Abstract
Immunothrombosis—immune-mediated activation of coagulation—is protective against pathogens, but excessive immunothrombosis can result in pathological thrombosis and multiorgan damage, as in severe coronavirus disease 2019 (COVID-19). The NACHT-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome produces major proinflammatory cytokines of the interleukin (IL)-1 family, IL-1β and IL-18, and induces pyroptotic cell death. Activation of the NLRP3 inflammasome pathway also promotes immunothrombotic programs including release of neutrophil extracellular traps and tissue factor by leukocytes, and prothrombotic responses by platelets and the vascular endothelium. NLRP3 inflammasome activation occurs in patients with COVID-19 pneumonia. In preclinical models, NLRP3 inflammasome pathway blockade restrains COVID-19-like hyperinflammation and pathology. Anakinra, recombinant human IL-1 receptor antagonist, showed safety and efficacy and is approved for the treatment of hypoxaemic COVID-19 patients with early signs of hyperinflammation. The non-selective NLRP3 inhibitor colchicine reduced hospitalization and death in a subgroup of COVID-19 outpatients but is not approved for the treatment of COVID-19. Additional COVID-19 trials testing NLRP3 inflammasome pathway blockers are inconclusive or ongoing. We herein outline the contribution of immunothrombosis to COVID-19-associated coagulopathy, and review preclinical and clinical evidence suggesting an engagement of the NLRP3 inflammasome pathway in the immunothrombotic pathogenesis of COVID-19. We also summarize current efforts to target the NLRP3 inflammasome pathway in COVID-19, and discuss challenges, unmet gaps, and the therapeutic potential that inflammasome-targeted strategies may provide for inflammation-driven thrombotic disorders including COVID-19.
Keywords: Coagulation, COVID-19, Endotheliopathy, Interleukin-1, NLRP3 inflammasome, Pyroptosis, Thrombosis
1. Introduction
Innate immunity is an essential first-line defence against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Dysregulated innate immune responses can be however detrimental, resulting in exaggerated release of proinflammatory cytokines that exacerbate tissue damage.1,2 Hyperinflammation in coronavirus disease 2019 (COVID-19) has been indeed linked to the development of acute respiratory distress syndrome (ARDS), thrombosis, and multiorgan injury.2,3
Inflammation and coagulation are closely intertwined.4,5 In order to limit pathogen replication and spread, innate immunity triggers the coagulation system including the blood coagulation cascade, platelets, and the vascular endothelium in an evolutionarily conserved process known as immunothrombosis.5–7 Nevertheless, excessive immunothrombosis can promote coagulopathy, leading to thrombosis and tissue ischaemia.5–7 The clinical relevance of immunothrombosis in COVID-19 may be attested by the increased incidence of macrovascular, primarily venous, and microvascular thrombosis observed in patients with severe disease.8–11 COVID-19 lungs exhibit diffuse alveolar damage with abundant inflammatory exudates and leukocyte infiltration next to endothelialitis, microangiopathy, and fibrin deposits.9–11 Several lines of evidence suggest that hyperinflammation contributes to excessive activation of immunothrombosis pathways sustaining the hypercoagulability, endotheliopathy, and thrombocytopathy occurring in COVID-19-associated coagulopathy (CAC).5–8,11
The intracellular innate immune receptor NACHT-, LRR-, and pyrin domain-containing protein 3 (NLRP3) is a regulator of inflammatory responses to sterile and non-sterile noxious stimuli including viruses.12,13 NLRP3 inflammasome activation leads to production of biologically active interleukin (IL)-1β and IL-18, two potent, primordial proinflammatory cytokines with prothrombotic activity, and can induce pyroptosis, a proinflammatory form of lytic cell death.12–14 Prompt and adequate activation of innate immunity is a prerequisite for adaptive immune responses, crucial to control infection, and to achieve immunogenicity following infection or vaccination.1 Nevertheless, dysregulated inflammatory responses, in part sustained by exuberant NLRP3 inflammasome activation, could be detrimental and may favour COVID-19 progression as well as the development of CAC and COVID-19-associated thrombosis.1–3,15,16
2. Immunothrombosis and CAC
Immunothrombosis, considered an intravascular effector of innate immunity, refers to complex networks of cellular interactions and molecular pathways aimed at limiting pathogen survival and spread.5,17 Conversely, thromboinflammation describes the immune-mediated activation of coagulation under sterile conditions.5,17 These processes are sustained by the activation of immune cells that release a number of proinflammatory mediators, with crucial contributions from platelets and the endothelium, that synergistically prime the activation of blood coagulation factors.5,17
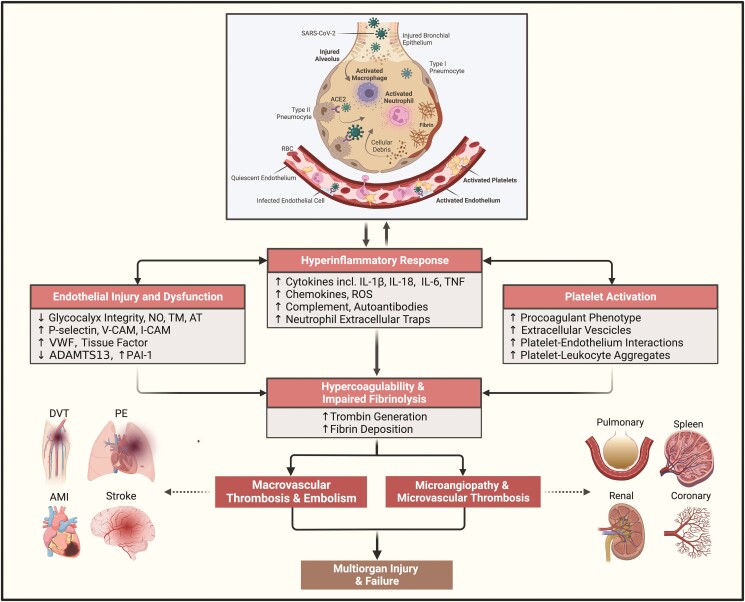
Distinctive features of CAC have been widely documented (Figure 1).7,18–20 These include overproduction of procoagulant factors such as tissue factor (TF), factors VIII and V, fibrinogen and von Willebrand factor (VWF), in parallel with downregulation of endogenous anticoagulants (e.g. TF pathway inhibitor, protein C, and antithrombin), resulting in a hypercoagulable milieu capable of increased thrombin generation.7,18–20 The increased release of proinflammatory mediators can affect leukocyte, endothelial, and platelet responses both locally (i.e. in the lungs) and systemically.5–7 Among multiple proinflammatory cytokines, IL-1β and downstream IL-6 can induce procoagulant programmes and suppress anticoagulant mechanisms in a concentration-dependent manner21–23 and have been implicated in the pathogenesis of CAC.6,15,16,24
Figure 1.
Overview of the pathogenetic mechanisms implicated in COVID-19-associated coagulopathy. Abbreviations: ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin Type 1 motifs, member 13; ACE2, angiotensin-converting enzyme 2; AMI, acute myocardial infarction; AT, antithrombin; DVT, deep vein thrombosis; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; PE, pulmonary embolism; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TM, thrombomodulin; TNF, tumour necrosis factor; VCAM-1, vascular cell adhesion molecule 1; VWF, von Willebrand factor. Figure created with BioRender.com.
Imbalance in fibrinolysis has also been described in CAC.7,20 The fibrinolytic agents tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) physiologically work to prevent fibrin accumulation.20 In severe COVID-19, increased levels of plasminogen activator inhibitor-1 and thrombin-activatable fibrinolysis inhibitor tend to outbalance tPA/uPA activity, with resultant intra-alveolar fibrin deposition.25–27 The extremely high levels of D-dimer observed in severe COVID-19 might not only reflect fibrinolytic activation secondary to clot formation but also be indicative of the intensity of the hyperinflammatory response, as proinflammatory cytokines including IL-1β and tumour necrosis factor (TNF) can induce endothelial expression of both fibrinolytic and antifibrinolytic molecules.20 Eventually, the net increase in intra-alveolar fibrin production can be considered the culmination of immunothrombosis since fibrin enables the entrapment of the invading virus.5–7,20
Endotheliopathy and thrombocytopathy are other major features of CAC.6,7,28 SARS-CoV-2 has the ability to directly infect the vascular endothelium decreasing its physiological antithrombotic properties. Activated endothelial cells can also release proinflammatory cytokines (e.g. IL-1α, IL-1β, IL-6, and TNF) acting in an autocrine and paracrine fashion to systemically induce a prothrombotic endothelial phenotype.6,7,28 Increased endothelial expression of TF and VWF facilitate the assembly of coagulation modules and recruitment of platelets.29,30 Studies have shown that increases in circulating VWF in COVID-19 patients are associated with consumption in ADAMTS13 (disintegrin and metalloproteinase with a thrombospondin Type 1 motif, member 13), resulting in the generation of ultralarge VWF multimers, enhanced platelet–endothelial interactions and thrombotic microangiopathy.30,31 Increased procoagulant platelets and platelet–monocyte aggregates were associated with worse COVID-19 prognosis.32,33 Plasma from COVID-19 patients was found to induce activation of naïve platelets. Platelets isolated from severely ill COVID-19 patients induced TF expression by monocytes from healthy donors, which was suppressed by inhibiting platelet P-selectin and integrin αIIb/β3, thus suggesting that altered platelet–endothelial and platelet–monocyte interactions contribute to CAC.32 Further experiments revealed that TF produced by SARS-CoV-2-infected cells potently activates platelets isolated from healthy subjects.34 This phenomenon required coagulation factors X, VII, and II and involved thrombin-mediated activation of platelet protease-activated receptors.34 Platelets from COVID-19 patients also exhibit procoagulant phenotypic and functional profiles, including constitutive expression of P-selectin, tendency to form aggregates with leukocytes, and enhanced release of procoagulant extracellular vesicles, proinflammatory cytokines, chemokines, and growth factors.33,35,36
Activated platelets also contribute to the generation of neutrophil extracellular traps (NETs).5,7,28,37 NETs are extruded web-like structures composed of a DNA backbone, histones, and proteolytic enzymes with antibacterial properties such as neutrophil elastase, myeloperoxidase, and cathepsin G.37 NETs promote thrombosis through multiple mechanisms including activation of blood coagulation factors XII and II, inhibition of anticoagulant pathways (e.g. TF pathway inhibitor, and antithrombin), and recruitment of platelets and leukocytes, and by providing a scaffold, resistant to fibrinolysis, for thrombus stability and enlargement.37 Increased circulating NETs-derived products including cell-free DNA, myeloperoxidase, and citrullinated histone H3 were detected in COVID-19 patients who developed thrombosis.38 Histopathology of COVID-19 organs also demonstrated thrombi enriched with abundant NETs and neutrophil–platelet aggregates.39–41 In addition, sera and neutrophils isolated from patients with severe COVID-19 displayed high basal NETs levels, and incubation of healthy neutrophils with COVID-19 plasma potently triggered NETosis.38–40,42
