Abstract
The Bcl2 family of proteins plays a significant role in regulation of apoptosis. In this study, the microtubule-damaging drugs paclitaxel, vincristine, and vinblastine induced Bcl2 hyperphosphorylation and apoptosis in MCF-7 and MDA-MB-231 cells and reduced Bcl2-Bax dimerization. Paclitaxel or vincristine induced increased expression of Bax, while overexpression of Bcl2 in these cell lines counteracted the effects of low doses of these drugs. In addition, paclitaxel- and vincristine-induced activation of cyclic AMP (cAMP)-dependent protein kinase (protein kinase A [PKA]) induced Bcl2 hyperphosphorylation and apoptosis, which were blocked by the PKA inhibitor Rp diastereomers of cAMP (Rp-cAMP). This finding suggests that activation of PKA due to microtubule damage is an important event in Bcl2 hyperphosphorylation and induction of apoptosis. These microtubule-damaging drugs caused growth arrest in G2-M phase of the cell cycle and had no effect on p53 induction, suggesting that hyperphosphorylation mediated inactivation of Bcl2 and apoptosis without the involvement of p53. By comparison, the DNA-damaging drugs methotrexate and doxorubicin had no effect on Bcl2 hyperphosphorylation but induced p53 expression. Interestingly, paclitaxel or vincristine induced activation of caspase 3 and cleavage of poly(ADP-ribose) polymerase downstream of Bcl2 hyperphosphorylation. These data suggest that there may be a signaling cascade induced by agents that disrupt or damage the cytoskeleton that is distinct from (i.e., p53 independent), but perhaps related to (i.e., involves kinase activation and leads to apoptosis), the cellular response to DNA damage.
Apoptosis plays an important role in a wide variety of physiological processes and, when dysregulated, contributes to the pathogenesis of many diseases, including cancer, autoimmunity, and neurodegenerative disorders (16, 41). While the regulation of apoptosis has been extensively studied, little is known about the mechanisms of cell survival or death. Among the growing number of genes that regulate apoptosis induced by a wide variety of stimuli is the Bcl2 family of genes (2, 21, 33). Some proteins within this family, including Bcl2 and Bcl-XL, inhibit apoptosis, while others, such as Bax and Bak, promote apoptosis. Bcl2 and related antiapoptotic proteins seem to dimerize with a proapoptotic molecule, Bax, and modulate the sensitivity of a cell to apoptosis (30, 35).
Apoptosis is an active and gene-directed form of cell death with well-characterized morphological and biochemical features (16). Recent evidence suggests that the activation of a cascade of cysteine proteases of the interleukin-1β-converting enzyme (ICE)/ced3 (caspase) family may exert a pivotal role in the execution of apoptosis (25). Mammalian cells express at least 10 such caspases, which cleave after aspartate residues (9). Overexpression of these caspases induces apoptosis, whereas their inhibition suppresses apoptosis (24, 25, 28). These caspases require proteolytic cleavage for their activation (10, 40). In turn, caspases cleave a variety of vital cellular substrate(s) including poly(ADP-ribose) polymerase (PARP) during apoptosis (39). Although PARP degradation has been observed (19), activation of caspase 3 by cleavage has not been demonstrated to occur during drug-induced apoptosis in breast cancer cells.
Recently, it has been shown that Bcl2 may protect cancer cells from apoptosis induced by a variety of anticancer agents (4, 11, 32). The precise mechanism of the Bcl2-induced multidrug resistance is unknown. Microtubule-stabilizing agents such as paclitaxel and docetaxel (11, 22) and microtubule-disrupting drugs such as vincristine, vinblastine, and colchicine have antimitotic and apoptosis-inducing activity (8). Human leukemic, breast cancer, and prostate cancer cells exposed to paclitaxel express a phosphorylated form of Bcl2 and undergo apoptosis, suggesting that phosphorylation of Bcl2 may inhibit Bcl2 function. In addition, Bcl2 phosphorylation appears to inhibit its binding to Bax, since less Bax was observed in an immunocomplex with Bcl2 in taxol-treated cancer cells (12).
The objectives of this study were (i) to determine if overexpression of Bcl2 in MCF-7 and MDA-MB-231 cells can protect the cell from apoptosis induced by paclitaxel or vincristine and (ii) to ascertain the functional consequences of the phosphorylation of Bcl2. We demonstrated that overexpression of Bcl2 counteracts the apoptotic effects of low doses of paclitaxel or vincristine but has no effect against high doses of these anticancer drugs. Furthermore, microtubule-damaging drugs (paclitaxel, vincristine, and vinblastine) induced apoptosis, caused growth arrest in G2-M phase of the cell cycle, induced caspase 3 activation as well as PARP degradation, but did not induce p53. In addition, microtubule-damaging drugs led to hyperphosphorylation of Bcl2 through protein kinase A (PKA), whereas DNA-damaging drugs did not. In contrast, DNA-damaging drugs induced p53 but had no effect on Bcl2 hyperphosphorylation, indicating that microtubule-damaging drugs induce apoptosis through a p53-independent mechanism. Interestingly, nocodazole, a reversible microtubule poison, does not activate PKA, does not induce Bcl2 hyperphosphorylation, and does not induce apoptosis. This finding suggests that activation of PKA due to microtubule damage is an important event in Bcl2 hyperphosphorylation and induction of apoptosis. However, some forms of microtubule damage activate a signal transduction pathway that ultimately leads to apoptosis.
MATERIALS AND METHODS
Reagents.
Paclitaxel (Taxus brevifolia), vincristine, vinblastine, nocodazole, methotrexate, doxorubicin, etoposide, vanadate (sodium orthovanadate), Hoechst 33258, cyclic AMP (cAMP), kemptide, and forskolin were purchased from Sigma Chemical Co., St. Louis, Mo. Anti-Bcl2 antibody and anti-p53 antibody were purchased from Oncogene Science, Uniondale, N.Y. Anti-Bax antibody and anti-Yama (caspase 3) antibody were purchased from Transduction Laboratories, Lexington, Ky. Anti-PARP antibody was purchased from Santa Cruz Biotechnology Inc., Santa Cruz, Calif. Enhanced chemiluminescence Western blot detection reagents were purchased from Amersham Life Sciences Inc., Arlington Heights, Ill. [32P]orthophosphoric acid (specific activity, 3,000 Ci/mmol) was purchased from NEN Life Science Products, Boston, Mass. l-[35S]methionine (specific activity, 1,000 Ci/mmol) and [γ-32P]ATP (specific activity, 3,000 Ci/mmol) were purchased from ICN Pharmaceuticals, Inc., Irvine, Calif. Protein phosphatase I (PPase I; catalytic subunit) and protein tyrosine phosphatase (PTPase) were purchased from Boehringer Mannheim, Indianapolis, Ind. Okadaic acid and phorbol 12-myristate 13-acetate (PMA) were purchased from GIBCO BRL, Grand Island, N.Y. PKA inhibitor Rp diastereomers of cAMP (Rp-cAMP) (TEA-salt) was purchased from Biolog Life Sciences Institute, La Jolla, Calif. Caspase inhibitors, N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethyl ketone (z-DEVD-fmk), and N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk) were purchased from Enzyme Systems Products, Livermore, Calif. The protein concentration was determined with the bicinchoninic acid reagent (Pierce, Rockford, Ill.).
