 2023;11:807. doi: 10.3390/pr11030807. [DOI] [Google Scholar]
- 20.Robens J.F., Dill G.S., Ward J.M., Joiner J.R., Griesemer R.A., Douglas J.F. Thirteen-Week Subchronic Toxicity Studies of Direct Blue 6, Direct Black 38, and Direct Brown 95 Dyes. Toxicol. Appl. Pharmacol. 1980;54:431–442. doi: 10.1016/0041-008X(80)90170-2. [DOI] [PubMed] [Google Scholar]
- 21.Humans I.W.G. Some Flame Retardants and Textile Chemicals, and Exposures in the Textile Manufacturing Industry. International Agency for Research on Cancer; Lyon, France: 1990. On the E. of C.R. to Disperse Yellow 3. [Google Scholar]
- 22.Mathieu-Denoncourt J., Martyniuk C.J., de Solla S.R., Balakrishnan V.K., Langlois V.S. Sediment Contaminated with the Azo Dye Disperse Yellow 7 Alters Cellular Stress-and Androgen-Related Transcription in Silurana Tropicalis Larvae. Environ. Sci. Technol. 2014;48:2952–2961. doi: 10.1021/es500263x. [DOI] [PubMed] [Google Scholar]
- 23.Chequer F.M.D., Angeli J.P.F., Ferraz E.R.A., Tsuboy M.S., Marcarini J.C., Mantovani M.S., de Oliveira D.P. The Azo Dyes Disperse Red 1 and Disperse Orange 1 Increase the Micronuclei Frequencies in Human Lymphocytes and in HepG2 Cells. Mutat. Res. Toxicol. Environ. Mutagen. 2009;676:83–86. doi: 10.1016/j.mrgentox.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz E.R.A., Grando M.D., Oliveira D.P. The Azo Dye Disperse Orange 1 Induces DNA Damage and Cytotoxic Effects but Does Not Cause Ecotoxic Effects in Daphnia Similis and Vibrio Fischeri. J. Hazard. Mater. 2011;192:628–633. doi: 10.1016/j.jhazmat.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 25.Feng J., Cerniglia C.E., Chen H. Toxicological Significance of Azo Dye Metabolism by Human Intestinal Microbiota. Front. Biosci. (Elite Ed.) 2012;4:568. doi: 10.2741/400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santhi P., Moses J.J. Study on Different Reducing Agents for Effective Vat Dyeing on Cotton Fabric. IJFTR. 2010;35:349–352. [Google Scholar]
- 27.Wang Y., Yi Q., Ding Y., Ji F., Wang N. Study on the Factors Influencing the Dyeing Performance of Cotton Fabric with Vat Dyes Based on Principal Component Analysis. J. Text. Inst. 2021;112:1460–1466. doi: 10.1080/00405000.2020.1824432. [DOI] [Google Scholar]
- 28.Mahapatra N.N. Textile Dyes. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 29.Dawood S., Sen T. Review on Dye Removal from Its Aqueous Solution into Alternative Cost Effective and Non-Conventional Adsorbents. J. Chem. Process Eng. 2014;1:104. [Google Scholar]
- 30.Verdon E., Andersen W.C. Chemical Analysis of Non-Antimicrobial Veterinary Drug Residues in Food. Wiley Online Library; Hoboken, NJ, USA: 2016. Certain Dyes as Pharmacologically Active Substances in Fish Farming and Other Aquaculture Products; pp. 497–548. [Google Scholar]
- 31.Sewu D.D., Lee D.S., Woo S.H., Kalderis D. Decolorization of Triarylmethane Dyes, Malachite Green, and Crystal Violet, by Sewage Sludge Biochar: Isotherm, Kinetics, and Adsorption Mechanism Comparison. Korean J. Chem. Eng. 2021;38:531–539. doi: 10.1007/s11814-020-0727-7. [DOI] [Google Scholar]
- 32.Pereira L., Alves M. Environmental Protection Strategies for Sustainable Development. Springer; Berlin/Heidelberg, Germany: 2012. Dyes—Environmental Impact and Remediation; pp. 111–162. [Google Scholar]
- 33.Perdue E.M., Ritchie J.D. Dissolved Organic Matter in Freshwaters. Treatise Geochem. 2003;5:605. [Google Scholar]
