Abstract
Instant rice is well-suited for ready-to-use applications as low-moisture, light-weight military ration and emergency food for our Armed Forces, offering longer shelf life with rapid rehydration characteristics. Present investigation demonstrated the effect of different salt pretreatment during soaking as precooking operation on the physico-chemical, cooking and rehydration kinetics of instant rice. Application of salt pretreatment reduced bulk density and damaged grain percentage, while enhanced the porosity, volume expansion percent, weight gain percentage, and rehydration characteristics. The grain elongation ratio was not affected significantly by the application of salt pretreatments; however, water uptake and chemical composition were significantly affected. Soaking pretreatment with 1% calcium chloride, followed by open pan cooking and subsequently freeze-thaw-dehydrating until attainment of 5–6% moisture content was found to be the optimal processing condition for developing instant rice with less than 2 min of rehydration time by mere addition of hot water. Modelling of water absorption behaviour revealed that both Peleg (R2 0.980–0.999) and Singh and Kulshrestha (R2 0.966–0.999) models fitted well.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-023-05877-y.
Keywords: Cooking quality, Instant rice, Kinetics, Porosity, Rehydration, Salt pretreatment
Introduction
Rice (Oryza sativa L.), a staple food globally, is mainly consumed in cooked form. India, with a production and productivity level of 118.73 million tonnes and 2705 kg/ha, respectively, during 2019–20 is the second largest global producer of rice, after China (GOI 2020). Due to change in lifestyle in present era of technology and increasing convenience, there is urgent need for ready-to-eat or instant convenience food products, which should be nutritious, delicious and as per the consumer expectations. Additionally, rations for Armed Forces majorly comprise of ready-to-eat or instant convenience food products that the troops can prepare just by warming or addition of hot water (Semwal et al. 2001). Rice is a staple food in larger part of the world, so convenience products based on rice with its adaptability for sweet and savoury palate, can play significant role to meet this necessity. Instant rice, which takes much lesser time to cook in comparison to cooking the rice in conventional way, also makes it a suitable choice for light- weight military rations and emergency foods.
Instant rice is prepared by first hydrating and/or precooking raw rice to gelatinize the starch; desired moisture content is achieved by then drying the treated rice. The structure of instant rice and its rehydration characteristics are highly influenced by the physico- chemical properties of rice (Sharma et al. 2022). Rice cultivars with wide variation in physico- chemical properties are traditionally consumed in Indian subcontinent. The majority of consumers in India prefer indica rice with intermediate amylose content (20–25%), to have their preferred non-sticky cooked rice. Different unit operations such as soaking, pre-treatment, boiling, freezing, drying, etc. involved in the production of instant rice, have been found to affect flavor, colour, and textural properties of instant rice (Agrawal et al. 2019; Chen et al. 2014; Sharma et al. 2022). Mujumdar (2004) opined that fluidized bed drying process is most suitable form of drying for particulates. Use of centrifugal fluidized bed drier is more popular and cost-effective method used in the preparation of instant products on industrial scale as it removes the moisture at a higher air velocity, lower temperature and in shorter time than conventional drying equipment, consequently leads to low energy requirement. The other available advanced methods of drying such as freeze drying, microwave and infrared drying are also effective; however, has limitation of higher energy requirement, high maintenance and higher production cost.
The established parameters of significance for good quality instant rice are white coloured, non-sticky and well separated cooked grains, less rehydration time, comparable organoleptic properties to freshly cooked rice, lower degree of broken or damaged grains, higher volume expansion ratio, and no hard core or ungelatinized center (Prasert and Suwannaporn 2009; Sripinyowanich and Noomhorm 2013). Some researchers (Durgrao et al. 2017; Sasmitaloka et al. 2019) have also highlighted the importance of physical properties of instant rice during design and selection of storage structures, and storage and processing equipment. Influence on colour attributes of processed instant rice plays an important role in sensory acceptability of the product.
Beneficial effects of some auxiliary chemicals like sodium citrate, sodium chloride, sodium bicarbonate, calcium chloride, etc. on sensory and textural properties of instant rice were reported by earlier researchers (Agrawal et al. 2019; Mridula et al. 2015). These auxiliary chemicals, particularly sodium citrate and calcium chloride are believed to enhance the water absorption by starch and improve the whiteness of the rice grain (Mridula et al. 2017; Hawa et al. 2022). The percentage of damaged and broken grains in instant rice is an index of the quality of product, which is affected by the type and cooking properties of rice. Moreover, low degree of damaged grains with presence of distinct, whole rice kernels is also visually appealing to the consumer (Bui et al. 2018). A comparative study of salt pretreatment including the types of salt and their effect on the quality attributes of instant rice are needed to ascertain their role. Therefore, the present study was designed to analyze two commonly used salts: sodium citrate and calcium chloride at various concentrations for their effect on quality attributes of instant rice such as percentage of damaged grains, physical attributes and rehydration characteristics. Besides, the rehydration kinetics of instant rice were also analysed by employing empirical mathematical models for better assessment of the rehydration process as these were considered efficient tools to design and optimize hydration as well as rehydration processes.
Materials and methods
Materials
Paddy samples of two rice cultivars viz., Pusa Basmati 1509 (PB1509) and Jeerakasala (JR) were procured from Indian Agricultural Research Institute (IARI), New Delhi and from a farmer from Kerala, respectively. The paddy samples were thoroughly cleaned to remove dust particles and dehusked by using a Satake Rubber Roll Dehusker followed by polishing in a Satake Polisher for further study.
Methods
Physicochemical properties of raw rice (or paddy)
Axial dimensions such as grain length, grain breadth and grain thickness of all the cultivars under study were measured using vernier caliper having a least count of 0.001 mm (Model: PR26, Aerospace). Bulk density was calculated by method of Wani et al. (2013). Tapped density, true density and porosity were calculated by the method of Meera et al. (2019). For measuring thousand kernel weight (TKW), counted 1000 rice grains were randomly selected and weighed using a precision electronic balance having an accuracy of 0.001 g (M/s Precisa, XB 220A, Precisa Instrument) (Varnamkhasti et al. 2008).
Colour of raw rice kernels were measured using Tri- stimulus Hunter Colorimeter (M/s Miniscan XE Plus, Model No. 45/O-S, Hunter Associates Laboratory, Inc. Reston, VA, USA) with D65 illuminator at an observer angle of 10° and colour coordinates L* (lightness), a* (redness-greenness), b* (yellowness-blueness) were determined. Whiteness was calculated as mentioned below:
The proximate composition (moisture, protein, fat, ash and fibre) of the rice samples were estimated by standard AOAC protocols (AOAC 2000). The carbohydrate content was estimated by difference method. Total starch content and amylose contents were estimated by Anthrone reagent and colorimetric methods, respectively (Sadasivam and Manickam 1992). Amylopectin content was determined by subtracting the amylose from total starch. Ratio of amylose to amylopectin (Am/Ap) was calculated by dividing the respective values of amylose by amylopectin for each cultivar.
Preparation of instant rice
Instant rice was prepared by the method described by Sharma et al. (2022) with slight modifications.
Pretreatment of Rice: Two-hundred-gram cleaned rice samples (free from dust particle) were soaked for one hour in 600 mL solutions with three concentrations (0.5, 1.0 and 1.5%) of sodium citrate and calcium chloride, individually. After soaking, rice was cooked in open pan. The cooked rice was washed four times with running water for removal of excess starch. The cooked rice was strained and was left to dry out surface moisture.
