Abstract
Background:
In Alzheimer’s disease (AD), the gradual accumulation of amyloid-β (Aβ) and tau proteins may underlie alterations in empathy.
Objective:
To assess whether tau aggregation in the medial temporal lobes related to differences in cognitive empathy (the ability to take others’ perspectives) and emotional empathy (the ability to experience others’ feelings) in AD.
Methods:
Older adults (n = 105) completed molecular Aβ positron emission tomography (PET) scans. Sixty-eight of the participants (35 women) were Aβ positive and symptomatic with diagnoses of mild cognitive impairment, dementia of the Alzheimer’s type, logopenic variant primary progressive aphasia, or posterior cortical atrophy. The remaining 37 (22 women) were asymptomatic Aβ negative healthy older controls. Using the Interpersonal Reactivity Index, we compared current levels of informant-rated cognitive empathy (Perspective-Taking subscale) and emotional empathy (Empathic Concern subscale) in the Aβ positive and negative participants. The Aβ positive participants also underwent molecular tau-PET scans, which were used to investigate whether regional tau burden in the bilateral medial temporal lobes related to empathy.
Results:
Aβ positive participants had lower perspective-taking and higher empathic concern than Aβ negative healthy controls. Medial temporal tau aggregation in the Aβ positive participants had divergent associations with cognitive and emotional empathy. Whereas greater tau burden in the amygdala predicted lower perspective-taking, greater tau burden in the entorhinal cortex predicted greater empathic concern. Tau burden in the parahippocampal cortex did not predict either form of empathy.
Conclusions:
Across AD clinical syndromes, medial temporal lobe tau aggregation is associated with lower perspective-taking yet higher empathic concern.
Keywords: Alzheimer’s disease, affective resonance, empathy, mentalization, social cognition, tau proteins
INTRODUCTION
In Alzheimer’s disease (AD), the progressive accumulation of amyloid-β (Aβ) plaques and tau neurofibrillary tangles [1–3] can manifest as several distinct clinical syndromes [4]. Although the topography of Aβ aggregation in the neocortex is diffuse and not consistently related to symptoms or disease progression [5–7], regional tau deposition closely aligns with cognitive deficits [8–13] and atrophy (e.g., [14–16]) and predicts functional decline [17] and clinical severity [18, 19] better than Aβ. In AD, tau first aggregates in the brainstem (e.g., locus coeruleus) [20, 21] and medial temporal lobes (MTL) [3, 22, 23]. In people with mild cognitive impairment (MCI)—the clinical phase that precedes functional impairment—and “typical” dementia of the Alzheimer’s type (DAT), MTL pathology often gives rise to episodic memory decline [24]. “Atypical” AD clinical syndromes emerge when pathological changes target other brain regions and cause deficits in language, visuospatial processing, or executive functioning [25, 26]. Whereas individuals with prominent language and phonological processing deficits may meet criteria for logopenic variant primary progressive aphasia (lvPPA) [27], those with predominant visual and spatial deficits may receive a diagnosis of posterior cortical atrophy (PCA) [28].
Cognitive symptoms in AD have been well characterized [24], but the changes in behavior and emotion that also arise remain poorly understood (e.g., [29–31]). Heightened social and emotional sensitivity may contribute to the neuropsychiatric symptoms that emerge in the symptomatic phase of AD [32–38] and to the gains in negative emotional reactivity, stress, and loneliness that characterize the preclinical period [39–42]. Neuropathological changes in AD may also affect how people understand or respond to others and thereby alter cognitive empathy (the ability to adopt the viewpoint of others) as well as emotional empathy (the ability to experience what others are feeling via physiological and motor mirroring systems) [43]. Tests of cognitive empathy often entail recognizing emotions in others [44], but cognitive empathy also includes perspective-taking abilities [45]. Although there is some evidence that people with AD have lower informant-reported perspective-taking than healthy older adults [29], it is unclear whether this impairment reflects more widespread cognitive deficits or problems with mentalizing per se [46]. There is accumulating evidence, in contrast, that emotional empathy climbs in the early stages of AD, even when people are cognitively asymptomatic. In our prior research, we have found elevations in two forms of emotional empathy—emotional contagion (a self-oriented form of emotional empathy that can be accompanied by feelings of distress) and empathic concern (an other-oriented form of emotional empathy that promotes prosocial actions) [47, 48]—in people on an AD trajectory. While emotional contagion is higher in MCI and AD than in healthy older adults [49], empathic concern increases more over time in cognitively healthy older adults with higher cortical Aβ than in those with lower levels [50].
In people with AD, tau deposition in the MTL may contribute to changes in empathy. Cognitive and emotional empathy have largely distinct neuroanatomical underpinnings [43, 51], but both rely on the MTL [51, 52]. Cognitive empathy, which allows individuals to step outside of their own minds and to take the perspective of another, often engages MTL structures (primarily the entorhinal cortex and hippocampus) as well as other regions in the default mode network [43, 51–56]. Tau pathology in the MTL, therefore, may disrupt perspective-taking as the default mode network declines in AD [53–55, 57–59] but have the opposite effect on emotional empathy. Unlike cognitive empathy, which does not necessarily elicit changes in subjective experience [51], emotional empathy evokes shared feeling states between people by activating the MTL (primarily the amygdala) and other structures in the salience network [51, 60], a system that supports emotion generation and interoception [61–64]. In early AD, default mode network dysfunction is accompanied by heightened salience network connectivity, a functional gain that relates to neuropsychiatric symptoms in the clinical phase [65, 66] and increasing empathic concern in the preclinical period [50]. While there is some evidence that early tau deposition in the MTL relates to heightened stress, anxiety, and depression [41, 42], whether MTL tau aggregation also relates to higher emotional empathy in AD is unknown.
Here, we investigated cognitive and emotional empathy in AD clinical syndromes and their associations with MTL tau pathology. As neuropathological changes in AD can give rise to a diverse set of syndromes that vary in the extent to which they affect the MTL [67, 68], we included participants with a wide range of symptoms who met criteria for MCI, DAT, lvPPA, or PCA. All participants with AD were in the very mild to mild clinical stages of disease and were Aβ positive (Aβ+) on molecular positron emission tomography (PET) imaging. Given that previous studies have found diminished cognitive empathy and enhanced emotional empathy in people along the AD continuum [29, 49, 50], we expected that symptomatic Aβ+ participants would have lower perspective-taking (a measure of cognitive empathy) yet higher empathic concern (a measure of emotional empathy) than Aβ negative (Aβ-) healthy older controls. While elevated Aβ accumulation may set the stage for empathy alterations in AD, regional tau aggregation may play a critical role in shaping empathy over the course of the disease. We hypothesized that, just as the default mode and salience networks show divergent functional changes in the setting of AD pathophysiology [69–71], tau aggregation in the MTL would also have opposing effects on empathy. We expected that greater tau burden in the entorhinal cortex and amygdala would relate to lower perspective-taking but higher empathic concern in Aβ+ participants. In contrast, we anticipated that tau burden in the parahippocampal cortex, an MTL region that is less involved in empathy but more involved with cognition [72], would not relate to cognitive or emotional empathy.
MATERIALS AND METHODS
Participants
A total of 105 older adults (48.0–85.5 years old) recruited from the University of California, San Francisco (UCSF) Memory and Aging Center participated in the present study. The sample included 68 symptomatic participants who met criteria for MCI (n = 14) [73], DAT (n = 33) [74], lvPPA (n = 10) [27], or PCA (n = 11) [28] and 37 healthy older controls. In the MCI group, 12 participants had an amnestic-predominant presentation, and two had a non-amnestic, multi-domain syndrome [73, 75]. The study was approved by the UCSF Human Research Protection Program, and informed consent was given by all participants, or their surrogates, before participating.
Participants underwent multidisciplinary diagnostic evaluations that included a neurological examination, neuropsychological testing, neuroimaging, and an informant-based assessment of daily functioning using the Clinical Dementia Rating Scale (CDR) [76]. The neuropsychological assessment included tests of episodic memory, executive functioning, visuospatial processing, language, and mood [77]. All symptomatic participants had CDR total scores of 1 or less, which suggests they were in the very mild to mild stages of impairment. The healthy controls underwent the same diagnostic evaluation as those in the symptomatic group. They were free of current psychiatric or neurological disorders, had a score of 0 on the CDR (indicating no functional impairment), and a score of 27 or greater on the Mini-Mental State Examination (MMSE), an assessment of overall mental status [78].
