Abstract
Purpose
Oxidative stress and mitochondrial dysfunction play central roles in reduced oocyte quality and infertility in obese patients. Mitochondria-targeted treatments containing co-enzyme Q10 such as mitoquinone (MitoQ) can increase mitochondrial antioxidative capacity; however, their safety and efficiency when supplemented to oocytes under lipotoxic conditions have not been described.
Methods
We tested the effect of different concentrations of MitoQ or its cationic carrier (TPP) (0, 0.1, 0.5, 1.0 μM each) during bovine oocyte IVM. Then, we tested the protective capacity of MitoQ (0.1 μM) against palmitic acid (PA)–induced lipotoxicity and mitochondrial dysfunction in oocytes.
Results
Exposure to MitoQ, or TPP only, at 1 μM significantly (P<0.05) reduced oocyte mitochondrial inner membrane potential (JC-1 staining) and resulted in reduced cleavage and blastocyst rates compared with solvent control. Lower concentrations of MitoQ or TPP had no effects on embryo development under control (PA-free) conditions. As expected, PA increased the levels of MMP and ROS in oocytes (CellROX staining) and reduced cleavage and blastocyst rates compared with the controls (P<0.05). These negative effects were ameliorated by 0.1 μM MitoQ. In contrast, 0.1 μM TPP alone had no protective effects. MitoQ also normalized the expression of HSP10 and TFAM, and partially normalized HSP60 in the produced blastocysts, indicating at least a partial alleviation of PA-induced mitochondrial stress.
Conclusion
Oocyte exposure to MitoQ may disturb mitochondrial bioenergetic functions and developmental capacity due to a TPP-induced cationic overload. A fine-tuned concentration of MitoQ can protect against lipotoxicity-induced mitochondrial stress during IVM and restore developmental competence and embryo quality.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02994-7.
Keywords: Oocyte, CoQ10, MitoQ, IVF, Embryo, Obesity, Oxidative stress
Introduction
Mitochondrial (MT) dysfunction in oocytes has been shown to play a central role in the pathogenesis of reduced oocyte quality and fertility in humans and animals [1]. Several metabolic diseases are associated with oocyte MT dysfunctions, such as obesity and type 2 diabetes [2, 3]. Exposure of oocytes to elevated concentrations of circulating lipotoxic free fatty acids (FFAs) and oxidative stress results in altered MT inner membrane potential (MMP) and impacts cellular bioenergetic and metabolic activities [4–7]. This has been demonstrated in high-fat diet–induced obese mouse models. Optimal ATP production in oocytes is essential to support several cytoplasmic and molecular changes during early development, including cytoskeletal dynamics, activation of different kinases, and cortical granule activation [8]. Suboptimal MT functions can thus lead to spindle malformation, failure of fertilization, polyspermy, embryo fragmentation, altered embryo kinetics, and embryo arrest, as well as reduced embryo quality [9, 10].
MT dysfunction in oocytes is associated with an increased intracellular accumulation of reactive oxygen species (ROS) which may have a causative or additive detrimental impact due to oxidative damage [11, 12]. Mitochondria are the main site of ROS production in the cell, and they are also the most vulnerable organelles to oxidative stress [13]. Using a well-established and validated bovine in vitro embryo production model, it was demonstrated that oocytes matured in vitro in the presence of elevated concentrations of FFAs (particularly palmitic acid, PA [14], mimicking the intrafollicular conditions seen in obese women [14, 15]) exhibit oxidative stress and increased MT MMP [16–18]. The relative abundance of several MT proteins particularly those involved in unfolded protein responses and redox regulation increase in these oocytes [18]. In this model, alteration in oocyte MT activity was also linked to abnormal epigenetic patterns in the surviving embryos, ultimately leading to deviant blastocyst metabolic and transcriptomic profiles [19, 20]. These alterations persist during post-hatching development even after transfer to a healthy uterine environment (as shown in an in vitro-in vivo bovine model [21]). Afterwards, persistent MT dysfunction and epigenetic alterations can increase the risk of miscarriage and neonatal and postnatal diseases [22].
Importantly, we have demonstrated that the endogenous cellular homeostatic mechanisms are not efficient in metabolically compromised oocytes and early embryos cultured in vitro [23]. Once cellular stress is elicited, external antioxidant supplementation becomes essential to support development to the blastocyst stage and to safeguard embryo quality [23, 24].
In human IVF clinics, MT dysfunction in oocytes and embryos is increasingly recognized as a main factor that determines the success rate of ART. Early and late pregnancy losses have been linked directly or indirectly to mitochondria-related factors [25–28]. Infertile women who seek ART treatments may already have reduced systemic antioxidant capacity making their oocytes more prone to oxidative stress and MT dysfunction during the ART procedures. A particularly vital example is the deficiency in CoQ10, which is common in humans in their late 30s [29]. CoQ10 is an integral component of the MT electron transport system and one of the most effective lipid antioxidants. Its abundance and function limit the generation of free radicals within the MT. The concentration of CoQ10 in the follicular fluid is positively correlated with oocyte maturation, embryo grade, and pregnancy rate in women undergoing ART [30, 31]. Obesity as such is strongly linked with systemic oxidative stress which can influence the ovarian microenvironment [32]. In addition, people who suffer from metabolic syndrome and hypercholesterolemia are likely to be treated with statins which have also been linked with systemic reduction of CoQ10 levels as a side effect [33]. The effects of lipotoxicity-induced MT dysfunction and oxidative stress together with the reduced antioxidant capacity and limited cellular homeostatic machinery can significantly impact oocyte MT functions and put subsequent embryo development at risk.
It might be difficult to modulate MT CoQ10 content and antioxidant capacity in oocytes by dietary supplementation. Studies using radioactive tracing show a high uptake in spleen, liver, and white blood cells, while adrenals, ovaries, thymus, and heart accumulate lower concentrations [34]. In contrast, a recent paper showed that MitoQ (a mitochondria targeted formulation of CoQ10) could ameliorate ovarian oxidative stress and granulosa cell steroidogenic functions in vivo in a DHEA-induced PCOS mouse model [35]. On the other hand, in vitro supplementation with MitoQ is increasingly suggested to improve ART success rates. Yet, the safety and efficiency of treating oocytes with MitoQ are under-investigated. In vitro oocyte maturation (IVM) is increasingly used in human ART [36, 37] to minimize the risk of ovarian hyperstimulation syndrome (OHSS). OHSS is a potential risk of IVF, especially in obese women [38]. IVM may thus form a potential window during which oocytes retrieved from obese women can be treated with MitoQ to support their MT functions and minimize cellular stress levels before fertilization. In addition, the ART procedures per se involve many factors that can increase oxidative and mitochondrial stress such as handling, exposure to light, ICSI, and suboptimal pH, temperature, and media composition [39]. Supplementation of antioxidants during IVM may increase the tolerance against suboptimal physico-chemical conditions, resulting in a better developmental competence.
Unfortunately, from the clinical side, there is a paucity of evidence favoring the protective qualities of antioxidants during IVM [40]. Non-targeted antioxidants (such as Trolox) which exert their action at the cytoplasmic level are not effective in protecting oocyte developmental competence when supplemented during IVM in the presence of lipotoxic concentrations of PA, nor in rescuing embryo development of the exposed oocytes when supplemented during in vitro embryo culture (IVC) [41]. Mitochondria-targeted antioxidants such as MitoQ can be more effective in supporting oocyte developmental competence. MitoQ (mitoquinone) is composed of CoQ10 conjugated to a lipophilic cation, triphenylphosphonium (TPP+), which has an affinity for the negatively charged mitochondrial membrane and markedly increases the accumulation of CoQ10 within the mitochondria [42]. We have shown that MitoQ can significantly improve blastocyst yields and quality when added during IVC of embryos derived from metabolically compromised oocytes [24]. In contrast, the efficiency of MitoQ supplementation during IVM and its potential protection against lipotoxicity and oxidative stress are underexplored.
In the present study, our primary hypothesis was that MitoQ can protect the oocytes against lipotoxicity during maturation and alleviate subsequent impact on early embryo development. To test this hypothesis, we used our well-established bovine IVM model where oocytes were exposed to pathophysiologically relevant lipotoxic concentration of PA (150 μM), in the presence or absence of MitoQ (1 μM, as effectively used during IVC in a previous study [24]). In the initial experiments, MitoQ (at 1 μM) was found to negatively impact oocyte developmental competence. Therefore, we also hypothesized that exposure of oocytes to the cationic carrier of MitoQ (TPP) can exert cytotoxic effects. Accordingly, a dose-response test was performed for both MitoQ and TPP, separately. Finally, the protective effects of an optimized concentration of MitoQ for IVM (0.1 μM) was tested in the presence of PA. The impact on oocyte mitochondrial MMP, ROS level cleavage, and blastocyst development as well as transcriptomic regulation of cellular stress in the blastomeres is reported.