Cooked rice was frozen at − 20 °C using air blast cooling in a blast freezer (Model 15LU2-300, M/s Hull Corporation Hatboro, USA). The frozen cooked rice was dried using the high velocity air stream (temperature: 70 °C up to moisture level about 5%) of fluidized bed dryer (Model 30 D, ChemecEng, Mumbai, India), that swiftly takes away moisture from the surface and avert sticking of kernels.
The control treatments with no soaking prior to cooking and soaking of rice in water for one hour without the addition of salts were performed.
After cooking, cooked rice were evaluated to check any effect of salt addition on the quality characteristics such as length expansion ratio, cooking time, volume expansion ratio, and gruel solid loss using the methods suggested by Chavan et al. (2018).
Quality evaluation of instant rice
Water activity
The water activity of instant rice samples was measured using a water activity meter (Model, Hygrolab 3; Make, Rotronic, Germany).
Bulk density
Instant rice was put in a 100 mL cylinder and tapped 25–30 times, and then weight of instant rice was recorded using electronic balance. Density was calculated as weight by volume of instant rice.
Degree of damaged grains
A representative sample of instant rice (10 g) was drawn for each rice cultivar and manually sorted for whole grains, broken grains and clumped grains. Each category was separately weighed. Total damaged grains were calculated by addition of both broken and clumped grains and percentage damaged grains were calculated.
Percentage volume expansion
Volume of instant rice before (Vr) and after rehydration (VR) were measured and the volume expansion % were calculated using following formula as suggested by Prasert and Suwannaporn (2009):
Colour properties
Colour values L*, a*, b* of dried instant rice kernels and whiteness were measured using the same methodology as used for raw rice. The total colour change (ΔE) was estimated from the following formulae:
where, L*: Lightness, a*: redness-greenness, and b*: yellowness-blueness.
Rehydration characteristics
Rehydration ratio and rehydration time
300 mL hot water (90 °C) was added to 100 g of instant rice placed in a stainless-steel vessel, covered and set aside until they attained the consistency of the cooked rice. Rehydration ratio was calculated as the ratio of weight of rehydrated sample to the weight of dehydrated sample. Rehydration time was estimated as the time required in attaining the consistency of cooked rice (Semwal et al. 2001).
Water gain percentage
Weight gain percentage was estimated by the method described by Agrawal et al. (2019) with slight modification. The dried instant rice sample (0.2 g) was put in 4 mL of heated water at 95 °C in a beaker for a period of 5 min. The samples were drawn at one-minute interval. The excess water was drained. The weight of rice before and after heating at each interval was determined using precision electronic weighing balance. Water gain percentage was calculated by dividing the gain in weight at each interval by initial dried rice weight, and multiplied by 100.
Rehydration kinetics of the instant rice samples were described by two empirical models: Peleg model (Peleg 1988) and Singh and Kulshrestha model (Singh and Kulshrestha 1987).
The moisture uptake kinetics data were fitted to Peleg’s Model:
| 1 |
where, M is the transient moisture content (% db), Mo is the initial moisture content (% db), k1 is the Peleg rate constant, k2 is the Peleg capacity constant, and t is time (minutes).
Singh and Kulshreshtha model proposed an empirical model that involves a single rate constant:
| 2 |
The linearized form of the Eq. 2 is described as:
| 3 |
where, m is the transient moisture content (% db), mo is the initial moisture content (% db), me is the equilibrium moisture content, k is the rate constant, and t is time (minutes).
Organoleptic properties
A group of semi-trained panellist’s evaluated organoleptic properties such as appearance, colour aroma, flavour and texture and overall acceptability (OAA) was worked out using 9-point Hedonic scale as per Mridula et al. (2017).
Statistical analysis
The mean data of three replications for each character were reported, unless otherwise stated. The replicated data was statistically analyzed for analysis of variance (ANOVA), using Statistical Package for Social Science (SPSS) software (SPSS, 2002). Coefficient of determination (R2), Root mean square error (RMSE) and Chi square test of goodness of fit were performed to compare observed and predicted values for empirical models for rehydration kinetics.
Results and discussion
Physico-chemical properties of different raw rice cultivars
Physico-chemical properties of rice cultivars affect the processing, handling, grading, marketability and end- product quality of milled rice. The different physico- chemical properties of rice cultivars are presented in Table 1. The grain length was observed as 5.53 mm for JR and 8.40 mm for PB1509, while grain breadth was observed as 1.70 mm for PB1509 to 2.20 mm for JR. Grain thickness was observed as 1.58 mm in both PB1509 and JR. Long slender PB1509 (23.74 g) depicted higher TKW than short grained JR (15.92 g). Grain hardness was observed as 6.62 N for PB1509 and 8.86 N for rice cultivar JR. The cultivar JR exhibited higher bulk density (874.43 kg/m3), tapped density (928.32 kg/m3), true density (1468.29 kg/m3) and porosity (36.77%), while PB1509 exhibited lower values for these characteristics. PB1509, being a long grain variety, depicted lower values for density characteristics as compared to short grained variety JR. The results are consistent with the earlier findings of Singh et al. (2005).
Table 1.
Physico-chemical, colour characteristics and proximate analysis of raw rice
| Characters | PB 1509 | JR | C.D.# | Characters | PB 1509 | JR | C.D.# | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||||
| GL (mm) | 8.40 | 0.02 | 5.53 | 0.01 | 0.06 | Moisture (%) | 11.56 | 0.04 | 11.91 | 0.11 | 0.33 |
| GB (mm) | 1.70 | 0.02 | 2.2 | 0.01 | 0.05 | Protein (%) | 7.90 | 0.05 | 7.79 | 0.02 | 0.04 |
| GT (mm) | 1.58 | 0.01 | 1.58 | 0.01 | - | Carbohydrate (%) | 79.08 | 0.02 | 79.05 | 0.01 | 0.29 |
| TKW (g) | 23.74 | 0.28 | 15.92 | 0.07 | 0.83 | Starch (%) | 75.59 | 0.08 | 79.12 | 0.40 | 1.15 |
| BD (kg/m3) | 769.48 | 12.61 | 874.43 | 11.24 | 48.16 | Amylose (%) | 23.30 | 0.27 | 20.19 | 0.42 | 1.43 |
| TD (kg/m3) | 904.26 | 9.54 | 928.32 | 14.16 | – | Amylopectin (%) | 52.29 | 0.28 | 58.93 | 0.34 | 0.65 |
| TrD (kg/m3) | 1359.5 | 19.36 | 1468.29 | 13.19 | 66.78 | Am/Ap ratio | 0.44 | 0.01 | 0.34 | 0.01 | 0.02 |
| Porosity (%) | 33.48 | 0.25 | 36.77 | 0.52 | 1.63 | Fat (%) | 0.75 | 0.02 | 0.48 | 0.02 | 0.48 |
| Hardness (N) | 6.62 | 0.02 | 8.86 | 0.01 | 0.71 | Fibre (%) | 0.20 | 0.02 | 0.32 | 0.02 | 0.07 |
| Ash (%) | 0.52 | 0.03 | 0.46 | 0.01 | – | ||||||
| Colour characteristics | |||||||||||
| L* | 58.61 | 0.18 | 61.86 | 0.03 | 0.52 | b* | 7.71 | 0.10 | 10.48 | 0.05 | 0.33 |
| a* | − 0.44 | 0.01 | − 1.39 | 0.02 | 0.06 | WI | 57.89 | 0.01 | 60.42 | 0.02 | 2,28 |
GL Grain length, GB Grain Breadth, GT Grain thickness, TKW Thousand kernel weight, BD Bulk density, TD Tapped density, TrD True density, L*: Lightness, a*: redness–greenness, b*: Yellowness-blueness, Am/Ap ratio: Amylose amylopectin ratio, # 5% level of probability
Estimates of colour values L* (lightness), a* (redness-greenness) and b* (yellowness-blueness) were observed as 58.61, -0.44 and 7.71 for PB1509, respectively, and 61.86, − 1.39 and 10.48 for JR, respectively. Slightly higher value of whiteness was depicted by JR as 60.42 in comparison of 57.89 for PB1509. The proximate composition of rice cultivars (Table 1) revealed higher protein (7.9%), fat (0.75%), ash (0.52%), amylose (23.3%) and amylose/amylopectin (0.44) were observed in case of cultivar PB1509. The rice cultivar JR showed relatively higher values for fibre (0.32%), starch (79.12%) and amylopectin (58.93%). The amount of carbohydrate calculated was 79.08% for PB1509 and 79.05% for JR.