Taken together, these and other observations suggest that hyperinflammatory responses occurring in severe COVID-19 can trigger excessive activation of immunothrombosis pathways contributing to endotheliopathy, thrombocytopathy, and hypercoagulability, eventually resulting in microvascular and macrovascular thrombosis.5–7
3. The NLRP3 inflammasome pathway
NOD-like receptors (NLRs) and Toll-like receptors (TLRs) are sets of phylogenetically conserved receptors, termed pattern recognition receptors (PRRs), that mediate innate immunity by binding pathogen-associated molecular-patterns (PAMPs) and damage-associated molecular-patterns (DAMPs).43 NLRs include the intracellular protein NLRP3.43 Upon stimulation, NLRP3, along with the apoptosis-associated speck-like protein containing a CARD (ASC) adaptor protein and caspase-1, form the NLRP3 inflammasome.44,45 This macromolecular effector complex produces active cytokines of the IL-1 family including IL-1β and IL-18 and can induce pyroptosis.12–14 NLRP3 is the most studied inflammasome; however, other inflammasomes (e.g. NLRP1 and AIM2) have been characterized and reviewed elsewhere.13
The sequence of events leading to assembly and activation of the NLRP3 inflammasome is finely regulated at multiple levels. In most cells, NLRP3 inflammasome activation may require two distinct signals:46 priming, which leads to the production of inflammasome components and substrates, and triggering, which culminates with inflammasome activation (see Supplementary material online, Figure S1).12,13 Priming is promoted by a wide array of PAMPs and DAMPs that activate PRRs such as TLRs or other receptors, resulting in the translocation of nuclear factor-κB (NF-κB) into the nucleus, promoting the transcription of inflammasome components and substrates.12,13 The second trigger of inflammasome assembly can be sourced by several stimuli including ATP-mediated activation of the purinoreceptor P2X7, reactive oxygen species (ROS) as well as mitochondrial and lysosomal destabilization.12,13 Altogether, these changes reduce intracellular potassium, which is detected by NLRP3 that oligomerizes to form the inflammasome.12,13 NLRP3 oligomers engage ASC into filaments enabling recruitment and subsequent autoactivation of caspase-1. Caspase-1 then cleaves pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18.12–14 Once released extracellularly, pro-IL-1β can also be processed by proteolytic enzymes into its mature form, independently of caspase-1.14,47 Conversely, pro-IL-1α lacks a caspase-1 processing site and is already active in its precursor form but can be cleaved into IL-1α by several enzymes that can augment its biological activity.48 When released upon necrotic cell death, IL-1α can serve as an ‘alarmin’ and induce IL-1β49,50 or function upon cell–cell contact when anchored to the cell membrane.51
Caspase-1 also cleaves gasdermin D (GSDMD) to produce N-terminal fragments that migrate to the cell membrane forming pores that enable extracellular release of IL-1β and IL-18.12–14 Activation of caspase-1 and GSDMD can also induce pyroptosis.12–14 Unlike other forms of programmed cell death, pyroptosis is characterized by cell swelling and rupture, with release of intracellular proinflammatory content. Pyroptosis can also occur through non-canonical inflammasome pathways involving caspase-4/caspase-5 in humans and caspase-11 in mice.52
Once released, IL-1β and IL-18 exert pleiotropic effects by binding, respectively, to the IL-1 receptor Type I (IL-1RI) and IL-18 receptor α (IL-18Rα) on target cells, leading to NF-κB activation and subsequent transcription of a wide array of proinflammatory genes.14,53 IL-1α also binds to IL-1RI.14,48 Endogenously occurring receptor inhibitors, namely, IL-1 receptor antagonist (IL-1Ra) and IL-18 binding protein (IL-18BP), can antagonize IL-1RI and IL-18Rα, respectively.14,53–55 Several other regulatory mechanisms that modulate NLRP3 inflammasome activation and IL-1 signaling are detailed elsewhere.12–14,43,48,52,53,56
4. NLRP3 inflammasome activation and hyperinflammation in COVID-19
Marked elevations in IL-6 and C-reactive protein (CRP), inflammatory biomarkers downstream of IL-1β widely used for cardiovascular risk stratification,57 have been observed in severely ill patients with COVID-19 and predict adverse outcomes.58,59 Increased levels of IL-1β were found in monocytes and sera from COVID-19 patients, however, they did not strictly correlate with disease severity.60–64 This could be related, at least in part, to the extremely short half-life of IL-1β and is also observed in other IL-1β-driven diseases.15,65 Because of its longer half-life, IL-1Ra, induced by IL-1β, may serve as a proxy of IL-1β activity.14,65,66 Serum IL-1Ra levels correlate with COVID-19 severity.63,67,68 Accordingly, analyses of bronchoalveolar lavages, reflecting the pulmonary microenvironment, detected marked IL-1β upregulation.69,70 Increased IL-1β expression was also demonstrated in the lungs of patients with fatal COVID-19.71,72 Unlike IL-1β, production of IL-18 strictly depends on inflammasome activation.14,53 Plasma IL-18 concentrations correlate with COVID-19 severity and predict mortality.63,67,68,73
Multiple studies provided direct evidence of NLRP3 inflammasome activation in patients with COVID-19.73–79 NLRP3 inflammasome genetic variants have been associated with worse disease severity,80 and single-cell transcriptome analysis of airway fluids from COVID-19 patients revealed elevated expression of inflammasome-related genes.81 Peripheral blood monocytes from COVID-19 patients exhibit distinctive features of inflammasome activation.73–75,79,82 Further experiments suggested that monocytes were most responsive to NLRP3 inflammasome stimulation in vitro, while selected subsets of neutrophils and granulocytes had defects in inflammasome activation, presumably reflecting immune exhaustion during advanced stages of COVID-19.78 Importantly, this inflammasome signature was shown to correlate with COVID-19 severity and predict adverse clinical evolution.78,83 Consistent with these observations, overexpression of inflammasome pathway components has been demonstrated in lung tissues from COVID-19 patients, primarily registering in leukocytes and, to a lesser extent, in pneumocytes and vascular endothelial cells.73,76,77,79
Cellular and animal experiments indicate that distinct SARS-CoV-2 constituents, including the spike protein, may trigger NLRP3 inflammasome activation in monocytes and macrophages, amplifying the inflammatory response and contributing to COVID-19 pathogenesis.64,79,84–88 The viral entry receptor angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine 2 (TMPRSS2), and Fcγ receptors were shown to mediate NLRP3 inflammasome activation following SARS-CoV-2 exposure, or, alternatively, SARS-CoV-2 can directly interact with the inflammasome intracellularly.85,86,89 One report suggested that SARS-CoV-2 RNA could induce monocyte IL-1β release through caspase-1/caspase-8, potassium efflux, and autophagy, independently from GSDMD and pyroptosis.86 In monocytes, the nucleocapsid protein of SARS-CoV-2 was found to directly bind NLRP3 monomers, facilitating inflammasome assembly and activation.89 Injection of SARS-CoV-2 nucleocapsid protein into mice markedly increased IL-1β and IL-6 plasma levels, resulting in aggravated lung injury and worse survival.89 Murine overexpression of viroporin ORF3a also induced robust NLRP3 activation, followed by GDSMD cleavage and IL-1β release.90 SARS-CoV-2 glycoproteins elicited similar effects in cultured human macrophages, which were suppressed in NLRP3-/GSDMD-knockout cells.91 Conversely, other studies found that monocyte exposure to either SARS-CoV-2 envelope or nucleocapsid proteins could exert inhibitory effects on the inflammasome.92,93 Since in vitro exposure to distinct SARS-CoV-2 constituents might elicit different cellular responses compared to exposure to the viable virus and that endotoxin contamination could not be excluded, additional studies using in vivo infection models are warranted. The effects of distinct SARS-CoV-2 variants on NLRP3 inflammasome activation and their clinical implications also remain to be elucidated.
Assembly of the NLRP3 inflammasome after SARS-CoV-2 exposure was also reported in epithelial and endothelial cells as well microglial and hematopoietic stem cells, suggesting that multiple cell lineages besides leukocytes can sustain hyperinflammation following SARS-CoV-2 infection, potentially contributing to the extra-pulmonary manifestations of COVID-19.77,85,90,94 Persistent inflammasome pathway activation is found in monocytes and macrophages derived from convalescent COVID-19 patients after several weeks from acute infection and in subjects recovering from mild disease.64,83 Additional studies are however required to evaluate the contribution of the NLRP3 inflammasome pathway to the pathogenesis of long COVID-19.
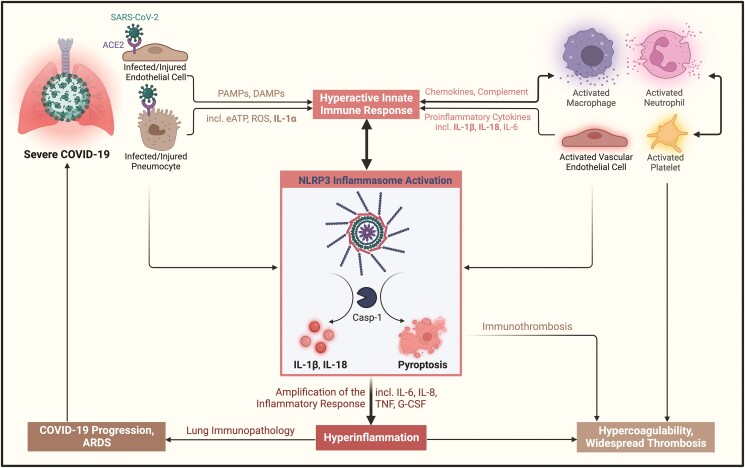
Besides direct SARS-CoV-2 cytotoxicity, indirect viral effects and host-related mechanisms may sustain NLRP3 inflammasome activation in COVID-19.15,16 Lysis of virus-infected cells induces massive generation of DAMPs including ATP, ROS, complement, and a plethora of proinflammatory mediators. Among these, IL-1α released from damaged epithelial and endothelial cells is a robust inducer of inflammatory responses by adjacent and remote cells.95,96 Such paracrine and endocrine mechanisms can therefore contribute to the systemic, inflammatory manifestations of COVID-19 (Figure 2).15,16 This notion might be supported by the fact that supernatants collected from SARS-CoV-2-infected epithelial cells triggered intense IL-1β production by naïve macrophages.97 In addition, patients with comorbidities such as diabetes, obesity, or heart disease, characterized by basal NLRP3 inflammasome activation, tend to be more prone to experience hyperinflammatory responses and adverse outcomes, suggesting that pre-existing inflammation may potentially prime a favourable substrate for inflammasome hyperactivation when infection occurs.15,16
Figure 2.
NLRP3 inflammasome pathway activation in COVID-19-associated hyperinflammation and lung immunopathology. Abbreviations: ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; Casp-1, caspase-1; DAMPs, damage-associated molecular patterns; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; eATP, extracellular ATP; PAMPs, pathogen-associated molecular-patterns; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Figure created with BioRender.com.
