Abstract
With the rising demand for in vitro fertilization (IVF) cycles, there is a growing need for innovative techniques to optimize procedure outcomes. One such technique is time-lapse system (TLS) for embryo incubation, which minimizes environmental changes in the embryo culture process. TLS also significantly advances predicting embryo quality, a crucial determinant of IVF cycle success. However, the current subjective nature of embryo assessments is due to inter- and intra-observer subjectivity, resulting in highly variable results. To address this challenge, reproductive medicine has gradually turned to artificial intelligence (AI) to establish a standardized and objective approach, aiming to achieve higher success rates. Extensive research is underway investigating the utilization of AI in TLS to predict multiple outcomes. These studies explore the application of popular AI algorithms, their specific implementations, and the achieved advancements in TLS. This review aims to provide an overview of the advances in AI algorithms and their particular applications within the context of TLS and the potential challenges and opportunities for further advancements in reproductive medicine.
Keywords: Artificial intelligence, Assisted reproductive technology, In vitro fertilization, Medical imaging, Neural networks, Time-lapse system
Introduction
The birth of Louis Brown in 1978, which marked the first successful implementation of in vitro fertilization (IVF), represents a significant milestone in assisted reproductive technology (ART), heralding a new era in the field of reproductive medicine [1]. Over time, advancements such as cryopreservation and preimplantation genetic test for aneuploidy (PGT-A) have been introduced, gradually improving assisted reproductive procedures for the 186 million infertile couples worldwide [2]. However, despite these advancements, accurately predicting the outcomes of an IVF cycle remains challenging, with clinical pregnancy rates varying between 7.6 and 51.1% in the USA [3]. Nevertheless, the emergence of time-lapse system (TLS) technology has provided valuable support for ART and has led to the identification of novel markers within the IVF procedure that can potentially enhance patient outcomes.
Researchers have discovered that TLS technology enables the identification of abnormal changes in embryonic development, such as the spontaneous collapse of the human blastocyst, which can indicate lower quality and higher rates of degeneration and aneuploidy [4]. By analyzing the relationship between oxygen consumption measurements and morphokinetics, researchers can observe the oxygen consumption during embryo cleavages and predict the potential for successful implantation [5]. TLS technology is currently being explored as a promising alternative to blastomere biopsy for PGT-A, as the latter has been found to hinder embryo compaction and blastulation [6].
In the realm of computer science, artificial intelligence (AI) techniques, including machine learning (ML) and neural networks, are being extensively researched. AI involves the design of intelligent computer systems that exhibit human-like characteristics such as language understanding, learning, reasoning, and problem-solving abilities [7]. The rapid progress in AI technology has led to numerous applications in the medical field, including pathology [8], dermatology [9], radiology [10], and oncology [11]. AI is increasingly enhancing diagnoses, prediction-based diagnoses, and clinical care. Recently, the World Health Organization (WHO) defined AI as a supportive tool to improve healthcare decision-making [12]. In the field of ART, AI has proven valuable in predicting embryo cell stages, blastocyst formation, and live births, thereby aiding in the selection of high-quality human blastocysts [13, 14]. Furthermore, AI is anticipated to have additional applications in automating the labeling of work pieces, developing optimal protocols for IVF stimulation, and quality control of culture conditions.
The availability of large amounts of image data has propelled the progress of AI applications in advancing TLS within reproductive medicine. AI’s ability to process vast quantities of image information has alleviated the workload and facilitated shared decision-making with embryologists [15]. However, it is crucial to prioritize the explainability of AI systems to gain a thorough understanding of the decision-making process involved in these applications. By ensuring transparency and explainability, clinicians can mitigate potential risks associated with the use of AI in ART and other critical domains. This review aims to provide an overview of recent AI algorithms, their application in TLS, and the potential future collaborations in this field.
Time-lapse system and AI requirements
Incubation and selection of embryos are critical stages in IVF process. However, traditional grading methods offer limited insights into embryonic morphology at specific time points. Monitoring developmental changes across multiple time points can provide a more comprehensive assessment of the embryo’s potential for successful implantation. Additionally, maintaining stability in the culture medium is crucial for ensuring stable embryo growth while evaluating their morphology in the laboratory setting. In 2008, the introduction of commercially available TLS systems with built-in cameras eliminated the need to transfer embryos between dishes and enabled continuous evaluation without disrupting the culture conditions [16]. In 2010, Wong et al. [17] pioneered the development of an algorithm that marked a significant breakthrough by predicting blastocyst formation on day 2 of embryo culture using TLS. This marked a transition from mere observation of human embryos in culture to harnessing TLS as a valuable tool for selection and prediction. TLS provided objective, precise, qualitative, and quantitative data without disrupting the delicate culture conditions.
However, the routine use of TLS in IVF clinics has stirred controversies. There is a lack of substantial evidence to differentiate between TLS and conventional incubation in terms of live births, miscarriages, stillbirths, or clinical pregnancies [18, 19]. Several factors contribute to this uncertainty. During the 3 to 5-day incubation period, TLS generates a substantial volume of digital images of embryos at regular intervals (typically every 10 to 20 min). Embryologists can review these images to assess division timings, temporal intervals for each cell stage transition, and identify embryos with abnormal cleavage patterns, which may not be suitable for transfer [20]. However, the manual process of reviewing and annotating the vast number of labeled time-lapse datasets is time-consuming and requires expertise and experience. Moreover, manual assessments introduce inter- and intra-individual variations, particularly between different laboratories. To address these challenges and standardize the process, computer-based algorithms with high accuracy and reliability are essential for automation.
Artificial intelligence (AI) tools are exceptionally adept at swiftly processing and analyzing vast amounts of data. Given the intricate nature of TLS techniques and the multitude of variables involved, AI techniques offer an ideal solution. Numerous algorithms have been employed in TLS to classify, predict, and rank reproductive medical data, aiming to improve the efficiency of IVF cycles by reducing failed transfers and miscarriages, ultimately leading to the successful birth of a healthy baby from the transfer of a single, euploid embryo [21].
Engage in AI models
AI technologies, specifically ML and neural networks, have gained widespread adoption in medical applications. These algorithms are designed to perform reasoning and map input-to-output data relationships. However, the training process of these computational models is often opaque, leading to their characterization as “black boxes.” Nevertheless, gaining an understanding of the underlying principles behind these models can reveal their internal mechanisms and enhance decision-making processes [22].
Machine learning
ML has emerged as the predominant approach within the field of AI for the development of valuable software, encompassing applications such as computer vision, image recognition, medical diagnosis, and prediction systems. ML leverages data and algorithms to mimic human learning, progressively improving precision. Through the training process, ML algorithms can automatically discern patterns within vast datasets, enabling them to generate predictions that prove instrumental in optimizing treatment procedures and forecasting pregnancy outcomes [23].
