Abstract
c-Jun N-terminal protein kinase (JNK) and p38, two distinct members of the mitogen-activated protein (MAP) kinase family, regulate gene expression in response to various extracellular stimuli, yet their physiological functions are not completely understood. In this report we show that JNK and p38 exerted opposing effects on the development of myocyte hypertrophy, which is an adaptive physiological process characterized by expression of embryonic genes and unique morphological changes. In rat neonatal ventricular myocytes, both JNK and p38 were stimulated by hypertrophic agonists like endothelin-1, phenylephrine, and leukemia inhibitory factor. Expression of MAP kinase kinase 6b (EE), a constitutive activator of p38, stimulated the expression of atrial natriuretic factor (ANF), which is a genetic marker of in vivo cardiac hypertrophy. Activation of p38 was required for ANF expression induced by the hypertrophic agonists. Furthermore, a specific p38 inhibitor, SB202190, significantly changed hypertrophic morphology induced by the agonists. Surprisingly, activation of JNK led to inhibition of ANF expression induced by MEK kinase 1 (MEKK1) and the hypertrophic agonists. MEKK1-induced ANF expression was also negatively regulated by expression of c-Jun. Our results demonstrate that p38 mediates, but JNK suppresses, the development of myocyte hypertrophy.
Cardiac hypertrophy is an adaptive physiological process (49). In response to various extracellular stimuli, myocardium adapts to increased workloads through the hypertrophy of individual terminally differentiated myocytes (6, 52). The hypertrophic response is characterized by an enlargement of individual cells, an increase in the content of contractile proteins such as myosin heavy chain (MHC), and expression of embryonic genes like that for atrial natriuretic factor (ANF) (6). The hypertrophic response is compensatory in nature, but sustained excessive workloads may lead to heart failure (52).
An important question in studying cardiac hypertrophy is how extracellular signals are transmitted into myocytes to induce the hypertrophic response. It has been shown that the proto-oncogene Ha-Ras, the Gαq-containing heterotrimeric G protein, and the interleukin-6 (IL-6) receptor gp130 are critical mediators in the hypertrophic response in vitro and in vivo (22, 26, 35), through activation of their downstream signaling pathways, such as mitogen-activated protein (MAP) kinase pathways.
MAP kinase is an essential part of cell signal transduction machinery and occupies a central position in cell growth, differentiation, and transformation (44). Several mammalian MAP kinases have been identified, including extracellular signal-regulated protein kinase (ERK) (4), c-Jun N-terminal protein kinase (JNK) (also known as SAPK, for stress-activated protein kinase) (12, 21, 34), and p38 (also known as Mkp2/CBSP) (19, 37, 59). The ERK pathway consists of Ha-Ras, Raf-1, MEK, and ERK (10, 11, 24, 31, 61, 73). ERK activity can be stimulated by a variety of extracellular stimuli and oncogenes (7). ERK in turn activates several cellular protein kinases (ribosomal S6 kinase, MAP kinase-activated protein kinase 2 [MAPAPK2], and MNK1) and nuclear transcription factor Elk (5, 14, 55, 64). Activation of the ERK pathway was shown to be necessary and sufficient to induce transformation in NIH 3T3 fibroblasts and to induce differentiation in PC12 chromocytoma cells (9, 42). The JNK pathway is composed of Ha-Ras, Rac/Cdc42Hs, MEKK, JNK kinase [JNKK], and JNK (8, 13, 23, 30, 36, 38, 41, 47, 48, 53, 60, 70). JNK is activated by various stimuli, including growth factors, cytokines, UV light irradiation, and oncogenes (29). Stimulation of JNK activity results in activation of several transcription factors such as c-Jun, ATF2, Elk, and Sap-1 (12, 18, 21, 28, 55, 72). The biological functions of the JNK pathway, however, remain to be elucidated. Several recent reports suggest that the JNK pathway may be involved in regulation of programmed cell death (apoptosis), but its precise role in apoptosis remains controversial (17, 40, 50, 74, 77). The signaling pathway that leads to p38 activation is still incomplete. The MAP kinase kinases (MKKs) for p38 are JNKK1, MKK3, and MKK6, and the MKK kinases may include Tak1 and Ask1 (20, 27, 39, 56, 75). p38 can be activated by proinflammatory cytokines such as IL-1 and tumor necrosis factor (TNF) and by environmental stresses such as UV irradiation and osmotic pressures (19, 37, 59). The biological functions of p38 have yet to be determined.
The role(s) of MAP kinase pathways in the development of the hypertrophic response has been studied extensively but is still far from conclusive. ERK can be activated by hypertrophic agonists such as endothelin-1 (ET-1), phenylephrine (PE), and angiotensin II in a Ha-Ras- and Raf-1-dependent manner (3, 54, 65, 67, 76). But the role played by the ERK pathway in the hypertrophic response is controversial. It was reported that ANF expression was stimulated by activated mutants of Raf-1, Raf-1(B × B), and MEK1, MEK1(ΔNED) (16, 67), suggesting that the ERK pathway is required for mediating the hypertrophic response. On the other hand, it was found that ANF expression was blocked by MEK1(ΔNED) and by the activation of ERK and was unaffected by a specific MEK1 inhibitor, PD098059 (54, 68, 69). In Ras transgenic mice, it was also found that ERK activation was not involved in the development of the hypertrophic response, including ANF expression (57). It is agreed, in general, that ERK activation is insufficient for transcriptional activation of the ANF gene (54, 57, 69).
Hypertrophic agonists such as PE also stimulated JNK activity (57, 69). In addition, MEKK1, the MKK kinase for JNK, was able to stimulate ANF expression (57, 69). But it is not clear whether activation of JNK is necessary and/or sufficient for the development of myocyte hypertrophy.
Here we show that p38 activity was stimulated by and required for ANF expression by hypertrophic agonists like ET-1, PE, and leukemia inhibitory factor (LIF) in rat neonatal ventricular myocytes. Activation of p38 by itself was also sufficient to stimulate ANF expression. In addition, the specific p38 inhibitor SB202190 significantly changed the hypertrophic morphology induced by ET-1 and LIF. In contrast, activation of JNK inhibited ANF expression induced by MEKK1 and the hypertrophic agonists. Our results demonstrate that p38 and JNK had opposing effects on the development of myocyte hypertrophy.
MATERIALS AND METHODS
Cell cultures.