Cells and culture conditions.
The human breast cancer cell lines MCF-7 and MDA-MB-231 and the prostate carcinoma cell line DU145 were obtained from the American Type Culture Collection, Rockville, Md. Cells were cultured in either Dulbecco modified Eagle medium (MCF-7) or RPMI 1640 (MDA-MB-231 and DU145) tissue culture medium (BioWhittaker Inc., Walkersville, Md.) supplemented with 10% fetal calf serum and a 1% penicillin-streptomycin mixture. Clones were obtained by limiting dilution.
Expression constructs and transfection.
Viable cells (2 × 106) were transfected with a mixture of Lipofectin reagent (GIBCO BRL) and either plasmid pSFFVneo-Bcl2 or plasmid pSFFV-neo. Transduced cells were selected in medium containing 1 mg of G418 (Geneticin; GIBCO BRL) per ml for 4 weeks. We selected six clones each of MCF-7 and MDA-MB-231 cells and observed little clonal variation in the observed effects. Lysates were evaluated for p26Bcl2 expression by immunoblot analysis (data not shown). MCF-7 cells were stably transfected with either vector alone or a CrmA expression construct (39). For glutathione S-transferase (GST)–Bcl2 fusion protein, full-length Bcl2 cDNA was cloned into pGEX-4T-2 expression vector (Pharmacia Biotech Inc., Piscataway, N.J.). This vector was expressed in bacteria, and recombinant fusion protein was purified from bacterial lysates by gravity-flow affinity chromatography using a Bulk GST purification kit (Pharmacia Biotech Inc.).
MTT assay.
Cells (103) were seeded into 96-well tissue culture plates. Viable cells were quantitated by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (7). In brief, cells were treated with anticancer drugs for 18 h (see the figure legends). Before harvesting, 20 μl of MTT solution (5 mg/ml) was added for 3 h to each well followed by 100 μl of 10% sodium dodecyl sulfate (SDS) and 0.01 N HCl. Viability was quantitated by measuring A570, using an enzyme-linked immunosorbent assay (ELISA) plate reader with a reference wavelength of 650 nm. The mean absorbance of four culture wells per time point was plotted. The percentage of cell survival was defined as (mean A650 of treated wells/A650 of untreated control wells) × 100.
In vitro and in vivo phosphorylation.
In vitro phosphorylation was performed on GST-Bcl2 protein. Briefly, 50 μg of GST-Bcl2 fusion protein was used in a final reaction volume of 50 μl with PKA buffer (10 mM Tris-HCl [pH 7.5], 0.25 mM dithiothreitol, 20 mM MgCl2, 100 μM ATP, 20 μCi [γ-32P]ATP). The reaction was initiated by the addition of PKA and incubated for 30 min at 30°C. Phosphorylated Bcl2 was resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (12% gel), transferred to nitrocellulose (NC) membranes, and visualized by autoradiography.
For in vivo phosphorylation, cells were incubated in medium for 4 h in the presence of 250 μCi of [32P]orthophosphoric acid per ml and further incubated with or without paclitaxel, vincristine, forskolin, okadaic acid, or vanadate for 4 h before harvesting. At the end of the labeling period, the cells were washed and harvested in ice-cold phosphate-buffered saline (PBS). The cells were lysed for 30 min on ice in 1 ml of radioimmunoprecipitation assay buffer (10 mM Tris-HCl [pH 7.5], 120 mM NaCl, 1% Nonidet P-40, 1% deoxycholate, 0.1% SDS). The lysates were centrifuged at 900 × g for 30 × gmin at 4°C. Bcl2 immunoprecipitation was conducted at 4°C on the resulting supernatant with the anti-Bcl2 antibody. After centrifugation and washing, the immunoprecipitated Bcl2 was resolved by SDS-PAGE (8 to 12% gel), electrotransferred onto NC filters, and autoradiographed.
Western blot analysis.
Cells (106) were plated in 10-cm-diameter dishes and incubated with drugs as described in the figure legends. Cells were washed twice with PBS, scraped, and centrifuged, and the cell pellets were lysed in lysis buffer (0.1 M NaCl, 5 mM MgCl2, 1% Nonidet P-40, 0.5% sodium deoxycholate, 20 mM Tris-HCl [pH 7.4]) containing proteolysis inhibitors, vortexed, passed through a 22-gauge needle for 10 min, allowed to stand for 30 min at 4°C, and centrifuged at 750 × g for 20 min; the resulting supernatants were used as cell lysates. Lysates were subjected to SDS-PAGE (12% gel), blotted onto an NC membrane, probed with antibody, and visualized with an enhanced chemiluminescence kit.
Immunoprecipitation.
Cells were washed twice with ice-cold PBS before harvesting and centrifuged, and the cell pellets were lysed in lysis buffer (see above). Fifty micrograms of total protein was immunoadsorbed with 1 μg of antibody, followed by protein A-Sepharose. The immunoadsorbed pellets were washed five times with washing buffer and finally resuspended in SDS sample buffer. The immunoprecipitates were subjected to SDS-PAGE, transferred onto an NC membrane, dried, and autoradiographed.
In vitro translation assay.
Hemagglutinin (HA)-tagged human Bax was obtained from J. M. Hardwick, Johns Hopkins School of Medicine, Baltimore, Md. (5). In brief, BglII fragments of HA-tagged human Bax were cloned into the BglII site of pSG5. In vitro translation of HA-tagged protein was performed as instructed by the manufacturer (TNT, T7 Quick Coupled Transcription/Translation System; Promega, Madison, Wis.).
cAMP-dependent protein kinase assay.
PKA was measured in cell pellets that were lysed and Dounce homogenized before the assay was performed with ATP, [γ-32P]ATP, MgCl2, kemptide, and Tris-HCl with or without cAMP (43). The incubation mixture was spotted on phosphocellulose filters and washed with phosphoric acid; then the radioactivity was measured. The PKA ratio is defined as the ratio of activity measured in the absence and in the presence of 8 μM cAMP. Since cAMP fully activates PKA, this ratio is a measure of how much PKA is activated relative to the total amount of PKA present.
Nuclear morphology.
To assay nuclear morphology (apoptotic nuclei), cells were washed with PBS, fixed with 70% ethanol for 1 h, and stained with 1 mM Hoechst 33258 for 30 min (7). The nuclear morphology of cells was visualized by a fluorescence microscope (Olympus BH2). Fluorescent nuclei were screened for normal morphology (unaltered chromatin), and apoptotic nuclei comprising those with fragmented (scattered) and condensed chromatin were counted. Apoptosis was expressed as the percentage of apoptotic nuclei/103 nuclei.
RESULTS
Induction of apoptosis by paclitaxel and vincristine in breast cancer cells.