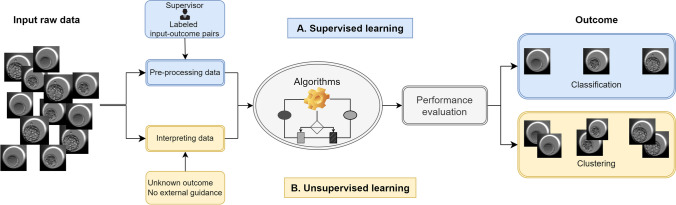
ML encompasses two prevalent models: supervised and unsupervised learning (Fig. 1). Supervised ML employs algorithms to classify or predict outcomes by analyzing new input, learning from labeled input-outcome pairs, and continuously improving its performance [24]. Numerous models have been developed for supervised learning, including decision trees, random forests, support vector machines, gradient boosting, and Bayesian classifiers. Selecting the most appropriate model from this diverse range can be a challenging task. Alternatively, the concept of ensemble learning has been explored in supervised learning, where “weak” classifiers—those with only slightly better than random guessing performance—are combined to achieve higher accuracy than individual classifiers alone [25]. In contrast, unsupervised learning refers to algorithms designed to identify patterns within datasets that lack classification or labeling information [26]. Algorithms employed in unsupervised learning, such as clustering, anomaly detection, and dimensionality reduction, can automatically group, label, and classify data points within these datasets without external guidance or supervision. Clustering, for example, has the ability to compress the entire feature set into cluster codes. This simplifies the handling of large datasets, as ML systems can efficiently process data groups using cluster identifiers. Consequently, the output data from clustering can serve as feature data for downstream ML systems.
Fig. 1.
Supervised and unsupervised learning: supervised learning involves using raw input data that requires human intervention to label or provide explicit guidance before being processed by model algorithms to generate the desired outcomes. On the other hand, unsupervised learning operates without external guidance, automatically classifying data points based on inherent patterns and structures
Neural networks
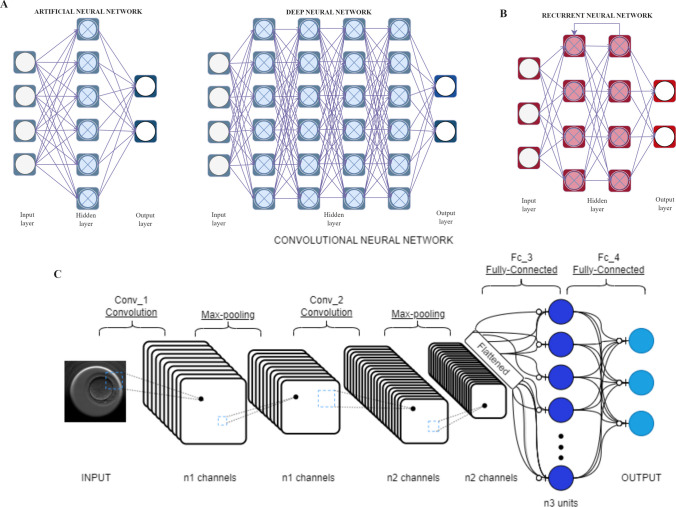
Neural networks, a subset of ML, draw inspiration from the architecture and dynamics of neuronal networks within the brain [27]. These networks consist of three essential layers: an input layer, one or more hidden layers, and an output layer. By propagating data through these interconnected layers, neural networks can learn from errors and autonomously make decisions without human intervention. Currently, three primary types of neural networks are utilized in the context of TLS: feedforward, recurrent, and convolutional neural networks (Fig. 2).
Fig. 2.
Neural networks: feedforward neural networks (FNN) consist of artificial neural networks (ANN) and deep neural networks (DNN) with one or multiple hidden layers, enabling them to process information in a unidirectional flow. Recurrent neural networks (RNN) possess the capability to learn from both past and future information in time series data. Convolutional neural networks (CNN) leverage convolutional operations to extract essential features from the data, followed by further processing using specific functions to generate appropriate outputs
Feedforward neural network
Feedforward neural networks (FNN) are designed to process information in a unidirectional manner, flowing from the input layer through the hidden layer(s), and ultimately reaching the output layer. This architecture ensures that there are no loops or cycles within the network, maintaining a clear direction of information flow [27]. Artificial neural networks (ANNs), a specific type of FNN, are computational models inspired by biological neural systems, particularly the human brain. ANNs are represented as a graph with nodes referred to as “neurons,” mimicking their biological counterparts.
ANNs offer numerous advantages, such as parallel processing capabilities, the ability to generate outputs with incomplete information, which is often encountered in reproductive data collection, and the capacity to extract hidden features and relationships without imposing restrictions on input and residual distributions. In comparison to traditional forecasting models, ANNs have demonstrated superior performance in predicting treatment outcomes [28]. However, ANNs require data to be translated into numerical values, which can influence their performance. Therefore, it is crucial to design an ANN that closely aligns with the characteristics of the input vectors, necessitating the user’s involvement in determining the representation scheme.
A deep neural network (DNN), also known as deep learning (DL), is an ANN with multiple hidden layers positioned between the input and output layers. The depth of the architecture is determined by the number of layers that perform non-linear operations in the learned function [29]. DL algorithms excel at handling both structured and unstructured data, including images, text, and audio. By automatically learning hierarchical representations of features, DL methods have the capacity to directly extract complex functions from data, eliminating the need for manual feature engineering [29]. However, the successful application of DL requires a comprehensive understanding of the domain and problem at hand to mitigate unintended consequences, such as biased models that discriminate against certain groups. Training DL models also demands significant computational resources, including powerful processors, ample memory, and substantial investments of time and financial resources. Despite these challenges, DNNs offer enhanced performance, efficiency, and accuracy. Notably, studies leveraging this network architecture have successfully constructed robust models for embryo selection in reproductive medicine [30, 31].
Recurrent neural network
The architecture of a recurrent neural network (RNN) can vary, encompassing fully interconnected or partially connected nets, and includes multilayer feedforward networks featuring separate input and output layers [32]. Unlike the independent layers of FNN, RNN architectures possess the ability to learn dependencies both forward and backward in time series data, making them suitable for problems involving sequences and temporal relationships [33]. For instance, predicting the next word in a sentence relies on the context and words that precede it.
