Abstract
Contact tracing forms a crucial part of the public-health toolbox in mitigating and understanding emergent pathogens and nascent disease outbreaks. Contact tracing in the United States was conducted during the pre-Omicron phase of the ongoing COVID-19 pandemic. This tracing relied on voluntary reporting and responses, often using rapid antigen tests due to lack of accessibility to PCR tests. These limitations, combined with SARS-CoV-2’s propensity for asymptomatic transmission, raise the question “how reliable was contact tracing for COVID-19 in the United States”? We answered this question using a Markov model to examine the efficiency with which transmission could be detected based on the design and response rates of contact tracing studies in the United States. Our results suggest that contact tracing protocols in the U.S. are unlikely to have identified more than 1.65% (95% uncertainty interval: 1.62-1.68%) of transmission events with PCR testing and 1.00% (95% uncertainty interval 0.98-1.02%) with rapid antigen testing. When considering a more robust contact tracing scenario, based on compliance rates in East Asia with PCR testing, this increases to 62.7% (95% uncertainty interval: 62.6-62.8%). We did not assume presence of asymptomatic transmission or superspreading, making our estimates upper bounds on the actual percentages traced. These findings highlight the limitations in interpretability for studies of SARS-CoV-2 disease spread based on U.S. contact tracing and underscore the vulnerability of the population to future disease outbreaks, for SARS-CoV-2 and other pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-18012-z.
Keywords: COVID-19, Contact tracing, SARS-CoV-2, Infectious disease epidemiology, Infectious disease transmission
Introduction
The management and control of infectious disease has been a signal modern achievement. Advances in epidemiological techniques pioneered during the 19th century established public health as a discipline. Overlapping with, but distinct from the medical establishment and the biopharmaceutical industry, modern public health organizations have sought to control disease using nonpharmaceutical interventions (NPIs).
Contact tracing is a cornerstone of the public-health response, particularly with emergent pathogens and nascent disease outbreaks [1]. Effective contact tracing facilitates estimates of epidemiological parameters describing disease spread. In the current COVID-19 pandemic, rigorous early studies relying on contact tracing revealed key epidemiological features of SARS-CoV-2 such as asymptomatic transmission [2, 3], superspreading [4], and aerosol transmission [5–9]. This provided a basis for projecting the future course of the outbreak and designing a public health response.
Effective contact tracing is also critical for limiting onward spread through the deployment of test-and-trace and isolation protocols. Many Asia-Pacific countries effectively limited SARS-CoV-2 community spread for the first two years of the pandemic, relying on contact tracing with isolation of contacts, including strict testing and isolation efforts at their borders. For example, South Korea used methods such as tracking credit card transactions and using closed circuit televisions to link contacts together [10]. In China, specifically Hubei, suspected contacts were placed under monitored house arrest throughout their quarantine period [11]. This strategy permitted high levels of within country contact and mobility while keeping case counts low [12–18].
In the U.S., contact tracing was primarily performed in the pre-Omicron era (late 2019-late 2021), and largely abandoned in early 2022 [19]. It has been widely recognized that contact tracing in the U.S. has not slowed disease transmission [20]. Part of the challenge has been that the process varied from state to state and relied on individual initiative and access to testing [19]. This meant that an individual typically must be symptomatic, voluntarily seek testing, and have their positive result reported to initiate contact tracing [21]. Public health officials initiated an investigation by asking the index case to identify their contacts, who in turn would be interviewed. The exposed contacts were monitored for symptoms and could choose to test for SARS-CoV-2 five days after exposure. If positive, the contact (now a secondary case) would be asked to name their contacts.
The process was largely voluntary, allowing for selection bias and many missed transmission chains. There was often no system for identifying close contacts whom the index case did not know personally. Many published papers noted that many named contacts were not successfully traced [22–24] and not all symptomatic contacts were willing to undergo testing [25]. A systematic surveillance-based cross-sectional study in the U.S. showed that 2 out of 3 index cases of COVID-19 were either not reached by tracers or declined to share contacts. Only 70% of named contacts agreed to be interviewed, and only 50% of those contacts were monitored, leading to an average of less than one contact per index case being monitored [26]. Additionally, the CDC-recommended 15 min of contact within six feet over a 24-hour period was somewhat arbitrary and never updated, even as evidence emerged indicating that COVID-19 could be transmitted through brief interactions.
The implications of these limitations in contact tracing are significant. The relatively high reproductive number for SARS-CoV-2 [27] would suggest that many transmission chains generated from a single index case went undetected. Additionally, asymptomatic transmission and superspreading behavior would also impact the efficacy of contact tracing for infection control and the generalizability of inferences made about transmission dynamics [28, 29].
In keeping with this voluntary and symptom-gated approach to contact tracing, there are many examples of minimally observed onward transmission in settings where transmission would be expected. This includes studies involving children with strong implications for policies related to schools. The results of studies investigating children and COVID-19 transmission have documented limited forward transmission, but this is often in context of significant mitigation strategies being in place or incomplete contact tracing [30–32]. During the initial omicron surge, when contact tracing was limited, schools struggled to remain open, reported high absenteeism rates, and in some cases, relied on the national guard to teach courses and due to incomplete contact tracing it was unclear what role children in schools played in transmission [33, 34]. In another case, two COVID-19 positive hairdressers in Missouri saw 139 clients over a ten-day period, with no reported onward transmission [24]. Notably, of the exposed clients, only ~ 75% (n = 104) responded to contact tracers’ requests for interviews, and only ~ 50% (n = 67) agreed to be tested. Biases in willingness to respond to interviews or participate in testing may have concealed many onward transmission events.
Another example that demonstrated the challenges in identifying both primary and secondary infections was the Sturgis motorcycle rally in August 2020. Following this 10-day event in Meade County, South Dakota (attended by approximately 460,000 persons [35] without [36–38] any mask-wearing requirements or other mitigating policies [39]), there was a wave of COVID-19 cases in Meade County, South Dakota. The counties outside of South Dakota that contributed the highest inflows of rally attendees experienced a 6.4–12.5% increase in COVID-19 cases relative to counties without inflows [40]. Despite evidence of population-level changes in COVID-19 case counts following the rally, the CDC and neighboring Minnesota Department of Health only identified 21 person-to-person transmission events [41]. Out of the 86 positive cases, only 41 reported being in close contact (defined as being within 6 feet of another person for ≥ 15 min) with other people, and they reported an average of 2.5 close contacts. Given the nature of the event, it is reasonable to assume that many cases and transmission events were not reported [42–44]. The CDC’s report does not specify how many of the 102 secondary contacts were tested, consistent with other U.S. contact-tracing studies [45, 46].
Examples such as these, coupled with the unique features of SARS-CoV-2 contact tracing in the U.S. during the early part of the pandemic raise the question “what was the efficiency of contact tracing, as it was implemented in the U.S.”? We answered this question using Markov Chain modeling to synthesize data from multiple sources of information on testing and contact tracing completeness to estimate the efficiency with which onward transmission could be detected. Our model-based approach sought to quantify two metrics of performance for contact tracing: (1) the percentage of all transmission pairs identified in a disease cluster/outbreak, and (2) the percentage of onward transmission events identified from a known index case. These metrics correspond broadly to the two contact-tracing scenarios described above, the Sturgis motorcycle rally study (seeking all transmission pairs) and the Missouri hairdressers (seeking onward transmission from a known index case). Our results suggest that contact tracing protocols in the United States are unlikely to have identified more than a small fraction of transmission events. We contrast this with a similar model run that incorporates data from Asian countries with more comprehensive contact tracing protocols in place, which yields a larger fraction of identified transmission events.
