Abstract
The globalization in business and tourism becomes crucial more and more for the economical sustainability of local communities. In the presence of an epidemic outbreak, there must be such a decision on the policy by the host community as whether to accept visitors or not, the number of acceptable visitors, or the condition for acceptable visitors. Making use of an SIRI type of mathematical model, we consider the influence of visitors on the spread of a reinfectious disease in a community, especially assuming that a certain proportion of accepted visitors are immune. The reinfectivity of disease here means that the immunity gained by either vaccination or recovery is imperfect. With the mathematical results obtained by our analysis on the model for such an epidemic dynamics of resident and visitor populations, we find that the acceptance of visitors could have a significant influence on the disease’s endemicity in the community, either suppressive or supportive.
Keywords: Epidemic dynamics, Mathematical model, Ordinary differential equations, Public health, Reinfection
Introduction
As the world becomes more of a global village with advances in technology and easier accessibility to different places, it is very crucial to consider side effects like the spread of diseases (Cossar 1994). The history of man is replete with stories of epidemics invading groups of people, sometimes resulting in mortality. In the long run, such diseases can disappear and recur in the future or become less deadly due to people getting immune. Some notable epidemics in history include the “Spanish” flu (1918–1919), the Black Deaths (1346–1350) which invaded Europe from Asia and recurred for three decades afterwards before getting eliminated (Brauer 2017), the SARS beginning with some infection on an airplane in 2003 (Wang and Wu 2018), and today’s pandemic situation with the COVID-19 since mid-December 2019 after the outbreak in China (CDC 2022; ECDC 2022; NIID 2022; WHO 2022). The exponentially increasing number of cases and large-scale spread of the emerging virus about the COVID-19 are being initiated and promoted by the human mobility in global and local scales (Walters et al. 2018; Du Toit 2020; Liu and Saif 2020; Munster et al. 2020; Phan et al. 2020; Ramaswamy et al. 2021; Zhang et al. 2022).
There have been many investigations concerning the effect of a people’s displacement due to social and political unrest as well as the natural migration of disease vectors to new areas on the epidemic outbreak, and especially conducted have been many theoretical/mathematical studies taking into account the possibility of individuals becoming infective during transportation and contributing significantly to transport-related infection (see Wilson (2010) and references therein; especially for the SARS virus transmission, see Wang (2014)). Not only the particular transportation with a long travel, but also the human quotidian mobility as a common phase of the human activity can be considered as one of relevant factors that could cause the spread of a transmissible disease such as influenza (WHO 2018; Seno 2020). So is the case of today’s pandemic of COVID-19 in local regions of every country (CDC 2022; ECDC 2022; WHO 2022). In the work presented by Parikh et al. (2013), a synthetic population model of the Washington DC metro area was extended to include leisure and business travelers classified as transients. The final size of the epidemic among residents was found to be remarkably higher when transients were included in the simulation of a flu-like disease outbreak. In considering the emerging diseases of wildlife, Tompkins et al. (2015) show that the key drivers of such diseases are agents from domestic sources and human-assisted exposure to infectious agents from wild populations. Talking about swine fever otherwise known as hog cholera, wild boar populations are known to serve as reservoir for the disease, thereby constituting a great challenge for domestic pig farmers, veterinarians and other stakeholders (Mur et al. 2018; Postel et al. 2018).
In this paper, we shall focus on the influence of temporal visitors in a community according to the endemicity of a transmissible disease spreading over the community. Since the globalization in business and tourism becomes crucial more and more for the economical sustainability of local communities, the condition about the acceptance of visitors would be an important part of the community’s policy for the public health about a spreading transmissible disease in and out of it. There must be such a decision on the policy by the host community as whether to accept visitors or not, the number of acceptable visitors, and the condition for acceptable visitors. Actually the importance of such a policy on the tourism regulation has been recognized more and more in the post-COVID-19 period (Rastegar et al. 2021; Volgger et al. 2021; Yan et al. 2021; Jones 2022; Okafor and Yan 2022). Making use of a mathematical model modifying the basic SIR model incorporating the regulated acceptance of an amount of temporal visitors in the community, which may be called an SIRI type of mathematical model, we shall try to consider the influence of visitors on the spread of a reinfectious disease in a community, especially assuming that a certain proportion of visitors are immune at the entry into the community.
The reinfectivity of disease in this paper means that the immunity gained by either vaccination or recovery is imperfect. For a spreading transmissible disease accompanied with a reinfectivity, the acceptance of visitors must influence the endemicity of such a disease in the community. Then the community’s policy must take account of the reinfection risk for both of residents and visitors. Actually there are transmissible diseases with a reinfectivity, including influenza (Davies et al. 1984; Hay et al. 2001; Earn et al. 2002; Price et al. 2022; Wang et al. 2022), pertussis (Hethcote 1999; van Boven et al. 2000), Lyme disease (Nadelman et al. 2012), hand, foot and mouth disease (Zhang et al. 2019), malaria (Arias et al. 2022; Rehman et al. 2022), tuberculosis (Vynnycky and Fine 1997; Horsburgh et al. 2022; Qiu et al. 2022), Ebola virus disease (MacIntyre and Chughtai 2016; Agusto 2017), chronic lung diseases (Yum et al. 2014), invasive pneumococcal disease (Lipsitch 1997), meningococcal disease (Gupta and Maiden 2001), and COVID-19 (Crawford 2022; Kumar et al. 2020; Le Page 2022; Mensah et al. 2022; Nguyen et al. 2022; Ren et al. 2022; Saad-Roy et al. 2022; Salzer et al. 2022; Shaheen et al. 2022), although the reinfectivity has been still requiring scientific researches to understand its kinetics and other nature.
According to Chowell et al. (2016), it is crucial to formulate reliable models that embody the basic transmission characteristics of specific pathogens and social scenarios. They further stated that improved models are required to capture the variation in early growth dynamics of real epidemics in order to gain better understanding of the dynamics as they reviewed trends in modeling and classifying early epidemic progression. Recently the mathematical epidemic dynamics models are being used to estimate or evaluate some epidemiological parameters and to predict the temporal variation in the morbidity about a spreading disease, making use of epidemiological data (Siettos and Russo 2013). So are particularly those on the COVID-19 spread (for example, Athayde and Alencar 2022; Kobayashi et al. 2020; Lin et al. 2022; Musa et al. 2022 ), however, this is not the case in our paper.
We are going to try to shed a light on the theoretical side about the influence of temporal visitors on the epidemic dynamics with a transmissible disease with a reinfectivity, since the acceptance of visitors under such an epidemic dynamics with the risk of reinfection could be a crucial factor to influence the endemicity in the community which must take a policy about the acceptance (Dansu and Seno 2019; Crawford 2022; Salzer et al. 2022). We shall consider one of the simplest epidemic dynamics models for a transmissible disease with reinfectivity, which is an SIRI type of mathematical model, taking into account the influence of temporal visitors accepted by a community. With the mathematical results obtained by our analysis on the model, it will be implied that the acceptance of visitors could have a significant influence on the disease’s endemicity in the community, either suppressive or supportive, depending on the risk of reinfection and the nature of accepted visitors.
Assumptions
We consider the spread of a transmissible disease during a short-term period, that is, a season after the community starts to accept visitors from the outside, satisfying the following assumptions on the epidemic dynamics:
The demographic change in the resident population is negligible in the season.
The fatality of disease on the resident and visitor populations is negligible in the season.
The community starts the acceptance of a number of temporal visitors from the outside in the season after a transmissible disease has already invaded in it.
The entry flow of visitors is constant, that is, the net entry rate is constant independently of time.
The exit of visitors from the community follows a constant per capita exit rate.
No infected visitor is accepted by the community (i.e., the perfect quarantine), so that every accepted visitor is susceptible or immune to the disease at the entry into the community.
A given proportion of visitors is immune at the entry into the community.
Only the susceptible residents can get the vaccination to become immune, and it is not available for any visitor staying in the community.
Immune visitor has a possibility to get reinfected (i.e., the imperfect or partial immunity) during its stay in the community, the same as the immune resident does.
Infected visitor has the same exit rate as the susceptible visitor, that is, we neglect any influence of the infection on the visitor's stay in the community.
Assumption H1 indicates a time-independent constant size of resident population during the season in which the epidemic dynamics is going on. We then ignore the death due to the transmissible disease under consideration in the epidemic dynamics too, as indicated by the assumption H2. Assumption H3 indicates that the community accepts the visitors, even undergoing the spread of a transmissible disease, since the fatality of the disease is negligible with the assumption H2. No disease invasion with the visitors is assumed, as indicated by the assumption H6. From the assumptions H4 and H6, the community carries out the perfect regulation for the visitors at the entry concerning the entry number and the quarantine. Assumption H5 mathematically means that the exit of a visitor from the community follows the homogeneous Poisson process. In a model with ordinary differential equations, it can be introduced with a constant exit rate per visitor. Assumption H7 is to reflect the situation of public health out of the community, applying the mean-field approximation for the proportion of immune visitors at the entry.
Since we assume that the community undergoes the disease spread, the assumption H8 gives the existence of a vaccination program for the residents, while it is not applied to the visitors. However, since the disease is reinfectious as assumed by the assumption H9, the immunity obtained by the vaccination or the recovery from the disease works only to reduce the risk of reinfection. Hence the state transition in terms of the disease follows the susceptible–infective–recovered/immunized–infective (SIRI) structure in our modeling, as used for example in Gomes et al. (2004, 2005), Gökaydin et al. (2007), Stollenwerk et al. (2007), Martins et al. (2009), Pinto et al. (2010), Song et al. (2011), Georgescu and Zhang (2013), Guo et al. (2014), Pagliara et al. (2018), Buonomo (2020), Ghosh et al. (2020), Wang (2021), Srivastava et al. (2022).
Remark that the assumed reinfection is not caused by the waning or loss of immunity, which must take a certain period after getting it by the infection or vaccination. As already mentioned in the introduction section, we assume instead the imperfectness of immunity obtained by the infection or vaccination. Hence we do not introduce any specific period or time scale to get reinfected after getting the immunity. Since the infection or vaccination must generate an immunity against the disease, the assumption H9 indicates that the immunity is imperfect or partial against the infection, for example, due to the multiplicity of pathogen types (e.g., mutated variants) (Gökaydin et al. 2007; Wang et al. 2022). Because the cross-immunity is well-known for such similar pathogens by the antigen for a type of pathogen, the reinfection may be suppressed or fail to induce an effective symptom to reproduce and discharge the pathogen to the environment.