A modified version of RNN called long short-term memory (LSTM) was developed exceling at modeling complex sequential data by preserving both short- and long-term dependencies, even in the presence of noise or incompressible input sequences [34]. However, LSTMs may not perform optimally with highly nonlinear or non-sequential data, as their strengths lie in capturing temporal relationships. Additionally, training LSTMs on large datasets can be time-consuming, as they require learning the parameters of LSTM cells, which can be computationally demanding. In the field of medical science, LSTM has demonstrated its superiority in handling temporal information. For example, Liao et al. [14] employed LSTM to learn cell stage information from consecutive frames of TLS videos and accurately predicted blastocyst formation by extracting morphokinetic embryo development parameters demonstrating the effectiveness of LSTM in capturing the temporal dynamics of embryo development. This approach highlights the potential of LSTM in extracting valuable insights from sequential data and its applicability in the field of reproductive medicine.
Convolutional neural network
CNN is a powerful DL model specifically designed for image recognition and classification tasks. It leverages the convolution operator, a fundamental concept in digital signal processing, to transform input information by convolving it with a filter, resulting in a new output signal. This process helps identify and isolate crucial features in the signal while filtering out irrelevant information.
CNNs consist of multiple layers, including convolutional, pooling, and fully connected layers, which collectively extract and process information from input images [35]. In the convolutional layers, convolutions between the input matrix and the filter generate units in a new layer, enabling two-dimensional feature extraction. Pooling calculates an expected value for each spatial region the filter passes through, effectively reducing the size of the image while preserving its essential contours. Once the matrix size is sufficiently reduced, it is flattened into a vector and fully connected between layers, employing the rectified linear unit (ReLU) activation function. The final layer of the CNN network, the last fully connected layer, contains the same number of units as the number of classes in the classification task. It applies the softmax activation function to compute the probability distribution across the classes.
Despite the effectiveness of CNNs in image recognition and classification, they face several challenges. The vanishing gradient problem becomes more pronounced with deeper layers, requiring a substantial amount of training data to prevent overfitting. Operations like pooling can significantly slow down the network, particularly when multiple layers are involved. Training CNNs can also be time-consuming if the computational resources lack robust data processing capabilities. However, the advancements in graphics processing unit (GPU) technology have played a significant role in overcoming these challenges and enabling the application of CNNs in analyzing and identifying features in digital images.
In recent studies, researchers have utilized multilayered CNNs ranging from 5 to 43 layers to classify embryos based on their morphological quality. Notably, the Xception model achieved the highest accuracy of 90.97% in these studies [36]. This research showcases the potential of CNNs in the field of reproductive medicine, where they can contribute to improved embryo classification and selection processes.
Clinical applications of AI technology
The primary objective of AI in TLS is to optimize the selection process of the most viable embryo for transfer, as this significantly impacts the final outcome of a pregnancy. AI technology plays a crucial role in aiding physicians by providing them with informed decision-making support and alleviating the substantial workload in busy IVF laboratories. Numerous research studies have been conducted to automate key steps in the analysis of TLS data, including segmentation, classification, prediction, and the exploration of novel findings related to human embryos. These efforts aim to develop end-to-end pipelines that enable the automated evaluation of raw TLS image sequences, ultimately leading to improved clinical pregnancy outcomes. In Table 1, a compilation of studies is provided, highlighting the application of AI techniques in various aspects of TLS and their impact on different outcomes. The majority of these articles utilize similar input data, such as embryo images, morphokinetic parameters, and clinical information, to develop and train their AI models.
Table 1.
Performance among AI based studies with TLS
| Author | Number of samples | Input data | Model | Main advancement | Performance |
|---|---|---|---|---|---|
| Automated segmentation/detection | |||||
| Zhao Xu et al. [37] | 24 embryos | Day-one embryo images | The hierarchical fully convolutional network (CNN) the generative adversarial network (GAN) | Automated day-one human embryo segmentation on three distinct features: zona pellucida (ZP), cytoplasm and pronucleus (PN) | The precisions of segmentation were that cytoplasm over 97%, PN over 84%, and ZP around 80% |
| Leahy, Jang et al. [38] | 989 embryos | Embryo images | ResNet101 (CNN) Inception-V3 (CNN) ResNeXt101Mask R-CNN & ResNet50 | Automate five key morphokinetic features extraction: Zona pellucida segmentation Fragmentation scoring Stage classification Cell object instance segmentation Pronucleus object instance segmentation | ZP accuracies between 93 and 99% Fragment scoring labels agree 88.9% of the time The developmental stage classifier with 87.9% accuracy mAP of 0.737 and 0.680 on cell object segmentation and pronucleus segmentation |
| Fukunaga, Sanami et al. [39] | 900 embryos | Embryo images | CNN | Automated pronuclei determination algorithms | The sensitivity rates of 0PN, 1PN, and 2PN were 99%, 82%, and 99% |
| Automated classification | |||||
| Zabari, Kan-Tor et al. [40] | 67,707 embryos | Embryo sequence images | U-Net & ResNet18 CNN | Automatic and standardized morphokinetic annotation of embryo development from time of pronuclei appearance to start of blastulation | R-square 0.994, 97% accuracy |
| Thirumalaraju, Kanakasabapathy et al. [36] | 2,440 embryos | Blastocyst stage images | Inception-V3 ResNet-50 Inception-ResNET-v2 NASNetLarge ResNeXt-101 ResNeXt-50 Xception | Evaluate popular CNN approaches in classifying blastocyst stage based on their morphological quality | Xception 90.97% accuracy |
| Malmsten, Zaninovic et al. [41] | 110,000 embryos | Cleavage stage images | Inception-V3 (CNN) | Up to 8-cell stage classification | 98.1% for up to 4-cell and 95.8% for up to 8-cell |
| Dirvanauskas, Maskeliunas et al. [42] | 10 embryos | Embryo images | AlexNet (CNN) Cecoc K-nearest neighbor (KNN) Decision tree (DT) Ensemble Discriminant Naïve Bayes | Automatically cleavage stage (up to 8-cell stage) classification, directly in the incubator machine | 93.58% accuracy for the task of eight-cell prediction |
| Raudonis, Paulauskaite-Taraseviciene et al. [43] | 300 embryos | Cleavage stage sequence images | AlexNet (CNN) VGG16 (CNN) | Embryo location detection and up to 4-cell stage classification | At least 90% accuracy in the detection of embryo location. Overall accuracy of over 92% for cell stages classification |
