Abstract
Background
There are conflicting data regarding baseline determinants of virological nonsuppression outcomes in persons with human immunodeficiency virus (HIV) starting antiretroviral treatment (ART). We evaluated the impact of different baseline variables in the RESPOND cohort.
Methods
We included treatment-naive participants aged ≥18 who initiated 3-drug ART, in 2014–2020. We assessed the odds of virological suppression (VS) at weeks 48 and 96 using logistic regression. Viral blips, low-level viremia (LLV), residual viremia (RV), and virological failure (VF) rates were assessed using Cox regression.
Results
Of 4310 eligible participants, 72% started integrase strand transfer inhibitor (INSTI)-based regimens. At 48 and 96 weeks, 91.0% and 93.3% achieved VS, respectively. At 48 weeks, Kaplan-Meier estimates of rates were 9.6% for viral blips, 2.1% for LLV, 22.2% for RV, and 2.1% for VF. Baseline HIV-1 RNA levels >100 000 copies/mL and CD4+ T-cell counts ≤200/µL were negatively associated with VS at weeks 48 (adjusted odds ratio, 0.51 [95% confidence interval, .39–.68] and .40 [.27–.58], respectively) and 96 and with significantly higher rates of blips, LLV, and RV. CD4+ T-cell counts ≤200/µL were associated with higher risk of VF (adjusted hazard ratio, 3.12 [95% confidence interval, 2.02–4.83]). Results were consistent in those starting INSTIs versus other regimens and those starting dolutegravir versus other INSTIs.
Conclusions
Initial high HIV-1 RNA and low CD4+ T-cell counts are associated with lower rates of VS at 48 and 96 weeks and higher rates of viral blips, LLV, and RV. Low baseline CD4+ T-cell counts are associated with higher VF rates. These associations remain with INSTI-based and specifically with dolutegravir-based regimens. These findings suggest that the impact of these baseline determinants is independent of the ART regimen initiated.
Keywords: blip, low-level viremia, residual viremia, virological failure, integrase inhibitors
High baseline human immunodeficiency virus RNA levelsand low baseline CD4 T-cell counts are associated with higher rates of viral blips, low-level viremia, and residual viremia in people treated with integrase inhibitors or specifically with dolutegravir-based regimens, suggesting an independent impact of these characteristics.
Antiretroviral treatment (ART) durably suppresses plasma human immunodeficiency virus (HIV) type 1 RNA to <50 copies/mL [1]. Virological nonsuppression outcomes including viral blips, persistent low-level viremia (LLV), residual viremia (RV), and virological failure (VF) hamper ART efficacy and may enable the selection of antiretroviral resistance and allow its transmission [2].
The lack of standardized definitions for LLV, VF [2–11], and RV [12, 13] has hindered the identification of baseline surrogate markers, with discordant results. The US Department of Health and Human Services guidelines define LLV as confirmed detectable HIV-1 RNA <200 copies/mL, VF as a confirmed viral load ≥200 copies/mL, and a viral blip as an isolated quantifiable HIV-1 RNA preceded and followed by virological suppression (VS) [14].
Virions can still be produced during ART-mediated suppression, with plasma HIV-1 RNA levels <20–50 copies/mL [15]. It is unclear whether this RV results from a combined or separated process of virus production by latently or long-lived HIV-infected cells and/or from virus replication in lymphoid tissue sanctuary sites. Some studies point to a relationship between pre-ART HIV-1 RNA, the size of established HIV-DNA reservoirs and the subsequent release of this detectable and persistent HIV-1 RNA in plasma during ART [16–20]. Viral blips could reflect the size of the reservoir [18, 21] and could predict LLV [18]. In addition, RV has been associated with viral blips and LLV [12]. Intriguingly, in some cohort studies, LLV with HIV-1 RNA 200–499 copies/mL was associated with increased risk of VF [5, 6, 9], whereas in those with LLVs of 50–199 copies/mL, this association was inconsistent [2–6, 11]. There is also discordance in the association between blips and VF [2, 13, 22–24].
Using data from a prospective multinational cohort consortium, we aimed to examine baseline factors associated with virological nonsuppression outcomes (blips, LLV, RV, and VF) in treatment-naive persons with HIV (PWH) who started a 3-drug ART regimen in the integrase strand transfer inhibitor (INSTI) era.
METHODS
Study Design and Data Sources
The International Cohort Consortium of Infectious Diseases (RESPOND) is a collaboration among 19 cohorts from Europe and Australia, using the HIV Cohorts Data Exchange Protocol for data collection (details at https://hicdep.org/) [25]. Clinical and demographic data were collected retrospectively back to 2012 at RESPOND enrollment and prospectively since 2017.
Study Population
Participants consented to share data according to local requirements. All cohorts had approval to share data with RESPOND according to national requirements. We included all ART-naive adults aged ≥18 years who started ART between 1 January 2014 and 31 December 2020, from 17 of 19 cohorts. Participants had a CD4+ T-cell count measured and a detectable plasma HIV-1 RNA value at ART initiation and a minimum follow-up time of 36 weeks.
Virological Outcome Definitions
VS was defined as HIV-1 RNA levels <50 copies/mL at weeks 48 and 96, with a 12-week window on either side; LLV, as the first of ≥ 2 consecutive plasma HIV-1 RNA measurements of 50–199 copies/mL, following VS; viral blip, as an isolated plasma HIV-1 RNA level of 50–199 copies/mL with previous and subsequent HIV-1 RNA levels <50 copies/mL, following VS; RV, as any detectable and quantifiable plasma HIV-1 RNA between 20 and 49 copies/mL, among participants with a HIV-1 RNA measurement with a limit of detection of 20 copies/mL, following VS; and VF, as the first of 2 consecutive plasma HIV-1 RNA measurements ≥50 copies/mL, with 1 measurement ≥200 copies/mL, following VS.
Statistical Methods
A descriptive analysis of participants’ demographic and immunovirological characteristics at ART initiation was carried out using frequency tables for categorical variables and median and interquartile range (IQR) for continuous variables. The outcomes were assessed in an intention-to-treat-exposed analysis including all participants starting their first ART regimen regardless of subsequent discontinuations and/or switches.
We used a logistic regression model to assess the impact of multiple baseline predictor variables on VS at weeks 48 and 96, expressed as adjusted odds ratios and 95% confidence intervals (CIs). Kaplan-Meier curves were used to estimate the time to viral blips, LLV, RV and VF, stratified by the third drug, and the comparison among curves was performed using log-rank tests. We performed a survival analysis using Cox regression to assess the impact of baseline variables on virological nonsuppression outcomes (ie, viral blips, LLV, RV, and VF). Associations were expressed as adjusted hazard ratios and 95% CIs.
Baseline variables were defined a priori. Models were adjusted for sex, age, year of ART initiation, race, hepatitis C (hepatitis C virus antibodies), European Australian region, prior AIDS-defining illness, HIV-1 RNA, CD4+ T-cell count and initial ART classes. The latter included: a 2–nucleos(t)ide reverse-transcriptase inhibitor (NRTI) backbone (abacavir-lamivudine, tenofovir disoproxil fumarate [TDF]–emtricitabine, tenofovir alafenamide [TAF]–emtricitabine) plus 1 of the following third agents: cobicistat- or ritonavir-boosted darunavir (protease inhibitor [PI]), rilpivirine (nonnucleoside reverse-transcriptase inhibitor [NNRTI]), and cobicistat-boosted elvitegravir, dolutegravir, or raltegravir (INSTI).
Sensitivity analyses were performed for viral blips, LLV, RV, and VF, restricted first only to participants who started treatment with INSTIs and further restricted to those who started dolutegravir versus other INSTIs. A category was included for missing data for confounders where required. Statistical analysis was performed using SAS software (Statistical Analysis Software), version 9.4. All tests were 2 tailed, and the significance level α was set at .05.
RESULTS
Baseline Characteristics
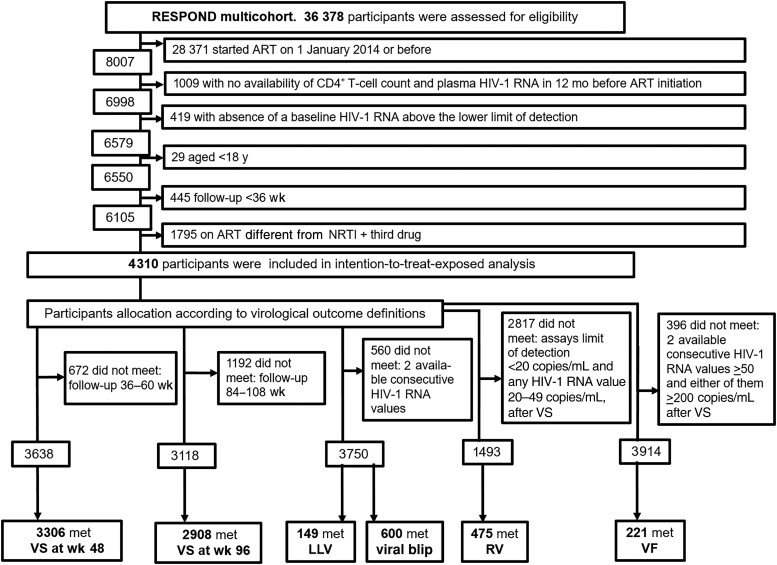
We included 4310 eligible ART-naive participants (Figure 1). Of these, 84% were male, 69.2% were white, 61.2% were men who had sex with men, 42.6% were from Central Europe, 89.8% were without prior AIDS, and 43.3% started ART in year 2014–2015. Their median age (IQR) was 38 ( 30–47) years, and 812 (18.8%) were >50 years old (Table 1). The median follow-up time (IQR) since starting ART was 3.8 (2.4–5.1) years, with 16 106 person-years of follow-up, with a median (IQR) of 8 (5–12) CD4+ T-cell counts and 10 (6–14) HIV-1 RNA measurements.
