Abstract
Forest production has great relevance in the Brazilian economy, characterized by several production sectors, including the production of seedlings. With the focus on maximizing the capacity of survival, development, and adaptation of seedlings, Trichoderma is highlighted as a potentially useful genus of microorganisms for promoting growth and higher product quality. In this sense, this review aims to describe the main mechanisms of fungi action in forest seedlings’ production. The different species of the genus Trichoderma have specific mechanisms of action, and the current scenario points to more advances in the number of species. The interaction process mediated by different mechanisms of action begins in the communication with plants, from the colonization process. After the interaction, chemical dialogues allow the plant to develop better because, from colonization, the forest seedlings can maximize height and increase shoot and root development. Fungi promote solubilization and availability of nutrients to seedlings, which show numerous benefits to the development. The use of beneficial microorganisms, such as fungi of the genus Trichoderma, has become a sustainable strategy to enhance seedling development, reducing the use of agrochemicals and industrial fertilizers.
Keywords: plant–microorganism interaction, synergism, growth promotion
1. Introduction
Forest production has great relevance in the Brazilian GDP, characterized by several production sectors, including seedlings. Despite the global scenario of the COVID-19 pandemic (SARS-CoV-2), the forest production chain showed resilience in the Brazilian market, with a growth of 7.5% in 2021, higher than the evolution of the national GDP [1]. This sector has great economic importance, intended for commercial plantations and recovery plans of degraded areas, which denotes the need to expand the capacity of seedling production and obtain higher production rates [2].
Despite the relevance of the forestry sector, in Brazil, there is still a great dependence on the import of inputs, with emphasis on synthetic fertilizers, which requires measures correlated with the reduction in the use of fertilizers and adequate practices to obtain success in the production of seedlings [3]. The production of woody plants promotes environmental and economic benefits by reducing the use of chemicals, including having less soil and water contamination, having sustainable production, spending less time on the formation of seedlings, and having higher-quality parameters [4].
To obtain high-quality seedlings, it is necessary to achieve high growth parameters arising from morphological and physiological attributes that reflect the development capacity and survival in the field. The morphological attributes are used to evaluate the ability of plant development, measured by height, the diameter of the neck, and root development. In contrast, the physiological attributes are obtained through the capacity of absorption of water and nutrients by the plant to provide important information on the performance of the species and efficiency in the production of seedlings, because vigorous seedlings with high field performance are defined in the initial phase of seedling establishment and development [5,6].
However, there are gaps in adopting technologies that increase biomass production with lower costs and production time. There are technologies aimed to improve the germination potential of seeds. To maximize the survival, development, and adaptation capacity of seedlings, the inoculation of microorganisms can improve growth and higher production quality [7,8].
When inoculated in seedlings and after the colonization of the root system by Trichoderma, changes occur in plant metabolism and increase root development, growth, and nutrition of plants [9]. The main mechanism of interaction between Trichoderma and seedlings occurs via chemical signaling, resulting from the production of compounds responsible for modifications in the transcriptome, proteome, and plant metabolome [10].
From there, the beneficial action of fungi begins via the modulation of molecular centers and prolonged systemic responses that stimulate plant development [11,12]. As there are broad benefits promoted by fungi of the genus Trichoderma in plant development, the objective of this review was to discuss the main mechanisms of action of fungi in the production of forest seedlings.
2. Fungi of the Genus Trichoderma: Beneficial Microorganisms
The genus Trichoderma is the imperfect phase of Hypocrea, belonging to the Kingdom Fungi, Phylum Ascomycota, Class Sordariomycetes, Order Hypocreales, and Family Hypocreaceae [13]. It comprises many species of free-living filamentous fungi in multiple ecosystems, from tropical to temperate regions, characterized by accelerated growth. They are considered highly active species in the soil, associated with the rhizosphere and the decomposition process of plant residues and wood, and are rarely associated with plant diseases [14].
The fungi Trichoderma spp. have bright green conidia and a repeatedly branched conidiophore, are opportunistic and avirulent plant symbionts, and have asexual reproduction by the production of conidia and chlamydospores and in wild habitats by ascospores [9]. In recent years, there has been an exponential increase in the number of species identified in the genus, and the current scenario points to further advances in the number of species [15].
3. Interaction Process: Trichoderma spp. and Forest Species
During the production process, the seedlings are generally subjected to several abiotic factors (climate, temperature, water availability) and/or biotic stress (phytopathogenic agents). These stresses may lead to considerable restrictions to obtain vigorous seedlings with high-quality indexes. The inoculation of Trichoderma fungi enhances acclimatization, resistance/tolerance through plant response mechanisms under adverse conditions and, above all, induces greater growth of seedlings [16,17,18].
The colonization of roots results in physical or biochemical responses, initiating chemical transmission, in which plants produce secondary metabolites (SM), constituting limiting factors for the invasion of the fungus in the cortical cell layers in the roots [19]. According to the barriers imposed by plants, Trichoderma fungi can produce strategies to “dribble” plant responses; this occurs via hormone production to establish a prolonged mutualistic association without the occurrence of obstacles that can make the root establishment and the symbiotic process unfeasible [10].
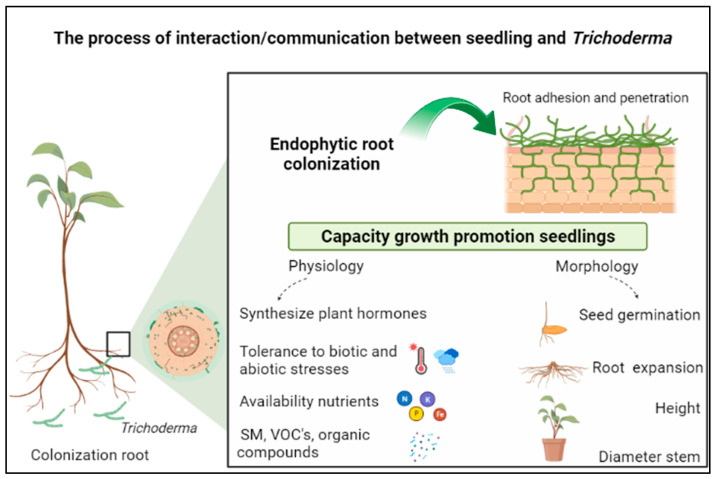
The interaction process between Trichoderma and plants is mediated by different mechanisms of action that begin in the communication with plants from the root colonization process. Figure 1 illustrates the stages of the interaction process between Trichoderma spp. and the host plant, starting via the release of root exudates, recognized by the fungus and responsible for chemotaxis (release of secondary metabolites). Subsequently, the fungus adheres to the root surface via protein action (hydrophobins) and produces enzymes responsible for cell degradation (cellulolytic, proteolytic, pectinolytic, and xylanolytic) and colonization of the root epidermis and cortex.
Figure 1.
Interaction processes between Trichoderma spp. and forest seedlings. Created in Biorender.
4. Potential Mechanisms of Interaction
Trichoderma fungi cause physiological changes and plant metabolism when colonizing the root system. From a practical point of view, the interaction is promising since it allows high-quality indexes of seedlings to be achieved, according to the release of compounds, which the root system will assimilate, solubilizing and absorbing nutrients by plants [9].
The ability to synthesize plant hormones is commonly observed in Trichoderma species [20,21]. Auxins, abscisic acid, cytokinin, ethylene, and gibberellins stimulate plant growth, especially in adverse conditions [22]. The biosynthesis of indole-3-acetic acid (IAA) performed by the fungus increases root development and production of secondary roots and root hairs [23].
The expansion of the root system occurs through endophytic colonization and the production of phytohormones in the induction of water and nutrient efficiency by plants, in addition to greater tolerance to biotic and abiotic stresses [24]. In the dynamic plant–microorganism communication process, the interaction with roots and other parts of the plant can be influenced by different factors, such as soil type, the potential of strains, and plant species [25].
4.1. Solubilization and Availability of Mineral Nutrients
Despite the high nutritional requirement of forest seedlings, Brazilian soils generally have low natural fertility and a high degree of weathering, which limit obtaining greater production potential since the low availability of nutrients in the soil reduces the efficiency of absorption and use of macro and micronutrients by plants [26]. Considering that the soil composition is a fundamental part of the forest production system, it is important to adopt management practices to maintain its physical–chemical–biological quality and obtain vigorous, productive, and profitable woody plants [27].
In general, the mineral nutrients available to plants cannot meet the appropriate demands in the production of vigorous seedlings; this factor is associated with the existence of and functions performed by microorganisms in the soil [28]. In this sense, a strategy to reduce the nutritional restriction to the production of seedlings becomes necessary to potentiate the production. Therefore, the adequate absorption of nutrients is important during the initial phase of seedling formation, with emphasis on phosphorus (P) because the insufficient nutrition restricts the production and quality of plants [29].
