Abstract
Purpose of Review
Prostate cancer (PCa) is amongst the most common cancers in men worldwide. Cardiovascular (CV) risk factors and CV disease (CVD) are common comorbidities in this patient population, posing a challenge for PCa-directed therapies which can cause or worsen CVRFs and CVDs. Herein, we summarize the approaches to prevent and manage CVD in patients with PCa receiving therapy.
Recent Findings
While patients with locally advanced and metastatic PCa benefit from hormonal therapy, these treatments can potentially cause CV toxicity. Androgen receptor targeting therapies, such as androgen deprivation therapy (ADT), can induce metabolic changes and directly impact cardiovascular function, thereby reducing cardiorespiratory fitness and increasing CV mortality. Moreover, more than half of the PCa patients have poorly controlled CV risk factors at baseline. Hence, there is an urgent need to address gaps in preventing and managing CVD in PCa patients.
Summary
Screening and optimizing CV risk factors and CVD in patients undergoing ADT are essential to reduce CV mortality, the leading non-cancer cause of death in PCa survivors. The risk of CV morbidity and mortality can be further mitigated by considering the patient’s cardiovascular risk profile when deciding the choice and duration of ADT. A multidisciplinary team-based approach is crucial to achieve the best outcomes for PCa patients undergoing therapy.
Keywords: Prostate cancer, Cardiovascular disease, Androgen deprivation therapy, GnRH, Gonadotropin-releasing hormone, Cardiotoxicity, GnRH agonist, GnRH antagonist
Introduction
Prostate cancer (Pca) is one of the leading cancers in men worldwide due to lifestyle, environmental factors, and improved detection through screening programs [1, 2]. With approximately 1.4 million new diagnoses and 375,000 deaths in 2020, Pca is the second most common cancer and the fifth leading cause of death among men worldwide [2]. Both, cardiovascular disease (CVD) and Pca share risk factors such as tobacco smoking, diabetes mellitus, metabolic syndrome, and obesity [3, 4]. While androgen receptor pathway inhibitors (ARPi) such as androgen deprivation therapy (ADT) in advanced Pca improve overall survival, they can induce further metabolic derangements and vascular disease, thereby reducing cardiorespiratory fitness and increasing cardiovascular (CV) mortality [5–8]. Furthermore, most patients with newly-diagnosed PCa have CV risk factors and CVD at baseline [6, 9–11]. Not surprisingly, the leading cause of noncancer death in PCa patients is CV in nature [12]. Given the high burden of CVD and CV risk factors in PCa patients, management of CVD is a key component of optimizing outcomes in men with PCa.
Shared Risk Factors and Co-Prevalence of Cardiovascular Disease and Prostate Cancer in Patients
Risk factors such as obesity, diabetes mellitus, tobacco usage, and a sedentary lifestyle are common to both PCa and CVD and may share biological mechanisms such as inflammation and oxidative stress [8, 9, 13, 14]. As a significant proportion of patients with newly diagnosed PCa have poorly controlled CV risk factors at baseline, this presents opportunities for optimizing CV risk factors prior to and during therapy [8–10, 13]. Furthermore, PCa therapy can exert direct CV toxic effects via hormonal regulation or indirect CV effects via metabolic derangement [15].
Overview of Therapy for Prostate Cancer
The prostate gland depends on androgens, specifically testosterone and its derivative dihydrotestosterone (DHT), for development and growth [16, 17]. As prostate tumor growth is androgen-driven, this led to the concept of androgen deprivation therapy (ADT), either by surgical or medical castration to block the production of androgens or to directly inhibit androgen receptors in the treatment of PCa [16, 17]. Broadly, there are four main types of pharmacological ADT: gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, androgen receptor inhibitors (ARI), and cytochrome P450 17A1 (CYP17) inhibitors, with many other AR targeting strategies currently being developed. Additional therapies used in advanced metastatic PCa include antimicrotubular agents such as docetaxel, which also have the anti-AR effect of decreasing the trafficking of the activated AR from the cytosol to the nucleus. Figure 1 provides an overview of the mechanistic action of the drugs used in the treatment of PCa and their CV side effect profile.
Fig. 1.