| Liu, Huang et al. [44] | 170 embryos | Embryo sequence images | ResNet50 Multi-task deep learning with dynamic programming | Embryo early development stage (up to 4-cell stage) automated classification from time-lapse videos | 86.9% accuracy |
| Khan, Gould et al. [45] | 265 embryos | Cleavage stage sequence images | AlexNet (CNN) | Automatically count the number of cells in the developing embryos up to the 5-cell stage | 91.05% average accuracy |
| Prediction: blastocyst quality | |||||
| Liao, Zhang et al. [14] | 10,432 embryos | Embryo sequence images | DenseNet201 Long short-term memory network (LSTM) The spatial–temporal ensemble model (STEM) Gradient boosting classifier | Predict blastocyst formation and usable blastocysts using TLM videos of the embryo’s first days | 78.2% accuracy and 0.82 AUC in predicting blastocyst formation, and achieved 71.9% accuracy and 0.79 AUC in predicting usable blastocysts |
| Coticchio, Fiorentino et al. [46] | 230 embryos | Embryo images | The k-nearest neighbor LSTM The hybrid ensemble classifier | Predicting blastocyst formation at early cleavage stages by detection of cytoplasm movement velocity and AI analysis | AI models with the blind operator classification, resulted in 82.6% accuracy |
| Giscard d'Estaing, Labrune et al. [13] | 891 embryos | Morphokinetic parameters | Logistic regression | A score issued from a ML system with self-improvement capacity able to predict embryo fate | DynScore AUC 0.638 in predicting blastocyst formation |
| Kan-Tor, Zabari et al. [47] | > 20 000 fresh embryos | Embryo images | DNN U-Net (CNN) Random forest Logistic regression | Early prediction of embryo blastulation and embryo implantation on day 3 | Blastulation prediction AUC 0.74 Implantation prediction AUC 0.71 |
| Khosravi, Kazemi et al. [30] | 10,148 embryos | Blastocyst stage images | Inception-V1 DNN (STORK) Decision tree | Accurately predict the quality of human blastocysts and help select the best single embryo for transfer | Predicts blastocyst quality with an AUC 0.987 |
| Prediction: implantation rate | |||||
| Theilgaard Lassen, Fly Kragh et al. [48] | 181,428 embryos | Embryo images | 3D CNN Logistic regression | Implantation rate | iDAScore v2.0 The AUCs on day 2, 3 and 5 + were 0.861, 0.872, and 0.954 for all embryos and 0.669, 0.621, and 0.707 for KID embryos |
| Duval, Nogueira et al. [49] | 9986 embryos | Embryo sequence images Clinical data | 3D CNN Gradient boosting | Multi-centric clinical data to better predict clinical pregnancy outcome | AUC 0.727 |
| Berntsen, Rimestad et al. [50] | 115,832 embryos | Embryo images | 3D (CNN) LSTM | Predict pregnancy in terms of fetal heartbeat (FH) | Automated iDAScore v1.0 model with AUC 0.67 for KID embryos all embryos with an AUC of 0.95 |
| Bori, Paya et al. [51] | 637 patients | Morphokinetic parameters | Artificial neural networks (ANN) | Novel embryo features capable of predicting implantation potential | AUC 0.77 & novel parameters in which blastocyst expanded diameter and trophectoderm cell cycle length had statistically different values in implanted and nonimplanted embryos |
| Tran, Cooke et al. [52] | 10,638 embryos | Embryo sequence images | DNN | Predict the probability of pregnancy with fetal heart (FH) from time-lapse videos | IVY model with AUC 0.93 |
| Milewski, Kuczyńska et al. [53] | 610 embryos | Morphokinetic parameters | Artificial neural networks (ANN) | Predicting implantation model based on morphokinetic information | AUC 0.71 |
| Prediction: live birth rate | |||||
| Yang, Peavey et al. [54] | 375 embryos | Morphokinetic parameters | Logistic regression, XGBoost, decision tree, and random forest K-means clustering | Predict clinical pregnancy and live birth rate | Random forest with the best AUC 0.69 and 0.64 in predicting implantation rate and live birth rate. Cluster of slowest morphokinetics had a higher pregnancy rate of 71% accuracy |
| Huang, Kosasa et al. [55] | 101 embryos | Blastocyst images | U-Net architecture (CNN) | Analysis of human blastocyst expansion from the unedited time-lapse image in selecting single blastocysts for transfer | The averaged expansion rate was significantly (P = 0.007) greater in euploid blastocysts that resulted in live births compared with those resulting in failure to give a live birth |
| Sawada, Sato et al. [56] | 470 embryos | Embryo images | Attention Branch Network (ABN) Residual neural network (ResNet56) | A confidence score based on embryo images that is useful for noninvasive selection of embryos that could result in live birth | AUC 0.642 |
| Prediction: ploidy status | |||||
| Zou, Pan et al. [31] | 773 embryos | Morphokinetic parameters Dysmorphism and irregular cleavages Blastocyst quality Clinical data | Decision tree, random forest, adaptive boosting (AdaBoost), Gradient boosting decision tree (GBDT), and gradient boosting (XGBoost) A novel hybrid DNN-LSTM | Euploid blastocysts Implantation prediction | GBDT model had the highest AUC 0.70 in euploidy prediction; AUC 0.78, accuracy 77% for implantation outcome of transferred euploid blastocysts |
| De Gheselle, Jacques et al. [57] | 539 embryos | Morphokinetic parameters Embryonic developmental feature Demographic and clinical data | Random forest classifier Gradient boosting classifier Support vector machine Multivariate logistic regression Naïve Bayes | Euploid blastocysts | The RFC model had the highest accuracy (71%) and AUC (0.75). Morphokinetic features ranked higher with tPB2, PN, and t7 showing the highest relative weight |
| Lee, Su et al. [58] | 690 embryos | Embryo videos | Two-Stream Inflated 3D ConvNet (I3D) | Ploidy status through raw time-lapse video | AUC 0.74 in discriminating between aneuploid embryos (group 1) and others (group 2, including euploid and mosaic embryos) |
| Huang, Tan et al. [59] | 1803 embryos | Blastocyst images & clinical data | 3D-resnet50 | Ploidy status | Predict euploid on the testing dataset with AUC of 0.80 |
Automated segmentation/detection
Segmentation is a crucial step in image analysis, particularly in the context of TLS sequences, as it enables the identification and assessment of embryo composition at each stage. However, manual segmentation is time-consuming and requires trained experts, posing challenges for medical workflows. Therefore, recent advancements focus on developing accurate automatic segmentation techniques that can seamlessly integrate into medical practices [37, 38].
In an IVF cycle incorporating TLS, embryos remain within the time-lapse system continuously from the time of intracytoplasmic sperm injection (ICSI) or fertilization until the day of transfer or cryopreservation. The confirmation of successful fertilization, indicated by the appearance of two pronuclei, usually occurs around 16–18 h after insemination or ICSI. Monitoring, recording, and evaluating this event is crucial to ensure normal fertilization. However, the time window for observing the appearance and disappearance of pronuclei is short, and their size and shape can be challenging to discern, particularly since the female pronucleus appears after the male pronucleus. Fukunaga et al. [39] developed a CNN automated pronuclei determination system that achieved high sensitivity rates in determining the number of pronuclei, with rates of 99% for 0PN, 82% for 1PN, and 99% for 2PN. Furthermore, the model accurately evaluated overlapping 2PN cases that are difficult to determine through microscopic observation alone. This high sensitivity can be valuable for embryologists in confirming fertilization.