The macronutrient phosphorus (P) in soils occurs in two forms: organic (plant decomposition) and inorganic (salts such as calcium (Ca), iron (Fe), and aluminum (Al)). However, most of it is in the inorganic and insoluble form, making its availability to plants unfeasible [30]. Phosphate rock is the world’s main P source; however, seedlings’ production depends on the continuous supply of phosphate fertilizers [31]. In this scenario, aiming at less dependence, there is a growing need to seek and adopt sustainable strategies capable of improving P availability [32].
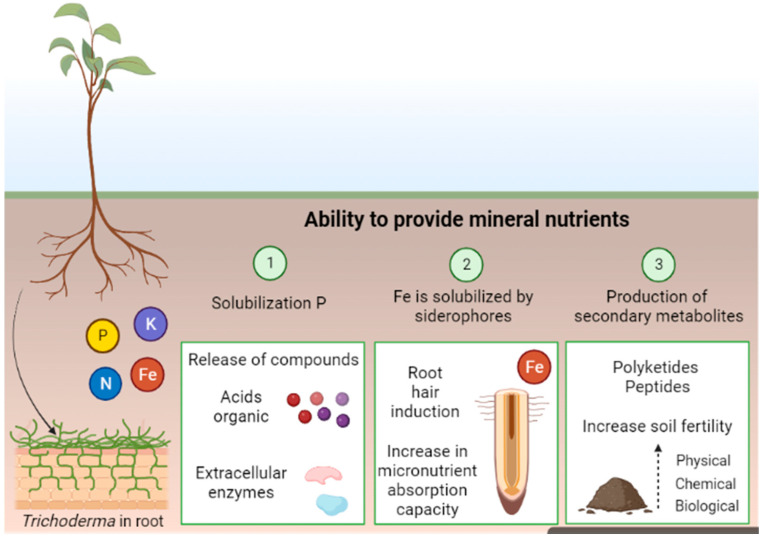
Fungi release substances such as volatile organic compounds (VOCs) and SM to form complexes with Fe (III) or reduce such element [33]. Fe is solubilized by siderophores and changes root morphology via induction of root hairs, which enables the absorption of this micronutrient, and this process is involved in several metabolic processes [34,35]. These mechanisms are the most common among Trichoderma fungi; however, the responses can be variable according to the capacity of each strain [36].
The nutritional flow system is mediated by complex interactions influenced by chemical reactions between the root system and Trichoderma fungi. The strains of the genus Trichoderma have different mechanisms of action on soil nutrients; however, the ability to solubilize and mineralize P is emphasized, transforming it into soluble forms for plant absorption [37,38]. Figure 2 illustrates the interaction process that is coordinated by multiple actions of microorganisms in the solubilization of P from biochemical mineralization (enzymatic release) and secretion of chemical complexes responsible for mineral solubilization (siderophores, protons, hydroxyl ions, organic acids), increasing P absorption by seedlings [39,40,41,42].
Figure 2.
The mechanisms by fungi of the genus Trichoderma in association with woody plant species. Created in Biorender.
In addition to organic compounds, the nutritional availability reaction can occur by producing secondary metabolites (SM), such as polyketides and peptides, which increase soil fertility [42]. It is worth mentioning that the greater the ability to obtain resources from the root system, the greater the development and survival of seedlings in the field [43]. Plants more efficient at absorbing water and nutrients can increase photosynthetic potential, achieving higher root systems [44].
4.2. Production of Organic Compounds, Secondary Metabolites, and Plant Hormones
The chemical signals released from the interaction between plant and Trichoderma produce complex association responses [45]. However, the effects obtained depend on the ability of Trichoderma strains to act on the production of chemical compounds, which promote biochemical changes in plants (Table 1).
Table 1.
Relationship between the production of chemical compounds by species of Trichoderma spp. and functions that promote growth.
| Species | Chemical Production | Activity | References |
|---|---|---|---|
|
T. asperellum; T. harzianum; T. viride; T. koningii |
Siderophores | Fe chelating agents, in this process, Fe3+ siderophores are recognized and absorbed by plants, adopting a key role in the availability of the micronutrient | [46,47,48] |
| T. harzianum | Terpenes | Provides signals to plants that trigger changes in growth | [49,50,51] |
| T. harzianum | Metabolites of isocyanate | Positive impact on the symbiosis process | [50] |
|
T. harzianum; T. koningii; T. viride |
Pyrones | 6-pentyl-2H-Piran-2-one (6-PP): chemical signaling via induction of auxin and ethylene formation, which modulates root architecture (formation of root hairs) Promotes seed germination and seedling development | [52,53,54] |
|
T. asperellum; T. harzianum; T. koningii; T. viride |
Synthesis of phytohormones | High rhizosphere competence. Performs the biosynthesis of indole-3-acetic acid (IAA), capable of modifying the root architecture and increasing root mass and rate of absorption of nutrients by the plant | [23,55] |
Furthermore, the production of chemical compounds is influenced by environmental conditions, and temperature, humidity, and soil pH are determining factors, considering that each species of Trichoderma produces different types of compounds and positively affects plant growth [49]. The VOCs belong to several chemical classes: mono and sesquiterpenes, alcohols, ketones, lactones, esters, phenols, thioesters, and cyclohexenes [56]. Fungi can produce different types of VOCs used to promote plant growth [57]. This process occurs from producing bioactive compounds and modulation of plant hormones, with benefits to increase root volume, plant biomass, and productivity [58,59].
5. Promoting the Growth of Forest Species
The development of seedlings depends on the adequate management of essential resources to plant functioning, and the phase of establishing of the seedlings has greater vulnerability to restrictive and adverse conditions (biotic and abiotic stresses) [60]. The use of potential microorganisms such as Trichoderma fungi can solve this problem and benefit the development of forest seedlings with mechanisms of action that improve plant growth, especially under adverse conditions. Under abiotic stress, Trichoderma benefits plant development by producing secondary metabolites, hydrolytic enzymes, phytohormones, and siderophores. Under biotic stresses, Trichoderma promotes biocontrol actions (parasitism, competition, and antagonism). In addition, members of this genus of fungi increase nutritional acquisition (production of secondary metabolites, phosphate solubilization, decomposition of organic matter) to promote greater availability of nutrients to plants.
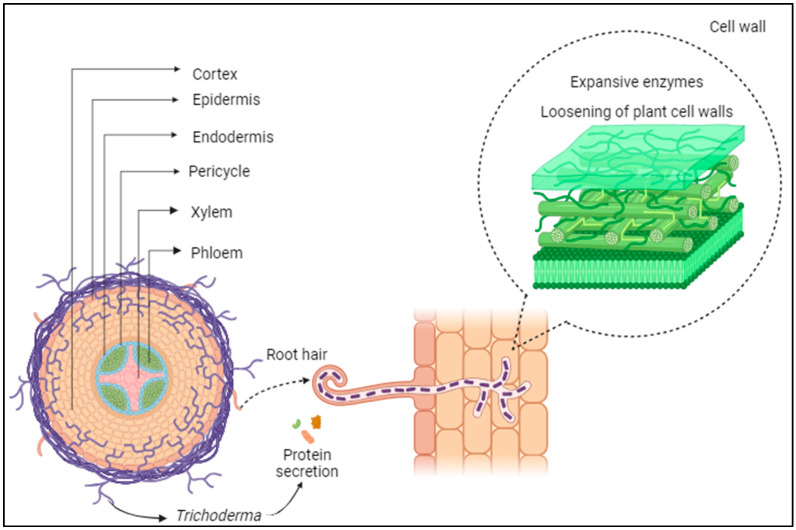
Figure 3 demonstrates that root colonization begins from the interaction of chemical signals between plant and fungus, composed of fixation, penetration, and root colonization, intermediated by the production of metabolites [46]. For the fungus to be able to colonize the roots, protein secretion must occur to loosen the plant cell wall and then facilitate root penetration and intercellular growth, limited to the epidermal layer and the external cortex [61]. The hydrophobic proteins, rich in cysteine, are involved in the initial bond with the root surface [62]; subsequently, the action of the expansive enzymes allows the loosening of plant cell walls through non-covalent interactions to maintain their integrity [63].
Figure 3.
Trichoderma in fixation, penetration, and root colonization. Created in Biorender.
As a mechanism of interaction, the plants provide sucrose to the fungi Trichoderma to optimize their development and consequent root colonization. From this stage, after the mediation by hydrophobic proteins occurs, the installation and adhesion of Trichoderma on the plant root surface of host plants begins. These steps are important to the functioning of cell communication processes, fungal morphogenesis, and adherence of hyphae to hydrophobic surfaces [54,64].
