Abstract
The neural circuits orchestrating complex behavioral response strategies to threat are not understood. In this issue of Neuron, Wang et al. establish the hypothalamic dorsal premammillary nucleus as a critical node that communicates with thalamic and midbrain regions to coordinate diverse escape strategies.
If fire breaks out while you are working in the lab, you are likely to avoid harm by adopting a cognitive approach that uses your spatial understanding of the building and accessible exit routes. However, if you perceive that there is no escape route, you may panic and adopt a less cerebral strategy, such as jumping out of the nearest window. Similarly, when foraging for resources, rodents must execute diverse escape strategies when faced with predatory threat. Indeed, animals show rapid and complex behavioral responses to threat that depend on context, intensity, and proximity (Perusini and Fanselow, 2015). Flight is an adaptive defensive response that is used by animals to avoid threats. Before initiating a flight response, animals must weigh the potential cost of staying in the current environment over the costs of escaping (Blank, 2018). Understanding how the brain controls complex responses to threat is important because maladaptive changes in the circuits mediating these responses may lead to pathological conditions.
The role of the hypothalamus in the regulation of flight from predatory threat is well-established (Wang, Chen and Lin, 2015; Silva, Gross and Gräff, 2016). The dorsal premammillary nucleus of the hypothalamus (PMd) is an important part of the medial hypothalamic defensive circuit that is involved in anti-predatory responses (Canteras et al., 2008). Furthermore, the PMd projects to the midbrain dorsolateral periaqueductal gray (dlPAG) and the anteromedial thalamic nucleus (amv)--regions implicated in the regulation of flight and spatial navigation, respectively (Canteras et al., 2008; Tovote et al., 2016). The PMd is therefore situated to influence complex and context-specific defensive responses to threat, but how this might be achieved was not known because most studies have used simplistic behavioral contexts that induce basic escape strategies. In this issue of Neuron, Wang et al. (2021) use a comprehensive array of complementary techniques to define the role of the PMd as a master coordinator of diverse escape strategies in a variety of more naturalistic environments, as well as in the presence of the panic-inducing agent CO2.
Using in-situ hybridization, the authors found that 87% of PMd neurons express both vesicular glutamate transporter 2 (VGLUT2) and cholecystokinin (cck). Because cck is more restricted to the PMd than VGLUT2, the authors used a cck-cre mouse line for their experiments. The authors first assess the effect of chemogenetic modulation of PMd-cck neuronal activity on escape behavior using three different threat assays—rat exposure, heated floor, and CO2 exposure. In the rat assay, the mouse is placed in a behavioral context with a live, but tethered rat at one end, and a complex escape route at the other end. Mice will naturally try to escape this environment because rats will kill and eat mice. Control experiments replace the live rat with a toy rat. Chemogenetic inhibition of PMd-cck cells decreased, while excitation increased, the number of escapes from the live rat yet there was no behavioral effect on the toy rat condition, suggesting selective control of PMd cells over escape responses to threat.
For the heated floor and CO2 exposure experiments, mice had to climb a cylinder wrapped in thin mesh to escape. Mice in the heated plate test had decreased or increased escape climbs, respectively, with chemogenetic inhibition or excitation of PMd-cck neurons. In contrast, exposure to CO2 induced primarily panic-related escape jumps, which decreased with inhibition and increased with excitation of PMd-cck cells. Collectively, these data suggest that PMd-cck activity is necessary and sufficient to regulate both context-specific escape strategies (during rat and hot plate experiments) as well as panic-related stereotypical escape jumping (during CO2 exposure, Figure 1).
Figure 1. Hypothalamic coordination in versatile flight from threat.