- 34.Ogawa H., Tanoue E. Dissolved Organic Matter in Oceanic Waters. J. Oceanogr. 2003;59:129–147. doi: 10.1023/A:1025528919771. [DOI] [Google Scholar]
- 35.Hu H.-Y., Du Y., Wu Q.-Y., Zhao X., Tang X., Chen Z. Differences in Dissolved Organic Matter between Reclaimed Water Source and Drinking Water Source. Sci. Total Environ. 2016;551–552:133–142. doi: 10.1016/j.scitotenv.2015.12.111. [DOI] [PubMed] [Google Scholar]
- 36.Longnecker K., Kujawinski E.B. Composition of Dissolved Organic Matter in Groundwater. Geochim. Cosmochim. Acta. 2011;75:2752–2761. doi: 10.1016/j.gca.2011.02.020. [DOI] [Google Scholar]
- 37.Kolya H., Kang C.-W. A New Approach for Agricultural Water Management Using Pillows Made from COVID-19 Waste Face Masks and Filled with a Hydrogel Polymer: Preliminary Studies. Agriculture. 2023;13:152. doi: 10.3390/agriculture13010152. [DOI] [Google Scholar]
- 38.Xiao Z., Xie X., Pi K., Gong J., Wang Y. Effects of Arsenic–Iron–Dissolved Organic Matter Interactions on Arsenic Mobilization: Insight from Column Experiments. J. Hydrol. 2023;616:128837. doi: 10.1016/j.jhydrol.2022.128837. [DOI] [Google Scholar]
- 39.Aiken G.R., Hsu-Kim H., Ryan J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011;45:3196–3201. doi: 10.1021/es103992s. [DOI] [PubMed] [Google Scholar]
- 40.Papadaki E.S., Chatzimitakos T., Athanasiadis V., Kalompatsios D., Bozinou E., Mitlianga P., Lalas S.I. Assessment of Humic and Fulvic Acid Sorbing Potential for Heavy Metals in Water. Foundations. 2023;3:788–804. doi: 10.3390/foundations3040044. [DOI] [Google Scholar]
- 41.Zeng J., Han G., Zhang S., Xiao X., Li Y., Gao X., Wang D., Qu R. Response of Dissolved Organic Carbon in Rainwater during Extreme Rainfall Period in Megacity: Status, Potential Source, and Deposition Flux. Sustain. Cities Soc. 2023;88:104299. doi: 10.1016/j.scs.2022.104299. [DOI] [Google Scholar]
- 42.Pouyat R.V., Szlavecz K., Yesilonis I.D., Groffman P.M., Schwarz K. Urban Ecosystem Ecology. American Society of Agronomy; Madison, WI, USA: 2010. Chemical, Physical, and Biological Characteristics of Urban Soils; pp. 119–152. Agronomy Monographs. [Google Scholar]
- 43.Singh J., Gupta P., Das A. Dyes from Textile Industry Wastewater as Emerging Contaminants in Agricultural Fields. Sustain. Agric. Rev. Emerg. Contam. Agric. 2021;50:109–129. ISBN 978-3-030-63249-6. [Google Scholar]
- 44.Bolan N.S., Adriano D.C., Kunhikrishnan A., James T., McDowell R., Senesi N. Dissolved Organic Matter: Biogeochemistry, Dynamics, and Environmental Significance in Soils. Adv. Agron. 2011;110:1–75. [Google Scholar]
- 45.Xiao R., Lei H., Zhang Y., Xiao Z., Yang G., Pan H., Hou Y., Yu J., Sun K., Dong Y. The Influence of Aerated Irrigation on the Evolution of Dissolved Organic Matter Based on Three-Dimensional Fluorescence Spectrum. Agronomy. 2023;13:980. doi: 10.3390/agronomy13040980. [DOI] [Google Scholar]
- 46.Kolya H., Kang C.-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers. 2023;15:3421. doi: 10.3390/polym15163421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolya H., Kang C.-W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability. 2023;15:9844. doi: 10.3390/su15129844. [DOI] [Google Scholar]
- 48.Mitra S., Chakraborty A.J., Tareq A.M., Emran T.B., Nainu F., Khusro A., Idris A.M., Khandaker M.U., Osman H., Alhumaydhi F.A., et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ.-Sci. 2022;34:101865. doi: 10.1016/j.jksus.2022.101865. [DOI] [Google Scholar]