Methods
Model
We created a Markov Model to represent the sequence of events in contact tracing depicted in Fig. 1. The model captures the steps in contact tracing beginning with identifying a primary infectious individual through testing and then engaging that person in contact tracing by accurately identifying their contacts. The final steps of the model details engaging the infected contact and completing testing. We focus on estimating the probability of identifying infected contacts only. Importantly, it should be noted that this model does not incorporate the possibility of asymptomatic transmission. Furthermore, as we note later in the limitations, we assume the contact tracing process to be instantaneous, thereby not allowing for further delays in the reporting of contacts due to timing lags in testing and transmission. We parameterize the model steps through literature review. We capture uncertainty in the transition parameters by sampling over a literature-informed uncertainty range of the parameters 10,000 times. When no data was available on a parameter value, we sweep over the range of [0,1].
Fig. 1.
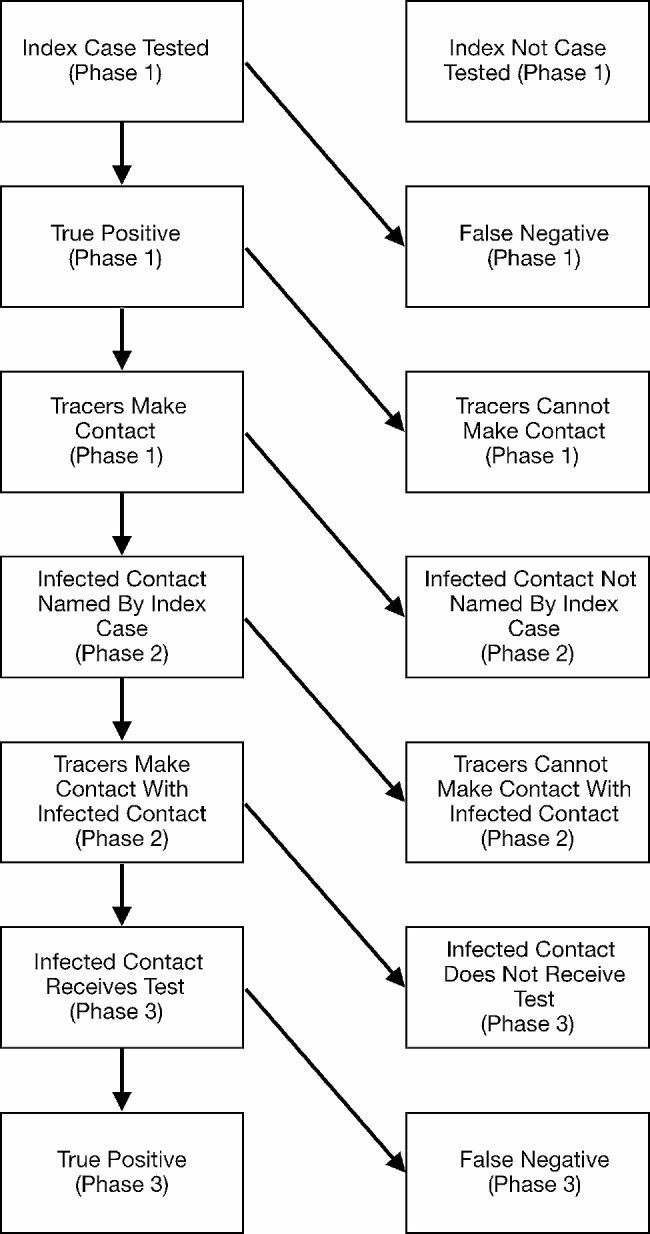
Schematic representation of steps required to correctly identify secondary cases of infected individuals. Each step is a binary variable and represents a point in the process where failure can occur. Steps 1–3 coincide with Phase 1 (identifying positive index cases). Steps 4 and 5 coincide with phase 2 (identifying contacts of positive cases). Finally, steps 6 and 7 coincide with Phase 3 (identifying positive cases among contacts)
Literature search and model parameterization
We reviewed the literature to produce estimates for each of the parameters of our model (Fig. 1). We used the search term “(covid 19 or covid-19 or covid19) AND (case investigation or contact tracing) AND (united states or US)” on PubMed to inform parameters that define the likelihood of individuals naming their close contacts and of those contacts responding to a contact tracing encounter and being tested. We collected data from contact tracing investigations that reported the proportion of positive cases that named contacts, the proportion of named contacts reached, the proportion of contacts that cooperated with tracers, and/or the proportion of contacts tested.
We also parameterized our model to represent a setting where stringent contact tracing was implemented. For this, we derived estimates from Taiwan and South Korea, which had rigorous contact tracing protocols during the initial phase of the pandemic using the search terms ‘(covid 19 or covid-19 or covid19) AND (case investigation or contact tracing) AND (taiwan)’ and ‘(covid 19 or covid-19 or covid19) AND (case investigation or contact tracing) AND (korea or south korea)’. We run the model separately for RAT and PCR tests.
The model can be divided into two distinct categories of parameters that contribute to the overall effectiveness of contact tracing: (1) efficiency of testing and (2) efficiency of contact tracing. Efficiency of testing includes the proportion of symptomatic people receiving testing, test sensitivity (RAT or PCR) [47, 48], and the proportion of contacts tested. Efficiency of contact tracing aggregates the probabilities that tracers contact a positive index case, a positive index case names contacts, and named contacts are traced. Both aggregate parameters take values between [0,1]. We plot all possible combinations of these parameters on a heatmap and identify the estimates obtained for the U.S.-based and ideal scenarios.
Code availability
The Markov Model was implemented in Python, and code for running the simulations and plotting the results are available in a Jupyter notebook on Github (https://github.com/Henry-Bayly/ContactTracingMarkovModel).
Results
Literature search
Our literature search of U.S. studies yielded 1,355 papers. Of these, the first 350 were reviewed to represent a random sample of the total papers found, as our goal was to conduct a representative and not comprehensive literature review. We excluded 325 papers that did not contain data relevant to the parameters required for our model, leaving twenty-five papers with information on contact tracing parameters. When multiple papers had values for the same parameter, for instance the probability of a case naming contacts, we assumed that the true value was uniformly distributed in the range of the reported values across the papers. All parameter values are shown in Table 1; sources of the parameters are shown in Tables S1-S6. We were unable to identify precise parameter estimates for the probability of a symptomatic person receiving testing and assume a uniform distribution over [0,1]. This parameter encapsulates the probability that an infected person experiencing symptoms will receive testing. Because this is a multifaceted question related to a person’s geographic location, socioeconomic status, and other factors impacting seeking testing and test availability, finding a comprehensive and representative estimate is difficult.
Table 1.