Figure 1.
International Cohort Consortium of Infectious Diseases (RESPOND) flow chart. Abbreviations: ART, antiretroviral treatment; HIV-1, human immunodeficiency virus type 1; LLV, low-level viremia; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; RV, residual viremia; VF, virological failure; VS, virological suppression.
Table 1.
Baseline Characteristics of Participants in the Intention-to-Treat Exposed Population
| Characteristic | Participants, No. (%) by ART Regimena | |||||
|---|---|---|---|---|---|---|
| Overall (n = 4310) | DRV Based (n = 641) | RPV Based (n = 555) | EVG/c Based (n = 771) | DTG Based (n = 1970) | RAL Based (n = 373) | |
| Sex | ||||||
| Male | 3614 (83.9) | 503 (78.5) | 465 (83.8) | 692 (89.8) | 1678 (85.2) | 276 (74.0) |
| Female | 696 (16.1) | 138 (21.5) | 90 (16.2) | 79 (10.2) | 292 (14.8) | 97 (26.0) |
| HIV transmission route | ||||||
| MSM | 2636 (61.2) | 327 (51.0) | 354 (63.8) | 543 (70.4) | 1223 (62.1) | 189 (50.7) |
| Heterosexual | 1206 (28.0) | 221 (34.5) | 161 (29.0) | 159 (20.6) | 543 (27.6) | 122 (32.7) |
| IDU | 208 (4.8) | 49 (7.6) | 22 (4.0) | 23 (3.0) | 94 (4.8) | 20 (5.4) |
| Other | 260 (6.0) | 44 (6.9) | 18 (3.2) | 46 (6.0) | 110 (5.6) | 42 (11.3) |
| Race | ||||||
| White | 2982 (69.2) | 419 (65.4) | 416 (75.0) | 481 (62.4) | 1423 (72.2) | 243 (65.1) |
| Other | 555 (12.9) | 82 (12.8) | 66 (11.9) | 63 (8.2) | 260 (13.2) | 84 (22.5) |
| Unknown | 773 (17.9) | 140 (21.8) | 73 (13.2) | 227 (29.4) | 287 (14.6) | 46 (12.3) |
| HBV (HBsAg) result | ||||||
| Negative | 3257 (75.6) | 457 (71.3) | 429 (77.3) | 569 (73.8) | 1579 (80.2) | 223 (59.8) |
| Positive | 113 (2.6) | 19 (3.0) | 17 (3.1) | 19 (2.5) | 45 (2.3) | 13 (3.5) |
| Unknown | 940 (21.8) | 165 (25.7) | 109 (19.6) | 183 (23.7) | 346 (17.6) | 137 (36.7) |
| HCV (antibody) result | ||||||
| Negative | 3077 (71.4) | 424 (66.1) | 429 (77.3) | 516 (66.9) | 1492 (75.7) | 216 (57.9) |
| Positive | 344 (8.0) | 66 (10.3) | 36 (6.5) | 52 (6.7) | 154 (7.8) | 36 (9.7) |
| Unknown | 889 (20.6) | 151 (23.6) | 90 (16.2) | 203 (26.3) | 324 (16.4) | 121 (32.4) |
| Region | ||||||
| Southern Europe | 1461 (33.9) | 209 (32.6) | 302 (54.4) | 309 (40.1) | 561 (28.5) | 80 (21.4) |
| Central Europe | 1835 (42.6) | 268 (41.8) | 201 (36.2) | 246 (31.9) | 1009 (51.2) | 111 (29.8) |
| Northern Europe or Australia | 609 (14.1) | 91 (14.2) | 32 (5.8) | 128 (16.6) | 210 (10.7) | 148 (39.7) |
| Eastern Europe | 405 (9.4) | 73 (11.4) | 20 (3.6) | 88 (11.4) | 190 (9.6) | 34 (9.1) |
| BMIb | ||||||
| ≤18 | 117 (2.7) | 19 (3.0) | 6 (1.1) | 20 (2.6) | 60 (3.0) | 12 (3.2) |
| 18.1–25 | 1693 (39.3) | 251 (39.2) | 233 (42.0) | 314 (40.7) | 824 (41.8) | 71 (19.0) |
| 25.1–30 | 598 (13.9) | 68 (10.6) | 74 (13.3) | 121 (15.7) | 305 (15.5) | 30 (8.0) |
| >30 | 184 (4.3) | 24 (3.7) | 32 (5.8) | 30 (3.9) | 89 (4.5) | 9 (2.4) |
| Smoking status | ||||||
| Never | 1173 (27.2) | 173 (27.0) | 135 (24.3) | 240 (31.1) | 562 (28.5) | 63 (16.9) |
| Current | 1416 (32.9) | 168 (26.2) | 188 (33.9) | 242 (31.4) | 759 (38.5) | 59 (15.8) |
| Previous | 178 (4.1) | 26 (4.1) | 25 (4.5) | 46 (6.0) | 77 (3.9) | 4 (1.1) |
| Unknown | 1543 (35.8) | 274 (42.7) | 207 (37.3) | 243 (31.5) | 572 (29.0) | 247 (66.2) |
| Prior AIDS | ||||||
| No | 3872 (89.8) | 562 (87.7) | 544 (98.0) | 721 (93.5) | 1751 (88.9) | 294 (78.8) |
| Yes | 438 (10.2) | 79 (12.3) | 11 (2.0) | 50 (6.5) | 219 (11.1) | 79 (21.2) |
| Age at ART initiation | ||||||
| ≤30 y | 1029 (23.9) | 142 (22.2) | 118 (21.3) | 184 (23.9) | 500 (25.4) | 85 (22.8) |
| 31–40 y | 1388 (32.2) | 211 (32.9) | 206 (37.1) | 251 (32.6) | 612 (31.1) | 108 (29.0) |
| 41–50 y | 1081 (25.1) | 171 (26.7) | 151 (27.2) | 192 (24.9) | 483 (24.5) | 84 (22.5) |
| >50 y | 812 (18.8) | 117 (18.3) | 80 (14.4) | 144 (18.7) | 375 (19.0) | 96 (25.7) |
| Age at ART initiation, median (IQR), y | 38 (30–47) | 38 (31–48) | 38 (31–46) | 38 (30–47) | 38 (30–47) | 39 (31–50) |
| Year of ART initiation | ||||||
| 2014–2015 | 1866 (43.3) | 407 (63.5) | 371 (66.8) | 350 (45.4) | 556 (28.2) | 182 (48.8) |
| 2016–2017 | 1627 (37.7) | 142 (22.2) | 140 (25.2) | 318 (41.2) | 899 (45.6) | 128 (34.3) |
| 2018–2019 | 817 (19.0) | 92 (14.4) | 44 (7.9) | 103 (13.4) | 515 (26.1) | 63 (16.9) |
| Baseline HIV viral load (HIV-1 RNA copies/mL)c | ||||||
| ≤10 000 | 971 (22.5) | 105 (16.4) | 234 (42.2) | 152 (19.7) | 403 (20.5) | 77 (20.6) |
| 10 001–99 999 | 1782 (41.3) | 241 (37.6) | 302 (54.4) | 349 (45.3) | 760 (38.6) | 130 (34.9) |
| 100 000–500 000 | 986 (22.9) | 181 (28.2) | 14 (2.5) | 198 (25.7) | 503 (25.5) | 90 (24.1) |
| >500 000 | 571 (13.2) | 114 (17.8) | 5 (0.9) | 72 (9.3) | 304 (15.4) | 76 (20.4) |
| HIV-1 RNA log10, median (IQR), copies/mL | 4.7 (4.1–5.3) | 4.9 (4.3–5.4) | 4.1 (3.6–4.5) | 4.7 (4.1–5.1) | 4.8 (4.1–5.3) | 4.9 (4.2–5.5) |
| Baseline CD4+ T-cell countc | ||||||
| ≤100/µL | 633 (14.7) | 148 (23.1) | 4 (0.7) | 75 (9.7) | 323 (16.4) | 83 (22.3) |
| 101–200/µL | 459 (10.6) | 81 (12.6) | 18 (3.2) | 70 (9.1) | 231 (11.7) | 59 (15.8) |
| 201–350/µL | 879 (20.4) | 147 (22.9) | 108 (19.5) | 164 (21.3) | 393 (19.9) | 67 (18.0) |
| 351–500/µL | 988 (22.9) | 122 (19.0) | 183 (33.0) | 205 (26.6) | 418 (21.2) | 60 (16.1) |
| >500/µL | 1351 (31.3) | 143 (22.3) | 242 (43.6) | 257 (33.3) | 605 (30.7) | 104 (27.9) |
| CD4+ T-cell count, median (IQR), cells/µL | 378 (199–560) | 293 (109–473) | 480 (359–633) | 404 (250–587) | 366 (175–554) | 300 (121–530) |
| Comorbid conditions | ||||||
| Hypertension | 740 (17.2) | 87 (13.6) | 87 (15.7) | 147 (19.1) | 392 (19.9) | 27 (7.2) |
| Diabetes mellitus | 105 (2.4) | 9 (1.4) | 12 (2.2) | 20 (2.6) | 49 (2.5) | 15 (4.0) |
| Prior CVD | 21 (0.5) | 4 (0.6) | 2 (0.4) | 2 (0.3) | 12 (0.6) | 1 (0.3) |
| Prior NADC | 38 (0.9) | 4 (0.6) | 5 (0.9) | 3 (0.4) | 21 (1.1) | 5 (1.3) |
| Prior ESLD | 6 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 4 (0.2) | 1 (0.3) |
| Prior CKD | 8 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 7 (0.4) | 0 (0.0) |
| Initial ART | ||||||
| NRTI | ||||||
| ABC-3TC | 908 (21.1) | 111 (17.3) | 0 (0.0) | 0 (0.0) | 797 (40.5) | 0 (0.0) |
