Abstract
Knowledge of female genital anatomy and physiology is often inadequate or incorrect among women. Precise patient–physician conversations can be inhibited by a reluctance or inability to speak accurately about the vulva and vagina, with the terms often being used interchangeably. There is a paucity of scientific evidence and clinical guidelines to support women and physicians in ensuring best practices in feminine hygiene. In this review, the unmet needs in the field are highlighted. Evidence is provided for the complex array of physiological and pathological systems, mechanisms and behaviours that either protect or, if inappropriate, predispose the vulva and vagina to infections, irritation or other conditions. The need for attention to perineal health is recommended, given the interdependence of perineal and vulvar microbiota and the risk of colonic pathogens reaching the vulva and the vagina. Differences in feminine hygiene practices can vary widely across the world and among varying age groups, and suboptimal habits (such as vaginal douching or the use of certain cleansers) can be associated with increased risks of vulvar and vaginal conditions. Critical areas for discussion when advising women on their intimate health include: advice surrounding aesthetic vulvar cosmetic trends (such as depilation and genital cosmetic surgery), bowel health and habits, and protection against sexually transmitted infections. Routine, once-daily (maximum twice-daily) washing of the vulva with a pH-balanced, mild cleanser is optimal, ideally soon after bowel voiding, when feasible. Due to the finely balanced ecosystems of the vulva, the vagina and the perineal area, a scientific and clinical perspective is essential when determining the most appropriate vulvar cleansers based on their components. Correct intimate care may contribute to improved genital and sexual health and overall well-being. An increased awareness of correct practices will empower women to be the advocates of their own intimate health.
Keywords: aesthetic gynaecology, female intimate hygiene, feminine cleansers, feminine hygiene guidelines, microbiota, STI protection, vulvar health
Introduction
Feminine hygiene is a sensitive and delicate issue, often overlooked in clinical conversations between women and their healthcare providers. Using the correct terminology when discussing female genital anatomy and health is of paramount importance, both among healthcare providers and in conversations with patients. 1 Indeed, the vulva and vagina are distinct organs with different characteristics, yet the terms are often used interchangeably. 2 This is incorrect and may lead to ineffective or ambiguous communication among healthcare providers and patients of all generations.1,2
From a practical point of view, there is a variety of feminine hygiene products available, and many women may use these as part of their daily cleansing routine.3,4 However, there is a paucity of studies and guidelines related to feminine care practices. 4 Therefore, this review focuses on the neglected topic of feminine hygiene. The first aim of this review is to examine how to improve clinical conversations with patients, with the hope of translating this into improved genital health and intimate well-being for women. The second aim is to summarize the available evidence on the most appropriate feminine hygiene behaviours.
The persisting language bias
Historically, scientific texts have referred mainly to the vagina when discussing female intimate health, rarely mentioning the vulva.5,6 More recently, the term ‘vulvovaginal’ has been adopted in scientific writing. The common use of ‘vulvovaginal’ to describe this whole region can perpetuate the confusion around the difference between the vulva and the vagina. Referring to the ‘vulva’ and the ‘vagina’ as two distinct anatomical structures is preferable as it represents a more accurate use of language and, more importantly, a recognition of the substantial anatomic, physiological and clinical differences between the two organs. Lack of appreciation of these differences, a reluctance to speak accurately and anatomically about genitalia, and a reliance on coy, euphemistic or derogatory slang can negatively impact communication between women and their healthcare providers, 2 and make it harder to precisely determine the site and cause of any gynaecological issues or complaints in these areas.
Specifically, the vagina forms part of the internal female genitalia, while the vulva describes the anatomical structures comprising the outer female genitalia (Figure 1, Table S1).1,7,8 The pelvic floor muscles are located underneath the vulva, perineal and perianal area, forming a hammock shape, which extends back from the pubic symphysis and pubic bone to the sacrococcygeal bone structures, hanging laterally to the ischial bones.9,10 The levator ani is composed of three key muscles: the puborectal, iliococcygeal and pubovisceral (pubococcygeus) muscles. 9 They support the pelvic organs (bladder, urethra, vagina and uterus, anus and rectum), and provide general support of the intra-abdominal contents.11,12 They contribute to continence of urine and faeces, and to the sexual functions of arousal, lubrication and orgasm.9,11 –13 The pelvic floor also contributes to the dynamics of vaginal delivery.9,14 Posterior to the vulva, the perineal skin and microbiota are underappreciated modulators of genital health.15 –23
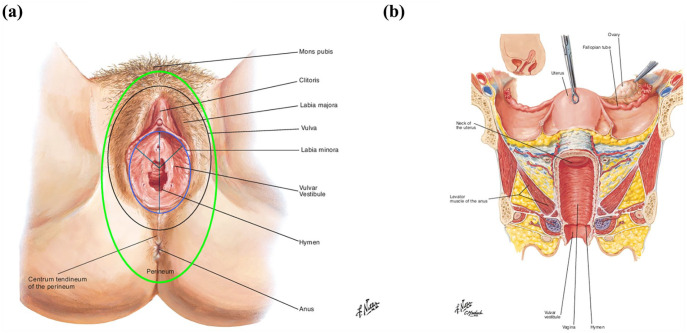
Figure 1.
(a) External genitalia: the vulva and the perineum. (b) Vertical plane of the internal and external female genitalia.
Adapted with permission of Elsevier. All rights reserved. www.netterimages.com.
The vulva includes part of the mons pubis, labia majora, labia minora, clitoris, vulvar vestibule, opening of the urethra and opening of the vagina (as indicated, the area within the black oval).
The vulvar vestibule includes the area between the lower part of the clitoris, the border of the small labia including the inner part of the small labia, remnants of the hymen and the small area around the urethra opening (as indicated, the area within the light blue oval).
The hymen includes the embryonic remnants separating the vulvar vestibule from the entrance of the vagina.
The perineum is a diamond-shaped region that includes the skin overlying the external genitalia, the urethral, vaginal, and anal orifices and the skin between the thighs (as indicated, the area within the light green oval).
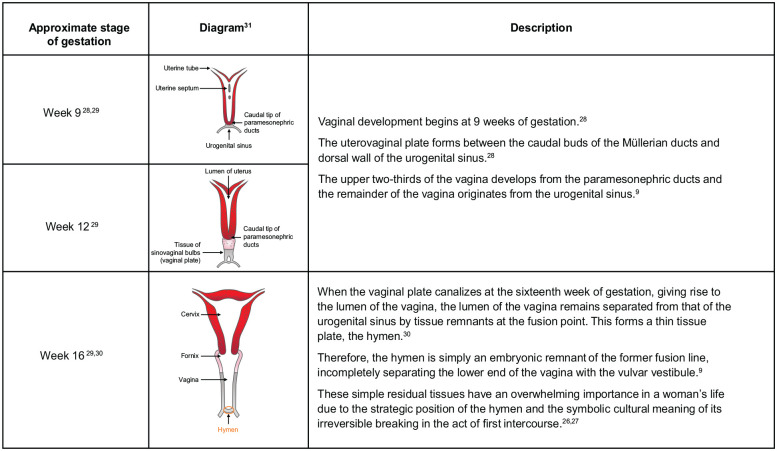
Notably, the vulva and vagina have also distinct embryological origins (Figure 2): the vulva develops from the urogenital sinus, the genital tubercle or phallus, the urogenital folds and the labioscrotal swellings. 24 The lower vagina (vulvar vestibule), external to the hymen, shares the same embryologic origins as the vulva. The upper two-thirds of the vaginal canal, above the hymen, develops from the Müllerian ducts.9,25 The hymen is an embryonic remnant of the former fusion line between the Müllerian ducts and the urogenital sinus. 9 Because of their strategic location, these simple residual tissues have an overwhelming cultural and sexual importance in a woman’s life.26,27
Figure 2.
The embryonic origins and importance of the hymen.9,26 –31
Diagram reproduced from: Kaur and Panneerselvam. Bicornuate Uterus. In: StatPearls [Internet]. Treasure Island (FL): StatPearls 31 under the terms of the Creative Commons Attribution 4.0 International Licence (http://creativecommons.org/licences/by/4.0/).
However, it is insufficient to consider only the vulva and the vagina: perineal health also plays an important part in intimate health. This is because the perineal area is vulnerable to contamination by microbes of faecal origin, especially following bowel voiding, given its close anatomic proximity. 22 Therefore, these three separate areas – the vulva, vagina and perineum – must be considered as distinct but synergistically acting players in the maintenance of intimate health.
Any professional recommendations for feminine hygiene should include guidance on behaviours encompassing all of these areas.
Key unmet needs in intimate care and hygiene practices
Intimate feminine hygiene practices can contribute to a woman’s overall well-being and can prevent or relieve many gynaecological issues.3,4 Notably, the vulva plays a key role in a woman’s sexuality (Table 1); a healthy vulva can contribute to sexual health.
Table 1.
The role of the vulva in sexuality.
| The vulva is rich with sensitive fibres 26 |
| The clitoris, along with the associated corpora cavernosa, 32 is the most innervated part of the vulva 9 and the main organ devoted to female genital arousal, pleasure, and orgasm 33 |
| The vestibular bulbs (counterpart of the corpora cavernosa of the penis) are thought to work closely with the corpora cavernosa and the clitoral tissues and nerves. During sexual arousal, the vestibular bulbs fill with blood. The resulting congestion exerts pressure on the corpus cavernosum of the clitoris and the glans of the clitoris. This pressure on the clitoral nerves and tissues is thought to induce a pleasant sensation during sexual arousal
34
Following menopause, the width of the vestibular bulbs reduces. 35 The corpora cavernosa undergo a progressive, age-dependent reduction from age 20 onwards. 36 In parallel, an age-related reduction of sexual hormones (including testosterone and DHEA) is observed,9,37 with DHEA values in people in their eighties and nineties sometimes as low as 10% to 20% of the original values encountered in young individuals38,39 |
| The vulvar vestibule and surrounding tissues become very congested during physiological sexual arousal, contributing to genital congestion and the formation of the so-called ‘orgasmic platform’ 9 |
| The controversial G-spot, now considered a component of the CUV complex, is hypothesized to mediate and contribute to the vaginally-activated orgasm, the climax obtained during vaginal penetration9,32,33,40 |
| The labia majora and minora engorge with blood and appear oedematous during sexual arousal 7 |
| Bartholin’s glands secrete a mucus-like substance to lubricate the internal part of the labia minora and the vestibular region during sexual intercourse; this is essential for preventing painful coitus and making penetration enjoyable7,9 |
| Skene’s glands release urethral secretions during sexual arousal 9 |
| The mons pubis provides cushioning during sexual intercourse and secretes pheromones to induce sexual attraction. 7 Resident physiological microbiota activate pheromonal substances produced by vulvar apocrine glands, 41 and pubic hair facilitates evaporation of pheromones 42 |
| Beneath the visible vulva, the muscular tissues (the three superficial trigonus muscles and the powerful levator ani) are key partners of pleasure (in addition to their roles in pelvic organ support and voluntary urinary and faecal continence) as they contract during the motor component of the orgasmic reflex. 9 A healthy and tonic pelvic floor is significantly associated with better arousal and orgasm. Hypoactivity of the muscles (low tone), relatively common after vaginal delivery, can lead to poor sexual function and lack of pleasure during coitus and orgasm, while hyperactivity (high tone) may be pathophysiologically linked to sexual pain disorders such as dyspareunia and vaginismus 9 |
| Adjacent to the vulva, effective maintenance of perineal hygiene could reduce the risk of UTIs and Candida albicans infection (thrush), as well as maintain the physiological microbiome,19 –21,23,43 all of which contribute to a healthy vulvar and vaginal microenvironment, an underappreciated contributor to pleasurable intercourse |
CUV: clitourethrovaginal; DHEA: dehydroepiandrosterone; UTI: urinary tract infection.