Table of parameters derived from literature that were used in the model
| Parameter (Step in the Model) | U.S. Probability Range | Comprehensive Contact Tracing Probability Range |
|---|---|---|
| Symptomatic case receives testing* | [0,1] | [0.90, 1.0] |
| COVID-19 test gives true positive* | [0.57, 0.83] (RAT) [48, 108] and [0.88, 0.92] (PCR) [47] | [0.57, 0.83] (RAT) and [0.88, 0.92] (PCR) |
| Tracers make contact with a positive case** [22, 26, 45, 46, 50, 109–113] | [0.41, 0.82] | [0.90, 1.0] |
| Positive case names any contacts**,a [22, 26, 45, 109, 110, 113, 114] | [0.17, 0.52] | [0.90, 1.0] |
| Tracers make contact with infected contacts of positive case** [22, 26, 46, 109, 110, 112–114] | [0.28, 0.85] | [0.90, 1.0] |
| Infected contacts of positive case get tested* [110, 114–118] | [0.19, 0.45] | [0.90, 1.0] |
| Contact’s COVID-19 test gives a true positive* | [0.57, 0.83] (RAT) [48, 108] and [0.88, 0.92] (PCR) [47] | [0.57, 0.83] (RAT) and [0.88, 0.92] (PCR) |
aThese values represent the probability that a positive case names any contacts at all. We use this as a proxy for the given step. Thus, our model represents an overestimate of the true efficacy of contact tracing
* parameter related to testing
**parameter related to tracing
We reviewed 225 papers from South Korea and Taiwan. However, we were unable to find studies quantifying contact tracing parameters related to the completeness of tracing. This appeared to be due to a much more comprehensive approach to contact tracing in these countries, negating the need to report on these parameters since it was assumed that reporting was nearly complete. For example, South Korea used traditional shoe-leather epidemiology along with large databases (global positioning system, credit card transactions, and closed-circuit television) [10] and in one study of 5,706 index cases an average of 9.9 contacts per index case were reported [10]. Taiwan similarly reported an average of 27.61 contacts per index case [49]. This contrasts with the US where the average number of non-household contacts reported in a large study was one for every three index cases [50]. Therefore, we hypothesized a range of [0.9, 1.0] for all parameters not associated with testing sensitivity in our ideal model setting, representing a more robust response to SARS-CoV-2.
Estimates of contact tracing efficacy
We examined the efficiency of contact tracing along the two dimensions described previously: (1) the percentage of all transmission pairs identified in a disease cluster/outbreak, and (2) the percentage of onward transmission events identified from a known index case.
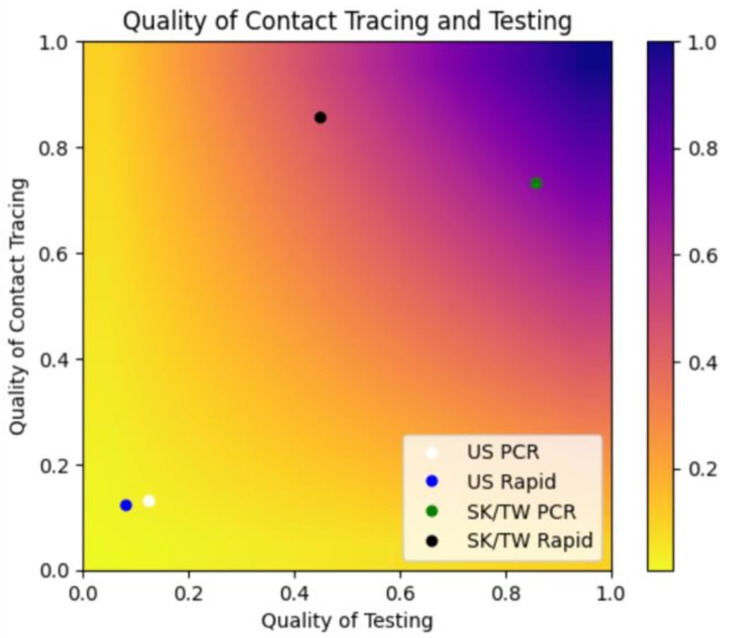
We estimate a 1.00% chance (95% uncertainty range: 0.98-1.02%) of identifying any transmission pair in the U.S. when RAT are the primary testing modality. More specifically, we estimate a 21.8% chance of identifying a positive index case, a 20.0% chance of identifying contacts given that the index case has been identified, and a 22.8% chance of identifying positive secondary cases given the index cases and contacts were identified. Using more sensitive, but less available, PCR tests, we estimate a 1.65% (95% uncertainty range: 1.62-1.68%) chance of identifying a transmission pair (Fig. 2; Table 2). Our model estimates a 27.7% chance of identifying a positive index case, a 19.8% chance of identifying contacts given that the index case has been identified, and a 29.2% chance of identifying positive cases given that the index cases and its contacts were correctly identified. By contrast, when we use a more robust scenario, based on data from East Asia, we estimate a 62.7% (95% uncertainty range: 62.6-62.8%) chance of identifying a given transmission pair when using PCR testing and 38.4% (95% uncertainty range: 38.2-38.6%) when using RAT (Figs. 2 and 3; Table 2).
Fig. 2.
Impact of contact tracing and testing on the probability of identifying a positive contact of an infected individual with COVID-19. Quality of testing refers to parts of the process relating to testing and aggregates the following probabilities: symptomatic people receiving testing, symptomatic index cases receive true positive test result (i.e. test sensitivity), contacts receive testing, contacts receive a true positive test result. Quality of contact tracing aggregates the probability of: tracers contact a positive index case, a positive index case names contacts, and tracers contact the contacts of the index case. This shows that if testing and contact tracing are done perfectly, we can expect to identify all contacts of infected individuals (illustrated through the colors of the heat map). The two circles in the lower left hand corner correspond to our United States estimates while the two in the upper half correspond to our simulations using estimates from South Korea and Taiwan, where there was stricter contact tracing
Table 2.
Probabilities of correctly identifying positive contacts of a given index case stratified by contact tracing style and by use of RAT/PCR tests. Values in parenthesis represent the 95% uncertainty intervals
| United States | Comprehensive Contact Tracing | |||
|---|---|---|---|---|
| RAT | PCR Tests | RAT | PCR Tests | |
| Percent of all transmission pairs in an outbreak | 1.00% (0.98-1.02%) | 1.65% (1.62-1.68%) | 38.4% (38.2-38.6%) | 62.7% (62.6-62.8%) |
| Percent of onward transmission from a known index case | 2.80% (2.77-2.84%) | 3.62% (3.58-3.66%) | 57.0% (56.9-57.1%) | 73.3% (73.2-73.4%) |
Fig. 3.
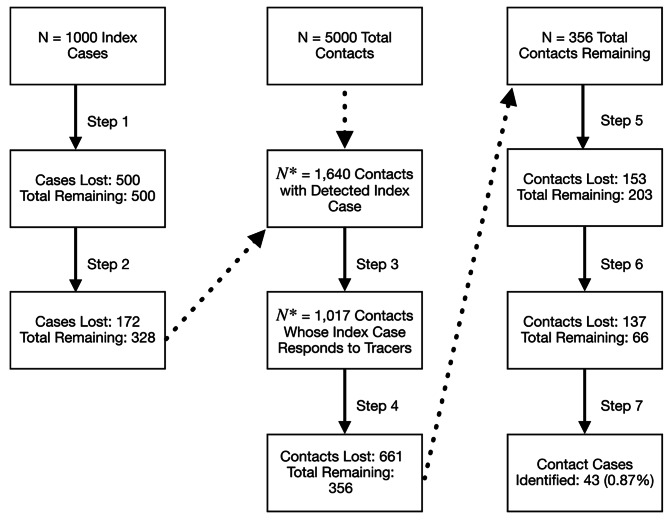
Illustration of Model results. We assume a hypothetical population of 1,000 infected people in a community with 5 unique infected contacts (no shared contacts). The figure depicts the results of our model, tracking the number of losses at each step. We split the tracking of ‘cases’ by first identifying how many index cases were identified. Then, with that information, we moved to correctly identifying positive contacts of those index cases. This example shows that if we had 1,000 people who infected 5 people each (5000 total infected contacts) and assuming the use of RAT, we would expect to correctly identify about 43 of the 5000 secondary cases
When an index case has been identified, we remove the steps 1 and 2 from our model. This effectively answers the question: given that we have knowledge of a truly positive index case, how likely are we to identify one of their positive contacts? In our U.S. based example, we estimate a 2.80% (95% uncertainty interval: 2.78-2.84%) and 3.62% (95% uncertainty interval: 3.58-3.66%) chance of identifying a positive contact of a known index case using RAT and PCR testing, respectively. In the stricter contact tracing setting, these increase to 57.0% (95% uncertainty interval: 57.1%-56.9%) for RAT and 73.3% (95% uncertainty interval: 73.2-73.4%) for PCR testing.
