Abstract
OBJECTIVES
Porcelain aorta complicates aortic valve replacement and is an indication for transcatheter approaches. No study has compared surgical and transcatheter valve replacement in the setting of porcelain aorta. We characterize porcelain aorta patients undergoing aortic valve replacement and the association of aortic calcification and outcomes.
METHODS
Patients undergoing aortic valve replacement with porcelain aorta were identified. Aortic calcium volume was determined using 3D computed tomography thresholding techniques. Propensity scoring was performed to assess the effect of surgical versus transcatheter approaches. Risk factors for composite major hospital complications (death, stroke and dialysis) were identified using random forest machine learning.
RESULTS
From January 2006 to January 2015, 164 patients with porcelain aorta underwent aortic valve replacement [105 (64%) surgical replacement, 59 (36%) transcatheter replacement]. Propensity scoring matched 29 pairs (49% of transcatheter patients). Before matching, 5-year survival was 41% [(43% surgical, 35% transcatheter, P(log-rank) = 0.9]. After matching, mortality for surgical versus transcatheter replacement was 3.4% (n = 1) vs 10% (n = 3), stroke 14% (n = 4) vs 3.4% (n = 1) and dialysis 6.9% (n = 2) versus 11% (n = 3). Matched 5-year survival was 40% after surgical replacement and 29% after transcatheter replacement [P(log-rank) = 0.4]. Total aortic calcium volume was greater in transcatheter than surgical patients [18 (8.0) vs 17 (7.7) ml] and was associated with more major hospital complications after either approach.
CONCLUSIONS
Surgical and transcatheter approaches are complementary options for aortic stenosis with porcelain aorta. Surgical valve replacement remains an effective treatment for patients requiring concomitant procedures. Quantifying aortic calcium volume is a helpful risk predictor in all patients with porcelain aorta.
Keywords: Aortic stenosis, Calcium volume quantification, Operative approach
Severe ascending aorta calcification (porcelain aorta) presents an operative challenge and substantial morbidity during surgical aortic valve replacement (SAVR) for aortic stenosis [1, 2].
INTRODUCTION
Severe ascending aorta calcification (porcelain aorta) presents an operative challenge and substantial morbidity during surgical aortic valve replacement (SAVR) for aortic stenosis [1, 2]. Calcification limits aortic clamping and cannulation options and increases embolic risk when the aorta is manipulated [3, 4]. Transcatheter aortic valve replacement (TAVR) has been recommended as a safe option for these patients [5, 6]. TAVR offers the benefit of isolated aortic valve replacement (AVR) without sternotomy or risks associated with cardiopulmonary bypass (CPB) and circulatory arrest. However, many patients with aortic stenosis and porcelain aorta have other cardiovascular conditions that may require multi-component operations. The paucity of clinical data within this limited population makes patient selection for either operative approach complex.
Due to the extent of calcium deposition in porcelain aorta and the perceived risk it confers, identifying risk factors associated with complications is imperative [1, 7]. Qualitative classification systems for porcelain aorta have been proposed, but their utility in predicting outcomes has not been assessed [8, 9]. Reproducible methods to quantitatively assess aortic calcification and its association with perioperative complications may improve patient selection and outcomes. We hypothesize aortic calcium volume is associated with outcomes. Thus, we aim to (i) characterize porcelain aorta patients undergoing AVR, (ii) determine the association of quantitative aortic calcification volume with postoperative complications and (iii) assess the impact of SAVR versus TAVR on intermediate-term outcomes.
PATIENTS AND METHODS
Ethics statement and patients
Patients were identified through comprehensive quality assurance registries in the Heart, Vascular and Thoracic Institute at Cleveland Clinic. We completed retrospective chart review with prospective follow-up of patients who presented to our institution from 1 January 2006 to 1 January 2015, encompassing the early era of TAVR. Use of these data for human research was approved by the institutional review board on 18 August 2014 (#14–973), with patient consent waived. Patients undergoing AVR with concomitant porcelain aorta were identified (Fig. 1A and Table 1). The strengthening the reporting of observational studies in epidemiology (STROBE) checklist is provided in Supplementary Material, Table S1.
Figure 1:
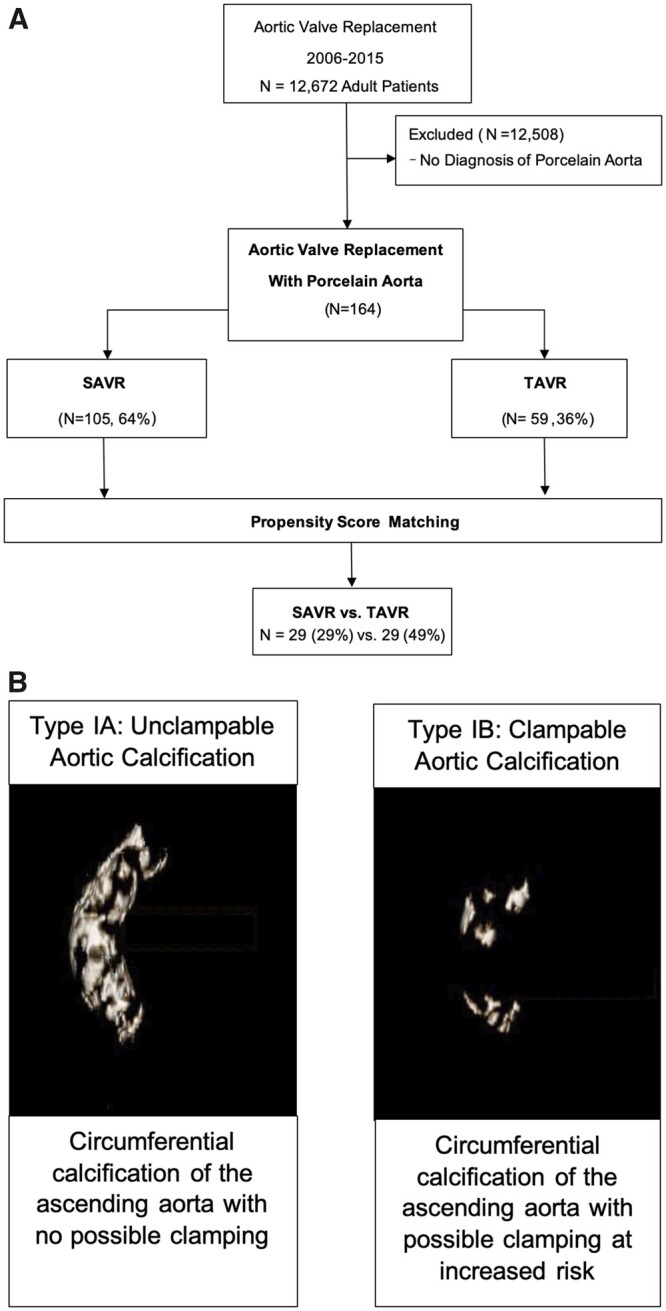
Study design and porcelain aorta. (A) Patients undergoing aortic valve replacement with a diagnosis of porcelain aorta. A total of 105 patients underwent surgical valve replacement and 59 underwent transcatheter valve replacement. Propensity score matching identified 29 pairs (29% of the surgical group, 49% of the transcatheter group). SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement. (B) Porcelain aorta is defined as the circumferential, or near circumferential, deposition of calcium within the ascending aorta. Subcategories, types IA and IB, can be defined based on ‘clampability’ of the aorta. Left panel: 3D reconstruction of an unclampable, type IA. Right panel: 3D reconstruction of a clampable, type IB.
