Abstract
BACKGROUND
We investigated patient outcomes in relation to their postoperative length of stay after minimally invasive valve surgery.
METHODS
All adults who survived elective, uncomplicated minimally invasive aortic or mitral valve surgery at a single center between 2012 and 2019 were classified by postoperative length of stay: early discharge (≤3 days) or late discharge (>3 days). The trend in early discharge was investigated over the study period, predictors of early discharge were identified using multivariate logistic regression modeling, and 1:1 propensity score matching was used to determine which patients in the late-discharge cohort had similar health to patients discharged early. Adjusted outcomes of 30-day mortality, readmission, and direct costs were analyzed.
RESULTS
Among 1262 consecutive patients undergoing minimally invasive valve surgery, 618 were elective and uncomplicated, 25% (n = 162) of whom were discharged early. The proportion of early-discharge patients increased over time (P for trend < .05). A history of congestive heart failure, stroke, or smoking and higher Society of Thoracic Surgeons predictive risk of mortality score negatively predicted early discharge (P < .05). Propensity score matching identified 101 (22%) late-discharge patients comparable with early-discharge patients. Adjusted 30-day mortality and readmission rates were comparable between cohorts. The median direct costs per patient ($20,046 vs $22,124, P < .05) were significantly lower in the early-discharge cohort.
CONCLUSIONS
In well-selected patients early discharge after minimally invasive valve surgery was associated with lower costs but comparable postoperative outcomes. About one-fifth of patients who remain in the hospital beyond postoperative day 3 may be candidates for earlier discharge.
Minimally invasive heart valve surgery has gained interest over the past decade because of improved perioperative outcomes including lower rates of postoperative complications, enhanced recovery, and superior cosmesis when compared with the conventional full sternotomy approach.1-5 Simultaneously implementation of Enhanced Recovery After Surgery (ERAS) protocols in the field of cardiac surgery (ERAS-C) has introduced opportunities for a higher quality perioperative experience and postoperative recovery for the surgical patient. Within ERAS-C programs patients are afforded decreased rates of postoperative complications, shorter durations of mechanical ventilation, improved postoperative gastrointestinal function, shorter intensive care unit stays, and fewer postoperative days until readiness for hospital discharge.6-9
Of the 3 studies reporting on reduced length of hospital stay after minimally invasive valve surgery in the context of ERAS-C protocol implementation, all concluded that the average patient is discharged between postoperative days 6 and 7.7,10,11 However investigations of outcomes for patients who are discharged within the first 3 days of recovery after minimally invasive aortic or mitral valve surgery are limited. The purpose of this study was to determine whether the rapid adoption of minimally invasive approaches to heart valve surgery concomitant with protocols that appropriately prioritize reduced postoperative length of hospital stay engender comparable short-term patient outcomes.
PATIENTS AND METHODS
This is a retrospective cohort study of adult patients (aged ≥ 18 years) who underwent isolated minimally invasive aortic or mitral valve surgery between January 2012 and December 2019 at a single academic medical center (Robert Wood Johnson University Hospital, New Brunswick, NJ). This center has an average annual surgical volume of 15,000 cases performed in 22 operating rooms; approximately 1600 are cardiac surgical procedures, with 1400 open cases and 200 transcatheter valves per year. All surgeries included in the study were performed by either of 2 cardiac surgeons (H.I. and L.Y.L.). The data source for the study was the cardiac surgery database of the academic medical center, developed according to The Society of Thoracic Surgeons (STS) Adult Cardiac Database version 2.81 definitions. Our database prospectively captures data on patient demographics, pre- and intraoperative clinical characteristics, and postoperative outcomes both in-hospital and after discharge. The study was approved by the Institutional Review Board at Rutgers Robert Wood Johnson Medical School.
Minimally invasive valve surgery patients included those who underwent aortic valve replacement or mitral valve repair or replacement through partial sternotomy or minithoracotomy. Patients were selected for minimally invasive valve surgery versus conventional full sternotomy based on shared decision-making between the surgeon and the patient. Patient characteristics such as body habitus did not preclude them from minimally invasive candidacy. Concomitant procedures requiring full sternotomy exposure, such as coronary artery bypass grafting or ascending aortic arch repair, eliminated the option of a minimally invasive approach; however such patients were dually excluded from the study because of failure to meet the inclusion criterion of an isolated valve procedure. Patients who were not admitted electively; encountered postoperative complications including atrial fibrillation, bleeding, echocardiographic valve dysfunction, reoperation, deep sternal infection, stroke, prolonged mechanical ventilation, or acute kidney injury; or died during hospitalization were excluded.
