Abstract
Objectives:
To determine the prevalence and correlates of treatment-resistant schizophrenia (TRS) through a systematic review and meta-analysis.
Methods:
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria, an electronic search was performed in PubMed and Embase through May 17, 2022. All study designs that assessed a minimum of 20 schizophrenia-spectrum patients and provided data on TRS prevalence or allowed its calculation were included. Estimates were produced using a random-effects model meta-analysis.
Results:
The TRS prevalence across 50 studies (n = 29,390) was 36.7% (95%CI 33.1-40.5, p < 0.0001). The prevalence ranged from 22% (95%CI 18.4-25.8) in first-episode to 39.5% (95%CI 32.2-47.0) in multiple-episode samples (Q = 18.27, p < 0.0001). Primary treatment resistance, defined as no response from the first episode, was 23.6% (95%CI 20.5-26.8) vs. 9.3% (95%CI 6.8-12.2) for later-onset/secondary (≥ 6 months after initial treatment response). Longer illness duration and recruitment from long-term hospitals or clozapine clinics were associated with higher prevalence estimates. In meta-regression analyses, older age and poor functioning predicted greater TRS. When including only studies with lower bias risk, the TRS prevalence was 28.4%.
Conclusion:
Different study designs and recruitment strategies accounted for most of the observed heterogeneity in TRS prevalence rates. The results point to early-onset and later-onset TRS as two separate disease pathways requiring clinical attention.
Registration number:
PROSPERO CRD42018092033.
Keywords: Schizophrenia, clozapine, treatment-resistant, prevalence, meta-analysis
Introduction
Articles, textbooks, and publications on schizophrenia often mention an approximate prevalence of treatment-resistant schizophrenia (TRS) of 30%.1 However, this statement is only based on clinical manuals or review papers, not on systematic evidence, essentially citing three main sources: 1) the 2004 American Psychiatric Association guideline,2 which reported a 10-30% prevalence, without references; 2) a 2007 review by Elkis,3 which reported a 20-30% prevalence based on two publications4,5; and 3) a comprehensive review published in 2001,6 which reported a TRS prevalence of up to 50% (based on two studies).7,8 Thus, the evidence base for TRS prevalence is minimal and is not based on comprehensive meta-analytic data, despite the fact that it is the most severe subtype of schizophrenia and has considerable implications on personal, health-care management, and societal levels.9,10
Furthermore, TRS may be the only clinical condition in schizophrenia with a single best-evidence treatment, i.e., clozapine.4,11 However, despite its clinical superiority for TRS, including symptom reduction, hospitalization risk, and all-cause as well as specific-cause mortality,12 clozapine is largely underutilized, which increases the disease burden and direct and indirect health care costs.13 Thus, a more precise estimate of TRS frequency could increase awareness, and thereby appropriate identification, of TRS, which could guide public policies regarding resource allocation and the cost-benefit of more frequent and earlier clozapine utilization, given the association between older patient age, delayed clozapine use, and poorer outcomes.14 More specifically, knowing the probability and correlates of TRS when assessing a patient may raise awareness and further increase diagnostic accuracy and earlier recognition of TRS.15
Moreover, emerging evidence indicates that TRS has a distinct biosignature from non-TRS,10,16 which has led some authors to recognize TRS as a subtype, rather than an stage, of schizophrenia.10 Additionally, even within TRS, a distinction has been made between early-onset/primary TRS (with no antipsychotic response) and later-onset/secondary TRS, which emerges after initial response to first-line, non-clozapine antipsychotics, although precise estimates of these potential TRS subtypes are unknown. Therefore, neurobiological research would also benefit from more precise estimates of the frequency of TRS and its potential subtypes.
A recent meta-analysis investigated the prevalence of TRS in first-episode schizophrenia (FES),17 reporting an overall TRS frequency of 24.4% (95%CI 19.5-30.0) across nine studies (n = 11,649). However, two studies on TRS relied on clozapine treatment as a definition for TRS (risking underestimation), while two studies considered non-remission as TRS, and in one study half of the patients only had prior failure to one antipsychotic (risking overestimation). Moreover, information on TRS correlates was limited to men. Thus, given these methodological considerations, the lack of any meta-analytic data regarding TRS in multi-episode patients, early-onset/primary vs. later-onset/secondary TRS, and TRS correlates beyond sex differences, as well as the importance and impact of TRS, we aimed to meta-analytically determine the prevalence of TRS and its correlates overall and in patient subgroups.
Methods
Systematic literature search and selection criteria
We searched PubMed and Embase from database inception to May 17, 2022, without language restrictions, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines18 (Table S1 (4.3MB, pdf) , available as online-only supplementary material), employing the following terms in all fields: (“first episode psychosis” OR schizophrenia OR “first episode” OR psychotic OR psychosis OR “schizoaffective disorder” OR schizoaffective) AND (“treatment resistant” OR treatment-resistant OR ultra-resistant OR resistant OR resistance OR “treatment refractory” OR treatment-refractory OR ultra-refractory OR refractory OR refractoriness OR unrespon* OR non-responsive OR “failed to respond” OR “failed to improve” OR “failure to respon*” OR “failure to improve” OR clozapine). Additionally, reference lists of included studies, systematic reviews, and meta-analyses were searched manually. Studies in languages other than English were assessed by native or bilingual reviewers and translated, if eligible, to extract relevant data. Finally, authors were contacted for additional information or unpublished data. Four authors (DR, ED, LF, and RC) independently performed a title and abstract screening of all studies. Each paper included in the full-text review phase was independently evaluated by two of the four authors (DR, ED, LF, and RC). After completing each phase, blinding was removed and any disagreement was resolved by consensus or consulting with two other investigators (AG and CUC). We included studies that: 1) reported on ≥ 20 patients aged ≥ 16 years (the lowest age for which the SCID-IV is validated) diagnosed with schizophrenia or schizoaffective disorder, regardless of diagnostic criteria; 2) provided information that yielded a TRS prevalence or allowed its estimation by calculating the rate of non-responding participants; 3) had longitudinal, prospective (interventional or observational), retrospective, case-control, or cross-sectional designs; and 4) presented original data published in a peer-reviewed journal. We excluded studies with inadequate TRS criteria (e.g., clozapine use as the sole proxy for TRS) or inadequate samples (e.g., only TRS patients). Case reports, case series, and non-peer reviewed publications were excluded as “wrong publication type.”
Studies enrolling individuals with diagnoses other than schizophrenia spectrum disorders (e.g., affective psychosis) were included, as long as separate TRS prevalence data could be extracted. When more than one study reported on the same sample, the larger was included in the meta-analysis.
Data extraction and quality assessment
Two out of four (DR, ED, LF, and RC) investigators independently extracted the data, including a comprehensive list of variables regarding study design, procedures, sample characteristics, and results (for a complete list, see the supplementary material).