For a simplification, the assumption H10 indicates that the exit of visitor is independent of whether the visitor is infected or not during the stay in the community. This assumption would be appropriate when the expected duration of the visitor is sufficiently shorter than the latent period, whereas it may be less appropriate when it is long. As assumed by the assumption H2, we consider a transmissible disease with little serious symptom, so that the assumption H10 would be applicable for visitors infected by such a disease.
Model
Generic model
With these assumptions given in the previous section, we shall consider the following model of ordinary differential equations (Fig. 1):
| 1 |
| 2 |
where , , and are the subpopulation sizes of susceptible, infective, and immune visitors respectively. Similarly , , and are the corresponding subpopulation sizes about the residents. The population sizes of residents and visitors staying in the community are denoted by and respectively. The resident population size N is constant independently of time t, as seen from for any t by the system (2). From the assumption H3 in Sect. 2, the community starts the acceptance of visitors from the outside in the considered season after a transmissible disease has already invaded in it. The visitor population size m could be reasonably assumed to be less than the population size of residents N: , whereas we shall not specifically assume so but consider the mathematically general case of m in the subsequent sections without any constraint except for . On the other hand, as given in Sect. 3.2, we will take an assumption on the visitor population size m accompanying with a confinement for the net entry rate of visitors .
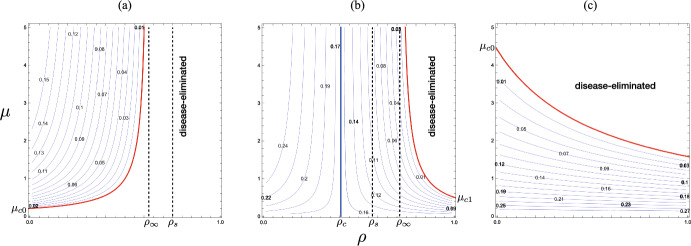
Fig. 1.
Scheme of the model for the epidemic dynamics in a community accepting temporal visitors, given by the system of (1) and (2)
All parameters are positive. Parameter is the proportion of immune visitors at the entry (). Proportion of visitors is susceptible at the entry. Parameter q is the per capita exit rate of visitor. Thus the expected duration of a visitor’s stay in the community is given by 1/q.
Parameter is the reinfection coefficient for immune resident and visitor, while is the infection coefficient for susceptible ones. Then the infection forces for the susceptible individual and the immune individual are respectively given by and by for both resident and visitor. Remark that, in the setup for our modeling, the visitors do not form any specific subcommunity distinct from the resident population. From the assumption H3, they are temporal visitors for tourism, business etc. For this setup, we could assume that most of visitors are independent of the others. Thus, for a mathematical simplification, the influence of their movement on the epidemic dynamics is introduced in the epidemic dynamics by the mean-field approximation. Further, although the visitors’ contribution to the infection forces would be different from the residents’ one because of the difference in the mobility/behevioral pattern, the infection forces have their same contributions in our modeling here. This modeling may be regarded as an oversimplification, though we think that our model would still worth being considered to get a cue for the discussion about the influence of visitors on the epidemic dynamics within a community.
From the assumption H9 in Sect. 2, our modeling assumes that the immunity is imperfect or partial against the infection. Because of the cross-immunity by the obtained antigen, we reasonably assume that in our model, so that the reinfection coefficient is not beyond the coefficient for susceptible . That is, the infection after the vaccination or recovery from the disease generally has a smaller likelihood than that for the susceptible. For the extremal case of , the vaccination or recovery does not work at all to reduce the risk of reinfection. For , the recovery and vaccination give the perfect immunity so that there is no likelihood of reinfection. Thus the parameter means an index for the likelihood of reinfection after the recovery or vaccination, so that it can be regarded as an index for the risk of reinfection. Remark here again that the reinfection in our modeling is assumed to be not due to the waning of immunity (like for the SIRS models) but due to the imperfect immunity, and hence also the vaccinated individual has a risk to get infected, as introduced by the assumption H9.
Parameter is the recovery rate of an infective individual, and the recovered individual gets immunity, which is however imperfect. Only the susceptible residents can get the vaccination, with rate , and it is not available for any visitor staying in the community. Since the vaccination is imperfect from the assumption H9, it works to reduce the risk of infection but is unable to protect the vaccinated individual from the infection.
Assumption for the visitor population size in the community
According to the dynamics for the visitor population (1), we have
where is the visitor population size at time t, the net entry rate of visitors, and q the per capita exit rate of visitor. Now let us consider the stationary situation with respect to the temporal change of visitor population size. This means that the number of visitors is assumed to be stationary, which may be regarded as a consequence of the regulation of their entry by the community, following the assumptions H4 and H6 given in Sect. 2. Therefore we mathematically assume the situation to satisfy that . Hence we put , and hereafter treat the visitor population size m as a positive constant.
The Initial Condition
Following the assumption of the stationary visitor population size with as given in Sect. 3.2, we have the following dynamics for the visitor population at the disease-free state:
where means the subpopulation size of immune visitors in the disease-free community. It can be easily found that this dynamics results in an eventual approach to the equilibrium state such that as for any non-negative initial condition with and . For this reason, let us assume the following initial condition for the epidemic dynamics with the model given by the system of (1) and (2):
| 3 |
where (a positive constant) with , and . This initial condition defines the situation when the community starts the acceptance of visitors from the outside, even under the existence of disease in it. The setup of this initial condition as our modeling follows the assumption H3 in Sect. 2.
Basic Reproduction Number
In the biological/epidemiological context, the basic reproduction number is defined as the expected number of new cases of infection caused by an infective individual within a population consisting of susceptible contacts only (for a useful review about the definition, the translation, and the practical application, see Delamater et al. (2019)). Such a situation can never occur for the epidemic dynamics in reality or even by any mathematical model. This is because the secondary infection itself changes the situation of population where the disease spreads. Increase of infectives must reduce the likelihood of the contact between an infective and an susceptible since the likelihood to contact the other infectives becomes less negligible. For this reason, the basic reproduction number must be mathematically defined to match the above conceptual definition in the biological/epidemiological context. Briefly saying, it is mathematically defined as the supremum of the expected number of new cases of infection caused by an infected individual at the stage of disease invasion in the community (Seno 2022). Following this definition in a biological/epidemiological sense, a mathematical theory can be used to derive the basic reproduction number about the mathematical model of epidemic dynamics, for example, as the spectral radius of a specific matrix, called the “next generation matrix”, for a system of ordinary differential equations governing an epidemic dynamics (see Diekmann et al. (2013) for a complete reference, or see Lewis et al. (2019) or van den Driessche (2017) for the further review). The choice of the derivation way of may depend on the nature of the model. As well known on the nature of the basic reproduction number, the invasion of a transmissible disease in the community is successful if , while it fails if . In this context, the invasion success means the increase of infectives in the early stage of the disease spread after a sufficiently small number of infectives appear in the community, and the invasion failure implies the decrease.
For our model given by the system of (1) and (2), we can derive the following formula of the basic reproduction number (Appendix A):
| 4 |
where , and for a convenience in the following arguments, we define , which is the basic reproduction number for the community when no visitor comes in (i.e., ). Note that this basic reproduction number is fundamental for the epidemic dynamics in the community after it starts the acceptance of visitors.
From the formula (4), we can immediately find that the basic reproduction number is less than 1 independently of the nature of accepted visitors if . Hence, if the disease fails its invasion in the community with before starting the acceptance of visitors, the number of infectives in the community cannot turn to increase in the early period after the acceptance of visitors starts. As we will see in the later sections of the analysis on our model, this is valid only in the early period after the acceptance of visitors starts.
As for the dependence of on the nature of accepted visitors, we note that is monotonically decreasing in terms of when the visitors contain some immune ones (i.e., ). Moreover, becomes smaller as the proportion of immune visitors at the entry gets larger. If any visitor is susceptible, that is, when , there is no contribution of the visitors to the basic reproduction number , that is, .
As an extremal case, if the immunity gained by the vaccination or recovery from the disease does not work at all to reduce the risk of reinfection, that is, if , the basic reproduction number is independent of the acceptance of visitors. This is easily understandable, since the reinfection is regarded as the same as the infection for the susceptible so that the immune individual is regarded as equivalent to the susceptible according to the epidemic dynamics when . Such an extreme case may be regarded as corresponding to an SIS type of the epidemic dynamics, where the state transition in terms of the disease follows the susceptible–infective–susceptible structure.
As the other extremal case, if the immunity is perfectly effective to make the immune individual unable to be reinfected, that is, if , the entry of immune visitors works to reduce the value of for the community. This extremal case may be regarded as corresponding to an SIR type of the epidemic dynamics, where the state transition in terms of the disease follows the susceptible–infective–removed structure.
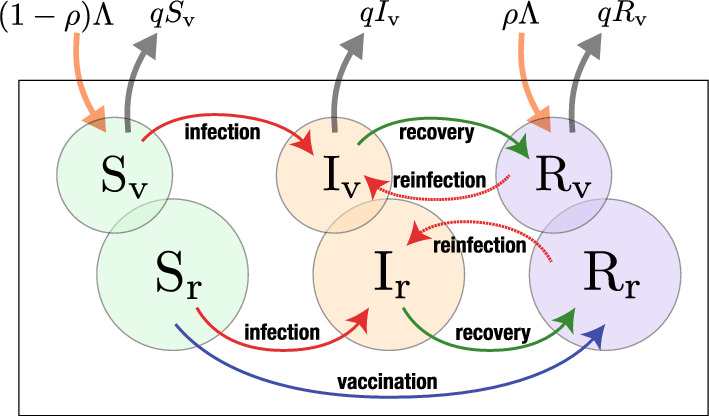
From these arguments with the basic reproduction number given by (4), we can get the following result on the influence of the acceptance of visitors in the early period after the community starts the acceptance of visitors (Fig. 2):
Fig. 2.
The dependence of the basic reproduction number given by (4) on parameters and . Numerically drawn with
Theorem 4.1
The acceptance of visitors influences the basic reproduction number given by (4) for the epidemic dynamics with the system of (1) and (2) as follows:
-
(i)
The acceptance of visitors makes smaller than if and only if the visitors contain some immune, and its decline becomes greater as the number of accepted visitors gets larger;
-
(ii)When , if
then independently of the number of accepted visitors; -
(iii)When , if and only if , the acceptance of visitors so many as
makes .