Feminine hygiene practices can vary widely across the world, and may be related to differences in cultural and social beliefs and religious practices. 3 Lifestyle factors, such as wearing tight-fitting clothing and underwear, pantyhose and panty liners, may affect the temperature, moisture levels or bacterial composition of the vulvar and vestibular area, or may even cause direct mechanical irritation, all of which could contribute to feminine itching, vulvodynia, Candidiasis, or other problems in the urogenital area.44–49 New or multiple sexual partners and unprotected sex also pose challenges to genital health such as increased risk of sexually transmitted infections (STIs) and bacterial vaginosis,50,51 and vaginal douching or the use of certain cleansers may be associated with increased risks of vulvar and vaginal diseases and infections. 3 However, only a handful of organizations have developed guidelines for vulvar and vaginal hygiene practices.52,53
In the ‘Break the Taboo’ survey conducted among 10,000 women across 10 countries, wide disparities regarding daily routines and perceptions were observed. 4 The proportion of women who engaged in daily intimate care practices varied widely from country to country (from 38% to 91%) and was highest in Italy, the Philippines, Portugal, Russia and Thailand. Intimate self-care was associated with many benefits for women’s physical and mental health, including the perception of being in good health (25% to 78%) and feeling attractive (19% to 47%).
In six out of 10 countries (the United Kingdom, Germany, France, Russia, Thailand and the Philippines), the practice of intimate self-care increased with age. 4 Younger generations were less likely to be comfortable discussing female genitalia compared with older women, 4 while another study reported that older women were more likely to use feminine deodorant spray and wet wipes. 54 This may seem dichotomous with the higher proportions of younger women having aesthetic procedures, for example vulvoplasty, 55 suggesting that younger women are more comfortable discussing intimate aesthetic procedures but not intimate health. However, this might stem from low genital self-image and dissatisfaction with female genitalia, often encountered by young women. 56 In addition, societal beauty ideals among adolescents, which are increasingly modulated by photograph-based social media platforms, can also affect genital self-image and desire for intimate aesthetic procedures. 57 In a cross-sectional study investigating genital self-image, 3.6% of women had severely low self-image, with 13.7% of women considering undergoing cosmetic genital surgery. 56 Moreover, better genital self-image was associated with a smaller protrusion of the labia minora. 56 Healthcare providers should educate their patients on the acceptance of normal genital diversity, to reduce body dissatisfaction and the fashion-driven request for intimate aesthetic procedures.
Speculatively, older women and postpartum women may be more concerned with feminine hygiene than younger women.54,58 Postpartum women have specific hygiene needs (Table S2) due to the many anatomical and functional changes associated with vaginal delivery. 58 Moreover, 20% of postpartum women with no obstetric antecedents complain of urinary urge incontinence, 24% report urinary stress incontinence and 14% report anal incontinence; obstetrics risk factors such as episiotomy and forceps delivery can further increase incontinence rates. 59 Incontinence is not only associated with hygienic issues but also psychosocial problems and reduced quality of life (QoL). 60 Incontinence can cause embarrassment, especially for women, because it can interfere with self-image and consequently self-esteem and sense of attractiveness.60,61 Meanwhile, 60% of menopausal women experience vaginal discomfort and 87% experience sexual dysfunction, with a significant negative impact on QoL. 62 Following menopause, various genital symptoms, sexual and urinary symptoms and signs, termed as ‘genitourinary syndrome of menopause’ (GSM), can have a profound impact on the QoL of post-menopausal women.63,64 These symptoms may cause women to seek further information or products to maintain their intimate health. Indeed, a cross-sectional study of postmenopausal women seeking routine gynaecological care reported that over half of women surveyed had used an over-the-counter product for vulvar and/or vaginal symptoms in the prior 3 months (including barrier treatments, topical anaesthetics, powders and antifungals), and one-third of women had used two or more products. 65 Non-hormonal therapy such as lubricants, moisturizers and vitamin E are currently used as first-line treatments for GSM; however, these do not address the underlying pathophysiology and related issues.64,66 In more severe cases, local hormonal therapy with prasterone, oestrogen and topical testosterone is considered.64,67 More specifically, local oestrogen products are considered the ‘gold standard’.64,68 Novel treatment modalities, including laser technologies, can also be used as alternative options, but further research is required to determine their viability before they are implemented as routine treatment.64,69 Healthcare providers should play a proactive role in the early diagnosis and treatment of GSM symptoms, with patient-tailored interventions.
The ‘Break the Taboo’ study highlights the vast differences across the world in intimate care regimes and perceptions. Most importantly, fewer than 50% of women in the survey reported being instructed or taught to practice intimate hygiene by their healthcare providers. 4 This highlights an unmet need and represents a requirement to educate healthcare providers to initiate discussions with patients on the benefits and importance of practicing intimate self-care routines.
The VuNet epidemiological study in female patients with chronic vulvar pain (vulvodynia) also highlighted a large unmet need in female intimate care. 70 Almost half of the women in the study (48%) had prolonged pain lasting 1 to 5 years before obtaining an accurate diagnosis. The authors proposed two reasons for this diagnostic delay: first, women may be unaware that their pain may have a name and a treatable cause; and second, healthcare providers may have difficulty diagnosing vulvodynia, as many are unfamiliar with the presentation of this condition. Thus, there is a need for greater awareness of common vulvar and vaginal conditions among both healthcare providers and patients. Common symptoms of vulvar and vaginal conditions and tools for diagnosis are presented in Tables S3A and S3B.
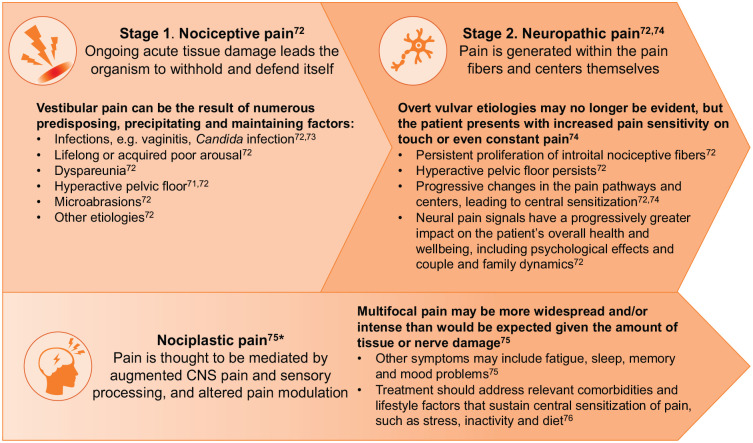
Of particular note is the presentation of vulvar vestibular pain, or vestibulodynia which, because of its complex aetiology and lack of characteristic histology, may erroneously lead to the pain being attributed to a psychosomatic disorder. 71 The complexity of vestibular pain pathogenesis is presented in Figure 3.
Figure 3.
The narrative of vestibular/vulvar pain pathogenesis.71–76
*Can occur in isolation or in combination with Stages 1 and 2. 76
The dynamic protection of the vulva and vagina
To understand the pathophysiology of common vulvar and vaginal conditions and how to prevent them, it is necessary to recognize the physiological mechanisms that protect the genitalia against infection.
‘What is essential is invisible to the eye’. In vulvar, vaginal and perineal health, this Antoine de Saint-Exupery quote is incredibly apt: in other words, by relying on only visible characteristics, we can be deceived and miss something’s true nature. A prime example of this is the vulva and vagina. The vulva is not only covered by skin, but by a complex array of structured dynamic systems that partially protect against infection and dryness. They include the complex vulvar ‘shield’ comprising the sophisticated vulvar microbiota, pubic hair and keratinized epithelial cells (Table 2). During the fertile period, the vagina, aside from the mucosa, is protected by its own unique microbiome (composed of lactobacillus species, among others), 3 an acidic pH, mucus and the immune system (Table 3) while the perineal area has its own unique skin composition and microbial considerations (Table 4).
Table 2.
Physiological barriers protecting the vulva.
| Vulvar protective barrier/characteristic | Description | Function |
|---|---|---|
| Microbiota | Species may include staphylococci, micrococci, diphtheroids, lactobacilli, streptococci, Gram-negative rods and yeasts (non-protective species of faecal origin may also be present) 3 | May reduce the proliferation of exogenous pathogens that cause vaginal and urinary tract infections 3 |
| pH | Vulvar pH could be expected to fall between the pH of the skin (pH 4.7) 3 and the vagina (normal pH 4.0–4.5)3,77 | Protects against sexually transmitted pathogens 78 |
| Pubic hair | Body hair found in the genital area 79 | Contributes to the microenvironment favouring evaporation of pheromones. 42 Moderately protects the genitalia from foreign bodies, bacteria and viruses, and protects skin from friction during intercourse that can lead to dryness, irritation and abrasions79,80 |
| Skin (epithelium) | Vulvar skin: differs from other skin sites in hydration, friction and permeability and is more susceptible to topical agents than forearm skin Vulvar vestibular skin: non-keratinized and therefore likely to be more permeable, and vulnerable to mechanical traumas (e.g. due to friction) than keratinized skin3,81 |
Provides a physical/mechanical biobarrier 82 |
| Epithelial cells | The cutaneous epithelium of the mons pubis and labia majora exhibits a keratinized, stratified, squamous structure with sweat glands, sebaceous glands and hair follicles. The degree of keratinisation decreases over the clitoris and the outer surface and inner two-thirds of the labia minora. The epidermis of this portion of the labia minora is markedly thinner than that of the labia majora and there are no hair follicles or sweat glands in women of reproductive age 81 | Provides a physical barrier 82 and acts as an immunocompetent tissue: Langerhans’ cells are the most common immune cell type in the vulva 81 |
Table 3.
Physiological barriers protecting the vagina.
| Vaginal protective barrier/characteristic | Description | Function |
|---|---|---|
| Microbiota | It was previously thought that a healthy vagina was dominated by Lactobacillus spp.; however, in some healthy women, lactobacilli are absent and replaced by other lactic acid-producing bacteria, such as Atopobium vaginae, Megasphaera spp., and/or Leptotrichia spp.