Methods
Experimental design
In the first set of experiments, MitoQ was added during bovine IVM at a concentration of 1 μM in the presence or absence of 150 μM of PA. A PA concentration of 150 μM is pathophysiologically relevant as measured in the FF of obese women [43] and validated in our previous studies [17, 18, 44]. A MitoQ concentration of 1 μM was effective during bovine embryo culture in our previous study [24]. Here, MitoQ 1 μM was found to be toxic for oocyte developmental competence. Therefore, a dose-response test was then performed for MitoQ and for its cationic carrier, TPP, separately at 0.0, 0.1, 0.5, and 1.0 μM each, to see if cytotoxicity is caused by the carrier and to check the effects at lower concentrations. The impact on oocyte mitochondrial MMP, ROS, and subsequent embryo development rates were examined. Lower concentrations of MitoQ or TPP only (0.1 μM and 0.5 μM) did not have a significant negative effect on mitochondrial functions and developmental competence of oocytes in the absence of a lipotoxic insult.
In the second stage of this study, the potential protective effects of MitoQ at a concentration of 0.1 μM were tested in the presence or absence of 150 μM of PA. TPP was also tested at the same concentration for comparison, to test if the beneficial effects are CoQ10-dependent. The effects on oocyte developmental competence, MMP, and ROS were examined. Finally, to check if MitoQ treatment during IVM can alleviate PA-induced cellular stress that normally persist during early embryo development, the expression of several cellular stress–related mRNA markers were examined in the resultant blastocysts.
The number of replicates and the number of oocytes per treatment per replicate used in each experiment are mentioned in the results. In vitro fertilization and embryo culture were performed following standard protocols in a solvent-free media in the absence of PA or MitoQ as described hereafter.
Collection of bovine cumulus oocyte complexes (COCs) from slaughterhouse ovaries
Bovine ovaries were collected from a local abattoir immediately after slaughter and transferred within 2–3 r to the laboratory in warm saline. Ovaries were rinsed in 70% ethanol and then in sterile saline containing kanamycin (125 μg/mL). Follicular fluid was aspirated from visible antral follicles (2–8 mm in diameter) with 18-G needles and 5-mL syringes and transferred to 15-mL conical tubes. The cellular precipitate was then mixed with sterile Wash-TALP media (Hepes-buffered Tyrode’s albumin lactate pyruvate media) and transferred to a searching dish. Only good-quality COCs with more than five layers of compact cumulus cells and dark homogenous ooplasm were selected under a stereomicroscope and further used in the experiments. Temperature throughout the collection and selection procedure was maintained at 37 °C either in a water bath or on warm stages.
In vitro maturation and treatments
Good-quality COCs were washed and incubated in 4-well dishes containing maturation media: TCM-199 media supplemented with gentamycin (50 μg/mL), sodium pyruvate (0.2 mM), cysteamine (0.1 mM), L-glutamine (0.4 mM), and murine epidermal growth factor (mEGF, 20 ng/ml) [19]. Bovine serum albumin (BSA, fatty acid free, 0.75%w/v) was also added as a carrier for PA. Mitoquinone (MitoQ, Cayman chemical, Sanbio B.V. Research & Diagnostics, Uden, Netherlands), its cationic carrier molecule, triphenylphosphonium (TPP), and PA were first dissolved in absolute ethanol (1000X stock solutions) and then added to the media as described in the experimental design [18]. Stock solutions were aliquoted under nitrogen gas, sealed and stored at −20 °C. An equivalent maximum final concentration of solvent was added in the control groups in all experiments (SCONT; 0.2% ethanol). This was previously proven not to influence developmental competence compared to solvent-free controls [17, 45]. COCs were matured in groups of 50±5 in 10-μL media per COC. Plates were equilibrated for at least 2 h before transferring the COCs. IVM incubation was for 24 h in 5% CO2 in humidified air at 38.5 °C.
Assessment of mitochondrial activity and intracellular reactive oxygen species in oocytes
Mitochondrial MMP intracellular ROS were determined in oocytes at 24 h of IVM (a random sample of eight to ten oocytes per treatment per replicate) using a combined fluorescence staining with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine iodide, Invitrogen) and CellROX™ Deep Red (Thermo Fisher) as validated by De Biasi, et al. [46] and described by Komatsu, et al. [47] and our previous study [18]. Briefly, oocytes were denuded of their surrounding cumulus cells by repeated pipetting in Wash-TALP. Denuded and partially denuded oocytes were incubated in Wash-TALP containing JC1 (5 μg/mL) and CellROX Deep Red (2.5 mM) (from 1000X stock solutions in DMSO) for 30 min at 37 °C. Then, they were washed twice and transferred to Wash-TALP droplets under equilibrated mineral oil in pre-warmed 35-mm glass bottom dishes. Stained oocytes were immediately examined under a Leica SP8 confocal microscope equipped with white laser source (Leica WLL) and enclosed in a pre-warmed chamber at 37 °C. Images of the oocytes or the surrounding few layers of CCs were acquired at excitation/emission 488/530 nm (green JC-1 monomers), 561/590 nm (yellow JC1-aggregates), and 644/665 nm (red, CellROX as a measure of ROS). The grayscale intensity was quantified using Image-J software. Mitochondrial activity (MMP) was calculated as a ratio of the grayscale intensity at 590 nm:530 nm. ROS content was presented as the grayscale intensity at 665 nm as such or as a ratio to active mitochondrial (intensity at 665/(intensity at 590/530)).
In vitro fertilization and in vitro embryo culture (IVC)
To determine embryo developmental competence, COCs were washed at 24 h of IVM and transferred to Fert-TALP medium containing 1 × 106 sperm/mL in 4-well plates (in pools of 80–100 COCs in 500 μL/well). For that, motile spermatozoa were selected from proven-fertile frozen bull semen by centrifugation through a Percoll gradient (45–90%) [45]. Straws from the same ejaculate was used in all experiments. COCs and sperms were co-incubated for 20 h in 5% CO2 in humidified air at 38.5 °C. Subsequently at 20 h or IVF, presumptive embryos were denuded by vortexing for 3 min and washed in Wash-TALP, then cultured in groups of 25±3 in 96-well plates in 75 μL/well of serum-free SOF medium containing 2% w/v BSA [18]. Embryos were incubated in 90% N2, 5% CO2, 5% O2, and 38.5 °C until day 8 post-fertilization (p.f.). IVF and IVC media were not supplemented with any treatments or solvent.
Embryo cleavage was assessed at 48 h p.f. by recording the proportions of cleaved embryos, good-quality embryos (with ≥ four cells and < 15% fragmentation) and fragmented embryos (with >15% fragmentation of the cellular mass). Blastocyst rates were recorded on days 7 and 8 p.f. and expressed as a proportion of the total number of oocytes or per cleaved embryos. Assessment was done under an inverted Olympus CKX41 microscope (Olympus, Aartselaar, Belgium) at 100× magnification.
Assessment of fertilization rate
In order to check if the effects induced by PA and MitoQ on cleavage and blastocyst rates are due to differences in fertilization rate, extra replicates were performed in which presumptive zygotes were collected at the end of IVF (24 h) for pronuclear staining. They were denuded of cumulus cells by vortexing, fixed in paraformaldehyde 4% (PFA 4%), and stained with Hoechst (1 μg/mL in PBS-PVP). Stained samples were mounted in DABCO, visualized under Olympus XI71 fluorescent microscope, and classified according to their nuclear morphology: the number of pronuclei or an arrest at metaphase I or II (MI, MII) or anaphase I (AI). At this stage, normal fertilized oocytes are expected to have two pronuclei.