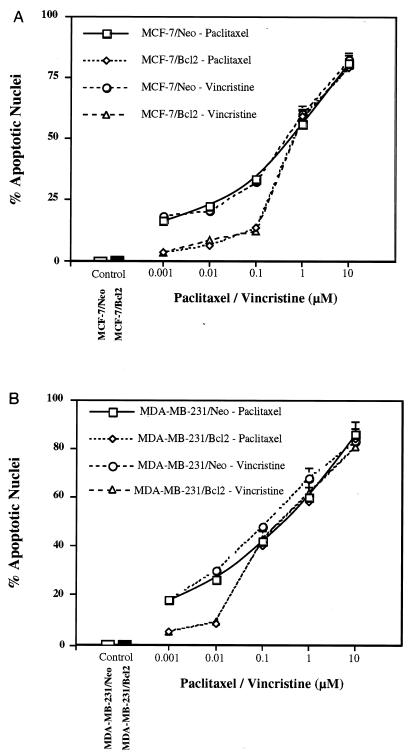
To evaluate the responsiveness of breast epithelial carcinoma to paclitaxel or vincristine, we first analyzed the viability of MCF-7 and MDA-MB-231 cells after exposure to various concentrations of drug. Both paclitaxel and vincristine inhibited viability of MCF-7 and MDA-MB-231 cells in a dose-dependent manner (data not shown). Similarly, paclitaxel or vincristine induced apoptosis in MCF-7 and MDA-MB-231 cells in a dose-dependent manner (Fig. 1). We next examined if overexpression of Bcl2 could counteract the apoptotic effects of paclitaxel or vincristine. The MDA-MB-231 cell line does not express endogenous Bcl2 as tested by Northern and Western blot analyses (data not shown). Overexpression of Bcl2 slightly attenuated the effect of low doses of paclitaxel or vincristine on apoptosis but had no effect on higher doses of these drugs (Fig. 1 and 2). In addition, paclitaxel or vincristine caused growth arrest at the G2-M stage of the cell cycle in MCF-7 and MDA-MB-231 cells (data not shown).
FIG. 1.
Induction of apoptosis by paclitaxel and vincristine in MCF-7 and MDA-MB-231 cells. Paclitaxel or vincristine induces apoptosis in MCF-7 (A) and MDA-MB-231 cells (B) in a dose-dependent manner, and overexpression of Bcl2 counteracts the effects of low doses of paclitaxel or vincristine. Cells were treated with paclitaxel or vincristine for 18 h and then visualized with Hoechst 33258 dye for apoptotic nuclei. Data (means ± standard errors) of quadruplicate determinations) are from one of three separate experiments that gave similar results.
FIG. 2.
Nuclear morphology of MDA-MB-231/Neo or MDA-MB-231/Bcl2 cells treated with paclitaxel or vincristine (0.1 μM). Cells were treated with paclitaxel or vincristine for 18 h and then visualized with Hoechst 33258 dye for apoptotic nuclei. Fluorescent nuclei were screened for normal morphology (unaltered chromatin), and apoptotic nuclei comprising those with fragmented (scattered) and condensed chromatin were counted. Apoptosis was expressed as the percentage of apoptotic nuclei per 103 nuclei.
Hyperphosphorylation of Bcl2 and induction of Bax expression by anticancer drugs.
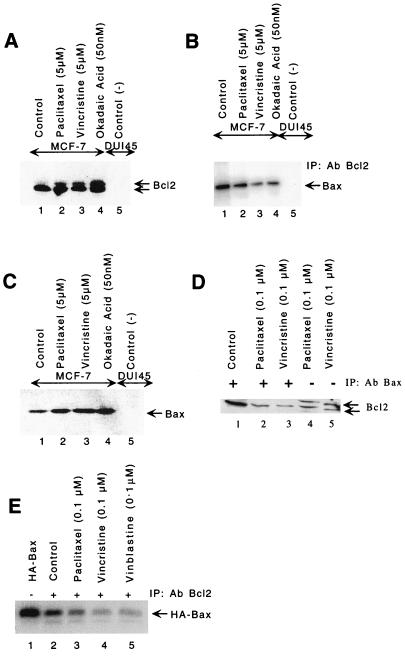
We next investigated the effects of paclitaxel, vincristine, or okadaic acid (a serine/threonine phosphatase inhibitor) on phosphorylation of Bcl2 and induction of Bax. Paclitaxel, vincristine, or okadaic acid induced Bcl2 hyperphosphorylation (caused a mobility shift) in MCF-7 cells (Fig. 3A, lanes 2 to 4). DU145, a prostate cancer cell line that does not express endogenous Bcl2 or Bax, was used as a negative control (Fig. 3A to C, lanes 5). Figure 3B clearly indicates more than 50% reduction of Bax protein in the immunocomplexes precipitated by anti-Bcl2 antibody in paclitaxel-, vincristine-, or okadaic-acid treated cells (Fig. 3B, lanes 2 to 4). We further confirmed this hypothesis by immunoprecipitating cell lysates with anti-Bax antibody and immunoblotting the complex with anti-Bcl2 antibody (Fig. 3D). Only hypophosphorylated Bcl2 was immunoprecipitated with anti-Bax antibody (Fig. 3D). These experiments suggest that hyperphosphorylated Bcl2 is incapable of forming heterodimers with Bax (Fig. 3B and D). We next examined if paclitaxel, vincristine, or okadaic acid could induce Bax protein in MCF-7 cells. Treatment of cells with paclitaxel, vincristine, or okadaic acid resulted in induction of Bax protein (Fig. 3C, lanes 2 to 4). Thus, these agents appear to exert both inhibitory effects on the antiapoptotic Bcl2 protein and stimulatory effects on the levels of the proapoptotic Bax protein.
FIG. 3.
Bcl2 hyperphosphorylation and induction of Bax in MCF-7 cells. Paclitaxel (5 μM), vincristine (5 μM), and okadaic acid (50 nM) induce Bcl2 hyperphosphorylation at 6 h (A) and Bax protein levels at 18 h (C). Cells were treated with drugs, and equal amounts of protein were resolved by SDS-PAGE (12% gel) followed by immunoblotting with anti-Bcl2 antibody (A) or anti-Bax antibody (C). (B) Less Bax heterodimerizes with hyperphosphorylated Bcl2 in cell treated with paclitaxel (5 μM), vincristine (5 μM), and okadaic acid (50 nM). Lanes 1 to 4, cell lysates from MCF-7 cells; lane 5, cell lysate from DU145 cells (Bcl2-negative prostate cancer cells). Proteins were immunoprecipitated (IP) with anti-Bcl2 antibody (Ab), resolved by SDS-PAGE (12% gel), and immunoblotted with anti-Bax antibody. (D) Only hypophosphorylated Bcl2 binds to Bax. Cells were treated with paclitaxel or vincristine for 18 h. Lanes 1 to 3, lysates were immunoprecipitated with anti-Bax antibody; lanes 4 and 5, lysates without immunoprecipitation. The blot was probed with anti-Bcl2 antibody. (E) Reduction in heterodimerization between hyperphosphorylated Bcl2 and in vitro-translated HA-tagged Bax. The HA-tagged Bax was cotranslated in vitro in the presence of [35S]methionine and then incubated with cell lysates treated with paclitaxel, vincristine, or vinblastine, immunoprecipitated with anti-Bcl2 antibody, resolved by SDS-PAGE (12% gel), and analyzed by autoradiography. The intensities of hyper- and hypophosphorylated bands were quantitated by densitometry using NIH image software.
