Abstract
Background
In the United States, influenza activity during the 2021–2022 season was modest and sufficient enough to estimate influenza vaccine effectiveness (VE) for the first time since the beginning of the coronavirus disease 2019 pandemic. We estimated influenza VE against laboratory-confirmed outpatient acute illness caused by predominant A(H3N2) viruses.
Methods
Between October 2021 and April 2022, research staff across 7 sites enrolled patients aged ≥6 months seeking outpatient care for acute respiratory illness with cough. Using a test-negative design, we assessed VE against influenza A(H3N2). Due to strong correlation between influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, participants who tested positive for SARS-CoV-2 were excluded from VE estimations. Estimates were adjusted for site, age, month of illness, race/ethnicity, and general health status.
Results
Among 6260 participants, 468 (7%) tested positive for influenza only, including 440 (94%) for A(H3N2). All 206 sequenced A(H3N2) viruses were characterized as belonging to genetic group 3C.2a1b subclade 2a.2, which has antigenic differences from the 2021–2022 season A(H3N2) vaccine component that belongs to clade 3C.2a1b subclade 2a.1. After excluding 1948 SARS-CoV-2–positive patients, 4312 patients were included in analyses of influenza VE; 2463 (57%) were vaccinated against influenza. Effectiveness against A(H3N2) for all ages was 36% (95% confidence interval, 20%–49%) overall.
Conclusions
Influenza vaccination in 2021–2022 provided protection against influenza A(H3N2)-related outpatient visits among young persons.
Keywords: influenza, vaccination, case control, test-negative
Influenza activity increased during 2021–2022 in the US for the first time since the COVID-19 pandemic, which allowed us to estimate influenza vaccine effectiveness. In the 2021–2022 season, influenza vaccines reduced the risk of influenza A(H3N2)-associated illness by about one-third.
Global influenza activity declined to historically low levels after the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in March 2020. In the United States (US), influenza activity increased in late 2021, with 2 distinct periods of influenza virus circulation. The first period began November 2021 and continued through mid-January 2022. Influenza activity declined from late December 2021 through late January 2022 during the rapid rise in the B.1.1.529 (Omicron) SARS-CoV-2 variant [1, 2]. In the second period, starting mid-January, influenza activity continued at a low level through June 2021, with a second peak from mid-March to mid-May, which was much later than previous influenza seasons. Despite the low to moderate level of influenza activity throughout the season, there were an estimated 8.0–13.0 million influenza illnesses, 3.7–6.1 million influenza-related medical visits, 82 000–170 000 hospitalizations, and 5000–14 000 influenza-associated deaths during 2021–2022 [3].
Annual influenza vaccination starting at 6 months of age is recommended in the US as the most effective means of mitigating and preventing influenza-associated illnesses and complications. Influenza vaccination is especially important during the ongoing coronavirus disease 2019 (COVID-19) pandemic to reduce the burden of medical visits and hospitalizations due to respiratory illness [4]. However, in November 2021, early influenza activity suggested potentially reduced vaccine effectiveness (VE) against predominant A(H3N2) viruses belonging to the 3C.2a1b.2a.2 subclade [1, 5]. Low global influenza circulation during the 2020–2021 influenza season limited selection of vaccine reference viruses for the 2021–2022 Northern Hemisphere influenza season [6]. Since 2004–2005, the Centers for Disease Control and Prevention (CDC) and collaborating study sites have produced annual estimates of influenza VE against laboratory-confirmed, mild to moderate (outpatient) medically attended acute respiratory infection (ARI) for every season except 2020–2021 due to very low influenza activity. Here, we report VE estimates for the 2021–2022 season against A(H3N2)-associated influenza illness, including VE estimates by age group and early- versus late-season influenza activity.
METHODS
Study Population
This study was conducted within the US Flu VE Network, which consists of participating health systems in 7 states: California, Michigan, Pennsylvania, Tennessee, Texas, Washington, and Wisconsin. Details of this network have been described previously [1, 7]. Between 4 October 2021 and 30 April 2022, research staff screened patients aged ≥6 months who had ARI with cough, fever/feverishness, or loss of taste or smell seeking outpatient medical care (ie, telehealth, primary care, urgent care, or emergency department) or clinical testing for SARS-CoV-2 ≤10 days after illness onset [8]. Research staff interviewed participants using standard questionnaires for data on patient demographics, symptoms, subjective general health status before illness onset, and self- or parent-reported receipt of the 2021–2022 seasonal influenza vaccine.
