Abstract
Recent contributions of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) regarding obstetrical perinatal interventions and neonatal delivery room practices include the following: the impact of multiple antepartum factors including maternal diabetes, hypertension, obesity and mode of delivery on outcomes of extremely preterm newborns, effects of delayed delivery interval for extremely preterm multiples, effects of antenatal steroids on preterm newborn outcomes and the impact of antenatal magnesium sulfate therapy on neurodevelopmental outcomes for extremely preterm infants. NRN studies also contribute important evidence for neonatal delivery room resuscitation guidelines including umbilical cord management and maintenance of euthermia immediately after birth. The updated NRN outcome calculator helps better counsel families regarding possible outcomes for the most immature newborns if resuscitation is attempted at birth. Thus, the NRN provides substantial information regarding effects of perinatal management on newborn infants.
Introduction
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) provides substantial information on perinatal management of newborn infants.1 This evidence has been used for the development of treatment recommendations from the International Liaison Committee on Resuscitation (ILCOR).2 The latter influences neonatal resuscitation guidelines across the globe. Major strengths of the NRN include collective expertise in research design, data collection, data analysis, and focus on important outcomes including neonatal mortality and morbidity with validated neurodevelopmental outcomes after discharge, up to an age that has progressively increased from 16–18 months to 22–26 months and even to childhood in several studies.
For many years the NRN has conducted landmark randomized controlled trials (RCTs) on delivery room (DR) management of very preterm infants. For example, the NRN Surfactant, Positive Pressure, and Oxygenation Randomized Trial (SUPPORT) compared continuous positive airway pressure (CPAP) versus endotracheal intubation in very preterm infants and led to a progressive practice change from routine intubation after birth to attempts at stabilization using CPAP in the DR.3 Meta-analysis showed that routine CPAP in the DR improves survival without bronchopulmonary dysplasia in these neonates and was subsequently incorporated into neonatal resuscitation guidelines.2 NRN RCTs of therapeutic cooling for hypoxic-ischemic encephalopathy in term and nearterm infants were landmark studies, which affected International Liaison Committee on Resuscitation guidelines and clinical practice around the globe.4
For decades, the NRN has prospectively collected data on very preterm infants in its comprehensive preterm database, which includes infants born alive at 401–1000 grams and <29 weeks gestational age (GA), thereby allowing comparison of serial changes in practices and outcomes.5 This database was recently expanded to include extremely small neonates and periviable fetuses, thereby allowing assessment of perinatal care effects on mortality and morbidity on this poorly studied and most fragile population. Collaboration with the Vermont-Oxford Neonatal Network (VON) led to external validation of the NRN prognostic tool for survival and morbidity of extreme preterm (EPT) infants, which is now used for perinatal counseling of periviable pregnancies.6 In addition, the NRN has collected observational perinatal data on a one-year cohort of moderate preterm infants (MPT); this provided an outstanding dataset on DR management for this population, for which only minimal data had been previously available.7,8
This current review will examine how the large cohort of newborns available to the NRN has allowed analysis of the impact of factors such as center, race/ethnicity, maternal age, and neonatal characteristics on outcomes. In addition we review how multiple NRN studies have assessed how maternal disease (e.g., pre-eclampsia), treatment (e.g., antenatal steroids), morbidity (e.g., obesity) and DR management (cord clamping or milking and maintenance of euthermia) affect perinatal outcomes.7,9–17
Effect of maternal factors on preterm newborn infant outcomes
Outcome of preterm infants in relation to maternal pre-pregnancy body mass index
Nearly a third of all women in the United States are obese.18 Obesity in women before pregnancy has been associated with multiple complications in women, fetuses and neonates, including risk of diabetes, gestational hypertension, fetal demise, birth injuries, preterm birth, and neonatal mortality.19,20 There is limited data on outcomes of neonates born preterm in relation to maternal weight status prior to pregnancy. Chawla and colleagues evaluated risk of mortality and major neonatal morbidities in relation to maternal weight and obesity among EPT infants.14 The study cohort included EPT infants (GA 220/7 to 286/7 weeks) born between January 2016 and June 2018 at NICHD NRN sites. Based on pre-pregnancy body mass index (BMI), mothers were classified as underweight (BMI < 18.5 kg/m2); normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2); and obese BMI ≥ 30 kg/m2. The primary outcome was survival to discharge without major morbidity, defined as presence of any of the following: grade III/IV intracranial hemorrhage (ICH), cystic periventricular leukomalacia (cPVL), late onset sepsis, stage ≥2 necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), or retinopathy of prematurity (ROP). Secondary outcomes were survival to hospital discharge, and survival without individual major neonatal morbidities.
A total of 2,415 infants were included in the analysis. There was a high prevalence of pre-pregnancy obesity (n=909, 38%), and overweight status (n= 568, 24%), with 938 (38%) mothers having normal/underweight status. There was no significant difference in the incidence of survival without significant morbidity among the three groups: 31% in the normal/underweight group, 28% in the overweight group, and 28% in the obese group (p=0.65). Survival at hospital discharge was lowest for neonates in the obese group (76%), as compared to 82% in the normal/underweight group, and 83% in the overweight group, (p=0.02). Each unit increase in the maternal BMI was associated with lower odds of neonatal survival at discharge [adjusted odds ratio (aOR) 0.98, 95% Confidence Interval (CI): 0.96–0.99, p <0.01]. Survival without cPVL was lowest in the obese group (driven primarily by the impact of maternal obesity on survival): 73% in the obese group, 79% in the normal/underweight group, and 80% in the overweight group, (p<0.01). Survival without NEC was also lowest in the obese group (driven primarily by the impact of maternal obesity on survival); 71% in the obese group, 77% in the normal/underweight group, and 76% in the overweight group (p=0.02). The authors concluded that preconception counseling and weight management for women may help improve survival and reduce major neonatal morbidities among EPT infants.
