Abstract
Women undergoing controlled ovarian hyperstimulation prior to in vitro fertilization (IVF) are treated using various protocols to induce multiple follicular growths. Complete failure of all oocytes to mature during IVF cycles is rare; however, it is a known cause of primary female infertility. Recently, pathogenic variations in a few genes have been identified in women with oocyte maturation defects; however, the underlying genetic causes remain largely unknown.
This study included a Turkish family comprising three sisters with recurring oocyte maturation arrest at the germinal vesicle stage after multiple ovarian stimulations. Exome sequencing revealed a homozygous missense variant (c.1037C>T, p.Ala346Val) in the EPAB gene (also known as PABPC1L) in all three affected sisters, which was either absent or heterozygous in the unaffected family members. Functional experiments confirming the pathogenicity of the variant were performed by transfecting HEK293T cells and demonstrated the instability and increased rate of proteolysis of the mutated PABPC1L/EPAB protein. The identified variant, located in the well-conserved fourth RNA recognition motif (RRM4), in silico 3D modelling suggested changes in the physical properties of the pathogenic variant of PABPC1L/EPAB.
Our findings validate PABPC1L/EPAB as an essential genetic contributor to the oocyte maturation process in humans and have direct implications for the genetic counselling of patients and their family members.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-03009-1.
Keywords: EPAB, PABPC1L, Immature oocyte, Female infertility, GV, Genetics
Introduction
Folliculogenesis is a complex process that starts with the recruitment of primordial follicles and ends with the ovulation of a fully competent oocyte able to sustain fertilization and early embryonic development until zygotic genome activation (ZGA) takes place [1]. The process starts in the fetal ovaries with the differentiation of post migratory primordial germ cells into primordial follicles which, after a few mitotic cycles, enter the initial stage of meiosis and get locked at the diplotene stage of prophase I. They then enter into a relatively quiescent metabolic stage, the dictyate stage, during which transcription is reinitiated [2]. At this point, they are referred to as germinal vesicle (GV) oocytes. At birth, all primordial follicles have reached the dictyate stage and, at puberty, a group of them undergo a phase of growth and differentiation in response to molecular signals. The oocytes however remain blocked at the diplotene stage. This growth stage involves a quantitative increase in oocyte cellular mass with fine-tuned qualitative changes. Folliculogenesis involves intercellular paracrine and intracellular autocrine controls, which are as important as the establishment, through meiosis, of haploidy, for fertilization and early embryonic development. During the final part of folliculogenesis, fully grown oocytes then undergo a maturation process. By convention, this phase starts when oocytes resume meiosis in response to luteinizing hormone, characterized by chromatin condensation and nuclear envelope breakdown, reaching the GV breakdown stage [3]. During this phase, transcription stops and oocyte maturation is dependent on cellular products (proteins, mRNA, rRNA) that have been stored during the dictyate stage. The latter also contributes to early embryonic development until ZGA takes place. Oocyte maturation includes cytoplasmic and meiotic processes, which are intrinsically linked. It involves mitochondria and endoplasmic reticulum reorganization and chromatin configuration changes corresponding to the different phases of meiosis. Meiosis I is completed by the extrusion of the first polar body. Oocytes then enter into the second meiotic division that will be arrested, for a second time, at the metaphase II stage (referred as MII oocytes) [4]. The ovulation process, which is tightly linked to oocyte maturation [5], allows the release of one MII oocyte at each menstruation cycle into the fallopian tube. Fertilization allows meiosis to resume. The second polar body will then be extruded [6].
Translation of these maternally stored mRNAs is tightly regulated during the maturation phase as well as the first embryonic division until ZGA. Poly(A) tail length is a critical regulator of the translation of maternal mRNAs, through dynamic polyadenylation and deadenylation. One of the best characterized actors of this process is the highly conserved embryonic poly(A)-binding protein (EPAB), also known as poly(A)-binding protein cytoplasmic 1 like (PABPCL1). PABPC1L/EPAB has been shown to be involved, at least in Xenopus oocytes, in preventing deadenylation [7], enhancing translation initiation [8], and acting in cytoplasmic polyadenylation processes [9]. The PABPC1L/EPAB protein is highly conserved from mammals to yeast [9–11].
In mice, Epab-deficient females are infertile, they fail to generate mature oocytes and display impaired cumulus expansion and ovulation, alongside abnormal spindle formation and chromosomal misalignment [12–15]. However, Epab−/− male mice are fertile with normal spermatogenesis [16].
In the present report, we identified, via whole exome sequencing (WES), a new homozygous missense variant in PABPC1L/EPAB in a Turkish consanguineous family with three sisters presenting with primary infertility caused by oocyte blockage at GV stage.
Our results confirm the link between PABPC1L/EPAB biallelic or homozygous variants and an oocyte/zygote/embryo maturation arrest (OZEMA) phenotype defined in Online Mendelian Inheritance in Man [OMIM-McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD) {accessed on 20/09/2021}] while opening new perspectives for patient care.
Materials and methods
Family description and sample collection
A consanguineous Turkish family comprising of four siblings, including three sisters with repeated oocyte arrest at the GV stage after multiple ovarian-stimulation cycles, and one brother of unknown fertility status, was identified by our collaborator clinic in Turkey (Fig. 1). Parents were first-degree cousins.
Fig. 1.
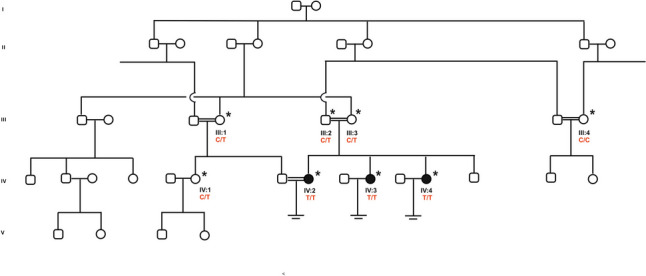
Turkish family with multiple loops of consanguinity comprising three sisters with oocyte maturation arrest. Filled symbols indicate affected members, and clear symbols indicate unaffected members. Asterisk (*) indicates available samples while arrows () indicate those sequenced by WES. Sanger sequencing confirmation and segregation are shown below the pedigrees. C/C: wild-type, C/T: heterozygous, T/T: homozygous
Our index patient (IV:2) was 36 years old, with 12 years of marriage without a pregnancy. She had one intra-uterine insemination (IUI) and one IVF cycle without success. A clinical investigation revealed a history of infertility in her two sisters. Blood samples from the index patient, her affected two sisters, parents, and available non-affected family members (eight samples in total, marked * in Fig. 1) were collected after obtaining written informed consent.