At enrollment, study staff collected nasal and/or oropharyngeal swab specimens (only nasal swab specimens were collected for children aged <2 years). Specimens were tested for influenza and SARS-CoV-2 using real-time reverse-transcription polymerase chain reaction (RT-PCR). Influenza-positive specimens with RT-PCR cycle threshold values <30 were sent to CDC for whole genome sequencing [9]. This activity was reviewed and approved by the CDC and each US Flu VE Network site's Institutional Review Board.
Vaccine Effectiveness Estimates
Vaccine effectiveness was estimated from logistic regression models using the test-negative design as 100% × (1 – adjusted odds ratio) [10]. We included participants who reported cough or fever/feverishness and were tested within 7 days of illness onset. Cases were patients testing positive for influenza by RT-PCR, and controls were patients testing negative for both influenza and SARS-CoV-2. Due to strong correlation between influenza and SARS-CoV-2 vaccination, participants who tested RT-PCR positive for SARS-CoV-2 infection were excluded from influenza VE estimation [11]. We conducted a sensitivity analysis including participants who tested positive for SARS-CoV-2.
Influenza vaccination status was determined using documentation from electronic sources, immunization information systems, and self-report of vaccination with a date of administration. Participants were considered vaccinated with receipt of 1 or more doses of any 2021–2022 seasonal influenza vaccine ≥14 days prior to illness onset. Using multivariable logistic regression, odds ratio estimates were adjusted for study site, age, month of illness onset, race/ethnicity, and general health status. A 95% confidence interval (CI) was calculated for each estimate. Stratified analyses were performed by age group where sample size permitted [12]. We also separately examined VE during 4 October 2021–15 January 2022, corresponding to the first period of seasonal influenza activity until widespread circulation of the SARS-CoV-2 Omicron variant, and 16 January–30 April 2022, during the second, extended period of influenza virus circulation. Analyses were conducted using SAS version 9.4 software (SAS Institute) and R software, version 4.0.2 (R Foundation for Statistical Computing).
RESULTS
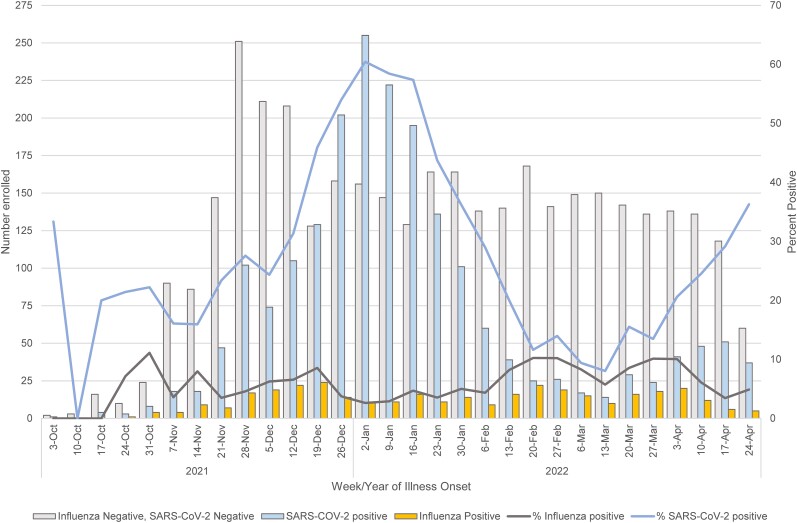
Among 7031 participants with ARI enrolled at the 7 study sites during 4 October 2021–30 April 2022, 787 (11%) were excluded due to influenza vaccination <14 days before illness onset (n = 143), missing testing or vaccination data (n = 628), or co-detection of influenza and SARS-CoV-2 (n = 16). Weekly influenza positivity ranged from 0% to <10% throughout the period; SARS-CoV-2 positivity ranged from 10% to 60%, with a peak in early to mid-January 2022 when the SARS-CoV-2 Omicron variant predominated (Figure 1). Among 6244 participants, 468 (7%) tested positive for influenza, 1948 (31%) tested positive for SARS-CoV-2, and 3844 (62%) tested negative for both influenza and SARS-CoV-2. Among influenza-positive participants, 440 (94%) were subtyped as A(H3N2), 2 (<1%) were A(H1N1)pdm09, and 26 (6%) were influenza A with no subtype result. No influenza B cases were detected. A total of 206 (47%) A(H3N2) viruses were characterized by whole genome sequencing; all belonged to genetic group 3C.2a1b subclade 2a.2 (full clade: 3C.2a1b.2a.2). The median age of influenza-positive cases (19 years) was younger than the median age of SARS-CoV-2–positive participants (37 years) or test-negative controls (33 years) (Table 1). The proportion of patients with influenza differed by study site, sex, age group, race/ethnicity, days from illness onset to enrollment, general health status, and presence of high-risk medical conditions (Table 1).