Cognitive and emotional empathy
Informants rated participants’ current levels of empathy using the Interpersonal Reactivity Index (IRI), a multidimensional empathy measure [79]. Informants assessed participants’ behavior using a scale of 1 (does not describe well) to 5 (describes well). Each IRI subscale contains scores ranging from 7 to 35 where higher scores indicate greater levels of empathy. Informant reports are a reliable technique to evaluate empathy and personality in individuals who are healthy [80, 81] and in those with dementia [29, 82].
In line with our prior work on AD [29, 49, 50], we used the Perspective-Taking IRI subscale as our measure of cognitive empathy and the Empathic Concern IRI subscale as our measure of emotional empathy. While the Perspective-Taking subscale measures the tendency to adopt others’ points of view (e.g., “Likely to try to understand others better by imagining how things look from their perspective”), the Empathic Concern subscale evaluates other-oriented feelings of compassion and concern (e.g., “Would show tender, concerned feelings for people less fortunate than them”).
Molecular PET scan acquisition and processing
Amyloid-PET scans
Participants underwent Aβ-PET imaging with either 11C-Pittsburgh compound B (PIB) or 18F-AV-45 (Florbetapir) PET ligands. The Aβ-PET scans were used to dichotomize Aβ levels into Aβ+ and Aβ-, which indicated the presence or absence of AD pathology following previous methods (e.g., [83, 84]). All participants in the symptomatic group were Aβ+ as determined by a visual read [83, 85] of their PIB (n = 67) or Florbetapir (n = 1) Aβ-PET scans at the time of their empathy assessment (mean [M] = 8.4 days after IRI, standard deviation [SD] = 239.3 days). All healthy controls were Aβ- on visual reads of their Florbetapir (n = 36) or PIB (n = 1) Aβ-PET scans. An additional 23 healthy controls had available empathy data but were Aβ+, and thus were excluded. For the Aβ- healthy controls, the Aβ-PET imaging occurred after the empathy assessment (M = 6.5 years after IRI; SD = 2.5 years), at which time they were still cognitively normal and functionally intact.
Tau-PET scans
Symptomatic participants also underwent 18F-AV-1451 (Flortaucipir) tau-PET imaging using established protocols [10, 86, 87], and these scans were used to correlate regional MTL tau aggregation with empathy. Standardized uptake value ratio (SUVR) values were calculated according to previous methods [10, 86, 87], using PET data acquired between 80- and 100-minutes post tracer injection, and using the inferior cerebellum grey matter as a reference region. Freesurfer segmentation [http://surfer.nmr.mgh.harvard.edu; 88] was performed to acquire regional values in right and left MTL regions of interest (i.e., entorhinal cortex, amygdala, and parahippocampal cortex) using the Desikan-Killiany atlas. All the included imaging data passed our visual quality control checks that assessed the magnetic resonance imaging (MRI) to PET scan registration and the Freesurfer segmentation. The data were not partial volume corrected because this entails making multiple assumptions, and our previous work has shown that SUVR values computed with and without partial volume correction are highly correlated (r = 0.98) [89].
We hypothesized that the entorhinal cortex and amygdala would relate to empathy in AD because these structures are hubs in networks that support empathy and typically have more tau signal than the parahippocampal cortex [43, 51–56, 60]. The parahippocampal cortex plays a central role in contextual associations [72] and, while it can be related to socioemotional behaviors (e.g., [90]), is less critical for empathy. Thus, we included the parahippocampal cortex as a control region within the MTL as we did not expect tau burden in this area would relate to empathy in AD. The tau-PET scans were obtained in close proximity to the Aβ-PET scans (M = 26.7 days after Aβ-PET scans, SD = 225.2 days) and empathy assessments (M = 35.1 days after IRI; SD = 106.0 days).
Analyses
All analyses were conducted in R v.4.0.3 [91].
Empathy analyses
We used multivariate linear regression analyses to compare the Aβ+ symptomatic and Aβ- healthy control groups on perspective-taking and empathic concern. Group (Aβ+ = 1, Aβ- = 0) was the independent variable, and the perspective-taking or empathic concern subscale score was the dependent variable. Covariates of non-interest included gender, age at IRI, and the contrasting IRI subscale score (i.e., the empathic concern score in the perspective-taking analysis and the perspective-taking score in the empathic concern analysis) to isolate the effects of cognitive or emotional empathy in each model.
We next conducted exploratory multivariate linear regression analyses to compare each clinical group (MCI, DAT, lvPPA, and PCA) to the healthy controls on perspective-taking and empathic concern (using the same covariates as above). Follow-up Type-II analyses of variance were conducted to determine the significance of the fixed effects. Results were considered significant if the p-values of the post hoc pairwise comparisons survived Bonferroni correction for four analyses (to account for pairwise comparisons between each clinical group and the healthy controls).
Tau and empathy analyses
We conducted forward-selection hierarchical regression models [92] using the MASS package in R [93] to examine associations between regional tau burden and empathy in the Aβ+ group. This approach avoids problems with multiple comparisons as it allowed the statistical program to determine the regions in which tau SUVR accounted for a significant amount of variance in empathy above and beyond the covariates of non-interest that were forced into the model in step one [94]. This is a conservative test of our hypothesis that the entorhinal cortex and amygdala are important for empathy because these regions must emerge as significant predictors from a group of candidate predictor regions (i.e., tau SUVR in the entorhinal cortex, amygdala, and parahippocampal cortex). To minimize collinearity between homologous regions and to assess potential effects of lateralization on emotion-related processes [95], we ran separate hierarchical regression models in each hemisphere. Covariates of non-interest included gender, age at IRI, CDR total score (to account for disease severity), and the contrasting IRI subscale (as described above). To account for the time interval between the tau-PET scan and IRI assessment, we computed a “time interval” variable (IRI date –tau-PET scan date) for each participant and included this variable as an additional covariate of non-interest in these analyses. Only variables that were significant predictors of empathy at p < 0.05 were eligible to enter the final regression model whereas variables that did not account for significant variance were excluded. All histograms and P–P plots of standardized residuals for the final regression models demonstrated normally distributed error terms, indicating that the assumptions of regression models were met. All variables in the final regression models demonstrated very weak collinearity with one another such that variance inflation factors < 2.0.
RESULTS
Aβ+ and Aβ- groups had similar demographics but different cognitive profiles
The Aβ+ symptomatic and Aβ- healthy control groups were similar in age, gender, and education (all p > 0.05). On average, the two groups were in their mid-sixties, highly educated, and predominantly White/European American. The Aβ+ participants were in the very mild to mild range of functional impairment, as measured by the CDR, and had lower scores on neuropsychological tests of episodic memory, language, visuospatial processing, and executive functioning than the Aβ- healthy controls. Depressive symptoms were minimal across the sample but somewhat higher in the Aβ+ than in the Aβ- group. Follow-up analyses that compared the MCI, DAT, lvPPA, and PCA groups to the Aβ- healthy controls showed expected cognitive profiles with milder deficits in MCI, episodic memory impairment in DAT, language difficulties in lvPPA, and visuospatial dysfunction in PCA. See Table 1.
Table 1.
