Abstract
Spinal cord injury (SCI) severely affects the quality of life and autonomy of patients, and effective treatments are currently lacking. Autophagy, an essential cellular metabolic process, plays a crucial role in neuroprotection and repair after SCI. Glycoprotein non-metastatic melanoma protein B (GPNMB) has been shown to promote neural regeneration and synapse reconstruction, potentially through the facilitation of autophagy. However, the specific role of GPNMB in autophagy after SCI is still unclear. In this study, we utilized the spinal cord transection method to establish SCI rats model and overexpressed GPNMB using adenoviral vectors. We assessed tissue damage using hematoxylin and eosin (H&E) and Nissl staining, and observed cell apoptosis using TUNEL staining. We evaluated the inflammatory response by measuring inflammatory factors using enzyme-linked immunosorbent assay (ELISA). In addition, we measured reactive oxygen species (ROS) levels using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), and assessed oxidative stress levels by measuring malondialdehyde (MDA) and glutathione (GSH) using ELISA. To evaluate autophagy levels, we performed immunofluorescence staining for the autophagy marker Beclin-1 and conducted Western blot analysis for autophagy-related proteins. We also assessed limb recovery through functional evaluation. Meanwhile, we induced cell injury using lipopolysaccharide (LPS) and added an autophagy inhibitor to verify the impact of GPNMB on SCI through autophagy modulation. The results demonstrated that GPNMB alleviated the inflammatory response, reduced oxidative stress levels, inhibited cell apoptosis, and promoted autophagy following SCI. Inhibiting autophagy reversed the effects of GPNMB. These findings suggest that GPNMB promotes neural injury repair after SCI, potentially through attenuating the inflammatory response, reducing oxidative stress, and inhibiting cell apoptosis.
Keywords: spinal cord injury, autophagy, GPNMB, inflammatory, ROS
Introduction
Spinal cord injury (SCI) is a debilitating condition characterized by significant motor and sensory impairments 1 . It poses a major global health concern, presenting significant challenges to both clinical medicine and fundamental research 2 . SCI imposes a considerable burden on affected individuals and society as a whole 3 . The prevalence of SCI is alarmingly high, with an estimated 2.5 million individuals worldwide currently affected by this condition 4 . Moreover, the annual incidence of new SCI cases is reported to exceed 130,000 5 . The pathological mechanisms involved in SCI encompass both primary and secondary injuries 6 . Primary injury refers to the immediate damage caused by trauma or other factors such as tumors or infection 7 . On the other hand, secondary injury involves a complex series of molecular events that lead to more severe damage to the spinal cord and its functions 8 . A critical factor in the prolonged deterioration of the spinal cord following injury is apoptosis, a process of programmed cell death 9 . Inhibition of neuronal apoptosis resulting from secondary injury has been identified as crucial for promoting neural functional recovery 10 .
Autophagy is a widely observed degradation process that operates within lysosomes and is crucial in a range of pathophysiological contexts11–13. Recent research has shed light on the important role of autophagy in SCI, including its regulation and the extent of autophagy flux 14 . These findings have significant implications, suggesting that targeting autophagy could potentially offer a novel neuroprotective strategy and provide a fresh perspective for the clinical management of SCI 15 . Studies have demonstrated that following SCI, there is an occurrence of neuroinflammation that can trigger autophagy. The activated autophagy, in turn, can partially mitigate the inflammatory response16,17. Simultaneously, activated cellular autophagy can eliminate a portion of oxidative stress products, leading to a reduction in cellular apoptosis 18 . Hence, regulating autophagy is an effective neurorecovery strategy after SCI.
Glycoprotein non-metastatic melanoma protein B (GPNMB) is a glycoprotein that spans the cell membrane and is abundantly present in various tissues and cell types. Its expression has been observed to be closely linked to essential biological processes, including cell proliferation, migration, survival, immune, and inflammatory responses 19 . A research study conducted an analysis of mRNA changes in the spinal cord following sciatic nerve injury using transcriptomics, the study observed a significant upregulation of GPNMB mRNA levels in the spinal cord after the injury, which was further confirmed through polymerase chain reaction (PCR) analysis 20 . In addition, proteomic analysis of rats with SCI showed an overlap with the differential gene expression observed in the transcriptomic analysis, indicating an increase in GPNMB expression in spinal cord samples following SCI 21 . GPNMB is upregulated after cerebral ischemia–reperfusion injury (IRI), and genomic overexpression of GPNMB significantly improves infarct volume and is neuroprotective against IRI through phosphorylation of ERK1/2 and Akt 22 . GPNMB attenuates Alzheimer’s disease and enhances autophagy through inhibition of mTOR signaling 23 . The above-mentioned studies indicate that GPNMB can exert a positive neuroprotective effect after injury. However, whether GPNMB can promote neuroregeneration after SCI and whether its specific mechanism is related to autophagy remains unclear. This study aims to investigate the specific role of GPNMB after SCI and its potential involvement in autophagy-mediated mechanisms through a combination of in vivo and in vitro experiments, with the goal of identifying new therapeutic targets and providing a theoretical basis for clinical treatment.
Materials and Methods
Animals
Healthy adult male Sprague-Dawley (SD) rats, aged 9 weeks and weighing between 220 and 240 g, were procured from Shanghai Bikai Biological Technology Co., Ltd. The rats were maintained under controlled conditions with ad libitum access to food and water. The housing environment was maintained at a temperature of 20°C–26°C, relative humidity of 40%–70%, and a 12-h light–dark cycle. All animal experiments complied with the ethical guidelines for animal experimentation and were approved by the Clinical Research and Laboratory Animal Ethics Committee, the First Affiliated Hospital of Sun Yat-sen University (No. 2023[216]). All experimental protocols strictly followed the guidelines outlined in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023).
