Abstract
Telomeres are located at the ends of linear chromosomes and play a critical role in maintaining genomic stability by preventing premature activation of DNA repair mechanisms. Because of exposure to various genotoxic agents, telomeres can undergo shortening and genetic changes. In mammalian cells, the basic DNA repair mechanisms, including base excision repair, nucleotide excision repair, double-strand break repair, and mismatch repair, function in repairing potential damages in telomeres. If these damages are not repaired correctly in time, the unfavorable results such as apoptosis, cell cycle arrest, and cancerous transition may occur. During lifespan, mammalian somatic cells, male and female germ cells, and preimplantation embryos experience a number of telomeric damages. Herein, we comprehensively reviewed the crosstalk between telomeres and the DNA repair mechanisms in the somatic cells, germ cells, and embryos. Infertility development resulting from possible defects in this crosstalk is also discussed in the light of existing studies.
Keywords: DNA damages in telomeres, DNA repair mechanisms, Oocytes, Spermatogenic cells, Embryos, Infertility
Introduction
Eukaryotic cells have linear chromosomes whose ends are protected from being recognized as DNA double-strand breaks (DSBs). This is ensured by a unique nucleoprotein complex consisting of telomeres and associated proteins [1]. Basically, telomeres play a central role in inhibiting inappropriate activation of the DNA damage response (DDR) so that genomic integrity is maintained. In case of shortening or various DNA damages in telomeres, the DDR pathway is activated to repair these sites correctly and timely using the DNA repair mechanisms, especially the DSB repair (DSBR) [2]. Otherwise, these cells may undergo apoptosis or cellular senescence [2–4].
During spermatogenesis, oogenesis, and early embryogenesis, telomeric shortening and DNA damages may appear due to exposing to various endogenous and exogenous genotoxic agents [5]. These telomeric issues must be repaired using a proper DNA repair mechanism. In these days, the crosstalk between telomeres and DNA repair mechanisms has been increasingly important in the aspect of how damaged or short telomeres recruit DNA repair proteins. In the following parts of this review, we will first introduce basic biological features of telomeres and DNA repair mechanisms, including DSBR, base excision repair (BER), nucleotide excision repair (NER), and mismatch repair (MMR), in mammalian cells. Subsequently, we will discuss the crosstalk between telomeres and these mechanisms in mammalian somatic cells, germ cells, and preimplantation embryos.
Telomere structure
Telomeres are repetitive noncoding DNA sequences and protect against chromosomal degradation and fusion, genomic instability, and inappropriate activation of the DDR signaling pathway [1, 6]. In mammals, telomeres consist of the tandem repeats of (5’-TTAGGG-3’)n interacting with some exclusive proteins, called the shelterin complex (Fig. 1A). The 3’ single-strand extension of the telomeres, which is about 50–400 nucleotides long, is called the G-tail or G-overhang. The G-tail folds back to form the telomere (T-loop) and the displacement (D-loop) loops, both of which function in preserving chromosome ends from being recognized as DSBs [6]. While the non-canonical secondary structure, the G-quadruplex loop (also known as the G4-loop), is formed by the folding of the guanine-rich G-tail site, the R-loop is formed by the hybridization of DNA:RNA structures [5, 7] (Fig. 1A). These R- and G4-loop may play a role in DNA repair and alternative lengthening of telomeres (ALT) [7, 8].
Fig. 1.
Schematic diagram of closed telomere structure and telomerase complex. A Telomeres consists of noncoding repeats (TTAGGG)n and the shelterin complex including TRF1, TRF2, POT1, TIN2, TPP1, and RAP1 proteins. The 3’ end of telomere folds back to create the basic loops, D-loop and T-loop, and also create the secondary structures, G4-loop and R-loop. B The enzyme telomerase is composed of the TERT and TERC subunits. The adaptor proteins such as dyskerin, NHP2, GAR, and NOP10 directly or indirectly interact with telomerase to provide physical and functional supports. The catalytic subunit TERT includes the three basic domains: N-terminal region, reverse transcriptase motifs, and C-terminal region. The TERC subunit serves as an RNA template in the synthesis of telomeres. It consists of the core, CR4/CR5, H/ACA scaRNA, and CR7 domains. All other details about telomere structure and telomerase complex as well as full names of the abbreviations are given in the text. This schematic diagram was created using the BioRender Program (BioRenderCompany, Toronto, Canada)
Various factors such as oxidative stress, biological aging, and lifestyle are known to cause telomere shortening and loop disruption [9]. Therefore, telomeres can occur two different states depending on their length: closed and open. In other words, telomeres in the closed state are able to maintain the normal structure (Fig. 1A). However, the open state occurs when telomere shortening reaches critical level, which is lengthened by the enzyme telomerase or ALT to bring it back into the closed state [5, 10].
Telomere elongation
Short telomeres in germ cells, embryos, stem cells, and activated lymphocytes can be elongated by adding telomeric repeats onto chromosome ends by the enzyme telomerase or ALT mechanism (reviewed in [1, 5]). Telomerase consists of two main components, telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC) as well as several adaptor proteins (Fig. 1B). The TERC subunit serves as a template for the synthesis of telomeric repeats and includes 451 nucleotides in humans [11]. The core site of TERC subunit, providing interaction with TERT subunit, contains the conserved region (CR) such as CR4/CR5, H/ACA box (also known as CR6/CR8), and CR7. The TERT subunit is composed of three major domains: N-terminal extension (NTE), central catalytic reverse transcriptase (RT), and C-terminal extension (CTE) [10]. The NTE domain further includes two domains: the basic N-terminal domain that recognizes telomeric extension, and the telomerase RNA-binding domain that binds to the TERC subunit [12]. Importantly, the RT domain of TERT provides a catalytic activity for adding telomeric repeats.
TERT expression exhibits a close correlation with telomerase activity [13] and is detected in germ cells, embryos, and stem cells [1]. Different from the somatic cells having no telomerase activity, telomeres can be elongated by telomerase in these cell types. While telomerase activity is at high levels in germ cells and blastocysts, it is at low levels in 1-cell, cleavage, and morula stage embryos (reviewed in [1, 5]). Because of showing low telomerase activity in these embryonic stages, telomere length seems to be maintained possibly using the ALT mechanism [14].
The ALT mechanism is commonly used in early embryos, cancerous, and neuroendocrine cells [15]. Indeed, these cells can be characterized by the presence of ALT-associated promyelocytic leukemia nuclear bodies [16]. With ALT mechanism, telomere lengthening is carried out rapidly using sister chromatids as a template. The molecular mechanisms controlling initiation and progression of ALT are not fully addressed yet in germ cells and embryos. However, studies on cancer cells and human cell lines showed that this mechanism is promoted in the presence of DNA damages [2, 17, 18]. Importantly, ALT mechanism is particularly activated in the cells having low or absence of telomerase activity. As cleavage stage embryos exhibit low telomerase activity, Liu and colleagues (2007) reported that 1-cell and cleavage-stage embryos elongate telomeres using ALT mechanism [14]. The long noncoding telomeric repeat-containing RNA (TERRA) contributes to regulating ALT mechanism through organizing R-loop formation [19, 20]. Therefore, TERRA expression was found at high levels in the embryos at cleavage stages [21]. This situation largely overlaps with the embryonic stages, in which ALT mechanism is highly activated.
In male germ cells during biological aging, despite low telomerase activity and increasing DNA damages, telomeres can be elongated possibly by the ALT mechanism [22, 23]. An increase in DNA damages may help to initiate this mechanism. Thus, having long telomeres in sperm cells of older men may be achieved by this mechanism [24] [25]. Thus, genomic integrity of resulting embryos can be ensured. Further studies on this subject are required to elucidate the intracellular signaling in controlling ALT mechanism and potential initiators of this mechanism in germ cells and embryos upon presence of DNA damages.
Telomere-associated proteins and their key functions
As a nucleoprotein complex, telomeres are composed of telomeric DNA and the shelterin complex. The shelterin complex is formed by assembly of the six proteins: telomeric repeat-binding factors 1 (TRF1) and 2 (TRF2), repressor/activator protein 1 (RAP1), protection of telomeres 1 (POT1), TRF1-interacting nuclear protein 2 (TIN2), and TIN2 and POT1 interaction protein 1 (TPP1) (Fig. 1A) [26]. The TRF1 and TRF2 proteins bind directly to double-strand telomeric repeats by comprising a homodimer or a tetramer. These proteins also interact with TIN2, which serves as a molecular bridge between them. The TIN2 protein further binds to the TPP1 protein that associates with the POT1 protein. Thus, all these interactions provide formation of a stable telomeric structure.