Group demographic and clinical data
| Measures | Groups | ||||||
| Aβ- | Aβ+ | Statistics | MCI | DAT | lvPPA | PCA | |
| Healthy | Symptomatic | (Aβ- versus Aβ+) | |||||
| Controls | Participants | ||||||
| n= | 37 | 68 | 14 | 33 | 10 | 11 | |
| Race/Ethnicity (n=) | |||||||
| Asian/Asian American | 0 | 3 | 0 | 3 | 0 | 0 | |
| Black/African American | 0 | 1 | 0 | 1 | 0 | 0 | |
| White/European American | 35 | 58 | 10 | 28 | 9 | 11 | |
| Other | 2 | 0 | 0 | 0 | 0 | 0 | |
| Gender (% female) | 59.5 | 51.5 | χ2(1, N = 105) = 0.62, p = 0.43 | 50.0 | 45.5e | 40.0e | 81.8c,d |
| Age at IRI: M (SD) | 68.1 (5.9) | 65.4 (9.1) | t(99.68) = 1.84, p = 0.07 | 69.3 (7.7)d,e | 67.6 (9.5)d,e | 59.6 (7.6)a,b,c | 59.0 (5.3)a,b,c |
| Education (years): M (SD) | 16.8 (1.6) | 16.7 (2.8) | t(101.95) = 0.10, p = 0.92 | 17.9 (3.3)e | 16.6 (2.5)e | 17.9 (2.4)e | 14.5 (1.9)a,b,c,d |
| Handedness (left/right/ambidextrous) | 2/35/0 | 5/45/1 | χ2(2, N = 105) = 1.34, p = 0.51 | 1/7/0 | 2/22/1 | 1/8/0 | 1/8/0 |
| Mini-Mental State Examination (/30): M (SD) | 29.5 (0.9) | 21.9 (6.0)a | t(70.99) = 10.19, p = 1.58 × 10–15 | 26.8 (3.1)a,c,d,e | 21.0 (5.5)a,b | 20.1 (8.3)a,b | 20.5 (5.0)a,b |
| Clinical Dementia Rating Scale Score (/3): M (SD) | 0.0 (0.0) | 0.7 (0.3)a | t(67.00) = –19.98, p = 6.29 x10–30 | 0.5 (0.0)c | 0.8 (0.2)a,b,d | 0.6 (0.4)a,c | 0.6 (0.3)a |
| Geriatric Depression Scale (/30): M (SD) | 1.8 (2.3) | 6.1 (4.1)a | t(89.43) = –6.37, p = 7.96 × 10–9 | 3.9 (2.4)a,c | 6.9 (4.5)a,b | 6.3 (4.6)a | 5.8 (3.6)a |
| Modified Trails Number Lines Correct: M (SD) | 14.0 (0.0) | 11.0 (5.2)a | t(47.00) = 4.09, p = 1.70 × 10–4 | 14.0 (0.0)c | 9.7 (5.8)a,b | 12.7 (3.3) | 8.8 (6.7) |
| Modified Trails Errors: M (SD) | 0.3 (0.6) | 0.8 (1.0)a | t(77.30) = –2.36, p = 0.02 | 0.3 (0.5)c | 1.0 (1.2)a,b | 0.5 (0.8) | 0.8 (0.8) |
| Phonemic Fluency: M (SD) | 14.3 (4.1) | 10.2 (5.8)a | t(82.23) = 4.00, p = 1.39 × 10–4 | 13.2 (2.9)c,d | 10.2 (6.3)a,b | 8.0 (5.4)a,b | 8.9 (6.2)a |
| Semantic Fluency: M (SD) | 22.1 (5.2) | 12.0 (6.4)a | t(85.71) = 8.52, p = 4.79 × 10–13 | 17.3 (5.1)a,c,d,e | 10.8 (5.7)a,b | 9.9 (7.6)a,b | 11.9 (6.1)a,b |
| Design Fluency Correct: M (SD) | 10.5 (3.4) | 6.3 (3.5)a | t(78.18) = 5.81, p = 1.29 × 10–7 | 9.1 (2.5)c,e | 5.9 (3.2)a,b,e | 8.1 (2.3)a,e | 2.7 (2.4)a,b,c,d |
| Repetition (/5): M (SD) | 4.8 (0.4) | 3.6 (1.5)a | t(80.82) = 6.10, p = 3.46 × 10–8 | 4.4 (1.1)d | 3.8 (1.3)a,d | 1.8 (1.5)a,b,c,e | 3.4 (1.3)a,d |
| Digit Span Backward: M (SD) | 5.5 (1.3) | 3.8 (1.5)a | t(79.81) = 5.80, p = 1.26 × 10–7 | 5.2 (1.1)c,d,e | 3.7 (1.3)a,b | 3.0 (1.0)a,b | 3.1 (1.6)a,b |
| Benson Figure Copy 10-Minute Recall (/17): M (SD) | 13.3 (1.9) | 4.3 (4.0)a | t(93.80) = 15.12, p = 6.96 × 10–27 | 6.8 (4.1)a,c | 2.7 (2.9) a,b,d | 6.4 (4.7)a,c | 4.3 (4.1)a |
| Benson Figure Copy (/17): M (SD) | 15.6 (0.8) | 12.3 (4.9)a | t(72.89) = 5.38, p = 8.76 × 10–7 | 14.6 (2.1)e | 12.9 (4.3)a,e | 13.6 (3.9)e | 6.1 (5.2)a,b,c,d |
| Modified Boston Naming Test Correct (total/15): M (SD) | 14.6 (0.6) | 11.6 (3.6)a | t(69.03) = 6.51, p = 1.00 × 10–8 | 13.9 (1.6)c,d,e | 12.1 (3.0)a,b | 9.8 (4.5)a,b | 9.3 (4.1)a,b |
Analyses included Pearson’s Chi-squared tests and Welch’s t-tests. aIndicates a significant pairwise difference in comparison with Aβ- healthy controls (p < 0.05). bIndicates a significant pairwise difference in comparison with MCI (p < 0.05). cIndicates a significant pairwise difference in comparison with DAT (p < 0.05). dIndicates a significant pairwise difference in comparison with lvPPA (p < 0.05). eIndicates a significant pairwise difference in comparison with PCA (p < 0.05). MCI, mild cognitive impairment; DAT, dementia of the Alzheimer’s type; lvPPA, logopenic variant primary progressive aphasia; PCA, posterior cortical atrophy.
Lower perspective-taking and higher empathic concern in the Aβ+ group
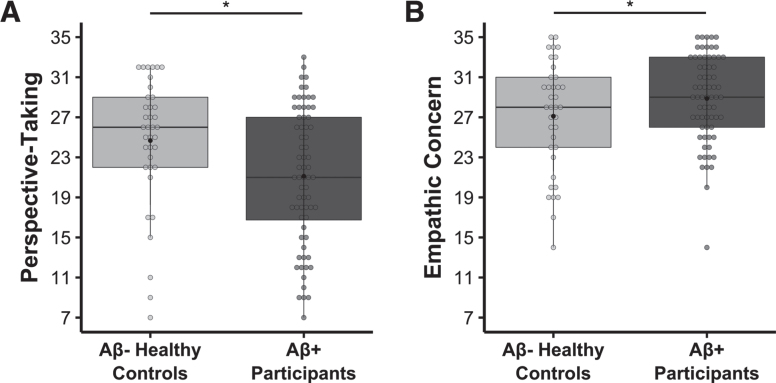
There was a main effect of group on both perspective-taking, b = –5.339, t(100) = –4.824, p = 5.039 × 10–6, model adjusted R2 = 0.431, n = 105 (Fig. 1A), and empathic concern, b = 3.550, t(100) = 4.925, p = 3.344 × 10–6, model adjusted R2 = 0.513, n = 105 (Fig. 1B), such that the Aβ+ participants had lower perspective-taking but higher empathic concern than the Aβ- healthy controls. There was also a main effect of gender in the empathic concern regression model, b = 3.202, t(100) = 4.609, p = 1.197 × 10–5, model adjusted R2 = 0.513, n = 105, where women had higher empathic concern than men, which is consistent with prior studies (e.g., [45, 79]). In addition, there was a main effect of perspective-taking in the empathic concern model, b = 0.397, t(100) = 7.628, p = 1.419 × 10–11, model adjusted R2 = 0.513, n = 105, and of empathic concern in the perspective-taking model, b = 0.927, t(100) = 7.628, p = 1.419 × 10–11, model adjusted R2 = 0.431, n = 105, which suggested a positive association between these two empathy subscales.
Fig. 1.
Lower perspective-taking, but higher empathic concern, in the Aβ+ symptomatic participants relative to the Aβ- healthy controls. Multivariate linear regression analyses found that, compared to Aβ- healthy controls, the Aβ+ symptomatic group had A) lower perspective-taking (b = –5.339, t(100) = –4.824, p = 5.039 × 10–6, model adjusted R2 = 0.431, n = 105) and B) higher empathic concern (b = 3.550, t(100) = 4.925, p = 3.344 × 10–6, model adjusted R2 = 0.513, n = 105). Covariates of non-interest in these models included age at IRI, gender, and the contrasting IRI subscale (i.e., empathic concern or perspective-taking). Raw perspective-taking scores and empathic concern scores are shown in the figure.
To investigate the role of gender in more detail, we conducted additional analyses to examine whether there was an interaction between group and gender. These analyses (which included all the same covariates as the previous models in addition to the interaction term between group and gender) revealed no interactions for either the perspective-taking, interaction b = 0.612, t(99) = 0.284, p = 0.777, model adjusted R2 = 0.426, n = 105, or empathic concern, interaction b = –1.636, t(99) = –1.173, p = 0.243, model adjusted R2 = 0.515, n = 105, models. All findings in the original analyses remained significant, however, including the main effect of group on perspective-taking, b = –5.696, t(99) = –3.391, p = 0.001, model adjusted R2 = 0.426, n = 105, and empathic concern, b = 4.467, t(99) = 4.206, p = 5.715 × 10–5, model adjusted R2 = 0.515, n = 105, such that Aβ+ participants had diminished perspective-taking but elevated empathic concern relative to the Aβ- healthy controls.