Table 1:
Preprocedure patient characteristics
| Label | Before matching |
After matching |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAVR, N = 105 |
TAVR, N = 59 |
SAVR, N = 29 |
TAVR, N = 29 |
|||||||
| N a | Count (%) or mean (SD) | N a | Count (%) or mean (SD) | SMD (%) |
N a | Count (%) or mean (SD) | N a | Count (%) or mean (SD) | SMD (%) | |
| Demographics (SD) | ||||||||||
| Age (years) | 105 | 73 (10) | 59 | 78 (10) | 45 | 29 | 77 (7.7) | 29 | 77 (9.7) | –2.9 |
| Sex (Female) | 105 | 60 (57) | 59 | 30 (51) | –13 | 29 | 15 (52) | 29 | 17 (59) | 14 |
| Body surface area (m2) | 103 | 1.9 (0.23) | 56 | 1.9 (0.20) | 21 | 27 | 1.9 (0.22) | 27 | 1.9 (0.21) | –12 |
| Comorbidities (%, SD) | ||||||||||
| Prior cardiovascular surgery | 105 | 30 (29) | 59 | 32 (54) | 54 | 29 | 12 (41) | 29 | 12 (41) | 0.0 |
| Prior myocardial infarction | 105 | 31 (30) | 59 | 20 (34) | 9.4 | 29 | 8 (28) | 29 | 9 (31) | 7.6 |
| Congestive heart failure | 105 | 41 (39) | 56 | 34 (61) | 44 | 29 | 15 (52) | 28 | 15 (54) | 3.7 |
| Left ventricular ejection fraction | 105 | 52 (10) | 58 | 52 (13) | –3.2 | 29 | 50 (12) | 28 | 51 (12) | 6.8 |
| Hypertension | 105 | 96 (91) | 56 | 45 (80) | –32 | 29 | 25 (86) | 28 | 23 (82) | –11 |
| Chronic obstructive pulmonary disease | 105 | 42 (40) | 59 | 31 (53) | 25 | 29 | 11 (38) | 29 | 19 (66) | 57 |
| Prior stroke | 105 | 27 (26) | 59 | 12 (20) | –13 | 29 | 7 (24) | 29 | 8 (28) | 7.9 |
| Peripheral artery disease | 105 | 27 (26) | 59 | 23 (39) | 29 | 29 | 12 (41) | 29 | 10 (34) | –14 |
| Carotid disease | 105 | 46 (44) | 59 | 8 (14) | –71 | 29 | 15 (52) | 29 | 4 (14) | –88 |
| Valve pathology features (SD, %) | ||||||||||
| Aortic stenosis | ||||||||||
| Aortic valve area (cm2) | 92 | 0.68 (0.18) | 55 | 0.61 (0.13) | –43 | 25 | 0.62 (0.17) | 27 | 0.62 (0.13) | 2.8 |
| Mean gradient (mmHg) | 100 | 42 (17) | 57 | 45 (16) | 13 | 28 | 47 (16) | 28 | 45 (17) | –13 |
| Aortic regurgitation (Y/N) | 105 | 69 (66) | 59 | 34 (58) | –17 | 29 | 21 (72) | 29 | 16 (55) | –36 |
Patients with data available.
SAVR: surgical aortic valve replacement; SD: standard deviation; SMD: standardized mean difference; TAVR: transcatheter aortic valve replacement.
‘Porcelain aorta’ defined
Porcelain aorta is circumferential or near circumferential calcification of the ascending aorta [9, 10]. A surgically relevant classification system for porcelain aorta, proposed by Amorim et al., subcategorizes patients qualitatively based on calcium distribution [8]. Type IA is severe circumferential ascending aortic calcification with no aortic clamping possible (herein termed ‘unclampable’). Type IB is severe circumferential ascending aortic calcification with possible aortic clamping, at increased risk (herein termed ‘clampable’, Fig. 1B). Preoperative computed tomography (CT) scans were reviewed to confirm circumferential or near-circumferential aortic calcification diagnostic of porcelain aorta, excluding patients lacking these criteria.
Aortic calcification characterization
Using 3D CT imaging reconstruction (TeraRecon, Actin, MA), aortas were qualitatively classified as clampable or nonclampable. The presence of continuous calcification (uninterrupted contiguous calcification) or diffuse calcification (non-continuous distinct calcium calcification), and involvement of arch branch vessels was noted (Table 2).
