Abstract
Cyclin C, a putative G1 cyclin, was originally isolated through its ability to complement a Saccharomyces cerevisiae strain lacking the G1 cyclin gene CLN1-3. Unlike cyclins D1 and E, the other two G1 cyclins obtained by the same approach and subsequently shown to play important roles during the G1/S transition, there is thus far no evidence to support the hypothesis that cyclin C is indeed critical for the promotion of cell cycle progression. In BAF-B03 cells, an interleukin 3 (IL-3)-dependent murine pro-B-cell line, cyclin C gene mRNA was induced at the G1/S phase upon IL-3 stimulation and reached a maximal level in the S phase. Enforced expression of exogenous cyclin C in this cell line failed to alter its growth properties. In the present study, we examined whether cyclin C is capable of cooperating with the cytokine-responsive immediate-early gene products c-Myc and c-Fos in the promotion of cell proliferation. We found that cyclin C is able to cooperate functionally with c-Myc, but not c-Fos, to induce both BAF-B03 cell proliferation in a cytokine-independent fashion and the formation of cell clusters. Furthermore, cyclin C was primarily responsible for the induction of cdc2 gene expression. Our data define a novel role for cyclin C in the regulation of both the G1/S and G2/M phases of the cell cycle, and this effect appears to be independent of the activity of CDK8 in the control of transcription.
Cyclins are a conserved family of proteins required for the activation of a class of protein kinases termed CDKs (cyclin-dependent kinases). Originally, cyclins were described as proteins whose abundance oscillated during the cell cycle (13). The first cyclin to be induced after mammalian cells are released from a quiescent state is a D-type cyclin. D-type cyclin (D1, D2, and D3) is generally highly growth factor inducible, is expressed continuously in response to growth factors, and may act as sensor of proliferative signals (32, 55). D-type cyclin appears in different combinations in various cell types (34, 40) and pairs primarily with CDK4 and CDK6 (33, 36). Microinjection of anti-cyclin D1 antibodies into normal fibroblasts during G1 prevents cells from entering the S phase, indicating that cyclin D1 is required for the G1/S transition (3, 51). Cyclin E is synthesized later in the G1 phase and forms a complex with CDK2 (11, 24). It is thought to act subsequently to D-type cyclins as a rate-limiting factor at the G1/S transition and to be required for the initiation of DNA replication (43, 47). Expression of cyclin A is induced shortly after that of cyclin E (50). Cyclin A assembles with both CDK2 and CDC2, and these complexes appear to play roles in both the S and G2 phases of the cell cycle (18, 46). Synthesis and destruction of cyclin B oscillate behind that of cyclin A during the cell cycle (37), and cyclin B-CDC2 complexes are thought to be important for promoting entry into the M phase (42).
Other cyclins have been identified, but their roles in cell cycle control, if any, remain to be determined. The best characterized of these distinct cyclins is cyclin H, which associates with CDK7, also known as CAK (CDK-activating kinase). CAK is able to phosphorylate the catalytic subunit of various CDKs at the residue equivalent to Thr161 of CDC2 to bring about activation of the different cyclin-CDK complexes (14, 15). However, the activity of CAK does not change in a cell cycle-dependent manner, and CAK activity is present even in quiescent cells. Interestingly, the cyclin H-CDK7 complex is also found within the basic transcription factor TFIIH and is capable of phosphorylating the C-terminal domain (CTD) of RNA polymerase II (Pol II) (57). It appears that the cyclin H-CDK7 complex may be involved in the processes of the cell cycle, transcription and DNA repair, although the precise role of this complex remains to be elucidated. Cyclin F was isolated by virtue of its ability to suppress the G1/S deficiency of a Saccharomyces cerevisiae cdc4 mutation. Its abundance is altered during the cell cycle and peaks in the G2 phase (2). Cyclin G has been suggested to be a transcriptional target of the p53 tumor suppressor gene, and perhaps it is also involved in DNA replication or repair (44, 58). Cyclin I has recently been cloned, but no function has been ascribed to it to date (41).
Cyclin C was originally isolated through its ability to complement an S. cerevisiae strain lacking the G1 cyclin gene CLN1-3 (26, 28, 29) and was assumed to be a G1 cyclin based on the finding that its expression increased during the G1 phase in mammalian cells. Although cyclins D1 and E, two other G1 cyclins, were identified by the same approach and were subsequently demonstrated to play important roles during the G1/S transition, thus far there have been no direct observations supporting a critical role for cyclin C in the promotion of cell cycle progression. However, several lines of evidence have implied that cyclin C may be important for the regulation of cell proliferation. For instance, (i) the highly conserved sequence similarity among different species (28) suggested that the function of cyclin C might also be conserved; (ii) other cyclins, such as D1, E, A, and B, which are capable of complementing yeast CLN deficiencies, have been proven to function in cell cycle control; and (iii) cyclin C is expressed in response to growth factor stimulation and oscillates throughout the cell cycle (29). Intriguingly, it has recently been reported that cyclin C binds to a novel CDK, CDK8 (59), and that this complex possesses kinase activity toward the CTD of RNA Pol II. It was further demonstrated that the cyclin C-CDK8 complex can associate in vivo with the large subunit of RNA Pol II (52), suggesting a potential role in the regulation of transcription. However, it is unclear whether the formation of a complex with CDK8 to regulate transcription represents the exclusive function of cyclin C.
As an approach to gain further insights into the potential roles of cyclin C in the regulation of cell cycle progression, we have examined the effect of overexpression of exogenous cyclin C on the growth properties of BAF-B03 cells, an interleukin-3 (IL-3)-dependent murine pro-B-cell line, particularly with respect to its ability, by itself or in cooperation with the other cytokine-responsive immediate-early gene products, c-Myc and c-Fos, to induce this cell line to be factor independent. Our experimental approach has allowed us to reveal that cyclin C, although insufficient by itself, is able to cooperate functionally with c-Myc, but not c-Fos, to promote both the proliferation of BAF-B03 cells in a cytokine-independent fashion and the formation of cell clusters. Moreover, we demonstrate that cyclin C is primarily responsible for the superinduction of cdc2 mRNA, a gene which is essential for G2/M-phase regulation. Our data represent the first observation of the functions of cyclin C in the regulation of the cell cycle and cell adhesion and suggest that cyclin C may play roles in regulating both the G1/S and G2/M phases of the cell cycle.
MATERIALS AND METHODS
Cells and cell culture.
BAF-B03, a subclone of the BA/F3 cell line, is a bone marrow-derived murine IL-3-dependent pro-B-cell line (7, 48). BC cells were established by transfection of a human cyclin C gene expression plasmid (Rc-cycC [10]; kindly provided by R. A. Weinberg) into BAF-B03 cells; BM cells were obtained by transfection of the human c-myc expression plasmid, pN-LTRmyc (4); BF cells are another line of BAF-B03-derived clones that were obtained by transfection of human c-fos (Rc-fos). BMC and BFC clones were established by transfection of a human cyclin C gene expression plasmid into pooled BM and BF cells, respectively. Drug-resistant clones were selected and designated BMC cells (for c-Myc and cyclin C coexpressing cells) and BFC cells (for c-Fos and cyclin C coexpressing cells), respectively. BEMC cells were obtained by cotransfection of Rc-cycC and pN-LTRmyc into BER2 cells (abbreviated here as BE) (56). B-MycER (BE-MycER) or B-Control (BE-Control) cells were established by retrovirus-mediated gene transfer of MycER or vector alone into BAF-B03 (B) or BER2 (BE) cells. BMC-k8WT and BMC-k8AMG cells were established by transfection of wild-type (pCMV-cdk8) and mutant (pCMV-cdk8AMG) plasmids of human CDK8 (both were tagged with a Myc epitope, MEQKLISEEDLNMN-M [CDK8], kindly provided by E. A. Nigg) into BMC cells (three mixed clones), respectively.