Ethical approval
Ethical approval was given by the Comité de Protection de la Personne (CPP) of Strasbourg University Hospital, France (CPP 09/40—WAC-2008–438 1W DC-2009-I 002).
DNA extraction
Genomic DNA was extracted from peripheral blood using Hibrigen DNA Extraction-Blood kit (Hibrigen, Istanbul, Turkey), according to the manufacturer’s instructions. DNA samples were suspended in water and quantified by nanodrop. Quality of DNA samples was checked on agarose gel.
WES and data analysis
Exome sequencing of patients IV:2, IV:3, and IV:4 (Fig. 1) was performed by Integragen Genomics (Integragen S.A., Evry, France). Library preparation, sequencing, and base calling were performed as described in [17].
Bioinformatic analysis was performed using IG Constitutional DNA pipeline V4.0. Poor quality reads were filtered out and good quality reads mapped onto the reference genome (GRCh38/hg38). Detected variants were annotated and ranked by VaRank [18].
Owing to multiple consanguineous loops within the family, we initially considered only homozygous variants shared by all affected individuals (IV:2, IV:3, and IV:4), not shared by other healthy individuals. However, we also considered possible autosomal dominant inheritance.
Further filtering was applied according to allele frequency in the largest available databases (filtering out variants with frequencies > 1%) including Genome Aggregation Database (gnomAD) [19] (http://gnomad.broadinstitute.org/) and 1000 Genomes Project (1000 g) [20] (https://www.internationalgenome.org/). SIFT [21] (https://sift.bii.a-star.edu.sg/), PolyPhen2 [22] (http://genetics.bwh.harvard.edu/pph2/), and MutationTaster2 [23] (http://www.mutationtaster.org/) were also used to predict pathogenicity of missense variants. We consulted available protein and RNA expression profiles from Human Protein Atlas [24] (http://www.proteinatlas.org) and AmaZonia [25] (http://amazonia.transcriptome.eu) to filter variants with possible roles in oocyte maturation. Available knockout (KO) animal models guided us to grade variations passing our filtering processes.
Copy number variants (CNV) were called using the CANOES program [26] and annotated using AnnotSV [27]. Adapted ACMG/AMP guidelines for single-gene CNV were used for interpretation of CNV [28].
Variant confirmation and gene–disease relationship scoring
The variant identified using WES analysis segregating across affected family members in the homozygous state was confirmed by Sanger sequencing. Primers to amplify EPAB’s exon 8 were designed using Primer3Plus online program, with specificity checked using online available programs In-SilicoPCR (https://genome.ucsc.edu/cgi-bin/hgPcr) and primer BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Amplification conditions and all primers are listed in Supplementary Table SI. DNA amplicons were then purified, and double-strand sequencing of each DNA fragment was performed by GATC Services, Eurofins Genomics (Ebersberg, Germany). Sequences were aligned on a reference sequence (GRCh38/hg38) using ApE plasmid editor in order to check variation(s). The gene–disease relationship (GDR) score for PABPC1L/EPAB was recalculated using the scoring sheet described in Van Der Kelen et al., which leads to the classification of genes as having definitive, strong, moderate, limited, or no evidence on infertility [29, 30].
Plasmids
The EPAB (PABPC1L) cDNAs expressing the Human PABPC1L/EPAB version 614 amino acid (aa) (EPAB short) inserted into the pENTR223 entry vector (DNASU, ID:HsCD00821641) was cloned by the Gateway® (Invitrogen) method into pDONR221 entry vector. The DNA sequence encoding the last 5 aa (YPPGA) of the EPAB long version was inserted using QuickChange II XL Site-Directed Mutagenesis kit (Agilent Technologies) to obtain pDONR221-EBAP. The p.Ala346Val missense variation was subsequently introduced using the same kit to obtain pDONR221-EPAB-A346V. Then these plasmids were recombined by the Gateway® (Invitrogen) method into pEGFP-C2 vector (Clontech, Takara) to obtain the pEGFP-C2-EPAB-A346V and pEGFP-C2-EPAB-WT plasmids. These plasmids were verified by Sanger sequencing (GATC Services, Eurofins Genomics).
HEK293T cell culture and transient transfection
HEK293T cell line was cultured in DMEM + Glutamax medium (Gibco) supplemented with 10% FCS and penicillin 100 UI/mL and streptomycin 100 μg/mL. U2OS cell line was cultured in McCoy’s 5A medium (Gibco) supplemented with 10% FCS and penicillin 100 UI/mL and streptomycin 100 μg/mL.
Vectors pEGFP-C2, pEGFP-C2-EPAB-WT, and pEGFP-C2-EPAB-A346V were transiently transfected in HEK293T and U2OS cells using X-tremeGENE 9 DNA transfection reagent as indicated (Sigma-Aldrich).
Cell extract and western blot
Transfected HEK293T cells were collected in PBS on ice 24 h post-transfection. The cellular pellets were lysed in RIPA buffer (10 mM Tris–Cl (pH 8.0), 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl) for 15 min at 4 °C and centrifuged at 16,000 g for 15 min at 4 °C. Fifteen micrograms of each extract were loaded and resolved on a 10% SDS-PAGE gel and detected by immunoblot using specific antibodies. Antibodies towards GFP (TP401), Beta-tubuline (TUB-2A2) were purchased from Torrey Pines Biolabs, Euromedex, respectively. For immunoblots, the concerned antibodies were diluted at 1000-fold.
RT-qPCR
Total RNA was extracted from transfected HEK293T cells using a GenElute Mammalian Total RNA Miniprep kit (Sigma) and reverse transcribed with SuperScript IV reverse transcriptase (Invitrogen). The quantitative PCR was done using the CFX384 real-time system (Biorad). The primers used for the GFP and GAPDH genes in qPCR were purchased from QIAGEN (Quantitect Primer Assay: EGFP_1_SG and Hs_GADPH_1_SG). The level of mRNA expression of the various genes analyzed was represented as the ratio of the values normalized with the housekeeping GAPDH mRNA.