Table 2:
Aortic calcification characteristics
| Label | Before matching |
After matching |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAVR, N = 105 |
TAVR, N = 59 |
SAVR, N = 29 |
TAVR, N = 29 |
|||||||
| Na | Count (%), mean (SD) | Na | Count (%), mean (SD) | SMD (%) |
Na | Count (%), mean (SD) | Na | Count (%), mean (SD) | SMD (%) | |
| Calcium volume (ml, SD) | ||||||||||
| Aortic root | 62 | 2.4 (1.6) | 39 | 3.1 (1.7) | 46 | 18 | 2.4 (1.3) | 19 | 2.5 (1.4) | 13 |
| Ascending aorta | 62 | 6.2 (3.5) | 39 | 6.8 (5.2) | 14 | 18 | 6.5 (3.4) | 19 | 7.2 (4.8) | 19 |
| Aortic arch | 62 | 5.8 (4.5) | 39 | 8.2 (5.7) | 47 | 18 | 7.7 (5.3) | 19 | 7.9 (5.1) | 4.3 |
| Total | 62 | 14.4 (6.9) | 39 | 18.1 (9.3) | 45 | 18 | 17 (7.7) | 19 | 18 (8.0) | 14 |
| Calcium classification (%) | ||||||||||
| Unclampable (IA) | 83 | 38 (46) | 53 | 24 (45) | –1.0 | 23 | 11 (48) | 26 | 14 (54) | 12 |
| Clampable (IB) | 83 | 45 (54) | 53 | 29 (55) | 1.0 | 23 | 12 (52) | 26 | 12 (46) | –12 |
| Calcium characteristics (%) | ||||||||||
| Regions of continuous calcification | 83 | 44 (53) | 53 | 29 (55) | 3.4 | 23 | 13 (57) | 26 | 16 (62) | 10 |
| Regions of diffuse calcification | 83 | 72 (87) | 53 | 44 (83) | –10 | 23 | 17 (74) | 26 | 21 (81) | 16 |
| Calcification involving arch-branch vessels | 83 | 76 (92) | 53 | 47 (89) | −9.7 | 23 | 20 (87) | 26 | 24 (92) | 18 |
Additional preoperative valvular characteristics are presented in Supplementary Material, Table S9.
Patients with data available.
SAVR: surgical aortic valve replacement; SD: standard deviation; SMD: standardized mean difference; TAVR: transcatheter aortic valve replacement.
Aortic calcium burden was quantified using a modified thresholding technique adapted from methods used for calcium scoring in CT coronary angiography [10]. Calcified structures were initially isolated by setting a lower and upper threshold (Fig. 2A). Lower thresholds were manually selected by visual examination in 25-unit increments to an upper threshold to ensure only calcified structures were selected, radiodensities outside these cut-offs were excluded (Fig. 2B). Region tools were used to select high-density calcium within the aorta (Fig. 2C). Volumetric analysis was performed on calcium within the aortic root, ascending aorta and aortic arch, yielding total aortic calcium volume in ml (Fig. 2D). Imaging reviewers were blinded to operative characteristics, conflicts were resolved by cardiovascular imaging specialists.
Figure 2:
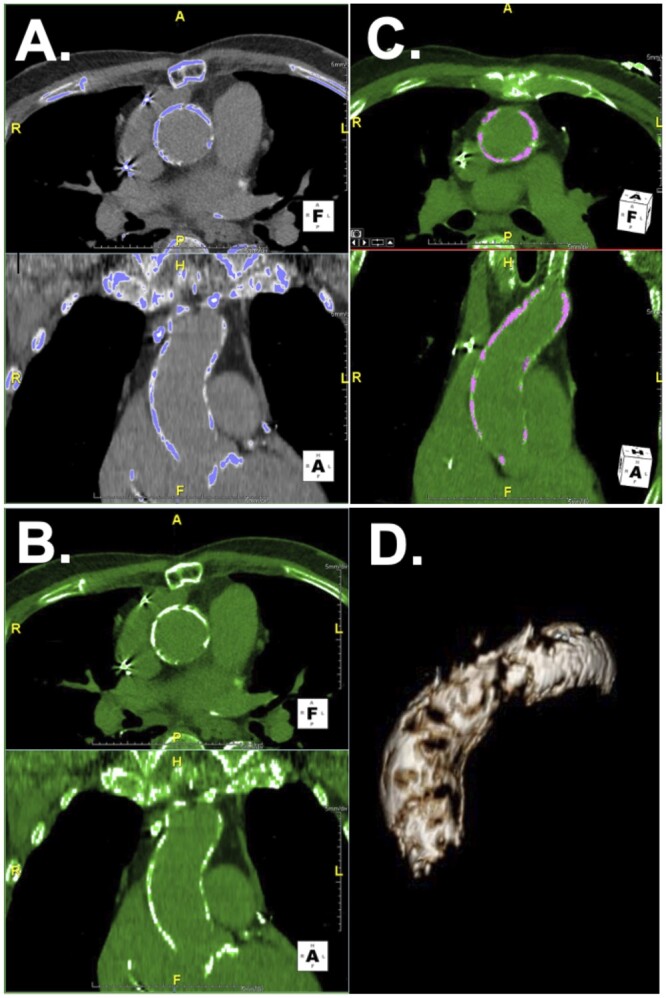
Modified calcium thresholding technique for determining quantitative aortic calcium volume. (A) Calcified structures were initially isolated by setting a lower threshold ranging from 300–700 Hounsfield units and an upper threshold of 2000 Hounsfield units to isolate areas of interest. (B) The lower threshold was selected by visual examination in 25-unit increments to ensure only calcified structures were selected. (C) Region tools were used to manually select high-density calcium within the aortic wall. (D) 3D reconstruction was implemented, and calcium volume measured using volumetric analysis.
End points
Endpoints were all-cause mortality and a composite ‘major hospital complication’ variable. Major hospital complication was defined as in-hospital mortality, renal failure requiring dialysis or stroke. A composite measure was utilized due to the low number of events. Mortality was assessed by Social Security Death Index, quality assurance registries and the electronic medical record.
Data analysis
Analyses were performed using SAS statistical software (SAS v9.4; SAS, Inc., Cary, NC) and R software 4.0.0. Categorical variables are summarized as frequencies and percentages; comparisons were made using chi-square test or Fisher’s exact test when fewer than 5 events were observed in either group. Continuous variables are summarized as mean (standard deviation) or for non-normal distributions as 15th, 50th (median) and 85th percentiles, and comparisons were made using the Wilcoxon rank-sum test.