3
The ability of lactobacilli to grow in biofilms has been demonstrated in vitro, suggesting that there may be a role for a physiological lactobacilli biofilm in the vagina83,84 |
Lactobacilli produce lactic acid, maintaining an acidic environment, and compete with exogenous pathogens to adhere to vaginal mucosa.3,78,85 Lactobacilli also produce H2O2 and bacteriocins, which suppress invasive bacteria3,85,86 |
| pH | Vaginal pH in women of reproductive age is ⩽4.5; this is mediated by the high presence of lactobacilli.3,87 Vaginal pH rises during menstruation 88 | Maintains vaginal eubiosis, 78 protects against sexually transmitted pathogens78,87 and inhibits the growth of anaerobic bacteria 86 |
| Skin (epithelium) | Vaginal skin is covered with a non-keratinized epithelial lining which, with oestrogen presence, is thick, with folds kept moist by fluid secreted through the vaginal wall and mucus from cervical and vestibular glands 3 | Provides a physical/mechanical biobarrier 82 |
| Epithelial cells | The lower part of the female reproductive tract is lined with multiple layers of stratified squamous epithelial cells. A lack of tight junctions in the lower part of the female reproductive tract may permit transition of pathogens to the intra-epithelial region, allowing them to come into contact with immune cells 82 | Provide a physical barrier against pathogens and microbial infections as well as secretions containing antimicrobial peptides, cytokines and chemokines which recruit and activate immune cells 82 |
| Vaginal secretions/mucus | Composed primarily of 90% to 95% water, inorganic and organic salts, urea, carbohydrates, mucins (particularly mucin-1), fatty acids, albumins, immunoglobulins, iron chelator, lysozyme and other macromolecules, leukocyte and epithelial debris82,85 | Prevents epithelial cells from direct contact with infectious agents 82 |
| Immune system | ||
| NAPs | Includes defensin, elafin, cathelicidin, secretory leukocyte protease inhibitor, lysozyme, and lactoferrin; produced by epithelial cells and neutrophils 82 | Destroy target microbial cells through abrogation of pH and ionic concentration gradients 82 |
| Pattern recognition receptors (TLRs) | Expressed on immune cells, including neutrophils, macrophages, dendritic cells, dermal endothelial cells and mucosal epithelial cells 82 | Detect microbial-associated molecular patterns and initiate intracellular signalling pathways in order to recruit the immune cells, secrete antimicrobial factors eradicating pathogens and facilitate adaptive immune responses 82 |
| Immune response | Includes cytokines and chemokines (secreted from epithelial cells) and inflammatory immune cells (macrophages, dendritic cells, natural killer cells, neutrophils), as well as resident epithelial cells and stromal fibroblasts 82 | Inflammatory immune cells that migrate into the genital tract, as well as resident epithelial cells and stromal fibroblasts, facilitating the immune response 82 |
NAP: natural antimicrobial peptide; spp.: species; TLR: toll-like receptor.
Table 4.
Physiological barriers protecting the perineal area.
| Perineal protective barrier/characteristic | Description | Function |
|---|---|---|
| Skin | The cutaneous perineum is a diamond-shaped region that includes the skin overlying the external genitalia, the urethral, vaginal, and anal orifices and the skin between the thighs. 89 The perineum skin, like the vulvar skin, has a keratinized, stratified squamous structure with sweat glands, sebaceous glands and hair follicles 3 | Provides a physical/mechanical biobarrier 82 |
| Microbiota | The normal microbiota of the intestinal tract is closely associated with human health. 16 However, given the proximity to the anal opening, facultatively pathogenic microorganisms (e.g. Escherichia coli, enterococci, Candida albicans) can frequently infiltrate the perineum. 22 E. coli may also cross the colonic mucosa (especially in women with IBS or constipation, which can be associated with ‘leaky gut syndrome’) and enter the blood stream and subsequently the bladder mucosa 23 | Imbalanced intestinal tract microbiota can cause a series of diseases, such as certain digestive system diseases, metabolic diseases, inflammatory bowel disease, IBS and autoimmune diseases. 16 Imbalanced perineal/perianal microbiota can cause skin diseases (e.g. eczema), 16 and vulvar and vaginal conditions such as vulvovaginitis and UTIs. 15 Comorbidity of lower urinary tract disorders and a range of colonic disorders has also been reported (e.g. IBS, inflammatory bowel disease, constipation overactive bladder, interstitial cystitis/painful bladder syndrome and faecal and urinary incontinence)90,91 |
IBS: irritable bowel syndrome; UTI: urinary tract infection.
The vaginal microbiota comprises microorganisms in a state of dynamic equilibrium, 3 modulated by hormonal and health status,3,92,93 and endogenous and exogenous factors (including diet).3,94 The predominant bacterial species comprising the healthy vaginal microbiota are lactobacilli (notably Lactobacillus crispatus, Lactobacillus iners, Lactobacillus gasseri and Lactobacillus jensenii).92,93 Lactobacilli produce lactic acid, maintaining an acidic environment, which inhibits the growth of pathogenic bacteria.3,92 When healthy, the microbiota acts as a dynamic barrier to protect against pathogenic invasion: lactobacilli bind to the surface of vaginal epithelial cells and compete with other microorganisms to prevent them from attaching to, and infecting, these cells.3,78,85 They also release soluble components that inhibit other bacteria from associating with the epithelial cell membrane.3,92 The vaginal microbiota fluctuates over a lifetime, during menarche, menses and pregnancy, in response to infection and in response to various external factors (e.g. hygiene practices, sexual intercourse, antibiotics, spermicides, hormonal contraceptives and hormone replacement therapy).3,92,93,95
The vulvar microbiome differs from the vaginal microbiome. Knowledge about the microbial composition of the vulva is in its infancy; however, it is anticipated to play a key role in overall genital health. 3 There is wide diversity in intra- and inter-individual vulvar microbiomes, with no single species common to all women.3,96 Similar to the vaginal microbiome, the vulvar microbiome may also affect the proliferation of exogenous pathogens that cause vaginal and urinary tract infections. 3
The vaginal and gut microbiota exist in a regulated, mutualistic relationship and influence the host female’s genital health. During the fertile period, both the vaginal and gut microbiota are modulated by sex hormones and can present a significant dysbiosis, a progressive disruption of microbiota equilibrium, after the menopause. 93 Moreover, the combined effects of diet and stress have been shown to impact the gut microbiota, resulting in dysbiosis. 97 Both acute and chronic stressors causing cortisol release can have profound effects on the gut microbiota, such as producing an inflammatory response, increasing the prevalence of pathogenic bacteria (dysbiosis) and enhancing gut barrier permeability, also referred to as ‘leaky gut syndrome’. 97 Subsequent movement of these pathogenic bacteria to the vagina can cause unfavourable gynaecological outcomes. 93 Moreover, psychological stress has been found to suppress cell-mediated immune responses that are important in inhibiting the growth of Candida albicans. 98 Stress and depression can also encourage unhealthy food choices, which indirectly facilitate the shift in bacterial species based on the micronutrient profile of the food consumed. 97 A high-saturated-fat diet, for example, can alter the vaginal microbiota by decreasing lactobacilli species dominance, which raises the risk of bacterial vaginosis. 99 In addition, Candida species growth is enhanced by sugar-rich diets, especially in patients with diabetes mellitus, increasing the risk of vulvovaginitis and cystitis.100,101
The microbiota can secrete an extracellular matrix, forming a ‘biofilm’: biofilms are structured communities of bacterial cells, often of different species, enclosed in a self-produced polymer matrix and adhered to an inert or living surface.83,102 Biofilms are often characterized by a complex internal architecture and can contain channels to allow the circulation of nutrients. 102 The ability of lactobacilli to grow in biofilms has been demonstrated in vitro, suggesting that there may also be a role for a physiological lactobacilli biofilm in the vagina.83,84 Lactobacilli can also counteract and inhibit various virulence factors that favour pathogens, including the production of toxins, biofilm formation, and host cell adhesion and invasion. 103
Many pathogenic bacteria can also form biofilms, which promote their survival and can limit the efficacy of antimicrobial or antibiotic treatment.104–108 Polymicrobial biofilms formed on vaginal epithelia play a crucial role in the pathogenesis of bacterial vaginosis. 102 The involvement of biofilm in a bacterial infection (e.g. in bacterial vaginosis) implies that the infection is difficult to treat and that the patient may experience relapses, as biofilms are less vulnerable to antimicrobial agents. 102 Biofilms are responsible for up to 80% of all human bacterial infections83,109 and are also produced by many clinically relevant fungi (such as Candida, Aspergillus and Cryptococcus).110,111
A range of endogenous and exogenous factors interact with the vaginal microbiota. Endogenous factors include oestrogen,3,112 cervical mucus, 113 pH3,78 and influences from commensal microbiota (such as those of intestinal origin, including Escherichia coli (E. coli)). 22 The vaginal and intestinal microbiota have an intense interplay in physiological and pathological conditions: irritable bowel syndrome (IBS) and constipation (associated with ‘leaky gut syndrome’) are intestinal conditions that may impact vaginal and vulvar microbiota.23,93,114 Indeed, a cross-sectional study of 1183 women with chronic vulvar pain found that IBS was the a common comorbidity present in 27.3% of women with chronic vulvar pain. 70 Exogenous factors include infection with invasive bacteria, 108 semen, 115 number of partners, 50 intrauterine devices, 116 antibiotics, 117 certain cleansing products or practices118,119 and diet. 94
An acidic vaginal pH (<4.5) maintains vaginal eubiosis, 78 protects against sexually transmitted pathogens,78,87 and inhibits the growth of anaerobic bacteria. 86 Vaginal pH fluctuates over a woman’s lifetime: for example, following menopause, as oestrogen levels fall, vaginal pH increases. 3 An increased vaginal pH, for example after menopause, is associated with increased infection rates and colonization with intestinal pathogenic microbes. 3 Conversely, vulvar pH could be expected to fall between the pH of the skin (estimated at pH 4.7) 3 and the vagina (normal pH 4.0–4.5), 77 with reports ranging from 3.8 to 4.2 across the menstrual cycle. 3 Vaginal pH can reach up to 5.2–6.7 during menstruation. 120
Therefore, the vagina and vulva are protected from infection by several mechanisms. However, many modern-day habits, including aesthetic practices and hygienic behaviours, can impair these protective functions on multiple levels.
The impact of aesthetic practices on intimate health
‘Beautifying’ practices for female genitalia are currently fashionable and are therefore on the increase. Yet these practices can be detrimental to a woman’s intimate health, and women should be cautioned not to mistake fashion for health before considering any ‘beautifying’ vulvar practices. Removal of pubic hair (pubic depilation) has become increasingly prevalent in recent years, with women citing a range of drivers such as increased satisfaction in their appearance, improved comfort and a perceived increase in cleanliness. 79 Perceived preference of their partner may also be one of the reasons women choose to remove pubic hair: in a demographic logistic regression analysis, men were more likely than women to report a preference for a pubic hair-free partner (60% vs 24%, respectively). 121 Furthermore, in college-age women (aged 18–24 years), those who were hair-free were more likely to have a history of vaginal intercourse, and receiving and giving oral sex, compared with those with some pubic hair (p < 0.01). 122 Although there is anecdotal evidence to suggest pubic depilation can improve sexual satisfaction for both partners, there have been no studies to quantify this effect. 123
Depilation or epilation (removing hair at the root, for example by waxing) can result in injury or irritation (lacerations, razor burn, folliculitis, microtears and epidermal abrasions). 79 Pubic hair also protects the skin covering the mons pubis, vulva and vagina during intercourse and therefore depilation can lead to dryness, irritation and abrasions. 79 Complete depilation is associated with an increased risk of vulvodynia, folliculitis and dermatitis,46,124 while preliminary data from a case-control study suggest that shaving and complete depilation of the labia majora may be associated with an increased risk of vulvar dysplasia or cancer, 125 although larger prospective controlled trials are required to confirm this finding. Women who shave the mons pubis area are 74% more likely to experience vulvodynia compared with those who shave only the bikini area. 46
Pubic hair removal also increases the risk of infection: pubic hair protects the vulva and vagina from irritants and pathogens. The act of waxing may also disturb the microcutaneous barrier, which is thought to increase the risk of pathogens penetrating the skin, including human papillomavirus and herpes simplex viruses, causing STIs. 79
Women who change their pubic hair status may alter the composition of their vulvar and vaginal microbiota: women in a ‘crossover’ group who switched from regular hair removal to no hair removal and vice versa had greater vaginal beta diversity (i.e. greater differences in microbial composition between samples) compared with controls who did not change their pubic hair status (p = 0.004). 126 Furthermore, pubic hair may play a role in sexual function by effectively facilitating the evaporation of pheromones, which are secreted from genital glands and activated by the vulvar microbiota (Table 5).