Abrogation of the NLRP3 inflammasome pathway suppresses immune hyperactivation and mitigates COVID-19 pathology.82–84,88–90,98 The selective NLRP3 blocker MCC950 inhibited spontaneous and lipopolysaccharide-induced secretion of IL-1β and IL-18 by monocytes isolated from COVID-19 patients. Similar effects were observed in monocytes isolated from COVID-19 patients receiving anakinra, a recombinant human IL-1Ra, blocking the activity of both IL-1α and IL-1β.82 In a ACE2 humanized mouse model of SARS-CoV-2 infection, genetic deletion or pharmacological inhibition of the NLRP3 inflammasome reduced proinflammatory cytokine release, cell death, and viral load, alleviating lung injury.98 In another study, Sefik et al. demonstrated that macrophage NLRP3 inflammasome activation is necessary for COVID-19-like immunopathology.88 The same group also showed that selective NLRP3 and caspase-1 inhibition reverses chronic lung injury but associates with higher viral titres.88 In addition, the NLRP3 inflammasome was shown to mediate the innate-adaptive immune crosstalk during SARS-CoV-2 infection and following mRNA vaccination.64,87 These observations collectively suggests that adequate NLRP3 inflammasome pathway activation aids in containing infection and achieving immune memory and vaccine immunogenicity, whereas excessive activation can sustain hyperinflammation and immunopathology. Additional research is necessary to clarify the precise triggers together with virus- and host-related factors regulating NLRP3 inflammasome pathway activation and to establish the exact contribution of distinct inflammasome pathway components to disease pathogenesis.15,16
5. NLRP3 inflammasome pathway activation as a potential pathogenetic mechanism contributing to immunothrombosis and CAC
The association between infection, inflammation, and thrombosis has long been established.4,5,99 In immunothrombosis, innate immune cells—primarily macrophages and neutrophils—interact with platelets and the endothelium to synergistically activate the coagulation system.4,5,17,99 Thrombosis is frequent in severe COVID-19 and affects prognosis.8–11,100,101 Increased levels of CRP at hospitalization independently associate with in-hospital venous thromboembolism, critical illness, and mortality.59,102 Higher pre-discharge CRP also predicts the occurrence of post-discharge deep vein thrombosis,103 suggesting that both acute and residually heightened inflammation may promote CAC, augmenting thrombotic risk in the short and longer terms.104,105
The prothrombotic effects of inflammasome-derived IL-1β and IL-18 have been widely recognized.106 IL-1β and IL-18 released by damaged cells increase vascular permeability and induce recruitment and transmigration of macrophages and neutrophils at the site of injury. In turn, IL-1β and IL-18 produced by leukocytes can activate the endothelium and enhance platelet recruitment, activation, and aggregation.106 In a feed-forward loop, activated endothelial cells and platelets can further secrete proinflammatory IL-1 cytokines sustaining immunothrombosis programmes.106 Although inflammasome activation and IL-1β are established players in arterial thrombosis,5,107 their roles in venous thrombosis under sterile- and pathogen-mediated inflammatory environments have only recently begun to be elucidated.99
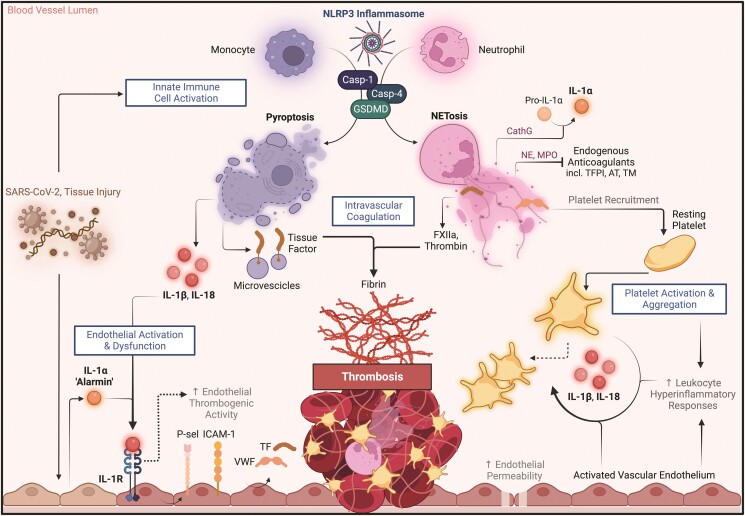
In macrophages, NLRP3 inflammasome activation and subsequent caspase-1 cleavage induces abundant release of highly procoagulant microvesicles containing TF (Figure 3).108 In a murine endotoxaemia model, inflammasome activation triggered, through caspase-1/caspase-11 and GSDMD, monocyte pyroptosis with subsequent TF release and widespread thrombosis.109 GSDMD membrane pore formation also favours intracellular calcium entry, which promotes externalization of phosphatidylserine and enhances TF procoagulant activity.110 Activation of the inflammasome, caspase-1/-11 and GSDMD might therefore represent IL-1-independent mechanisms sustaining immunothrombosis. This concept might be supported by the extreme levels of TF-rich extracellular vesicles found in the plasma of COVID-19 patients, which positively correlated with the intensity of the inflammatory response and mortality.29,111,112 A role for caspase-11 and its human homolog caspase-4 in promoting immunothrombosis in COVID-19 has been identified. Compared to GSDMD-deficient and wide-type mice, SARS-CoV-2-infected mice lacking caspase-11 exhibited restrained lung neutrophil recruitment and expression of IL-1β and IL-6 and were protected from vascular damage including thickening, obliteration, angiogenesis, and neovascularization.113 Additionally, caspase-11 deficiency drastically reduced pulmonary expression of VWF, while preserving Kruppel-like factor 2, an endothelial transcription factor that maintains vascular integrity.113 Mice deficient in caspase-11 also exhibited more pronounced defects in neutrophil movement and function and suppressed NETosis.113
Figure 3.
Proposed role of NLRP3 inflammasome pathway activation in COVID-19-associated coagulopathy and immunothrombosis. Abbreviations: AT, antithrombin; FXIIa, factor XIIa; CathG, cathepsin G; GSDMD, gasdermin D; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; IL-1R, interleukin-1 receptor; MPO, myeloperoxidase; NE, neutrophil elastase; NET, neutrophil extracellular trap; P-sel, P-selectin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TF, tissue factor; TFPI, tissue factor pathway inhibitor; TM, thrombomodulin; VWF, von Willebrand factor. Figure created with BioRender.com.
NETs centrally contribute to the immunothrombotic pathogenesis of microvascular and macrovascular thrombosis.5,7,37–39,99 Activation of the NLRP3 inflammasome pathway in neutrophils can induce, through GSDMD, the release of NETs.114 In turn, NETs sustain inflammasome activation and production of IL-1β and IL-18.114–117 NLRP3 inflammasome assembly was demonstrated in neutrophils derived from peripheral blood and tracheal aspirates of subjects with severe COVID-19.118 NLRP3 inflammasome mostly assembled in neutrophils with intact polylobulated nuclei, correlating with histone H3 citrullination and localizing with the microtubule organizing centre long before NETosis, which suggests an upstream role for the inflammasome in NETs formation during severe COVID-19.118 In another study, blood neutrophils from COVID-19 patients displayed elevated expression of active caspase-1/caspase-4 and GSDMD, which accumulated in the plasma membrane and NETs, with higher expression detected in severely versus moderately ill patients.81 A positive relationship between GSDMD and NETs levels was also found in the sera of these patients.81 Importantly, pharmacological inhibition of caspases or GSDMD abrogated NETs release by SARS-CoV-2-infected neutrophils.81 Notably, abrogation of the NLRP3 inflammasome pathway was previously shown to drastically reduce NETosis and thrombogenesis in murine vein thrombosis models.119,120 IL-1α also contributes to enhance NETs-induced endothelial activation and thrombogenicity by inducing the expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and TF, which is abolished in the presence of antibodies against IL-1α or IL-1Ra.121 Interestingly, cathepsin G released with NETs potentiated the prothrombotic endothelial effects of IL-1α by cleaving the pro-IL-1α precursor into its mature form.121
Endothelial cells and platelets represent, with innate immune cells, key cellular drivers of immunothrombosis.28,99 These cells also constitute major compartments for NLRP3 assembly and critical targets for IL-1 activity.122–125 Activation of the NLRP3 inflammasome was shown to induce, through TLR4 and Bruton’s tyrosine kinase, platelet activation, and aggregation, leading to thrombus formation.126,127 Platelet–monocyte interactions engage reciprocal activation loops feeding immunothrombosis in COVID-19.32,33 Monocytes from patients with severe COVID-19 displayed enhanced platelet binding and IL-1β secretion in response to P-selectin and fibrinogen.33 Platelets also potentiate monocyte immunothrombotic responses including cytokines release and TF expression during SARS-CoV-2 infection.33 In particular, platelet adhesion was proposed by Hottz et al. as an upstream event inducing proinflammatory cytokine secretion and TF expression, while TF sustains immunothrombosis by triggering additional IL-1β and TNF release.33
Endothelial NLRP3 inflammasome activation and proinflammatory IL-1 cytokines are also able to promote vascular permeability and dysfunction.128 Exposure of healthy human endothelial cells of different anatomical origins to exosomes isolated from COVID-19 patients induces robust increases in mRNA expression of NLRP3, caspase-1 and IL-1β, with activated caspase-1 being detected in the culture medium, suggesting that endothelial NLRP3 inflammasome activation contributes to the systemic endotheliopathy characteristic of CAC.129 Proinflammatory IL-1 cytokines reportedly enhance the endothelial expression of adhesion molecules (e.g. P-selectin, ICAM-1, and VCAM-1) and procoagulant factors (e.g. VWF and TF) and reduce anticoagulant molecules (e.g. protein C and thrombomodulin).21,22 In turn, TF and fibrin produced during thrombogenesis can work to potentiate IL-1-driven endothelial activation (Figure 3).130,131 Importantly, interrupting this vicious circuit by abrogation of the NLRP3 inflammasome pathway or IL-1 signalling significantly reduces thrombus formation and propagation in preclinical models of venous thrombosis.119,120,132–136
6. NLRP3 inflammasome pathway blockade in COVID-19
The NLRP3 inflammasome and IL-1 cytokines play a central role in the pathogenesis of several rheumatologic and cardiovascular diseases.65,107,137–139 Agents targeting IL-1 are clinically available for the treatment of multiple inflammatory disorders.65,138,139 Early in the pandemic, IL-1 blockers were repurposed for COVID-19. Initial data from observational studies suggested that anakinra and the anti-IL-1β monoclonal antibody canakinumab could blunt hyperinflammation associated with COVID-19, possibly improving outcomes.140–142
Trials with anakinra yielded overall favourable yet heterogeneous results (Table 1).143–147 Findings from studies including the REMAP-CAP multiplatform trial showed that anakinra was safe and well tolerated but did not provide additional benefit over usual care in hospitalized patients with severe-to-critical COVID-19.143–145 In the SAVE trial, addition of anakinra to usual care reduced progression to severe respiratory failure at Day 14 and mortality at Day 30 in patients with moderate-to-severe COVID-19 and elevated soluble urokinase plasminogen activator receptor (suPAR), an early indicator of hyperinflammation and predictor of COVID-19 progression.146 A larger suPAR-guided trial with anakinra (SAVE-MORE) later showed that anakinra significantly shortened hospital stay and improved 28-day clinical status and mortality compared to placebo in COVID-19 patients without invasive mechanical ventilation.147
Table 1.
