Abstract
Accumulating evidence suggests that the insect and mammalian innate immune response is mediated by homologous regulatory components. Proinflammatory cytokines and bacterial lipopolysaccharide stimulate mammalian immunity by activating transcription factors such as NF-κB and AP-1. One of the responses evoked by these stimuli is the initiation of a kinase cascade that leads to the phosphorylation of p38 mitogen-activated protein (MAP) kinase on Thr and Tyr within the motif Thr-Gly-Tyr, which is located within subdomain VIII. We have investigated the possible involvement of the p38 MAP kinase pathway in the Drosophila immune response. Two genes that are highly homologous to the mammalian p38 MAP kinase were molecularly cloned and characterized. Furthermore, genes that encode two novel Drosophila MAP kinase kinases, D-MKK3 and D-MKK4, were identified. D-MKK3 is an efficient activator of both Drosophila p38 MAP kinases, while D-MKK4 is an activator of D-JNK but not D-p38. These data establish that Drosophila indeed possesses a conserved p38 MAP kinase signaling pathway. We have examined the role of the D-p38 MAP kinases in the regulation of insect immunity. The results revealed that one of the functions of D-p38 is to attenuate antimicrobial peptide gene expression following exposure to lipopolysaccharide.
Upon bacterial or fungal infection, insects synthesize and secrete into the hemolymph a spectrum of antimicrobial peptides which function synergistically to lyse the invading microorganisms (6, 29, 30, 32). Antimicrobial peptides such as attacin, cecropin, diptericin, defensin, lysozyme, and drosomycin have been isolated from different insects (6, 29, 30, 32). The insect immune response has been proposed to be similar to vertebrate innate immunity, which includes the activation of macrophages and the induction of the acute-phase response during infection and injury (3, 40, 53, 70). This contention is supported by the isolation of cecropin from pig intestine (44) and defensin from neutrophils and macrophages (46).
To facilitate the molecular genetic analysis of insect immunity, the genes coding for a number of antimicrobial peptides were cloned from Drosophila melanogaster (2, 14, 18, 42, 75). By using these molecular clones as probes, several laboratories have demonstrated that at least some of these genes are regulated by transcription factors related to NF-κB, NF-IL6, and GATA (17–19, 34, 36, 37). Furthermore, the bacterial cell wall component lipopolysaccharide (LPS), a potent mitogen of mammalian lymphoid cells and inducer of NF-κB and AP-1 activities (13, 25, 52, 58, 72), can efficiently induce insect immunity gene expression (17, 34, 60, 63). Therefore, it is likely that insects and mammals employ homologous signaling molecules in self-defense processes. This notion is further supported by the molecular cloning of Dif, the Dorsal-related immunity factor, and Relish, which belong to the Rel/NF-κB family of transcription factors (16, 35, 73). Experimental analyses suggest that the Rel-related factors are activated upon infection and regulate at least a subset of antimicrobial peptide genes (16, 21, 35, 48, 49, 57). The modulation of Dif and Dorsal activities depends on the transmembrane protein Toll and its intracellular signaling components, which are strikingly similar to those of the interleukin 1 (IL-1) receptor that regulates NF-κB activity (4, 34). In addition, a human homolog of Toll has recently been cloned and shown to be involved in human innate immunity (53, 54). Together, these data provide strong support for a conserved regulatory mechanism in the immune response of different species.
The regulation of AP-1 and related proteins in mammalian immunity requires a series of mitogen-activated protein kinase (MAPK) pathways (33, 38, 74). The MAPK signal transduction pathways are conserved from yeast to mammals (10, 65). Exposure of cells to growth factors, cytokines, or environmental stress leads to the activation of MAPK kinases, which activate the MAPKs by dual phosphorylation on the Thr-X-Tyr motif located within subdomain VIII (1, 11, 58). The activated MAPKs may then regulate the activity of transcription factors, such as ATF2, Elk-1, and c-Jun, to control gene expression.
Several MAPK pathways have been defined in mammalian systems. Following stimulation, the c-Jun N-terminal kinase (JNK) phosphorylates c-Jun and ATF2, leading to elevated transcriptional activities (74). The p38 MAPK, when activated, phosphorylates different but overlapping groups of protein substrates (58). The p38 MAPK was cloned based on its tyrosine phosphorylation after LPS treatment and on its binding to an anti-inflammatory drug (26, 45, 62). Further experiments suggested that p38 participates not only in the inflammatory response but also in stress-induced signaling (43), cell proliferation (55, 68), and apoptosis (39, 41, 77).
Since insect and mammalian immune responses may have similar signaling molecules, we have investigated the involvement of conserved MAPK pathways in insect immunity. Previous experiments identified a Drosophila JNK MAPK signaling pathway (61, 66) and demonstrated that D-JNK is activated during the immune response (66). The p38 MAPK pathway may also be employed in insect self-defense processes. Indeed, we found two genes, D-p38a and D-p38b, that encode proteins highly related to the mammalian p38 MAPK. Two upstream MAPK kinases, related to MKK3 (12) and MKK4 (12, 50, 64), have also been identified in the Drosophila genome. The Drosophila p38 MAPKs can phosphorylate ATF2 and, to a lesser extent, D-Jun. These kinase activities can be activated by MAPK kinase D-MKK3. The anti-inflammatory compound SB203580, which has been shown to inhibit the activity of the mammalian p38 MAPK and the production of cytokines (45), inhibits the activities of both D-p38 kinases. Treatment of cultured Drosophila cells with SB203580 enhanced LPS-induced expression of antimicrobial peptide genes. In addition, overexpression of D-p38a in transgenic animals suppresses the LPS induction of immunity genes. These data, together with biochemical evidence that LPS activates D-p38 MAPK, indicate that the D-p38 MAPKs may be involved in insect immunity, perhaps by mediating down regulation of immunity gene expression after prolonged induction. These results demonstrate that insects possess a conserved p38 MAPK pathway and that the utilization of this signaling pathway in inflammatory responses is of ancient evolutionary origin.
MATERIALS AND METHODS
Molecular cloning.
To clone D-p38a, the degenerate oligonucleotide primers 5′-GCNGTNGTNCGNGGNACNAA(C/T)ATGCA-3′ and 5′-TANCCNGTCAT(C/T)TC(A/G)TT(C/T)TCNGTNGG-3′ were employed for PCR amplification of a 0- to 4-h embryonic cDNA library (7). The reaction was performed for 30 cycles with an annealing temperature of 42°C. A single 400-bp DNA product was obtained and blunt end cloned into pBluescript KS(+) at the EcoRV site. DNA sequencing identified a fragment that corresponds to a p38-related protein kinase. This PCR fragment was used as a probe to screen the embryonic cDNA library, and six independent clones were isolated. Most of these clones corresponded to probably full-length cDNA by comparison with the size of the mRNA transcript on Northern blots. Two clones were sequenced completely, and the deduced amino acid sequence is shown in Fig. 1. The genomic region of D-p38a was also obtained from a λ library and analyzed. Sequence comparison showed that the gene contains only one intron of 80 bp, located in the 5′ untranslated region (UTR). The D-p38b genomic sequence, deposited by the Berkeley Drosophila Genome Project, was identified in GenBank (accession no. AC000616) by using the D-p38a sequence and the BLAST algorithm (National Center for Biotechnology Information). The similarity between the two D-p38 genes is restricted to the protein-coding region.