Moreover, we examined the degree to which each study used the diagnostic criteria of the Treatment Response and Resistance in Psychosis (TRRIP) Working Group,1 including psychopathology (assessment, severity, duration, functioning), treatment (assessment of past response, duration, dosage, number of antipsychotics, current adherence), symptom domains, time course, and clozapine-treatment resistance, quantifying and summing up the 11 TRRIP criteria (i.e., criterion not fulfilled: score = 0, minimum requirement: score = 1; optimum requirement: score = 2).
Study quality was assessed with a modified version of the Newcastle-Ottawa Scale,19 which has five domains: 1) sample representativeness; 2) sample size; 3) non-respondents (TRS criteria fulfillment); 4) ascertainment of TRS (prospective vs. retrospective evaluation); 5) and statistical quality (for full information regarding scoring) (Table S2 (4.3MB, pdf) , available as online-only supplementary material). Each study was independently judged as low risk of bias (≥ 3 points) or high risk of bias (< 3 points) by three investigators (ED, LF, and RC). Any inconsistencies were resolved by discussion or adjudication by a fourth reviewer (AG).
Data synthesis and analysis
The protocol for this review was preregistered in PROSPERO (CRD42018092033 at http://www.crd.york.ac.uk/PROSPERO), and updated on March 13, 2019, prior to data extraction to allow broader calculation methods for TRS. For each study, we calculated the TRS prevalence via two datasets: intent-to-treat (ITT) and observed cases (OC). Within those analyses, we also calculated a more conservative (MC) and less conservative (LC) TRS estimate, depending on whether or not dropouts were counted as TRS (Figure S1 (4.3MB, pdf) ). We decided a priori that if no significant differences between these four estimates (ITT MC, ITT LC, OC MC, and OC LC) were found, the most conservative prevalence (ITT MC) would be used in further analysis. Identical procedures were used to calculate the prevalence of early-onset/primary and later-onset/secondary TRS.
Clopper-Pearson (or exact binomial) CIs for individual studies were calculated by modeling proportion data using a beta-binomial distribution.20 We adopted an approximate likelihood approach, using the Freeman-Tukey double arcsine transformation to compute the pooled estimates and perform the back-transformation for stabilizing variances, enabling the inclusion of studies with proportions near 0 or 1.21 Furthermore, 95% CIs of pooled estimates were calculated using the Wald method.22 For overall prevalences, we adopted the DerSimonian and Laird random effects model23 with the heterogeneity estimate being taken from the inverse variance model due to the expected heterogeneity of the results. For early-onset/primary and later-onset/secondary TRS estimates, we used both fixed and random effects models. Between-study heterogeneity was assessed using the I2 and chi-square tests.
Publication bias was assessed with the Peters test24 and visual inspection of funnel plots. The final pooled results and 95% CIs in the forest plots were back-transformed for ease of interpretation. We also evaluated the influence of individual studies on the overall prevalence by serially excluding each study in a sensitivity analysis.
In pre-specified subgroup analyses, we determined whether TRS prevalences differed according to: continent, study design, diagnostic criteria of schizophrenia (classification system), specific schizophrenia-spectrum diagnoses, illness phase (first- vs. multiple-episode samples), participant “chronicity” defined (yes vs. no), studies enrolling only previous nonresponders to one antipsychotic (yes vs. no), studies exclusively enrolling symptomatic subjects (yes vs. no), illness duration, minimum antipsychotic trial duration, follow-up duration, or treatment setting.
We also performed univariate random-effects meta-regression analyses to assess the effect of continuous variables on TRS prevalence: publication year, mean age, % male, race/ethnicity, illness duration, age at onset, antipsychotic dose, total Positive and Negative Syndrome Scale (PANSS) score (final score for experimental and longitudinal studies), total Clinical Global Impressions-Severity scale score, total Global Assessment of Functioning score, duration of untreated psychosis, % of clozapine users, total % of PANSS change as a response criterion, dropout rate, and TRRIP criteria fulfillment (individual items and total score).
Analyses were performed using the meta package in R version 3.5. All statistical tests were two-sided with an alpha of 0.05.
Results
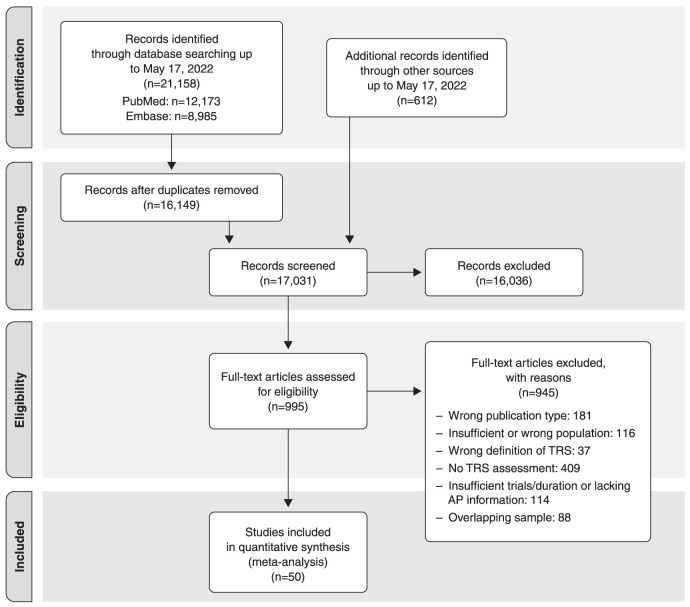
The search results are summarized in Figure 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. AP = antipsychotic; TRS = treatment-resistant schizophrenia.
Patient characteristics
The 50 independent studies7,8,16,25-71 included in the quantitative synthesis were published between 1975 and 2022 and were from every continent, although 357,8,16,25,27,28,31,34-44,46,47,50-53,56-65,70 were from high-income countries and only 1026,30,45,48,49,54,55,66,68,69 were from upper-middle-income countries. The five multicenter studies were from high- and upper-middle-income countries.29,32,33,67,71 The list of excluded full-text records, including the reasons for exclusion, are shown in Table S3 (4.3MB, pdf) , available as online-only supplementary material.
Altogether, 29,390 participants were included in the meta-analysis (mean age = 38.3 [SD, 6.2] years, 64.8 [SD, 12.1%] males). Sociodemographic and clinical characteristics for the complete sample and for patients with TRS are shown in Table 1.