BMC, BEMC, BMC-k8WT, and BMC-k8AMG cells were maintained in RPMI 1640 medium (Nissui) containing 0.03% glutamine (Wako), 100 μg of kanamycin per ml, 10 mM HEPES, 10 mM modified Eagle’s medium (MEM) nonessential amino acids solution (Gibco), and 100 mM MEM sodium pyruvate solution (Gibco) supplemented with 10% (vol/vol) fetal calf serum (FCS; JRH Biosciences); other cells were cultured in the same medium containing 10% (vol/vol) WEHI-3B cell supernatant as a source of IL-3. (WEHI-3B cells were plated at 105/ml and cultured with RPMI 1640 medium for 2 to 3 days at 37°C until the cells were close to confluent; the supernatants were then harvested and filtered.) To examine whether the autocrine production of growth factors is occurring, BMC cells were plated at 105/ml and cultured with RPMI 1640 medium for 2 to 3 days at 37°C until the cells were close to confluent; filtered supernatants were then used to culture other cells. To analyze gene expression, cells were synchronized in the G1 phase by depriving them of cytokines for 15 h and restimulating them with IL-3 (10% [vol/vol] WEHI-3B cell supernatant).
DNA transfection.
Plasmid DNAs were transfected into cells by an electroporation procedure as described previously (9), except for B-MycER (BE-MycER) or B-Control (BE-Control) cells, which were established by retrovirus-mediated gene transfer (25, 31) with MycER in the retroviral packaging GP+E-86 cell line. Selection was initiated 48 h after retrovirus-mediated gene transfer and 24 h after DNA transfection, with 2 mg of G418 per ml for BC, BMC, and BFC cells; 1 mg of hygromycin per ml for BM and BF cells; or 0.75 μg of puromycin per ml for BEMC, BMC-k8WT, BMC-k8AMG, B-MycER (BE-MycER), or B-Control (BE-Control) cells. Drug-resistant clones were either pooled or subsequently cloned by limiting dilution as described previously (38).
Cell cycle analysis.
Cell cycle analysis was performed according to the protocol recommended by JASCO. In brief, cells were harvested and washed with phosphate-buffered saline (PBS) and then fixed in 50% methanol at −20°C overnight. After being washed with 30% methanol (−20°C) and ice-cold PBS, samples were treated with 1 mg of RNase per ml (0.2 M PBS [pH 7.2]) at 37°C for 30 min. Following a wash with PBS, samples were stained in PBS solution containing 50 μg of propidium iodide per ml at 4°C for 2 h. The fluorescence intensity of each cell nucleus was measured by flow cytometry (Cyto ACE-300; JASCO). The percentages of cells in each phase of the cell cycle were determined by analysis with software provided with the Cyto ACE-300 flow cytometer.
Preparation of probe DNA.
The probe DNAs for the cyclin D1, D2, D3, C, and E genes were prepared as follows. For the cyclin D1 gene, a 1.2-kb HindIII fragment was excised from Rc-cycD1 (10). For the cyclin D3 gene, an ∼1.0-kb EcoRI and XbaI fragment was excised from pBluescript-cycD3. For the cyclin C gene, a 0.9-kb HindIII and XbaI fragment was excised from Rc-cycC (10). For the cyclin E gene, an ∼0.6-kb C-terminal fragment was obtained by PCR (8). Probes for the cyclin D2, A, and B and cdc2, cdk2, and c-myc genes were prepared as described previously (56).
RNA extraction and Northern blot analysis.
Cells were harvested at the indicated time points, and total RNA was prepared by guanidinium thiocyanate-CsCl centrifugation or by using the ISOGEN RNA preparation kit according to the manufacturer’s instructions (Wako). Ten micrograms of RNA was electrophoresed through 1% agarose formaldehyde gels and transferred onto nylon membranes. Probes were labeled with [α-32P]dCTP by using the Multiprimer labeling kit (Amersham) and were hybridized as described previously (38). Specific activity was approximately 106 cpm/ng for all probe DNAs. 28S rRNA was visualized by the staining of a filter with methylene blue. In some cases, membranes were rehybridized after the previous probe had been removed.
Western blot analysis.
Cells (5 × 106) were harvested and solubilized in lysis buffer (50 mM Tris-HCl [pH 7.4], 0.5% [vol/vol] Nonidet P-40, 150 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml) by sonication. Soluble lysates were separated by centrifugation at 10,000 × g for 10 min. Protein was quantified with the Bio-Rad DC protein assay kit. For Western blot analysis, samples containing equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide (10%) gel electrophoresis. Separated proteins were transferred onto polyvinylidene difluoride membranes (Immobilon; Millipore). After being blocked with TBST-milk (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.5% [vol/vol] Tween 20, 5% nonfat dry milk), membranes were incubated with anti-human c-Myc, -c-Fos (Santa Cruz), -cyclin C, and -CDK8 antibodies (kindly provided by E. Lees) (1:1,000 dilution in TBST–0.5% milk) overnight at 4°C. The membranes were then washed with TBST and incubated with fluorescein isothiocyanate-conjugated secondary antibodies (1:3,000 dilution in TBST–0.5% milk) for 1 h at room temperature. After three washes in TBST, proteins were detected with the ECL (enhanced chemiluminescence) kit according to the manufacturer’s instructions (Amersham).
Luciferase assay.
Twenty-five micrograms of human cyclin A- and cdc2 promoter-luciferase reporter plasmids (provided by T. L. Born) were cotransfected with a pRL-TK control plasmid (25:1) into different cell lines (2 × 105 cells) by the DEAE-dextran method as described previously (22). The transfected cells were divided into two dishes and then cultured in RPMI 1640 supplemented with 10% FCS for 12 h. Growth factor-starved cells were stimulated with culture medium alone or with mouse IL-3 (10% [vol/vol] WEHI-3B-conditioned medium) for an additional 10 h as described previously (23). Preparation of cell extracts and detection of luciferase activity were carried out with the dual-luciferase reporter assay system kit according to the manufacturer’s instructions (Promega). Equal amounts of lysate were subjected to analysis, and amounts of protein were determined with the Bio-Rad DC protein assay kit.
Actin polymerization.
The presence of F-actin was detected as described previously (1). In brief, cells (106/ml) were fixed on slides and then permeabilized. F-actin was detected by being stained with rhodamine-phalloidin and was analyzed with a Nikon Microphot-FX microscope.
Nuclear run-on assay.
BAF-B03 and BMC cells were cultured in RPMI 1640 medium containing 10% WEHI-3B supernatant as a source of IL-3. Some of the cells were harvested, and the other cells were washed with PBS three times to get rid of IL-3, cultured in complete RPMI 1640 for 12 h, and then harvested. A total of 5 × 107 cells were washed with ice-cold PBS, and pellets were suspended in 4 ml of ice-cold sucrose buffer I (0.32 M sucrose, 3 mM CaCl2, 2 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-Cl [pH 8.0], 1 mM dithiothreitol, 0.5% [vol/vol] Nonidet P-40). After the cells had been uniformly lysed, 4 μl of sucrose buffer II (2 M sucrose, 5 mM magnesium acetate, 0.1 mM EDTA, 10 mM Tris-Cl [pH 8.0], 1 mM dithiothreitol) was added, and then this mixture was layered onto the sucrose cushion in polyallomer SW41 tube. The gradient was centrifuged at 30,000 × g for 45 min, the supernatant was removed, and then the nuclear pellets were resuspended with glycerol storage buffer and stored in liquid nitrogen. The run-on transcription assay was performed as described elsewhere (19). A total of 5 × 106 cpm of 32P-labeled transcripts per ml was hybridized to human cdc2 and human β-actin DNA immobilized on nitrocellulose. The radioactivity was visualized by autoradiography.
Cell growth assay and viability assay.
For the cell growth assay, factor-starved cells were cultured at a density of 5 × 105 cells/ml in RPMI 1640 supplemented with 10% FCS and with 10% (vol/vol) WEHI-3B-conditioned medium or without WEHI-3B-conditioned medium for factor-independent cells. The culture medium was changed every other day. For the cell viability assay, factor-starved cells were cultured in RPMI 1640 supplemented with 10% FCS without WEHI-3B-conditioned medium. In both assays, viable cell numbers were determined by trypan blue exclusion assay as described previously (39).
RESULTS
Cyclin C gene mRNA was induced at the G1/S phase and peaked in the S phase upon IL-3 stimulation of BAF-B03 cells.