Main clinical trials targeting the NLRP3 inflammasome pathway in patients with COVID-19
| Trial | Sample size | Study design | Intervention | Main inclusion criteria | Main findings | NCT/PMID number | |||
|---|---|---|---|---|---|---|---|---|---|
| R | DB | PC | M | ||||||
| Anakinra | |||||||||
| CORIMUNO-ANA-1 (Cohort Multiple Randomized Controlled Trials Open-Label of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients-Anakinra trial) | 114 | X | X | Anakinra 400 mg/day IV on Days 1–3, followed by 200 mg on day 4 and 100 mg on day 5 | Hospitalized patients with severe COVID-19 with CRP ≥25 mg/L (59 received anakinra) | Anakinra did not significantly reduce MV or death at Day 4 and Day 14; no significant increase in AEs; trial interrupted prematurely | 04341584 | ||
| COV-AID (Treatment of COVID-19 Patients with Anti-interleukin Drugs) | 342 | X | X | Anakinra 100 mg daily SC for 28 days or until discharge | Hospitalized patients with severe COVID-19 and laboratory signs of cytokine release syndrome (112 received anakinra) | Anakinra did not shorten time to clinical improvement; SAEs events similar across study arms | 04330638 | ||
| REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-Acquired Pneumonia) | 2216 | X | X | Anakinra 300 mg IV on Day 1, followed by 400 mg/day for 14 days or until free from IMV or discharge from ICU | Hospitalized patients with critical COVID-19 (378 received anakinra) | Anakinra did not reduce median organ support-free days or mortality | 02735707 | ||
| SAVE (suPAR-Guided Anakinra Treatment for Management of Severe Respiratory Failure by COVID-19) | 260 | X | Anakinra 100 mg/day SC for 10 days | Hospitalized patients with moderate-to-severe COVID-19 with suPAR ≥6 ng/mL (130 received anakinra) | Anakinra significantly reduced progression to severe respiratory failure at Day 14 and mortality at Day 30 | 04357366 | |||
| SAVE-MORE (suPAR-Guided Anakinra Treatment for Management of Severe Respiratory Failure by COVID-19) | 594 | X | X | X | X | Anakinra 100 mg/day SC for 10 days | Hospitalized patients with moderate-to-severe COVID-19 with suPAR ≥6 ng/mL (405 received anakinra) | Anakinra significantly shortened hospital stay and improved clinical status and survival at Day 28; fewer AEs in the anakinra arm | 04680949 |
| Canakinumab | |||||||||
| Three C (Canakinumab in COVID-19 Cardiac Injury) | 45 | X | X | X | Canakinumab 600 mg or 300 mg as single dose IV on Day 1 | Hospitalized patients with moderate-to-critical COVID-19, elevated troponin and CRP >50 mg/L (15 received canakinumab 600 mg, 14 canakinumab 300 mg) | Canakinumab did not shorten time to clinical improvement at Day 14; AEs similar between groups | 04365153 | |
| CanCovDia (Canakinumab in Patients with COVID-19 and Type 2 Diabetes) | 116 | X | X | X | X | Canakinumab (body weight adapted dose of 450–750 mg) as single dose IV on Day 1 | Hospitalized patients with Type 2 diabetes and a BMI >25 kg/m2 (58 received canakinumab) | Canakinumab did reduce the primary composite outcome (unmatched win-ratio approach based on length of survival, ventilation, ICU stay, and hospitalization at Day 29); reduced number of anti-diabetes drugs, IL-6, and hs-CRP; SAEs similar between two arms | 04510493 |
| CAN-COVID (Study of Efficacy and Safety of Canakinumab Treatment for CRS in Participants with COVID-19-Induced Pneumonia) | 448 | X | X | X | X | Canakinumab 450, 600, or 750 mg (depending on body weight) as a single IV dose on Day 1 | Hospitalized patients with severe COVID-19 and CRP ≥20 mg/L or ferritin ≥600 µg/L (225 received canakinumab) | Canakinumab did not increase survival without IMV at Day 29 but was associated with improvement in the composite of death, IMV or use rescue therapy with tocilizumab or anakinra; fewer SAEs in canakinumab arm | 04362813 |
| Colchicine | |||||||||
| RECOVERY (Randomised Evaluation of COVID-19 Therapy) | 11,340 | X | X | Colchicine 1 mg PO at randomization and 0.5 mg after 12 h, followed by 1 mg/day for 10 days or until discharge | Hospitalized patients with moderate-to-severe COVID-19 (5610 received colchicine) | Colchicine did not reduce 28-day mortality, progression to IMV or death, or time to discharge | 04381936 | ||
| ECLA PHRI COLCOVID (Effects of Colchicine on Moderate/High-risk Hospitalized COVID-19 Patients) | 1279 | X | X | Colchicine 1.5 mg PO at randomization and 0.5 mg within 2 hours, followed by 1 mg/day for 14 days or until discharge | Hospitalized patients with moderate-to-severe COVID-19 (640 received colchicine) | Colchicine did not significantly reduce MV or 28-day mortality; diarrhoea more frequent with colchicine | 04328480 | ||
| COL-COVID (Trial to Study the Benefit of Colchicine in Patients with COVID-19) | 102 | X | Colchicine 1.5 mg PO within 48 h from hospitalization, followed by 1 mg/day for 1 week and 0.5 mg/day for 28 days | Hospitalized patients with moderate-to-severe COVID-19 (52 received colchicine) | Colchicine did not improve clinical status at Days 14 and 28 but associated with a lower risk of clinical deterioration after adjustment for risk factors and concomitant therapies | 04350320 | |||
| COLORIT (COLchicine versus Ruxolitinib and Secukinumab in Open-Label Prospective Randomized Trial in Patients with COVID-19) | 43 | X | Colchicine 1 mg/day PO for 1–3 days, followed by 0.5 mg/day for 14 days | Hospitalized patients with moderate-to-severe COVID-19 (21 received colchicine) | Colchicine improved clinical status at Day 12 or at hospital discharge and associated with reduced CPR | 33734043 | |||
| Effects of colchicine for moderate to severe COVID-19: a randomized, double-blinded, placebo-controlled clinical trial | 74 | X | X | X | Colchicine 1.5 mg/day PO for 5 days, followed by 1 mg/day for 5 days | Hospitalized patients with moderate-to-severe COVID-19 (36 received colchicine) | Colchicine shortened duration of oxygen therapy at Day 7 and hospital stay, and associated with reduced CPR; diarrhoea more frequent with colchicine | 33542047 | |
| GRECCO (The Greek Study in the Effects of Colchicine in Covid-19 Complications Prevention) | 105 | X | X | Colchicine 1.5 mg PO and 0.5 mg after 1 h, following by 1 mg/day until hospital discharge or up to 21 days | Hospitalized patients with moderate-to-severe COVID-19 (55 received colchicine) | Colchicine improved time to clinical deterioration; no significant differences in CRP and troponin between the two study arms; AEs similar, except for diarrhoea (more frequent with colchicine) | 04326790 | ||
| ColCORONA (Colchicine Coronavirus SARS-CoV2 Trial) | 4488 | X | X | X | X | Colchicine 1 mg/day PO for the first 3 days, followed by 0.5 mg/day for 27 days | Outpatients with mild-to-moderate COVID-19 with high risk for progression (2235 received colchicine) | Colchicine reduced the composite of death or hospital admission a subgroup of patients with laboratory-confirmed COVID-19; diarrhoea more frequent in the colchicine arm, SAEs, and pneumonia less frequent | 04322682 |
| Selective NLRP3 Inhibitor | |||||||||
| Study of Efficacy and Safety of DV890 in Patients with COVID-19 Pneumonia | 143 | X | X | DFV890 50 mg PO twice daily for 14 days | Hospitalized patients with COVID-19 pneumonia, impaired respiratory function, APACHE II score ≥10, CRP ≥20 mg/L, and/or ferritin ≥600 µ/L (71 received DFV890) | DFV890 not superior to SoC alone in reducing the primary endpoint (APACHE II score at Day 14), but associated with faster viral clearance, improved clinical status, reduced mechanical ventilation-free survival, and death at Day 28 | 04382053 | ||
Note: COVID-19 severity refers to the World Health Organization severity classification (https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2). The individual trial-specific criteria for disease severity classification as well as detailed descriptions of study inclusion/exclusion criteria, design, and findings can be found on https://clinicaltrials.gov/and in relative publications.
Abbreviations: AEs, adverse events; CRP, C-reactive protein; DB, double-blind; IMV, invasive mechanical ventilation; IV, intravenously; M, multicentre; MV, mechanical ventilation; PC, placebo-controlled; PO, per os; R, randomized; SAEs, severe adverse events; SC, subcutaneously, SoC, standard-of-care.
Canakinumab was also tested in COVID-19.148–150 In the proof-of-concept Three C study enrolling 45 moderate-to-critically ill hospitalized COVID-19 patients with myocardial injury and elevated CRP, canakinumab was safe but did not significantly reduce time to clinical improvement.148 In the CanCovDia trial, the effects of canakinumab versus placebo with regard to a composite of length of survival, ventilation, intensive care unit stay, and hospitalization were evaluated among 116 hospitalized COVID-19 patients with Type 2 diabetes and body mass index >25 kg/m2.149 In this study, addition of canakinumab to standard-of-care did not result in a statistically significant improvement in the primary outcome, while reducing systemic inflammation and the number of glucose-lowering drugs.149 In the CAN-COVID trial that randomized 454 severely ill COVID-19 patients to receive canakinumab or placebo, canakinumab did not reduce progression to invasive mechanical ventilation at Day 29.150 However, canakinumab was associated with a significant reduction of the composite of death, invasive mechanical ventilation, or use of other immunomodulators as rescue therapy, thus suggesting a biological efficacy.150
Colchicine, currently employed for the treatment of several inflammatory disorders including gout and pericarditis, is an alkaloid extracted from the Colchicum autumnale.151 Originally only thought to block microtubule polymerization and leukocyte chemotaxis, colchicine possesses numerous anti-inflammatory effects including inhibition of NLRP3 inflammasome assembly and activation at clinically relevant concentrations, being regarded to as a non-selective inflammasome inhibitor.151–154 The colchicine arm in the RECOVERY trial was interrupted prematurely due to futility.155 In the ECLA PHRI COLCOVID trial, colchicine did not significantly decrease mechanical ventilation or 28-day mortality in severely ill COVID-19 patients.156 Conversely, in the GRECCO study, colchicine improved time to clinical deterioration in patients with moderate-to-severe COVID-19.157 In another small, placebo-controlled trial including moderately-to-severely ill subjects, colchicine shortened the duration of oxygen therapy and hospital stay.158 The ColCORONA trial that enrolled 4488 outpatients with COVID-19, diagnosed by either polymerase chain reaction testing or clinical criteria, at risk for progression, clinical improvement did not reach statistical significance possibly due to the low number of events.159 Although not meeting the study primary endpoint, in a prespecified analysis considering only patients with laboratory-confirmed COVID-19 (n = 4159), colchicine significantly reduced the composite of death and hospital admission compared to placebo.159
Multiple selective NLRP3 inflammasome blockers have been developed and tested in preclinical and early phase clinical studies, showing potential for efficacy across a wide range of inflammatory disorders.137–139,160–162 Preclinical studies indicated that selective NLRP3 inflammasome inhibition mitigates COVID-19-like immune overactivation and pathology, suggesting that such therapeutic approach could be promising in humans.82–84,88–90,98 In a recently completed phase 2a trial enrolling 143 hospitalized COVID-19 patients with impaired respiratory function, the selective NLRP3 inhibitor DFV890 did not reduce the primary endpoint of APACHE II score at Day 14 (or on day-of-discharge, whichever came first) as compared to standard-of-care alone.163 DFV890 was however well tolerated with no safety concerns and associated with faster viral clearance, improved mechanical ventilation-free survival, and fewer fatal events at Day 28.163 Additional trials testing NLRP3 inflammasome pathway inhibitors in COVID-19 are ongoing.