Table 1. Sociodemographic and clinical characteristics of the included subjects (whole sample and patients with TRS).
| Whole sample (n=28,392) | TRS subjects (n=8,838) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean (SD) | Median | Range | Number of studies with data | Number of subjects with data | Mean (SD) | Median | Range | Number of studies with data | Number of subjects with data |
| Age (years) | 38.3 (6.2) | 38.3 | 22.2-48.9 | 36 | 15,657 | 40.1 (4.6) | 39.2 | 32.9-49.7 | 16 | 2096 |
| Male (%) | 64.8 (12.1) | 64.8 | 42.0-100.0 | 38 | 22,794 | 64.7 (13.6) | 64.1 | 40.6-88.9 | 19 | 2638 |
| Race (%) | ||||||||||
| White | 66.4 (18.0) | 67.8 | 32.5-100.0 | 11 | 10,606 | 75.7 (28.6) | 82.8 | 44.1-100.0 | 3 | 663 |
| Black | 22.2 (15.8) | 21.2 | 0.0-50.4 | 8 | 8309 | 21.9 (29.8) | 9.9 | 0.0-55.9 | 3 | 663 |
| Asian | 4.9 (4.9) | 3.6 | 0.0-13.0 | 6 | 7432 | 0.0 (0.0) | 0.0 | 0.0 | 3 | 663 |
| Illness duration (years) | 14.3 (5.3) | 14.8 | 1.0-27.5 | 17 | 3806 | 16.2 (4.8) | 16.3 | 10.1-26.6 | 9 | 1064 |
| Age at onset (years) | 23.1 (1.8) | 23.1 | 20.5-26.9 | 17 | 4950 | 22.3 (1.7) | 22.4 | 19.4-24.4 | 13 | 1897 |
| DUP (months) | 11.6 (8.4) | 11.6 | 0.6-25.2 | 7 | 2817 | 14.4 (9.2) | 16.2 | 0.8-25.7 | 5 | 926 |
| PANSS total – final (score) | 70.0 (16.5) | 67.0 | 43.0-100.7 | 12 | 2769 | 88.6 (15.3) | 91.7 | 66.8-105.7 | 8 | 760 |
| GAF – final (score) | 54.5 (3.3) | 54.3 | 50.9-58.2 | 4 | 559 | 42.6 (4.4) | 42.9 | 37.5-47.4 | 4 | 213 |
| CGI – final (score) | 4.1 (0.7) | 3.9 | 3.2-5.3 | 9 | 2078 | 4.4 (0.8) | 4.4 | 3.7-5.8 | 6 | 557 |
| AP final dose – CPZ eq. (mg/day) | 713.0 (344.7) | 594.9 | 408.7-1.512.1 | 12 | 8506 | 839.0 (491.6) | 679.1 | 441.2-1.556.5 | 4 | 325 |
| Clozapine use (%) | 25.5 (19.0) | 25.5 | 0.0-65.6 | 22 | 18,025 | 67.2 (37.6) | 54.8 | 12.8-100.0 | 19 | 5550 |
| Ultra-TRS (%) | - | - | - | - | - | 25.6 (6.0) | 25.6 | 16.7-33.3 | 8 | 2762 |
AP = antipsychotic; CGI = Clinical Global Impressions; CPZ eq. = chlorpromazine equivalents; DUP = duration of untreated psychosis; GAF = Global Assessment of Functioning; PANSS = Positive and Negative Syndrome Scale; TRS = treatment-resistant schizophrenia.
Overall prevalence of treatment-resistant schizophrenia
Neither the ITT and OC estimates nor the MC and LC analyses yielded statistically significant differences for TRS prevalence (Table S4 (4.3MB, pdf) , available as online-only supplementary material). The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study40 only allowed a single estimate (OC LC). Due to the CATIE study’s large sample size, prospective TRS determination, and its relevance, further analyses were conducted by pooling this estimate with the prevalence estimates calculated from the MC ITT method of the other 49 studies (indicated as ITT MC2 in Table S4 (4.3MB, pdf) ).
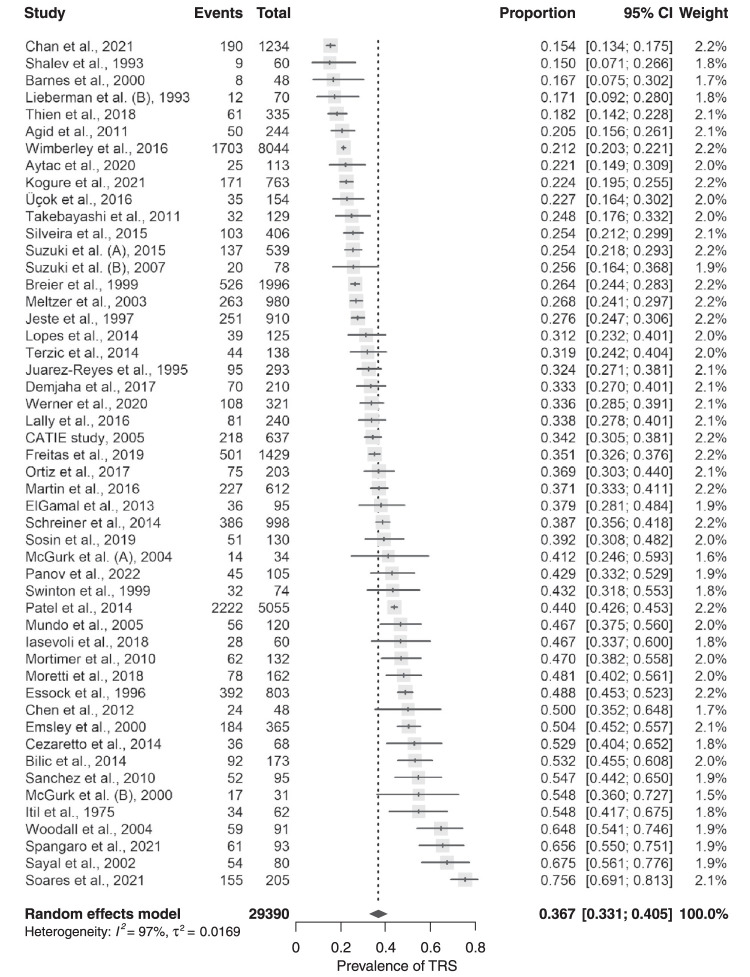
Thus, across all studies, the pooled TRS prevalence was 36.7% (95%CI 33.1-40.5), ranging from 15.4% (95%CI 13.4-17.5)69 to 75.6% (95%CI 69.1-81.3),68 with considerable between-study heterogeneity (I2 = 97.3%, p < 0.0001) (Table S4 (4.3MB, pdf) and Figure 2).
Figure 2. Forest plot of treatment-resistant schizophrenia (TRS) prevalence across all studies (intent-to-treat [ITT]; more conservative [MC2]).
Characteristics of the individual studies can be found in Table S5 (4.3MB, pdf) .
Prospectively assessed early-onset/primary and late-onset/secondary treatment-resistant schizophrenia
Only two longitudinal FES cohorts provided data on early-onset/primary vs. later-onset/secondary TRS prevalence, one with schizophrenia patients38 and another that also included patients diagnosed with unspecified psychotic disorders.16 Both defined later-onset/secondary TRS as resistance appearing ≥ 6 months after an initial treatment response. Early-onset/primary TRS prevalence could be calculated from another FES study.25 The pooled prevalence of early-onset/primary TRS was 23.6% (95%CI 20.5-26.8; I2 = 36.5%, p = 0.207; n = 694), while that for late-onset/secondary TRS was 9.3% (95%CI 6.8-12.2; I2 = 0, p = 0.613; n = 450) (Table S6 (4.3MB, pdf) and Figure S2 (4.3MB, pdf) , both available as online-only supplementary material).