Gene expression analysis
Day 8 blastocysts from every treatment were washed and transferred to a 1.5-mL vial in minimal volume of PBS, and immediately snap frozen in liquid nitrogen and stored at −80 °C. RNA extraction was carried out using PicoPureTM RNA Isolation Kit (Thermo Fisher Sci.). RNA concentration and integrity were examined using a NanoDropTM (Thermo) and an Agilent Bioanalyzer (RIN≥7). Total RNA was treated with DNase and reverse transcribed (50 ng RNA/sample) using Sensiscript RT kits (QIAGEN) following manufacturer instructions. Quantitative polymerase chain reaction (Real-Time PCR; qPCR) using SYBR Green was used to study the expression of genes related to oxidative stress (SOD2, GPx1, and CAT), mitochondrial UPR (HSP10 and HSP60), endoplasmic reticulum UPRer (ATF4 and ATF6), and mitochondrial biogenesis (TFAM). The quantification was normalized using the geometric mean of three housekeeping genes (18S, YWHAZ, and H2A), and the relative fold change of each gene was calculated using the 2^(-∆∆CT) method, as described by Pfaffl [48]. Primers were designed using Primer-Blast based on NCBI Reference Sequences of Bos taurus and are all intron flanking (supplementary table 1).
Statistical analysis
Statistical analyses were performed using IBM Statistics SPSS 26 (for Windows, Chicago, IL, USA) using data derived from at least three replicates. Categorical data, such as fertilization, cleavage, and blastocyst rates, were compared using a binary logistic regression model. Numerical data, cell counts, gene expression data, JC1, and CellROX staining quantification were checked for equal variance and normality of distribution, and means were compared using ANOVA. The interaction between treatment and replicate effects was tested and excluded from the final model if it was not significant (which was the case in all experiments). A Bonferroni post hoc test was performed to account for multiple familial comparisons. Data were considered significantly different at P values ≤ 0.05, and higher P values up to 0.1 were described as tendencies.
Results
Effects of high concentration of MitoQ (1 μM) during oocyte maturation in the presence or absence of metabolic stress conditions
Exposure of COCs to 150 μM of PA significantly reduced cleavage rate, proportion of good-quality embryos at 48 h, and blastocyst rates at day 8 (P<0.05). Unexpectedly, supplementation of 1 μM MitoQ during 24-h IVM was found to be toxic for oocyte developmental competence. Cleavage rate was significantly reduced, and the percentage of good-quality embryos (≥four cells at 48 h p.i.) and blastocysts (at day 8) were surprisingly very low compared to the control group (P < 0.05). Surprisingly, when added with PA during IVM, MitoQ (1 μM) increased the negative impact on development compared to PA only. Fig. 1
Fig. 1.
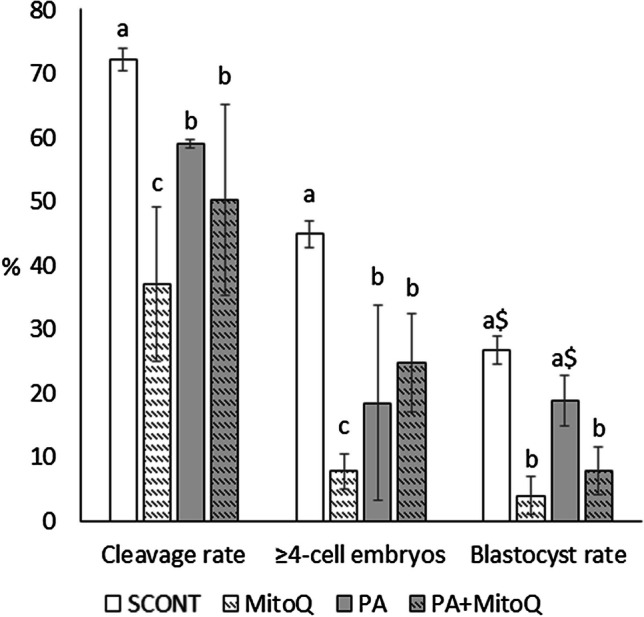
The effect of PA (150 μM) and/or MitoQ (1 μM) supplementation during IVM on early embryo development. Total cleavage and 4-cell-plus embryo rates were recorded at 48-h post-fertilization, while blastocyst rates were recorded at day 8. Bars with difference alphabets are significantly different at P<0.05. The $ sign indicates a tendency (P<0.1) compared to the corresponding control group. Data are shown as mean percentage ± SEM from three replicates using a total of 735 oocytes (157–198 oocytes per treatment)
Concentration-dependent effects of MitoQ and TPP during IVM on embryo development
We examined if the effects of MitoQ during IVM are concentration dependent and if the detrimental effects of 1 μM MitoQ on developmental competence observed above are caused by TPP. Treatment with MitoQ at 0.1 μM or 0.5 μM during IVM did not significantly affect embryo cleavage and development to the blastocyst stage compared to the non-supplemented solvent control (SCONT) (Fig. 2A). The negative impact of adding 1.0 μM of MitoQ was confirmed (P < 0.05). TPP was found to exert very similar concentration-dependent effects on cleavage and blastocyst rates (Fig. 2B). The negative effects at 0.5 μM and 1 μM of TPP were larger, since relatively less proportions of blastocysts could survive.
Fig. 2.

The effect of different concentrations of MitoQ (a) and TPP (b) (0, 0.1, 0.5, and 1.0 μM) during IVM on early embryo development. Bars with difference alphabets are significantly different at P<0.05. Data are shown as mean percentage ± SEM from three replicates each, using a total of 705 oocytes in the MitoQ experiment and 872 oocytes in the TPP experiment (165–187 oocytes per treatment)
Concentration-dependent effects of MitoQ and TPP during IVM on mitochondrial functions in oocytes
As a next step, we hypothesized that since TPP is a strong cationic compound it may interfere with mitochondrial functions at high concentrations and may also alter ROS production. Therefore, we tested the impact of adding different concentrations of MitoQ and TPP during IVM on mitochondrial MMP and intracellular levels of ROS.
Low concentrations of TPP only and MitoQ (at 0.1 and 0.5 μM) did not have any significant influence on MMP (Fig. 3A) and ROS (Fig. 3B) levels (P>0.1), whereas the highest concentration of TPP and MitoQ significantly reduced MMP. High concentration of TPP only, but not MitoQ, also significantly reduced ROS concentrations. The impact on MMP was higher than the impact on ROS; hence, an increased ROS per active mitochondria was noticed (P<0.05 for MitoQ 1 μM vs SCONT).
Fig. 3.
