Abstract
Background and Aims
Cannabis and its various derivatives are commonly used for both recreational and medicinal purposes. Cannabinoids have been shown to have anti‐inflammatory properties. Inflammation is an important component of wound healing and the effect of cannabinoids on wound healing has become a recent topic of investigation. The objective of this article is to perform a comprehensive review of the literature to summarize the effects of cannabinoids on wound healing of the skin and to guide future avenues of research.
Methods
A comprehensive literature review was performed to evaluate the effects of cannabinoids on cutaneous wound healing.
Results
Cannabinoids appear to improve skin wound healing through a variety of mechanisms. This is supported through a variety of in vitro and animal studies. Animal studies suggest application of cannabinoids may improve the healing of postsurgical and chronic wounds. There are few human studies which evaluate the effects of cannabinoids on wound healing and many of these are case series and observational studies. They do suggest cannabinoids may have some benefit. However, definitive conclusions cannot be drawn from them.
Conclusion
While further human studies are needed, topical application of cannabinoids may be a potential therapeutic option for postsurgical and chronic wounds.
Keywords: cannabidiol, cannabinoids, cannabis, endocannabinoid, wound healing
1. INTRODUCTION
Cannabis has been cultivated throughout history since 8000 BC and has been utilized for medicine, textiles, rope, paper, and recreation. In the 19th century, it was even prescribed by physicians for gastrointestinal issues and nausea. 1 , 2 , 3 However, during the 20th century, significant restrictions were implemented which limited availability for recreational use and the ability to test the medical applications of cannabis. 4 However, since the 1990s, there has been a movement towards legalization of both medical and recreational cannabis use. With this, there has been an increase in the number of cannabis users in the United States and its use has even surpassed tobacco use with 16% of Americans reporting they currently smoke marijuana. 5 , 6
Today, cannabis is a term that is often used interchangeably with marijuana. However, this is an incorrect attribution. Although the precise taxonomy has not been agreed upon, the plant used in the development of marijuana is considered either a species (Cannabis indica) in the larger Cannabis genus, or a subspecies (C. sativa subsp. indica) of the Cannabis sativa species. The plant responsible for cannabis derived products is recognized as either the species Cannabis sativa in the former taxonomy or C. sativa subsp. sativa in the latter. 7 , 8 For the purposes of this review, we will accept the former taxonomy for simplicity's sake, recognizing the ongoing debate in this field.
Regardless of the taxonomy, a vast array of chemicals has been identified from cannabis plants. Of these, the cannabinoids have been an area of great interest. These cannabinoids include delta‐9 tetrahydrocannabinol (THC), the psychoactive compound that is predominantly found in the C. indica plant and is the focus of regulations, as well as cannabidiol (CBD), predominantly found in the C. sativa plant, which is the focus of industrial uses and nonpsychotropic medical research. 9 , 10
While the systemic effects of cannabis have been well studied, there is a dearth of literature addressing the effects on the wound healing process. 11 , 12 , 13 , 14 Cannabis and its components have been shown to have anti‐inflammatory properties. 15 As inflammation is an important component within the process of wound healing, the effect of cannabis and its constituents on wound healing has become a topic of renewed investigation. 16
The endocannabinoid system is centered around the cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors. Traditionally, CB1 was reported to be found within the central nervous system while CB2 was located within peripheral tissues and immune cells. 17 However, recent studies have shown this is not always the case and CB1 receptors may also be found peripherally. 18 Additionally, while there are numerous natural cannabinoids which have been isolated and studied, there has been a movement to develop selective CB1 and CB2 agonists and antagonists which has furthered the understanding of how cannabinoids could play a role in cellular homeostasis. 19 , 20 This review describes a variety of CB1 and CB2 ligands including the CB1 agonist, arachidonoyl‐chloro‐ethanolamide (ACEA), the CB2 agonists JWH015, JWH133, and GP1a and the nonspecific CB receptor agonist WIN55,212‐2. Additionally, there are some compounds that interact with multiple receptors such as VCE‐004.8, which is a CB2 and PPARγ agonist. Finally, there are a variety of antagonists such as AM251 and AM630, which are CB1 and CB2 receptor antagonists, respectively (Table 1). These compounds can have varying actions on the CB receptors leading to complex downstream effects. However, the increased understanding of the roles of the CB1 and CB2 and the development of selective receptor ligands have allowed for further understanding of the effect of cannabinoids on the wound healing process.