Cooking properties
Cooking time for PB1509 and JR without soaking was observed as 20 and 22 min, respectively. Both rice cultivars JR and PB1509 fall in the category of intermediate amylose rice type, which is considered as the rice type generally selected for superior cooking quality. However, variable cooking properties were observed in both rice cultivars, which could be attributed to the genetic composition of the grain, gelatinization temperature, etc. PB1509 exhibited higher amylose content and lower cooking time, which was consistent with earlier findings (Singh et al. 2005). Cooking time decreased by 2 min in both rice cultivars after the soaking pretreatment of rice in water. In general, cooking time decreased with the addition of salt at all level of concentrations, irrespective of its type. With addition of salts, further reduction in cooking time was observed up to 12 and 15 min for PB1509 and JR, respectively (Table 2). In comparison to soaking pretreatment, maximum reduction of cooking time by 6 min (33%) and 5 min (25%) for PB1509 and JR, respectively, were observed at higher concentration of salt. Percentage gruel solid loss decreased from 4.51 to 3.15% and 4.83 to 4.22% for PB1509 and JR, respectively with salt pretreatment of sodium citrate. Reduction in cooking time and percentage gruel solid loss was consistent with the increase in concentration of salt during soaking. Soaking pretreatment with different salts might have induced the changes in the rice starch, and more soluble components of rice in cooking water ultimately resulted in lesser solid loss. The lower pH of the soaking solution of sodium citrate might have influenced the cooking time of rice by affecting the diffusion of water into the kernel and degree of gelatinization (Mridula et al. 2017). Pretreatment with sodium citrate salt solution during the soaking process modified the protein structure of the rice by disruption as well as disintegration. This disruption in protein structure imparted higher porosity to the instant rice (Hawa et al. 2022). In case of corn grain, soaking with sodium citrate resulted into more porous structure, with more exposed starch cell walls to trap the water into granules (Yulianti et al. 2021).
Table 2.
Effect of different pretreatments on cooking properties of rice cultivars
| Treatment | WUR | LER | %GSL | VER | CT (min) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | |
| PB1509 | ||||||||||
| W/O S | 179.24 | 0.56 | 1.94 | 0.004 | 4.51 | 0.06 | 1.25 | 0.004 | 20.00 | 0.19 |
| W S | 185.29 | 0.39 | 1.94 | 0.004 | 4.46 | 0.08 | 1.51 | 0.007 | 18.00 | 0.23 |
| SC 0.5% | 202.15 | 1.26 | 1.96 | 0.005 | 3.62 | 0.07 | 1.47 | 0.007 | 15.00 | 0.14 |
| SC 1.0% | 201.18 | 0.45 | 1.96 | 0.005 | 3.15 | 0.05 | 1.49 | 0.014 | 15.00 | 0.19 |
| SC 1.5% | 199.79 | 0.67 | 1.97 | 0.004 | 3.36 | 0.07 | 1.47 | 0.007 | 12.00 | 0.29 |
| CC 0.5% | 203.26 | 0.52 | 1.96 | 0.004 | 3.32 | 0.07 | 1.47 | 0.007 | 13.00 | 0.10 |
| CC 1.0% | 215.11 | 0.68 | 1.97 | 0.004 | 3.21 | 0.05 | 1.47 | 0.007 | 13.00 | 0.22 |
| CC 1.5% | 208.65 | 0.77 | 1.98 | 0.004 | 3.55 | 0.08 | 1.47 | 0.007 | 12.00 | 0.18 |
| JR | ||||||||||
| W/O S | 185.41 | 0.60 | 1.09 | 0.010 | 4.83 | 0.11 | 1.07 | 0.009 | 22.00 | 0.12 |
| WS | 191.22 | 0.24 | 1.1 | 0.001 | 4.62 | 0.10 | 1.36 | 0.031 | 20.00 | 0.10 |
| SC 0.5% | 197.68 | 0.66 | 1.11 | 0.002 | 4.58 | 0.08 | 1.39 | 0.027 | 21.00 | 0.27 |
| SC 1.0% | 201.29 | 0.35 | 1.12 | 0.001 | 4.34 | 0.06 | 1.37 | 0.027 | 18.00 | 0.19 |
| SC 1.5% | 212.77 | 0.58 | 1.13 | 0.004 | 4.22 | 0.09 | 1.36 | 0.031 | 17.00 | 0.14 |
| CC 0.5% | 210.21 | 0.38 | 1.12 | 0.002 | 4.39 | 0.14 | 1.41 | 0.015 | 17.00 | 0.23 |
| CC 1.0% | 213.39 | 0.59 | 1.13 | 0.005 | 4.28 | 0.14 | 1.39 | 0.027 | 16.00 | 0.27 |
| CC 1.5% | 211.27 | 0.46 | 1.14 | 0.002 | 4.35 | 0.09 | 1.39 | 0.027 | 15.00 | 0.14 |
| C.D.# | 1.77 | 0.01 | 0.25 | 0.05 | 0.57 | |||||
| SE(m) | 0.61 | 0.01 | 0.09 | 0.02 | 0.20 | |||||
| SE(d) | 0.87 | 0.01 | 0.12 | 0.03 | 0.28 | |||||
| C.V | 0.58 | 0.38 | 3.73 | 2.33 | 2.05 | |||||
WUR Water uptake ratio, LER Length expansion ratio, % GSL Percent gruel solid ratio, VER Volume expansion ratio, CT Cooking time, W/OS Without soaking, WS With soaking, SC Sodium citrate, CC Calcium chloride, # 5% level of probability
Length expansion ratio and volume expansion ratio were observed as 1.94 and 1.25 for PB1509, and 1.09 and 1.07 for JR, respectively, for the rice cooked without pretreatments. Length expansion ratio and volume expansion ratio were affected after the soaking pretreatment with and without addition of salts. With increasing concentration of sodium citrate and calcium chloride during soaking, Length expansion ratio increased coherently/ consistently and ranged from 1.96 to 1.98 and 1.11 to 1.14 for PB1509 and JR, respectively. Volume expansion ratio ranged from 1.47 to 1.51 and 1.36 to 1.41 for PB1509 and JR, respectively.
Water uptake ratio observed for PB1509 and JR without soaking pretreatment were 179.24 and 185.41, respectively, which increased after the soaking and salt pretreatment. Soaking pretreatment decreased the tendency of the rice kernel to be disintegrated or mutilated by the internal osmotic pressure, which leads to bursting of rice kernel during the cooking operation and thus leaching of starch takes place. Highest water uptake ratio of 215.11% for PB1509 and 213.39% for JR was observed with 1% level of calcium chloride salt pretreatment.