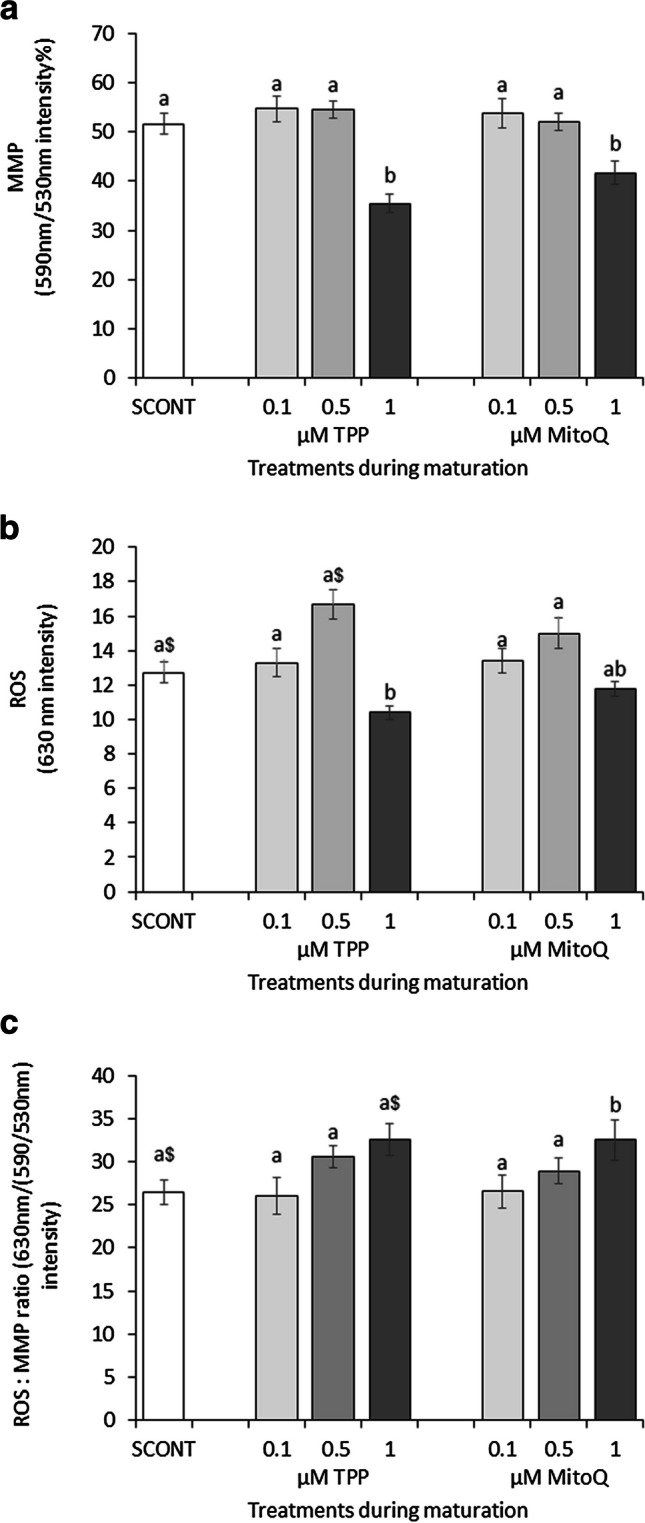
The effect of different concentrations of MitoQ and TPP (0 (solvent control, SCONT), 0.1, 0.5, and 1.0 μM) during IVM on a mitochondrial inner membrane potential (MMP) and b intracellular reactive oxygen species (ROS) concentrations in oocytes. This was determined using JC-1 and CellROX Deep Red staining, respectively, and confocal laser scanning microscopy. c The ratio of ROS per active mitochondria is also presented. Bars with difference alphabets are significantly different at P<0.05 within each treatment category (TPP or MitoQ) and compared to SCONT. The $ sign denotes a tendency (0.05< P <0.1) compared to the SCONT. Data are shown as mean percentage ± SEM from three replicates each, using on average 25 oocytes per treatment
Protective effects of MitoQ (0.1 μM) during oocyte maturation under metabolic stress conditions on oocyte developmental competence
Based on the previous experiment, MitoQ concentration of 0.1 μM was considered optimal. The next experiment aimed to test if MitoQ at 0.1 μM can protect the oocyte from the lipotoxic impact of PA. To limit the number of treatment groups, the effect of TPP was not tested further. Exposure of COCs to a high PA concentration (150 μM) during IVM in the absence of MitoQ significantly reduced total cleavage rate and the proportion of 4-cell plus embryos, and increased embryo fragmentation at 48 h. PA also decreased total blastocyst rates at day 8 (Table 1). On the other hand, supplementation of MitoQ in the PA+MitoQ group completely protected oocyte developmental capacity as it resulted in restored cleavage and blastocyst rates compared to the control (P>0.1). In contrast, TPP supplementation in the presence of PA (PA+TPP) did not have significant protective effects.
Table 1.
Early embryo development after exposure of COCs to high concentration of palmitic acid (PA; 150 μM) during IVM in the presence or absence of MitoQ or TPP (0.1 μM) compared to a solvent control (SCONT)
| SCONT | PA | PA+TPP 0.1 μM | PA+MitoQ 0.1 μM | |
|---|---|---|---|---|
| Total COCs (n) | 275 | 272 | 267 | 268 |
| Cleaved embryos (%) | 190 (69.0%)a | 154 (56.6%)b | 149 (55.8%)b | 186 (69.4%)a |
| 4-cell stage or more (%) | 137 (49.8%)a | 93 (34.1%)b | 97 (36.3%)b | 136 (50.7%)a |
| Fragmented embryos (%) | 11 (4%)a | 24 (8.8%)b | 22 (8.2%)b | 14 (5.2%)a |
| Blastocysts (% from total) | 66 (24%)a | 38 (13.9%)b | 54 (20.2%)ab | 65 (24.2%)a |
| Blastocysts (% from cleaved) | 66 (34.7%)a | 38 (24.6%)a* | 54 (36.2%)a | 65 (34.9%)a |
Data is presented as proportions (%). Different superscripts indicate significant differences at P<0.05 within the same row. *Significant from control if Bonferroni correction is not applied
Extra replicates were performed to check if the increase in early embryo development in the MitoQ-supplemented group originates from an increased fertilization rate. Presumptive zygotes were collected at the end of the IVF period (24 h), fixed in PFA 4%, and stained with Hoechst to assess their nuclear morphology. We found that in the PA-exposed group, the percentage of zygotes with two pronuclei was significantly reduced, and the percentage of non-fertilized oocytes (arrested at MII) was doubled compared to SCONT (P<0.05). Treatment with MitoQ during IVM alleviated these effects and resulted in about 20% increase in normal fertilization rate (Table 2).
Table 2.
Fertilization rate after exposure of COCs to high concentration of palmitic acid (PA; 150 μM) during IVM in the presence or absence of MitoQ (0.1 μM) compared to a solvent control (SCONT)
| 2PN | 1PN | >2PN | MII | MI-AI | Degenerated | |
|---|---|---|---|---|---|---|
| SCONT | 66/86 (76.7%)a |
5/86 (5.8%) |
6/86 (7%) |
8/86 (9.3%) ab$ |
1/86 (1.2%) |
0/86 (0%) |
| PA | 52/83 (62.7%)b |
3/83 (3.6%) |
9/83 (10.8%) |
16/83 (19.3%)b$ |
2/83 (2.4%) |
1/83 (1.2%) |
|
PA+MitoQ 0.1 μM |
81/100 (81%)a |
2/100 (2%) |
10/100 (10%) |
5/100 (5%)a |
2/100 (2%) |
0/100 (0%) |
Data is presented as proportions (%). Different superscripts indicate significant differences at P < 0.05 within the same column. PN, pronuclei; MII, metaphase II; MI, metaphase I; AI, anaphase I. Proportions with $ sign tend to be different (P=0.062)
Protective effects of MitoQ (0.1 μM) during oocyte maturation under lipotoxic conditions on mitochondrial activity and oxidative stress in oocytes
To test if the protective effects of MitoQ on oocyte developmental capacity is mediated through regulation of mitochondrial functions, MMP and ROS levels were measured in the treated oocytes at the end of maturation, after removal of the cumulus cells. As expected, in the absence of MitoQ, PA increased mitochondrial MMP and ROS per active mitochondria (P<0.05 compared to SCONT). These effects were mitigated by MitoQ (P>0.1 compared to SCONT) (Fig. 4).
Fig. 4.
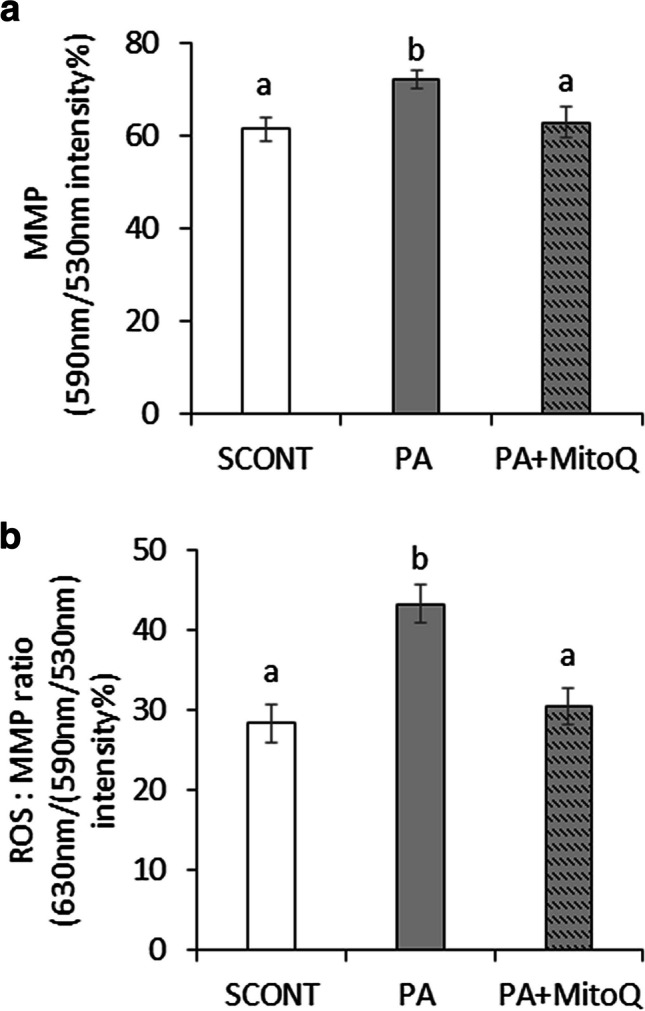
The effect of PA (150 μM) during IVM in the presence or absence of MitoQ (0.1 μM) on (a) mitochondrial MMP and (b) the ratio of ROS per active mitochondria in oocytes at 24 h of IVM. Bars with difference alphabets are significantly different at P<0.05. Data are shown as mean percentage ± SEM from three replicates each, using 20 oocytes per treatment
Protective effects of MitoQ (0.1 μM) supplementation on marker genes of cellular stress in blastocysts
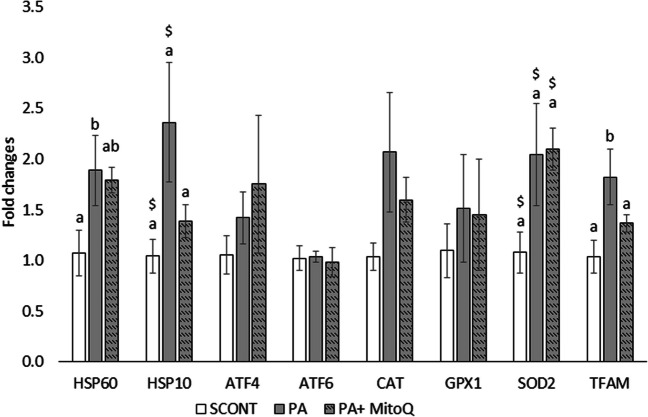
Finally, the expression of genes related to cellular stress was examined in day 8 blastocysts produced after PA exposure during IVM in the presence or absence of 0.1 μM MitoQ. Blastocysts produced from PA oocytes exhibited significantly increased expression of mitochondrial HSP60 and a tendency to a higher expression of the mitochondrial HSP10. These effects were partially normalized when MitoQ was also added during IVM (Fig. 5). PA had no effect on blastocyst expression of the ER stress markers ATF4 and ATF6 and only resulted in tendency for an increased SOD2 expression but not GPX1. SOD2 expression was not normalized with MitoQ. PA significantly increased TFAM expression, an effect which was alleviated by MitoQ.