Table 1.
Table listing various cannabinoid agonists and antagonists.
| Arachidonoyl‐chloro‐ethanolamide (ACEA) | CB1 agonist |
|---|---|
| JWH015 | CB2 agonist |
| JWH133 | CB2 agonist |
| GP1a | CB2 agonist |
| WIN55,212‐2 | Nonspecific CB receptor ligand |
| VCE‐004.8 | CB2 and PPARγ agonist |
| AM251 | CB1 antagonist |
| AM630 | CB2 antagonist |
The goal of this review article is to perform a comprehensive review of the literature including in vitro, animal, and human studies, and to summarize the effects of cannabinoids on wound healing of the skin to guide potential future avenues of translational research.
2. METHODS
A comprehensive review of the literature was performed using OVID Medline. Search terms included wound healing or wound inflammation and cannabis, Cannabis sativa, cannabidiol, cannabinoids, tetrahydrocannabinol, marijuana, hashish, or hemp. Primary basic science, animal, and human studies were identified, and abstracts were reviewed by the primary authors. Review articles and studies evaluating wound healing unrelated to the skin were excluded. Only articles in English and full text articles were included for evaluation (Figure 1). The included articles were evaluated by the authors and summarized below.
Figure 1.
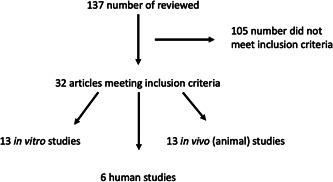
Diagram of articles reviewed.
3. RESULTS
A total of 32 articles met the inclusion criteria. There were 13 in vitro studies, 13 in vivo studies, and six human studies (Tables 2, 3, 4). The studies are summarized below.
Table 2.
Table summarizing the in vitro studies evaluated in this review article.
| In vitro studies | |||
|---|---|---|---|
| Year | Author | Cannabinoid type | Findings |
| 2013 | Ramot et al. 21 | ACEA and AM251 | Keratin 6, a protein which regulates keratinocyte migration, is altered by CB1 agonists and antagonists |
| 2015 | Styrczewska et al. 22 | Flax fiber extract: CBD, phytosterol, and unsaturated fatty acids | Flax fiber extract showed improved wound healing, decreased inflammation, and promotion of the extracellular matrix after scratch test assays on human skin cells |
| 2017 | Bort et al. 23 | JWH015 | CB2 agonist reduced proinflammatory markers, increased anti‐inflammatory markers, and led to faster scratch gap closure |
| 2018 | Ruhl et al. 24 | CBD | Multipotent mesenchymal stromal cells treated with CBD showed reduced oxidative stress |
| 2019 | Sangiovanni et al. 25 | CBD and cannabis sativa extract | Skin cells treated with these compounds showed decreased proinflammatory cytokine release and downregulation of genes which were overexpressed with TNF‐α treatment |
| 2020 | Ruhl et al. 26 | WIN55,212‐2 and JWH133 | Multipotent mesenchymal stromal cells treated with a nonspecific CB receptor ligand and a CB2 agonist had varying effects on their differentiation which, in turn, may affect wound healing |
| 2020 | Correia‐Sa et al. 27 | N/A | Obtained skin samples from patients who underwent body contouring surgery: noted lower levels of anandamide found in patients who developed hypertrophic scars |
| 2021 | Ruhl et al. 28 | anandamide, 2‐arachidonoyl‐glycerol, and JWH133 | Macrophage and mesenchymal stromal cells treated with endocannabinoids showed downregulation of their inflammatory responses |
| 2021 | Correia‐Sa et al. 29 | ACEA and AM251 | Skin samples from abdominoplasty patients treated with CB1 agonist and antagonist noted that the agonist showed increased collagen deposition and reepithelialization |
| 2021 | Correia‐Sa et al. 29 | JWH133 and AM630 | Fibroblast cells treated with a CB2 agonist and antagonist showed varying effects on collagen deposition depending on whether cells were treated with TGF‐β |
| 2021 | Atalay et al. 30 | CBD | Keratinocytes treated with H2O2 led to a proinflammatory state which was partially attenuated by CBD |
| 2021 | Miller et al. 31 | THC and CBD | Multiple cells lines treated with THC and CBD showed improved cell migration and specifically porcine primary fibroblasts showed faster wound closure |
| 2021 | Antezana et al. 32 | Cannabis sativa extract | Collagen hydrogels loaded with silver nanoparticles and C. sativa extract provided a matrix for tissue growth and showed improved biocompatibility compared to similar products without C. sativa extract |
Abbreviations: TGF, transforming growth factor; TNF, tumor necrosis factor.