- 49.Basu N., Bastiansz A., Dórea J.G., Fujimura M., Horvat M., Shroff E., Weihe P., Zastenskaya I. Our Evolved Understanding of the Human Health Risks of Mercury. Ambio. 2023;52:877–896. doi: 10.1007/s13280-023-01831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao D., Wang P., Zhao F.-J. Dietary Cadmium Exposure, Risks to Human Health and Mitigation Strategies. Crit. Rev. Environ. Sci. Technol. 2023;53:939–963. doi: 10.1080/10643389.2022.2099192. [DOI] [Google Scholar]
- 51.Muzaffar S., Khan J., Srivastava R., Gorbatyuk M.S., Athar M. Mechanistic Understanding of the Toxic Effects of Arsenic and Warfare Arsenicals on Human Health and Environment. Cell Biol. Toxicol. 2023;39:85–110. doi: 10.1007/s10565-022-09710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolya H., Hashitsume K., Kang C.-W. Recent Advances in Colorimetric Detection of Arsenic Using Metal-Based Nanoparticles. Toxics. 2021;9:143. doi: 10.3390/toxics9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arato I., Giovagnoli S., Di Michele A., Bellucci C., Lilli C., Aglietti M.C., Bartolini D., Gambelunghe A., Muzi G., Calvitti M. Nickel Oxide Nanoparticles Exposure as a Risk Factor for Male Infertility:“In Vitro” Effects on Porcine Pre-Pubertal Sertoli Cells. Front. Endocrinol. 2023;14:1063916. doi: 10.3389/fendo.2023.1063916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pei X., Jiang H., Li C., Li D., Tang S. Oxidative Stress-Related Canonical Pyroptosis Pathway, as a Target of Liver Toxicity Triggered by Zinc Oxide Nanoparticles. J. Hazard. Mater. 2023;442:130039. doi: 10.1016/j.jhazmat.2022.130039. [DOI] [PubMed] [Google Scholar]
- 55.Genchi G., Lauria G., Catalano A., Sinicropi M.S., Carocci A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023;24:2633. doi: 10.3390/ijms24032633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan Y., Yang Y., Zhang Z., Xing Y., Li H. Toxicity of Titanium Dioxide Nanoparticles on the Histology, Liver Physiological and Metabolism, and Intestinal Microbiota of Grouper. Mar. Pollut. Bull. 2023;187:114600. doi: 10.1016/j.marpolbul.2023.114600. [DOI] [PubMed] [Google Scholar]
- 57.Pourahmad J., Salami M., Zarei M.H. Comparative Toxic Effect of Bulk Copper Oxide (CuO) and CuO Nanoparticles on Human Red Blood Cells. Biol. Trace Elem. Res. 2023;201:149–155. doi: 10.1007/s12011-022-03149-y. [DOI] [PubMed] [Google Scholar]
- 58.Georgaki M.-N., Charalambous M., Kazakis N., Talias M.A., Georgakis C., Papamitsou T., Mytiglaki C. Chromium in Water and Carcinogenic Human Health Risk. Environments. 2023;10:33. doi: 10.3390/environments10020033. [DOI] [Google Scholar]
- 59.Ahmad J., Wahab R., Siddiqui M.A., Saquib Q., Al-Khedhairy A.A. Synthesis, Characterization of Vanadium Oxide Nanostructures and Their Cytotoxic Activities in Human Cell Lines. J. King Saud Univ. 2023;35:102856. doi: 10.1016/j.jksus.2023.102856. [DOI] [Google Scholar]
- 60.Farkas B., Vojtková H., Farkas Z., Pangallo D., Kasak P., Lupini A., Kim H., Urík M., Matúš P. Involvement of Bacterial and Fungal Extracellular Products in Transformation of Manganese-Bearing Minerals and Its Environmental Impact. Int. J. Mol. Sci. 2023;24:9215. doi: 10.3390/ijms24119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iavicoli I., Leso V. Handbook on the Toxicology of Metals. Elsevier; Amsterdam, The Netherlands: 2022. Rhodium; pp. 691–728. [Google Scholar]
- 62.Filella M. Tantalum in the Environment. Earth-Sci. Rev. 2017;173:122–140. doi: 10.1016/j.earscirev.2017.07.002. [DOI] [Google Scholar]
