Abstract
Fusarium species are common plant pathogens that cause serious crop losses worldwide. Fusarium spp. colonize not only the main host plants, crops, but also alternative hosts. The effectiveness of fungicide use in disease management ranges from very successful to possibly promoting the growth of the pathogen. Triazole fungicides are widely used to control these pathogens due to their broad-spectrum activity and systemic nature. This paper reviews the sensitivity of 40 Fusarium strains isolated from weeds, non-gramineous plants, and spring wheat to metconazole, prothioconazole, and tebuconazole. The effect of fungicides was determined by the percentage inhibition of F. graminearum, F. culmorum, F. sporotrichioides, and F. avenaceum fungal mycelial growth. The 50% effective concentration (EC50) values of all isolates on metconazole were lower than 2.9 mg L−1, prothioconazole EC50 ranged from 0.12 to 23.6 mg L−1, and tebuconazole ranged from 0.09 to 15.6 mg L−1. At 0.00025–0.025 mg L−1, the fungicides were ineffective, except for the growth of the F. avenaceum species. It was observed that isolates from weeds were more sensitive to low concentrations of fungicide than isolates from crop plants. In general, information is scarce regarding the comparison of fungicide resistance in Fusarium isolates from weed and crop plants, making this study an additional contribution to the existing knowledge base.
Keywords: fungicides, chemical control, Fusarium, mycelium growth inhibition, weed, non-gramineous plants, EC50
1. Introduction
Fusarium head blight (FHB), also known as scab, is a devastating disease of small-grain cereals worldwide [1]. This crop disease is still one of the main and economically most important diseases. Fusarium species affect a range of plant parts, such as roots, stems, and heads [2,3]. The most prevalent Fusarium species that cause FHB are F. graminearum, followed by F. culmorum and F. avenaceum [3]. These species are capable of producing mycotoxins such as zearalenone, deoxynivalenol (DON), T-2 toxin, and others, which reduce the quality and yield of the affected crops, making them unfit for human or animal consumption due to contamination with mycotoxins [4,5]. In Lithuania, F. graminearum, F. culmorum, F. avenaceum, and F. sporotrichioides are most associated with the disease [6]. Apart from the main host plants (wheat, barley, etc.), these pathogens are often found in other plants, such as crop weeds, grasses, and non-cultivated plants [7,8,9,10]. In order to ensure food safety and control economic loss, the management of FHB disease is a necessary aspect. Chemical control is increasingly being chosen for managing FHB disease due to the lack of disease-resistant varieties and effective biological control methods for the field [11].
In recent years, fungicides, such as triazoles, have been commonly used to control FHB by preventing the growth and spread of the Fusarium fungi [12,13]. Metconazole, prothioconazole, and tebuconazole are fungicides used in agriculture to control various fungal diseases, including FHB [14,15,16]. Triazole fungicides have been shown to be effective in inhibiting Fusarium species growth both in vitro and in vivo. In vitro studies have shown that triazole fungicides can inhibit the mycelial growth, spore germination, and toxin production in Fusarium species [17]. Through field trials, Paul et al. [18] showed that triazole-based fungicides are effective in controlling FHB and DON [19].
The effectiveness of triazole fungicides in inhibiting the growth of Fusarium species depends on several factors, including the type of fungicide, the timing and frequency of application, the susceptibility of the pathogen, and environmental conditions [15,20]. However, it has been extensively recorded and discussed that combining several management strategies was generally more effective against FHB disease severity than using just one strategy [12,21,22]. As well, the use of triazole fungicides can have potential risks, including the development of fungicide resistance, the accumulation of toxic residues in crops, and impacts on non-target organisms such as beneficial insects and soil microorganisms [23]. Since triazole fungicides have been used for controlling different pathogens on crops for long periods, some Fusarium species showed tolerance to these chemicals [24]. Despite this, it is not clear how Fusarium strains isolated from alternative plants (weeds), which, in our previous studies, showed the ability to cause very intense FHB disease [6] produce mycotoxins [25] and reduce grain yield [26], respond to fungicides. Finally, it is valuable to monitor the sensitivity of Fusarium populations from different sources to fungicides as part of integrated disease and weed management to implement control strategies.
This study aims to evaluate and compare the susceptibility of Fusarium species (F. graminearum, F. culmorum, F. avenaceum, and F. sporotrichioides) from weeds (scentless false mayweed, field pansy, shepherd’s purse, meadow grass, wild buckwheat) from crops (oilseed rape, field pea, sugar beet) and main host-plant spring wheat to triazole group fungicides (metconazole, prothioconazole, and tebuconazole) in vitro.
2. Materials and Methods
2.1. Isolation of Fusarium Spp. from Plants
The study was carried out at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry, in 2021 and 2022. Plant samples were collected at the same time from five cropping system fields located in the middle of Lithuania (55°23′50″ N, 23°51′40″ E). Plants (crops and weeds) were collected during BBCH 65–79 (from the full flowering stage till the development of fruit), except sugar beet, which was taken at BBCH 38 (rosette growth stage). Fusarium strains were isolated from spring wheat (Triticum aestivum), field pea (Pisum sativum), sugar beet (Beta vulgaris), oilseed rape (Brassica napus), and weeds (Viola arvensis, Fallopia convolvulus, Poa annua, Capsella bursa-pastoris, and Tripleurospermum inodorum) according to [9]. Fusarium fungi were isolated from all morphological parts of the plant, including roots (r), crowns (c), stems (s), leaves (l), florets (fl), pods (p), and fruits (f). Only isolates from spring wheat were isolated from spikes. The plants were cut (1 cm in size) and sterilized for 3 min in 1% sodium hypochlorite (NaClO) solution and then rinsed 3 times in sterile distilled water (SDW) and dried on sterile filter paper in a laminar. Different parts of the plant were placed on potato dextrose agar (PDA, Merck) medium supplemented with 130 mg L−1 streptomycin sulfate, and the plates were incubated at 22 ± 2 °C in the dark for 2–4 days. The Fusarium fungi that appeared were purified via PDA and grown on a Spezieller Nährstoffarmer Agar medium (SNA) at 25 ± 2 °C for 10–30 days until spore mass formation. Single spores were picked and transferred onto PDA to obtain pure cultures for subsequent DNA extraction. Spore suspensions were prepared for further studies according to Suproniene et al. [9] and stored at −80 °C.