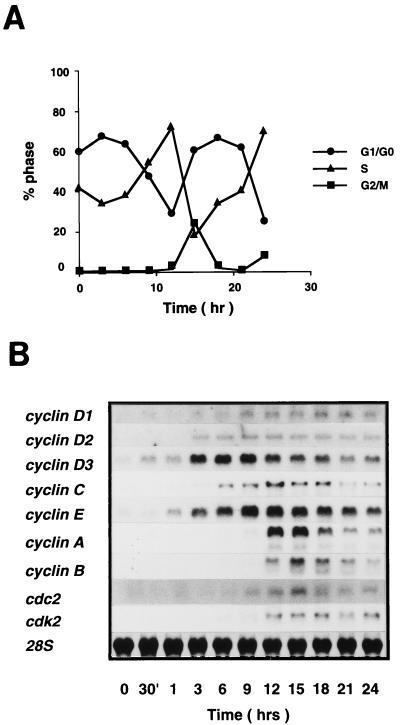
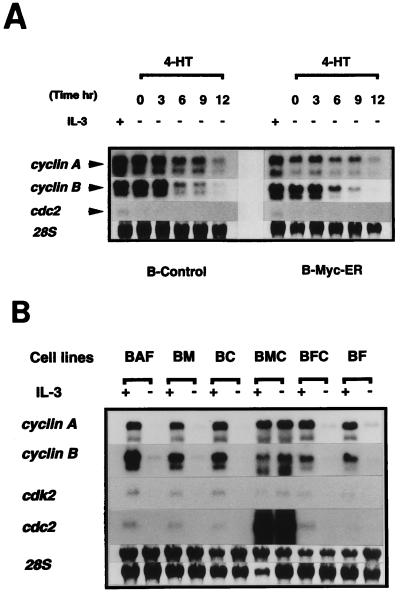
To study the potential role of cyclin C in the regulation of the hematopoietic cell cycle, the pattern of cyclin C mRNA induction upon cytokine stimulation was examined. Cell cycle progression of BAF-B03 cells was first monitored under our experimental conditions. When cells were starved of IL-3 for 15 h, the cells arrested in the early G1 phase. Upon IL-3 stimulation, the cells began to replicate their DNA within 9 h, the percentage of cells in the S phase reached maximum around 12 h, and the cells entered the G2/M phase at 15 h. By 18 h, the majority of cells had returned to G1 (Fig. 1A). RNA blot analysis was next performed to determine in which phase the cyclin C gene was induced compared to other cyclin, cdc2, or cdk2 genes. As summarized in Fig. 1B, mRNAs for all cyclin, cdc2, and cdk2 genes could be induced upon IL-3 stimulation. Expression of the cyclin C gene was induced at the mid- to late G1 phase, reached a peak during the S phase, and remained high throughout the G2/M phase, suggesting that cyclin C may play roles at the G1/S- and/or G2/M-phase transitions. Mitotic cyclin A and B, cdc2, and cdk2 genes were induced to maximal levels at the G2/M phase, while the cyclin D3 gene was induced during the early G1 phase. The cyclin E, D1, and D2 genes were induced rather later in the G1 phase than the cyclin D3 gene was.
FIG. 1.
(A) Cell cycle analysis of BAF-B03 cells following IL-3 stimulation. Cells were synchronized by growth factor starvation for 15 h and restimulated with IL-3. Samples were harvested at various times after stimulation, stained with propidium iodide, and analyzed by flow cytometry as described in Materials and Methods. The calculated percentages of cells at each phase are plotted. (B) Differential expression of cyclin and cdc2 family kinase genes in BAF-B03-derived transformants stimulated with IL-3. Stimulated cells were harvested at various times as indicated, and total RNA extracted from them was subjected to RNA blot analysis as described in Materials and Methods. Membranes were stained with methylene blue to detect 28S rRNA and the membrane used for hybridization with the cyclin C gene, followed by reprobing with the cyclin B gene, is shown to confirm that levels of 28S rRNA remained essentially identical.
Constitutive coexpression of cyclin C with c-Myc, but not c-Fos, induced proliferation of BAF-B03-derived cells in a cytokine-independent fashion and induced formation of cell clusters.
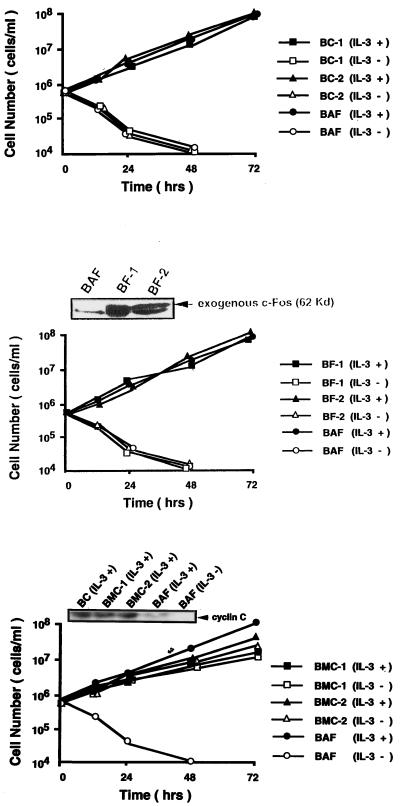
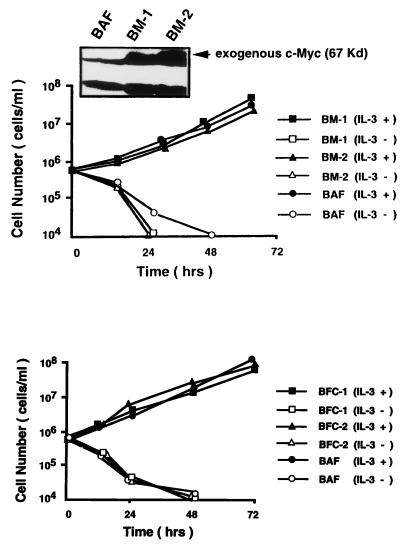
To investigate further the role of cyclin C in hematopoietic cell proliferation, we transfected a human cyclin C gene expression plasmid into BAF-B03 cells and neo+ neomycin-resistant clones were selected and pooled (BC cells). Similarly, either human c-myc or human c-fos expression plasmid was transfected into BAF-B03 cells along with a hygromycin resistance gene, and hygromycin-resistant clones were established; these were termed BM and BF, respectively. Expression of exogenous c-Myc or c-Fos was assessed by Western blot analysis (Fig. 2). Compared to IL-3-stimulated parental BAF-B03 cells, around an eight-fold-increased amount of total c-Myc in BM cells was detectable by Northern blot analysis (data not shown). BC, BM, and BF cells still required IL-3 for cell growth, and their growth rates were unchanged. In the absence of IL-3, accelerated cell death was observed in BM cells, whereas BC and BF cells displayed essentially the same survival characteristics as the parental BAF-B03 cells. These results indicate that expression of cyclin C, c-Myc, or c-Fos alone is insufficient to promote BAF-B03 cell proliferation in the absence of IL-3. We next examined whether cyclin C is able to cooperate with c-Myc and c-Fos, which have been proven to be targets of the distinct IL-3 signaling pathway (54), to promote cell proliferation. For this purpose, the human cyclin C gene was transfected into pooled BM and BF cells, and G418-resistant clones were selected and designated BMC and BFC, respectively. These cells were then cultured in complete RPMI 1640 medium supplemented with 10% (vol/vol) FCS in the absence of IL-3. In parallel, cells transfected with vector alone were cultured under the same conditions. Interestingly, whereas neither the parental BC or BFC cells nor the mock-transfected cells (data not shown) could proliferate unless they were stimulated with IL-3, the BMC cells were able to grow in a cytokine-independent fashion, indicating that cyclin C cooperates with c-Myc to promote hematopoietic cell proliferation. Overexpression of cyclin C in both BC and BMC cells was confirmed by Western blot analysis with anti-cyclin C antibody (kindly provided by E. Lee). Around four- to fivefold-increased levels relative to that of BAF-B03 cells stimulated with IL-3 were observed (Fig. 2). BMC cells were able to proliferate for more than 6 months under our culture conditions. In all experiments, more than 10 independent stable clones were established and analyzed. Representative results from three mixed clones are presented.
FIG. 2.