Table 3.
Table summarizing the in vivo studies evaluated in this review article.
| In vivo studies | |||
|---|---|---|---|
| Year | Author | Cannabinoid type | Findings |
| 2012 | Zheng et al. 33 | N/A | Created wounds on mice: CB2 was present at baseline in uninjured tissue and its expression increased after wound injury and peaked at 3–5 days |
| 2016 | Li et al. 34 | Intraperitoneal GP1a and AM630 | Mice wounds after treatment with a CB2 agonist showed less collagen, decreased skin thickness, and downregulation of fibrosis genes compared to an antagonist |
| 2016 | Wang et al. 35 | Intraperitoneal GP1a and AM630 | Mice wounds after treatment with a CB2 agonist showed less wound contraction, accelerated reepithelialization, thinner dermal scar with more slender collagen fibers while the antagonist showed delayed reepithelialization |
| 2016 | Wohlman et al. 36 | N/A | Mice wounds treated with sulfur and nitrogen mustard led to upregulation of CB1 and CB2 and findings suggested CB1 may regulate keratinocyte proliferation whereas CB2 may regulate keratinocyte differentiation |
| 2016 | Del Rio et al. 37 | Intraperitoneal VCE‐004.8 or AM630 | A CB2 and PPARγ agonist reduced collagen synthesis, deposition, and myofibroblast differentiation and reduced skin fibrosis when mice were treated with bleomycin (to create an animal model for systemic scleroderma) |
| 2016 | Mehrabani et al. 38 | Topical “New Formula” (included hemp oil) | Mice burn wounds treated with new formula showed increased wound contraction and decreased epithelialization time |
| 2018 | Du et al. 39 | Intraperitoneal JWH133, GP1a, and AM630 | CB2 led to an anti‐inflammatory response by inhibiting proinflammatory M1 macrophages rather than increasing activity of anti‐inflammatory M2 macrophages. |
| 2018 | Klein et al. 40 | Intraperitoneal CBD | Mice wounds after CBD treatment showed lower inflammatory infiltrate on day 3. On day 7, they noted increased tissue organization and marked epithelial changes |
| 2019 | Koyama et al. 41 | Topical beta‐caryophyllene | Mice wounds treated with CB2 ligand showed improved reepithelialization. However, other findings suggested these may not be directly related to CB2 activation. |
| 2020 | Casares et al. 42 | Topical CBD | Mice skin treated with topical CBD showed increased levels of HMOX1 and wound repair keratins in mice skin |
| 2020 | McIver et al. 43 | Topical CBD | Horse wounds treated with either unique manuka factor versus topical CBD showed no difference in healing rates |
| 2021 | Ruhl et al. 24 | N/A | CB1 knockout mice showed delayed wound healing and CB2 knockout mice had increased proinflammatory cytokines but no changes to tissue regeneration |
| 2021 | Zhao et al. 44 | Topical GP1a hydrogel | GP1a hydrogel to mice wounds decreased inflammation and fibrogenesis and increased wound closure and reepithelialization |
Table 4.
Table summarizing the human studies evaluated in this review article.