2.2. Identification of Fusarium Spp.
Fusarium species were isolated and identified by colony morphology and spore shape using a light microscope, as described by Leslie and Summerell [27]. DNA for PCR was extracted from 1- to 2-week-old mycelia using a ZR Fungal/Bacterial DNA MiniPrep kit (Zymo Research, Irvine, CA, USA) according to Sneideris et al. [28]. All morphologically identified cultures of Fusarium species were verified by species-specific PCR using the primer pairs (F. avenaceum: J1AF/R; F. culmorum: FC01F/R; F. graminearum: Fg16F/R; and F. sporotrichioides: AF330109CF/R) reported by Demeke et al. [29] and using conventional end-point PCR. The PCR reactions were performed in mixtures containing 2.5 μL of 10× PCR buffer (provided with the polymerase; Applied Biosystems, Waltham, MA, USA), 0.5 μL of dNTP Mix (10 mM each) (Thermo Fisher Scientific Baltics, Vilnius, Lithuania), 0.5 μL of each 25 μM primer, 1.25 U of AmpliTaq Gold polymerase (Applied Biosystems), 1 μL of extracted DNA template, and nuclease-free water up to a total volume of 25 μL. The thermocycling conditions consisted of initial denaturation and polymerase activation at 95 °C for 10 min; then, 38 cycles of 95 °C for 40 s, 55 to 62 °C for 30 s, and 72 °C for 55 s; followed by a final extension at 72 °C for 10 min. The annealing temperature was selected for each primer pair according to their original description [9,28,30].
Fusarium species were selected based on isolated species from the plant parts. However, we lost strains of F. avenaceum from oilseed rape, sugar beet, and field pea during storage and these were therefore not included. All Fusarium isolates and number per host plant are listed in Table 1.
Table 1.
Information on Fusarium isolates selected for sensitivity test to fungicides.
| Isolate Number per Host Plant | Isolates per Fusarium Species | |
|---|---|---|
| Weed | ||
| Scentless mayweed | 1 FG, 1 FC, 1 FA, 1 FS | F. graminearum (FG)—13 |
| Shepherd‘s purse | 1 FG, 1 FC, 1 FA, 1 FS | F. culmorum (FC)—12 |
| Field pansy | 1 FG, 1 FC, 1 FA, 1 FS | F. avenaceum (FA)—6 |
| Wild buckwheat | 1 FG, 1 FC, 1 FA, 1 FS | F. sporotrichioides (FS)—9 |
| Meadow grass | 1 FC and 1 FA | Total: 40 isolates |
| Crops | ||
| Oilseed rape | 1 FG, 1 FC, 1 FS | |
| Field pea | 2 FG, 2 FC and 1 FS | |
| Sugar beet | 2 FC, 1 FG, 1 FS | |
| Spring wheat | 5 FG, 2 FC, 1 FA, 2 FS | |
2.3. Preparation of Fungicide Solutions
Three different fungicides were used in this study: metconazole (Pestanal, Sigma Aldrich, JAV), prothioconazole (Carbosynth Limited, Dallas, TX, USA), and tebuconazole (Santa Cruz Biotechnology, Inc., San Diego, CA, USA). The initial fungicide solution was prepared with a concentration of 1000 mg L−1. Briefly, 10 mg of fungicide was weighed and dissolved in 0.5 mL of 70% ethanol. After dissolution, it was diluted with sterile distilled water to 10 mL. Tenfold dilutions in sterile distilled water were made from this initial fungicide stock solution.
The PDA (potato dextrose agar) medium was prepared according to the manufacturer’s instructions. The medium was prepared in 200 mL bottles, autoclaved at 121 °C 2 atm pressure for 20 min, and cooled in a water bath until 50 °C in temperature. After cooling, 1 mL of the prepared fungicide solutions of six concentrations and control-distilled water was added to the medium (with constant stirring). The final concentration gradients in the PDA media were: 0 (control), 0.00025; 0.0025; 0.025; 0.25; 2.5 and 25 mg L−1 of each fungicide. A medium with different concentrations of fungicides was poured under aseptic conditions into 90 mm diameter Petri dishes and left to solidify at room temperature. Each fungicide was tested against fungal species strains in four replicates.
2.4. Mycelial Growth Inhibition Assay
Sensitivity tests of thirteen F. graminearum, twelve F. culmorum, six F. avenaceum, and nine F. sporotrichioides strains to metconazole, prothioconazole, and tebuconazole were determined by a mycelial growth inhibition method, as described in previous studies [17,31]. All Fusarium isolates were derived from frozen (−80 °C) spore suspensions and grown on PDA medium for 2–3 days. Later, single cultures were transferred to new PDA plates. The 10 mm mycelial plug from the edge of seven-day-old Fusarium colonies was transferred face down to the centers of the prepared PDA plates containing different concentrations of fungicides. The treated plates were incubated at 25 ± 2 °C. The mycelial radial growth (mm) was measured by the crossing method [32] for 3 days until the fungus nearly covered the control plate. The inhibition caused by each fungicide concentration was expressed as a percentage value. We improved the formula [33] slightly by subtracting the diameter of the transferred mycelial plug for data normalization. The percent inhibition was calculated as follows (1):
| Percent growth inhibition (%) = ((dc − dt)/(dc − 10)) × 100%, | (1) |
where dc—colony diameter of control plate (without fungicide), dt—colony diameter in fungicide treated plate, and 10—is the added 10 mm mycelial plug.
The raw data of the experiment are reported in the Supplementary Materials (Table S1).
2.5. Determination of Fungicide Effective Concentration (EC50) and Statistical Analyses
The effective metconazole, prothioconazole, and tebuconazole concentrations, which reduced mycelial growth by 50% (EC50 values), were determined for each Fusarium strain using the ‘drc’ (Dose–Response Curve) package [34] and nonlinear four-parameter log-logistic model (LL.4) [35] in R (version 4.2.3). One-way ANOVA was performed to determine the significance level of each fungicide’s EC50 to Fusarium isolates. The research data were processed by Tukey’s HSD (honestly significant difference) test (p = 0.05) to compare significant differences between isolates. Mean ± SEM (standard error of the mean) was used to describe the variability of measurements. Box-and-whisker plots were made using geom_boxplot function ggplot2 library in R studio to visualize the data (Figure 1 and Figure 2).
Figure 1.
Mycelial growth inhibition (%) of four Fusarium species isolated from various plants at different concentrations of metconazole, prothioconazole, and tebuconazole. Fs—F. sporotrichioides, Fg—F. graminearum, Fc—F. culmorum, Fa—F. avenaceum. Concentrations: 1—0.00025, 2—0.0025, 3—0.025, 4—0.25, 5—2.5, 6—25 mg L−1. The black circle indicates the median inhibition values of all Fusarium isolates from the indicated host plant. The blue bar shows a range from the minimum to maximum values. Error bars indicate ± SE.
Figure 2.
Mycelial growth inhibition (%) of all Fusarium isolates under the influence of different fungicides and their concentrations. The concentrations of fungicides indicated on the Y-axis are 1—0.00025, 2—0.0025, 3—0.025, 4—0.25, 5—2.5, 6—25 mg L−1. The black circle indicates the median inhibition values of all Fusarium isolates from the indicated host plant. The blue bar shows a range from the minimum to maximum values. Error bars indicate ±SE.