Outcomes of extremely preterm infants born to adolescent mothers
There is scarcity of data on the neurodevelopmental outcomes of preterm infants born to adolescent mothers. Hoffman and colleagues evaluated the developmental and behavioral outcomes of EPT infants born to adolescent mothers (age <20 years) and explored the role of social and home constructs of these infants on neurodevelopmental outcomes evaluated at 18 to 22 months corrected age.13 Extremely preterm infants <27 weeks GA born between January 2008 and June 2011 at NICHD NRN sites, who underwent comprehensive neurologic and developmental evaluation, including Bayley Scales of Infant Development (BSID-III) and Brief Infant Social Emotional Assessment (BITSEA) were included in the analysis. The primary outcomes were BSID-III composite language and cognitive scores. Secondary outcomes were BITSEA scores, neurodevelopmental impairment, growth parameters and rehospitalization rates.
During the study period, 1,934 patients met the inclusion criteria; 211 were born to adolescent mothers and 1,723 were born to adult mothers. The mean age of adolescent cohort was 17.7 ±1.3 years, and the mean age of the adult cohort was 28.6 ±5.8 years. Adolescent mothers and their infants were more likely to have public insurance (75% vs. 48%, p<0.001), more likely to have received services from a visiting nurse (54% vs. 46%, p=0.02), and to be under state supervision (7% vs. 3%, p=0.01). The risk of rehospitalization was higher among infants born to adolescent mothers (56% vs. 46%, p=0.005). There was no significant difference in the BSID-III cognitive, language and motor composite scores or in the rate of neurologic impairment between the two groups. Infants of adolescent mothers had higher BITSEA/Problem Scale scores than those of adult women (mean 14.8 vs. 12.1, p <0.001), suggesting increased behavior and social problems. There was no significant difference in the weight and length at follow-up examination. However, infants born to adolescent mothers had smaller head size at 18 to 22 months of age, compared to infants born to adult mothers (46.4 ±2.1 cm vs. 46.8 ±2.3 cm, p=0.03).
The study concluded that EPT infants born to adolescent mothers experience many social and environmental risks and experience higher rates of behavioral problems. Access to comprehensive follow-up and coordinated care for both adolescent mothers and their children may improve their outcomes.
Racial disparities among extremely preterm infants in the United States
There are limited data regarding the role of race/ethnicity on survival, major morbidities, and neurodevelopmental outcomes of EPT infants over time. Travers and colleagues assessed whether racial/ethnic differences in hospital mortality, major morbidities and utilization of key care practices changed over time among EPT infants.15 This cohort study included EPT infants (GA 220/7 to 276/7 weeks) born between 2002 and 2016 at 25 different NRN sites. Classification of race/ethnicity was based on self-report by parents.
A total of 20,092 EPT infants (mean GA 25.1 ±1.5 weeks) were included of which 8,331 (41.5%) were black infants, 3701 (18.4%) were Hispanic infants, and 8,060 (40.1%) were white infants. Hospital mortality decreased over time among infants of all races and ethnicity (black infants from 35% to 24%, Hispanic infants from 32% to 27%, and white infants from 30% to 22%; p = 0.59 for race × year interaction). The rates of late-onset sepsis were higher among black infants and Hispanic infants in the beginning, but were similar to white infants in recent years (from 37% to 24%, from 45% to 23%, and from 36% to 25%, respectively, p =0.02 for race × year interaction). As a marker of utilization of care practices, exposure to antenatal steroids among black infants and Hispanic infants were initially lower and increased at a faster rate over time as compared with white infants (from 72% to 90%, from 73% to 83%, and from 86% to 90%, respectively; p=0.01 for race × year interaction). Similarly, the rates of cesarean delivery among black infants and Hispanic infants were initially lower and increased at a faster rate over time as compared with white infants (from 45% to 59%, from 49% to 59%, and from 62% to 63%, respectively; p =0.03 for race × year interaction). Thus, there has been narrowing of racial/ethnic disparities in some important neonatal care practices, including the use of antenatal corticosteroids, cesarean delivery and less late onset sepsis.
Effect of antenatal corticosteroids
Use of antenatal steroids coupled with magnesium sulfate therapy
Antenatal therapies such as antenatal corticosteroids (ANS) and magnesium sulfate improve neonatal outcomes in preterm infants.21,22 While magnesium sulfate reduces cerebral palsy and gross motor dysfunction,22 administration of ANS reduces mortality, respiratory distress syndrome, NEC, ICH and systemic infections in the first 48 hours of life.21 Given these data, a large prospective observational NRN study conducted by Gentle et al investigated the long term outcomes after the utilization of a combination of these two therapies in 3093 infants born between 220/7-266/7 weeks GA.23 Neurodevelopmental follow up was assessed at 18–26 months corrected age using the BSID-III. Infants exposed to both therapies had lower composite rates of severe neurodevelopmental impairment (NDI) or death (36.3%) as compared to either intervention alone [44.3%, adjusted odds ratio (aOR) 0.73; 95% CI 0.58–0.91 for ANS; 53%; aOR 0.49; 95% CI 0.29–0.82 for magnesium sulfate) or neither therapy (48.2%; aOR 0.66; 95% CI 0.49–0.89)].23 When death and NDI were assessed separately, the notable reduction in death persisted in the combination group however, NDI among survivors did not differ between exposure groups. This study differed from a meta-analysis of magnesium sulfate exposure alone which did not demonstrate an effect on the composite outcome of NDI or death.22 However, only a minority of patients included in the meta-analysis were less than 30 weeks of gestation and the combination of magnesium sulfate with steroids was not addressed. Of note, in Gentle’s large multicenter NRN study, fewer babies received magnesium sulfate than antenatal steroids.23 The reason for this is unclear, but given the decreased composite outcome of death or NDI when antenatal steroids are given in combination with magnesium sulfate, this information could influence obstetrical practice and encourage the use of both drugs in combination.