- 63.Almutairi B., Ali D., Alyami N., Alothman N.S., Alakhtani S., Alarifi S. Tantalum Doped TiO2 Nanoparticles Induced Cytotoxicity and DNA Damage through ROS Generation in Human Neuroblastoma Cells. J. King Saud Univ.-Sci. 2021;33:101546. doi: 10.1016/j.jksus.2021.101546. [DOI] [Google Scholar]
- 64.Turkez H., Cakmak B., Celik K. Evaluation of the Potential in Vivo Genotoxicity of Tungsten (VI) Oxide Nanopowder for Human Health. Key Eng. Mater. 2013;543:89–92. doi: 10.4028/www.scientific.net/KEM.543.89. [DOI] [Google Scholar]
- 65.Dogra V., Kaur G., Kumar R., Kumar S. Toxicity Assessment of Palladium Oxide Nanoparticles Derived from Metallosurfactants Using Multi Assay Techniques in Allium Sativum. Colloids Surf. B Biointerfaces. 2020;187:110752. doi: 10.1016/j.colsurfb.2019.110752. [DOI] [PubMed] [Google Scholar]
- 66.Brown A.L., Winter H., Goforth A.M., Sahay G., Sun C. Facile Synthesis of Ligand-Free Iridium Nanoparticles and Their In Vitro Biocompatibility. Nanoscale Res. Lett. 2018;13:208. doi: 10.1186/s11671-018-2621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith I.C., Carson B.L., Ferguson T.L. Osmium: An Appraisal of Environmental Exposure. Environ. Health Perspect. 1974;8:201–213. doi: 10.1289/ehp.748201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pawlak J., Łodyga-Chruścińska E., Chrustowicz J. Fate of Platinum Metals in the Environment. J. Trace Elem. Med. Biol. 2014;28:247–254. doi: 10.1016/j.jtemb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Field J.A., Luna-Velasco A., Boitano S.A., Shadman F., Ratner B.D., Barnes C., Sierra-Alvarez R. Cytotoxicity and Physicochemical Properties of Hafnium Oxide Nanoparticles. Chemosphere. 2011;84:1401–1407. doi: 10.1016/j.chemosphere.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 70.Haase A.A., Bauer E.B., Kühn F.E., Crans D.C. Speciation and Toxicity of Rhenium Salts, Organometallics and Coordination Complexes. Coord. Chem. Rev. 2019;394:135–161. doi: 10.1016/j.ccr.2019.05.012. [DOI] [Google Scholar]
- 71.Dsouki N.A., de Lima M.P., Corazzini R., Gáscon T.M., Azzalis L.A., Junqueira V.B.C., Feder D., Fonseca F.L.A. Cytotoxic, Hematologic and Histologic Effects of Niobium Pentoxide in Swiss Mice. J. Mater. Sci. Mater. Med. 2014;25:1301–1305. doi: 10.1007/s10856-014-5153-0. [DOI] [PubMed] [Google Scholar]
- 72.Chen X.-L., Xu Y.-M., Lau A.T.Y. Toxic Metals in the Regulation of Epithelial–Mesenchymal Plasticity: Demons or Angels? Cancer Cell Int. 2022;22:237. doi: 10.1186/s12935-022-02638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slama H.B., Chenari Bouket A., Pourhassan Z., Alenezi F.N., Silini A., Cherif-Silini H., Oszako T., Luptakova L., Golińska P., Belbahri L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021;11:6255. doi: 10.3390/app11146255. [DOI] [Google Scholar]
- 74.Wu S., Peng L., Fu F., Feng Y., He J., Wang H. Response Surface Methodology Approach for Dyeing Process Optimization of Ayous (Triplochiton scleroxylon) Wood with Acid Dye. Eur. J. Wood Wood Prod. 2023;81:1045–1058. doi: 10.1007/s00107-023-01939-2. [DOI] [Google Scholar]
- 75.Maneval W.E. Staining Bacteria and Yeasts with Acid Dyes. Stain Technol. 1941;16:13–19. doi: 10.3109/10520294109106189. [DOI] [Google Scholar]
- 76.İyim T.B., Güçlü G. Removal of Basic Dyes from Aqueous Solutions Using Natural Clay. Desalination. 2009;249:1377–1379. doi: 10.1016/j.desal.2009.06.020. [DOI] [Google Scholar]
- 77.Chung K.-T. Azo Dyes and Human Health: A Review. J. Environ. Sci. Health Part C. 2016;34:233–261. doi: 10.1080/10590501.2016.1236602. [DOI] [PubMed] [Google Scholar]