3. Results
3.1. Mycelial Growth Inhibition Assay
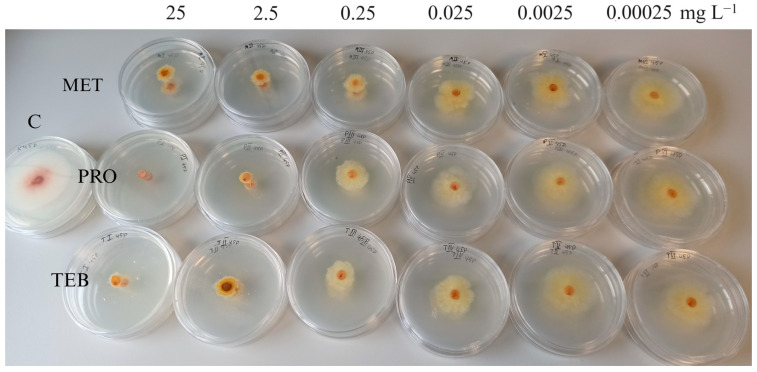
An in vitro sensitivity test of metconazole, prothioconazole, and tebuconazole for four different Fusarium species (F. graminearum, F. avenaceum, F. sporotrichioides, and F. culmorum) isolated from weeds, crops, and spring wheat showed different results. We noticed that F. graminearum and F. culmorum species, which usually cause FHB in Lithuania, were relatively less sensitive to triazoles than other species (Figure 1). Isolates of F. avenaceum, even at low doses, were effectively suppressed. However, negative inhibition values were also detected for this species, while the F. sporotrichioides isolates were shown to be the most sensitive to all triazoles (Figure 3).
Figure 3.
Mycelial growth inhibition of Fusarium sporotrichioides strain (45P) from wheat by different concentrations of metconazole (MET), prothioconazole (PRO), and tebuconazole (TEB). C—control plate without fungicides.
Comparing the mycelial growth inhibition of four Fusarium species obtained from different plants, the least susceptible were the Fusarium isolates from sugar beet and oilseed rape plants (Figure 2). The Fusarium isolates from weed (shepherd’s purse, field pansy, and scentless false mayweed) differed in sensitivity and were least resistant to all fungicides. Also, we noticed that the susceptibility of isolates from meadow grass to fungicides did not vary gradually with concentration. The resistance of these weed isolates to fungicides ranged from weak to complete susceptibility, even at low doses.
3.2. Determination of EC50 Values for Fusarium Isolates
The estimation of the fungicide EC50 values for all Fusarium isolates revealed that metconazole was significantly more efficient in comparison to prothioconazole and tebuconazole. However, no differences were found in the EC50 values between prothioconazole and tebuconazole (Table 2).
Table 2.
Differences in effective fungicide concentrations that reduce mycelial growth by 50% (EC50) between Fusarium species, their source, and fungicides.
| Source | Mean EC50 (mg L−1) 1 | Fusarium Species | Mean EC50 (mg L−1) 1 |
|---|---|---|---|
| Oilseed rape | 8.851 a | F. graminearum | 7.035 a |
| Sugar beet | 8.075 a | F. culmorum | 5.770 ab |
| Wild buckwheat | 6.852 ab | F. avenaceum | 4.194 bc |
| Field pea | 6.762 ab | F. sporotrichioides | 2.561 c |
| Spring wheat | 4.205 bc | Fungicide | Mean EC50 (mg L−1) 2 |
| Meadow grass | 4.196 bc | Metconazole | 0.952 b |
| Shepherd’s purse | 3.291 c | Prothioconazole | 9.315 a |
| Field pansy | 3.229 c | Tebuconazole | 8.315 a |
| Scentless false mayweed | 3.078 |
1 Means within column followed by different letters are significantly different according to Tukey’s HSD test at the p < 0.001 level. 2 Significance at p < 0.05 level.
Generally, the EC50 values of all isolates on metconazole were lower than 2.9 mg L−1, while prothioconazole EC50 ranged from 0.12 to 23.6 mg L−1 and tebuconazole ranged from 0.09 to 15.6 mg L−1 (Table 3, Table 4 and Table 5). The EC50 values for metconazole were 0.3–2.2 mg L−1 for F. avenaceum, 0.18–1.6 mg L−1 for F. culmorum, 0.18–2.9 mg L−1 for F. graminearum, and 0.05–1.9 mg L−1 for F. sporotrichioides. The EC50 values for prothioconazole were significantly higher: 0.12–16.8 mg L−1 for F. avenaceum, 2.4–21.4 mg L−1 for F. culmorum, 2.2–22.9 mg L−1 for F. graminearum, and 0.15–23.5 mg L−1 for F. sporotrichioides. The tebuconazole EC50 values ranged from 1.1 to 15.1 mg L−1 for F. avenaceum, 1.1–3.6 mg L−1 for F. culmorum, 2.6–25.6 mg L−1 for F. graminearum, and 0.09–5.4 mg L−1 for F. sporotrichioides.
Table 3.
Effective concentration of metconazole, prothioconazole, and tebuconazole fungicides that reduces mycelial growth by 50% (EC50) for each Fusarium isolate obtained from weeds.