Discussion
Since its emergence in humans more than three years ago, SARS-CoV-2 has overwhelmed public health institutions globally. The virus still exerts an enormous mortality and morbidity burden worldwide, with nearly 7 million reported deaths thus far [51]. Underlying this is the systematic failure of contact tracing, which was abandoned by most states in the United States by early 2022 [19] and contraindicated in CDC guidance for communities with “sustained ongoing transmission” of COVID-19 [21]. The Lancet Commission report on COVID-19 [52] and several reviews [20, 53, 54] have highlighted this shortcoming in pandemic response.
Our work takes this a step further by quantifying the extent to which contact tracing failed to identify transmission events in the U.S. and in a more robust contact tracing setting based on East Asian data. Our results demonstrate that the voluntary steps in contact tracing, i.e. seeking testing and interacting with contact tracers, reduced the efficiency dramatically, with fewer than 2% of transmission events identified compared to 62.7% in a setting where testing and compliance with tracing was higher.
Contact tracing formed the basis of modern epidemiological practice, dating back to the investigation of the 1854 Broad Street Cholera outbreak in Britain [55] that led to both a mechanistic understanding of cholera transmission [56] and successful control of the outbreak. A more recent example of highly successful and proactive contact tracing by public health authorities was the effective suppression of monkeypox in the Western US in 2003 [57]. Notably, during the current pandemic, many other countries (such as China, Japan, South Korea, Taiwan, Vietnam and Singapore) were successful at implementing contact tracing in the first two years of the pandemic [14–18].
The impact of poor contact tracing in the U.S. has undermined our understanding of the transmission potential of SARS-CoV-2. For example, the argument that schools do not contribute to SARS-CoV-2 transmission was based in part on the lack of detection of transmission chains in a school setting. Numerous publications showed a lack of contact-traced chains of transmission in a school setting [58–62], while in effect lacking a positive control for the ability to identify onward chains of transmission [63]. It is now clear that SARS-CoV-2 is readily transmitted in schools [64–72], particularly when robust mitigation measures are not in place [73, 74]. Indeed, dramatic increases in case detection rates have been observed in studies that relied on surveillance testing, rather than contact tracing [70]. Additionally, this led to the conclusion that the most common source of transmission was gatherings in the home, but it is unclear if this is a consequence of household contacts being easiest to identify or a result of many transmission studies being conducted in settings with strict shut downs, where households were one of the few places where transmission could occur [75]. Also, reports from the West have pointed to a lack of detected transmission chains in air travel [76–78]. These reports are contradicted by careful contact tracing studies from other countries, which have clearly demonstrated person-to-person transmission in flight [79–81], even when robust mitigation measures were in place [82].
Our work has several limitations. We have assumed instantaneous contact tracing, ignoring the impact of tracing delays on infection control which has a significant impact on contact tracing effectiveness against transmission [83, 84] due to short incubation period of SARS-CoV-2 [85]. Instead, our estimates for contact tracing effectiveness apply to the informativeness of contact tracing studies, and they form an upper bound for the effectiveness of contact tracing as a transmission prevention measure. We do not account for asymptomatic transmission, again making our estimates an upper bound. The percent of asymptomatic COVID-19 cases is estimated to be anywhere between 1.6% and 56.5% [86–92], with these cases having a relative reduced infectiousness of 0 to 62% [86–93]. This would mean our estimated 1.65% of transmission pairs identified with PCR testing could be as low as 0.9%, assuming no asymptomatic index cases are identified. We also have not accounted for superspreading, which has been estimated to be a significant feature in COVID-19 transmission [90–92]. This implies that missing a superspreading index case would have tremendous impact on downstream contact tracing efforts and that there is significant stochasticity [93]. We only consider the probability of identifying infected contacts, but it is ideal to also identify uninfected contacts accurately. Finally, we estimate the probability of naming an infected contact using data describing the probability of naming any contacts at all (rather than the probability of naming any given contact). This means that our final estimated probabilities are upper bounds of the true values. Despite representing an upper bound, our contact tracing estimates suggest that U.S. contact tracing studies fail to identify the majority of transmission pairs and onward transmission events. This severely limits the inferences that can be drawn from such studies.
Our work points to several key lessons for future public health efforts. First, compliance is a key driver of contact tracing effectiveness. Methods to improve compliance will be crucial for future contact tracing efforts- whether using technological approaches (such as mobile phone or surveillance-camera based tracing) or by making changes to the legal framework around public health efforts (see Supplementary Information S2 for a more on this topic).
Second, there is a pressing need for innovation, to develop contact tracing methodologies that are more resistant to noncompliance. One such approach may be backward contact tracing, which seeks to identify who infected the detected case. Here when contact tracing is executed backward to identify the source of infection (parent), the more offspring (infections) a parent has produced, the more frequently the parent shows up as a contact. Model-based analysis suggests that a backwards contact tracing approach does not require sampling a network at such a large scale as forward tracing [94, 95] to understand transmission dynamics, and addresses the problem of low compliance. This approach has been proposed by others for COVID-19 [96–98], and has been empirically shown to be effective, particularly in identifying superspreading events [99], however this is unlikely to be as helpful in reducing transmission.
Third, public health responses to future outbreaks must include educate the public about behaviors with health outcomes, including creating a normative framework around contact tracing compliance. Consistent messaging about limiting transmission and contact tracing are key as has been noted by the Lancet Commission [52], among others [100, 101]. This could include reframing messaging to reduce stigma that has often been associated with contact tracing [102] and which undermines contact tracing efficacy [103]. During the HIV epidemic, contact tracers emphasized the index case’s personal responsibility towards the health of their sexual partners [1]. It also includes addressing misinformation, which led many to believe that COVID-19 was a “hoax” [104] and public health measures were overreactions [105].
Finally, we have shown that testing availability and accuracy create a critical gap in contact tracing efforts. Considering only the steps for testing accuracy and cases/contacts receiving testing in our model we find that only 12.4% of possible cases could be identified with RAT, the most available testing modality. To effectively manage future outbreaks, tests need to be sensitive, provide rapid results, and be readily available.