Several factors, including pronuclei size, zona pellucida (ZP) properties, and embryo fragmentation, correlate positively with embryo development. These characteristics play a vital role in assessing the embryo’s “score” during the early stage. Zhao et al. [37] and Leahy et al. [38] employed CNNs for image analysis to extract valuable features during the pronuclear and cleavage stages, demonstrating consistent results in high-accuracy segmentation (Fig. 3). Leahy et al. [38] further developed a pipeline of five CNNs to expedite the measurement of biologically relevant features that could assist in embryo selection.
Fig. 3.
Segmentation model. An automated segmentation model utilizing convolutional neural networks (CNN) to produce precise pattern annotations for image object segmentation
Morphology assessment remains the most commonly used method for evaluating embryo quality. Traditionally, embryo quality was manually graded based on intrinsic morphological features such as cell size, density, and expansion level. However, there is a growing interest in automating the grading process. Huang et al. [55] utilized a DNN on time-lapse images to analyze human blastocysts, employing an optimized U-Net for the segmentation task. This software accurately identified the irregular shape of the trophectoderm in focused images, enabling precise measurement of the total enclosed area and subsequent analysis of human blastocysts.
Automated classification
The process of assigning labels to unlabeled groups is known as classification. While classifying embryo cells is not a new challenge, there is a continuous need for high accuracy and performance, particularly when aiming for autonomous performance within TLS. Classification involves labeling from a finite set of classes, with two primary types: binary classification, which distinguishes between two classes, and multiclass classification, which identifies three or more distinct classes [36, 42].
In the evaluation of embryo quality, especially in the case of human embryos, images often contain substantial noise and overlapping cells in the background, making traditional cell counting methods based on manually crafted features inadequate for handling large datasets. Consequently, researchers have actively sought automatic and accurate methods for cell number classification in the early stages of embryo development, providing valuable information for embryologists (Fig. 4). Khan et al. [45] proposed a framework for automated cell counting up to the 5-cell stage in human embryo development. Their approach utilized a CNN model consisting of eight layers, including five convolutional and three fully connected layers, with a softmax function generating a distribution across five class labels. The framework achieved an average accuracy of 91.05% for cell classification. Other researchers have also explored the use of various algorithms, such as MTDL and VGG16, for cleavage cell number classification, obtaining comparable results [43, 44].
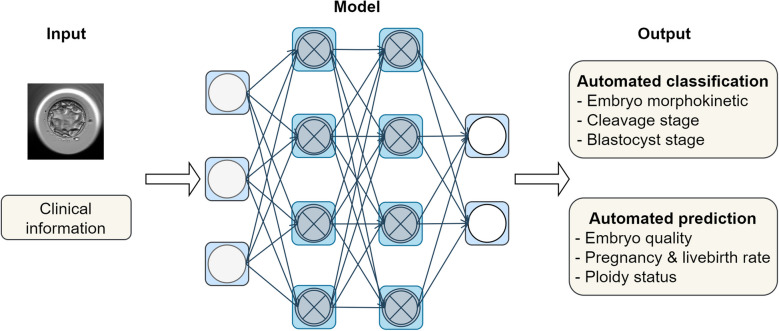
Fig. 4.
Classification and prediction model. The artificial intelligence model can be applied to various types of input data to generate the desired outcomes in classification and prediction tasks
As the number of cells in an embryo increases, accurately determining the cell count during division becomes more complex and challenging due to overlapping cells, requiring a more robust model and advanced optimization strategies. To address this issue, Malmsten et al. [41] proposed a novel method that utilizes DL to classify embryo images based on different cell division stages, up to 8 cells, specifically for human embryos. Their approach integrated advanced DL models and latent domain knowledge, enabling highly accurate predictions of cell division stages. Initially, the Inception-V3 model was trained using a publicly available dataset of mouse embryos, followed by the development of an optimization strategy using multiple images from the TLS sequence. Subsequently, the model was adapted and applied to a private dataset of human embryos, achieving accuracies of 98.1% and 95.8% for up to 4-cell and 8-cell stages, respectively.
In 2023, Zabari and colleagues [40] developed a CNN-based model that automatically annotates morphokinetic events from pronuclei appearance to the beginning of blastulation with high accuracy and standardization. The model outperformed previous automated morphokinetic annotation tools [60], achieving an R-square of 0.994 and 97% accuracy. Additionally, they utilized unsupervised clustering of high-quality embryos to investigate the morphokinetic heterogeneity of preimplantation development, independent of maternal age and blastulation rate. Comparative analysis of transfer versus implantation rates revealed differences between embryo clusters, primarily characterized by poor synchronization of the third meiotic cell-cleavage cycle. This study is expected to improve the integration of morphokinetic-based decision support tools in IVF treatments and enhance our understanding of preimplantation heterogeneity.
Automated prediction
Prediction methods involve utilizing learned outcomes to create models capable of anticipating values for new data. While classification focuses on categorizing data into classes, prediction aims to fit a shape that closely approximates the data. In the context of embryo transfer, accurate prediction of developmental potential and suitable embryos for cultivation is essential to enhance fertility, minimize economic loss, and reduce pregnancy-related complications. Automated prediction approaches are gaining interest in clinical settings to achieve these goals [13, 46].
In 2019, Khosravi et al. [30] conducted a robust study using an AI approach based on the STORK framework, which employed Google’s Inception-V1 architecture model. This study aimed to predict the quality of blastocysts from a vast collection of over 10,000 human embryos. The model was trained with labeled images of blastocyst stages, and STORK achieved accurate predictions of high or low quality blastocysts with an AUC of over 0.98.
Both Kan-Tor et al. [47] and Liao et al. [14] attempted to build models for predicting the likelihood of blastocyst formation and estimating the implantation rate in the 3-day embryo stage. Kan-Tor et al. [47] utilized an automated segmentation technique with U-Net to segment embryo images, which were then fed into a DNN model. In contrast, Liao et al. [14] combined a cell-counting algorithm with a long short-term memory (LSTM) network to create a temporal stream model. This model converted video data into numerical information and learned the duration and dynamics of cell numbers, enabling the prediction of blastocyst formation based on morphokinetic parameters.