The result (i) in Theorem 4.1 means that the acceptance of visitors does not help the spread of a transmissible disease as long as , and instead it could work to suppress the spread if the community accepts a sufficiently large number of visitors with a sufficiently large proportion of immune, as indicated by the results (ii) and (iii).
Note that these arguments and result are about the effect of the acceptance of visitors on the temporal change of the number of infectives only in the early period after the community starts the acceptance of visitors. They cannot be necessarily applied for its later temporal change. It may be possible that the number of infectives turns to increase in a later period, independently of what final state the epidemic dynamics approaches, as we will actually see in the later sections of the analysis on our model.
Non-Dimensional Transformation of the System
Since the population sizes of visitors and residents are assumed constant independently of time, the above six dimensional system of (1) and (2) can be mathematically reduced to the following closed four dimensional one, making use of and :
Now we apply the following transformation of variables and parameters for this four dimensional system:
and then we can derive the following non-dimensinalized system:
| 5 |
where as before. Remark that the symbol is formally introduced now as a dimensionless parameter for the non-dimensionalized system given by (5), while its meaning is given in Sect. 4 as the basic reproduction number for the community when no visitor comes in. The initial condition (3) now becomes
with , where we set Rr0 = 0 for a simplicity that there is no immune resident at the initial, which will not affect any mathematical result obtained in our analysis on the model. In the following sections, we shall analyze the non-dimensionalized system (5) to investigate the nature of the epidemic dynamics by our model with the system of (1) and (2).
Dynamics Without Reinfection
In this section, we consider the system without reinfection, that is, with , while we will consider our model only with in the subsequent sections. For the system (5) with , we can easily find that and as . In other words, since the epidemic dynamics for the resident population is governed by an SIR model with the continuous vaccination measure for the susceptibles, the disease eventually disappears in the resident population, and the residents come to make no contribution to the epidemic dynamics. This means that, for our model of (1) and (2) without reinfection, no endemic state can be established as long as the community does not accept any visitor from the outside. Thus we consider the case of hereafter in this section, when the community accepts the visitors.
By the local stability analysis with the eigenvalues of Jacobi matrix at the equilibrium, we can easily find that the endemic equilibrium with
| 6 |
is locally asymptotically stable when it exists. Then we can get the following theorem on the epidemic dynamics given by (5) with (Appendix B):
Theorem 6.1
For the system (5) with , if and only if the condition
| 7 |
is satisfied, the endemic equilibrium with (6) uniquely exists, and it is globally asymptotically stable. Otherwise, the disease-eliminated equilibrium is globally asymptotically stable.
See Fig. 3(a, b) for numerical examples.
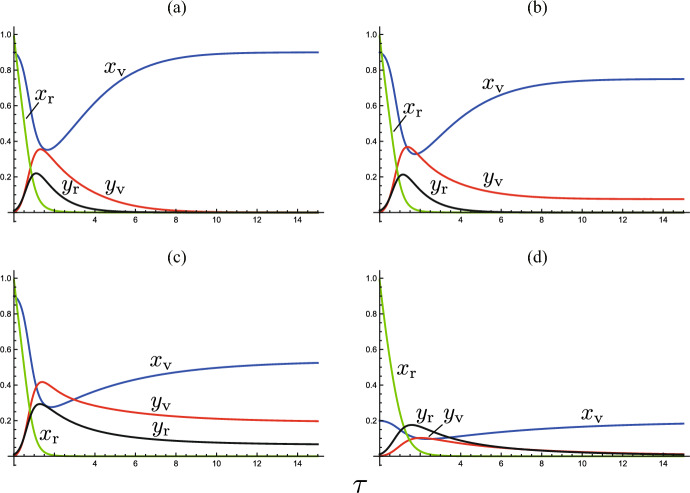
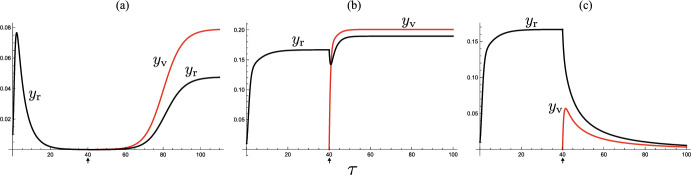
Fig. 3.
Temporal variations by the system (5). Numerically drawn with a (); b (); c (); d (); and commonly ; ; ; . In a, d, the system approaches the disease-eliminated equilibrium, and in b, c, it approaches the endemic equilibrium
From the basic reproduction number given by (4) with , we can easily find that if the condition (7) is satisfied, while the inverse does not necessarily hold. Hence we can get the following result:
Corollary 6.1.1
Even when the disease successfully invades in the community with , the disease without reinfection eventually gets eliminated unless the condition (7) is satisfied.
When the condition (7) is unsatisfied with , the number of infectives increases at the initial stage of disease spread in the community, and then it eventually turns to decrease toward zero, as numerically exemplified in Fig. 3(a).
For , the condition (7) cannot be satisfied:
Corollary 6.1.2
If the community accepts only immune visitors, the epidemic dynamics without reinfection necessarily approaches the disease-eliminated equilibrium.
Therefore, when the reinfection is impossible/negligible, the acceptance of visitors with a high immune proportion at the entry does not cause the endemicity of disease.
We must remark that the endemic equilibrium is sustained only by the visitor subpopulation, while no resident contributes to the epidemic dynamics at the equilibrium because all residents have eventually become immune by the past infection or vaccination [Fig. 3(a, b)]. From Theorem 6.1, for the disease with a sufficiently high infectivity, the acceptance of many visitors with a sufficiently small immune proportion at the entry can induce such an apparent endemicity in the community. Therefore, as seen in Fig. 3(a, b), when the community successfully controls and reduces the number of visitors to make sufficiently small, or if the community suspends accepting the visitors, the endemic state can be disrupted, and then the disease gets eliminated in the community. However, as we will see in the subsequent sections, if the disease is accompanied by a reinfectivity, this could not be the case [Fig. 3(c, d)].
Dynamics with no Visitor
Next we consider the model of (1) and (2) with reinfection, that is, with , when the community does not accept any visitor from the outside. Thus we analyze the following system derived from (5) with and :
| 8 |
As already mentioned in Sect. 4, the basic reproduction number for this epidemic dynamics is defined as . It is easy to find that there are two feasible equilibria for this system of : the disease-eliminated equilibtium and the endemic equilibrium . The endemic equilibrium exists when and only when .
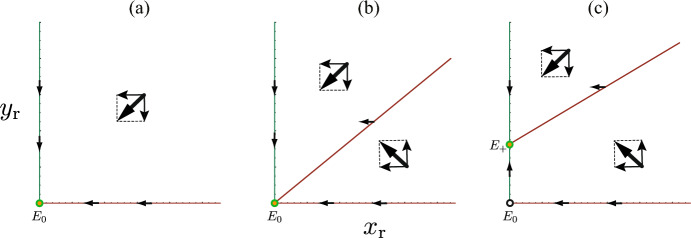
Making use of the local stability analysis for the equilibrium of the system (8), we can easily find that the equilibrium is locally asymptotically stable if , and unstable if . The endemic equilibrium is locally asymptotically stable whenever it exists. Taking account of the result on the local stability of equilibria, the isocline method for the two dimensional system (8) can further give the following result (see Fig. 4):
Fig. 4.
Application of the isocline method for the system with no visitor (8) when a ; b ; (c)
Theorem 7.1
For the system (8) with no visitor,
-
(i)
if and only if , the disease-eliminated equilibrium is globally asymptotically stable;
-
(ii)
if and only if , the endemic equilibrium exists and is globally asymptotically stable, while is unstable.
This result was shown for a mathematically equivalent SIRI model in Gomes et al. (2005).
From this result, we find that, even with the basic reproduction number , the community approaches the disease-eliminated equilibrium if with . In such a case, the number of infectives increases at the initial stage of disease spread in the community, and then it eventually turns to decrease toward zero. If and only if the basic reproduction number is sufficiently large as , the disease becomes endemic in the community.
As shown in the previous section, no endemicity arises in the community with a non-reinfectious disease when no visitor is accepted. Now the result obtained in this section indicates that the endemicity of a disease can arise in the community even with no visitor if the disease has both sufficiently high infectivity () and sufficiently high reinfectivity ().
Dynamics with Visitors
Disease-Eliminated Equilibrium
For the system (5) with visitors, we can get the following result on the local stability of the disease-eliminated equilibrium (Appendix C):
Theorem 8.1
The disease-eliminated equilibrium is unstable if
| 9 |
while it is locally asymptotically stable if the inverse inequality of (9) is satisfied.
It can be easily seen that the condition (9) becomes equivalent to (7) as . This shows a mathematical consistency of Theorem 8.1 to Theorem 6.1.
Moreover we can prove the following result about the relation of the basic reproduction number defined by (4) to the condition (9) (Appendix D):
Corollary 8.1.1
When , the disease-eliminated equilibrium is locally asymptotically stable.
This result is consistent with the biological/epidemiological meaning of the basic reproduction number with respect to the invasion success of a disease in a population, which was described in Sect. 4 and references therein. Under the condition that at the initial stage of a disease invasion in a population, the number of infectives is expected to decrease toward the disease elimination. In a sense of epidemic dynamics, such a decline of the infective subpopulation toward the elimination must follow the locally asymptotic stability of the disease-free equilibrium (as referred in most literatures), which corresponds here to the disease-eliminated equilibrium . The result of Corollary 8.1.1 shows this consistency.
Endemic Equilibrium
From the equations of (5), if the endemic equilibrium with and exists for , then it must satisfy that
| 10 |
and . In contrast, at the endemic equilibrium for , we have
| 11 |
and , , instead of (10). We can obtain the following result on the existence of (Appendix E):
Theorem 8.2
The endemic equilibrium uniquely exists if and only if the condition (9) is satisfied.
Hence, when the disease-eliminated equilibrium is locally asymptotically stable, the endemic equilibrium does not exist, and when is unstable, uniquely exists.
For the local stability of the endemic equilibrium for (5), we can get the following result:
Theorem 8.3
When the endemic equilibrium exists, it is locally asymptotically stable.