One common feature among the Bcl2 family members is that they often homodimerize or heterodimerize with other family members (35). To assess the effectiveness of dimerization of hyperphosphorylated Bcl2 with Bax, we incubated the 35S-labeled in vitro-translated HA-tagged Bax with lysates from cells treated with paclitaxel, vincristine, or vinblastine, followed by immunoprecipitation with anti-Bcl2 antibody. Immunocomplexes were resolved by SDS-PAGE. Amount of Bcl2 binding with HA-tagged Bax was decreased in lysates from cells treated with paclitaxel, vincristine, or vinblastine (Fig. 3E, lanes 3 to 5), suggesting that hyperphosphorylated Bcl2 was unable to bind with HA-tagged Bax. Thus, microtubule-acting chemotherapeutic agents and okadaic acid led to the hyperphosphorylation of Bcl2, its dissociation from Bax, and the induction of Bax protein. The net result of this sequence of events is an increase in either free Bax protein or Bax homodimers and the initiation of an apoptosis pathway.
cAMP-dependent protein kinase is involved in Bcl2 phosphorylation.
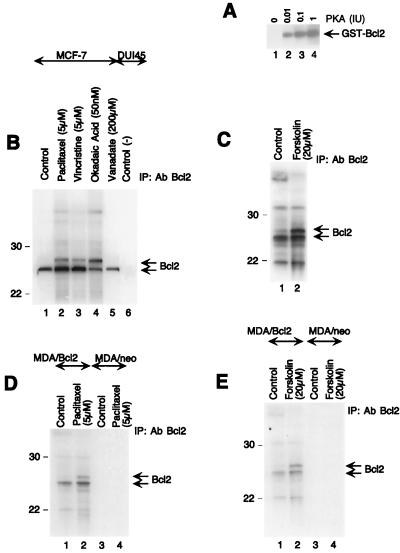
We next examined if the cAMP-dependent protein kinase (PKA) pathway is involved in Bcl2 phosphorylation. Forskolin, an adenylate cyclase activator and 8-Cl-cAMP, a site selective cAMP analog, induced Bcl2 hyperphosphorylation in MCF-7 cells (Fig. 4A). Since MDA-MB-231 cells do not natively express Bcl2, it was of interest to determine if MDA-MB-231 cells transfected to overexpress Bcl2 would respond similarly to these drugs. Paclitaxel, forskolin, or okadaic acid induced Bcl2 hyperphosphorylation in MDA/Bcl2 (Bcl2 overexpressing MDA-MB-231 cells) (Fig. 4B). We also examined whether PKC was involved in Bcl2 phosphorylation by using a phorbol ester, PMA. PMA did not cause any change in a band mobility shift of Bcl2 (Fig. 4B). Thus, PKA rather than PKC appears to hyperphosphorylate Bcl2 even when Bcl2 is overexpressed.
FIG. 4.
Involvement of PKA, but not PKC, in Bcl2 hyperphosphorylation. (A) Paclitaxel (1 μM), vincristine (5 μM), okadaic acid (50 nM), forskolin (20 μM), and 8-Cl-cAMP (5 μM) induce Bcl2 hyperphosphorylation in MCF-7 cells at 18 h, whereas vanadate (200 μM) and PMA (60 ng/ml) do not. (B) MDA-MB-231 cells were transfected with either Neo (MDA/neo) or Bcl2 (MDA/Bcl2) expression vector. Paclitaxel (5 μM), forskolin (20 μM), and okadaic acid (50 nM) induce hyperphosphorylation of Bcl2 at 18 h in MDA/Bcl2 cells, whereas PMA (60 ng/ml) does not. Note that MDA/Neo cells express an undetectable level of Bcl2. (C) Bcl2 hyperphosphorylated protein is dephosphorylated by PPase I but not by PTPase in MCF-7 cells. Cells were treated with either paclitaxel (1 μM) or vanadate (200 μM) for 18 h. Lane 1, control (untreated); lane 2, paclitaxel (1 μM); lane 3, cell lysate from paclitaxel (1 μM)-treated cells incubated with PTPase; lane 4, cell lysate from paclitaxel (1 μM)-treated cells incubated with PPase I; lane 5, cell lysate from vanadate (200 μM)-treated cells. (D) PKA inhibitor, Rp-cAMP blocks paclitaxel-induced Bcl2 hyperphosphorylation in MCF-7 cells. Cells were treated with paclitaxel, Rp-cAMP, or both for 18 h. Lane 1, control; lane 2, Rp-cAMP (100 μM); lane 3, paclitaxel (0.1 μM); lane 4, paclitaxel (0.1 μM) and Rp-cAMP (100 μM). Proteins were resolved by SDS-PAGE (8 to 20% gradient gel) and immunoblotted with anti-Bcl2 antibody.
Since Bcl2 hyperphosphorylation was observed in cells treated with okadaic acid and paclitaxel but not by vanadate, it was of interest to evaluate whether hyperphosphorylated Bcl2 could be dephosphorylated by protein phosphatases. MCF-7 cells were treated with paclitaxel, and cell extracts were incubated with either PTPase (Fig. 4C, lane 3) or PPase I (lane 4). The hyperphosphorylated band of Bcl2 protein is dephosphorylated by PPase I (Fig. 4C, lane 4) but not by PTPase in MCF-7 cells (Fig. 4C, lane 3). Thus, in vivo hyperphosphorylated Bcl2 appears to be phosphorylated on serine/threonine residues.
We next examined the involvement of PKA in paclitaxel-induced Bcl2 hyperphosphorylation by using the PKA inhibitor Rp-cAMP (Fig. 4D). The Rp-cAMP blocked paclitaxel-induced Bcl2 hyperphosphorylation in MCF-7 cells (Fig. 4D, lane 4), suggesting that PKA may play a role in paclitaxel-induced Bcl2 hyperphosphorylation.
In vitro and in vivo phosphorylation of Bcl2.
We tested whether Bcl2 could serve as a substrate for PKA in vitro. GST-Bcl2 was incubated in the presence of [γ-32P]ATP and PKA (see Materials and Methods). The phosphorylated protein was resolved by SDS-PAGE (12% gel), electrotransferred onto NC filters, and autoradiographed. PKA phosphorylated the Bcl2 protein in vitro (Fig. 5A, lanes 2 to 4).
FIG. 5.