In more detail, TRF2 plays a key role in preventing telomeric ends from being recognized as DNA breaks or from undesired recombination events [27]. For this purpose, TRF2 inhibits the ataxia-telangiectasia mutated (ATM) kinase, a mediator of the DDR activation, possibly by promoting T-loop formation [27, 28]. There are two probable models for this issue. The first model argues that TRF2 hinders recruitment of the MRN complex and KU70/80 heterodimers so that ATM and then cNHEJ activations are blocked [2, 29]. Consistently, TRF2 deficiency causes conversion of T-loop into a linear form [28], and thus ATM kinase and then cNHEJ pathway are stimulated [30]. Indeed, deletion of the Trf2 gene in mouse embryonic fibroblasts (MEFs) results in accumulation of the DNA damage-response factors, 53BP1 and gamma-H2AX (γH2AX), and ultimately ATM kinase activation occurs [31]. TRF2 deficiency additionally causes growth arrest, resulting in cellular senescence [32], and increases of p53- and ATM-dependent apoptosis [33], as in the case of telomere attrition [34].
In the second model, TRF2 inhibits the interaction between ATM kinase and the MRN complex at G2/M checkpoint so that phosphorylation of ATM at S1981 residue is hampered [29]. In addition, TRF2 is capable of blocking activation of CHK2, which is a mediator of checkpoint response and senescence by physical interaction [35]. TRF2 further inhibits cNHEJ by forming a complex with RAP1, thereby binding of KU70/80 to telomere ends is prevented [29]. However, the RAP1 protein solely associates with TRF2 to prevent undesirable ATM kinase and cNHEJ actions [36]. RAP1 also contributes to hindering chromosome fusion, inhibiting activation of DSBR to telomeres, and controlling telomere length [26]. Taken together, TRF2 contributes to protecting chromosome ends from being recognized as DNA damages by promoting T-loop formation and inhibiting activation of the ATM-CHK2 signaling. It creates a complex with RAP1 to execute some of these functions. Different from the TRF1 and TRF2 proteins, POT1 establishes an interaction only with the 3’ single-strand telomeric site. Like TRF2, but in a different way, POT1 represses the ATR kinase activity by disallowing the interaction of replication protein A (RPA) with single-strand DNA as well as with 9-1-1 clamp, TopBP1, and ATRIP proteins [37]. As expected, depletion of POT1 results in activation of ATR kinase [38]. Furthermore, conditional deletion of Pot1a in mice entailed an increase in sister chromatid exchanges, chromosomal instability, early embryonic lethality, and aberrant homologous recombination (HR) at telomeres [39]. Likewise, Yang et al. [40] also revealed that POT1 deficiency causes apoptosis, chromosomal abnormalities, and cellular senescence in human fibroblasts [40]. Telomeric single-strand breaks (SSBs) were also observed in the case of POT1 deficiency. Thus, these breaks may lead to telomere dysfunction and induction of DSBR pathways, especially single-strand annealing (SSA) so that chromosome end protection could be no longer sustained.
As explained above, the T-loop structure, TRF2, and POT1 proteins provide important contributions to maintaining telomeric integrity through blocking DSBR. A previous study by our group showed that TRF2 and POT1 expression decreased in human ovaries during aging [41]. Decreased levels of these proteins may be one of the reasons for increased DNA damages and thereby infertility with aging because of changed telomeric integrity. Similarly, Pirzada and colleagues reported that downregulation of the TRF2 and TPP1 gene expression in aborted fetus material may be associated with recurrent pregnancy loss owing to telomere shortening and disrupted T-loop structure [42]. In addition to these shelterin proteins, the other telomeric proteins such as RAP1 seem to have an indirect role in this protection. All these findings suggest that shelterin complex has an indispensable role in continuation of fertility and telomeric integrity in germ cells and embryos.
As is known, unpreserved telomeres can undergo shortening that makes them vulnerable to endonucleolytic activities [43] so that the DSBR pathways such as HR and cNHEJ are activated through inducing the ATR and ATM kinases [44]. Improper activation of these pathways may pose a threat to telomeres. Therefore, they are efficiently repressed by the shelterin complex proteins such as TRF2 and POT1. As TRF2 contributes to repressing the ATM kinase signaling and cNHEJ activation at telomeres by ensuring integrity of T-loop [45, 46], its loss causes activation of ATM kinase and subsequently formation of telomere dysfunction-induced foci (TIF) [29]. TIF contains the DDR components such as γH2AX, MDC1, MRN complex, and 53BP1. As a result, the DSBR pathways, especially cNHEJ and HR, are stimulated. In summary, shelterin complex has crucial roles in maintaining structural integrity, telomere length regulation, and preventing inappropriate activation of DDR pathways through promoting loop formation [37, 47]. Indeed, telomeres are protected from DDR activation by means of T- and D-loop as well as the shelterin complex components, especially TRF2 and POT1. Potential damages in telomeres and disassociation of shelterin complex can cause telomere shortening and unwinding of telomeric loops, which may result in cellular senescence, genomic instability, and activation of DNA repair mechanisms, especially DSBR.
DSB repair mechanism
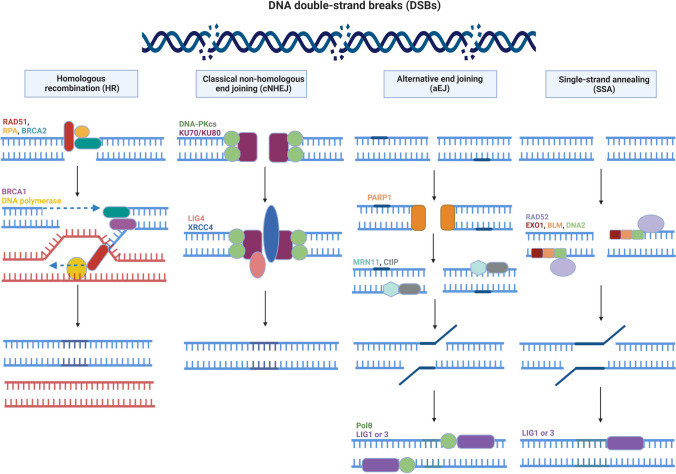
Exogenous and endogenous genotoxic agents are known to cause DSBs. A common exogenous factor is ionizing radiation exposure that arises from environmental mutagens and chemotherapeutic drugs, whereas endogenous factors are aberrant topoisomerase activity and DNA replication errors [48]. In the case of DSBs, at least one of the following DSBR pathways: HR, cNHEJ, alternative end joining (aEJ), and/or SSA is activated (review in [23]) (Fig. 2). While the HR and SSA pathways only work in the S and G2 phases of cell cycle, the cNHEJ and aEJ pathways serve in the G1, S, and G2 phases [49].
Fig. 2.
The DNA double-strand break (DSB) repair mechanisms in mammalian cells. DSBs deriving from various genotoxic agents such as ionization radiation can be repaired by the following pathways: homologous recombination (HR), classical non-homologous end joining (cNHEJ), alternative end joining (aEJ), and single-strand annealing (SSA). While the HR-based pathway uses sister or non-sister chromatids as a template to repair DSBs in an error-free manner, cNHEJ joins broken ends in an error-prone manner as aEJ pathway does. The SSA pathway specifically joins free ends, including small microhomology sites. Functional features of all proteins participating in these pathways are elaborately explained in the text. Notably, we used the same color in typing and labeling corresponding icons, and full names of all the abbreviations are given in the text. This figure was created using the BioRender program (BioRender, Toronto, Canada)
The HR-based DSBR pathway uses sister or non-sister chromatids as a template to correctly repair DBSs to maintain genomic integrity [50]. It is worth noting that HR, which is a basic process utilized in DSBR, further plays an important role in the ALT mechanism to facilitate elongation of telomeres in meiotic cells [51]. Three main steps can be distinguished during HR repair. First, a 3’ single-strand DNA end is created by a nucleolytic degradation of the 5’ strand. This is catalyzed by the MRN complex (consisting of MRE11, RAD50, and NBS1) that shows an endonuclease activity. In the second step, the single-strand DNA end is covered by RPA filaments. Subsequently, RPA is replaced with the RAD51 protein in a BRCA1/BRCA2-dependent process to promote the recombinase reaction using homologous DNA as a template. Finally, genomic integrity is restored by ligating the free ends with a specific ligase (Fig. 2).