Table 5.
Sex matters: the importance of vulvar pheromones.
| • Like many other animals, humans use olfactory signals (via pheromones) for the transmission of biologically relevant information between members of the same species 41 |
| • Body odour can vary highly between individuals and is influenced by the MHC. In this regard, body odour is thought to influence reproductive competition, allowing individuals to select the most appropriate mate from an immune system point of view
127
• The main producers of human pheromones are the apocrine glands, which are located in the pubic region and axillae 41 |
| • At sexual maturation, the apocrine glands produce steroidal secretions derived from 16-androstenes via testosterone 41 |
| • Apocrine secretions are initially odourless; however, aerobic coryneform bacteria on the skin transform them into odorous pheromones, androstenone and androstenol
41
• In the vagina, aliphatic acids (also known as copulins) are secreted; their odour varies with the menstrual cycle 41 |
| • Human sociosexual interactions – driven by emotions such as fear, anxiety and sexual attraction – are influenced by pheromones, even if they are not consciously detected: there is increasing evidence to support this 128 |
| • One study, in which male volunteers were asked to record the occurrence of sociosexual behaviours before and after daily application of a male pheromone or placebo, showed a significantly higher proportion of participants using pheromones reporting an increase from baseline in ‘sexual intercourse’ and ‘sleeping next to a romantic partner’ compared with those using a placebo 42 |
| • Therefore, the overall impact of the modern-day goals of cleanliness and odourlessness on our everyday social lives and human reproductive success, as well as on anatomic/reproductive health, must be considered41,127 |
MHC: major histocompatibility complex.
‘Aesthetic gynaecology’ has dramatically increased in recent years,129,130 with over a third of procedures attributed to purely aesthetic motivations. 129 Vaginal rejuvenation is used to describe different surgical interventions aimed at decreasing the average diameter of the vagina (i.e. after vaginal delivery), either through excisions or laser technology, with novel methods (use of silicone threads or hyaluronic acid filler injections) under investigation. 131
Labiaplasty is the most common genital surgery performed for aesthetic reasons, with the goal of improving the appearance of the vulva and removing tissues that are obstructive during sexual intercourse. 130 Nearly all women who request labiaplasty have labia minora within the range of diversity expected. 132 Vulvar lightening procedures, to address perceived areas of hyperpigmentation using chemical agents or non-surgical laser techniques, are also commonly offered.130,133
There are concerns that removal of the highly sensitive and vascularised tissues of the labia may actually reduce or impair sexual function and pleasure (Table S4). 132 The American College of Obstetricians and Gynaecologists has previously advised: ‘Women should be informed about the lack of data supporting the efficacy of these procedures [vaginal rejuvenation, designer vaginoplasty, revirgination, G-spot amplification] and their potential complications, including infection, altered sensation, dyspareunia, adhesions and scarring’. 134
Women should be encouraged to think critically before following these trends in genital aesthetic practices, as they likely would for trends concerning other parts of their body: for example, arguably fewer people would follow suit if it became fashionable for women to shave their heads. Therefore, in matters concerning intimate health, the medical authority of the physician over the aesthetician should be emphasized. An effort to increase women’s awareness of the impact of these practices is required.
Why don’t gynaecologists look backwards?
When considering intimate health, it is insufficient to consider only the vulva and the vagina: perineal and perianal health also play a huge part in intimate health. While urinary tract infections, bacterial vaginosis and yeast vaginitis are caused by multiple factors, frequently the source of these infections is passive transfer of bacteria and yeast from the anal opening along the short distance to the perineum. 22 After defecation, it is relatively easy for bacteria and yeast to transfer to the vulva and vagina, where they may survive and thrive. 22
Early studies have shown that facultatively pathogenic E. coli, enterococci, Candida albicans and other pathogens that infect the bladder and vagina ascend from the anus and replicate in the vaginal vault. 22 In one study, E. coli was identified in cultures from 77% of patients with recurrent urinary tract infections: the faecal microbiota acts as an optimal reservoir for uropathogenic E. coli strains. 23 Furthermore, E. coli may cross the colonic mucosa (especially in women with IBS or constipation, which can be associated with ‘leaky gut’ syndrome) and enter the blood stream and subsequently the bladder mucosa. 23 Comorbidity of lower urinary tract disorders and a range of colonic disorders has been reported (including IBS, inflammatory bowel disease, overactive bladder, interstitial cystitis/painful bladder syndrome and faecal and urinary incontinence).90,91
More recently, it has been demonstrated that diverse urinary E. coli isolates not only adhere to, but also invade, vaginal cells, suggesting that E. coli can establish a vaginal intracellular reservoir, where it may reside and evade extracellular stressors prior to causing an ascending infection. 135 In addition, in women suffering with haemorrhoids, leakage of fluid can contribute to a warm, moist perianal region: this constitutes an optimal growth medium for pathogenic microbes such as Candida albicans (thrush). 20
A shorter anogenital distance has been hypothesized to induce more frequent postnatal episodes of faecal microbiota contamination of the vulva and vagina. The resulting cervicovaginal microbial dysbiosis has been predicted to sustain subclinical inflammation processes and increase the risk of developing subclinical endometriosis, resulting in impaired fertility. 17
Furthermore, habitual use of warm-water cleaning toilets or ‘bidet toilets’ (widely used in Japan) aggravates the vaginal microbiome, partially by facilitating opportunistic infection of faecal bacteria and other microorganisms.18,19 This manifests as declining detection of normal bacteria (lactobacilli) and increasing detection of faecal bacteria (E. coli, Enterococcus faecalis and Enterobacter aerogenesis) and other ubiquitous pathogens (e.g. Streptococcus agalactiae, Staphylococcus aureus and other Streptococcus species). 19 The use of bidet toilets has also been associated with vulvar pruritis, 136 which may be indicative of more specific causes of itching, such as lichen sclerosus. 137
It has also been suggested that the direction of wiping post-micturition can facilitate the migration of faecal bacteria to the urogenital area: wiping back-to-front is associated with a greater risk of developing urinary tract infection than wiping front-to-back. 21 Similarly after bowel voiding, a back-to-front direction of wiping is associated with an increased risk of urinary tract infection. 43
In addition, a tightened pelvic floor is associated with pelvic floor hyperactivity and contributes to urethral mechanical trauma if intercourse occurs without adequate lubrication, thus increasing the risk of post-coital urethritis and cystitis. 138 Rehabilitation treatment to relax the pelvic floor with electromyographic biofeedback or hands-on physiotherapy is recommended. 138
This highlights the need to educate women on correct personal hygiene practices in order to prevent disease and improve women’s health, and underscores the importance of adopting a broader vision of intimate health, encompassing the vulva, vagina and perineal area and muscles. To this author’s knowledge, there are no published guidelines, consensuses or recommendations for perineal/perianal hygiene, reflecting an unmet need in this area. As part of the general gynaecological evaluation, physicians may consider including a perineal inspection and discussing a patient’s bowel health and habits. It is also important to consider the role of the perineal area when developing guidance for women’s intimate health. This may include guidance on the direction of post-micturition and post-defecation wiping, and the most appropriate time for cleansing the vulva: theoretically, cleansing the vulva immediately after bowel voiding, if feasible, could prevent contamination with faecal bacteria and reduce the risk of infection (however, prospective studies are required to confirm this). Without addressing these factors, there is a risk of excluding this area of hygiene and undermining all other efforts to improve feminine genital health.
The importance of protection from STIs
Another unmet need in women’s awareness of their intimate health is the need to consistently use protection against STIs. Condoms are one of the most commonly used contraceptive methods in the world and are the only available method that protects against both pregnancy and STIs. 139
The Royal College of Obstetricians and Gynaecologists advise that the consistent and correct use of condoms is the most efficient means of protecting against human immunodeficiency virus (HIV) and other STIs during intercourse, including during oral and anal intercourse. 140 However, condoms are often only perceived as a contraceptive method and not as a key method of protection against STIs: in a systematic review in school-going adolescents in Europe, despite knowing that use of condoms helps protect against contracting an STI, some adolescents still regarded condoms primarily as an interim method of contraception before using the pill. 141
In addition, many women report discomfort following condom use due to latex allergies. 142 Exposure to latex can lead to an immunoglobulin E-mediated allergic reaction – from contact urticaria to anaphylaxis – and, more frequently, to a type IV allergic reaction. 143
One study reported a lower proportion of women using condoms consistently compared with men; 144 conversely, Ruiz-Palomino et al. 145 found that a higher percentage of males versus females use condoms inconsistently. Consistent condom use was predicted by marital status (not being married), having greater confidence negotiating condom use with their partners, having safer preparatory sexual behaviours (e.g. recent purchases of condoms) and not using condoms only when practicing abstinence. Living with a partner and having a lack of risk perception (e.g. risk of acquired immune deficiency syndrome (AIDS)) significantly predicted inconsistent condom use. 146
Furthermore, heterosexual women who have receptive anal intercourse are less likely to report consistent condom use compared with men who have sex with men.147,148 This represents a greater risk of contracting STIs in women than in men: given the low rates of condom use during these behaviours and the reportedly high proportions of people who partake in anal intercourse (33% of women and 38% of men), 148 it is important that recommendations for sexual risk assessments are followed.148,149 It may be beneficial for physicians to initiate conversations with women on the risk of STI and HIV acquisition during sex, including oral and anal intercourse.
The impact of hygienic behaviours on intimate health
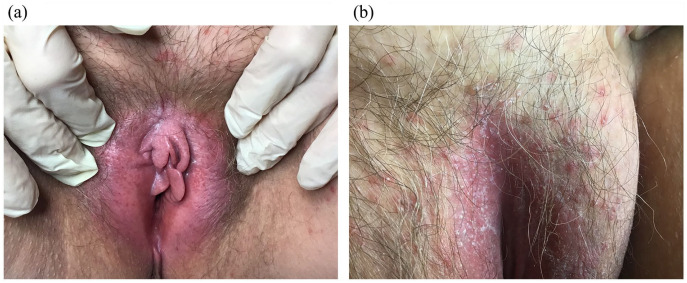
Many myths or ‘fake news’ circulate around feminine hygiene and intimate care: for example, the need to wash excessively, perform vaginal douching, and use soaps, shower gels, scrubs, deodorants and over-the-counter preparations. However, washing too frequently can impair the vulvar and perineal skin microenvironments, causing irritation and exacerbating existing conditions. 53 Soaps, shower gels, scrubs, bubble baths, deodorants, baby wipes and douches may contain skin irritants and some available over-the-counter preparations, such as baby or nappy creams, herbal creams (tea tree oil, aloe vera) and thrush treatments, may aggravate allergies and worsen existing symptoms.52,53 Examples of common vulvar conditions caused by inappropriate hygienic behaviours are shown in Figure 4(a) and (b).
Figure 4.
Common vulvar conditions caused by inappropriate hygienic behaviours. (a) Vulvitis caused by repeated use of cosmetics on the vulva of a diabetic woman; (b) Pustular mycosis and diaper dermatitis.