Time course of GFP-positive cell detection
HEK293T and U2OS cell lines were seeded in six-well plates. The following day, the cells were transfected using vectors PEGF-C2, pEGF-C2-EPAB-WT, or pEGF-C2-EPAB-A346V. GFP-positive cells were monitored over a 40-h period and recorded every 2 h using Incucyte (Essenbioscience). To assess the impact of proteasome-dependent proteolysis, transfected cells were treated, 8 h post-transfection, with either proteasome inhibitor MG132 at 20 μM (Sigma-Aldrich) or the corresponding vehicle (EtOH). Similarly, GFP-positive cells were monitored and recorded every 2 h using Incucyte (Essenbioscience). Data from three biological replicate series, each with three technical replicate wells, were used for plotting. For each time point, 16 images per well were collected using a 20-fold magnification objective.
Sequence alignment protocol
The sequences from the seven human PAB proteins, PABPC1L, PABPC1, PABPC3, PABPC4, PABPC5, PABPN1, and PABPN1L, were retrieved from Uniprot database entries [31]. Orthologs were searched with Orthoinspector in 700 eukaryotic species [32]. For each PAB protein, orthologs were aligned with PipeAlign2 [33] and sequence errors in the alignments were weighted with the SIBIS tool [34]. Conservation metrics (identity and similarity) were calculated taking taxonomy (phylogenetic distance) into account. The Jensen-Shannon divergence scores [35, 36] were calculated for both As from the AxxA motif.
In silico protein modeling of PABPC1L/EPAB
Since a high-resolution structure of EPAB has not yet been determined, homology modeling was used to obtain a 3D structure model. The FASTA sequence (619 aa) and domain organization were retrieved from the Q4VXU2 (PAP1L_HUMAN) Uniprot database entry [31]. To identify previously determined structures of poly(A)-binding proteins, BLAST searches (protein Basic Local Alignment Search Tool, BLAST (Altschul et al., 1990) http://blast.ncbi.nlm.nih.gov/Blast.cgi) were performed against the Protein Data Bank (PDB, [37] http://www.rcsb.org/pdb/home/home.do) using the PABPC1L/EPAB sequence as a query. The best hit (expect value 2e-152, 56.8% identity, and 72% coverage) was obtained for the cryoEM structure of a yeast poly(A)-binding protein which is the structure of a PAB with the longest sequence and contains the four RRMs. The following hits corresponded to structures of only part of PABPC1 (1 or 2 RRM or PABC domain). The sequences of EPAB, PABPC1, and PABP_YEAST were then aligned using Pipealign2. To analyze the effect of the amino acid substitution, the full-length wild-type RRM4 and mutated (A346V) RRM4 of PABPC1L/EPAB 3D structures were predicted using Phyre2 (Protein Homology/Analogy Recognition Engine V2.0, Phyre2 [38], http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index). We focused on RRM4, which contains the variant p.Ala346Val. The complex of RRM4 with RNA was modeled using the PABP_YEAST structure in complex with RNA (PDB code 6R5K) [39].
Sequence motif analysis of the AxxA motif in all RRM with known structures
We identified 1281 structures from the PFam PF00076 (RRM_1) entry corresponding to 257 RRM domains of 185 protein sequences (Uniprot entries). The 257 RRM sequences were aligned with Pipealign2 and statistics calculated for the amino acid present in the first and last position of the AxxA motif.
Prediction of the protein stability change upon single variation
The integrated predictor, iStable [40] (http://predictor.nchu.edu.tw/iStable/), was used to predict the effect of the variant p.Ala346Val of the AxxA motif on the structure of the four RRM of PAB_YEAST using the structure of the PDB code 6R5K and EPAB_HUMAN using the PDB codes 4F02 and 2D9P. We also performed the prediction using the sequence of PABPC1L/EPAB.
Results
Clinical findings
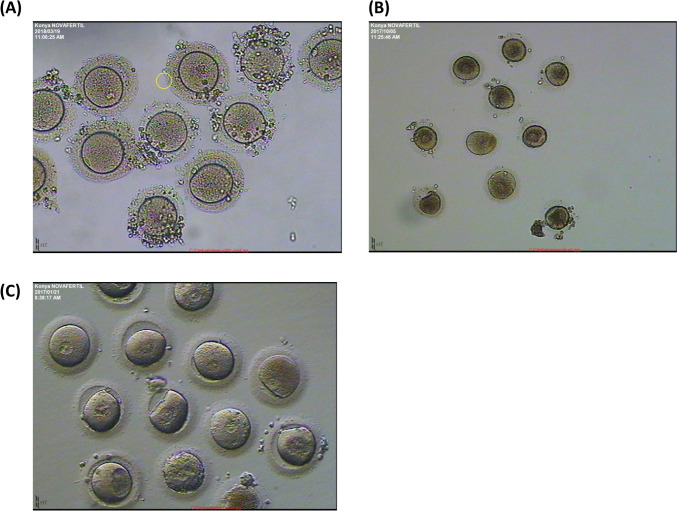
All three sisters had a normal karyotype, regular menstruation cycle, and hormonal dosages within the normal range; however, their sibling (IV:5) was diagnosed with Down syndrome. Index patient (IV:2), after an unsuccessful IUI, had one IVF cycle where all the 18 collected oocytes were at the GV stage, none of them matures even after an extended period of culture in vitro (see Supplementary Table SII). Her sister, IV:3, had three IUI before four IVF cycles, from which we got precise information for three of them, the fourth one having been done at another IVF center. Out of the three cycles, she produced a total of 35 oocytes (11 + 13 + 11) all at the GV stage. She reported that during the fourth cycle, an intra-cytoplasmic sperm injection (ICSI) was performed and one embryo transferred, but no pregnancy ensued. The third sister, IV:4, had three IVF cycles with a total of 32 oocytes (21 + 1 + 10) collected, 27 of them were immature at GV stage and 5 degenerated. For the third cycle, a prolonged culture of the collected oocytes was performed, but none of the 10 GV oocytes reached MII stage (culture conditions are given in Supplementary Table SII). The oocytes displayed morphological abnormalities such as an elongated shape and increased cytoplasmic granularity. Figure 2 illustrates the morphology of the denuded oocytes for each sister. Additional information about stimulation cycles is provided in Supplementary Table SII.
Fig. 2.
Collected oocytes after denudation from A IV:2, B IV:3, and C IV:4. All oocytes were at GV stage and failed to proceed to maturation even after extended culture in vitro
Identification of pathogenic variant in the PABPC1L/EPAB gene
One affected sister (IV:3) was screened via our infertility panel, particularly to study the two known genes at that time causing oocyte maturation arrest phenotypes, namely TUBB8 and PATL2 [41]. No pathogenic or likely pathogenic variants were identified.