Subgroup analyses
Studies that only included patients with FES found a lower TRS prevalence (22%, 95%CI 18.4-25.8; n = 10,579)16,25,27,38,39,62,64,66,69 than those that only included multi-episode patients (39.5%, 95%CI 32.2-57.0; n = 4,685)8,29,31,34,36,40,43,49,70 (Q = 17.7, p < 0.0001).
There were also significant differences in TRS prevalence according to illness chronicity, study continent, TRS criteria, antipsychotic switching due to intolerance, diagnostic criteria for schizophrenia, treatment setting, illness duration, follow-up duration, and decade of publication (Table S7 (4.3MB, pdf) ).
There were no significant differences regarding diagnosis, study design, use of clozapine as a criterion for TRS, TRS estimate type (original study vs. calculated), inpatient vs. outpatient setting, exclusive recruitment of symptomatic patients, exclusive inclusion of non-responders to a previous trial, response measurement (scale vs. clinical evaluation), or minimum duration of antipsychotic trial (Table S7 (4.3MB, pdf) ).
Meta-regression analysis
In the meta-regression analyses, there were significant associations between higher TRS prevalence, older age (0.0066, p = 0.0436),7,25-31,33-38,40,42-47,49-51,53,55,56,58-61,65-68,70 higher percentage of clozapine users (0.0056, p = 0.0003),25,30,31,33,34,38,39,41,43,45,46,50,54,56,59-61,64-68 lower Global Assessment of Functioning score (-0.0260, p = 0.0324),45,55,60,61 and lower total TRRIP score (-0.0143, p = 0.0059),7,8,16,25-71 as well as three specific TRRIP criteria: less fulfillment of current symptom severity threshold (-0.0641, p = 0.00462),7,8,16,25-71 less fulfillment of minimum functional impairment (-0.0439, p = 0.0376),7,8,16,25-71 and time course (-0.0634, p = 0.0326)7,8,16,25-71 (Table S8 (4.3MB, pdf) ).
TRS prevalence was not significantly associated with publication year, sex, illness duration, age at illness onset, endpoint total PANSS score, endpoint Clinical Global Impressions-Severity score, % of PANSS change as a response criterion (≥ 20, ≥ 30, ≥ 40, ≥ 50%), dropout rate, race/ethnicity, or final antipsychotic dose (Table S8 (4.3MB, pdf) ).
Sensitivity analyses
According to the sensitivity analysis, the pooled TRS prevalence of schizophrenia-spectrum disorders varied from 35.9% (95% CI 32.4-39.5; I2 = 97.1%, p < 0.0001)68 to 37.3% (95% CI 33.7-41; I2 = 97%, p ≤ 0.0001)69 after serially excluding each study (Figure S3 (4.3MB, pdf) and Table S9 (4.3MB, pdf) ). No individual study had a disproportionate influence on the pooled prevalence.
Publication bias
Peters’ test indicated a small-study effect on TRS prevalence (p = 0.044) (Table S4 (4.3MB, pdf) ), as did visual inspection of the funnel-plot (Figure S4 (4.3MB, pdf) ): smaller studies (< 200 participants) (40.9% [95% CI 35.1-46.8], n = 2,568)26-28,30,31,34,35,39,41,43-47,49,51-53,55-57,59-61,65,66,70 had higher TRS prevalence estimates than larger studies (32.8% [95% CI 27.9-37.6], n = 26,822)7,8,16,25,29,32,33,36-38,40,42,48,50,54,58,62-64,67-69,71 (Q = 4.61, p = 0.032).
Risk of bias within and between studies
Low risk of bias studies found a significantly lower TRS prevalence (28.4%, 95% CI 23.9-33.0)7,16,25,26,32,33,36,38-40,48,50,53,54,57,58,60,62,66,67,69,71 than high risk of bias studies (44.35%, 95% CI 38.29-50·50)8,27-31,34,35,37,41-47,49,51,52,55,56,59,61,63-65,68,70 (Q = 17.1, p < 0.0001). The results for individual scores and subgroup analyses across studies for each criterion and total scores are shown in Tables S10 and S11 (4.3MB, pdf) , respectively. Additionally, a sensitivity analysis was performed by excluding high risk of bias studies (Figure S5 (4.3MB, pdf) ).
Discussion
The main results of this comprehensive meta-analysis included: 1) an overall TRS prevalence of 36.7%; 2) significantly lower TRS among FES than multiple-episode patients (22% vs. 39.5%); 3) early-onset/primary and later-onset/secondary TRS rates of 23.6% and 9.3%, respectively, based on limited evidence from only three studies; and 4) higher TRS frequency was significantly associated with older age and greater illness severity and chronicity, as well as with the treatment setting of more chronically ill patients and clozapine use. However, there were no significant differences between men and women, as had been reported in a FES sample, and TRS frequency did not vary according to year of study/publication.
The overall TRS prevalence in this meta-analysis was higher than previous estimates in clinical manuals, non-systematic reviews, or individual studies, which were generally around 30%. Our finding of 36.7% summarizes evidence from 50 independent studies and 29,390 participants.
The 28.4% TRS prevalence found after excluding high-risk of bias studies reflects the predominance of higher-quality FES studies, which had a lower TRS prevalence, larger samples, and prospective evaluations. However, this database is limited and further studies are needed to more conclusively determine the TRS frequency from such sources, including studies characterizing patients with validated TRS criteria and not health care records alone.
While we rigorously avoided using clozapine use as a proxy for TRS (risking underestimation) or including nonresponders in the definition of TRS (risking overestimation), we did not restrict the analyses to studies specifically targeting TRS estimates, although we required calculable TRS frequencies. As expected, TRS frequencies were highly heterogeneous, reinforcing the need for subgroup analysis. Unsurprisingly, the lowest prevalence (22%) was in first-episode psychosis samples. Eight16,25,38,39,62,64,66,69 out of nine FES studies were longitudinal, but the estimates were still heterogeneous, ranging from 15.469 to 33.7%,38 which might be explained by variations in recruitment, TRS criteria, non-response criteria, and follow-up duration.
In a recently published meta-analysis focusing exclusively on first-episode cohorts,17 the TRS prevalence (not counting three mixed first-episode psychosis samples) was 24.4% (95%CI 19.5-30.0) across nine studies and 11,649 participants. The authors included only cohort studies and used different inclusion criteria from our meta-analysis (allowing studies that defined TRS according to clozapine use and/or including non-remission patients who are not necessary treatment-resistant), and found no significant TRS correlates besides male sex,17 which was not replicated in this meta-analysis.