Communication between forest seedlings and Trichoderma is complex and dynamic, based on the exchange and perception of chemical signals [65]. The extensive dialogue in the early stages of interaction releases the exudation of important compounds such as volatile organic compounds, secondary metabolites, and plant hormones [66]. Such substances greatly influence the seedling formation process, emphasizing the benefits from the seed germination process to the emergence of seedlings and seedling formation with high quality [7].
From there, the endophytic colonization begins, where the microorganism assists the development and growth of seedlings through the emission of chemical signals that allow the best plant development due to the symbiosis process. Consequently, shoot and root biomass are improved; seedlings also have greater resistance and responses to stress conditions [67,68].
Studies with the inoculation of Trichoderma in species of forest seedlings have shown positive effects on its development. In seedlings of Acacia auriculiformis, inoculation with strains of Trichoderma sp. increased plant total dry biomass compared to those not inoculated with the fungus [69]. In the production of argan seedlings (Agrania spinosa), after root colonization by Trichoderma, benefits were observed in the growth parameters through greater seed germination potential, root development, and plant height [70].
VOCs regulate the hormonal concentration of seedlings, which reflects in higher biomass production and yield through increased root volume [71], through the ability to induce better redistribution of auxins in the roots, and growth of seedlings [72]. The potential of SM infers significant gains in forest seedlings through increased seedling production [73]. In Acacia mangium, the inoculation of T. viride improved seedling dry matter, which can be explained by the higher absorption capacity of Fe by plants through the production of siderophores, VOCs, and secretion of hydrolytic enzymes [74,75].
Phytohormones (IAA, cytokinins, gibberellins) associated with SM synthesized by Trichoderma allow the increase of plant height and root development, as observed in Camellia sinensis [76]. In Bougainvillea spectabilis, soil treated with T. longibrachiatum stimulated root production in cuttings through the production of indole-3-butyric acid (IBA) and α-naphthalene acetic acid (NAA); such auxin regulators contribute to the development of morphological characteristics of seedlings, with emphasis on root development [77]. T. virens in oil palm seedlings (Elaeis guineensis) contributed to the increase in the production of growth factors and phytohormones by both isolates and consequent effective growth [78].
Trichoderma strains exude SM in olive seedlings (Olea europaea), which increases height, leaf area, stem diameter, and seedling clearance in nursery conditions [79,80]. One of the most promising functions performed by secondary metabolites refers to the multiplication of plant cells, in which the action performed by Trichoderma strains correlated with higher rates of germination speed and germination percentages and factors for obtaining seedlings with high morphological and physiological parameters [81].
The greater root development is one of the main effects provided by Trichoderma sp. because the growth of seedlings is optimized through the greater capacity of root exploration, which influences the wide capacity of absorption of macro and micronutrients of the soil [82]. The development of seedlings is optimized by the proper use of macro and micronutrients, with emphasis on nitrogen (N), phosphorus (P), potassium (K), and iron (Fe), required in the seedling formation stage, through changes in the anchoring of the root system or exudation of metabolites [83].
The root is the organ most susceptible to environmental variations, constituting a barrier to the survival of seedlings [84]. Some species of the genus Trichoderma are capable of excreting metabolites, auxin analogs, and other protein compounds around the root system, promoting the increase of primary and secondary roots, as well as stimulating the production of root hairs, which increase absorption of nutrients [85].
The fungi sequester the phosphate in the unavailable form in the soil through their mycelium and then release it to the seedlings in the readily available form [86]. This mechanism was observed in Hevea brasiliensis, where Trichoderma promoted the solubilization of insoluble phosphate into available phosphate due to the release of organic acid (citric acid), which promoted the development of rubber tree seedlings in the nursery [87,88].
Stimulating the development of the root system, height, and stem diameter contributes to the production of forest seedlings, with stem diameter in the seedling production phase being one of the desirable factors for reducing seedling time in nurseries [89]. In Euterpe oleracea seedlings inoculated with Trichoderma, there was interaction and consequent increases in stem diameter, which explains a greater capacity for survival of seedlings in the field [90].
Macro and micronutrients are involved in photosynthesis because such activity requires a series of chemical and physiological steps related to the adequate supply of nutrients [91]. As the interaction between Trichoderma and plant promotion occurs, seedling photosynthesis and total water content of Quercus robur L. avoid the reduction of energy during water transpiration [92]. In addition, reports are showing that photosynthetic capacity is high due to gene regulation developed by Trichoderma sp. to make the seedlings more resistant to adverse conditions and achieve higher-quality indexes [93].
During the initial stage of development of forest seedlings, they require adequate energy for greater efficiency in producing photoassimilates. During the growth of seedlings, the energy requirement is increased [94]. Plants synthesize carbohydrates through photosynthesis and obtain the energy content necessary for the breathing process, which is fundamental to the conduction of physiological activities for maintenance and growth of seedlings [95]. These strategies corroborate the success in the silvicultural sector destined to produce seedlings, providing favorable conditions to obtain more productive seedlings, a decisive stage for the good development of forest stands.
Practical Examples of Trichoderma in the Formation of Forest Seedlings
The use of Trichoderma spp. in soil treatment is revealed as an alternative of great technological innovation, constituting a mechanism that promotes distinct gains in forest seedlings’ development, quality, and growth. The effectiveness in promoting plant growth comes from species capable of establishing lasting interactions with the plant, considering that the association is highly variable, whether in the function of the fungus species, development conditions, inoculum rate, or type of formulation [96]. Table 2 reports the symbiotic association between different species of Trichoderma and woody species capable of maximizing growth promotion.
Table 2.
Relationship of growth promotion from the association between Trichoderma sp. and seedlings of forest species.
| Trichoderma sp. | Forest Species | Effects | References |
|---|---|---|---|
| T. harzianum | Abroma augusta | Height and stem diameter | [97] |
| T. harzianum; T. lignorum; T. koningii | Acacia mangium | Height, stem diameter; biomass and root volume | [98] |
| T. asperelloides; T. harzianum | Bauhinia forficata | Height, stem diameter, and chlorophyll content | [99] |
| T. asperelloides | Cabralea canjerana | Height, biomass, and root system | [100] |
| T. harzianum | Cedrela fissilis | Height, biomass, and root system | [101] |
| T. strigosellum | E. urophylla | Height, number of leaves, and biomass | [102] |
| T. asperellum | Enterolobium schomburgkii | Height and stem diameter | [103] |
| T. harzianum | Malus hupehensis | Biomass and root system | [104] |
| T. asperellum | Theobroma cacao | Height and root system | [105] |
The increase in dry mass is associated with the highest percentage of survival of seedlings at the time of transplanting, making seedlings tolerant to water restrictions, mediated by the ability to change the environment and promote prolonged mutualistic association [10,106]. The morphology of the seedlings directly affects their production potential since both the growth in the shoot and the root architecture depend upon the availability of nutrients in the soil [107]. The close relationship with plants ensures that Trichoderma fungi, when colonizing the root system, promote changes in plant metabolism, affecting plant growth and nutrition, the development of the root system, and the biocontrol of pathogens [9].
The action of microorganisms allows obtaining seedlings with a root system and well-developed shoot, which are determining factors for survival and desirable development in the seedling production process [108]. Furthermore, the increase up to 200% in the total plant biomass in inoculated plants indicates that the inoculation of Trichoderma is a promising method to produce seedlings at the commercial level [109]. The advantages of the use of potential microorganisms are due to the benefits at the physiological level, such as the increase in the photosynthetic potential, greater efficiency, and absorption of water and nutrients, which influence the arrangement of the morphological attributes of the seedlings [100,110].
Currently, the need to reduce the use of agrochemicals is increasing in sustainable agriculture. This plant–microorganism interaction is viable because, in addition to promoting the productivity of forest seedlings, growth promoters allow adding value to the product, making it more competitive and lower cost to the producer [55]. Remarkably, plant growth-promoting substances ensure improvements in the seed germination process and the development and quality of seedlings [8].
According to the morpho and physiological characteristics of the main forest species cultivated in Brazil, synergism via interactions with beneficial microorganisms becomes a useful tool for new microbial quality of the soil and forest production [111]. Since plant responses to fungi actions are broad, it is necessary to evaluate different conditions conducive to plant–microorganism interactions to improve silvicultural techniques for promoting seedling growth [112].
Promoting the growth of forest species through association with Trichoderma is shown to be a viable alternative for the sustainable production of seedlings in Brazil, which is capable of reducing the cost of production via less dependence on mineral fertilizers, has lower risks of environmental contamination, as well as speeds up the process of permanence in forest nurseries and possibly makes the seedlings more capable of being established in the field, according to improvements in the root system.