We next conducted a follow-up exploratory analysis of perspective-taking that compared each of the Aβ+ clinical syndromes to the Aβ- healthy controls. In this model (which included the same covariates as our original analyses), there was a main effect of group, F(4, 97) = 7.571, p = 2.374 × 10–5, model adjusted R2 = 0.449, n = 105. Bonferroni-corrected post hoc analyses revealed lower perspective-taking in the DAT, t(97) = –4.651, pBONFERRONI = 4.183 × 10–5, Cohen’s d = 1.157; lvPPA, t(97) = –4.176, pBONFERRONI = 2.590 × 10–4, Cohen’s d = 1.579; and PCA, t(97) = –2.658, pBONFERRONI = 0.037, Cohen’s d = 0.974, groups than in Aβ- healthy controls (Supplementary Figure 1A). Perspective-taking in the MCI and Aβ- healthy control groups did not differ, t(97) = –1.726, pBONFERRONI = 0.350, Cohen’s d = 0.556, however. There was also a main effect of empathic concern, F(1, 97) = 56.497, p = 2.817 × 10–11, model adjusted R2 = 0.449, n = 105, in line with the previous analysis.
In a similar follow-up exploratory analysis of empathic concern, there was also a main effect of group, F(4, 97) = 6.437, p = 1.228 × 10–4, model adjusted R2 = 0.507, n = 105. Bonferroni-corrected post hoc analyses revealed higher empathic concern in the MCI, t(97) = 2.825, pBONFERRONI = 0.023, Cohen’s d = 0.889; DAT, t(97) = 4.763, pBONFERRONI = 2.673 × 10–5, Cohen’s d = 1.180; and lvPPA, t(97) = 2.996, pBONFERRONI = 0.014, Cohen’s d = 1.177, groups than in the Aβ- healthy controls (Supplementary Figure 1B). Empathic concern in the PCA and Aβ- healthy control groups did not differ, however, t(97) = 2.095, pBONFERRONI = 0.155, Cohen’s d = 0.778. Additional main effects included gender, F(1, 97) = 21.850, p = 9.518 × 10–6, model adjusted R2 = 0.507, n = 105, and perspective-taking, F(1, 97) = 56.497, p = 2.817 × 10–11, model adjusted R2 = 0.507, n = 105, consistent with the previous analysis.
MTL tau burden had divergent associations with perspective-taking and empathic concern in Aβ+ participants
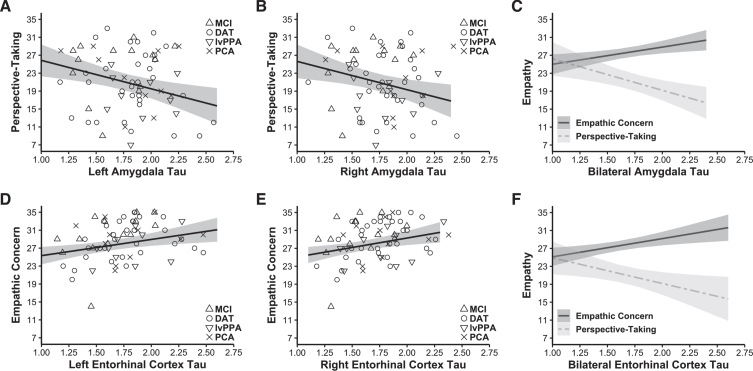
Forward-selection hierarchical regression models in the Aβ+ group revealed that greater tau burden in the left amygdala (final model b = –6.349, final model coefficient p = 0.003, final model R2 = 0.514, R2 change from preliminary model = 0.074, final model adjusted R2 = 0.466, n = 68; Fig. 2A) and right amygdala (final model b = –6.305, final model coefficient p = 0.009, final model R2 = 0.501, R2 change from preliminary model = 0.060, final model adjusted R2 = 0.451, n = 68; Fig. 2B) was associated with lower perspective-taking. Other main effects included empathic concern and the time interval between participants’ tau-PET scans and IRI (significant in both models), which suggested that a greater time interval between the IRI assessments and the tau-PET scans related to lower perspective-taking. See Table 2. Tau burden in neither the left nor the right parahippocampal cortex predicted perspective-taking.
Fig. 2.
Tau burden in the amygdala had a negative association with perspective-taking, but tau burden in the entorhinal cortex had a positive association with empathic concern. Forward-selection hierarchical regression models conducted in the Aβ+ group found tau burden in the MTL was differentially related to empathy. Greater tau burden in the A) left (final model b = –6.349, final model coefficient p = 0.003, final model adjusted R2 = 0.466, n = 68) and B) right amygdala (final model b = –6.305, final model coefficient p = 0.009, final model adjusted R2 = 0.451, n = 68) related to lower perspective-taking. In contrast, greater tau burden in the D) left (final model b = 3.610, final model coefficient p = 0.007, final model adjusted R2 = 0.518, n = 68) and E) right entorhinal cortex (final model b = 4.217, final model coefficient p = 0.006, final model adjusted R2 = 0.520, n = 68) was associated with greater empathic concern. Panels C and F indicate perspective-taking and empathic concern as they relate to tau burden in the bilateral amygdala and entorhinal cortex (tau burden in these bilateral regions were calculated as weighted averages using the number of voxels present in each MTL region). Covariates of non-interest in these analyses included gender, age at IRI, time interval in days between the tau-PET scan and IRI, CDR total score, and the contrasting IRI subscale (i.e., empathic concern or perspective-taking). Plotted regressions reflect the predicted fits from the analysis models, while the scatterplots indicate raw data grouped by diagnosis. MCI, mild cognitive impairment; DAT, dementia of the Alzheimer’s type; lvPPA, logopenic variant primary progressive aphasia; PCA, posterior cortical atrophy.
Table 2.
Forward-selection hierarchical regression analyses assessing the impact of tau burden in the left and right amygdala on perspective-taking. Gender is coded such that men = 0 and women = 1
| Left Amygdala | Right Amygdala | |||||
| Estimates | Confidence Interval | p | Estimates | Confidence Interval | p | |
| Intercept | 5.354 | –8.719 –19.428 | 0.450 | 4.607 | –9.796 –19.009 | 0.525 |
| Tau-PET SUVR | –6.349 | –10.526 ––2.171 | 0.003 | –6.305 | –10.963 ––1.646 | 0.009 |
| Age | –0.029 | –0.170 –0.112 | 0.682 | –0.027 | –0.170 –0.116 | 0.706 |
| Gender | 0.735 | –2.088 –3.559 | 0.604 | 0.665 | –2.200 –3.530 | 0.644 |
| Empathic Concern | 1.065 | 0.747 –1.382 | <0.001 | 1.059 | 0.737 –1.381 | <0.001 |
| IRI-PET Interval | –0.015 | –0.027 ––0.002 | 0.020 | –0.014 | –0.027 ––0.002 | 0.024 |
| CDR Score | –3.515 | –7.903 –0.874 | 0.114 | –2.753 | –7.311 –1.804 | 0.232 |
Forward-selection hierarchical regression models in the Aβ+ group found that greater tau burden in the left entorhinal cortex (final model b = 3.610, final model coefficient p = 0.007, final model R2 = 0.561, R2 change from preliminary model = 0.056, final model adjusted R2 = 0.518, n = 68; Fig. 2D) and right entorhinal cortex (final model b = 4.217, final model coefficient p = 0.006, final model R2 = 0.563, R2 change from preliminary model = 0.059, final model adjusted R2 = 0.520, n = 68; Fig. 2E) was associated with greater empathic concern. Additional main effects included perspective-taking (significant in both models) and gender (significant in the left hemisphere model), which suggested that higher perspective-taking was associated with higher empathic concern. See Table 3. Tau burden in neither the left nor the right parahippocampal cortex predicted empathic concern.
Table 3.