- 78.Chequer F.M.D., Dorta D.J., de Oliveira D.P. Azo Dyes and Their Metabolites: Does the Discharge of the Azo Dye into Water Bodies Represent Human and Ecological Risks. Adv. Treat. Text. Effl. 2011;48:28–48. [Google Scholar]
- 79.Geldasa F.T., Kebede M.A., Shura M.W., Hone F.G. Experimental and Computational Study of Metal Oxide Nanoparticles for the Photocatalytic Degradation of Organic Pollutants: A Review. RSC Adv. 2023;13:18404–18442. doi: 10.1039/D3RA01505J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velusamy S., Roy A., Sundaram S., Kumar Mallick T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021;21:1570–1610. doi: 10.1002/tcr.202000153. [DOI] [PubMed] [Google Scholar]
- 81.Sudarshan S., Harikrishnan S., RathiBhuvaneswari G., Alamelu V., Aanand S., Rajasekar A., Govarthanan M. Impact of Textile Dyes on Human Health and Bioremediation of Textile Industry Effluent Using Microorganisms: Current Status and Future Prospects. J. Appl. Microbiol. 2023;134:lxac064. doi: 10.1093/jambio/lxac064. [DOI] [PubMed] [Google Scholar]
- 82.Lechuga M., Avila-Sierra A., Lobato-Guarnido I., García-López A.I., Ríos F., Fernández-Serrano M. Mitigating the Skin Irritation Potential of Mixtures of Anionic and Non-Ionic Surfactants by Incorporating Low-Toxicity Silica Nanoparticles. J. Mol. Liq. 2023;383:122021. doi: 10.1016/j.molliq.2023.122021. [DOI] [Google Scholar]
- 83.Hemashenpagam N., Selvajeyanthi S. Nanohybrid Materials for Treatment of Textiles Dyes. Springer; Berlin/Heidelberg, Germany: 2023. Textile Dyes and Their Effect on Human Beings; pp. 41–60. [Google Scholar]
- 84.Gharavi-Nakhjavani M.S., Niazi A., Hosseini H., Aminzare M., Dizaji R., Tajdar-Oranj B., Mirza Alizadeh A. Malachite Green and Leucomalachite Green in Fish: A Global Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. 2023;30:48911–48927. doi: 10.1007/s11356-023-26372-z. [DOI] [PubMed] [Google Scholar]
- 85.Muthukumar M., Sargunamani D., Senthilkumar M., Selvakumar N. Studies on Decolouration, Toxicity and the Possibility for Recycling of Acid Dye Effluents Using Ozone Treatment. Dye Pigment. 2005;64:39–44. doi: 10.1016/j.dyepig.2004.03.012. [DOI] [Google Scholar]
- 86.Kanwal S., Irfan A., Al-Hussain S.A., Sharif G., Mumtaz A., Batool F., Zaki M.E.A. Fabrication of Composites of Sodium Alginate with Guar Gum and Iron Coated Activated Alumina for the Purification of Water from Direct Blue 86. Coatings. 2023;13:103. doi: 10.3390/coatings13010103. [DOI] [Google Scholar]
- 87.Wang W., Zhao P., Hu Y., Zan R. Application of Weak Magnetic Field Coupling with Zero-Valent Iron for Remediation of Groundwater and Wastewater: A Review. J. Clean. Prod. 2020;262:121341. doi: 10.1016/j.jclepro.2020.121341. [DOI] [Google Scholar]
- 88.Zhou Y., Zhou L., Zhang Y., Zhu G., Qin B., Jang K.-S., Spencer R.G.M., Kothawala D.N., Jeppesen E., Brookes J.D., et al. Unraveling the Role of Anthropogenic and Natural Drivers in Shaping the Molecular Composition and Biolability of Dissolved Organic Matter in Non-Pristine Lakes. Environ. Sci. Technol. 2022;56:4655–4664. doi: 10.1021/acs.est.1c08003. [DOI] [PubMed] [Google Scholar]
- 89.Covich A.P. In: Energy Flow and Ecosystems. 2nd ed. Levin S.A.B.T.-E., editor. Academic Press; Waltham, MA, USA: 2013. pp. 237–249. [Google Scholar]