FIG. 1.
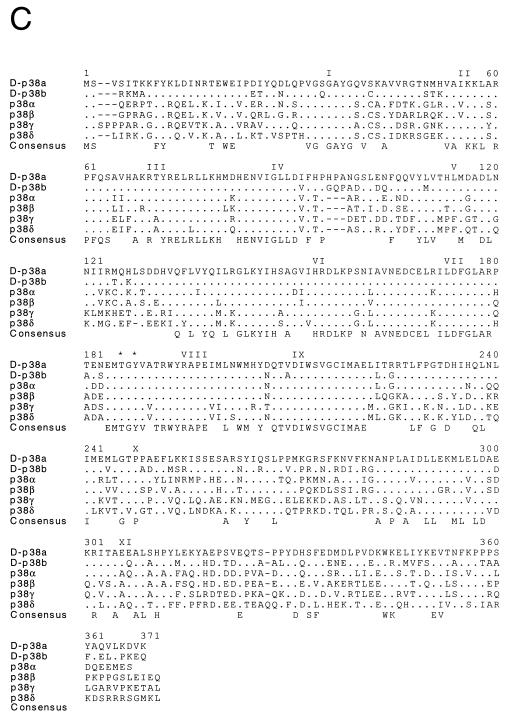
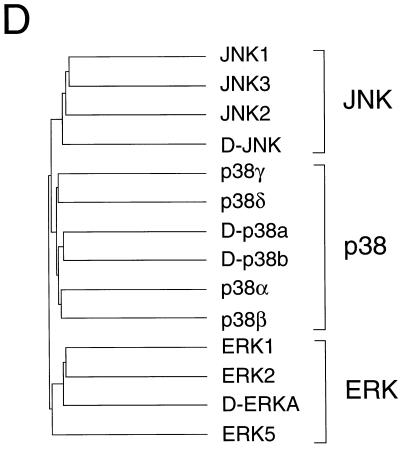
Molecular cloning of two Drosophila p38 MAPKs. (A and B) The nucleotide sequences of the D-p38a (A) and D-p38b (B) genomic clones are presented. The exon structure of the D-p38a gene and the primary sequence of the D-p38a protein kinase were deduced from the sequences of cDNA clones. The sequences are numbered starting with nucleotide 1 of exon 1. Two exons were identified in the D-p38a gene; one exon included the 5′ UTR, and the second exon included the complete coding region. The intron is udnerlined. A single exon containing the coding region of D-p38b was identified from the genomic sequence. The sequence of the D-p38b gene is numbered arbitrarily upstream of the initiation codon. The primary sequence encoded by each exon is illustrated in the single-letter code (italics). Termination codons are indicated by asterisks. (C) The D-p38 primary sequences were compared with those of human MAPKs (JNK1, JNK2, JNK3, p38α, p38β, p38γ, p38δ, ERK1, ERK2, and ERK5) and Drosophila MAPKs (D-JNK and D-ERKA) by using the PILE-UP program (version 9.0; Wisconsin Genetics Computer Group). The protein sequences are presented in the single-letter code. Gaps introduced into the sequences to optimize the alignments are represented by dashes. Identical residues are indicated by dots. The sites of activating phosphorylation on Thr and Tyr are indicated by asterisks. Conserved kinase subdomains I to XI are illustrated. (D) The relationship between members of the human and Drosophila MAPK groups is presented as a dendrogram created by the unweighted pair-group method using arithmetic averages (PILE-UP program).
The PCR amplification of D-MKK3 and D-MKK4 was similar to that of D-p38a, except that the annealing temperature was 47°C. The degenerate oligonucleotides 5′-TGGATITG(C/T)ATGGA(A/G)(C/T)TIATG-3′ and 5′-(G/A)TCIATIC(G/T)(T/C)IGGIGCCAT(G/A)TA-3′ (where I is inosine) were used as primers. These amplimers were designed based on conserved regions of the mammalian MAPK kinase group. The PCR product (approximately 400 bp) was cloned into the pBluescript vector. The sequence of two clones was similar to that of mammalian MKK3, and that of three clones was similar to that of mammalian MKK4. These cloned PCR fragments were used to screen an embryonic cDNA library to isolate full-length cDNA. Genomic clones corresponding to the two MAPK kinases were also isolated and sequenced.
Embryo in situ hybridization.
Collections of wild-type embryos ranging from 0 to 16 h were rinsed in a Triton X-100 NaCl solution (0.03% Triton X-100, 50 mM NaCl), dechorionated in bleach for 3 min, and fixed with vigorous shaking in 4 ml of heptane–4 ml of 4.6% formaldehyde–0.5× phosphate-buffered saline–25 mM EGTA for 25 min. The embryos in the heptane layer were mixed with 2 volumes of methanol, devitellinized by shaking for 1 min, washed with three changes each of methanol, and washed with ethanol. The ethanol-washed embryos were soaked in xylene for 30 min, rinsed with ethanol, postfixed in 5% formaldehyde in 1× phosphate-buffered saline–0.1% Tween 80 (PBT), washed in PBT, and digested with proteinase K (4 μg/ml) for 5 min. The embryos were washed, postfixed again, and prehybridized (in 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]–50% deionized formamide–100-μg/ml salmon sperm DNA–50-μg/ml heparin–0.1% Tween) at 55°C for 1 h. Hybridization was carried out in the same buffer, at the same temperature, with digoxigenin-labeled antisense RNA probes for 18 h. The hybridized embryos were washed extensively with hybridization buffer at 55°C and then with PBT at room temperature and the incubated with an alkaline phosphatase-conjugated antidigoxigenin antibody (Boehringer Mannheim) at 4°C overnight. The embryos were washed with PBT, and the expression patterns were visualized by incubation with staining solution (100 mM NaCl, 50 mM MgCl2, 100 mM Tris [pH 9.5], 0.1% Tween 80) containing nitroblue tetrazolium and X-phosphate (Boehringer Mannheim) as substrates. The stained embryos were mounted in Permount (Fisher) and photographed under differential interference contrast optics using a Zeiss Axiophot microscope.
Protein purification and kinase assays.