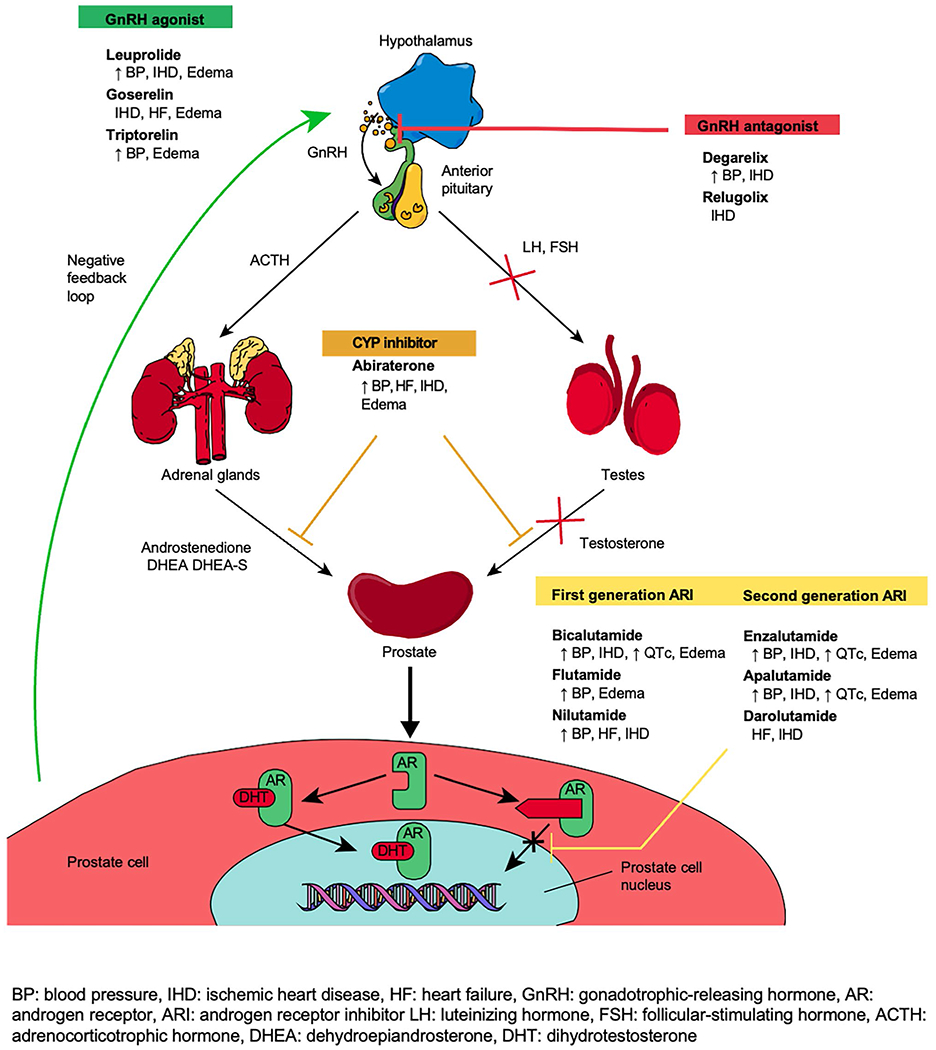
Mechanism of action and selected cardiovascular toxicities of commonly used medications in prostate cancer [57, 59–62]
GnRH agonists (i.e., leuprolide, goserelin, and triptorelin) work by binding to GnRH receptors in the anterior pituitary gland, resulting in the release of GnRH; this triggers transient luteinizing hormone (LH) and follicular-stimulating hormone (FSH) surge which paradoxically increases testosterone production initially and, in turn, activates a negative hormonal feedback loop to downregulate GnRH receptors, thus reducing LH and FSH and testosterone production within 1 to 2 weeks (Fig. 1) [17, 18]. On the contrary, GnRH antagonists (i.e. degarelix) bind to GnRH receptors in the anterior pituitary gland, rapidly suppressing LH, FSH, and testosterone production within 1 to 2 days. ARI (i.e., enzalutamide, bicalutamide) competitively inhibits dihydrotestosterone binding to the androgen receptor at the androgen binding site. Lastly, CYP17 inhibitors (i.e. abiraterone) prevent the final conversion step of cholesterol to testosterone, thereby decreasing androgen production in all tissues including in prostate cancer cells [17–19].
While the staging and treatment regimen for PCa are beyond the scope of this paper, staging can be described as localized (low-risk, intermediate-risk, high-risk) disease and advanced metastatic disease based on prostate-specific antigen (PSA) levels, the Gleason scoring, and other sites of involvement. With advances in treatment, the 5-year survival in patients with localized PCa approaches 99%, while metastatic disease remains incurable with a 5-year survival of only 32% in the United States [20, 21].
Briefly, treatment modalities for localized low-risk PCa include active surveillance, delayed ADT, radiotherapy with electron beam radiotherapy or brachytherapy, and radical prostatectomy. For localized intermediate-risk patients, active surveillance, radical prostatectomy, neoadjuvant ADT, and radiotherapy can be considered. In patients with high-risk localized and locally advanced PCa, radical prostatectomy and pelvic lymphadenectomy, or neoadjuvant ADT, electron beam radiotherapy and adjuvant ADT have been used. For patients with advanced, metastatic, or recurrent disease, treatment paradigms are rapidly evolving. Continuous ADT remains the backbone of therapy, with the addition of various other approved drugs including ARPIs, chemotherapy, PARP inhibitors, radioligand therapy, and immunotherapy [22, 23]. Figure 2 provides an overview of the treatment strategies in PCa.
Fig. 2.
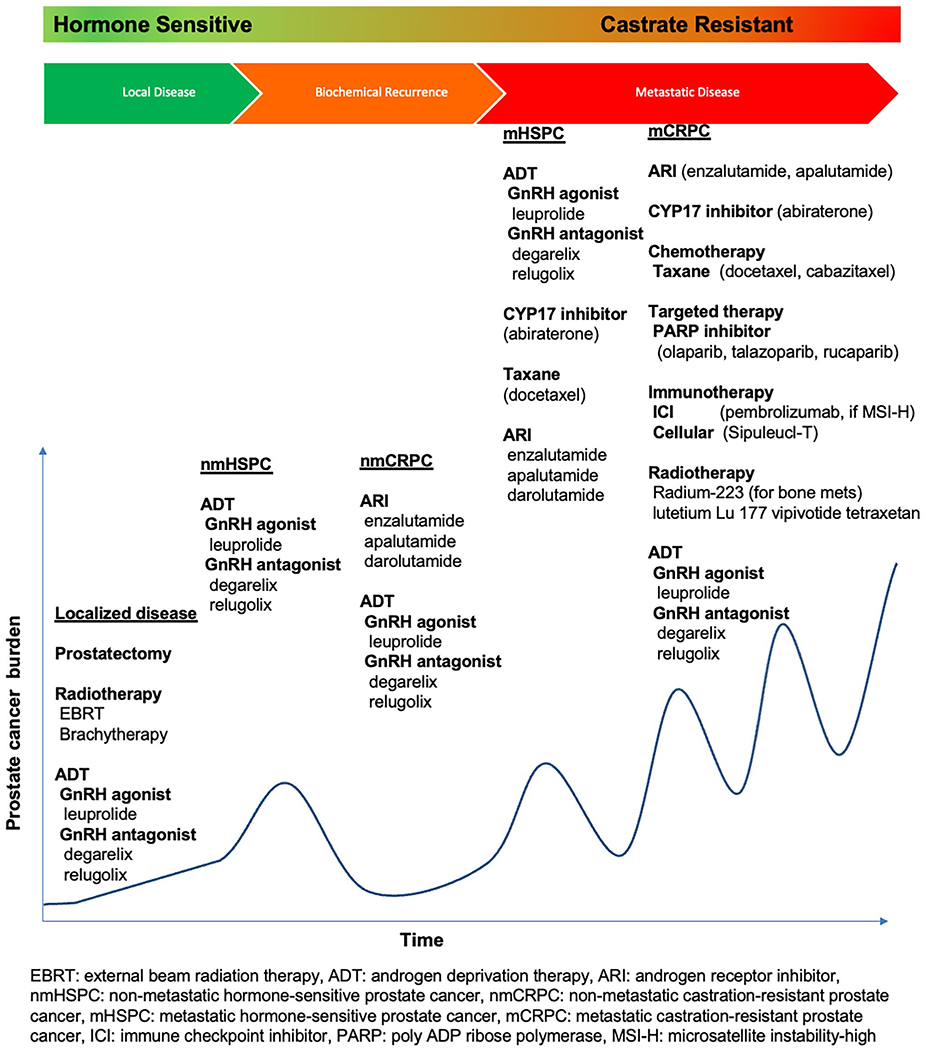
Overview of treatment strategies in prostate cancer [22, 63, 64•]
Effects of Androgen Deprivation Therapy on the Heart
While ADT has proven to be effective in PCa, it can cause adverse CV effects. Androgens such as testosterone have CV protective effects by increasing catecholamine-induced lipolysis, and reducing lipoprotein lipase activity, and triglyceride uptake in visceral adipose tissue, thereby maintaining lean body mass and improving insulin sensitivity. Indeed, men with low testosterone levels were found to have higher incidences of diabetes mellitus, dyslipidaemia, heart failure, and coronary artery disease [17, 24].