| Isolate | Code | Source | Fusarium Species | EC50 (mg L−1) 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Met | SEM | Pro | SEM | Teb | SEM | ||||
| 1P | TI1118c | Tripleurospermum inodorum | F. avenaceum | 2.203 ab | 1.936 | 7.672 b | 16.309 | 4.886 a | 5.549 |
| 4P | VA1110f | Viola arvensis | 0.867 bc | 0.559 | 4.918 bc | 5.040 | 5.247 a | 7.791 | |
| 6P | CBP1149c | Capsella bursa-pastoris | 0.307 c | 0.205 | 1.834 bc | 1.756 | 1.149 a | 1.270 | |
| 7P | PA1126s | Poa annua | 0.949 bc | 2.011 | 0.126 c | 2.273 | 3.162 a | 3.807 | |
| 9P | FC1178fl | Fallopia convolvulus | 1.925 ab | 1.707 | 16.879 a | 17.108 | 15.134 a | 3.159 | |
| 14P | TI1330r2 | Tripleurospermum inodorum | F. culmorum | 0.265 c | 0.004 | 2.968 bc | 0.092 | 1.197 a | 0.034 |
| 16P | VA1164f | Viola arvensis | 0.273 c | 0.133 | 15.501 a | 18.611 | 2.364 a | 1.690 | |
| 18P | CBP1401r | Capsella bursa-pastoris | 0.365 c | 0.053 | 18.868 a | 33.419 | 3.691 a | 0.154 | |
| 19P | PA1129c | Poa annua | 0.187 c | 0.241 | 18.756 a | 43.118 | 1.996 a | 69.548 | |
| 21P | FC1088r | Fallopia convolvulus | 0.219 c | 0.134 | 18.214 a | 46.024 | 3.333 a | 0.230 | |
| 25P | TI1120c | Tripleurospermum inodorum | F. graminearum | 0.302 c | 0.009 | 5.808 bc | 2.376 | 10.418 a | 2.271 |
| 28P | VA541s | Viola arvensis | 0.189 c | 0.023 | 5.384 bc | 64.189 | 3.636 a | 2.224 | |
| 29P | CBP1151f | Capsella bursa-pastoris | 0.290 c | 0.002 | 6.339 bc | 0.166 | 6.194 a | 1.490 | |
| 33P | FC144r | Fallopia convolvulus | 2.796 a | 0.011 | 7.911 b | 0.287 | 15.657 a | 0.060 | |
| 38P | TI1135l | Tripleurospermum inodorum | F. sporotrichioides | 0.455 c | 0.127 | 0.262 c | 0.099 | 0.500 a | 0.063 |
| 39P | VA1107f | Viola arvensis | 0.057 c | 0.026 | 0.154 c | 0.030 | 0.164 a | 0.026 | |
| 42P | CBP1148f | Capsella bursa-pastoris | 0.107 c | 0.039 | 0.157 c | 0.025 | 0.187 a | 0.058 | |
| 43P | FC1089c | Fallopia convolvulus | 0.089 c | 0.042 | 0.191 c | 0.015 | 0.167 a | 0.032 | |
1 Means within the column followed by different letters differ significantly according to HSD test (p < 0.05). Met—metconazole, Pro—prothioconazole, Teb—tebuconazole, SEM—standard error of the mean. Letters in code means part of plant: roots (r), crowns (c), stems (s), leaves (l), florets (fl), and fruits (f).
Table 4.
Effective concentration of metconazole, prothioconazole, and tebuconazole fungicides that reduces mycelial growth by 50% (EC50) for each Fusarium isolate obtained from crops.
| Isolate | Code | Source | Fusarium Species | EC50 (mg L−1) 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Met | SEM | Pro | SEM | Teb | SEM | ||||
| 14N | BN26r | Brassica napus | F. culmorum | 0.871 ef | 0.037 | 21.409 abc | 0.091 | 2.399 a | 0.075 |
| 16N | PS7r | Pisum sativum | 1.677 cd | 0.020 | 2.994 g | 0.049 | 2.346 a | 0.004 | |
| 18N | PS23r | Pisum sativum | 0.275 g | 0.066 | 18.148 cde | 0.187 | 1.740 a | 4.958 | |
| 20N | BV15.1l | Beta vulgaris | 0.829 f | 0.053 | 19.608 abcd | 0.152 | 1.969 a | 0.427 | |
| 21N | BV18.1s | Beta vulgaris | 0.212 g | 0.024 | 18.988 bcd | 0.165 | 1.412 a | 0.323 | |
| 27N | BN425l | Brassica napus | F. graminearum | 2.140 b | 0.010 | 15.889 de | 0.006 | 5.945 a | 0.173 |
| 28N | 5PS3p3-2 | Pisum sativum | 1.912 bc | 0.016 | 5.190 fg | 0.045 | 4.259 a | 0.137 | |
| 30N | 5PS3p3-1 | Pisum sativum | 2.144 b | 0.043 | 22.909 ab | 0.046 | 25.623 a | 5.008 | |
| 31N | BV7L6 | Beta vulgaris | 2.812 a | 0.012 | 14.487 e | 0.223 | 22.824 a | 0.089 | |
| 37N | BN9fl1 | Brassica napus | F. sporotrichioides | 1.938 bc | 0.472 | 23.590 a | 71.746 | 5.480 a | 2.019 |
| 42N | PS37s | Pisum sativum | 1.293 de | 0.451 | 6.618 fg | 1.206 | 4.304 a | 1.848 | |
| 43N | BV33.2s | Beta vulgaris | 0.852 ef | 0.230 | 8.708 f | 2.172 | 4.199 a | 1.745 | |
1 Means within column followed by different letters differ significantly according to HSD test (p < 0.05). Met—metconazole, Pro—prothioconazole, Teb—tebuconazole, SEM—standard error of the mean. Letter in code means part of plant: roots (r), stems (s), leaves (l), florets (fl), pods (p).
Table 5.
Effective concentration of metconazole, prothioconazole, and tebuconazole fungicides that reduces mycelial growth by 50% (EC50) for each Fusarium isolate obtained from main host-plant spring wheat.
| Isolate | Code | Source | Fusarium Species | EC50 (mg L−1) 1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Met | SEM | Pro | SEM | Teb | SEM | ||||
| 23N | 8SW5SP2 | Triticum aestivum | F. culmorum | 0.383 bc | 0.005 | 2.406 def | 0.003 | 1.190 cd | 0.058 |
| 23P | 8SWTG5SP4 | 0.217 bc | 0.102 | 19.001 a | 69.432 | 1.461 cd | 0.576 | ||
| 34N | 6SW4SP1 | F. graminearum | 0.325 bc | 0.014 | 5.110 cde | 1.092 | 11.402 ab | 1.496 | |
| 35N | 6SW5SP1 | 0.312 bc | 0.066 | 4.415 cde | 8.570 | 10.155 ab | 0.516 | ||
| 36N | 6SW5SP19 | 0.224 bc | 0.001 | 2.201 ef | 2.677 | 2.674 cd | 1.703 | ||
| 35P | 6SW4SP1 | 2.796 a | 0.011 | 14.718 b | 0.169 | 6.383 bc | 0.111 | ||
| 36P | 6SW5SP1 | 2.967 a | 0.017 | 3.396 cde | 0.019 | 16.232a | 0.213 | ||
| 45N | 9SW5SP17-1 | F. sporotrichioides | 0.851 bc | 0.124 | 5.281 cd | 0.909 | 3.219 cd | 1.281 | |
| 45P | 9SW5SP17-2 | 0.057 c | 0.029 | 0.175 f | 0.055 | 0.094 d | 0.021 | ||
| 12P | 8VK4V10 | F. avenaceum | 1.154 b | 1.813 | 5.428 c | 6.294 | 1.932 cd | 14.249 | |
1 Means within column followed by different letters differ significantly according to HSD test (p < 0.05). Met—metconazole, Pro—prothioconazole, Teb—tebuconazole, SEM—standard error of the mean.