SCI Animal Model and Treatment
In the GPNMB gene overexpression experiment in rats, the AAV2/9-CMV-GPNMB-WPRE-pA expression vector (AAV-GPNMB) and its negative control (AAV-NC) provided by Shanghai TaiTu Biotechnology Co., Ltd. was utilized. Twenty SD rats were randomly divided into four groups: a sham surgery group (Sham), an SCI group, an SCI group given AAV-NC (SCI + NC), and an SCI group given AAV-GPNMB (SCI + GPNMB). The rat model of SCI was established using the spinal cord transection method 24 . In summary, rats in the SCI group received abdominal injection of 10% aqueous chloral hydrate (3.5 ml/kg) for anesthesia and were positioned prone, securely fixed to the operating table. After disinfecting the skin and hair in the incision area, we made a longitudinal incision of 1–1.5 cm in the midline of the back, centered around the T10 vertebra. We then performed blunt dissection of the bilateral vertebral muscles, achieving hemostasis through compression. Removing the vertebral lamina exposed the T9–T11 vertebrae, revealing the spinal cord. At the T10 level, we used microsurgical scissors to transect a 2-mm section of the spinal cord, which was subsequently extracted. The sham group underwent the same surgical procedure without causing any spinal cord damage.
Rats in the SCI + NC and SCI + GPNMB groups were given intraspinal injections 30 min after SCI. A microsyringe (Hamilton) was employed to connect the rats to the World Precision Instruments (WPI) microinjection pump system along with its controller. A 5-μl virus solution was injected into the spinal cord sheath at the T9 a rate of 1 μl/min. Hemostasis was ensured after the injection, and layered suturing was performed. The rats were then placed in a warm environment until they regained consciousness. Subsequently, penicillin sodium (400,000 U/rat) was subcutaneously injected twice daily for 5 consecutive days to prevent bacterial infection. Manual bladder voiding was conducted twice daily until natural urination was restored.
At the end of the experiment, rats were anesthetized by intraperitoneal injection of 10% chloral hydrate, restrained in the supine position, and the thoracic cavity was exposed. A catheter was threaded via the ascending aorta into the heart, through which 250 ml of physiological saline was swiftly flushed for 5 min. Then, a 4% paraformaldehyde solution was infused from the left ventricle to the ascending aorta, with an incrementally changing infusion rate. The perfusion was kept steady for 30 min, after which the spinal cord was extracted. Centering upon the transverse section of the spinal cord, extract spinal cord tissue approximately 2 cm in length for subsequent research.
Basso, Beattie, and Bresnahan Test
The Basso, Beattie, and Bresnahan (BBB) test is a comprehensive assessment of hindlimb motor function in rats, utilizing a scale ranging from 0 to 21 25 . A score of 0 represents complete paralysis of the hindlimbs, while 21 indicates unimpaired locomotion. Motor function was evaluated using the BBB score before and at 1, 3, 7, 14, 21, and 28 days following SCI. The assessment includes multiple aspects of hind limb movements such as joint movement, weight-bearing capacity, stepping patterns, coordination, paw positioning, trunk stability, and tail control within an open-field environment. The test was performed by two experimenter who were blinded to the experimental conditions. Each rat was observed for over 3 min, with one experimenter dedicated to recording the score. Prior to the test, the rats were examined to establish their baseline motor function. The scores from each testing session were then averaged to provide an overall measure of performance. This standardized protocol ensured the accuracy and consistency of the evaluation procedure.
Louisville Swimming Scale Test
The Louisville Swimming Scale (LSS) is an 18-point scale (0–17) with three ranges: 0–5, 6–11, and 12–1726. Rats were evaluated 1 day before and at post-injury days 7, 14, 21, and 28. This assessment aims to evaluate swimming performance based on three primary components: forelimb dependency, hindlimb activity, and body position. In normal rats, swimming relies entirely on hindlimb propulsion, with forelimbs used sporadically for steering. The trunk remains at an angle of 20° or less to the water surface, and hindlimbs kick alternately to propel the animal through the water. In contrast, rats with moderately severe spinal cord injuries exhibit complete reliance on their forelimbs for swimming, displaying a tail-down body angle greater than 45° and/or rotational instability of 45° or more around the long axis. According to the LSS, animals scoring in the 0–5 category are poor swimmers heavily dependent on their forelimbs for forward propulsion with minimal hindlimb movement. Animals scoring in the 6–11 range are intermediate swimmers with intermittent to frequent hindlimb movement, some residual reliance on forelimbs, mild trunk instability, and a mild body angle (21°–45°, tail-down from horizontal) during forward locomotion. Animals scoring 12 and higher exhibit consistent hindlimb movement, little to no forelimb dependency, minimal trunk instability, and frequent to consistent alternating hindlimb movement.
Grid Walking Test
The grid walking test is a method used to evaluate hindlimb placement control in animals after brain or SCI 27 . The rats were evaluated on day 28 of the experiment. It involves placing the animals on a horizontal or inclined grid with a distance of 2.5 cm between the bars. The animals are trained to find food and water above the grid, and behavioral data such as the number of hindlimb slips, footstep sounds, and time to traverse the distance are recorded.
Hematoxylin and Eosin and Nissl Staining
The excised spinal cord was immersed in 4% paraformaldehyde overnight, with approximately 0.5 cm of spinal cord preserved on both sides of the transverse section. The tissue was embedded in paraffin and cut into 4- to 6-μm-thick slices using a microtome. The 4 μm sections were stained with hematoxylin and eosin (H&E) staining solution. The 6 μm sections were deparaffinized and stained with Nissl staining solution (Beyotime, Shanghai) at a temperature of 50°C–60°C. Tissue pathology images were obtained using an optical microscope (Leica, Germany).
Cell Culture and Treatment
PC12 cells (CRL-1721, Shanghai Cell Biology Institute) were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 5% fetal bovine serum (FBS), 1% antibiotics, and 10% horse serum at 37°C in a humidified atmosphere with 5% CO2. After cell attachment, the medium was replaced with differentiation medium containing nerve growth factor (NGF), horse serum, and antibiotics. The cells were cultured for 7 days to obtain differentiated PC12 cells. For stimulation experiments, PC12 cells were seeded in a six-well plate and treated with 5 μg/ml of lipopolysaccharide (LPS) (L2630, Sigma-Aldrich) for 12 h. Cells treated with phosphate-buffered saline (PBS; P4474, Sigma-Aldrich) were used as the control. The cells in the LPS + NC group, LPS + GPNMB group, and LPS + GPNMB + 3-MA group were transfected with the corresponding virus vector using Lipofectamine 3000 (Sigma-Aldrich, St. Louis, MO). After 48 h, the cells were treated with LPS for 12 h. To inhibit the activity of autophagy, specific cell cultures were supplemented with 1 mM of 3-MA (189490, Sigma-Aldrich).