6. Potential Microorganisms: Co-Inoculation Capacity
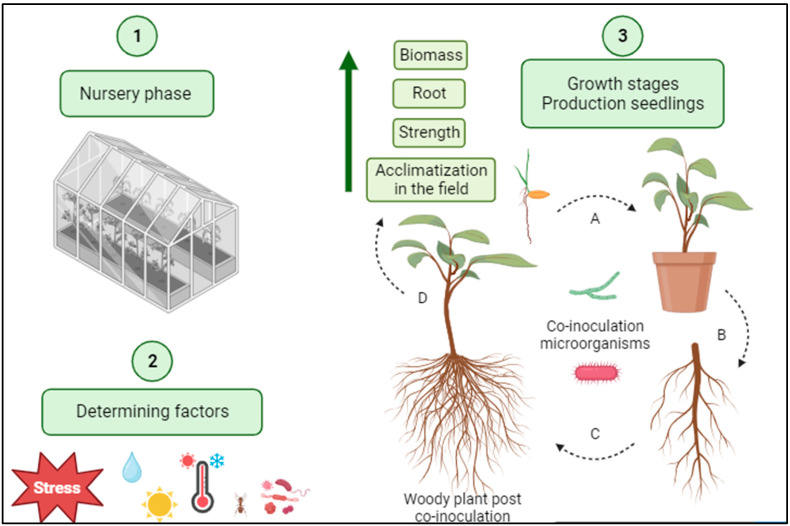
During the nursery phase (production of forest seedlings), several factors can influence positively or negatively the production capacity of plants, so it is essential to insert viable technologies to increase plant production (Figure 4). The system of “consortium” (combination of two or more microorganisms) segmented through the co-inoculation of microorganisms is a valuable alternative in the seedling development stage [113].
Figure 4.
Capacity of microorganisms’ co-inoculation in the production process of forest seedlings. Stages: (1) Nursery phase mediated for (2) determining factors (biotic and abiotic stress), (3) growth stages in production seedlings co-inoculation ((A) better germination, (B) growth seedling, (C) root development, (D) fortified root, appearance of secondary roots and root hairs), as a result, obtaining seedlings with greater biomass, root system, strength and acclimatization in the field. Created in Biorender.
Despite the positive effects on the production and growth parameters of arboreal plants, biopromotion via double inoculation is variable, depending on the compatibility between such microorganisms and the amount of inoculum applied to avoid competition. Furthermore, the co-inoculation with fungi and bacteria can promote an antagonistic effect, which is undesirable in the silvicultural system production and sustainable development [114,115].
The interaction process between plant and microorganism is complex, which reinforces the need to evaluate the different effects of microbial consortium on plant development and forest seedling production. In contrast, each species presents specific characteristics and responses to interaction promoting additive/synergistic or antagonistic effects [77,116]. The beneficial effects of co-inoculation are observed in improvements of germination, vigor, root morphogenesis, photosynthetic capacity, and high biomass indexes, as well as enabling soil maintenance and ecological balance [117,118].
The success of communication and interaction between plants and microorganisms is observed in several species; in this sense, the evaluation of the potential of the process of co-inoculation between fungi and bacteria is a promising tool for the development of forest seedlings, as observed in the interaction between Ambispora leptoticha; Azotobacter chroococcum and T. harzianum under large-scale nursery conditions increasing teak biomass (Tectona grandis) [110]. In addition, in [119], some authors also obtained success for producing teak seedlings under co-inoculation with mycorrhizal fungi and rhizobacteria. In palm seedlings (Elaeis guineensis), the co-inoculation of Bacillus cereus and T. asperellum increased root growth and promoted greater plant development through phosphate solubilization [120].
Among the limiting factors to silvicultural development, the initial stages of seedling formation are highlighted to avoid a low increase in biomass and restrictions in the root system. Such factors have a direct association with the low nutrient content in the substrate used for the growth of seedlings; considering such a restriction, studies revealed that the co-inoculation of Rhizoglomus fasciculatum (arbuscular mycorrhizal fungus), Mortierella sp. (phosphate solubilizing fungus), and Azospirillum brasilense (plant growth promoter bacteria) promoted greater potential in the development and higher quality of seedlings [121].
Positive results were obtained through the consortium between microorganisms (R. fasciculatus, A. chroococcum, B. coagulans, and T. harzianum) in the production of seedlings of Dalbergia sissoo, where plants associated with microorganisms obtained high rates in all growth parameters, with good establishment and vigorous seedlings in the field [122]. Through the multifaceted action of microorganisms, co-inoculation is characterized as a promising method for the growth and development of seedlings [119].
The co-inoculation of mycorrhizal fungi (R. irregulares; Funneliformis mosseae and Claroideoglomus etunicatum) and T. harzianum increased shoot and root systems of apple trees, which led to a reduction in the need for nursery replanting, which are improvements resulting from the action of microorganisms in soil quality [123]. The mechanisms involved in promoting plant development are broad and have different facets under the conditions of cultivation, cultivated species, and action of the microorganism (synthesis of nutrients, phytohormones, mobilization of soil compounds), which influence and ensure that seedlings can develop in good conditions [124].
There are several microorganisms used as inoculum in tree seedlings, such as mycorrhizal arbuscular [125], growth-promoting bacteria [126], rhizobia [127], and Trichoderma [82]. These microorganisms improve root and shoot development and nutrient content in plants. The advantage of inoculating Trichoderma is that this genus is associated with plant growth, bioremediation, and the production of secondary metabolites.
7. Considerations and Future Perspectives
The inoculation of Trichoderma for seedling production in forest species promotes several benefits to plant development; in addition to the low production cost, it is a simple and effective practice, which stimulates root development, promotes greater nutritional absorption capacity, and increases plant biomass, which are determinant for obtaining more vigorous seedlings and greater economic yield.
Adopting beneficial microorganisms, such as fungi of the genus Trichoderma, is demonstrated not only as a viable strategy to produce seedlings of forest species but also as a sustainable alternative and recovery plan for degraded environments, from the considerable reduction in the use of agrochemicals and industrial fertilizers.
Another major advantage associated with the promotion of forest species through interaction with potential microorganisms is that most plant–microorganism interactions are observed in annual species, with few studies in the forest sector. There is a gap of understanding the different mechanisms of action of the symbiotic process, such as metabolic activity and mechanism of interaction with plants and other microorganisms, to increase the use of potential microorganisms in the silvicultural sector.
Although the great relevance and capacity to promote growth, there are still questions to be elucidated, such as the mechanisms involved in the solubilization of nutrients in potential native forest species, which would possibly enable its large-scale production.
Information correlated with the action of microorganisms in the silvicultural sector is still scarce, which requires greater attention.
Acknowledgments
To Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the PhD fellowship granted to the first author.