7. Targeting the NLRP3 inflammasome pathway in COVID-19: challenges, unmet gaps, and future perspectives
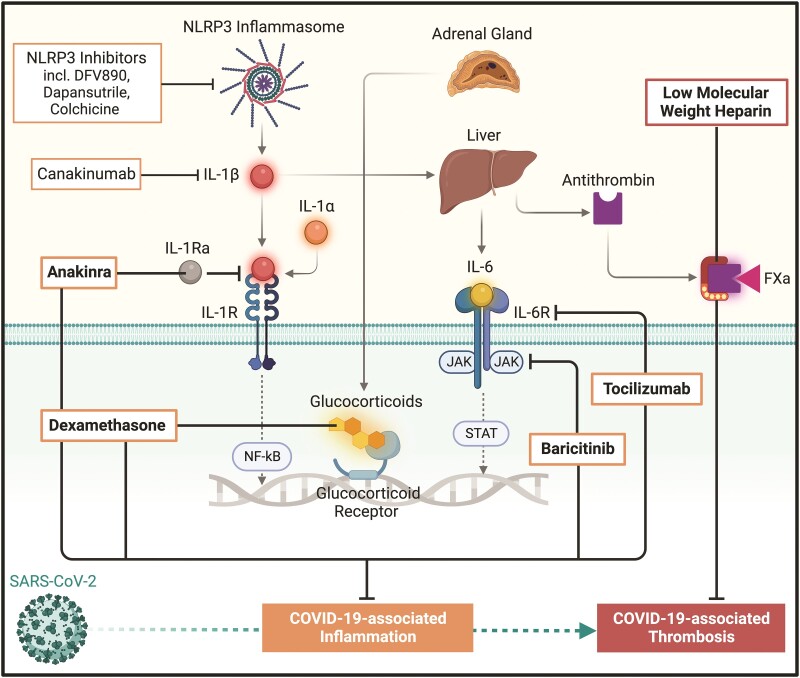
Most trials to date indicate that targeting the NLRP3 inflammasome pathway can be safe and well tolerated in patients with COVID-19.143–150,156–159,163 Mixed results on the efficacy of these interventions have been however obtained, with some studies showing little or no clinical benefits.143–145,150,155 As most trials were conceived during the initial stages of the pandemic, it is possible that the fewer-than-expected number of events made some of these studies underpowered.164 Yet, the heterogenous concomitant use of drugs later proven to be effective including antivirals, glucocorticoids, IL-6 receptor blockers, and low-molecular-weight heparins, as either background or rescue therapies, may have blurred potential signals for clinical efficacy.164,165 Notwithstanding these limitations, some trials provided positive results.146,147,157–159 The European Medicines Agency recommended anakinra for selected hypoxaemic COVID-19 patients with elevated suPAR levels,166 and the US Food and Drug Administration recently authorized its emergency use.167 Colchicine prevented hospitalization and death in a selected subgroup of ambulatory patients with laboratory-confirmed, mild-to-moderate COVID-19 and slowed clinical deterioration in some patients with moderate-to-severe COVID-19.157–159 Taken together, these data may prompt important considerations when addressing hyperinflammation in COVID-19. For instance, anti-inflammatory therapies could be detrimental if administered too early in the course of disease, as they may favour viral replication and reduce immune competence and memory. By contrast, delayed immunomodulation may be ineffective when massive cytokine release and organ injury already occurred. The inflammasome and IL-1 cytokines occupy an apical role in the inflammatory cascade (Figure 4).57,138 In critical disease, where immune overactivation is likely sustained by several proinflammatory cytokines, targeting a single, upstream inflammatory pathway could be insufficient to blunt hyperinflammation and improve clinical outcomes. This might partially explain the benefits of glucocorticoids (e.g. dexamethasone), IL-6 receptor inhibitors (e.g. tocilizumab), and Janus kinase (JAK) inhibitors (e.g. baricitinib) with broader anti-inflammatory effects165 and those of early NLRP3 inflammasome pathway blockade, which were not observed in critically ill patients. Identifying the optimal therapeutic window and refining strategies to select patient subgroups most likely responsive to NLRP3 inflammasome pathway inhibition remain major challenges warranting further investigation.
Figure 4.
Pharmacological agents addressing COVID-19-associated inflammation and thrombosis. Abbreviations: FXa, factor Xa; IL, interleukin; IL-1R, interleukin-1 receptor; IL-6R, interleukin-6 receptor; IL-1Ra, interleukin-1 receptor antagonist; JAK, Janus kinase; NF-κB, nuclear factor-kappa B; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; STAT, signal transducer and activator of transcription protein. Figure created with BioRender.com.
In recent years, seminal studies including the CANTOS, COLCOT, and LoDoCo2 trials have demonstrated antithrombotic effects by targeting the NLRP3/IL-1β axis with canakinumab or colchicine.168–170 Notably, these agents provided significant antithrombotic protection on top of conventional antithrombotic drugs, without increasing the risk of bleeding.168–170 COVID-19 trials testing inflammasome pathway blockade primarily focused on assessing inflammatory biomarkers and respiratory function. Although organ failure might be used as an indirect indicator of microvascular thrombosis, macrovascular thromboembolic events were not uniformly reported (mostly as safety outcomes) in these trials, with lack of systematic use of diagnostic strategies to detect thrombosis and standardized thromboprophylaxis regimens. In the GRECCO trial, colchicine was associated with reduced D-dimer.157 A secondary analysis of the REMAP-CAP trial found that anakinra reduced major thromboembolic events comprising myocardial infarction, pulmonary embolism, ischaemic stroke, and systemic arterial embolism.143 However, the overall low number of thromboembolic events recorded across the above-mentioned trials—generally much lower than in COVID-19 anticoagulation trials171—may not have allowed to capture clinically significant antithrombotic signals. Adequately designed studies are therefore needed to specifically assess the effects of NLRP3 inflammasome pathway inhibition on CAC and COVID-19-associated thrombosis.
Activation of the NLRP3 inflammasome pathway can exert prothrombotic effects through both IL-1-dependent and IL-1-independent mechanisms.15 It is therefore important to consider that anti-IL-1 agents may not completely address inflammasome-mediated, IL-1-independent immunothrombotic responses. Additionally, individual IL-1 blockers may exert distinct clinical effects owing to their different mechanism of action (e.g. IL-1α/IL-1β inhibition with anakinra versus IL-1β inhibition with canakinumab) as well as to their different pharmacokinetic and pharmacodynamic properties.138,139 It is also possible to consider that selective NLRP3 inhibitors might target the NLRP3 inflammasome more effectively than non-selective NLRP3 inhibitors and other immunomodulatory agents, with potential clinical implications on immunothrombosis and CAC.83,84 Future investigations should also address whether NLRP3 inflammasome inhibitors and IL-1 blockers could be used in synergy with other anti-inflammatory and anticoagulant drugs, potentially maximizing their benefits on inflammation and thrombosis without compromising safety. Importantly, additional studies are needed to evaluate the clinical usefulness of immunomodulatory agents including NLRP3 inflammasome pathway blockers with regard to vaccination, the evolving landscape of SARS-CoV-2 variants, and long COVID-19.
8. Concluding remarks
Inflammation and thrombosis are key features of severe COVID-19. Activation of the NLRP3 inflammasome pathway occurs in COVID-19 patients and is implicated in the inflammatory pathogenesis of the disease. The NLRP3 inflammasome pathway may also promote the hyperactivation of immunothrombosis programmes including generation of NETs and TF, as well as prothrombotic endothelial and platelet responses. Clinical trials testing the IL-1 inhibitors anakinra and canakinumab, the non-selective NLRP3 inhibitor colchicine, and the selective NLRP3 inhibitor DFV890 in COVID-19 patients showed reassuring results in terms of safety and tolerability and overall a signal for efficacy. Blockade of IL-6 signalling, downstream of IL-1, also reduced mortality in severe COVID-19 pneumonia. Anakinra and tocilizumab are now approved for the treatment of hypoxaemic COVID-19 patients with hyperinflammation. Further investigation is warranted to better characterize the exact mechanisms driving NLRP3 inflammasome pathway activation and its effects on CAC and COVID-19-associated thrombosis. The possibility that targeting the NLRP3 inflammasome pathway may simultaneously address inflammation and thrombosis might offer novel therapeutic perspectives for multiple inflammation-driven thrombotic disorders including COVID-19.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Contributor Information
Nicola Potere, Department of Medicine and Ageing Sciences, ‘G. d’Annunzio’ University, Via Luigi Polacchi 11, Chieti 66100, Italy.
Evan Garrad, Laboratory of Vascular Thrombosis and Inflammation, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA; University of Missouri School of Medicine, Columbia, MO, USA.
Yogendra Kanthi, Laboratory of Vascular Thrombosis and Inflammation, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
Marcello Di Nisio, Department of Medicine and Ageing Sciences, ‘G. d’Annunzio’ University, Via Luigi Polacchi 11, Chieti 66100, Italy.
Gilles Kaplanski, Aix-Marseille Université, INSERM, INRAE, Marseille, France; Division of Internal Medicine and Clinical Immunology, Assistance Publique - Hôpitaux de Marseille, Hôpital Conception, Aix-Marseille Université, Marseille, France.
Aldo Bonaventura, Department of Internal Medicine, Medicina Generale 1, Medical Center, Ospedale di Circolo e Fondazione Macchi, ASST Sette Laghi, Varese, Italy.
Jean Marie Connors, Division of Hematology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Raffaele De Caterina, University Cardiology Division, Pisa University Hospital, Pisa, Italy; Chair and Postgraduate School of Cardiology, University of Pisa, Pisa, Italy; Fondazione Villa Serena per la Ricerca, Città Sant’Angelo, Pescara, Italy.
Antonio Abbate, Robert M. Berne Cardiovascular Research Center, Department of Medicine, Division of Cardiovascular Medicine, University of Virginia, 415 Lane Rd (MR5), PO Box 801394, Charlottesville, VA 22903, USA.
Author contributions
N.P., Y.K., M.D.N., E.G., G.K., A.B., J.M.C., R.D.C., and A.A.
Funding
None.
Data availability
No new data were generated or analysed in support of this work.