Propensity matching
To address confounding caused by differing characteristics between SAVR and TAVR patients, propensity score matching was utilized. For incomplete observations, we used five-fold multiple imputation using a Markov chain Monte Carlo technique, assuming missing at random [11]. A parsimonious model was then developed, to which preprocedural variables were added to create a matching score (Supplementary Material, Table S2) [12]. Based on each of the complete datasets, we estimated an average propensity score for each patient (Supplementary Material, Section S2 and S3). TAVR cases whose propensity scores deviated more than 0.2 from SAVR cases were considered unmatched. We accepted a higher calliper width than the usual 0.1 to capture more matched pairs [13].
Survival and composite complication risk factors
Survival was estimated nonparametrically by the Kaplan–Meier estimator and parametrically by temporal decomposition [14]. Random forest was used to identify predictors of composite major hospital complications using the variables listed in Supplementary Material, Section S4, implementing a forest of 5000 regression trees from a subset of 7 randomly selected variables at each split [15]. On-the-fly imputation was used to impute missing data [16]. Random forest variable importance was used to hierarchically order covariates in relation to predicted major hospital complication [17]. Partial dependency plots were used to describe the relationship between covariates of interest and the response by risk-adjusting for all other covariates [18].
RESULTS
Patients
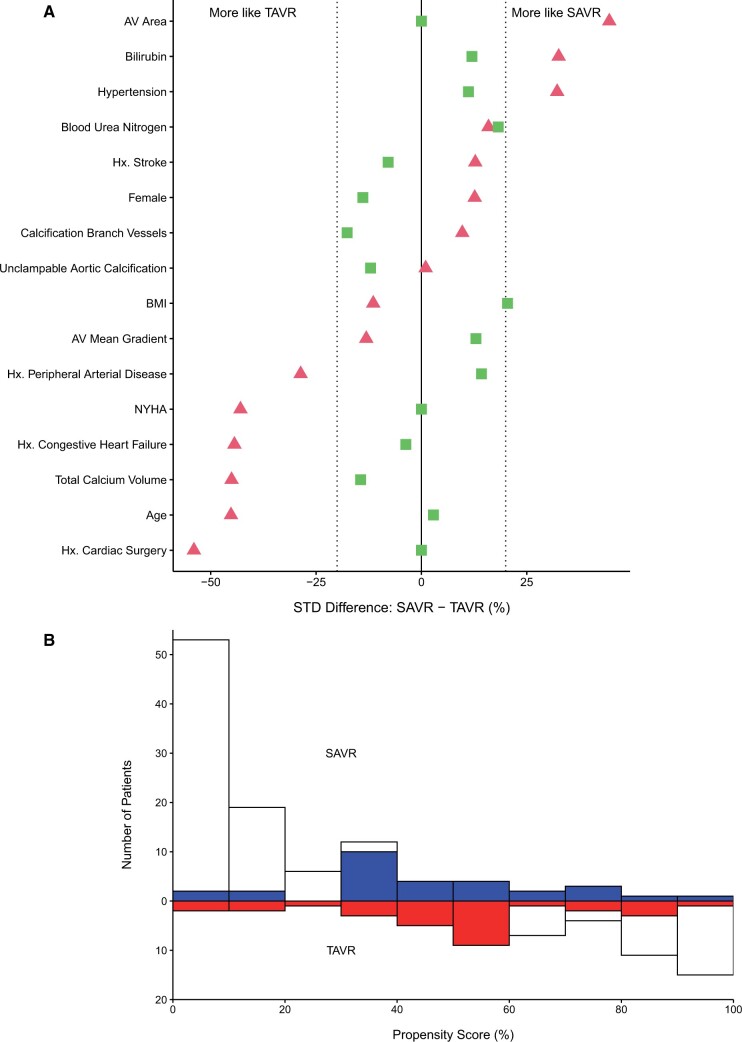
From 1 January /2006 to 1 January 2015, 12 672 patients underwent AVR. Of these, 164 (1.3%) with aortic valve stenosis, and no history of thoracic aortic surgery, underwent AVR in the setting of porcelain aorta [105 (64%) SAVR; 59 (36%) TAVR, Fig. 1A]. Mean age was 73 (10) years in the SAVR group and 78 (10) years in the TAVR group (Table 1). Propensity scoring based on 17 preprocedural variables, including aortic calcium volume, yielded 29 matched pairs, accounting for 49% of TAVR patients (Fig. 3). Carotid disease, aortic regurgitation and chronic obstructive pulmonary disease remained higher in SAVR patients after matching (Table 1).
Figure 3:
Propensity score matching results for comparing outcomes of SAVR versus TAVR. AV: aortic valve; BMI: body mass index; Hx: history; NYHA: New York Heart Association Symptom Class; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement. (A) Covariable balance for selected variables before (triangles) and after (squares) matching, contrasting characteristics of patients undergoing SAVR and TAVR. Values on the horizontal axis represent standardized difference. Triangles to the left of zero (negative) represent TAVR-like characteristics; triangles to the right of zero (positive) represent SAVR-like characteristics. (B) Mirror histogram of the distribution of propensity scores for SAVR (bars above zero line) and TAVR (bars below zero line) approaches. Shaded areas represent 29 matched patient pairs. Unshaded areas represent unmatched patients (SAVR, N = 76; TAVR, N = 30).
Preoperative CTs were available for calcification assessment in 136 (83%) patients. Missing images were primarily due to the incompatibility of outside imaging systems with analysis software, prohibiting data collection.
The median follow-up was 1.1 years, with 18% of survivors followed >5 years. Completeness of follow-up is presented in Supplementary Material, Fig. S1.
Aortic calcification
Qualitative classifications
Overall, qualitative calcification review demonstrated that 38 (46%) SAVR aortas were unclampable and 45 (54%) were clampable. In TAVR patients, 24 (45%) aortas were unclampable, and 29 (55%) were clampable (Table 2).
After matching, 11 (48%) SAVR patients had unclampable aortas; 14 (54%) TAVR aortas were unclampable. Twelve (52%) SAVR patients had clampable calcification, as did 12 (46%) TAVR patients.
Quantitative measurements
Overall, the mean aortic root calcium volume was 2.7 (1.7) ml, ascending aortic calcium volume 6.5 (4.2) ml, and aortic arch calcium volume 6.7 (5.1) ml (Table 2). Mean total calcium volume in patients undergoing TAVR was 18 (9.3) ml, and those undergoing SAVR was 14 (6.9) ml. Total aortic calcium volume after matching in patients who underwent SAVR was 17 (7.7) ml, and 18 (8.0) ml in matched TAVR patients (Table 2).