Further along these lines, prospective studies have demonstrated that ADT is associated with obesity, increased serum low-density lipoprotein (LDL), and decreased insulin sensitivity. This had led to a joint statement by the American Heart Association, American Cancer Society, and American Urologic Association in 2010 on the potential CV risks from ADT and the need for early CV risk optimization to mitigate CVD morbidity and mortality in PCa patients receiving ADT [25]. In the same year, the US Food and Drug Administration (FDA) recommended that all patients on GnRH agonists should be monitored for the development of diabetes and CVD, and that physicians should actively manage CV risk factors [26]. Recent meta-analyses have corroborate the metabolic dimensions of PCa therapies and shown that ADT resulted in a weight gain of between 0.6 and 3.8%, an increase in total cholesterol of between 3.2 and 10.6%, an increase in triglycerides of between 3.8 and 46.6%, an increase in LDL of between 0.3% and 14.8%, an increase in fasting glucose of between 0.3 and 3.9%, and an increase in hemoglobinA1c (HbA1c) of up to 3.0% [6, 27]. ADT, particularly ARI such as enzalutamide, may also be associated with QTc prolongation on electrocardiogram (ECG), potentially increasing the risk of arrhythmias such as torsade de pointes [5, 18, 28••]. Testosterone is required for ventricular repolarisation, thereby normalizing the QTc interval on ECG [5, 18]; consequently, ARI therapy-related depletion leads to QTc prolongation.
In a large Scottish registry, ADT was associated with a 30% increase in CV events compared to non-treated PCa patients; the increase in CV events was associated with the use of GnRH agonists [29]. Compared to GnRH antagonists, GnRH agonists seem to have a higher rate of CV events based on a meta-analysis of real-world observational studies [3]. In the HERO trial, the use of relugolix (an oral GnRH antagonist) was associated with a 54% lower risk of CV events compared to leuprolide (a GnRH agonist); however, this study was not designed to study CV events, and the events were not adjudicated by a cardiologist [30]. In contrast, the PRONOUNCE trial was specifically designed to investigate the CV safety of GnRH antagonists versus agonists. Unfortunately, it was terminated after only 545 of 900 planned accruals due to slower-than-expected enrolment and fewer-than-expected primary outcome CV events. At the time of study termination, there was no significant difference in major adverse cardiovascular events after 12 months of ADT (hazard ratio, 1.28 [95% CI, 0.59–2.79]; P = 0.53) [31]. One plausible hypothesis from this trial may, henceforth, be that CV event rates might be low and not significantly different between GnRH agonists and antagonists once PCa patients are carefully cared for from a CV perspective. ARIs such as enzalutamide are associated with an increased risk of hypertension, whereas the CYP17 inhibitor, abiraterone, was associated with an increased risk of both CV events and hypertension [18]. Also, adverse cardiac events occurred more frequently in older patients 75 years and older receiving treatment with enzalutamide or bicalutamide for metastatic PCa [32].
Compared with single hormonal therapy, combination hormonal therapy in PCa is associated with an increased risk of cardiovascular events [33]. A retrospective analysis of a pharmacovigilance database found that ARI and abiraterone were associated with an increased risk of hospitalization requiring levels of hypertension and heart failure events when used in combination with GnRH antagonist [33].
Proposed ABCDE Approach to Preventing and Managing Cardiovascular Disease in Patients with Prostate Cancer (Fig. 3)
Fig. 3.