Forward-selection hierarchical regression analyses assessing the impact of tau burden in the left and right entorhinal cortex on empathic concern. Gender is coded such that men = 0 and women = 1
| Left Entorhinal Cortex | Right Entorhinal Cortex | |||||
| Estimates | Confidence Interval | p | Estimates | Confidence Interval | p | |
| Intercept | 10.120 | 2.267 – 17.973 | 0.012 | 9.448 | 1.383 – 17.514 | 0.022 |
| Tau-PET SUVR | 3.610 | 1.024 – 6.196 | 0.007 | 4.217 | 1.270 – 7.164 | 0.006 |
| Age | 0.048 | –0.037 – 0.132 | 0.263 | 0.051 | –0.033 – 0.136 | 0.228 |
| Gender | 1.722 | 0.060 – 3.384 | 0.043 | 1.531 | –0.171 – 3.232 | 0.077 |
| Perspective-Taking | 0.379 | 0.263 – 0.495 | <0.001 | 0.380 | 0.264 – 0.495 | <0.001 |
| IRI-PET Interval | 0.007 | –0.001 – 0.015 | 0.072 | 0.007 | –0.001 – 0.014 | 0.073 |
| CDR Score | 0.975 | –1.739 – 3.689 | 0.475 | 0.347 | –2.401 – 3.094 | 0.802 |
DISCUSSION
The present study uncovered novel associations between MTL tau burden and empathy in symptomatic Aβ+ participants. Compared to their cognitively healthy Aβ- counterparts, Aβ+ participants with a broad range of cognitive symptoms had diminished perspective-taking but greater empathic concern. In exploratory analyses of the different clinical syndromes, perspective-taking was lower in DAT, lvPPA, and PCA (but not MCI) than in Aβ- healthy controls while empathic concern in MCI, DAT, and lvPPA (but not PCA) was higher. In the Aβ+ participants, regional tau burden had opposing associations with empathy such that greater MTL tau pathology was associated with lower cognitive empathy yet higher emotional empathy. While greater MTL tau aggregation in the amygdala related to lower perspective-taking, greater MTL tau aggregation in the entorhinal cortex related to higher empathic concern. Tau aggregation in the parahippocampal cortex, however, was not associated with either form of empathy.
These findings build on our previous studies that revealed enhanced emotional empathy in AD [49, 50]. Although perspective-taking may decline in people with AD [29] as they lose their ability to take another’s point of view [96, 97], emotional empathy—a more automatic form of empathy that does not require higher-order cognition—climbs. Unlike emotional contagion, which can elicit self-oriented feelings of distress or being overwhelmed during negative emotional situations [48], empathic concern focuses attention outward onto the needs of others and promotes prosocial actions such as helping and consolation [43, 47]. In a prior study, we found greater gains in empathic concern over time in Aβ+ than Aβ- cognitively healthy older adults [50], which suggested there are enhancements in this form of emotional empathy in the preclinical stages of AD. In the present study, we expand on this work by showing that empathic concern is also higher in the clinical phase of AD. In general, symptomatic Aβ+ participants had higher empathic concern than Aβ- healthy controls, and follow-up pairwise comparisons revealed this difference was driven primarily by elevations in the MCI, DAT, and lvPPA groups. Although the emotional empathy enhancement in PCA failed to reach significance, our results suggest heightened emotional empathy is a common feature of AD pathophysiology.
Our results suggest that, during the early symptomatic phase of AD, tau deposition in the MTL contributes to alterations in empathy. The MTL plays important roles in empathy and other socioemotional processes including perception of emotional and social cues [98–101], emotion generation [63, 102], interpersonal sensitivity [103], and affiliative behavior [104]. Often a prominent site of early tau pathology in DAT and MCI [3, 22, 23], the MTL is also affected in lvPPA and PCA, albeit to a lesser extent [10, 105, 106]. Taken together with our prior study that found empathic concern gains in cognitively healthy Aβ+ older adults [50], our research suggests that Aβ and tau may both contribute to emotional empathy increases in people on an AD trajectory. Previous research has suggested that Aβ and tau interact through both local and remote connections and that Aβ deposition facilitates the spread of tau beyond the MTL [107]. Just as the synergistic effects of tau and Aβ lead to cognitive decline [108], they may also drive empathy alterations in AD. We speculate that tau accumulation in the MTL may impede perspective-taking as the default mode network declines in AD [109–113] but accentuate empathic concern, a form of emotional empathy that depends on the salience network [51, 60]. Our findings are consistent with a picture of early AD in which neuropathological changes in the MTL and its connected networks [65, 66, 114] lead to heightened social sensitivity and emotional empathy.
The present study has several important limitations to consider. First, our data were cross-sectional, and we were unable to determine how longitudinal tau aggregation relates to changes in empathy over time. Neurofibrillary tau tangles in the MTL arise early in the AD pathophysiological cascade [115–119] and then spread to other vulnerable regions [107, 120–122]. Within the MTL, tau first aggregates in the entorhinal cortex before spreading into the hippocampus, amygdala, and parahippocampal cortex and then affecting cerebral neocortical regions [3, 22, 23]. As tau in the entorhinal cortex was associated with elevated empathic concern, and tau in the amygdala was associated with lower perspective-taking, our results suggest the possibility that emotional empathy begins to increase before cognitive empathy declines in those on an AD course. Future longitudinal studies will be needed to investigate this question in detail, however. Second, we quantified tau deposition in MTL regions of interest, but these structures are comprised of smaller subregions that differ in their functions and connections [116, 123]. Whereas the basolateral amygdala plays an important role in evaluating affective and social information [124], the central nucleus generates affective responses via its connections to subcortical pattern generators [125–129]. Human and non-human animal studies of the entorhinal cortex have found anatomical subdivisions [130–132] such that the medial and lateral portions have different functions [133–136] and connectivity patterns [135, 137–139]. As the limited spatial resolution of PET did not allow us to differentiate between amygdala nuclei or entorhinal subregions, we were unable to determine whether tau burden in these areas had unique associations with perspective-taking or empathic concern. More fine-grained functional or structural MRI analyses may help to resolve the anatomical correlates of empathy change in AD. Third, as we could not include task-free neuroimaging in our study, we could not test whether expected changes in default mode network or salience network functional connectivity related to MTL tau burden and empathy in this sample. Additional research is warranted to address this question and to elucidate the neural mechanisms underlying cognitive and empathy alterations in AD.
With recent advances in AD biomarkers, studies can now link cognitive and behavioral measures with in vivo neuropathological changes in the brain and body. Relatively little is known about emotions and social behavior in typical and atypical AD syndromes [140], but our findings contribute to an emerging picture of the empathy changes that characterize early AD. The results of the present study suggest that lower perspective-taking and higher empathic concern may be common features of AD pathophysiology. As disease-modifying therapies become increasingly available for people with AD, new clinical outcomes will be needed to assess improvement, stability, or decline. Empathy may be an overlooked area of change in AD that, if evaluated with rigor, could help to expedite detection and to improve monitoring by offering an additional window into disease progression.
Supplementary Material
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-230367.
FUNDING
This study was supported by the Larry L. Hillblom Foundation, the UCSF Alzheimer’s Disease Research Center (P30AG062422, P50AG023501), the John Douglas French Alzheimer’s Foundation, and grants from the National Institute on Aging (K23AG040127, K99AG065501, R01AG057204, and R01AG073244).
CONFLICT OF INTEREST
Dr. Rabinovici receives research support from Avid Radiopharmaceuticals, GE Healthcare, and Life Molecular Imaging; has received consulting fees or speaking honoraria from Axon Neurosciences, Avid Radiopharmaceuticals, GE Healthcare, Johnson & Johnson, Roche, Eisai, Genentech, and Merck; and is an associate editor of JAMA Neurology. Dr. Seeley received consulting fees or speaking honoraria from BridgeBio, Corcept Therapeutics, Biogen Idec, Bristol Myers-Squibb, Guidepoint Global, and GLG Council.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
Data in the present study are conditionally available upon request through the UCSF Memory and Aging Center Resource Request Form (https://memory.ucsf.edu/resources/data).