Images courtesy of Professor Franco Anglana, MD, and used with permission.
Vaginal douching may alter the normal vaginal microbiome, predisposing women to dysbiosis, including bacterial vaginosis, infections from colonic pathogens and STIs.3,119,150 Vaginal douching has also been associated with an increased risk of pelvic inflammatory disease, endometriosis, cervical cancer and adverse pregnancy outcomes.3,52,150 It should be noted that these associations may not necessarily be causative; for example, women may be more inclined to self-prescribe vaginal douching in response to bacterial vaginosis to reduce vaginal odour and irritation, or in response to other symptoms such as menstrual bleeding. 119 One study found that douching, but not other feminine hygiene behaviours (including type of underwear, menstrual protection and use of a hygiene spray), was significantly associated with bacterial vaginosis, and suggested that this provided evidence that douching may be causally associated with bacterial vaginosis rather than simply a response to bacterial vaginosis symptoms. 119 However, prospective studies are required to determine if douching is a causal factor for dysbiosis or infection or if it is simply a common, self-prescribed behaviour among women at higher risk for these conditions. 52
Wearing tight-fitting garments can also impact intimate health: the concept that feminine itching and urogenital issues are associated with tight-fitting clothing has been reported in several studies.44,45,47,48,151 Tight-fitting clothing can impede perspiration and increase body temperature, stimulating the proliferation of pathogens. 151 In addition, wearing tight-fitting clothing was also found to be associated with increased risk of vulvodynia. 46 Speculatively, an increased likelihood of vulvar skin microabrasions could be caused by the rigid seam adjacent to the genitals, which can worsen vulvar pain. This risk may be further increased when the skin has been subject to inappropriate or incorrect cleansing or depilation.
In contrast, routine, once-daily washing of the vulva is desirable to prevent accumulation of vaginal discharge, sweat, urine and to prevent faecal contamination and body odour.3,52,53 Theoretically, twice-daily washing of the vulva may be desirable in the case of multiple daily bowel voiding, such as in women with IBS. The Royal College of Obstetricians and Gynaecologists’ advice for women with vulval skin conditions includes avoiding irritants, using a soap substitute and cleaning the vulval area only once a day, 53 while the Middle East and central Asia guidelines on female genital hygiene similarly recommend daily cleansing with a hypoallergenic, mild detergent. 52 Women reporting the use of soaps, gels or washes to cleanse the vulva are less likely to have vulvodynia compared with women reporting the use of water only. 46 In a randomized study in breast cancer survivors who experienced menopause after chemotherapy or endocrine therapy, a vaginal pH-balanced gel decreased vaginal pH and improved vaginal dryness and dyspareunia compared with placebo gel. 152
There has been a rise in the availability of intimate hygiene products for cleanliness and odour control; however, some of these may modify the pH in the vulva and vagina, with effects on the physiological bacterial species required for protection against infection. 3 Hence, the mainstay of vulvar cleaning is the selection of a mild detergent that is capable of enhancing skin homeostasis with a physiologic pH of 4.2–5.6. 52
It is important that the use of hygiene products and their composition be considered during the gynaecologist’s evaluation, particularly when patients present with a vulvar burning sensation. Instructions should be given concerning the avoidance of irritants, such as perfumed soaps, bubble baths, hygiene sprays, lubricants and spermicides,143,153 which patients may self-prescribe in an attempt to treat odours, itching, pain, dryness or other symptoms. Some components of over-the-counter products can also contain allergens (e.g. balsam of Perù in scented soaps and propylene glycol in lubricants), 143 and material used for menstrual pads can also be allergenic.154,155 These factors can mediate genital contact dermatitis (see Figure 4(b)),143,154,155 which may further perpetuate any existing vulvar symptoms. Reusable menstrual products, such as period underwear and menstrual cups, are potential alternatives to menstrual pads. 156 However, washing and drying the period underwear may demand more effort and time. In addition, women may need to practice and use the menstrual cup on a regular basis before they are comfortable using it. 157
Given these potential adverse effects and the widespread use of over-the-counter feminine hygiene products, 65 such products should be more adequately tested by the suppliers to ensure that they do not cause vulvar skin irritation, sensitisation or pH or microbiota imbalances.
Evidence base for specific feminine wash components
Feminine washes must respect the individual needs of the vulva and avoid disrupting its natural balance. As noted above, feminine washing should ideally be limited to once per day, but for women required to wash more regularly (e.g. those with IBS with frequent diarrhoea), a product that respects the unique vulvar microenvironment is necessary to maintain the microbiome balance and avoid dryness and exacerbation of any existing issues. 3
Of the feminine wash products available, many claim to have efficacy in odour-neutralizing, moisturizing or antibacterial activity. 3 Common ingredients include lactic acid, glycerine, and a variety of natural extracts. 3
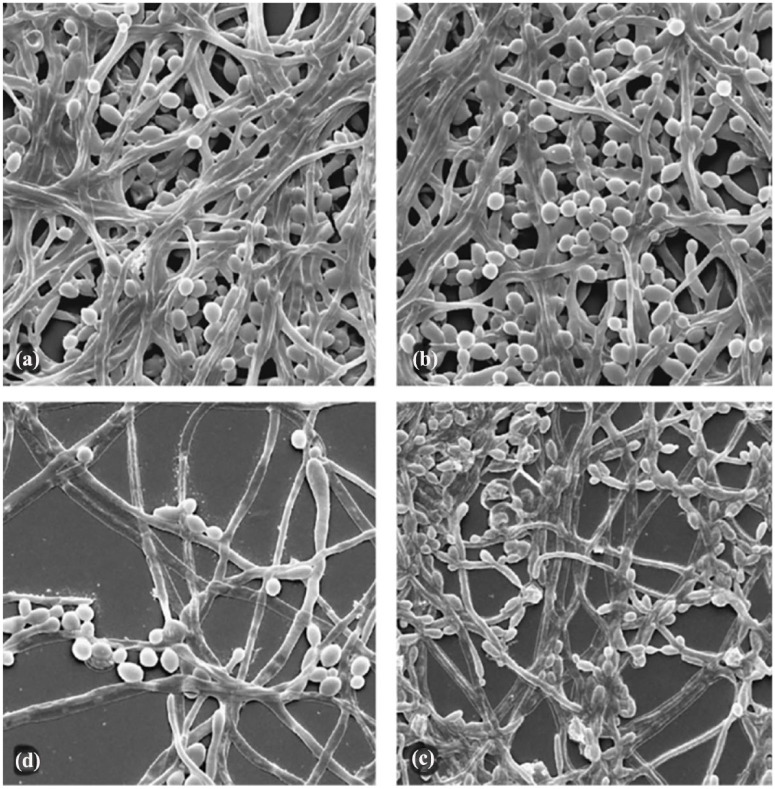
One such natural extract is thymol, a major component of thyme oil. Thymol is an emerging constituent of feminine hygiene products due to its phenolic structure and resulting pharmacological properties, including selective antimicrobial and antifungal effects that may contribute to maintenance of natural microbiota.158,159 It is proven to inhibit pathogenic microbial growth160,161 and pathogenic biofilm formation, including Candida albicans (Figure 5) and Gardnerella vaginalis.158,159 Thymol has been shown to exert not only antibacterial and antifungal action, but also anti-inflammatory activity through elastase inhibition. 162
Figure 5.
Scanning electron microscopy images of mature Candida albicans biofilm. (a) Before thymol incubation, the mature biofilm consists of a very dense multi-layered network of yeast cells and filamentous forms. (b) After incubation with 1/2× the minimum inhibitory concentration (MIC) of thymol for 24 h, only slight changes were visible in comparison with the control. (c) Incubation with thymol 1× MIC induced visible changes in biofilm structure. (d) Incubation with thymol 2× MIC clearly inhibited all of the elements and greatly damaged the filamentous forms. Magnification 1250×.
Reprinted from Int J Antimicrob Agents, Vol 31(5), Braga PC, Culici M, Alfieri M, et al., Thymol inhibits Candida albicans biofilm formation and mature biofilm, p472–477, Copyright (2008), with permission from Elsevier. 158
The inclusion of lactic acid in feminine hygiene products has been shown to promote vulvar and vaginal health through maintaining an acidic pH,78,163 while glycerine is used widely in dermatological interventions as a skin conditioning agent.164,165 Working as a humectant, glycerine attracts water from the atmosphere or deeper in the dermis to maintain hydration levels in the upper epidermis layers.164,165 Including glycerine in feminine washes is thought to moisturize and protect against over-drying of the vulvar skin. 166
For odour control, anti-oxidant ketoglutaric acid 167 may be included in feminine washes: its anti-oxidant activity could potentially result in oxidation of the amines associated with malodour,168–170 exerting anti-odourant properties.
Based on this evidence, an acid pH thymol-containing cleansing wash, with glycerine, ketoglutaric acid and lactic acid has been developed. 166 In pregnant and postpartum women, including those with vulvar and vaginal candidiasis or bacterial vaginosis, the acid pH thymol-containing cleansing wash was shown to reduce pH and relieve vulvar and vaginal discomfort, and also significantly improved sexual disturbances, including desire, lubrication, arousal and orgasm. 171 Also, in a study of healthy women, this acid pH thymol-containing cleansing wash was compared with an alternative lactic acid-based wash and was associated with a smaller reduction in skin hydration of the labia majora and minora skin. 166 The acid pH thymol-containing cleanser wash contains several moisturizing ingredients, such as humectant glycerine and emollient oat milk, which may have contributed to maintaining skin hydration; the alternative wash contained sodium lauryl sulphate and sodium laurate as anionic surfactants, which are known to irritate the skin and make it more vulnerable to injury leading to transepidermal water loss. 166
As our knowledge of female physiology is becoming more extensive and accurate, cleansers are being developed with the goal of satisfying the emerging needs of the vulva, most notably maintenance of the microbiome, hydration and pH regulation. The thymol-containing cleansing wash described above (also containing glycerine, ketoglutaric acid and lactic acid) has the capability to satisfy many of these emerging needs, by reducing pH, maintaining skin hydration, relieving vulvar and vaginal discomfort and improving sexual disturbances.166,171 The thymol-containing cleansing wash therefore represents an appropriate cleansing option for maintaining the delicate and complex equilibrium of the vulva. Given our evolving knowledge of feminine hygiene and intimate care, there is a need for rigorous evidence-based scientific and clinical studies to determine further appropriate components for feminine wash products.
Limitations
Although this review provides an important overview of feminine hygiene-related discussions and behaviours, it is subject to several limitations associated with a narrative review. First, as no pre-established search methodologies were utilized, the included studies, recommendations and conclusions are subject to selection bias and further relevant studies may not have been identified. Further, while overall findings from a large number of studies are discussed, no pooled analyses or direct comparisons of the included studies were performed, due to the variety of outcomes and studies included, and to provide a comprehensive vision that will be useful in daily clinical practice with patients. With this perspective, a comprehensive narrative review of the current situation is provided, which identifies areas for improvement of women’s genital health and intimate well-being.