To further explore the potential genetic cause of female infertility in this family, DNA of the three affected sisters (IV:2, IV:3, and IV:4) was analyzed using WES. At least 4.8 GB of DNA sequence was generated with a mean depth of 63X and > 99.2% of the target exome represented with > 25-fold coverage (see Supplementary Table SIII).
Given the consanguinity loops, we favored a recessive mode of transmission and thus tracked homozygous variants shared by all three sisters. However, we did not exclude possible dominant or X-linked transmission; after a first round of analysis, we also checked for heterozygous and hemizygous variants. Data analysis revealed five homozygous variants in four genes (see Supplementary Table SIV). No pathogenic CNV shared by the three sisters was found related to the phenotype studied.
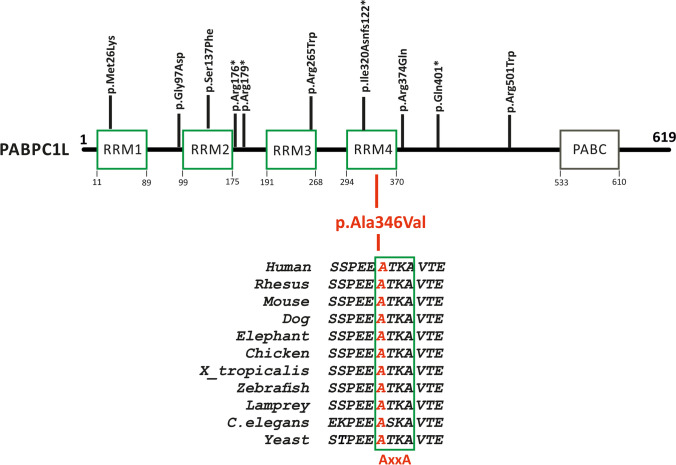
Our filtering process retained EPAB also known as PABPC1L (NM_001372179) as the most plausible candidate gene due to the existence of a mouse KO model with a female sterility phenotype similar to the one observed in our patients. PABPC1L/EPAB maps to chromosome 20 and consists of 15 exons. The gene encodes a 619aa protein with five highly conserved domains, including four RNA recognition motifs (RRMs 1 to 4) and a poly(A)-binding C-terminal (PABC) domain (Fig. 3). In humans, PABPC1L/EPAB expression is not restricted to ovaries, but the full-length protein is abundantly expressed in oocytes, while an alternatively spliced form lacking the first 58 base pairs of exon 8 is predominantly expressed in somatic tissues [12]. Human PABPC1L/EPAB shares 77% identity and 84% similarity, and 72% identity and 83% similarity with mouse and Xenopus orthologues (where it was first described) [42]. It shares 57% identity and 75% similarity to the unique Saccharomyces cerevisiae PAB1 orthologue.
Fig. 3.
Schematic representation of the PABPC1L/EPAB protein domains and conservation around the identified variation. RRM1–4, RNA recognition motif (RRM-Pfam: PF00076); PABC, poly(A)-binding protein C-terminal (PABC-Pfam: PF00658) domain. The pathogenic variations identified so far are shown on top, and the identified variation in this study is marked in red in the bottom part; the amino acid conservation within the species is shown below the protein representation
Variant confirmation, screening, and GDR scoring
A homozygous missense variation c.1037C > T, p.Ala346Val, in exon 8 of PABPC1L/EPAB, was identified in all three sisters. This variant is extremely rare, only listed 59 times as heterozygous in gnomAD v3.1 (25 females and 34 male) for an allele frequency of 0.00002627 (accessed on 06/011/2023). No homozygous cases were observed in any public databases. All bioinformatics programs used predicted the variant as deleterious (see Supplementary Table SIV). The variant, and its segregation in the family, was confirmed through Sanger sequencing for all the available family members, which included both parents, a female fertile cousin (IV:1) and two fertile aunts (III:1; III:4). The variant co-segregated with the infertility phenotype. The three sisters were homozygous for the variant, while the parents were carriers as well as the fertile cousin and one aunt. The other fertile aunt was homozygous wild type.
PABPC1L/EPAB GDR was then recalculated, considering the recently published data [43] [44] and the variant described in this report, PABPC1L/EPAB, now reached a score of 16 points changing the classification from limited to definitive (see scoring sheet in Supplementary data). The recently reported patients and the identification of the variants in the patients as well as the functional data described here improved its classification from “strong” to “definitive.”
The EPAB-Ala346Val variant presents a lower stability in human cells
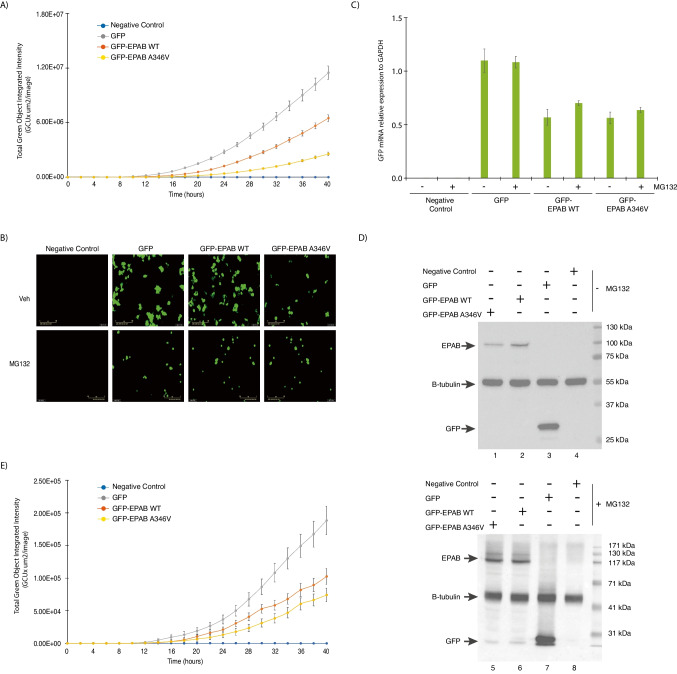
In order to establish the pathogenicity of the identified variant, we investigated the impact of the substitution (A346V) on expression and stability of the PABPC1L/EPAB protein in human cells. HEK293T cells that do not express endogenous PABPC1L/EPAB were transiently transfected with vectors expressing either GFP, GFP-EPAB WT, or GFP-EPAB A346V. The expression of EPAB WT and A346V was followed by live-cell imaging, monitoring, and recording every 2 h GFP-positive cells for a period of 40 h (Fig. 4A and B). The number and the signal intensity for the different GFP-positive cells continuously increased along the time course with the highest and more rapid kinetics for GFP-expressing cells. However, the dynamic accumulation and GFP intensity were significantly lower for GFP-EBAP A346V compared to the GFP-EPAB WT (Fig. 4A and B). Similar results were obtained using the U2OS cell line (Supplementary Fig. 1). Western blot analysis validated the expression of the different proteins. Moreover, we detected a lower level of expression of the variant compared to the GFP-EPAB WT corroborating the live-cell imaging observations. Importantly, the relative mRNA expression of both EPAB WT and EPAB A346V was similar (Fig. 4C and D). Altogether, these data suggest that the substitution A346V makes the protein more sensitive to proteolysis. We then blocked proteasome-dependent proteolysis by treating transfected HEK293T cells with MG132 inhibitor and repeated the live-cell imaging analysis. We observed that the inhibition of proteolysis correlated with a comparable accumulation and GFP intensity for GFP-EBAP A346V and GFP-EPAB WT (Fig. 4E and B). The relative mRNA expression and protein detection were also similar for both EPAB WT and EPAB A346V (Fig. 4C and D). Taken together, these results indicated that the EPAB-A346V variant presents poorer stability in human cells.