Proliferation profiles for BAF-B03-derived cells. For growth assay, synchronized BAF-B03, BC, BM, BF, and BFC cells were plated at 5 × 105 cells/ml in the presence of IL-3. For viability assays, IL-3-cultured cells were washed three times with PBS and plated at 5 × 105 cells/ml in the absence of IL-3. Factor-independent BMC cells were plated at 5 × 105 cells/ml in the absence of IL-3 after being washed with PBS. The concentration of viable cells was counted at various times after plating and is represented on a logarithmic scale. Expression of c-Myc and c-Fos from two clones of BM and BF cells and expression of cyclin C from two clones of BMC and pooled BC cells were assessed by Western blotting (inset panels).
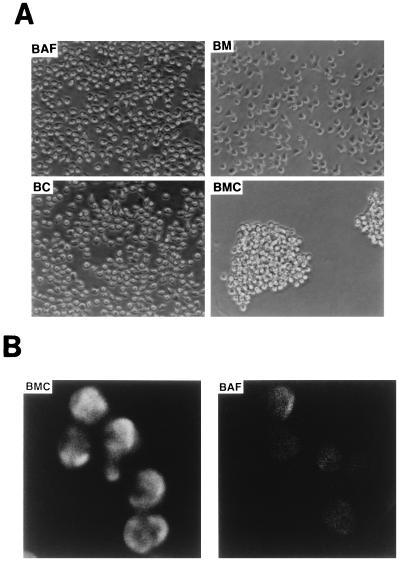
Interestingly, unlike parental BAF-B03, BM, or BC cells, which present as single cells in suspension, BMC cells formed clusters and aggregates (Fig. 3A), regardless of the IL-3, and remarkable actin polymerization was observed in BMC cells (Fig. 3B), but not in BAF-B03, BM, or BC cells (data not shown), suggesting that the cellular adhesion molecule(s) on BMC cells is activated.
FIG. 3.
(A) Morphological properties of BAF-B03-derived cells. BAF-B03, BM, and BC cells were cultured in the presence of IL-3, while BMC cells were cultured in the absence of IL-3. Addition of IL-3 did not affect the formation of cell clusters of BMC cells. (B) Induction of actin polymerization in BMC cells. BAF-B03 (IL-3 positive) and BMC (IL-3 negative) cells were stained with rhodamine-phalloidin and to detect F-actin.
We next examined the possibility that the cytokine-independent cell growth of BMC cells could be a result of autocrine production of growth factors by these cells. However, supernatants from BMC cells did not support the proliferation of BAF-B03 cells (data not shown), indicating that cell proliferation is directly mediated by coexpression of cyclin C and c-Myc, rather than by induction of endogenous growth factors. It is also well known that Bcl-2, an antiapoptotic protein, is able to cooperate with c-Myc to immortalize pre-B cells (60) and to induce proliferation of BAF-B03-derived cells in a cytokine-independent fashion (38). Therefore, we also examined whether coexpression of cyclin C and c-Myc could induce expression of endogenous bcl-2 or the related bcl-xL genes and found that expression of bcl-2 or bcl-xL genes is not induced in BMC cells in the absence of IL-3 (data not shown). Thus, neither induction of endogenous growth factor genes nor induction of bcl-2 and the related bcl-xL genes was responsible for the cooperative effects of cyclin C and c-Myc on promoting cell proliferation.
The function of cyclin C in the regulation of cell cycle progression is independent of CDK8.
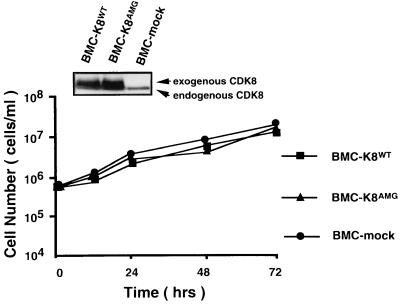
Recent findings that cyclin C could associate with CDK8 (59) led us to address the question of whether the role of cyclin C in promoting the cell cycle is dependent on CDK8, although the fact that the amount of cyclin C-CDK8 complex and its activity were constant throughout the cell cycle did not support a critical role of CDK8 in regulating the cell cycle. We first examined the amounts of CDK8 in both BMC and parental BAF-B03 cells and also tested whether expression of CDK8 changed following IL-3 stimulation. We found that the amounts of CDK8 did not increase in BMC cells compared to other cells, suggesting that ectopically expressed cyclin C can function without a concomitant increase in the expression of CDK8 (data not shown). Consistent with previous observations, expression of CDK8 was essentially the same in both growing and growth-arrested BAF-B03 cells (data not shown). Next, we performed an in vitro kinase assay to compare the abilities of the CDK8 in BMC and parental cells. In agreement with the results represented by the protein levels of CDK8, the activity of CDK8 in phosphorylation of the CTD of RNA Pol II also remained constant (data not shown), implying that overexpression of cyclin C did not up-regulate the activity of CDK8. The existence of comparable levels of CDK8 activity in growth-arrested BAF-B03 cells suggested that this activity may not be important for cell cycle progression. To investigate more directly whether the role of cyclin C in the regulation cell proliferation is dependent on CDK8, we used a catalytically inactive CDK8 mutant (kindly provided by E. A. Nigg), in which the Asp (D) residue in the DMG motif within kinase subdomain VII was replaced by Ala (A), and like wild-type CDK8, this mutant was still able to form a complex with cyclin C in vitro (20a). This mutant was transfected into BMC cells, and so we obtained several BMC-k8AMG clones. As a control, a wild-type CDK8 and an empty vector were also employed in our transfection experiments, and the resultant cells were termed BMC-k8WT and BMC-mock, respectively. Expression of exogenous CDK8 was confirmed by using anti-CDK8 antibody (kindly provided by E. Lee) (Fig. 4). To avoid the possibility that expression of a catalytically inactive mutant of CDK8 may affect the role of cyclin C in cell proliferation and may induce the death of cytokine-independent BMC cells, drug selection was carried out in the presence of IL-3. For each kind of transfectant, at least three independent clones were obtained, and the results from a representative clone are presented. As shown in Fig. 4, the properties of both the cytokine-independent growth and cell adhesion (unpublished data) of BMC cells were not affected by ectopic expression of either the catalytically inactive mutant or wild-type CDK8, suggesting that the functions of cyclin C in the regulation of cell cycle progression and cell adhesion are independent of CDK8 activity.
FIG. 4.
Effects of the catalytically inactive CDK8 on the growth property of BMC cells. Proliferation profiles for BMC-k8WT, BMC-k8AMG, and BMC-mock cells are shown. Factor-independent BMC-k8WT, BMC-k8AMG, and BMC-mock cells were plated at 5 × 105 cells/ml in the absence of IL-3 after being washed with PBS. The concentration of viable cells was counted at various times after plating and is represented on a logarithmic scale. Expression of CDK8 was detected by anti-CDK8 antibody (inset panels). Upper bands are Myc-tagged exogenous CDK8, and lower bands are endogenous CDK8.
Cyclin C, in concert with c-Myc, was able to induce expression of mitotic cyclin mRNAs and to superinduce expression of cdc2 mRNA.