REFERENCES
- [1]. Fox N, Warrington E, Freeborough P, Hartikainen P, Kennedy A, Stevens J, Rossor MN (1996) Presymptomatic hippocampal atrophy in Alzheimer’s disease: A longitudinal MRI study. Brain 119, 2001–2007. [DOI] [PubMed] [Google Scholar]
- [2]. Hampel H, Bürger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K (2008) Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement 4, 38–48. [DOI] [PubMed] [Google Scholar]
- [3]. Braak H, Braak E (1991) Neuropathological stageing of alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [4]. Lehmann M, Madison CM, Ghosh PM, Seeley WW, Mormino E, Greicius MD, Gorno-Tempini ML, Kramer JH, Miller BL, Jagust WJ, Rabinovici GD (2013) Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer’s disease. Pro Natl Acad Sci U S A 110, 11606–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Chetelat G, La Joie R, Villain N, Perrotin A, De La Sayette V, Eustache F, Vandenberghe R (2013) Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin 2, 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, Dekosky ST, Halligan EM, Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Jack CR Jr., Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC, Alzheimer’s Disease Neuroimaging Initiativve (2009) Serial PIB And MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain 132, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Okamura N, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Harada R, Yates P, Pejoska S, Kudo Y, Masters CL, Yanai K, Rowe CC, Villemagne VL (2014) Non-invasive assessment of Alzheimer’s disease neurofibrillary pathology using 18F-THK5105 PET. Brain 137, 1762–1771. [DOI] [PubMed] [Google Scholar]
- [9]. Villemagne VL, Furumoto S, Fodero-Tavoletti MT, Mulligan RS, Hodges J, Harada R, Yates P, Piguet O, Pejoska S, Dore V, Yanai K, Masters CL, Kudo Y, Rowe CC, Okamura N (2014) In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging 41, 816–826. [DOI] [PubMed] [Google Scholar]
- [10]. Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD (2016) Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60, 1495–1500. [DOI] [PubMed] [Google Scholar]
- [12]. Brier MR, Gordon B, Friedrichsen K, Mccarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TL, Ances BM (2016) Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 8, 338ra366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Bucci M, Chiotis K, Nordberg A, Alzheimer’s Disease Neuroimaging Initiative (2021) Alzheimer’s disease profiled by fluid and imaging markers: Tau PET best predicts cognitive decline. Mol Psychiatry 26, 5888–5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Marks SM, Lockhart SN, Baker SL, Jagust WJ (2017) Tau and beta-amyloid are associated with medial temporal lobe structure, function, and memory encoding in normal aging. J Neurosci 37, 3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J, Chaudhary K, Edwards L, Iaccarino L, Janabi M, Lesman-Segev OH, Miller ZA, Perry DC, O’Neil JP, Pham J, Rojas JC, Rosen HJ, Seeley WW, Tsai RM, Miller BL, Jagust WJ, Rabinovici GD (2020) Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. , eaau. Sci Transl Med 12, 5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Gordon BA, Mccullough A, Mishra S, Blazey TM, Su Y, Christensen J, Dincer A, Jackson K, Hornbeck RC, Morris JC, Ances BM, Benzinger TLS (2018) Cross-sectional and longitudinal atrophy is preferentially associated with tau rather than amyloid beta positron emission tomography pathology. Alzheimers Dement (Amst) 10, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Bolton CJ, Khan OA, Liu D, Moore EE, Pechman KR, Blennow K, Zetterberg H, Hohman TJ, Gifford KA, Jefferson AL (2021) Baseline plasma total tau predicts longitudinal cognitive and functional decline in aging adults. Alzheimers Dement 17, E055665. [Google Scholar]
- [18]. Biel D, Brendel M, Rubinski A, Buerger K, Janowitz D, Dichgans M, Franzmeier N, Alzheimer’s Disease Neuroimaging Initiative (2021) Tau-PET and in vivo Braak-staging as prognostic markers of future cognitive decline in cognitively normal to demented individuals. Alzheimers Res Ther 13, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Chiotis K, Savitcheva I, Poulakis K, Saint-Aubert L, Wall A, Antoni G, Nordberg A (2021) [(18)F]THK5317 imaging as a tool for predicting prospective cognitive decline in Alzheimer’s disease. Mol Psychiatry 26, 5875–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Jacobs HIL, Becker JA, Kwong K, Engels-Dominguez N, Prokopiou PC, Papp KV, Properzi M, Hampton OL, d’Oleire Uquillas F, Sanchez JS, Rentz DM, El Fakhri G, Normandin MD, Price JC, Bennett DA, Sperling RA, Johnson KA (2021) In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci Transl Med 13, eabj2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, Leite RP, Diehl Rodriguez R, Mejia MB, Rub U, Farfel JM, De Lucena Ferretti-Rebustini RE, Nascimento CF, Nitrini R, Pasquallucci CA, Jacob-Filho W, Miller B, Seeley WW, Heinsen H, Grinberg LT (2017) Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol 43, 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16, 271–278; Discussion 278-284. [DOI] [PubMed] [Google Scholar]
- [24]. Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939. [DOI] [PubMed] [Google Scholar]
- [25]. Von Gunten A, Bouras C, Kovari E, Giannakopoulos P, Hof PR (2006) Neural substrates of cognitive and behavioral deficits in atypical Alzheimer’s disease. Brain Res Rev 51, 176–211. [DOI] [PubMed] [Google Scholar]
- [26]. Warren JD, Fletcher PD, Golden HL (2012) The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol 8, 451–464. [DOI] [PubMed] [Google Scholar]
- [27]. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, Van Der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, De Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suarez Gonzalez A, Tang-Wai DF, Yong KXX, Carrillo M, Fox NC, Alzheimer’s Association ISTAART Atypical Alzheimer’s Disease and Associated Syndromes Professional Interest Area (2017) Consensus classification of posterior cortical atrophy. Alzheimers Dement 13, 870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL (2006) Structural anatomy of empathy in neurodegenerative disease. Brain 129, 2945–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Fernandez-Duque D, Hodges SD, Baird JA, Black SE (2010) Empathy in frontotemporal dementia and Alzheimer’s disease. J Clin Exp Neuropsychol 32, 289–298. [DOI] [PubMed] [Google Scholar]
- [31]. Lyketsos CG, Olin J (2002) Depression in Alzheimer’s disease: Overview and treatment. Biol Psychiatry 52, 243–252. [DOI] [PubMed] [Google Scholar]
- [32]. Apostolova LG, Cummings JL (2008) Neuropsychiatric manifestations in mild cognitive impairment: A systematic review of the literature. Dement Geriatr Cogn Disord 25, 115–126. [DOI] [PubMed] [Google Scholar]
- [33]. Monastero R, Mangialasche F, Camarda C, Ercolani S, Camarda R (2009) A systematic review of neuropsychiatric symptoms in mild cognitive impairment. J Alzheimers Dis 18, 11–30. [DOI] [PubMed] [Google Scholar]
- [34]. Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, Smith GE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA (2008) Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: Population-based study. Arch Gen Psychiatry 65, 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Spalletta G, Musicco M, Padovani A, Perri R, Fadda L, Canonico V, Trequattrini A, Pettenati C, Caltagirone C, Palmer K (2010) Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry 18, 1026–1035. [DOI] [PubMed] [Google Scholar]
- [36]. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, Dekosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. JAMA 288, 1475–1483. [DOI] [PubMed] [Google Scholar]
- [37]. Suarez-Gonzalez A, Crutch SJ, Franco-Macias E, Gil-Neciga E (2016) Neuropsychiatric symptoms in posterior cortical atrophy and Alzheimer disease. J Geriatr Psychiatry Neurol 29, 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Modirrousta M, Price BH, Dickerson BC (2013) Neuropsychiatric symptoms in primary progressive aphasia: Phenomenology, pathophysiology, and approach to assessment and treatment. Neurodegener Dis Manag 3, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, Johnson KA, Sperling RA, Study HAB (2018) Longitudinal association of amyloid beta and anxious-depressive symptoms in cognitively normal older adults. Am J Psychiatry 175, 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, Johnson KA, Sperling RA (2016) Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry 73, 1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Terracciano A, Bilgel M, Aschwanden D, Luchetti M, Stephan Y, Moghekar AR, Wong DF, Ferrucci L, Sutin AR, Resnick SM (2022) Personality associations with amyloid and tau: Results from the Baltimore Longitudinal Study of Aging and meta-analysis. Biol Psychiatry 91, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Binette AP, Vachon-Presseau E, Morris J, Bateman R, Benzinger T, Collins DL, Poirier J, Breitner JCS, Villeneuve S, Dominantly Inherited Alzheimer Network (DIAN); PREVENT-AD Research Group (2021) Amyloid and tau pathology associations with personality traits, neuropsychiatric symptoms, and cognitive lifestyle in the preclinical phases of sporadic and autosomal dominant Alzheimer’s disease. Biol Psychiatry 89, 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Decety J, Jackson PL (2004) The functional architecture of human empathy. Behav Cogn Neurosci Rev 3, 71–100. [DOI] [PubMed] [Google Scholar]
- [44]. Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, Levenson RW (2015) Emotion recognition in frontotemporal dementia and Alzheimer’s disease: A new film-based assessment. Emotion 15, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Davis MH (1980) A multidimensional approach to individual differences in empathy. Thesis. The University of Texas at Austin.