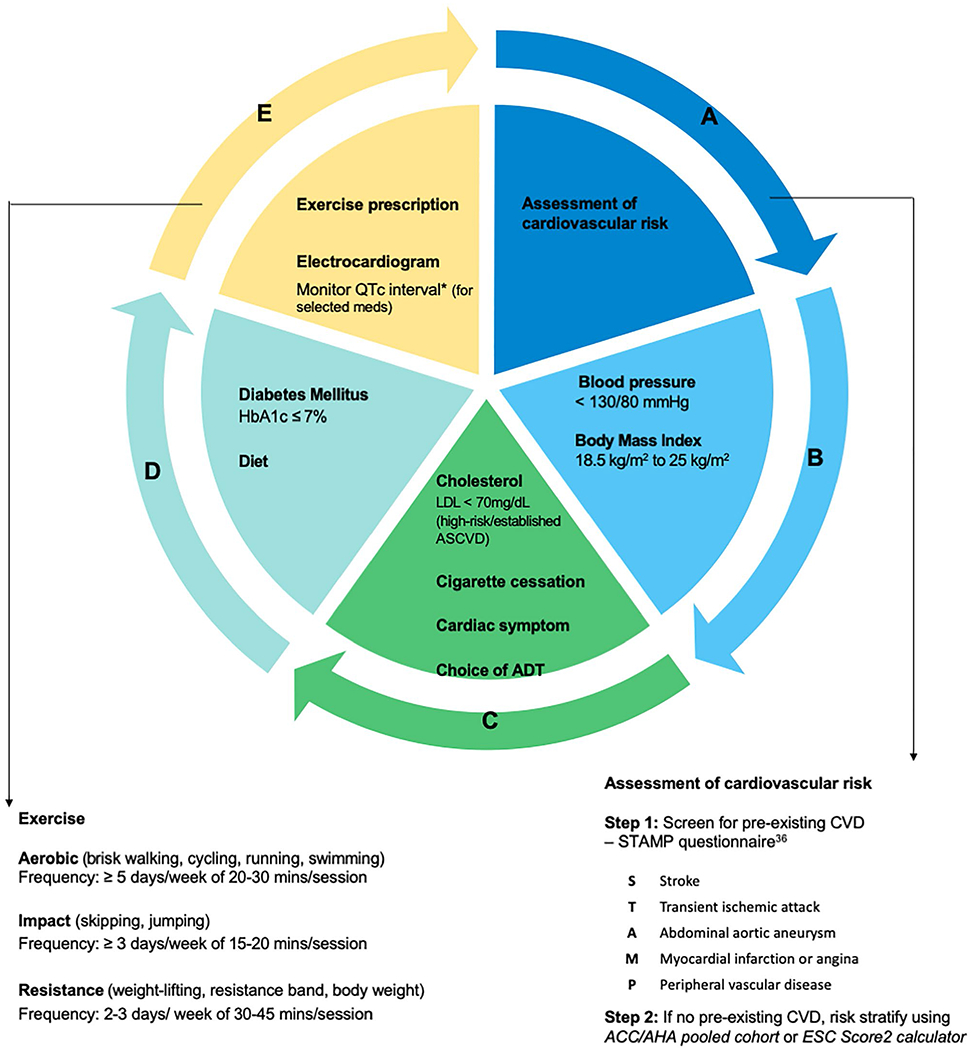
A proposed ABCDE approach to managing cardiovascular disease in prostate cancer patients [26, 34, 36]
In order to help providers and patients, we have expanded on the ABCDE framework developed by Guan et al. [34] which can be used in the clinical setting to prevent and manage CV risk factors in patients with PCa.
Assessment of cardiovascular risk
All PCa patients starting ADT should have baseline CV risk stratification, which should be completed promptly without delaying treatment [35•].
The initial clinic evaluation should include the following:
Vitals: body mass index (BMI) including height and weight, blood pressure.
Comprehensive history comprising cardiac symptoms such as chest pain or shortness of breath on exertion; leg swelling; past medical history: STAMP (see below) questionnaire and lifestyle factors: tobacco use, exercise intensity, and frequency.
Investigations: ECG, HbA1c and/or fasting glucose, and lipid profile
Medication use, including previous chemotherapy (if any)
Patients with pre-existing atherosclerotic cardiovascular disease (ASCVD) the STAMP questionnaire (stroke, transient ischemic attack, abdominal aortic aneurysm, myocardial infarction or angina, and peripheral vascular disease) PENDING [36] allows for further risk stratification (Fig. 3). In patients without established ASCVD, a risk calculator can be used to estimate the 10-year ASCVD risk. Although ASCVD risk calculators have not been validated in the cancer population or those 80 years or older, some of the population-based risk calculators that can be used include the ACC/AHA pooled cohort risk calculator (https://tools.acc.org/ascvd-risk-estimator-plus/) or the ESC Score2/Score2-OP (https://www.heartscore.org/). Based on this, PCa patients can be stratified into low ASCVD risk (if the 10-year ASCVD risk score < 10%), moderate ASCVD risk (if the 10-year ASCVD risk score ≥ 10% to < 20%), and high ASCVD risk (if 10-year ASCVD risk score > 20% or if the patient has known pre-existing ASCVD) [35•]. PCa patients with high ASCVD risk or have established ASCVD with poorly controlled CV risk factors or active cardiac symptoms can be referred to a cardio-oncologist for further assessment and optimization.
Previous studies have demonstrated that two-thirds of PCa patients are at high ASCVD risk,[8] with more than half of them having poorly controlled CV risk factors at baseline [6, 9–11]. Optimizing control and prevention of CV risk factors may reduce CV mortality and morbidity, which is the leading non-cancer cause of death in PCa survivors [12]. In patients with established ASCVD, optimal control of CV risk factors will reduce the risk of recurrent CV events such as myocardial infarction.
Apart from cardiovascular risk stratification, the various cardiovascular risk factors should be optimized before, during, and after therapy in PCa patients.