The cNHEJ and aEJ join two free DNA ends rapidly without using a template DNA sequence; therefore, they work in an error-prone manner [49]. cNHEJ is initiated after binding of KU70-KU80 (also known as XRCC6-XRCC5) heterodimer or homodimer to broken ends [52, 53]. Nucleated KU dimer recruits other cNHEJ-related factors, including DNA-dependent protein kinase, catalytic subunit (DNA-PKcs), DNA ligase 4 (LIG4), and the scaffolding factors such as XRCC4 [54]. Thus, the two ends become closely aligned with the non-catalytic function of XRCC4-LIG4 and DNA-PKcs activity, and ultimately the free ends are ligated [55] (Fig. 2). Notably, the aEJ and SSA pathways are rarely used to join free ends, showing distinct conformational structures [23].
If cNHEJ does not work, aEJ pathway is activated [49, 56]. A microhomology site ranging from 2 to 4 bp and some critical proteins, including PARP1, CtIP, and the MRN complex, are essential for aEJ repair [57]. While PARP1 promotes this repair, the MRN complex (after activated by CtIP) resects the 5’ end with own endonuclease activity to generate a 3’ overhang ranging from 15 to 100 nucleotides [58]. Then, Polθ makes synthesis of missing nucleotides using other strand as a template. Finally, the free ends are sealed using the ligase enzyme I (LIG1) or III (LIG3).
The SSA pathway is involved in repairing damaged sites having more than 20 bp non-conservative homology [45] (Fig. 2). For microhomology creation, CtIP and the MRN complex work together to create a 3’ single-strand DNA tail composed of 15–100 nucleotides, which is further extended by the nuclease EXO1, BLM, or DNA2 [59]. Meanwhile, RAD52 contributes to annealing single-strand DNA tails, showing complementary sites. After elimination of unannealed and non-homologous portions at the 3’ single-strand DNA tails, the free ends are ligated by LIG1 or LIG3 [56].
DSB repair at telomeres in somatic cells, germ cells, and embryos
Maintenance of the structure and length of telomeres and protection from inappropriate DNA repair activation are of great importance for reproductive continuity [10]. During this process, especially HR and cNHEJ are involved in somatic cells, germ cells, and embryos based on the cell cycle stage and DNA break types [60–62]. Genomic integrity in oocytes is mainly affected from long duration at meiotic arrest following meiotic recombination at the fetal period [63]. DSBs occur physiologically in the processes of consecutive mitotic divisions of oogonia and early phase of first meiosis. At these periods, the other factors such as oxidative stress, reactive oxygen species (ROS), and biological aging can also lead to DSBs in developing oocytes and surrounding somatic cells [61].
Despite presence of DSBs, ATM kinase expression in germinal vesicle (GV) oocytes was found at lower levels compared to the other oocyte stages and early embryos [61, 64]. In the aspect of telomeres, reduced ATM kinase expression may be associated with the presence of long telomeres that forms closed telomere state in GV oocytes so that ATM kinase activity may be inhibited. However, it is worth noting that GV oocytes involve the DNA repair proteins that may be synthesized from maternal mRNAs [62, 65]. Nevertheless, potential DSBs at telomeres are repaired through HR pathway at the end of the first meiotic prophase even though it may become slowly [66]. In metaphase II (MII) oocytes, DNA repair pathways like HR and cNHEJ are in an active state. Consistently, mouse and human oocytes highly express the HR and cNHEJ genes [65, 67].
Unless DSBs are repaired correctly and timely in oocytes during folliculogenesis, it may lead to onset of certain infertility disorders such as premature ovarian insufficiency (POI) [68]. Huang et al. [69] have reported that animal models exhibiting meiotic arrest and DSB accumulation in their oocytes showed POI-like phenotypes and variations in the HR-related genes, such as BRCA1, BRCA2, RAD51, and STAG3 [69]. Lower telomerase activity and telomere length were also found in the granulosa cells of POI patients when compared to controls [70–72]. These results suggest that changed expression of the DSB-related genes contributes to alternating telomere length, which may lead to developing infertility in POI patients. Similarly, increased DSB levels and the other DNA damages, induced by hydrogen peroxide (H2O2), were discovered to cause development of polycystic ovary syndrome (PCOS) [73]. In analysis of telomere length and telomerase activity in human oocytes and leukocytes, while telomerase activity was at higher levels in the GV and MII oocytes, telomere length in leukocytes was lowered in PCOS group compared to the control group [74]. These findings promote researchers to perform further studies to examine the potential connection between telomere length and DSBR pathways.
After fertilization, zygotes undergo consecutive mitotic divisions in oviducts, in which embryos undergo ROS that causes DNA damages [61, 75]. The DSB damages in embryos can be repaired through the cNHEJ and HR pathways. Indeed, Derijck et al. [76] showed an activation of both pathways in mouse zygotes. Another study demonstrated that these pathways are also activated in human MII oocytes and blastocysts [67]. Thus, integrity of genome and telomeres is preserved in oocytes and embryos. Excessively activating these DSBR mechanisms may result in further telomere shortening in embryos. Such telomere dysfunction may be associated with aneuploidy formation [77].
In early meiotic cells of male mice, cNHEJ is repressed in the case of HR repair activity [78]. SSBs and DSBs in early embryos due to chromatin remodelling are repaired by the SSA and other DSB pathways, respectively. All these repair proteins are translated from maternal mRNAs because embryonic genome activation takes place at 4–8 cell stages in humans [60]. Cytodifferentiation in spermatids is a complex remodelling process that includes replacement of majority of histones with protamines [79]. This results in DNA compaction, which is accomplished by the temporary creation of SSBs and DSBs in the nuclear genome of sperm cells. These DNA breaks largely occurring at the early spermiogenesis stage [79] are repaired in spermatids through the aEJ mechanism since haploid cells cannot use HR-based repair [80].
In primary spermatocytes, DSBs formed by SPO11 are repaired by HR pathway during telomere movement to provide chromosome pairing and segregation at the first meiotic prophase [81]. However, cNHEJ pathway, being activated in the G1 phase, is downregulated at early prophase. A recent study has examined the kinetics of in vivo-generated DSBs and their repair at the telomeres of late prophase I chromosomes up to 12 h following 0.5 Gy whole-body gamma irradiation using wild-type and cNHEJ-deficient (severe combination immunodeficient) male mice [82]. Overlapping of γH2AX foci with the telomere repeat signal in late pachytene and diplotene spermatocytes showed telomeric DNA damages. Repair of these DSBs throughout the first hour following irradiation in wild-type mice was faster than in the controls. This repair may be largely carried out by the cNHEJ pathway during late prophase I. These findings suggest that cNHEJ pathway mediates DSBR at meiotic telomeres of late prophase I chromosomes. This may also be associated with unique chromatin organization at meiotic telomeres, which is opposite to the compact and largely inaccessible telomeres in somatic cells [83]. Consequently, cNHEJ is an essential mechanism for repairing unscheduled and genotoxic DSBs in the primary spermatocytes at late prophase I stage in order to successfully complete meiosis [82].
Strong γH2AX staining was found in the spermatocytes and the oocytes at leptotene or zygotene stage, and γH2AX colocalization with RPA was detected in the pachytene spermatocytes [84]. This finding demonstrates that following recombination, DSBR works in spermatocytes whereas it is uncommon and occurs more slowly in oocytes.
When Brca2 is conditionally deleted or RAD51 is inhibited in MEFs, it results in telomere shortening and fragmentation [85]. This suggests that BRCA2-mediated HR process is essential for maintaining telomere length and preserving its integrity during normal cell growth. Accordingly, mouse mammary tumors lacking BRCA2 exhibited telomere dysfunction, and human breast tumors with BRCA2 mutations had shorter telomeres when compared to those with the BRCA1 mutations [85]. This phenotypic effect may derive from the vital role of BRCA2 in preserving genomic integrity by participating in telomere maintenance and DNA repair [85]. Likewise, BRCA1 participates in DSBR and regulation of telomere length [86]. Thus, it has a crucial role in regulating ovarian aging and in oocyte survival [87].