Cell Viability Assay
After the incubation period, the treated PC12 cells were washed and transferred to a new 96-well plate. RPMI-1640 medium (100 µl) was added to each well, followed by the addition of 10 µl of CCK-8 reagent. The plate was then incubated at 37°C for a specified period. The cell viability was assessed by measuring the absorbance at a wavelength of 450 nm using a microplate reader. This methodology allowed the evaluation of cell viability in the treated PC12 cells using the CCK-8 assay, providing insights into the impact of the experimental treatments on cell survival.
Reactive Oxygen Species Assay
The ROS (reactive oxygen species) assay kit (CA1410, Solarbio) was used to measure the levels of ROS in the spinal cord and PC12 cells of each group. The spinal cord was placed in pancreatic enzyme solution and mixed thoroughly using a pipette for 2 min to ensure a homogeneous mixture. The homogenate was then filtered through a 200-mesh sieve to obtain a cell suspension. The tissue cell suspensions and PC12 cell suspension were incubated with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, 10 μM) at 37°C for 25 min. The mixture was inverted every 3–5 min to ensure complete contact between the probe and the cells. The samples were imaged under a microscope, such as one from Olympus, Tokyo, Japan. Quantitative analysis of ROS levels was performed using image processing software, such as ImageJ v1.53c, developed by Schneider et al. at the NIH, Bethesda, Maryland, USA in 2012.
Western Blotting
Spinal cord tissues were homogenized with RIPA (radioimmunoprecipitation assay) lysate containing protease inhibitors, and PC12 cells were treated with RIPA lysate containing protease inhibitors for 15 minutes. After centrifugation, the supernatant were collected to quantify the protein concentration using the BCA (bicinchoninic acid) protein quantification kit. Mix the supernatant with loading buffer and denature the proteins. Perform sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel electrophoresis with two stages. Transfer the proteins to a membrane and block with 5% skim milk. Incubate the membrane overnight at 4°C with the primary antibodies (LC3, 1:2,000, Abcam; Beclin-1, 1:2,500, CST; p62, 1:3,000, CST; BCL-2, 1:1,000, CST; Bax, 1:2,000, CST). Then, incubate the membrane for 2 h at room temperature with the appropriate secondary antibody. Develop the membrane in the dark and capture gel images using a gel imaging system.
Enzyme-Linked Immunosorbent Assay
The levels of malondialdehyde (MDA) and glutathione (GSH) in spinal cord tissues and PC12 cells were determined using the Lipid Peroxidation MDA Assay Kit (88-3909-22, eBioscience) and GSH Assay Kit (88-50600-22, eBioscience) according to the manufacturer’s instructions. Tissue homogenate samples and cell supernatants were added to microtiter plate wells containing specific capture antibodies. Wash and add enzyme-labeled detection antibodies. After washing, add substrate to initiate enzyme reaction. Measure absorbance at 450 nm for quantification.
TUNEL Staining
The TUNEL Staining Kit from Wuhan Sanying Biotechnology Co., Ltd. was used to detect neuronal death in injured spinal cords. Paraffin slices of spinal cord tissue were deparaffinized with DNase-free proteinase K for 15 min. PC12 cells were washed with PBS and fixed with 4% paraformaldehyde for 30 min. PBS was washed and cells were resuspended by adding PBS containing 0.3% Triton X-100 and incubated at room temperature for 5 min. Sections and cells were washed and added to TUNEL assay solution and incubated at for 1 h at 37°C in the dark. After counterstaining with DAPI (4′,6-diamidino-2-phenylindole), the sections were observed under a fluorescence microscope.
Immunofluorescence Staining
After the sections have been pretreated with xylene and sodium citrate, they are incubated at 37°C in PBS containing 10% normal bovine serum and 0.1% Triton X-100 for 1 h. The cells were fixed with 4% neutral formaldehyde at 4°C for 15 min, the fixative was removed, and the cells were washed 3 times with pre-chilled Tris-buffered saline (TBS) buffer at 4°C. The sections and cells were completely covered with 5% normal goat serum, and the slices were placed in a humid chamber, while the cell culture plate was sealed and placed in a constant temperature and humidity incubator at 37°C for 30 min. Next, the appropriate primary antibodies, NeuN (1:2,000 dilution) and Beclin-1 (1:1,500 dilution), are added to the sections and incubated overnight at 4°C. The sections are then washed three times with PBS at room temperature. Subsequently, the corresponding secondary antibodies (1:1,000 dilution) are applied and incubated at 37°C, avoiding exposure to light, for 1 h. After three additional washes with PBS, each for 5 min, the sections are stained with DAPI (0.25 mg/ml) for 10 min to visualize the cell nuclei. Fluorescent images are captured using an Olympus CKX53 inverted microscope (Olympus, Japan) at the boundaries of the normal and damaged regions in the corresponding anatomical area. Assuming at least three images with regions of interest are analyzed using J software, the data obtained can be processed using SPSS 26 software to generate statistical graphs.
Statistical Analyses
Statistical analyses were performed using SPSS 26.0 (SPSS, Chicago, IL). The data were reported as mean ± standard deviation (SD). Comparisons between two independent groups were assessed using an independent-samples t-test. Comparisons among more than three groups were evaluated using one-way analysis of variance (ANOVA) with the least significant difference (LSD) post hoc test, assuming equal variances. A significance level of P < 0.05 was used to determine statistical significance.
Results
GPNMB Promotes Motor Function Recovery After SCI
We utilized the adeno-associated virus (AAV)-mediated cDNA injection technique to introduce GPNMB into the site of SCI. Through protein analysis, we observed a higher expression level of GPNMB in the SCI + GPNMB group compared with the SCI + NC group (Fig. 1A), confirming the success of our method. Assessing the motor function, we noted a significant decline in BBB scores for the SCI group compared with the sham group, indicating the pronounced impact of SCI on motor function. However, on the seventh day, the SCI + GPNMB group exhibited a notable improvement compared with the SCI + NC group (P < 0.001; Fig. 1C). In terms of LSS scores, a significant increase was observed on the 14th day specifically in the SCI + GPNMB group (P < 0.001; Fig. 1D). Finally, on the 28th day, during the grid error experiment, the SCI + GPNMB group demonstrated significantly reduced error rates compared with the SCI + NC group (P < 0.001; Fig. 1E). These findings highlight that overexpressing GPNMB can facilitate the recovery of motor function and play a beneficial role in SCI.
Figure 1.