Fig. 4.
The PABPC1L/EPAB carrying the p.Ala346Val variant is unstable in human cells. Quantification of transfected HEK293T cells expressing GFP, GFP-EPAB WT, GFP-EPAB A346V in absence (A) or presence of proteasome inhibitor MG132 (E). GFP-positive cells were monitored and recorded every 2 h using Incucyte (Essenbioscience) during 48 h. Data from three biological replicate series, each with three technical replicate wells, were used for plotting. B GFP-positive cells images obtained from 48 h live-imaging follow-up performed using Incucyte on transfected HEK293T cells expressing GFP, GFP-EPAB WT, GFP-EPAB A346V in absence (upper panels) or presence of proteasome inhibitor MG132 (lower panels). C Relative mRNA expression of GFP from total RNA extracts collected 24 h after transfection of HEK293T cells with p-EGFP-C2, p-EGFP-C2-EPAB WT, and p-EGFP-C2-EPAB A346V vectors. Error bars represent the SD of quadruplicates. D Relative protein expression, analyzed by western blotting, of GFP, GFP-EPAB WT, GFP-EPAB A346V detected using GFP antibodies and normalized by beta-tubulin from whole-cell extract prepared 24 h after transfection of HEK293T cells with p-EGFP-C2, p-EGFP-C2-EPAB WT, and p-EGFP-C2-EPAB A346V vectors in absence (upper panel) or presence of proteasome inhibitor MG132 (lower panel)
Effect of the p.Ala346Val variant on EPAB structure and properties
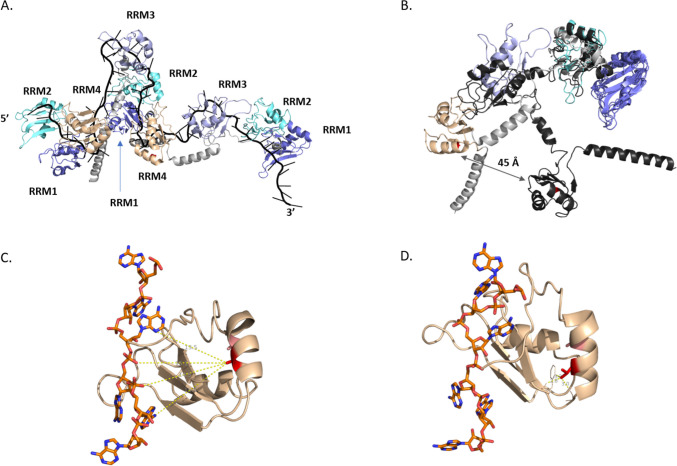
We further explored the possible effects of the identified substitution (Ala346Val) on the protein structure. For this purpose, we analyzed the 3D structure of the PABPC1L/EPAB protein by homology modeling. PABPC1L/EPAB comprises four RRMs (Fig. 5A), responsible for binding to RNA, as well as a C-terminal poly(A)-binding protein (PABC) domain which is critical for protein–protein interactions (Fig. 3). The typical architecture of an RRM domain is a four-stranded antiparallel β-sheet with two alpha helices packed on the same side [45] (Fig. 5). As the regions between the RRMs are highly flexible (Fig. 5B), the conformation of the full-length model is not relevant. The substitution Ala346Val takes place at the first alanine of the highly conserved AxxA motif found in all RRM of poly(A)-binding (PAB) proteins (conservation of nearly 95% for both A, with Jensen-Shannon divergence scores between 0.46 and 0.66) (Fig. 5-Table SI).
Fig. 5.
In silico protein modeling of EPAB. A Structure of three protomers (two with the four RRM and one with the first two RRM) of the yeast poly(A)-binding protein epab yeast in complex with poly(A) RNA (in black) (extracted form structure with PDB code 6R5K). B Superimposition on RRM1 of the three protomers represented in A, showing the high flexibility of epab yeast. The distance between the two Cβ of the first alanine of the AxxA motif is 10 Å for RRM3 (not shown) and 45 Å for RRM4 (shown by the black arrow). C Model of PABPC1L RRM4 wild type. The distances to the poly(A) RNA (cpk colors) are represented. D Model of PABPC1L RRM4 with the A346V variation. The network of interactions made by the valine side chain is represented
To check if this motif is conserved throughout the RRM domain in other protein families, we analyzed the sequences of all RRM with known structures (Fig. S1). Sixty-two percent of the sequences have an AxxA motif at this position (between 70 and 83% when considering each A alone, e.g., for Axxx and for xxxA). Less than 8% of the sequences possess a VxxA motif. The substitution Ala346Val is localized at the internal face of α-helix 2 of RRM4, at the opposite side of the RNA-binding interface (distance between the methyl group and the sugar-phosphate backbone is approximatively 14 Å, Fig. 5C). The alanine side chain points towards the N-terminal part of the RRM (notably to Val294) without making any interaction. Interestingly, when a valine substitutes the alanine at position 346, the side chain of the valine makes a network of interactions, notably with the N-terminal part of strand β1 and the loop β3-H2, stabilizing the RRM structure, as observed in the experimental structures or in the model of PABPC1L/EPAB RRM4 with the A to V substitution (Fig. 5D). The findings are in agreement with the prediction of the effect of the missense on the structure of RRM using the integrated predictor IStable. The predictions show that the effect is not the same in the four RRM modules (Table SII). RRM4 is the only RRM module for which protein stability is increased with the variant. This increase could be explained by interactions forming through the side chain of the valine that are not possible with an alanine. Worth noting, when looking closer at the Jensen-Shannon divergence scores of all the PAB-RRM AxxA motifs, RRM4 possesses the most conserved AxxA motif (especially for the four PAB proteins with the same architecture as PABPC1L/EPAB, namely PABPC1, PABPC3, PABPC4, and EPAB), suggesting that a modification of this motif may affect the stability of the full-length structure. Taken together, the study of the conformational consequences of Ala346Val substitution supports the hypothesis of a rigidification of RRM4 that diminishes the global flexibility of the full-length protein. This may hinder RNA recognition (Fig. 5).