In vitro and in vivo hyperphosphorylation of Bcl2. (A) PKA (0.01, 0.1, and 1 IU) induces Bcl2 hyperphosphorylation in vitro. GST-Bcl2 fusion protein was incubated in the presence or absence of PKA with [32P]ATP, subjected to SDS-PAGE (12% gel), and autoradiographed (see Materials and Methods). (B and C) Paclitaxel (5 μM), vincristine (5 μM), okadaic acid (50 nM), and forskolin (20 μM) hyperphosphorylate Bcl2 at 4 h in MCF-7 cells in vivo, whereas vanadate (200 μM) does not. (D and E) Paclitaxel (5 μM) and forskolin (20 μM) induce Bcl2 hyperphosphorylation at 4 h in MDA/Bcl2 cells in vivo. Cells were incubated in medium for 4 h in the presence of [32P]orthophosphoric acid and further incubated with or without drugs for 4 h before being harvested. At the end of the labeling period, the cells were washed, harvested, and lysed in radioimmunoprecipitation assay buffer (see Materials and Methods). Cell lysates were immunoprecipitated (IP) with anti-Bcl2 antibody (Ab), and immunoprecipitated Bcl2 was resolved by SDS-PAGE (8 to 20% gradient gel), electrotransferred onto NC filters, and autoradiographed. Bcl2 bands were further confirmed by the Western blot analysis using the same membrane (data not shown). The experiments whose results are shown in panels B to E were carried out by SDS-PAGE on an 8 to 20% gradient gel, which gave better resolution between hyperphosphorylated and hypophosphorylated Bcl2 bands than the SDS–12% polyacrylamide gel used in the rest of the experiments. Note that a small amount of Bcl2 was labeled even in untreated control cells. Sizes are indicated in kilodaltons.
Since PKA phosphorylated Bcl2 in vitro, it was of interest to examine if the PKA pathway is involved in vivo phosphorylation. MCF-7 cells were incubated in the presence of [32P]orthophosphoric acid and treated with or without paclitaxel, vincristine, okadaic acid, vanadate, or forskolin (Fig. 5B and C). The cells were lysed and immunoprecipitated with anti-Bcl2 antibody. The immunoprecipitated Bcl2 was resolved by SDS-PAGE (8 to 20% gradient gel), electrotransferred onto NC filters, and autoradiographed. Paclitaxel, vincristine, okadaic acid, or forskolin induced Bcl2 hyperphosphorylation in MCF-7 cells in vivo (Fig. 5B, lanes 2 to 4; Fig. 5C, lane 2). In contrast, vanadate did not hyperphosphorylate Bcl2 in MCF-7 cells (Fig. 5, lane 5). DU145 cells were used as a negative control (Fig. 5B, lane 6).
To assess whether the pathway for Bcl2 hyperphosphorylation is intact in vivo in Bcl2-negative breast cancer cells, we transfected MDA-MB-231 cells with wild-type human Bcl2. The cells were subsequently labeled with [32P]orthophosphoric acid and treated with paclitaxel or forskolin. Cellular extracts were prepared and immunoprecipitated with an anti-Bcl2 antibody. Labeled proteins were resolved by SDS-PAGE (8 to 20% gradient gel) and visualized by autoradiography (Fig. 5D, lane 2; Fig. 5E, lane 2). Paclitaxel or forskolin induced Bcl2 hyperphosphorylation in MDA/Bcl2 cells in vivo.
Since PKA inhibitor Rp-cAMP blocked paclitaxel-induced Bcl2 hyperphosphorylation, it was of interest to examine if Rp-cAMP would also block paclitaxel-induced apoptosis. Interestingly, Rp-cAMP blocked paclitaxel-induced apoptosis in MCF-7 cells (data not shown). These observations confirm that PKA plays a significant role in paclitaxel-induced Bcl2 hyperphosphorylation and apoptosis.
Activation of PKA by paclitaxel and vincristine.
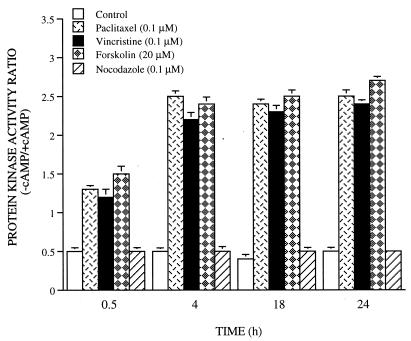
To determine whether changes in the catalytic activity of PKA are involved following microtubule damage, we examined phosphotransferase activity in cells during paclitaxel, vincristine, nocodazole, and forskolin treatment, using kemptide as a substrate in the presence and absence of a saturating concentration of cAMP (43). This method of assay gives accurate determination of the relative levels of dissociated catalytic subunit activity and total catalytic activity. The PKA activity ratio, the ratio of activity in the absence of added cAMP to that in the presence of cAMP, measures the degree of free catalytic subunit release. As shown in Fig. 6, paclitaxel, vincristine, or forskolin induced PKA activation as early as 30 min. In contrast, nocodazole, a drug that reversibly blocks microtubule polymerization, had no effect on PKA activation (Fig. 6) and Bcl2 hyperphosphorylation (data not shown). In our previous studies, we have shown that the PKA activity ratio remained elevated during 48 h of 8-Cl-cAMP treatment in MCF-7TH cells (36). These results show that paclitaxel and vincristine treatment resulted in an increase in release of free catalytic subunit of PKA.
FIG. 6.
Activation of PKA by paclitaxel, vincristine, and forskolin in MCF-7 cells. Cells were treated with paclitaxel (0.1 μM), vincristine (0.1 μM), forskolin (20 μM), or nocodazole (0.1 μM) and harvested at 0.5, 4, 18, and 24 h. Lysates were assayed for phosphotransferase activity, and the PKA ratio was calculated as described in Materials and Methods. Data (means ± standard errors of quadruplicate determinations) are from one of three separate experiments that gave similar results.
Microtubule-damaging drugs hyperphosphorylate Bcl2, whereas DNA-damaging drugs do not.
Because paclitaxel induces tubulin polymerization and forms extremely stable and nonfunctional microtubules (20), it was of an interest to examine if drugs inducing microtubule depolymerization also induce Bcl2 hyperphosphorylation. Vincristine and vinblastine, two vinca alkaloids that bind monomeric tubulin and prevent microtubule assembly (34), also induced Bcl2 hyperphosphorylation in MCF-7 cells (Fig. 7A). By comparison, the DNA-damaging agents methotrexate and doxorubicin failed to induce Bcl2 hyperphosphorylation (did not cause a band shift) (Fig. 7A). In addition, etoposide, which stabilizes covalent complexes between topoisomerase II and genomic DNA, did not induce Bcl2 hyperphosphorylation. Similarly, when lysate of [32P]orthophosphoric acid-labeled cells exposed to doxorubicin was immunoprecipitated with anti-Bcl2 antibody and resolved by SDS-PAGE (8 to 20% gradient gel), no hyperphosphorylated band was detected (data not shown). On the basis of these data, it appears that drugs that damage microtubule integrity induce loss of Bcl2 antiapoptotic function through hyperphosphorylation, whereas DNA-damaging drugs do not. This has been recently shown to occur in 697 leukemia cells and PC-3 prostate carcinoma cells (11).