This theorem can be proved by the eigenvalue analysis on the Jacobi matrix for (5) at the endemic equilibrium , applying the Routh-Hurwitz criterion (Appendix F). Although we could not get any analytical result on the global stability of the endemic equilibrium , our numerical calculations imply that it is globally asymptotically stable when it exists. We then have the mathematical consistency of Theorems 8.1, 8.2, and 8.3 to Theorem 6.1 as for the case with no reinfection, .
Influence of the Acceptance of Visitors
Shift in Endemicity
As the important preliminary for our analysis on the model, we can easily find the following features of defined in (9):
.
is monotonically increasing in terms of for any .
is
for any positive and if and only if .
is
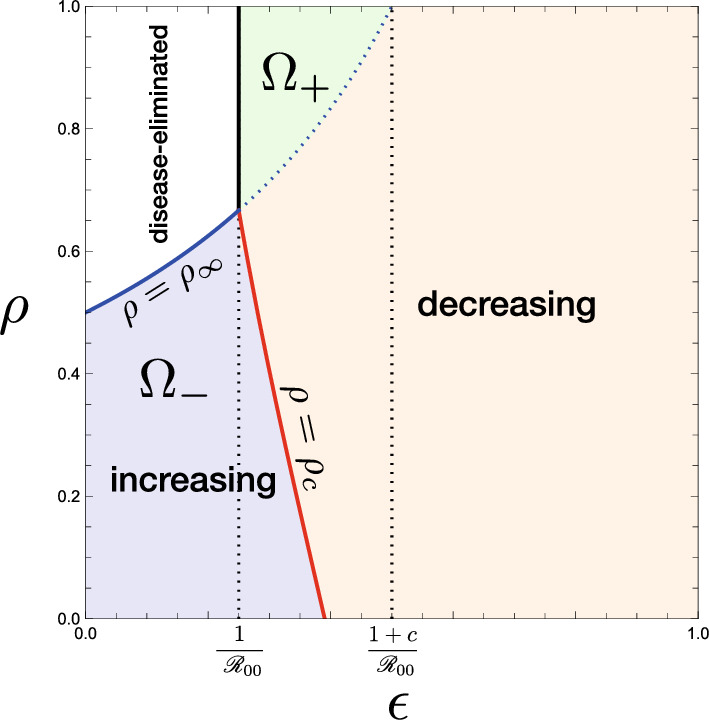
Then from these mathematical features of , and Theorems 8.1, 8.2, and 8.3, we can get the following result on the disease endemicity in the community accepting the visitors (see Figs. 5 and 6):
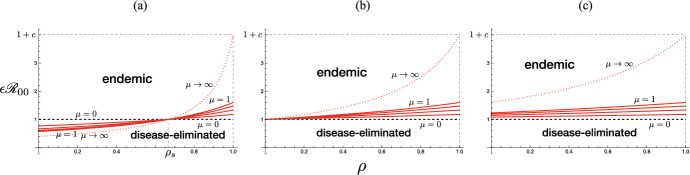
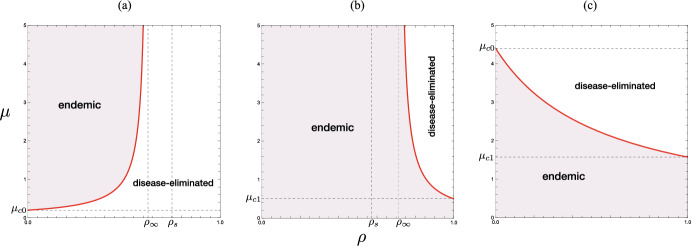
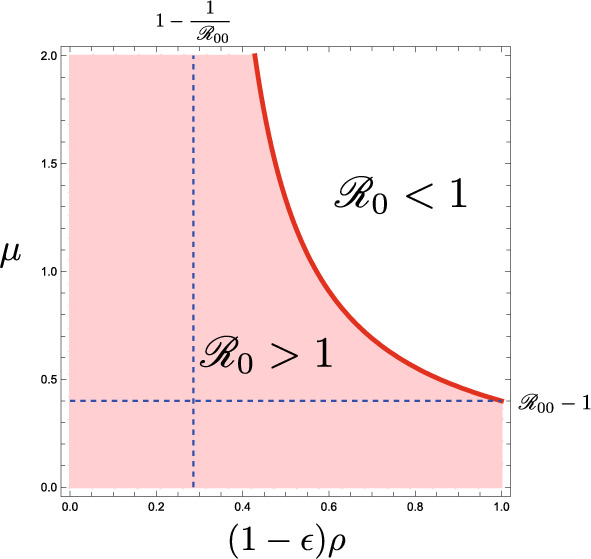
Fig. 5.
Parameter region and boundary indicated by the condition (9). The boundary curve is given by . a ; b ; c . Numerically drawn with a ; b ; c , commonly for . Solid curves are for in each figure. Dotted curve indicates
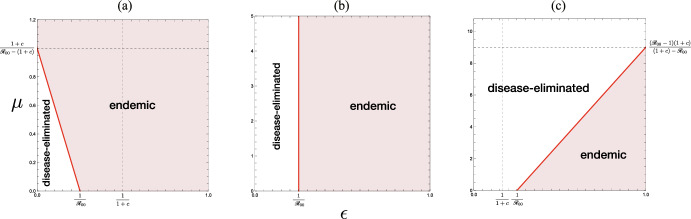
Fig. 6.
-dependence of the endemicity, derived from the condition (12) in Theorem 9.1. Numerically drawn for a ; b ; c , commonly with . For the region , the acceptance of visitors may change the endemic situation of the community for the disease-eliminated equilibrium as described in Theorem 9.2, while, for the region , it may drive the situation of the community approaching the disease-eliminated equilibrium toward the endemic equilibrium as described in Theorem 9.3. For the region out of and , the endemicity is independent of whether the community accepts visitors or not
Theorem 9.1
Independently of whether the community accepts the visitors or not, it approaches an endemic equilibrium if while it approaches the disease-eliminated equilibrium if , where
Only when
| 12 |
the endemicity could significantly depend on the acceptance of visitors.
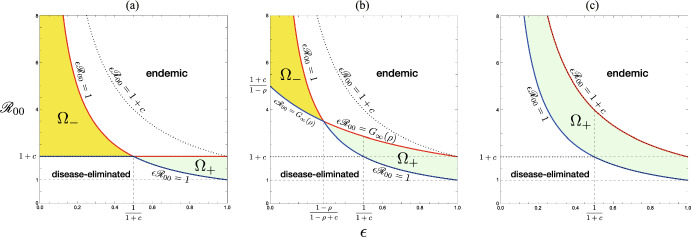
The inequality (12) gives a necessary condition for which the acceptance of visitors could change the epidemic situation in the community from the endemic equilibrium to the disease-eliminated equilibrium or vice versa. The corresponding parameter regions are shown as and in Fig. 6. Numerical examples of such a change of endemicity by the acceptance of visitors are given in Fig. 7(a, c).
Fig. 7.
Temporal variations of infective subpopulations and by the systems (5) and (8). Numerically drawn for model (8) until and model (5) for , with a (; ); b (; ); c (; ); and commonly ; ; ; ; . In a and c, the endemicity is changed before and after starting the acceptance of visitors, while in b the system remains at an endemic state before and after it
Further from the monotonicity of in terms of and as described in the above, we find the following result on the condition with respect to the influence of the acceptance of visitors on the endemicity in the community:
Corollary 9.1.1
Independently of whether the community accepts the visitors or not, it approaches an endemic equilibrium if
while it approaches the disease-eliminated equilibrium if
Only when
| 13 |
the endemicity could significantly depend on the acceptance of visitors.
As seen in Figs. 5 and 6, such an influence to cause a change of endemicity depends on the nature of accepted visitors (i.e., the number, the immune proportion, and the duration of stay).
Moreover, from the features of described in the above, we find that, if , the disease is endemic independently of how many visitors the community accepts even under the condition (13), as seen in Fig. 5. Thus, in comparison to the result for the community with no visitor (i.e., ) given by Theorem 7.1, we can get the following result (see Fig. 6):
Theorem 9.2
Suppose that the disease was endemic under the condition that before the community accepts visitors. If the community accepts visitors with an immune proportion
| 14 |
the disease remains endemic independently of how many visitors are accepted. If the community accepts visitors with an immune proportion , then the acceptance of visitors so many as
| 15 |
makes the community approach the disease-eliminated equilibrium. Even if , the acceptance of visitors with does not sufficiently shift the endemicity, and the disease remains endemic.
The critical value satisfies the equation . The latter case of in Theorem 9.2 corresponds to the parameter region in Fig. 6. Figure 8(b) shows a numerical example of the -dependence in such a case when .
Fig. 8.
-dependence of the endemicity, derived from the condition (9) with the results given by Theorems 8.1, 8.2, and 8.3: a, b ; c . Numerically drawn for a (); b (); c (), each of which satisfies the condition (13) in Corollary 9.1.1
Since defined by (15) is monotonically decreasing in terms of when , we note that
| 16 |
where for . Then we get the following corollary:
Corollary 9.2.1
When , if the community accepts visitors few enough to have , the disease remains endemic independently of how much proportion of visitors is immune at the entry.
This result is numerically pointed out in Fig. 8(b, c).
Moreover we note that, when so that defined by (14) is negative, the first case in Theorem 9.2 does not occur. Then the -dependence becomes as shown by Fig. 8(c), where there is a finite value of beyond which the community approaches the disease-eliminated equilibrium, independently of the immune proportion in the visitors at the entry:
| 17 |
Corollary 9.2.2
When , if the community accepts visitors so many as , the disease tends to get eliminated independently of how much proportion of visitors is immune at the entry. When , only the acceptance of visitors with and can change the endemicity and lead the community to the disease-eliminated equilibrium.
Hence the value gives a sufficient number of accepted visitors which is effective to suppress the disease spread in the community when . See the numerical examples in Fig. 7(b, c).
In contrast, when the risk of reinfection is so weak as , the acceptance of visitors may cause the opposite influence on the epidemic dynamics, as numerically indicated by Fig. 8(a):
Theorem 9.3
Suppose that the disease was getting eliminated under the condition that before the community accepts visitors. If the community accepts visitors with an immune proportion , the disease keeps getting eliminated independently of how many visitors are accepted. If the community accepts visitors with an immune proportion , then the acceptance of visitors so many as induces the endemicity, and the disease becomes endemic. Even if , the acceptance of visitors so few as does not induce the endemicity, and the disease keeps getting eliminated.