The RAD54 protein is also involved in HR pathway, and its deficiency in MEFs led to reduced HR activity [88]. The RAD54-deficient mice had considerably shorter telomeres compared to wild-type mice, showing crucial role of RAD54 in maintaining telomere length. Additionally, more frequent fusions at chromosome ends including telomeres in RAD54-deficient MEFs indicate that this protein might mediate protection of telomeres from potential fusions [88]. RAD51D, essential for RAD54-mediated DSBR, localizes at telomeres in both meiotic and somatic cells [89]. When RAD51D is absent in telomerase-positive MEFs, it results in shortening of telomeric repeats [89]. Additionally, the chromosomal abnormalities such as telomeric end-to-end fusions were detected. Suppressing RAD51D synthesis in human cell lines using siRNA resulted in telomere erosion and chromosome fusion [89]. All these results demonstrated that RAD51D is required for maintaining genomic stability and telomere integrity. In mouse models, the impact of telomerase deficiency on expression of the DNA repair proteins, including PARP1, KU86, and DNA-PKcs, was investigated in the context of cancer and aging [90]. Absence of PARP1 in combination with telomerase deficiency showed no significant influence on the rate of telomere shortening and telomeric integrity when compared to the mice having only telomerase deficiency [90]. PARP1 seems to play a minor role in telomere maintenance in the loss of telomerase. In contrast, deficiencies of telomerase and KU86 or DNA-PKcs caused an accelerated loss of viability in comparison to only telomerase deficiency [90]. This phenotype may be resulted from defective cell proliferation and other age-related health problems. All these abnormalities may be associated with defective DSBR, which resulted in increased DNA breaks and fragments, abnormalities caused by defective telomeric integrity, i.e., telomere-telomere fusions [91]. Supportingly, deletion of the Ku70, Ku80, or both genes led to similar aging phenotypes without increasing cancer rates [91]. Taken together, changes in the cNHEJ and HR repair components as well as in telomere maintenance mechanism contribute to emerging various aging phenotypes. As a result, telomere-associated proteins ensure integrity of telomeres by protecting them from undesired DNA repair pathways. Although telomeric dysfunction is known to trigger DNA repair response, molecular biological details of this crosstalk have not yet been elucidated. Future studies would discover more interactions between the DSBR pathway components and telomeric proteins and/or telomerase components (especially the TERT protein).
The other DNA repair mechanisms at telomeres in somatic cells, germ cells, and embryos
Nucleotide excision repair
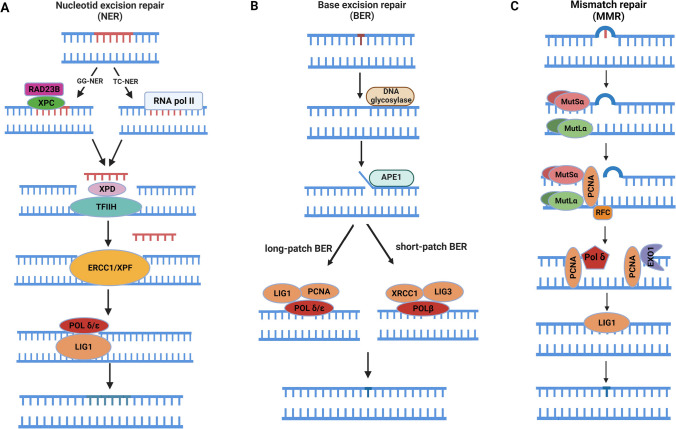
The NER mechanism acts in repairing the damages such as ultraviolet (UV)-induced pyrimidine dimers, bulky inserts, lesions formed by oxidative stress, and cross-links in DNA chains [92]. These damages can disrupt helical DNA structure at telomeres [93]. Once mammalian cells encounter these damages, NER excises 24–32 nucleotides in length from the DNA sequence involving damaged sites with a high accuracy rate. To accomplish this repair, approximately 30 proteins work together [94] (Fig. 3A).
Fig. 3.
The basic DNA repair pathways with the exception of DNA double-strand break repair (DSBR, presented in Fig. 2) in mammalian cells. A Nucleotide excision repair (NER) includes two subpathways: global genome (GG) and transcriptional-coupled (TC). Damaged or modified nucleotides especially due to ultraviolet light (UV) are repaired by this mechanism after excising approximately 30 nucleotides. B Base excision repair (BER). It is primarily responsible for removing small and non-helical base lesions from genome. C DNA mismatch repair (MMR). This pathway plays a critical role in maintaining genomic integrity through repairing mismatched bases. MMR also fixes minor insertions and deletions, arisen from potential errors during DNA replication. Functional properties of the proteins participating in these pathways are elaborately addressed in the text. The full names of all the abbreviations are given in the text. This figure was created using the BioRender program (BioRender, Toronto, Canada)
The NER mechanism is essential in somatic cells, germ cells, and early embryos to prevent mutation formation, which could lead to malformations and genetic defects in the resulting offspring. Excision of a ∼30 nucleotide-long patch including DNA damage is a unique step of this repair mechanism and is therefore defined as an unscheduled DNA synthesis. Gap-filling DNA synthesis measures the excision size and repairs efficiency [95]. Basically, NER consists of two pathways separated dependently on recognizing the damages: global genome NER (GG-NER) and transcription-coupled NER (TC-NER). Each pathway is able to recognize different lesions in DNA [94, 96]. For example, the GG-NER pathway detects and eliminates bulky damages in whole genome. Scanning of DNA for detecting these damages is carried out by the complex, composed of XPC-RAD23B proteins. Among NER proteins, RAD23B was shown to be expressed in mouse oocytes [97]. Moreover, NER activity was determined in human MII oocytes and blastocysts [67].
The TC-NER exclusively repairs the damages blocking transcriptional activity. TC-NER mechanism first ceases RNA polymerase II at the damaged site. After that, the Cockayne syndrome factors A (CSA) and B (CSB) introduce this site. These factors further create a gap to provide an easy access to the repair-related proteins. In both pathways, general transcription factor IIH, composed of XPB and CPD helicases, unwind about 30 base pairs including damaged site. The relaxed DNA helix allows the protein A to bind XPA for recognizing this region. Following that, the endonucleases XPG and XPF/ERCC1 cleave and remove the lesion [98]. Finally, DNA polymerase delta (Pol δ) and epsilon (Pol ε) enzymes synthesize new DNA strands, and LIG1 ligates the free ends.
In normal conditions, the shelterin complex interacts with the XPF-ERCC1 complex [99, 100] to inhibit unfavorable NER activation to telomeres. However, when large DNA lesions and UV ray-derived pyrimidine dimers occur [101–103], NER is activated with the permission of shelterin complex components. Consistently, in the case of TRF2 inhibition, XPF-ERCC1 recruits at the damaged telomeric sites [104]. We also think that expression levels of shelterin members or their conformation may undergo changes to stimulate NER when it is necessary.
The XPB, XPC, and XPD proteins taking part in NER are involved in the protection of telomeres against oxidative stress [103]. These proteins contribute to resecting oxidation-induced DNA lesions in order to ensure telomeric integrity [105]. As expected, mutations in the NER helicases XPB and XPD in the cells derived from patients with xeroderma pigmentosum (XP) showed high rates of hydrogen peroxide-induced telomere loss and end-to-end fusion rates [105, 106]. In a different study, Xpc−/− or Xpc−/− and Terc−/− double knockout mice exhibited excessively long telomeres possibly resulting from over activation of ALT mechanism, suggesting that XPC may have a role in suppressing recombination events among telomeres [107]. The TC-NER-related two genes (CSA and CSB) also participate in telomere maintenance [108]. Indeed, CSB facilitates interaction with the TRF2 protein [108]. In other words, CSB physically binds to TRF2 and therefore is observed at telomeres in human 293T cells [108]. Accordingly, CSB-deficient human 293T cells showed an excessive loss and fragility in their telomeres [108]. The CSB and RAD52 proteins play crucial roles in efficiently repairing ROS-induced DSBs in telomeres as demonstrated in the U2OS, BJ, HeLa, and 293 cell lines [7]. RAD52 functions in telomere repair by recruiting POLD3, a crucial protein in break-induced DNA replication (BIR). The ROS-induced telomeric R-loop also facilitates repair of telomeric DSBs via the CSB-RAD52-POLD3-mediated BIR pathway so that telomeres can be safeguarded from ROS-derived damages [109]. It is worth noting that no remarkable difference in telomere length was discovered in XPF mutation in humans and Ercc1 loss in mice [99]. Moreover, there was no change detected in the sister chromatid exchange at telomeres in the absence of ERCC1-XPF complex [110].
According to the literature analysis, a limited number of studies showed that NER mechanism functions in repairing certain damages in telomeres to maintain telomeric structure and length. Changed expression and genetic variants in the NER genes may lead to telomeric attrition. Further analysis of the mechanistic relationship between telomeres and NER components especially in germ cells and embryos may contribute to understanding its importance in producing high quality gametes and embryos.