Discussion
Complete failure of all oocytes to mature is very rare, but a recognized cause of primary female infertility. Despite recent efforts to uncover the genetic aetiology of female infertility and in particular genetic causes of defective oocyte maturation, OMIM lists only 19 genes responsible for oocyte/zygote/embryo maturation defects. In the present report, we identified a homozygous variant c.1037C > T, p.Ala346Val in EPAB (gene known also as PABPC1L) in three sisters from a consanguineous Turkish family using a WES strategy. EPAB is a highly conserved poly(A)-binding protein well described functionally in yeast, Xenopus, and the mouse. In yeast, PAB1 (EPAB ortholog) plays a key role in translational control and regulation of cell growth, through its influence on the length of the poly(A) tail [46]. In Xenopus and mouse, EPAB is essential in the dynamical control of the poly(A) length during oocyte maturation. It has detrimental function in the control of maternally stored mRNA translation, through either dependent or independent polyadenylation translational activation during oocyte maturation [42]. In both Xenopus and the mouse, EPAB is specifically expressed in the oocyte and, for mice, less strongly in the testis [14]. However, in contrast with the transcriptional regulation in Xenopus and mouse, oocyte- and early embryo-specific expression of PABPC1L/EPAB in humans is regulated by a post-transcriptional mechanism. Indeed, in humans, PABPC1L/EPAB mRNA, in addition to its presence in oocytes and pre-implantation embryos, is also detected in multiple somatic tissues including brain, pancreas, liver, thymus, and lung [12, 42]. However, in somatic tissues as well as 8-cell and blastocyst stage human embryos, where PABPC1 expression is initiated, alternative splicing takes place eliminating the first 58 bp of exon 8, resulting in the production of a truncated protein lacking the essential PAB domain. The full-length PABPC1L/EPAB mRNA is almost undetectable in somatic tissues, only being expressed in oocytes and early embryos prior to ZGA [12].
The identified variation, c.1037C > T, p.Ala346Val, is located in one of the four RNA recognition domains (RRM4, Fig. 3), predicted as disease causing/deleterious using different online tools. Pathogenicity of the identified variant was assessed by functional studies using transient transfection HEK293T cells. These experiments clearly showed that the A346V substitution affects protein expression as well as its stability. Treatment of transfected cells with a proteasome inhibitor demonstrated that the A346V substitution makes the PABPC1L/EPAB protein more sensitive to proteolysis. In order to further sustain this finding, we also tried to develop a humanized yeast model. Since the human EPAB-WT was not able to restore the yeast mutant phenotype, this model turned out to be irrelevant (see Supplementary data). This is an interesting point to note. Even with a very high degree of conservation, some model species cannot be used to study human protein functions.
From a structural point of view, RRM4 has been shown to be functionally different, at least in terms of RNA binding properties [47]. In the cryoEM structure of the yeast poly(A) RNP, RRM4 of the yeast Pab1 protomer interacts with the RRM1–RRM2 module of the adjacent protomer (Fig. 5A) [39]. Whereas a valine residue at this position could be observed in rare orthologous sequences, these observations suggest that, through reduction of the global flexibility of the protein, the consequence of the substitution is a rigidification of the protein structure. This may explain the instability of PABPC1L/EPAB and its faster proteolysis.
Three recent reports describe eight women in total, from seven different families, suffering from oocyte/zygote/embryo arrest and presenting variants in PABPC1L/EPAB [43, 44, 48]. Heterozygotes of both sexes were fertile [43]. Since only biallelic or homozygous variants were found in affected women and one heterozygous woman was fertile, these results confirm the recessive mode of transmission we describe here. Altogether, the patients identified as carrying variants in PABPC1L/EPAB display a variable phenotype, which fluctuates from complete oocyte maturation arrest, where all the oocytes picked up are at the GV stage—as for the three sisters presented here—to a mixture of oocytes at the different stages of maturation (GV, MI, or MII stages). In all cases where MII oocytes where treated by ICSI, either an absence of fertilization was observed or an early embryonic arrest of development [43].
This variability of the phenotype observed in the case of PABPC1L/EPAB has been observed with other genes. Indeed, this is the case for the first identified gene responsible for an oocyte maturation arrest phenotype, TUBB8, which turns out, as for PATL2 or TRIP13, to exhibit a variable phenotype. This can be explained by the type of variants affecting the protein, which can have a more or less drastic effect or by variable susceptibility linked to the genetic background of the affected individual. This is an important point since it underlines that the limit between oocyte maturation arrest, fertilization failure, and early embryonic development arrest is not clear cut. In a diagnostic perspective, it will be important to keep in mind that genes involved in both phenotypes must be analyzed in case of oocyte maturation arrest, fertilization failure, or early embryonic arrest. This is now well accepted such that the OMIM database has redefined these phenotypes as OZEMA.
The description of new patients warrants the re-evaluation of the involvement of the validity of PABPC1L/EPAB in female infertility. For this purpose, we recalculated the GDR score. Using all the data available, the score of PABPC1L/EPAB is now 16 points, instead of 5.5 points. Such a level of evidence fully justifies to add PABPC1L/EPAB in a collection of genetic diagnostic tools for female infertility with OZEMA.