| TDF-FTC | 2417 (56.1) | 463 (72.2) | 418 (75.3) | 462 (59.9) | 728 (37.0) | 346 (92.8) |
| TAF-FTC | 985 (22.9) | 67 (10.5) | 137 (24.7) | 309 (40.1) | 445 (22.6) | 27 (7.2) |
| Third drug | ||||||
| DRV | 641 (14.9) | … | … | … | … | … |
| RPV | 555 (12.9) | … | … | … | … | … |
| EVG/c | 771 (17.9) | … | … | … | … | … |
| DTG | 1970 (45.7) | … | … | … | … | … |
| RAL | 373 (8.7) | … | … | … | … | … |
| Booster | ||||||
| None | 2898 (67.2) | 0 (0.0) | 555 (100.0) | 0 (0.0) | 1970 (100.0) | 373 (100.0) |
| Cobicistat | 899 (20.9) | 128 (20.0) | 0 (0.0) | 771 (100.0) | 0 (0.0) | 0 (0.0) |
| Ritonavir | 513 (11.9) | 513 (80.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; AIDS, acquired immunodeficiency syndrome, referred as AIDS-defining illness; ART, antiretroviral treatment; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; DRV, darunavir; DTG, dolutegravir; ESLD, end-stage liver disease; EVG/c, cobicistat-boosted elvitegravir; FTC, emtricitabine; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug user; IQR, interquartile range; MSM, men who have sex with men; NADC, non–AIDS-defining cancer; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; RAL, raltegravir RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Data represent no. (%) of participants unless otherwise specified.
BMI calculated as weight in kilograms divided by height in meters squared.
Baseline CD4+ T-cell count and HIV-1 RNA level were defined as the last measurement in the 12 months preceding the ART initiation date, and, where this was not available, the first measurement up to 14 days after ART initiation.
The median (IQR) CD4+ T-cell count was 378/µL (199–560/µL). Overall, 1971 participants (45.7%) had CD4+ T-cell counts ≤350/µL at presentation, and 1092 (25.3%) had severe immunosuppression (CD4+ T-cell count, ≤200/µL); 36.1% had HIV-1 RNA levels ≥100 000 copies/mL. Overall, 72.3% of participants initiated an INSTI-based regimen, of whom 1970 (63.3%) started dolutegravir (Table 1).
Virological Outcomes
VS at weeks 48 and 96 was achieved in 3306 of 3638 (90.9% [95% CI, 89.9%–91.8%) and 2908 of 3118 (93.3% [92.4%–94.1%]) participants, respectively. At 48 weeks, Kaplan-Meier estimates of the proportions were 9.6% (95%, CI, 8.7%–10.5%) for viral blips, 2.1% (1.6%–2.5%) for LLV, 22.2% (20.0%–24.3%) for RV, and 2.1% (1.7%–2.6%) for VF.
Virological Suppression
In multivariate analysis, darunavir (vs dolutegravir), baseline HIV-1 RNA levels >100 000 copies/mL and CD4+ T-cell counts ≤350/µL at ART initiation were associated with significantly lower VS rates at week 48 (Table 2). At week 96, abacavir-lamivudine (vs TDF-emtricitabine), raltegravir (vs dolutegravir), HIV-1-RNA levels >100 000 copies/mL, and CD4+ T-cell counts ≤350/µL were associated with significantly lower VS rates (Table 2).
Table 2.
Multivariate Logistic Regression Analysis of Variables Associated With Virological Suppression at Weeks 48 and 96, for All Participants
| Variable | Virological Suppression wk 48 | Virological Suppression at wk 96 | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Sex | ||||
| Male | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Female | 1.16 (.83–1.61) | .40 | .81 (.56–1.18) | .27 |
| Race | ||||
| White | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Other | .80 (.55–1.16) | .23 | .80 (.51–1.26) | .34 |
| HCV | ||||
| Negative | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Positive | .46 (.32–.68) | <.001a | .64 (.38–1.05) | .08 |
| Region | ||||
| Southern Europe | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Central Europe | .87 (.65–1.15) | .32 | 1.00 (.71–1.41) | .99 |
| Northern Europe or Australia | .90 (.60–1.34) | .60 | 1.30 (.80–2.12) | .29 |
| Eastern Europe | .74 (.45–1.21) | .23 | .88 (.44–1.75) | .72 |
| Baseline HIV viral load (HIV-1 RNA copies/mL)b | ||||
| ≤10 000 | 1.19 (.80–1.76) | .39 | 1.41 (.87–2.27) | .16 |
| 10 001–100 000 | 1.0 (Reference) | … | 1.0 (Reference) | … |
| >100 000 | .51 (.39–.68) | <.001a | .69 (.49–.97) | .03a |
| Baseline CD4+ T-cell countb | ||||
| ≤200/µL | .40 (.27–.58) | <.001a | .35 (.22–.55) | <.001a |
| 201–350/µL | .58 (.39–.84) | <.001a | .48 (.30–.76) | <.001a |
| 351–500/µL | .91 (.60–1.38) | .66 | 1.13 (.67–1.93) | .64 |
| >500/µL | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Prior AIDS | ||||
| No | 1.0 (Reference) | … | 1.0 (Reference) | … |
| Yes | .72 (.52–1.00) | .05 | .73 (.48–1.12) | .15 |
| Age at ART initiation | ||||
| ≤30 y | 1.0 (Reference) | … | 1.0 (Reference) | … |
| 31–40 y | .95 (.68–1.34) | .78 | 1.04 (.68–1.58) | .85 |
| 41–50 y | 1.17 (.81–1.67) | .40 | 1.40 (.88–2.21) | .15 |
| >50 y | .87 (.60–1.25) | .44 | .77 (.50–1.20) | .26 |
| Year of ART initiation | ||||
| 2014–2015 | 1.17 (.87–1.58) | .30 | .93 (.65–1.34) | .70 |
| 2016–2017 | 1.0 (Reference) | … | 1.0 (Reference) | … |
| 2018–2019 | .74 (.55–1.11) | .14 | .68 (.42–1.08) | .10 |
| NRTI | ||||
| ABC-3TC | .85 (.60–1.19) | .34 | .46 (.30–.69) | <.001a |
| TDF-FTC | 1.0 (Reference) | … | 1.0 (Reference) | … |
| TAF-FTC | 1.19 (.84–1.69) | .33 | .97 (.61–1.53) | .88 |
| Third drug | ||||
| DRV | .63 (.45–.87) | .01a | .65 (.43–1.00) | .05 |
| RPV | .99 (.57–1.71) | .96 | .66 (.34–1.26) | .20 |
| EVG/c | 1.08 (.72–1.62) | .70 | .66 (.41–1.07) | .09 |
| DTG | 1.0 (Reference) | … | 1.0 (Reference) | … |
| RAL | .79 (.51–1.23) | .29 | .52 (.29–.90) | .02a |
Abbreviations: 3TC, lamivudine; ABC, abacavir; AIDS, acquired immunodeficiency syndrome, referred as AIDS-defining illness; ART, antiretroviral treatment; CI, confidence interval; DRV, darunavir; DTG, dolutegravir; EVG/c, cobicistat-boosted elvitegravir; FTC, emtricitabine; HCV, hepatitis C virus infection; HIV, human immunodeficiency virus; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; OR, odds ratio;
RAL, raltegravir; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Significant at P < .05.
Baseline CD4+ T-cell count and HIV-1 RNA level were defined as the last measurement in the 12 months preceding ART initiation date and, where this was not available, the first measurement up to 14 days after ART initiation.