Quality attributes of instant rice
Water activity, bulk density and volume expansion
Water activity value ranged from 0.40 to 0.50 amongst all the instant rice samples, which indicated that instant rice samples were efficiently dried for safe storage. Bulk density and percent volume expansion ranged from 464.22 to 490.00 kg/m3 and 152.15 to 160.92% for PB1509, while 625.18 to 642.80 kg/m3 and 132.15 to 139.81%, for JR respectively (Table 3). Instant rice prepared from short grain JR had higher bulk density as compared to instant rice from PB1509. After the soaking and salt pretreatment, bulk density decreased, in general, with decrease in concentration of salts added in soaking water. During the freezing process, ice crystal formation occurs which result in breakdown of colloidal starch structure, and thus leads to setting of porous structure (Sripinyowanich and Noomhorm 2011). Lower density products have higher porosity as shown in previous studies (Sasmitaloka et al. 2019; Sharma et al. 2022). Food systems undergo volumetric changes (shrinkage) due to water loss during dehydration process (Gulati and Datta 2015). Percent volume expansion increased after the soaking and salt pretreatment of rice. PB1509 depicted higher increase (from 156.21 to 162.97%) percent volume expansion than JR (from 136.25 to 139.81%). Volume expansion is affected by water absorption. Higher porosity and water absorption in a food matrix tends to result into higher volume expansion. Using a centrifugal fluidized bed drying process caused puffing on volume expansion of the rice structure and lead to greater porosity. The puffing phenomenon consequently developed from the swift expansion of water vapour in the granule. Amongst all treated samples, lower bulk density was observed for instant rice pretreated with calcium chloride salt in both rice cultivars. The lowest bulk density and highest percent volume expansion for both cultivars were observed in instant rice pretreated with 1% concentration of calcium chloride.
Table 3.
Effect of different pretreatments on quality attributes of instant rice
| Treatment | BD (kg/m3) | VE% | WA | DG (%) | RR | RT (min) | L* | a* | b* | WI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | |
| PB1509 | ||||||||||||||||||||
| W/O S | 490.00 | 4.87 | 152.15 | 0.51 | 0.50 | 0.01 | 22.15 | 2.57 | 3.28 | 0.04 | 5.00 | 0.02 | 55.67 | 0.30 | − 0.48 | 0.02 | 5.91 | 0.07 | 55.28 | 0.28 |
| W/ S | 485.21 | 6.88 | 153.15 | 0.69 | 0.49 | 0.01 | 20.62 | 1.27 | 3.29 | 0.03 | 4.73 | 0.19 | 55.81 | 0.08 | − 0.47 | 0.01 | 5.76 | 0.18 | 55.43 | 0.09 |
| SC 0.5% | 472.16 | 6.45 | 158.29 | 0.86 | 0.47 | 0.01 | 17.82 | 0.99 | 3.32 | 0.18 | 2.00 | 0.03 | 56.19 | 0.09 | − 0.41 | 0.02 | 5.70 | 0.16 | 55.81 | 0.12 |
| SC 1.0% | 475.21 | 5.84 | 156.21 | 0.56 | 0.48 | 0.01 | 18.37 | 1.26 | 3.31 | 0.14 | 2.50 | 0.03 | 56.27 | 0.13 | − 0.43 | 0.01 | 5.73 | 0.10 | 55.86 | 0.13 |
| SC 1.5% | 481.75 | 6.20 | 155.87 | 0.56 | 0.47 | 0.01 | 18.89 | 1.29 | 3.30 | 0.17 | 3.50 | 0.06 | 56.68 | 0.13 | − 0.46 | 0.01 | 5.76 | 0.16 | 56.29 | 0.15 |
| CC 0.5% | 470.22 | 5.47 | 158.51 | 0.29 | 0.46 | 0.02 | 17.15 | 0.96 | 3.32 | 0.26 | 2.00 | 0.06 | 58.12 | 0.05 | − 0.32 | 0.01 | 5.02 | 0.08 | 57.81 | 0.04 |
| CC 1.0% | 464.22 | 6.68 | 160.92 | 0.58 | 0.40 | 0.01 | 16.21 | 1.61 | 3.34 | 0.20 | 1.50 | 0.03 | 59.71 | 0.12 | − 0.35 | 0.01 | 5.12 | 0.70 | 59.38 | 0.12 |
| CC 1.5% | 471.15 | 4.05 | 157.18 | 0.55 | 0.49 | 0.01 | 16.89 | 0.99 | 3.33 | 0.46 | 2.00 | 0.09 | 58.65 | 0.09 | − 0.39 | 0.01 | 5.25 | 0.14 | 58.44 | 0.08 |
| JR | ||||||||||||||||||||
| W/O S | 642.80 | 18.24 | 132.15 | 0.10 | 0.46 | 0.01 | 5.30 | 0.57 | 3.48 | 0.05 | 3.00 | 0.09 | 61.7 | 0.12 | − 0.87 | 0.03 | 5.25 | 0.08 | 61.35 | 0.13 |
| W/ S | 640.95 | 5.95 | 135.86 | 0.26 | 0.47 | 0.02 | 5.12 | 0.23 | 3.48 | 0.04 | 2.83 | 0.30 | 60.15 | 0.08 | − 0.86 | 0.01 | 4.81 | 0.08 | 59.85 | 0.07 |
| SC 0.5% | 631.86 | 6.65 | 137.01 | 0.24 | 0.47 | 0.01 | 5.11 | 0.26 | 3.52 | 0.01 | 2.00 | 0.03 | 61.87 | 0.03 | − 0.85 | 0.02 | 5.12 | 0.06 | 61.51 | 0.01 |
| SC 1.0% | 632.91 | 3.92 | 138.29 | 0.79 | 0.49 | 0.01 | 5.12 | 0.05 | 3.53 | 0.01 | 2.50 | 0.09 | 62.65 | 0.14 | − 0.86 | 0.02 | 4.89 | 0.09 | 62.32 | 0.18 |
| SC 1.5% | 637.15 | 4.76 | 136.25 | 0.66 | 0.49 | 0.01 | 5.27 | 0.20 | 3.50 | 0.02 | 2.73 | 0.19 | 60.59 | 0.08 | − 0.88 | 0.01 | 3.75 | 0.04 | 60.40 | 0.08 |
| CC 0.5% | 628.22 | 5.33 | 139.22 | 0.16 | 0.46 | 0.02 | 5.09 | 0.04 | 3.54 | 0.03 | 2.00 | 0.10 | 62.68 | 0.23 | − 0.82 | 0.01 | 2.76 | 0.02 | 62.56 | 0.20 |
| CC 1.0% | 625.18 | 6.55 | 139.81 | 0.44 | 0.47 | 0.01 | 4.69 | 0.24 | 3.53 | 0.01 | 2.00 | 0.06 | 62.07 | 0.32 | − 0.86 | 0.02 | 3.19 | 0.12 | 61.92 | 0.32 |
| CC 1.5% | 630.76 | 6.51 | 137.53 | 0.94 | 0.48 | 0.01 | 5.05 | 0.11 | 3.52 | 0.02 | 2.00 | 0.03 | 61.92 | 0.14 | − 0.87 | 0.01 | 3.28 | 0.09 | 61.76 | 0.13 |
| C.D.# | 20.97 | 1.79 | 0.01 | 3.02 | N/A | 0.32 | 0.45 | 0.05 | 0.31 | 0.44 | ||||||||||
| SE(m) | 7.25 | 0.62 | 0.02 | 1.05 | 0.16 | 0.11 | 0.16 | 0.02 | 0.11 | 0.15 | ||||||||||
| SE(d) | 10.25 | 0.87 | 0.01 | 1.48 | 0.23 | 0.16 | 0.22 | 0.02 | 0.15 | 0.22 | ||||||||||
| C.V | 2.265 | 0.73 | 0.01 | 15.33 | 8.06 | 7.29 | 0.45 | 4.29 | 3.81 | 0.45 | ||||||||||
BD, Bulk density; VE%, Volume expansion percentage; WA, Water activity; RR, Rehydration ratio; RT, Rehydration time; L*, Lightness; a*, redness-greenness; b* Yellowness–blueness; WI, Whiteness; W/OS, Without soaking; WS, With soaking; SC, Sodium citrate; CC, Calcium chloride; # 5% level of probability
Degree of damaged grains
The proportion of damaged grains varied from 16.21 to 22.15% and 4.69 to 5.30% for PB1509 and JR, respectively. Grains of cultivar PB1509 were more swollen and cooked softer, thus were more prone to damage during handling as compared to short grain cultivar JR. Higher clumping was observed in case of instant rice from long grained PB1509 due to more breakage, which led to sticking of grains together. After soaking and salt pretreatment, reduction in percentage of damaged grains were observed in both cultivars. The reduction in damaged grains is more pronounced in PB1509, which reduced from 22.15 to 20.62% in water soaked treatment, and further reduced to 18.89 to 16.21% after different salt pretreatments. In case of cultivar JR, this reduction is of lower magnitude as it reduced from 5.30 to 5.12 in water soaked treatment and further reduced to 5.27 to 4.69% after different salt treatments. The instant rice pretreated with calcium chloride exhibited lower percentage of damaged grains in comparison to instant rice pretreated with sodium citrate for both rice cultivars. Lowest damaged grains percentage (16.21% for PB1509 and 4.69% for JR) was observed after soaking pretreatment with addition of 1% calcium chloride (Table 3). Addition of calcium chloride to the soak water deter adhesion between the grains, which would cause mutilation if the grains were forced apart.