In addition to replicating the phenotype observed in our patients, the EPAB KO mouse model also indicates a role of EPAB in cumulus expansion and ovulation dysfunction [13]. Most probably because scoring cumulus or oocyte before decoronization for the ICSI procedure is not part of the routine of IVF laboratories, we did not obtain any information about any abnormalities in the cumulus collected during the ovarian puncture. Since these patients were stimulated and the oocytes collected by ovarian puncture, we have no information about the ovulation process in these patients. Female mice have a normal oestrous cycle, suggesting a normal hormonal cycle; however, this was not formally checked, fitting with the observation that the three sisters did not present any hormonal abnormalities.
Furthermore, it has also been shown that homozygous EPAB mutant mice present a miss-organization of the oocyte meiotic spindle microtubules [13]. Therefore, given the presence of a brother with trisomy 21 in this family, further investigation into whether heterozygous variants in human PABPC1L/EPAB affect chromosome segregation would be interesting with potential consequences for genetic counselling.
Guzeloglu-Kayisli et al. tried to rescue the mouse oocyte maturation phenotype by injecting the Epab mRNA into denuded GV stage Epab−/− oocytes without any success [13], suggesting that EPAB is required at an earlier stage during oogenesis compared to WEE2, another OZEMA gene defect, which was successfully rescued [49]. Lowther and Mehlmann then tested if injection of Epab mRNA into prenatal follicle enclosed oocytes isolated from Epab−/− mice. Such approach restores follicle and oocyte growth. These in vitro maturated oocytes were not tested for their ability to sustain fertilization and/or early embryonic development. However, even with this improved strategy, gene therapy applications in human patients do not appear currently realistic [10]. Another therapeutic strategy suggested by Wang et al. showed that the Mos-MAPK pathway is abnormally activated in mouse mutated oocytes [43] raising the question: could a treatment with a Mos-MAPK inhibitor help restore oocyte maturation?
In conclusion, our results provide novel understanding of the pathogenesis of oocyte maturation arrest with identification and further characterization of a novel genetic cause of GV arrest in our patients. Our results have consequences for the investigation and management of women with infertility, in addition, opening up new genetic counselling considerations for affected families with PABPC1L/EPAB variants. For the moment, and until some success is achieved in gene therapy trials, these patients should be orientated towards egg donation, when possible. Male mice deficient for EPAB are fertile [16], which can be considered reassuring for brothers of affected females; however, this remains to be verified in humans.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like also to thank Dr. Robert Drillien for his critical reading of this manuscript.
Author contribution
OO data analysis, variation confirmation; ASG recruitment of patients, collection of clinical data and samples; UB DNA extraction, quality and quantity control; JM supervised the bioinformatics analysis; ER performed the assays using humanized yeast cells and analyzed the data; NLM and RL performed the HEK293T cell assays, ER performed the yeast experiments, and KC performed the sequence alignments and analysis; CM performed the 3D modeling and analysis; SV supervised all the study. OO and SV designed and wrote the first draft of the manuscript. All authors contributed to the revision process and agreed to the last version of the manuscript.
Funding
The study was funded by Fondation Maladie Rares (High Throughput Sequencing and Rare Diseases–2018, “GenOmics of rare diseases”).
Data availability
Data generated in this study are included in the following article and corresponding supplementary data. The raw sequencing data generated in the course of this study are not publicly available due to research protocol restrictions with patient informed consent not covering this data distribution. The identified variant has been submitted to ClinVar (VCV001172840.1) (https://www.ncbi.nlm.nih.gov/clinvar).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Erickson G. Follicle growth and development | GLOWM [Internet]. 2008 [cited 2021 Jun 28]. Available from: http://www.glowm.com/section-view/heading/follicle-growth-and-development/item/288
- 2.Johnson MH, Everitt B. Essential reproduction. In: Blackwell Wiley., editor. Sperm and eggs chapter. Wiley-Blackwell; 2000. pp. 175–188. [Google Scholar]
- 3.Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol. 2017;79:237–260. doi: 10.1146/annurev-physiol-022516-034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21:427–454. doi: 10.1093/humupd/dmv011. [DOI] [PubMed] [Google Scholar]
- 5.Robker RL, Hennebold JD, Russell DL. Coordination of ovulation and oocyte maturation: a good egg at the right time. Endocrinology. 2018;159:3209–3218. doi: 10.1210/en.2018-00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- 7.Voeltz GK, Ongkasuwan J, Standart N, Steitz JA. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15:774–788. doi: 10.1101/gad.872201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkie GS, Gautier P, Lawson D, Gray NK. Embryonic poly(A)-binding protein stimulates translation in germ cells. Mol Cell Biol. 2005;25:2060–2071. doi: 10.1128/MCB.25.5.2060-2071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Richter JD. RINGO/cdk1 and CPEB mediate poly(A) tail stabilization and translational regulation by ePAB. Genes Dev. 2007;21:2571–2579. doi: 10.1101/gad.1593007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowther KM, Mehlmann LM. Embryonic poly(A)-binding protein is required during early stages of mouse oocyte development for chromatin organization, transcriptional silencing, and meiotic competence. Biol Reprod. 2015;93:43. doi: 10.1095/biolreprod.115.131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pushpa K, Kumar GA, Subramaniam K. Signaling-Mediated Control of Cell Division, From Oogenesis to Oocyte-to-Embryo Development. Results and Problems in Cell Differentiation vol 59. In: Arur S, editor. Translational Control of Germ Cell Decisions. Cham: Springer; 2017. pp. 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzeloglu-Kayisli O, Lalioti MD, Babayev E, Torrealday S, Karakaya C, Seli E. Human embryonic poly(A)-binding protein (EPAB) alternative splicing is differentially regulated in human oocytes and embryos. Mol Hum Reprod. 2014;20:59–65. doi: 10.1093/molehr/gat061. [DOI] [PubMed] [Google Scholar]