Isolates 1P, 9P (F. avenaceum), and 33P (F. graminearum) obtained from weeds had a significantly higher mean of metconazole EC50 than the other isolates. The EC50 values recorded on prothioconazole were higher than on metconazole, except in the case of the 38P (F. sporotrichioides) and 7P (F. avenaceum) isolates. Prothioconazole showed the lowest growth inhibition of isolate 9P (F. avenaceum), 16P, 18P, 19P, and 21P (F. culmorum). However, the tebuconazole EC50 values did not show any significant difference between the isolates from weeds (p > 0.05). The isolates of F. avenaceum were less sensitive to metconazole than the isolates of F. culmorum, F. graminearum, and F. sporotrichioides. Meanwhile, four F. culmorum and one F. avenaceum isolate were the least susceptible to prothioconazole. In contrast, the F. sporotrichioides isolates were the most sensitive to all fungicides.
The average EC50 values of the Fusarium isolates obtained from crops were slightly higher than the values of those from weeds. In this case, the isolates of all Fusarium species were less sensitive to prothioconazole, and the EC50 varied from 2.9 to 23.6 mg L−1. The most effective fungicide was metconazole, with EC50 values ranging from 0.21 to 2.8 mg L−1. The F. culmorum isolates appeared to be the most sensitive to metconazole compared to the Fusarium species from crops. In contrast, the average prothioconazole EC50 values obtained from this species were the highest.
The Fusarium isolates from wheat were best inhibited by metconazole: the EC50 ranged from 0.05 to 2.9 mg L−1. Only 35P and 36P F. graminearum isolates on metconazole significantly differed from the others. The EC50 values of prothioconazole were the highest for the F. culmorum 23P isolate, while the most sensitive to this fungicide was F. sporotrichioides isolate 45P. The EC50 values for tebuconazole differed among the Fusarium species. The least sensitive were three out of five F. graminearum isolates, with EC50 values of 10.1, 11.4, and 16.2 mg L−1.
4. Discussion
We evaluated the effect of triazole fungicides on different Fusarium species isolated from weeds, crops, and spring wheat. Our study was based on the inhibition of Fusarium mycelial growth in vitro at six different concentrations of metconazole, prothioconazole, and tebuconazole. Metconazole was found to be the most efficient fungicide against Fusarium. This fungicide inhibited the mycelial growth of F. graminearum isolates from weeds and crops, namely, F. culmorum and F. sporotrichioides from crop plants, by 100% at a 25 mg L−1 concentration. However, metconazole at 25 mg L−1 showed a significantly weaker effect on F. sporotrichioides, F. avenaceum, and F. culmorum weed strains at 82%, 93%, and 94%, respectively. Similar to our findings, Ivic et al. [36] conducted a study that showed that metconazole at 10 mg L−1 inhibited F. graminearum growth by 100%, while tebuconazole at the same concentration exhibited an 87.8% inhibition. At low concentrations (0.00025–0.025 mg L−1), the F. graminearum species proved completely resistant to metconazole. These findings are consistent with several reports that have shown that the susceptibility of Fusarium to antifungal agents varies between species [37,38,39]. The mycelial growth of F. graminearum and F. avenaceum species was least inhibited by tebuconazole. These results corroborate the findings of Paul et al. [18] that demonstrated that metconazole and prothioconazole were significantly more effective for reducing FHB than tebuconazole. However, prothioconazole was less effective than tebuconazole for F. sporotrichioides strains from crops. Meanwhile, the growth of isolates of this species from weeds was most effectively inhibited by prothioconazole, followed by tebuconazole and metconazole. The growth of F. culmorum was most effectively inhibited by metconazole and tebuconazole. Additionally, it was observed that, in some cases, azoles more strongly inhibited the growth of Fusarium isolates from weeds than from crop plants. No data were found regarding the susceptibility of Fusarium strains isolated from alternative host plants (weeds) to fungicides.
One interesting finding is that strains of F. avenaceum species were sensitive even to low metconazole concentrations. Some isolates were inhibited up to 82% at 0.00025 mg L−1, while others showed even better growth than the control. These findings demonstrate F. avenaceum species’ huge genetic variety with different levels of resistance to fungicides, and are in agreement with those of Falcão et al. [40] and Gaviria-Rivera et al. [41]. Surprisingly, negative inhibition was observed in the F. avenaceum isolate from spring wheat, whereas the lowest resistance (high growth inhibition) was found in F. avenaceum isolates from meadow grass. Recent studies [42] indicated that the most aggressive F. avenaceum isolate was the least fungicide-sensitive. In our case, the tests of F. culmorum and F. avenaceum from weeds’ sensitivity to fungicides showed that some isolates at 0.00025–0.25 mg L−1 had negative inhibition. This corroborates with other studies [43] suggesting that low concentrations of the active substance of fungicide complex (one of them being tebuconazole) stimulate the accumulation of F. culmorum fungal biomass compared to controls. Cendoya et al. [13] found that sub-lethal doses of fungicides can enhance not only Fusarium growth, but also the production of mycotoxins. It is believed that, under certain conditions, fungicides act as stress factors, inducing the production of mycotoxins until the growth of the fungus is inhibited [40,44].
The study of the effective concentration of the fungicide, which inhibits fungal mycelial growth by 50%, showed that the susceptibility varied among the isolates and species. Fusarium isolates were most sensitive to the fungicide metconazole: the EC50 values were lower than 2.9 mg L−1. The EC50 values for prothioconazole ranged from 0.12 to 23.6 mg L−1 overall, and for tebuconazole, from 0.09 to 15.6 mg L−1. In general, the fungicide acts very differently even in isolates of the same Fusarium species. For example, F. graminearum isolate 35P from spring wheat required a prothioconazole concentration of 14.7 mg L−1 to reduce its growth by 50%, whereas isolate 36P of the same species required only 3.4 mg L−1. In a US study, Anderson et al. [45] showed that 45 F. graminearum isolates collected between 2000 and 2014 had mean EC50 values of 0.0405 and 0.3311 mL L−1 for metconazole and tebuconazole, respectively. Our study obtained higher average values of these fungicides against F. graminearum: 1.478 mg L−1 for metconazole and 10.877 mg L−1 for tebuconazole. Similarly, Liuyuan et al. [46] showed that metconazole was the most effective among other seven fungicides (difenoconazole, epoxiconazole, carbendazim, phenamacril, pydiflumetofan, tebuconazole, and prothioconazole) in inhibiting the growth of F. graminearum with an average EC50 value of 0.032 mg L−1, whereas prothioconazole’s EC50 values were the highest (average 0.55 mL L−1). In our case, the EC50 values of prothioconazole for F. graminearum ranged from 2.2 to 22.9 mg L−1. This study showed that the Fusarium isolates from weeds (scentless false mayweed, field pansy, shepherd’s purse, meadow grass) were more susceptible to fungicides than the isolates from crops. The EC50 values of metconazole for F. avenaceum ranged from 0.3 to 2.203 mL L−1. Meanwhile, the EC50 values for F. culmorum (0.187–0.365 mL L−1), F graminearum (0.189–0.302 mL L−1) (except 33P), and F. sporotrichioides (0.057–0.455 mL L−1) were not statistically significantly different between weed species. Metconazole’s EC50 values obtained for isolates from crops (sugar beet, field pea, and oilseed rape) were higher: from 0.275 to 1.677 mL L−1 for F. culmorum, 1.9–2.812 mL L−1 for F. graminearum, and 0.852–1.9 mL L−1 for F. sporotrichioides. Generally, the Fusarium isolates from sugar beet and oilseed rape required higher concentrations of fungicides to inhibit all Fusarium species’ growth, whereas the lowest EC50 values were obtained with isolates from weeds. We speculate that Fusarium isolates residing in crops are less sensitive to fungicides due to the development of partial resistance due to their constant use on cultivated plants. According to de Chaves et al. [24], fungicide use on a broad scale has the potential to select resistant strains, which enhances the production of mycelium. Our hypothesis that isolates from spring wheat would be the least susceptible to fungicides due to their seasonal application on these plants was not confirmed. The EC50 values for the isolates from spring wheat were significantly lower than those from crop plants, but higher than or similar to those from weeds.