Apart from soaking and salt pretreatment, the post cooking processes also contributed for reducing sticking and damage during the preparation. The cooked rice was frozen prior to drying in a fluidized bed drier, which reduced stickiness and provided better dry flow characteristics than conventional drying. The freezing pretreatment toughened the gel structures reducing the stickiness of the starch-glutelin gel complexes, which results from the pre- cooking process. The glutelins become hydrated to a considerable degree during the freezing pretreatment. The coagulation releases free water into the voids, which are opened by coagulation and shrinkage, and the liberated water migrate and increase the size of any ice crystal nuclei, which are present. The freezing process thus liberates water from these gels while partially denaturing the protein lattice. This sufficiently reduced the tendency of the individual grains to stick together, and to mash and break at the slightest pressure. The effect of freezing as a pretreatment on the porosity of instant rice has been reported in our earlier study (Sharma et al. 2022), wherein slow freezing at − 20 °C promoted the growth of large ice crystals during the freezing process, which created larger pore size voids, thus decreasing the bulk density, and increasing the water absorption and percent volume expansion of the instant rice.
Fluidized bed drying caused puffing on volume expansion which possibly led to enhanced porosity. The individual frozen cooked rice kernels moved separately from each other through the flowing hot air of fluidized bed drier and thereby effective absorption of heat by the kernels. The high velocity air stream of fluidized bed drier rapidly carries moisture away from the surface and prevents grains from sticking together, thus inhibiting clumping and damage of rice kernels (Sharma et al. 2022). Centrifugal force restrained the particles from clumping in a high velocity air stream and gives homogenous fluidisation, avoided scorching or surface heat damage, minimised clumps and thus ensured efficient moisture removal.
Colour properties
L*, a* and b* values varied from 55.67 to 59.71, − 0.32 to − 0.48 and 5.02 to 5.91, respectively, for PB1509, and 60.15 to 62.68, − 0.82 to -0.88 and 2.76 to 5.12, respectively, for JR. Colour values (L*, a* and b*) of instant rice samples were affected by the salt pretreatments during soaking. Whiteness varied from 55.28 to 59.38 and 59.85 to 62.58 for PB1509 and JR, respectively. After the chemical pretreatment, there was improvement in the whiteness of the rice grains (Table 3) in both the cultivars. Amongst the two salts, instant rice pretreated with calcium chloride exhibited higher values for L* and whiteness, in comparison to sodium citrate pretreated instant rice. Instant rice pretreated with calcium chloride exhibited improved whiteness for both rice cultivars. The maximum whiteness was observed for both the cultivars in pretreated samples with 1% calcium chloride. The enhancement of whiteness may be due to the pretreatment with salts that inhibited Maillard reaction. The chemicals sodium citrate and calcium chloride improved degree of gelatinization, water absorption index and rehydration ratio of white rice. Addition of chemicals decreased the cooking time of rice, thereby, reducing the heat treatment required which lowers degree of Maillard reaction (Le et al. 2014; Wang et al. 2013). Total colour change () varied from 0.20–4.11 and 0.21–2.67 for PB1509 and JR, respectively (Fig. 1). The total colour change of instant rice developed by soaking in water and soaking in 0.5% sodium citrate solution were the lowest colour change observed amongst all pretreatments.
Fig. 1.
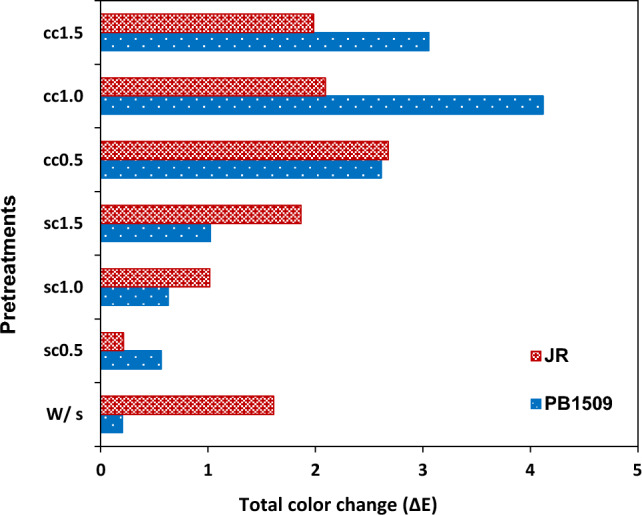
Effect of salt pretreatment with varying concentration (0.5, 1.0 and 1.5%) of SC and CC on total colour change of instant rice. Note: WS: With soaking, SC: Sodium citrate, CC: Calcium chloride
Rehydration characteristics
Rehydration ratio and rehydration time
Rehydration ratio and rehydration time varied from 3.28 to 3.34 and 1.5 to 5 min for PB1509, respectively, while 3.48 to 3.54 and 2 to 3 min, respectively, for JR. After the soaking and salt pretreatment, rehydration ratio increased for both the varieties. Higher rehydration ratio was observed with calcium chloride pretreatment in both PB1509 (3.32 to 3.34) and JR (3.52 to 3.54). Hawa et al. (2022) also observed increase in rehydration ratio of instant rice after salt treatment. Rehydration time decreased after the application of pretreatments, with maximum decrease observed in basmati cultivar PB1509. After the soaking pretreatment with sodium citrate, reduction in rehydration time up to 2 min was observed for both rice cultivars. Calcium chloride pretreatment was more effective as compared to sodium citrate pretreatment in increasing the rehydration ratio while reducing the rehydration time for instant rice prepared from both rice cultivars. Minimum rehydration time was observed in instant rice samples of both cultivars prepared with soaking in 1% solution of calcium chloride. Pretreatment with salts effectively reduced the rehydration time by increasing the porosity and water absorption process of the instant rice samples. The results are in agreement with Mridula et al. (2017) and Hawa et al. (2022). Calcium chloride (1%) pretreated instant rice of cultivar PB1509 depicted lowest rehydration time of 1.5 min by mere addition of hot water. Armed Forces have to operate in difficult terrains and under adverse conditions where cooking of these products becomes difficult. Any reduction in cooking time of rice is of immense help to the consumers and Armed Forces. Armed Forces all over the world depend largely on quick cooking convenience foods for their emergency and operational rations, which are easy to reconstitute, light in weight, calorie dense and have long shelf life.