The study population was divided into 2 groups according to postoperative length of stay. Patients who were discharged ≤ 3 days after their operation were classified as early discharge, whereas those who stayed > 3 days were classified as late discharge. Three postoperative days was chosen based on the median length of stay after the implementation of an ERAS-C protocol at our center.
The temporal trend in minimally invasive valve surgical case volume and the incidence of early discharge across the 8-year study period were investigated. The distribution of postoperative length of stay across the early and late halves of the study period, reflecting eras before and after implementation of an ERAS-C protocol, was described. Baseline demographic and clinical characteristics were retrieved and compared between patients of the early- and late-discharge cohorts. Independent predictors of early discharge were identified through statistical modeling. Patient outcomes of all-cause mortality within 30 days of surgery, readmission by postoperative day 30, and direct procedure costs were evaluated. Direct costs were defined as all costs associated with the surgical procedure and perioperative hospital stay, including operative room time, supplies used in the operating room, recovery unit, cardiovascular intensive care unit, hospital floor supplies, direct labor, and room and board. Finally we calculated the proportion of patients in the late-discharge cohort of similar health to early-discharge patients to estimate the proportion of the population that could have been discharged earlier after surgery; the trend in this proportion was plotted over time.
Upon statistical analysis, continuous and categorical variables are presented as medians with corresponding interquartile ranges (IQR; 25th to 75th percentiles) and frequencies with corresponding percentages, respectively, and compared using the Mann-Whitney or χ2 and Fisher’s exact tests. A stepwise logistic regression model with backward selection using the minimum Akaike information criterion was developed to identify predictors of early discharge. Variables included in the model were age, gender, race, STS predictive risk of mortality (PROM), current cigarette smoking, diabetes mellitus, hypertension, hyperlipidemia, chronic kidney disease requiring hemodialysis, congestive heart failure, diagnosed cerebrovascular disease, and history of stroke. An odds ratio (OR) and its 95% confidence interval (CI) were established for each covariate.
To identify a group of patients of similar health to those with early discharge, the resulting logistic equation was used to calculate the probability of each patient from the late-discharge cohort being discharged early. Using the propensity score early-discharge patients were matched 1:1 to late-discharge patients using a greedy matching strategy. The adequacy of the propensity model was checked by comparing covariates before and after matching. A standardized difference of <10% between the 2 groups indicated a successful match. The Cochrane-Armitage test was used to determine the significance of the 8-year trends in early discharge and in the proportion of late-discharge patients who matched to early-discharge counterparts.
Statistical significance was accepted at P < .05. The JMP statistical software version 15.0 (SAS Institute Inc, Cary, NC) and Stata statistical software version 16.0 (Stata Corp LLC, College Station, TX) were used for the analysis.
RESULTS
During the study period 1262 patients underwent minimally invasive aortic or mitral valve surgery. Six hundred forty-four patients, including 353 nonelective, 257 complicated, and 34 who died before hospital discharge, were excluded from the study. The final study cohort consisted of 618 patients of whom 162 (26.2%) were discharged early. Sixty-three percent (n = 128) of cases were performed through a right minithoracotomy and 37% (n = 74) through a partial sternotomy. The median length of stay was 3 days (IQR, 2-3) versus 5 days (IQR, 4-6) for the early- and late-discharge cohorts, respectively (P < .05).