| Human studies | |||
|---|---|---|---|
| Year | Author | Cannabinoid type | Findings |
| 2018 | Chelliah et al. 45 | Topical CBD | Case series: patients with epidermolysis bullosa had improved symptoms |
| 2019 | Palmieri et al. 46 | Topical CBD | Retrospective review: patients with various cutaneous disorders had improved symptoms |
| 2020 | Maida et al. 47 | Topical CBD, terpenes, and flavonoids | Prospective cohort study: patients with nonuremic calciphylaxis leg ulcers who failed other treatments had a good response |
| 2021 | Maida et al. 48 | Topical CBD, terpenes, and flavonoids | Prospective cohort study: patients with venous leg ulcers who failed other treatments had a good response |
| 2021 | Diaz et al. 49 | Oral THC and CBD oils | Case report: patient with pressure ulcer refractory to treatment for 5 years improved incidentally after starting CBD and THC oil |
| 2021 | Schrader et al. 50 | Topical, oral, and inhaled cannabinoids | Cross‐sectional survey: patients with epidermolysis bullosa had improvement in their symptoms |
3.1. In vitro studies
Many studies have evaluated the effects of cannabinoids and CB receptor ligands on keratinocyte and fibroblast cells lines. Sangiovanni et al. 25 treated keratinocyte and fibroblast cell lines with tumor necrosis factor‐α (TNF‐α) and either C. sativa or cannabidiol (CBD) and evaluated the effects of the proinflammatory stimulus. They noted that C. sativa inhibited the proinflammatory cytokine release to varying degrees whereas CBD showed some inhibition but not to the same degree as C. sativa. Finally, they showed that both compounds were able to downregulate genes which were overexpressed after TNF‐α treatment. 25 Miller et al. 31 treated bone marrow‐derived stem cells, adipose‐derived stem cells, and porcine primary fibroblasts with THC and CBD and noted improved cell migration. Additionally, they found when porcine primary fibroblasts where exposed to CBD, there was a 75% faster wound closure. 31 Ramot et al. 21 found the expression of keratin 6, a protein which downregulates keratinocyte migration, decreased when keratinocytes were treated with arachidonoyl‐chloro‐ethanolamide (ACEA), a CB1 agonist, and increased when keratinocytes were treated with a CB1 antagonist. They hypothesized the effects of CB1 stimulation on keratin 6 could be exploited to modulate reepithelialization in wound healing. 21 Bort et al. 23 showed that JWH015, a CB2 agonist, reduced proinflammatory cytokines and increased anti‐inflammatory compounds, such as transforming growth factor‐β (TGF‐β), in keratinocytes and fibroblasts treated with lipopolysaccharide, and induced a faster gap closure during a scratch test assay. The cells were treated with CB1 and CB2 antagonists and they noted these effects were likely modulated through both receptors. Additionally, they utilized porcine skin as an ex vivo skin model and found that JWH015 had sustained and low transdermal distribution which may have promising therapeutic potential. 23
Ruhl et al. 24 treated cells with lipopolysaccharides and observed that co‐treatment with CBD reduced the oxidative stress on cells. Lipopolysaccharides also inhibited further cell differentiation, and this was attenuated by CBD. 24 They then evaluated the effects of WIN55,212‐2, a nonspecific CB receptor ligand, and JWH133, a CB₂ agonist, on multipotent mesenchymal stromal cells from subcutaneous adipose tissue and noted these compounds had varying effects on their differentiation. Interestingly, they also noted that both WIN55,212‐2 and JWH133 increased hepatocyte growth factor which is known to stimulate migration and proliferation of keratinocytes and is important in cutaneous wound healing. 26 They then treated macrophages and mesenchymal stromal cells with lipopolysaccharides and evaluated the inflammatory response after treatment with the endocannabinoids anandamide, 2‐arachidonoyl‐glycerol, and JWH133 and noted they decreased the inflammatory response of M1 macrophages. This decrease was less significant in activated mesenchymal stromal cells. 28
Correia‐Sa et al. 27 collected skin samples from patients who underwent body contouring surgery and noted lower levels of anandamide were found in hypertrophic scars compared to normal scars. They suggested that reduced anandamide is potentially related to increased inflammation or a prolonged inflammatory phase which predisposed patients to scar hypertrophy. 27 They then collected skin samples from abdominoplasty patients and treated cells with ACEA and AM251, a CB1 antagonist. They noted increased collagen deposition with ACEA and decreased collagen deposition with AM251. Additionally, they performed ex vivo reepithelization studies and noted ACEA speeds reepithelialization. 29 This same group then evaluated the effects of TGF‐β on fibroblast cells and treated these cells with JWH133 and AM630, a CB2 antagonist. They noted TGF‐β increased α‐SMA expression, which is a marker for myofibroblast differentiation, and increased expression of CB2. Both JWH133 and AM630 led to decreased collagen deposition and α‐SMA expression after exposure to TGF‐β. When the fibroblasts were not treated with TGF‐β, only AM630 led to decreased collagen deposition and α‐SMA expression. This study showed the complex interplay of CB2 on the wound healing process. 51
Styrczewska et al. 22 performed scratch test assays with hydrophobic flax fiber extract, which contained CBD, and demonstrated improved wound healing via inhibition of chronic inflammation and promotion of extracellular matrix remodeling and skin cell migration. The authors suggested the improvement is secondary to two components: presence of CBD and the content of phytosterol. Atalay et al. 30 treated keratinocytes with hydrogen peroxide which led to a proinflammatory state which was partially attenuated by CBD.