To create glutathione S-transferase (GST) fusion constructs, D-p38a was PCR amplified with 5′-TGTGGGATCCTCAAGCAACACCATGGACTATAAGGACGATGACGACAAATCAGTGT CCATTACAAAA-3′ and 5′-TGTGGAGCTCTCACTTTACATCCTTTAG-3′ as the primers and the cDNA clone as the template. The DNA product was digested with BamHI and SacI and ligated into the same sites of Escherichia coli expression vector pGSTag (15). D-p38b was amplified by using 5′-GGCGGATCCGCCACCATGGCCAAATTCTACAAGCTG-3′ and 5′-GCGTCTAGATTACTGCTCTTTGGGCAGGA-3′ as the primers and wild-type genomic DNA as the template. The PCR product was digested with BamHI and XbaI and inserted into the pGSTag vector. The expression constructs were introduced into E. coli BL21(DE3), and the cultures were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The GST fusion proteins were purified with glutathione-agarose columns (67). The purified proteins were employed for kinase assays. Approximately 0.5 μg of D-p38a, 0.1 μg of D-p38b, 0.5 μg of D-JNK (66), and 0.1 μg of protein kinase A (PKA; Sigma) were used for each reaction. The phosphorylation reactions were initiated by addition of 1 μg of substrate protein (ATF2 [58], D-JUN [66], myelin basic protein [Sigma], or casein [Sigma]) and 1 pmol of [γ-32P]ATP (10 mCi/mmol) in a final volume of 20 μl of buffer (25 mM HEPES, [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 25 μM ATP, 0.1 mM Na3VO4, 0.5 mM dithiothreitol [DTT]). When the SB203580 compound (Calbiochem) was used, it was dissolved in dimethyl sulfoxide (DMSO) and added to the reaction mixture containing the protein kinases prior to addition of the substrates. The reactions were terminated after 30 min of incubation at 30°C by addition of sodium dodecyl sulfate (SDS) sample buffer. The phosphorylation of substrate proteins was examined by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography.
Cell culture.
While some Schneider S2 subcultures show no response to LPS stimulation (57, 63), the S2* cells (57) in our laboratory show significant induction of the Cecropin and Attacin genes. On the other hand, Diptericin, Drosomycin, and Defensin are not as inducible in this cultured S2* cell line (data not shown). S2* cells were grown in Schneider’s medium supplemented with 10% heat-inactivated fetal calf serum, 1× Glutamax I, and 1× Pen-strep (GIBCO). Cells were treated with 1/1,000 of the cell culture volume of either DMSO or SB203580 (dissolved in DMSO). Following 1 h of treatment, LPS was added to 5 μg/ml. Samples were taken at different times for RNA isolation.
Tissue culture expression vectors were constructed by PCR amplification of the D-p38 cDNA with oligonucleotide primers. A FLAG epitope was inserted between codons 1 and 2 of the D-p38 cDNA. D-p38a was cloned into the pPA-Actin5C vector (27) between the BamHI and SacI sites, while D-p38b was cloned into the BamHI and EcoRV sites. D-MKK3 was PCR amplified, without the FLAG sequence, and cloned into the BamHI and SacI sites of the pPA-Actin5C vector; D-MKK4 was similarly cloned into the EcoRV site. S2* cells were transfected by using the Lipofectin method (Life Technologies Inc.) with 1 μg each of the D-p38 MAPK and D-MKK expression vectors. After 48 h, the cells were serum deprived for 4 h, treated with LPS, and harvested.
Immunoprecipitation and protein kinase assays.
Cells were solubilized with buffer A (20 mM Tris [pH 7.5], 10% glycerol, 1% Triton X-100, 0.137 M NaCl, 25 mM β-glycerophosphate, 2 mM EDTA, 0.5 mM DTT, 1 mM Na3VO4, 2 mM Na pyrophosphate, 10 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride). The extracts were centrifuged at 15,000 × g (15 min at 4°C). Epitope-tagged protein kinases were immunoprecipitated by incubation for 2 h at 4°C with the M2 Flag monoclonal antibody (IBI-Kodak) bound to protein G-Sepharose (Pharmacia-LKB Biotechnology, Inc.). The immunoprecipitates were washed twice with buffer A and twice with kinase buffer (25 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 0.5 mM DTT, 0.1 mM Na3VO4).
Protein kinase assays were performed with protein kinase immunoprecipitates. The reactions were initiated by addition of 1 μg of a recombinant GST-ATF2 fusion protein and 50 μM [γ-32P]ATP (10 Ci/mmol) in a final volume of 40 μl of kinase buffer. The reactions were terminated after 30 min at 30°C by addition of Laemmli sample buffer. Phosphorylation of ATF2 was examined after SDS-PAGE by autoradiography and PhosphorImager analysis.
Fly stocks.
Canton S or yw flies were used as the wild-type stock. The heat shock D-p38a construct was generated by inserting the D-p38a cDNA into the pCaSpeR-hs vector between the BglII and NotI sites (69). Transgenic flies were generated by P-element-mediated transformation using the yw stock. The lines that contain transgenes on the second or the third chromosomes were crossed to double balancer stocks and interbred to generate a line that contains four copies of the heat shock transgene. All fly stocks were maintained at 25°C on standard yeast-cornmeal-agar media. Heat shock at 37°C for 1 h was used to induce gene expression. After the heat shock, the larvae were allowed a 30-min recovery period before a bacterial challenge. This challenge was performed by pricking third-instar larvae with a thin glass needle previously dipped into a saturated E. coli bacterial culture. RNA was isolated at various time points after exposure to bacteria.
RNA preparation and Northern analysis.
Total RNA was prepared with Tri-Reagent (containing phenol and guanidinium thiocyanate) in accordance with the protocol furnished by Molecular Research Center Inc. (Cincinnati, Ohio). Essentially, the cells or the larvae were homogenized in Tri-Reagent and the total RNA was precipitated with isopropanol. For Northern blotting experiments, approximately 10 μg of total RNA was separated on a formaldehyde-agarose gel and blotted onto a GeneScreen Plus membrane (NEN Life Science). Hybridization was performed at 45°C in 50% formamide–2× SSC–1% SDS–200-μg/ml salmon sperm DNA–1% nonfat dry milk. After 20 h of hybridization, the filter was washed in 0.2× SSC–0.1% SDS. The hybridized probe was detected by autoradiography.
Nucleotide sequence accession numbers.
The nucleotide sequences described in this report have been deposited in GenBank and assigned accession no. AF035546, AF035547, AF035548, AF035549, AF035550, AF035551, and AF035552.
RESULTS
Molecular cloning of Drosophila p38 MAPKs.
The mammalian ERK, JNK, and p38 MAPKs are characterized by distinct tripeptide phosphorylation motifs that mediate activation of the MAPKs. The dual phosphorylation motifs of ERK, JNK, and p38 are TEY, TPY, and TGY, respectively. Drosophila homologs of ERK (5, 8) and JNK (61, 66) have been previously identified. However, a Drosophila homolog of the mammalian p38 MAPK has not been reported. To identify possible homologs of the p38 MAPK in Drosophila, we designed degenerate oligonucleotides to amplify MAPK-related sequences from a Drosophila embryonic cDNA library (7). Sequence analysis led to the identification of one group of cDNAs (D-p38a) related to the mammalian p38 MAPK. The full-length D-p38a cDNA was subsequently isolated, and sequence analysis demonstrated the presence of an open reading frame of 366 amino acids (Fig. 1). D-p38a shares 66% amino acid identity with human p38α (Fig. 1C). The protein includes the 11 conserved kinase subdomains and the characteristic TGY dual phosphorylation motif in the activation loop. We also cloned and analyzed the genomic locus of D-p38a. The gene contains only one 80-bp intron, which is located in the 5′ UTR (Fig. 1A).