Unmatched SAVR patients had a total calcium volume of 14 (6.5) ml, an arch calcium volume of 5.0 (3.9) ml and root calcium volume of 2.4 (1.8) ml (Supplementary Material, Table S3). Unmatched TAVR patients had a total calcium volume of 19 (11) ml, an arch calcium volume of 8.4 (6.3) ml, and a root calcium volume of 3.7 (1.8) ml (Supplementary Material, Table S4).
Operative characteristics
Patients who were older, had more severe preoperative heart failure and underwent AVR more recently were more likely to undergo TAVR (Supplementary Material, Table S2). Details regarding surgical cannulation strategies and transcatheter valve deployment are listed in Supplementary Material, Table S5.
TAVR was most commonly performed transapically (n = 31, 53%), followed by transfemorally (n = 23, 39%) and transaortically (n = 5, 7%, Supplementary Material, Table S4). Two patients (3.3%) underwent concomitant coronary angioplasty. Four patients (6.8%) experienced unplanned use of CPB (Supplementary Material, Table S5). Two of which required postoperative intra-aortic balloon pump support, and the remaining were weaned of CPB. No TAVR patients were converted to sternotomy.
Eighty (76%) SAVR patients underwent non-aortic or non-valvular concomitant procedures. Most SAVR patients underwent concomitant cardiac procedures: 65 (62%) coronary artery bypass grafting, 32 (30%) mitral valve repair or replacement, 15 (14%) tricuspid valve repair and 7 (6.7%) surgical ablations for atrial fibrillation. Six (10%) TAVR patients also had multi-component procedures. (Supplementary Material, Table S6).
In-hospital outcomes
Twenty-eight (18%) patients had a composite major hospital complication. Before matching, 9 (8.6%) in-hospital deaths occurred after SAVR and 5 (8.5%) after TAVR; 5 (4.8%) strokes occurred after SAVR and 1 (1.7%) after TAVR; and renal failure requiring dialysis occurred in 15 (15%) patients after SAVR and 5 (8.9%) after TAVR. Minor postoperative complications (atrial fibrillation, prolonged ventilation, need for blood products and length of stay) are reported in Table 3.
Table 3:
Complications and postprocedure course
| Label | Before matching |
After matching |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAVR, N = 105 |
TAVR, N = 59 |
SAVR, N = 29 |
TAVR, N = 29 |
|||||||
| N | Count (%), mean (SD), median (15th, 80th percentile) | N | Count (%), mean (SD), median (15th, 80th percentile) | P-Value | N | Count (%), mean (SD), median (15th, 80th percentile) | N | Count (%), mean (SD), median (15th, 80th percentile) | P-Value | |
| Major hospital complications (%) | ||||||||||
| In-hospital death | 105 | 9 (8.6) | 59 | 5 (8.5) | >0.9 | 29 | 1 (3.4) | 29 | 3 (10) | 0.6 |
| Stroke | 105 | 5 (4.8) | 59 | 1 (1.7) | 0.4 | 29 | 4 (14) | 29 | 1 (3.4) | 0.4 |
| Renal failure (requiring dialysis) | 102 | 15 (15) | 56 | 5 (8.9) | 0.3 | 29 | 2 (6.9) | 28 | 3 (11) | 0.7 |
| Composite major hospital complicationa | 104 | 21 (20) | 56 | 7 (13) | 0.2 | 29 | 4 (14) | 28 | 4 (14) | >0.9 |
| Minor hospital complications (%, percentile) | ||||||||||
| Prolonged ventilation (>24 h) | 105 | 57 (54) | 59 | 9 (15) | <0.0001 | 29 | 16 (55) | 29 | 4 (14) | 0.002 |
| Postoperative atrial fibrillation | 84 | 36 (43) | 46 | 4 (8.7) | <0.0001 | 24 | 11 (46) | 23 | 3 (13) | 0.02 |
| Required postoperative blood products | 105 | 82 (78) | 59 | 23 (39) | <0.0001 | 29 | 25 (86) | 29 | 11 (38) | 0.0002 |
| Any postoperative red blood cells given | 105 | 76 (72) | 59 | 23 (39) | <0.0001 | 29 | 23 (79) | 29 | 11 (38) | 0.001 |
| Length of stay (percentile) | ||||||||||
| ICU length of stay (h) | 105 | 137 (43, 464) | 59 | 70 (26, 216) | 0.0006 | 29 | 107 (44, 436) | 29 | 71 (25, 188) | 0.04 |
| Hospital length of stay (days) | 105 | 15 (7.3, 31) | 59 | 8.0 (4.0, 17) | <0.0001 | 29 | 19 (8.8, 42) | 29 | 7.9 (3.6, 14) | <0.0001 |
| Operative length of stay (days) | 105 | 12 (7.1, 28) | 59 | 7.1 (3.1, 13) | <0.0001 | 29 | 12 (7.6, 36) | 29 | 7.0 (2.7, 12) | 0.0002 |
aComposite major hospital complication is the added total incidence of in-hospital death, stroke and renal failure requiring dialysis.
ICU: intensive care unit; SAVR: surgical aortic valve replacement; SD: standard deviation; TAVR: transcatheter aortic valve replacement.
Sixteen (12%) patients classified as having an unclampable ascending aorta experienced a major hospital complication, and 8 (6.1%) patients classified with a clampable aorta experienced the same (Supplementary Material, Table S7).