Viral Blips Analysis
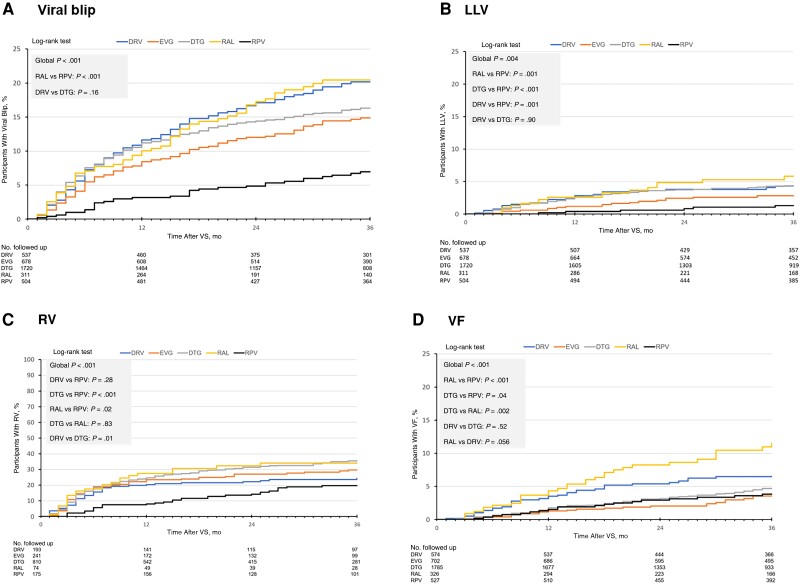
In the time-to-blip analysis (Figure 2A), differences among third drugs favored rilpivirine (P < .001) with a time to blip longer than raltegravir (P < .001). Darunavir and dolutegravir had similar times to blip (P = .16).
Figure 2.
Time-to-virological outcomes estimated by Kaplan-Meier curves. A. Time-to-viral blip following virological suppression. B. Time-to-low-level viremia following virological suppression. C. Time-to-residual viremia following virological suppression. D. Time-to-virological failure following virological suppression. Abbreviations: DTG, dolutegravir; DRV, darunavir; EVG, elvitegravir; FU, follow-up; LLV, low-level viremia; RAL, raltegravir; RPV, rilpivirine; RV, residual viremia; VF, virological failure; VS, virological suppression.
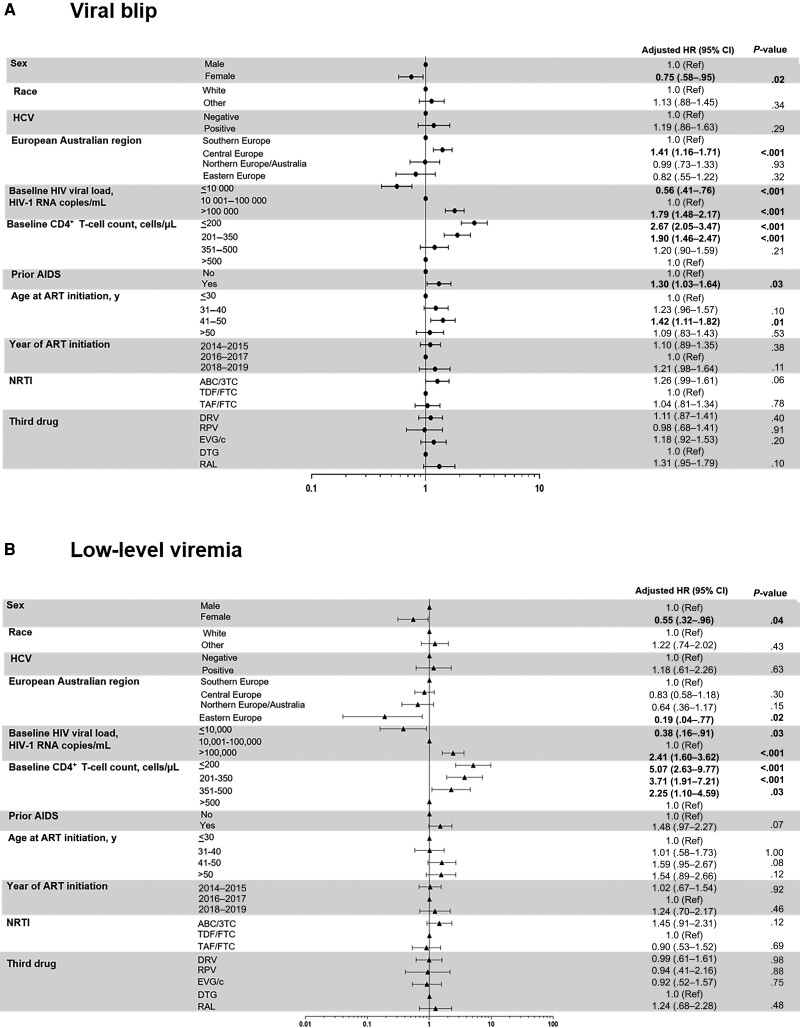
Female sex was associated with a lower blip incidence in multivariate analysis. Factors associated with a higher rate of blips were age 41–50 years, Central European region, prior AIDS, and CD4+ T-cell count ≤350/µL. Baseline HIV-1 RNA levels paralleled blip incidence, with values ≤10 000 copies/mL associated with lower rates, whereas those >100 000 copies/mL had the highest blip risk. We found no association between NRTIs or the third drug and blip incidence (Figure 3A).
Figure 3.
Multivariate analysis of viral blip (A) and low-level viremia (B) rates. Statistically significant values of variables are highlighted in bold. Abbreviations: 3TC, lamivudine; ABC, abacavir; AIDS, acquired immunodeficiency syndrome, referred as AIDS-defining illness; ART, antiretroviral treatment; CI, confidence interval; DRV, darunavir; DTG, dolutegravir; EVG/c, cobicistat-boosted elvitegravir; FTC, emtricitabine; HCV, hepatitis C virus; HIV, human immunonodeficiency virus; HR, hazard ratio; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; RAL, raltegravir; Ref, reference; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Within the subset initiating any INSTI-based regimen, female sex, age 41–50 years, Central European region, HIV-1 RNA level >100 000 copies/mL and CD4+ T-cell count ≤350/µL remained associated with blips. The same analysis restricted to dolutegravir-based regimens showed an association between HIV-1 RNA levels and CD4+ T-cell counts and blips (Supplementary Tables 1 and 2).
LLV Analysis
In the time-to-LLV analysis (Figure 2B), differences among all third drugs favored rilpivirine (P = .004) overall, with a longer time than raltegravir (P = .001) or dolutegravir (P < .001) and similar results as for darunavir and dolutegravir (P = .90).
Female sex and Eastern European region were associated with lower LLV rates in multivariate analysis. Baseline HIV-1 RNA levels ≤10 000 copies/mL were associated with lower LLV rates and levels >100 000 copies/mL with the highest rates. CD4+ T-cell counts ≤500/µL were associated with a higher LLV risk. We found no association between NRTIs or the third drugs and LLV (Figure 3B).
These associations remained in the subset receiving INSTI-based regimens (female sex, Eastern European region, HIV-1 RNA level >100 000 copies/mL, CD4+ T-cell count ≤350/µL). In the dolutegravir subset, HIV-1 RNA levels >100 000 copies/mL and CD4+ T-cell counts ≤500/µL remained associated with LLV (Supplementary Tables 1 and 2).
RV Analysis
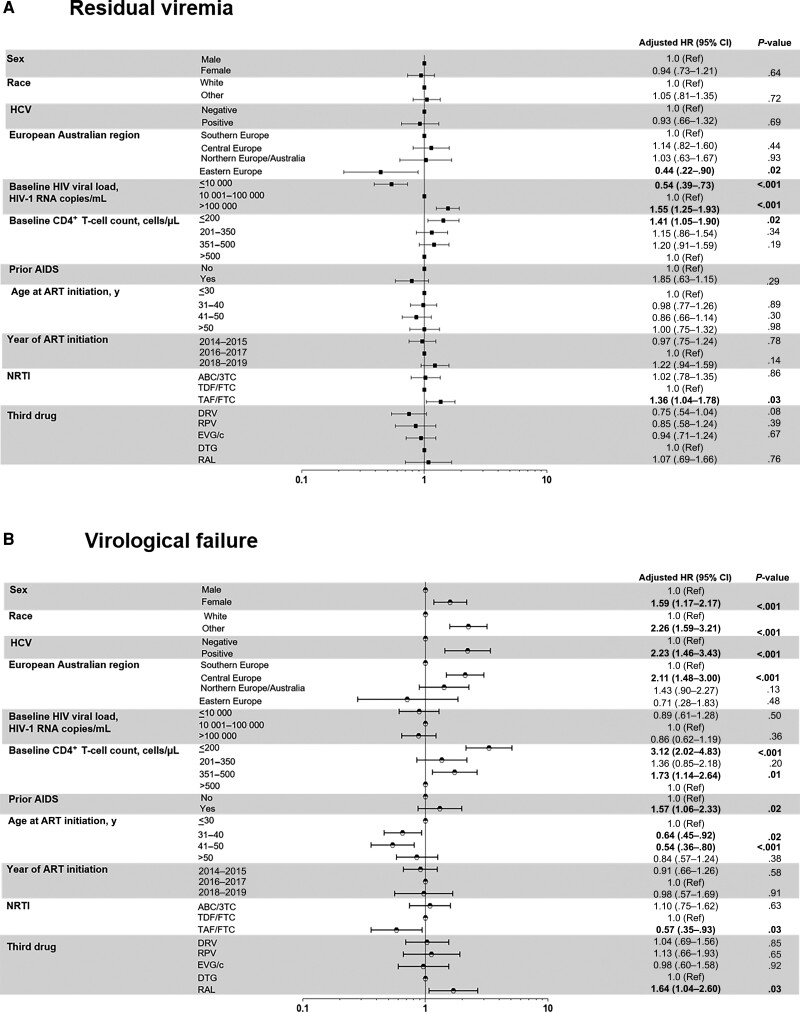
The time-to-RV analysis across third drugs favored rilpivirine (P < .001) (Figure 2C). Darunavir showed a similar time to RV as rilpivirine (P = .28) and a longer time than dolutegravir (P = .01). Eastern European region was associated with lower RV rates in multivariate analysis. TAF-emtricitabine (vs TDF-emtricitabine) was associated with a higher RV incidence. Baseline HIV-1 RNA levels ≤10 000 copies/mL were associated with a lower RV rate, whereas those >100 000 copies/mL and CD4+ T-cell counts ≤200/µL were associated with the highest RV rates (Figure 4A). Within the subset treated with INSTI and specifically dolutegravir, Eastern European region and HIV-1 RNA levels, but not CD4+ T-cell counts, remained associated with RV (Supplementary Tables 1 and 2).