Our previous study [6] evaluated FHB disease severity, AUDPC, and 1000-grain weight in wheat infected with these Fusarium isolates, and results showed that F. graminearum isolate 5SP3p3-1 (30N) caused the highest FHB severity (70%). In our investigation, this most pathogenic isolate was the least sensitive to the fungicide tebuconazole (EC50 25.6 mg L−1) and had the second highest EC50 value for prothioconazole among all isolates. Meanwhile, the lowest severity of the disease was recorded in the F. avenaceum and F. sporotrichioides species, averaging 12.6% and 11.9%, respectively. According to our sensitivity test, we found that F. sporotrichioides and some isolates of F. avenaceum were the most susceptible to fungicides. From the aforementioned study, the 18P (CBP1401r) F. culmorum isolate from weed caused the highest FHB disease intensity and 1000-grain weight loss compared to the control. In our case, the 18P isolate had the highest EC50 values of all fungicides compared to the other F. culmorum isolates from weeds. Another highly pathogenic strain of F. culmorum (21P) required a high concentration of prothioconazole for growth inhibition with an EC50 value of 18.214 mg L−1. This suggests that highly aggressive strains require higher fungicide doses to reduce growth and are less sensitive to fungicides.
Our results indicate a partial development of resistance to triazole fungicides in Fusarium strains residing in cultivated plants compared to those found in weeds. Metconazole, prothioconazole, and tebuconazole are commonly used; however, we recommend avoiding the consecutive use of the same fungicides in the same field against these pathogens. Employing a strategy of mixing or alternating different fungicides can help mitigate the risk of developing resistant fungal strains [47]. We propose considering the use of metconazole alone or in combination with other fungicides to enhance the efficacy against Fusarium head blight (FHB) disease.
5. Conclusions
In conclusion, this study showed substantial differences in the sensitivity of Fusarium species isolated from different plants to metconazole, prothioconazole, and tebuconazole. We determined that F. graminearum and F. culmorum exhibit high resistance to triazole concentrations less than 2.5 mg L−1. Besides this, our data may be useful for understanding fungicides’ effect on the growth inhibition of different Fusarium species (isolated from weeds, crops, and spring wheat). However, this study was only conducted in vitro, so further field studies are needed to carry out susceptibility studies from the perspective of changing times and conditions.
Acknowledgments
This study was supported by the long-term research program ‘Harmful Organisms in Agro and Forest Ecosystems’ implemented by the Lithuanian Research Centre for Agriculture and Forestry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens13020160/s1, Table S1: Mycelial radial growth and growth inhibition (%).
Author Contributions
Conceptualization, N.M., R.Ž., S.S., G.K. and E.Z.; methodology, N.M., S.S., G.K. and R.Ž.; software, N.M.; validation, N.M., R.Ž., S.S., G.K. and E.Z.; formal analysis, R.Ž.; investigation, R.Ž.; resources, G.K.; data curation, R.Ž., N.M. and S.S.; writing—original draft preparation, N.M.; writing—review and editing, N.M., S.S., E.Z., R.Ž. and G.K.; visualization, N.M.; supervision, S.S.; project administration, G.K. and S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dweba C.C., Figlan S., Shimelis H.A., Motaung T.E., Sydenham S., Mwadzingeni L., Tsilo T.J. Fusarium Head Blight of Wheat: Pathogenesis and Control Strategies. Crop Prot. 2017;91:114–122. doi: 10.1016/j.cropro.2016.10.002. [DOI] [Google Scholar]
- 2.Parry D.W., Jenkinson P., McLeod L. Fusarium Ear Blight (Scab) in Small Grain Cereals? A Review. Plant Pathol. 1995;44:207–238. doi: 10.1111/j.1365-3059.1995.tb02773.x. [DOI] [Google Scholar]
- 3.Birr T., Hasler M., Verreet J.A., Klink H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms. 2020;8:617. doi: 10.3390/microorganisms8040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champeil A., Doré T., Fourbet J.F. Fusarium Head Blight: Epidemiological Origin of the Effects of Cultural Practices on Head Blight Attacks and the Production of Mycotoxins by Fusarium in Wheat Grains. Plant Sci. 2004;166:1389–1415. doi: 10.1016/j.plantsci.2004.02.004. [DOI] [Google Scholar]
- 5.Ekwomadu T.I., Akinola S.A., Mwanza M. Fusarium Mycotoxins, Their Metabolites (Free, Emerging, and Masked), Food Safety Concerns, and Health Impacts. Int. J. Environ. Res. Public Health. 2021;18:11741. doi: 10.3390/ijerph182211741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavtrikovienė E., Gorash A., Kadžienė G., Matelionienė N., Supronienė S. Pathogenicity of Asymptomatically Residing Fusarium Species in Non-Gramineous Plants and Weeds to Spring Wheat under Greenhouse Conditions. Pathogens. 2022;11:1467. doi: 10.3390/pathogens11121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkinson P., Parry D.W. Isolation of Fusarium Species from Common Broad-Leaved Weeds and Their Pathogenicity to Winter Wheat. Mycol. Res. 1994;98:776–780. doi: 10.1016/S0953-7562(09)81054-X. [DOI] [Google Scholar]
- 8.Postic J., Cosic J., Vrandecic K., Jurkovic D., Saleh A.A., Leslie J.F. Diversity of Fusarium Species Isolated from Weeds and Plant Debris in Croatia. J. Phytopathol. 2012;160:76–81. doi: 10.1111/j.1439-0434.2011.01863.x. [DOI] [Google Scholar]