GPNMB promotes motor function recovery after SCI. (A, B) Western blot detected the expressions of GPNMB. BBB scores (C), LSS scores (D), and grid error (E) were used to assess the motor function of rat limbs. BBB: Basso, Beattie, and Bresnahan; GPNMB: glycoprotein non-metastatic melanoma protein B; LSS: Louisville Swimming Scale; NC: negative control; SCI: . Compared with the sham group, *P < 0.05, **P < 0.01, ***P < 0.001, and when compared with the SCI group, #P < 0.05; ###P < 0.001.
GPNMB Ameliorates Spinal Cord Tissue Damage in Rats After SCI
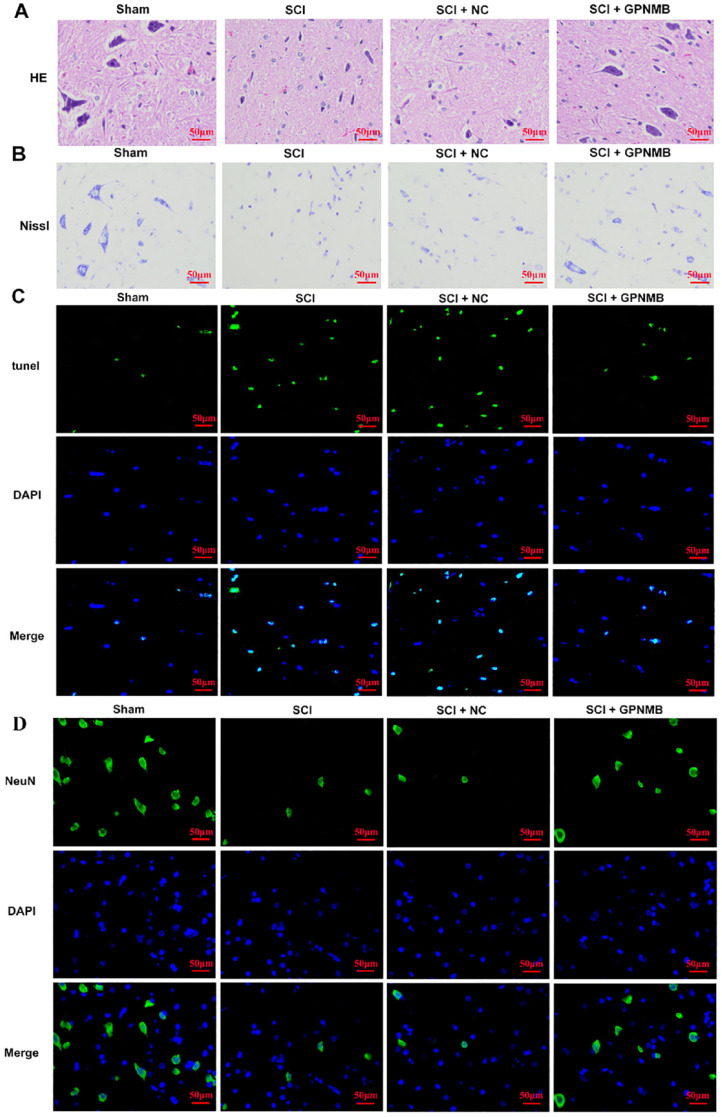
Autophagy can function as an adaptive response to mitigate cellular stress and enhance cell viability, thereby suppressing apoptosis 28 . To evaluate tissue damage and recovery, we examined rat spinal cord tissue sections using H&E and Nissl staining. Compared with the sham group, the SCI group exhibited structural disarray, infiltration of inflammatory cells, nuclear condensation of neurons, and translucent areas indicating dissolution of central Nissl bodies. However, when compared with the SCI + NC group, the SCI + GPNMB group showed reduced neuronal wrinkling, significantly decreased necrotic areas and vacuoles, and a notable reduction in inflammatory cell infiltration (Fig. 2A, B). Furthermore, TUNEL staining revealed an increase in apoptotic cell count in the SCI group, whereas the SCI + GPNMB group exhibited a significant decrease in apoptotic cells compared with the SCI + NC group (Fig. 2C). Conversely, immunofluorescence (IF) detection of neuronal survival demonstrated a higher number of surviving neurons in the SCI + GPNMB group compared with the SCI + NC group (Fig. 2D). These findings suggest that GPNMB may promote cell survival by inhibiting apoptosis.
Figure 2.
GPNMB ameliorates spinal cord tissue damage in rats after SCI. H&E (A) and Nissl (B) staining were used to evaluate the extent of spinal cord tissue damage in rats. TUNEL staining (C) was performed to assess neuronal apoptosis. Immunofluorescence staining of NeuN (D) was conducted to evaluate neuronal survival. DAPI: 4′,6-diamidino-2-phenylindole; H&E: hematoxylin and eosin; GPNMB: glycoprotein non-metastatic melanoma protein B; NC: negative control; SCI: spinal cord injury.
GPNMB Alleviates Neuroinflammation and Oxidative Stress in Rats After SCI
Inflammatory responses and oxidative stress can activate the autophagy pathway, and at the same time, the autophagy pathway can regulate the extent of inflammatory responses and oxidative stress 29 . To assess neuroinflammation in rats after injury, we used ELISA to measure the levels of inflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6. Compared with the sham group, the SCI group showed a significant inflammatory response. When compared with the SCI + NC group, treatment with GPNMB demonstrated a reduction in inflammation at the site of injury (P < 0.001, P < 0.01, P < 0.001; Fig. 3A). Furthermore, we used the DCFH-DA assay kit to detect ROS and assessed MDA and GSH levels using corresponding assay kits. The results showed that compared with the sham group, the SCI group exhibited significantly increased ROS activity, whereas the SCI + GPNMB group demonstrated a significant decrease in ROS activity compared with the SCI + NC group (Fig. 3B). MDA levels were consistent with ROS measurements (P < 0.001; Fig. 3C), indicating a reduction in oxidative stress. In addition, GSH levels were significantly increased in the SCI + GPNMB group compared with the SCI + NC group (P < 0.001; Fig. 3C). These findings suggest that GPNMB can alleviate neuroinflammation and oxidative stress.
Figure 3.