Currently, achieving successful implantation is the ultimate goal, and researchers aim to develop robust, practical, and accurate models for clinical use [49, 53]. Significant progress has been made in supporting the selection of 3-day and 5-day embryos using traditional algorithm-based approaches like KIDScore D3 [61] and KIDScore D5 v.3 [62]. More recently, DL-based models such as iDAScore v1 [50] and iDAScore v2.0 [48] have been introduced. These models analyze time-lapse sequences without user input and provide reliable rankings of embryos according to their likelihood of implantation in both cleavage and blastocyst stages. They improve the consistency of embryo grading and save time compared to conventional evaluation methods. Tran et al. [52] also developed the IVY model, which predicts the likelihood of pregnancy with fetal heart using time-lapse videos, achieving an AUC of 0.93. However, controversies have arisen regarding this algorithm due to imbalances in the training model and classification criteria [63].
The journey from successful fertilization to live birth is a complex process influenced not only by selecting a “perfect” embryo but also by considering the clinical factors of the mother throughout pregnancy. Attempts to predict live birth rates have been made but have not yielded impressive results [54, 56]. Ploidy status, an important outcome for the success of an IVF cycle, is currently confirmed through invasive biopsies using PGT-A. However, with the development of TLS, noninvasive screening for ploidy status is expected to be facilitated by AI [31, 58].
Huang et al. [59] have developed models to predict euploid blastocysts by considering various embryo features and other factors contributing to the success of IVF treatment, including clinical data. The euploid prediction algorithm (EPA) achieved an AUC of 0.8 by optimizing time-lapse videos from specific periods. More recently, De Gheselle et al. [57] utilized a similar approach and analyzed the correlation between features and the target variable in a study on the ploidy prediction algorithm. Their results indicated that morphokinetic features, such as tPB2, PN, and t7, had the highest relative weight, while demographic and clinical parameters, such as sperm characteristics, the woman’s age at the onset of treatment, and the total dose of gonadotropins, also had significant weight.
Challenges and potential future applications AI with TLS
Current limitations/challenges
The application of AI in TLS presents several hurdles, including issues with transparency, small sample sizes, and the absence of external validation. These challenges collectively limit the efficacy of these models in producing reliable outcomes. The lack of transparency arises from the hidden decision-making processes within the model, making it challenging to discern the criteria used to predict outcomes. To build trust and understanding, enhancing transparency in AI models is imperative. On the other hand, AI algorithms heavily rely on large amounts of high-quality data for training and accurate predictions. However, acquiring comprehensive and standardized datasets in the field of ART is challenging due to the unique development patterns exhibited by each embryo, which complicates establishing consistent and standardized AI models. Variations in morphology, cell division rates, and quality further impact prediction accuracy. AI algorithms must accurately analyze and interpret the intricate morphological changes occurring during embryo development, including subtle differences and subjective assessments or uncommon patterns, which can be challenging. Privacy concerns and the limited availability of embryos for analysis in individual centers or research studies raise concerns about data sharing, potentially leading to biased or less accurate predictions. Extensive validation studies and regulatory approvals are necessary to demonstrate AI-based systems’ safety, efficacy, and cost-effectiveness for their widespread adoption in ART centers. Keeping pace with evolving technologies and research findings challenges ensuring optimal performance and accuracy.
Potential future works
Despite the challenges, AI holds significant potential in conjunction with TLS for embryo evaluation in ART. Researchers have conducted numerous studies over the past 5 years to develop and enhance AI-assisted TLS techniques, leading to improved accuracy. Novel markers such as cytoplasmic movements and blastocyst expansion rate have been investigated to select higher-quality embryos [46, 51, 55]. The current emphasis on noninvasive diagnostics presents an opportunity to uncover novel and stable markers for assessing ploidy and implantation rates, consequently enhancing the likelihood of healthier live births as the quality of imaging equipment, including cameras and microscopes, advances. The utilization of improved imaging technology, such as higher-resolution cameras and enhanced time-lapse imaging, offers the potential to obtain more detailed and precise information concerning embryo development. By integrating these techniques with advanced image analysis algorithms, there is a possibility of augmenting the capacity to interpret the intricate morphological changes occurring during embryo development accurately. In subsequent actions, automated segmentation could be employed to assess the relative size of blastomeres and identify dividing planes, which are believed to influence the following development stages. Uneven cleavage, characterized by unequal-sized cells, has been associated with an increased risk of multinucleation and aneuploidy due to an uneven distribution of cytoplasmic molecules such as proteins and mRNAs [64, 65]. In-depth investigations into the field of the embryo culture environment, by leveraging automated evaluation of embryo morphodynamics, AI can analyze and make informed decisions regarding adjustments and modifications to the personalized culture platform. Furthermore, integrating supplementary data, such as multi-omics data, with morphological data offers the potential to yield a more comprehensive understanding of embryo development and augment the precision of predictions.
One potential solution to address the lack of transparency is the implementation of explainable AI (XAI). XAI techniques enable human users to comprehend and trust the results and outputs generated by ML algorithms. Approaches like Local Interpretable Model-Agnostic Explanations (LIME) and DeepLIFT can explain the predictions made by ML algorithms and establish a traceable link between each activated neuron, respectively [66]. This would aid embryologists in understanding and trusting the system while facilitating patient interpretation of the results [67]. Large-scale, prospective validation studies involving multiple clinics and diverse patient populations are essential to provide more robust evidence of AI-based systems’ clinical utility and effectiveness in ART. Collaborative approaches like federated learning (FL) can facilitate data sharing while preserving privacy, benefiting smaller hospitals with limited resources. In FL, a central server creates a shared model and distributes it to clients who use their datasets to train the model and send updated parameters back to the server. By sharing trained parameters, privacy is preserved. Moreover, clients contribute computational power for training, reducing the storage capacity and computational resources required by the server. Smaller hospitals with limited training data and computational resources can also benefit from collaborative FL approaches [68]. Establishing clear regulatory pathways and involving clinicians and researchers in developing and refining AI systems are crucial for their safe and responsible integration into clinical practice. Continuous model improvement based on new scientific knowledge, emerging technologies, and feedback from clinical users should be implemented to ensure the relevance and accuracy of AI systems over time.
Conclusion
This review paper aims to provide a comprehensive overview of AI and its current applications in TLS within the context of reproductive medicine. With the proliferation of large-scale datasets and advancements in precision medicine, the role of AI in this field is poised for significant expansion. Integrating AI into TLS has the potential to revolutionize clinical workflows by automating repetitive tasks and delivering expert-level performance in assessing crucial information related to embryo implantation potential. In order to propel the field forward, it is imperative to develop new methods and techniques for explaining AI outcomes, as well as foster enhanced communication and collaboration among centers to improve the reliability and accuracy of algorithms. As TLS and AI systems continue to evolve, AI-based TLS holds promise for enhancing the likelihood of successful live births through more effective and accurate embryo evaluation and prediction algorithms.
Funding
This work is supported by the National Science and Technology Council, Taiwan [grant numbers MOST110-2221-E-038–001-MY2 and MOST111-2628-E-038–002-MY3].