Fig. 5.
Effect of PA exposure during IVM in the presence or absence of MitoQ (0.1 μM) on the mRNA expression of cellular stress–related genes in blastocysts at day 8. Data are derived from three replicates using extracts from at least ten blastocysts per treatment per replicate. Data were normalized based on the expression levels of three housekeeping genes (18S, H2A, and YWHAZ) and presented as fold changes relative to the control group. Bars with different alphabets at P < 0.05 based on the ANOVA results followed by an LSD pairwise comparisons. The $ sign denotes a tendency (0.05< P <0.1) compared to the SCONT
Discussion
The aim of this study was to test the effects of different concentrations of mitoquinone (MitoQ) and the potential cytotoxic effects of its carrier, TPP, during oocyte IVM. We also aimed to test the capacity of optimal concentration of MitoQ to protect the oocyte against PA-induced mitochondrial stress and to enhance developmental competence.
In the first experiments, using 1 μM of MitoQ during IVM significantly reduced cleavage and blastocyst rates, where only about 5% of the formed zygotes could survive. In contrast, in a previous study [24], using the same MitoQ concentration during IVC did not compromise developmental competence and effectively rescued blastocyst development of PA-exposed bovine oocytes. Here, we found that exposure to only TPP at 1 μM induced the same cytotoxic effects. As mentioned above, MitoQ is composed of the ubiquinone moiety of the endogenous antioxidant CoQ10 [49] loaded on a strong lipophilic cationic carrier; triphenylphosphonium (TPP), which enables accumulation of the ubiquinone at the mitochondrial matrix at a 100- to 500-fold higher concentration compared to the cytoplasm [50]. The inner mitochondrial membrane (IMM) possesses a distinctive negative potential that arises from the movement of protons required for ATP synthesis. The presence of immobile internal negative charges on the IMM favors the transport of cations. This negative charge is therefore used to enable the delivery of molecules conjugated to phosphonium cations as carriers [51]. However, this negative charge is also important to regulate the transportation of other cations across the mitochondria membranes, following the Nernstian behavior of the equilibrium of electrode potential [52]. This involves the primary intracellular cations, namely, potassium (K+) and sodium (Na+). The movement of these monovalent cations (osmolytes) is accompanied by the movement of water, contributing to the regulatory mechanism of mitochondrial volume. Furthermore, the negative charge of the IMM also regulates the transport of the divalent cations, calcium (Ca2+), and magnesium (Mg2+) into the mitochondrial matrix [52]. We may thus expect that any cationic overload can disturb mitochondrial functions. This can be demonstrated for example by the fact that calcium overload has been shown to inhibit mitochondrial oxidative phosphorylation when it exceeds a certain threshold [53]. This was associated with detrimental changes in mitochondrial MMP and bioenergetic pathways, leading to lower ATP production rates. In the dose-response experiments performed here, we illustrated that exposure of the oocytes to high MitoQ concentration (1 μM) resulted in a significant reduction in the MMP. The exact effect could be replicated by exposure to the same concentration of TPP. This suggests that the high concentration of TPP and MitoQ resulted in a cationic overload on the mitochondria. The reduction in MMP was detrimental for the oocyte developmental capacity as it significantly reduced cleavage and blastocyst rates. As mentioned in the introduction, MMP in oocytes is crucial for several processes during oocyte maturation and subsequent embryo development, including energy provision, cytoskeletal dynamics and intracellular transport, spindle formation and cell division, and phosphorylation and enzymatic activation. The reduction in MMP by TPP overload is expected to impact these vital processes, explaining the reduction in subsequent development rates.
In addition, calcium oscillations play a crucial role in oocyte cortical granule reaction, fertilization, and prevention of polyspermia. Upon fertilization, the intracellular concentration of calcium ion significantly increases in the oocyte, which initiates the cortical granule reaction to block polyspermy [54]. Therefore, in addition to MMP- and ATP-mediated effects, the reduction in cleavage rate after exposure to high MitoQ and TPP concentrations can be mediated by altering the mitochondrial transport of other intracellular cations like calcium in the oocyte. A previous study have demonstrated that TPP itself inhibits the Na /Ca2 exchanger in the mitochondrial membranes and thus increases intra-mitochondrial calcium levels. The degree of inhibition was greater with TPP compared to MitoQ [55]. While this has not been examined in oocytes, we see that the negative impact of TPP on development is more severe compared to MitoQ (Fig. 1).
We have previously shown that 1 μM of MitoQ supplementation during IVC (from 22 h to day 8 post fertilization) resulted in a significant reduction in ROS levels together with a significant increase in MMP both in the absence or presence of metabolic stress [24]. The contrast with the effects shown here during IVM (where both MMP and ROS are reduced) suggests that mitochondria are more sensitive to TPP and MitoQ treatments during oocyte maturation compared to embryos during in vitro culture. Despite the fact that oocyte mitochondria are immature and possess undeveloped cristae, it has been shown that 65% of the immature (GV) oocyte basal respiration is used for mitochondrial respiration and ATP production via oxidative phosphorylation [56]. This slightly decreases during maturation (to about 50%) [56] and further decreases during early embryo development. Early embryos are known to favor low mitochondrial activity (in accordance with the quite embryo hypothesis) and gradually switch to Warburg metabolism (aerobic glycolysis) [57]. A recent study in our laboratory using a Seahorse Bioanalyzer has shown that over 85% of the ATP production rate by denuded mature oocytes is linked to oxygen consumption (i.e., mitochondrial), whereas only about 15% could be linked to the increase in extracellular acidification rate (glycolytic) [20]. We have also noticed that the intensity of J-aggregate staining in mature oocytes (indicative for a relatively high MMP) is relatively higher than J-aggregate intensity at the 4-cell stage (see supplementary figure 1). Taken together, a higher dependence on mitochondrial bioenergetic functions during oocyte maturation may make the oocytes more sensitive to the TPP cationic overload compared to early embryos. In addition, if disruption of calcium homeostasis plays a role, as suggested above, this can be another reason why developmental competence is more affected if exposure to the cationic overload occur before fertilization.