Another highly homologous p38 DNA sequence deposited by the Drosophila Genome Project was identified by searching GenBank with the D-p38a sequence. This genomic DNA sequence contains an open reading frame (D-p38b) which also does not contain an intron and encodes a putative protein of 365 amino acids (Fig. 1B). The deduced amino acid sequence is 75% identical to that of D-p38a (Fig. 1C). Therefore, the Drosophila genome contains at least two genes homologous to human p38 MAPK.
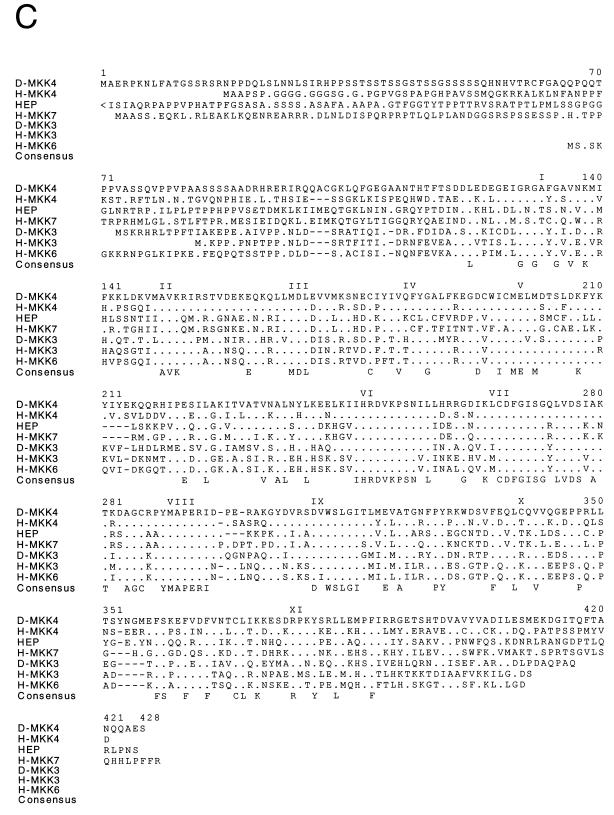
The activity of human p38 MAPKs is regulated by upstream kinases, which include MKK3 and MKK6 (12, 59). Another upstream human kinase, MKK4, has been shown to phosphorylate and activate both JNK and p38 (12). We therefore investigated whether similar upstream kinases are present in Drosophila. The MKKs contain highly conserved motifs and constitute a family of proteins (12, 59). We used degenerate oligonucleotides to amplify MKK-related sequences from a Drosophila embryonic cDNA library by PCR. Two novel protein kinases were identified. Sequence analysis of full-length cDNAs led to the identification of homologs of human MKK3 and MKK4 (H-MKK3 and H-MKK4) (Fig. 2). The sequence of D-MKK3 is 53% identical to that of H-MKK3. The identity between D-MKK4 and H-MKK4 is 50%. Analysis of the genomic sequences demonstrated that both D-MKK genes contain a small number of introns in the coding region (Fig. 2A and B).
FIG. 2.
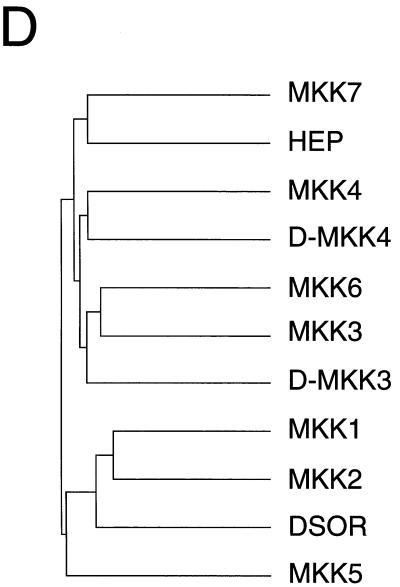
Molecular cloning of two Drosophila MAPK kinases. (A and B) The nucleotide sequences of D-MKK4 (A) and D-MKK3 (B) genomic clones are presented. The exon structures of the D-MKK4 and D-MKK3 genes and the primary sequences of the D-MKK4 and D-MKK3 protein kinases were deduced from the sequences of cDNA clones. The sequences are numbered starting with nucleotide 1 of exon 1. Both genes consist of five exons. The primary sequence encoded by each exon is illustrated in the single-letter code (italics). Termination codons are indicated by asterisks. For D-MKK4, DNA polymorphisms were detected at codon 111 (GCC/GGC; Ala/Gly), codon 112 (GCT/GCC; Ala/Ala), and codon 140 (ATC/ACC; Ile/Thr). (C) The D-MKK4 and D-MKK3 primary sequences were compared with human MAPK kinases (MKK1, MKK2, MKK3, MKK4, MKK5, MKK6, and MKK7) and Drosophila MAPK kinases (HEP and DSOR) by using the PILE-UP program (version 9.0; Wisconsin Genetics Computer Group). The protein sequences are presented in the single-letter code. Gaps introduced into the sequences to optimize the alignment are represented by dashes. Identical residues are indicated by periods. Conserved kinase subdomains I to XI are illustrated. (D) The relationship between members of the human and Drosophila MAPK kinase groups is presented as a dendrogram created by the unweighted pair-group method using arithmetic averages (PILE-UP program).
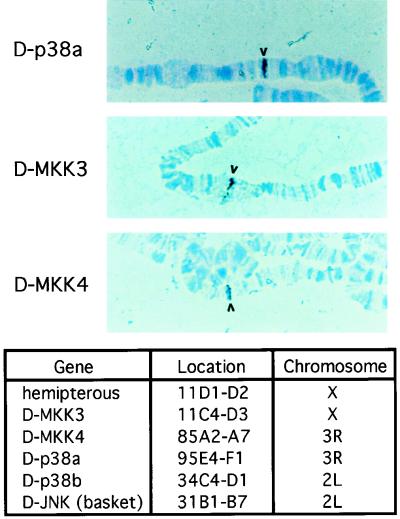
To facilitate future genetic analysis of these kinases, we performed chromosome in situ hybridization to map the cytological locations of these genes. The results demonstrated that these genes are located in distinct regions of the Drosophila genome. The D-p38a gene was mapped to a single site at 95E4-F1 on the third chromosome. D-p38b was located at 34C4-D1 on the second chromosome. The D-MKK3 and D-MKK4 genes were found at 11C4-D3 (X chromosome) and 85A2-A7 (third chromosome), respectively. Figure 3 summarizes the cytological locations of the stress-activated MAPKs and MKKs.
FIG. 3.
Cytological localization of the p38 MAPK and MKK genes. Chromosome in situ hybridization was performed with digoxigenin-labeled cDNA probes on samples prepared from wild-type larval salivary glands. After hybridization with the probes, the samples were incubated with antidigoxigenin antibodies, which were conjugated with horseradish peroxidase. The hybridized probe, visualized with a peroxidase reaction, is indicated by the arrowheads. The locations of the genes for the MAPKs and the upstream kinases are summarized in the table.