Author Contributions
Conceptualization, N.C.d.F.F. and M.L.G.R.; methodology, N.C.d.F.F.; software, N.C.d.F.F.; validation, N.C.d.F.F. and M.L.G.R.; formal analysis, M.L.G.R.; investigation, N.C.d.F.F., M.L.G.R. and A.G.; resources, N.C.d.F.F.; data curation, N.C.d.F.F., M.L.G.R. and A.G.; writing—original draft preparation, N.C.d.F.F., M.L.G.R. and A.G.; writing—review and editing, N.C.d.F.F. and M.L.G.R.; visualization, N.C.d.F.F. and M.L.G.R.; supervision, M.L.G.R.; project administration, N.C.d.F.F., M.L.G.R. and A.G.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors acknowledge the financial support of the Federal District Research Support Foundation. Fundação de Apoio à Pesquisa do Distrito Federal—FAPDF (Notice 03/2023—FAPDF/Process n° 00193-00002115/2023-87).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.IBÁ . Brazilian Tree Industry: Annual Report 2022. Indústria Brasileira de Árvores-IBÁ; Brasília, Brazil: 2022. 96p [Google Scholar]
- 2.Maximo Y.I., Hassegawa M., Verkerk P.J., Missio A.L. Forest bioeconomy in Brazil: Potential innovative products from the forest sector. Land. 2022;11:1297. doi: 10.3390/land11081297. [DOI] [Google Scholar]
- 3.Yang H., Liu Y., Liu J., Meng J., Hu X., Tao S. Improving the imbalanced global supply chain of phosphorus fertilizers. Earth’s Future. 2019;7:638–651. doi: 10.1029/2018EF001005. [DOI] [Google Scholar]
- 4.Grossnickle S.C., Macdonald J.E. Seedling quality: History, application, and plant attributes. Forests. 2018;9:283. doi: 10.3390/f9050283. [DOI] [Google Scholar]
- 5.Lopes P.R.M., Barretto V.C.M., Montagnolli R.N., Ferreira P.H.F. Production of eucalyptus seedlings using alternative substrates. Rev. Eng. Agric. REVENG. 2021;29:236–244. doi: 10.13083/reveng.v29i1.11236. [DOI] [Google Scholar]
- 6.Peccatti A., Rovedder A.P.M., Steffen G.P.K., Maldaner J., Missio E.L., Witt C.S., Dalcul L.P. Effect of Trichoderma spp. on the propagation of Maytenus ilicifolia Mart. ex Reissek. J. Agric. Sci. 2019;11:435–442. doi: 10.5539/jas.v11n3p435. [DOI] [Google Scholar]
- 7.Riikonen J., Luoranen J. Seedling production and the field performance of seedlings. Forests. 2018;9:740. doi: 10.3390/f9120740. [DOI] [Google Scholar]
- 8.Vinale F., Sivasithamparam K., Ghisalberti E.L., Marra R., Woo S.L., Lorito M. Trichoderma-plant-pathogen interactions. Soil Biol. Bioch. 2008;40:1–10. doi: 10.1016/j.soilbio.2007.07.002. [DOI] [Google Scholar]
- 9.Khan M.R., Mohiddin F.A. Crop Improvement through Microbial Biotechnology. Volume 13. Elsevier; Amsterdam, The Netherlands: 2018. Trichoderma: Its multifarious utility in crop improvement; pp. 263–291. [DOI] [Google Scholar]
- 10.Kashyap P.L., Rai P., Srivastava A.K., Kumar S. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 2017;33:155. doi: 10.1007/s11274-017-2319-1. [DOI] [PubMed] [Google Scholar]
- 11.Alfiky A., Weisskopf L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi. 2021;7:61. doi: 10.3390/jof7010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morán-Diez M.E., Alba Á.E.M., Rubio M.B., Hermosa R., Monte E. Trichoderma and the plant heritable priming responses. J. Fungi. 2021;7:318. doi: 10.3390/jof7040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waghunde R.R., Shelake R.M., Sabalpara A.N. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016;11:1952–1965. doi: 10.5897/AJAR2015.10584. [DOI] [Google Scholar]
- 14.Banerjee S., Heijden M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2022;21:6–20. doi: 10.1038/s41579-022-00779-w. [DOI] [PubMed] [Google Scholar]
- 15.Cai F., Druzhinina I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021;107:1–69. doi: 10.1007/s13225-020-00464-4. [DOI] [Google Scholar]
- 16.Osakabe Y., Kawaoka A., Nishikubo N., Osakabe K. Responses to environmental stresses in woody plants: Key to survive and longevity. J. Plant Res. 2012;125:1–10. doi: 10.1007/s10265-011-0446-6. [DOI] [PubMed] [Google Scholar]
- 17.Puglielli G., Laanisto L., Gori A., Cardoso A.A. Woody plant adaptations to multiple abiotic stressors: Where are we? Flora. 2023;299:152221. doi: 10.1016/j.flora.2023.152221. [DOI] [Google Scholar]
- 18.Qin X., Xu J., An X., Yang J., Wang Y., Dou M., Wang M., Huang F., Fu Y. Insight of endophytic fungi promoting the growth and development of woody plants. Crit. Rev. Biotechnol. 2023;1:1–22. doi: 10.1080/07388551.2022.2129579. [DOI] [PubMed] [Google Scholar]
- 19.Prudencio O.G.R., Castro M.D., Rivera M.E., López M.C.G., Moreno S.J., Flores S.C. Trichoderma in the rhizosphere. In: Singh H.B., Vaishnav A., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Volume 1. Elsevier; Amsterdam, The Netherlands: 2020. pp. 3–38. [DOI] [Google Scholar]
- 20.Guzmán P.G., Troncoso M.D.P., Monfil V.O., Estrella A.H. Trichoderma species: Versatile plant symbionts. Phytopathology. 2018;109:6–16. doi: 10.1094/PHYTO-07-18-0218-RVW. [DOI] [PubMed] [Google Scholar]
- 21.Singh A., Shukla N., Kabadwal B.C., Tewari A.K., Kumar J. Review on Plant-Trichoderma-Pathogen Interaction. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:2382–2397. doi: 10.20546/ijcmas.2018.702.291. [DOI] [Google Scholar]
- 22.Devi R., Kaur T., Kour D., Rana K.L., Yadav A., Yadav A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020;5:21–47. doi: 10.21608/mb.2020.32802.1016. [DOI] [Google Scholar]
- 23.Soldan A., Watzlawick L.F., Botelho R.V., Faria C.M.D.R., Maia A.J. Development of forestry species inoculated with Trichoderma spp. fertilized with rock phosphate. Floresta e Ambiente. 2018;25:e20160643. doi: 10.1590/2179-8087.064316. [DOI] [Google Scholar]
- 24.Vassileva M., Mendes G.D.O., Deriu M.A., Benedetto G.D., Peregrin E.F., Mocali S., Martos V., Vassilev N. Fungi, P-solubilization, and plant nutrition. Microorganisms. 2022;10:1716. doi: 10.3390/microorganisms10091716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho Filho M.R., Martins I., Peixoto G.H.S., Muniz P.H.P.C., Carvalho D.D.C., Mello S.C.M. Biological control of leaf spot and growth promotion of eucalyptus plants by Trichoderma spp. J. Agric. Sci. 2018;10:459–467. doi: 10.5539/jas.v10n9p459. [DOI] [Google Scholar]
- 26.Reis D.N., Silva F.G., Santana R.C., Oliveira T.C., Freiberger M.B., Silva F.B., Müller C. Growth, physiology and nutrient use efficiency in Eugenia dysenterica DC under varying rates of nitrogen and phosphorus. Plants. 2020;9:722. doi: 10.3390/plants9060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar M., Poonam A., Ahmad S., Singh R.P. Plant growth promoting microbes: Diverse roles for sustainable and ecofriendly agriculture. Energy Nexus. 2022;7:100133. doi: 10.1016/j.nexus.2022.100133. [DOI] [Google Scholar]
- 28.Zhao M., Zhao J., Yuan J., Hale L., Wen T., Huang Q., Shen Q. Root exsudate drive soil microbe nutrient feedbacks in response to plant growth. Plant Cell Environ. 2020;44:613–628. doi: 10.1111/pce.13928. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y., Song Y., Zhao L., Chen P., Bu C., Liu P., Zhang D. The genetic basis of phosphorus utilization efficiency in plants provide new insight into woody perennial plants improvement. Int. J. Mol. Sci. 2022;23:2353. doi: 10.3390/ijms23042353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divjot K.O.U.R., Rana K.L., Tanvir K.A.U.R., Yadav N., Yadav A.N., Kumar M., Saxena A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and-mobilizing microbes: A review. Pedosphere. 2021;31:43–75. doi: 10.1016/S1002-0160(20)60057-1. [DOI] [Google Scholar]
- 31.Morales M., Medina S.E.L., Morán J.N., Quevedo A., Ratti M.F. Nematophagous fungi: A review of their phosphorus solubilization potential. Microorganisms. 2023;11:137. doi: 10.3390/microorganisms11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhu N., Borkar S., Garg S. Phosphate solubilization by microorganisms: Overview, mechanisms, applications and advances. In: Meena S.N., Naik M.M., editors. Advances in Biological Science Research. Volume 1. Springer; Dona Paula, Goa, India: 2019. pp. 161–176. [DOI] [Google Scholar]