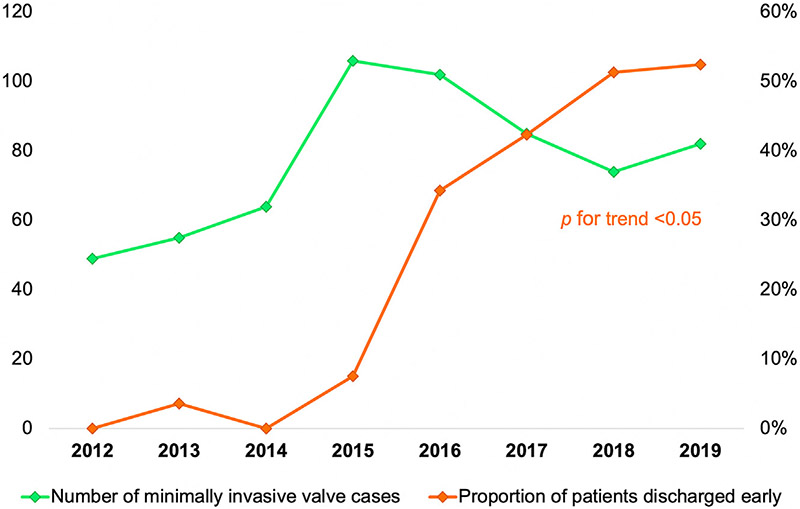
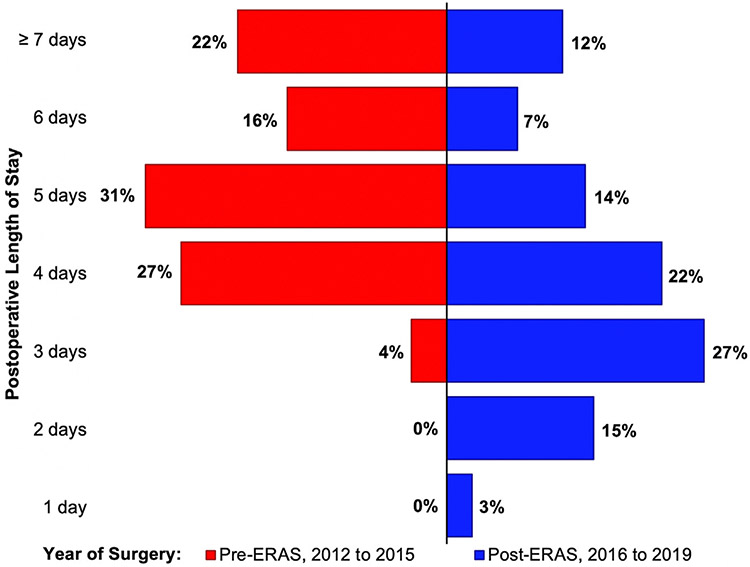
Minimally invasive valve surgical case volume tended to increase over the study period. In 2016 the incidence of early discharge after minimally invasive valve surgery increased abruptly; the proportion of patients discharged early continued to increase each year thereafter (P for trend < .05) (Figure 1). Relative to the early years of the study period before ERAS-C protocol implementation, postoperative length of stay tended toward fewer hospital days during the latter half of the study period (Figure 2).
FIGURE 1.
Eight-year trend in the elective, uncomplicated, minimally invasive left heart valve surgical volume (left-sided y-axis) and proportion of these patients discharged early (≤3-day postoperative length of stay; right-sided y-axis) at a single academic medical center.
FIGURE 2.
Proportion of minimally invasive valve surgery patients distributed by postoperative length of stay, comparing operations performed between 2012 and 2015 (before implementation of an Enhanced Recovery After Surgery [ERAS] protocol) with those performed between 2016 and 2019 (after the implementation of this protocol).
Baseline and predischarge characteristics of the study cohorts are presented in Table 1. Before matching early-discharge patients were younger (67 vs 69 years, P < .05) and less likely to have a history of atrial fibrillation, congestive heart failure, stroke, or hypertension (P < .05). The patients discharged early also had lower STS PROM scores (1.02% vs 1.72%, P < .05). After propensity score matching within the total study population, 101 patients were in each discharge cohort with no differences in baseline demographic or clinical characteristics (Table 1).
TABLE 1.