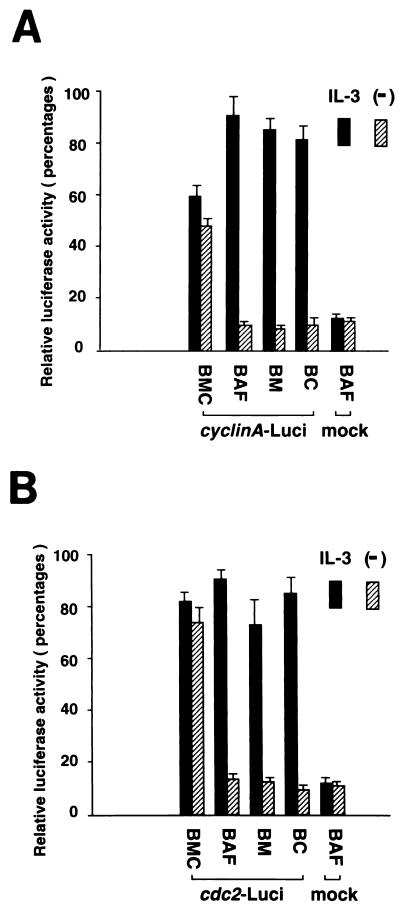
The molecular mechanism underlying the cooperative effect of c-Myc and cyclin C in promoting cell cycle progression remained unclear. Given that BMC cells proliferate by passing through the G2/M phase without stimulation by growth factors, it was assumed that regulators of the G2/M phase are induced or activated in these cells. To explore a possible connection between cyclin C, c-Myc, and G2/M-phase regulators, such as mitotic cyclins and cdc2 family kinases, we first tested whether c-Myc or cyclin C alone was able to induce the expression of the mitotic cyclin A and B, cdc2, and cdk2 genes. To investigate a possible link between c-Myc and these G2/M-phase regulators, we infected BAF-B03 or BER2, a BAF-B03-derived cell line expressing epidermal growth factor receptor (EGFR), with a retroviral vector bearing a cDNA encoding a chimeric molecule, MycER, in which c-Myc was fused with a mutated estrogen receptor (ER) (53) to yield B-MycER or BE-MycER cells, respectively. In this inducible system, the chimeric protein is constitutively expressed in an inactive state, but it can be activated upon treatment with the synthetic compound 4-hydroxytamoxifen (4-HT [250 nM]; RBI). As a control, cells infected with a mock vector were also established. To confirm that the MycER could be activated by stimulation with 4-HT, a viability assay for B-MycER cells and a growth assay for BE-MycER cells were performed. In the absence of IL-3, addition of 4-HT slightly accelerated apoptosis of B-MycER cells and rendered BE-MycER cells capable of proliferating in the presence of EGF (data not shown), consistent with the previous observations that ectopic expression of c-Myc alone accelerated cell apoptosis, yet in combination with EGF stimulation, promoted BER2-derived cell cycle progression beyond the S phase in the absence of IL-3 (56), confirming the utility of this system. B-Myc-ER and control cells were cultured in the absence of IL-3 for 3 h prior to addition of 4-HT to shut down IL-3-induced signals in order to avoid any signal transduction which might cooperate with c-Myc and confuse the results. As shown in Fig. 5A, activation of the MycER in B-MycER cells by 4-HT failed to induce any observable expression of cyclin A and B, cdc2, and cdk2 gene mRNAs (essentially identical results were obtained for BE-MycER cells [data not shown]). We also examined the effect of constitutive overexpression of c-Myc on the induction of these G2/M regulators by using BM cells, and, similarly, the function of cyclin C in the regulation of the expression of the cyclin A and B, cdc2, and cdk2 genes was examined with BC cells, in which cyclin C was constitutively expressed. As shown in Fig. 5B, no increase in expression of cyclin A and B, cdc2, and cdk2 gene mRNAs was detected in either BM or BC cells compared to parental BAF-B03 cells. In contrast, expression of these G2/M-phase genes in BMC cells was dramatically induced, to a level comparable to that observed in IL-3-stimulated parental BAF-B03 cells. In particular, cdc2 mRNA was superinduced approximately 15-fold. Moreover, similar results were obtained from transient promoter-driven gene induction experiments. Human cyclin A and cdc2 gene promoter-luciferase reporter plasmids were cotransfected with a pRL-TK control plasmid into different cell lines, and transfectants were either left unstimulated or were stimulated with IL-3. As shown in Fig. 6, enforced expression of neither cyclin C alone nor c-Myc alone was capable of inducing cyclin A and cdc2 promoter activation, whereas high-level activation was achieved by coexpression of cyclin C and c-Myc, even in the absence of IL-3 stimulation. The slightly weaker activation of the cyclin A promoter in BMC cells compared with other cells might reflect an asynchronized state of this cell line because of its resistance to growth factor starvation. Moreover, the increased amount of cdc2 mRNA in BMC cells seemed to reflect transcriptional activation rather than an increased stability of the transcripts, because a relatively strong activation of the cdc2 promoter in comparison to that of the cyclin A promoter was observed in the asynchronized BMC cells. Taken together, these results indicated that while neither c-Myc nor cyclin C alone was sufficient to induce the expression of mitotic cyclin, cdc2, or cdk2 gene mRNAs, cyclin C, in concert with c-Myc, could bring about induction of these G2/M-phase regulators to drive cell cycle progression.
FIG. 5.
(A) Effect of conditional activation of c-Myc on the induction of mitotic cyclin and cdc2 family kinase gene mRNAs. B-MycER and B-Control cells were starved for 3 h in the absence of IL-3 and stimulated with 250 nM 4-HT. Total RNA extracted from various states of cells was subjected to Northern blot analysis. 28S rRNA stained with methylene blue is shown. Membranes were reprobed following dehybridization. (B) Differential expression of mitotic cyclin and cdc2 family kinase gene mRNAs in BAF-B03-derived transformants in the presence or absence (∼12 h) of IL-3. Cells were harvested, and total RNA was extracted and subjected to Northern blot analysis. 28S rRNA stained with methylene blue is shown. Membranes were reprobed following dehybridization. The upper 28S panel shows membrane hybridized with cyclin A and cdc2 gene probes, respectively; the lower panel was hybridized with cyclin B and cdk2 gene probes.
FIG. 6.
Effect of coexpression of cyclin C and c-Myc on cyclin A (A) and cdc2 (B) gene promoter activation. Various cell lines were transfected with reporter plasmids by the DEAE-dextran method. Luciferase (Luci) activities were measured with a dual-luciferase reporter assay system kit according to the manufacturer’s instructions (Promega) and are shown as percentages. Essentially identical results were obtained in three separate experiments.
Cyclin C, but not cyclin E or D3, could mediate superinduction of cdc2 by cooperation with c-Myc.
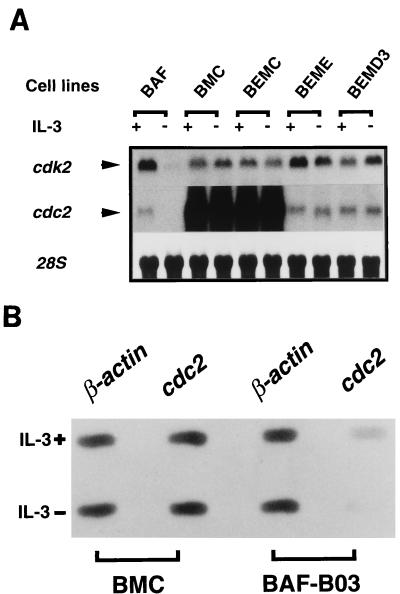
The finding that cdc2 mRNA was selectively superinduced in BMC cells prompted us to explore the potential relationship between c-Myc, cyclin C, and CDC2. We were particularly interested in examining whether cyclin C was primarily responsible for superinduction of the cdc2 gene, since the relationship between c-Myc and CDC2 has been extensively studied (5, 16). To this end, we took advantage of several previously established cell lines (20a) in which exogenous human c-Myc was coexpressed ectopically with human cyclin E (BEME cells) or cyclin D3 (BEMD3 cells) in BAF-B03-derived BER2 cells. Both BEME and BEMD3 cells could proliferate in a factor-independent fashion. It should be noted that the exogenous human EGFR responds primarily to human EGF rather than any other factors in the medium. In fact, ectopically expressed EGFR was not phosphorylated under our culture conditions (data not shown). Moreover, to exclude any possible effects emanating from the EGFR, BEMC cells were also established by cotransfection of cyclin C and c-Myc into BER2 cells. (BEMC cells also exhibited factor-independent growth and formed cell clusters to a very similar degree to BMC cells [data not shown].) Thus, the difference between BMC or BEMC and BEME or BEMD3 cells would primarily reflect a unique function of cyclin C. As shown in Fig. 7, superinduction of cdc2 mRNA, as opposed to cdk2 mRNA, was only observed in the cyclin C-transfected cells, indicating that superinduction of the cdc2 gene depends on the expression of cyclin C. Since constitutive overexpression of cyclin C alone failed to elicit up-regulation of the expression of cdc2 mRNA or activate the cdc2 promoter, c-Myc also seemed to be required for this process (see Discussion). We also examined the protein levels and histone H1 kinase activities of CDC2 in these cells. Unlike cdc2 mRNA levels, neither CDC2 protein expression nor CDC2 histone H1 kinase activity was drastically up-regulated compared to that in other cell lines (data not shown). To further address the mechanism of drastically increased cdc2 mRNA in BMC cells, we performed a nuclear run-on assay to examine whether cooperation of cyclin C and c-Myc affects transcription of the cdc2 gene. Figure 7B showed that transcription of cdc2 mRNA almost stopped in parental BAF-B03 cells after withdrawal of IL-3 for 12 h, but still occurred in BMC cells at a rate comparable to that when stimulated by IL-3, suggesting that superinduction of the cdc2 gene in BMC cells is, at least partly, due to increased transcriptional rates of cdc2 mRNA.