- 33.López G.N., Lawry R., Aquino J.F.E., Mendoza-Mendoza A. Chemical communication between Trichoderma and plants. In: Singh H.B., Vaishnav A., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Volume 1. Elsevier; Amsterdam, The Netherlands: 2020. pp. 109–139. [DOI] [Google Scholar]
- 34.Chen M., Liu Q., Gao S.S., Young A.E., Jacobsen S.E., Tang Y. Genome mining and biosynthesis of a polyketide from a biofertilizer fungus that can facilitate reductive iron assimilation in plant. Proc. Natl. Acad. Sci. USA. 2019;116:5499–5504. doi: 10.1073/pnas.1819998116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aishwarya S., Viswanath H.S., Singh A., Singh R. Biosolubilization of different nutrients by Trichoderma spp. and their mechanisms involved: A Review. Int. J. Adv. Sci. Technol. 2020;7:34–39. [Google Scholar]
- 36.Núñez J.A.D., Lobo M.B. Application of microorganisms in forest plant. In: Inamuddin A.M.I., Boddula R., Rezakazemi M., editors. Biofertilizers. Wiley; Cambridge, MA, USA: 2021. pp. 265–287. [DOI] [Google Scholar]
- 37.Prasad A., Dixit M., Meena S.K., Kumar A. Qualitative and quantitative estimation for phosphate solubilizing ability of Trichoderma isolates: A natural soil health enhancer. Mater. Today Proc. 2021;81:360–366. doi: 10.1016/j.matpr.2021.03.305. [DOI] [Google Scholar]
- 38.Yu Z., Wang Z., Zhang Y., Wang Y., Liu Z. Biocontrol and growth-promoting effect of Trichoderma asperellum TaspHu1 isolate from Juglans mandshurica rhizosphere soil. Microbiol. Res. 2021;242:126596. doi: 10.1016/j.micres.2020.126596. [DOI] [PubMed] [Google Scholar]
- 39.Mäkelä P.S., Wasonga D.O., Hernandez A.S., Santanen A. Seedling growth and phosphorus uptake in response to different phosphorus sources. Agronomy. 2020;10:1089. doi: 10.3390/agronomy10081089. [DOI] [Google Scholar]
- 40.Ali S., Khan M.J., Anjum M.M., Khan G.R., Ali N. Trichoderma harzianum modulates phosphate and micronutrient solubilization in the rhizosphere. Gesunde Pflanz. 2022;74:853–862. doi: 10.1007/s10343-022-00643-0. [DOI] [Google Scholar]
- 41.Silva L.I.D., Pereira M.C., Carvalho A.M.X.D., Buttrós V.H., Pasqual M., Dória J. Phosphorus-solubilizing microorganisms: A key to sustainable agriculture. Agriculture. 2023;13:462. doi: 10.3390/agriculture13020462. [DOI] [Google Scholar]
- 42.Yu C., Luo X. Trichoderma koningiopsis controls Fusarium oxysporum causing damping-off in Pinus massoniana seedlings by regulating active oxygen metabolism, osmotic potential, and the rhizosphere microbiome. Biol. Control. 2020;150:104352. doi: 10.1016/j.biocontrol.2020.104352. [DOI] [Google Scholar]
- 43.Cabal C., Martínez-García R., Castro Aguilar A., Valladares F., Pacala S.W. The exploitative segregation of plant roots. Science. 2020;370:1197–1199. doi: 10.1126/science.aba9877. [DOI] [PubMed] [Google Scholar]
- 44.Kulmann M.S.S., Arruda W.S., Vitto B.B., Souza R.O.S., Berghetti Á.L.P., Tarouco C.P., Brunetto G. Morphological and physiological parameters influence the use efficiency of nitrogen and phosphorus by Eucalyptus seedlings. New For. 2022;53:431–448. doi: 10.1007/s11056-021-09864-z. [DOI] [Google Scholar]
- 45.Wang N.Q., Kong C.H., Wang P., Meiners S.J. Root exsudate signals in plant—Plant interactions. Plant Cell Environ. 2021;44:1044–1058. doi: 10.1111/pce.13892. [DOI] [PubMed] [Google Scholar]
- 46.Vinale F., Sivasithamparam K., Ghisalberti E.L., Ruocco M., Woo S., Lorito M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012;7:1934578X1200701133. doi: 10.1177/1934578X1200701133. [DOI] [PubMed] [Google Scholar]
- 47.Lehner S.M., Atanasova L., Neumann N.K., Krska R., Lemmens M., Druzhinina I.S., Schuhmacher R. Isotope-assisted screening for iron-containing metabolites reveals a high degree of diversity among known and unknown siderophores produced by Trichoderma spp. Appl. Environ. Microbiol. 2013;79:18–31. doi: 10.1128/AEM.02339-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trivedi P., Leach J.E., Tringe S.G., Sa T., Singh B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020;18:607–621. doi: 10.1038/s41579-020-0412-1. [DOI] [PubMed] [Google Scholar]
- 49.Lee S., Yap M., Behringer G., Hung R., Bennett J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016;3:7. doi: 10.1186/s40694-016-0025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeilinger S., Gruber S., Bansal R., Mukherjee P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016;30:74–90. doi: 10.1016/j.fbr.2016.05.001. [DOI] [Google Scholar]
- 51.Salwan R., Rialch N., Sharma V. Bioactive volatile metabolites of Trichoderma: An overview. In: Singh H.B., Keswani C., Reddy M.S., editors. Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms. Volume 1. Springer; Singapore: 2019. pp. 87–111. [DOI] [Google Scholar]
- 52.Valdespino C.A.R., Flores S.C., Monfil V.O. Trichoderma as a model to study effector-like molecules. Front. Microbiol. 2019;10:1030. doi: 10.3389/fmicb.2019.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolli S.C., Adusumilli N. Trichoderma-Its paramount role in agriculture. In: Singh H.B., Vaishnav A., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Volume 1. Elsevier; Amsterdam, The Netherlands: 2020. pp. 69–83. [DOI] [Google Scholar]
- 54.Tyśkiewicz R., Nowak A., Ozimek E., Jaroszuk-Ściseł J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022;23:2329. doi: 10.3390/ijms23042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chagas L.F.B., Chagas Júnior A.F., Castro H.G. Phosphate solubilization capacity and indole acetic acid production. Braz. J. Agric. V. 2017;92:176–185. doi: 10.37856/bja.v92i2.3221. [DOI] [Google Scholar]
- 56.Nieto-Jacobo M.F., Steyaert J.M., Salazar-Badillo F.B., Nguyen D.V., Rostás M., Braithwaite M., Mendoza-Mendoza A. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017;8:102. doi: 10.3389/fpls.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharifi R., Ryu C.M. Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 2018;122:349–358. doi: 10.1093/aob/mcy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minerdi D., Maggini V., Fani R. Volatile organic compounds: From figurants to leading actors in fungal symbiosis. FEMS Microbiol. Ecol. 2021;97:fiab067. doi: 10.1093/femsec/fiab067. [DOI] [PubMed] [Google Scholar]
- 59.Vinale F., Nigro M., Sivasithamparam K., Flematti G., Ghisalberti E.L., Ruocco M., Lorito M. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013;347:123–129. doi: 10.1111/1574-6968.12231. [DOI] [PubMed] [Google Scholar]
- 60.Boonman C.C., Van Langevelde F., Oliveras I., Couédon J., Luijken N., Martini D., Veenendaal E.M. On the importance of root traits in seedlings of tropical tree species. New Phytol. 2020;227:156–167. doi: 10.1111/nph.16370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukherjee M., Mukherjee P.K., Horwitz B.A., Zachow C., Berg G., Zeilinger S. Trichoderma–plant–pathogen interactions: Advances in genetics of biological control. Indian J. Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brotman Y., Landau U., Inostroza Á.C., Takayuki T., Fernie A.R., Chet I., Willmitzer L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013;9:e1003221. doi: 10.1371/annotation/8b818c15-3fe0-4e56-9be2-e44fd1ed3fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brotman Y., Briff E., Viterbo A., Chet I. Role of swollenin, an expansin-like protein from Trichoderma, in plant root colonization. Plant Physiol. 2008;147:779–789. doi: 10.1104/pp.108.116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendoza-Mendoza A., Zaid R., Lawry R., Hermosa R., Monte E., Horwitz B.A., Mukherjee P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. Rev. 2018;32:62–85. doi: 10.1016/j.fbr.2017.12.001. [DOI] [Google Scholar]