Baseline Demographic and Clinical Characteristics of Minimally Invasive Valve Surgery Patients and Types of Valve Procedures Performed
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Patient Characteristics | Early Discharge (≤3 Days Postoperatively) (n = 162) |
Late Discharge (>3 Days Postoperatively) (n = 455) |
P | Early Discharge (≤3 Days Postoperatively) (n = 101) |
Late Discharge (>3 Days Postoperatively) (n = 101) |
P |
| Demographics | ||||||
| Age, y | 67 [58-73] | 69 [62-79] | <.001 | 68 [58-74] | 66 [61-74] | .75 |
| Male sex | 86 (53.1) | 245 (53.9) | .87 | 62 (61.4) | 63 (62.4) | .89 |
| Non-White race | 30 (18.5) | 88 (19.3) | .82 | 15 (14.9) | 18 (17.8) | .57 |
| Comorbidities | ||||||
| Atrial fibrillation | 35 (21.6) | 155 (34.2) | .003 | 22 (21.8) | 25 (24.8) | .62 |
| Body mass index | 27.2 [24.2-31.4] | 28.3 [24.4-32.1] | .14 | 27.3 [24.6-32.8] | 27.3 [23.8-31.0] | .65 |
| Chronic kidney disease requiring dialysis | 1 (0.6) | 12 (2.6) | .20 | 0 (0) | 2 (2.0) | .50 |
| Congestive heart failure | 21 (13.0) | 137 (30.1) | <.001 | 13 (12.9) | 16 (15.8) | .55 |
| Cigarette smokinga | 5 (5.0) | 36 (9.0) | .19 | 5 (5.0) | 4 (4.0) | >.99 |
| Diabetes mellitus | 34 (21.0) | 123 (27.0) | .13 | 24 (23.8) | 23 (22.8) | .87 |
| Hyperlipidemia | 121 (74.7) | 352 (77.4) | .49 | 71 (70.3) | 77 (76.2) | .34 |
| Hypertension | 120 (74.1) | 373 (82.0) | .03 | 82 (81.2) | 82 (81.2) | >.99 |
| Prior stroke | 3 (1.85) | 33 (7.25) | .01 | 2 (2.0) | 1 (1.0) | >.99 |
| Society of Thoracic Surgeons predictive risk of mortality score, % | 1.02 [0.63-1.69] | 1.72 [0.92-3.37] | <.001 | 1.02 [0.66-1.68] | 1.20 [0.70-1.98] | .29 |
| Valve procedure | .04 | .87 | ||||
| Aortic valve replacement | 109 (67.3) | 307 (67.5) | 72 (71.3) | 69 (68.3) | ||
| Mitral valve replacement | 8 (4.9) | 50 (11.0) | 3 (3.0) | 4 (4.0) | ||
| Mitral valve repair | 45 (27.8) | 98 (21.5) | 26 (25.7) | 28 (27.7) | ||
Current cigarette smoking at time of preoperative clinic visit. Values are median [interquartile range] or n (%).
Through multivariate logistic regression modeling a history of congestive heart failure (OR, 0.35; 95% CI, 0.21-0.56; P < .05), prior stroke (OR, 0.24; 95% CI, 0.06-0.69; P < .05), current cigarette smoking (OR, 0.59; 95% CI, 0.41-0.86; P < .05), and higher STS PROM score (OR, 0.69; 95% CI, 0.59-0.77; P < .05) were associated with lower odds of early discharge. During the study period no 30-day postoperative deaths occurred in either cohort. Before matching, rates of readmission at postoperative day 30 (10.5% vs 11.0%, P = .86) did not differ between early- and late-discharge cohorts, and these similarities persisted after matching in all patients (13.9% vs 13.9%, P > .99) and matching within procedure-specific subpopulations for those undergoing aortic valve replacement (16.7% [n = 12] vs 14.5% [n = 10], P = .72) or mitral valve surgery (6.9% [n = 2] vs 12.5% [n = 4], P = .67). Conversely median direct costs per patient ($19,732 [IQR, 17,291-21,632] vs $22,481 [IQR, 19,593-25,962]) were significantly lower for those with early discharge than those who were discharged late (P < .05). The differences in cost persisted after propensity score matching in all patients ($20,046 [IQR, 17,307.05-21,336.99 vs $22,124 [IQR, 18,249-24,769], P < .05) and in those who underwent mitral valve surgery ($15,881 [IQR, 14,855-17,986] vs $21,340 [IQR, 17,272-24,016], P < .05) or aortic valve replacement ($20,654 [IQR, 19,239-22,144] vs $22,332 [IQR, 19,897-25,186], P < .05).