Rat neonatal ventricular myocytes (referred to hereafter in this work as myocytes, for simplicity) were prepared from the hearts of 1- to 3-day-old Sprague-Dawley rats, as described previously (62). Ventricles were separated from atrial tissue and washed briefly in digestion solution (116 mM NaCl, 20 mM HEPES, 1 mM NaH2PO4, 5.5 mM glucose, 5.4 mM KCl, 0.8 mM MgSO4 [pH 7.35], collagenase [95 U/ml], and pancreatin [0.6 mg/ml]). The myocytes were dissociated in fresh digestion buffer and collected by centrifugation. The isolated cells, which were a mixture of myocytes and nonmyocyte fibroblasts, were suspended in plating media (Dulbecco’s modified Eagle’s medium and medium 199 at a ratio of 4:1) and plated onto 150-mm-diameter noncoated culture dishes for 1 h to reduce contaminated cardiac fibroblasts, which often constitute 5% of the cardiac cells and can attach to noncoated culture dishes. After a 1-h incubation, unattached cells were collected and more than 95% of the cells were myocytes as determined by cell morphology and myosin staining. Myocytes were purified by Percoll gradient, replated at a density of 1.5 × 105 in plates precoated with 1% gelatin or 1 × 105 in 25-mm-diameter etched coverslips and grown in plating media. After 24 h, the media were replaced by serum-free media (DMEM and medium 199; 4:1), supplemented with 2 mM glutamine, penicillin (100 U/ml), and 100 streptomycin (100 mg/ml).
cDNA constructs.
Expression vectors of MEKKΔ, JNK1, JNK1(APF), JNKK1, JNKK1(AA), p38, p38(Y→F), MKK6b, MKK6b(EE), c-Jun, c-Jun(A63), GAL4–c-Jun, GAL4–c-Jun(AA63/73), GAL4-Elk, and GAL4-Elk (AA383/389) have been described elsewhere (19, 20, 21, 25, 39, 41, 54). Reporter genes of ANF-luciferase (ANF-Luc), in which the full-length rat ANF promoter (at position −3003) was fused to firefly luciferase gene, and 5×GAL4-Luc, in which the GAL4 DNA-binding domain was fused to the luciferase gene, have also been described previously (39, 41, 62).
Fusion proteins.
Glutathione-S-transferase (GST)–c-Jun, GST-ATF2, and GST-hsp27 were purified on glutathione-agarose, as described previously (39, 48).
Transfections.
Myocytes were transiently transfected with mammalian expression vectors encoding p38, p38(Y→F), MKK6b, MKK6b(EE), MEKKΔ, JNK1, JNK1(APF), JNKK1, JNKK1(AA), c-Jun, c-Jun(A63), GAL4-c-Jun, GAL4–c-Jun(AA63/73), GAL4-Elk, GAL4-Elk(AA383/389), and appropriate luciferase reporter genes by using Tfx-20 (Promega), according to the manufacturer’s procedure. After transfection, cells were washed three times and maintained in serum-free medium with or without ET-1 (10 nM), PE (50 μM), and LIF (1 nM) in the absence or presence of specific p38 inhibitor SB202190 (20 μM), or the specific MEK1 inhibitor PD098059 (50 μM) for 48 h. Luciferase assays were performed as described before (39).
Immunofluorescence staining.
Purified cardiac myocytes (104 cells/cm2) were cultured in serum-free medium in the presence or absence of 10 nM ET-1 or 1 nM LIF and in the presence or absence of the specific p38 inhibitor SB202190 (10 μM) for 4 days. The cells were then washed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 30 min at 25°C, and washed three times. Immunostaining was performed as described previously (62). Anti-α-MHC monoclonal antibody (a gift from Jim Lin, University of Iowa, Iowa City) was used to identify cardiac myocytes.
Kinase assays.
JNK, p38, ERK, and MAPKAPK2 assays were performed as described previously (39, 41, 59). Briefly, myocytes were serum starved for 24 h and then treated with or without agonists for various times as indicated in the figure legends. The cells were harvested in lysis buffer and clarified by centrifugation. Endogenous JNK, p38, ERK, and MAPKAPK2 were immunoprecipitated with their specific antibodies for 3 h at 4°C. The activity of the immune complex was assayed at 30°C for 30 min in 30 μl of kinase buffer (39) in the presence of 10 μM ATP–10 μCi of [γ-32P]ATP (10 Ci/mmol) with appropriate substrates, as indicated in the figure legends. The reactions were terminated with Laemmli sample buffer. The proteins were resolved by sodium dodecyl sulfate–13% polyacrylamide gel electrophoresis followed by autoradiography. The phosphorylated proteins were quantitated by a PhosphoImager.
RESULTS
Activation of p38 kinase activity in myocytes.
p38 can be activated by various extracellular stimuli, including proinflammatory cytokines IL-1 and TNF, and environmental stress, such as UV irradiation (1, 19, 37, 59). Since the hypertrophic response in myocytes is a unique type of cellular stress response (1, 51), we tested whether hypertrophic agonists can also stimulate p38 activity in myocytes.
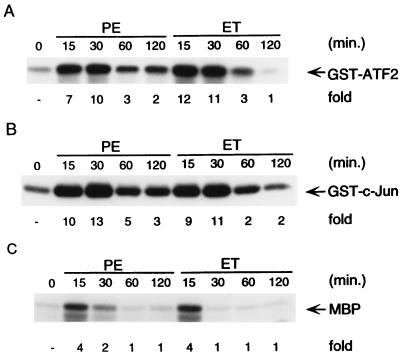
Myocytes were isolated and grown in serum-free media for 24 h as described previously (62). The cells were treated with ET-1 and PE for various times as indicated (Fig. 1A). Endogenous p38 was immunoprecipitated with anti-p38 antibody (PharMingen, Inc.) and its activity was measured in immune complex kinase assays with GST-ATF2 as a substrate, as described previously (41, 47). We found that p38 activity was significantly stimulated by ET-1 and PE, and the stimulation lasted a few hours (Fig. 1A). p38 activity was also mildly stimulated by LIF (data not shown). This result demonstrates that p38 activity can also be stimulated by stimuli that induce myocyte hypertrophy.
FIG. 1.