Base excision repair
BER is a conserved repair mechanism being employed from bacteria to humans. It is responsible for repairing many DNA damages deriving from endogenous and exogenous agents such as deamination, depurination, oxidation, and alkylation [111]. These damages are recognized by the specific enzymes, DNA glycosylases [112]. The DNA glycosylase superfamily, uracil DNA glycosylase, includes four lesion-specific enzymes as follows: uracil DNA N-glycosylase (UNG), thymine DNA glycosylase, single-strand-selective mono-functional uracil-DNA glycosylase 1, and methyl-CpG-binding domain 4 [113]. Another enzyme type participating in this process is apurinic/apyrimidinic endonuclease (APE, also known as REF1, HAP1, or APEX), which removes bases from lesion site [112]. All these enzymes can excise covalent bonds between deoxyribose sugar and incorrectly placed nitrogenous bases so that apurinic or apyrimidinic sites are created. After that, one of the two subpathways of BER is activated according to the number of nucleotides to be added. While the short patch repair (SPR) subpathway enables addition of 1–2 nucleotides, the long patch repair (LPR) subpathway can add 2–8 nucleotides [108] (Fig. 3B).
In the SPR subpathway, one or two nucleotides are excised by a proper glycosylase enzyme, and then APE removes these nucleotide(s) from DNA strand to facilitate initiation of DNA synthesis. Then, polymerase β correctly incorporates the missing nucleotides to apurinic or apyrimidinic site(s). Eventually, ligation of free ends is carried out by LIG3. The LPR subpathway acts together with the fundamental proteins, including replication factor C (RFC), DNA polymerase, and PCNA. At the end, free ends are sealed by LIG1. While SPR is the dominant process during replication, LPR works especially at post-replicative period, mainly initiated by UNG2 [114] or NEIL1 [115]. SPR holds at an active state in proliferating and non-proliferating cells [116], but LPR serves mainly in proliferating cells [117].
Most oxidative stress-derived lesions at telomeres are mainly repaired by BER mechanism [9, 118]. The long TTAGGG repeats of telomeres make them more susceptible to lesions upon exposure to oxidative stress, which may result in telomere shortening [119, 120]. Increased oxidative stress with advanced age may also cause telomere dysfunction in oocytes [121]. The most commonly encountered oxidative damage is 8-oxo-7,8-dihydroguanine (8-oxoG) formation because telomere repeats contain a large number of guanines [9, 122]. The 8-oxoG can disrupt binding of TRF1 and TRF2 proteins to telomeres [123]. In normal conditions, 8-oxoG is cleaved by OGG1, and then other BER components complete the repair process [124]. Expectedly, acute 8-oxoG formation in the cells lacking OGG1 causes shortening, fragility, and replication stress at telomeres [125, 126]. As observed in OGG1 deficiency, loss of UNG in mouse hematopoietic cells results in uracil accumulation in telomeres, which causes telomeric fragility [127]. Replacement of thymine residues with uracil weakens the interaction between POT1 and TPP1 proteins in vitro, which may result in structural disassociation in telomeres [127]. The other guanosine oxidization 8-OH2dG (8-hydroxy 2-deoxyguanosine) occurs in telomeres of human sperm cells [61, 128]. Importantly, 8-OH2dG might cause a minor shortening of telomeres due to the action of OGG1 to eliminate it [9]. As a result, all guanine derivatives and other damages in telomeres are mainly repaired by the BER mechanism to maintain telomeric integrity [125].
Loss of DNA glycosylase NTH1 removing thymine derivatives results in insufficient repair of oxidative DNA damages so that oxidized base lesions accumulate at telomeres [129]. This finding demonstrates that NTH1 plays an important role in repairing oxidative damages appearing in telomeres [129]. As mentioned above, the apuric/apyrimidinic endonuclease APE1 protects telomeres from oxidative stress in cooperating with TRF2 protein [130–132]. Accordingly, a partial depletion of Ape1 gene expression caused telomere shortening in BJ-hTERT and HeLa human cell lines [132] because APE1 recruits the factors necessary for BER at telomeres and contributes to the stability of TRF2 [132, 133]. It was reported that APE1 expression exhibits an inverse relationship with cellular senescence in human fibroblasts [134]. This is exemplified by the fact that when APE1 is depleted, both cellular senescence and accumulation of DNA damages are increased. Conditional deletion of Apex1 in mice led to cellular senescence and other aging characteristics, becoming evident at later ages [134]. These aging-related phenotypes may occur due to telomere dysfunction, arising from APE1 deficiency.
Overall, BER mechanism is essential for repairing base adducts and modifications at telomeres, and also for synthesis of missing bases. Thus, telomeric integrity is sustained during lifespan. Further studies on determining intracellular signaling mechanisms regulating BER component levels around telomeres are required, especially in germ cells and embryos.
Mismatch repair
The MMR mechanism is a preserved cellular process ranging from bacteria to humans, and recognizes and repairs mismatched bases [135]. A mismatch between bases likely arises from errors in DNA replication and genetic recombination processes as well as from chemical and physical damages. The mismatch damages appearing owing to consecutive mitotic divisions in early spermatogenesis are also fixed by this mechanism [60, 136].
The four key proteins playing a role in introducing mismatched sites in mammals are mutL homolog 1 (MLH1), mutS homolog 2 (MSH2), mutS homolog 6 (MSH6), and post-meiotic segregation increased 2 (PMS2). The human homologs of E. coli MutS proteins are hMSH2, hMSH3, hMSH5, and hMSH6, while MutL homologs are referred to as hMLH1, hPMS1 (also known as hMLH2), hMLH3, and hPMS2 (also known as hMLH4) [137]. The heterodimers forming between MSH2 and MSH6 or MSH2 and MSH3 bind to mismatched bases while checking DNA strands immediately after undergoing replication [135]. MMR is a bidirectional (including excision and re-synthesis) process that is initiated at the 3’- or 5’-strand. Taken together, this process consists of four basic steps: (i) recognition of mismatches by MSHs, (ii) recruitment of MLHs by ATP-bound MSHs, which then binds to DNA strand segment where excision begins, (iii) cut of DNA strand, and (iv) re-synthesis of excision gap by a replicative DNA polymerase using remaining DNA strand as a template [138].
The heterodimer, MSH2-MSH6, detects a single base mismatch and insertion-deletion in dinucleotides whereas MSH2-MSH3 defines larger insertion-deletion loops involving more than ~13 nucleotides in length [139]. The MLH1-PMS2 complex physically interacts with the clamp loader subunit of Pol δ and brings it to the MMR site. Thus, the errors that escape from polymerase proofreading in the first place are corrected by Pol δ later [140]. For this purpose, the interactions between MutL, MutS, and PCNA (involved in replication) promote activation of MutL to cut the mismatched region in an ATP-dependent manner from newly synthesized strand (Fig. 3C).
The MMR mechanism also works at telomeres to remove unfavorable modified nucleotides that may result in a partial telomeric loss [141]. Consistently, the MLH1 expression was detected at high levels in the telomeres of dog spermatocytes [142]. Telomere length in the leukocytes of cancer patients carrying MMR-related gene mutations was shorter than controls [143]. As observed in normal human cells [144], an accelerated telomere shortening was found in human lung fibroblasts, in which the key component of MMR, hMSH2, was downregulated [145]. In other words, a remarkable decrease in MMR activity may cause telomere shortening in these cells. Although telomere length and telomerase activity were at normal levels in the Msh2−/− mouse tissues and embryonic fibroblasts, there were an increase in the chromosomal aneuploidy, defective mitotic spindle organization, and unequal chromosome separation [146]. The basic role of MSH2 seems to show species-dependent difference in telomere length regulation. All findings on MMR mechanism suggest that its components exhibit different effects at telomeres in case of their expressional changes. The length and structural integrity of telomeres may alter dependent on cumulative effects of all DNA repair mechanisms, at least in certain conditions.