Conclusion
A summary of key points covered in this review article is shown in Table S5. Correct intimate care may contribute to improved genital and sexual health; however, a lack of knowledge of the correct behaviours can result in inappropriate aesthetic, hygiene and sexual practices, and impairment of the dynamic protective mechanisms of the vulva and vagina. There is a need for prospective clinical studies to determine appropriate components for feminine wash products and to inform evidence-based guidelines for best practice in female genital health. The goal is to offer women the awareness and knowledge to enable them to be the advocates of their own intimate health, contributing to the prevention of genital pain and other symptoms that may arise from inappropriate intimate care. There is a need for discussion of intimate care issues to become a routine part of clinical conversations with patients. The younger the patient is when these discussions begin, the better the chances are of maximizing the lifelong benefits of self-protecting genital hygiene. It is essential to recognize that also in intimate care, education is key.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455057231223716 for Maintaining vulvar, vaginal and perineal health: Clinical considerations by Alessandra Graziottin in Women’s Health
Acknowledgments
The author acknowledges the Alessandra Graziottin Foundation (non-profit organization) for supporting research and clinical studies on vulvar health and vulvar pain. Medical writing support for the development of this manuscript, under the direction of the author, was provided by Bonnie Nicholson, PhD, of Ashfield MedComms, an Inizio company, and funded by Meda Pharma S.p.A., a Viatris company.
Footnotes
ORCID iD: Alessandra Graziottin  https://orcid.org/0000-0001-7973-424X
https://orcid.org/0000-0001-7973-424X
Supplemental material: Supplemental material for this article is available online.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Alessandra Graziottin: Conceptualization; Supervision; Writing – review & editing.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Publication fees and medical writing support for the development of this manuscript, under the direction of the author, was funded by Meda Pharma S.p.A., a Viatris company.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: Prof. Alessandra Graziottin has participated in speakers’ bureaus for Alfasigma, Astellas, Bayer, Gedeon-Richter, Lolipharm, Shionogi and Uriach; participated in advisory boards for Alfasigma, Astellas, Bayer, Mylan, Uriach and Viatris; and served as a consultant for Alfasigma, Astellas, Bayer, Fagron, Lolipharm, Mylan, Shionogi, Uriach and Viatris.
Availability of data and materials: Not applicable.
References
- 1. Hill AJ, Balgobin S, Mishra K, et al. Recommended standardized anatomic terminology of the posterior female pelvis and vulva based on a structured medical literature review. Am J Obstet Gynecol 2021; 225: 169.e1–169.e16. [DOI] [PubMed] [Google Scholar]
- 2. Braun V, Kitzinger C. ‘Snatch’, ‘hole’, or ‘honey-pot’? Semantic categories and the problem of nonspecificity in female genital slang. J Sex Res 2001; 38: 146–158. [Google Scholar]
- 3. Chen Y, Bruning E, Rubino J, et al. Role of female intimate hygiene in vulvovaginal health: global hygiene practices and product usage. Womens Health 2017; 13: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murina PF, Graziottin A, Bagot O, et al. Real-world practices and attitudes towards intimate self-care: results from an international women’s survey. J Gynecol Obstet Hum Reprod 2021; 50(10): 102192. [DOI] [PubMed] [Google Scholar]
- 5. Hoch Z. Vaginal erotic sensitivity by sexological examination. Acta Obstet Gynecol Scand 1986; 65: 767–773. [DOI] [PubMed] [Google Scholar]
- 6. Ottesen B, Pedersen B, Nielsen J, et al. Vasoactive intestinal polypeptide (VIP) provokes vaginal lubrication in normal women. Peptides 1987; 8(5): 797–800. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen JD, Duong H. Anatomy, abdomen and pelvis, female external genitalia. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC, (2022, accessed February 2024). https://www.ncbi.nlm.nih.gov/books/NBK547703/ [PubMed] [Google Scholar]
- 8. Yavagal S, de Farias TF, Medina CA, et al. Normal vulvovaginal, perineal, and pelvic anatomy with reconstructive considerations. Semin Plast Surg 2011; 25(2): 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graziottin A, Gambini D. Anatomy and physiology of genital organs – women. Handb Clin Neurol 2015; 130: 39–60. [DOI] [PubMed] [Google Scholar]
- 10. Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterol Clin North Am 2008; 37(3): 493–509, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grimes WR, Stratton M. Pelvic floor dysfunction. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC, (2021, accessed February 2024). https://www.ncbi.nlm.nih.gov/books/NBK559246/ [PubMed] [Google Scholar]
- 12. Shafik A. Vagino-levator reflex: description of a reflex and its role in sexual performance. Euro J Obstetr Gynecol Reprod Biol 1995; 60: 161–164. [DOI] [PubMed] [Google Scholar]
- 13. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J 2015; 26(7): 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashton-Miller JA, Delancey JO. On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng 2009; 11: 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gorbachinsky I, Sherertz R, Russell G, et al. Altered perineal microbiome is associated with vulvovaginitis and urinary tract infection in preadolescent girls. Ther Adv Urol 2014; 6(6): 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma M, Lu H, Yang Z, et al. Differences in microbiota between acute and chronic perianal eczema. Medicine (Baltimore) 2021; 100: e25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. García-Peñarrubia P, Ruiz-Alcaraz AJ, Martínez-Esparza M, et al. Hypothetical roadmap towards endometriosis: prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Human Reprod Updat 2020; 26: 214–246. [DOI] [PubMed] [Google Scholar]
- 18. Kim YM, Kim JY, Lee MY, et al. Prospective study of bidet toilet use: association of abnormal vaginal colonization and preterm birth in high-risk pregnant women. J Obstet Gynaecol Res 2019; 45(6): 1134–1142. [DOI] [PubMed] [Google Scholar]
- 19. Ogino M, Iino K, Minoura S. Habitual use of warm-water cleaning toilets is related to the aggravation of vaginal microflora. J Obstet Gynaecol Res 2010; 36(5): 1071–1074. [DOI] [PubMed] [Google Scholar]
- 20. Orlay G. Haemorrhoids – a review. Austral Fam Phys 2003; 32: 523–526. [PubMed] [Google Scholar]
- 21. Persad S, Watermeyer S, Griffiths A, et al. Association between urinary tract infection and postmicturition wiping habit. Acta Obstet Gynecol Scand 2006; 85(11): 1395–1396. [DOI] [PubMed] [Google Scholar]
- 22. Reid G. Probiotic Lactobacilli for urogenital health in women. J Clin Gastroenterol 2008; 42: S234–236. [DOI] [PubMed] [Google Scholar]
- 23. Salonia A, Clementi MC, Graziottin A, et al. Secondary provoked vestibulodynia in sexually active women with uncomplicated recurrent urinary tract infections. J Sex Med 2013; 10(9): 2265–2273. [DOI] [PubMed] [Google Scholar]
- 24. Puppo V. Embryology and anatomy of the vulva: the female orgasm and women’s sexual health. Eur J Obstet Gynecol Reprod Biol 2011; 154(1): 3–8. [DOI] [PubMed] [Google Scholar]
- 25. Bulun SE. Chapter 17 – physiology and pathology of the female reproductive axis. In: Melmed S, Polonsky KS, Larsen PR, et al. (eds) Williams textbook of endocrinology. 13th ed. Philadelphia, PA: Elsevier, 2016, pp. 589–663. [Google Scholar]
- 26. Graziottin A. Vaginal biological and sexual health – the unmet needs. Climacteric 2015; 18(Suppl. 1): 9–12. [DOI] [PubMed] [Google Scholar]
- 27. Holtzman D, Kulish N. Nevermore: the hymen and the loss of virginity. J Am Psychoanal Assoc 1996; 44(Suppl.): 303–332. [PubMed] [Google Scholar]
- 28. McVeigh E. Reproductive surgery. In: Wells D, Coward K. (eds) Textbook of clinical embryology. Cambridge: Cambridge University Press, 2013, pp. 337–345. [Google Scholar]
- 29. Robboy SJ, Kurita T, Baskin L, et al. New insights into human female reproductive tract development. Differentiation 2017; 97: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keefe DL, Winkler N. Chapter 1 – embryology. In: Sokol AI, Sokol ER. (eds) General gynecology. Philadelphia, PA: Mosby, 2007, pp. 1–20. [Google Scholar]
- 31. Kaur P, Panneerselvam D. Bicornuate uterus. In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC, (2022, accessed February 2024). https://www.ncbi.nlm.nih.gov/books/NBK559246/ [PubMed] [Google Scholar]
- 32. Jannini EA, Buisson O, Rubio-Casillas A. Beyond the G-spot: clitourethrovaginal complex anatomy in female orgasm. Nat Rev Urol 2014; 11: 531–538. [DOI] [PubMed] [Google Scholar]
- 33. Jannini EA, Rubio-Casillas A, Whipple B, et al. Female orgasm(s): one, two, several. J Sex Med 2012; 9: 956–965. [DOI] [PubMed] [Google Scholar]
- 34. Palacios S. Expression of androgen receptors in the structures of vulvovaginal tissue. Menopause 2020; 27(11): 1336–1342. [DOI] [PubMed] [Google Scholar]
- 35. Woodard TL, Diamond MP. Physiologic measures of sexual function in women: a review. Fertil Steril 2009; 92(1): 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tarcan T, Park K, Goldstein I, et al. Histomorphometric analysis of age-related structural changes in human clitoral cavernosal tissue. J Urol 1999; 161: 940–944. [PubMed] [Google Scholar]
- 37. Venkatesh KK, Cu-Uvin S. Anatomic and hormonal changes in the female reproductive tract immune environment during the life cycle: implications for HIV/STI prevention research. Am J Reprod Immunol 2014; 71(6): 495–504. [DOI] [PubMed] [Google Scholar]
- 38. Samaras N, Samaras D, Frangos E, et al. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation Res 2013; 16: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baulieu EE. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab 1996; 81: 3147–3151. [DOI] [PubMed] [Google Scholar]
- 40. Jannini EA, Whipple B, Kingsberg SA, et al. Who’s afraid of the G-spot? J Sex Med 2010; 7: 25–34. [DOI] [PubMed] [Google Scholar]
- 41. Grammer K, Fink B, Neave N. Human pheromones and sexual attraction. Euro J Obstetr Gynecol Reprod Biol 2005; 118: 135–142. [DOI] [PubMed] [Google Scholar]
- 42. Mostafa T, Khouly GE, Hassan A. Pheromones in sex and reproduction: do they have a role in humans? J Adv Res 2012; 3: 1–9. [Google Scholar]
- 43. Mishra B, Srivastava R, Agarwal J, et al. Behavioral and psychosocial risk factors associated with first and recurrent cystitis in Indian women: a case-control study. Ind J Commun Med 2016; 41: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elegbe IA, Botu M. A preliminary study on dressing patterns and incidence of candidiasis. Am J Public Health 1982; 72(2): 176–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foxman B, Frerichs RR. Epidemiology of urinary tract infection: II. Diet, clothing, and urination habits. Am J Pub Heal 1985; 75: 1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klann AM, Rosenberg J, Wang T, et al. Exploring hygienic behaviors and vulvodynia. J Low Genital Tract Dis 2019; 23: 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reed BD. Risk factors for Candida vulvovaginitis. Obstetr Gynecol Surv 1992; 47: 551–560. [DOI] [PubMed] [Google Scholar]
- 48. Runeman B, Rybo G, Forsgren-Brusk U, et al. The vulvar skin microenvironment: impact of tight-fitting underwear on microclimate, pH and microflora. Acta Dermato-Venereol 2005; 85: 118–122. [DOI] [PubMed] [Google Scholar]
- 49. Runeman B, Rybo G, Larkö O, et al. The vulva skin microclimate: influence of panty liners on temperature, humidity and pH. Acta Derm Venereol 2003; 83(2): 88–92. [DOI] [PubMed] [Google Scholar]
- 50. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Inf Dis 2008; 47: 1426–1435. [DOI] [PubMed] [Google Scholar]
- 51. Masters NT, George WH, Davis KC, et al. Women’s unprotected sex intentions: roles of sexual victimization, intoxication, and partner perception. J Sex Res 2014; 51(5): 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arab H, Almadani L, Tahlak M, et al. The Middle East and Central Asia guidelines on female genital hygiene. BMJ Middle East 2011; 19: 99–106. [Google Scholar]
- 53. Royal College of Obstetricians and Gynaecologists. Skin conditions of the vulva, https://www.rcog.org.uk/en/patients/patient-leaflets/skin-conditions-of-the-vulva/ (2013, accessed January 2022).