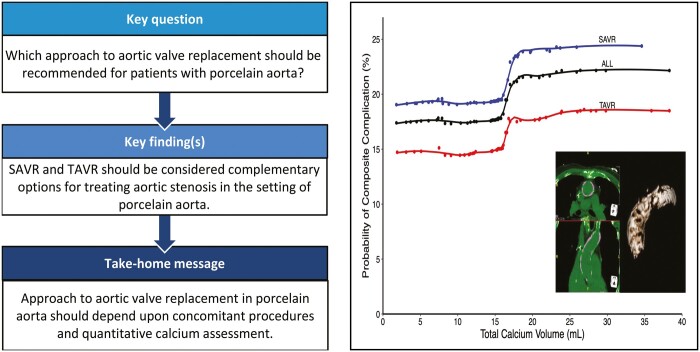
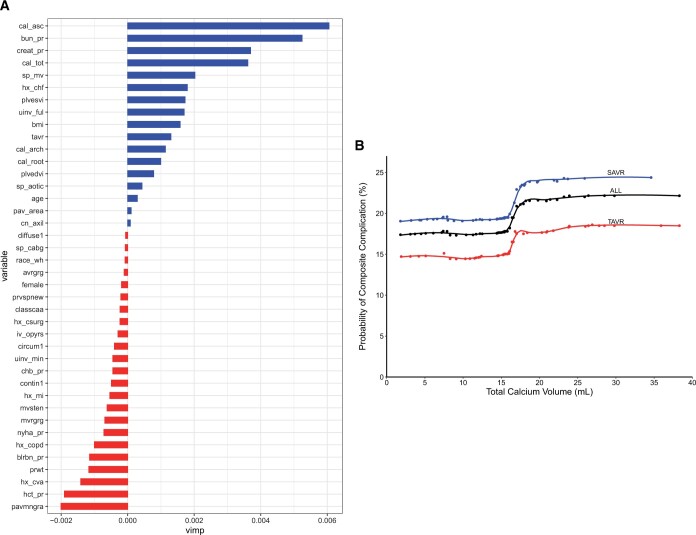
Total aortic calcium volume and ascending aortic calcium volume contributed most to prediction of a major hospital complication (Fig. 4A). Partial-dependence risk-adjusted plots between total calcium score and predicted probability of hospital complication demonstrated an inflection point at 16 ml of total aortic calcium volume (Fig. 4B).
Figure 4:
Representative risk of major hospital complication is associated with aortic calcium volume and regionality. (A) Variable of importance plot. Variables with larger values are more important to the random forest model in predicting a major hospital complication. Variable acronyms/names are available in Supplementary Material, Table S8. (B) Partial-dependence risk-adjusted graph of association between total calcium score and predicted probabilities of composite major hospital complication (death, stroke, renal failure requiring dialysis). Risk-adjusted estimates are based on random forest classification. Symbols represent ensemble averages (probabilities) across total calcium volume, and solid line depicts smoothing spline curves of these probabilities. AV: aortic valve; BMI: body mass index; Hx: history; NYHA: New York Heart Association Symptom Class; SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
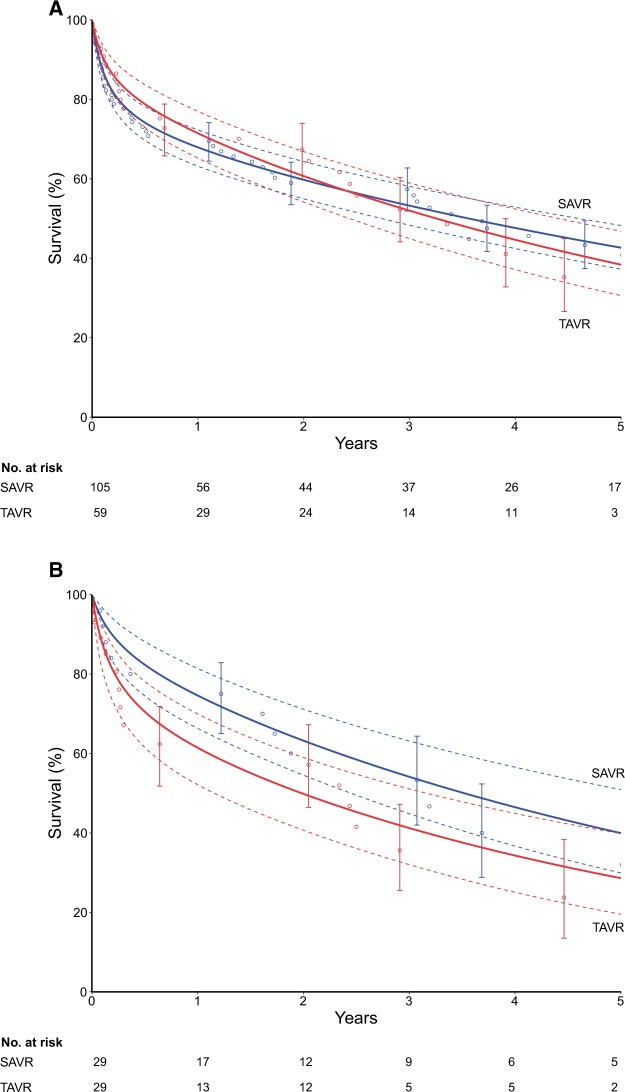
Survival
Overall 5-year survival was 41% (43% SAVR, 35% TAVR; Fig. 5A). After matching, survival in the SAVR group was 88%, 75%, 54% and 40% at 90 days, 1, 3 and 5 years, respectively; after TAVR, survival was 79%, 61%, 41% and 29% at the same intervals [P(log rank) = 0.4, Fig. 5B].
Figure 5:
Overall and matched survival following valve replacement in the setting of porcelain aorta. (A) Survival by surgical approaches [SAVR (blue) versus TAVR (red)] in the overall cohort. (B) Survival by surgical approaches [SAVR (blue) versus TAVR (red)] in the matched cohort. Each symbol represents a death, and vertical bars are asymmetric 68% confidence limits equivalent to ±1 standard error. Solid line depicts the parametric estimates of survival enclosed within dashed 68% confidence bands. SAVR: surgical aortic valve replacement; TAVR: transcatheter aortic valve replacement.
Unmatched SAVR patients had survival of 77%, 67%, 56% and 44% at 90 days, 1, 3 and 5 years, respectively. Unmatched TAVR patients had survival of 88%, 83%, 72% and 48% at 90 days, 1, 3 and 5 years, respectively (Supplementary Material, Fig. S2).
DISCUSSION
Principal findings
In-hospital mortality, major hospital complications and survival following SAVR and TAVR in the setting of porcelain aorta were similar before and after matching. Quantitative assessment of aortic calcium volume and an unclampable ascending aorta were associated with risk of major hospital complications independent of operative approach. Notable heterogenicity between these populations remained after matching. This analysis demonstrates that the optimal approach and outcomes depend on the presence of concomitant disease and calcium volume, respectively.