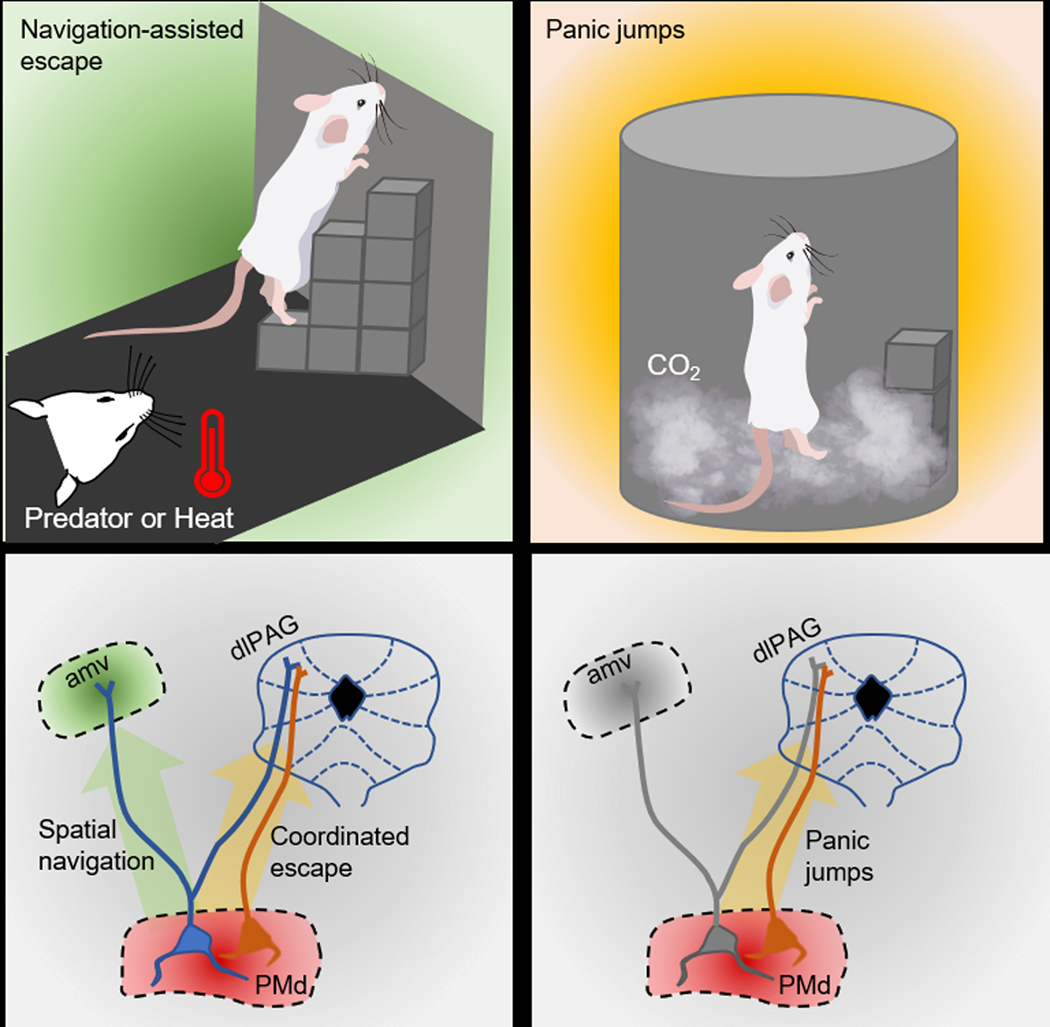
The dorsal premammillary nucleus of the hypothalamus (PMd) projects to the dorsolateral periaqueductal gray (dlPAG) and the anteromedial thalamic nucleus (amv). Wang et al. (2021) show that exposing mice to imminent threats, such as a predator or heat, activates both the PMd→dlPAG and PMd→amv pathways, resulting in coordinated navigation-assisted escape (left side). The panic-inducing agent CO2 activates only the PMd→dlPAG pathway, which elicits panic jumps (right side).
Interestingly, chemogenetic excitation of the dorsomedial hypothalamus and dorsomedial part of ventromedial hypothalamus (VMH), which are known to contribute to panic- and predator-induced defensive behavior, did not alter context-specific escape in the rat exposure assay. However, optogenetic stimulation of steroidogenic factor 1-expressing neurons in the VMH did induce, along with freezing behavior, some panic jumping and escape from a novel, threat-free enclosure. This is consistent with a previous study that demonstrated context-dependent somatomotor and autonomic responses with optogenetic stimulation of this VMH population (Wang, Chen and Lin, 2015). Thus, although other areas of the hypothalamus may not alone be sufficient to induce context-specific escape, they may contribute under certain conditions. Optogenetic and chemogenetic loss-of-function experiments are needed to test this possibility.
Having determined the importance of PMd-cck neurons in executing various escape strategies from threats, the authors then used optogenetic stimulation of PMd-cck cells to induce escape behavior from complex environments in the absence of threat. Optogenetic activation of PMd-cck cells in an empty, inescapable box elicited escape jumping behavior. Remarkably, PMd-cck stimulation produced escape, but not jumping, in all mice exposed to novel environments with different escape routes. Furthermore, using a nose poke negative reinforcement paradigm and a real-time place avoidance paradigm, it was determined that optogenetic stimulation of PMd-cck neurons is aversive. Stimulation also elicited sympathetic responses, such as pupil dilation and increased heart rate. Taken together, these data suggest that PMd-cck cells facilitate context-specific escape by controlling the behavioral, psychological, and physiological responses associated with threat exposure.
Wang et al. (2021) then used open-source miniature microscopes to perform deep-brain imaging of calcium activity to determine the neuronal correlates of escape in the PMd. Neural states were defined in an unbiased way by using an unsupervised hidden Markov model, and these states were remarkably accurate in predicting escape. Further analysis of these data demonstrated that PMd-cck cells in general were highly active before, during and after escape in all three threat assays. However, in neurons specifically activated by escape, the proportion of peaks occurring prior to escape onset was higher for the rat exposure and heated floor assays than during CO2 exposure. Thus, PMd-cck escape-specific cells are more engaged prior to context-specific escapes than to panic-related jumping. While convincing, these data analyses do not reveal the extent to which the same neurons are activated by distinct forms of threat. This information would reveal how PMd neuronal subpopulations are recruited under various escape responses and could be addressed by registering cells across sessions in future experiments.