- 90.Chacón L., Reyes L., Rivera-Montero L., Barrantes K. Chapter 5—Transport, Fate, and Bioavailability of Emerging Pollutants in Soil, Sediment, and Wastewater Treatment Plants: Potential Environmental Impacts. In: Sarma H., Dominguez D.C., Lee W.-Y.B.T.-E.C., editors. Emerging Contaminants in the Environment. Elsevier; Amsterdam, The Netherlands: 2022. pp. 111–136. [Google Scholar]
- 91.Xu J.-W., Ding Y.-D., Li S.-L., Mao R. Amount and Biodegradation of Dissolved Organic Matter Leached from Tree Branches and Roots in Subtropical Plantations of China. For. Ecol. Manag. 2021;484:118944. doi: 10.1016/j.foreco.2021.118944. [DOI] [Google Scholar]
- 92.Xenopoulos M.A., Barnes R.T., Boodoo K.S., Butman D., Catalán N., D’Amario S.C., Fasching C., Kothawala D.N., Pisani O., Solomon C.T., et al. How Humans Alter Dissolved Organic Matter Composition in Freshwater: Relevance for the Earth’s Biogeochemistry. Biogeochemistry. 2021;154:323–348. doi: 10.1007/s10533-021-00753-3. [DOI] [Google Scholar]
- 93.Barbosa Jr F., Rocha B.A., Souza M.C.O., Bocato M.Z., Azevedo L.F., Adeyemi J.A., Santana A., Campiglia A.D. Polycyclic Aromatic Hydrocarbons (PAHs): Updated Aspects of Their Determination, Kinetics in the Human Body, and Toxicity. J. Toxicol. Environ. Health Part B. 2023;26:28–65. doi: 10.1080/10937404.2022.2164390. [DOI] [PubMed] [Google Scholar]
- 94.Emoyoma U.O., Ezejiofor A.N., Frazzoli C., Bocca B., Ekhator O.C., Onyena A.P., Udom G.J., Orisakwe O.E. Polycyclic Aromatic Hydrocarbons in Fish (Fresh and Dried) and Public Health in Nigeria: A Systematic Review. Int. J. Environ. Health Res. 2023:1–16. doi: 10.1080/09603123.2023.2230915. [DOI] [PubMed] [Google Scholar]
- 95.Chakraborty B.K. Effect of Pesticide and Heavy Metal Toxicants on Fish and Human Health. J. Crop Weed. 2023;19:1–7. doi: 10.22271/09746315.2023.v19.i1.1669. [DOI] [Google Scholar]
- 96.Chatterjee N., Kim C., Im J., Kim S., Choi J. Mixture and Individual Effects of Benzene, Toluene, and Formaldehyde in Zebrafish (Danio Rerio) Development: Metabolomics, Epigenetics, and Behavioral Approaches. Environ. Toxicol. Pharmacol. 2023;97:104031. doi: 10.1016/j.etap.2022.104031. [DOI] [PubMed] [Google Scholar]
- 97.Ball A.L., Solan M.E., Franco M.E., Lavado R. Comparative Cytotoxicity Induced by Parabens and Their Halogenated Byproducts in Human and Fish Cell Lines. Drug Chem. Toxicol. 2023;46:786–794. doi: 10.1080/01480545.2022.2100900. [DOI] [PubMed] [Google Scholar]
- 98.Zhang K., Chang S., Fu Q., Sun X., Fan Y., Zhang M., Tu X., Qadeer A. Occurrence and Risk Assessment of Volatile Organic Compounds in Multiple Drinking Water Sources in the Yangtze River Delta Region, China. Ecotoxicol. Environ. Saf. 2021;225:112741. doi: 10.1016/j.ecoenv.2021.112741. [DOI] [PubMed] [Google Scholar]
- 99.Brinkmann M., Petersen S., Pelletier A., Bryshun L., Schaefer N., Barnes M., Doig L., Strickert G., Jardine T. Multiple Lines of Evidence to Assess Risk from Dioxins and Dioxin-like Chemicals in Sediment and Fish from Waterbodies along a Large Prairie River. J. Soils Sediments. 2023;24:414–424. doi: 10.1007/s11368-023-03620-w. [DOI] [Google Scholar]
- 100.Rodriguez C., Cook A., Devine B., Van Buynder P., Lugg R., Linge K., Weinstein P. Dioxins, Furans and PCBs in Recycled Water for Indirect Potable Reuse. Int. J. Environ. Res. Public Health. 2008;5:356–367. doi: 10.3390/ijerph5050356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dueñas-Moreno J., Vázquez-Tapia I., Mora A., Cervantes-Avilés P., Mahlknecht J., Capparelli M.V., Kumar M., Wang C. Occurrence, Ecological and Health Risk Assessment of Phthalates in a Polluted Urban River Used for Agricultural Land Irrigation in Central Mexico. Environ. Res. 2024;240:117454. doi: 10.1016/j.envres.2023.117454. [DOI] [PubMed] [Google Scholar]