The work presented here adds to the growing body of literature [26, 106, 107] highlighting the poor performance of contact tracing in the West during the ongoing pandemic and suggests practical fixes for this problem, as we have described. In its absence, public health is forced to rely on population-wide measures for disease spread and will not be able to fine-tune its responses to match the situation. If we are to improve our response to the current crisis, or to others in the future, we must improve our ability to deliver this key function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
LFW and AC conceived the study. HB carried performed the literature review and analysis. LFW, AC and HB wrote the paper. All authors participated in discussions on the direction of the paper and research and read and approved the final paper.
Funding
LFW and HB were funded by NIH award number R35GM14182. The NIH had not involvement in the design or analysis of the study.
Data availability
All data and code are available at https://github.com/Henry-Bayly/ContactTracingMarkovModel.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Arijit Chakravarty and Laura F White contributed equally to this work.
References
- 1.Brandt AM. The history of contact tracing and the Future of Public Health. Am J Public Health Am Public Health Association. 2022;112(8):1097–9. doi: 10.2105/AJPH.2022.306949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 3.Sun K, Wang W, Gao L, et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Volume 371. Science. American Association for the Advancement of Science; 2021. p. eabe2424. 6526. [DOI] [PMC free article] [PubMed]
- 4.Endo A, Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Abbott S, Kucharski AJ, Funk S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. doi: 10.12688/wellcomeopenres.15842.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J, Gu J, Li K, et al. COVID-19 Outbreak Associated with Air Conditioning in Restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628–31. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichler N, Thornley C, Swadi T, et al. Transmission of severe Acute Respiratory Syndrome Coronavirus 2 during Border Quarantine and Air Travel, New Zealand (Aotearoa) Emerg Infect Dis. 2021;27(5):1274–8. doi: 10.3201/eid2705.210514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon K-S, Park J-I, Park YJ, Jung D-M, Ryu K-W, Lee J-H. Evidence of Long-Distance Droplet transmission of SARS-CoV-2 by Direct Air Flow in a Restaurant in Korea. J Korean Med Sci. 2020;35(46):e415. doi: 10.3346/jkms.2020.35.e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020;189(4):1143–4. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Li C, Dong H et al. Airborne Transmission of COVID-19: epidemiologic evidence from two outbreak investigations. SSRN Electron J. 2020.
- 10.Park YJ, Choe YJ, Park O, et al. Contact tracing during Coronavirus Disease Outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465–8. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier BF, Brockmann D. Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China. Science. 2020;368(6492):742–6. doi: 10.1126/science.abb4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu N, Cheng K-W, Qamar N, Huang K-C, Johnson JA. Weathering COVID-19 storm: successful control measures of five Asian countries. Am J Infect Control. 2020;48(7):851–2. doi: 10.1016/j.ajic.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Jiang H, Wang Q, Yang M, Chen Y, Jiang Q. Use of contact tracing, isolation, and mass testing to control transmission of covid-19 in China. BMJ. 2021;375:n2330. doi: 10.1136/bmj.n2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Guerche-Séblain C, Chakir L, Nageshwaran G, et al. Experience from five Asia-Pacific countries during the first wave of the COVID-19 pandemic: mitigation strategies and epidemiology outcomes. Travel Med Infect Dis. 2021;44:102171. doi: 10.1016/j.tmaid.2021.102171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jefferies S, French N, Gilkison C, et al. COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study. Lancet Public Health. 2020;5(11):e612–23. doi: 10.1016/S2468-2667(20)30225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Nguyen H, Lan Nguyen H, Thi Minh Dao A, et al. The COVID-19 pandemic in Australia: public health responses, opportunities and challenges. Int J Health Plann Manage. 2022;37(1):5–13. doi: 10.1002/hpm.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S-C. Taiwan’s experience in fighting COVID-19. Nat Immunol. 2021;22(4):393–4. doi: 10.1038/s41590-021-00908-2. [DOI] [PubMed] [Google Scholar]
- 18.Van Nguyen Q, Cao DA, Nghiem SH. Spread of COVID-19 and policy responses in Vietnam: an overview. Int J Infect Dis IJID off Publ Int Soc Infect Dis. 2021;103:157–61. doi: 10.1016/j.ijid.2020.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feliciano M. State Approaches to Contact Tracing during the COVID-19 Pandemic [Internet]. Natl. Acad. State Health Policy. 2022 [cited 2022 Nov 26]. Available from: https://www.nashp.org/state-approaches-to-contact-tracing-covid-19/.
- 20.Clark E, Chiao EY, Amirian ES. Why contact tracing efforts have failed to curb Coronavirus Disease 2019 (COVID-19) transmission in much of the United States. Clin Infect Dis off Publ Infect Dis Soc Am. 2021;72(9):e415–9. doi: 10.1093/cid/ciaa1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Health Departments [Internet]. Cent. Dis. Control Prev. 2020 [cited 2022 Nov 26]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/contact-tracing.html.
- 22.Lash RR, Donovan CV, Fleischauer AT, et al. COVID-19 contact tracing in two counties - North Carolina, June-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1360–3. doi: 10.15585/mmwr.mm6938e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadeem R. The Challenges of Contact Tracing as U.S. Battles COVID-19 [Internet]. Pew Res. Cent. Internet Sci. Tech. 2020 [cited 2022 Nov 26]. Available from: https://www.pewresearch.org/internet/2020/10/30/the-challenges-of-contact-tracing-as-u-s-battles-covid-19/.
- 24.Hendrix MJ, Walde C, Findley K, Trotman R. Absence of Apparent Transmission of SARS-CoV-2 from two stylists after exposure at a Hair Salon with a Universal Face Covering Policy - Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(28):930–2. doi: 10.15585/mmwr.mm6928e2. [DOI] [PubMed] [Google Scholar]
- 25.Doyle T, Kendrick K, Troelstrup T, et al. COVID-19 in primary and secondary school settings during the First Semester of School reopening - Florida, August-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(12):437–41. doi: 10.15585/mmwr.mm7012e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lash RR, Moonan PK, Byers BL, et al. COVID-19 Case Investigation and contact tracing in the US, 2020. JAMA Netw Open. 2021;4(6):e2115850. doi: 10.1001/jamanetworkopen.2021.15850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alimohamadi Y, Taghdir M, Sepandi M. Estimate of the Basic Reproduction Number for COVID-19: a systematic review and Meta-analysis. J Prev Med Pub Health Korean Soc Prev Med. 2020;53(3):151–7. doi: 10.3961/jpmph.20.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajo K, Nishiura H. Exploring secondary SARS-CoV-2 transmission from asymptomatic cases using contact tracing data. Theor Biol Med Model. 2021;18(1):12. doi: 10.1186/s12976-021-00144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinoshita R, Anzai A, Jung S-M, et al. Containment, contact tracing and asymptomatic transmission of Novel Coronavirus Disease (COVID-19): a modelling study. J Clin Med. 2020;9(10):3125. doi: 10.3390/jcm9103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and Meta-analysis. JAMA Pediatr. 2021;175(2):143–56. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Choe YJ, Lee J, et al. Role of children in household transmission of COVID-19. Arch Dis Child. 2021;106(7):709–11. doi: 10.1136/archdischild-2020-319910. [DOI] [PubMed] [Google Scholar]
- 32.Yung CF, Kam K, Nadua KD, et al. Novel coronavirus 2019 transmission risk in Educational Settings. Clin Infect Dis. 2021;72(6):1055–8. doi: 10.1093/cid/ciaa794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz S, Gewertz C. Omicron Is Making a Mess of Instruction, Even Where Schools Are Open. Educ Week [Internet]. 2022 [cited 2023 Feb 20];. Available from: https://www.edweek.org/teaching-learning/omicron-is-making-a-mess-of-instruction-even-where-schools-are-open/2022/01.