References
- 1. Diamond MS, Kanneganti T-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat Immunol 2022;23:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res 2016;118:1392–1408. [DOI] [PubMed] [Google Scholar]
- 5. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol 2021;18:666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 2021;21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, Gralinski LE, Rondina MT, van de Veerdonk FL, Hoffmeister KM, Griffin JH, Nugent D, Moon K, Morrissey JH. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol 2022;22:639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen H-R, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020;173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Höchter D, Keppler O, Teupser D, Zwißler B, von Bergwelt-Baildon M, Kääb S, Massberg S, Pekayvaz K, Stark K. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 2020;142:1176–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 2019;19:477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 2016;16:407–420. [DOI] [PubMed] [Google Scholar]
- 14. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 2018;281:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vora SM, Lieberman J, Wu H. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol 2021;21:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Potere N, Del Buono MG, Caricchio R, Cremer PC, Vecchié A, Porreca E, Dalla Gasperina D, Dentali F, Abbate A, Bonaventura A. Interleukin-1 and the NLRP3 inflammasome in COVID-19: pathogenetic and therapeutic implications. EBioMedicine 2022;85:104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 18. Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care 2020;24:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leentjens J, van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents—lessons after 1 year. Lancet Haematol 2021;8:e524–e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vincent J-L, Levi M, Hunt BJ. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir Med 2022;10:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med 1984;160:618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nawroth PP, Handley DA, Esmon CT, Stern DM. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci 1986;83:3460–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bester J, Pretorius E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Reports 2016;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Potere N, Batticciotto A, Vecchié A, Porreca E, Cappelli A, Abbate A, Dentali F, Bonaventura A. The role of IL-6 and IL-6 blockade in COVID-19. Expert Rev Clin Immunol 2021;17:601–618. [DOI] [PubMed] [Google Scholar]
- 25. Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, David JS, Bonnet A, Negrier C, Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost 2020;18:2215–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, Madison JA, Knight JS, Kanthi Y, Lawrence DA. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep 2021;11:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bachler M, Bösch J, Stürzel DP, Hell T, Giebl A, Ströhle M, Klein SJ, Schäfer V, Lehner GF, Joannidis M, Thomé C. Impaired fibrinolysis in critically ill COVID-19 patients. Br J Anaesth 2021;126:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI, Halene S, Martin KA, Chun HJ, Hwa J. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol 2021;18:194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guervilly C, Bonifay A, Burtey S, Sabatier F, Cauchois R, Abdili E, Arnaud L, Lano G, Pietri L, Robert T, Velier M, Papazian L, Albanese J, Kaplanski G, Dignat-George F, Lacroix R. Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19. Blood Adv 2021;5:628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward SE, Fogarty H, Karampini E, Lavin M, Schneppenheim S, Dittmer R, Morrin H, Glavey S, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Mallon PW, Curley GF, Baker RI Budde U, O’Sullivan JM, O’Donnell JS; Irish COVID-19 Vasculopathy Study (iCVS) investigators . ADAMTS13 Regulation of VWF multimer distribution in severe COVID-19. J Thromb Haemost 2021;19:1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mancini I, Baronciani L, Artoni A, Colpani P, Biganzoli M, Cozzi G, Novembrino C, Boscolo Anzoletti M, De Zan V, Pagliari MT, Gualtierotti R, Aliberti S, Panigada M, Grasselli G, Blasi F, Peyvandi F. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost 2021;19:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pão CRR, Righy C, Franco S, Souza TML, Kurtz P, Bozza FA, Bozza PT. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 2020;136:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hottz ED, Martins-Gonçalves R, Palhinha L, Azevedo-Quintanilha IG, de Campos MM, Sacramento CQ, Temerozo JR, Soares VC, Dias SSG, Teixeira L, Castro Í, Righy C, Souza TML, Kurtz P, Andrade BB, Nakaya HI, Monteiro RQ, Bozza FA, Bozza PT. Platelet-monocyte interaction amplifies thromboinflammation through tissue factor signaling in COVID-19. Blood Adv 2022;6:5085–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puhm F, Allaeys I, Lacasse E, Dubuc I, Galipeau Y, Zaid Y, Khalki L, Belleannée C, Durocher Y, Brisson AR, Wolberg AS, Langlois M-A, Flamand L, Boilard E. Platelet activation by SARS-CoV-2 implicates the release of active tissue factor by infected cells. Blood Adv 2022;6:3593–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taus F, Salvagno G, Canè S, Fava C, Mazzaferri F, Carrara E, Petrova V, Barouni RM, Dima F, Dalbeni A, Romano S, Poli G, Benati M, De Nitto S, Mansueto G, Iezzi M, Tacconelli E, Lippi G, Bronte V, Minuz P. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler Thromb Vasc Biol 2020;40:2975–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, Limami Y, Zaid N, Sadki K, Ben El Haj R, Mahir W, Belayachi L, Belefquih B, Benouda A, Cheikh Ae, Langlois M-A, Cherrah Y, Flamand L, Guessous F, Boilard E. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ Res 2020;127:1404–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ackermann M, Anders HJ, Bilyy R, Bowlin GL, Daniel C, De Lorenzo R, Egeblad M, Henneck T, Hidalgo A, Hoffmann M, Hohberger B, Kanthi Y, Kaplan MJ, Knight JS, Knopf J, Kolaczkowska E, Kubes P, Leppkes M, Mahajan A, Manfredi AA, Maueröder C, Maugeri N, Mitroulis I, Muñoz LE, Narasaraju T, Naschberger E, Neeli I, Ng LG, Radic MZ, Ritis K, Rovere-Querini P, Schapher M, Schauer C, Simon H-U, Singh J, Skendros P, Stark K, Stürzl M, van der Vlag J, Vandenabeele P, Vitkov L, von Köckritz-Blickwede M, Yanginlar C, Yousefi S, Zarbock A, Schett G, Herrmann M. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ 2021;28:3125–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zuo Y, Zuo M, Yalavarthi S, Gockman K, Madison JA, Shi H, Woodard W, Lezak SP, Lugogo NL, Knight JS, Kanthi Y. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis 2021;51:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD, Yost CC. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020;136:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetité D, Tavares LA, Paiva IM, Rosales R, Colón D, Martins R, Castro IA, Almeida GM, Lopes MIF, Benatti MN, Bonjorno LP, Giannini MC, Luppino-Assad R, Almeida SL, Vilar F, Santana R, Bollela VR, Auxiliadora-Martins M, Borges M, Miranda CH, Pazin-Filho A, da Silva LLP, Cunha LD, Zamboni DS, Dal-Pizzol F, Leiria LO, Siyuan L, Batah S, Fabro A, Mauad T, Dolhnikoff M, Duarte-Neto A, Saldiva P, Cunha TM, Alves-Filho JC, Arruda E, Louzada-Junior P, Oliveira RD, Cunha FQ. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med 2020;217:e20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blasco A, Coronado MJ, Hernández-Terciado F, Martín P, Royuela A, Ramil E, García D, Goicolea J, Del Trigo M, Ortega J, Escudier JM, Silva L, Bellas C. Assessment of neutrophil extracellular traps in coronary thrombus of a case series of patients with COVID-19 and myocardial infarction. JAMA Cardiol 2021;6:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair CN, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps in COVID-19. JCI Insight 2020;5:e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009;22:240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoffman HM, Gregory SG, Mueller JL, Tresierras M, Broide DH, Wanderer AA, Kolodner RD. Fine structure mapping of CIAS1: identification of an ancestral haplotype and a common FCAS mutation, L353P. Hum Genet 2003;112:209–216. [DOI] [PubMed] [Google Scholar]
- 45. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-wells autoinflammatory disorder. Immunity 2004;20:319–325. [DOI] [PubMed] [Google Scholar]
- 46. Netea MG, Nold-Petry CA, Nold MF, Joosten LAB, Opitz B, van der Meer JHM, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JWM, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009;113:2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fantuzzi G, Ku G, Harding MW, Livingston DJ, Sipe JD, Kuida K, Flavell RA, Dinarello CA. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol 1997;158:1818–1824. [PubMed] [Google Scholar]
- 48. Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, Sota J, Dinarello CA. Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev 2021;20:102763. [DOI] [PubMed] [Google Scholar]
- 49. Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 2007;13:851–856. [DOI] [PubMed] [Google Scholar]
- 50. Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. The interleukin-1α precursor is biologically active and is likely a key Alarmin in the IL-1 family of cytokines. Front Immunol 2013;4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci USA 1985;82:1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Downs KP, Nguyen H, Dorfleutner A, Stehlik C. An overview of the non-canonical inflammasome. Mol Aspects Med 2020;76:100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev 2018;281:138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nicklin MJH, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med 2000;191:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity 1999;10:127–136. [DOI] [PubMed] [Google Scholar]
- 56. Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 2019;50:778–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016;118:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, Alcaraz-Serna A, Mateu-Albero T, Rodríguez-Cortes P, Sánchez-Cerrillo I, Esparcia L, Martínez-Fleta P, López-Sanz C, Gabrie L, del Campo Guerola L, Suárez-Fernández C, Ancochea J, Canabal A, Albert P, Rodríguez-Serrano DA, Aguilar JM, del Arco C, de los Santos I, García-Fraile L, de la Cámara R, Serra JM, Ramírez E, Alonso T, Landete P, Soriano JB, Martín-Gayo E, Fraile Torres A, Zurita Cruz ND, García-Vicuña R, Cardeñoso L, Sánchez-Madrid F, Alfranca A, Muñoz-Calleja C, González-Álvaro I; REINMUN-COVID Group . IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J Allergy Clin Immunol 2021;147:72–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, Berger JS. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J 2021;42:2270–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Obaid A, Lu-Culligan A, Nelson A, Brito A, Nunez A, Martin A, Watkins A, Geng B, Kalinich C, Harden C, Todeasa C, Jensen C, Kim D, McDonald D, Shepard D, Courchaine E, White EB, Song E, Silva E, Kudo E, DeIuliis G, Rahming H, Park H-J, Matos I, Nouws J, Valdez J, Fauver J, Lim J, Rose K-A, Anastasio K, Brower K, Glick L, Sharma L, Sewanan L, Knaggs L, Minasyan M, Batsu M, Petrone M, Kuang M, Nakahata M, Campbell M, Linehan M, Askenase MH, Simonov M, Smolgovsky M, Sonnert N, Naushad N, Vijayakumar P, Martinello R, Datta R, Handoko R, Bermejo S, Prophet S, Bickerton S, Velazquez S, Alpert T, Rice T, Khoury-Hanold W, Peng X, Yang Y, Cao Y, Strong Y, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020;584:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Theobald SJ, Simonis A, Georgomanolis T, Kreer C, Zehner M, Eisfeld HS, Albert MC, Chhen J, Motameny S, Erger F, Fischer J. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol Med 2021;13:e14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002;13:323–340. [DOI] [PubMed] [Google Scholar]
- 67. Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, Xu B, Dai Y, Li X, Zhang C, Peng Y, Feng Y, Li A, Hu Z, Xiang H, Ogg G, Ho L-P, McMichael A, Jin R, Knight JC, Dong T, Zhang Y. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI insight 2020;5:e139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Satış H, Özger HS, Aysert Yıldız P, Hızel K, Gulbahar Ö, Erbaş G, Aygencel G, Tunccan OG, Öztürk MA, Dizbay M, Tufan A. Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19. Cytokine 2021;137:155302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu G, Qi F, Li H, Yang Q, Wang H, Wang X, Liu X, Zhao J, Liao X, Liu Y, Liu L. The differential immune responses to COVID-19 in peripheral and lung revealed by single-cell RNA sequencing. Cell Discov 2020;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;26:842–844. [DOI] [PubMed] [Google Scholar]