Commentary
The difficulty of assessing ideal operative approaches in porcelain aorta is evident in the current literature. Porcelain aorta was the most common indication (15% of patients) for TAVR in the technically inoperable arm of the PARTNER I trial [1]. Additionally, in the OBSERVANT trial, porcelain aorta was the 2nd most common reason patients underwent TAVR as opposed to SAVR [19]. Outside clinical trials, management of aortic stenosis in porcelain aorta is debated [3, 6, 20]. Variation in cohort characteristics and limited occurrence of porcelain aorta make a prospective clinical trial impractical. Propensity score matching attempts to compare surgical and transcatheter interventions in a more similar subset of heterogenous patients.
The heterogeneity of porcelain aorta patients makes perioperative evaluation, risk stratification and analysis difficult. With an overall 5-year mortality of 8.5%, the complexity of these interventions, contextualized by severe calcification, conveys greater risk than either routine or reoperative AVR [21]. Balancing the risks and benefits of choosing SAVR in this population is difficult. Assessment based on qualitative disease alone is insufficient [2, 6, 8]. There is no standardized process to quantify atherosclerotic disease of the aorta preoperatively in patients being evaluated for SAVR or TAVR [22]. Our approach to preoperative calcium quantification revealed that unclampable calcification and higher total aortic calcium volume were associated with an increased probability of major hospital complications. Quantifying aortic calcium volume may facilitate better operative planning and direct patients towards a more appropriate therapy including continued medical therapy when symptoms are mild. For example, older patients with higher calcium volumes but a clampable aorta who may not tolerate postoperative complications could benefit from TAVR or best medical therapy.
Analyses before and after matching demonstrated similar results, emphasizing the utility of SAVR with proper patient selection. Comparing unmatched and matched SAVR groups identified patients who may potentially benefit from TAVR over SAVR, or vice versa. Without an observed difference in mortality and a lower morbidity, our analysis preferentially suggests TAVR for isolated AVR even with a clampable aorta. However, patients with lower calcium volumes and clampable aortas may tolerate SAVR, and require it in the setting of concomitant disease. In the context of postoperative complications, improving the tools for patient selection is critical for providing optimal treatment. Quantitative calcium assessment is useful for aiding in patient selection.
Limitations
Patient heterogenicity remains a significant problem in both clinical management and this analysis. Despite propensity matching, SAVR patients had more comorbidities and underwent more concomitant procedures. These populations remain difficult to compare, necessitating our widened matching strategy and unmatched comparisons. Additionally, the small sample size reduces our ability to detect differences between groups. While propensity score matching provides some insight to comparability of these heterogenous populations, the independent effect of operative approach on valve replacement remains difficult to determine.
Our imaging analysis was limited by inconsistent CT slice thickness and contrast protocols. Manual selection of calcium thresholding during analysis was aimed at decreasing the possibility of contrast or other high radiodensity structures being included in the volumetric calculations. Institution-specific protocolized methods for selecting the threshold windows is recommended, reducing variability.
Since this cohort was treated, advancements have improved safety and efficacy of both SAVR and TAVR, further emphasizing the utility of both procedures. Improved understanding and techniques of hypothermic circulatory arrest and antegrade brain perfusion have led to greater adoption during SAVR [23]. In combination with the ‘no-touch’ technique, axillary cannulation or double arterial cannulation (with the addition of a femoral in-flow cannula) provides safer options for reducing postoperative complications [24].
CONCLUSION
Patients with porcelain aorta must be evaluated carefully when considering intervention, regardless of the procedural approach. While TAVR offers a lower risk of postoperative complications, higher calcium volume remains an independent predictor of postoperative complications. TAVR should represent the treatment of choice in porcelain aorta for isolated AVR. For patients requiring concomitant procedures, SAVR can be performed with similar occurrence of composite major hospital complications and 5-year survival. The absence of a ‘clampable zone’ and increased total calcium volume are associated with increased morbidity and mortality. To improve patient selection, quantitative methods of determining aortic calcium volume should be considered in all patients with porcelain aorta.
Supplementary Material
Glossary
ABBREVIATIONS
- AVR
Aortic valve replacement
- CPB
Cardiopulmonary bypass
- CT
Computed tomography
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
Contributor Information
Benjamin Kramer, Aortic Valve Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Heart, Vascular, and Thoracic Institute, Cleveland, OH, USA.
Andrew M Vekstein, Aortic Valve Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Heart, Vascular, and Thoracic Institute, Cleveland, OH, USA.
Paul D Bishop, Department of Vascular Surgery, Cleveland Clinic, Cleveland, OH, USA.
Ashley Lowry, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, USA.
Douglas R Johnston, Aortic Valve Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Heart, Vascular, and Thoracic Institute, Cleveland, OH, USA.
Samir Kapadia, Aortic Valve Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Vascular Surgery, Cleveland Clinic, Cleveland, OH, USA.
Amar Krishnaswamy, Aortic Valve Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Vascular Surgery, Cleveland Clinic, Cleveland, OH, USA.
Eugene H Blackstone, Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Heart, Vascular, and Thoracic Institute, Cleveland, OH, USA; Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, USA.
Eric E Roselli, Aortic Valve Center, Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Thoracic and Cardiovascular Surgery, Cleveland Clinic, Heart, Vascular, and Thoracic Institute, Cleveland, OH, USA.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This study was supported by the High-Risk Cardiovascular Research Philanthropy Fund and The Stephens Family Endowed Chair. Dr Eric E. Roselli is the principal investigator and recipient of these grants. Dr Benjamin Kramer is the recipient of support from the National Heart Lung and Blood Institute (NHLBI) as a Clinical Research Scholar of the Cardiothoracic Surgical Trials Network, National Institute of Health (NIH) grant HL088955.
Conflict of interest: Eric E. Roselli discloses relationships with Cook, Artivion, Edwards Lifesciences, W.L. Gore, Medtronic and Terumo Aortic as a speaker, consultant and investigator. The other authors report no conflicts of interest.
DATA AVAILABILITY
The data underlying this article are within and in its online supplementary material, and data may be shared on reasonable request to the corresponding author.