Weight gain%
The weight gain % as affected by different pretreatments is shown in supplementary information. The perusal of results revealed that at the beginning, water absorption increased rapidly and then became constant. After the pretreatments, water absorption was found to increase. The instant rice preparation process involving addition of chemicals during soaking, freezing and drying pretreatment modified the starch, internal structure of rice, and loosening of the protein structure, which further enhanced the absorption rate (Agrawal et al. 2019; Sharma et al. 2022; Hawa et al. 2022). Sodium citrate in the soak water acts principally to modify the protein structure of the rice by disruption and disintegration (Hawa et al. 2022). The modification of starch and/ or protein by the action of these salts leads to facilitated diffusion of water by the starch component during the gelatinization process (Hawa et al. 2022; Mridula et al. 2017). The freeze-thaw-dehydration process caused the creation of cracks inside the structure due to which gelatinization process increases. The slow freezing process at − 20 °C created large ice crystals and lead to highly porous structure after drying. Higher porosity of instant rice samples leads to higher water absorption rate.
Rehydration kinetics
Peleg model (Peleg 1988) and Singh and Kulshrestha model (Singh and Kulshrestha 1987) were used to determine moisture uptake kinetics. Linear regression was employed to determine all the model’s parameters (k1 and k2 for Peleg model, and k and me for Singh and Kulshrestha model) as shown in Table 4, and supplementary information. Peleg model (Peleg 1988) depicted that k1 is the initial rehydration value, and k2 is related to the maximum water absorption capacity or the equilibrium moisture content. After the pretreatment with salt during soaking of rice, lower values for k2 were observed; this indicated higher water absorption capacity after pretreatment with salts. The lowest values of k2 were observed at 0.5% of calcium chloride for cultivar JR, and at 0.5 and 1% of calcium chloride for cultivar PB1509. The k1 and k2 values obtained from Eq. 1 were used to estimate moisture content at any time. The comparison of experimental and predicted values depicted a good agreement as indicated by the high R2 values (0.980–0.999) (Table 4). The difference between the experimental data and the predicted Peleg model data was not significantly different based on lower Chi-square values at p ≤ 0.05.
Table 4.
Parameters of rehydration kinetics of instant rice estimated by Peleg Model and Singh and Kulshrestha Model
| Treatment | Peleg model | Singh and Kulshrestha model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | k2 | R2 | RMSE | χ2 | k | me | R2 | RMSE | χ2 | |
| PB1509 | ||||||||||
| W/O S | 0.009 | 0.003 | 0.984 | 9.691 | 4.030 | 0.440 | 304.210 | 0.971 | 14.217 | 6.390 |
| W S | 0.008 | 0.003 | 0.980 | 11.071 | 5.491 | 0.583 | 286.920 | 0.966 | 15.041 | 7.248 |
| SC 0.5% | 0.004 | 0.003 | 0.995 | 6.671 | 1.314 | 0.571 | 417.800 | 0.993 | 8.830 | 1.714 |
| SC 1.0% | 0.003 | 0.002 | 0.987 | 13.043 | 4.492 | 0.500 | 477.230 | 0.981 | 15.892 | 5.196 |
| SC 1.5% | 0.005 | 0.002 | 0.994 | 7.268 | 1.613 | 0.400 | 455.680 | 0.992 | 9.737 | 2.360 |
| CC 0.5% | 0.003 | 0.002 | 0.999 | 3.156 | 0.240 | 0.724 | 477.380 | 0.999 | 3.188 | 0.183 |
| CC 1.0% | 0.002 | 0.002 | 0.999 | 4.676 | 0.411 | 0.840 | 477.260 | 0.999 | 3.502 | 0.220 |
| CC 1.5% | 0.003 | 0.002 | 0.998 | 8.505 | 1.585 | 0.656 | 477.360 | 0.994 | 9.522 | 1.765 |
| JR | ||||||||||
| W/O S | 0.003 | 0.003 | 0.998 | 3.282 | 0.253 | 0.844 | 371.440 | 0.998 | 4.085 | 0.398 |
| WS | 0.002 | 0.003 | 0.999 | 4.223 | 0.408 | 0.813 | 385.620 | 0.999 | 3.931 | 0.326 |
| SC 0.5% | 0.004 | 0.003 | 0.991 | 9.589 | 2.684 | 0.610 | 401.070 | 0.988 | 10.992 | 3.187 |
| SC 1.0% | 0.004 | 0.003 | 0.990 | 9.833 | 2.836 | 0.595 | 401.070 | 0.987 | 11.545 | 3.463 |
| SC 1.5% | 0.004 | 0.003 | 0.991 | 9.484 | 2.687 | 0.581 | 401.120 | 0.988 | 11.077 | 3.166 |
| CC 0.5% | 0.006 | 0.002 | 0.997 | 6.235 | 1.224 | 0.269 | 556.580 | 0.995 | 7.374 | 1.466 |
| CC 1.0% | 0.002 | 0.003 | 0.997 | 6.469 | 0.983 | 1.739 | 371.470 | 0.997 | 6.856 | 1.054 |
| CC 1.5% | 0.002 | 0.003 | 0.995 | 4.462 | 0.431 | 1.083 | 385.710 | 0.998 | 4.908 | 0.485 |
W/OS, Without soaking; WS, With soaking; SC, Sodium citrate; CC, Calcium chloride; k, Rate constant; k1, Peleg rate constant; k2, Peleg capacity constant; me, Equilibrium moisture constant; R2, Coefficient of determination; RMSE, Root mean square error
Singh and Kulshrestha model is useful for estimation of equilibrium moisture content without prolonged experimentation. The values of equilibrium moisture content estimated by the Singh and Kulshrestha model were close to those observed by the extrapolation of linear plot of rate of change of moisture content v/s moisture content. The rate constant k and me calculated from Singh and Kulshrestha model from equation was used to estimate moisture content at any time (Table 4). The comparison of experimental and predicted values depicted a good agreement as indicated by the high R2 value in Singh and Kulshrestha Model (0.966–0.999) (Table 4). The results are in agreement with the study of Puspitowati and Driscoll (2007) as well as of Rhim et al. (2011). Both empirical models evaluated for rehydration kinetics of instant rice exhibited high R2 values and showed good fit for the study.