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;312(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. Atlanta, GA: U.S. Dept of Healthand Human Services. 2021.
- 4.Cimadomo D, et al. Human blastocyst spontaneous collapse is associated with worse morphological quality and higher degeneration and aneuploidy rates: a comprehensive analysis standardized through artificial intelligence. Hum Reprod. 2022;37(10):2291–2306. doi: 10.1093/humrep/deac175. [DOI] [PubMed] [Google Scholar]
- 5.Tejera A, et al. Combination of metabolism measurement and a time-lapse system provides an embryo selection method based on oxygen uptake and chronology of cytokinesis timing. Fertil Steril. 2016;106(1):119–126.e2. doi: 10.1016/j.fertnstert.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Bar-El L, et al. Blastomere biopsy for PGD delays embryo compaction and blastulation: a time-lapse microscopic analysis. J Assist Reprod Genet. 2016;33(11):1449–1457. doi: 10.1007/s10815-016-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winston PH. Artificial intelligence. 3rd ed. Addison-Wesley Longman Publishing Co., Inc. 1992.
- 8.Kim I, et al. Application of Artificial intelligence in pathology: trends and challenges. Diagnostics. 2022;12(11):2794. doi: 10.3390/diagnostics12112794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlessinger DI et al. Artificial intelligence and dermatology: opportunities, challenges, and future directions. Semin Cutan Med Surg. 2019;38(1):E31–37. [DOI] [PubMed]
- 10.Teramoto A. Application of artificial intelligence in radiology. Gan To Kagaku Ryoho. 2019;46(3):418–422. [PubMed] [Google Scholar]
- 11.Hamamoto R, et al. Application of artificial intelligence technology in oncology: towards the establishment of precision medicine. Cancers. 2020;12(12):3532. doi: 10.3390/cancers12123532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Guidance. Ethics and governance of artificial intelligence for health. World Health Organization. 2021.
- 13.Giscard d'Estaing S, et al. A machine learning system with reinforcement capacity for predicting the fate of an ART embryo. Syst Biol Reprod Med. 2021;67(1):64–78. doi: 10.1080/19396368.2020.1822953. [DOI] [PubMed] [Google Scholar]
- 14.Liao Q, et al. Development of deep learning algorithms for predicting blastocyst formation and quality by time-lapse monitoring. Commun Biol. 2021;4(1):415. doi: 10.1038/s42003-021-01937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trolice MP, Curchoe C, Quaas AM. Artificial intelligence—the future is now. J Assist Reprod Genet. 2021;38:1607–1612. doi: 10.1007/s10815-021-02272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung AS, Son WY, Dahan MH. Time-lapse imaging of embryos: current evidence supporting its use. Expert Rev Med Devices. 2016;13(10):881–883. doi: 10.1080/17434440.2016.1230015. [DOI] [PubMed] [Google Scholar]
- 17.Wong CC, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong S, et al. Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev. 2019;5. [DOI] [PMC free article] [PubMed]
- 19.Racowsky C, Kovacs P, Martins WP. A critical appraisal of time-lapse imaging for embryo selection: where are we and where do we need to go? J Assist Reprod Genet. 2015;32:1025–1030. doi: 10.1007/s10815-015-0510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan YL, et al. Abnormally cleaving embryos are able to produce live births: a time-lapse study. J Assist Reprod Genet. 2016;33(3):379–385. doi: 10.1007/s10815-015-0632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curchoe CL, Bormann CL. Artificial intelligence and machine learning for human reproduction and embryology presented at ASRM and ESHRE 2018. J Assist Reprod Genet. 2019;36(4):591–600. doi: 10.1007/s10815-019-01408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik A, et al. Ten simple rules for engaging with artificial intelligence in biomedicine. PLoS Comput Biol. 2021;17(2):e1008531. doi: 10.1371/journal.pcbi.1008531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, et al. Artificial intelligence in reproductive medicine. Reproduction. 2019;158(4):R139–R154. doi: 10.1530/REP-18-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caruana R, Niculescu-Mizil A. An empirical comparison of supervised learning algorithms. in Proceedings of the 23rd international conference on Machine learning. (ICML '06). Association for Computing Machinery, New York, NY, USA. 2006:161–168.
- 25.Sagi O, Rokach L. Ensemble learning: A survey. Wiley Interdiscip Rev Data Min Knowl Discov. 2018;8(4):e1249. [Google Scholar]
- 26.Barlow HB. Unsupervised Learning. Neural Comput. 1989;1(3):295–311. [Google Scholar]
- 27.Mehlig B. Machine learning with neural networks: an introduction for scientists and engineers. Cambridge: Cambridge University Press; 2021. [Google Scholar]
- 28.Milewski R, et al. Comparison of artificial neural networks and logistic regression analysis in pregnancy prediction using the in vitro fertilization treatment. Studies in Logic, Grammar and Rhetoric. 2013;35(1):39–48. [Google Scholar]
- 29.Bengio Y. Learning deep architectures for AI. Found trends® Mach Learn. 2009;2(1):1–127. [Google Scholar]
- 30.Khosravi P, et al. Deep learning enables robust assessment and selection of human blastocysts after in vitro fertilization. NPJ Digit Med. 2019;2:21. doi: 10.1038/s41746-019-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou Y, et al. Can the combination of time-lapse parameters and clinical features predict embryonic ploidy status or implantation? Reprod Biomed Online. 2022;45(4):643–651. doi: 10.1016/j.rbmo.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Connor JT, Martin RD, Atlas LE. Recurrent neural networks and robust time series prediction. IEEE Trans Neural Networks. 1994;5(2):240–254. doi: 10.1109/72.279188. [DOI] [PubMed] [Google Scholar]
- 33.Scherpf M, et al. Predicting sepsis with a recurrent neural network using the MIMIC III database. Comput Biol Med. 2019;113:103395. doi: 10.1016/j.compbiomed.2019.103395. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, et al. Long short-term memory model–a deep learning approach for medical data with irregularity in cancer predication with tumor markers. Comput Biol Med. 2022;144:105362. doi: 10.1016/j.compbiomed.2022.105362. [DOI] [PubMed] [Google Scholar]
- 35.Le NQK, et al. DeepETC: A deep convolutional neural network architecture for investigating and classifying electron transport chain's complexes. Neurocomputing. 2020;375:71–79. [Google Scholar]
- 36.Thirumalaraju P, et al. Evaluation of deep convolutional neural networks in classifying human embryo images based on their morphological quality. Heliyon. 2021;7(2):e06298. doi: 10.1016/j.heliyon.2021.e06298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao M, et al. Application of convolutional neural network on early human embryo segmentation during in vitro fertilization. J Cell Mol Med. 2021;25(5):2633–2644. doi: 10.1111/jcmm.16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leahy BD, et al. Automated measurements of key morphological features of human embryos for IVF. Med Image Comput Comput Assist Interv. 2020;12265:25–35. doi: 10.1007/978-3-030-59722-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukunaga N, et al. Development of an automated two pronuclei detection system on time-lapse embryo images using deep learning techniques. Reprod Med Biol. 2020;19(3):286–294. doi: 10.1002/rmb2.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabari N, et al. Delineating the heterogeneity of embryo preimplantation development using automated and accurate morphokinetic annotation. J Assist Reprod Genet. 2023;40(6):1391–1406. doi: 10.1007/s10815-023-02806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malmsten J, et al. Automated cell division classification in early mouse and human embryos using convolutional neural networks. Neural Comput Appl. 2020;33(7):2217–2228. [Google Scholar]
- 42.Dirvanauskas D, et al. Embryo development stage prediction algorithm for automated time lapse incubators. Comput Methods Programs Biomed. 2019;177:161–174. doi: 10.1016/j.cmpb.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 43.Raudonis V, et al. Towards the automation of early-stage human embryo development detection. Biomed Eng Online. 2019;18(1):120. doi: 10.1186/s12938-019-0738-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, et al. Multi-task deep learning with dynamic programming for embryo early development stage classification from time-lapse videos. IEEE Access. 2019;7:122153–122163. [Google Scholar]
- 45.Khan A, Gould S, Salzmann M. Deep convolutional neural networks for human embryonic cell counting. In Computer Vision–ECCV 2016 Workshops: Amsterdam, The Netherlands, Springer International Publishing. 2016;14:339–348.