The situation considered in this theorem corresponds to the parameter region in Fig. 6, and is numerically exemplified by Fig. 7(a). Theorem 9.3 indicates that, if the proportion of immune visitors is so low as , there exists the upper threshold for the number of accepted visitors to suppress the revival of the disease spread after starting the acceptance of visitors in the community where the disease was getting eliminated.
Acceptance of Only Immune Visitors
When the community accepts only immune visitors, that is, when with , is necessarily greater than 1 and monotonically increasing in terms of . Then, from Theorem 9.2, we can find that only the acceptance of visitors so many as can induce the disease-eliminated equilibrium in the community where the disease was endemic before starting the acceptance of visitors. As defined by (16), the critical value depends on the risk of reinfection, and then, from Theorems 9.1 and 9.2, we can obtain the following result (see Figs. 8 and 9):
Fig. 9.
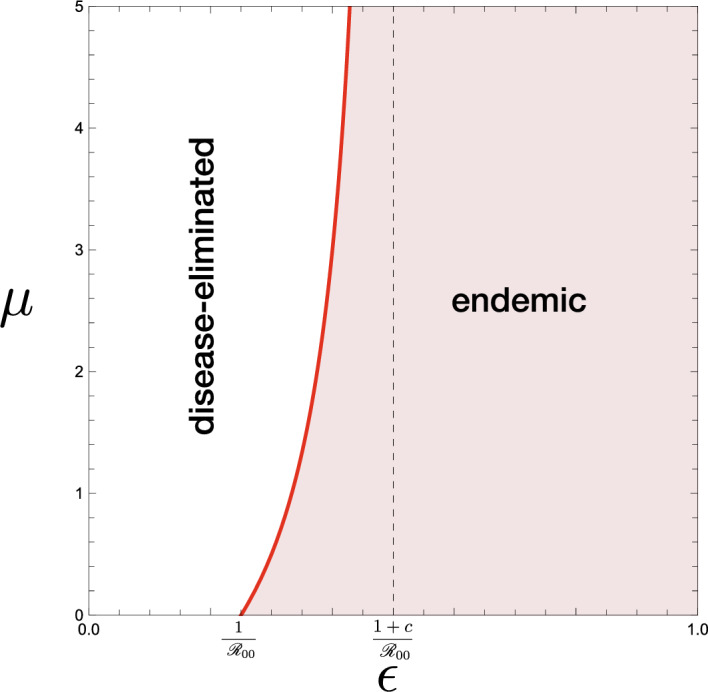
Parameter region and boundary indicated by the condition (9) with the results given by Theorems 8.1, 8.2, and 8.3 when the community accepts only immune visitors with . Numerically drawn with and . Refer to Sect. 9.2
Corollary 9.2.3
Suppose that the disease was endemic under the condition that before the community accepts only immune visitors. If , the endemicity remains independently of how many visitors the community accepts. If , the acceptance of visitors so many as is effective to make the disease eliminated.
Therefore the community under an endemic situation could have a preferable influence to suppress the endemicity by accepting only immune visitors only when the reinfectivity is sufficiently low as indicated by Fig. 9.
If the community was approaching the disease-eliminated equilibrium with the risk of reinfection so low as , the community keeps approaching the disease-eliminated equilibrium even after starting the acceptance of only immune visitors, independently of how many visitors the community accepts.
Acceptance of Only Susceptible Visitors
Now let us consider the case where all visitors accepted by the community are susceptible, that is, when with . Then, from Theorem 9.3, we find three different cases according to the influence of the acceptance of visitors as shown in Fig. 10, taking account of the features of given in Sect. 9.1. We can get the following result:
Fig. 10.
Parameter region and boundary indicated by the condition the condition (9) with the results given by Theorems 8.1, 8.2, and 8.3 when all visitors accepted by the community is susceptible with : a ; b ; c . Numerically drawn with a ; b ; c , and commonly . Refer to Sect. 9.3
Corollary 9.3.1
When the disease was getting eliminated under the condition that , the acceptance of only susceptible visitors induces
In contrast, when the disease was endemic under the condition that , the acceptance of only susceptible visitors induces
The critical value is defined by (17).
Therefore the acceptance of only susceptible visitors could have the counter effect according to the endemicity, depending on the infectivity of disease. Only for a moderately high infectious disease such that , the acceptance of only susceptible visitors so many as can lead the community to the disease-eliminated equilibrium. For the disease with a low reinfectivity such that , the acceptance of only susceptible visitors so many as can lead the community to the endemic equilibrium.
Further, as indicated by Fig. 10, we find that there is a sufficient value of which determines the epidemic situation in the community:
Corollary 9.3.2
If , the acceptance of only susceptible visitors so many as
necessarily makes the disease endemic. In contrast, if , the acceptance of only susceptible visitors so many as
necessarily makes the disease eliminated.
The former case means an unpreferable influence of the sufficiently large number of visitors for the community with the spread of a highly infectious disease, while the latter does a preferable influence for the community with the spread of a moderately infectious disease.
Change in Endemic Size
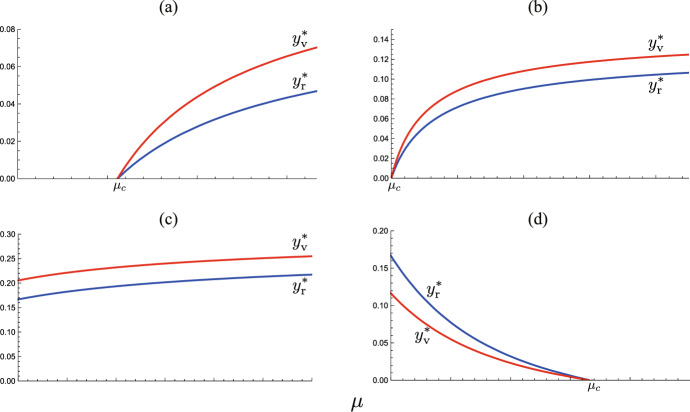
Figure 11 shows the numerically drawn -dependence of endemic sizes and at the endemic equilibrium determined by (10). As the figure implies, the endemic size necessarily has a monotonic dependence on the number of accepted visitors, represented now by , about which we can get the following analytical result (Appendix G):
Fig. 11.
-dependence of endemic sizes. Numerically drawn by (10) with a (, ); b (, , ), c (, , ), d (, , ), and commonly ;
Theorem 9.4
The endemic sizes , , and the total endemic size
| 18 |
are monotonically increasing in terms of if and only if or
| 19 |
It can be easily found that when . See the numerically drawn -dependence of the endemic size in Fig. 12.
Fig. 12.
-dependence of the endemic size . Numerically drawn contour maps for three cases correspond to those in Fig. 8: a ; b and ; c and , where the parameter values are respectively the same as in Fig. 8
We remark that, as shown in Sect. 9.2, the endemic equilibrium for exists only when . Then we can find the following result too (Appendix H):
Corollary 9.4.1
When the community accepts only immune visitors (i.e., ), the endemic size is monotonically decreasing in terms of .
This result may be regarded as included in Theorem 9.4 because the condition given in Theorem 9.4 can never hold when . We can see the numerical examples in Fig. 12(b, c).
When defined by (19) is non-positive with , any cannot be smaller than , so that the endemic size is necessarily monotonically decreasing in terms of :
Corollary 9.4.2
If , the endemic size is necessarily monotonically decreasing in terms of .
The numerical example of Fig. 12(c) illustrates the case.
For the critical case of with , we can derive the explicit values at the endemic equilibrium from (10) (Appendix G):
| 20 |
Hence the endemic sizes are independent of the number of accepted visitors in this case:
Corollary 9.4.3
For with , the endemic sizes , , and the total endemic size are determined independently of .
A numerical example is given in Fig. 12(b). We note it necessary for with that , that is, . Moreover, the case of Corollary 9.4.3 can appear only when .
Additionally we can find the following relations among the specific values , , and for the immune proportion of accepted visitors at the entry:
Corollary 9.4.4
It holds that and .
The proof is easy by calculating the differences and to show them positive. Numerical calculation of Fig. 12(b) demonstrates this result.
Consequently as indicated by Fig. 13, the larger number of accepted visitors makes the endemic size bigger only when the immune proportion of accepted visitors at the entry is sufficiently small under the epidemic situation with a sufficiently low risk of infection.
Fig. 13.
Classification of the parameter region of according to the -dependence of the change in the endemic size. Numerically drawn with and . Regions correspond to those in Fig. 6
Concluding Remarks
The results of our model imply that the acceptance of temporal visitors from the outside may induce a significant change of the epidemic state in the community. Contrary to an intuitive expectation, the acceptance of visitors does not necessarily make the epidemic situation worse in the community. Only when the reinfectivity of the disease is sufficiently weak, the acceptance of visitors may induce the endemicity if the community accepts the visitors with a sufficiently low immune proportion. Furthermore, when the reinfectivity is high, the acceptance of a sufficiently large number of visitors may induce the elimination of the disease if the community can regulate to accept the visitors with a sufficiently high immune proportion.
The visitors certainly play a role of recruitment of hosts for the infectious disease spread in the community. The visitors with a higher susceptible proportion could be regarded as a larger supply of susceptible individuals in the community, and they subsequently provide a fast recruitment of infectives. In contrast, the visitors with a high immune proportion cause only a slow recruitment of infectives with the reinfection. For these reasons, the influence of the visitor acceptance on the epidemic dynamics with a reinfectious disease must depend on the immune proportion in the visitors at the entry. On the other hand, the entry of many visitors could induce a dilution of the infective density in the community at the same time, which is regarded as an advantageous influence of the visitor acceptance against the disease spread. In the epidemic dynamics with our model, a balance of these counteractive factors of the visitor acceptance with respect to the disease spread could significantly affect the consequence of epidemic dynamics in the community.
As a result, a preferable acceptance of visitors must be regulated to have a sufficiently large immune proportion according to the public health in the community. In this sense, the best policy for the visitor acceptance would be to allow the entry only for the immune visitors. From the results on our model, such an acceptance of only immune visitors may lower the endemic size, and further suppress the endemicity to induce the elimination of the disease spread in the community.