- 102.Prasad G.S., Rout S.K., Malik M.M., Karmakar S., Amin A., Ahmad I. Occurrence of Xenoestrogen Alkylphenols (Octylphenols and Nonylphenol) and Its Impact on the Aquatic Ecosystem. In: Rather M.A., Amin A., Hajam Y.A., Jamwal A., Ahmad I., editors. Xenobiotics in Aquatic Animals: Reproductive and Developmental Impacts. Springer Nature; Singapore: 2023. pp. 275–284. [Google Scholar]
- 103.Kumar V., Sharma N., Sharma P., Pasrija R., Kaur K., Umesh M., Thazeem B. Toxicity Analysis of Endocrine Disrupting Pesticides on Non-Target Organisms: A Critical Analysis on Toxicity Mechanisms. Toxicol. Appl. Pharmacol. 2023;474:116623. doi: 10.1016/j.taap.2023.116623. [DOI] [PubMed] [Google Scholar]
- 104.Singh G., Singh A., Singh P., Gupta A., Shukla R., Mishra V.K. Chapter 29—Sources, Fate, and Impact of Pharmaceutical and Personal Care Products in the Environment and Their Different Treatment Technologies. In: Kumar A., Singh V.K., Singh P., Mishra V.K.B.T.-M.M.R., editors. Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing; Sawston, UK: 2021. pp. 391–407. [Google Scholar]
- 105.Thorel E., Clergeaud F., Jaugeon L., Rodrigues A.M.S., Lucas J., Stien D., Lebaron P. Effect of 10 UV Filters on the Brine Shrimp Artemia Salina and the Marine Microalga Tetraselmis sp. Toxics. 2020;8:29. doi: 10.3390/toxics8020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hamid N., Junaid M., Manzoor R., Sultan M., Chuan O.M., Wang J. An Integrated Assessment of Ecological and Human Health Risks of Per- and Polyfluoroalkyl Substances through Toxicity Prediction Approaches. Sci. Total Environ. 2023;905:167213. doi: 10.1016/j.scitotenv.2023.167213. [DOI] [PubMed] [Google Scholar]
- 107.Lee J., Lee S., Jiang X. Cyanobacterial Toxins in Freshwater and Food: Important Sources of Exposure to Humans. Annu. Rev. Food Sci. Technol. 2017;8:281–304. doi: 10.1146/annurev-food-030216-030116. [DOI] [PubMed] [Google Scholar]
- 108.Ma F., Wan Y., Yuan G., Meng L., Dong Z., Hu J. Occurrence and Source of Nitrosamines and Secondary Amines in Groundwater and Its Adjacent Jialu River Basin, China. Environ. Sci. Technol. 2012;46:3236–3243. doi: 10.1021/es204520b. [DOI] [PubMed] [Google Scholar]
- 109.Rudneva I.I., Omel’chenko S.O. Nitrosamines in Aquatic Ecosystems: Sources, Formation, Toxicity, Environmental Risk (Review). 2. Content In Aquatic Biota, Biological Effects and Risk Assessment. Water Resour. 2021;48:291–299. doi: 10.1134/S0097807821020135. [DOI] [Google Scholar]
- 110.Wilburn W., Guha S., Beni R. FDA Ban on Triclosan Leads to Major Changes in Levels of Trihalomethanes in Drinking Water Sources across the United States. Voice Publ. 2023;9:173–195. doi: 10.4236/vp.2023.93015. [DOI] [Google Scholar]
- 111.Wang Q., Lam J.C.W., Han J., Wang X., Guo Y., Lam P.K.S., Zhou B. Developmental Exposure to the Organophosphorus Flame Retardant Tris(1,3-Dichloro-2-Propyl) Phosphate: Estrogenic Activity, Endocrine Disruption and Reproductive Effects on Zebrafish. Aquat. Toxicol. 2015;160:163–171. doi: 10.1016/j.aquatox.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Lan Y., Gao X., Xu H., Li M. 20 Years of Polybrominated Diphenyl Ethers on Toxicity Assessments. Water Res. 2024;249:121007. doi: 10.1016/j.watres.2023.121007. [DOI] [PubMed] [Google Scholar]