Organoleptic properties
The post- cooking processes severely alter the organoleptic properties of instant rice. The chemical composition of rice is known to affect the rice taste quality, especially amylose content. During soaking, amylose and short-chain amylopectin are the major components to leach out, and are known to affect the textural attributes of rice. All the instant rice samples were accepted by the panellist. Although, a slight variation was observed in the overall acceptability scores among instant rice prepared with different salt pretreatment as compared to the control sample without any pretreatment (Table 5). Among different salt pretreatments, highest overall acceptability scores (8.3 and 7.7 for PB 1509 and JR, respectively) were found for calcium chloride (1% concentration). Similarly, for visual appearance, flavour, aroma and texture also pretretated rice sample with calcium chloride (1%) emerged as best. In general, for all the organoleptic properties pretreated rice with calcium chloride gave better scores than the sodium citrate pretreated rice in both the cultivars. Pretreatment with calcium chloride did not show any remarkable difference in the visual appearance, flavour and texture of the rehydrated instant rice and freshly prepared cooked rice. No agglomeration was observed and individual grains could be recognized in all of the instant rice samples. Mridula et al. (2017) also observed the normal appearance of instant rice, prepared by soaking in calcium chloride and sodium citrate solution. During the freezing process in preparation of instant rice, development of larger ice crystal led to larger pore size after drying and produce spongy and soft textured grains after rehydration (Arunyanart and Charoenrein 2008; Sharma et al. 2022).
Table 5.
Effect of different pretreatments on organoleptic characteristics of rehydrated instant rice
| Treatment | Aroma | Flavour | Appearance | Texture | OAA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | |
| PB1509 | ||||||||||
| W/O S | 7.10 | 0.18 | 8.40 | 0.16 | 8.20 | 0.13 | 8.30 | 0.15 | 8.00 | 0.30 |
| WS | 6.80 | 0.13 | 8.30 | 0.15 | 8.50 | 0.17 | 8.30 | 0.15 | 7.98 | 0.39 |
| SC 0.5% | 6.80 | 0.20 | 7.50 | 0.17 | 7.60 | 0.16 | 7.90 | 0.18 | 7.45 | 0.23 |
| SC 1.0% | 6.80 | 0.20 | 7.50 | 0.17 | 7.50 | 0.17 | 7.50 | 0.17 | 7.33 | 0.18 |
| SC 1.5% | 6.80 | 0.20 | 7.10 | 0.18 | 7.20 | 0.13 | 7.40 | 0.16 | 7.13 | 0.13 |
| CC 0.5% | 7.00 | 0.21 | 7.10 | 0.18 | 7.30 | 0.21 | 7.00 | 0.21 | 7.10 | 0.07 |
| CC 1.0% | 6.70 | 0.26 | 7.10 | 0.10 | 7.20 | 0.13 | 7.30 | 0.15 | 7.08 | 0.13 |
| CC 1.5% | 7.00 | 0.21 | 7.30 | 0.15 | 7.10 | 0.18 | 7.00 | 0.21 | 7.10 | 0.07 |
| JR | ||||||||||
| W/O S | 7.30 | 0.21 | 8.30 | 0.15 | 7.60 | 0.16 | 7.00 | 0.15 | 7.55 | 0.28 |
| WS | 7.00 | 0.15 | 8.30 | 0.15 | 7.60 | 0.16 | 7.20 | 0.20 | 7.53 | 0.29 |
| SC 0.5% | 6.80 | 0.20 | 7.50 | 0.17 | 7.20 | 0.20 | 7.00 | 0.15 | 7.13 | 0.15 |
| SC 1.0% | 7.00 | 0.26 | 7.30 | 0.15 | 7.00 | 0.15 | 7.10 | 0.23 | 7.10 | 0.07 |
| SC 1.5% | 6.80 | 0.20 | 7.30 | 0.15 | 7.10 | 0.18 | 7.20 | 0.20 | 7.10 | 0.11 |
| CC 0.5% | 6.90 | 0.23 | 7.10 | 0.18 | 7.00 | 0.15 | 7.00 | 0.26 | 7.00 | 0.04 |
| CC 1.0% | 7.20 | 0.20 | 7.10 | 0.18 | 7.10 | 0.23 | 7.10 | 0.28 | 7.13 | 0.03 |
| CC 1.5% | 6.90 | 0.18 | 7.00 | 0.15 | 7.00 | 0.21 | 7.10 | 0.18 | 7.00 | 0.04 |
| C.D | N/A | 0.45 | 0.49 | 0.54 | 0.54 | |||||
| SE(m) | 0.20 | 0.16 | 0.17 | 0.19 | 0.19 | |||||
| SE(d) | 0.29 | 0.23 | 0.25 | 0.27 | 0.26 | |||||
| C.V | 9.32 | 6.75 | 7.43 | 8.35 | 5.21 | |||||
W/OS Without soaking, WS With soaking, SC Sodium citrate, CC Calcium chloride, OAA Overall acceptability
Conclusion
The present study assesses the effect of salt pretreatment of rice prior to cooking for preparation of instant rice, and evaluates their effect on its quality. After the application of pretreatments, significant change in cooking quality attributes of rice were observed. Reduction in cooking time while increase in water uptake by the rice grains were observed after the pretreatments. Application of soaking and salt pretreatments significantly improved the physical, rehydration and organoleptic characteristics of instant rice. Salt pretreatment reduced degree of damaged grains and rehydration time of instant rice. Both sodium citrate and calcium chloride salts proved to be effective in improving the water absorption rate, volume expansion and porosity of instant rice samples. Calcium chloride pretreatment proved to be more effective than sodium citrate pretreatment for development of instant rice with improved quality characteristics such as lower bulk density, percentage of damaged grains and rehydration time while higher porosity, whiteness, water absorption and rehydration ratio. Soaking pretreatment with 1% calcium chloride for developing instant rice by freeze-thaw-dehydration process significantly reduced the rehydration time below 2 min. Such reduced rehydration time for instant rice is useful for ready-to-use applications as well as in development of various instant rice based preparations such as Pulav, Biryani, etc. Instant rice with lower rehydration time, besides having the similar appearance of conventionally cooked rice, is of immense value for those situated at high altitude, especially for our Armed Forces. Additionally, the process for the preparation of instant rice adopted is user friendly, cost effective, requires no special working appliances, and, hence, can be easily adopted at industrial scale. Use of soaking treatment with 1% calcium chloride during preparation of instant rice could be recommended to the food industries dealing with convenience and ready-to-eat food products. Both Peleg, and Singh and Kulshrestha Models fitted well to the water absorption process of instant rice during rehydration.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Director, Defence Food Research Laboratory (DFRL), Defence Research and Development Organization (DRDO), Mysore, India for providing us with all facilities and financial assistance to carry out the research work.
Author’s contributions
SS conceived, carried out the experiments and wrote the manuscript; ADS provided guidance, supervised the work, and edited the manuscript; SPS assisted during the experimentation; TGR assisted during the experimentation; DDW provided the resources and facilities.
Funding
Defence Food Research Laboratory, DRDO, Mysore.
Availability of data and material
Supplementary Material.