FIG. 7.
(A) Superinduction of cdc2 mRNA in cells coexpressing cyclin C and c-Myc. Total RNA was extracted from cells cultured in the presence or absence (∼12 h) of IL-3 and subjected to Northern blot analysis of cdc2 and cdk2 expression as described in Materials and Methods. 28S rRNA stained with methylene blue is shown. Membranes were reprobed following dehybridization. (B) Transcriptional analysis of the cdc2 gene in BMC and BAF-B03 cells under different conditions. cdc2 gene plasmids bound to nitrocellulose were hybridized with 32P-labelled run-on transcripts from nuclei isolated from BMC and BAF-B03 cells IL-3 stimulated or IL-3 deprived for 12 h. As a control, detection of β-actin transcript is shown.
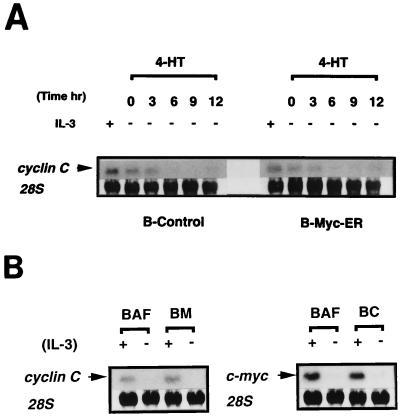
Expression of cyclin C and c-myc was not mutually affected.
We further investigated whether cyclin C and c-Myc can augment each other or act in distinct signaling pathways. Considering the fact that following IL-3 stimulation, c-myc was induced earlier than the cyclin C gene, c-Myc is likely to act, if at all, upstream of cyclin C. Hence, we examined the effect of conditional activation of MycER on the induction of cyclin C gene mRNA. As shown in Fig. 8A, upon 4-HT stimulation, there was no detectable up-regulation of cyclin C gene expression. Similarly, expression of cyclin C gene mRNA was not enhanced in stable BM cells (Fig. 8B), suggesting that the cyclin C gene is not a target gene of c-Myc. On the other hand, the expression of the c-myc gene was not significantly affected by the constitutive expression of cyclin C. Moreover, the fact, that BC cells were more resistant to factor-starvation-induced apoptosis than BM cells did not support the idea that cyclin C could augment the c-Myc signaling pathway. Therefore, it was suggested that the synergistic action of cyclin C and c-Myc in promoting cell cycle progression reflects a functional cooperation of both molecules rather than a mutual activation of expression of the genes coding for these proteins.
FIG. 8.
(A) Effect of conditional activation of c-Myc on the induction of cyclin C gene mRNA. The experiment was carried out as described in the legend to Fig. 5A. (B) Effect of constitutive expression of cyclin C on the expression of the c-myc gene and vice versa. BC and BM cells cultured in the presence or absence (∼12 h) of IL-3 were harvested, and total RNA was prepared and subjected to Northern blot analysis of either the c-myc or cyclin C gene as described in Materials and Methods.
DISCUSSION
Cyclin C appears to have multiple functions.
In the present study, we describe a novel role for cyclin C in the regulation of hematopoietic cell cycle progression by cooperation with c-Myc. Furthermore, we found that cyclin C, but not cyclin E or D3, was responsible for the superinduction of cdc2 mRNA. Thus, our data, obtained by utilizing a hematopoietic cell line, BAF-B03, nonetheless provide direct evidence that cyclin C is indeed important for the regulation of cell cycle progression. Recently, it has been demonstrated that cyclin C is capable of associating with a unique cyclin-dependent protein kinase, CDK8, and it has been proposed that the cyclin C-CDK8 complex may regulate transcription. However, it remains unclear whether the role of cyclin C in the promotion of cell proliferation is mediated through formation of a complex with CDK8 to regulate transcription. The finding that CDK8 expression and kinase activity toward CTD of RNA Pol II are not up-regulated by constitutive expression of cyclin C suggests that the effect of ectopically expressed cyclin C on cell cycle progression may be independent of CDK8. In fact, it has been reported by others that the amount of cyclin C-CDK8 complex and its ability to phosphorylate CTD of RNA Pol II remain constant throughout the cell cycle (52), which is in accordance with our results. Interestingly, it has been shown that cyclin C can assemble with other molecules in addition to CDK8; however, the nature of such molecules remains unknown (52). Moreover, SRB11-SRB10, a putative yeast homolog of mammalian cyclin C-CDK8, has not been demonstrated to be critical for yeast cell cycle control (30, 45). The fact that the cyclin C-CDK8 complex is unable to exhibit kinase activity in vitro toward any of the canonical CDK substrates tested (i.e., histone H1 and pRb) (52) also indicates that this complex does not share similarity with any other CDKs or CAKs that have been shown to be critical for cell cycle control. Importantly, our observations that enforced expression of the catalytically inactive mutant of CDK8 in the BMC cells fails to reverse or alleviate either the cytokine-independent proliferation or homotypic aggregation (30b) of BMC cells indicate more directly that CDK8 activity is not required for the function of cyclin C. Taken together, we suggest that cyclin C plays a role in the regulation of cell proliferation through a CDK8-independent mechanism, and formation of a complex with CDK8 to regulate transcription may represent a distinct function of cyclin C.
In the context of transcriptional regulation, unlike CDK8, CDC2, associating with and phosphorylating the CTD of RNA Pol II, appears to be important for regulation of cell proliferation. Hence, our results may represent a novel mechanism for cyclin C to be involved in the control of cell cycle progression, partly through the regulation of the cdc2 gene. CDC2 kinase triggers the entry of mammalian cells into mitosis, the only cell cycle phase in which transcription is globally repressed. It has already been demonstrated that CDC2 kinase is able to phosphorylate components of the RNA Pol II transcription machinery, including CTD of RNA Pol II, and such phosphorylation is sufficient to inhibit transcription (6, 17). Furthermore, it has been reported that CDC2 can destabilize the RNA Pol II preinitiation complex (62). Thus, the biological significance of CDC2 association with and phosphorylation of CTD of RNA Pol II correlates well with its well-established role in cell cycle control. We hypothesize that the function of cyclin C in the regulation of cell cycle progression is, at least in part, mediated by modulation of CDC2 rather than association with CDK8. Further study will be required to elucidate the precise function(s) of the cyclin C-CDK8 complex. In particular, it is of importance to examine (i) whether CDK8 is indeed a major functional partner of cyclin C, (ii) whether there are other targets besides CTD of RNA Pol II, (iii) whether the cyclin C-CDK8 complex is specifically responsible for the induction of the cdc2 gene or other cell cycle regulators, and (iv) whether phosphorylation of CTD by CDC2 and cyclin C-CDK8 is distinct.
In addition, the findings that all clones coexpressing c-Myc and cyclin C form cell clusters and aggregates and that F-actin polymerization is induced in these cells suggest that an adhesion molecule(s) on BMC cells may be activated. In fact, we found that integrin VLA-4 (very late antigen 4) is activated, that its counterreceptor, VCAM-1 (vascular cell adhesion molecule-1), is induced on the BMC cells, and that this VLA-4–VCAM-1 pair is primarily responsible for mediating homotypic aggregation of BMC cells (30b). Intriguingly, BMC cells exhibit a drastically decreased cell adhesion property following treatment with anti-α4 integrin blocking antibody, but do not undergo apoptosis and can still proliferate in the absence of cytokine, which suggests that cytokine-independent growth of BMC cells is not tightly dependent on cell adhesion and that cyclin C thus may function directly to promote cell cycle progression rather than via regulation of cell adhesion, which can supply survival signals to cooperate with c-Myc. This is likely because BAF-B03 cells are not adhesion dependent and both BEME and BEMD3 cells can proliferate without cell adhesion. The fact that neither BEME nor BEMD3 cells acquire similar cell-adhesive properties indicates that cyclin C, but not cyclin E or D3, is responsible for inducing such adhesion properties in cooperation with c-Myc. Furthermore, ectopic expression of CDC2 in BM cells fails to induce the formation of cell clusters even in the presence of IL-3, suggesting that CDC2 is not responsible for the activation of a putative adhesion molecule(s). Collectively, our observations indicate that cyclin C also plays a role in the regulation of cell adhesion.