Comparison of the expression of the p38 MAPKs and MKKs in Drosophila.
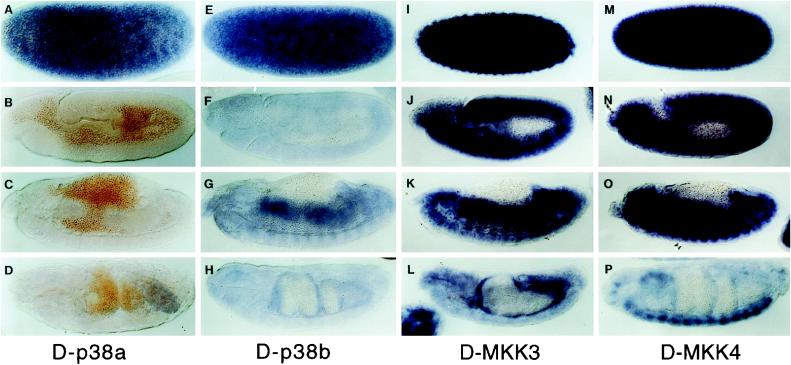
The expression patterns of the protein kinases were examined in the developing embryo to gain information about the possible functional relationship among the MAPK and MKK isoforms. The two D-p38 genes exhibited different embryonic expression patterns. The D-p38a mRNA expression was predominantly at the preblastoderm stage, indicating a high level of maternal deposition (Fig. 4A). Zygotic expression was less than the detectable level during most of the embryonic development (Fig. 4B and C). Northern analysis, however, demonstrated the presence of low mRNA levels throughout development (data not shown). At stage 16, there was a low level of staining in the posterior region, which may correspond to the developing hindgut (Fig. 4D). The preblastoderm staining indicates that D-p38a may participate in early embryonic development.
FIG. 4.
Embryonic mRNA expression patterns of the p38 MAPKs and MKKs. Digoxigenin-labeled cDNA probes were incubated with wild-type embryos, and the hybridized probe was visualized with an alkaline phosphatase reaction. Panels A, E, I, and M show stage 2, before pole cell formation and zygotic gene expression. Panels B, F, J, and N show stage 10, when the germ band has been fully extended. Panels C, G, K, and O show stage 13, when the germ band has been retracted and the midguts have been fused. Panels D, H, L, and P show stage 15, when the ventral nerve cord starts to retract.
The D-p38b gene is expressed throughout embryonic development (Fig. 4E to H). There is a high level of maternal deposition, and at later stages, zygotic expression is present in most of the tissues. At midembryogenesis, higher levels of mRNA were detected in the developing anterior and posterior midguts (Fig. 4G). The expression pattern of D-p38b is very similar to that of the two D-MKKs, which also have high maternal deposition in early embryos and zygotic expression in the midguts. One noteworthy difference between the two D-MKKs is that D-MKK4 expression is sustained in the ventral nerve cord (Fig. 4P), while D-MKK3 expression is less detectable in this tissue but is more prominent in the midgut (Fig. 3L). Previous studies have demonstrated that D-JNK expression is present in the ventral nerve cord (66), suggesting a functional relationship with D-MKK4 in this tissue. Since each of these kinases is deposited maternally, they may all be involved in early development. At later stages, D-p38b may be the primary mediator of the p38 MAPK pathway, particularly in the developing midgut.
D-MKK3 and LPS increase D-p38 MAPK activity.
We examined the protein kinase activities of D-p38a and D-p38b by using an in vitro assay (Fig. 5). The two D-p38 MAPKs were expressed as GST fusion proteins in bacteria and purified. In vitro kinase assays demonstrated that both D-p38a and D-p38b phosphorylate D-Jun and mammalian ATF2. The D-p38 isoforms can also phosphorylate myelin basic protein, which is phosphorylated more effectively by PKA (Fig. 5). Previous studies demonstrated that the mammalian p38 MAPK efficiently phosphorylates ATF2 but not c-Jun (58). In contrast, mammalian JNK recognizes both substrates with similar specificities (22, 23). Our results revealed that while D-p38 can phosphorylate ATF2, D-Jun also serves as a substrate but to a lesser extent. On the other hand, D-JNK recognized both substrates with the same efficiency (Fig. 5).
FIG. 5.
In vitro phosphorylation of substrates by the D-p38 kinases. The substrate proteins indicated at the top were incubated with the kinases indicated to the left of the panels. The proteins in the reaction were resolved by SDS-PAGE, and the autoradiogram of a representative experiment is presented. MBP, myelin basic protein.
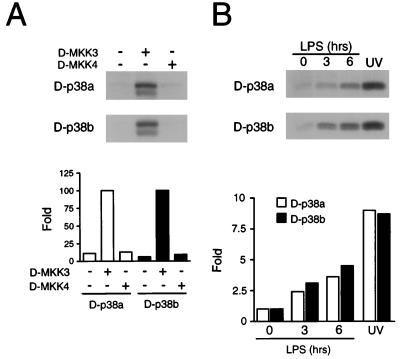
We tested the regulation of D-p38 MAPK activity by MAPK kinases D-MKK3 and D-MKK4. The cDNA were placed under the control of the Actin5C promoter, and different combinations of the plasmids were cotransfected into Schneider S2* cells (57). The FLAG epitope-tagged D-p38 MAPKs were immunoprecipitated, and their kinase activity was measured in vitro with ATF2 as the substrate. The results demonstrated that D-MKK3 is an efficient activator of both D-p38a and D-p38b (Fig. 6A). Cotransfection of D-MKK4 under similar conditions did not activate the D-p38 MAPKs (Fig. 6A) but did cause activation of D-JNK (data not shown). The possible role of D-MKK4 in the activation of D-p38 requires further investigation.
FIG. 6.
Regulation of D-p38 activity. (A) The ability of D-MKK3 and D-MKK4 to activate D-p38a and D-p38b MAPKs was tested in cotransfection assays. S2* cells were transfected with the FLAG epitope-tagged D-p38a or D-p38b MAPK together with an Actin5C empty vector or an expression vector encoding D-MKK3 or D-MKK4. D-p38 MAPK activity was measured in an immune complex kinase assay using ATF2 as the substrate (top). D-p38 MAPK activity was quantitated by PhosphorImager analysis and is presented as relative protein kinase activity (bottom). (B) The D-p38 MAPKs are activated by LPS. S2* cells transfected with epitope-tagged D-p38a or D-p38b were treated with LPS (5 μg/ml) for 3 or 6 h or with UV-C (80 J/m2). D-p38 MAPK activity was measured in an immune complex kinase assay using ATF2 as the substrate (top). D-p38 MAPK activity was quantitated by PhosphorImager analysis, and the results are presented as relative protein kinase activity (bottom).
We also examined the regulation of D-p38 by extracellular stimuli. Epitope-tagged D-p38 MAPKs were expressed in S2* cells, which were then treated with bacterial LPS. The D-p38 MAPK activity was measured in an immune complex assay with ATF2 as the substrate. It was found that LPS increased the kinase activity of both D-p38 isoforms. Control experiments demonstrated that the exposure of S2* cells to UV light also activated the MAPKs (Fig. 6B).