- 13.Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, Sasson I, Ilbay O, Sakkas D, et al. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J. 2012;446:47–58. doi: 10.1042/BJ20120467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci USA. 2005;102:367–372. doi: 10.1073/pnas.0408378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C-R, Lowther KM, Lalioti MD, Seli E. Embryonic poly(A)-binding protein (EPAB) is required for granulosa cell EGF signaling and cumulus expansion in female mice. Endocrinology. 2016;157:405–416. doi: 10.1210/en.2015-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozturk S, Guzeloglu-Kayisli O, Lowther KM, Lalioti MD, Sakkas D, Seli E. Epab is dispensable for mouse spermatogenesis and male fertility. Mol Reprod Dev. 2014;81:390. doi: 10.1002/mrd.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okutman Ö, Demirel C, Tülek F, Pfister V, Büyük U, Muller J, et al. Homozygous splice site mutation in ZP1 causes familial oocyte maturation defect. Genes (Basel) 2020;11:E382. doi: 10.3390/genes11040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geoffroy V, Pizot C, Redin C, Piton A, Vasli N, Stoetzel C, et al. VaRank: a simple and powerful tool for ranking genetic variants. PeerJ. 2015;3:e796. doi: 10.7717/peerj.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim N-L, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit7.20. doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 24.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 25.Le Carrour T, Assou S, Tondeur S, Lhermitte L, Lamb N, Reme T, et al. Amazonia!: an online resource to google and visualize public human whole genome expression data. TOBIOIJ. 2010;4:5–10. [Google Scholar]
- 26.Backenroth D, Homsy J, Murillo LR, Glessner J, Lin E, Brueckner M, et al. CANOES: detecting rare copy number variants from whole exome sequencing data. Nucleic Acids Res. 2014;42:e97. doi: 10.1093/nar/gku345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geoffroy V, Herenger Y, Kress A, Stoetzel C, Piton A, Dollfus H, et al. AnnotSV: an integrated tool for structural variations annotation. Bioinformatics. 2018;34:3572–3574. doi: 10.1093/bioinformatics/bty304. [DOI] [PubMed] [Google Scholar]
- 28.Brandt T, Sack LM, Arjona D, Tan D, Mei H, Cui H, et al. Adapting ACMG/AMP sequence variant classification guidelines for single-gene copy number variants. Genet Med. 2020;22:336–344. doi: 10.1038/s41436-019-0655-2. [DOI] [PubMed] [Google Scholar]
- 29.Van Der Kelen A, Okutman Ö, Javey E, Serdarogullari M, Janssens C, Ghosh MS, et al. A systematic review and evidence assessment of monogenic gene-disease relationships in human female infertility and differences in sex development. Hum Reprod Update. 2023;29:218–232. doi: 10.1093/humupd/dmac044. [DOI] [PubMed] [Google Scholar]
- 30.Smith ED, Radtke K, Rossi M, Shinde DN, Darabi S, El-Khechen D, et al. Classification of Genes: Standardized Clinical Validity Assessment of Gene-Disease Associations Aids Diagnostic Exome Analysis and Reclassifications. Hum Mutat. 2017;38:600–608. doi: 10.1002/humu.23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nevers Y, Kress A, Defosset A, Ripp R, Linard B, Thompson JD, et al. OrthoInspector 3.0: open portal for comparative genomics. Nucleic Acids Res. 2019;47:D411–8. doi: 10.1093/nar/gky1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plewniak F, Bianchetti L, Brelivet Y, Carles A, Chalmel F, Lecompte O, et al. PipeAlign: a new toolkit for protein family analysis. Nucleic Acids Res. 2003;31:3829–3832. doi: 10.1093/nar/gkg518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khenoussi W, Vanhoutrève R, Poch O, Thompson JD. SIBIS: a Bayesian model for inconsistent protein sequence estimation. Bioinformatics. 2014;30:2432–2439. doi: 10.1093/bioinformatics/btu329. [DOI] [PubMed] [Google Scholar]
- 35.Capra JA, Singh M. Predicting functionally important residues from sequence conservation. Bioinformatics. 2007;23:1875–1882. doi: 10.1093/bioinformatics/btm270. [DOI] [PubMed] [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 37.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäfer IB, Yamashita M, Schuller JM, Schüssler S, Reichelt P, Strauss M, et al. Molecular basis for poly(A) RNP architecture and recognition by the Pan2-Pan3 Deadenylase. Cell. 2019;177:1619–1631.e21. doi: 10.1016/j.cell.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C-W, Lin J, Chu Y-W. iStable: off-the-shelf predictor integration for predicting protein stability changes. BMC Bioinformatics. 2013;14(Suppl 2):S5. doi: 10.1186/1471-2105-14-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okutman O, Tarabeux J, Muller J, Viville S. Evaluation of a custom design gene panel as a diagnostic tool for human non-syndromic infertility. Genes (Basel) 2021;12:410. doi: 10.3390/genes12030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guzeloglu-Kayisli O, Pauli S, Demir H, Lalioti MD, Sakkas D, Seli E. Identification and characterization of human embryonic poly(A) binding protein (EPAB) Mol Hum Reprod. 2008;14:581–588. doi: 10.1093/molehr/gan047. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Guo J, Shi J, Li Q, Chen B, Pan Z, et al. Bi-allelic pathogenic variants in PABPC1L cause oocyte maturation arrest and female infertility. EMBO Mol Med. 2023;15:e17177. doi: 10.15252/emmm.202217177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Zhou R, Lu X, Dai S, Liu M, Jiang C, Yang Y, Shen Y, Wang Y, Liu H. Identification of nonfunctional PABPC1L causing oocyte maturation abnormalities and early embryonic arrest in female primary infertility. Clin Genet. 2023;104:648–658. doi: 10.1111/cge.14425. [DOI] [PubMed] [Google Scholar]
- 45.Daubner GM, Cléry A, Allain FH-T. RRM-RNA recognition: NMR or crystallography ...and new findings. Curr Opin Struct Biol. 2013;23:100–8. doi: 10.1016/j.sbi.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Sachs AB, Davis RW. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989;58:857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- 47.Yao G, Chiang Y-C, Zhang C, Lee DJ, Laue TM, Denis CL. PAB1 self-association precludes its binding to poly(A), thereby accelerating CCR4 deadenylation in vivo. Mol Cell Biol. 2007;27:6243–6253. doi: 10.1128/MCB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maddirevula S, Awartani K, Coskun S, AlNaim LF, Ibrahim N, Abdulwahab F, et al. A genomics approach to females with infertility and recurrent pregnancy loss. Hum Genet. 2020;139:605–613. doi: 10.1007/s00439-020-02143-5. [DOI] [PubMed] [Google Scholar]
- 49.Sang Q, Li B, Kuang Y, Wang X, Zhang Z, Chen B, et al. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet. 2018;102:649–657. doi: 10.1016/j.ajhg.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated in this study are included in the following article and corresponding supplementary data. The raw sequencing data generated in the course of this study are not publicly available due to research protocol restrictions with patient informed consent not covering this data distribution. The identified variant has been submitted to ClinVar (VCV001172840.1) (https://www.ncbi.nlm.nih.gov/clinvar).