Author contributions
Benjamin Kramer: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Andrew M. Vekstein: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Paul Bishop: Conceptualization; Data curation; Methodology; Resources; Supervision; Writing—review & editing. Ashley Lowry: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Visualization; Writing—review & editing. Douglas R. Johnston: Conceptualization; Resources; Supervision; Writing—review & editing. Samir Kapadia: Conceptualization; Methodology; Resources; Supervision; Writing—review & editing. Amar Krishnaswamy: Conceptualization; Methodology; Resources; Supervision; Writing—review & editing. Eugene H. Blackstone: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—review & editing. Eric E. Roselli: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Gianni D. Angelini and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 36th EACTS Annual Meeting, during the ‘Hot Topics in Transcatheter Valve Treatment’ section, Milan, Italy, October 8, 2022.
REFERENCES
- 1. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Porcelain aorta: a comprehensive review. Circulation 2015;131:827–36. [DOI] [PubMed] [Google Scholar]
- 2. LeMaire SA. Individualized treatment strategies for patients with aortic valve disease and porcelain aorta. J Thorac Cardiovasc Surg 2015;149:134–6. [DOI] [PubMed] [Google Scholar]
- 3. Urbanski PP, Luehr M, Bartolomeo D, Diegeler R, De Paulis A, Esposito R et al. Multicentre analysis of current strategies and outcomes in open aortic arch surgery: heterogeneity is still an issue. Eur J Cardiothorac Surg 2016;50:249–55. [DOI] [PubMed] [Google Scholar]
- 4. Djaiani G, Fedorko L, Borger M, Mikulis D, Carroll J, Cheng D et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke 2004;35:e356–8. [DOI] [PubMed] [Google Scholar]
- 5. Zahn R, Schiele R, Gerckens U, Linke A, Sievert H, Kahlert P et al. Transcatheter aortic valve implantation in patients with “porcelain” aorta (from a Multicenter Real World Registry). Am J Cardiol 2013;111:602–8. [DOI] [PubMed] [Google Scholar]
- 6. Idrees J, Roselli EE, Raza S, Krishnaswamy A, Mick S, Kapadia S et al. Aborted sternotomy due to unexpected porcelain aorta: does transcatheter aortic valve replacement offer an alternative choice? J Thorac Cardiovasc Surg 2015;149:131–4. [DOI] [PubMed] [Google Scholar]
- 7. Michelena HI, Rihal CS, Enriquez-Sarano M. Porcelain aorta. Eur Heart J 2011;32:2303. [DOI] [PubMed] [Google Scholar]
- 8. Amorim PA, Penov K, Lehmkuhl L, Haensig M, Mohr FW, Rastan AJ. Not all porcelain is the same: classification of circular aortic calcifications (porcelain aorta) according to the impact on therapeutic approach. Thorac Cardiovasc Surg 2013;61:559–63. [DOI] [PubMed] [Google Scholar]
- 9. Asami M, Bernhard B, Demirel C, Okuno T, Stortecky S, Heg D et al. Clinical outcomes following transcatheter aortic valve implantation in patients with porcelain aorta. J Cardiovasc Comput Tomogr 2022;16:215–21. [DOI] [PubMed] [Google Scholar]
- 10. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 11. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY, USA: John Wiley & Sons, 2004. [Google Scholar]
- 12. Rajeswaran J, Blackstone EH. Identifying risk factors: challenges of separating signal from noise. J Thorac Cardiovasc Surg 2017;153:1136–8. [DOI] [PubMed] [Google Scholar]
- 13. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blackstone EH, Naftel DC, Turner ME Jr. The decomposition of time-varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc 1986;81:615–24. [Google Scholar]
- 15. Ishwaran H, Kogalur U. Random forests for survival, regression and classification (RF-SRC). R Package Version, 2017,2. [Google Scholar]
- 16. Tang F, Ishwaran H. Random forest missing data algorithms. Stat Anal Data Min 2017;10:363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishwaran H. Variable importance in binary regression trees and forests. Electron J Stat 2007;1:519–37. [Google Scholar]
- 18. Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat 2001;29:1189–232. [Google Scholar]
- 19. Tarantini G, Fovino N, D'Errigo L, Rosato P, Barbanti S, Tamburino M et al Factors influencing the choice between transcatheter and surgical treatment of severe aortic stenosis in patients younger than 80 years: results from the OBSERVANT study. Catheter Cardiovasc Interv 2020;95:E186–95. [DOI] [PubMed] [Google Scholar]
- 20. Carrel T, Vogt PR. Porcelain aorta does not mean inoperability but needs special strategies. Interact Cardiovasc Thorac Surg 2022;35:ivac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onorati F, Biancari F, Feo D, Mariscalco M, Messina G, Santarpino A et al Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative†. Eur J Cardiothorac Surg 2014;47:269–80. [DOI] [PubMed] [Google Scholar]
- 22. Zingone B, Rauber E, Gatti G, Pappalardo A, Benussi B, Dreas L et al. The impact of epiaortic ultrasonographic scanning on the risk of perioperative stroke. Eur J Cardiothorac Surg 2006;29:720–8. [DOI] [PubMed] [Google Scholar]
- 23. Isoda S, Osako M, Kimura T, Nishimura K, Yamanaka N, Nakamura S et al. A stepwise aortic clamp procedure to treat porcelain aorta associated with aortic valve stenosis and hemodialysis. Ann Thorac Cardiovasc Surg 2014;20 Suppl:725–9. [DOI] [PubMed] [Google Scholar]
- 24. De Paulis R, Maselli D, Scaffa R, Nardella S. Double-arterial cannulation for aortic valve replacement with porcelain aorta. Eur J Cardiothorac Surg 2009;36:769–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are within and in its online supplementary material, and data may be shared on reasonable request to the corresponding author.
Author contributions
Benjamin Kramer: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Andrew M. Vekstein: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft; Writing—review & editing. Paul Bishop: Conceptualization; Data curation; Methodology; Resources; Supervision; Writing—review & editing. Ashley Lowry: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Visualization; Writing—review & editing. Douglas R. Johnston: Conceptualization; Resources; Supervision; Writing—review & editing. Samir Kapadia: Conceptualization; Methodology; Resources; Supervision; Writing—review & editing. Amar Krishnaswamy: Conceptualization; Methodology; Resources; Supervision; Writing—review & editing. Eugene H. Blackstone: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—review & editing. Eric E. Roselli: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Gianni D. Angelini and the other anonymous reviewer(s) for their contribution to the peer review process of this article.