Figure 1.
Distribution of participants by test result and percentage positivity by week of onset, 4 October 2021–30 April 2022. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Characteristics of Participants With Medically Attended Acute Respiratory Infection by Influenza and Severe Acute Respiratory Syndrome Coronavirus 2 Test Result, 4 October 2021–30 April 2022a
| Characteristic | Influenza Positive (n = 468) |
SARS-CoV-2 Positive (n = 1932) |
Test-Negative Controlsb (n = 3844) |
P Valuec |
|---|---|---|---|---|
| Study site | <.01 | |||
| ȃCalifornia | 12 (2.6) | 274 (14.2) | 479 (12.5) | |
| ȃMichigan | 26 (5.6) | 211 (10.9) | 298 (7.8) | |
| ȃPennsylvania | 43 (9.2) | 193 (10.0) | 463 (12.0) | |
| ȃTennessee | 83 (17.7) | 266 (13.8) | 567 (14.8) | |
| ȃTexas | 144 (30.8) | 200 (10.4) | 797 (20.7) | |
| ȃWashington | 8 (1.7) | 197 (10.2) | 410 (10.7) | |
| ȃWisconsin | 152 (32.5) | 591 (30.6) | 830 (21.6) | |
| Sex | <.01 | |||
| ȃMale | 209 (44.7) | 820 (42.4) | 1478 (38.4) | |
| ȃFemale | 259 (55.3) | 1109 (57.4) | 2362 (61.4) | |
| Age, y, median (IQR) | 19.0 (10.0–35.0) | 37.0 (25.0–52.0) | 33.0 (15.0–51.0) | <.01 |
| Age group | <.01 | |||
| ȃ6 mo–8 y | 102 (21.8) | 107 (5.5) | 622 (16.2) | |
| ȃ9–17 y | 121 (25.9) | 192 (9.9) | 499 (13.0) | |
| ȃ18–49 y | 178 (38.0) | 1071 (55.4) | 1685 (43.8) | |
| ȃ50–64 y | 43 (9.2) | 366 (18.9) | 626 (16.3) | |
| ȃ≥65 y | 24 (5.1) | 196 (10.1) | 412 (10.7) | |
| Race/ethnicity | <.01 | |||
| White, non-Hispanic | 265 (56.6) | 1148 (59.4) | 2179 (56.7) | |
| Black, non-Hispanic | 24 (5.1) | 115 (6.0) | 205 (5.3) | |
| Hispanic, any race | 79 (16.9) | 209 (10.8) | 531 (13.8) | |
| Other, non-Hispanic | 23 (4.9) | 188 (9.7) | 366 (9.5) | |
| Unknown | 77 (16.5) | 272 (14.1) | 563 (14.6) | |
| Illness onset to enrollment, d | <.01 | |||
| <3 | 261 (55.8) | 1053 (54.5) | 1658 (43.1) | |
| 3–4 | 142 (30.3) | 561 (29.0) | 1310 (34.1) | |
| 5–7 | 65 (13.9) | 318 (16.5) | 876 (22.8) | |
| General self-reported health status | <.01 | |||
| Excellent | 168 (35.9) | 471 (24.4) | 1017 (26.5) | |
| Very good | 159 (34.0) | 802 (41.5) | 1459 (38.0) | |
| Good | 115 (24.6) | 526 (27.2) | 1009 (26.2) | |
| Fair/poor | 25 (5.3) | 131 (6.8) | 349 (9.1) | |
| ≥1 high-risk medical condition | <.01 | |||
| ȃNo | 390 (83.3) | 1440 (74.5) | 2876 (74.8) | |
| Yesd | 75 (16.0) | 463 (24.0) | 900 (23.4) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sixteen participants were excluded due to co-detection of influenza and SARS-CoV-2.