- 65.Cornejo H.A., Macías-Rodríguez L., Del-Val E.K., Larsen J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016;92:fiw036. doi: 10.1093/femsec/fiw036. [DOI] [PubMed] [Google Scholar]
- 66.Santoyo G., Guzmán-Guzmán P., Parra-Cota F.I., Santos-Villalobos S.D.L., Orozco-Mosqueda M., Glick B.R. Plant growth stimulation by microbial consortia. Agronomy. 2021;11:219. doi: 10.3390/agronomy11020219. [DOI] [Google Scholar]
- 67.Poveda J., Eugui D., Abril-Urias P. Could Trichoderma be a plant pathogen? Successful root colonization. In: Sharma A., Sharma P., editors. Trichoderma. Volume 1. Springer; Singapore: 2020. pp. 35–59. [DOI] [Google Scholar]
- 68.Afzal I., Sabir A., Sikandar S. Trichoderma: Biodiversity, abundances, and biotechnological applications. In: Yadav A.N., editor. Recent Trends in Mycological Research. Volume 1. Springer; Cham, Switzerland: 2021. pp. 293–315. [DOI] [Google Scholar]
- 69.Raghu H.B., Ashwin R., Ravi J.E., Bagyaraj D.J. Enhancing plant quality and outplanting growth of Acacia auriculiformis in dry wasteland plantations by inoculating a selected microbial consortium in the nursery. Can. J. For. Res. 2020;50:736–741. doi: 10.1139/cjfr-2019-0335. [DOI] [Google Scholar]
- 70.Sellal Z., Touhami A.O., Chliyeh M., Mouden N., Selmaoui K., Dahmani J., Douira A. Effect of seeds treatment with Trichoderma harzianum on argan plants growth. Plant Cell Biotechnol. Mol. Biol. 2020;21:69–77. [Google Scholar]
- 71.Poveda J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021;168:104118. doi: 10.1016/j.apsoil.2021.104118. [DOI] [Google Scholar]
- 72.González Pérez E., Ortega Amaro M.A., Salazar Badillo F.B., Bautista E., Douterlungne D., Bremont J.F.J. The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 2018;8:16427. doi: 10.1038/s41598-018-34500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simamora M., Basyuni M., Lisnawita L. Potency of secondary metabolites of Trichoderma asperellum and Pseudomonas fluorescens in the growth of cocoa plants affected by vascular streak dieback. Biodiversitas J. Biol. Divers. 2021;22:1–6. doi: 10.13057/biodiv/d220511. [DOI] [Google Scholar]
- 74.Suryantini R., Wulandari R.S. Effectiveness of Trichoderma viride (T2) to the growth of Acacia mangium seedlings. J. Adv. Agric. Technol. 2017;4:1–4. doi: 10.18178/joaat.4.4.364-367. [DOI] [Google Scholar]
- 75.López A.C., Alvarenga A.E., Zapata P.D., Luna M.F., Villalba L.L. Trichoderma spp. from Misiones, Argentina: Effective fungi to promote plant growth of the regional crop Ilex paraguariensis St. Hil. Mycology. 2019;10:210–221. doi: 10.1080/21501203.2019.1606860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shang J., Liu B., Xu Z. Efficacy of Trichoderma asperellum TC01 against anthracnose and growth promotion of Camellia sinensis seedlings. Biol. Control. 2020;143:104205. doi: 10.1016/j.biocontrol.2020.104205. [DOI] [Google Scholar]
- 77.Adebayo A.G., Kareem K.T., Olatunji M.T., Shokalu A.O., Akintoye H.A., James I.E. Effects of Trichoderma longibrachiatum (NGJ167) and compost on early growth of Bougainvillea spectabilis. Ornam. Hortic. 2020;26:614–620. doi: 10.1590/2447-536x.v26i4.2097. [DOI] [Google Scholar]
- 78.Paudzai F.A.M., Sundram S., Yusof M.T., Angel L., Hashim A.M., Abdullah S.N.A. Induced systemic resistance and promotion of plant growth in oil palm seedlings by endophytic Trichoderma virens. J. Oil Palm Res. 2019;31:572–581. doi: 10.21894/jopr.2019.0031. [DOI] [Google Scholar]
- 79.Dini I., Pascale M., Staropoli A., Marra R., Vinale F. Effect of selected Trichoderma strains and metabolites on olive drupes. Appl. Sci. 2021;11:8710. doi: 10.3390/app11188710. [DOI] [Google Scholar]
- 80.Vaio C., Testa A., Cirillo A., Conti S. Slow-release fertilization and Trichoderma harzianum-based biostimulant for the nursery production of young olive trees (Olea europaea L.) Agron. Res. 2021;19:1–15. doi: 10.15159/ar.21.143. [DOI] [Google Scholar]
- 81.Santos M.F., Santos L.E., Costa D.L., Vieira T.A., Lustosa D.C. Trichoderma spp. on treatment of Handroanthus serratifolius seeds: Effect on seedling germination and development. Heliyon. 2020;6:E04044. doi: 10.1016/j.heliyon.2020.e04044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zin N.A., Badaluddin N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020;65:168–178. doi: 10.1016/j.aoas.2020.09.003. [DOI] [Google Scholar]
- 83.Gorai P.S., Barman S., Gond S.K., Mandal N.C. Trichoderma. In: Amaresan N., Annapurna K., Sankaranarayanan A., Kumar M.S., Kumar K., editors. Beneficial Microbes in Agro-Ecology. Volume 1. Academic Press; Cambridge, MA, USA: 2020. pp. 571–591. [DOI] [Google Scholar]
- 84.Guignabert A., Augusto L., Gonzalez M., Chipeaux C., Delerue F. Complex biotic interactions mediated by shrubs: Revisiting the stress gradient hypothesis and consequences for tree seedling survival. J. Appl. Ecol. 2020;57:1341–1350. doi: 10.1111/1365-2664.13641. [DOI] [Google Scholar]
- 85.Singh B.N., Dwivedi P., Sarma B.K., Singh G.S., Singh H.B. A novel function of N-signaling in plants with special reference to Trichoderma interaction influencing plant growth, nitrogen use efficiency, and cross talk with plant hormones. 3 Biotech. 2019;9:109. doi: 10.1007/s13205-019-1638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapri A., Tewari L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010;41:787–795. doi: 10.1590/S1517-83822010005000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Promwee A., Issarakraisila M., Intana W., Chamswarng C., Yenjit P. Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma strains. J. Agric. Sci. 2014;6:8. doi: 10.5539/jas.v6n9p8. [DOI] [Google Scholar]
- 88.Halifu S., Deng X., Song X., Song R. Effects of two Trichoderma strains on plant growth, rhizosphere soil nutrients, and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests. 2019;10:758. doi: 10.3390/f10090758. [DOI] [Google Scholar]
- 89.Santos J.M.R.D., Taniguchi C.A.K., Silva C.D.F.B.D., Natale W., Artur A.G. Trichoderma in the promotion of growth and nutrition of dwarf cashew rootstock. Revista Ciência Agronômica. 2021;52:1–9. doi: 10.5935/1806-6690.20210053. [DOI] [Google Scholar]
- 90.Campos B.F., Araújo A.J.C., Felsemburgh C.A., Vieira T.A., Lustosa D.C. Trichoderma contributes to the germination and seedling development of açaí palm. Agriculture. 2020;10:456. doi: 10.3390/agriculture10100456. [DOI] [Google Scholar]
- 91.Mo Q., Li Z.A., Sayer E.J., Lambers H., Li Y., Zou B.I., Wang F. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct. Ecol. 2019;33:503–513. doi: 10.1111/1365-2435.13252. [DOI] [Google Scholar]
- 92.Oszako T., Voitka D., Stocki M., Stocka N., Nowakowska J.A., Linkiewicz A., Malewski T. Trichoderma asperellum efficiently protects Quercus robur leaves against Erysiphe alphitoides. Eur. J. Plant Pathol. 2021;159:295–308. doi: 10.1007/s10658-020-02162-y. [DOI] [Google Scholar]
- 93.Harman G.E., Doni F., Khadka R.B., Uphoff N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021;130:529–546. doi: 10.1111/jam.14368. [DOI] [PubMed] [Google Scholar]
- 94.West P.W. Do increasing respiratory costs explain the decline with age of forest growth rate? J. For. Res. 2020;31:693–712. doi: 10.1007/s11676-019-01020-w. [DOI] [Google Scholar]
- 95.Akaji Y., Inoue T., Tomimatsu H., Kawanishi A. Photosynthesis, respiration, and growth patterns of Rhizophora stylosa seedlings in relation to growth temperature. Trees. 2019;33:1041–1049. doi: 10.1007/s00468-019-01840-7. [DOI] [Google Scholar]
- 96.Woo S.L., Hermosa R., Lorito M., Monte E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2022;1:312–326. doi: 10.1038/s41579-022-00819-5. [DOI] [PubMed] [Google Scholar]
- 97.Parkash V., Gaur A., Agnihotri R., Aggarwal A. Trichoderma harzianum Rifai: A beneficial fungus for growth and development of Abroma augusta L. seedlings with other microbial bioinoculants. In: Shah M.M., Sharif U., Buhari T.R., editors. Trichoderma-The Most Widely Used Fungicide. Volume 1. IntechOpen; London, UK: 2019. p. 91. [DOI] [Google Scholar]