Figure 4.
Multivariate analysis of residual viremia (A) and virological failure (B) rates. Statistically significant values of variables ares highlighted in bold. Abbreviations: 3TC, lamivudine; ABC, abacavir; AIDS, acquired immunodeficiency syndrome, referred as AIDS-defining illness; ART, antiretroviral treatment; CI, confidence interval; DTG, dolutegravir; DRV, darunavir;, dolutegravir; EVG/c, cobicistat-boosted elvitegravir; FTC, emtricitabine; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; NRTI, nucleos(t)ide reverse-transcriptase inhibitor; RAL, raltegravir; Ref, reference; RPV, rilpivirine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
VF Analysis
In the time-to-VF analysis (Figure 2D), differences among third drugs again favored rilpivirine (P < .001). Raltegravir had a shorter time to VF than rilpivirine (P < .001), dolutegravir (P = .002), and darunavir (borderline significance, P = .056).
In multivariate analysis, factors associated with higher VF rates (Figure 4B) were female sex, nonwhite race, chronic hepatitis C virus, prior AIDS, and Central European region, whereas age 31–50 years was associated with lower VF rates. Low baseline CD4+ T-cell counts (≤200/µL or 351–500/µL) were associated with higher VF rates (Figure 4B), but, intriguingly, HIV-1 RNA levels were not. Raltegravir use was associated with higher VF rates in multivariate analysis compared to dolutegravir, whereas rilpivirine was not. TAF-emtricitabine (vs TDF-emtricitabine) was associated with lower rates of VF.
The subset of any INSTI- and dolutegravir-based regimen showed an increased risk of VF associated with lower CD4+ T-cell count. No association was found with HIV-1 RNA levels or TAF-emtricitabine (Supplementary Tables 1 and 2).
DISCUSSION
PWH who initiated ART beyond 2014 in the multinational prospective RESPOND cohort, with 72% of participants receiving INSTI-based regimens, had high VS rates at weeks 48 and 96 (91.0% and 93.3%, respectively). Using stringent definitions for virological nonsuppression outcomes, at 48 weeks the proportions with viral blips, LLV, RV, and VF were 9.6%, 2.1%, 22.2%, and 2.1%, respectively.
High baseline HIV-1-RNA and low CD4+ counts were strongly associated with lower rates of VS at 48 and 96 weeks. The use of darunavir (vs dolutegravir) was associated with a significantly lower probability of VS at week 48, but this association was lost at week 96. PIs have slower initial viral load decay kinetics compared with INSTIs, particularly with high baseline HIV-1 RNA levels, as shown in randomized clinical trials [26, 27] and cohort studies [28]. In our study, abacavir-lamivudine, higher HIV-1 RNA and lower CD4+ T-cell counts were associated with lower rates of VS at 96 weeks. Abacavir-lamivudine was associated with a significantly shorter time to VF than TDF-emtricitabine combined with either boosted atazanavir or efavirenz in the AIDS Clinical Trials Group A5202, in strata of HIV-1 RNA levels ≥100 000 copies/mL and CD4+ T-cell counts <200/µL [29]. However, this has not been reproduced in pivotal dolutegravir studies in initial treatment [26, 30].
We found a significant association between high baseline plasma HIV-1 RNA level or low CD4+ T-cell count and the blip incidence in the overall cohort and in participants starting an INSTI- and dolutegravir-based regimen. This is consistent with findings in previous cohorts [23, 24]. In turn, blip rates were higher with PI-based and lower with INSTI-based ART. However, there could have been a channeling prescription bias of PIs to higher-risk PWH based on their perceived higher barrier to resistance [24]. We found no association between viral blips and NRTI or third drug types in our analysis. These data are consistent with results from a randomized trial comparing dolutegravir with ritonavir-boosted darunavir [26].
A significant association between high baseline plasma HIV-1 RNA level and low CD4+ T-cell count was also seen with LLV overall, with any INSTI- and dolutegravir-based regimens. These findings are in agreement with those in a Spanish cohort [31] showing that a plasma HIV-1 RNA level >100 000 copies/mL was an independent predictor of LLV, an association that remained for participants starting any INSTI-based regimen. Conversely, other cohorts have reported a higher risk of LLV with PI-based than with NNRTI- and INSTI-based regimens [6]. However, these analyses had a low proportion of darunavir use among PIs (most participants received atazanavir or lopinavir) [6]. It is likely that the risk of LLV could be different for darunavir versus other PIs. In our study the only PI included was darunavir, the only currently recommended PI [1, 14].
The rate of RV in our study (22.2%) was similar to that described in a Dutch cohort (24.7%) [12]. High baseline HIV-1 RNA level and low CD4+ T-cell count were also associated with increased rates of RV. In our analysis, the association with HIV-1 RNA level remained in participants receiving any INSTI and dolutegravir in particular, consistent with findings in a previous French cohort [32].
Interestingly, while low CD4+ T-cell counts were significantly associated with VF, high baseline HIV-1 RNA levels were not. This finding is consistent with findings in a French cohort [11], with no relationship between baseline HIV-1 RNA level and VF. In addition, HIV-1 RNA >100 000 copies/mL did not affect risk of VF in a European cohort [28]. These results differ from those observed in a Spanish cohort [31] in which a HIV-1 RNA >100 000 copies/mL was a consistent predictor of VF. Different definitions of VF and time points for HIV-1 RNA measurements could lead to noncomparable results. Similarly, other cohorts [33, 34] found a higher risk of VF with HIV-1 RNA levels ≥100 000 copies/mL on INSTI-based regimens. However, because both cohorts used thresholds of 50 copies/mL for VF, many VFs could have been LLV. Another recent analysis has found an association between baseline HIV-1 RNA level and VF (hazard ratio, 1.1 [95% CI, 1.0–1.2]) [2], but for a broader definition of VF.
In our analysis, compared to TDF, TAF was associated with higher rates of RV but a lower risk of VF. TAF showed superior virological efficacy compared with TDF in a clinical trial [35]; however, there were no data on its impact on RV.
Raltegravir had a significantly shorter time to VF than rilpivirine, darunavir, or dolutegravir. This association remained significant in multivariate analysis. This is consistent with findings of the SPRING-2 trial, showing fewer participants who met protocol-defined VF with dolutegravir versus raltegravir [30]. Indeed, raltegravir showed lower VS rates at week 96 in our multivariate analysis. Unmeasured residual confounding could exist regarding raltegravir dosing notwithstanding. Raltegravir once daily is associated with higher rates of VF, particularly with high HIV-1 RNA and low CD4+ T-cell counts, but we did not have access to dosing data for raltegravir in RESPOND [36, 37]. In a recent European cohort analysis, INSTI- and NNRTI-based ART had similar rates of VF, whereas PI-based ART was associated with an increased risk of VF [2]. However, not every drug within a class was assessed owing to the limited number of virological outcome events.
Results were consistent regarding HIV-1 RNA levels and CD4+ T-cell counts in those starting INSTIs versus other ART classes or those starting dolutegravir (compared with other individual INSTIs), for every virological nonsuppression outcome. These findings strongly suggest that HIV-1 RNA level and CD4+ T-cell count are baseline determinants associated with long-term consequences, including higher rates of viral blips, LLV, and RV, independent of the ART regimen initiated. Alternatively, VF was associated only with a low baseline CD4+ T-cell count. All of these findings indicate that the interaction between the HIV reservoir established before ART initiation and the rates of viral blips, LLV, and RV seems to be closely related to baseline HIV-1 RNA level but not to the type of ART administered, including the second-generation INSTIs [18, 38–40]. HIV-1 integrated into silent chromosomal sites in the deep latency of clonally expanded infected T cells can harbor defective proviruses and, less likely, intact (replication-competent) viruses, which are not affected by ART [41, 42]. Nonsuppressible RV has been associated with large HIV reservoir size [43, 44]. In addition, the nadir CD4+ T-cell count was inversely correlated with levels of both cell-associated DNA and cell-associated RNA in a pooled analysis of AIDS Clinical Trials Group treatment interruption studies [38].