It is also important to notice that MitoQ did not reduce ROS levels in the oocytes under control conditions (in the absence of lipotoxic metabolic stress). This was evident when using low concentrations of MitoQ (0.1 and 0.5 μM). The reduction in ROS observed with 1 μM MitoQ appears to be secondary to the reduction in MMP, as it could be replicated using TPP only. It is known that ROS production requires a high membrane potential [58]. The accumulation of positively charged TPP in the mitochondria can decrease the MMP, lowering the ROS production. This mechanism has been illustrated by both MitoQ and TPP in isolated mitochondria [50]. Nevertheless, it is expected that once the MitoQ accumulates within the mitochondria, the ubiquinone moiety should be reduced by accepting two electrons from complex ӏ or ӏӏ of the ETC, to be then converted to ubiquinol, which is the active antioxidant form [59]. Ubiquinol can scavenge or detoxify ROS, as it is oxidized back to ubiquinone. This process is repeated and has been shown to significantly reduce ROS production, inhibiting downstream lipid peroxidation and oxidative stress after MitoQ administration [59]. Here, this was not evident under stress-free conditions. Similarly, many studies have demonstrated that supplementation of other antioxidants, such as vitamin C, vitamin E, and Trolox, had no beneficial effects on oocyte maturation under standard conditions [60, 61]. High concentrations of antioxidants during IVM may even block spontaneous resumption of meiosis because a minimal concentration of ROS is needed to stimulate vital signaling and activate regulatory molecules required for development [62]. Nevertheless, we noticed that while 0.5 μM TPP increased ROS levels (potentially due to disruption of ion transport across the IMM), ROS levels in oocytes treated with 0.5 μM MitoQ were not altered. This shows that the ubiquinone moiety may still be able to attenuate ROS production under stress conditions.
Knowing that low concentrations of MitoQ (especially 0.1 μM) do not impact oocyte developmental competence and may attenuate ROS production under stress conditions, we could proceed with the second set of experiments to examine the potential protective effects of MitoQ against PA-induced lipotoxicity during oocyte maturation. PA is the most predominant FFA in the follicular fluid, and its abundance is negatively correlated with positive pregnancy outcomes in women undergoing ART [14, 63]. Several studies from our laboratory and by others have illustrated that in vitro exposure of bovine COCs during IVM to a pathophysiological concentration (150 μM) of PA resulted in an increased cumulus cell apoptosis, increased oocyte MMP and ROS, and reduced oocyte developmental competence [16–18]. Here, these detrimental effects of PA were confirmed, ultimately leading to about 10% reduction in blastocyst rate.
The toxic effects of PA on oocyte developmental competence can be mediated via several mechanisms. For example, PA has been associated with ceramide accumulation in oocytes, downregulating the AMPK/SIRT3 pathway and consequently inducing mitochondrial protein hyper-acetylation and mitochondrial defects [11]. In addition, a comprehensive proteomic analysis was performed in our laboratory to study the changes that occur in oocytes (after removal of their surrounding cumulus cells) after a 24-h exposure to 150 μM of PA during IVM. There, we could show that 30% of the differentially regulated proteins in the oocytes were mitochondrial proteins, and that the top canonical pathways affected were related to mitochondrial dysfunction, unfolded protein responses, and redox regulatory mechanisms [18]. Likewise, here we show that PA significantly increased MMP and almost doubled intracellular ROS levels in the exposed oocytes. The increased MMP is probably a metabolic response to the increased availability of FFAs (PA) which undergo β-oxidation and increase mitochondrial rates of oxidative phosphorylation [64]. Increased MMP and ROS were also reported in oocytes collected from inbred and outbred diet-induced obese mouse models after feeding an obesogenic diet [5, 12, 65]. This illustrates the relevance of our model to search for strategies to support oocyte quality in obese patients.
In addition, MMP and ROS levels are clearly connected, since the ETC in mitochondria is the main source of ROS production [58]. We repeatedly notice that the CellROX Deep Red staining used to quantify ROS is clearly colocalized with the mitochondria stained with JC-1 (see Suppl Fig. 1). This justifies our aim to use a mitochondrial targeted antioxidant to provide protection under these conditions. Low concentration of MitoQ (0.1 μM) could significantly reduce embryo fragmentation rate and completely restore the PA-induced reduction in cleavage and blastocyst rates. This beneficial effect could not be achieved using the same concentration of TPP, which shows that it was driven by the antioxidant capacity of MitoQ. We illustrated that the increased rates of embryo development to the blastocyst stage in the PA+MitoQ (0.1 μM) group originated from an increased fertilization rate. We could also illustrate that this beneficial effect is mediated at least in part through normalizing oocyte MMP and ROS levels, which were both significantly lowered in oocytes treated with PA+MitoQ compared to those exposed to PA only. Increased embryo fragmentation and reduced development are strongly linked to mitochondrial dysfunction and high ROS production in early embryos [66]. A previous study showed that the use of antioxidants that are not targeted to the mitochondria, such as Trolox, during IVM in the presence of PA, could not reduce MMP and ROS back to normal levels and did not rescue subsequent early embryo development [41]. In contrast, a recent study showed that supplementation with mitochondrial targeted antioxidants such as MitoQ and BGP-15 during IVM normalizes mitochondrial MMP and reverses the spindle and chromosomal abnormalities which occur in oocytes collected from reproductively aged female mice [67]. Deficiency of CoQ10 in oocytes is suggested to be the main reason for the high rates of aneuploidy in aged infertile patients (Bentov et al. 2014). Therefore, specific targeting of the mitochondria with antioxidants appears to be more effective.
Furthermore, we examined the expression of several cellular stress–related marker genes to check if cellular homeostasis in the blastocysts is completely reestablished or to identify carry-over effects that might have not been fully restored by the MitoQ treatment during IVM. As expected, exposure to elevated concentration of PA during IVM resulted in an increase or a tendency to increase mitochondrial UPR markers (HSP60 and HSP10), catalase, SOD2, and TFAM. Markers of ER stress were not affected. This is mostly in line with our previously published data [24]. mtHSP60 and mtHSP10 are known to be upregulated in conditions of mitochondrial stress due to altered MMP, altered OXPHOS, or accumulations of misfolded proteins [68]. These chaperones belong to the heat shock protein 70 (HSP70) family and are both necessary for protein import and to support protein folding [69]. Increased TFAM expression may imply that mitochondrial biogenesis might be altered [70]. The increased expression of SOD2 illustrates a persistently high level of oxidative stress during embryo development despite the fact that both fertilization and culture took place in FFA-free (i.e., standard, stress-free) culture condition. It has been previously suggested that early embryos do not have the machinery to establish cellular homeostasis [23], hence the need for exogenous support. Here, the protective effects of MitoQ (0.1 μM) during IVM were associated with a partial or complete normalization of the expression level of most of these markers showing no carryover persistent cell stress. Surprisingly, SOD2 expression was persistently high. Extending the MitoQ support also during IVC may further support embryo redox regulatory mechanisms.
While these results are generated in a bovine IVM model, the data provided here can be useful for human IVF application. Testing the safety and effectiveness of new compounds on human oocyte developmental competence is difficult due to the limited availability of biological material and due to the ethical concerns. Unlike murine models, bovine oocytes share several physiologic characteristics with human oocytes. For example, humans and cows are both mono-ovulatory species, having comparable oocyte characteristics, such as oocyte diameter, the time to reach the 2-cell stage, blastocysts and hatching, the duration of oocyte maturation, lipid content, energy metabolism, and a similar timing of genome activation [71, 72]. Nevertheless, translatability of the data provided here to the human IVF applications should only be done following the necessary validation studies.
In conclusion, the use of MitoQ during oocyte IVM should be done with caution. The cationic carrier of MitoQ appears to disturb oocyte mitochondrial bioenergetic functions and negatively impact early embryo development. An optimal concentration of MitoQ during IVM can support mitochondrial bioenergetic functions and protect the oocyte against oxidative stress, leading to enhanced developmental competence and lower cellular stress levels in the produced blastocysts. These effects are specific to the CoQ10 content of MitoQ since the carrier molecule TPP had no protective effects. On the other hand, MitoQ has no beneficial effects on oocytes in the absence of lipotoxicity-induced cellular stress.
Supplementary information
(DOCX 5.06 MB)
(DOCX 14.5 KB)
Acknowledgements
The authors would like to acknowledge ACAM, the microscopy core facility of the University of Antwerp, for the use of their microscopy facilities. The Leica SP8 microscope used in this publication was funded by a Medium-scale research infrastructure grant (GOH4216N) of the FWO.
Author contributions
WFAM contributed with the main project idea, conducted the experiments, analyzed the data, and wrote the manuscript. O.M-E performed the confocal microscopy and image analysis. IP helped with the optimization of microscopy and image data analysis. J.L.M.R supervised the project and critically read and edited the manuscript.