Antenatal steroid use for periviable births
Management of EPT birth at the cusp of viability requires much collaboration among obstetricians, neonatologists and parents during this difficult decision making process.24 Current obstetrical guidelines recommend the use of antenatal corticosteroids in threatened preterm birth between 24–34 weeks of gestation.25,26 In recent years, given the evolution of neonatal practices, increasing numbers of infants born at 22–23 weeks of gestation are being resuscitated.27 However, there remains a dearth of substantial clinical data supporting the use of antenatal corticosteroids in this cohort of infants.21 A large observational study conducted by Rysavy et al investigated live births at 220/7 - 266/7 weeks gestation in NICHD NRN centers and included follow up at 18–22 months corrected age.12 The purpose of this study was to describe the discordance in antenatal steroid use and resuscitation, where one and not the other was offered at different GAs and its correlation with infant survival and development. The authors found discordant antenatal corticosteroid use and resuscitation (where one and not the other occurred) were more frequent in the 22–23 weeks GA group (rate ratio [95% CI] at 22 weeks: 1.7 [1.3–2.2]; 23 weeks: 2.6 [2.2–3.2] as compared to older GAs. Interestingly, among infants born at 23 weeks, if the ANS rate was adjusted to the hospital average for 25-week GA neonates (89.2%), infant survival was projected to increase by 7.1% (95% CI 5.4–8.8%) and survival without impairment by 6.4% (95% CI 4.7–8.1%). However, this projection was not replicated in the 22-week GA group. This suggests there may be value in routine use of ANS in situations where resuscitation of 23-week GA infants is contemplated. Randomized trials of ANS for these earliest gestations would be needed to prove this definitively.
Antenatal steroid use and outcomes in extremely preterm multiple gestation infants
While the practicality and efficacy of a single course of antenatal corticosteroids administered 24 hours to 7 days before delivery has been established in singleton pregnancies before 34 weeks,21 data on the effect of ANS on multiple gestation has been limited and conflicting. A study of 6925 premature multiples born at NICHD NRN centers between 22–28 weeks of gestation explored the effect of exposure to ANS and demonstrated lower in-hospital mortality [adjusted relative risk (ARR) 0.87, 95% CI 0.78–0.96] but no decrease in risk for composite NDI or death (aRR 0.93, 95% CI 0.84–1.03).9 This is in contrast to a sub-group analysis of preterm multiples in a meta-analysis conducted by Roberts et al21 and an earlier single center study by Hackman et al28 which both showed no difference in mortality in multiples exposed to ANS as compared to those who were not. Differences in the effects of ANS on multiples as compared to singleton pregnancies may be due to differing pharmacokinetics of the drug in singleton versus multiple gestations. Data remain conflicting regarding the pharmacokinetics of ANS in multiple gestation pregnancies. One study demonstrated a shorter half-life and greater clearance of ANS in twin pregnancies suggesting the need for a higher dose in multiples to achieve effective therapeutic levels,29 while another suggested adequacy of the current dose.30
On further subgroup analysis in the NRN study,9 small for GA (SGA) infants exposed to ANS had higher risk for mortality and the composite outcome of NDI or mortality (aRR 1.4, 95% CI 1.02–1.93 and aRR 1.62, 95% CI 1.22–2.16 respectively) than their AGA counterparts. While this could be explained by the fact that SGA infants have higher levels of endogenous steroids due to stress and thus may have altered responses to ANS31 this finding was not seen in other studies such as one from the Vermont Oxford Network32 which actually showed decreased death in SGA infants exposed to ANS. An earlier study33 using NRN data demonstrated no significant differences in death or NDI in SGA infants. These conflicting data warrant further investigation.
Differential exposure to antenatal corticosteroids
Antenatal corticosteroids reduce mortality and morbidity in preterm infants.21,34 However, most of these data come from comparisons between infants who received either a full course of ANS or no ANS exposure. Most studies probing outcomes of ANS exposed infants do not evaluate partial courses separately from the group who received full courses or no ANS.35,36 A complete course of ANS is defined as 2 intramuscular doses of betamethasone given 24 hours apart (the more commonly used regimen) or 4 doses of dexamethasone given 12 hours apart. Due to time constraints or maternal/fetal factors, it is not always possible for a course of ANS to be completed prior to birth. Data are sparse regarding the comparative effects of differential exposure to steroids.
A subgroup analysis of a previous NRN study by Carlo et al found benefits after a partial course of ANS on the composite outcome of death or NDI.33 This prompted larger NRN studies specifically investigating the dose dependent effects of ANS on death or neurodevelopmental outcomes of premature infants.7,10,11 Using the NRN GDB, Chawla et al conducted an observational cohort study of infants born between 22–27 weeks. These infants were categorized into 3 separate groups-no ANS exposure, partial ANS exposure and complete ANS exposure, and the infants were followed to 18–22 months corrected age for the primary outcome of death or NDI.10 NDI was defined as any of the following: moderate to severe cerebral palsy, a cognitive score <85 on the BSID-III, blindness or deafness. Mortality was decreased most significantly in the group exposed to a full course of ANS (aOR 0.66, 95% CI 0.55–0.79, full course vs. no steroids), although even a partial course was also shown to be advantageous over no exposure to ANS (aOR 0.76, 95% CI 0.62–0.93). Moreover, rates of the primary outcome (death or NDI) were also decreased in the full ANS group (aOR 0.63, 95% CI 0.53–0.76) and partial ANS groups (aOR 0.77, 95% CI 0.63–0.95) compared with the no ANS group. Intact survival without significant NDI, blindness or hearing loss was also highest in the complete ANS group and lowest in the unexposed group.10 Although the mechanism of the beneficial effects of ANS is currently unknown, preclinical studies point to a potential pathway which could involve the protective effects of ANS on cerebral blood flow by increasing cerebrovascular resistance37 and reducing blood brain barrier permeability,38 thus reducing ICH and cPVL.