- 9.Suproniene S., Kadziene G., Irzykowski W., Sneideris D., Ivanauskas A., Sakalauskas S., Serbiak P., Svegzda P., Kelpsiene J., Pranaitiene S., et al. Asymptomatic Weeds Are Frequently Colonised by Pathogenic Species of Fusarium in Cereal-based Crop Rotations. Weed Res. 2019;59:312–323. doi: 10.1111/wre.12367. [DOI] [Google Scholar]
- 10.Gerling M., Pätzig M., Hempel L., Büttner C., Müller M.E.H. Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi. Agronomy. 2022;12:823. doi: 10.3390/agronomy12040823. [DOI] [Google Scholar]
- 11.Figueroa M., Hammond-Kosack K.E., Solomon P.S. A Review of Wheat Diseases—A Field Perspective. Mol. Plant Pathol. 2018;19:1523–1536. doi: 10.1111/mpp.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegulo S.N., Baenziger P.S., Hernandez Nopsa J., Bockus W.W., Hallen-Adams H. Management of Fusarium Head Blight of Wheat and Barley. Crop Prot. 2015;73:100–107. doi: 10.1016/j.cropro.2015.02.025. [DOI] [Google Scholar]
- 13.Cendoya E., Nichea M.J., del Pilar Monge M., Zachetti V.G.L., Chiacchiera S.M., Ramirez M.L. Effect of Fungicides Commonly Used for Fusarium Head Blight Management on Growth and Fumonisin Production by Fusarium proliferatum. Rev. Argent. Microbiol. 2021;53:64–74. doi: 10.1016/j.ram.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Haidukowski M., Visconti A., Perrone G., Vanadia S., Pancaldi D., Covarelli L., Balestrazzi R., Pascale M. Effect of Prothioconazole-Based Fungicides on Fusarium Head Blight, Grain Yield and Deoxynivalenol Accumulation in Wheat under Field Conditions. Phytopathol. Mediterr. 2012;51:236–246. [Google Scholar]
- 15.Tini F., Beccari G., Onofri A., Ciavatta E., Gardiner D.M., Covarelli L. Fungicides May Have Differential Efficacies towards the Main Causal Agents of Fusarium Head Blight of Wheat. Pest Manag. Sci. 2020;76:3738–3748. doi: 10.1002/ps.5923. [DOI] [PubMed] [Google Scholar]
- 16.Somma S., Scarpino V., Quaranta F., Logrieco A.F., Reyneri A., Blandino M., Moretti A. Impact of Fungicide Application to Control T-2 and HT-2 Toxin Contamination and Related Fusarium sporotrichioides and F. Langsethiae Producing Species in Durum Wheat. Crop Prot. 2022;159:106020. doi: 10.1016/j.cropro.2022.106020. [DOI] [Google Scholar]
- 17.Masiello M., Somma S., Ghionna V., Logrieco A.F., Moretti A. In Vitro and in Field Response of Different Fungicides against Aspergillus flavus and Fusarium Species Causing Ear Rot Disease of Maize. Toxins. 2019;11:11. doi: 10.3390/toxins11010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul P.A., Lipps P.E., Hershman D.E., McMullen M.P., Draper M.A., Madden L.V. Efficacy of Triazole-Based Fungicides for Fusarium Head Blight and Deoxynivalenol Control in Wheat: A Multivariate Meta-Analysis. Phytopathology. 2008;98:999–1011. doi: 10.1094/PHYTO-98-9-0999. [DOI] [PubMed] [Google Scholar]
- 19.Jørgensen L.N., Heick T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation—Is It Indispensable? Front. Cell Infect. Microbiol. 2021;11:806. doi: 10.3389/fcimb.2021.730297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Domínguez E., Meriggi P., Ruggeri M., Rossi V. Efficacy of Fungicides against Fusarium Head Blight Depends on the Timing Relative to Infection Rather than on Wheat Growth Stage. Agronomy. 2021;11:1549. doi: 10.3390/agronomy11081549. [DOI] [Google Scholar]
- 21.D’Angelo D.L., Bradley C.A., Ames K.A., Willyerd K.T., Madden L.V., Paul P.A. Efficacy of Fungicide Applications during and after Anthesis against Fusarium Head Blight and Deoxynivalenol in Soft Red Winter Wheat. Plant Dis. 2014;98:1387–1397. doi: 10.1094/PDIS-01-14-0091-RE. [DOI] [PubMed] [Google Scholar]
- 22.Mcmullen M., Halley S., Schatz B., Meyer S., Jordahl J., Ransom J. Integrated Strategies for Fusarium Head Blight Management in the United States. Cereal Res. Commun. 2008;36:563–568. doi: 10.1556/CRC.36.2008.Suppl.B.45. [DOI] [Google Scholar]
- 23.Ribas e Ribas A.D., Spolti P., Del Ponte E.M., Donato K.Z., Schrekker H., Fuentefria A.M. Is the Emergence of Fungal Resistance to Medical Triazoles Related to Their Use in the Agroecosystems? A Mini Review. Braz. J. Microbiol. 2016;47:793–799. doi: 10.1016/j.bjm.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Chaves M.A., Reginatto P., da Costa B.S., de Paschoal R.I., Teixeira M.L., Fuentefria A.M. Fungicide Resistance in Fusarium graminearum Species Complex. Curr. Microbiol. 2022;79:62. doi: 10.1007/s00284-021-02759-4. [DOI] [PubMed] [Google Scholar]
- 25.Janaviciene S., Venslovas E., Kadziene G., Matelioniene N., Berzina Z., Bartkevics V., Suproniene S. Diversity of Mycotoxins Produced by Fusarium Strains Infecting Weeds. Toxins. 2023;15:420. doi: 10.3390/toxins15070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matelionienė N., Supronienė S., Shamshitov A., Zavtrikovienė E., Janavičienė S., Kadžienė G. Weeds in Cereal Crop Rotations May Host Fusarium Species That Cause Fusarium Head Blight and Grain Weight Losses in Wheat. Agronomy. 2022;12:2741. doi: 10.3390/agronomy12112741. [DOI] [Google Scholar]
- 27.Leslie J.F., Summerell B.A. In: The Fusarium Laboratory Manual. Leslie J.F., Summerell B.A., editors. Wiley; Hoboken, NJ, USA: 2006. [Google Scholar]
- 28.Sneideris D., Ivanauskas A., Prakas P., Butkauskas D., Treikale O., Kadziene G., Rasiukeviciute N., Kelpsiene J., Suproniene S. Population Structure of Fusarium graminearum Isolated from Different Sources in One Area over the Course of Three Years. Phytopathology. 2020;110:1312–1318. doi: 10.1094/PHYTO-08-19-0298-R. [DOI] [PubMed] [Google Scholar]