Data availability
Raw data files are available at Marei, Waleed (2023), “MitoQ during IVM”, Mendeley Data, V1, doi: 10.17632/htrsvbjfk5.1.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adhikari D, Lee IW, Yuen WS, Carroll J. Oocyte mitochondria-key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol Reprod. 2022;106:366–377. doi: 10.1093/biolre/ioac024. [DOI] [PubMed] [Google Scholar]
- 2.Wu LL, Norman RJ, Robker RL. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Reprod Fertil Dev. 2011;24:29–34. doi: 10.1071/RD11904. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–1612. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681–691. doi: 10.1242/dev.114850. [DOI] [PubMed] [Google Scholar]
- 5.Marei WFA, Smits A, Mohey-Elsaeed O, Pintelon I, Ginneberge D, Bols PEJ, Moerloose K, Leroy J. Differential effects of high fat diet-induced obesity on oocyte mitochondrial functions in inbred and outbred mice. Sci Rep. 2020;10:9806. doi: 10.1038/s41598-020-66702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smits A, Marei WFA, Moorkens K, Bols PEJ, De Neubourg D, Leroy J. Obese outbred mice only partially benefit from diet normalization or calorie restriction as preconception care interventions to improve metabolic health and oocyte quality. Hum Reprod. 2022;37:2867–2884. doi: 10.1093/humrep/deac226. [DOI] [PubMed] [Google Scholar]
- 7.Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, Chi M, Cusumano A, Scheaffer S, Moley KH. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 2016;16:1–8. doi: 10.1016/j.celrep.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Thouas GA, Trounson AO, Wolvetang EJ, Jones GM. Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol Reprod. 2004;71:1936–1942. doi: 10.1095/biolreprod.104.033589. [DOI] [PubMed] [Google Scholar]
- 10.Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10:23–32. doi: 10.1093/molehr/gah004. [DOI] [PubMed] [Google Scholar]
- 11.Itami N, Shirasuna K, Kuwayama T, Iwata H. Palmitic acid induces ceramide accumulation, mitochondrial protein hyperacetylation, and mitochondrial dysfunction in porcine oocytes. Biol Reprod. 2018;98:644–653. doi: 10.1093/biolre/ioy023. [DOI] [PubMed] [Google Scholar]
- 12.Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. doi: 10.1371/journal.pone.0010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:446–452. doi: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, Hashemi Karooee SF. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis. 2017;16:18. doi: 10.1186/s12944-016-0396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valckx SD, De Bie J, Michiels ED, Goovaerts IG, Punjabi U, Ramos-Ibeas P, Gutierrez-Adan A, Bols PE, Leroy JL. The effect of human follicular fluid on bovine oocyte developmental competence and embryo quality. Reprod Biomed Online. 2015;30:203–207. doi: 10.1016/j.rbmo.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Aardema H, Vos PL, Lolicato F, Roelen BA, Knijn HM, Vaandrager AB, Helms JB, Gadella BM. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol Reprod. 2011;85:62–69. doi: 10.1095/biolreprod.110.088815. [DOI] [PubMed] [Google Scholar]
- 17.Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, Bols PE, Leroy JL. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS One. 2011;6:e23183. doi: 10.1371/journal.pone.0023183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marei WFA, Van Raemdonck G, Baggerman G, Bols PEJ, Leroy J. Proteomic changes in oocytes after in vitro maturation in lipotoxic conditions are different from those in cumulus cells. Sci Rep. 2019;9:3673. doi: 10.1038/s41598-019-40122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmet KL, Van Hoeck V, Gagne D, Fournier E, Thakur A, O'Doherty AM, Walsh CP, Sirard MA, Bols PE, Leroy JL. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genomics. 2016;17:1004. doi: 10.1186/s12864-016-3366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meulders B, Leroy JLMR, De Keersmaeker L, Bols PEJ, Marei WFA. Inhibition of mitochondrial ATP production during <i>in vitro</i> maturation of bovine oocytes alters DNA methylation patterns in mature oocytes and resulting embryos. Reprod Fertil Dev. 2023;35:125–126. doi: 10.1071/RDv35n2Ab2. [DOI] [Google Scholar]
- 21.Desmet KLJ, Marei WFA, Richard C, Sprangers K, Beemster GTS, Meysman P, Laukens K, Declerck K, Vanden Berghe W, Bols PEJ, Hue I, Leroy J. Oocyte maturation under lipotoxic conditions induces carryover transcriptomic and functional alterations during post-hatching development of good-quality blastocysts: novel insights from a bovine embryo-transfer model. Hum Reprod. 2020;35:293–307. doi: 10.1093/humrep/dez248. [DOI] [PubMed] [Google Scholar]
- 22.Bruna de Lima C, Cristina Dos Santos E, Sirard MA. DOHaD: A menagerie of adaptations and perspectives: the interplay between early embryo metabolism and mitoepigenetic programming of development. Reproduction. 2023;166:F15–F26. doi: 10.1530/REP-22-0424. [DOI] [PubMed] [Google Scholar]
- 23.Marei WFA, Leroy J. Cellular stress responses in oocytes: molecular changes and clinical implications. Adv Exp Med Biol. 2022;1387:171–189. doi: 10.1007/5584_2021_690. [DOI] [PubMed] [Google Scholar]
- 24.Marei WFA, Van den Bosch L, Pintelon I, Mohey-Elsaeed O, Bols PEJ, Leroy J. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model. Hum Reprod. 2019;34:1984–1998. doi: 10.1093/humrep/dez161. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Seli E. Mitochondria as a biomarker for IVF outcome. Reproduction. 2019;157:R235–R242. doi: 10.1530/REP-18-0580. [DOI] [PubMed] [Google Scholar]
- 26.Hu X-Q, Zhang L. Hypoxia and mitochondrial dysfunction in pregnancy complications. Antioxidants. 2021;10:405. doi: 10.3390/antiox10030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AN, Wang X, Thomas DG, Tatum RE, Booz GW, Cunningham MW., Jr The role of mitochondrial dysfunction in preeclampsia: causative factor or collateral damage? Am J Hypertens. 2021;34:442–452. doi: 10.1093/ajh/hpab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv. 2007;62:335–347. doi: 10.1097/01.ogx.0000261644.89300.df. [DOI] [PubMed] [Google Scholar]
- 29.Miles MV, Horn PS, Tang PH, Morrison JA, Miles L, DeGrauw T, Pesce AJ. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;347:139–144. doi: 10.1016/j.cccn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Turi A, Giannubilo SR, Bruge F, Principi F, Battistoni S, Santoni F, Tranquilli AL, Littarru G, Tiano L. Coenzyme Q10 content in follicular fluid and its relationship with oocyte fertilization and embryo grading. Arch Gynecol Obstet. 2012;285:1173–1176. doi: 10.1007/s00404-011-2169-2. [DOI] [PubMed] [Google Scholar]
- 31.Akarsu S, Gode F, Isik AZ, Dikmen ZG, Tekindal MA. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J Assist Reprod Genet. 2017;34:599–605. doi: 10.1007/s10815-017-0882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballesteros-Guzman AK, Carrasco-Legleu CE, Levario-Carrillo M, Chavez-Corral DV, Sanchez-Ramirez B, Marinelarena-Carrillo EO, Guerrero-Salgado F, Reza-Lopez SA. Prepregnancy obesity, maternal dietary intake, and oxidative stress biomarkers in the fetomaternal unit. Biomed Res Int. 2019;2019:5070453. doi: 10.1155/2019/5070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deichmann R, Lavie C, Andrews S. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010;10:16–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Turunen M, Appelkvist EL, Sindelar P, Dallner G. Blood concentration of coenzyme Q(10) increases in rats when esterified forms are administered. J Nutr. 1999;129:2113–2118. doi: 10.1093/jn/129.12.2113. [DOI] [PubMed] [Google Scholar]
- 35.Kyei G, Sobhani A, Nekonam S, Shabani M, Ebrahimi F, Qasemi M, Salahi E, Fardin A. Assessing the effect of MitoQ(10) and Vitamin D3 on ovarian oxidative stress, steroidogenesis and histomorphology in DHEA induced PCOS mouse model. Heliyon. 2020;6:e04279. doi: 10.1016/j.heliyon.2020.e04279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong X, Li H, Zhao Y. The improvement and clinical application of human oocyte in vitro maturation (IVM) Reprod Sci. 2022;29:2127–2135. doi: 10.1007/s43032-021-00613-3. [DOI] [PubMed] [Google Scholar]