Some authors also evaluated how CBD may assist in modulating wound healing dressings. Antezana et al. 32 developed collagen hydrogels loaded with silver nanoparticles and C. sativa extract. They found this compound reduced bacterial growth and provided a matrix that allowed for tissue growth. The silver nanoparticles and collagen gels were shown to be toxic to human cell lines; however, with the addition of C. sativa there was improved biocompatibility due to decreased cell toxicity and continued antimicrobial activity. 32
3.2. Animal studies
Ruhl et al. 52 evaluated CB1 and CB2 knockout mice by creating a wound which was analyzed over a 2‐week period. They found CB2 knockout mice had increased proinflammatory cytokines but no changes in tissue regeneration. CB1 knockout mice showed delayed wound closure early in the healing process suggesting that while previously not considered as present within the immune system, CB1 does play a role in modulating inflammation during wound healing. 52
Zheng et al. 33 created a 1.5 cm incision on mice and evaluated these wounds at variable time intervals for the for the timing of CB2 expression. They found that while CB2 was present at baseline within uninjured tissue, its expression within inflammatory cells significantly increased after wound injury. They found macrophages and myofibroblasts with CB2 reached their maximum numbers at 3 and 5 days, respectively. 33 They then evaluated the effects of CB2 activation on fibrogenesis. They created two 6 mm wounds on mice dorsum and subsequently treated the mice with GP1a, a CB2 agonist, AM630, and a control, intraperitoneally. They found mice treated with GP1a had less collagen deposition, more slender collagen fibers, and decreased skin thickness whereas those treated with AM630 showed opposing results. Additionally, the GP1a group showed decreased expression of cytokines involved in fibrogenesis and downregulation of fibrosis associated genes whereas the AM630 group showed increased levels of these cytokines and upregulation of these genes. 34 In another study, they evaluated the effects of GP1a and AM630 on wound inflammation and reepithelialization after wounds were created on mice. When treated with AM630, there was delayed reepithelization. When treated with the GP1a, they noted decreased wound contraction, accelerated reepithelialization, thinner dermal scar with more slender collagen fibers, increased interferon‐γ, and decreased epidermal hypertrophy, granulation tissue, inflammatory infiltration, and expression of inflammatory proteins. Additionally, there was more epithelial to mesenchymal transition with increased ability for cell migration. Overall, these findings suggest CB2 receptor activation attenuates wound inflammation and fibrogenesis. 35 In 2018, they created cutaneous wounds on mice and treated them with JWH133, GP1a, and AM630 to evaluate the specific macrophage response noted in the wound bed. They noted that while both M1 and M2 macrophages were present within the wound bed after injury, M1 macrophages were seen earlier in the injury process whereas M2 macrophages were more present in the later stages. They also found the presence of M1 macrophages was likely related to expression of the CB2 and suggested its activation led to an anti‐inflammatory response by inhibiting the proinflammatory M1 macrophages rather than increasing the activity of the anti‐inflammatory M2 macrophages. 39 Overall, the studies from this group describe the critical role that CB2 activation plays in improved wound healing.