GPNMB alleviates neuroinflammation and oxidative stress in rats after SCI. ELISA (A) was employed to quantify the expression levels of TNF-α, IL-1β, and IL-6 in spinal cord tissue. The DCFH-DA assay kit (B) was utilized to detect ROS. The expression levels of MDA and GSH were determined using MDA and GSH assay kits, respectively (C). DCFH-DA: 2′,7′-dichlorodihydrofluorescein diacetate; ELISA: enzyme-linked immunosorbent assay; GPNMB: glycoprotein non-metastatic melanoma protein B; GSH: glutathione; IL: interleukin; MDA: malondialdehyde; NC: negative control; ROS: reactive oxygen species; SCI: spinal cord injury; TNF-α: tumor necrosis factor alpha. Compared with the sham group, ***P < 0.001, and when compared with the SCI group, ##P < 0.01, ##P < 0.001.
GPNMB Promotes Autophagy Levels in Rats After SCI
To assess the autophagic activity after SCI, we evaluated the fluorescence of Beclin-1 and measured the protein levels of autophagic markers (LC3II, Beclin-1) and autophagic substrate protein (p62). IF analysis showed enhanced fluorescence intensity of Beclin-1 (red fluorescence) in the SCI group compared with the sham group (Fig. 4A). In addition, a stronger fluorescence signal was observed in the SCI + GPNMB group compared with the SCI + NC group. Western blot analysis was conducted to measure the levels of LC3II, Beclin-1, and p62 proteins (Fig. 4B). The results demonstrated that the optical density (OD) value of LC3II and Beclin-1 was significantly higher in the SCI group compared with the sham group (P < 0.001, P < 0.05; Fig. 4C, D), whereas the OD value of p62 was significantly lower in the SCI group compared with the sham group (P < 0.001; Fig. 4E). When compared with the SCI + NC group, the SCI + GPNMB group showed a significant increase in the levels of LC3II and Beclin-1 (P < 0.001, P < 0.001; Fig. 4C, D), and a significant decrease in p62 levels (P < 0.01; Fig. 4E). These results indicate that GPNMB not only increases the levels of autophagic markers but also reduces the burden of autophagic substrate, potentially due to the overall increase in autophagic activity induced after SCI.
Figure 4.
GPNMB promotes autophagy levels in rats after SCI. The expression levels of Beclin-1 (A) were assessed using immunofluorescence detection. The protein expression levels of LC3II, Beclin-1, and p62 (B–E) were determined using Western blot analysis. DAPI: 4′,6-diamidino-2-phenylindole; H&E: hematoxylin and eosin; GPNMB: glycoprotein non-metastatic melanoma protein B; NC: negative control; SCI: spinal cord injury. Compared with the sham group, *P < 0.05, **P < 0.01, ***P < 0.001, and when compared with the SCI group, ##P < 0.01, ###P < 0.001.
GPNMB Modulates the Levels of Autophagy in Response to Cellular Damage
To elucidate the regulatory role of GPNMB on autophagy following cellular damage, we employed an LPS-induced cell injury model and administered the GPNMB inhibitor, 3-MA. This approach aimed to determine the suppressive effects of GPNMB on autophagy. Preliminary analysis using IF indicated increased Beclin-1 expression after LPS-induced injury. Compared with the LPS control group, the LPS + GPNMB group demonstrated a significant upregulation in Beclin-1 expression. In contrast, the LPS + GPNMB+3-MA group displayed a decrease in autophagy levels (Fig. 5A). Concurrently, Western blot results corroborated these observations. The LPS + GPNMB + 3-MA group exhibited a notable reduction in LC3II and Beclin-1 expression compared with the LPS + GPNMB group (P < 0.05, Fig. 5C, D), while p62 levels exhibited an upward trend (P < 0.05; Fig. 5E). These findings suggest that GPNMB enhances autophagy at the cellular level, and its inhibitors can mitigate this effect.
Figure 5.
GPNMB modulates the levels of autophagy in response to cellular damage. Immunofluorescence was used to detect the expression levels of Beclin-1 in the presence or absence of 3-MA (5). Western blot analysis was conducted to assess the expression levels of LC3II, Beclin-1, and p62 in the presence or absence of 3-MA (B–E). GPNMB: glycoprotein non-metastatic melanoma protein B; LPS: lipopolysaccharide; NC: negative control. Compared with the control group, **P < 0.01, compared with the LPS group, ##P < 0.01, ###P < 0.001, and when compared with the LPS + GPNMB group, @P < 0.05.
GPNMB Reduces LPS-Induced Cell Damage by Enhancing Autophagy
Next, we validated the effects of GPNMB on autophagy, apoptosis, neuroinflammation, and oxidative stress at the cellular level. First, we used LPS-induced cell damage to simulate animal SCI. We added the LPS + GPNMB + 3-MA group to reverse-validate the role of GPNMB in SCI. Cell viability assays showed that the LPS group exhibited decreased viability compared with the control group, while the LPS + GPNMB group showed increased viability compared with the LPS + NC group. However, the LPS + GPNMB + 3-MA group (P < 0.05, Fig. 6A) exhibited decreased viability compared with the LPS + GPNMB group. TUNEL staining depicted consistent results with the cell viability assay, reflecting the extent of cellular apoptosis (P < 0.001, Fig. 6B). Through the detection of autophagy-related markers, we found that the LPS group promoted and enhanced autophagy. The LPS + GPNMB group exhibited even stronger autophagy, while the LPS + GPNMB + 3-MA group demonstrated a weakened trend (P < 0.05, Fig. 6C). Subsequently, we measured the levels of neuroinflammatory factors (TNF-α, IL-1β, and IL-6) and observed an elevation in the LPS group compared with the control group. In contrast, the LPS + GPNMB group displayed reduced levels compared with the LPS + NC group. However, the LPS + GPNMB + 3-MA group exhibited increased levels compared with the LPS + GPNMB group (P < 0.01, P < 0.001, P < 0.05, Fig. 6D). Finally, we measured markers related to oxidative stress (ROS, MDA, and GSH). The results indicated that ROS (Fig. 6E) and MDA (p < 0.05, Fig. 6F) levels were consistent with the inflammatory factors. Conversely, GSH levels were decreased in the LPS group compared with the control group, increased in the LPS + GPNMB group compared with the LPS + NC group, and decreased again in the LPS + GPNMB + 3-MA group (P < 0.05, Fig. 6F) compared with the LPS + GPNMB group. These experimental findings suggest that GPNMB mitigates LPS-induced cellular damage by enhancing autophagy.
Figure 6.