In contrast, when the community was on the way to the disease-eliminated equilibrium before starting the acceptance of visitors, the acceptance of visitors without any epidemiological regulation may cause the revival of the disease spread in the community. Such a case would occur by reduced cautiousness of the disease before starting the acceptance of visitors, which could be caused by the reason that the number of infective residents became rather small in comparison to that at the outbreak.
Our model could be regarded as a consideration on the epidemic dynamics in a season. In this sense, the number of visitors may be beyond the number of residents in the community (i.e., ), as some popular touristic local places like Venice in the vacation season, or a certain place attracting visitors like a newly found gold mine. As another example, we could consider a community accepting many evacuees from a certain calamity. Even though the number of visitors would be smaller than the number of residents in most cases (i.e., ), our results imply that the influence of the visitor acceptance could depend on the infectivity and reinfectivity in the epidemic dynamics, and the regulation on the epidemiological nature of accepted visitors.
Since the infectivity and reinfectivity are not only determined by the nature of disease itself but also by social custom, sanitary condition, and people’s behavior (Ferguson et al. 2005; Heymann 2005; Funk et al. 2009; Perisic and Bauch 2009; Manfredi and D’Onofrio 2013; Bavel et al. 2020; Kapitány-Fövény and Sulyok 2020; Thu et al. 2020), the influence of the visitor acceptance could depend also on social factors in the community which accepts the visitors under the epidemic dynamics. Such social factors could be affected by the situation of disease spread during the epidemic dynamics in the community. For example, some strategic/non-strategic transmission of information about the disease spread or a public health campaign to prevent the further disease spread could alter people’s social behavior, and subsequently the risk of infection/reinfection. Hence if the infectivity and reinfectivity would be changeable in the epidemic dynamics, the influence of the visitor acceptance must be qualitatively changed.
The results from our model imply such a possibility that a shift of the infectivity and/or reinfectivity to the weaker would induce an epidemic situation in which the acceptance of visitors causes the increase in the epidemic size or the revival of disease spread even with the endemicity. If so, there would be repetitive revivals of disease spread in the community, driven by a temporal shift of the infectivity and/or reinfectivity which could bring a feedback influence on the policy to control the disease spread in the community. Such theoretical/mathematical researches on the relation between the disease spread and the nature of hosts are interesting and require further development.
Acknowledgements
We greatly appreciate valuable comments and suggestions of an anonymous reviewer and the editor to improve and finalize the manuscript. The author YX was supported by JST SPRING, Grant Number JPMJSP2114. The author HS was supported in part by JSPS KAKENHI Grant Number 18K03407.
Appendix A Derivation of
In our model, the new cases consist of residents and visitors. Therefore, from the conceptual definition and the mathematical feature of the basic reproduction number, we shall derive it here from the following conditions:
for the initial condition (3) with . This is because the basic reproduction number is defined as the expected number of new infectives produced by one infective individual in an environment consisting of only susceptibles. Following the assumption H3 in Sect. 2, we shall adopt the initial condition (3) in order to define the basic reproduction number. Then, in place of the above conditions, we can use the followings:
for the initial condition (3) with . Remark that, in this context about the situation to define the basic reproduction number of our model, the initial infective must be a resident, which matches the assumption H3 in Sect. 2. Then, making use of (3), we have
Thus we find that
Consequently we can define the basic reproduction number as follows:
This formula can be rewritten as given by (4) to clarify the meaning.
Appendix B Proof for Theorem 6.1
From the arguments in the first paragraph of Sect. 6, we find that the dynamics given by (5) with necessarily approaches the dynamics with the following limiting system in terms of the visitor population:
| B1 |
The feasible equilibria are and with (6). The former corresponds to the disease-eliminated equilibrium for the system (5), , and so does the latter to the endemic equilibrium . The endemic equilibrium can exist when and only when the condition (7) is satisfied. By the local stability analysis with the eigenvalues of the Jacobi matrix at the equilibrium, we can easily find that the endemic equilibrium is locally asymptotically stable when it exists. In the following part, we shall consider its global stability.
First we set the following mathematical result on the boundedness for the solution of the system (B1):
Lemma B.1
For any initial condition in the domain
| B2 |
the solution of (B1) stays in D for any .
Proof
We can obtain the following features from (B1) for the initial condition :
The first inequality indicates that cannot reach 0 from the initial value . The second equation indicates that is necessarily positive for any and . Then, from
for , we can find that cannot become for any and initial condition in D.
Lemma B.1 means that the domain D is invariant for the dynamics given by (B1). Further, when the endemic equilibrium exists, satisfying the condition (7), we find from (6) that
Hence we have the following result:
Lemma B.2
When exists, it must belong to the domain D defined by (B2).
When the condition (7) is not satisfied, that is, when the endemic equilibrium does not exist, we can easily find that the disease-eliminated equilibrium is globally asymptotically stable, making use of the isocline method shown by Fig. 14(a). In contrast, as seen from Fig. 14(b), when the condition (7) is satisfied and the endemic equilibrium exists, the stability cannot be determined only by the isocline method.
Fig. 14.
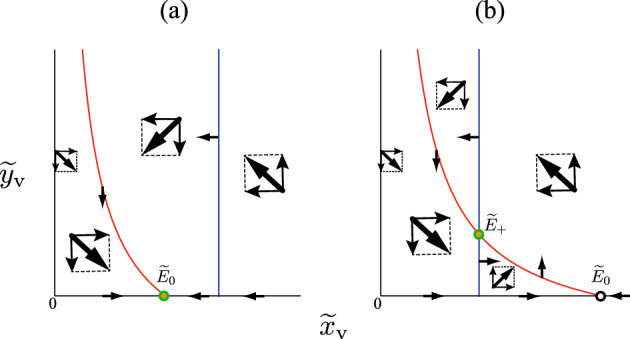
Application of the isocline method for the system (B1) when the condition (7) is (a) not satisfied; (b) satisfied
When the endemic equilibrium exists, let us consider the following function of in the domain D defined by Lemma B.1:
| B3 |
It can be easily found that and for any in D. Further, making use of (B1), we can derive
which becomes zero only for , and negative for any in D. These features of V indicates that it is a Lyapunov function according to the endemic equilibrium for the system (B1). Therefore, when the endemic equilibrium exists for the system (B1), it is globally asymptotically stable with respect to the dynamics given by (B1) with the initial condition in D. Since the dynamics of (5) with must eventually approach that of (B1), this result shows the global stability of the endemic equilibrium for (5).
Appendix C Proof for Theorem 8.1
The characteristic equation with the Jacobi matrix according to the disease-eliminated equilibrium for the system (5) becomes
Hence we have two negative eigenvalues and with the other two given by the roots of the equation that the above determinant is equal to zero. Both of them have negative real parts if and only if
that is,
Therefore we find that the second inequality gives the necessary and sufficient condition that every eigenvalue has a negative real part. It can be expressed as the inverse inequality of (9). At the same time, we can find that, if the inverse of the above second inequality is satisfied, there exists an eigenvalue with a positive real part. Then the disease-eliminated equilibrium is unstable.
Appendix D Proof for Corollary 8.1.1
It is sufficient to show that when . From the definition of defined by (4), we have if and only if
Then we can derive
Therefore, we find that when , and then the inverse equality of (9) is satisfied.
Appendix E Proof for Theorem 8.2
First we consider the case of . From (11), we can derive the following equations about and :
| E4 |
The function is monotonically decreasing in terms of , and . Hence, if and only if , the equation has a unique positive root . We remark from the first equation of (E4) that is uniquely determined for each positive . Therefore, if and only if , the endemic equilibrium exists when . It is easy to show that the condition that is necessary and sufficient to make (9) hold with .
Next, from (10) in the case of and , we can derive the equation in terms of with
| E5 |
Since for the endemic equilibrium, we have . We can easily find that is monotonically increasing in terms of . Further as . Hence the equation necessarily has a unique positive root if and only if , which can be easily proved to be equivalent to the condition (9). With , the equilibrium value is uniquely determined by .
On the other hand, with the second equation of (10), we can derive
Then we can easily find that g(x) is concave with and , so that for . This result indicates that, if the equation has a unique positive root , the value is reasonably determined by the second equation of (10) such that . Moreover, by the third equation of (10), the value is reasonably determined at the same time such that . Finally, from these arguments, if and only if , which is equivalent to the condition (9), the endemic equilibrium uniquely exists when .
Appendix F Proof for Theorem 8.3
The Jacobi matrix about the endemic equilibrium becomes
where
Then the characteristic polynomial for the eigenvalue about can be obtained as with
| F6 |
where
Thus we have a negative eigenvalue and the cubic equation given by (F6) to determine the other three eigenvalues for .
Every coefficient of is positive: , , and . Since , we have
and subsequently find that
| F7 |
Consequently from the Routh-Hurwitz criterion, we can find that all roots of have negative real parts. Therefore it has been proved that every eigenvalue for has negative real part. This result shows Theorem 8.3 about the local stability of when it exists.
Appendix G Proof for Theorem 9.4 and Corollary 9.4.3
As shown in the proof for Theorem 8.2 (Appendix E), the endemic size can be determined by the unique positive root of the equation with (E5) for under the condition (9). From the equation , we can derive
| G8 |
with
First for , we find from (G8) that for any and . Since , we then have . Lastly we can get the following result:
Lemma G.1
is monotonically decreasing in terms of when and .
Next for , the partial derivative (G8) indicates that if and only if , while if and only if . Since is monotonically increasing in terms of , such that for and for under the condition (9), we find that if and only if , while if and only if . Since is independent of , this result indicates that the sign of is determined independently of . Hence we obtain the following lemma:
Lemma G.2
is monotonic in terms of when .
Now we can derive
| G9 |
Thus we can result that if and only if where is defined by (19). That is, if and only if . Lastly we have
Lemma G.3
is monotonically decreasing in terms of if and only if when and .
It can be easily found that when .
In the critical case of with , we have in (G9), which means that and subsequently . Actually, from , we find that , the endemic sizes , , and defined by (18) are independent of , as given by (20) which are derived by (10). This result gives Corollary 9.4.3.
On the other hand, from (10), we can easily find that the endemic sizes , , and are monotonically decreasing in terms of . Therefore, from Lemma G.2, we can get the following lemma:
Lemma G.4
The endemic sizes , , and are monotonic in terms of when .
Consequently, from Lemmas G.1, G.2, G.3, and G.4, we can obtain the result of Theorem 9.4 for .