- 46.Coticchio G, et al. Cytoplasmic movements of the early human embryo: imaging and artificial intelligence to predict blastocyst development. Reprod Biomed Online. 2021;42(3):521–528. doi: 10.1016/j.rbmo.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Kan-Tor Y, et al. Automated evaluation of human embryo blastulation and implantation potential using deep-learning. Advanced Intelligent Systems. 2020;2(10):2000080. [Google Scholar]
- 48.Theilgaard Lassen J, et al. Development and validation of deep learning based embryo selection across multiple days of transfer. Sci Rep. 2023;13:1. doi: 10.1038/s41598-023-31136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duval A, et al. A hybrid artificial intelligence model leverages multi-centric clinical data to improve fetal heart rate pregnancy prediction across time-lapse systems. Hum Reprod. 2023;38(4):596–608. [DOI] [PMC free article] [PubMed]
- 50.Berntsen J, et al. Robust and generalizable embryo selection based on artificial intelligence and time-lapse image sequences. PLoS ONE. 2022;17(2):e0262661. doi: 10.1371/journal.pone.0262661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bori L, et al. Novel and conventional embryo parameters as input data for artificial neural networks: an artificial intelligence model applied for prediction of the implantation potential. Fertil Steril. 2020;114(6):1232–1241. doi: 10.1016/j.fertnstert.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Tran D, et al. Deep learning as a predictive tool for fetal heart pregnancy following time-lapse incubation and blastocyst transfer. Hum Reprod. 2019;34(6):1011–1018. doi: 10.1093/humrep/dez064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milewski R, et al. How much information about embryo implantation potential is included in morphokinetic data? A prediction model based on artificial neural networks and principal component analysis. Adv Med Sci. 2017;62(1):202–206. doi: 10.1016/j.advms.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Yang L, et al. Development of a dynamic machine learning algorithm to predict clinical pregnancy and live birth rate with embryo morphokinetics. F S Rep. 2022;3(2):116–123. doi: 10.1016/j.xfre.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang TTF, et al. Deep learning neural network analysis of human blastocyst expansion from time-lapse image files. Reprod Biomed Online. 2021;42(6):1075–1085. doi: 10.1016/j.rbmo.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Sawada Y, et al. Evaluation of artificial intelligence using time-lapse images of IVF embryos to predict live birth. Reprod Biomed Online. 2021;43(5):843–852. doi: 10.1016/j.rbmo.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 57.De Gheselle S, et al. Machine learning for prediction of euploidy in human embryos: in search of the best-performing model and predictive features. Fertil Steril. 2022;117(4):738–746. doi: 10.1016/j.fertnstert.2021.11.029. [DOI] [PubMed] [Google Scholar]
- 58.Lee CI, et al. End-to-end deep learning for recognition of ploidy status using time-lapse videos. J Assist Reprod Genet. 2021;38(7):1655–1663. doi: 10.1007/s10815-021-02228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang B, et al. An artificial intelligence model (euploid prediction algorithm) can predict embryo ploidy status based on time-lapse data. Reprod Biol Endocrinol. 2021;19(1):185. doi: 10.1186/s12958-021-00864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feyeux M, et al. Development of automated annotation software for human embryo morphokinetics. Hum Reprod. 2020;35(3):557–564. doi: 10.1093/humrep/deaa001. [DOI] [PubMed] [Google Scholar]
- 61.Petersen BM, et al. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Hum Reprod. 2016;31(10):2231–2244. doi: 10.1093/humrep/dew188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vitrolife. KIDScore D5 decision support tool. 2019. Available from: https://www.vitrolife.com/globalassets/support-documents/tech-notes/technote_kidscore-d5_v3_v3.pdf. Accessed 10 June 2023.
- 63.Kan-Tor Y, Ben-Meir A, Buxboim A. Can deep learning automatically predict fetal heart pregnancy with almost perfect accuracy? Hum Reprod. 2020;35(6):1473. doi: 10.1093/humrep/deaa083. [DOI] [PubMed] [Google Scholar]
- 64.Prados FJ, et al. The cleavage stage embryo. Hum Reprod. 2012;27(1):i50–i71. doi: 10.1093/humrep/des224. [DOI] [PubMed] [Google Scholar]
- 65.Hardarson T, et al. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001;16(2):313–318. doi: 10.1093/humrep/16.2.313. [DOI] [PubMed] [Google Scholar]
- 66.Barredo Arrieta A, et al. Explainable Artificial Intelligence (XAI): concepts, taxonomies, opportunities and challenges toward responsible AI. Information Fusion. 2020;58:82–115. [Google Scholar]
- 67.Liu C-F, et al. Does AI explainability affect physicians’ intention to use AI? Int J Med Inform. 2022;168:104884. doi: 10.1016/j.ijmedinf.2022.104884. [DOI] [PubMed] [Google Scholar]
- 68.Narmadha K, Varalakshmi P. Federated learning in healthcare: a privacy preserving approach. Challenges of Trustable AI and Added-Value on Health. IOS Press. 2022:194. [DOI] [PubMed]