Klein et al. 40 created oral tongue ulcers in rats and treated them with varying concentrations of CBD and a control, intraperitoneally. They grossly and pathologically analyzed the wound at days 3 and 7. They noted no gross difference between the groups. However, they found that at day 3 the groups treated with CBD had a significantly lower level of inflammatory infiltrate compared to the vehicle groups, but this change was not noted on day 7. Interestingly, they noted increased tissue organization at day 7 and more marked epithelial changes in the CBD group suggesting its favorable effects on reepithelization. 40
Zhao et al. 44 created a hydrogel containing Gp1a and administered this locally to 4 mm punch wounds created on mice. They found after treatment with the Gp1a hydrogel, there was increased levels of CB2 expression 4 and 8 days after surgery suggesting the hydrogel allowed for gradual release of Gp1a over 8 days. Additionally, the Gp1a hydrogel group showed decreased messenger RNA (mRNA) expression of inflammatory cytokines, fibrosis, skin thickness, and mRNA and protein levels of collagen I compared to the control. Finally, the Gp1a hydrogel group showed faster wound healing, longer epithelial sheets, and increased levels of protein markers characteristic of epithelial‐mesenchymal transition. 44
Koyama et al. 41 treated mice wounds with beta‐caryophyllene, a compound present in herbs and spices and a ligand of CB2. They noted improved reepithelialization, increased markers of reepithelization, and increased cell proliferation throughout the wound bed. However, while some of their experiments suggested these changes may be related to CB2 activation, others suggested they may not be directly related to CB2 activation given that CB2 was downregulated in the beta‐caryophyllene group. 41 Wohlman et al. 36 evaluated the effects of sulfur mustard and nitrogen mustard on mice. Administration of the mustard compounds led to epidermal hyperplasia and upregulation of CB1, CB2, PPARα, and fatty acid amid hydrolase (FAAH). They noted there was a significant increase in these proteins immediately after exposure to the vesicants, and the increase in CB1, CB2, and PPARα was persistent in the hyperplastic epidermis. Their results suggested CB1 may be important in regulating keratinocyte proliferation given it was upregulated in proliferating cells whereas CB2 may regulate keratinocyte differentiation as it was upregulated within sebaceous glands. 36 Del Rio 37 developed a CB2 and PPARγ agonist, VCE‐004.8. They showed TGF‐β induced collagen synthesis, deposition, and myofibroblast differentiation were decreased when cells were pretreated with VCE‐004.8 and subsequently stimulated with TGF‐β. Interestingly, scratch test assays showed that pretreatment with VCE‐004.8 attenuated wound closure induced by TGF‐β, further emphasizing the complex interplay of cannabinoid receptors and wound healing. To create an animal model of systemic scleroderma, they treated mice with bleomycin injections which increased dermal thickness and collagen content and they showed treatment with VCE‐004.8 reduced skin fibrosis. Whereas when pretreated with a CB2 or PPARγ antagonist before treatment with VCE‐004.8, a reduced antifibrotic response was noted, suggesting the observed effect is dependent of both PPARγ and CB2. 37 Casares et al. 42 evaluated the effects of CBD on HMOX1 which has several important antioxidant and anti‐inflammatory properties. When keratinocyte cells were treated with CBD, there was noted to be increased HMOX1 which was likely secondary to BACH1 degradation. They also performed in vivo studies on mice using topical CBD which showed increased levels of HMOX1 and wound repair keratins in mice skin. 42
Mehrabani et al. 38 described the beneficial effects of “new formula” (NF), a combination of sesame, wild pistachio, hemp, and walnut oil, on burn wounds in mice. They treated the burns with either nothing, NF, or silver sulfadiazine and noted the NF group had significantly increased wound contraction and decreased epithelialization time compared to the other groups. 38 McIver et al. 43 treated open wounds created on limbs of horses with either unique manuka factor (UMF) 5 alone (which has minimal antimicrobial activity), UMF 20 manuka honey (which has superior antimicrobial activity), saline, or UMF 5 mixed with 1% CBD. They found no difference in healing rates. They postulated that since all horses had at least one wound treated with CBD, the CBD had a systemic anti‐inflammatory effect leading to similar would healing times; however, no further testing was conducted to validate this hypothesis. 43
3.3. Human studies
There were limited studies which examined the effects of cannabinoids on humans and wound healing. The articles identified were mainly observational studies and case reports.