In the current study, the time to virological nonsuppression outcomes (viral blips, LLV, RV, and VF) was significantly and consistently longer for rilpivirine. The fact that this association disappeared after adjustment reflects the imbalance in baseline characteristics between the different treatments. The use of rilpivirine plus 2 NRTIs has been approved only for PWH with HIV-1 RNA levels <100 000 copies/mL and is not recommended for those with CD4+ T-cell counts <200/µL, supporting a likely channeling prescription bias, as it is preferentially prescribed in PWH with characteristics associated with better virological outcomes [14, 45]. The Italian Italian cohort naive antiretrovirals cohort compared rilpivirine- and INSTI-based first-line regimens in participants with HIV-1 RNA levels <100 000 copies/mL and CD4+ T-cell counts >200/µL and found no differences in virological rebound rates [46].
We identified higher rates of VF in nonwhite PHW. This association probably reflects higher rates of immigration, socioeconomic deprivation, and lower treatment adherence rates in this group [47].
Our study has limitations. RESPOND does not systematically collect data on HIV subtypes and genotypic resistance analysis, which could affect the choice of initial ART or the virological outcome. The second-generation INSTI bictegravir, the 2-drug regimen dolutegravir-lamivudine and the new NNRTI doravirine were not included owing to an insufficient number of participants or short follow-up. Despite adjustment for a wide range of variables, confounding by indication and residual uncontrolled confounders might still introduce unknown biases in drug comparisons.
A strength of the current study is the inclusion of a large number of INSTI-based participants and the ability to compare individual drugs within ART classes. In addition, we describe virological nonsuppression outcomes not explored in randomized trials, using strict definitions.
In conclusion, baseline plasma HIV-1 RNA levels >100 000copies/mL and CD4+ T-cell counts ≤350/µL were associated with lower rates of VS at 48 and 96 weeks, and higher rates of viral blips, RV, and LLV. CD4+ T-cell counts ≤200/µL were associated with a higher risk of VF. Importantly, the association between HIV-1 RNA levels or CD4+ T-cell count and these virological outcomes persisted in participants initiating INSTI-based and specifically dolutegravir-based regimens. These data suggest that baseline HIV-1 RNA levels and CD4+ T-cell counts are determinants associated with virological nonsuppression outcomes regardless of the antiretroviral regimen initiated and point to underlying mechanisms established before ART initiation, likely focused on the HIV reservoir size. Further research is warranted to explore the impact of bictegravir-emtricitabine-TAF, doravirine, and INSTI-based 2-drug regimens on long-term virological nonsuppression outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Hortensia Álvarez, Department of Internal Medicine, Infectious Diseases Unit, Complexo Hospitalario Universitario de Ferrol, Ferrol, SERGAS-A Coruña, Spain; Department of Biochemistry, Genetics and Immunology, Universidade de Vigo, Vigo, Spain.
Amanda Mocroft, CHIP, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; Centre for Clinical Research, Epidemiology, Modelling and Evaluation, Institute for Global Health, University College London, London, United Kingdom.
Lene Ryom, CHIP, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; Department of Infectious Diseases, Hvidovre University Hospital, Copenhagen, Denmark.
Bastian Neesgaard, CHIP, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Simon Edwards, Department of HIV, Mortimer Market Centre, London, United Kingdom.
Veronica Svedhem, Department of Medicine, Medical Unit Infectious Diseases, Karolinska University Hospital, Karolinska Institutet, Huddinge, Sweden.
Huldrych F Günthard, Department of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich and Institute of Medical Virology, University of Zurich, Zurich, Switzerland.
Robert Zangerle, Austrian HIV Cohort Study, Medizinische Universität Innsbruck, Innsbruck, Austria.
Colette Smith, The Royal Free HIV Cohort Study, Royal Free Hospital, University College London, London, United Kingdom.
Antonella Castagna, San Raffaele Scientific Institute, Università Vita-Salute San Raffaele, Milano, Italy.
Antonella d’Arminio Monforte, Italian Cohort Naive Antiretrovirals (ICONA), ASST Santi Paolo e Carlo, Milano, Italy.
Ferdinand Wit, AIDS Therapy Evaluation in the Netherlands (ATHENA) cohort, HIV Monitoring Foundation, Amsterdam, The Netherlands.
Melanie Stecher, Division of Infectious Diseases, Department I of Internal Medicine, Medical Faculty and University Hospital Cologne, University of Cologne, Cologne, Germany.
Clara Lehman, Division of Infectious Diseases, Department I of Internal Medicine, Medical Faculty and University Hospital Cologne, University of Cologne, Cologne, Germany.
Cristina Mussini, Modena HIV Cohort, Università degli Studi di Modena, Modena, Italy.
Eric Fontas, Nice HIV Cohort, Université Côte d´Azur et Centre Hospitalier Universitaire, Nice, France.
Eva González, PISCIS Cohort Study, Centre Estudis Epidemologics de ITS i VIH de Catalunya, Badalona, Spain.
Jan-Christian Wasmuth, Medical Department, University Hospital Bonn, Bonn, Germany.
Anders Sönnerborg, Swedish InfCare HIV Cohort, Karolinska University Hospital, Stockholm, Sweden.
Stéphane De Wit, CHU Saint-Pierre, Université Libre de Bruxelles, Brussels, Belgium.
Nikoloz Chkhartishvili, Georgian National AIDS Health Information System, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia.
Christoph Stephan, Frankfurt HIV Cohort Study, University Hospital Frankfurt, Goethe-University, Infectious Diseases Unit, Frankfurt, Germany.
Kathy Petoumenos, The Kirby Institute, University of New South Wales, Sydney, Australia.
Nadine Jaschinski, CHIP, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Vani Vannappagari, ViiV Healthcare, Research Triangle Park, North Carolina, USA.
Joel Gallant, Gilead Sciences, Foster City, California, USA.
Lital Young, Merck Sharp & Dohme, Luzern, Switzerland.
Alain Volny Anne, European AIDS Treatment Group, Brussels, Belgium.
Lauren Greenberg, CHIP, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; Centre for Clinical Research, Epidemiology, Modelling and Evaluation, Institute for Global Health, University College London, London, United Kingdom.
Raquel Martín-Iguacel, Infectious Diseases Department, Odense University Hospital, Odense, Denmark.
Eva Poveda, Group of Virology and Pathogenesis, Galicia Sur Health Research Institute (IIS Galicia Sur)–Complexo Hospitalario Universitario de Vigo, Vigo, SERGAS-UVigo, Spain.
Josep M Llibre, Infectious Diseases Division and Fight Infections Foundation, University Hospital Germans Trias i Pujol, Barcelona, Spain.