- [46]. Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M (2016) Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer’s disease and the behavioral-variant of frontotemporal dementia. J Alzheimers Dis 53, 801–816. [DOI] [PubMed] [Google Scholar]
- [47]. Batson CD, Duncan BD, Ackerman P, Buckley T, Birch K (1981) Is empathic emotion a source of altruistic motivation? J Pers Soc Psychol 40, 290–302. [Google Scholar]
- [48]. Hatfield E, Cacioppo JT, Rapson RL (1993) Emotional contagion. Curr Dir Psychol Sci 2, 96–100. [Google Scholar]
- [49]. Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP (2013) Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc Natl Acad Sci U S A 110, 9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Chow TE, Veziris CR, La Joie R, Lee AJ, Brown JA, Yokoyama JS, Rankin KP, Kramer JH, Miller BL, Rabinovici GD, Seeley WW, Sturm VE (2023) Increasing empathic concern relates to salience network hyperconnectivity in cognitively healthy older adults with elevated amyloid-beta burden. Neuroimage Clin 37, 103282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Zaki J, Ochsner KN (2012) The neuroscience of empathy: Progress, pitfalls and promise. Nat Neurosci 15, 675–680. [DOI] [PubMed] [Google Scholar]
- [52]. Spreng RN, Mar RA, Kim ASN (2009) The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci 21, 489–510. [DOI] [PubMed] [Google Scholar]
- [53]. Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD (2009) Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38, 433–447. [DOI] [PubMed] [Google Scholar]
- [56]. Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF (2012) On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci 6, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- [59]. Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, Frisoni GB (2016) Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev 30, 25–48. [DOI] [PubMed] [Google Scholar]
- [60]. Singer T, Klimecki OM (2014) Empathy and compassion. Curr Biol 24, R875–R878. [DOI] [PubMed] [Google Scholar]
- [61]. Menon V, Uddin LQ (2010) Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Leech R, Sharp DJ (2014) Damage to the salience network and interactions with the default mode network. J Neurosci 34, 10798–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Hermans EJ, Van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, Van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernandez G (2011) Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334, 1151–1153. [DOI] [PubMed] [Google Scholar]
- [65]. Balthazar ML, Pereira FR, Lopes TM, Da Silva EL, Coan AC, Campos BM, Duncan NW, Stella F, Northoff G, Damasceno BP (2014) Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum Brain Mapp 35, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Quaranta D, Di Tella S, Marra C, Gaudino S, L’Abbate F, Silveri MC (2022) Neuroanatomical correlates of semantic features of narrative speech in semantic and logopenic variants of primary progressive aphasia. Brain Sci 12, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Firth NC, Primativo S, Marinescu RV, Shakespeare TJ, Suarez-Gonzalez A, Lehmann M, Carton A, Ocal D, Pavisic I, Paterson RW, Slattery CF, Foulkes AJM, Ridha BH, Gil-Neciga E, Oxtoby NP, Young AL, Modat M, Cardoso MJ, Ourselin S, Ryan NS, Miller BL, Rabinovici GD, Warrington EK, Rossor MN, Fox NC, Warren JD, Alexander DC, Schott JM, Yong KXX, Crutch SJ (2019) Longitudinal neuroanatomical and cognitive progression of posterior cortical atrophy. Brain 142, 2082–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Agosta F, Canu E, Leocadi M, Calderaro D, Magnani G, Caroppo P, Prioni S, Tremolizzo L, Appollonio I, Silani V, Kostic V, Valsasina P, Filippi M (2020) Brain functional connectivity disruption in a large cohort of patients with primary progressive aphasia. Neurology 94 (15 Supplement), 1517. [Google Scholar]
- [70]. Fredericks CA, Brown JA, Deng J, Kramer A, Ossenkoppele R, Rankin K, Kramer JH, Miller BL, Rabinovici GD, Seeley WW (2019) Intrinsic connectivity networks in posterior cortical atrophy: A role for the pulvinar? Neuroimage Clin 21, 101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Isella V, Villa G, Mapelli C, Ferri F, Appollonio IM, Ferrarese C (2015) The neuropsychiatric profile of posterior cortical atrophy. J Geriatr Psychiatry Neurol 28, 136–144. [DOI] [PubMed] [Google Scholar]
- [72]. Aminoff EM, Kveraga K, Bar M (2013) The role of the parahippocampal cortex in cognition. Trends Cogn Sci 17, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Albert MS, Dekosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Mckhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute On Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, De Leon M, Decarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, Van Duijn C, Visser P, Petersen RC (2004) Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256, 240–246. [DOI] [PubMed] [Google Scholar]
- [76]. Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. [DOI] [PubMed] [Google Scholar]
- [77]. Kramer JH, Jurik J, Sharon JS, Rankin KP, Rosen HJ, Johnson JK, Miller BL (2003) Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 16, 211–218. [DOI] [PubMed] [Google Scholar]
- [78]. Folstein MF, Folstein SE, Mchugh PR (1975) “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [79]. Davis MH (1983) Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol 44, 113. [Google Scholar]
- [80]. Konrath S (2013) A critical analysis of the Interpersonal Reactivity Index. Mededportal Directory and Repository of Educational Assessment Measures (DREAM).
- [81]. Saroglou V, Pichon I, Trompette L, Verschueren M, Dernelle R (2005) Prosocial behavior and religion: New evidence based on projective measures and peer ratings. J Sci Study Relig 44, 323–348. [Google Scholar]
- [82]. Strauss ME, Pasupathi M, Chatterjee A (1993) Concordance between observers in descriptions of personality change in Alzheimer’s disease. Psychol Aging 8, 475. [DOI] [PubMed] [Google Scholar]
- [83]. Rabinovici GD, Furst AJ, O’Neil JP, Racine CA, Mormino EC, Baker SL, Chetty S, Patel P, Pagliaro TA, Klunk WE, Mathis CA, Rosen HJ, Miller BL, Jagust WJ (2007) 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 68, 1205–1212. [DOI] [PubMed] [Google Scholar]
- [84]. Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Arora A, Carpenter AP, Flitter ML, Joshi AD, Krautkramer MJ, Lu M, Mintun MA, Skovronsky DM, Group A-AS (2012) Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: A prospective cohort study. Lancet Neurol 11, 669–678. [DOI] [PubMed] [Google Scholar]
- [85]. Chetelat G, Arbizu J, Barthel H, Garibotto V, Law I, Morbelli S, Van De Giessen E, Agosta F, Barkhof F, Brooks DJ, Carrillo MC, Dubois B, Fjell AM, Frisoni GB, Hansson O, Herholz K, Hutton BF, Jack CR Jr., Lammertsma AA, Landau SM, Minoshima S, Nobili F, Nordberg A, Ossenkoppele R, Oyen WJG, Perani D, Rabinovici GD, Scheltens P, Villemagne VL, Zetterberg H, Drzezga A (2020) Amyloid-PET and (18)F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol 19, 951–962. [DOI] [PubMed] [Google Scholar]
- [86]. Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ (2016) PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87]. Ranasinghe KG, Verma P, Cai C, Xie X, Kudo K, Gao X, Lerner H, Mizuiri D, Strom A, Iaccarino L, La Joie R, Miller BL, Gorno-Tempini ML, Rankin KP, Jagust WJ, Vossel K, Rabinovici GD, Raj A, Nagarajan SS (2022) Altered excitatory and inhibitory neuronal subpopulation parameters are distinctly associated with tau and amyloid in Alzheimer’s disease. Elife 11, e77850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- [89]. Edwards L, La Joie R, Iaccarino L, Strom A, Baker SL, Casaletto KB, Cobigo Y, Grant H, Kim M, Kramer JH, Mellinger TJ, Pham J, Possin KL, Rosen HJ, Soleimani-Meigooni DN, Wolf A, Miller BL, Rabinovici GD (2021) Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer’s continuum: Greater tau-PET retention in females. Neurobiol Aging 105, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Rankin KP, Salazar A, Gorno-Tempini ML, Sollberger M, Wilson SM, Pavlic D, Stanley CM, Glenn S, Weiner MW, Miller BL (2009) Detecting sarcasm from paralinguistic cues: Anatomic and cognitive correlates in neurodegenerative disease. Neuroimage 47, 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. R Core Team (2020), R Foundation For Statistical Computing, Vienna, Austria. URL https://www.r-project.org/
- [92]. Akaike H (1998) Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike, Springer, pp. 199-213.
- [93]. Venables WN, Ripley BD (2002) Modern Applied Statistics with S. Fourth Edition. Springer, New York.