- 34.Long C. Omicron Exacerbating School Staff Shortages| NEA [Internet]. [cited 2023 Feb 20]. Available from: https://www.nea.org/advocating-for-change/new-from-nea/omicron-exacerbating-school-staff-shortages.
- 35.2020 Sturgis Rally Final Vehicle Counts [Internet]. [cited 2022 Nov 26]. Available from: https://news.sd.gov/newsitem.aspx?id=27174.
- 36.Blistein J. Freedom-Loving People: Behind the Scenes at That Controversial Smash Mouth Show in South Dakota [Internet]. Roll. Stone. 2020 [cited 2022 Nov 26]. Available from: https://www.rollingstone.com/music/music-news/sturgis-covid-19-biker-fest-smash-mouth-1043040/.
- 37.Even the Official Motorcycle Brand of the Sturgis Rally Thinks the Mass Gathering. Is Too Risky [Internet]. [cited 2022 Nov 26]. Available from: https://news.yahoo.com/even-official-motorcycle-brand-sturgis091043595.html?guce_referrer=aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnLw&guce_referrer_sig=AQAAAE-Mbd02M7hUYmmufo9DeJ5BTNfsZOc1Z4B0b2yq9vvRmYegzua-r6eYCg3WWyq8Hfffeq2WkWB6sR3jX1eeVKlMA_74t-hd-vLk798wTDPvXiizPqVBjiNMviJblOYBHfn-7EGUdE_4PeQ7N-eYEcch3PJ4C_8Tsi26OG7TPWAT&guccounter=2.
- 38.It’s literally impossible to stop.: Sturgis, South Dakota, braces as hundreds of thousands of bikers arrived in the middle of a pandemic [Internet]. [cited 2022 Nov 26]. Available from: https://news.yahoo.com/literally-impossible-stop-sturgis-south-231030345.html.
- 39.Sturgis Motorcycle Rally [Internet]. Wikipedia. 2022 [cited 2022 Nov 26]. Available from: https://en.wikipedia.org/w/index.php?title=Sturgis_Motorcycle_Rally&oldid=1120591558
- 40.Dave D, McNichols D, Sabia JJ. The contagion externality of a superspreading event: the Sturgis Motorcycle Rally and COVID-19. South Econ J. 2021;87(3):769–807. doi: 10.1002/soej.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Firestone MJ. COVID-19 Outbreak Associated with a 10-Day Motorcycle Rally in a Neighboring State — Minnesota, August–September 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2022 Nov 26]; 69. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6947e1.htm. [DOI] [PMC free article] [PubMed]
- 42.Harleys everywhere, masks nowhere.: Sturgis expects crowd of 250,000 [Internet]. Chic. Sun-Times. 2020 [cited 2022 Nov 26]. Available from: https://chicago.suntimes.com/coronavirus/2020/8/7/21359318/sturgis-motorcycle-harleys-everywhere-masks-nowhere.
- 43.Rapier G. If I die from the virus, it was just meant to be: 250,000 descend upon tiny South Dakota town for world-famous motorcycle rally [Internet]. Bus. Insid. [cited 2022 Nov 26]. Available from: https://www.businessinsider.com/sturgis-motorcycle-rally-kicks-off-despite-surging-coronavirus-cases-2020-8.
- 44.2020 Sturgis Motorcycle Rally. attracts thousands with no mask requirements amid pandemic [Internet]. [cited 2022 Nov 26]. Available from: https://www.usatoday.com/picture-gallery/news/nation/2020/08/09/2020-sturgis-motorcycle-rally-draws-thousands-no-mask-requirements-covid-19-coronavirus/3331908001/.
- 45.Bonacci RA, Manahan LM, Miller JS, et al. COVID-19 contact tracing outcomes in Washington State, August and October 2020. Front Public Health. 2021;9:782296. doi: 10.3389/fpubh.2021.782296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelby T, Schenck C, Weeks B, et al. Lessons learned from COVID-19 contact tracing during a Public Health emergency: a prospective implementation study. Front Public Health. 2021;9:721952. doi: 10.3389/fpubh.2021.721952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kortela E, Kirjavainen V, Ahava MJ, et al. Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients. PLoS ONE. 2021;16(5):e0251661. doi: 10.1371/journal.pone.0251661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.F S-R SJ. P J, P B, M N. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int J Infect Dis IJID Off Publ Int Soc Infect Dis [Internet]. Int J Infect Dis; 2021 [cited 2022 Nov 26]; 109. Available from: https://pubmed.ncbi.nlm.nih.gov/34242764/. [DOI] [PMC free article] [PubMed]
- 49.Cheng H-Y, Jian S-W, Liu D-P, et al. Contact tracing Assessment of COVID-19 Transmission dynamics in Taiwan and Risk at different exposure periods before and after Symptom Onset. JAMA Intern Med. 2020;180(9):1156–63. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hood JE, Kubiak RW, Avoundjian T, et al. A multifaceted evaluation of a COVID-19 contact tracing program in King County, Washington. J Public Health Manag Pract JPHMP. 2022;28(4):334–43. doi: 10.1097/PHH.0000000000001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO Coronavirus (COVID-19.) Dashboard [Internet]. [cited 2023 Jan 15]. Available from: https://covid19.who.int.
- 52.The Lancet Commission on lessons for the future from the COVID-19. pandemic - The Lancet [Internet]. [cited 2022 Nov 26]. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)01585-9/fulltext.
- 53.Lewis D. Why many countries failed at COVID contact-tracing — but some got it right. Nature. 2020;588(7838):384–7. doi: 10.1038/d41586-020-03518-4. [DOI] [PubMed] [Google Scholar]
- 54.Kwon O. Evidence of the importance of contact tracing in fighting COVID-19. Epidemiol Health. 2022;44:e2022006. doi: 10.4178/epih.e2022006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tulchinsky TH. John Snow, Cholera, the Broad Street Pump; Waterborne diseases then and now. Case Stud Public Health. 2018;:77–99.
- 56.Competing Theories of Cholera [Internet]. [cited 2022 Nov 27]. Available from: https://www.ph.ucla.edu/epi/snow/choleratheories.html.
- 57.CDC. Monkeypox in the U.S. [Internet]. Cent. Dis. Control Prev. 2022 [cited 2022 Nov 27]. Available from: https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html.
- 58.COVID-19 in children. and the role of school settings in transmission - second update [Internet]. Eur. Cent. Dis. Prev. Control. 2021 [cited 2022 Nov 26]. Available from: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission.
- 59.Hershow RB, Low. SARS-CoV-2 Transmission in Elementary Schools — Salt Lake County, Utah, December 3, 2020–January 31, 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2021 [cited 2022 Nov 26]; 70. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7012e3.htm. [DOI] [PMC free article] [PubMed]
- 60.Falk A, Benda A, Falk P, Steffen S, Wallace Z, Høeg TB. COVID-19 cases and transmission in 17 K-12 schools - Wood County, Wisconsin, August 31-November 29, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):136–40. doi: 10.15585/mmwr.mm7004e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandini S, Rainisio M, Iannuzzo ML, Bellerba F, Cecconi F, Scorrano L. No evidence of association between schools and SARS-CoV-2 second wave in Italy [Internet]. medRxiv; 2020 [cited 2022 Nov 26]. p. 2020.12.16.20248134. Available from: https://www.medrxiv.org/content/10.1101/2020.12.16.20248134v1.