Blood Pressure Control and Body Mass Index (BMI) Target
Blood Pressure Control
Around half of the patients with newly diagnosed PCa have pre-existing hypertension [8]. In patients with PCa, a systolic blood pressure of > 150 mmHg was associated with a 49% increased risk of all-cause mortality compared to those with normal blood pressure [37]. The use of abiraterone or enzalutamide significantly increases the risk of all-grade hypertension by 10.5 and 26.2%, respectively [38] and of high-grade hypertension by 6.9% and 4.8%, respectively [38]. Hyperaldosteronism has been discussed with the use of abiraterone, whereas the mechanism(s) underlying hypertension with enzalutamide remain unclear [39]. In patients with normal blood pressure, ADT use was associated with a 1.78-fold increased risk of developing new-onset hypertension [40]. Depending on the baseline blood pressure, treatment of hypertension can be accomplished through lifestyle and/or pharmacological interventions [41]. As PCa patients on ADT are at higher risk of CV complications and worsening CV risk factor control, a blood pressure goal of < 130/80 mmHg can be considered in the absence of contraindications. Four primary classes of antihypertensive medications exist: thiazide diuretics, angiotensin-converting receptor enzyme inhibitors/angiotensin receptor blockers, beta blockers, and calcium channel blockers [41]. The choice of initial pharmacological treatment will depend on the patient’s preference and comorbidities. For instance, patients with diabetes mellitus and hypertension may benefit from angiotensin-converting receptor enzyme inhibitors as first-line treatment in the absence of advanced renal impairment [3], whereas, in hypertensive PCa patients on abiraterone therapy (which carries a higher risk of edema), calcium channel blockers should be avoided if possible. Salt restriction and aldosterone antagosists take a greater role in these patients.
Body Mass Index Target
A high BMI in PCa patients is associated with an increased risk of advanced disease, disease progression, and prostate cancer-specific mortality [42]. Compared with PCa patients with a normal weight, any unit increase in BMI is associated with an approximately 10% increased risk of prostate cancer-specific mortality, and a BMI of ≥ 30 kg/m2 associates with a nearly twofold increased risk of death from PCa [42]. It is, therefore, prudent for PCa patients to maintain a normal BMI of 18.5 to 24.9 kg/m2 primarily through structured programs comprising regular self-monitoring of food intake, physical activity, and weight [43]. In addition, regular exercise is important to maintain lean muscle mass in PCa patients undergoing ADT. In the obese general population, a clinically meaningful weight loss of≥ 5% led to moderate improvement in blood pressure, LDL, triglyceride, and glucose control [43].
Cholesterol Control, Cigarette Cessation, and Choice of Androgen Deprivation Therapy
Cholesterol Control
ADT is associated with an increase in total cholesterol of between 3.2% and 10.6%, an increase in triglycerides of between 3.8% and 46.6%, and an increase in LDL of 0.3% to 14.8%; this is possibly mediated by testosterone depletion, leading to decreased lipolysis [6, 27]. In PCa patients with established ASCVD or with high ASCVD risk and >75 years of age they should be on a high-intensity statin if tolerated to reduce LDL by ≥ 50% and achieve an LDL target of < 70 mg/dL (1.8 mmol/L). Adjunct therapy with ezetimibe or PCSSK9-inhibitor can be considered. In those with moderate ASCVD risk aged below and 75 years of age or younger, a moderate-intensity statin can be considered to reduce LDL by 30–49%. Although there is insufficient data to recommend statin use in patients age above 75 years of age, it is reasonable to continue or a statin if they have high ASCVD risk or established ASCVD [44], especially in PCa patients undergoing ADT. While not conclusive, some observational studies have suggested a lower risk of fatal PCa among statin users [45].
Cigarette Cessation
Current smokers with PCa have higher cancer-specific mortality, higher risk of metastasis, and increased risk of biochemical recurrence [46]. In PCa patients, smoking prior to diagnosis was associated with a 61% increased risk of PCa mortality and biochemical recurrence [42]. Therefore, physicians are encouraged to ask all PCa patients about tobacco use, advise smokers to quit, assess a smoker’s readiness to quit, and assist smokers to quit. A combination of pharmacotherapy and behavioral intervention is an effective strategy for cigarette cessation [47].
Choice of Androgen Deprivation Therapy
In patients with established ASCVD or at high ASCVD risk, the use of GnRH antagonists may be preferred over GnRH agonists, owing to a higher observed incidence of CV events in the latter group [28••]. There was also a higher risk of CV hospitalization in PCa patients treated with abiraterone compared to enzalutamide [48]. As outlined above, the use of abiraterone and enzalutamide significantly increases the risk of all-grade and high-grade hypertension by 10.5 and 26.2%, respectively [38]. In PCa patients with diabetes mellitus, the use of abiraterone (which requires combination therapy with steroids) can cause hyperglycaemia and worsen diabetic control. In patients requiring anticoagulation with direct oral anticoagulants (DOAC), physicians should be aware of potential drug interactions with PCa therapy; for instance, enzalutamide, which is a potent CYP3A4 enzyme inducer, should not be used together with apixaban or rivaroxaban [49]. Longer duration of ADT exposure (> 6 months) in PCa patients has been associated with increased CV mortality [7]. Prescribing physicians should consider the presence of CVD, CV risk factors such as hypertension, diabetes, and potential drug interactions when deciding the type and duration of ADT, while at the same time, balancing it against the cancer treatment efficacy.
Diabetes Mellitus
PCa patients with pre-existing diabetes have a 37% higher risk of all-cause mortality compared to those without diabetes [50]. In the absence of specific guidelines for glycaemic control in PCa, diabetes should be managed by currently available guidelines to achieve an HbA1c goal of ≤ 7%. In type 2 diabetes mellitus, antidiabetic medications work by increasing insulin secretion (sulfonylureas, incretin), improving insulin sensitivity (biguanides), or increasing glucose excretion (sodium-glucose co-transporter-2 (SGLT2) inhibitors). As anti-diabetic medications have not been shown to increase the risk of complications in PCa patients, the choice of pharmacotherapy should be based on patient factors (e.g. cost, route of administration, and risk of hypoglycaemia) and the presence of comorbidities (e.g. CVD, chronic kidney disease or obesity) [50–52]. Medications such as SGLT2 inhibitors and glucagon-like-peptide-1 receptor agonists (GLP-1RA) can have beneficial CV effects in PCa patients with established ASCVD or are at high risk of ASCVD.