Participants who tested negative for both influenza and SARS-CoV-2.
P value from χ2 test comparing proportions by test result.
Self-reported presence of at least 1 high-risk medical condition including heart disease, lung disease, diabetes, cancer, liver or kidney disease, or immunosuppression.
After excluding SARS-CoV-2–positive patients who were tested for influenza, 4312 patients were included in analyses of influenza VE; 2463 (57%) were vaccinated against influenza (Table 2). Of the 2463 vaccinated individuals, 251 (10%) self-reported having received a vaccine that was not documented in available electronic records. Compared to unvaccinated participants, participants who received influenza vaccine were older, more likely to be non-Hispanic white, more likely to have received 3 or more COVID-19 vaccinations, and more likely to report having at least 1 high-risk medical condition (Table 2).
Table 2.
Characteristics of Participants by Influenza Vaccination Status, 4 October 2021–30 April 2022a
| Characteristic | Vaccinateda (n = 2463) | Unvaccinated (n = 1849) | P Valueb |
|---|---|---|---|
| Study site | <.01 | ||
| ȃCalifornia | 333 (13.5) | 158 (8.5) | |
| ȃMichigan | 257 (10.4) | 67 (3.6) | |
| ȃPennsylvania | 278 (11.3) | 228 (12.3) | |
| ȃTennessee | 371 (15.1) | 279 (15.1) | |
| ȃTexas | 351 (14.3) | 590 (31.9) | |
| ȃWashington | 290 (11.8) | 128 (6.9) | |
| ȃWisconsin | 583 (23.7) | 399 (21.6) | |
| Sex | <.01 | ||
| ȃMale | 919 (37.3) | 768 (41.5) | |
| ȃFemale | 1542 (62.6) | 1079 (58.4) | |
| Age, y, median (IQR) | 36.0 (15.0–56.0) | 25.0 (12.0–41.0) | <.01 |
| Age group | <.01 | ||
| ȃ6 mo–8 y | 394 (16.0) | 330 (17.8) | |
| ȃ9–17 y | 255 (10.4) | 365 (19.7) | |
| ȃ18–49 y | 1010 (41.0) | 853 (46.1) | |
| ȃ50–44 y | 454 (18.4) | 215 (11.6) | |
| ȃ≥65 y | 350 (14.2) | 86 (4.7) | |
| Race/ethnicity | <.01 | ||
| White, non-Hispanic | 1483 (60.2) | 961 (52.0) | |
| Black, non-Hispanic | 93 (3.8) | 136 (7.4) | |
| Hispanic, any race | 278 (11.3) | 332 (18.0) | |
| Other, non-Hispanic | 243 (9.9) | 146 (7.9) | |
| Unknown | 366 (14.9) | 274 (14.8) | |
| Illness onset to enrollment, d | .44 | ||
| <3 | 1112 (45.1) | 807 (43.6) | |
| 3–4 | 810 (32.9) | 642 (34.7) | |
| 5–7 | 541 (22.0) | 400 (21.6) | |
| General self-reported health status | .06 | ||
| Excellent | 655 (26.6) | 530 (28.7) | |
| Very good | 942 (38.2) | 676 (36.6) | |
| Good | 637 (25.9) | 487 (26.3) | |
| Fair/poor | 221 (9.0) | 153 (8.3) | |
| ≥1 high-risk medical condition present | <.01 | ||
| ȃNo | 1764 (71.6) | 1502 (81.2) | |
| Yesc | 653 (26.5) | 322 (17.4) | |
| COVID-19 vaccination status | |||
| ȃ0 doses | 211 (8.6) | 666 (36.0) | <.01 |
| 1–2 doses | 652 (26.5) | 679 (36.7) | <.01 |
| ≥3 doses | 1299 (52.7) | 268 (14.5) | <.01 |
| Influenza rRT-PCR result | |||
| ȃNegative | 2265 (92.0) | 1579 (85.4) | <.01 |
| ȃInfluenza A | 198 (8.0) | 270 (14.6) | |
| ȃA(H1N1)pdm09 | 0 (0) | 2 (0.1) | |
| ȃA(H3N2) | 182 (7.4) | 258 (14.0) | |
| ȃInfluenza B | 0 (0) | 0 (0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; rRT-PCR, real-time reverse-transcription polymerase chain reaction.