GPNMB reduces LPS-induced cell damage by enhancing autophagy. CCK-8 assay was used to measure cell viability in the presence or absence of 3-MA. (A). TUNEL assay was used to assess cell apoptosis in the presence or absence of 3-MA (B). Western blot analysis was performed to evaluate the expression levels of Bcl-2 and Bax in the presence or absence of 3-MA (C). ELISA was used to measure the expression levels of TNF-α, IL-1β, and IL-6 in the presence or absence of 3-MA (D). The DCFH-DA assay kit was utilized to detect ROS in the presence or absence of 3-MA (E). The expression levels of MDA and GSH were determined using MDA and GSH assay kits in the presence or absence of 3-MA, respectively (F). DCFH-DA: 2′,7′-dichlorodihydrofluorescein diacetate; ELISA: enzyme-linked immunosorbent assay; GPNMB: glycoprotein non-metastatic melanoma protein B; GSH: glutathione; LPS: lipopolysaccharide; MDA: malondialdehyde; NC: negative control; ROS: reactive oxygen species. Compared with the control group, ***P < 0.001, compared with the LPS group, ###P < 0.001, and when compared with the LPS + GPNMB group, @P < 0.05, @@P < 0.01, @@@P < 0.001.
Discussion
The study investigated whether GPNMB improves neural injury in SCI by promoting autophagy through in vivo and in vitro experiments. The results indicated that GPNMB improves motor function in rats with SCI by mediating autophagy, reducing inflammation, apoptosis, and oxidative stress levels in the spinal cord and PC12 cells. This suggests that GPNMB may be a promising therapeutic target for SCI.
The main clinical features of SCI are characterized by limited capacity for neuronal regeneration and a complex pathological progression. When the nervous system is injured, immune cells and inflammatory cells are activated, leading to the production of inflammatory mediators and triggering an inflammatory response that leads to inflammatory damage to neural tissue and cell apoptosis 30 . Researchers found that the proliferation and activation of glial cells are increased in SCI mice, accompanied by the up-regulation of pro-inflammatory cytokines. Intrathecal injection of human menstrual blood-derived endometrial stem cells (MenSCs) alleviates the inflammatory microenvironment in mice with SCI and promotes the recovery of nerve function 31 . In addition, neurological tissue damage and inflammation can lead to oxidative stress. Excessive ROS, in turn, can lead to cell apoptosis and inflammation 32 . Research has shown a close relationship between inflammation and oxidative stress. Biological markers of oxidative stress can regulate inflammatory response in SCI through multiple signaling pathways 33 . For instance, in rats with traumatic spinal cord injury (TSCI), the expression of proteins such as NOX-1, NOX-2, TLR-4, nuclear factor kappa B (NF-κB), IL-1β, and TNF-α in the spinal cord is elevated, indicating the presence of oxidative stress and an inflammatory response 34 . Resveratrol can facilitate the repair of spinal cord injuries by reducing oxidative stress and inflammatory response35,36. During the experimental research on the SCI rats, this study observed an increase in the levels of inflammatory factors, ROS, MDA, and apoptosis, a decrease in GSH levels. These findings indicated that inflammation and oxidative stress play important roles in secondary injury following neural damage and are crucial for the treatment of SCI.
In addition, previous studies have found that GPNMB shows increased expression after SCI and exhibits beneficial neuroprotective effects in the injury20,22. In the present study, both in vivo and in vitro experiments observed that GPNMB can reduce levels of inflammatory factors, eliminate the toxic metabolite MDA associated with oxidative stress, increase GSH content, and inhibit cell apoptosis. These results are consistent with the aforementioned studies. Meanwhile, in the assessment of motor function, rats in the GPNMB group showed better motor performance in terms of BBB score, LSS score, and grid-walking. This indicates that GPNMB can alleviate oxidative stress and inflammatory response, inhibit apoptosis, and improve limb motor function in SCI.
Kanno et al. first reported the overexpression of autophagy proteins Beclin-1 and LC3II in experimental SCI 37 . Since then, the autophagy mechanism and its impact on the pathological process of SCI have become the focus of many studies. In this study, it was found that the levels of autophagy were higher in the rats of the SCI group and LPS-induced PC12 cells compared with the sham group and control group, indicating the activation of autophagy due to inflammation and oxidative stress response after injury, consistent with the previous literature 38 . In the SCI + GPNMB group, we found that autophagy was further enhancement. To investigate this, the study conducted in-depth research by adding autophagy inhibitors in cell experiments. The results showed that the addition of autophagy inhibitors decreased the expression of autophagy markers, indicating the reversal of GPNMB-mediated autophagy. Simultaneously, inflammation, oxidative stress and apoptosis were increased in the LPS + GPNMB + 3-MA group as the level of autophagy decreased. This suggested that the regulation of inflammation, oxidative stress, and apoptosis in PC12 cells by GPNMB is closely related to its enhancement of autophagy.
Furthermore, research has shown that the total flavonoids of hawthorn leaves can promote the recovery of motor function in rats with SCI and exert neuroprotective effects in spinal motor neurons by stimulating autophagy 39 . In renal ischemia, GPNMB participates in cell protection by recruiting autophagy protein LC3 to phagosomes 40 . In IRI, the expression of GPNMB was upregulated. Overexpression of GPNMB has been shown to significantly improve infarct volume by activating autophagy 23 . Combining the results of this experiment, it is evident that GPNMB can alleviate oxidative stress and inflammatory responses, inhibit cell apoptosis, promote tissue repair in SCI, and therefore improve motor function in SCI rats by activating autophagy.
Moreover, although our research has provided new insights into the role of GPNMB in SCI, there are still some shortcomings that need to be addressed. Firstly, in animal experiments, some rats were not included in the subsequent studies due to issues with the recovery after spinal cord transection surgery. This resulted in a smaller sample size for each group (N = 5). In addition, the specific mechanisms by which GPNMB regulates autophagy and its detailed role in SCI recovery remain unclear. Future research could consider exploring the classic signaling pathways of autophagy, such as mTOR, to further investigate the regulatory mechanism of GPNMB in autophagy during SCI. A deeper understanding of these aspects will contribute to a more comprehensive understanding of the role of GPNMB in SCI recovery and its optimized application. Further research is needed to address these issues and evaluate the potential and effectiveness of GPNMB in clinical treatment of SCI.