for the RESPOND (International Cohort Consortium of Infectious Diseases) Study Group:
F Wit, M v d Valk, M Hillebregt, K Petoumenos, M Law, D Byonanebye, J Hutchinson, R Zangerle, H Appoyer, J Vera, A Clarke, B Broster, L Barbour, S De Wit, M Delforge, J Begovac, G Wandeler, C Stephan, M Bucht, N Chkhartishvili, O Chokoshvili, A d’Arminio Monforte, A Rodano, A Tavelli, I Fanti, C Mussini, V Borghi, C Pradier, E Fontas, K Dollet, C Caissotti, J Casabona, J M Miro, C Smith, F Lampe, M Johnson, F Burns, C Chaloner, A Castagna, A Lazzarin, A Poli, A Sönnerborg, K Falconer, V Svedhem, H F Günthard, B Ledergerber, H Bucher, K Kusejko, J C Wasmuth, J Rockstroh, J J Vehreschild, G Fätkenheuer, M Scherer, N Schulze, B Franke, L Ryom, M Law, J Rooney, I McNicholl, V Vannappagari, H Garges, K Petoumenos, G Wandeler, R Zangerle, C Smith, S De Wit, J Lundgren, H F Günthard, L Young, R Campo, J Lundgren, H F Günthard, J Kowalska, D Raben, L Ryom, A Mocroft, J Rockstroh, L Peters, O Kirk, D Podlekareva, A Volny Anne, N Dedes, E D Williams, N Chkhartishvili, R Zangerle, K Petoumenos, M Law, F Wit, C Necsoi, G Wandeler, C Stephan, C Pradier, A d’Arminio Monforte, C Mussini, A Bruguera, H Bucher, A Sönnerborg, J J Vehreschild, J C Wasmuth, C Smith, A Castagna, J Vera, J Begovac, J Rooney, I McNicholl, V Vannappagari, H Garges, L Young, R Campo, L Ryom, A Mocroft, B Neesgaard, L Greenberg, N Jaschinski, L Bansi-Matharu, V Svedhem-Johansson, F Wit, K Grabmeier-Pfistershammer, R Zangerle, J Hoy, M Bloch, D Braun, A Calmy, G Schüttfort, M Youle, S De Wit, C Mussini, S Zona, A Castagna, A Antinori, N Chkhartishvili, N Bolokadze, E Fontas, K Dollet, C Pradier, J M Miro, J M Llibre, J J Vehreschild, C Schwarze-Zander, J C Wasmuth, J Rockstroh, K Petoumenos, M Law, C Duvivier, G Dragovic, R Radoi, C Oprea, M Vasylyev, J Kowalska, R Matulionyte, V Mulabdic, G Marchetti, E Kuzovatova, N Coppola, J Begovac, I Aho, S Martini, H Bucher, A Harxhi, T Wæhre, A Pharris, A Vassilenko, G Fätkenheuer, J Bogner, A Maagaard, E Jablonowska, D Elbirt, G Marrone, C Leen, C Wyen, M Kundro, C Hathleberger, A Pelchen-Matthews, D Byonanebye, O Fursa, A Roen, L Dahlerup-Rasmussen, N Dedes, E Dixon Williams, J Gallant, D Thorpe, V Vannappagari, H Garges, J M Arduino, P Sklar, Alain Volny Anne, Nikos Dedes, Luis Mendão, Esther Dixon Williams, J F Larsen, B Neesgaard, N Jaschinski, O Fursa, O Valdemaier, A Timiryasova, L Ryom, L Peters, M L Jakobsen, C Kraef, M Gardizi, D Raben, T W Elsing, L Ramesh Kumar, S Shahi, K Andersen, J Reekie, A Mocroft, L Greenberg, L Bansi-Matharu, A Pelchen-Matthews, K Petoumenos, D Byonanebye, E Tusch, A Roen, and W Bannister
Notes
International Cohort Consortium of Infectious Disease (RESPOND) study group members. AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA): F. Wit, M. v. d. Valk, and M. Hillebregt (Stichting HIV Monitoring (SHM), Amsterdam, Netherlands). Australian HIV Observational Database (AHOD): K. Petoumenos, M. Law, D. Byonanebye, and J. Hutchinson (University of New South Wales, Sydney, Australia). Austrian HIV Cohort Study: R. Zangerle and H. Appoyer (Medizinische Universität Innsbruck, Innsbruch, Austria). Brighton HIV Cohort: J. Vera, A. Clarke, B. Broster, and L. Barbour (Brighton, United Kingdom). CHU Saint-Pierre: S. De Wit and M. Delforge (CHU Saint Pierre, Université Libre de Bruxelles, Brussels, Belgium). Croatian HIV Cohort: J. Begovac (University Hospital of Infectious Diseases, Zagreb, Croatia). EuroSIDA Cohort: G. Wandeler (CHIP, Rigshospitalet, RegionH Copenhagen, Denmark). Frankfurt HIV Cohort Study: C. Stephan and M. Bucht (Johann Wolfgang Goethe University Hospital, Frankfurt, Germany). Georgian National AIDS Health Information System: N. Chkhartishvili and O. Chokoshvili (Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia). Italian cohort naive antiretrovirals (ICONA): A. d’Arminio Monforte, A. Rodano, and A. Tavelli (ASST Santi Paolo e Carlo, Milano, Italy); I. Fanti (Icona Foundation, Milano, Italy). Modena HIV Cohort: C. Mussini and V. Borghi (Università degli Studi di Modena, Modena, Italy). Nice HIV Cohort: C. Pradier, E. Fontas, K. Dollet, and C. Caissotti (Université C’te d’Azur, Centre Hospitalier Universitaire de Nice, Department of Public Health, UR2CA, Nice, France). PISCIS Cohort Study: J. Casabona and J. M. Miro (Centre Estudis Epidemiologics de ITS I VIH de Catalunya, Badalona, Spain). Royal Free Hospital Cohort: C. Smith, F. Lampe, M. Johnson, F. Burns, and C. Chaloner (Royal Free Hospital, University College London, London, United Kingdom). San Raffaele Scientific Institute: A. Castagna, A. Lazzarin, and A. Poli (Università Vita-Salute San Raffaele, Milano, Italy). Swedish InfCare HIV Cohort: A. Sönnerborg, K. Falconer, and V. Svedhem (Karolinska University Hospital, Stockholm, Sweden). Swiss HIV Cohort Study: H. F. Günthard, B. Ledergerber, H. Bucher, and K. Kusejko (University of Zurich, Zurich, Switzerland). University Hospital Bonn: J. C. Wasmuth and J. Rockstroh (Bonn, Germany). University Hospital Cologne: J. J. Vehreschild, G. Fätkenheuer, M. Scherer, N. Schulze, and B. Franke (Cologne, Germany).
RESPOND committees and staff. Executive committee: L. Ryom (chair), M. Law (chair), J. Rooney, I. McNicholl, V. Vannappagari, H. Garges, K. Petoumenos, G. Wandeler, R. Zangerle, C. Smith, S. De Wit, J. Lundgren, H. F. Günthard, L. Young, and R. Campo. Scientific steering committee: J. Lundgren (chair), H. F. Günthard (chair), J. Kowalska, D. Raben, L. Ryom, A. Mocroft, J. Rockstroh, L. Peters, O. Kirk, D. Podlekareva, A. Volny Anne, N. Dedes, E. D. Williams, N. Chkhartishvili, R. Zangerle, K. Petoumenos, M. Law, F. Wit, C. Necsoi, G. Wandeler, C. Stephan, C. Pradier, A. d’Arminio Monforte, C. Mussini, A. Bruguera, H. Bucher, A. Sönnerborg, J. J. Vehreschild, J. C. Wasmuth, C. Smith, A. Castagna, J. Vera, J. Begovac, J. Rooney, I. McNicholl, V. Vannappagari, H. Garges, L. Young, and R. Campo. Outcomes with antiretroviral treatment scientific interest group: L. Ryom, A. Mocroft, B. Neesgaard, L. Greenberg, N. Jaschinski, L. Bansi-Matharu, V. Svedhem-Johansson, F. Wit, K. Grabmeier-Pfistershammer, R. Zangerle, J. Hoy, M. Bloch, D. Braun, A. Calmy, G. Schüttfort, M. Youle, S. De Wit, C. Mussini, S. Zona, A. Castagna, A. Antinori, N. Chkhartishvili, N. Bolokadze, E. Fontas, K. Dollet, C. Pradier, J. M. Miro, J. M. Llibre, J.J. Vehreschild, C. Schwarze-Zander, J. C. Wasmuth, J. Rockstroh, K. Petoumenos, M. Law, C. Duvivier, G. Dragovic, R. Radoi, C. Oprea, M. Vasylyev, J. Kowalska, R. Matulionyte, V. Mulabdic, G. Marchetti, E. Kuzovatova, N. Coppola, J. Begovac, I. Aho, S. Martini, H. Bucher, A. Harxhi, T. Wæhre, A. Pharris, A. Vassilenko, G. Fätkenheuer, J. Bogner, A. Maagaard, E. Jablonowska, D. Elbirt, G. Marrone, C. Leen, C. Wyen, M. Kundro, C. Hathleberger, A. Pelchen-Matthews, D. Byonanebye, O. Fursa, A. Roen, L. Dahlerup-Rasmussen, N. Dedes, E. Dixon Williams, J. Gallant, D. Thorpe, V. Vannappagari, H. Garges, J. M. Arduino, and P. Sklar. Community representatives: Alain Volny Anne, Nikos Dedes, and Luis Mendão (European AIDS Treatment Group); Esther Dixon Williams (United Kingdom). Coordinating center staff: J. F. Larsen, B. Neesgaard, N. Jaschinski, O. Fursa, O. Valdemaier, A. Timiryasova, L. Ryom, L. Peters, M. L. Jakobsen, C. Kraef, M. Gardizi, and D. Raben. Data management staff: T. W. Elsing, L. Ramesh Kumar, S. Shahi, and K. Andersen. Statistical staff: J. Reekie, A. Mocroft, L. Greenberg, L. Bansi-Matharu, A. Pelchen-Matthews, K. Petoumenos, D. Byonanebye, E. Tusch, A. Roen, and W. Bannister.
Author Contributions. H. A. and J. M. L. contributed to the conception and design of the study and drafting of the manuscript. A. M. performed the acquisition of data and statistical analysis. All authors contributed to the collection of data, interpretation of the results, and revision of the manuscript and approved the final version of the manuscript.
Disclaimer. Per International Cohort Consortium of Infectious Disease (RESPOND) governance (https://chip.dk/Portals/0/files/RESPOND/Study%20documents/RESPOND%20governance%20and%20procedures_v6_2020SEP30.pdf?ver=2020-10-20-163958-080), funders of the study were academic collaborators and included as coauthors if they met the ICJME criteria. Funders were not in a position to veto study design, data collection, data analysis, data interpretation, and/or the writing of the manuscript.