Another prospective observational study using the NRN Generic Database conducted by Travers et al11 corroborated the benefits of exposure to even an incomplete course of steroids. This study included extremely low birth weight infants born between 220/7 - 286/7 days GA and analyzed the rate of death and the rate of physiological BPD by 36 weeks post-menstrual age by level of exposure to antenatal steroids. Death rates were lower amongst infants exposed to any steroids (any steroids vs. no steroids; 22.7% vs. 41.5%; aRR 0.71, p<0.0001). Rates of BPD in survivors were not significantly changed regardless of level of ANS exposure.
An observational cohort NRN study compared short term outcomes of moderately preterm infants (290/7-336/7 GA) exposed to no ANS, a partial course of ANS or a complete course of ANS.7 The authors reported a graded response to the ANS with a complete ANS course associated with lowest rates of surfactant treated respiratory disease and ICH compared to the partial ANS group or no ANS group in the moderate preterm infants.
These data strongly suggest improved neonatal outcomes even in those infants exposed only to partial courses of ANS7,10,11 and support the administration of ANS even in scenarios where it is unlikely that the ANS course will be completed prior to birth due to time constraints.
Periviability
Periviable birth is often defined as occurring between 200/7 and 256/7 weeks of gestation.39 Infants born at these extremely early GAs have high mortality rates, and survivors have high risk for disability. Outcomes data about infants born at extremely early GAs are essential for informing care practices as well as defining a research agenda to improve the outcomes of these vulnerable infants. The NICHD NRN routinely utilizes both the generic database of inborn infants <29 weeks GA and the follow-up database of two-year corrected age outcomes of inborn infants <27 weeks GA to provide critical outcome data for infants born at the lowest GA and birth weights. Serial reports of outcomes from the NRN databases have distinct advantages over comparisons across different cohorts because the composition of the NRN is relatively stable over time, criteria for inclusion in the databases are consistent, and approaches to ascertainment of outcomes are standardized. As such, relevant shifts in important patient outcomes as a result of changes in care practices and population characteristics over time may be easier to measure.
Survival at periviable GA was nearly zero in the 1940s, and survival has steadily increased since that time.40 Serial reports from the NRN have been instrumental in demonstrating the clear decreases in mortality for this group of infants. Two of the most recent large reports from the NRN, published in 2015 and 2022, demonstrate continued gains, particularly among infants born at 23 and 24 weeks of gestation (Fig. 1).5,41 In the most recent cohort, among infants born 2008–2012, 49.4% of all infants born at 23 weeks GA and 69.9% of infants born at 24 weeks GA survived to discharge or remained in the hospital one year after birth.41 Yet, survival is not the only objective and not the only outcome reported by the NRN. Rates of survival to discharge without neonatal morbidities and rates of survival to childhood without disability remain important considerations when caring for infants born at the limits of viability. In contrast to the clear decreases in mortality, rates of neurodevelopmental morbidities among survivors of periviable birth have not decreased markedly in recent years. A 2017 report from the NICHD NRN focused on the neurodevelopmental outcomes of infants born at 22–24 weeks of gestation.42 Compared to earlier epochs, infants born at 23 and 24 weeks in 2008–2011 had a higher chance of survival without NDI at 18–22 months corrected age. Equally importantly, however, rates of survival with NDI did not decrease over the same period. Unfortunately, use of the BSID-III in the later years, as compared to the Bayley Scales of Infant Development-2nd Edition in the earlier years, make these comparisons more challenging to interpret.