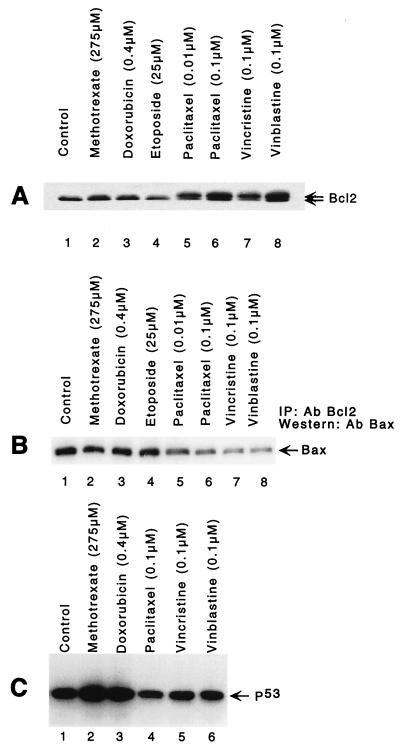
FIG. 7.
Comparison of microtubule- and DNA-damaging anticancer drugs in MCF-7 cells. (A) The microtubule-damaging drugs paclitaxel (0.01 and 0.1 μM), vincristine (0.1 μM), and vinblastine (0.1 μM) induce Bcl2 hyperphosphorylation at 6 h, whereas the DNA-damaging drugs methotrexate (275 μM) and doxorubicin (0.4 μM) do not (no change in a band mobility shift). Cell lysates were fractionated by SDS-PAGE (12% gel) and immunoblotted with anti-Bcl2 antibody. (B) Lower contents of Bax protein in the immunocomplex isolated from paclitaxel (0.01 and 0.1 μM)-, vincristine (0.1 μM)-, and vinblastine (0.1 μM)-treated cells by immunoprecipitation (IP) with anti-Bcl2 antibody (Ab). Proteins were immunoprecipitated with anti-Bcl2 antibody, resolved by SDS-PAGE (12% gel), and immunoblotted with anti-Bax antibody. (C) DNA-damaging anticancer drugs methotrexate (275 μM) and doxorubicin (0.4 μM) induce p53 (lanes 2 and 3), whereas paclitaxel (0.1 μM), vincristine (0.1 μM), and vinblastine (0.1 μM) do not. Cells were treated with drugs for 16 h, harvested, and lysed, and equal amounts of protein were resolved by SDS-PAGE (12% gel) followed by immunoblotting with anti-p53 antibody.
We next compared the amounts of Bax bound to Bcl2 in cells treated with microtubule- and DNA-damaging anticancer drugs. Only microtubule-damaging drugs that hyperphosphorylated Bcl2 caused a significant reduction of Bax in the immunocomplexes precipitated by Bcl2 antibody (Fig. 7B, lanes 5 to 8). This result suggests that microtubule-destabilizing drugs act similarly to paclitaxel.
We further investigated the effects of microtubule-damaging drugs that hyperphosphorylate Bcl2 on induction of p53 protein. The DNA-damaging agents methotrexate and doxorubicin induced p53 in MCF-7 cells (Fig. 7C, lanes 2 and 3), whereas the microtubule-damaging drugs paclitaxel, vincristine, and vinblastine had no effect on p53 expression (Fig. 7C, lanes 4 to 6).
Induction of caspase 3 activation and PARP cleavage by microtubule-damaging drugs.
The effects of paclitaxel, vincristine, and vinblastine on caspase 3 protein as well as PARP levels were examined in MCF-7 and MDA-MB-231 cells (data not shown). All three drugs induced activation of caspase 3 by cleaving it to a 20-kDa subunit (data not shown). Similarly, this has been shown to occur when caspase 3 is activated by cleavage (45). In addition to caspase 3 cleavage and activation, following treatment of MCF-7 cells with paclitaxel, vincristine, or vinblastine for 18 h, p116PARP was degraded into an 85-kDa fragment (data not shown). Both the cleavage and activation of caspase 3 as well as the degradation of PARP were not observed following treatment with paclitaxel (0.01 to 10 μM) for 6 h (data not shown), the time at which Bcl2 begins to undergo hyperphosphorylation. In addition, paclitaxel-induced effects of caspase 3 and PARP were detected at 12 h of exposure to paclitaxel (data not shown), suggesting that the activation of caspase 3 protease and PARP degradation precedes the DNA fragmentation of apoptosis.
It has been shown that caspases play significant roles in execution of apoptosis induced by chemotherapeutic drugs, and inactivation of these caspases by cowpox virus gene product CrmA could block drug-induced apoptosis (9, 40). We therefore examined the effects of CrmA on paclitaxel- or vincristine-induced PARP degradation and apoptosis in MCF-7 cells. Stably transfected CrmA protected cells against paclitaxel- or vincristine-induced PARP degradation and apoptosis (data not shown). However, Bcl2 was phosphorylated in these cells (data not shown). In addition, a caspase inhibitor z-DEVD-fmk was able to block paclitaxel- or vincristine-induced caspase 3 activation in MDA-MB-231 cells (data not shown). Interestingly, caspase inhibitors z-DEVD-fmk and z-VAD-fmk were effective in blocking paclitaxel- or vincristine-induced apoptosis (as measured by nucleosome ELISA; Oncogene Research Products, Cambridge, Mass.) in MDA-MB-231 cells (data not shown). In contrast, nocodazole, a reversible inhibitor of microtubule polymerization, does not activate PKA (as shown above) and does not induce apoptosis (data not shown). These caspase inhibitors also blocked paclitaxel- or vincristine-induced PARP cleavage in MDA-MB-231 cells (data not shown).
DISCUSSION
Recent evidence suggests that inactivation of the antiapoptotic function of Bcl2 by phosphorylation occurs after treatment with the microtubule-stabilizing anticancer drug paclitaxel or the phosphatase inhibitor okadaic acid (1, 11, 14). Results of the present study extend these previous findings and demonstrate that the microtubule-disrupting drugs induce apoptosis in MCF-7 cells that is associated with Bcl2 hyperphosphorylation and loss of function while having no effect on p53. In contrast, DNA-damaging drugs have no effect on Bcl2 hyperphosphorylation but induce p53, suggesting that the actions of microtubule-damaging drugs on apoptosis are exerted independently of the p53 pathway. Interestingly, hyperphosphorylation occurred on serine/threonine residues since PPase I treatment resulted in dephosphorylation of Bcl2. Furthermore, this is the first study showing that PKA may be involved in Bcl2 hyperphosphorylation. In addition, time course experiments demonstrate that paclitaxel and vincristine induce caspase 3 activation as well as PARP degradation downstream of Bcl2 hyperphosphorylation.
It has been demonstrated previously that Bcl2 is phosphorylated on serine residues, and of the 17 serine residues present in Bcl2, several could be the potential sites of phosphorylation by different kinases (13, 31). It is not yet known whether the same sites are hyperphosphorylated by microtubule-damaging-drugs and PKA. Thus, the effect of hyperphosphorylation on Bcl2 function may depend on the site of phosphorylation. Further studies are under way to identify the site(s) of hyperphosphorylation in Bcl2 protein by these drugs and define the relevant kinases.