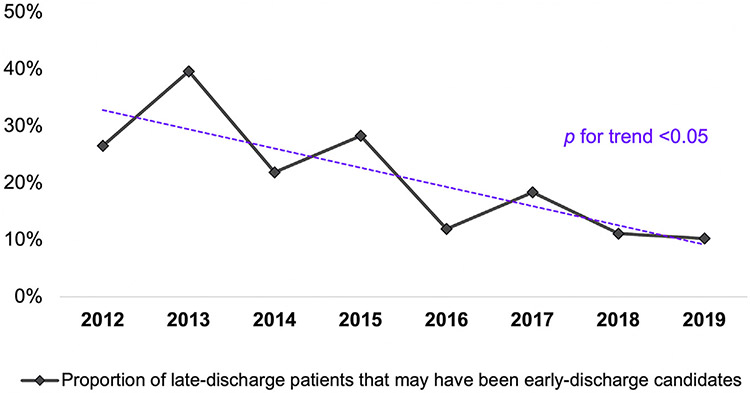
After propensity score matching 101 patients (22%) in the late-discharge cohort had similar baseline characteristics as those in the early-discharge cohort. Stratified by study year this proportion decreased over time (P for trend < .05) (Figure 3).
FIGURE 3.
Eight-year change in the proportion of all patients discharged late (>3 days postoperatively) after minimally invasive valve surgery whose baseline demographic and clinical characteristics matched those of early-discharge (≤3 days postoperatively) patients.
COMMENT
Findings from this single-center study of patients who underwent minimally invasive aortic or mitral valve surgery between 2012 and 2019 exhibit a growing proportion of patients discharged within a few days of their operation. The model produced by logistic regression showed higher STS PROM scores to be associated with lower odds of early discharge, supporting the utility of the STS risk score not only for prediction of perioperative mortality but also of readiness for timely discharge after minimally invasive valve surgery. Propensity score matching by demographic and clinical characteristics demonstrated early discharge to be safe, with no greater readmission rates, and lower cost as compared with late discharge after the third postoperative day. After implementing an ERAS-C protocol in 2016, patients of similar health to those discharged early less often experienced a prolonged postoperative hospital stay.
Before discharge after valve surgery patients at our study center must meet the following criteria: no new dysrhythmia, or if a new atrial fibrillation occurs the rhythm is controlled and the patient anticoagulated; no oxygen dependency; able to tolerate physical activity commensurate with activities of daily living; and a clear chest x-ray. The trend toward a shorter length of stay after minimally invasive cardiac surgery reflects clinical practice changes implemented at our study center in 2016, whereas simultaneous shifts toward reducing postoperative hospital stays have been rather ubiquitous across cardiac surgery centers. Fast-track protocols in cardiac surgery as early as 1994 were followed by the adoption of ERAS protocols at numerous cardiac surgery centers worldwide in the late 2010s.6,12 At our center the integration of ERAS-C elements into the perioperative care of cardiac surgery patients began in 2016. This called on the multidisciplinary cardiac surgical care team and included practice changes such as regional nerve blocks for multimodal pain control, immediate extubation on the operating room table, minimal use of invasive lines, 6-hour transfer from the intensive care unit to the floor, and early ambulation.
In 2018 Li and colleagues8 conducted the only prospective randomized clinical trial of an ERAS-C protocol. Defining readiness for discharge as a stable cardiac rhythm, adequate pain control, tolerating a diet and mobilization, passing stool and urine, without chest tubes and with uncomplicated wound healing, normal laboratory tests, normothermia, and echocardiographic confirmation of good valvular function, this trial found ERAS-C to be associated with a shorter postoperative timeline to discharge readiness; however, the trial failed to find an association between ERAS-C and reduced postoperative length of hospital stay.8 A systematic review of fast-track protocols in cardiac surgery by Wong and colleagues13 produced similarly negative results, finding no significant reduction in length of hospital stay. Furthermore although the literature has yet to describe the effect of condensed discharge timelines on patient outcomes after minimally invasive valve surgery, Cowper and associates14 reported no greater rates of 60-day readmission or mortality in patients discharged early (3-6 days postoperatively) after coronary artery bypass grafting.