An anti-inflammatory drug that inhibits D-p38 MAPK also modulates insect immunity gene expression.
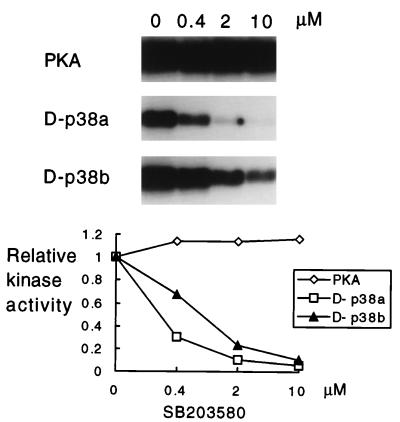
SB203580 is a pyridinyl-imidazole compound that binds to the human p38 MAPK and inhibits the production of IL-1 and tumor necrosis factor by monocytes during inflammation (45). The bacterially expressed kinases were first incubated either with the solvent DMSO or with various concentrations of the drug prior to the measurement of protein kinase activity. The results demonstrate that this anti-inflammatory drug is an efficient inhibitor of both D-p38 MAPK isoforms (Fig. 7). The inhibitory effect is similar to that on mammalian p38α, since micromolar concentrations are sufficient to almost abolish protein kinase activity (45).
FIG. 7.
Inhibition of D-p38 by the pyridinyl-imidazole compound SB203580. The ATF2 protein was used as the substrate for all of the assays, with PKA, D-p38a, or D-p38b as the kinase, as indicated to the left. The compound was added to the reaction mixture containing the kinase prior to addition of the substrate. Various concentrations of the drug or the DMSO solvent (0 μM) were added to the reaction mixture. The proteins in the reaction mixture were resolved by SDS-PAGE, and the autoradiogram of a representative experiment is presented. The graph at the bottom is the result of quantitation, by PhosphorImager analysis (Molecular Dynamics), of the experiment shown at the top.
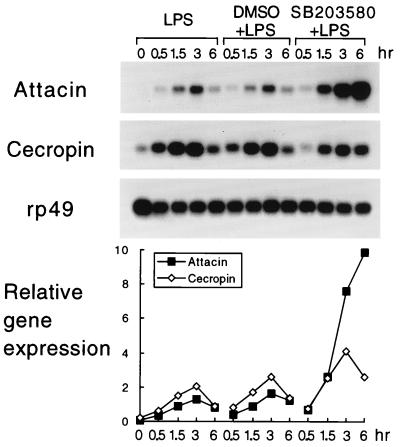
Induction of the insect immune response leads to the production of antimicrobial peptides. This inductive event can be mimicked in tissue culture (57, 63). To test the hypothesis that the D-p38 MAPKs regulate insect immunity, we incubated S2* cells (57) in medium that contained either solvent (DMSO) or drug (SB203580) prior to LPS induction. With LPS treatment alone, the mRNA levels of two antibacterial peptide genes, Attacin and Cecropin, were induced by more than 10-fold within 3 h (Fig. 8). The expression of Attacin and Cecropin returned to lower levels after 6 h. In the presence of SB203580, the early phase of induction of these two genes was similar to that of the DMSO control. During the later phase, 3 to 6 h after LPS treatment, when the gene expression should be reduced, Attacin was induced to a much higher level and Cecropin induction was modestly elevated. These results suggest that the targets of the drug, presumably the D-p38 protein kinases, are involved in the down regulation of immunity gene expression. We surmise that D-p38 is involved in the attenuation of immunity gene expression during the late phase of infection. Such negative regulation may be employed to attenuate immunity gene expression to avoid overactivation, which may be harmful to the host.
FIG. 8.
The drug SB203580 potentiates immunity gene expression. S2* cells were treated with LPS and SB203580 as indicated. Total RNA was isolated and assayed by Northern blotting (top). Control experiments designed to examined RNA loading were performed by using a ribosomal protein rp49 probe. The level of immunity gene mRNA expression relative to that of rp49, determined by quantitation of the hybridization signals, is presented at the bottom.
Overexpression of D-p38a blocks antimicrobial peptide gene expression.
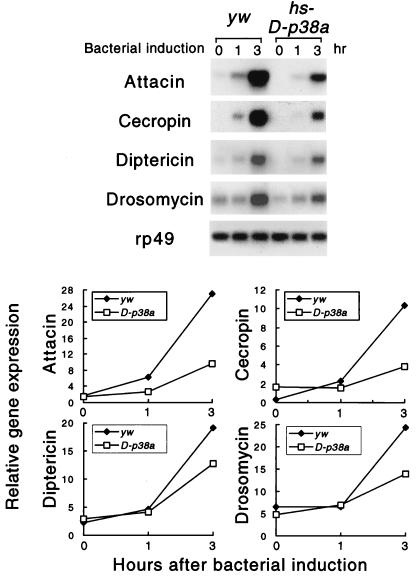
To test whether the D-p38 protein kinases function in vivo as attenuators of immunity gene expression, we generated transgenic flies that express the D-p38a cDNA under the control of the heat shock promoter. A fly strain with four copies of the transgene was established through genetic manipulation. The transgenic larvae, or the control parental yw animals, were heat shocked at 37°C to induce the expression of D-p38a. After heat shock, the animals were challenged by bacterial injection. Total RNA was isolated, and immunity gene expression was examined. The parental strain showed normal induction of Attacin and Cecropin. Within 3 h, the mRNA levels were more than 10-fold higher than those in the unchallenged animals. On the other hand, overexpression of D-p38a caused significantly reduced mRNA levels of both Cecropin and Attacin (Fig. 9). The expression of the antifungal peptide gene Drosomycin was also examined, and it appeared to be affected to a lesser extent, while the expression of another antibacterial peptide gene, Diptericin, was only modestly decreased. These results suggest that the p38 MAPK pathway is used to attenuate the induced expression of a selective group of immunity genes in vivo.
FIG. 9.
Overexpression of D-p38 inhibits immunity gene expression in vivo. Transgenic hs-D-p38a or yw larvae were heat shocked and induced with bacteria for the amounts of time indicated. RNA was isolated and analyzed by Northern blotting (top). The ratio of immunity gene to rp49 mRNA levels is presented graphically at the bottom.
DISCUSSION
The p38 MAPK pathway has been implicated in the mediation of stress signals. Proinflammatory cytokines, osmotic shock, and bacterial LPS can induce p38 MAPK activity, which leads to increased cytokine production (43, 74). The p38 MAPKs are also involved in the control of other cellular processes, such as apoptosis and proliferation (39, 41, 55, 68, 77).