Recently, it has been shown that binding of the angiotensin type 2 receptor leads to dephosphorylation of Bcl2 and apoptosis by activation of mitogen-activated protein kinase phosphatase 1 in PC12 cells (17). Furthermore, overexpression of c-Jun N-terminal kinase antagonizes an antiapoptotic action of Bcl2 in N18TG cells (31). Contrary to our findings and those of others (1, 13), it has been reported that interleukin-3 (IL-3) and erythropoietin, or the PKC activator bryostatin 1, not only suppress apoptosis but also stimulate the phosphorylation of Bcl2 in murine IL-3-dependent NSF/N1.H7 cells (18, 26). In addition, it has been reported that Bcl2 is phosphorylated on serine 70 after IL-3 or bryostatin stimulation, and Bcl2 phosphorylation is required for its antiapoptotic function. The differences observed with activators of PKA or PKC, and subsequent Bcl2 hyperphosphorylation by these drugs, may be due to differences in cell types. It is likely that multiple kinases are involved in Bcl2 hyper- or hypophosphorylation, and the specific sites of phosphorylation may determine whether Bcl2 loses or gains its antiapoptotic function.
The actions of cAMP in the regulation of various cellular functions, including cell proliferation, differentiation, and gene induction, through the activation of PKA are well known (20). We and others have recently demonstrated that down-regulation of PKA type I and up-regulation of PKA type II by cAMP analog 8-Cl-cAMP and RIα antisense induce apoptosis in several cancer cell lines (6, 7, 23, 37). Recently, synergistic inhibition of growth and induction of apoptosis by 8-Cl-cAMP and paclitaxel, cisplatin, or retinoic acid in several human cancer cells has been demonstrated (37, 42). Furthermore, intracytoplasmic microinjection of purified PKA catalytic subunit commits the cells to death (48). In support of this hypothesis, PKA type II has been found to be associated with mammalian centromeres (44), and upon microtubule disruption by paclitaxel, vincristine, or vinblastine, activated PKA would hyperphosphorylate Bcl2, leading to apoptosis. Interestingly, nocodazole acts like vincristine to block microtubule polymerization but does not activate PKA, does not induce Bcl2 hyperphosphorylation, and does not lead to apoptosis. This finding suggests that activation of PKA due to microtubule damage is an important event in Bcl2 hyperphosphorylation and induction of apoptosis.
Apoptosis can be selectively triggered in tumor cells in response to several stimuli, including anticancer drugs and Fas antigen (15, 27). Aborted apoptosis in neoplastic cells could be a critical factor in resistance both to natural defenses and to clinical therapy, particularly in cases of recurrent disease (14), and the mechanisms underlying this phenomenon have yet to be fully elucidated. Apoptosis induction in mammalian cells is controlled by an equilibrium between suppressor and inducer gene products (29). Dysregulated expression of Bcl2 is believed to contribute to neoplastic cell expansion via an antiapoptotic effect which enhances cell survival rather than by accelerating rates of cellular proliferation. Although the mode by which Bcl2 affects the process of cell death is not fully understood, recent studies indicate that the Bcl2 protein binds to other proteins with which it has amino acid sequence homology, including Bax, Bcl-XL, Bcl-XS, Mcl-1, Bik, and Bad (2, 3, 30, 35, 47). The functional significance of many of these Bcl2 family protein-protein interactions remains unclear. However, the heterodimerization of Bcl2 with Bax appears crucial in preventing Bax-mediated apoptosis (30). Bax appears to antagonize Bcl2 function, abrogating the ability of Bcl2 to prolong cell survival. In the present study, less Bax was immunoprecipitated with Bcl2 antibody in paclitaxel-, vincristine-, or vinblastine-treated cells, indicating that hyperphosphorylated Bcl2 is less able to form heterodimers with Bax. Similarly, the treatment of prostate cancer cells results in the phosphorylation of Bcl2 and in programmed cell death of cancer cells concomitantly with a reduction of heterodimer complexes with Bax (12). Thus, cell viability will be maintained either by activating apoptosis-suppressing proteins or by inhibiting apoptosis-inducing proteins.
Consistent with these observations, overexpression of Bcl2 in MCF-7 and MDA-MB-231 cell lines counteracted the effects of low doses of paclitaxel or vincristine on cell survival, whereas high doses of paclitaxel or vincristine inhibited survival in both Neo- and Bcl2-overexpressing breast cancer cell lines. A previous report also has shown that Bcl2 overexpression inhibits low dose paclitaxel-induced apoptosis and significantly improves the survival of human leukemic cells (38).
It is now clear that the proteolytic degradation of specific substrates is involved in many of the morphological and biochemical features of apoptosis (25, 40, 45). Activation of the cysteine proteases belonging to the ICE/ced-3 family is part of the cascade of protease activity that results in apoptosis (10, 45). We have demonstrated that paclitaxel- and vincristine-induced apoptosis of breast cancer cells activates at least one such protease encoded by the caspase 3 (CPP32β/Yama) gene. Caspase 3 was cleaved and activated, leading to PARP degradation. The cowpox virus CrmA and caspase inhibitors z-DEVD-fmk and z-VAD-fmk were able to protect cells from paclitaxel- or vincristine-induced apoptosis and PARP degradation. Similarly, CrmA-expressing Rat-1 fibroblasts were protected from death activated by serum withdrawal (46). Overexpression of CrmA also prevented apoptosis due to engagement of the Fas (CD95) receptor or to exposure to tumor necrosis factor alpha (9, 40). Time course experiments demonstrated that the caspase 3 activation and PARP degradation occur downstream of Bcl2 phosphorylation.
Based on our data and those of others (1, 11), it is clear that drugs that affect microtubule integrity by inhibiting polymerization or depolymerization induce loss of Bcl2 antiapoptotic function through hyperphosphorylation, whereas anticancer drugs that damage DNA do not. These experiments may provide a rationale for the use of the anticancer drugs, paclitaxel, vincristine, and vinblastine for the treatment of Bcl2-positive cancers, such as follicular lymphoma and breast, prostate, and ovarian cancer. Furthermore, these data suggest that there may be a cytoskeletal damage response pathway just as there seems to be a DNA damage response pathway. Analysis of the nature of the signaling cascade induced by the cytoskeleton-damaging agents is under way. Taken together, these studies not only increase our understanding of Bcl2 function but also elucidate general mechanisms through which intracellular signals can mediate regulatory functions relevant to disease states.
ACKNOWLEDGMENTS
We are grateful to J. M. Hardwick (Johns Hopkins School of Medicine, Baltimore, Md.) for providing HA-tagged Bax expression plasmid. We thank Jim Kenny, Tony Passaniti, and Charles Filburn for critically reviewing the manuscript. We also thank Francis J. Chrest for his technical help.
This work was supported partly by a fellowship of the National Research Council, National Academy of Sciences, to R.K.S.