Exercise Prescription
Exercise has been demonstrated to reduce CV risk, improve diabetic control, and reduce obesity in general, and to improve strength, fitness, and mental wellness in cancer patients [42, 53, 54]. In particular, exercise has been shown to improve cancer-specific quality of life, fatigue, and exercise capacity in PCa patients with advanced disease who are on ADT [53, 54], suggesting a role for exercise to alleviate ADT-related adverse effects. There is also a trend that supervised exercise program in PCa patients can reduce recurrence, all-cause, and prostate-cancer-specific mortality [26]. Furthermore, in patients with localized PCa under active surveillance, the ERASE trial found that high-intensity interval exercise (HIIT) increased cardiorespiratory fitness levels and decreased both PSA levels and tumor growth [55]. Other studies confirm that a sedentary lifetsyle is associated with an increased risk of more advanced PCa [56].
Henceforth, physicians managing PCa patients who are sedentary should consider recommending exercise or referral to an exercise physiologist with expertise in working with cancer patients. Supervised exercise is essential for tailored exercise prescriptions to minimize the risk of injury, to maximize patient engagement, and to attain the desired therapeutic benefits [26]. In the absence of contraindications such as bone metastasis, uncontrolled hypertension, or active CVD, moderate-to-high-intensity aerobic, resistance, and impact exercises are recommended in the PCa population [26]. Figure 3 provides an overview of the suggested exercises in PCa patients [26].
Monitoring of Cardiovascular Risk Factors During Prostate Cancer Treatment
Regular HbA1c and/or fasting glucose monitoring during ADT therapy is an essential. Non-diabetic PCa patients treated with ADT are at almost 60% increased risk of developing diabetes mellitus, whereas, in PCa patients with established diabetes mellitus, ADT is associated with higher HbA1c values [36]. Enzalutamide has an increased risk of hypertension, whereas the CYP17 inhibitor, whereas abiraterone has an increased risk of cardiac events and hypertension [18]. There is also the risk of worsening cholesterol control in PCa patients on ADT. As such, we recommend monitoring lipid profile, HbA1c or fasting glucose, ECG, and blood pressure every 6–12 months (or more frequently as required to reach the target goal) [57].
Management of Cardiovascular Events During Prostate Cancer Treatment
PCa patients are at increased risk of CV events due to shared risk factors and ADT, against the backdrop of a pro-inflammatory state which can cause accelerated atherosclerosis and plaque rupture [28••]. As the 5-year survival rates approach 99% in non-metastatic PCa [20], patients who develop cardiac symptoms such as exertional chest pain, decreased effort tolerance, or heart failure should be managed according to existing guidelines. Physicians should have a low threshold for further evaluation since clinical presentations such as myocardial infarction can sometimes be confounded by cancer or treatment-related side effects [28••]. In cancer patients with a good prognosis who present with an acute coronary syndrome, an invasive strategy with coronary revascularisation should be considered. With appropriate treatment, the 30-day all-cause mortality after acute myocardial infarction can be given lower in PCa patients compared to the general population [58].
Prostate Cancer Survivorship
Patients who have been successfully treated for PCa should continue to maintain a healthy lifestyle and have an annual assessment of CV risk factors; this could potentially reduce both cancer recurrence and the risk of succumbing to CVD. During visits to primary care or the urologist, patients should be opportunistically screened for cardiac symptoms. The presence of cardiac symptoms such as decreased effort tolerance, exertional chest pain, shortness of breath, or leg swelling should prompt referral to a cardiologist for further assessment. Maintaining an ideal BMI, regular physical exercise, avoidance of tobacco and a healthy diet can help reduce cancer recurrence.
Multidisciplinary Team-Based Approach in Managing Prostate Cancer Patients
Given the wide-ranging effects of ADT in PCa patients, it is important to utilize a multi-disciplinary approach to achieve the best possible outcomes. Urologists or oncologists managing PCa could consider involving cardiooncologists, endocrinologists, primary care physicians, and allied health including nurses, dieticians, and sports physiologists to achieve the ideal CV risk factor control.
Conclusions
PCa is the most common malignancy among men worldwide. It shares many risk factors with CVD, inhibition of the androgen receptor pathway substantially increases the risk of developing or worsening CV risk factors and CV events. Screening and optimization of CV risk factors before, during, and after cancer treatment is pivotal in reducing CV mortality, the leading non-cancer cause of deaths in PCa patients. A holistic multidisciplinary ABCDE approach is important to achieve the best possible outcomes for PCa patients undergoing therapy.
Funding
Joerg Herrmann reports grants from the National Institutes of Health and Miami Heart Research Institute.
Conflict of Interest
Choon Ta Ng reports consultancy fees from AstraZeneca and Ferring Pharmaceuticals. Hilda M. Gonzalez Bonilla has nothing to disclose. Alan Bryce reports honoraria from Elsevier, Fallon Medica, Horizon CME, PRIME education, MJH Life Sciences, Research to Practice; consultancy fees from Merck, Astellas, AstraZeneca, Novartis AG, Pfizer, Myovant, Lantheus, Foundation Medicine, and Carden Jennings; and grants from Janssen. Parminder Singh reports consultancy fees from Aveno Pharmaceuticals, Bayer Healthcare, Curio Science LLC, EMD Soreno, Inc., Janssen Research & Development, LLC, Medscape from WebMD, and Seattle Genetics. Joerg Herrmann reports consultancy fees from Pfizer (Advisory Board) and Elsevier (for Editorial work).