In summary, this study conducted an in-depth exploration of the role of GPNMB in regulating autophagy after SCI. Our findings revealed that GPNMB is a significant molecular effector that plays a critical role in the functional recovery following SCI in rats. Our data support that GPNMB may improve neurologic function in rats after SCI by modulating the process of autophagy. However, further investigation is needed to understand the mechanisms by which GPNMB mediates autophagy.
Footnotes
Author Contributions: Xixi Li, Jiakun Xu, and Weijie Su designed and performed experiments. Luoxi Su, Xiangkun Chen, and Jia Yang analyzed the data. Xunxun Lin and Lixuan Yang confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript.
Availability of Data and Materials: The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Ethical Approval: All animal experiments complied with the ethical guidelines for animal experimentation and were approved by the Clinical Research and Laboratory Animal Ethics Committee, the First Affiliated Hospital of Sun Yat-sen University (No. 2023[216]).
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: (1) Guangdong Provincial Regional Joint Fund—Youth Fund, 2020A1515110625. (2) Guangzhou Science and Technology Program (Research and Development of Innovative Drugs and Medical Devices), 201903010093. (3) National Natural Science Foundation of China, 82073049.
ORCID iD: Xixi Li  https://orcid.org/0009-0007-9759-8967
https://orcid.org/0009-0007-9759-8967
References
- 1. Barros NA, Aidar FJ, Marcal AC, Santos JL, de Souza RF, Menezes JL, Gomes MZ, de Matos DG, Neves EB, Carneiro ALG, de Almeida-Neto PF, et al. Effects of resistance training on oxidative stress markers and muscle damage in spinal cord injured rats. Biology. 2022;11(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu X, Wu L, Wen Y, Liu J, Li H, Zhang Y, Wang Z, Ding J, Zeng Z, Xia H. Hippocampal mitochondrial abnormalities induced the dendritic complexity reduction and cognitive decline in a rat model of spinal cord injury. Oxid Med Cell Longev. 2022;2022:9253916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang C, Zhang L, Ndong JC, Hettinghouse A, Sun G, Chen C, Zhang C, Liu R, Liu CJ. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J Neuroinflammation. 2019;16(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Zhao H, Zhang A, Zhao C, Mei Z, Yao H, Fan Z, Liang D. Identifying a novel KLF2/lncRNA SNHG12/miR-494-3p/RAD23B axis in Spare Nerve Injury-induced neuropathic pain. Cell Death Discov. 2022;8(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krotov V, Medvediev V, Abdallah I, Bozhenko A, Tatarchuk M, Ishchenko Y, Pichkur L, Savosko S, Tsymbaliuk V, Kopach O, Voitenko N. Phenotypes of motor deficit and pain after experimental spinal cord injury. Bioengineering. 2022;9(6):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He C, Xiao J, Ye Y, Huang S, Zhong Y, Liu L, Liu W, Liu S. Long non-coding RNA-small nucleolar RNA host gene 7 regulates inflammatory responses following spinal cord injury by regulating the microRNA-449a/TNF-alpha-induced protein 3-interacting protein 2 axis. Bioengineered. 2022;13(4):10215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niu SP, Zhang YJ, Han N, Yin XF, Zhang DY, Kou YH. Identification of four differentially expressed genes associated with acute and chronic spinal cord injury based on bioinformatics data. Neural Regen Res. 2021;16(5):865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabirzhanov B, Matyas J, Coll-Miro M, Yu LL, Faden AI, Stoica BA, Wu J. Inhibition of microRNA-711 limits angiopoietin-1 and Akt changes, tissue damage, and motor dysfunction after contusive spinal cord injury in mice. Cell Death Dis. 2019;10(11):839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ban D, Yu P, Xiang Z, Liu Y. TNF-like weak inducer of apoptosis / nuclear factor κB axis feedback loop promotes spinal cord injury by inducing astrocyte activation. Bioengineered. 2022;13(5):11503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han W, Li Y, Cheng J, Zhang J, Chen D, Fang M, Xiang G, Wu Y, Zhang H, Xu K, Wang H, et al. Sitagliptin improves functional recovery via GLP-1R-induced anti-apoptosis and facilitation of axonal regeneration after spinal cord injury. J Cell Mol Med. 2020;24(15):8687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lan T, Wu Y, Zhang Y, Li S, Zhu Z, Wang L, Mao X, Li Y, Fan C, Wang W, Yu SY. Agomelatine rescues lipopolysaccharide-induced neural injury and depression-like behaviors via suppression of the Galphai-2-PKA-ASK1 signaling pathway. J Neuroinflammation. 2022;19(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee DH, Park JS, Lee YS, Han J, Lee DK, Kwon SW, Han DH, Lee YH, Bae SH. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy. 2020;16(11):1949–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Wei M, Fan J, Yan W, Zha X, Song H, Wan R, Yin Y, Wang W. Ischemia-induced upregulation of autophagy preludes dysfunctional lysosomal storage and associated synaptic impairments in neurons. Autophagy. 2021;17(6):1519–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu S, Ying Y, Ye L, Ying W, Ye J, Wu Q, Chen M, Zhu H, Li X, Dou H, Xu H, et al. Systemic administration of fibroblast growth factor 21 improves the recovery of spinal cord injury (SCI) in rats and attenuates SCI-induced autophagy. Front Pharmacol. 2020;11:628369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang D, Zhu D, Wang F, Zhu JC, Zhai X, Yuan Y, Li CX. Therapeutic effect of regulating autophagy in spinal cord injury: a network meta-analysis of direct and indirect comparisons. Neural Regen Res. 2020;15(6):1120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Lei Z, Ritzel RM, He J, Li H, Choi HMC, Lipinski MM, Wu J. Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice. Theranostics. 2022;12(12):5364–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiong Y, Xia Y, Deng J, Yan X, Ke J, Zhan J, Zhang Z, Wang Y. Direct peritoneal resuscitation with pyruvate protects the spinal cord and induces autophagy via regulating PHD2 in a rat model of spinal cord ischemia-reperfusion injury. Oxid Med Cell Longev. 2020;2020:4909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esnault S, Fichtinger PS, Barretto KT, Fogerty FJ, Bernau K, Mosher DF, Mathur SK, Sandbo N, Jarjour NN. Autophagy protects against eosinophil cytolysis and release of DNA. Cells. 2022;11(11):1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saade M, Araujo de, Souza G, Scavone C, Kinoshita PF. The role of GPNMB in inflammation. Front Immunol. 2021;12:674739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weng J, Li DD, Jiang BG, Yin XF. Temporal changes in the spinal cord transcriptome after peripheral nerve injury. Neural Regen Res. 2020;15(7):1360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao XQ, Liu ZY, Chen JY, Huang ZC, Liu JH, Sun BH, Zhu QA, Ding RT, Chen JT. Proteomics and bioinformatics reveal insights into neuroinflammation in the acute to subacute phases in rat models of spinal cord contusion injury. FASEB J. 2021;35(7):e21735. [DOI] [PubMed] [Google Scholar]