Appendix H Proof for Corollary 9.4.1
In case of , the unique positive root of with (E4) gives the endemic size under the condition (9) with (Appendix E). Then, from the equation , we can derive
| H10 |
When , we have for any . Therefore we obtain the following result:
Lemma H.1
The endemic sizes and are monotonically decreasing in terms of when and .
From the first equation in (E4), we note that the endemic size is monotonically increasing in terms of .
When , if and only if , while if and only if . Since is monotonically decreasing in terms of , such that for and for . Hence, if and only if , while if and only if . Now we can derive
for . Therefore we have the following result:
Lemma H.2
The endemic sizes and are monotonically decreasing in terms of when and .
Consequently, from Lemmas H.1 and H.2, the endemic sizes and are monotonically decreasing in terms of when .
Author Contributions
Conceptualization: YX, HS. Numerical simulation: IA, TISI, EFS, HS. Mathematical analysis: YX, TISI, HS. Visualization: HS. Writing-original draft: YX, HS. Writing-review & editing: HS.
Funding
Not applicable.
Data Availability
Not applicable.
Declarations
Conflict of interest
The authors state that there are no conflicts in interest.
Ethical Approval
Not applicable.
Consent to Participate
All authors agree to participate in this research project.
Consent for Publication
All authors approve publication of manuscript in a present form.
Code Availability
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agusto FB. Mathematical model of Ebola transmission dynamics with relapse and reinfection. Math Biosci. 2017;283:48–59. doi: 10.1016/j.mbs.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Arias CF, Acosta FJ, Fernandez-Arias C. Killing the competition: a theoretical framework for liver-stage malaria. Open Biol. 2022;12:210341. doi: 10.1098/rsob.210341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athayde GM, Alencar AP. Forecasting Covid-19 in the United Kingdom: a dynamic SIRD model. PlOS one. 2022;17(8):e0271577. doi: 10.1371/journal.pone.0271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavel JJ, Baicker K, Boggio PS, Capraro V, Cichocka A, Cikara M, Crockett MJ, Crum AJ, Douglas KM, Druckman JN, Drury J. Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav. 2020;4(5):460–471. doi: 10.1038/s41562-020-0884-z. [DOI] [PubMed] [Google Scholar]
- Brauer F. Mathematical epidemiology: past, present, and future. Infect Dis Model. 2017;2:113–127. doi: 10.1016/j.idm.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomo B. Effects of information-dependent vaccination behavior on coronavirus outbreak: insights from a SIRI model. Ricerche di Mat. 2020;69:483–499. doi: 10.1007/s11587-020-00506-8. [DOI] [Google Scholar]
- Centre for Disease Control and Prevention (CDC) (2022) COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 21 June 2022
- Chowell G, Sattenspiel L, Bansal S, Viboud C. Mathematical models to characterize early epidemic growth: a review. Phys Life Rev. 2016;18:66–97. doi: 10.1016/j.plrev.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossar JH. Influence of travel and disease: an historical perspective. J Travel Med. 1994;1(1):36–39. doi: 10.1111/j.1708-8305.1994.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Crawford NW. Importance of understanding the reinfection risk of COVID-19 in children. Lancet Child Adolesc Health. 2022;6(6):355–357. doi: 10.1016/S2352-4642(22)00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansu EJ, Seno H. A model for epidemic dynamics in a community with visitor subpopulation. J Theor Biol. 2019;478:115–27. doi: 10.1016/j.jtbi.2019.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JR, Grilli EA, Smith AJ. Influenza a: infection and reinfection. Epidemiol Infect. 1984;92(1):125–127. doi: 10.1017/S002217240006410X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater PL, Street EJ, Leslie TF, Yang YT, Jacobsen KH. Complexity of the basic reproduction number (Ro) Emerg Infect Dis. 2019;25(1):1–4. doi: 10.3201/eid2501.171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek JAP, Britton T. Mathematical tools for understanding infectious disease dynamics. Princeton series in theoretical and computational biology. Princeton: Princeton University Press; 2013. [Google Scholar]
- Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18(3):123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earn DJ, Dushoff J, Levin SA. Ecology and evolution of the flu. Trends Ecol Evol. 2002;17(7):334–340. doi: 10.1016/S0169-5347(02)02502-8. [DOI] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) (2022) COVID-19. https://www.ecdc.europa.eu/en/covid-19. Accessed 21 June 2022
- Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- Funk S, Gilad E, Watkins C, Jansen VA. The spread of awareness and its impact on epidemic outbreaks. Proc Natl Acad Sci. 2009;106(16):6872–6877. doi: 10.1073/pnas.0810762106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu P, Zhang H. A Lyapunov functional for a SIRI model with nonlinear incidence of infection and relapse. Appl Math Comput. 2013;219(16):8496–8507. doi: 10.1016/j.amc.2013.02.044. [DOI] [Google Scholar]
- Ghosh M, Olaniyi S, Obabiyi OS. Mathematical analysis of reinfection and relapse in malaria dynamics. Appl Math Comput. 2020;373:125044. doi: 10.1016/j.amc.2020.125044. [DOI] [Google Scholar]
- Gökaydin D, Oliveira-Martins JB, Gordo I, Gomes MG. The reinfection threshold regulates pathogen diversity: the case of influenza. J R Soc Interface. 2007;4(12):137–142. doi: 10.1098/rsif.2006.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MG, White LJ, Medley GF. Infection, reinfection, and vaccination under suboptimal immune protection: epidemiological perspectives. J Theor Biol. 2004;228(4):539–549. doi: 10.1016/j.jtbi.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Gomes MG, White LJ, Medley GF. The reinfection threshold. J Theor Biol. 2005;236(1):111–113. doi: 10.1016/j.jtbi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Guo P, Yang X, Yang Z. Dynamical behaviors of an SIRI epidemic model with nonlinear incidence and latent period. Adv Differ Equ. 2014;2014:164. doi: 10.1186/1687-1847-2014-164. [DOI] [Google Scholar]
- Gupta S, Maiden MC. Exploring the evolution of diversity in pathogen populations. Trends Microbiol. 2001;9(4):181–185. doi: 10.1016/S0966-842X(01)01986-2. [DOI] [PubMed] [Google Scholar]
- Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356(1416):1861–1870. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethcote HW. Simulations of pertussis epidemiology in the United States: effects of adult booster vaccinations. Math Biosci. 1999;158(1):47–73. doi: 10.1016/S0025-5564(99)00004-8. [DOI] [PubMed] [Google Scholar]
- Heymann DL. Social, behavioural and environmental factors and their impact on infectious disease outbreaks. J Publ Health Policy. 2005;26(1):133–139. doi: 10.1057/palgrave.jphp.3200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh CR, Jr, Jo Y, Nichols B, Jenkins HE, Russell CA, White LF. Contribution of reinfection to annual rate of tuberculosis infection (ARI) and iIncidence of tuberculosis disease. Clin Infect Dis Ciac. 2022;451:1058–4838. doi: 10.1093/cid/ciac451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A review of the UK’s tourism recovery plans post COVID-19. Athens J Tour. 2022;9(1):9–18. doi: 10.30958/ajt.9-1-1. [DOI] [Google Scholar]
- Kapitány-Fövény M, Sulyok M. Social markers of a pandemic: modeling the association between cultural norms and COVID-19 spread data. Humanit Soc Sci Commun. 2020;7(1):1–9. doi: 10.1057/s41599-020-00590-z. [DOI] [Google Scholar]
- Kobayashi G, Sugasawa S, Tamae H, Ozu T. Predicting intervention effect for COVID-19 in Japan: state space modeling approach. BioSci Trends. 2020;14(3):174–181. doi: 10.5582/bst.2020.03133. [DOI] [PubMed] [Google Scholar]
- Kumar A, Shiwalkar N, Shaikh JD, Kaur R, Leite AFV, Persaud P, Kichloo A, Rodríguez-Morales AJ, Sah R (2020) Reinfection after SARS-CoV-2 infection: A looming concern. Journal of Experimental Biology and Agricultural Sciences 8(Spl-1- SARS-CoV-2): S114–S118. 10.18006/2020.8(Spl-1-SARS-CoV-2).S114.S118 [DOI]
- Le Page M. The risks from covid-19 reinfection. New Sci. 2022;255(3394):21. doi: 10.1016/S0262-4079(22)01208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MA, Shuai Z, van den Driessche P. A general theory for target reproduction numbers with applications to ecology and epidemiology. J Math Biol. 2019;78:2317–2339. doi: 10.1007/s00285-019-01345-4. [DOI] [PubMed] [Google Scholar]
- Lin L, Zhao Y, Chen B, He D. Multiple COVID-19 waves and vaccination effectiveness in the united states. Int J Environ Res Publ Health. 2022;19(4):2282. doi: 10.3390/ijerph19042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci USA. 1997;94(12):6571–6576. doi: 10.1073/pnas.94.12.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-L, Saif L. Emerging viruses without borders: the Wuhan coronavirus. Viruses. 2020;12:130. doi: 10.3390/v12020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre CR, Chughtai AA. Recurrence and reinfection – a new paradigm for the management of Ebola virus disease. Int J Infect Dis. 2016;43:58–61. doi: 10.1016/j.ijid.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Manfredi P, D’Onofrio A (2013) Modeling the interplay between human behavior and the spread of infectious diseases. Springer Science & Business Media New York. 10.1007/978-1-4614-5474-8
- Martins J, Pinto A, Stollenwerk N. A scaling analysis in the SIRI epidemiological model. J Biol Dyn. 2009;3(5):479–496. doi: 10.1080/17513750802601058. [DOI] [PubMed] [Google Scholar]
- Mensah AA, Lacy J, Stowe J, Seghezzo G, Sachdeva R, Simmons R, Bukasa A, O’Boyle S, Andrews N, Ramsay M, Campbell H, Brown K. Disease severity during SARS-COV-2 reinfection: a nationwide study. J Infect. 2022;84(4):542–550. doi: 10.1016/j.jinf.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China – Key questions for impact assessment. New Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- Mur L, Sánchez-Vizcaíno JM, Fernández-Carrión E, Jurado C, Rolesu S, Feliziani F, Laddomada A, Martínez-López B. Understanding African Swine Fever infection dynamics in Sardinia using a spatially explicit transmission model in domestic pig farms. Transbound Emerg Dis. 2018;65:123–134. doi: 10.1111/tbed.12636. [DOI] [PubMed] [Google Scholar]