Activation of p38, JNK, and ERK in myocytes. (A) Myocytes were grown in serum-free medium for 24 h and then treated with ET-1 (10 nM) or PE (50 μM) for the indicated times or left untreated. The cells were harvested after stimulation, and whole-cell extracts were prepared. Endogenous p38 was immunoprecipitated from cell extracts (30 μg) with anti-p38 antibody, and its activity was measured in immune complex kinase assays with GST-ATF2 (2 μg) as a substrate. (B) Endogenous JNK was immunoprecipitated from cell extracts (30 μg) with anti-JNK1 antibody, and its activity was measured in immune complex kinase assays with GST–c-Jun (2 μg) as a substrate. (C) Endogenous ERK was immunoprecipitated from cell extracts (30 μg) with anti-ERK antibody, and its activity was measured in immune complex kinase assays with MBP (4 μg) as a substrate. For all panels, fold stimulation is indicated below each lane.
We also examined the regulation of JNK and ERK activities in myocytes by ET-1 and PE. Endogenous JNK was immunoprecipitated with anti-JNK antibody (PharMingen, Inc.) and its activity was measured in immune complex kinase assays with GST–c-Jun as a substrate, as described previously (39, 41). Like p38, JNK activity was stimulated by ET-1 and PE and the activation lasted a few hours (Fig. 1B). JNK activity was also mildly stimulated by LIF (data not shown). This result is consistent with and reinforces earlier reports that PE was able to stimulate JNK activity in myocytes (57, 69).
In contrast, endogenous ERK activity was stimulated by ET-1 and PE transiently, as measured by immune complex kinase assays using anti-ERK antibody (Upstate Biotechnology Inc.) with MBP (myelin basic protein) as a substrate (Fig. 1C), in agreement with an earlier report (3).
Activation of p38 results in stimulation of transcription factor Elk activity in myocytes.
In several cell systems, p38 activity can be stimulated by coexpression of p38 with MKK6b, or by expression of constitutively activated MKK6b(EE), in which the activating phosphorylation residues Ser207 and Thr211 were replaced by glutamic acids (25). The activation of p38 leads to the stimulation of transcription factor Elk activity (56). Using transcription assays, we tested whether the activation of p38 can lead to the stimulation of Elk activity in myocytes.
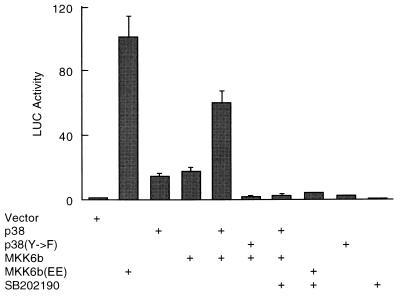
Myocytes were transfected with expression vectors of p38, MKK6b, or MKK6b(EE) or empty vector, along with a GAL4-Elk fusion protein, in which the Elk transactivation domain was fused to the GAL4 DNA-binding domain (43). Expression of MKK6b(EE), or coexpression of p38 with MKK6b stimulated GAL4-Elk activity manyfold, as measured by a 5×GAL4-Luc reporter gene (Fig. 2A). In contrast, coexpression of p38 with MKK6b did not stimulate the activity of the GAL4-Elk(AA383/389) mutant, in which both p38 phosphorylation residues Ser383 and Ser389 were replaced with alanines (Fig. 2A). The stimulation of GAL4-Elk activity is dependent on p38 activation, since it was inhibited by the specific p38 inhibitor SB202190 (Fig. 2A). Coexpression of p38 and MKK6b also stimulated p38 kinase activity, as measured in immune complex kinase assays with GST-ATF2 as a substrate (Fig. 2B). These results demonstrate that activation of p38 is sufficient to stimulate its reporter gene expression in myocytes.
FIG. 2.
Activation of p38 stimulates gene transcription in myocytes. (A) Myocytes were cotransfected with a 5×GAL4-Luc reporter plasmid (1 μg/plate) and expression vectors encoding p38, MKK6b, and/or MKK6b(EE) (300 ng each) and/or GAL4-Elk or GAL4-Elk(Ala383/389) (10 ng each) in the presence (+) or absence of the specific p38 inhibitor SB202190 (20 μM) as indicated. After 48 h, cells were harvested, and luciferase activity was determined and normalized to the protein content of each extract. Luciferase activity expressed by cells transfected with pSRα was given an arbitrary value of 1. The results are presented as means ± standard errors (error bars) and represent six individual experiments. (B) Myocytes were transfected with expression vectors encoding MKK6b, M2-Flag-tagged p38, or empty vector (3 μg each) as indicated. M2-p38 was immunoprecipitated with anti-M2 antibody (Kodak Inc.), and its activity was measured as described in the legend to Fig. 1A.
Activation of p38 by itself is sufficient to induce ANF expression.
ANF expression is an important genetic marker of cardiac hypertrophy in vitro and in vivo (6). To determine the effect of p38 activation on ANF expression, we tested whether p38 activation was able to stimulate ANF promoter activity.
Myocytes were transfected with an ANF-Luc reporter gene along with expression vectors encoding MKK6b(EE) or empty vector. Expression of MKK6b(EE) stimulated ANF-Luc activity manyfold (Fig. 3), yet this result does not rule out the possibility that MKK6b(EE) may stimulate ANF expression in a p38-independent manner, as it does in the induction of T-lymphocyte apoptosis (25). Therefore, we tested whether MKK6b(EE)-induced ANF expression is due to activation of p38.
FIG. 3.
Activation of p38 by itself is sufficient to stimulate ANF expression in myocytes. Myocytes were cotransfected with the ANF-Luc reporter plasmid (1.5 μg/plate) and expression vectors encoding p38, p38(Y→F), MKK6b, and/or MKK6b(EE) (300 ng each), in the presence (+) or absence of the specific p38 inhibitor SB202190 (20 μM) as indicated. After 48 h, the cells were harvested and luciferase activity was determined and normalized to the protein content of each extract. Luciferase activity expressed by cells transfected with pSRα was given an arbitrary value of 1. The results are presented as means ± standard errors (error bars) and represent six individual experiments.
Myocytes were transfected with the ANF-Luc reporter gene along with expression vectors encoding p38, MKK6b, or empty vector. Expression of p38 or MKK6b alone was able to stimulate ANF-Luc activity mildly (Fig. 3). Coexpression of p38 and MKK6b together stimulated ANF-Luc activity manyfold (Fig. 3). The effect of MKK6b is dependent on p38 activation, since MKK6b failed to stimulate ANF-Luc activity when it was cotransfected with an inactive p38(Y→F) mutant, in which one of the activating phosphorylation residues, Tyr182, was replaced by phenylalanine (Fig. 3). Furthermore, the specific p38 inhibitor SB202190 completely blocked the effect of p38/MKK6b and MKK6b(EE) (Fig. 3). The inhibition was specific, since the dose response of ANF promoter inhibition was well correlated with the dose response of p38 inhibition to SB202190 (data not shown). These results demonstrate that activation of p38 by itself is sufficient to stimulate ANF expression.
p38 is required for stimulation of ANF expression by hypertrophic agonists.