Conclusion
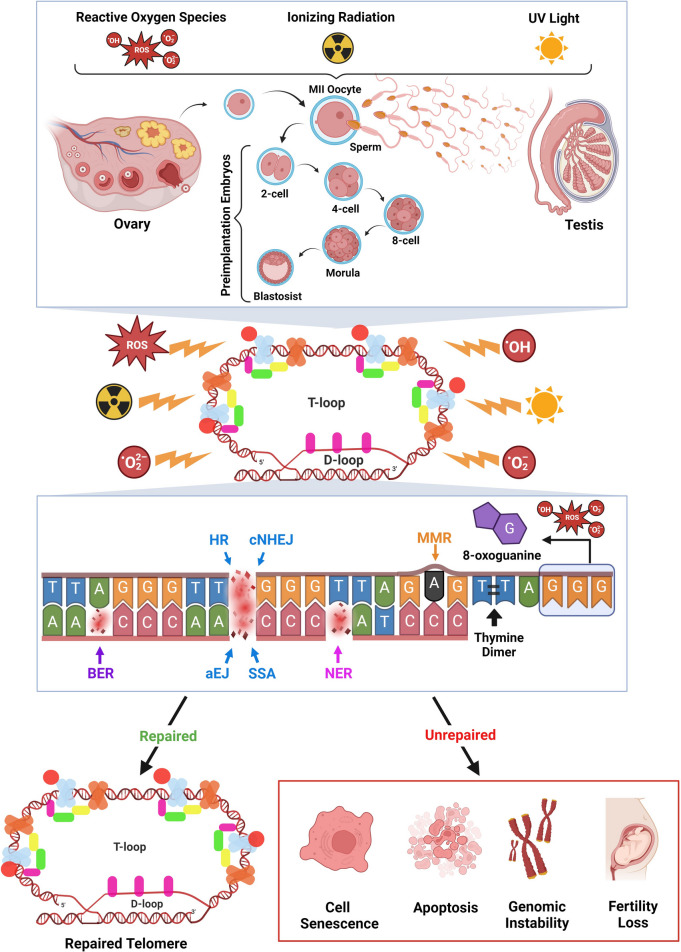
Telomeric DNA damages in somatic cells, germ cells, and early embryos are mainly repaired by specific DNA repair mechanisms, including DSBR, BER, NER, and MMR (Fig. 4). Possible defects in these repair mechanisms due to genetic and/or epigenetic variations may lead to telomere shortening, which can lead to genetic instabilities, cellular senescence, and fertility loss (Fig. 4). Further studies are required to elucidate mechanistic background of the crosstalk between DNA repair mechanisms and telomeres, especially in oocytes, spermatogenic cells, and embryos. After addressing these unknown points, new therapeutic strategies may be developed to produce gametes and embryos with high quality.
Fig. 4.
Potential telomere DNA damages and their repairs in mammalian germ cells and preimplantation embryos. Ionizing radiation, ultraviolet (UV) light, and various ROS are able to cause damages in telomeric DNA such as 8-oxoguanine, thymine dimers, mismatched bases, DSBs, and other lesions. Dependent on damage types, they can be repaired by DNA double-strand break repair (DSBR) pathways [including homologous recombination (HR), classical non-homologous end joining (cNHEJ), alternative end joining (aEJ), and single-strand annealing (SSA)], base excision repair (BER), nucleotide excision repair (NER), or mismatch repair (MMR). Thus, telomeres bring into the closed state, showing a robust integrity. If telomeric damages are not repaired efficiently and timely, the unfavorable outcomes such as cell senescence, apoptosis, genomic instability, and fertility loss may occur. This figure was created using the BioRender Program (BioRenderCompany, Toronto, Canada)
Author contributions
SO, BT, and GT designed the study. BT and GT wrote the manuscript and created all the figures. SO critically read and revised the manuscript.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Betul Tire and Gunel Talibova contributed to the manuscript equally.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ozturk S, Sozen B, Demir N. Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod. 2014;20(1):15–30. doi: 10.1093/molehr/gat055. [DOI] [PubMed] [Google Scholar]
- 2.Doksani Y. The response to DNA damage at telomeric repeats and ıts consequences for telomere function. Genes (Basel). 2019;10(4):318. [DOI] [PMC free article] [PubMed]
- 3.Longhese MP. DNA damage response at functional and dysfunctional telomeres. Genes Dev. 2008;22(2):125–140. doi: 10.1101/gad.1626908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doksani Y, de Lange T. The role of double-strand break repair pathways at functional and dysfunctional telomeres. Cold Spring Harb Perspect Biol. 2014;6(12):a016576. doi: 10.1101/cshperspect.a016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tire B, Ozturk S. Potential effects of assisted reproductive technology on telomere length and telomerase activity in human oocytes and early embryos. J Ovarian Res. 2023;16(1):130. doi: 10.1186/s13048-023-01211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11(3):171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan J, Lan L. The DNA secondary structures at telomeres and genome instability. Cell Biosci. 2020;10:47. doi: 10.1186/s13578-020-00409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent T, Clynes D. Alternative lengthening of telomeres: lessons to be learned from telomeric DNA double-strand break repair. Genes (Basel). 2021;12(11):1734. [DOI] [PMC free article] [PubMed]
- 9.Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019;177:37–45. doi: 10.1016/j.mad.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes AC, Oliveira PF, Sousa M. Shedding light into the relevance of telomeres in human reproduction and male factor infertilitydagger. Biol Reprod. 2019;100(2):318–330. doi: 10.1093/biolre/ioy215. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Kim NK, Feigon J. Architecture of human telomerase RNA. Proc Natl Acad Sci U S A. 2011;108(51):20325–20332. doi: 10.1073/pnas.1100279108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyatt HD, West SC, Beattie TL. InTERTpreting telomerase structure and function. Nucleic Acids Res. 2010;38(17):5609–5622. doi: 10.1093/nar/gkq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong L, et al. mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mech Dev. 2000;97(1-2):109–116. doi: 10.1016/s0925-4773(00)00423-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, et al. Telomere lengthening early in development. Nat Cell Biol. 2007;9(12):1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 15.Heaphy CM, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179(4):1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 17.Mao P, et al. Homologous recombination-dependent repair of telomeric DSBs in proliferating human cells. Nat Commun. 2016;7:12154. doi: 10.1038/ncomms12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fasching CL, et al. DNA damage induces alternative lengthening of telomeres (ALT) associated promyelocytic leukemia bodies that preferentially associate with linear telomeric DNA. Cancer Res. 2007;67(15):7072–7077. doi: 10.1158/0008-5472.CAN-07-1556. [DOI] [PubMed] [Google Scholar]
- 19.Azzalin CM, et al. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318(5851):798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 20.Rocca MS, Foresta C, Ferlin A. Telomere length: lights and shadows on their role in human reproduction. Biol Reprod. 2019;100(2):305–317. doi: 10.1093/biolre/ioy208. [DOI] [PubMed] [Google Scholar]
- 21.Kordowitzki P, et al. Dynamics of telomeric repeat-containing RNA expression in early embryonic cleavage stages with regards to maternal age. Aging (Albany NY). 2020;12(16):15906–15917. doi: 10.18632/aging.103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozturk S. Telomerase activity and telomere length in male germ cells. Biol Reprod. 2015;92(2):53. doi: 10.1095/biolreprod.114.124008. [DOI] [PubMed] [Google Scholar]
- 23.Talibova G, Bilmez Y, Ozturk S. DNA double-strand break repair in male germ cells during spermatogenesis and its association with male infertility development. DNA Repair (Amst). 2022;118:103386. doi: 10.1016/j.dnarep.2022.103386. [DOI] [PubMed] [Google Scholar]
- 24.Antunes DM, et al. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32(11):1685–1690. doi: 10.1007/s10815-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stindl R. The paradox of longer sperm telomeres in older men’s testes: a birth-cohort effect caused by transgenerational telomere erosion in the female germline. Mol Cytogenet. 2016;9:12. doi: 10.1186/s13039-016-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 27.Imran SAM, et al. The ıntra- and extra-telomeric role of TRF2 in the DNA damage response. Int J Mol Sci. 2021;22(18):9900. [DOI] [PMC free article] [PubMed]
- 28.Doksani Y, et al. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013;155(2):345–356. doi: 10.1016/j.cell.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feuerhahn S, et al. No DDRama at chromosome ends: TRF2 takes centre stage. Trends Biochem Sci. 2015;40(5):275–285. doi: 10.1016/j.tibs.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Ruis P, Boulton SJ. The end protection problem-an unexpected twist in the tail. Genes Dev. 2021;35(1-2):1–21. doi: 10.1101/gad.344044.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7(7):712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 32.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92(3):401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 33.Karlseder J, et al. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283(5406):1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Epel E. Stress and telomere shortening: ınsights from cellular mechanisms. Ageing Res Rev. 2022;73:101507. doi: 10.1016/j.arr.2021.101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buscemi G, et al. The shelterin protein TRF2 inhibits Chk2 activity at telomeres in the absence of DNA damage. Curr Biol. 2009;19(10):874–879. doi: 10.1016/j.cub.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 36.Sfeir A, et al. Loss of Rap1 induces telomere recombination in the absence of NHEJ or a DNA damage signal. Science. 2010;327(5973):1657–1661. doi: 10.1126/science.1185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Lange T. Shelterin-mediated telomere protection. Annu Rev Genet. 2018;52:223–247. doi: 10.1146/annurev-genet-032918-021921. [DOI] [PubMed] [Google Scholar]