- 29.Demeke T., Clear R.M., Patrick S.K., Gaba D. Species-Specific PCR-Based Assays for the Detection of Fusarium Species and a Comparison with the Whole Seed Agar Plate Method and Trichothecene Analysis. Int. J. Food Microbiol. 2005;103:271–284. doi: 10.1016/j.ijfoodmicro.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Suproniene S., Kadziene G., Irzykowski W., Sneideris D., Ivanauskas A., Sakalauskas S., Serbiak P., Svegzda P., Auskalniene O., Jedryczka M. Weed Species within Cereal Crop Rotations Can Serve as Alternative Hosts for Fusarium graminearum Causing Fusarium Head Blight of Wheat. Fungal. Ecol. 2019;37:30–37. doi: 10.1016/j.funeco.2018.10.002. [DOI] [Google Scholar]
- 31.Duan Y., Tao X., Zhao H., Xiao X., Li M., Wang J., Zhou M. Activity of Demethylation Inhibitor Fungicide Metconazole on Chinese Fusarium Graminearum Species Complex and Its Application in Carbendazim-Resistance Management of Fusarium Head Blight in Wheat. Plant Dis. 2019;103:929–937. doi: 10.1094/PDIS-09-18-1592-RE. [DOI] [PubMed] [Google Scholar]
- 32.Antoine B., Kouabenan A., Mohamed D., Gaston K., Fernand K., Seydou T., Koné N., Pacôme K., Daouda K. Analysis of Sclerotinia Expression Due to Sclerotium rolfsii Fungus in Market Gardening Crops in the Different Agroecological Zones of Côte d’Ivoire. J. Agric. Ecol. Res. Int. 2016;6:1–12. doi: 10.9734/JAERI/2016/23681. [DOI] [Google Scholar]
- 33.Vincent J.M. Distortion of Fungal Hyphæ in the Presence of Certain Inhibitors. Nature. 1947;159:850. doi: 10.1038/159850b0. [DOI] [PubMed] [Google Scholar]
- 34.Lindl T., Lewandowski B., Schreyögg S., Stäudte A. An Evaluation of the In Vitro Cytotoxicities of 50 Chemicals by Using an Electrical Current Exclusion Method versus the Neutral Red Uptake and MTT Assays. Altern. Lab. Anim. 2005;33:591–601. doi: 10.1177/026119290503300614. [DOI] [PubMed] [Google Scholar]
- 35.Wang R., Zhang H., Sun L., Qi G., Chen S., Zhao X. Microbial Community Composition Is Related to Soil Biological and Chemical Properties and Bacterial Wilt Outbreak. Sci. Rep. 2017;7:343. doi: 10.1038/s41598-017-00472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivic D., Sever Z., Kuzmanovska B. In Vitro Sensitivity of Fusarium graminearum, F. Avenaceum and F. Verticillioides to Carbendazim, Tebuconazole, Flutriafol, Metconazole and Prochloraz. Pestic. Fitomedicina. 2011;26:35–42. doi: 10.2298/PIF1101035I. [DOI] [Google Scholar]
- 37.Müllenborn C., Steiner U., Ludwig M., Oerke E.C. Effect of Fungicides on the Complex of Fusarium Species and Saprophytic Fungi Colonizing Wheat Kernels. Eur. J. Plant Pathol. 2008;120:157–166. doi: 10.1007/s10658-007-9204-y. [DOI] [Google Scholar]
- 38.Al-Hatmi A.M.S., Van Diepeningen A.D., Curfs-Breuker I., Sybren de Hoog G., Meis J.F. Specific Antifungal Susceptibility Profiles of Opportunists in the Fusarium Fujikuroi Complex. J. Antimicrob. Chemother. 2014;70:1068–1071. doi: 10.1093/jac/dku505. [DOI] [PubMed] [Google Scholar]
- 39.Blaize M., Normand A.C., Imbert S., Al-Hatmi A.M.S., Chryssanthou E., Cassaing S., Schuttler C., Hasseine L., Mahinc C., Costa D., et al. Antifungal Susceptibility of 182 Fusarium Species Isolates from 20 European Centers: Comparison between Eucast and Gradient Concentration Strip Methods. Antimicrob. Agents Chemother. 2021;65:12. doi: 10.1128/AAC.01495-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falcão V.C.A., Ono M.A., de Ávila Miguel T., Vizoni E., Hirooka E.Y., Ono E.Y.S. Fusarium verticillioides: Evaluation of Fumonisin Production and Effect of Fungicides on In Vitro Inhibition of Mycelial Growth. Mycopathologia. 2011;171:77–84. doi: 10.1007/s11046-010-9339-9. [DOI] [PubMed] [Google Scholar]
- 41.Gaviria-Rivera A., Giraldo-López A., Cano-Restrepo L.E. In Vitro Antifungal Susceptibility of Clinical Isolates of Fusarium from Colombia. Rev. Salud Publica. 2017;19:800–805. doi: 10.15446/rsap.v19n6.54654. [DOI] [PubMed] [Google Scholar]
- 42.Garmendia G., Pattarino L., Negrín C., Martínez-Silveira A., Pereyra S., Ward T.J., Vero S. Species Composition, Toxigenic Potential and Aggressiveness of Fusarium Isolates Causing Head Blight of Barley in Uruguay. Food Microbiol. 2018;76:426–433. doi: 10.1016/j.fm.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Orina A., Gavrilova O., Gagkaeva T. The Effect of Fungicides on Growth of Fusarium Fungi in Vitro. BIO Web Conf. 2020;18:00022. doi: 10.1051/bioconf/20201800022. [DOI] [Google Scholar]
- 44.Kulik T., LŁojko M., Jestoi M., Perkowski J. Sublethal Concentrations of Azoles Induce Tri Transcript Levels and Trichothecene Production in Fusarium graminearum. FEMS Microbiol. Lett. 2012;335:58–67. doi: 10.1111/j.1574-6968.2012.02637.x. [DOI] [PubMed] [Google Scholar]
- 45.Anderson N.R., Freije A.N., Bergstrom G.C., Bradley C.A., Cowger C., Faske T., Hollier C., Kleczewski N., Padgett G.B., Paul P., et al. Sensitivity of Fusarium graminearum to Metconazole and Tebuconazole Fungicides Before and After Widespread Use in Wheat in the United States. Plant Health Prog. 2020;21:85–90. doi: 10.1094/PHP-11-19-0083-RS. [DOI] [Google Scholar]
- 46.Liuyuan F.U., Jinpeng C., Shuan W., Jia J., Zhiping C.H.E., Yuee T., Genqiang C., Shengming L.I.U. Baseline Sensitivity of Fusarium graminearum to Difenoconazole and Sensitivity Correlation to Other Fungicides. Chin. J. Pestic. Sci. / Nongyaoxue Xuebao. 2021;23:694–702. [Google Scholar]
- 47.Corkley I., Fraaije B., Hawkins N. Fungicide Resistance Management: Maximizing the Effective Life of Plant Protection Products. Plant Pathol. 2022;71:150–169. doi: 10.1111/ppa.13467. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.