- 37.Smitz JE, Thompson JG, Gilchrist RB. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med. 2011;29:24–37. doi: 10.1055/s-0030-1268701. [DOI] [PubMed] [Google Scholar]
- 38.Sun B, Ma Y, Li L, Hu L, Wang F, Zhang Y, Dai S, Sun Y. Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front Endocrinol (Lausanne). 2020;11:615957. doi: 10.3389/fendo.2020.615957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 2006;86:503–512. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 40.Budani MC, Tiboni GM. Effects of supplementation with natural antioxidants on oocytes and preimplantation embryos. Antioxidants (Basel). 2020;9 10.3390/antiox9070612. [DOI] [PMC free article] [PubMed]
- 41.De Bie J, Smits A, Marei WFA, Leroy J. Capacity of Trolox to improve the development and quality of metabolically compromised bovine oocytes and embryos invitro during different windows of development. Reprod Fertil Dev. 2021;33:291–304. doi: 10.1071/RD20194. [DOI] [PubMed] [Google Scholar]
- 42.Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol. 2013;1:86–93. doi: 10.1016/j.redox.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valckx SD, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, Bols PE, Leroy JL. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol. 2014;12:13. doi: 10.1186/1477-7827-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, Genicot G, Van Soom A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–495. doi: 10.1530/rep.1.00735. [DOI] [PubMed] [Google Scholar]
- 45.Marei WFA, De Bie J, Mohey-Elsaeed O, Wydooghe E, Bols PEJ, Leroy J. Alpha-linolenic acid protects the developmental capacity of bovine cumulus-oocyte complexes matured under lipotoxic conditions in vitro. Biol Reprod. 2017;96:1181–1196. doi: 10.1093/biolre/iox046. [DOI] [PubMed] [Google Scholar]
- 46.De Biasi S, Gibellini L, Cossarizza A. Uncompensated polychromatic analysis of mitochondrial membrane potential using JC-1 and Multilaser Excitation. Curr Protoc Cytom. 2015;72 10.1002/0471142956.cy0732s72. [DOI] [PubMed]
- 47.Komatsu K, Iwase A, Mawatari M, Wang J, Yamashita M, Kikkawa F. Mitochondrial membrane potential in 2-cell stage embryos correlates with the success of preimplantation development. Reproduction. 2014;147:627–638. doi: 10.1530/REP-13-0288. [DOI] [PubMed] [Google Scholar]
- 48.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatti JS, Kumar S, Vijayan M, Bhatti GK, Reddy PH. Therapeutic strategies for mitochondrial dysfunction and oxidative stress in age-related metabolic disorders. Prog Mol Biol Transl Sci. 2017;146:13–46. doi: 10.1016/bs.pmbts.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Cocheme HM, Kelso GF, James AM, Ross MF, Trnka J, Mahendiran T, Asin-Cayuela J, Blaikie FH, Manas AR, Porteous CM, Adlam VJ, Smith RA, et al. Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion. 2007;7(Suppl):S94–102. doi: 10.1016/j.mito.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Pala L, Senn HM, Caldwell ST, Prime TA, Warrington S, Bright TP, Prag HA, Wilson C, Murphy MP, Hartley RC. Enhancing the mitochondrial uptake of phosphonium cations by carboxylic acid incorporation. Frontiers. Chemistry. 2020:8. 10.3389/fchem.2020.00783. [DOI] [PMC free article] [PubMed]
- 52.Austin S, Nowikovsky K. Mitochondrial osmoregulation in evolution, cation transport and metabolism. Biochim Biophys Acta Bioenerg. 2021;1862:148368. doi: 10.1016/j.bbabio.2021.148368. [DOI] [PubMed] [Google Scholar]
- 53.Strubbe-Rivera JO, Schrad JR, Pavlov EV, Conway JF, Parent KN, Bazil JN. The mitochondrial permeability transition phenomenon elucidated by cryo-EM reveals the genuine impact of calcium overload on mitochondrial structure and function. Sci Rep. 2021;11:1037. doi: 10.1038/s41598-020-80398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrinol. 2011;9:149. doi: 10.1186/1477-7827-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leo S, Szabadkai G, Rizzuto R. The mitochondrial antioxidants MitoE(2) and MitoQ(10) increase mitochondrial Ca(2+) load upon cell stimulation by inhibiting Ca(2+) efflux from the organelle. Ann N Y Acad Sci. 2008;1147:264–274. doi: 10.1196/annals.1427.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugimura S, Matoba S, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Konishi K, Imai K. Oxidative phosphorylation-linked respiration in individual bovine oocytes. J Reprod Dev. 2012;58:636–641. doi: 10.1262/jrd.2012-082. [DOI] [PubMed] [Google Scholar]
- 57.Krisher RL, Prather RS. A role for the Warburg effect in preimplantation embryo development: metabolic modification to support rapid cell proliferation. Mol Reprod Dev. 2012;79:311–320. doi: 10.1002/mrd.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei YH, Lu CY, Wei CY, Ma YS, Lee HC. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Phys. 2001;44:1–11. [PubMed] [Google Scholar]
- 59.Kelso GF, Porteous CM, Hughes G, Ledgerwood EC, Gane AM, Smith RA, Murphy MP. Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann N Y Acad Sci. 2002;959:263–274. doi: 10.1111/j.1749-6632.2002.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 60.Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in vitro maturation. Biol Reprod. 2001;65:1800–1806. doi: 10.1095/biolreprod65.6.1800. [DOI] [PubMed] [Google Scholar]
- 61.Takami M, Preston SL, Toyloy VA, Behrman HR. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. Am J Phys. 1999;276:E684–E688. doi: 10.1152/ajpendo.1999.276.4.E684. [DOI] [PubMed] [Google Scholar]
- 62.Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14:345–357. doi: 10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valckx SD, Van Hoeck V, Arias-Alvarez M, Maillo V, Lopez-Cardona AP, Gutierrez-Adan A, Berth M, Cortvrindt R, Bols PE, Leroy JL. Elevated non-esterified fatty acid concentrations during in vitro murine follicle growth alter follicular physiology and reduce oocyte developmental competence. Fertil Steril. 2014;102:1769–1776. doi: 10.1016/j.fertnstert.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Liverini G. Skeletal muscle oxidative capacity in rats fed high-fat diet. Int J Obes Relat Metab Disord. 2002;26:65–72. doi: 10.1038/sj.ijo.0801844. [DOI] [PubMed] [Google Scholar]
- 65.Smits A, Marei WFA, De Neubourg D, Leroy J. Diet normalization or caloric restriction as a preconception care strategy to improve metabolic health and oocyte quality in obese outbred mice. Reprod Biol Endocrinol. 2021;19(166) 10.1186/s12958-021-00848-4. [DOI] [PMC free article] [PubMed]
- 66.Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- 67.Al-Zubaidi U, Adhikari D, Cinar O, Zhang QH, Yuen WS, Murphy MP, Rombauts L, Robker RL, Carroll J. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum Reprod. 2021;36:771–784. doi: 10.1093/humrep/deaa300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu F, Liu F. Mitochondrial stress: a bridge between mitochondrial dysfunction and metabolic diseases? Cell Signal. 2011;23:1528–1533. doi: 10.1016/j.cellsig.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang SG, Dimitrova MN, Ortega J, Ginsburg A, Maurizi MR. Human mitochondrial ClpP is a stable heptamer that assembles into a tetradecamer in the presence of ClpX. J Biol Chem. 2005;280:35424–35432. doi: 10.1074/jbc.M507240200. [DOI] [PubMed] [Google Scholar]
- 70.Antelman J, Manandhar G, Yi YJ, Li R, Whitworth KM, Sutovsky M, Agca C, Prather RS, Sutovsky P. Expression of mitochondrial transcription factor A (TFAM) during porcine gametogenesis and preimplantation embryo development. J Cell Physiol. 2008;217:529–543. doi: 10.1002/jcp.21528. [DOI] [PubMed] [Google Scholar]
- 71.Campbell BK, Souza C, Gong J, Webb R, Kendall N, Marsters P, Robinson G, Mitchell A, Telfer EE, Baird DT. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod Suppl. 2003;61:429–443. [PubMed] [Google Scholar]
- 72.Santos RR, Schoevers EJ, Roelen BA. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol. 2014;12:117. doi: 10.1186/1477-7827-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 5.06 MB)
(DOCX 14.5 KB)
Data Availability Statement
Raw data files are available at Marei, Waleed (2023), “MitoQ during IVM”, Mendeley Data, V1, doi: 10.17632/htrsvbjfk5.1.