- 62.Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. 2021;147(4):e2020048090. doi: 10.1542/peds.2020-048090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson KE, Lachmann M, Stoddard M et al. Detecting in-school transmission of SARS-CoV-2 from case ratios and documented clusters. MedRxiv Prepr Serv Health Sci. 2021;:2021.04.26.21256136.
- 64.White LF, Murray EJ, Chakravarty A. The role of schools in driving SARS-CoV-2 transmission: not just an open-and-shut case. Cell Rep Med. 2022;3(3):100556. doi: 10.1016/j.xcrm.2022.100556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manica M, Poletti P, Deandrea S et al. Estimating SARS-CoV-2 transmission in educational settings: A retrospective cohort study. Influenza Other Respir Viruses [Internet]. [cited 2022 Nov 29]; n/a(n/a). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/irv.13049. [DOI] [PMC free article] [PubMed]
- 66.Meuris C, Kremer C, Geerinck A, et al. Transmission of SARS-CoV-2 after COVID-19 screening and Mitigation Measures for Primary School Children Attending School in Liège, Belgium. JAMA Netw Open. 2021;4(10):e2128757. doi: 10.1001/jamanetworkopen.2021.28757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres JP, Piñera C, De La Maza V, et al. Severe Acute Respiratory Syndrome Coronavirus 2 antibody prevalence in blood in a large School Community subject to a Coronavirus Disease 2019 outbreak: a cross-sectional study. Clin Infect Dis off Publ Infect Dis Soc Am. 2021;73(2):e458–65. doi: 10.1093/cid/ciaa955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gold JAW, Gettings JR, Kimball A, et al. Clusters of SARS-CoV-2 infection among Elementary School educators and students in one School District - Georgia, December 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):289–92. doi: 10.15585/mmwr.mm7008e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontanet A, Tondeur L, Grant R, et al. SARS-CoV-2 infection in schools in a northern French city: a retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2021;26(15):2001695. doi: 10.2807/1560-7917.ES.2021.26.15.2001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crowe J, Schnaubelt AT, SchmidtBonne S, et al. Assessment of a program for SARS-CoV-2 screening and Environmental Monitoring in an Urban Public School District. JAMA Netw Open. 2021;4(9):e2126447. doi: 10.1001/jamanetworkopen.2021.26447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buja A, Zabeo F, Cristofori V, et al. Opening schools and trends in SARS-CoV-2 transmission in European Countries. Int J Public Health. 2021;66:1604076. doi: 10.3389/ijph.2021.1604076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Llupià A, Borràs-Santos A, Guinovart C, Utzet M, Moriña D, Puig J. SARS-CoV-2 transmission in students of public schools of Catalonia (Spain) after a month of reopening. PLoS ONE. 2021;16(5):e0251593. doi: 10.1371/journal.pone.0251593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theuring S, Thielecke M, van Loon W, et al. SARS-CoV-2 infection and transmission in school settings during the second COVID-19 wave: a cross-sectional study, Berlin, Germany, November 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2021;26(34):2100184. doi: 10.2807/1560-7917.ES.2021.26.34.2100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cowger TL, Murray EJ, Clarke J, et al. Lifting Universal Masking in Schools — Covid-19 incidence among students and staff. N Engl J Med Mass Med Soc. 2022;387(21):1935–46. doi: 10.1056/NEJMoa2211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lei H, Xu X, Xiao S, Wu X, Shu Y. Household transmission of COVID-19-a systematic review and meta-analysis. J Infect. Elsevier; 2020; 81(6):979–997. [DOI] [PMC free article] [PubMed]
- 76.Nir-Paz R, Grotto I, Strolov I, et al. Absence of in-flight transmission of SARS-CoV-2 likely due to use of face masks on board. J Travel Med. 2020;27(8):taaa117. doi: 10.1093/jtm/taaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saretzki C, Bergmann O, Dahmann P, et al. Are small airplanes safe with regards to COVID-19 transmission? J Travel Med. 2021;28(7):taab105. doi: 10.1093/jtm/taab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pombal R, Hosegood I, Powell D. Risk of COVID-19 during Air Travel. JAMA. 2020;324(17):1798. doi: 10.1001/jama.2020.19108. [DOI] [PubMed] [Google Scholar]
- 79.Guo Q, Wang J, Estill J, et al. Risk of COVID-19 transmission aboard aircraft: an Epidemiological Analysis Based on the National Health Information Platform. Int J Infect Dis IJID off Publ Int Soc Infect Dis. 2022;118:270–6. doi: 10.1016/j.ijid.2022.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khanh NC, Thai PQ, Quach H-L, et al. Transmission of SARS-CoV 2 during Long-Haul Flight. Emerg Infect Dis. 2020;26(11):2617–24. doi: 10.3201/eid2611.203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang N, Shen Y, Shi C, et al. In-flight transmission cluster of COVID-19: a retrospective case series. Infect Dis Lond Engl. 2020;52(12):891–901. doi: 10.1080/23744235.2020.1800814. [DOI] [PubMed] [Google Scholar]
- 82.Bae SH, Shin H, Koo H-Y, Lee SW, Yang JM, Yon DK. Asymptomatic transmission of SARS-CoV-2 on Evacuation Flight. Emerg Infect Dis. 2020;26(11):2705–8. doi: 10.3201/eid2611.203353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kretzschmar ME, Rozhnova G, Bootsma MCJ, van Boven M, van de Wijgert JHHM, Bonten MJM. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5(8):e452–9. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson KE, Stoddard M, Nolan RP, White DE, Hochberg N, Chakravarty A. This time is different: model-based evaluation of the implications of SARS-CoV-2 infection kinetics for disease control [Internet]. medRxiv; 2020 [cited 2022 Nov 29]. p. 2020.08.19.20177550. Available from: https://www.medrxiv.org/content/10.1101/2020.08.19.20177550v2.
- 85.Quantifying SARS-. CoV-2 transmission suggests epidemic control with digital contact tracing| Science [Internet]. [cited 2022 Nov 26]. Available from: https://www.science.org/doi/10.1126/science.abb6936?cookieSet=1. [DOI] [PMC free article] [PubMed]
- 86.Tan J, Ge Y, Martinez L, et al. Transmission roles of symptomatic and asymptomatic COVID-19 cases: a modelling study. Epidemiol Infect. 2022;150:e171. doi: 10.1017/S0950268822001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Just how contagious. is asymptomatic Covid-19? [Internet]. [cited 2023 Mar 8]. Available from: https://www.advisory.com/Daily-Briefing/2022/06/02/covid-transmission.