Code availability
Not available.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The research has been conducted in an ethical and responsible manner. The submitted work is original and has not been published elsewhere. The manuscript shall not be submitted to any other Journal.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrawal S, Raigar RK, Mishra HN. Effect of combined microwave, hot air, and vacuum treatments on cooking characteristics of rice. J Food Process Eng. 2019;42(4):e13038. doi: 10.1111/jfpe.13038. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 17. Washington: The Association of Official Analytical Chemists; 2000. [Google Scholar]
- Arunyanart T, Charoenrein S. Effect of sucrose on the freeze–thaw stability of rice starch gels: correlation with microstructure and freezable water. Carbohyd Polym. 2008;74(3):514–518. doi: 10.1016/j.carbpol.2008.04.002. [DOI] [Google Scholar]
- Bui LT, Coad RA, Stanley RA. Properties of rehydrated freeze-dried rice as a function of processing treatments. LWT Food Sci Technol. 2018;91:143–150. doi: 10.1016/j.lwt.2018.01.039. [DOI] [Google Scholar]
- Chavan P, Sharma SR, Mittal TC, Mahajan G, Gupta SK. Effect of parboiling technique on physico-chemical and nutritional characteristics of basmati rice. Agric Res J. 2018;55(3):490–499. doi: 10.5958/2395-146X.2018.00089.3. [DOI] [Google Scholar]
- Chen X, Qian P, Zhang XJ, Liu FN, Lu RR. Improving instant rice quality by novel combined drying. Dry Technol. 2014;32(12):1448–1456. doi: 10.1080/07373937.2014.900503. [DOI] [Google Scholar]
- Durgrao MNV, Deshpande HW, Syed IH. Studies on suitability of Indian rice variety for preparation of instant rice. J Pharmacogn Phytochem. 2017;6(6):1425–1429. [Google Scholar]
- GOI (2020) Agricultural Statistics at a Glance 2020 Government of India Ministry of Agriculture and Farmers Welfare, Department of Agriculture, Cooperation and Farmers Welfare Directorate of Economics and Statistics
- Gulati T, Datta AK. Mechanistic understanding of case-hardening and texture development during drying of food materials. J Food Eng. 2015;166:119–138. doi: 10.1016/j.jfoodeng.2015.05.031. [DOI] [Google Scholar]
- Hawa LC, Rhomadhona W, Putranto AW. Physicochemical characteristics of instant boiled rice: study of sodium citrate concentration and soaking time. Jurnal Teknik Pertanian Lampung (j Agricultural Eng) 2022;11(4):561–573. doi: 10.23960/jtep-l.v11i4.561-573. [DOI] [Google Scholar]
- Le TQ, Songsermpong S, Rumpagaporn P, Suwanagul A, Wallapa S. Microwave heating for accelerated aging of paddy and white rice. Aust J Crop Sci. 2014;8(9):1348–1358. [Google Scholar]
- Meera K, Smita M, Haripriya S. Varietal distinctness in physical and engineering properties of paddy and brown rice from southern India. J Food Sci Technol. 2019;56(3):1473–1483. doi: 10.1007/s13197-019-03631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mridula D, Kaur H, Goswami D, Gupta RK, Gurumayum S, Tyagi SK. Development of quick cooking rice: application of salts and enzyme pretreatments. Proc Nat Acad Sci India Sect B Biol Sci. 2017;87(3):637–646. doi: 10.1007/s40011-015-0625-7. [DOI] [Google Scholar]
- Mujumdar AS. Research and development in drying: recent trends and future prospects. Dry Technol. 2004;22(1–2):1–26. doi: 10.1081/DRT-120028201. [DOI] [Google Scholar]
- Peleg M. An empirical model for the description of moisture sorption curves. J Food Sci. 1988;53:1216–1217. doi: 10.1111/j.1365-2621.1988.tb13565.x. [DOI] [Google Scholar]
- Prasert W, Suwannaporn P. Optimization of instant jasmine rice process and its physicochemical properties. J Food Eng. 2009;95(1):54–61. doi: 10.1016/j.jfoodeng.2009.04.008. [DOI] [Google Scholar]
- Puspitowati S, Driscoll RH. Effect of degree of gelatinization on the rheology and rehydration kinetics of instant rice produced by freeze drying. Int J Food Prop. 2007;10(3):445–453. doi: 10.1080/10942910600871289. [DOI] [Google Scholar]
- Rhim JW, Koh S, Kim JM. Effect of freezing temperature on rehydration and water vapour adsorption characteristics of freeze-dried rice porridge. J Food Eng. 2011;104(4):484–491. doi: 10.1016/j.jfoodeng.2010.08.010. [DOI] [Google Scholar]
- Sadasivam S and Manickam A (1992) Biochemical Methods for Agricultural Sciences. Wiley Eastern Ltd, New Delhi
- Sasmitaloka KS, Widowati S, Sukasih E. Effect of freezing temperature and duration on physicochemical characteristics of instant rice. IOP Conf Ser Earth Environ Sci. 2019;309(1):012043. doi: 10.1088/1755-1315/309/1/012043. [DOI] [Google Scholar]
- Semwal AD, Sharma GK, Patki PE, Padmashree A, Arya SS. Studies on development and storage stability of instant vegetable pulav mix. J Food Sci Technol. 2001;38(3):231–234. [Google Scholar]
- Sharma S, Semwal AD, Srihari SP, Govindraj T, Wadikar DD. Impact of physico-chemical variation in different rice cultivars and freezing pretreatment for retaining better rehydration characteristics of instant rice. Int J Trop Agr. 2022;40(1–2):71–84. [Google Scholar]
- Singh BPN, Kulshrestha SP. Kinetics of water adsorption by soybean and pigeonpea grains. J Food Sci. 1987;52(6):1538–1541. doi: 10.1111/j.1365-2621.1987.tb05874.x. [DOI] [Google Scholar]
- Singh N, Kaur L, Sodhi NS, Sekhon KS. Physicochemical, cooking and textural properties of milled rice from different Indian rice cultivars. Food Chem. 2005;89(2):253–259. doi: 10.1016/j.foodchem.2004.02.032. [DOI] [Google Scholar]
- Sripinyowanich J, Noomhorm A. A new model and quality of unfrozen and frozen cooked rice dried in a microwave vibro-fluidized bed dryer. Dry Technol. 2011;29(7):735–748. doi: 10.1080/07373937.2010.535399. [DOI] [Google Scholar]
- Sripinyowanich J, Noomhorm A. Effects of freezing pretreatment, microwave-assisted vibro-fluidized bed drying and drying temperature on instant rice production and quality. J Food Process Preserv. 2013;37(4):314–324. doi: 10.1111/j.1745-4549.2011.00651.x. [DOI] [Google Scholar]
- Varnamkhasti MG, Mobli H, Jafari A, Keyhani AR, Soltanabadi MH, Rafiee S, Kheiralipour K. Some physical properties of rough rice (Oryza sativa L) grain. J Cereal Sci. 2008;47(3):496–501. doi: 10.1016/j.jcs.2007.05.014. [DOI] [Google Scholar]
- Wang JP, An HZ, Jin ZY, Xie ZJ, Zhuang HN, Kim JM. Emulsifiers and thickeners on extrusion-cooked instant rice product. J Food Sci Technol. 2013;50(4):655–666. doi: 10.1007/s13197-011-0400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani IA, Sogi DS, Wani AA, Gill BS. Physico-chemical and functional properties of flours from Indian kidney bean (Phaseolus vulgaris L) cultivars. LWT Food Sci Technol. 2013;53(1):278–284. doi: 10.1016/j.lwt.2013.02.006. [DOI] [Google Scholar]
- Yulianti LE, Setiaboma W, Hakim NA, Widowati E, Afifah N, Ekafitri R. The effect of beans types and soaking time on the characteristics of Indonesian traditional food" Instant Bose". Food Sci Technol. 2021;42:e19621. doi: 10.1590/fst.19621. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary Material.
Not available.