- 22. Nakano Y, Suzuki Y, Takagi T, Kitashoji A, Ono Y, Tsuruma K, Yoshimura S, Shimazawa M, Iwama T, Hara H. Glycoprotein nonmetastatic melanoma protein B (GPNMB) as a novel neuroprotective factor in cerebral ischemia-reperfusion injury. Neuroscience. 2014;277:123–31. [DOI] [PubMed] [Google Scholar]
- 23. Zhu Z, Liu Y, Li X, Zhang L, Liu H, Cui Y, Wang Y, Zhao D. GPNMB mitigates Alzheimer’s disease and enhances autophagy via suppressing the mTOR signal. Neurosci Lett. 2022;767:136300. [DOI] [PubMed] [Google Scholar]
- 24. He L, Chang Q, Zhang Y, Guan X, Ma Z, Chen X, Liu W, Li Y, Feng H. MiR-155-5p aggravated astrocyte activation and glial scarring in a spinal cord injury model by inhibiting Ndfip1 expression and PTEN nuclear translocation. Neurochem Res. 2023;48(6):1912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y, Wu L, Shi M, Zeng D, Hu R, Wu X, Han S, He K, Xu H, Shao X, Ma R. Electroacupuncture inhibits NLRP3 activation by regulating CMPK2 after spinal cord injury. Front Immunol. 2022;13:788556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Y, Li P, Ju C, Zuo X, Li X, Ding T, Liang Z, Zhang J, Li K, Wang X, Zhu Z, et al. Photobiomodulation attenuates neurotoxic polarization of macrophages by inhibiting the Notch1-HIF-1alpha/NF-kappaB signalling pathway in mice with spinal cord injury. Front Immunol. 2022;13:816952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xue MT, Sheng WJ, Song X, Shi YJ, Geng ZJ, Shen L, Wang R, Lu HZ, Hu JG. Atractylenolide III ameliorates spinal cord injury in rats by modulating microglial/macrophage polarization. CNS Neurosci Ther. 2022;28(7):1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gu Y, Chen D, Zhou L, Zhao X, Lin J, Lin B, Lin T, Chen Z, Chen Z, Wang Z, Liu W. Lysine-specific demethylase 1 inhibition enhances autophagy and attenuates early-stage post-spinal cord injury apoptosis. Cell Death Discov. 2021;7(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee H, Jiang X, Perwaiz I, Yu P, Wang J, Wang Y, Huttemann M, Felder RA, Sibley DR, Polster BM, Rozyyev S, et al. Dopamine D(5) receptor-mediated decreases in mitochondrial reactive oxygen species production are cAMP and autophagy dependent. Hypertens Res. 2021;44(6):628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mao Y, Du J, Chen X, Al Mamun A, Cao L, Yang Y, Mubwandarikwa J, Zaeem M, Zhang W, Chen Y, Dai Y, et al. Maltol promotes mitophagy and inhibits oxidative stress via the Nrf2/PINK1/Parkin pathway after spinal cord injury. Oxid Med Cell Longev. 2022;2022:1337630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi Y, Liu Y, Zhang B, Li X, Lin J, Yang C. Human menstrual blood-derived endometrial stem cells promote functional recovery by improving the inflammatory microenvironment in a mouse spinal cord injury model. Cell Transplant. 2023;32:9636897231154579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang ML, Kang YM, Li XG, Su Q, Li HB, Liu KL, Fu LY, Saahene RO, Li Y, Tan H, Yu X-J. Central blockade of NLRP3 reduces blood pressure via regulating inflammation microenvironment and neurohormonal excitation in salt-induced prehypertensive rats. J Neuroinflammation. 2018;15(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu R, Yu RJ, Yang C, Wang Q, Xuan Y, Wang Z, He Z, Xu Y, Kou L, Zhao YZ, Yao Q, et al. Evaluation of the hepatoprotective effect of naringenin loaded nanoparticles against acetaminophen overdose toxicity. Drug Deliv. 2022;29(1):3256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yin TC, Shao PL, Chen KH, Lin KC, Chiang JY, Sung PH, Wu SC, Li YC, Yip HK, Lee MS. Synergic effect of combined therapy of hyperbaric oxygen and adipose-derived mesenchymal stem cells on improving locomotor recovery after acute traumatic spinal cord injury in rat mainly through downregulating inflammatory and cell-stress signalings. Cell Transplant. 2022;31:9636897221133821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Z, Yao X, Sun B, Jiang W, Liao C, Dai X, Chen Y, Chen J, Ding R. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-kappaB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic Biol Med. 2021;168:142–54. [DOI] [PubMed] [Google Scholar]
- 36. Cai L, Gao L, Zhang G, Zeng H, Wu X, Tan X, Qian C, Chen G. DJ-1 alleviates neuroinflammation and the related blood-spinal cord barrier destruction by suppressing NLRP3 inflammasome activation via SOCS1/Rac1/ROS pathway in a rat model of traumatic spinal cord injury. J Clin Med. 2022;11(13):3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kanno H, Ozawa H, Sekiguchi A, Itoi E. The role of autophagy in spinal cord injury. Autophagy. 2009;5(3):390–2. [DOI] [PubMed] [Google Scholar]
- 38. Wu C, Chen H, Zhuang R, Zhang H, Wang Y, Hu X, Xu Y, Li J, Li Y, Wang X, Xu H, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 2021;17(4):1138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Q, Liu M, Nong H, Zhang Y, Bai Y, Liu P, Zong S, Zeng G. Total flavonoids of hawthorn leaves protect spinal motor neurons via promotion of autophagy after spinal cord injury. Front Pharmacol. 2022;13:925568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li B, Castano AP, Hudson TE, Nowlin BT, Lin SL, Bonventre JV, Swanson KD, Duffield JS. The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair. FASEB J. 2010;24(12):4767–81. [DOI] [PMC free article] [PubMed] [Google Scholar]