Fig. 1 –
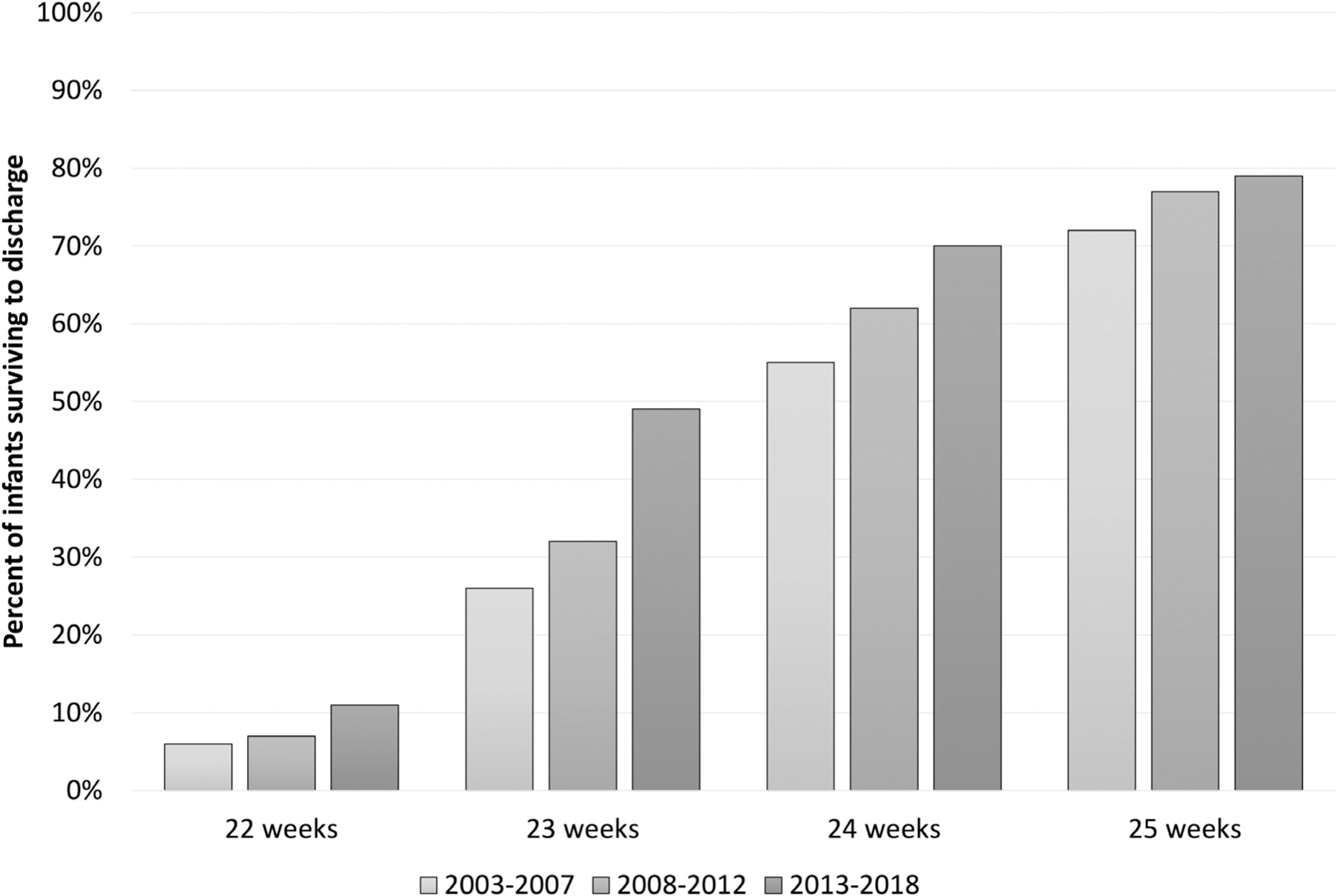
Percent of liveborn infants who survived to hospital discharge in the NICHD NRN, by gestational age.5,41
The most recent NRN report about neurodevelopmental outcomes of periviable infants demonstrates stable rates of survival without NDI (Fig. 2, panel A) and higher rates of survival with NDI in infants born at 22–24 weeks of gestation in 2013–2016 (followed in 2015–2019) as compared to those born in 2008–2011. Notably, this includes modestly higher rates of cognitive delay (BSID-III cognitive composite score <85) and higher rates of moderate or severe cerebral palsy [Gross Motor Function Classification System (GMFCS) level 2–5] among infants born 22–24 weeks of gestation than in the prior report (Fig. 2, panel B).41,42 Infants born in 2008–2011 were evaluated at 18–22 months corrected age, whereas those born in 2013–2016 were evaluated at 22–26 months corrected age, which could have influenced these findings. An additional study by NICHD NRN investigators included infants born between these epochs and followed at 18–26 months corrected age 2011–2015.43 This study excluded infants with congenital anomalies and reported somewhat lower rates of cognitive delay (35%) and similar rates of GMFCS level 2–5 (16%) among infants born 22–24 weeks GA and seen in follow-up.
Fig. 2 –
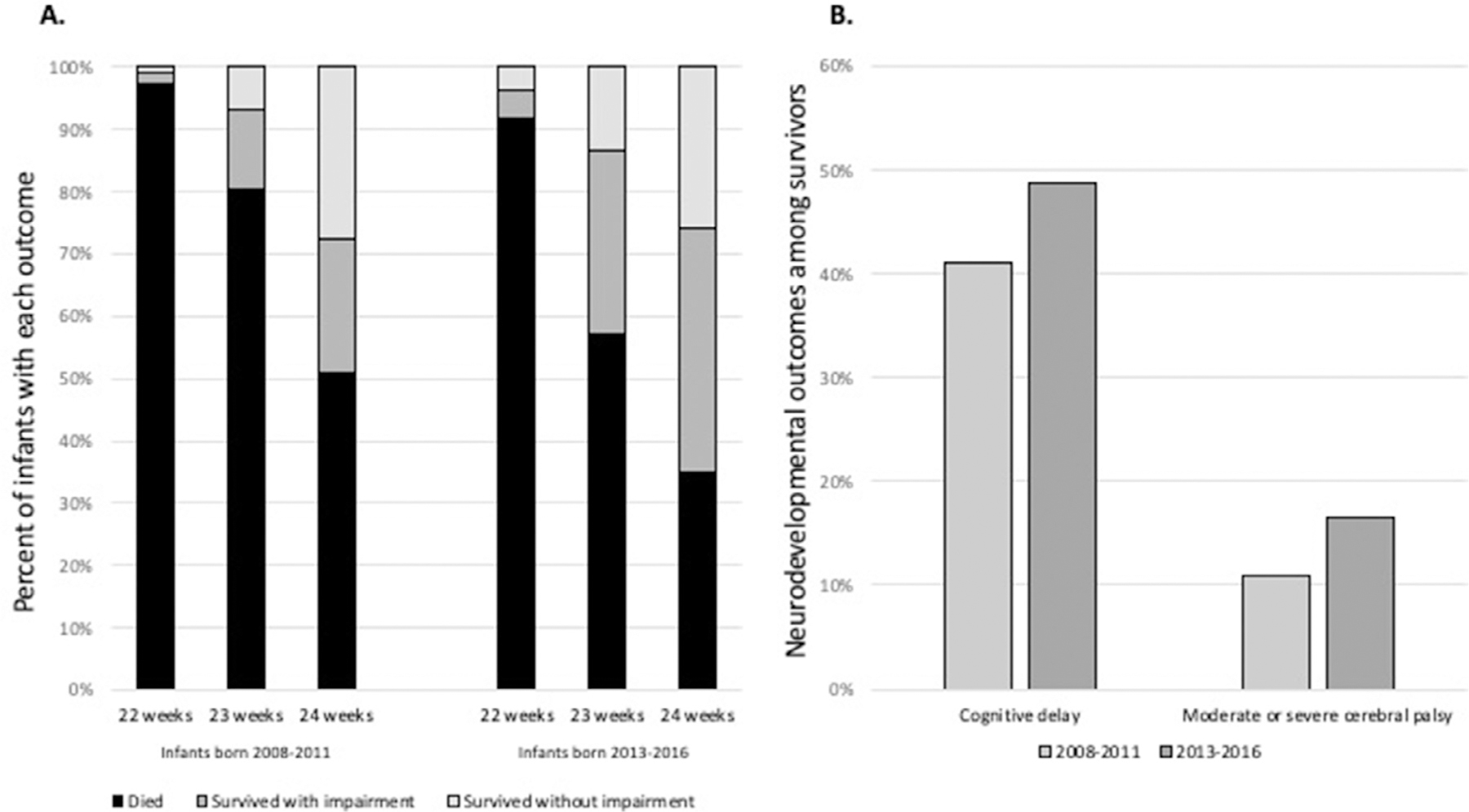
Death, survival with neurodevelopmental impairment, and survival without neurodevelopmental impairment (A) and selected neurodevelopmental morbidities among survivors (B) among infants born at 22–24 weeks gestational age in the NICHD NRN, 2008–2011 and 2013–2016. Infants born in 2008–2011 were followed at 18–22 months corrected age; infants born in 2013–2018 were followed at 22–26 months corrected age. Cognitive delay was defined as <85 on the Bayley Scales of Infant and Toddler, 3rd Edition. Moderate or severe cerebral palsy was defined as Gross Motor Function Classification System level 2–5.41,42
While cognitive development and GMFCS level are critical outcomes for periviable infants and families, other medical and developmental outcomes may have significant impacts on health-related quality of life. Again, the NICHD NRN has capitalized on the long-standing GDB and follow-up registries to provide critical data about these outcomes. The NRN has reported rates of medical resource utilization at 18–22 months corrected age, by birth GA and level of developmental impairment at follow-up.44 For example, among infants born at 22–23 weeks gestation who survived to follow-up at 18–22 months, 60% have been rehospitalized, 21% were on medications for asthma or bronchopulmonary dysplasia, 50% were receiving physical or occupational therapy, and 5% were receiving tube feedings.
Lastly, the NRN provides an opportunity to provide robust data about the small numbers of infants born at the most extreme limits of viability. A recent publication reported the outcomes of infants with birth weight <400 grams and 22–26 weeks of gestation.45 Of 205 infants, 101 (49.3%) received active treatment at birth and 26 (12.7%) survived to discharge. Two of these infants died between discharge and 2 years corrected age. Among those seen in follow-up during the study period, 14 of 19 (74%) had moderate or severe neurodevelopmental impairment. Medical problems were frequent: 63% (12 of 19) had been re-hospitalized since discharge, 63% (12 of 19) had growth problems, 42% (8 of 19) used medical equipment, and 26% (5 of 19) had vision impairment at 18–26 months corrected age. The granular medical and developmental data reported for these relatively uncommon but important infants are essential for thoughtful clinical care and counselling of families who face imminent delivery of an extremely small infant or decisions around provision of life-sustaining medical treatment. An on-going study, the All Birth Cohort, is collecting data on all births between 20–28 weeks estimated GA at NRN centers including not just live births but stillbirths as well and will provide even more understanding in the coming years.