REFERENCES
- 1.Blagosklonny M V, Giannakakou P, El-Deiry W S, Kingston D G I, Higgs P I, Neckers L, Fojo T. Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death. Cancer Res. 1997;57:130–135. [PubMed] [Google Scholar]
- 2.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 3.Boyd J M, Gallo G J, Elangovan B, Houghton A B, Malstrom S, Avery B J, Ebb R G, Subramanian T, Chittenden T, Lutz R J, Chinnadurai G. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 4.Carson D A, Ribeiro J M. Apoptosis and disease. Lancet. 1993;341:1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- 5.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho-Chung Y S. Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approach to therapy. Cancer Res. 1990;50:7093–7100. [PubMed] [Google Scholar]
- 7.Cho-Chung Y S, Nesterova M, Kondrashin A, Noguchi K, Srivastava R K, Pepe S. Antisense-protein kinase A: a single-gene-based therapeutic approach. Antisense Nucleic Acid Drug Dev. 1997;7:217–223. doi: 10.1089/oli.1.1997.7.217. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson K L, Goolsby G L, Kiener P A, Wahl A F. Activation of p34cdc2 coincident with taxol-induced apoptosis. Cell Growth Differ. 1994;5:1041–1050. [PubMed] [Google Scholar]
- 9.Enari M, Hug H, Nagata S. Involvement of ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes-Alnemri T, Litwack G, Alnemri E S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian-1β-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 11.Haldar S, Basu A, Croce C M. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- 12.Haldar S, Chintapalli J, Croce C M. Taxol-induced bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 13.Haldar S, Jena N, Croce C M. Inactivation of bcl-2 by phosphorylation. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison D J. Molecular mechanism of drug resistance in tumors. J Pathol. 1995;175:7–12. doi: 10.1002/path.1711750103. [DOI] [PubMed] [Google Scholar]
- 15.Hickman J A. Apoptosis induced by anti-cancer drugs. Cancer Metastasis Res. 1992;11:121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 16.Hockenbery D. Defining apoptosis. Am J Pathol. 1995;146:16–19. [PMC free article] [PubMed] [Google Scholar]
- 17.Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau V J. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Deng X, Carr B, May W S. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann S H, Desnoyers S, Ottaviano Y, Davidson N E, Poirier G G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- 20.Krebs E G, Beavo J A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;88:923–939. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 22.Kumar N. Taxol-induced polymerization of purified tubulin. J Biol Chem. 1981;256:10435–10441. [PubMed] [Google Scholar]
- 23.Lanotte M, Riviere J B, Hermouet S, Houge G, Wintermyr O K, Gjertsen B T, Doskeland S O. Programmed cell death (apoptosis) is induced rapidly and with positive cooperativity by activation of cyclic adenosine monophosphate-kinase I in a myeloid leukemia cell line. J Cell Physiol. 1991;146:73–80. doi: 10.1002/jcp.1041460110. [DOI] [PubMed] [Google Scholar]
- 24.Lazebnik Y A, Kaufmann S H, Desnoyers S, Poirier G G, Earnshaw W C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 25.Martin S J, Green D R. Protease activation during apoptosis. Death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 26.May W S, Tyler P G, Ito T, Armstrong D K, Qatsha K A, Davidson N E. Interleukin-3 and bryostatin-1 mediate hyperphosphorylation of Bcl2α in association with suppression of apoptosis. J Biol Chem. 1994;269:26865–26870. [PubMed] [Google Scholar]
- 27.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin Y, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 29.Oltvai Z N, Korsmeyer S J. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79:189–192. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 30.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 31.Park J, Kim I, Oh Y U, Lee K-W, Pyung-Lim H, Choi E-J. Activation of c-Jun N-terminal Kinase antagonizes an antiapoptotic action of Bcl2. J Biol Chem. 1997;272:16725–16728. doi: 10.1074/jbc.272.27.16725. [DOI] [PubMed] [Google Scholar]
- 32.Pietenpol J A, Papadopoulos N, Markowitz S, Willson J K V, Kinzler K W, Vogelstein B. Paradoxical inhibition of solid tumor cell growth by Bcl2. Cancer Res. 1994;54:3714–3717. [PubMed] [Google Scholar]
- 33.Reed J C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sackett D I. Vinca site agents induce structural changes in tubulin different from and antagonistic to changes induced by colchicine site agents. Biochemistry. 1995;34:7010–7019. doi: 10.1021/bi00021a012. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Hanada M, Bodrag S, Irie S, Iwame N, Boise L H, Thompson C B, Colemia S, Fong I, Wary H-G, Reed J C. Interaction among members of the bcl-2 protein family analyzed with a yeast two-hybrid system. Proc Natl Acad Sci USA. 1994;91:9238–9242. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scala S, Budilon A, Zhan Z, Cho-Chung Y S, Jefferson J, Tsokos M, Bates S E. Downregulation of mdr-1 expression by 8-Cl-cAMP in multidrug resistant MCF-7 human breast cancer cells. J Clin Invest. 1995;96:1026–1034. doi: 10.1172/JCI118088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava R K, Srivastava A R, Cho-Chung Y S. Synergistic effects of 8-Cl-cAMP and retinoic acid on induction of apoptosis in Ewing’s sarcoma CHP-100 cells. Clin Cancer Res. 1998;4:755–761. [PubMed] [Google Scholar]
- 38.Tang C, Willingam M C, Reed J C, Miyashita T, Ray S, Ponnatpur V, Huang Y, Mahoney M E, Bullock G, Bhalla K. High levels of p26Bcl2 oncoprotein related taxol-induced apoptosis in human pre-B leukemia cells. Leukemia (Baltimore) 1994;8:1960–1969. [PubMed] [Google Scholar]
- 39.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus CrmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 40.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng S, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32β, a mammalian homolog of Ced-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 41.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science (Washington, DC) 1995;267:1456–1460. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 42.Tortora G, Isernia G D, Sandomenico C, Bianco R, Pomatico G, Pepe S, Bianco A R, Ciardiello F. Synergistic inhibition of growth and induction of apoptosis by 8-cloro-cAMP and paclitaxel or cisplatin in human cancer cells. Cancer Res. 1997;57:5107–5111. [PubMed] [Google Scholar]
- 43.Tortora G, Yokozaki H, Pepe S, Clair T, Cho-Chung Y S. Differentiation of HL-60 leukemia by type I regulatory subunit antisense oligodeoxynucleotide of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1991;88:2011–2015. doi: 10.1073/pnas.88.5.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tournier S, Raynaud F, Gerbaud P, Lohmann S M, Doree M, Evain-Brion D. Association of type II cAMP-dependent protein kinase with p34cdc2 protein kinase in human fibroblasts. J Biol Chem. 1991;28:19018–19022. [PubMed] [Google Scholar]
- 45.Vaux D L, Haecker G, Strasser A. An evolutionary perspective on apoptosis. Cell. 1994;76:777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Miura M, Bergeron L, Zhu H, Yuan L. Ich-1, an ICE/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994;78:739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- 47.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 48.Vintermyr O K, Gjersten B T, Lanotte M, Doskeland S O. Microinjected catalytic subunit of cAMP-dependent protein kinase induces apoptosis in myeloid leukemia (IPC-81) cells. Exp Cell Res. 1993;206:157–161. doi: 10.1006/excr.1993.1132. [DOI] [PubMed] [Google Scholar]