- 71. Li S, Zhang Y, Guan Z, Li H, Ye M, Chen X, Shen J, Zhou Y, Shi Z-L, Zhou P, Peng K. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther 2020;5:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang J, Wu H, Yao XH, Zhang D, Zhou Y, Fu B, Wang W, Li H, Wang Z, Hu Z, Ren Y, Sun R, Tian Z, Bian X, Wei H. Pyroptotic macrophages stimulate the SARS-CoV-2-associated cytokine storm. Cell Mol Immunol 2021;18:1305–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MHF, Silva CMS, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SCL, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 2021;218:e20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ferreira AC, Soares VC, de Azevedo-Quintanilha IG, Dias SDSG, Fintelman-Rodrigues N, Sacramento CQ, Mattos M, de Freitas CS, Temerozo JR, Teixeira L, Damaceno Hottz E, Barreto EA, Pão CRR, Palhinha L, Miranda M, Bou-Habib DC, Bozza FA, Bozza PT, Souza TML. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell death Discov 2021;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zheng J, Wang Y, Li K, Meyerholz DK, Allamargot C, Perlman S. Severe acute respiratory syndrome coronavirus 2-induced immune activation and death of monocyte-derived human macrophages and dendritic cells. J Infect Dis 2021;223:785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannatà A, Pillappa R, Sinagra G, Nana-Sinkam P, Sime P, Abbate A. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res 2021;70:7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Paul O, Tao JQ, West E, Litzky L, Feldman M, Montone K, Rajapakse C, Bermudez C, Chatterjee S. Pulmonary vascular inflammation with fatal coronavirus disease 2019 (COVID-19): possible role for the NLRP3 inflammasome. Respir Res 2022;23:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Courjon J, Dufies O, Robert A, Bailly L, Torre C, Chirio D, Contenti J, Vitale S, Loubatier C, Doye A, Pomares-Estran C, Gonfrier G, Lotte R, Munro P, Visvikis O, Dellamonica J, Giordanengo V, Carles M, Yvan-Charvet L, Ivanov S, Auberger P, Jacquel A, Boyer L. Heterogeneous NLRP3 inflammasome signature in circulating myeloid cells as a biomarker of COVID-19 severity. Blood Adv 2021;5:1523–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Junqueira C, Crespo Â, Ranjbar S, de Lacerda LB, Lewandrowski M, Ingber J, Parry B, Ravid S, Clark S, Schrimpf MR, Ho F, Beakes C, Margolin J, Russell N, Kays K, Boucau J, Das Adhikari U, Vora SM, Leger V, Gehrke L, Henderson LA, Janssen E, Kwon D, Sander C, Abraham J, Goldberg MB, Wu H, Mehta G, Bell S, Goldfeld AE, Filbin MR, Lieberman J. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022;606:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maes M, Del Tedesco Junior WL, Lozovoy MAB, Mori MTE, Danelli T, de Almeida ERD, Tejo AM, Tano ZN, Reiche EMV, Simão ANC. In COVID-19, NLRP3 inflammasome genetic variants are associated with critical disease and these effects are partly mediated by the sickness symptom complex: a nomothetic network approach. Mol Psychiatry 2022;27:1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Silva CMS, Wanderley CWS, Veras FP, Gonçalves AV, Lima MHF, Toller-Kawahisa JE, Gomes GF, Nascimento DC, Monteiro VVS, Paiva IM, Almeida CJLR, Caetité DB, Silva JC, Lopes MIF, Bonjorno LP, Giannini MC, Amaral NB, Benatti MN, Santana RC, Damasceno LEA, Silva BMS, Schneider AH, Castro IMS, Silva JCS, Vasconcelos AP, Gonçalves TT, Batah SS, Rodrigues TS, Costa VF, Pontelli MC, Martins RB, Martins TV, Espósito DLA, Cebinelli GCM, da Fonseca BAL, Leiria LOS, Cunha LD, Arruda E, Nakaia HI, Fabro AT, Oliveira RDR, Zamboni DS, Louzada-Junior P, Cunha TM, Alves-Filho JCF, Cunha FQ. Gasdermin-D activation by SARS-CoV-2 triggers NET and mediate COVID-19 immunopathology. Crit Care 2022;26:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bertoni A, Penco F, Mollica H, Bocca P, Prigione I, Corcione A, Cangelosi D, Schena F, Del Zotto G, Amaro A, Paladino N, Pontali E, Feasi M, Signa S, Bustaffa M, Caorsi R, Palmeri S, Contini P, De Palma R, Pfeffer U, Uva P, Rubartelli A, Gattorno M, Volpi S. Spontaneous NLRP3 inflammasome-driven IL-1-β secretion is induced in severe COVID-19 patients and responds to anakinra treatment. J Allergy Clin Immunol 2022;150:796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lage SL, Amaral EP, Hilligan KL, Laidlaw E, Rupert A, Namasivayan S, Rocco J, Galindo F, Kellogg A, Kumar P, Poon R, Wortmann GW, Shannon JP, Hickman HD, Lisco A, Manion M, Sher A, Sereti I. Persistent oxidative stress and inflammasome activation in CD14highCD16- monocytes from COVID-19 patients. Front Immunol 2022;12:799558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Olajide OA, Iwuanyanwu VU, Lepiarz-Raba I, Al-Hindawi AA. Induction of exaggerated cytokine production in human peripheral blood mononuclear cells by a recombinant SARS-CoV-2 spike glycoprotein S1 and its inhibition by dexamethasone. Inflammation 2021;44:1865–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W, Kucia M. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45− precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the nlrp3 inflammasome. Stem Cell Rev Reports 2021;17:266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Campbell GR, To RK, Hanna J, Spector SA. SARS-CoV-2, SARS-CoV-1, and HIV-1 derived ssRNA sequences activate the NLRP3 inflammasome in human macrophages through a non-classical pathway. iScience 2021;24:102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Theobald SJ, Simonis A, Mudler JM, Göbel U, Acton R, Kohlhas V, Albert M-C, Hellmann A-M, Malin JJ, Winter S, Hallek M, Walczak H, Nguyen P-H, Koch M, Rybniker J. Spleen tyrosine kinase mediates innate and adaptive immune crosstalk in SARS-CoV-2 mRNA vaccination. EMBO Mol Med 2022;14:e15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, Brewer J. R, Han A, Steach HR, Israelow B, Blackburn HN, Velazquez SE, Chen YG, Halene S, Iwasaki A, Meffre E, Nussenzweig M, Lieberman J, Wilen CB, Kluger Y, Flavell RA. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022;606:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, Xiao F, Wang Z, Wang J, Jia Y, Wang W, Wan P, Zhang J, Chen W, Lei Zi, Chen X, Luo Z, Zhang Q, Xu M, Li G, Li Y, Wu J. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun 2021;12:4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xu H, Akinyemi IA, Chitre SA, Loeb JC, Lednicky JA, McIntosh MT, Bhaduri-McIntosh S. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology 2022;568:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Eisfeld HS, Simonis A, Winter S, Chhen J, Ströh LJ, Krey T, Koch M, Theobald SJ, Rybniker J. Viral glycoproteins induce NLRP3 inflammasome activation and pyroptosis in macrophages. Viruses 2021;13:2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yalcinkaya M, Liu W, Islam MN, Kotini AG, Gusarova GA, Fidler TP, Papapetrou EP, Bhattacharya J, Wang N, Tall AR. Modulation of the NLRP3 inflammasome by Sars-CoV-2 envelope protein. Sci Rep 2021;11:24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ma J, Zhu F, Zhao M, Shao F, Yu D, Ma J, Zhang X, Li W, Qian Y, Zhang Y, Jiang D. SARS-Cov-2 nucleocapsid suppresses host pyroptosis by blocking gasdermin D cleavage. EMBO J 2021;40:e108249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Albornoz EA, Amarilla AA, Modhiran N, Parker S, Li XX, Wijesundara DK, Aguado J, Zamora AP, McMillan CLD, Liang B, Peng NYG, Sng JDJ, Saima FT, Fung JN, Lee JD, Paramitha D, Parry R, Avumegah MS, Isaacs A, Lo MW, Miranda-Chacon Z, Bradshaw D, Salinas-Rebolledo C, Rajapakse NW, Wolvetang Ernst J., Munro TP, Rojas-Fernandez A, Young PR, Stacey KJ, Khromykh AA, Chappell KJ, Watterson D, Woodruff TM. SARS-CoV-2 drives NLRP3 inflammasome activation in human microglia through spike protein. Mol Psychiatry. 2022. doi: 10.1038/s41380-022-01831-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc Natl Acad Sci USA 2011;108:20684–20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA., Apte RN. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 2011;187:4835–4843. [DOI] [PubMed] [Google Scholar]
- 97. Xia B, Shen X, He Y, Pan X, Liu F-L, Wang Y, Yang F, Fang S, Wu Y, Duan Z, Zuo X, Xie Z, Jiang X, Xu L, Chi H, Li S, Meng Q, Zhou H, Zhou Y, Cheng X, Xin X, Jin L, Zhang H-L, Yu D-D, Li M-H, Feng X-L, Chen J, Jiang H, Xiao G, Zheng Y-T, Zhang L-K, Shen J, Li J, Gao Z. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res 2021;31:847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zeng J, Xie X, Feng XL, Xu L, Han JB, Yu D, Zou QC, Liu Q, Li X, Ma G, Li MH. Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. EBioMedicine 2022;75:103803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Colling ME, Tourdot BE, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res 2021;128:2017–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, Le Mao R, Rodríguez C, Hunt BJ, Monreal M. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest 2021;159:1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine 2020;29-30:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dujardin RWG, Hilderink BN, Haksteen WE, Middeldorp S, Vlaar APJ, Thachil J, Müller MCA, Juffermans NP. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb Res 2020;196:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li P, Zhao W, Kaatz S, Latack K, Schultz L, Poisson L. Factors associated with risk of postdischarge thrombosis in patients with COVID-19. JAMA Netw Open 2021;4:e2135397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Katsoularis I, Fonseca-Rodríguez O, Farrington P, Jerndal H, Lundevaller EH, Sund M, Lindmark K, Fors Connolly A-M. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: nationwide self-controlled cases series and matched cohort study. BMJ 2022;377:e069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chanchal S, Mishra A, Singh MK, Ashraf MZ. Understanding inflammatory responses in the manifestation of prothrombotic phenotypes. Front Cell Dev Biol 2020;873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018;15:203–214. [DOI] [PubMed] [Google Scholar]
- 108. Rothmeier AS, Marchese P, Petrich BG, Furlan-Freguia C, Ginsberg MH, Ruggeri ZM, Ruf W. Caspase-1–mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest 2015;125:1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, Shi J, Li X-A, Daugherty A, Smyth SS, Kirchhofer D, Shiroishi T, Shao F, Mackman N, Wei Y, Li Z. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 2019;50:1401–1411.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, Wu J, Wang Z, Liu Y, Chen F, Xiao X, Mackman N, Billiar TR, Han J, Lu B. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity 2019;51:983–996.e6. [DOI] [PubMed] [Google Scholar]
- 111. Balbi C, Burrello J, Bolis S, Lazzarini E, Biemmi V, Pianezzi E, Burrello A, Caporali E, Grazioli LG, Martinetti G, Fusi-Schmidhauser T. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine 2021;67:103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rosell A, Havervall S, Von Meijenfeldt F, Hisada Y, Aguilera K, Grover SP, Lisman T, Mackman N, Thålin C. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report. Arterioscler Thromb Vasc Biol 2021;41:878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Eltobgy MM, Zani A, Kenney AD, Estfanous S, Kim E, Badr A, Carafice C, Daily K, Whitham O, Pietrzak M, Webb A, Kawahara J, Eddy AC, Denz P, Lu M, Kc M, Peeples ME, Li J, Zhu J, Que J, Robinson R, Rosas Mejia O, Rayner RE, Hall-Stoodley L, Seveau S, Gavrilin MA, Zhang X, Thomas J, Kohlmeier JE, Suthar MS, Oltz E, Tedeschi A, Robledo-Avila FH, Partida-Sanchez S, Hemann EA, Abdelrazik E, Forero A, Nimjee SM, Boyaka PN, Cormet-Boyaka E, Yount JS, Amer AO. Caspase-4/11 exacerbates disease severity in SARS-CoV-2 infection by promoting inflammation and immunothrombosis. Proc Natl Acad Sci USA 2022;119:e2202012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 2018;3:eaar6676. [DOI] [PubMed] [Google Scholar]
- 115. Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap–associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 2013;190:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Clancy DM, Henry CM, Sullivan GP, Martin SJ. Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines. FEBS J 2017;284:1712–1725. [DOI] [PubMed] [Google Scholar]