- 54. Czerwinski BS. Variation in feminine hygiene practices – as a function of age. J Obstet Gynecol Neonatal Nurs 2000; 29(6): 625–633. [DOI] [PubMed] [Google Scholar]
- 55. Ampt AJ, Roach V, Roberts CL. Vulvoplasty in New South Wales, 2001–2013: a population-based record linkage study. Med J Aust 2016; 205: 365–369. [DOI] [PubMed] [Google Scholar]
- 56. Hustad IB, Malmqvist K, Ivanova E, et al. Does size matter? Genital self-image, genital size, pornography use and openness toward cosmetic genital surgery in 3503 Swedish men and women. J Sex Med 2022; 19(9): 1378–1386. [DOI] [PubMed] [Google Scholar]
- 57. Vandenbosch L, Fardouly J, Tiggemann M. Social media and body image: recent trends and future directions. Curr Opin Psychol 2022; 45: 101289. [DOI] [PubMed] [Google Scholar]
- 58. Simon EG, Laffon M. Maternal care after vaginal delivery and management of complications in immediate post-partum – guidelines for clinical practice. J Gynecol Obstet Biol Reprod (Paris) 2015; 44(10): 1101–1110. [DOI] [PubMed] [Google Scholar]
- 59. Casey BM, Schaffer JI, Bloom SL, et al. Obstetric antecedents for postpartum pelvic floor dysfunction. Am J Obstet Gynecol 2005; 192: 16551662. [DOI] [PubMed] [Google Scholar]
- 60. Corrado B, Giardulli B, Polito F, et al. The impact of urinary incontinence on quality of life: a cross-sectional study in the Metropolitan City of Naples. Geriatrics (Basel) 2020; 5: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moossdorff-Steinhauser HFA, Berghmans BCM, Spaanderman MEA, et al. Prevalence, incidence and bothersomeness of urinary incontinence between 6 weeks and 1 year post-partum: a systematic review and meta-analysis. Int Urogynecol J 2021; 32: 1675–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen TM, Do TTT, Tran TN, et al. Exercise and quality of life in women with menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health 2020; 17: 7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim HK, Kang SY, Chung YJ, et al. The recent review of the genitourinary syndrome of menopause. J Menopausal Med 2015; 21(2): 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Angelou K, Grigoriadis T, Diakosavvas M, et al. The genitourinary syndrome of menopause: an overview of the recent data. Cureus 2020; 12: e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Erekson EA, Martin DK, Brousseau EC, et al. Over-the-counter treatments and perineal hygiene in postmenopausal women. Menopause 2014; 21(3): 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Simon JA, Ferenczy A, Black D, et al. Efficacy, tolerability, and endometrial safety of ospemifene compared with current therapies for the treatment of vulvovaginal atrophy: a systematic literature review and network meta-analysis. Menopause (New York, NY) 2023; 30: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Graziottin A. Topical testosterone cream: dosing, safety, efficacy and rationale of use. J Plastic Pathol Dermatol 2022; 18: 193–203. [Google Scholar]
- 68. Sarmento ACA, Kamilos MF, Costa APF, et al. Use of moisturizers and lubricants for vulvovaginal atrophy. Front Reprod Health 2021; 3: 781353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mortensen OE, Christensen SE, Løkkegaard E. The evidence behind the use of LASER for genitourinary syndrome of menopause, vulvovaginal atrophy, urinary incontinence and lichen sclerosus: a state-of-the-art review. Acta Obstet Gynecol Scand 2022; 101(6): 657–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Graziottin A, Murina F, Gambini D, et al. Vulvar pain: the revealing scenario of leading comorbidities in 1183 cases. Eur J Obstet Gynecol Reprod Biol 2020; 252: 50–55. [DOI] [PubMed] [Google Scholar]
- 71. Munday P, Buchan A. Vulval vestibulitis. BMJ 2004; 328: 1214–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Graziottin A, Brotto LA. Vulvar vestibulitis syndrome: a clinical approach. J Sex Marital Ther 2004; 30(3): 125–139. [DOI] [PubMed] [Google Scholar]
- 73. Edwards SK, Bates CM, Lewis F, et al. 2014 UK national guideline on the management of vulval conditions. Int J Std AIDS 2014; 26: 611–624. [DOI] [PubMed] [Google Scholar]
- 74. Dhar R, Nunns D. Vulvodynia management. Obstet Gynaecol Reprod Med 2009; 19: 175–177. [Google Scholar]
- 75. Fitzcharles MA, Cohen SP, Clauw DJ, et al. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 2021; 397: 2098–2110. [DOI] [PubMed] [Google Scholar]
- 76. Nijs J, Lahousse A, Kapreli E, et al. Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J Clin Med 2021; 10: 3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lin YP, Chen WC, Cheng CM, et al. Vaginal pH value for clinical diagnosis and treatment of common vaginitis. Diagnostics (Basel) 2021; 11: 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tachedjian G, Aldunate M, Bradshaw CS, et al. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol 2017; 168(9–10): 782–792. [DOI] [PubMed] [Google Scholar]
- 79. Hodges AL, Holland AC. Prevention and treatment of injuries and infections related to pubic hair removal. Nurs Womens Health 2017; 21(4): 313–317. [DOI] [PubMed] [Google Scholar]
- 80. Stone N, Graham CA, Baysal I. Women’s engagement in pubic hair removal: motivations and associated factors. Int J Sex Health 2017; 29: 89–96. [Google Scholar]
- 81. Farage M, Maibach HI. The vulvar epithelium differs from the skin: implications for cutaneous testing to address topical vulvar exposures. Cont Derm 2004; 51(4): 201–209. [DOI] [PubMed] [Google Scholar]
- 82. Amjadi F, Salehi E, Mehdizadeh M, et al. Role of the innate immunity in female reproductive tract. Adv Biomed Res 2014; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Graziottin A. Contraception containing estradiol valerate and dienogest – advantages, adherence and user satisfaction. Minerva Ginecol 2014; 66(5): 479–495. [PubMed] [Google Scholar]
- 84. Carvalho FM, Teixeira-Santos R, Mergulhão FJM, et al. Effect of lactobacillus plantarum biofilms on the adhesion of Escherichia coli to urinary tract devices. Antibiotics (Basel, Switzerland) 2021; 10: 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boris S, Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2000; 2(5): 543–546. [DOI] [PubMed] [Google Scholar]
- 86. Graver MA, Wade JJ. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob 2011; 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miller EA, Beasley DE, Dunn RR, et al. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique? Front Microbiol 2016; 7: 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Farage M, Maibach H. Lifetime changes in the vulva and vagina. Arch Gynecol Obstet 2006; 273: 195–202. [DOI] [PubMed] [Google Scholar]
- 89. Carr D, Pootrakul L, Harmon J, et al. Cutaneous malignancies of the perineum. Clin Obstet Gynecol 2015; 58(1): 158–171. [DOI] [PubMed] [Google Scholar]
- 90. Grundy L, Brierley SM. Cross-organ sensitization between the colon and bladder: to pee or not to pee? Am J Physiol Gastrointest Liver Physiol 2018; 314: G301–G308. [DOI] [PubMed] [Google Scholar]
- 91. Graziottin A. Medical and sexual comorbidities: when the integrative approach is key. Sexologies 2008; 17: S21–S22. [Google Scholar]
- 92. Witkin SS, Linhares IM. Why do lactobacilli dominate the human vaginal microbiota. BJOG 2017; 124(4): 606–611. [DOI] [PubMed] [Google Scholar]
- 93. Amabebe E, Anumba DOC. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front Immunol 2020; 11: 2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barrientos-Durán A, Fuentes-López A, de Salazar A, et al. Reviewing the composition of vaginal microbiota: inclusion of nutrition and probiotic factors in the maintenance of eubiosis. Nutrients 2020; 12: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 2017; 129(4): 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brown CJ, Wong M, Davis CC, et al. Preliminary characterization of the normal microbiota of the human vulva using cultivation-independent methods. J Med Microbiol 2007; 56: 271–276. [DOI] [PubMed] [Google Scholar]
- 97. Madison A, Kiecolt-Glaser JK. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci 2019; 28: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Núñez MJ, Balboa J, Riveiro P, et al. Effects of psychological stress and alprazolam on development of oral candidiasis in rats. Clin Diagn Lab Immunol 2002; 9: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neggers YH, Nansel TR, Andrews WW, et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr 2007; 137(9): 2128–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rodrigues CF, Rodrigues ME, Henriques M., Candida sp. infections in patients with diabetes mellitus. J Clin Med 2019; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Van Ende M, Wijnants S, Van Dijck P. Sugar sensing and signaling in candida albicans and candida glabrata. Front Microbiol 2019; 10: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hardy L, Cerca N, Jespers V, et al. Bacterial biofilms in the vagina. Res Microbiol 2017; 168: 865–874. [DOI] [PubMed] [Google Scholar]
- 103. Colautti A, Orecchia E, Comi G, et al. Lactobacilli, a weapon to counteract pathogens through the inhibition of their virulence factors. J Bacteriol 2022; 204: e0027222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bartoletti R, Cai T, Nesi G, et al. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study. World J Urol 2014; 32(3): 737–742. [DOI] [PubMed] [Google Scholar]
- 105. Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 1996; 60(3): 539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kovalenko T, Tishchenko M, Vovk O, et al. The influence of contraception on vaginal microbiocenosis condition. Georgian Med News 2020: 40–44. [PubMed] [Google Scholar]
- 107. Post JC, Hiller NL, Nistico L, et al. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg 2007; 15(5): 347–351. [DOI] [PubMed] [Google Scholar]
- 108. Sabbatini S, Monari C, Ballet N, et al. Anti-biofilm properties of Saccharomyces cerevisiae CNCM I-3856 and Lacticaseibacillus rhamnosus ATCC 53103 probiotics against G. Vaginalis. Microorganisms 2020; 8: 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 2012; 272(6): 541–561. [DOI] [PubMed] [Google Scholar]
- 110. Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog 2012; 8: e1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pohl CH. Recent advances and opportunities in the study of Candida albicans polymicrobial biofilms. Front Cell Infect Microbiol 2022; 12: 836379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kaur H, Merchant M, Haque MM, et al. Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological lifecycle. Front Microbiol 2020; 11: 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol 2017; 595: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Petricevic L, Domig KJ, Nierscher FJ, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep 2014; 4: 5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mändar R, Punab M, Borovkova N, et al. Complementary seminovaginal microbiome in couples. Res Microbiol 2015; 166(5): 440–447. [DOI] [PubMed] [Google Scholar]