Footnotes
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ng CT, Tan LL, Sohn IS, Gonzalez Bonilla H, Oka T, Yinchoncharoen T, et al. Advancing cardio-oncology in Asia. Korean Circ J. 2023;53(2):69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 3.Davey P, Alexandrou K. Assessment and mitigation of cardiovascular risk for prostate cancer patients: a review of the evidence. Int J Clin Pract. 2022;2022:2976811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4(6):877–92. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal M, Canan T, Glover G, Thareja N, Akhondi A, Rosenberg J. Cardiovascular effects of androgen deprivation therapy in prostate cancer. Curr Oncol Rep. 2019;21(10):91. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuzuka K, Arai Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int J Urol. 2018;25(1):45–53. [DOI] [PubMed] [Google Scholar]
- 7.Gong J, Payne D, Caron J, Bay CP, McGregor BA, Hainer J, et al. Reduced cardiorespiratory fitness and increased cardiovascular mortality after prolonged androgen deprivation therapy for prostate cancer. JACC CardioOncol. 2020;2(4):553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leong DP, Fradet V, Shayegan B, Duceppe E, Siemens R, Niazi T, et al. Cardiovascular risk in men with prostate cancer: insights from the RADICAL PC study. J Urol. 2020;203(6):1109–16. [DOI] [PubMed] [Google Scholar]
- 9.Klimis H, Pinthus JH, Aghel N, Duceppe E, Fradet V, Brown I, et al. The burden of uncontrolled cardiovascular risk factors in men with prostate cancer: a RADICAL-PC analysis. JACC CardioOncol. 2023;5(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Parikh RB, Hubbard RA, Cashy J, Takvorian SU, Vaughn DJ, et al. Assessment and management of cardiovascular risk factors among US veterans with prostate cancer. JAMA Netw Open. 2021;4(2):e210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virgo KS, Rumble RB, de Wit R, Mendelson DS, Smith TJ, Taplin ME, et al. Initial Management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO Guideline Update. J Clin Oncol. 2021;39(11):1274–305. [DOI] [PubMed] [Google Scholar]
- 12.Weiner AB, Li EV, Desai AS, Press DJ, Schaeffer EM. Cause of death during prostate cancer survivorship: a contemporary. US population-based analysis Cancer. 2021;127(16):2895–904. [DOI] [PubMed] [Google Scholar]
- 13.Hassan MA, Telvizian T, Abohelwa M, Mukherji D, Skouri H. Cardiac risk factors and events in patients with prostate cancer commencing androgen deprivation therapy: analysis from a tertiary care centre in the Middle East. Ecancermedicalscience. 2022;16:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moustsen IR, Larsen SB, Duun-Henriksen AK, Tjonneland A, Kjaer SK, Brasso K, et al. Risk of cardiovascular events in men treated for prostate cancer compared with prostate cancer-free men. Br J Cancer. 2019;120(11):1067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. 2021;42(3):354–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gheorghe GS, Hodorogea AS, Ciobanu A, Nanea IT, Gheorghe ACD. Androgen deprivation therapy, hypogonadism and cardiovascular toxicity in men with advanced prostate cancer. Curr Oncol. 2021;28(5):3331–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu JR, Duncan MS, Morgans AK, Brown JD, Meijers WC, Freiberg MS, et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer: contemporary meta-analyses. Arterioscler Thromb Vasc Biol. 2020;40(3):e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryce A, Ryan CJ. Development and clinical utility of abiraterone acetate as an androgen synthesis inhibitor. Clin Pharmacol Ther. 2012;91(1):101–8. [DOI] [PubMed] [Google Scholar]
- 20.Pascale M, Azinwi CN, Marongiu B, Pesce G, Stoffel F, Roggero E. The outcome of prostate cancer patients treated with curative intent strongly depends on survival after metastatic progression. BMC Cancer. 2017;17(1):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 22.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–34. [DOI] [PubMed] [Google Scholar]
- 23.Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. NCCN Guidelines(R) Insights: prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288–98. [DOI] [PubMed] [Google Scholar]
- 24.Kaushik M, Sontineni SP, Hunter C. Cardiovascular disease and androgens: a review. Int J Cardiol. 2010;142(1):8–14. [DOI] [PubMed] [Google Scholar]
- 25.Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121(6):833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Administration UFaD. FDA drug safety communication: ongoing safety review of GnRH agonists and possible increased risk of diabetes and certain cardiovascular diseases. 2010. Available from: https://cacmap.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-ongoing-safety-review-gnrh-agonists-and-possible-increased-risk. [Google Scholar]
- 27.Sawazaki H, Araki D, Kitamura Y, Yagi K. Metabolic changes with degarelix vs leuprolide plus bicalutamide in patients with prostate cancer: a randomized clinical study. World J Urol. 2020;38(6):1465–71. [DOI] [PubMed] [Google Scholar]
- 28.Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361. [DOI] [PubMed] [Google Scholar]; •• This is the first cardio-oncology guidelines, which covers recommendations on cardiovascular complications during treatment for prostate cancer.
- 29.Cardwell CR, O’Sullivan JM, Jain S, Harbinson MT, Cook MB, Hicks BM, et al. The Risk of cardiovascular disease in prostate cancer patients receiving androgen deprivation therapies. Epidemiology. 2020;31(3):432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–96. [DOI] [PubMed] [Google Scholar]
- 31.Lopes RD, Higano CS, Slovin SF, Nelson AJ, Bigelow R, Sorensen PS, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144(16):1295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siemens DR, Klotz L, Heidenreich A, Chowdhury S, Villers A, Baron B, et al. Efficacy and safety of enzalutamide vs bicalutamide in younger and older patients with metastatic castration resistant prostate cancer in the TERRAIN trial. J Urol. 2018;199(1):147–54. [DOI] [PubMed] [Google Scholar]
- 33.Zhang KW, Reimers MA, Calaway AC, Fradley MG, Ponsky L, Garcia JA, et al. Cardiovascular events in men with prostate cancer receiving hormone therapy: an analysis of the FDA adverse event reporting system (FAERS). J Urol. 2021;206(3):613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan J, Khambhati J, Jones LW, Morgans A, Allaf M, Penson DF, et al. Cardiology patient page. ABCDE Steps for Heart and Vascular Wellness Following a Prostate Cancer Diagnosis. Circulation. 2015;132(18):e218–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This position stattement provides suggested baseline cardiovascular screening and risk stratitfication prior to commencing prostate cancer therapy.