Delivery room management
The NRN consistently contributes evidence to inform DR practices of preterm infants. Recent work has focused on two topics: DR management for moderate preterm infants and placental transfusion for EPT infants.
Moderately preterm infants (born from 29–33 weeks’ gestation) represent a large proportion of preterm infants admitted to the neonatal intensive care unit (NICU) each year, yet this population is relatively understudied compared with EPT infants. The NRN’s time-limited observational study of MPT infants from 2012–2013 provided valuable data to better understand DR interventions and outcomes for MPT infants.
Bajaj et al categorized 7,014 MPT infants into 5 groups according to the most intense DR intervention received.8 This study demonstrated that MPT infants receive more DR intervention than infants born late preterm or term, with 24% infants receiving routine care (no additional support), 32.5% oxygen and/or continuous positive airway pressure, 26.1% bag and mask ventilation, 14.7% endotracheal intubation, and 2.7% cardiopulmonary resuscitation (chest compressions and/or epinephrine). Factors associated with less likelihood of all levels of DR intervention included increasing GA, exposure to antenatal steroids, and prolonged rupture of membranes. After adjusting for important baseline characteristics, increased intensity of DR interventions was independently associated with mortality and NICU care (longer duration of respiratory and nutritional support, longer length of stay).
Defining the incidence of DR interventions across a multicenter setting is an important contribution to the resuscitation literature. These data help inform hospital resuscitation guidelines for moderate preterm infants to ensure teams are optimally staffed and prepared to support neonatal transition for these infants immediately after birth.
The NRN previously demonstrated that hypothermia and hyperthermia on NICU admission are associated with morbidity and mortality for EPT infants.46 More recently, Laptook et al assessed NICU admission temperatures for 5,818 MPT infants and 3,213 EPT infants born in 2012–2013.47 The temperature distribution significantly differed between these cohorts; a greater proportion of MPT infants were normothermic on admission (36.5°C-37.5°C)48 compared with EPT infants (57.3% vs 52.9%). Both hypothermia and hyperthermia were more common among EPT infants. Admission temperature was inversely associated with in-hospital mortality across the entire cohort (EPT and MPT infants).
Importantly, the authors also found that NICU admission temperatures for EPT were generally higher when compared with a cohort of EPT infants born a decade earlier. Twice as many EPT infants in the contemporary era were normothermic on admission (»25% in the older cohort vs »53% in the recent cohort), and the proportion of infants with admission temperatures >37.5°C more than tripled (2%−6.1%). These data suggest the efficacy of efforts to prevent hypothermia in the DR for EPT infants but also emphasize the need to carefully monitor temperatures to avoid hyperthermia.
Umbilical cord management in the delivery room
Optimal umbilical cord management practices have been the focus of many RCTs. Some trials suggest placental transfusion practices such as delayed cord clamping or cord milking reduces the risk of mortality and/or morbidity for preterm infants, but these results are not consistent.49 Further, DR interventional trials often rely on antenatal consent, resulting in selected study populations that may not represent all preterm infants.50 The NRN conducted multiple observational studies to identify the impact of umbilical cord management practices on mortality and morbidity for EPT infants enrolled in the GDB. This line of work adds an important contribution of clinical data from non-research settings to the evolving literature.