In this report, we demonstrate the conservation of some components of the mammalian p38 MAPK pathway in Drosophila. Two D-p38 genes and two genes related to mammalian upstream kinases, MKK3 and MKK4, have been identified. Previous studies demonstrated that Hemipterous is an activator of D-JNK (66) and is homologous to MKK4 (20). Detailed comparison of all of the MKKs (Fig. 2D), however, indicates that Hemipterous is more closely related to the newly identified mammalian isoform MKK7 (31, 51, 56, 71), which exclusively activates JNK. Therefore, it is likely that Hemipterous is a functional homolog of MKK7, while D-MKK4 and D-MKK3 are functional homologs of the respective mammalian kinases. Our experimental results demonstrated that D-MKK3 can, indeed, activate both D-p38 isoforms, suggesting a conservation of the functions of these signaling components. Using cell culture and transgenic assays, we found that one of the possible functions of the D-p38 pathway is to down regulate insect immunity gene expression after prolonged infection. These results further support the notion that insect antimicrobial response and human innate immunity share an evolutionary origin.
One of the well-characterized Drosophila signaling pathways involved in the self-defense response is the Toll-Dif/Dorsal pathway (4, 34). Toll is the transmembrane protein that transmits the extracellular signal to regulate Dorsal nuclear transport in the early embryo (28). The intracellular components that mediate the signal include Tube, Pelle, and Cactus. Recent evidence demonstrates that this signaling cassette is also involved in the induction of some immunity genes, particularly the antifungal peptide gene Drosomycin (49). The Drosophila Toll signaling molecules are strikingly similar to those in the mammalian IL-1R–NF-κB pathway, which participate in the inflammatory response (34, 52, 73). Furthermore, a human Toll homolog has recently been identified and shown to be able to induce cytokine production in monocytic cells, probably through the activation of NF-κB (53, 54). Therefore, the Toll pathway is employed for self-defense in many organisms that have diverged evolutionarily. However, although much evidence points to the notion that Toll signaling mediates innate immunity in both vertebrates and invertebrates, genetic analysis suggests that only a subset of Drosophila immunity genes requires this pathway (49). A recent study showed that a Toll-like transmembrane protein, 18-Wheeler, is required for the induction of Attacin and, to some extent, Cecropin gene expression (76). Moreover, the mutation immune deficiency (imd), localized to the 55C-F region, exhibits a specific defect in the induction of antibacterial, but not antifungal, genes (9, 47). It was therefore proposed that multiple signaling pathways are involved in regulating the repertoire of immunity genes in insects (47, 49, 76).
To search for other parallel pathways that regulate Drosophila immunity, we cloned a number of kinases that are homologs of mammalian protein kinases that have been shown to participate in stress-induced responses (66, this study). Previous results indicated that the MAPK D-JNK is activated by immune challenge in cultured cells (66). However, due to the embryonic lethality caused by mutations in the D-JNK gene (61, 66), no genetic data have been obtained on the in vivo function of D-JNK in insect immunity. In this paper, we report the analysis of the D-p38 MAPK pathway. MAPK p38 has been implicated in the process of inflammation, as human p38 was found to be the target of the anti-inflammatory drug SB203580, which inhibits the production of IL-1 and tumor necrosis factor (45).
By using transgenic flies and pharmacological assays, we demonstrated that D-p38 MAPKs are involved in mediating antimicrobial responses in Drosophila. However, while we expected that the kinases might positively mediate immunity signaling, experimental results demonstrated that D-p38 may function as a negative regulator. Inhibition of D-p38 by the drug SB203580 leads to increased expression of antimicrobial peptide genes in the later phase of induction by LPS. The drug treatment caused no enhancement of immunity gene expression in the early phase (Fig. 7), suggesting that the D-p38 protein kinases do not function as constitutive repressors. Meanwhile, overexpression of D-p38a in transgenic animals caused inhibition of immunity gene induction. Therefore, it is conceivable that one of the functions of the D-p38 MAPK is to mediate attenuation of immunity gene expression at late phases of infection. This mechanism is analogous to the attenuation of cytokine production during inflammation, which may otherwise lead to septic shock.
Repressive cross-regulation of MAPKs has been identified in the yeast osmosensing pathway, which involves the yeast p38 homolog HOG1, and in the mating type pathway (24). It may be that the LPS-activated D-p38 signaling pathway serves as a repressor for yet another Drosophila MAPK which positively mediates the LPS effect. This MAPK should not be sensitive to treatment with pyridinyl-imidazole drugs. Genetic analysis will help to resolve the in vivo functions of these protein kinases.
ACKNOWLEDGMENTS
We thank Dan Hultmark for providing the Attacin cDNA probe, Ylva Engstrom for the Cecropin cDNA probe, Kirugaval Hemavathy for help with S2 cell culture, and Catherine Tournier for the MKK amplimers.
This publication was made possible by NIH grants GM53269 (to Y.T.I.) and CA65861 (to R.J.D.). Y.T.I. is a recipient of the Scholar Award of the Leukemia Society of America. R.J.D. is an investigator of the Howard Hughes Medical Institute.
ADDENDUM IN PROOF
The sequence of D-p38a was recently reported by S.-J. Han et al. (J. Biol. Chem. 273:369–374).
REFERENCES
- 1.Anderson N G, Maller J L, Tonks N K, Sturgill T W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651–652. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- 2.Asling B, Dushay M, Hultmark D. Identification of early genes in the Drosophila immune response by PCR-based differential display: the Attacin A gene and the evolution of attacin-like proteins. Insect Biochem Mol Biol. 1995;25:511–518. doi: 10.1016/0965-1748(94)00091-c. [DOI] [PubMed] [Google Scholar]
- 3.Baumann H, Prowse K R, Marinkovic S, Won K A, Jahreis G P. Stimulation of hepatic acute-phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280–295. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- 4.Belvin M P, Anderson K V. A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 5.Biggs W H, Zavitz K H, Dickson B, van der Straten A, Brunner D, Hafen E, Zipursky S L. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994;13:1628–1635. doi: 10.1002/j.1460-2075.1994.tb06426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 7.Brown N H, Kafatos F C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988;203:425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- 8.Brunner D, Oellers N, Szabad J, Biggs W H, Zipursky S L, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- 9.Corbo J C, Levine M. Characterization of an immunodeficiency mutant in Drosophila. Mech Dev. 1996;55:211–220. doi: 10.1016/0925-4773(96)00506-0. [DOI] [PubMed] [Google Scholar]
- 10.Davis R J. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 11.Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 12.Dérijard B, Raingeaud J, Barrett T, Wu I-H, Han J, Ulevitch R J, Davis R J. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Guerra M J M, Velasco M, Martin-Sanz P, Bosca L. Evidence for common mechanisms in the transcriptional control of type II nitric oxide synthase in isolated hepatocytes. Requirement of NF-kappaB activation after stimulation with bacterial cell products and phorbol esters. J Biol Chem. 1996;271:30114–30120. doi: 10.1074/jbc.271.47.30114. [DOI] [PubMed] [Google Scholar]
- 14.Dimarcq J L, Hoffmann D, Meister M, Bulet P, Lanot R, Reichhart J-M, Hoffmann J A. Characterization and transcriptional profiles of a Drosophila gene encoding an insect defensin: a study in insect immunity. Eur J Biochem. 1994;221:201–209. doi: 10.1111/j.1432-1033.1994.tb18730.x. [DOI] [PubMed] [Google Scholar]
- 15.Dressler H, Ron D. pGSTag—a versatile bacterial expression plasmid for enzymatic labelling of recombinant proteins. BioTechniques. 1992;13:866–869. [PubMed] [Google Scholar]