- 36.Kenk M, Gregoire JC, Cote MA, Connelly KA, Davis MK, Dresser G, et al. Optimizing screening and management of cardiovascular health in prostate cancer: a review. Can Urol Assoc J. 2020;14(9):E458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stikbakke E, Schirmer H, Knutsen T, Stoyten M, Wilsgaard T, Giovannucci EL, et al. Systolic and diastolic blood pressure, prostate cancer risk, treatment, and survival. The PROCA-life study Cancer Med. 2022;11(4):1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645–53. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Wu S. Risk of hypertension in cancer patients treated with abiraterone: a meta-analysis. Clin Hypertens. 2019;25:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakarla M, Ausaja Gambo M, Yousri Salama M, Haidar Ismail N, Tavalla P, Uppal P, et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer patients: a systematic review. Cureus. 2022;14(6):e26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. [DOI] [PubMed] [Google Scholar]
- 42.Peisch SF, Van Blarigan EL, Chan JM, Stampfer MJ, Kenfield SA. Prostate cancer progression and mortality: a review of diet and lifestyle factors. World J Urol. 2017;35(6):867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–209. [DOI] [PubMed] [Google Scholar]
- 45.Craig EL, Stopsack KH, Evergren E, Penn LZ, Freedland SJ, Hamilton RJ, et al. Statins and prostate cancer-hype or hope? The epidemiological perspective. Prostate Cancer Prostatic Dis. 2022;25(4):641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foerster B, Pozo C, Abufaraj M, Mari A, Kimura S, D’Andrea D, et al. Association of smoking status with recurrence, metastasis, and mortality among patients with localized prostate cancer undergoing prostatectomy or radiotherapy: a systematic review and meta-analysis. JAMA Oncol. 2018;4(7):953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332–65. [DOI] [PubMed] [Google Scholar]
- 48.Keating NL. Cardiovascular and metabolic diagnoses associated with novel hormonal agents for prostate cancer in nontrial populations. J Natl Cancer Inst. 2022;114(8):1057–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shatzel JJ, Daughety MM, Olson SR, Beer TM, DeLoughery TG. Management of Anticoagulation in patients with prostate cancer receiving enzalutamide. J Oncol Pract. 2017;13(11):720–7. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Giovannucci E, Jeon JY. Diabetes and mortality in patients with prostate cancer: a meta-analysis. Springerplus. 2016;5(1):1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic Targets: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S97–S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knura M, Garczorz W, Borek A, Drzymala F, Rachwal K, George K, et al. The influence of anti-diabetic drugs on prostate cancer. Cancers (Basel). 2021;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourke L, Smith D, Steed L, Hooper R, Carter A, Catto J, et al. Exercise for men with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703. [DOI] [PubMed] [Google Scholar]
- 54.Hayes BD, Brady L, Pollak M, Finn SP. Exercise and prostate cancer: evidence and proposed mechanisms for disease modification. Cancer Epidemiol Biomarkers Prev. 2016;25(9):1281–8. [DOI] [PubMed] [Google Scholar]
- 55.Kang DW, Fairey AS, Boule NG, Field CJ, Wharton SA, Courneya KS. Effects of exercise on cardiorespiratory fitness and biochemical progression in men with localized prostate cancer Under active surveillance: the ERASE randomized clinical trial. JAMA Oncol. 2021;7(10):1487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger FF, Leitzmann MF, Hillreiner A, Sedlmeier AM, Prokopidi-Danisch ME, Burger M, et al. Sedentary behavior and prostate cancer: a systematic review and meta-analysis of prospective cohort studies. Cancer Prev Res (Phila). 2019;12(10):675–88. [DOI] [PubMed] [Google Scholar]
- 57.Okwuosa TM, Morgans A, Rhee JW, Reding KW, Maliski S, Plana JC, et al. Impact of hormonal therapies for treatment of hormone-dependent cancers (Breast and Prostate) on the cardiovascular system: effects and modifications: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2021;14(3):e000082. [DOI] [PubMed] [Google Scholar]
- 58.Forster RB, Kjellstadli C, Myklebust TA, Egeland G, Sulo G, Bjorge T, et al. Treatment and 30-day mortality after myocardial infarction in prostate cancer patients: a population-based study from Norway. Cardiology. 2023;148(1):83–92. [DOI] [PubMed] [Google Scholar]
- 59.DailyMed [Internet]. Available from: https://www.dailymed.nlm.nih.gov/dailymed/index.cfm.
- 60.Joerg H Cardio-oncology practice manual: a companion to Braunwald’s Heart Disease: Elsevier; 2023. [Google Scholar]
- 61.UpToDate Online [Internet]. [cited 1 March 2023]. Available from: https://www.uptodate.com/.
- 62.Rosario DJ, Davey P, Green J, Greene D, Turner B, Payne H, et al. The role of gonadotrophin-releasing hormone antagonists in the treatment of patients with advanced hormone-dependent prostate cancer in the UK. World J Urol. 2016;34(12):1601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilk M, Wasko-Grabowska A, Szmit S. Cardiovascular complications of prostate cancer treatment. Front Pharmacol. 2020;11:555475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abou D, Benabdallah N, Jiang W, Peng L, Zhang H, Villmer A, et al. Prostate cancer theranostics - an overview. Front Oncol. 2020;10:884. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article provides an overview of the therapies used in prostate cancer.