Participants who tested positive for severe acute respiratory syndrome coronavirus 2 are excluded.
P value from χ2 test comparing proportions by test result.
Self-reported presence of at least 1 high-risk medical condition including heart disease, lung disease, diabetes, cancer, liver or kidney disease, or immunosuppression.
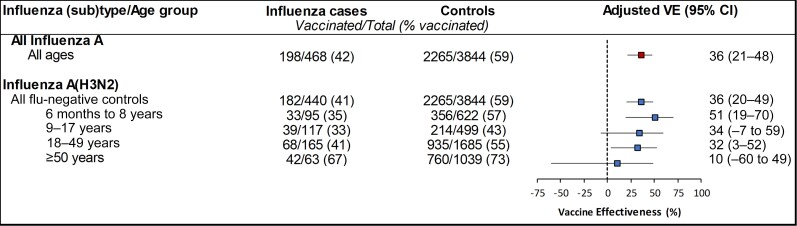
For all ages combined, VE against influenza A was 36% (95% CI: 21%–48%) and 36% (95% CI: 20%–49%) for A(H3N2), specifically. VE against A(H3N2) varied by age from 51% (95% CI: 19%–70%) among patients aged 6 months–8 years, 32% (95% CI: 3%–52%) among adults aged 18–49 years, and 10% (95% CI: −60% to 49%) among adults aged ≥50 years (Figure 2). We were underpowered to detect a statistically significant VE of 30% in all age groups [12], but the number of cases among older adults aged ≥50 years was particularly sparse.
Figure 2.
Vaccine effectiveness against outpatient all influenza A- and A(H3N2)-associated illness, 4 October 2021–30 April 2022. Adjusted for study site, age, month of illness onset, race/ethnicity, and self-rated general health status. Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
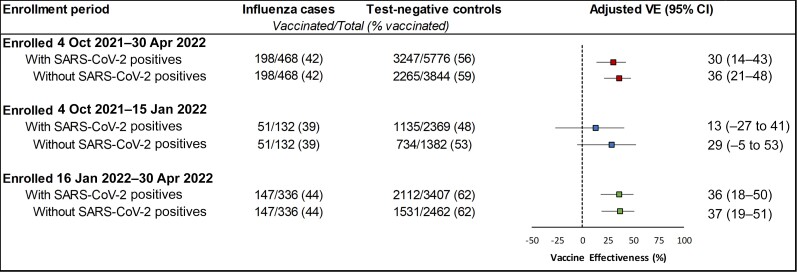
In a sensitivity analysis including participants who tested positive for SARS-CoV-2 at enrollment, VE was slightly lower (30% [95% CI: 14%–43%]) for the full enrollment period. There was no statistically significant difference observed between VE among those enrolled on or before 15 January 2022 (29% [95% CI: −5% to 53%]) compared to those enrolled after 15 January 2022 (37% [95% CI: 19%–51%]). However, VE point estimates slightly increased after SARS-CoV-2 positives were excluded (Figure 3).
Figure 3.
Vaccine effectiveness against outpatient influenza A(H3N2)-associated illness by time since vaccination, 4 October 2021–30 April 2022, United States. Adjusted for study site, age, month of illness onset, race/ethnicity, and self-rated general health status and included all participants aged ≥6 mo. Abbreviations: CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE, vaccine effectiveness.
DISCUSSION
For the 2021–2022 influenza season, influenza A(H3N2) viruses predominated, although virus circulation remained relatively low in comparison to the prevalence of SARS-CoV-2 Omicron variant and subvariants (B.1.1.529/BA.1/BA.2). Influenza vaccines were 36% effective against A(H3N2)-related illnesses among all participants <50 years of age. However, we were unable to detect statistically significant protection against laboratory-confirmed influenza among adults aged 50 years and older. Our findings align with findings from other studies of VE against outpatient outcomes from the 2021-22 season [13, 14]. In general, detection of statistically significant VE below 30% with high vaccine coverage requires a larger sample size than we were able to enroll this season [12].