In our meta-analysis, which also included multi-episode patients, this population had the highest proportions of TRS, averaging 40% and reaching 55%.43 Around one-third of the studies recruited patients from specialized centers with more severe patients. Notably, population-based studies, which reported lower TRS prevalences, likely due to non-selected samples, also disproportionately considered antipsychotic switching due to adverse effects as failed trials. This methodological feature may explain the unexpected finding that studies which considered intolerability-related treatment failure as TRS25-27,29,31,34,35,40,41,47,53,57,64,65,69 reported a lower TRS prevalence than those that did not.7,8,16,28,30,32,33,36-39,42-46,48-52,54-56,58-63,66-68,70,71
The likely heterogeneous nature of TRS is also reflected in our results. One patient group never responded to currently available postsynaptic anti-dopaminergic agents, with up to 24% of the schizophrenia patients having early-onset/primary TRS. This TRS subtype might be related to hyper-glutamatergic rather than hyper-dopaminergic pathology.10 A second TRS group initially responded to antidopaminergic drugs but developed TRS over time, which, in our limited prospective data, corresponded to 9% of schizophrenia patients. This likely occurred after relapse,72 and/or was related to substance abuse.73 While this 24% vs. 9% ratio may suggest that more than two-thirds of TRS is early-onset/primary TRS, prospective data were very limited and of short duration. The pooled TRS prevalence of 22% in FES patients vs. 40% in multi-episode patients, or 36.7% of TRS overall suggests something like a 1:1 ratio of early onset/primary vs. later onset/secondary TRS. Nevertheless, more data are needed to substantiate this and identify potential risk factors and the biological underpinnings of early-onset/primary vs. later-onset/secondary TRS.
Significant correlates of TRS frequency in our analysis included older age and illness chronicity, reinforcing the idea that compromised neural regeneration74 or illness progression triggers TRS. Lower Global Assessment of Functioning scores were associated with higher TRS rates, confirming an association between poor functioning and TRS. Additionally, lower total TRRIP scores were associated with higher TRS prevalence, likely because non-first episode studies with higher TRS rates are less likely to define TRS using high-quality criteria.
Several limitations should be considered when interpreting these results. First, although we excluded studies whose definitions of TRS were based on clozapine use or included patients who were not in remission, we did not require detailed TRS criteria, and only 21 studies7,8,16,25,31,38,39,41,46,49,50,52-54,57,59,62-64,68,69 were designed to determine TRS prevalence. Second, we identified seven standardized but heterogeneous TRS criteria,1,2,4,6,75-77 which likely explains the wide-ranging prevalences in addition to differences in treatment setting, populations, etc. We found significant TRS prevalence differences in the subgroup analysis, which is probably explained by the lower TRS prevalence of a single study27 that applied Brenner et al.’s76 criterion in first-episode psychosis patients, which contrasts with the higher TRS prevalence in studies that utilized other TRS criteria2,4,77 and included participants with longer illness duration. Third, because antipsychotic adherence was not objectively assessed in 41 studies,7,8,26-33,35-37,39-47,49-51,53-56,58-60,63-70,71 we were unable to differentiate between true TRS vs. pseudo-resistance due to (partial) non-adherence. Among the included studies, one reported an adherence rate of 80% based solely on pill counts and patient and caregiver feedback.25 Four studies exclusively enrolled patients with a documented history of good medication adherence according to medical reports, chart reviews, or the accounts of relatives.16,48,61,70 Two studies confirmed medication adherence by enrolling patients who had been hospitalized.28,52 One study examined medication adherence by analyzing the dose/serum ratio of antipsychotic treatment, finding a median (interquartile range) of 0.53 in 65 of 108 treatment-resistant patients, although data on serum concentration from previous trials were not available.63 Only one study34 addressed pseudo-resistance via a 4-week prospective trial, finding that 12 (36.4%) of 33 putative TRS patients had pseudo-resistance. Due to the lack of reliable information on medication adherence, its impact on TRS prevalence could not be assessed, and it should be considered a potential confounding factor. Hence, the TRS prevalence may be overestimated. Fourth, alcohol and/or substance dependence and misuse posed another challenge: despite excluding diagnoses of substance-induced psychosis, the available data could not be analyzed due to insufficient information, potentially leading to overestimation of TRS prevalence. Twenty-five studies provided no data on alcohol and/or substance dependence and misuse.8,16,25-29,31,32,35,39,41,43-45,48-51,54,55,57,59,61,65 Eight studies used alcohol/substance misuse as an exclusion criterion.7,37,47,53,56,58,60,70 Seven studies found no significant differences in alcohol/substance misuse between TRS and non-TRS samples.30,33,34,38,42,46,68 Three studies only reported history of alcohol/substance abuse.36,52,63 Six studies presented data on current alcohol or substance abuse, which ranged from 3 to 30%.40,64,66,67,69,71 One study62 reported a 58.5% prevalence of comorbid substance abuse in a sample of first-episode psychosis (n = 544), although it did not provide specific data on individuals with schizophrenia spectrum psychosis (335 non-TRS vs. 61 TRS). Fifth, six studies29,32,34,40,70,71 were antipsychotic trials that recruited patients unresponsive to a previous trial, which probably increased the number of participants with TRS, considering that there is an increased likelihood of non-response after a first inefficacious antipsychotic trial. However, a subgroup analysis found no difference between studies that only recruited patients in their second trial vs. those that did not, although other sample characteristics, including recruitment strategies, might have influenced this finding. Sixth, most studies had a cross-sectional, observational design. Thirty-two7,8,26,27,30,31,33,36,37,41-48,50-52,54-56,58,59,61,63-65,67-69 of the 50 studies assessed the treatment data retrospectively, such as chart reviews and patient or informant reports, which could have influenced the results. Finally, there was considerable heterogeneity in the primary outcome (I2 = 97.3%), and despite conducting subgroup and sensitivity analyses, we were unable to ascertain the underlying source of this heterogeneity. Our hypothesis is that it is due to a combination of factors rather than isolated variables, since most studies were not designed specifically to investigate TRS prevalence. These factors could include study design, treatment setting, recruitment strategies, population characteristics, criteria used to define TRS, and methods of TRS calculation. However, due to the limited number of studies in each subgroup, we were unable to perform these analyses. Furthermore, our search strategy focused on a specific outcome (treatment resistance), which may not have been exhaustive and could have contributed to publication bias. Consequently, given these limitations, caution should be exercised when interpreting the results of this meta-analysis. This highlights the need for further studies specifically designed to determine the prevalence of TRS.