- 88.Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A. 2020;117(30):17513–5. doi: 10.1073/pnas.2008373117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muller CP. Do asymptomatic carriers of SARS-COV-2 transmit the virus? Lancet Reg Health– Eur [Internet]. Elsevier; 2021 [cited 2023 Mar 8]; 4. Available from: https://www.thelancet.com/journals/lanepe/article/PIIS26667762(21)00059-4/fulltext. [DOI] [PMC free article] [PubMed]
- 90.Chen PZ, Koopmans M, Fisman DN, Gu FX. Understanding why superspreading drives the COVID-19 pandemic but not the H1N1 pandemic. Lancet Infect Dis Elsevier. 2021;21(9):1203–4. doi: 10.1016/S1473-3099(21)00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lakdawala SS, Menachery VD. Catch me if you can: Superspreading of COVID-19. Trends Microbiol. 2021;29(10):919–29. doi: 10.1016/j.tim.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chopping the tail.: How preventing superspreading can help to maintain COVID-19 control - ScienceDirect [Internet]. [cited 2022 Dec 1]. Available from: https://www.sciencedirect.com/science/article/pii/S1755436520300487. [DOI] [PMC free article] [PubMed]
- 93.Gupta M, Parameswaran GG, Sra MS, et al. Contact tracing of COVID-19 in Karnataka, India: Superspreading and determinants of infectiousness and symptomatic infection. PLoS ONE. 2022;17(7):e0270789. doi: 10.1371/journal.pone.0270789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kojaku S, Hébert-Dufresne L, Mones E, Lehmann S, Ahn Y-Y. The effectiveness of backward contact tracing in networks. Nat Phys. 2021;17:652–8. doi: 10.1038/s41567-021-01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.A National Plan. To Enable Comprehensive COVID-19 Case Finding and contact tracing in the US.:16.
- 96.Empirical evidence on the efficiency of backward contact tracing in COVID. -19| Nature Communications [Internet]. [cited 2022 Nov 29]. Available from: https://www.nature.com/articles/s41467-022-32531-6. [DOI] [PMC free article] [PubMed]
- 97.Bradshaw WJ, Alley EC, Huggins JH, Lloyd AL, Esvelt KM. Bidirectional contact tracing could dramatically improve COVID-19 control. Nat Commun Nat Publishing Group. 2021;12(1):232. doi: 10.1038/s41467-020-20325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.S0523_Oxford_-_Backwards_contact_tracing. pdf [Internet]. [cited 2022 Nov 29]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1048768/S0523_Oxford_-_Backwards_contact_tracing.pdf.
- 99.Endo A, Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Leclerc QJ, et al. Implication of backward contact tracing in the presence of overdispersed transmission in COVID-19 outbreaks. Wellcome Open Res. 2020;5:239. doi: 10.12688/wellcomeopenres.16344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amara PS, Platt JE, Raj M, Nong P. Learning about COVID-19: sources of information, public trust, and contact tracing during the pandemic. BMC Public Health. 2022;22(1):1348. doi: 10.1186/s12889-022-13731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samuel G, Lucivero F, Johnson S, Diedericks H. Ecologies of Public Trust: the NHS COVID-19 contact tracing app. J Bioethical Inq. 2021;18(4):595–608. doi: 10.1007/s11673-021-10127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brandt AM. No magic bullet: a Social History of Venereal Disease in the United States since 1880- 35th Anniversary Edition. Oxford University Press; 2020.
- 103.El-Sadr WM, Platt J, Bernitz M, Reyes M. Contact tracing: barriers and facilitators. Am J Public Health Am Public Health Association. 2022;112(7):1025–33. doi: 10.2105/AJPH.2022.306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanase L-M, Kerr J, Freeman ALJ, Schneider CR. COVID-19 risk perception and hoax beliefs in the US immediately before and after the announcement of President Trump’s diagnosis. R Soc Open Sci Royal Soc; 9(8):212013. [DOI] [PMC free article] [PubMed]
- 105.Nadeem R, Lack of Preparedness Among Top Reactions Americans Have to Public Health Officials’ COVID-19 Response [Internet]. Pew Res. Cent. Sci. Soc. 2022 [cited 2022 Nov 26]. Available from: https://www.pewresearch.org/science/2022/10/05/lack-of-preparedness-among-top-reactions-americans-have-to-public-health-officials-covid-19-response/.
- 106.Using a household-. structured branching process to analyse contact tracing in the SARS-CoV-2 pandemic| Philosophical Transactions of the Royal Society B: Biological Sciences [Internet]. [cited 2022 Nov 29]. 10.1098/rstb.2020.0267. [DOI] [PMC free article] [PubMed]
- 107.Davis EL, Lucas TCD, Borlase A, et al. Contact tracing is an imperfect tool for controlling COVID-19 transmission and relies on population adherence. Nat Commun Nat Publishing Group. 2021;12(1):5412. doi: 10.1038/s41467-021-25531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chu VT, Schwartz NG, Donnelly MAP, et al. Comparison of Home Antigen Testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med. 2022;182(7):701–9. doi: 10.1001/jamainternmed.2022.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miller JS, Bonacci RA, Lash RR, et al. COVID-19 Case Investigation and contact tracing in Central Washington State, June-July 2020. J Community Health. 2021;46(5):918–21. doi: 10.1007/s10900-021-00974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stargel A, Taylor MM, Zansky S, Spencer K, Hogben M, Shultz A. Case Investigation and contact tracing efforts from Health departments in the United States, November 2020–December 2021. Clin Infect Dis off Publ Infect Dis Soc Am. 2022;:ciac442. [DOI] [PMC free article] [PubMed]
- 111.Kalyanaraman N, Fraser MR, Containing COVID-19 through contact tracing. Public Health Rep. 2020;136(1):32–8. doi: 10.1177/0033354920967910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spencer KD, Chung CL, Stargel A, et al. COVID-19 case investigation and contact tracing efforts from Health departments - United States, June 25-July 24, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(3):83–7. doi: 10.15585/mmwr.mm7003a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kanu FA, Smith EE, Offutt-Powell T, Hong R, Dinh T-H, Pevzner E. Declines in SARS-CoV-2 transmission, hospitalizations, and Mortality after implementation of Mitigation Measures— Delaware, March–June 2020. Morb Mortal Wkly Rep. 2020;69(45):1691–4. doi: 10.15585/mmwr.mm6945e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sachdev DD, Brosnan HK, Reid MJA, et al. Outcomes of contact tracing in San Francisco, California—Test and Trace during Shelter-in-place. JAMA Intern Med. 2021;181(3):381–3. doi: 10.1001/jamainternmed.2020.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sachdev DD, Chew Ng R, Sankaran M, et al. Contact-tracing outcomes among Household contacts of fully vaccinated Coronavirus Disease 2019 (COVID-19) patients: San Francisco, California, 29 January-2 July 2021. Clin Infect Dis off Publ Infect Dis Soc Am. 2022;75(1):e267–75. doi: 10.1093/cid/ciab1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jones A, Fialkowski V, Prinzing L, Trites J, Kelso P, Levine M. Assessment of Day-7 Postexposure Testing of Asymptomatic contacts of COVID-19 patients to Evaluate Early Release from Quarantine - Vermont, May-November 2020. MMWR Morb Mortal Wkly Rep. 2021;70(1):12–3. doi: 10.15585/mmwr.mm7001a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matthias J. Notes from the Field: COVID-19 Case Investigation and Contact Tracing Program — Spirit Lake Tribe, North Dakota, September–November 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2021 [cited 2022 Nov 27]; 70. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7014a4.htm. [DOI] [PMC free article] [PubMed]
- 118.Atherstone C, Siegel M, Schmitt-Matzen E, et al. SARS-CoV-2 Transmission Associated with High School Wrestling tournaments - Florida, December 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):141–3. doi: 10.15585/mmwr.mm7004e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code are available at https://github.com/Henry-Bayly/ContactTracingMarkovModel.