- 38.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448(7157):1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126(1):49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q, Zheng YL, Harris CC. POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol. 2005;25(3):1070–1080. doi: 10.1128/MCB.25.3.1070-1080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uysal F, et al. Decreased expression of TERT and telomeric proteins as human ovaries age may cause telomere shortening. J Assist Reprod Genet. 2021;38(2):429–441. doi: 10.1007/s10815-020-01932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirzada RH, et al. Role of TRF2 and TPP1 regulation in idiopathic recurrent pregnancy loss. Int J Biol Macromol. 2019;127:306–310. doi: 10.1016/j.ijbiomac.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama H, et al. Preferential binding to branched DNA strands and strand-annealing activity of the human Rad51B, Rad51C, Rad51D and Xrcc2 protein complex. Nucleic Acids Res. 2004;32(8):2556–2565. doi: 10.1093/nar/gkh578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doksani Y, de Lange T. Telomere-ınternal double-strand breaks are repaired by homologous recombination and PARP1/Lig3-dependent end-joining. Cell Rep. 2016;17(6):1646–1656. doi: 10.1016/j.celrep.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhargava R, Onyango DO, Stark JM. Regulation of single-strand annealing and its role in genome maintenance. Trends Genet. 2016;32(9):566–575. doi: 10.1016/j.tig.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kazda A, et al. Chromosome end protection by blunt-ended telomeres. Genes Dev. 2012;26(15):1703–1713. doi: 10.1101/gad.194944.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 48.Mehta A, Haber JE. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb Perspect Biol. 2014;6(9):a016428. doi: 10.1101/cshperspect.a016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scully R, et al. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol. 2019;20(11):698–714. doi: 10.1038/s41580-019-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West SC. Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol. 2003;4(6):435–445. doi: 10.1038/nrm1127. [DOI] [PubMed] [Google Scholar]
- 51.Henson JD, et al. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21(4):598–610. doi: 10.1038/sj.onc.1205058. [DOI] [PubMed] [Google Scholar]
- 52.Britton S, Coates J, Jackson SP. A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol. 2013;202(3):579–595. doi: 10.1083/jcb.201303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue X, et al. DNA-PKcs: a multi-faceted player in DNA damage response. Front Genet. 2020;11:607428. doi: 10.3389/fgene.2020.607428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlieb TM, Jackson SP. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 55.Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66(6):801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sfeir A, Symington LS. Microhomology-mediated end joining: a back-up survival mechanism or dedicated pathway? Trends Biochem Sci. 2015;40(11):701–714. doi: 10.1016/j.tibs.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang HHY, et al. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18(8):495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Z, et al. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134(6):981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Rodriguez A, et al. DNA damage and repair in human reproductive cells. Int J Mol Sci. 2018;20(1):31. [DOI] [PMC free article] [PubMed]
- 61.Musson R, et al. DNA damage in preimplantation embryos and gametes: specification, clinical relevance and repair strategies. Hum Reprod Update. 2022;28(3):376–399. doi: 10.1093/humupd/dmab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin JH, et al. DNA damage and repair in the female germline: contributions to ART. Hum Reprod Update. 2019;25(2):180–201. doi: 10.1093/humupd/dmy040. [DOI] [PubMed] [Google Scholar]
- 63.Hajkova P, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117(1-2):15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 64.Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol. 2012;22(11):989–994. doi: 10.1016/j.cub.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 65.Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol. 2004;272(2):483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Huang Y, Roig I. Genetic control of meiosis surveillance mechanisms in mammals. Front Cell Dev Biol. 2023;11:1127440. doi: 10.3389/fcell.2023.1127440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaroudi S, et al. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod. 2009;24(10):2649–2655. doi: 10.1093/humrep/dep224. [DOI] [PubMed] [Google Scholar]
- 68.Veitia RA. Primary ovarian insufficiency, meiosis and DNA repair. Biomed J. 2020;43(2):115–123. doi: 10.1016/j.bj.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C, Guo T, Qin Y. Meiotic recombination defects and premature ovarian ınsufficiency. Front Cell Dev Biol. 2021;9:652407. doi: 10.3389/fcell.2021.652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Butts S, et al. Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J Clin Endocrinol Metab. 2009;94(12):4835–4843. doi: 10.1210/jc.2008-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toupance S, et al. Ovarian telomerase and female fertility. Biomedicines. 2021;9:7. doi: 10.3390/biomedicines9070842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fattet AJ, et al. Telomere length in granulosa cells and leukocytes: a potential marker of female fertility? A systematic review of the literature. J Ovarian Res. 2020;13(1):96. doi: 10.1186/s13048-020-00702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dinger Y, et al. DNA damage, DNA susceptibility to oxidation and glutathione level in women with polycystic ovary syndrome. Scand J Clin Lab Invest. 2005;65(8):721–8. doi: 10.1080/00365510500375263. [DOI] [PubMed] [Google Scholar]
- 74.Pedroso DCC, et al. Telomere length and telomerase activity in ımmature oocytes and cumulus cells of women with polycystic ovary syndrome. Reprod Sci. 2020;27(6):1293–1303. doi: 10.1007/s43032-019-00120-6. [DOI] [PubMed] [Google Scholar]
- 75.Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online. 2003;6(1):84–96. doi: 10.1016/s1472-6483(10)62060-3. [DOI] [PubMed] [Google Scholar]
- 76.Derijck A, et al. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17(13):1922–1937. doi: 10.1093/hmg/ddn090. [DOI] [PubMed] [Google Scholar]
- 77.Treff NR, et al. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011;7(6):e1002161. doi: 10.1371/journal.pgen.1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goedecke W, et al. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet. 1999;23(2):194–198. doi: 10.1038/13821. [DOI] [PubMed] [Google Scholar]
- 79.Gouraud A, et al. “Breaking news” from spermatids. Basic Clin Androl. 2013;23:11. doi: 10.1186/2051-4190-23-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmed EA, Scherthan H, de Rooij DG. DNA double strand break response and limited repair capacity in mouse elongated spermatids. Int J Mol Sci. 2015;16(12):29923–29935. doi: 10.3390/ijms161226214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmed EA, et al. Ku70 and non-homologous end joining protect testicular cells from DNA damage. J Cell Sci. 2013;126(Pt 14):3095–3104. doi: 10.1242/jcs.122788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed EA, Rosemann M, Scherthan H. NHEJ contributes to the fast repair of radiation-induced DNA double-strand breaks at late prophase I telomeres. Health Phys. 2018;115(1):102–107. doi: 10.1097/HP.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 83.Siderakis M, Tarsounas M. Telomere regulation and function during meiosis. Chromosome Res. 2007;15(5):667–679. doi: 10.1007/s10577-007-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roig I, et al. Female-specific features of recombinational double-stranded DNA repair in relation to synapsis and telomere dynamics in human oocytes. Chromosoma. 2004;113(1):22–33. doi: 10.1007/s00412-004-0290-8. [DOI] [PubMed] [Google Scholar]
- 85.Badie S, et al. BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol. 2010;17(12):1461–1469. doi: 10.1038/nsmb.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosen EM. BRCA1 in the DNA damage response and at telomeres. Front Genet. 2013;4:85. doi: 10.3389/fgene.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Titus S, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaco I, et al. Role of mammalian Rad54 in telomere length maintenance. Mol Cell Biol. 2003;23(16):5572–5580. doi: 10.1128/MCB.23.16.5572-5580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarsounas M, et al. Telomere maintenance requires the RAD51D recombination/repair protein. Cell. 2004;117(3):337–347. doi: 10.1016/s0092-8674(04)00337-x. [DOI] [PubMed] [Google Scholar]
- 90.Espejel S, et al. Impact of telomerase ablation on organismal viability, aging, and tumorigenesis in mice lacking the DNA repair proteins PARP-1, Ku86, or DNA-PKcs. J Cell Biol. 2004;167(4):627–638. doi: 10.1083/jcb.200407178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, et al. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol. 2007;27(23):8205–8214. doi: 10.1128/MCB.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iyama T, Wilson DM., 3rd DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst). 2013;12(8):620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillet LC, Scharer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev. 2006;106(2):253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- 94.Petruseva IO, Evdokimov AN, Lavrik OI. Molecular mechanism of global genome nucleotide excision repair. Acta Naturae. 2014;6(1):23–34. [PMC free article] [PubMed] [Google Scholar]