Lower VE among older adults compared with younger persons has been observed in previous seasons, especially against A(H3N2) viruses [15]. The A(H3N2) reference virus selected for egg-based and cell- or recombinant-based 2021–2022 influenza vaccines, A/Cambodia/e0826360/2020 belonged to clade 3C.2a1b subclade 2a.1, whereas almost all influenza A(H3N2) viruses circulating throughout the US during November 2021–April 2022 belonged to the 3C.2a1b subclade 2a.2. While these 2 subclades were genetically similar, there were antigenic differences detected between circulating viruses and the cell- and egg-grown vaccine component, based on postinfection ferret antibody cross-reactivity [6]. It is unclear whether older adults responded differently than younger persons to the mismatched A(H3N2) vaccine component or if there were differences in cross-protective antibodies. Alternatively, influenza-positive older patients may have differed in unmeasured characteristics from those testing negative for influenza and SARS-CoV-2 in ways that affected VE estimates. It is also unclear whether egg-adapted changes in A(H3N2) viruses used in vaccine production or whether boosting antibodies specific to egg-adapted viruses may have affected VE, although statistically significant VE was observed among young children and adults aged 18–49 years. At the time of this report, we were unable to calculate VE by vaccine type because complete vaccination data including vaccine type were not yet available. In recent influenza seasons, uptake of recombinant influenza vaccine and cell-culture influenza vaccine has increased among US Flu VE Network participants including older adults, who also may receive high-dose egg-based influenza vaccine [16].
SARS-CoV-2–positive participants were excluded from estimates of influenza VE to remove potential bias resulting from positive correlation between COVID-19 vaccination and influenza vaccination [11]. A previous study carried out nearly a decade ago has shown that the test-negative design produced similar estimates of influenza VE when test-negative controls included participants who tested positive for other respiratory viruses [17]. However, in that study, vaccines were not available for the other respiratory viruses detected. In our study, SARS-CoV-2–positive participants were less likely to receive influenza vaccine compared to those who tested negative for SARS-CoV-2. This resulted in lower estimated influenza VE when SARS-CoV-2–positive participants were included. The effect of this bias was greatest during the period of high COVID-19 prevalence. Estimated midseason influenza VE from October 2021 to February 2022 was 14% (95% CI: −17% to 37%) [1]. After removing SARS-CoV-2–positive, influenza-negative controls, we estimate the corrected VE for this period was approximately 30%. Over the entire study period of October 2021–April 2022, removal of SARS-CoV-2–positive participants increased VE point estimates >5% but <10%.
Several limitations of our study should be considered. First, the validity of observational VE studies depends on accurate classification of vaccination status and influenza infection [10]. Vaccination status at 6 of 7 sites included plausible self-report rather than medical record documentation, which might result in misclassification of influenza vaccination status for some patients. Second, healthcare-seeking behavior has changed during the COVID-19 pandemic, and enrollment of patients with outpatient illness from COVID-19 testing sites might have affected results in uncertain ways. The test-negative design for estimating influenza VE requires validation when multiple vaccine-preventable respiratory viruses are co-circulating. Finally, VE estimates in this report are specific to the prevention of outpatient influenza illness rather than to more severe influenza outcomes (eg, hospitalization, intensive care unit admission, or death), which other study designs may be able to address.
In conclusion, influenza vaccination in 2021–2022 reduced outpatient medically attended acute respiratory illness with cough due to influenza A(H3N2) viruses by approximately one-third overall. Protection afforded by vaccination was comparable to previous A(H3N2)-dominant seasons before the COVID-19 pandemic [15]. To provide better antigenic match to the A(H3N2) viruses that circulated during the 2021–2022 season, the A(H3N2) component for 2022–2023 Northern Hemisphere influenza vaccines was updated to include reference viruses representing the 3C.2a1b.2a.2 subclade. If subclade 2a.2-like viruses continue to circulate, the updated A(H3N2) vaccine component representing the 3C.2a1b.2a.2 subclade may provide improved protection among subclade 2a.2-like viruses during the 2022–2023 influenza season.