- [94]. Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW (2013) Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci 8, 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Silberman EK, Weingartner H (1986) Hemispheric lateralization of functions related to emotion. Brain Cogn 5, 322–353. [DOI] [PubMed] [Google Scholar]
- [96]. De Lucena AT, Bhalla RK, Belfort Almeida Dos Santos TT, Dourado MCN (2020) The Relationship between theory of mind and cognition in Alzheimer’s disease: A systematic review. J Clin Exp Neuropsychol 42, 223–239. [DOI] [PubMed] [Google Scholar]
- [97]. Dodich A, Cerami C, Crespi C, Canessa N, Lettieri G, Iannaccone S, Marcone A, Cappa SF, Cacioppo JT (2016) Differential impairment of cognitive and affective mentalizing abilities in neurodegenerative dementias: Evidence from behavioral variant of frontotemporal dementia, Alzheimer’s disease, and mild cognitive impairment. J Alzheimers Dis 50, 1011–1022. [DOI] [PubMed] [Google Scholar]
- [98]. Adolphs R, Tranel D, Damasio H, Damasio A (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672. [DOI] [PubMed] [Google Scholar]
- [99]. Adolphs R (2009) The social brain: Neural basis of social knowledge. Annu Rev Psychol 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Skuse D (2006) Genetic influences on the neural basis of social cognition. Philos Trans R Soc Lond B Biol Sci 361, 2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ (1996) A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383, 812–815. [DOI] [PubMed] [Google Scholar]
- [102]. Phelps EA (2006) Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol 57, 27–53. [DOI] [PubMed] [Google Scholar]
- [103]. Shdo SM, Ranasinghe KG, Gola KA, Mielke CJ, Sukhanov PV, Miller BL, Rankin KP (2018) Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia 116, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104]. Meunier M, Cirilli L, Bachevalier J (2006) Responses to affective stimuli in monkeys with entorhinal or perirhinal cortex lesions. J Neurosci 26, 7718–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Petersen C, Nolan AL, De Paula França Resende E, Miller Z, Ehrenberg AJ, Gorno-Tempini ML, Rosen HJ, Kramer JH, Spina S, Rabinovici GD (2019) Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol 138, 597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106]. Nasrallah IM, Chen YJ, Hsieh MK, Phillips JS, Ternes K, Stockbower GE, Sheline Y, Mcmillan CT, Grossman M, Wolk DA (2018) (18)F-flortaucipir PET/MRI correlations in nonamnestic and amnestic variants of Alzheimer disease. J Nucl Med 59, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Lee WJ, Brown JA, Kim HR, La Joie R, Cho H, Lyoo CH, Rabinovici GD, Seong JK, Seeley WW, Alzheimer’s Disease Neuroimaging Initiative (2022) Regional Abeta-tau interactions promote onset and acceleration of Alzheimer’s disease tau spreading. Neuron 110, 1932–1943 E1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K (2019) Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol 76, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. King-Robson J, Wilson H, Politis M, Alzheimer’s Disease Neuroimaging Initiative (2021) Associations between amyloid and tau pathology, and connectome alterations, in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis 82, 541–560. [DOI] [PubMed] [Google Scholar]
- [110]. Sepulcre J, Sabuncu MR, Li Q, El Fakhri G, Sperling R, Johnson KA (2017) Tau and amyloid beta proteins distinctively associate to functional network changes in the aging brain. Alzheimers Dement 13, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Grajski KA, Bressler SL, Alzheimer’s Disease Neuroimaging Initiative (2019) Differential medial temporal lobe and default-mode network functional connectivity and morphometric changes in Alzheimer’s disease. Neuroimage Clin 23, 101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. Cope TE, Rittman T, Borchert RJ, Jones PS, Vatansever D, Allinson K, Passamonti L, Vazquez Rodriguez P, Bevan-Jones WR, O’Brien JT, Rowe JB (2018) Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain 141, 550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci U S A 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Canuet L, Pusil S, Lopez ME, Bajo R, Pineda-Pardo JA, Cuesta P, Galvez G, Gaztelu JM, Lourido D, Garcia-Ribas G, Maestu F (2015) Network disruption and cerebrospinal fluid amyloid-beta and phospho-tau levels in mild cognitive impairment. J Neurosci 35, 10325–10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Braak H, Del Tredici K (2012) Alzheimer’s disease: Pathogenesis and prevention. Alzheimers Dement 8, 227–233. [DOI] [PubMed] [Google Scholar]
- [116]. Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA (2014) Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci 17, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Moreno H, Wu WE, Lee T, Brickman A, Mayeux R, Brown TR, Small SA (2007) Imaging the Aβ-related neurotoxicity of Alzheimer disease. Arch Neurol 64, 1467–1477. [DOI] [PubMed] [Google Scholar]
- [118]. Dickerson BC, Goncharova I, Sullivan M, Forchetti C, Wilson R, Bennett D, Beckett LA, Detoledo-Morrell L (2001) MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging 22, 747–754. [DOI] [PubMed] [Google Scholar]
- [119]. Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hänninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala E-L (2004) Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging 25, 303–310. [DOI] [PubMed] [Google Scholar]
- [120]. Franzmeier N, Neitzel J, Rubinski A, Smith R, Strandberg O, Ossenkoppele R, Hansson O, Ewers M, Alzheimer’s Disease Neuroimaging I (2020) Functional brain architecture is associated with the rate of tau accumulation in Alzheimer’s disease. Nat Commun 11, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Pasquini L, Rahmani F, Maleki-Balajoo S, La Joie R, Zarei M, Sorg C, Drzezga A, Tahmasian M (2019) Medial temporal lobe disconnection and hyperexcitability across Alzheimer’s disease stages. J Alzheimers Dis Rep 3, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122]. Adams JN, Maass A, Harrison TM, Baker SL, Jagust WJ (2019) Cortical tau deposition follows patterns of entorhinal functional connectivity in aging. Elife 8, E49132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Gallagher M, Chiba AA (1996) The amygdala and emotion. Curr Opin Neurobiol 6, 221–227. [DOI] [PubMed] [Google Scholar]
- [124]. Rosenkranz JA, Grace AA (2002) Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22, 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Wellman LL, Forcelli PA, Aguilar BL, Malkova L (2016) Bidirectional control of social behavior by activity within basolateral and central amygdala of primates. J Neurosci 36, 8746–8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Yang Y, Wang JZ (2017) From structure to behavior in basolateral amygdala-hippocampus circuits. Front Neural Circuits 11, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127]. Weera MM, Shackett RS, Kramer HM, Middleton JW, Gilpin NW (2021) Central amygdala projections to lateral hypothalamus mediate avoidance behavior in rats. J Neurosci 41, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128]. Fadok JP, Markovic M, Tovote P, Luthi A (2018) New perspectives on central amygdala function. Curr Opin Neurobiol 49, 141–147. [DOI] [PubMed] [Google Scholar]
- [129]. Yu K, Garcia Da Silva P, Albeanu DF, Li B (2016) Central amygdala somatostatin neurons gate passive and active defensive behaviors. J Neurosci 36, 6488–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Montchal ME, Reagh ZM, Yassa MA (2019) Precise temporal memories are supported by the lateral entorhinal cortex in humans. Nat Neurosci 22, 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Reagh ZM, Yassa MA (2014) Object and spatial mnemonic interference differentially engage lateral and medial entorhinal cortex in humans. Proc Natl Acad Sci U S A 111, E4264–E4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132]. Schultz H, Sommer T, Peters J (2012) Direct evidence for domain-sensitive functional subregions in human entorhinal cortex. J Neurosci 32, 4716–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Hargreaves EL, Rao G, Lee I, Knierim JJ (2005) Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308, 1792–1794. [DOI] [PubMed] [Google Scholar]
- [134]. Save E, Sargolini F (2017) Disentangling the role of the MEC and LEC in the processing of spatial and non-spatial information: Contribution of lesion studies. Front Syst Neurosci 11, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135]. Canto CB, Wouterlood FG, Witter MP (2008) What does anatomical organization of entorhinal cortex tell us? Neural Plast 2008, 381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Witter MP, Amaral DG (1991) Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol 307, 437–459. [DOI] [PubMed] [Google Scholar]
- [137]. Kerr KM, Agster KL, Furtak SC, Burwell RD (2007) Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus 17, 697–708. [DOI] [PubMed] [Google Scholar]
- [138]. Maass A, Berron D, Libby LA, Ranganath C, Düzel E (2015) Functional subregions of the human entorhinal cortex. Elife 4, E06426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139]. Schröder TN, Haak KV, Jimenez NIZ, Beckmann CF, Doeller CF (2015) Functional topography of the human entorhinal cortex. Elife 4, E06738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140]. Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, Garcia AM (2019) More than words: Social cognition across variants of primary progressive aphasia. Neurosci Biobehav Rev 100, 263–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data in the present study are conditionally available upon request through the UCSF Memory and Aging Center Resource Request Form (https://memory.ucsf.edu/resources/data).