- 95.Wienholz F, Vermeulen W, Marteijn JA. Amplification of unscheduled DNA synthesis signal enables fluorescence-based single cell quantification of transcription-coupled nucleotide excision repair. Nucleic Acids Res. 2017;45(9):e68. doi: 10.1093/nar/gkw1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18(4):357–365. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- 97.Wang S, et al. Proteome of mouse oocytes at different developmental stages. Proc Natl Acad Sci U S A. 2010;107(41):17639–17644. doi: 10.1073/pnas.1013185107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31(3):309–319. doi: 10.1016/j.rbmo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 99.Zhu XD, et al. ERCC1/XPF removes the 3’ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell. 2003;12(6):1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- 100.de Lange T. Telomere biology and DNA repair: enemies with benefits. FEBS Lett. 2010;584(17):3673–3674. doi: 10.1016/j.febslet.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 101.Rochette PJ, Brash DE. Human telomeres are hypersensitive to UV-induced DNA damage and refractory to repair. PLoS Genet. 2010;6(4):e1000926. doi: 10.1371/journal.pgen.1000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci U S A. 1995;92(1):258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fouquerel E, Opresko PL. Convergence of The nobel fields of telomere biology and DNA repair. Photochem Photobiol. 2017;93(1):229–237. doi: 10.1111/php.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y, Mitchell TR, Zhu XD. Human XPF controls TRF2 and telomere length maintenance through distinctive mechanisms. Mech Ageing Dev. 2008;129(10):602–10. doi: 10.1016/j.mad.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Ting AP, et al. Telomere attrition and genomic instability in xeroderma pigmentosum type-b deficient fibroblasts under oxidative stress. J Cell Mol Med. 2010;14(1-2):403–416. doi: 10.1111/j.1582-4934.2009.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gopalakrishnan K, et al. Hydrogen peroxide induced genomic instability in nucleotide excision repair-deficient lymphoblastoid cells. Genome Integr. 2010;1(1):16. doi: 10.1186/2041-9414-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stout GJ, Blasco MA. Telomere length and telomerase activity impact the UV sensitivity syndrome xeroderma pigmentosum C. Cancer Res. 2013;73(6):1844–1854. doi: 10.1158/0008-5472.CAN-12-3125. [DOI] [PubMed] [Google Scholar]
- 108.Batenburg NL, et al. Cockayne syndrome group B protein interacts with TRF2 and regulates telomere length and stability. Nucleic Acids Res. 2012;40(19):9661–9674. doi: 10.1093/nar/gks745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan J, et al. An R-loop-initiated CSB-RAD52-POLD3 pathway suppresses ROS-induced telomeric DNA breaks. Nucleic Acids Res. 2020;48(3):1285–1300. doi: 10.1093/nar/gkz1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hagelstrom RT, et al. Hyper telomere recombination accelerates replicative senescence and may promote premature aging. Proc Natl Acad Sci U S A. 2010;107(36):15768–15773. doi: 10.1073/pnas.1006338107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wallace SS. Base excision repair: a critical player in many games. DNA Repair (Amst). 2014;19:14–26. doi: 10.1016/j.dnarep.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim YJ, Wilson DM., 3rd Overview of base excision repair biochemistry. Curr Mol Pharmacol. 2012;5(1):3–13. doi: 10.2174/1874467211205010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schormann N, Ricciardi R, Chattopadhyay DJPs. Uracil-DNA glycosylases—structural and functional perspectives on an essential family of DNA repair enzymes. Protein Sci. 2014;23(12):1667–85. [DOI] [PMC free article] [PubMed]
- 114.Otterlei M, et al. Post-replicative base excision repair in replication foci. EMBO J. 1999;18(13):3834–44. [DOI] [PMC free article] [PubMed]
- 115.Hegde ML, et al. Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease 1. J Biol Chem. 2008;283(40):27028–37. [DOI] [PMC free article] [PubMed]
- 116.Krokan HE, Bjoras M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012583. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Svilar D, et al. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. 2011;14(12):2491–2507. doi: 10.1089/ars.2010.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jia P, Her C, Chai W. DNA excision repair at telomeres. DNA Repair (Amst). 2015;36:137–145. doi: 10.1016/j.dnarep.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 120.Saretzki G, Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Ann N Y Acad Sci. 2002;959:24–29. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 121.Kordowitzki P. Oxidative stress induces telomere dysfunction and shortening in human oocytes of advanced age donors. Cells. 2021;10(8):1866. [DOI] [PMC free article] [PubMed]
- 122.Oikawa S, Tada-Oikawa S, Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40(15):4763–4768. doi: 10.1021/bi002721g. [DOI] [PubMed] [Google Scholar]
- 123.Opresko PL, et al. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol Cell. 2004;14(6):763–774. doi: 10.1016/j.molcel.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 124.Fouquerel E, et al. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol Cell. 2019;75(1):117–130 e6. doi: 10.1016/j.molcel.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Z, et al. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet. 2010;6(5):e1000951. doi: 10.1371/journal.pgen.1000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rhee DB, et al. Factors that influence telomeric oxidative base damage and repair by DNA glycosylase OGG1. DNA Repair (Amst). 2011;10(1):34–44. doi: 10.1016/j.dnarep.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vallabhaneni H, et al. Defective repair of uracil causes telomere defects in mouse hematopoietic cells. J Biol Chem. 2015;290(9):5502–5511. doi: 10.1074/jbc.M114.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Benoff S, et al. Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers) Mol Med. 2009;15(7-8):248–262. doi: 10.2119/molmed.2008.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vallabhaneni H, et al. Defective repair of oxidative base lesions by the DNA glycosylase Nth1 associates with multiple telomere defects. PLoS Genet. 2013;9(7):e1003639. doi: 10.1371/journal.pgen.1003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dejardin J, Kingston RE. Purification of proteins associated with specific genomic loci. Cell. 2009;136(1):175–186. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee OH, et al. Genome-wide YFP fluorescence complementation screen identifies new regulators for telomere signaling in human cells. Mol Cell Proteomics. 2011;10(2):M110 001628. doi: 10.1074/mcp.M110.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Madlener S, et al. Essential role for mammalian apurinic/apyrimidinic (AP) endonuclease Ape1/Ref-1 in telomere maintenance. Proc Natl Acad Sci U S A. 2013;110(44):17844–17849. doi: 10.1073/pnas.1304784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Opresko PL, et al. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33(4):1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li M, et al. APE1 deficiency promotes cellular senescence and premature aging features. Nucleic Acids Res. 2018;46(11):5664–5677. doi: 10.1093/nar/gky326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiricny J. Postreplicative mismatch repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012633. doi: 10.1101/cshperspect.a012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ioannou D, et al. Impact of sperm DNA chromatin in the clinic. J Assist Reprod Genet. 2016;33(2):157–166. doi: 10.1007/s10815-015-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Amaral-Silva GK, et al. Mismatch repair system proteins in oral benign and malignant lesions. J Oral Pathol Med. 2017;46(4):241–245. doi: 10.1111/jop.12484. [DOI] [PubMed] [Google Scholar]
- 138.Martin-Lopez JV, Fishel R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Fam Cancer. 2013;12(2):159–168. doi: 10.1007/s10689-013-9635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brown MW, et al. Dynamic DNA binding licenses a repair factor to bypass roadblocks in search of DNA lesions. Nat Commun. 2016;7:10607. doi: 10.1038/ncomms10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pecina-Slaus N, et al. Mismatch repair pathway, genome stability and cancer. Front Mol Biosci. 2020;7:122. doi: 10.3389/fmolb.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Siegl-Cachedenier I, et al. Deficient mismatch repair improves organismal fitness and survival of mice with dysfunctional telomeres. Genes Dev. 2007;21(17):2234–2247. doi: 10.1101/gad.430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Basheva EA, Bidau CJ, Borodin PM. General pattern of meiotic recombination in male dogs estimated by MLH1 and RAD51 immunolocalization. Chromosome Res. 2008;16(5):709–719. doi: 10.1007/s10577-008-1221-y. [DOI] [PubMed] [Google Scholar]
- 143.Segui N, et al. Telomere length and genetic anticipation in Lynch syndrome. PLoS One. 2013;8(4):e61286. doi: 10.1371/journal.pone.0061286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mendez-Bermudez A, Royle NJ. Deficiency in DNA mismatch repair increases the rate of telomere shortening in normal human cells. Hum Mutat. 2011;32(8):939–946. doi: 10.1002/humu.21522. [DOI] [PubMed] [Google Scholar]
- 145.Rampazzo E, et al. Relationship between telomere shortening, genetic instability, and site of tumour origin in colorectal cancers. Br J Cancer. 2010;102(8):1300–1305. doi: 10.1038/sj.bjc.6605644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Campbell MR, et al. Msh2 deficiency leads to chromosomal abnormalities, centrosome amplification, and telomere capping defect. Oncogene. 2006;25(17):2531–2536. doi: 10.1038/sj.onc.1209277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.