Cyclin C may function at both the G1/S and G2/M transitions.
Cell proliferation is primarily regulated in the G1 phase of the cell cycle. Growth factors act throughout the G1 phase by binding to specific cell surface receptors, which in turn trigger signaling cascades that ultimately govern cell growth. Late in G1, growth factor-induced signals converge on the cell cycle machinery, thereby driving the cell cycle beyond the restriction point. Once the cell cycle passes through this point, cells become refractory to growth factor-induced signals (49) and instead come to rely upon the intrinsic cell cycle machinery to regulate progression through subsequent phases. It is generally believed that regulation of cell cycle passage through the restriction point in late G1 is particularly important in the acquisition of factor independence.
Myc is the product of a growth factor-induced immediate-early gene, and its role in the regulation of the G1 phase of the cell cycle has been extensively studied (12, 20, 21). In our study, enforced expression of c-Myc alone in BAF-B03 cells accelerates apoptosis rather than promoting cell proliferation in the absence of cytokine stimulation. The result indicates that c-Myc alone is insufficient to drive BAF-B03 cell to complete an entire cell cycle, and an additional signal(s), which functions either to block apoptosis or to promote cell cycle progression, is required to cooperate with c-Myc to promote complete cell cycle progression. Since overexpression of cyclin C neither retards apoptosis nor induces bcl-2 gene expression, it is likely that cyclin C is important in promoting passage of cells through the restriction point in the late G1 phase and allowing them to become refractory to growth factor-induced signals. The kinetics of cyclin C mRNA induction seems to be in accordance with this idea, since induction occurs at the G1/S transition and peaks in the S phase. Another piece of evidence supporting an important role for cyclin C in the G1/S transition comes from its functional similarity to cyclins D and E. Since cyclins D1 and E were identified by the same complementation approach used for cyclin C, we have investigated whether D-type or E cyclins could functionally substitute for cyclin C to cooperate with c-Myc (unpublished observations). Since cyclin D3 may function as the major D-type cyclin in this cell line because the D3 gene is induced early and strongly while the D1 gene is only induced weakly and late after IL-3 stimulation (Fig. 1B), we examined cyclin D3 instead of D1. Cotransfection of c-myc with either human cyclin D3 or E expression plasmids into BER2 cells demonstrated that both cyclin D3 and cyclin E can synergize with c-Myc to promote cell proliferation in the absence of IL-3 (BEMD3 and BEME cells), implying that cyclin C has, at least in part, a role similar to that of cyclins D and E, two well-known G1 cyclins. Further study will be required to elucidate the exact mechanism by which cyclin C acts at the G1/S transition.
On the other hand, our results also suggest that the role of cyclin C is not limited to the G1/S phase. Superinduction of the cdc2 gene observed in BMC cells suggests that constitutive expression of c-Myc is a prerequisite, since superinduction of the cdc2 gene does not occur in BC cells while cyclin C is primarily responsible for superinduction of the cdc2 gene, because cooperation of cyclin D3 or E with c-Myc failed to elicit superinduction of cdc2 gene expression. Thus, the roles of cyclin C do not appear to be monophasic; cyclin C seems to be required for passage through the restriction point in late G1 by collaboration with c-Myc and again for the induction of the cdc2 gene, which is a key regulator in the G2/M phase. Interestingly, ectopic expression of CDC2 in BM cells revealed that CDC2 was unable to replace cyclin C to cooperate with c-Myc (unpublished observation), suggesting, in turn, that an important role of cyclin C at the G1/S transition is required for promoting cell cycle progression, although we could not exclude the possibility that induction of cdc2 is just one of mechanisms by which cyclin C regulates the G2/M phase.
In comparison with the superinduced cdc2 mRNA, our data show that the protein level and kinase activity of CDC2 in BMC and BEMC cells are not dramatically increased. This is consistent with previous reports that the levels of CDC2 protein remained more or less constant (35, 61), despite the fact that expression of cdc2 was cell cycle regulated (27, 35). It is possible that an excess of cell cycle regulators may be harmful, and cells favor maintaining an optimal balance. It has been reported that the abundance of CDC2 is coordinately regulated by its synthesis and degradation. Once synthesis is activated, a concurrent mechanism of degradation is also activated, and the half-life of the protein is reduced (35). Superinduction of the cdc2 gene in BMC cells is at least partly due to an increased transcription rate; however, the significance of superinduction of the cdc2 gene remains obscure, since the relatively low levels of cdc2 mRNA induction observed in BEME and BEMD3 cells are sufficient for cell cycle progression. It has been suggested that newly synthesized CDC2 is required for progression through each cell cycle (35, 61). This may be particularly important for cells such as BMC cells that proliferate in the absence of growth factor. Under such conditions, several negative regulators, such as CDK inhibitors (CKIs), or Wee-1 family protein kinases, may be activated, and cells need newly synthesized CDC2 to compensate for the inactivated or rapidly degraded “old” CDC2, while cyclins E and D3 may utilize different mechanisms to overcome these problems and to regulate the proliferation of BEME and BEMD3 cells. In fact, it has been shown that different CKIs can associate with cyclin E-CDK2 or cyclin D-CDK4/6 complexes. Therefore, ectopically expressed cyclins E and D3 may be able to compete against the activities of CKI, while cyclin C may be unable to do so, and thus turns to utilize other mechanisms, such as superinduction of cdc2. Further studies will be required to understand the precise mechanism by which cyclin C regulates the superinduction of cdc2 in cooperation with c-Myc and the significance of this phenomenon.
ACKNOWLEDGMENTS
We are grateful to R. A. Weinberg for human cyclin C, E, D1, and D3 expression plasmids (Rc-cycC, Rc-cycE, Rc-cycD1 and Rc-cycD3, respectively); Y. Nakabeppu for human c-fos plasmid; E. A. Nigg for pCMV-myc-tagged human cdk8 and pCMV-myc-tagged cdk8AMG mutant plasmids; B. Rudolph for MycER and mock retroviral packaging GP+E-86 cells; E. Lee for anti-human cyclin C and anti-CDK8 antibodies; and T. L. Born for cyclin A and cdc2 promoter-luciferase reporter genes. We also thank A. Kukula for critical reading of the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas provided by the Ministry of Education, Science, Sports and Culture, Japan; Nippon Boehringer Ingelheim Co., Ltd., Kawanishi Pharma Research Institute; the Kato Memorial Bioscience Foundation; and The Naito Foundation. Z.-J. L. was supported by a Grant-in-Aid for Japan Society for the Promotion of Science Fellows.