Financial support. The International Cohort Consortium of Infectious Disease (RESPOND) is supported by ViiV Healthcare, Gilead Sciences, and Merck Sharp & Dohme. Additional support has been provided by participating cohorts contributing in-kind data and/or statistical support: the Austrian HIV Cohort Study, the Australian HIV Observational Database, CHU Saint-Pierre, University Hospital Cologne, EuroSIDA, the Frankfurt HIV Cohort Study, the Georgian National AIDS Health Information System, the Modena HIV Cohort, the San Raffaele Scientific Institute, the Swiss HIV Cohort Study, the AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA), and the Royal Free HIV Cohort Study. The Australian HIV Observational Database is further supported by the US National Institutes of Health (grant U01-AI069907) and the National Health and Medical Research Council, Australia (grant GNT1050874), and the Swiss HIV Cohort Study is supported by the Swiss National Science Foundation.
References
- 1. European AIDS Clinical Society . Guidelines: version 11. 1. 2022. Available at: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf. Accessed 14 November 2022.
- 2. Elvstam O, Malmborn K, Elén S, et al. Virologic failure following low-level viremia and viral blips during antiretroviral therapy: results from a European multicenter cohort. Clin Infect Dis 2023; 76:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laprise C, de Pokomandy A, Baril JG, et al. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57:1489–96. [DOI] [PubMed] [Google Scholar]
- 4. Swenson LC, Min JE, Woods CK, et al. HIV Drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS 2014; 28:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antiretroviral Therapy Cohort Collaboration (ART-CC); Vandenhende MA, Ingle S, May M, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29:373–83. [DOI] [PubMed] [Google Scholar]
- 6. Bernal E, Gómez JM, Jarrín I, et al. Low-level viremia is associated with clinical progression in HIV-infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr 2018; 78:329–37. [DOI] [PubMed] [Google Scholar]
- 7. Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18:188–97. [DOI] [PubMed] [Google Scholar]
- 8. Joya C, Won SH, Schofield C, et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 2019; 69:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming J, Mathews WC, Rutstein RM, et al. Low level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS 2019; 33:2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esber A, Polyak C, Kiweewa F, et al. Persistent low-level viremia predicts subsequent virologic failure: is it time to change the third 90? Clin Infect Dis 2019; 69:805–12. [DOI] [PubMed] [Google Scholar]
- 11. Cuzin L, Flandre P, Allavena C, et al. Low-level viral loads and virological failure in the integrase strand transfer era. J Antimicrob Chemother 2023; 78:1111–6. [DOI] [PubMed] [Google Scholar]
- 12. Hofstra LM, Mudrikova T, Stam AJ, et al. Residual viremia is preceding viral blips and persistent low-level viremia in treated HIV-1 patients. PLoS One 2014; 9:e110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pernas B, Grandal M, Pertega S, et al. Any impact of blips and low-level viraemia episodes among HIV-infected patients with sustained virological suppression on ART? J Antimicrob Chemother 2016; 71:1051–5. [DOI] [PubMed] [Google Scholar]
- 14. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. 2022. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. Accessed 2 October 2022.
- 15. Aamer HA, Mc Clure J, Ko D, et al. Cells producing residual viremia during antiretroviral treatment appear to contribute to rebound viremia following interruption of treatment. PLoS Pathog 2020; 16:e1008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobin NH, Learn GH, Holte SE, et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 2005; 79:9625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wirden M, Todesco E, Valantin MA, et al. Low-level HIV-1 viraemia in patients on HAART: risk factors and management in clinical practice. J Antimicrob Chemother 2015; 70:2347–53. [DOI] [PubMed] [Google Scholar]
- 18. Bachmann N, von Siebenthal C, Vongrad V, et al. Determinants of HIV-1 reservoir size and long-term dynamics during suppressive ART. Nat Commun 2019; 10:3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorenzo-Redondo R, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature 2016; 530:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vancoillie L, Hebberecht L, Dauwe K, et al. Longitudinal sequencing of HIV-1 infected patients with low-level viremia for years while on ART shows no indications for genetic evolution of the virus. Virology 2017; 510:185–93. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki K, Levert A, Yeung J, et al. HIV-1 viral blips are associated with repeated and increasingly high levels of cell-associated HIV-1 RNA transcriptional activity. AIDS 2021; 35:2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Young J, Rickenbach M, Calmy A, et al. Transient detectable viremia and the risk of viral rebound in patients from the Swiss HIV cohort study. BMC Infect Dis 2015; 15:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sörstedt E, Nilsson S, Blaxhult A, et al. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis 2016; 16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dijkstra S, Hofstra LM, Mudrikova T, et al. Lower incidence of HIV-1 blips observed during integrase inhibitor-based combination antiretroviral therapy. J Acquir Immune Defic Syndr 2022; 89:575–82. [DOI] [PubMed] [Google Scholar]
- 25. The RESPOND Study Group . How to RESPOND to modern challenges for people living with HIV: a profile for a new cohort consortium. Microorganisms 2020; 8:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 27. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus Abacavir-Lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 28. Sörstedt E, Tetens MM, Nilsson S, et al. Impact of pre-antiretroviral treatment HIV-RNA on time to successful virological suppression and subsequent virological failure—two nationwide, population-based cohort studies. AIDS 2023; 37:279–86. [DOI] [PubMed] [Google Scholar]
- 29. Sax PE, Tierney C, Collier AC, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med 2009; 361:2230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 31. Álvarez H, Rava M, Martínez C, et al. Predictors of low-level HIV viremia and virological failure in the era of integrase inhibitors: a Spanish nationwide cohort. HIV Med 2022; 23:825–36. [DOI] [PubMed] [Google Scholar]
- 32. Lambert-Niclot S, Boyd A, Fofana D, et al. INSTI-based triple regimens in treatment-naïve HIV-infected patients are associated with HIV-RNA viral load suppression at ultralow levels. Open Forum Infect Dis 2019; 6:ofz177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pyngottu A, Scherrer AU, Kouyos R, et al. Predictors of virological failure and time to viral suppression of first-line integrase inhibitor-based antiretroviral treatment. Clin Infect Dis 2021; 73:e2134-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossetti B, Fabbiani M, Di Carlo D, et al. Effectiveness of integrase strand transfer inhibitor-based regimens in HIV-infected treatment-naive individuals: results from a European multi-cohort study. J Antimicrob Chemother 2021; 76:2394–9. [DOI] [PubMed] [Google Scholar]
- 35. Arribas JR, Thompson M, Sax PE, et al. Brief report: randomized, double-blind comparison of tenofovir alafenamide (TAF) vs tenofovir disoproxil fumarate (TDF), each coformulated with elvitegravir, cobicistat and emtricitabine (E/C/F) for initial HIV-1 treatment: week 144 results. J Acquir Immune Defic Syndr 2017; 75:211–8. [DOI] [PubMed] [Google Scholar]
- 36. Eron JJ, Rockstroh JK, Reynes J, et al. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect Dis 2011; 11:907–15. [DOI] [PubMed] [Google Scholar]
- 37. Cahn P, Kaplan R, Sax PE, et al. Raltegravir 1200 mg once daily versus raltegravir 400 mg twice daily, with tenofovir disoproxil fumarate and emtricitabine, for previously untreated HIV-1 infection: a randomised, double-blind, parallel-group, phase 3, non-inferiority trial. Lancet HIV. 2017; 4:e486–94. [DOI] [PubMed] [Google Scholar]
- 38. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Halvas EK, Joseph KW, Brandt LD, et al. HIV-1 viremia not suppressible by antiretroviral therapy can originate from large T cell clones producing infectious virus. J Clin Invest 2020; 130:5847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seiger KW, Lian X, Parsons E, et al. Selection of intact HIV-1 proviruses in deep latency during long-term ART. Presented at: Conference on Retroviruses and Opportunistic Infections (CROI), Hybrid conference (virtual and in-person in Denver, Colorado); 12–16 February 2022 with symposia on 22–24 February 2022. Abstract 68.
- 42. Dufour C, Gantner P, Fromentin R, Chomont N. The multifaceted nature of HIV latency. J Clin Invest 2020; 130:3381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mohammadi A, Etemad B, Zhang X, et al. Viral and host mediators of persistent low-level viremia. Presented at Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington; 19–22 February 2023. Abstract 137.
- 44. White JA, Wu F, Yasin S, et al. Clonally expanded HIV-1 proviruses with 5′-leader defects can give rise to nonsuppressible residual viremia. J Clin Invest 2023; 133:e165245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Porter DP, Kulkarni R, Garner W, et al. Viral blips were infrequent in treatment-naive adults treated with rilpivirine/emtricitabine/tenofovir DF or efavirenz/emtricitabine/tenofovir DF through 96 weeks. Antivir Ther 2017; 22:495–502. [DOI] [PubMed] [Google Scholar]
- 46. Gagliardini R, Gianotti N, Maggiolo F, et al. Durability of rilpivirine-based versus integrase inhibitor-based regimens in a large cohort of naïve HIV-infected patients starting antiretroviral therapy. Int J Antimicrob Agents 2021; 58:106406. [DOI] [PubMed] [Google Scholar]
- 47. Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis 2000; 30(suppl 2):S171–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.