It is known that ET-1 and PE can induce expression of ANF in myocytes (6). Here we show that they also stimulate p38 activity (Fig. 1A) and that p38 activation by itself was sufficient to stimulate ANF promoter activity (Fig. 3). Therefore, we tested whether activation of p38 is required for ANF expression induced by these agonists.
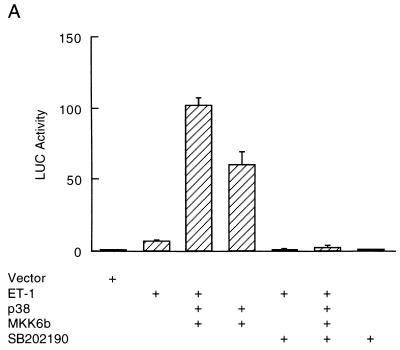
Myocytes were cotransfected with the ANF-Luc reporter gene, along with expression vectors of p38, MKK6b, or empty vector. The cells were then treated with or without the hypertrophic agonists, as indicated (Fig. 4). Treatment with ET-1 increased ANF-Luc activity sevenfold (Fig. 4A). Coexpression of p38 with MKK6b potentiated the effect of ET-1, leading to 110-fold activation (Fig. 4A). The potentiation by p38/MKK6b was shown in a synergistic manner, since coexpression of p38 with MKK6b caused only a 60-fold activation (Fig. 4A). However, p38 may act in a parallel but separate signaling pathway from the ET-1 signaling pathway, leading to stimulation of ANF-Luc activity. Therefore, we examined whether the specific p38 inhibitor SB202190 can inhibit ET-1-induced ANF expression. Treatment of myocytes with SB202190 completely inhibited the stimulation of ANF-Luc activity by ET-1 (Fig. 4A), suggesting that p38 acts downstream of ET-1. The mechanism of potentiation is likely due to posttranslational modification of p38 and MKK6, since ET-1 stimulated p38 activity (Fig. 1A) but did not significantly affect p38 expression (data not shown).
FIG. 4.
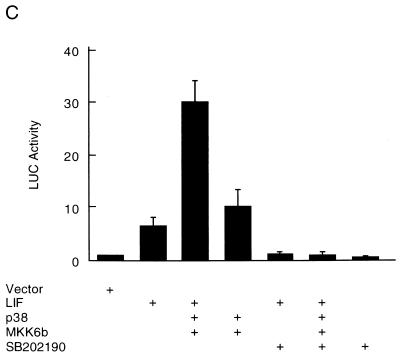
p38 mediates the stimulatory effects of ET-1, PE, and LIF on ANF expression in myocytes. Myocytes were transfected with the ANF-Luc reporter plasmid (1.5 μg/plate) and expression vectors encoding p38 and MKK6b (300 ng each). The cells were treated with hypertrophic agonists ET-1 (10 nM) (A), PE (50 μM) (B), or LIF (1 nM) (C), in the presence (+) or absence of the specific p38 inhibitor SB202190 (20 μM), as indicated. The ANF-Luc activity was determined as described in the legend to Fig. 3. The results are presented as means ± standard errors (error bars) and represent six individual experiments.
In parallel experiments, coexpression of p38 with MKK6b also potentiated the stimulation of ANF-Luc activity by PE and LIF (Fig. 4B and C), and this stimulation was also blocked by the specific p38 inhibitor SB202190 (Fig. 4B and C). These results demonstrate that the p38 pathway is one of the signaling pathways that mediate the effects of hypertrophic agonists on ANF expression.
Inhibition of p38 activity changes the hypertrophic morphology of myocytes.
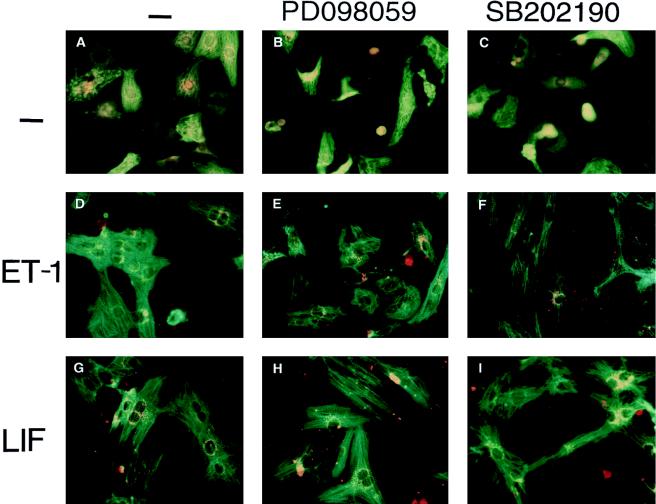
Myocytes were treated with or without ET-1 and LIF in the presence or absence of the specific p38 inhibitor SB202190 or the specific MEK1 inhibitor PD098059 as indicated (Fig. 5). The morphologic changes were examined by immunostaining, as described previously (38, 62). Treatment of myocytes with ET-1 or LIF led to an increase in cell size and the accumulation and assembly of individual MHC into organized sarcomeric units (Fig. 5D and G). The specific p38 inhibitor SB202190 significantly changed the hypertrophic morphology induced by ET-1 and LIF; the cells became thinner and more elongated instead of enlarged, and the assembly of myofilament appears in much disarray (Fig. 5F and I). The specific p38 inhibitor SB202190 also changed PE-induced hypertrophic morphology (data not shown). In contrast, the specific MEK1 inhibitor PD098059 had no significant effect on the hypertrophic morphology induced by ET-1 and LIF (Fig. 5E and H), in agreement with an earlier report (66). This data indicates that p38 activation may also be involved in the hypertrophic morphology changes.
FIG. 5.
Inhibition of p38 activity changes the hypertrophic morphology induced by ET-1 and LIF in myocytes. Myocytes were either left untreated (A to C) or were treated with ET-1 (10 nM) (D to F) or LIF (1 nM) (G to I), in the presence or absence (−) of the specific MEK1 inhibitor PD098059 (50 μM) (B, E, and H) or the specific p38 inhibitor SB202190 (10 μM) (C, F, and I), as indicated. After 48 h, the cells were stained with anti-α-MHC monoclonal antibody, followed by fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin (green). ANF polyclonal antibody was recognized by rhodamine-conjugated anti-rabbit immunoglobulin G (orange).