Kumbhat et al studied 3116 infants born <29 weeks’ GA without anomalies between 2016–2017 who received active treatment after delivery.16 Mortality and in-hospital outcomes were compared between infants who received any form of placental transfusion (delayed cord clamping or umbilical cord milking) and infants treated with immediate cord clamping. In this cohort, placental transfusion occurred in 40% of infants and was not associated with the primary composite outcome of in-hospital mortality or morbidity (defined as severe brain injury, NEC, late onset sepsis, BPD, or severe ROP). However, placental transfusion was independently associated with a reduction in the adjusted odds of mortality before 36 weeks’ post-menstrual age (aOR 0.71, 95% CI 0.55 to 0.92). Subsequently, Kumbhat et al directly compared outcomes following two common placental transfusion practices (delayed cord clamping vs. umbilical cord milking) among 1834 infants born before <29 weeks’ gestation.17 There was no difference between groups in the adjusted odds of the primary outcome, a composite of in-hospital mortality by 36 weeks post-menstrual age or severe IVH. However, severe IVH was more frequent amongst infants exposed to umbilical cord milking (19.8%) compared with those who received delayed cord clamping (11.8%), aOR 1.70 (95% CI 1.20, 2.43). These results were consistent with the results of the largest RCT on this topic,51 providing more evidence suggesting that umbilical cord milking should be avoided among EPT infants. Ongoing work is focused on identifying the impact of umbilical cord management on neurodevelopmental outcomes for EPT infants.
Conclusion
The NICHD NRN has contributed significantly to our understanding of how antepartum factors and perinatal management of mothers and newborn infants affect neonatal outcomes. Many questions remain as to best perinatal practices. The NRN remains well equipped and poised to address current perinatal questions both now and in the future.
Funding support
Supported in part by National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) cooperative agreements: U10 HD021373, UG1 HD021364, UG1 HD021385, UG1 HD027851, UG1 HD027853, UG1 HD027856, UG1 HD027880,UG1 HD027904, UG1 HD034216, UG1 HD036790, UG1 HD040492, UG1 HD040689, UG1 HD053089, UG1 HD053109, UG1 HD068244, UG1 HD068270, UG1 HD068278, UG1 HD068263, UG1 HD068284; UG1 HD087226, and UG1 HD087229 and National Center for Advancing Translational Sciences (NCATS) contracts UL1 TR000006, UL1 TR000041, UL1 TR000042, UL1 TR000077, UL1 TR000093, UL1 TR000105, UL1 TR000442, UL1 TR000454, and UL1 TR001117.
Abbreviation:
- ANS
Antenatal corticosteroids
- aOR
Adjusted odds ratio
- ARR
Adjusted relative risk
- BITSEA
Brief Infant Social Emotional Assessment
- BMI
Body mass index
- BPD
Bronchopulmonary dysplasia
- BSID-III
Bayley Scales of Infant Development-III
- CI
Confidence interval
- CPAP
Continuous positive airway pressure
- cPVL
Cystic periventricular leukomalacia
- DR
Delivery room
- EPT
Extreme preterm
- GA
Gestational age
- GDB
Generic Database study
- GMFCS
Gross Motor Function Classification System
- ICH
Intracranial hemorrhage
- MPT
Moderate preterm infants
- NEC
Necrotizing enterocolitis
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NICU
Neonatal intensive care unit
- NDI
Neurodevelopmental impairment
- NRN
Neonatal Research Network
- OR
Odds ratio
- RCT
Randomized controlled trial
- ROP
Retinopathy of prematurity
- SGA
Small for gestational age
- SUPPORT
Surfactant, Positive Pressure, and Oxygenation Randomized Trial
- VON
Vermont-Oxford Neonatal Network
Footnotes
Disclosures
The authors have no conflicts to disclose.
While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government.
REFERENCES
- 1.Chawla S, Foglia EE, Kapadia V, Wyckoff MH. Perinatal management: what has been learned through the network? Semin Perinatol 2016;40(6):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz K, Lee CHC, Escobedo MB, et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2021;147(Suppl 1). [DOI] [PubMed] [Google Scholar]
- 3.SSGotEKSNNR Network, Finer NN, Carlo WA, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010;362(21):1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoehn T, Hansmann G, Buhrer C, et al. Therapeutic hypothermia in neonates. Review of current clinical data, ILCOR recommendations and suggestions for implementation in neonatal intensive care units. Resuscitation 2008;78(1):7–12. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rysavy MA, Horbar JD, Bell EF, et al. Assessment of an updated neonatal research network extremely preterm birth outcome model in the Vermont Oxford Network. JAMA Pediatr 2020;174(5):e196294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla S, Natarajan G, Chowdhury D, et al. Neonatal morbidities among moderately preterm infants with and without exposure to antenatal corticosteroids. Am J Perinatol 2018;35(12):1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj M, Natarajan G, Shankaran S, et al. Delivery room resuscitation and short-term outcomes in moderately preterm infants. J Pediatr 2018;195:33–38.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boghossian NS, McDonald SA, Bell EF, et al. Association of antenatal corticosteroids with mortality, morbidity, and neurodevelopmental outcomes in extremely preterm multiple gestation infants. JAMA Pediatr 2016;170(6):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla S, Natarajan G, Shankaran S, et al. Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr 2016;170(12):1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travers CP, Carlo WA, McDonald SA, et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol 2018; 218(1):130..: e1—e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rysavy MA, Bell EF, Iams JD, et al. Discordance in antenatal corticosteroid use and resuscitation following extremely preterm birth. J Pediatr 2019;208:156–162.e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman L, Bann C, Higgins R, Vohr B. Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Developmental outcomes of extremely preterm infants born to adolescent mothers. Pediatrics 2015;135(6):1082–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla S, Laptook AR, Smith EA, et al. In-hospital mortality and morbidity among extremely preterm infants in relation to maternal body mass index. J Perinatol 2021;41(5):1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Travers CP, Carlo WA, McDonald SA, et al. Racial/ethnic disparities among extremely preterm infants in the United States From 2002 to 2016. JAMA Netw Open 2020;3(6):e206757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumbhat N, Eggleston B, Davis AS, et al. Placental transfusion and short-term outcomes among extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2021;106(1):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumbhat N, Eggleston B, Davis AS, et al. Umbilical cord milking vs delayed cord clamping and associations with in-hospital outcomes among extremely premature infants. J Pediatr 2021;232:87–94.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309(22):2362–2370. [DOI] [PubMed] [Google Scholar]