Nevertheless, despite these limitations, this is the first comprehensive meta-analysis on the prevalence and correlates of TRS in schizophrenia-spectrum disorders. We found that roughly 20% of FES patients and 40% of multi-episode patients with schizophrenia have TRS, which did not significantly change over time despite therapeutic advancements, thus making them candidates for clozapine.1,9 Several correlates of higher TRS rates emerged, including older age, longer illness duration, poor functioning, and recruitment from treatment settings with more chronic/severely ill patients. The results of this meta-analysis can inform clinical care, clinical and neurobiological research, as well as health care policy and resource allocation. First, clinicians should consider the reported frequencies of TRS in FES and multi-episode schizophrenia patients and perform routine screening, given its magnitude and impact. Second, specific protocols and services for early identification and treatment of TRS should be developed and prioritized. These should include training about clozapine as the most cost-effective treatment for TRS patients,13,78 which remains underused,79 especially in FES.15 Third, in clinical practice and research, standardized criteria for TRS should be used, such as those proposed by the TRRIP consensus.1 Fourth, population-based and longitudinal studies that use TRRIP consensus criteria are needed, including comprehensive epidemiological approaches to characterizing TRS and the distinction between early-onset/primary and later-onset/secondary TRS. Finally, given the high prevalence of TRS and the fact that clozapine, which is underutilized, is the only available evidence-based pharmacological treatment for this patient population, increased effort should be made to develop novel medications with specific efficacy for TRS.
Disclosure
RB has received research grants from Janssen Cilag, Novartis, and Roche; has been a forum consultant for Janssen, Novartis, and Roche; and has participated in speaker bureaus for Ache, Janssen, Lundbeck, and Novartis. CUC has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris; has provided expert testimony for Janssen and Otsuka; has served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; has received grant support from Janssen and Takeda; has received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, and Quantic. AG has been a consultant and/or advisor to or has received honoraria from Aché, Daiichi-Sankyo, Torrent, Bayer, Cristalia, and Janssen. ARB has received in-kind support from Flow Neuroscience and MagVenture, unrelated to the present study. The other authors report no conflicts of interest.
Acknowledgements
ARB has received grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2018/10861-7, 2019/06009-6), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; CNPq-1B), Universidade de São Paulo productivity support (Programa de Incentivo à Produtividade Acadêmica [PIPA-A]), and Newton Advanced Fellowship (NAFR 2012\1010). RB has received research grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), CNPq, and FAPESP (2016/022465 and 2011/50740-5).
The authors would like to thank Felipe Arcadepani for his relevant contribution to this study, as well as all fellow investigators who provided additional information to this meta-analysis regarding their original studies: Andrew Martin, Dmitriy Sosin, Gerardo Araújo Filho, Herbert Y. Meltzer, Jeffrey A. Lieberman, Katherine Beck, Oliver Howes, Robert McCutcheon, Scott Stroup, Susan M. Essock, Tea Terzic, and Vanessa K. Ota.
Footnotes
How to cite this article: Diniz E, Fonseca L, Rocha D, Trevizol A, Cerqueira R, Ortiz B, et al. Treatment resistance in schizophrenia: a meta-analysis of prevalence and correlates. Braz J Psychiatry. 2023;45:448-458. http://doi.org/10.47626/1516-4446-2023-3126
References
- 1.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174:216. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161:1–56. [PubMed] [Google Scholar]
- 3.Elkis H. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2007;30:511–33. doi: 10.1016/j.psc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 5.Miller A, McEvoy J, Jeste D, Marder S. Treatment of chronic schizophrenia. In: Lieberman J, Stroup T, Perkins D, editors. Textbook of schizophrenia. Washington, DC: American Psychiatric Publishing; 2006. pp. 365–81. [Google Scholar]
- 6.Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biol Psychiatry. 2001;50:898–911. doi: 10.1016/s0006-3223(01)01271-9. [DOI] [PubMed] [Google Scholar]
- 7.Juarez-Reyes MG, Shumway M, Battle C, Bacchetti P, Hansen MS, Hargreaves WA. Effects of stringent criteria on eligibility for clozapine among public mental health clients. Psychiatr Serv. 1995;46:801–6. doi: 10.1176/ps.46.8.801. [DOI] [PubMed] [Google Scholar]
- 8.Essock SM, Hargreaves WA, Dohm FA, Goethe J, Carver L, Hipshman L. Clozapine eligibility among state hospital patients. Schizophr Bull. 1996;22:15–25. doi: 10.1093/schbul/22.1.15. [DOI] [PubMed] [Google Scholar]
- 9.Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer JP, Marder S, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80:18com12123. doi: 10.4088/JCP.18com12123. [DOI] [PubMed] [Google Scholar]
- 10.Potkin SG, Kane JM, Correll CU, Lindenmayer JP, Agid O, Marder SR, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6:1. doi: 10.1038/s41537-019-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209:385–92. doi: 10.1192/bjp.bp.115.177261. [DOI] [PubMed] [Google Scholar]
- 12.Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74:686–93. doi: 10.1001/jamapsychiatry.2017.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gören JL, Rose AJ, Smith EG, Ney JP. The business case for expanded clozapine utilization. Psychiatr Serv. 2016;67:1197–205. doi: 10.1176/appi.ps.201500507. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen J, Nielsen RE, Correll CU. Predictors of clozapine response in patients with treatment-refractory schizophrenia: results from a Danish Register Study. J Clin Psychopharmacol. 2012;32:678–83. doi: 10.1097/JCP.0b013e318267b3cd. [DOI] [PubMed] [Google Scholar]
- 15.Correll CU, Brevig T, Brain C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry. 2019;19:362. doi: 10.1186/s12888-019-2318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47:1981–9. doi: 10.1017/S0033291717000435. [DOI] [PubMed] [Google Scholar]
- 17.Siskind D, Orr S, Sinha S, Yu O, Brijball B, Warren N, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry. 2022;220:115–20. doi: 10.1192/bjp.2021.61. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 21.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Statist. 1950;21:607–11. [Google Scholar]