- 113.Lee J.-D., Chiou T.-H., Zhang H.-J., Chao H.-R., Chen K.-Y., Gou Y.-Y., Huang C.-E., Lin S.-L., Wang L.-C. Persistent Halogenated Organic Pollutants in Deep-Water-Deposited Particulates from South China Sea. Toxics. 2023;11:968. doi: 10.3390/toxics11120968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Russo C., Maugeri A., Albergamo A., Dugo G., Navarra M., Cirmi S. Protective Effects of a Red Grape Juice Extract against Bisphenol A-Induced Toxicity in Human Umbilical Vein Endothelial Cells. Toxics. 2023;11:391. doi: 10.3390/toxics11040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Elizalde-Velázquez G.A., Gómez-Oliván L.M., Herrera-Vázquez S.E., Rosales-Pérez K.E., SanJuan-Reyes N., García-Medina S., Galar-Martínez M. Acute Exposure to Realistic Concentrations of Bisphenol—A Trigger Health Damage in Fish: Blood Parameters, Gene Expression, Oxidative Stress. Aquat. Toxicol. 2023;261:106610. doi: 10.1016/j.aquatox.2023.106610. [DOI] [PubMed] [Google Scholar]
- 116.Kim D.-H., Choi S., Park J., Kim K., Oh J.-E. Phenolic Compounds in the Freshwater Environment in South Korea: Occurrence and Tissue-Specific Distribution. Sci. Total Environ. 2023;905:166914. doi: 10.1016/j.scitotenv.2023.166914. [DOI] [PubMed] [Google Scholar]
- 117.Caporossi L., Viganò P., Paci E., Capanna S., Alteri A., Campo G., Pigini D., De Rosa M., Tranfo G., Papaleo B. Female Reproductive Health and Exposure to Phthalates and Bisphenol A: A Cross Sectional Study. Toxics. 2021;9:299. doi: 10.3390/toxics9110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santos S.G., Souza M.C.O., Barbosa-Junior F., Prodocimo M.M., Marcondes F.R., Almeida W., Cestari M.M., Souza-Bastos L.R., Martino-Andrade A.J., Guiloski I.C. Evaluation of the Toxicity of Di-Iso-Pentyl-Phthalate (DiPeP) Using the Fish Danio Rerio as an Experimental Model. Environ. Sci. Pollut. Res. 2023;30:27996–28009. doi: 10.1007/s11356-022-24071-9. [DOI] [PubMed] [Google Scholar]
- 119.Makame K.R., Masese S.N., Ádám B., Nagy K. Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells. Toxics. 2023;11:976. doi: 10.3390/toxics11120976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azadikhah D., Varcheh M., Yalsuyi A.M., Forouhar Vajargah M., Mansouri Chorehi M., Faggio C. Hematological and Histopathological Changes of Juvenile Grass Carp (Ctenopharyngodon idella) Exposed to Lethal and Sublethal Concentrations of Roundup (Glyphosate 41% SL) Aquac. Res. 2023;2023:4351307. doi: 10.1155/2023/4351307. [DOI] [Google Scholar]
- 121.Shen M., Hu Y., Zhao K., Li C., Liu B., Li M., Lyu C., Sun L., Zhong S. Occurrence, Bioaccumulation, Metabolism and Ecotoxicity of Fluoroquinolones in the Aquatic Environment: A Review. Toxics. 2023;11:966. doi: 10.3390/toxics11120966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geremia E., Muscari Tomajoli M.T., Murano C., Petito A., Fasciolo G. The Impact of Micro-and Nanoplastics on Aquatic Organisms: Mechanisms of Oxidative Stress and Implications for Human Health—A Review. Environments. 2023;10:161. doi: 10.3390/environments10090161. [DOI] [Google Scholar]
- 123.Jesus F., Tremblay L.A. Key Challenges to the Effective Management of Pollutants in Water and Sediment. Toxics. 2022;10:219. doi: 10.3390/toxics10050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shannon M.A., Bohn P.W., Elimelech M., Georgiadis J.G., Mariñas B.J., Mayes A.M. Science and Technology for Water Purification in the Coming Decades. Nature. 2008;452:301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]