Activation of JNK blocks ANF expression induced by MEKK1 and the hypertrophic agonists.
The fact that JNK can be activated by hypertrophic agonists (Fig. 1B) suggests that the JNK pathway is involved in the hypertrophic response. We tested this possibility by using MEKKΔ, an NH2-terminal truncated version of MEKK1 which functions as a constitutive activator of JNK (48), since there is no known constitutively active JNK or JNKK (37a).
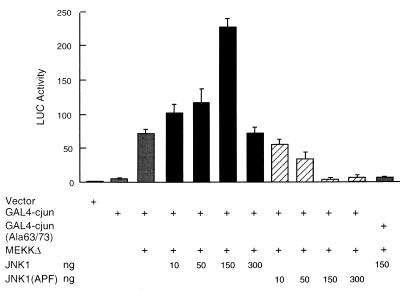
We examined whether MEKKΔ was able to stimulate c-Jun transcription activity in myocytes, as it does in other cells (39, 41). Myocytes were cotransfected with expression vectors encoding MEKKΔ, JNK1, JNK1(APF), or empty vector along with a GAL4–c-Jun fusion protein, which consists of the GAL4 DNA binding domain and the c-Jun transactivation domain (Fig. 6). JNK1(APF) is a kinase-deficient mutant, in which the activating phosphorylation residues Thr183 and Tyr185 were replaced by alanine and phenylalanine, respectively (12, 41). Expression of MEKKΔ-stimulated GAL4–c-Jun transcription activity manyfold, as measured by the GAL4-Luc reporter gene (Fig. 6). As expected, the effect of MEKKΔ was potentiated by cotransfected wild-type JNK1 and inhibited by the inactive JNK1(APF) mutant, indicating that MEKK stimulates c-Jun transcription activity in a JNK-dependent manner in myocytes. The effect of JNK1 on MEKKΔ-induced GAL–4-c-Jun-driven expression appeared to be biphasic. In small amounts (10 to 150 ng), JNK1 potentiated the effect of MEKKΔ on GAL4–c-Jun-driven expression. However, in larger amounts (300 ng), JNK1 failed to continue to potentiate the effect of MEKKΔ.
FIG. 6.
Stimulation of c-Jun transcription activity by MEKK1 in myocytes is through the JNK pathway. Myocytes were transfected with the 5×GAL4-Luc reporter plasmid (1 μg/plate), GAL4–c-Jun or GAL4–c-Jun(Ala63/73) (10 ng each), and expression vector encoding MEKKΔ (10 ng) or JNK1 or JNK1 (APF) (amounts as shown) as indicated. The GAL4-Luc activity was determined as described in the legend to Fig. 2. The results are presented as means ± standard errors (error bars) and represent six individual experiments, except that the data on 300 ng of JNK1 represent three individual experiments.
Then, we examined the effect of MEKKΔ on ANF expression in myocytes by measuring ANF promoter activity. Expression of MEKKΔ stimulated ANF expression manyfold, as measured by ANF-Luc activity (Fig. 7A), in agreement with earlier reports (57, 69). However, this result does not rule out the possibility that MEKKΔ can stimulate ANF expression in a JNK-independent manner, as it does in the induction of c-myc and Sap-1 (29, 72). Therefore, we tested whether the effect of MEKK is due to the activation of JNK.
FIG. 7.
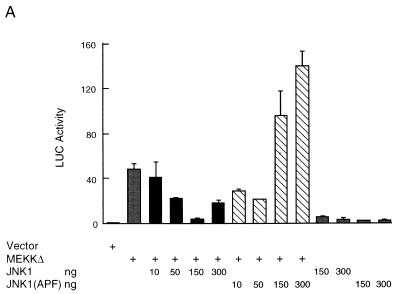
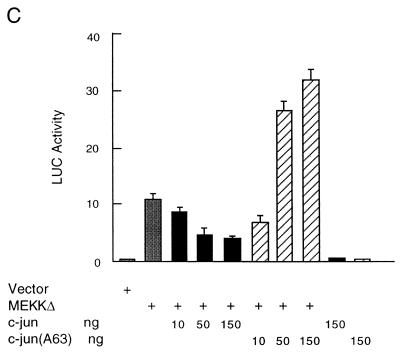
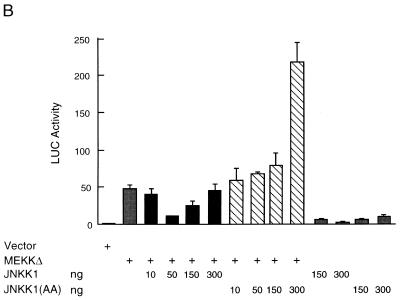
Activation of JNK inhibits MEKK1-induced ANF expression. (A) Myocytes were transfected with the ANF-Luc reporter plasmid (1.5 μg/plate) and expression vector encoding MEKKΔ (10 ng) or JNK1 or JNK1 (APF) (amounts as shown) as indicated. (B) Myocytes were transfected with an ANF-Luc reporter plasmid (1.5 μg/plate) and expression vectors encoding MEKKΔ (10 ng) and/or JNKK1 or JNKK1 (AA) (amounts as shown) as indicated. The results are presented as means ± standard errors (error bars) and represent six individual experiments. (C) Myocytes were transfected with the ANF-Luc reporter plasmid (1.5 μg/plate) and expression vectors encoding MEKKΔ (10 ng) and/or c-Jun or c-Jun (A63) (amounts as shown), or empty vector as indicated. The results are presented as means ± standard errors (error bars) and represent three individual experiments. For all panels, the ANF-Luc activity was measured as described in the legend to Fig. 3.
Myocytes were transfected with the ANF-Luc reporter gene, along with expression vectors encoding MEKKΔ, JNK1, JNK1(APF), JNKK1, JNKK1(AA), or empty vector. The JNKK1(AA) mutant is an interfering mutant, in which the activating phosphorylation residues Ser257 and Thr261 were replaced with alanines (39, 41). Surprisingly, we found that the effect of MEKKΔ on ANF expression was inhibited, rather than potentiated, by cotransfected wild-type JNK1 (Fig. 7A). Conversely, the effect of MEKKΔ was potentiated by the inactive JNK1(APF) mutant (Fig. 7A). The effect of MEKKΔ was also inhibited by wild-type JNKK1 and potentiated by the inactive JNKK1(AA) mutant (Fig. 7B). Both inhibition and potentiation are shown in a dose-dependent manner (Fig. 7A and B). Interestingly, the effect of wild-type JNK1 (and JNKK1) on MEKKΔ-induced ANF expression also appeared to be biphasic. In small amounts (10 to 150 ng), JNK1 (and JNKK1) inhibited the MEKKΔ effect. But in larger amounts (300 ng), JNK1 (JNKK1) failed to continue to suppress the MEKKΔ effect.