- 22.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 25.Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72:1439–44. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- 26.Aytac HM, Ozdilli K, Tuncel FC, Pehlivan M, Pehlivan S. Tumor necrosis factor-alpha (TNF-α) –238 G/A polymorphism is associated with the treatment resistance and attempted suicide in schizophrenia. Immunol Invest. 2022;51:368–80. doi: 10.1080/08820139.2020.1832115. [DOI] [PubMed] [Google Scholar]
- 27.Barnes TR, Hutton SB, Chapman MJ, Mutsatsa S, Puri BK, Joyce EM. West London first-episode study of schizophrenia. Clinical correlates of duration of untreated psychosis. Br J Psychiatry. 2000;177:207–11. doi: 10.1192/bjp.177.3.207. [DOI] [PubMed] [Google Scholar]
- 28.Bilic P, Jukic V, Vilibic M, Savic A, Bozina N. Treatment-resistant schizophrenia and DAT and SERT polymorphisms. Gene. 2014;543:125–32. doi: 10.1016/j.gene.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 29.Breier A, Hamilton SH. Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Biol Psychiatry. 1999;45:403–11. doi: 10.1016/s0006-3223(98)00291-1. [DOI] [PubMed] [Google Scholar]
- 30.Cezaretto M, Silva EF de SF, Ambrizzi A, de Biase VED, Silva E de F, da Cruz EMTN, et al. Perfil clínico e sociodemográfico de pacientes com esquizofrenia refratária tratados em um centro terciário. J Bras Psiquiatr. 2014;63:185–90. [Google Scholar]
- 31.ElGamal M, ElTayebani M, Fathi S. Schizophrenia resistance (is there a difference?) Egypt J Psychiatry. 2013;34:51–60. [Google Scholar]
- 32.Emsley RA, Raniwalla J, Bailey PJ, Jones AM. A comparison of the effects of quetiapine (‘seroquel’) and haloperidol in schizophrenic patients with a history of and a demonstrated, partial response to conventional antipsychotic treatment. PRIZE Study Group. Int Clin Psychopharmacol. 2000;15:121–31. doi: 10.1097/00004850-200015030-00001. [DOI] [PubMed] [Google Scholar]
- 33.Freitas R, Dos Santos B, Altamura C, Bernasconi C, Corral R, Evans J, et al. Can the Positive and Negative Syndrome Scale (PANSS) differentiate treatment-resistant from non-treatment-resistant schizophrenia? A factor analytic investigation based on data from the Pattern cohort study. Psychiatry Res. 2019;276:210–7. doi: 10.1016/j.psychres.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Iasevoli F, D’Ambrosio L, Notar Francesco D, Razzino E, Buonaguro EF, Giordano S, et al. Clinical evaluation of functional capacity in treatment resistant schizophrenia patients: comparison and differences with non-resistant schizophrenia patients. Schizophr Res. 2018;202:217–25. doi: 10.1016/j.schres.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 35.Itil TM, Marasa J, Saletu B, Davis S, Mucciardi AN. Computerized EEG: predictor of outcome in schizophrenia. J Nerv Ment Dis. 1975;160:118–203. [PubMed] [Google Scholar]
- 36.Jeste DV, Klausner M, Brecher M, Clyde C, Jones R. A clinical evaluation of risperidone in the treatment of schizophrenia: a 10-week, open-label, multicenter trial. ARCS Study Group. Assessment of Risperdal in a Clinical Setting. Psychopharmacology (Berl) 1997;131:239–47. doi: 10.1007/s002130050289. [DOI] [PubMed] [Google Scholar]
- 37.Kogure M, Kanahara N, Miyazawa A, Oishi K, Nakata Y, Oda Y, et al. Interacting roles of COMT and GAD1 genes in patients with treatment-resistant schizophrenia: a genetic association study of schizophrenia patients and healthy controls. J Mol Neurosci. 2021;71:2575–82. doi: 10.1007/s12031-021-01866-y. [DOI] [PubMed] [Google Scholar]
- 38.Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016;46:3231–40. doi: 10.1017/S0033291716002014. [DOI] [PubMed] [Google Scholar]
- 39.Lieberman J, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S, et al. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry. 1993;50:369–76. doi: 10.1001/archpsyc.1993.01820170047006. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman JA. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: efficacy, safety and cost outcomes of CATIE and other trials. J Clin Psychiatry. 2007;68:e04. doi: 10.4088/jcp.0207e04. [DOI] [PubMed] [Google Scholar]
- 41.Lopes AT, Gilluley P, Veisi M, Patel S, Sukhwal S, Dow J, et al. Management of treatment resistant schizophrenia in medium secure care. Prog Neurol Psychiatry. 2014;18:27–32. [Google Scholar]
- 42.Martin AK, Mowry B. Increased rare duplication burden genomewide in patients with treatment-resistant schizophrenia. Psychol Med. 2016;46:469–76. doi: 10.1017/S0033291715001701. [DOI] [PubMed] [Google Scholar]
- 43.McGurk SR, Meltzer HY. The role of cognition in vocational functioning in schizophrenia. Schizophr Res. 2000;45:175–84. doi: 10.1016/s0920-9964(99)00198-x. [DOI] [PubMed] [Google Scholar]
- 44.McGurk SR, Lee MA, Jayathilake K, Meltzer HY. Cognitive effects of olanzapine treatment in schizophrenia. MedGenMed. 2004;6:27. [PMC free article] [PubMed] [Google Scholar]
- 45.Moretti PN, Ota VK, Gouvea ES, Pedrini M, Santoro ML, Talarico F, et al. Accessing gene expression in treatment-resistant schizophrenia. Mol Neurobiol. 2018;55:7000–8. doi: 10.1007/s12035-018-0876-4. [DOI] [PubMed] [Google Scholar]
- 46.Mortimer AM, Singh P, Shepherd CJ, Puthiryackal J. Clozapine for treatment-resistant schizophrenia: National Institute of Clinical Excellence (NICE) guidance in the real world. Clin Schizophr Relat Psychoses. 2010;4:49–55. doi: 10.3371/CSRP.4.1.4. [DOI] [PubMed] [Google Scholar]
- 47.Mundo E, Altamura AC, Vismara S, Zanardini R, Bignotti S, Randazzo R, et al. MCP-1 gene (SCYA2) and schizophrenia: a case-control association study. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:1–4. doi: 10.1002/ajmg.b.30100. [DOI] [PubMed] [Google Scholar]
- 48.Ortiz BB, Eden FDM, de Souza ASR, Teciano CA, de Lima DM, Noto C, et al. New evidence in support of staging approaches in schizophrenia: differences in clinical profiles between first episode, early stage, and late stage. Compr Psychiatry. 2017;73:93–6. doi: 10.1016/j.comppsych.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Panov GP. Early markers in resistant schizophrenia: effect of the first antipsychotic drug. Diagnostics (Basel) 2022;12:803. doi: 10.3390/diagnostics12040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel MX, Bishara D, Jayakumar S, Zalewska K, Shiers D, Crawford MJ, et al. Quality of prescribing for schizophrenia: evidence from a national audit in England and Wales. Eur Neuropsychopharmacol. 2014;24:499–509. doi: 10.1016/j.euroneuro.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Sánchez P, Ojeda N, Elizagárate E, Peña J, Ballesteros J, Yoller AB, et al. Attention deficits and response to drug therapy in patients with treatment-resistant schizophrenia: results through confirmatory factor analysis. Rev Psiquiatr Salud Ment. 2010;3:40–9. doi: 10.1016/j.rpsm.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Sayal K, Maden A. The treatment and security needs of patients in special hospitals: views of referring and accepting teams. Crim Behav Ment Health. 2002;12:244–53. doi: 10.1002/cbm.503. [DOI] [PubMed] [Google Scholar]