Contributor Information
Ashley M Price, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Brendan Flannery, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
H Keipp Talbot, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carlos G Grijalva, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Karen J Wernli, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, USA.
C Hallie Phillips, Kaiser Permanente Washington Health Research Institute, Seattle, Washington, USA.
Arnold S Monto, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Emily T Martin, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Edward A Belongia, Marshfield Clinic Research Institute, Wisconsin, USA.
Huong Q McLean, Marshfield Clinic Research Institute, Wisconsin, USA.
Manjusha Gaglani, Department of Pediatrics, Baylor Scott & White Health, USA; Department of Medical Education, Texas A&M University College of Medicine, Temple, Texas, USA.
Manohar Mutnal, Department of Pediatrics, Baylor Scott & White Health, USA; Department of Medical Education, Texas A&M University College of Medicine, Temple, Texas, USA.
Krissy Moehling Geffel, Department of Family Medicine, University of Pittsburgh Schools of the Health Sciences and University of Pittsburgh Medical Center, Pittsburgh, Pannsylvania, USA.
Mary Patricia Nowalk, Department of Family Medicine, University of Pittsburgh Schools of the Health Sciences and University of Pittsburgh Medical Center, Pittsburgh, Pannsylvania, USA.
Sara Y Tartof, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California, USA.
Ana Florea, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, California, USA.
Callie McLean, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Sara S Kim, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Manish M Patel, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Jessie R Chung, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. M. G. reports support for this work from the CDC (US Flu VE Network BAA contract). E. T. M., A. S. M., M. P. N., and H. K. T. report a grant in support of this work from the CDC. H. Q. M. reports a research grant from the CDC. C. H. P. and K. J. W. report a contract from the CDC (75D30121C11909) for data collection for this study. S. Y. T. reports grant support paid directly to institution from the CDC.
References
- 1. Chung JR, Kim SS, Kondor RJ, et al. Interim estimates of 2021–22 seasonal influenza vaccine effectiveness—United States, February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022–23 influenza vaccine—United States, 2021–22 season. MMWR Morb Mortal Wkly Rep 2022; 71:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . 2021–2022 U.S. flu season: preliminary in-season burden estimates. 2022. Available at: https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm. Accessed 23 August 2022.
- 4. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Recomm Rep 2021; 70:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delahoy MJ, Mortenson L, Bauman L, et al. Influenza A(H3N2) outbreak on a university campus—Michigan, October–November 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1712–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Recommendations for influenza vaccine composition . Available at: https://www.who.int/teams/global-influenza-programme/vaccines/who-recommendations. Accessed 23 August 2022.
- 7. Kim SS, Chung JR, Talbot HK, et al. Effectiveness of two and three mRNA COVID-19 vaccine doses against Omicron- and Delta-related outpatient illness among adults, October 2021–February 2022. Influenza Other Respir Viruses 2022; 16:975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung JR, Kim SS, Jackson ML, et al. Clinical symptoms among ambulatory patients tested for SARS-CoV-2. Open Forum Infect Dis 2021; 8:ofaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 11. Doll MK, Pettigrew SM, Ma J, Verma A. Effects of confounding bias in COVID-19 and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis 2022; 75:e564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung JR, Flannery B, Kim SS, et al. Sample size considerations for mid-season estimates from a large influenza vaccine effectiveness network in the United States. Vaccine 2021; 39:3324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S, Chuang ES, Sabaiduc S, et al. Influenza vaccine effectiveness against A (H3N2) during the delayed 2021/22 epidemic in Canada. Euro Surveill 2022; 27:2200720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kissling E, Pozo F, Martínez-Baz I, et al. Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021–2022 I-MOVE primary care multicentre study. Influenza Other Respi Viruses 2022; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis 2016; 16:942–51. [DOI] [PubMed] [Google Scholar]
- 16. Wu MJ, Chung JR, Kim SS, et al. Influenza vaccination coverage among persons seeking outpatient medical care for acute respiratory illness in five states in the United States, 2011–2012 through 2018–2019. Vaccine 2021; 39:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57:789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]