- 98.Stewart A., Hill R. Applications of Trichoderma in plant growth promotion. In: Gupta V.G., Schmoll M., Estrella A.H., Upadhyay R.S., Druzhinina I., Tuohy M., editors. Biotechnology and Biology of Trichoderma. Volume 1. Elsevier; Amsterdam, The Netherlands: 2014. pp. 415–428. [DOI] [Google Scholar]
- 99.Peccatti A., Rovedder A.P.M., Steffen G.P.K., Maldaner J., Camargo B., Dalcul L.P., Neeuenschwander F. Biological inputs in promoting the growth of Bauhinia forficata Link. seedlings. Ciência Florest. 2020;30:367–379. doi: 10.5902/1980509833322. [DOI] [Google Scholar]
- 100.Griebeler A.M., Araujo M.M., Tabaldi L.A., Steffen G.P., Turchetto F., Rorato D.G., Barbosa F.M., Berghetti A.L.P., Nhantumbo L.S., Lima M.S. Type of container and Trichoderma spp. inoculation enhance the performance of tree species in enrichment planting. Ecol. Eng. 2021;169:106317. doi: 10.1016/j.ecoleng.2021.106317. [DOI] [Google Scholar]
- 101.Díaz T.S., González L.C. Efecto bioestimulante de Trichoderma harzianum Rifai en posturas de Leucaena, Cedro y Samán. Colomb. For. 2018;21:81–90. doi: 10.14483/2256201X.11744. [DOI] [Google Scholar]
- 102.Batista K.O.M., Silva D.V., Nascimento V.L., Souza D.J. Effects of Trichoderma strigosellum in Eucalyptus urophylla development and leaf-cutting ant behavior. J. Fungi. 2022;8:15. doi: 10.3390/jof8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santos M.F., Costa D.L., Vieira T.A., Lustosa D.C. Effect of’ Trichoderma spp. fungus for production of seedlings in Enterolobium schomburgkii (Benth.) Benth. Aust. J. Crop Sci. 2019;13:1706–1711. doi: 10.21475/ajcs.19.13.10.p2023. [DOI] [Google Scholar]
- 104.Zhang R., Yan Z., Wang Y., Chen X., Yin C., Mao Z. Effects of Trichoderma harzianum fertilizer on the soil environment of Malus hupehensis Rehd. seedlings under replant conditions. HortScience. 2021;56:1073–1079. doi: 10.21273/HORTSCI15970-21. [DOI] [Google Scholar]
- 105.Sousa W.N., Brito N.F., Felsemburgh C.A., Vieira T.A., Lustosa D.C. Evaluation of Trichoderma spp. isolates in cocoa seed treatment and seedling production. Plants. 2021;10:1964. doi: 10.3390/plants10091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grossnickle S.C. Why seedlings survive: Influence of plant attributes. New For. 2012;43:711–738. doi: 10.1007/s11056-012-9336-6. [DOI] [Google Scholar]
- 107.Toca A., Moler E., Nelson A., Jacobs D.F. Environmental conditions in the nursery regulate root system development and architecture of forest tree seedlings: A systematic review. New For. 2022;53:1113–1143. doi: 10.1007/s11056-022-09944-8. [DOI] [Google Scholar]
- 108.Ferreira N.C.F., Rocha E.C., Rodrigues F., Santos S.X., Oliveira T.A.S., Duarte E.A.A., Carvalho D.D.C. Trichoderma spp. in growth promotion of Jacaranda mimosifolia D. Don. J. Agric. Stud. 2021;9:335–346. doi: 10.5296/jas.v9i2.18410. [DOI] [Google Scholar]
- 109.Raghu H.B., Ashwin R., Ravi J.E., Bagyaraj D.J. Microbial consortium improved growth and performance of teak (Tectona grandis Lf.) in nursery and field trials. Biol. Sci. 2020;90:903–909. doi: 10.1007/s40011-019-01163-0. [DOI] [Google Scholar]
- 110.Oliveira H.B., Rocha E., Teles T., Florentino L.A. Microbial activity in the agricultural and forestry system. Res. Soc. Dev. 2022;11:e56211226184. doi: 10.33448/rsd-v11i2.26184. [DOI] [Google Scholar]
- 111.Chagas Júnior A.F., Dias P.C., Santos G.R., Ribeiro A.S.N., Sousa K.Â.O., Chagas L.F.B. Trichoderma as a growth promoter in Astronium urundeuva (M. Allemão) Engl. Sci. Plena. 2022;18:1–10. doi: 10.14808/sci.plena.2022.056201. [DOI] [Google Scholar]
- 112.Liu W.Y.Y., Poobathy R. Biofertilizer utilization in forestry. In: Inamuddin A.M.I., Boddula R., Rezakazemi M., editors. Biofertilizers. Volume 1. Wiley; Cambridge, MA, USA: 2021. pp. 1–37. [DOI] [Google Scholar]
- 113.Avila G.M.A., Gabardo G., Clock D.C., Lima Júnior O.S. Use of efficient microorganisms in agriculture. Res. Soc. Dev. 2021;10:e40610817515. doi: 10.33448/rsd-v10i8.17515. [DOI] [Google Scholar]
- 114.Poveda J., Eugui D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control. 2022;176:105100. doi: 10.1016/j.biocontrol.2022.105100. [DOI] [Google Scholar]
- 115.Hakim S., Naqqash T., Nawaz M.S., Laraib I., Siddique M.J., Zia R., Mirza M.S., Imran A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021;5:617157. doi: 10.3389/fsufs.2021.617157. [DOI] [Google Scholar]
- 116.Dabrowska G.B., Garstecka Z., Trejgell A., Dąbrowski H.P., Konieczna W., Szyp-Borowska I. The impact of forest fungi on promoting growth and development of Brassica napus L. Agronomy. 2021;11:2475. doi: 10.3390/agronomy11122475. [DOI] [Google Scholar]
- 117.Antoszewski M., Mierek-Adamska A., Dabrowska G.B. The importance of microorganisms for sustainable agriculture-A review. Metabolites. 2022;12:1100. doi: 10.3390/metabo12111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaiya L., Gavinlertvatana P., Teaumroong N., Pathom-Aree W., Chaiyasen A., Sungthong R., Lumyong S. Enhancing teak (Tectona grandis) seedling growth by rhizosphere microbes: A sustainable way to optimize agroforestry. Microorganisms. 2021;9:990. doi: 10.3390/microorganisms9091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Syafiq T.H.T.M., Nusaibah S.A., Rafii M.Y. Effectiveness of bioinoculants Bacillus cereus and Trichoderma asperellum as oil palm seedlings growth promoters. Pertanika J. Trop. Agric. Sci. 2021;44:157–170. doi: 10.47836/pjtas.44.1.09. [DOI] [Google Scholar]
- 120.Aguirre M.I.H., Vega W.O., Peláez J.L.D. Co-inoculation with beneficial soil microorganisms promoted growth and quality of Tabebuia rosea seedlings. For. Sci. 2022;68:95–103. doi: 10.1093/forsci/fxab050. [DOI] [Google Scholar]
- 121.Bettegowda R.H., Nanjundappa A., Revanna A., Manchegowda H.K., Ravi J.E., Bagyaraj D.J. Selected microbial consortia promotes Dalbergia sissoo growth in the large-scale nursery and wastelands in a semi-arid region in India. J. For. Res. 2021;26:448–454. doi: 10.1080/13416979.2021.1955439. [DOI] [Google Scholar]
- 122.Zydlik Z., Zydlik P., Wieczorek R. The effects of bioinoculants based on mycorrhizal and Trichoderma spp. fungi in an apple tree nursery under replantation conditions. Agronomy. 2021;11:2355. doi: 10.3390/agronomy11112355. [DOI] [Google Scholar]
- 123.Macías Rodríguez L., Contreras Cornejo H.A., Adame Garnica S.G., Del Val E., Larsen J. The interactions of Trichoderma at multiple trophic levels: Inter-kingdom communication. Microbiol. Res. 2020;240:126552. doi: 10.1016/j.micres.2020.126552. [DOI] [PubMed] [Google Scholar]
- 124.Chaudhary P., Xu M., Ahamad L., Chaudhary A., Kumar G., Adeleke B.S., Verma K.K., Hu D.-M., Širić I., Kumar P., et al. Application of synthetic consortia for improvement of soil fertility, pollution remediation, and agricultural productivity: A Review. Agronomy. 2023;13:643. doi: 10.3390/agronomy13030643. [DOI] [Google Scholar]
- 125.Zhang Z., Mallik A., Zhang J., Huang Y., Zhou L. Effects of arbuscular mycorrhizal fungi on inoculated seedling growth and rhizosphere soil aggregates. Soil Tillage Res. 2019;194:104340. doi: 10.1016/j.still.2019.104340. [DOI] [Google Scholar]
- 126.Zhang P., Dumroese R.K., Pinto J.R. Organic or inorganic nitrogen and rhizobia inoculation provide synergistic growth response of a leguminous forb and tree. Front. Plant Sci. 2019;10:1308. doi: 10.3389/fpls.2019.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chaín J.M., Tubert E., Graciano C., Castagno L.N., Recchi M., Pieckenstain F.L., Estrella M.J., Gudesblat G., Amodeo G., Baroli I. Growth promotion and protection from drought in Eucalyptus grandis seedlings inoculated with beneficial bacteria embedded in a superabsorbent polymer. Sci. Rep. 2020;10:18221. doi: 10.1038/s41598-020-75212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.