REFERENCES
- 1.Adams D H, Harvath L, Bottaro D P, Interrante R, Catalano G, Tanaka Y, Strain A, Hubscher S G, Shaw S. Hepatocyte growth factor and macrophage inflammatory protein 1β: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc Natl Acad Sci USA. 1994;91:7144–7148. doi: 10.1073/pnas.91.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai C, Richman R, Elledge S J. Human cyclin F. EMBO J. 1994;13:6087–6098. doi: 10.1002/j.1460-2075.1994.tb06955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 4.Battey J, Moulding C, Taub R, Murphy W, Stewart T, Potter H, Lenoir G, Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983;34:779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- 5.Born T L, Frost J A, Schönthal A, Prendergast G C, Feramisco J R. c-Myc cooperates with activated Ras to induce the cdc2 promoter. Mol Cell Biol. 1994;14:5710–5718. doi: 10.1128/mcb.14.9.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cisek L J, Corden J L. Phosphorylation of RNA polymerase by the murine homologue of the cell-cycle control protein cdc2. Nature. 1989;339:679–684. doi: 10.1038/339679a0. [DOI] [PubMed] [Google Scholar]
- 7.Collins M K, Downward J, Miyajima A, Maruyama K, Arai K, Mulligan R C. Transfer of functional EGF receptors to an IL-3-dependent cell line. J Cell Physiol. 1988;137:293–298. doi: 10.1002/jcp.1041370212. [DOI] [PubMed] [Google Scholar]
- 8.Damjanov I, Shan J, Wang R F, Damjanov A, DeLoia J A. Molecular cloning and characterization of murine cyclin E. Biochem Biophys Res Commun. 1994;201:994–1000. doi: 10.1006/bbrc.1994.1800. [DOI] [PubMed] [Google Scholar]
- 9.Doi T, Hatakeyama M, Minamoto S, Kono T, Mori H, Taniguchi T. Human interleukin 2 (IL-2) receptor β chain allows transduction of IL-2-induced proliferation signal(s) in a murine cell line. Eur J Immunol. 1989;19:2375–2378. doi: 10.1002/eji.1830191229. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 11.Dulic V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 12.Eilers M, Picard D, Yamamoto K R, Bishop J M. Chimaeras of MYC oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989;340:66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- 13.Evans T, Rosenthal E T, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 14.Fesquet D, Labbe J C, Derancourt J, Capony J P, Galas S, Girard F, Lorca T, Shuttleworth J, Doree M, Cavadore J C. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993;12:3111–3121. doi: 10.1002/j.1460-2075.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher R P, Morgan D O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994;78:713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 16.Furukawa Y, Piwnica-Worms H, Ernst T J, Kanakura Y, Griffin J D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990;250:805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- 17.Gebara M M, Sayre M H, Corden J L. Phosphorylation of the carboxy-terminal repeat domain in RNA polymerase II by cyclin-dependent kinases is sufficient to inhibit transcription. J Cell Biochem. 1997;64:390–402. [PubMed] [Google Scholar]
- 18.Girard F, Strausfeld U, Fernandez A, Lamb N J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg M E, Ziff E B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984;311:433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- 20.Heikkila R, Schwab G, Wickstrom E, Loke S L, Pluznik D H, Watt R, Neckers L M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. Nature. 1987;328:445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- 20a.Jaquenoud, M., J. P. Tassan, and E. A. Nigg. Personal communication.
- 21.Karn J, Watson J V, Lowe A D, Green S M, Vedeckis W. Regulation of cell cycle duration by c-myc levels. Oncogene. 1989;4:773–787. [PubMed] [Google Scholar]
- 22.Kawahara A, Minami Y, Taniguchi T. Evidence for a critical role for the cytoplasmic region of the interleukin 2 (IL-2) receptor γ chain in IL-2, IL-4, and IL-7 signalling. Mol Cell Biol. 1994;14:5433–5440. doi: 10.1128/mcb.14.8.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawahara A, Minami Y, Miyazaki T, Ihle J N, Taniguchi T. Critical role of the interleukin 2 (IL-2) receptor γ-chain-associated Jak3 in the IL-2 induced c-fos and c-myc, but not bcl-2, gene induction. Proc Natl Acad Sci USA. 1995;92:8724–8728. doi: 10.1073/pnas.92.19.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koff A, Giordano A, Desai D, Yamashita K, Harper J W, Elledge S, Nishimoto T, Morgan D O, Franza B R, Roberts J M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 25.Kuhr T, Dougherty G J, Klingemann H G. Transfer of the tumor necrosis factor alpha gene into hematopoietic progenitor cells as a model for site-specific cytokine delivery after marrow transplantation. Blood. 1994;84:2966–2970. [PubMed] [Google Scholar]
- 26.Lahue E E, Smith A V, Orr-Weaver T L. A novel cyclin gene from Drosophila complements CLN function in yeast. Genes Dev. 1991;5:2166–2175. doi: 10.1101/gad.5.12a.2166. [DOI] [PubMed] [Google Scholar]
- 27.Lee M G, Norbury C J, Spurr N K, Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988;333:676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- 28.Leopold P, O’Farrell P H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991;66:1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lew D J, Dulic V, Reed S I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 30.Liao S-M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, van Vuuren H J, Young R A. A kinase-cyclin pair in the RNA polymerase II holoenzyme. Nature. 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 30a.Liu, Z.-J., and T. Taniguchi. Unpublished data.
- 30b.Liu, Z.-J., et al. Submitted for publication.
- 31.Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 33.Matsushime H, Ewen M E, Strom D K, Kato J Y, Hanks S K, Roussel M F, Sherr C J. Identification and properties of an atypical catalytic subunit (p34PSKJ3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 34.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGowan C H, Russell P, Reed S I. Periodic biosynthesis of the human M-phase promoting factor catalytic subunit p34 during the cell cycle. Mol Cell Biol. 1990;10:3847–3851. doi: 10.1128/mcb.10.7.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minshull J, Golsteyn R, Hill C S, Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990;9:2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyazaki T, Liu Z-J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian E L, Perlmutter R M, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki T, Liu Z-J, Taniguchi T. Selective cooperation of HTLV-1 encoded p40tax-2 with cellular oncoproteins in the induction of hematopoietic cell proliferation. Oncogene. 1996;12:2403–2408. [PubMed] [Google Scholar]
- 40.Motokura T, Keyomarsi K, Kronenberg H M, Arnold A. Cloning and characterization of human cyclin D3, a cDNA closely related in sequence to the PRAD1/cyclin D1 proto-oncogene. J Biol Chem. 1992;267:20412–20415. [PubMed] [Google Scholar]
- 41.Nakamura T, Sanokawa R, Sasaki Y F, Ayusawa D, Oishi M, Mori N. Cyclin I: a new cyclin encoded by a gene isolated from human brain. Exp Cell Res. 1995;221:534–542. doi: 10.1006/excr.1995.1406. [DOI] [PubMed] [Google Scholar]
- 42.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 43.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto K, Beach D. Cyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J. 1994;13:4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Neill E M, O’Shea E K. Transcriptional regulation. Cyclins in initiation. Nature. 1995;374:121–122. doi: 10.1038/374121a0. [DOI] [PubMed] [Google Scholar]
- 46.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagano M, Pepperkok R, Lukas J, Baldin V, Ansorge W, Bartek J, Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 49.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 50.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 51.Quelle D E, Ashmun R A, Shurtleff S A, Kato J Y, Bar S D, Roussel M F, Shree C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 52.Rickert P, Seghezzi W, Shanahan F, Cho H, Lees E. Cyclin C/CDK8 is a novel CTD kinase associated with RNA polymerase II. Oncogene. 1996;12:2631–2640. [PubMed] [Google Scholar]
- 53.Rudolph B, Saffrich R, Zwicker J, Henglein B, Muller R, Ansorge W, Eilers M. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 1996;15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- 54.Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992;11:3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherr C J. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 56.Shibuya H, Yoneyama M, Ninomiya-Tsuji J, Matsumoto K, Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992;70:57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- 57.Shiekhattar R, Mermelstein F, Fisher R P, Drapkin R, Dynlacht B, Wessling H C, Morgan D O, Reinberg D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature. 1995;374:283–287. doi: 10.1038/374283a0. [DOI] [PubMed] [Google Scholar]
- 58.Tamura K, Kanaoka Y, Jinno S, Nagata A, Ogiso Y, Shimizu K, Hayakawa T, Nojima H, Okayama H. Cyclin G: a new mammalian cyclin with homology to fission yeast Cig1. Oncogene. 1993;8:2113–2118. [PubMed] [Google Scholar]
- 59.Tassan J-P, Jaquenoud M, Leopold P, Schultz S J, Nigg E A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc Natl Acad Sci USA. 1995;92:8871–8875. doi: 10.1073/pnas.92.19.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaux D L, Cory S, Adams J M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 61.Welch P J, Wang J Y. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. Proc Natl Acad Sci USA. 1992;89:3093–3097. doi: 10.1073/pnas.89.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zawel L, Lu H, Cisek L J, Corden J L, Reinberg D. The cycling of RNA polymerase II during transcription. Cold Spring Harbor Symp Quant Biol. 1993;58:187–198. doi: 10.1101/sqb.1993.058.01.023. [DOI] [PubMed] [Google Scholar]