The inhibitory effect of JNK on MEKKΔ-induced ANF expression appears to be mediated by c-Jun. As shown in Fig. 7C, coexpression of c-Jun resulted in suppression of MEKKΔ-induced ANF expression in a dose-dependent manner. Taken together, these results indicate that stimulation of ANF expression by MEKKΔ is independent of JNK. In fact, activation of the JNK pathway negatively regulated MEKKΔ-induced ANF expression.
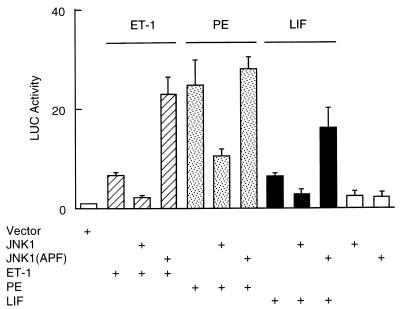
Consistent with the findings above, JNK also negatively regulated ANF expression induced by hypertrophic agonists like ET-1, PE, and LIF. Transfection of wild-type JNK1 inhibited the ANF expression (Fig. 8). Conversely, transfection of the inactive JNK1(APF) mutant potentiated the ANF expression (Fig. 8).
FIG. 8.
Activation of JNK inhibits ANF expression induced by ET-1, PE, and LIF. Myocytes were transfected with the ANF-Luc reporter plasmid (1.5 μg/plate) and expression vectors encoding JNK1 or JNK1 (APF) (150 ng each). The cells were left untreated or were treated with the hypertrophic agonists as indicated. The ANF-Luc activity was measured as described in the legend to Fig. 3. The results are presented as means ± standard errors (error bars) and represent six individual experiments.
DISCUSSION
In this report we demonstrate that JNK and p38 MAP kinases have opposing effects on cardiomyocyte hypertrophy. Activation of p38 is necessary and sufficient for ANF expression and is probably involved in the induction of the hypertrophic morphology changes. On the other hand, activation of JNK suppresses ANF expression induced by MEKK1 and hypertrophic stimuli such as PE and ET-1.
The conclusion that the p38 pathway may be required for the development of myocyte hypertrophy is based on the following four lines of evidence.
First, p38 activity was stimulated by hypertrophic agonists, such as ET-1 and PE (Fig. 1A). The fact that activation of p38 lasted a few hours suggests that it is a relevant event in the development of myocyte hypertrophy. This is in agreement with an earlier report that in vivo, p38 was activated by ischemia and/or reperfusion in isolated rat hearts (2). In contrast, the activation of ERK was transient (Fig. 1C), in agreement with the previous report that the ERK pathway may not be directly involved in the hypertrophic response (54).
Second, expression of MKK6b(EE), the constitutive activator of p38, stimulated ANF expression (Fig. 3). The effect of MKK6b(EE) was dependent on activation of p38, since coexpression of MKK6b with p38, but not the inactive p38(Y→F) mutant, stimulated ANF expression (Fig. 3). This result was further supported by the finding that the specific p38 inhibitor SB202190 completely blocked the effect of MKK6b (Fig. 3).
Third, p38 was required for the induction of ANF expression by the hypertrophic agonists, including ET-1, PE, and LIF, though it remains to be determined whether p38 is also required for the development of other forms of myocyte hypertrophy. The effects of the hypertrophic agonists were potentiated by coexpression of p38 with MKK6b, and blocked by the specific p38 inhibitor SB202190 (Fig. 4). In myocytes, both ET-1 and PE induce ANF expression and secretion through yet-to-be defined signaling pathways which involve G proteins (Gαq and Gαi) (32, 46, 63). The identification of p38 as a signaling mediator that acts downstream of ET-1 and PE provides a model system for further elucidation of ET-1 and PE signaling pathways. LIF is a member of the IL-6 family of cytokines and exerts its biological effects through binding to the heterodimers formed between its receptor and gp130 (22). It was reported that the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway may act downstream of LIF to induce the hypertrophic response in myocytes (62). It will be of interest to determine the possible interplay between the p38 pathway and the JAK/STAT pathway in regard to the development of myocyte hypertrophy.
Fourth, the specific p38 inhibitor SB202190 significantly changed the hypertrophic morphology induced by ET-1 and LIF in myocytes (Fig. 5). This result suggests that p38 not only is required for expression of ANF but also may be involved in the hypertrophic morphology changes. This finding is of particular interest since little is known about signaling pathways that mediate the morphology changes induced by hypertrophic agonists. It is not known how p38 influences the hypertrophic morphology changes. Some candidates that may be involved are Rac and Cdc42Hs. We and others have found that p38 can be activated by the oncoproteins Rac and Cdc42Hs (33, 58), both of which are important mediators in actin reorganization in many other cell systems (58). Rac and Cdc42Hs also stimulated ANF expression in myocytes (49a). It remains to be demonstrated whether Rac and Cdc42Hs are also involved in the hypertrophic morphology changes, and whether their effects are dependent on p38 activation.
Our conclusion is further supported by recent reports that hypertrophic morphology changes and the expression of ANF gene were induced by recombinant adenoviruses of constitutively active MKK6(EE) and suppressed by the specific p38 inhibitor SB202190 (71, 78). The mechanisms by which p38 mediates myocyte hypertrophy are currently under investigation.
The role of the JNK pathway in the development of myocyte hypertrophy is complicated. Like p38, JNK activity was stimulated by hypertrophic agonists, including ET-1 and PE (Fig. 1B). Expression of a small amount (10 ng) of JNK activator, MEKKΔ, was sufficient to induce ANF expression (Fig. 7). These results are in agreement with previous reports (57, 69), suggesting that the JNK pathway is required for the development of myocyte hypertrophy. Surprisingly, we found that MEKKΔ-induced ANF expression was not dependent on JNK activation, since it was blocked by coexpression of wild-type JNK1 but potentiated by the inactive JNK1(APF) mutant (Fig. 7A). On the other hand, stimulation of c-Jun transcription activity by MEKK was dependent on JNK activation (Fig. 6).