- 117. Hudock KM, Collins MS, Imbrogno M, Snowball J, Kramer EL, Brewington JJ, Gollomp K, McCarthy C, Ostmann AJ, Kopras EJ, Davidson CR, Srdiharan A, Arumugam P, Sengupta S, Xu Y, Worthen GS, Trapnell BC, Clancy JP. Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 2020;319:L137–L147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Aymonnier K, Ng J, Fredenburgh LE, Zambrano-Vera K, Münzer P, Gutch S, Fukui S, Desjardins M, Subramaniam M, Baron RM, Raby BA, Perrella MA, Lederer JA, Wagner DD. Inflammasome activation in neutrophils of patients with severe COVID-19. Blood Adv 2022;6:2001–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Campos J, Ponomaryov T, de Prendergast A, Whitworth K, Smith CW, Khan AO, Kavanagh D, Brill A. Neutrophil extracellular traps and inflammasomes cooperatively promote venous thrombosis in mice. Blood Adv 2021;5:2319–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Münzer P, Negro R, Fukui S, Di Meglio L, Aymonnier K, Chu L, Cherpokova D, Gutch S, Sorvillo N, Shi L, Magupalli VG. NLRP3 inflammasome assembly in neutrophils is supported by PAD4 and promotes NETosis under sterile conditions. Front Immunol 2021;12:683803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol 2018;38:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Libby PE, Ordovas JM, Auger KR, Robbins AH, Birinyi LK, Dinarello CA. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol 1986;124:179. [PMC free article] [PubMed] [Google Scholar]
- 123. Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol 2001;154:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Brown GT, McIntyre TM. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. J Immunol 2011;1865489–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hottz ED, Lopes JF, Freitas C, Valls-de-Souza R, Oliveira MF, Bozza MT, Da Poian AT, Weyrich AS, Zimmerman GA, Bozza FA, Bozza PT. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013;122:3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Murthy P, Durco F, Miller-Ocuin JL, Takedai T, Shankar S, Liang X, Liu X, Cui X, Sachdev U, Rath D, Lotze MT, Zeh HJ, Gawaz M, Weber AN, Vogel S. The NLRP3 inflammasome and Bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochem Biophys Res Commun 2017;483:230–236. [DOI] [PubMed] [Google Scholar]
- 127. Vogel S, Murthy P, Cui X, Lotze MT, Zeh HJ, Sachdev U. TLR4-dependent Upregulation of the platelet NLRP3 inflammasome promotes platelet aggregation in a murine model of hindlimb ischemia. Biochem Biophys Res Commun 2019;508:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bai B, Yang Y, Wang Q, Li M, Tian C, Liu Y, Aung LHH, Li P-f, Yu T, Chu X-m. NLRP3 Inflammasome in endothelial dysfunction. Cell Death Dis 2020;11:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sur S, Steele R, Isbell TS, Ray R, Ray RB. Circulatory exosomes from COVID-19 patients trigger NLRP3 inflammasome in endothelial cells. MBio 2022;13:e0095122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR. Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J Transl Med 2005;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sahni A, Guo M, Sahni SK, Francis CW. Interleukin-1beta but not IL-1alpha binds to fibrinogen and fibrin and has enhanced activity in the bound form. Blood 2004;104:409–414. [DOI] [PubMed] [Google Scholar]
- 132. Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, Khan N, Nair V, Bajaj N, Sharma M, Ashraf MZ. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci USA 2017;114:4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhang Y, Cui J, Zhang G, Wu C, Abdel-Latif A, Smyth SS, Shiroishi T, Mackman N, Wei Y, Tao M, Li Z. Inflammasome activation promotes venous thrombosis through pyroptosis. Blood Adv 2021;5:2619–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yadav V, Chi L, Zhao R, Tourdot BE, Yalavarthi S, Jacobs BN, Banka A, Liao H, Koonse S, Anyanwu AC, Visovatti SH, Holinstat MA, Kahlenberg JM, Knight JS, Pinsky DJ, Kanthi Y. ENTPD-1 disrupts inflammasome IL-1β-driven venous thrombosis. J Clin Invest 2019;129:2872–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Abu-Fanne R, Stepanova V, Litvinov RI, Abdeen S, Bdeir K, Higazi M, Maraga E, Nagaswami C, Mukhitov AR, Weisel JW, Cines DB, Higazi AA-R. Neutrophil α-defensins promote thrombosis in vivo by altering fibrin formation, structure, and stability. Blood 2019;133:481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Potere N, Abbate A, Kanthi Y, Carrier M, Toldo S, Porreca E, Di Nisio M. Inflammasome signaling, thromboinflammation, and venous thromboembolism. JACC Basic Transl Sci 2023. doi: 10.1016/j.jacbts.2023.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mauro AG, Bonaventura A, Vecchié A, Mezzaroma E, Carbone S, Narayan P, Potere N, Cannatà A, Paolini JF, Bussani R, Montecucco F, Sinagra G, Van Tassel BW, Abbate A, Toldo S. The role of NLRP3 inflammasome in pericarditis: potential for therapeutic approaches. JACC Basic to Transl Sci 2021;6:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res 2020;126:1260–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Toldo S, Mezzaroma E, Buckley LF, Potere N, Di Nisio M, Biondi-Zoccai G, Van Tassell BW, Abbate A. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol Ther 2021;236:108053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, Jean R, Fouche L, Bornet C, Pauly V, Mazodier K, Pestre V, Jarrot P-A, Dinarello CA, Kaplanski G. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci USA 2020;117:18951–18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, Castiglioni B, Din CT, Boffini N, Tomelleri A. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020;2:e325–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ucciferri C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, Vecchiet J, Falasca K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol 2020;2:e457–ee458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. REMAP-CAP Investigators, Derde LPG, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, Beasley R. Effectiveness of tocilizumab, sarilumab, and anakinra for critically ill patients with COVID-19 the REMAP-CAP COVID-19 immune modulation therapy domain randomized clinical trial. medRxiv. 2021:2021.06.18.21259133. [Google Scholar]
- 144. Declercq J, Van Damme KFA, De Leeuw E, Maes B, Bosteels C, Tavernier SJ, De Buyser S, Colman R, Hites M, Verschelden G, Fivez T. Effect of anti-interleukin drugs in patients with COVID-19 and signs of cytokine release syndrome (COV-AID): a factorial, randomised, controlled trial. Lancet Respir Med 2021;9:1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mariette X, Hermine O, Resche-Rigon M, Porcher R, Ravaud P, Bureau S, Dougados M, Tibi A, Azoulay E, Cadranel J, Emmerich J. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med 2021;9:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kyriazopoulou E, Panagopoulos P, Metallidis S, Dalekos GN, Poulakou G, Gatselis N, Karakike E, Saridaki M, Loli G, Stefos A, Prasianaki D, Georgiadou S, Tsachouridou O, Petrakis V, Tsiakos K, Kosmidou M, Lygoura V, Dareioti M, Milionis H, Papanikolaou IC, Akinosoglou K, Myrodia D-M, Gravvani A, Stamou A, Gkavogianni T, Katrini K, Marantos T, Trontzas IP, Syrigos K, Chatzis L, Chatzis S, Vechlidis N, Avgoustou C, Chalvatzis S, Kyprianou M, van der Meer JW, Eugen-Olsen J, Netea MG, Giamarellos-Bourboulis EJ. An open label trial of anakinra to prevent respiratory failure in COVID-19. Elife 2021;10:e66125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia D-M, Argyraki A, Cardellino CS, Koliakou K, Katsigianni E-I, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, Giamarellos-Bourboulis EJ. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021;27:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cremer PC, Sheng CC, Sahoo D, Dugar S, Prada RA, Wang TKM, Hassan OKA, Hernandez-Montfort J, Wolinsky DA, Culver DA, Rajendram P, Duggal A, Brennan DM, Wolski KE, Lincoff AM, Nissen SE, Menon V. Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation. Eur Hear J Open 2021;1:oeab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hepprich M, Mudry JM, Gregoriano C, Jornayvaz FR, Carballo S, Wojtusciszyn A, Bart PA, Chiche JD, Fischli S, Baumgartner T, Cavelti-Weder C. Canakinumab in patients with COVID-19 and type 2 diabetes - A multicentre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine 2022;53:101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, Arduino R, Fomina D, Bogdanov R, Stepanenko T, Ruiz-Seco P, Gónzalez-García A, Chen Y, Li Y, Whelan S, Noviello S, CAN-COVID Investigators . Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA 2021;326:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Leung YY, Yao Hui LL, Kraus VB. Colchicine-update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015;45:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, Akira S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol 2013;14:454–460. [DOI] [PubMed] [Google Scholar]
- 153. Robertson S, Martínez GJ, Payet CA, Barraclough JY, Celermajer DS, Bursill C, Patel S. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond) 2016;130:1237–1246. [DOI] [PubMed] [Google Scholar]
- 154. Silvis MJM, Fiolet ATL, Opstal TSJ, Dekker M, Suquilanda D, Zivkovic M, Duyvendak M, The SHK, Timmers L, Bax WA, Mosterd A, Cornel JH, de Kleijn DPV. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: a LoDoCo2 biomarker substudy. Atherosclerosis 2021;334:93–100. [DOI] [PubMed] [Google Scholar]
- 155. RECOVERY Collaborative Group . Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med 2021;9:1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Diaz R, Orlandini A, Castellana N, Caccavo A, Corral P, Corral G, Chacón C, Lamelas P, Botto F, Díaz ML, Domínguez JM. Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial. JAMA Netw Open 2021;4:e2141328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, Metallidis S, Sianos G, Baltagiannis S, Panagopoulos P, Dolianitis K. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open 2020;3:e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, Dib SM, Gigante SL, Benatti MN, Rezek UC, Emrich-Filho LL, Sousa BA. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021;7:e001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tardif JC, Bouabdallaoui N, L’Allier PL, Gaudet D, Shah B, Pillinger MH, Lopez-Sendon J, da Luz P, Verret L, Audet S, Dupuis J. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med 2021;9:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lonnemann N, Hosseini S, Marchetti C, Skouras DB, Stefanoni D, D’Alessandro A, Dinarello CA, Korte M. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 2020;117:32145–32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Klück V, Jansen TLTA, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, Schraa K, Cleophas MCP, Scribner CL, Skouras DB, Marchetti C, Dinarello CA, Joosten LAB. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol 2020;2:e270–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wohlford GF, Van Tassell BW, Billingsley HE, Kadariya D, Canada JM, Carbone S, Mihalick VL, Bonaventura A, Vecchié A, Chiabrando JG, Bressi E. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J Cardiovasc Pharmacol 2020;77:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Madurka I, Vishnevsky A, Soriano JB, Gans SJ, Ore DJS, Rendon A, Ulrik CS, Bhatnagar S, Krishnamurthy S, Mc Harry K, Welte T, Fernandez AA, Mehes B, Meiser K, Gatlik E, Sommer U, Junge G, Rezende E; Study group . DFV890: a new oral NLRP3 inhibitor-tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function. Infection 2022;51:641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Moeller WJ, Potere N, Bonaventura A, Vecchiè A, Sedhai YR, Caricchio R, Abbate A. Use of placebo in clinical trials in COVID-19 pandemic times: considerations on pros, cons, challenges and limitations. Minerva Med. 2021;113:1000–1007. [DOI] [PubMed] [Google Scholar]
- 165. NIH COVID-19 Treatment Guidelines . Hospitalized Adults: Therapeutic Management. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/hospitalized-adults--therapeutic-management/ (29 November 2022, date last accessed).
- 166. European Medicines Agency . EMA Recommends Approval for Use of Kineret in Adults with COVID-19. . https://www.ema.europa.eu/en/news/ema-recommends-approval-use-kineret-adults-covid-19 (29 November 2022, date last accessed).
- 167. U.S. Food and Drug Administration . EUA 109 Kineret LOA 11082022. https://www.fda.gov/media/163081/download
- 168. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF., Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 169. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 170. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu X-F, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL; LoDoCo2 Trial Investigators . Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–1847. [DOI] [PubMed] [Google Scholar]
- 171. Farkouh ME, Stone GW, Lala A, Bagiella E, Moreno PR, Nadkarni GN, Ben-Yehuda O, Granada JF, Dressler O, Tinuoye EO, Granada C, Bustamante J, Peyra C, Godoy LC, Palacios IF, Fuster V. Anticoagulation in patients with COVID-19: JACC review topic of the week. J Am Coll Cardiol 2022;79:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this work.