- 116. Achilles SL, Austin MN, Meyn LA, et al. Impact of contraceptive initiation on vaginal microbiota. Am J Obstet Gynecol 2018; 218(6): 622.e1–622.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ahrens P, Andersen LO, Lilje B, et al. Changes in the vaginal microbiota following antibiotic treatment for Mycoplasma genitalium, Chlamydia trachomatis and bacterial vaginosis. PLoS ONE 2020; 15: e0236036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jenkins A, Money D, O’Doherty KC. Is the vaginal cleansing product industry causing harm to women. Expert Rev Anti Infect Ther 2021; 19(3): 267–269. [DOI] [PubMed] [Google Scholar]
- 119. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis 2010; 37(2): 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wagner G, Ottesen B. Vaginal physiology during menstruation. Ann Intern Med 1982; 96(6, Pt. 2): 921–923. [DOI] [PubMed] [Google Scholar]
- 121. Butler SM, Smith NK, Collazo E, et al. Pubic hair preferences, reasons for removal, and associated genital symptoms: comparisons between men and women. J Sex Med 2015; 12: 48–58. [DOI] [PubMed] [Google Scholar]
- 122. DeMaria AL, Sundstrom B, McInnis SM, et al. Perceptions and correlates of pubic hair removal and grooming among college-aged women: a mixed methods approach. Sex Health 2016; 13(3): 248–256. [DOI] [PubMed] [Google Scholar]
- 123. Ramsey S, Sweeney C, Fraser M, et al. Pubic hair and sexuality: a review. J Sex Med 2009; 6(8): 2102–2110. [DOI] [PubMed] [Google Scholar]
- 124. Stenson AL, Leclair C. To shave or not to shave? A series of periclitoral masses associated with depilatory techniques and a review of the literature. J Lower Genital Tract Dis 2018; 22: 412–414. [DOI] [PubMed] [Google Scholar]
- 125. Schild-Suhren M, Soliman AA, Malik E. Pubic hair shaving is correlated to vulvar dysplasia and inflammation: a case-control study. Infect Dis Obstet Gynecol 2017; 2017: 9350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Geynisman-Tan J, Kenton K, Tavathia M, et al. Bare versus hair: do pubic hair grooming preferences dictate the urogenital microbiome? Fem Pelvic Med Reconstruct Surg 2021; 27: 532–537. [DOI] [PubMed] [Google Scholar]
- 127. Wedekind C, Seebeck T, Bettens F, et al. MHC-dependent mate preferences in humans. Proc Royal Soc 1995; 260: 245–249. [DOI] [PubMed] [Google Scholar]
- 128. Yang L, Comninos AN, Dhillo WS. Intrinsic links among sex, emotion, and reproduction. Cell Mol Life Sci 2018; 75: 2197–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lowenstein L, Salonia A, Shechter A, et al. Physicians’ attitude toward female genital plastic surgery: a multinational survey. J Sex Med 2014; 11(1): 33–39. [DOI] [PubMed] [Google Scholar]
- 130. Güneş A, Alinsod RM. A mini-review of aesthetic gynecology and leading gynecology associations’ approaches to this issue. Turk J Obstet Gynecol 2018; 15(2): 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Barbara G, Facchin F, Buggio L, et al. Vaginal rejuvenation: current perspectives. Int J Womens Health 2017; 9: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Lowe J, Black KI. Female genital cosmetic surgery. Aust N Z J Obstet Gynaecol 2021; 61: 325–327. [DOI] [PubMed] [Google Scholar]
- 133. Preti M, Vieira-Baptista P, Digesu GA, et al. The clinical role of LASER for vulvar and vaginal treatments in gynecology and female urology: an ICS/ISSVD best practice consensus document. J Low Genit Tract Dis 2019; 23: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Committe on Gynecologic Practice and American College of Obstetricians and Gynecologists. ACOG committee opinion no. 378: vaginal ‘rejuvenation’ and cosmetic vaginal procedures. Obstet Gynecol 2007; 110: 737–738. [DOI] [PubMed] [Google Scholar]
- 135. Brannon JR, Dunigan TL, Beebout CJ, et al. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat Commun 2020; 11: 2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Catarsi L, Troiano G, Bagnoli A, et al. Unpleasant side effects due to bidet toilet use. Eur J Public Health 2018; 28: 325.29444220 [Google Scholar]
- 137. Kirtschig G. Lichen sclerosus-presentation, diagnosis and management. Deutsches Arzteblatt International 2016; 113: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Graziottin A. Recurrent cystitis after intercourse: why the gynaecologist has a say. In: Studd J, Seang L, Chervenak F. (eds) Current progress in obstetrics and gynaecology. Mumbai, India: Kothari Medical Subscription Services Pvt Ltd, 2014, pp. 319–336. [Google Scholar]
- 139. Higgins JA, Smith NK. The sexual acceptability of contraception: reviewing the literature and building a new concept. J Sex Res 2016; 53(4–5): 417–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Royal College of Obstetricians and Gynaecologists. Faculty of sexual & reproductive healthcare clinical guidance: barrier methods for contraception and STI prevention, https://www.fsrh.org/standards-and-guidance/documents/ceuguidancebarriermethodscontraceptionsdi/ (2015, accessed August 2022).
- 141. Samkange-Zeeb FN, Spallek L, Zeeb H. Awareness and knowledge of sexually transmitted diseases (STDs) among school-going adolescents in Europe: a systematic review of published literature. BMC Public Health 2011; 11: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Liccardi G, Senna G, Rotiroti G, et al. Intimate behavior and allergy: a narrative review. Ann Allergy Asthma Immunol 2007; 99(5): 394–400. [DOI] [PubMed] [Google Scholar]
- 143. Caminati M, Giorgis V, Palterer B, et al. Allergy and sexual behaviours: an update. Clin Rev Allergy Immunol 2017; 56: 269–277. [DOI] [PubMed] [Google Scholar]
- 144. Islam N, Laugen C. Gender differences in depression and condom use among sexually active Canadians. J Affect Disord 2015; 174: 511–515. [DOI] [PubMed] [Google Scholar]
- 145. Ruiz-Palomino E, Gil-Llario MD, Giménez-García C, et al. Explanatory psychological factors of inconsistently condom use among Spanish university students: gender differences. Spanish J Psychol 2020; 23: e12. [DOI] [PubMed] [Google Scholar]
- 146. Costa EC, Oliveira R, Ferreira D, et al. Predictors of consistent condom use among Portuguese women attending family planning clinics. AIDS Care 2016; 28(1): 119–123. [DOI] [PubMed] [Google Scholar]
- 147. DʼAnna LH, Warner L, Margolis AD, et al. Consistency of condom use during receptive anal intercourse among women and men who have sex with men: findings from the safe in the city behavioral study. Sex Transm Dis 2015; 42(7): 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Maierhofer CN, Lancaster KE, Turner AN. Anal sex is more common than having a twitter account in the United States. Sexual Trans Dis 2018; 45: 783–785. [DOI] [PubMed] [Google Scholar]
- 149. Habel MA, Leichliter JS, Dittus PJ, et al. Heterosexual anal and oral sex in adolescents and adults in the United States, 2011-2015. Sex Transm Dis 2018; 45(12): 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cottrell BH. An updated review of evidence to discourage douching. MCN Am J Matern Child Nurs 2010; 35(2): 102–107; quiz 108. [DOI] [PubMed] [Google Scholar]
- 151. Guaschino S, Benvenuti C. SOPHY project: an observational study of vaginal pH and lifestyle in women of different ages and in different physiopathological conditions. Part I. Miner Ginecol 2008; 60(5): 353–362. [PubMed] [Google Scholar]
- 152. Lee YK, Chung HH, Kim JW, et al. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: a randomized controlled trial. Obstet Gynecol 2011; 117(4): 922–927. [DOI] [PubMed] [Google Scholar]
- 153. Bergeron S, Reed BD, Wesselmann U, et al. Vulvodynia. Nat Rev Dis Primers 2020; 6: 36. [DOI] [PubMed] [Google Scholar]
- 154. Wiwanitkit V. Sanitary pad dermatitis. Indian J Dermatol 2009; 54: 391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rademaker M. Allergic contact dermatitis to a sanitary pad. Australas J Dermatol 2004; 45: 234–235. [DOI] [PubMed] [Google Scholar]
- 156. Ramsay C, Hennegan J, Douglass CH, et al. Reusable period products: use and perceptions among young people in Victoria, Australia. BMC Women’s Health 2023; 23: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Singh R, Agarwal M, Sinha S, et al. Study of adaptability and efficacy of menstrual cups in managing menstrual health and hygiene: a descriptive longitudinal study. Cureus 2022; 14: e29690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Braga PC, Culici M, Alfieri M, et al. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int J Antimicrob Agents 2008; 31(5): 472–477. [DOI] [PubMed] [Google Scholar]
- 159. Braga PC, Dal Sasso M, Culici M, et al. Inhibitory activity of thymol on native and mature Gardnerella vaginalis biofilms: in vitro study. Arzneimittelforschung 2010; 60(11): 675–681. [DOI] [PubMed] [Google Scholar]
- 160. Didry N, Dubreuil L, Pinkas M. Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm Acta Helv 1994; 69(1): 25–28. [DOI] [PubMed] [Google Scholar]
- 161. Lambert RJ, Skandamis PN, Coote PJ, et al. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 2001; 91(3): 453–462. [DOI] [PubMed] [Google Scholar]
- 162. Braga PC, Dal Sasso M, Culici M, et al. Anti-inflammatory activity of thymol: inhibitory effect on the release of human neutrophil elastase. Pharmacology 2006; 77(3): 130–136. [DOI] [PubMed] [Google Scholar]
- 163. Kim JM, Park YJ. Probiotics in the prevention and treatment of postmenopausal vaginal infections: review article. J Menopausal Med 2017; 23(3): 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chularojanamontri L, Tuchinda P, Kulthanan K, et al. Moisturizers for acne: what are their constituents. J Clin Aesthet Dermatol 2014; 7(5): 36–44. [PMC free article] [PubMed] [Google Scholar]
- 165. Becker LC, Bergfeld WF, Belsito DV, et al. Safety assessment of glycerin as used in cosmetics. Int J Toxicol 2019; 38(3_suppl.): 6S–22S. [DOI] [PubMed] [Google Scholar]
- 166. Murina F, Caimi C, Felice R, et al. Characterization of female intimate hygiene practices and vulvar health: a randomized double-blind controlled trial. J Cosmet Dermatol 2020; 19(10): 2721–2726. [DOI] [PubMed] [Google Scholar]
- 167. Liu S, He L, Yao K. The antioxidative function of alpha-ketoglutarate and its applications. Biomed Res Int 2018; 2018: 3408467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Borgogna JC, Shardell MD, Santori EK, et al. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: a cross-sectional analysis. BJOG Int J Obstetr Gynaecol 2020; 127: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yeoman CJ, Thomas SM, Miller ME, et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS ONE 2013; 8(2): e56111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Srinivasan S, Morgan MT, Fiedler TL, et al. Metabolic signatures of bacterial vaginosis. mBio 2015; 6: e00204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Guaschino S, Benvenuti C. and SOPHY Study Group. SOPHY project: an observational study of vaginal pH, lifestyle and correct intimate hygiene in women of different ages and in different physiopathological conditions. Minerva Ginecol 2008; 60(5): 353–362. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455057231223716 for Maintaining vulvar, vaginal and perineal health: Clinical considerations by Alessandra Graziottin in Women’s Health