The inhibitory effect of JNK1 on MEKKΔ-induced ANF expression appears to be mediated by c-Jun, because coexpression of c-Jun also suppressed MEKKΔ-induced ANF expression in a dose-dependent manner (Fig. 7B). This finding is in agreement with the previous report that expression of c-Jun suppressed PE-induced ANF promoter activity and the c-Jun N-terminal transactivation domain was sufficient for this inhibition (45). Although the precise mechanisms by which c-Jun suppresses MEKKΔ-induced ANF expression remain to be determined, it is plausible that phosphorylation of c-Jun on its N-terminal transactivation domain by activated JNK is required for c-Jun to interact with yet-to-be-identified limiting cardiac cofactors, leading to inhibition of ANF expression.
JNK1 exerted opposing effects on MEKKΔ-induced GAL4–c-Jun-driven expression and ANF expression. In small amounts (10 to 150 ng), JNK1 potentiated MEKKΔ-induced GAL4–c-Jun-driven expression but suppressed MEKKΔ-induced ANF expression. However, in larger amounts (300 ng), JNK1 did not continue to potentiate MEKKΔ-induced GAL4–c-Jun-driven expression and also failed to suppress MEKKΔ-induced ANF expression. It appears that 300 ng of JNK1 failed to continue to potentiate the effect of MEKK on GAL4–c-Jun due to the “squelching” effect (15). Overexpressed JNK1 (300 ng) may titrate GAL4–c-Jun and other limiting cofactors for transcription, resulting in much less promoter-bound GAL4–c-Jun and cofactors and decreased GAL4–c-Jun-driven expression. Since 300 ng of JNK1 failed to stimulate c-Jun-driven expression, it can no longer suppress MEKKΔ-induced ANF expression (Fig. 7A).
The conclusion that activation of the JNK pathway results in inhibition of MEKK-induced ANF expression in myocytes appears to be contradictory to the earlier report that MEKK stimulates ANF expression in a JNK-dependent manner (69). We do not completely understand this discrepancy. However, it is noteworthy that in the earlier report the amount of interfering mutants of JNKK, JNK, and c-Jun employed to inhibit MEKK-induced ANF expression was quite large (3 μg of each per 35-mm-diameter plate) and that the interference was not shown to be dose dependent (69). In transcription assays, cotransfection of large amounts of expression vectors may lead to repression of ANF-Luc expression. In the same earlier report, it was also found that higher amounts of MEKK expression vector (0.5, 1, or 3 μg) caused suppression of ANF expression, though small amounts of MEKK (1, 10, or 100 ng) potentiated ANF expression (69). In the experiments described in this report, the effect of JNK (or JNKK) expression vectors was dose dependent and occurred in a range of smaller quantities of plasmid (10 to 300 ng per 21-mm-diameter plate) (Fig. 7). In addition, activation of JNK also inhibited ANF expression induced by hypertrophic agonists (Fig. 8). It is likely that activation of JNK is a cellular stress response to hypertrophic stimuli and functions as a negative feedback on ANF expression induced by MEKK or the hypertrophic agonists.
The signaling pathway that mediates the effect of MEKK1 on ANF expression remains to be determined. The effect of MEKKΔ can be blocked by the specific p38 inhibitor SB202190 and the specific MEK1 inhibitor PD098059, but there is no clear correlation between activation of p38 or ERK with the effect of MEKKΔ (49a). Further studies are needed to elucidate the mechanism of the MEKK effect on ANF expression.
JNK and p38 are usually coregulated by extracellular stimuli. It was thought that these two MAP kinases may have similar functions. Our results, for the first time, demonstrate that activation of JNK and p38 has opposing effects on an important physiological event, myocyte hypertrophy. Activation of p38 induced ANF expression and was probably involved in the hypertrophic morphology changes, while activation of JNK inhibited ANF expression. This observation may provide an opportunity to investigate how differential effects can be induced by similarly regulated protein kinases.
ACKNOWLEDGMENTS
We thank M. Karin, J. Han, K. R. Chien, G. Johnson, M. Cobb, A. Yee, S. Pelech, and J. Lin for the different plasmids and antibodies that made this work possible. We also thank J. DiDonato and J. Frost for helpful discussions.
This work was supported by American Heart Association Scientist Development Grant 9630261N and National Institutes of Health grant CA73740 (to A.L.).
REFERENCES
- 1.Anversa P, Beghi C, Kikkawa Y, Olivetti G. Myocardial response to infarction in the rat. Morphometric measurement of infarct size and myocyte cellular hypertrophy. Am J Pathol. 1985;118:484–492. [PMC free article] [PubMed] [Google Scholar]
- 2.Bogoyevitch M A, Gillespie-Brown J, Ketterman A J, Fuller S J, Ben-Levy R, Ashworth A, Marshall C J, Sugden P H. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–173. doi: 10.1161/01.res.79.2.162. [DOI] [PubMed] [Google Scholar]
- 3.Bogoyevitch M A, Glennon P E, Andersson M B, Clerk A, Lazou A, Marshall C J, Parker P J, Sugden P H. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 4.Boulton T G, Yancopoulos G D, Gregory J S, Slaughter C, Moomaw C, Hsu J, Cobb M H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990;249:64–65. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- 5.Chen R-H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien K R, Knowlton K U, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 7.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 8.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 9.Cowley S, Paterson H, Kemp P, Marchall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Crews C M, Alessandrini A, Erikson R L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 11.Dent P, Haser W, Haystead T A, Vincent L A, Roberts T M, Sturgill T W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3. Science. 1992;257:1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- 12.Derijard B, Hibi M, Wu I H, Barret T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill G, Ptashne M. Mutants of GAL4 protein altered in an activation function. Cell. 1987;51:121–126. doi: 10.1016/0092-8674(87)90016-x. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie-Brown J, Fuller S J, Bogoyevitch M A, Cowley S, Sugden P H. The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J Biol Chem. 1995;270:28092–28096. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 17.Goillot E, Raingeaud J, Ranger A, Tepper R I, Davis R J, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Campbell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Lee J-D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 21.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 22.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA. 1995;92:4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland P M, Suzanne M, Campbell J S, Noselli S, Cooper J A. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 24.Howe L R, Leevers S J, Gomez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 26.Hunter J J, Tanaka N, Rockman H A, Ross J, Jr, Chien K R. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 27.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 28.Janknecht R, Hunter T. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 1997;16:1620–1627. doi: 10.1093/emboj/16.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson N L, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;27:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 30.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 31.Khokhlatchev A, Xu S, English J, Wu P, Schaefer E, Cobb M H. Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria. Efficient synthesis of active protein kinases. J Biol Chem. 1997;272:11057–11062. doi: 10.1074/jbc.272.17.11057. [DOI] [PubMed] [Google Scholar]
- 32.Knowlton K U, Michel M C, Itani M, Shubeita H E, Ishihara K, Brown J H, Chien K R. The alpha 1A-adrenergic receptor subtype mediates biochemical, molecular and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem. 1993;268:15374–15380. [PubMed] [Google Scholar]
- 33.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kyriakis J M, Banerjee P, Nikolakaki E, Dai E A, Rubie T, Ahmad M F, Avruch J, Woodgt J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 35.LaMorte V J, Thorburn J, Absher D, Spiegel A, Brown J H, Chien K R, Feramisco J R, Knowlton K U. Gq- and ras-dependent pathways mediate hypertrophy of neonatal rat ventricular myocytes following alpha 1-adrenergic stimulation. J Biol Chem. 1994;269:13490–13496. [PubMed] [Google Scholar]
- 36.Lange-Carter C A, Johnson G L. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994;265:1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- 37.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughllin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 37a.Lin, A. Unpublished results.