- 53.Shalev A, Hermesh H, Rothberg J, Munitz H. Poor neuroleptic response in acutely exacerbated schizophrenic patients. Acta Psychiatr Scand. 1993;87:86–91. doi: 10.1111/j.1600-0447.1993.tb03335.x. [DOI] [PubMed] [Google Scholar]
- 54.Silveira AS de A, Rocha DMLV, Attüx CR de F, Daltio CS, da Silva LA, Elkis H, et al. Patterns of clozapine and other antipsychotics prescriptions in patients with treatment-resistant schizophrenia in community mental health centers in São Paulo, Brazil. Arch Clin Psychiatry (São Paulo) 2015;42:165–70. [Google Scholar]
- 55.Sosin D, Ivashchenko D, Sozaeva Z, Ryzhikova K, Fadeeva V, Chomskaya V, et al. Cognitive impairment in patients with treatment resistant schizophrenia: associations with DRD2, DRD3, HTR2A, BDNF and CYP2D6 genetic polymorphisms. Neurol Psychiatry Brain Res. 2019;33:48–55. [Google Scholar]
- 56.Spangaro M, Martini F, Bechi M, Buonocore M, Agostoni G, Cocchi F, et al. Longitudinal course of cognition in schizophrenia: does treatment resistance play a role? J Psychiatr Res. 2021;141:346–52. doi: 10.1016/j.jpsychires.2021.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T, Uchida H, Watanabe K, Nomura K, Takeuchi H, Tomita M, et al. How effective is it to sequentially switch among Olanzapine, Quetiapine and Risperidone?--A randomized, open-label study of algorithm-based antipsychotic treatment to patients with symptomatic schizophrenia in the real-world clinical setting. Psychopharmacology (Berl) 2007;195:285–95. doi: 10.1007/s00213-007-0872-2. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki T, Kanahara N, Yamanaka H, Takase M, Kimura H, Watanabe H, et al. Dopamine supersensitivity psychosis as a pivotal factor in treatment-resistant schizophrenia. Psychiatry Res. 2015;227:278–82. doi: 10.1016/j.psychres.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Swinton M, Ahmed AG. Reasons for non-prescription of clozapine in treatment-resistant schizophrenia. Crim Behav Ment Health. 1999;9:207–14. [Google Scholar]
- 60.Takebayashi Y, Ohnuma T, Hanzawa R, Shibata N, Maeshima H, Baba H, et al. No genetic association between SLC7A10 and Japanese patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1965–8. doi: 10.1016/j.pnpbp.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Terzić T, Kastelic M, Dolžan V, Plesničar BK. Genetic variability testing of neurodevelopmental genes in schizophrenic patients. J Mol Neurosci. 2015;56:205–11. doi: 10.1007/s12031-014-0482-5. [DOI] [PubMed] [Google Scholar]
- 62.Thien K, Bowtell M, Eaton S, Bardell-Williams M, Downey L, Ratheesh A, et al. Clozapine use in early psychosis. Schizophr Res. 2018;199:374–9. doi: 10.1016/j.schres.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 63.Werner MCF, Wirgenes KV, Haram M, Bettella F, Lunding SH, Rødevand L, et al. Indicated association between polygenic risk score and treatment-resistance in a naturalistic sample of patients with schizophrenia spectrum disorders. Schizophr Res. 2020;218:55–62. doi: 10.1016/j.schres.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 64.Wimberley T, Støvring H, Sørensen HJ, Horsdal HT, MacCabe JH, Gasse C. Predictors of treatment resistance in patients with schizophrenia: a population-based cohort study. Lancet Psychiatry. 2016;3:358–66. doi: 10.1016/S2215-0366(15)00575-1. [DOI] [PubMed] [Google Scholar]
- 65.Woodall AA, Menkes DB, Trevelyan TR, Lanceley CP. A study of clozapine and long-term hospitalisation rates. Psychiatric Bull. 2004;28:285–8. [Google Scholar]
- 66.Üçok A, Çıkrıkçılı U, Ergül C, Tabak Ö, Salaj A, Karabulut S, et al. Correlates of clozapine use after a first episode of schizophrenia: results from a long-term prospective study. CNS Drugs. 2016;30:997–1006. doi: 10.1007/s40263-016-0358-z. [DOI] [PubMed] [Google Scholar]
- 67.Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia—erratum. Arch Gen Psychiatry. 2003;60:735. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 68.Soares D de S, Carvalho DR, Ribeiro MDT, Diniz EJB, Rêgo AF., Neto Prevalence and predictors of treatment-resistant schizophrenia in a tertiary hospital in Northeast Brazil. Trends Psychiatry Psychother. 2021;43:270–7. doi: 10.47626/2237-6089-2020-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan SKW, Chan HYV, Honer WG, Bastiampillai T, Suen YN, Yeung WS, et al. Predictors of treatment-resistant and clozapine-resistant schizophrenia: a 12-year follow-up study of first-episode schizophrenia-spectrum disorders. Schizophr Bull. 2021;47:485–94. doi: 10.1093/schbul/sbaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JJ, Chan HY, Chen CH, Gau SSF, Hwu HG. Risperidone and olanzapine versus another first generation antipsychotic in patients with schizophrenia inadequately responsive to first generation antipsychotics. Pharmacopsychiatry. 2012;45:64–71. doi: 10.1055/s-0031-1291293. [DOI] [PubMed] [Google Scholar]
- 71.Schreiner A, Lahaye M, Peuskens J, Naber D, Dilbaz N, Millet B, et al. Paliperidone extended-release in patients with non-acute schizophrenia previously unsuccessfully treated with other oral antipsychotics. Expert Opin Pharmacother. 2014;15:593–603. doi: 10.1517/14656566.2014.884071. [DOI] [PubMed] [Google Scholar]
- 72.Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in Treatment Resistant Schizophrenia (TRS) Front Psychiatry. 2019;9:757. doi: 10.3389/fpsyt.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taipale H, Tanskanen A, Correll CU, Tiihonen J. Real-world effectiveness of antipsychotic doses for relapse prevention in patients with first-episode schizophrenia in Finland: a nationwide, register-based cohort study. Lancet Psychiatry. 2022;9:271–9. doi: 10.1016/S2215-0366(22)00015-3. [DOI] [PubMed] [Google Scholar]
- 74.Falkai P, Rossner MJ, Schulze TG, Hasan A, Brzózka MM, Malchow B, et al. Kraepelin revisited: schizophrenia from degeneration to failed regeneration. Mol Psychiatry. 2015;20:671–6. doi: 10.1038/mp.2015.35. [DOI] [PubMed] [Google Scholar]
- 75.Jobson KO, Schatzberg A. The international psychopharmacology algorithm project (IPAP) Psychopharmacol Bull. 1998;34:347. [PubMed] [Google Scholar]
- 76.Brenner HD, Dencker SJ, Goldstein MJ, Hubbard JW, Keegan DL, Kruger G, et al. Defining treatment refractoriness in schizophrenia. Schizophr Bull. 1990;16:551–61. doi: 10.1093/schbul/16.4.551. [DOI] [PubMed] [Google Scholar]
- 77.National Institute of Health and Clinical Excellence (NICE) London: NICE; 2014. Psychosis and schizophrenia in adults: treatment and management. Clinical Guideline 178. The NICE guidelines on treatment and management. [Google Scholar]
- 78.Revicki DA, Luce BR, Weschler JM, Brown RE, Adler MA. Cost-effectiveness of clozapine for treatment-resistant schizophrenic patients. Hosp Community Psychiatry. 1990;41:850–4. doi: 10.1176/ps.41.8.850. [DOI] [PubMed] [Google Scholar]
- 79.Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136:37–51. doi: 10.1111/acps.12742. [DOI] [PubMed] [Google Scholar]