Our study thus highlights 2 important findings. First early discharge within 3 days after minimally invasive valve surgery is not only feasible but is reaching predominance, indicated by more than half of patients discharged on this postoperative timeline in the years after ERAS-C protocol implementation. Second our study suggests that this discharge timeline is accompanied by no greater short-term morbidity yet offers the advantage of greater affordability. A safe ≤3-day discharge timeline approaches that reported after transcatheter valve procedures, therefore making minimally invasive surgery a more comparable treatment option for patients with valvular pathology.15
Our patients discharged within 3 days of surgery encountered significantly lower costs than their matched late-discharge counterparts. This cost difference of approximately 2000 US dollars per patient is likely attributable to greater resource utilization during additional days on the hospital floor for late-discharge patients. Although previous reports have estimated a similar cost reduction for valve surgery performed through a minimally invasive incision relative to a full sternotomy, the relationship between cost and early discharge has not been well described in the minimally invasive valve population.16
Scrutinizing the propensity score-matched study cohorts revealed that about one-fifth of patients (22%) who remained in the hospital beyond postoperative day 3 may have been candidates for early discharge. In addition the distribution of these late-discharge patients across the study years depicts the persistence of these likely missed opportunities for expedited discharge to home (Figure 3). Our results presented here suggest that a large proportion of patients discharged late before ERAS-C protocol implementation in 2016 could have had reduced lengths of stay without compromise to their postoperative recovery. After the integration of a formal ERAS-C protocol in 2016 we observed stable global minima in the proportions of patients with what may have been unnecessarily prolonged postoperative lengths of stay. Expectedly this temporality mirrors the spike in crude incidence of early discharge; we propose that the common practice of early discharge enabled appropriate, timely discharge timelines for more patients (Figure 1). Optimizing postoperative hospital stay is important to clinical practice because of comparable outcomes paired with lower costs; so too, patients should be shielded from undue risks of hospitalization, such as nosocomial infection and postoperative delirium.17,18
There are several limitations to this study. First its retrospective, observational design may endure inherent bias through the lack of randomization, control group, and a priori data field selection. A future prospective study conducted with a sample size in accordance with an a priori power calculation would allow for the detection of more minute differences in postoperative outcomes between cohorts. Second the primary outcome of 30-day mortality could not be compared between cohorts because of low event rates in the study population. A multicenter study of a larger population would allow scrutiny of the effect of early discharge on short-term mortality after minimally invasive valve surgery.
In conclusion reduced postoperative length of stay is becoming increasingly common at cardiac surgery centers in tandem with the rise in prevalence of minimally invasive valve surgery and ERAS-C protocols. The patients in this study faced no excess short-term morbidity but had greater affordability of care with expedited discharge timelines. We suggest that early discharge within 3 days of minimally invasive valve surgery is safe and feasible in well-selected patients. In addition after the implementation of an ERAS-C protocol, early-discharge rates increased, and patients appropriate for early discharge more often experienced a postoperative hospital stay congruent with this timeline. We should strive to afford patients this opportunity for a high-quality, efficient postoperative recovery, a pursuit that may be facilitated by the continuation of ERAS-C protocols. Future studies are warranted to understand the definitive role of ERAS-C in expedited discharge timelines, how such timelines affect postdischarge postoperative mortality, early-discharge patient selection, and patient preferences and quality of life specific to length of hospital stay after minimally invasive valve surgery.
ABTS Announcement for Maintenance of Certification.
The American Board of Thoracic Surgery’s Maintenance of Certification (MOC) program was adopted 14 years ago. Since that time, the Board has continuously evaluated the overall process, based upon internal discussions and input from our Diplomates.
The input resulted in our decision to migrate from a purely knowledge-based, multiple-choice exam at a Pearson Testing Center to a mastery learning process using an online SESATS format. Diplomates enrolled in the 10-year MOC process now fulfill their Part III requirement by following the instructions on the ABTS website and conveniently completing the exam online at their home or office. Diplomates choose their exam module (General Thoracic, Adult Cardiac, Cardiothoracic, or Congenital) by indicating their preference within the 10-year online application.
The exam is tailored to one’s practice—for example, if your practice is 100% adult cardiac, you may choose the Adult Cardiac Exam, which will only have adult cardiac and some critical care questions on your exam. The MOC exam is composed of 100 multiple-choice questions. The Board and MOC Committee believe that reading the critique provided after each question is key to the learning process.
Diplomates with approved applications will be able to take the MOC exam online during the months of September and October 2021. For those Diplomates who have used SESATS in the past, the process of working through the questions is the same. While SESATS is a helpful resource, it is not required.
The goal of this exam is to provide a learning opportunity using judgment, knowledge, and decision-making skills. The Board sincerely hopes that this new MOC exam format is viewed favorably by our Diplomates.
The ABTS staff thank you for embracing the primary principle of MOC—life-long learning, which is consistent with our obligation to the public trust.