- 16.Dushay M S, Asling B, Hultmark D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc Natl Acad Sci USA. 1996;93:10343–10347. doi: 10.1073/pnas.93.19.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engstrom Y, Kadalayil L, Sun S-C, Samakovlis C, Hultmark D, Faye I. κB-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993;232:327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- 18.Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert W F, Hetru C, Hoffmann J A. Insect immunity. septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994;269:33159–33163. [PubMed] [Google Scholar]
- 19.Georgel P, Meister M, Kappler C, Lemaitre B, Reichhart J-M, Hoffmann J A. Insect immunity: the diptericin promoter contains multiple functional regulatory sequences homologous to mammalian acute-phase response elements. Biochem Biophys Res Commun. 1993;197:508–517. doi: 10.1006/bbrc.1993.2508. [DOI] [PubMed] [Google Scholar]
- 20.Glise B, Bourbon H, Noselli S. hemipterous encodes a novel MAP kinase kinase required for epithelial cell sheet movement. Cell. 1995;83:451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 21.Gross I, Georgel P, Kappler C, Reichhart J M, Hoffmann J A. Drosophila immunity: a comparative analysis of the Rel proteins Dorsal and Dif in the induction of the genes encoding diptericin and cecropin. Nucleic Acids Res. 1996;24:1238–1245. doi: 10.1093/nar/24.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Barrett T, Whitmarsh A J, Sluss H K, Cavanagh J, Davis R J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Campbell D, Dérijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 24.Hall J P, Cherkasova V, Elion E, Gustin M C, Winter E. The osmoregulatory pathway represses mating pathway activity in Saccharomyces cerevisiae: isolation of a Fus3 mutant that is insensitive to the repression mechanism. Mol Cell Biol. 1996;16:6715–6723. doi: 10.1128/mcb.16.12.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hambleton J, Weinstein S L, Lem L, DeFranco A L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Lee J-D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 27.Han K, Manley J L. Functional domains of the Drosophila engrailed protein. EMBO J. 1993;12:2723–2733. doi: 10.1002/j.1460-2075.1993.tb05934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto C, Hudson K L, Anderson K V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1989;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann J A. Innate immunity of insects. Curr Opin Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann J A, Reichhart J-M, Hetru C. Innate immunity in higher insects. Curr Opin Immunol. 1996;8:8–13. doi: 10.1016/s0952-7915(96)80098-7. [DOI] [PubMed] [Google Scholar]
- 31.Holland P M, Suzanne M, Campbell J S, Noselli S, Cooper J A. MKK7 is a stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 32.Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993;9:178–193. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- 33.Ip, Y. T., and R. J. Davis. Signal transduction by the c-Jun NH2-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol., in press. [DOI] [PubMed]
- 34.Ip Y T, Levine M. Molecular genetics of Drosophila immunity. Curr Opin Genet Dev. 1994;4:672–677. doi: 10.1016/0959-437x(94)90133-n. [DOI] [PubMed] [Google Scholar]
- 35.Ip Y T, Reach M, Engstrom Y, Kadalayil L, Cai H, Gonzalez-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- 36.Kadalayil L, Petersen U-M, Engstrom Y. Adjacent GATA and κB-like motifs regulate the expression of a Drosophila immune gene. Nucleic Acids Res. 1997;25:1233–1239. doi: 10.1093/nar/25.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kappler C, Meister M, Lagueux M, Gateff E, Hoffmann J A, Reichhart J-M. Insect immunity. Two 17 bp repeats nesting a κB-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenged Drosophila. EMBO J. 1993;12:1561–1568. doi: 10.1002/j.1460-2075.1993.tb05800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki H, Morooka T, Shimohama S, Kimura J, Hirano T, Gotoh Y, Nishida E. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–18521. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 40.Koj A. Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317:84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 41.Kummer J L, Rao P K, Heidenreich K A. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]
- 42.Kylsten P, Samakovlis C, Hultmark D. The cecropin locus in Drosophila: a compact gene cluster involved in the response to infection. EMBO J. 1990;9:217–224. doi: 10.1002/j.1460-2075.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kyriakis J M, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 44.Lee J, Boman A, Chuanxin S, Andersson M, Jornvall H, Mutt V, Boman H G. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci USA. 1989;86:9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;327:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 46.Lehrer R I, Ganz T, Selsted M E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991;64:229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- 47.Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart J-M, Hoffmann J A. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart J-M, Hoffmann J A. Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J. 1995;14:536–545. doi: 10.1002/j.1460-2075.1995.tb07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemaitre B, Nicolas E, Michaut L, Reichhart J M, Hoffmann J A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 50.Lin A, Minden A, Martinetto H, Claret F-X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 51.Lu X, Nemoto S, Lin A. Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J Biol Chem. 1997;272:24751–24754. doi: 10.1074/jbc.272.40.24751. [DOI] [PubMed] [Google Scholar]
- 52.Mackman N, Brand K, Edgington T S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991;174:1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medzhitov R, Janeway C A., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 54.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 55.Molnar A, Theodoras A M, Zon L I, Kyriakis J M. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–13235. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 56.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen U-M, Bjorklund G, Ip Y T, Engstrom Y. The dorsal-related immunity factor, Dif, is a sequence-specific trans-activator of Drosophila Cecropin gene expression. EMBO J. 1995;14:3146–3158. doi: 10.1002/j.1460-2075.1995.tb07317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 59.Raingeaud J, Whitmarsh A J, Barrett T, Dérijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reichhart J-M, Meister M, Dimarcq J-C, Zachary D, Hoffmann D, Ruiz C, Richards G, Hoffmann J A. Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J. 1992;11:1469–1477. doi: 10.1002/j.1460-2075.1992.tb05191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riesgo-Escovar J R, Jenni M, Fritz A, Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- 62.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 63.Samakovlis C, Asling B, Boman H G, Gateff E, Hultmark D. In vitro induction of cecropin genes—an immune response in a Drosophila blood cell line. Biochem Biophys Res Commun. 1992;188:1169–1175. doi: 10.1016/0006-291x(92)91354-s. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 65.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 66.Sluss H K, Han Z, Barrett T, Goberdhan D C I, Wilson C, Davis R J, Ip Y T. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10:2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 67.Smith S B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 68.Spector M S, Auer K L, Jarvis W D, Ishac E J, Gao B, Kunos G, Dent P. Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556–3565. doi: 10.1128/mcb.17.7.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thummel C S, Pirrotta V. New pCaSpeR P-element vectors. Dros Inf Serv. 1992;71:150. [Google Scholar]
- 70.Tilg H, Dinarello C A, Mier J W. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997;18:428–432. doi: 10.1016/s0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 71.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 73.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 74.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 75.Wicker C, Reichhart J-M, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann J A. Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem. 1990;265:22493–22498. [PubMed] [Google Scholar]
- 76.Williams M J, Rodriguez A, Kimbrell D A, Eldon E D. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 1997;16:6120–6130. doi: 10.1093/emboj/16.20.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]