The next important aspect in this study was to investigate how the PMd-cck population influences downstream nuclei to orchestrate escape responses. The authors found that 94% of PMd-cck cells are connected to the dlPAG via monosynaptic glutamatergic excitatory projections. A smaller fraction of PMd neurons project only to the amv, and a large population (98%) of the amv-projecting neurons send collaterals to the dlPAG. A dual-site fiber photometry approach was used to simultaneously record Ca2+ activity in PMd-cck neurons and, in separate groups, also the dlPAG or amv. Remarkably, neuronal activity in the PMd and dlPAG was strongly correlated during all types of threat compared to control conditions, while PMd and amv activity was significantly correlated only under conditions requiring context-specific escape. This suggests that the PMd→dlPAG pathway may be involved in executing generalized threat-induced flight, while the PMd→amv pathway operates under only spatial navigation-assisted escape behaviors.
The authors tested this hypothesis by using fiber photometry to record Ca2+ transients in PMd-cck axonal terminals in either the dlPAG or amv. Consistent with the dual-site correlational analysis, PMd-cck axons in the amv were activated during the rat exposure and heated plate assays, but not during CO2 exposure. Axons terminating in the dlPAG, however, were activated during escape in all assays. Finally, optogenetic inhibition at PMd→dlPAG terminals decreased escape responses induced by all threats, while inhibition of PMd→amv terminals decreased escape in the rat and heated floor assays, without altering stereotyped jumping in the CO2 assay.
The findings presented in this novel study clearly demonstrate that the PMd is a critical node in a brain-wide network controlling escape strategies, especially those that require spatial navigation. Prior studies have examined the role of neuronal circuits in the central nucleus of amygdala (Fadok et al., 2017), thalamic reticular nucleus (Dong et al., 2019), and the ventromedial hypothalamus (Wang, Chen and Lin, 2015) in the control of simple, or panic-like, flight responses. Additionally, other regions of the hypothalamus, which facilitate escape jumping and avoidance, are connected to the PMd (Canteras et al., 2008). Future studies will need to investigate whether these regions might coordinate with the PMd to elicit diverse escape responses in complex environments.
The current study by Wang et al. investigated unconditioned responses to innately threatening stimuli; however, animals must also use learned sensory information and past experience to make survival decisions. It would be interesting to know how PMd circuits operate in more familiar environments, especially those in which fear learning has occurred. Furthermore, wild animals must make a series of spatial navigation assessments while escaping threat, thus, it would be interesting to test how the PMd coordinates escape requiring a complex series of decisions. Finally, because PMd stimulation induces negative valences states, it might also be involved in preflight risk assessment behavior, approach-avoidance conflict, and other behaviors indicative of a negative valence state.
References
- Blank DA (2018) ‘Escaping behavior in goitered gazelle’, Behavioural Processes. Elsevier, 147(December 2017), pp. 38–47. doi: 10.1016/j.beproc.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Canteras NS et al. (2008) ‘Sensing danger through the olfactory system: The role of the hypothalamic dorsal premammillary nucleus’, Neuroscience and Biobehavioral Reviews, 32(7), pp. 1228–1235. doi: 10.1016/j.neubiorev.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Dong P. et al. (2019) ‘A novel cortico-intrathalamic circuit for flight behavior’, Nature Neuroscience. Springer US, 22(6), pp. 941–949. doi: 10.1038/s41593-019-0391-6. [DOI] [PubMed] [Google Scholar]
- Fadok JP et al. (2017) ‘A competitive inhibitory circuit for selection of active and passive fear responses’, Nature. Nature Publishing Group, 542(7639), pp. 96–99. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- Perusini JN and Fanselow MS (2015) ‘Neurobehavioral perspectives on the distinction between fear and anxiety’, Learning and Memory, 22(9), pp. 417–425. doi: 10.1101/lm.039180.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva BA, Gross CT and Gräff J. (2016) ‘The neural circuits of innate fear: Detection, integration, action, and memorization’, Learning and Memory, 23(10), pp. 544–555. doi: 10.1101/lm.042812.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P. et al. (2016) ‘Midbrain circuits for defensive behaviour’, Nature. Nature Publishing Group, 534(7606), pp. 206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen IZ and Lin D. (2015) ‘Collateral Pathways from the Ventromedial Hypothalamus Mediate Defensive Behaviors’, Neuron. Elsevier Inc., 85(6), pp. 1344–1358. doi: 10.1016/j.neuron.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. et al. (2021) ‘Coordination of escape and spatial navigation circuits orchestrates versatile flight from threats’, Neuron. Elsevier Inc., pp. 1–13. doi: 10.1016/j.neuron.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