- Musa SS, Yusuf A, Zhao S, Abdullahi ZU, Abu-Odah H, Saad FT, Adamu L, He D. Transmission dynamics of COVID-19 pandemic with combined effects of relapse, reinfection and environmental contribution: a modeling analysis. Res Phys. 2022;38:105653. doi: 10.1016/j.rinp.2022.105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelman RB, Hanincová K, Mukherjee P, Liveris D, Nowakowski J, McKenna D, Brisson D, Cooper D, Bittker S, Madison G, Holmgren D, Schwartz I, Wormser GP. Differentiation of reinfection from relapse in recurrent Lyme disease. New Engl J Med. 2012;367(20):1883–1890. doi: 10.1056/NEJMoa1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Infectious Diseases (NIID) (2022) Evaluation of the latest infection status, etc. https://www.niid.go.jp/niid/en/2019-ncov-e.html. Accessed 21 June 2022
- Nguyen NN, Houhamdi L, Hoang VT, Delerce J, Delorme L, Colson P, Brouqui P, Fournier P-E, Raoult D, Gautret P. SARS-CoV-2 reinfection and COVID-19 severity. Emerg Microbes Infect. 2022;11(1):894–901. doi: 10.1080/22221751.2022.2052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor L, Yan E. Covid-19 vaccines, rules, deaths, and tourism recovery. Ann Tour Res. 2022;95:103424. doi: 10.1016/j.annals.2022.103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara R, Dey B, Leonard NE. Bistability and resurgent epidemics in reinfection models. IEEE Control Syst Lett. 2018;2(2):290–295. doi: 10.1109/LCSYS.2018.2832063. [DOI] [Google Scholar]
- Parikh N, Youssef M, Swarup S, Eubank S (2013) Modeling the effect of transient populations on epidemics in Washington DC. Scientific Reports 3, Article 3152, 8pp. 10.1038/srep03152 [DOI] [PMC free article] [PubMed]
- Perisic A, Bauch CT. Social contact networks and disease eradicability under voluntary vaccination. PLoS Comput Biol. 2009;5(2):e1000280. doi: 10.1371/journal.pcbi.1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan LT, Le HQ, Cao TM. Importation and human-to-human transmission of a novel coronavirus in Vietnam. New Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Aguiar M, Martins J, Stollenwerk N. Dynamics of epidemiological models. Acta Biotheor. 2010;58:381–389. doi: 10.1007/s10441-010-9116-7. [DOI] [PubMed] [Google Scholar]
- Postel A, Austermann-Busch S, Petrov A, Moennig V, Becher P. Epidemiology, diagnosis and control of classical swine fever: Recent developments and future challenges. Transbound Emerg Dis. 2018;65:248–261. doi: 10.1111/tbed.12676. [DOI] [PubMed] [Google Scholar]
- Price O, Birrell F, Mifsud E, Sullivan S. Epidemiology of repeat influenza infection in Queensland, Australia, 2005–2017. Epidemiol Infect. 2022;150:E144. doi: 10.1017/S0950268822001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu B, Wu Z, Tao B, Li Z, Song H, Tian D, Wu J, Zhan M, Wang J. Risk factors for types of recurrent tuberculosis (reactivation versus reinfection): a global systematic review and meta-analysis. Int J Infect Dis. 2022;116:14–20. doi: 10.1016/j.ijid.2021.12.344. [DOI] [PubMed] [Google Scholar]
- Ramaswamy H, Oberai AA, Yortsos YC. A comprehensive spatial-temporal infection model. Chem Eng Sci. 2021;233:116347. doi: 10.1016/j.ces.2020.116347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegar R, Higgins-Desbiolles F, Ruhanen L. COVID-19 and a justice framework to guide tourism recovery. Ann Tour Res. 2021;91:103161. doi: 10.1016/j.annals.2021.103161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Singh R, Singh J. Mathematical analysis of multi-compartmental malaria transmission model with reinfection. Chaos, Solitons Fractals. 2022;163:112527. doi: 10.1016/j.chaos.2022.112527. [DOI] [Google Scholar]
- Ren X, Zhou J, Guo J, Hao C, Zheng M, Zhang R, Huang Q, Yao X, Li R, Jin Y. Reinfection in patients with COVID-19: a systematic review. Global Health Res Policy. 2022;7:12. doi: 10.1186/s41256-022-00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad-Roy CM, Metcalf JE, Grenfell BT. Immuno-epidemiology and the predictability of viral evolution. Science. 2022;376(6598):1161–1162. doi: 10.1126/science.abn9410. [DOI] [PubMed] [Google Scholar]
- Salzer HJ, Neuböck M, Heldt S, Haug I, Paar C, Lamprecht B. Emerging COVID-19 reinfection four months after primary SARS-CoV-2 infection. Wien Med Wochenschr. 2022;172(3):74–76. doi: 10.1007/s10354-021-00813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno H. An SIS model for the epidemic dynamics with two phases of the human day-to-day activity. J Math Biol. 2020;80(7):2109–2140. doi: 10.1007/s00285-020-01491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno H (2022) A Primer on Population Dynamics Modeling: Basic Ideas for Mathematical Formulation. Theoretical Biology series, Springer Singapore, Singapore. 10.1007/978-981-19-6016-1
- Shaheen NA, Sambas R, Alenezi M, Alharbi NK, Aldibasi O, Bosaeed M. COVID-19 reinfection: a multicenter retrospective study in Saudi Arabia. Ann Thorac Med. 2022;17(2):81–86. doi: 10.4103/atm.atm_74_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siettos CI, Russo L. Mathematical modeling of infectious disease dynamics. Virulence. 2013;4(4):295–306. doi: 10.4161/viru.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LP, Jin Z, Sun GQ. Reinfection induced disease in a spatial SIRI model. J Biol Phys. 2011;37:133–140. doi: 10.1007/s10867-010-9204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Sonu & Srivastava PK, Nonlinear dynamics of a SIRI model incorporating the impact of information and saturated treatment with optimal control. Eur Phys J Plus. 2022;137(9):1028. doi: 10.1140/epjp/s13360-022-03201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollenwerk N, Martins J, Pinto A. The phase transition lines in pair approximation for the basic reinfection model SIRI. Phys Lett A. 2007;371(5–6):379–388. doi: 10.1016/j.physleta.2007.06.040. [DOI] [Google Scholar]
- Thu TP, Ngoc PN, Hai NM. Effect of the social distancing measures on the spread of COVID-19 in 10 highly infected countries. Sci Total Environ. 2020;742:140430. doi: 10.1016/j.scitotenv.2020.140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DM, Carver S, Jones ME, Krkošek M, Skerratt LF. Emerging infectious diseases of wildlife: a critical perspective. Trends Parasitol. 2015;31(4):149–159. doi: 10.1016/j.pt.2015.01.007. [DOI] [PubMed] [Google Scholar]
- van Boven M, de Melker HE, Schellekens JF, Kretzschmar M. Waning immunity and sub-clinical infection in an epidemic model: implications for pertussis in The Netherlands. Math Biosci. 2000;164(2):161–182. doi: 10.1016/S0025-5564(00)00009-2. [DOI] [PubMed] [Google Scholar]
- van den Driessche P. Reproduction numbers of infectious disease models. Infect Dis Model. 2017;2:288–303. doi: 10.1016/j.idm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgger M, Taplin R, Aebli A. Recovery of domestic tourism during the COVID-19 pandemic: an experimental comparison of interventions. J Hosp Tour Manag. 2021;48:428–440. doi: 10.1016/j.jhtm.2021.07.015. [DOI] [Google Scholar]
- Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119(2):183–201. doi: 10.1017/S0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CE, Meslé MMI, Hall IM. Modelling the global spread of diseases: a review of current practice and capability. Epidemics. 2018;25:1–8. doi: 10.1016/j.epidem.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang L, Xu Y, He W, Zhang C, Bi F, Tan Y, Ning C. Epidemiology of influenza virus reinfection in Guangxi, China: a retrospective analysis of a nine-year influenza surveillance data: Characteristics of influenza virus reinfection. Int J Infect Dis. 2022;120:135–41. doi: 10.1016/j.ijid.2022.04.045. [DOI] [PubMed] [Google Scholar]
- Wang K-Y. How change of public transportation usage reveals fear of the SARS virus in a city. PloS one. 2014;9(3):e89405. doi: 10.1016/j.ijid.2022.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu JT. Characterizing the dynamics underlying global spread of epidemics. Nat Commun. 2018;9(1):218. doi: 10.1038/s41467-017-02344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S (2021) Dynamic Assessment of Population by Using SIRI Model. In: 4th International Conference on Biometric Engineering and Applications 2021 May 25 (pp. 93-97). 10.1145/3476779.3476795
- Wilson ME (2010) Global travel and emerging infections. Infectious Disease Movement in a Borderless World: Workshop Summary, 90–104
- World Health Organization (WHO) (2018) Managing epidemics: key facts about major deadly diseases. World Health Organization. https://apps.who.int/iris/handle/10665/272442 License: CC BY-NC-SA 3.0 IGO
- World Health Organization (WHO) (2022) Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 21 June 2022
- Yan H, Wei H, Wei M. Exploring tourism recovery in the post-covid-19 period: An evolutionary game theory approach. Sustainability. 2021;13(16):9162. doi: 10.3390/su13169162. [DOI] [Google Scholar]
- Yum H-K, Park I-N, Shin B-M, Choi S-J. Recurrent Pseudomonas aeruginosa Infection in Chronic Lung Diseases: relapse or Reinfection? Tuberc Respir Dis. 2014;77(4):172–177. doi: 10.4046/trd.2014.77.4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang S, Hu T, Fu X, Wang X, Hu Y, Halloran B, Li Z, Cui Y, Liu H, Liu Z, Bao S. Human mobility and COVID-19 transmission: a systematic review and future directions. Ann GIS. 2022 doi: 10.1080/19475683.2022.2041725. [DOI] [Google Scholar]
- Zhang Q, Zhou M, Yang Y, You E, Wu J, Zhang W, Jin J, Huang F. Short-term effects of extreme meteorological factors on childhood hand, foot, and mouth disease reinfection in Hefei, China: a distributed lag non-linear analysis. Sci Total Environ. 2019;653:839–848. doi: 10.1016/j.scitotenv.2018.10.349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.