Acknowledgments
The authors acknowledge Susette Coyle and Marie Macor from the Rutgers Robert Wood Johnson Medical School, Department of Surgery, Division of Surgical Sciences, for their critical assistance in maintenance of the academic medical center’s cardiac surgery database.
Footnotes
Presented at the Fifty-seventh Annual Meeting of The Society of Thoracic Surgeons, Virtual Meeting, Jan 29-31, 2021.
REFERENCES
- 1.Downs EA, Johnston LE, LaPar DJ, et al. Minimally invasive mitral valve surgery provides excellent outcomes without increased cost: a multi-institutional analysis. Ann Thorac Surg. 2016;102:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang C, Raza S, Altarabsheh SE, et al. Minimally invasive approaches to surgical aortic valve replacement: a meta-analysis. Ann Thorac Surg. 2018;106:1881–1889. [DOI] [PubMed] [Google Scholar]
- 3.Bruno P, Cammertoni F, Rosenhek R, et al. Improved patient recovery with minimally invasive aortic valve surgery: a propensity-matched study. Innovations. 2019;14:419–427. [DOI] [PubMed] [Google Scholar]
- 4.Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2008;34:943–952. [DOI] [PubMed] [Google Scholar]
- 5.Al Otaibi A, Gupta S, Belley-Cote EP, et al. Mini-thoracotomy vs. conventional sternotomy mitral valve surgery: a systematic review and meta-analysis. J Cardiovasc Surg. 2017;58:489–496. [DOI] [PubMed] [Google Scholar]
- 6.Baxter R, Squiers J, Conner W, et al. Enhanced recovery after surgery: a narrative review of its application in cardiac surgery. Ann Thorac Surg. 2020;109:1937–1944. [DOI] [PubMed] [Google Scholar]
- 7.Zaouter C, Oses P, Assatourian S, et al. Reduced length of hospital stay for cardiac surgery-implementing an optimized perioperative pathway: prospective evaluation of an Enhanced Recovery After Surgery program designed for mini-invasive aortic valve replacement. J Cardiothorac Vasc Anesth. 2019;33:3010–3019. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Zhang J, Gan TJ, et al. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: a randomized clinical trial. Eur J Cardiothorac Surg. 2018;54:491–497. [DOI] [PubMed] [Google Scholar]
- 9.Fleming IO, Garratt C, Guha R, et al. Aggregation of marginal gains in cardiac surgery: feasibility of a perioperative care bundle for enhanced recovery in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2016;30:665–670. [DOI] [PubMed] [Google Scholar]
- 10.Petersen J, Kloth B, Konertz J, et al. Economic impact of enhanced recovery after surgery protocol in minimally invasive cardiac surgery. BMC Health Serv Res. 2021;21:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubitz JC, Schulte-Uentrop L, Zoellner C, et al. Establishment of an enhanced recovery after surgery protocol in minimally invasive heart valve surgery. PLoS One. 2020;15:e0231378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman RM, Rousou JA, Flack JE III, et al. Fast-track recovery of the coronary bypass patient. Ann Thorac Surg. 1994;58:1742–1746. [DOI] [PubMed] [Google Scholar]
- 13.Wong WT, Lai VK, Chee YE, Lee A. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database System Rev. 2016;9:CD003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowper PA, DeLong ER, Hannan EL, et al. Is early too early? Effect of shorter stays after bypass surgery. Ann Thorac Surg. 2007;83:100–107. [DOI] [PubMed] [Google Scholar]
- 15.Barbanti M, Capranzano P, Ohno Y, et al. Early discharge after transfemoral transcatheter aortic valve implantation. Heart. 2015;101:1485–1490. [DOI] [PubMed] [Google Scholar]
- 16.Ghanta RK, Lapar DJ, Kern JA, et al. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: a real-world multi-institutional analysis. J Thorac Cardiovasc Surg. 2015;149:1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchita M, Khan SH, Perkins AJ, et al. Perioperative risk factors for postoperative delirium in patients undergoing esophagectomy. Ann Thorac Surg. 2019;108:190–195. [DOI] [PubMed] [Google Scholar]
- 18.Loke HY, Kyaw WM, Chen MIC, Lim JW, Ang B, Chow A. Length of stay and odds of MRSA acquisition: a dose-response relationship? Epidemiol Infect. 2019;147:e223. [DOI] [PMC free article] [PubMed] [Google Scholar]