- 20.Johansson S, Villamor E, Altman M, Bonamy AK, Granath F, Cnattingius S. Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. BMJ 2014;349:g6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 2009;(1): CD004661. [DOI] [PubMed]
- 23.Gentle SJ, Carlo WA, Tan S, et al. Association of antenatal corticosteroids and magnesium sulfate therapy with neurodevelopmental outcome in extremely preterm children. Obstet Gynecol 2020;135(6):1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings J. Committee On F, Newborn. Antenatal Counseling Regarding Resuscitation and Intensive Care Before 25 Weeks of Gestation. Pediatrics 2015;136(3):588–595. [DOI] [PubMed] [Google Scholar]
- 25.Stock SJ, Thomson AJ, Papworth S, Royal College of O, Gynaecologists. Antenatal corticosteroids to reduce neonatal morbidity and mortality: Green-top Guideline No. 74 February 2022: Green-top Guideline No. 74. BJOG 2022. [DOI] [PubMed]
- 26.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement 1994;12(2):1–24. [PubMed] [Google Scholar]
- 27.Rysavy MA, Li L, Bell EF, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med 2015;372(19):1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacking D, Watkins A, Fraser S, Wolfe R, Nolan T. Respiratory distress syndrome and antenatal corticosteroid treatment in premature twins. Arch Dis Child Fetal Neonatal Ed 2001;85(1): F77–F78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballabh P, Lo ES, Kumari J, et al. Pharmacokinetics of betamethasone in twin and singleton pregnancy. Clin Pharmacol Ther 2002;71(1):39–45. [DOI] [PubMed] [Google Scholar]
- 30.Mulder EJ, Derks JB, Visser GH. Effects of antenatal betamethasone administration on fetal heart rate and behavior in twin pregnancy. Pediatr Res 2004;56(1):35–39. [DOI] [PubMed] [Google Scholar]
- 31.Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther 1988;3(3):158–164. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2000;182(1 Pt 1):198–206. [DOI] [PubMed] [Google Scholar]
- 33.Carlo WA, McDonald SA, Fanaroff AA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA 2011;306(21):2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol 1990;97(1):11–25. [DOI] [PubMed] [Google Scholar]
- 35.Doyle LW, Kitchen WH, Ford GW, Rickards AL, Kelly EA. Antenatal steroid therapy and 5-year outcome of extremely low birth weight infants. Obstet Gynecol 1989;73(5 Pt 1):743–746. [PubMed] [Google Scholar]
- 36.Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Short and long-term effects of antenatal corticosteroids assessed in a cohort of 7,827 children born preterm. Acta Obstet Gynecol Scand 2009;88(8):933–938. [DOI] [PubMed] [Google Scholar]
- 37.Lohle M, Muller T, Wicher C, et al. Betamethasone effects on fetal sheep cerebral blood flow are not dependent on maturation of cerebrovascular system and pituitary-adrenal axis. J Physiol 2005;564(Pt 2):575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stonestreet BS, Petersson KH, Sadowska GB, Pettigrew KD, Patlak CS. Antenatal steroids decrease blood-brain barrier permeability in the ovine fetus. Am J Physiol 1999;276(2):R283–R289. [DOI] [PubMed] [Google Scholar]
- 39.American College of O, Gynecologists, the Society for Maternal-Fetal M. Periviable birth: Interim update. Am J Obstet Gynecol 2016;215(2):B2–B12.e11. [DOI] [PubMed] [Google Scholar]
- 40.Patel RM, Rysavy MA, Bell EF, Tyson JE. Survival of infants born at periviable gestational ages. Clin Perinatol 2017;44(2):287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA 2022;327(3):248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younge N, Goldstein RF, Bann CM, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med 2017;376(7):617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams-Chapman I, Heyne RJ, DeMauro SB, et al. Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics 2018;141(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rysavy MA, Colaizy TT, Bann CM, et al. The relationship of neurodevelopmental impairment to concurrent early childhood outcomes of extremely preterm infants. J Perinatol 2021;41(9):2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brumbaugh JE, Hansen NI, Bell EF, et al. Outcomes of extremely preterm infants with birth weight less than 400 g. JAMA Pediatr 2019;173(5):434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laptook AR, Salhab W, Bhaskar B, Neonatal Research N. Admission temperature of low birth weight infants: predictors and associated morbidities. Pediatrics 2007;119(3):e643–e649. [DOI] [PubMed] [Google Scholar]
- 47.Laptook AR, Bell EF, Shankaran S, et al. Admission temperature and associated mortality and morbidity among moderately and extremely preterm infants. J Pediatr 2018;192:53–59. e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uxa F Thermal control of the newborn. Kangaroo 1994;3(1):67–68. [PubMed] [Google Scholar]
- 49.Seidler AL, Gyte GML, Rabe H, et al. Umbilical cord management for newborns <34 weeks’ gestation: a meta-analysis. Pediatrics 2021;147(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foglia EE, Owen LS, Kirpalani H. Delivery room research: when does poor quality evidence become an ethical issue? Pediatrics 2015;135(5):e1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katheria A, Reister F, Essers J, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA 2019;322:1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]