- 38.Lin A, Frost J, Deng T, Smeal T, Al-Alawi N, Kikkaw U, Hunter T, Brenner D, Karin M. Casein kinase II is a negative regulator of c-Jun DNA binding and AP-1 activity. Cell. 1992;70:777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Minden A, Martinetto H, Claret F X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 41.Lu X, Nemoto S, Lin A. Identification of JNK-activating kinase 2 as an activator of JNK but not p38. J Biol Chem. 1997;272:24751–24754. doi: 10.1074/jbc.272.40.24751. [DOI] [PubMed] [Google Scholar]
- 42.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Wound G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 43.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 44.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1994;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 45.McBride K, Robitaille L, Tremblay S, Argentin S, Nemer M. fos/jun repression of cardiac-specific transcription in quiescent and growth-stimulated myocytes is targeted at a tissue-specific cis element. Mol Cell Biol. 1993;13:600–612. doi: 10.1128/mcb.13.1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonough P M, Brown J H, Glembotski C C. Phenylephrine and endothelin differentially stimulate cardiac PI hydrolysis and ANF expression. Am J Physiol. 1993;264:625–630. doi: 10.1152/ajpheart.1993.264.2.H625. [DOI] [PubMed] [Google Scholar]
- 47.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 48.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 49.Morgan H E, Gordon E E, Kira Y, Chua B H L, Russo L A, Peterson C J, McDermott P J, Watson P A. Biochemical mechanisms of cardiac hypertrophy. Annu Rev Physiol. 1987;49:533–543. doi: 10.1146/annurev.ph.49.030187.002533. [DOI] [PubMed] [Google Scholar]
- 49a.Nemoto, S., and A. Lin. Unpublished results.
- 50.Nishina H, Fischer K D, Radvanyi L, Shahinian A, Hakem R, Rubie A E, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 51.Olivetti G, Capasso J M, Meggs L G, Sonnenblick E H, Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 1991;68:856–869. doi: 10.1161/01.res.68.3.856. [DOI] [PubMed] [Google Scholar]
- 52.Olson E N, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–676. doi: 10.1126/science.272.5262.671. [DOI] [PubMed] [Google Scholar]
- 53.Pombo C M, Kehrl J H, Sanchez I, Katz P, Avruch J, Zon L I, Woodgett J R, Force T, Kyriakis J M. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature. 1995;377:750–754. doi: 10.1038/377750a0. [DOI] [PubMed] [Google Scholar]
- 54.Post G R, Goldstein D, Thuerauf D J, Glembotski C C, Brown J H. Dissociation of p44 and p42 mitogen-activated protein kinase activation from receptor-induced hypertrophy in neonatal rat ventricular myocytes. J Biol Chem. 1996;271:8452–8457. doi: 10.1074/jbc.271.14.8452. [DOI] [PubMed] [Google Scholar]
- 55.Price M A, Rogers A E, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez M T, Sah V P, Zhao X L, Hunter J J, Chien K R, Brown J H. The MEKK-JNK pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 58.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 59.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 61.Seger R, Seger D, Lozeman F J, Ahn N G, Graves L M, Campbell J S, Ericsson L, Harrylock M, Jensen A M, Krebs E G. Human T-cell mitogen-activated protein kinase kinases are related to yeast signal transduction kinases. J Biol Chem. 1992;267:25628–25631. [PubMed] [Google Scholar]
- 62.Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown J H, Chien K R. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1997;272:5783–5791. doi: 10.1074/jbc.272.9.5783. [DOI] [PubMed] [Google Scholar]
- 63.Shubeita H E, McDonough P M, Harris A, Knowlton K U, Glembotski C, Brown J H, Chien K R. Endothelin induction of sarcomere assembly and cardiac gene expression in ventricular myocytes: a paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990;265:20555–20562. [PubMed] [Google Scholar]
- 64.Stokoe D, Campbell D G, Nakielny S, Hidaka H, Leevers S J, Marshall C, Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thorburn A, Thorburn J, Chen S Y, Powers S, Shubeita H E, Feramisco J R, Chien K R. HRas-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993;268:2244–2249. [PubMed] [Google Scholar]
- 66.Thorburn J, Frost J A, Thorburn A. Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle cell hypertrophy. J Cell Biol. 1994;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thorburn J, McMahon M M, Thorburn A. Raf-1 kinase activity is necessary and sufficient for gene expression changes but not sufficient for cellular morphology changes associated with cardiac myocyte hypertrophy. J Biol Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- 68.Thorburn J, Carlson M, Mansour S J, Chien K R, Ahn N G, Thorburn A. Inhibition of a signaling pathway in cardiac muscle cells by active mitogen-activated protein kinase kinase. Mol Biol Cell. 1995;6:1479–1490. doi: 10.1091/mbc.6.11.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Huang S, Sah V P, Ross J, Jr, Brown J H, Han J, Chien K R. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 72.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 73.Wood K W, Sarnecki C, Roberts T M, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 74.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 75.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 76.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, Maemura K, Kurihara H, Aikawa R, Takano H, Yazaki Y. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–3228. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 77.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zechner D, Thuerauf D J, Hanford D S, McDonough P M, Glembotski C C. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]