Diaz et al. 49 published a case report where they described a patient who was treated with oral cannabis oil, which included a combination of CBD and THC, to treat a chronic pressure ulcer. Specifically, the patient was administered a CBD‐dominant oil once daily and two different THC‐dominant oils twice daily. They noted significant improvement in the healing of the pressure ulcer at 2 weeks and almost complete closure by 2 months. A foam padded dressing was the only other intervention applied which may have confounded the effect of the cannabis oil treatment. 49 Maida et al. 47 , 48 discussed topical cannabis‐based medicines (TCBMs) which had varying amounts of THC, THCA (delta‐9 tetrahydrocannabinolic acid), and CBD. They have published two case series describing the treatment of venous stasis ulcers and nonuremic calciphylaxis ulcers with topical TCBMs. In the venous leg ulcer study, they treated 14 patients with 16 recalcitrant leg ulcers with TCBMs and compression bandaging. They reported complete closure in 11 patient and 13 wounds within a median of 34 days. In the nonuremic calciphylaxis leg ulcer study, two patients with multiple recalcitrant ulcers were treated with TCBMs. Both patients had complete closure of their wounds within a mean of 76.3 days. Although they reported a possible benefit in both studies, their study design did not include a control group making it difficult to determine if the TCBMs led to the wound closure. 47 , 48
In another case series, three patients self‐initiated various topical formulations of CBD to treat epidermolysis bullosa (EB) lesions. The formulations were not consistent among the cases nor was the application standardized. The patients were being treated with a variety of ineffective wound care measures, and after starting topical CBD, they reported significant improvement of pain, with reduction of opioid use as an endpoint, and concluded there was acceleration in wound healing. However, this was anecdotal and not based on objective measures. 45 In an international cross‐sectional study by Schraeder et al., 50 a survey was sent out to EB patients and their caretakers. Seventy‐one respondents reported utilizing both topical and oral CBD in varying concentrations and in conjunction with other remedies. Pain and pruritus were self‐reported to decrease by 3 points on a numerical rating scale from 0 to 10 (p < 0.001 for both) after CBD use. Most respondents reported CBD use improved their overall EB symptoms (95%), pain (94%), pruritus (91%), wound healing (81%), and decreased the use of pain medication (79%). 50 Palmieri et al. 46 retrospectively evaluated the effects of CBD ointment administered to patients 20 patients with moderate to severe psoriasis (five patients), atopic dermatitis (five patents), and the resulting scars from these disorders (10 patients). They were treated with an organic skin care ointment which contained CBD oil twice daily for 3 months. They objectively measured skin hydration, transepidermal water loss, and skin elasticity and noted improvements in all parameters. Additionally, they reported improvement in cutaneous blemishes and scars and fewer pustules and papules. Finally, they noted significant improvement in patient's Psoriasis Area Severity Index scores. 46
4. DISCUSSION
From some of the earliest recorded history, the medicinal use of cannabis and its derivatives has been an area of keen interest. Although many historical reports exist to profess claims of the capabilities of these compounds, only more recently has the scientific process been applied to determine what, if any, role they may have. Here, we reviewed the available literature on cannabis and its derivatives as they relate to cutaneous wound healing.
In vitro studies have offered a variety of targets in which cannabis containing compounds may influence wound healing. Overall, these studies suggest a positive effect on wound healing by cannabinoids. One study even utilized human skin samples and noted hypertrophic scars had lower levels of anandamide when compared to those with normal scars. 27 Perhaps the most compelling of target based on these studies is CB2 and its modulation of the acute phase inflammatory response. Later animal studies offered a potential explanation for this observation through the decreased expression of M1 macrophages in early wound healing when the wound was exposed to CB2 agonists. 39 Several subsequent studies have corroborated the beneficial effects seen with CB2 agonists in wound healing. In addition to its effect on early inflammatory markers, CB2 agonists appear to improve wound reepithelialization as well as lead to a decrease in fibrosis and epidermal hypertrophy. 29 , 34 , 35 , 37 , 38 Although the available human studies addressing cannabinoids and skin wound healing have promising results, the relative paucity of more robust study methods (e.g., randomized control trials, prospective case‐control studies, etc.) limits their applicability. Nonetheless, these studies serve as valuable proof of concept reports that future, more robust, studies may build upon.
In addition to discerning the physiologic method of action of cannabinoids and wound healing, another important avenue for research lies in medication delivery. Many of the animal studies reviewed here utilized intraperitoneal injection to deliver the experimental drug, even though systemic application may not be the ideal delivery mechanism to examine more localized effects. These systemic administrations increase technical difficulty as well as introduce unnecessary side effects for subjects. Topical and local application can be performed by several methods including oils, ointment, paste, local injection after wound closure, or impregnation into dressings. Additionally, some formulations may allow for sustained drug delivery. For example, Zhao et al. 44 developed a hydrogel which required only one application. If further human studies do show improved wound healing after cannabinoid application, optimizing drug delivery methods will be critical to improved outcomes.
As cannabis and cannabinoid‐related products become more pervasive in the lives of patients, it is important for researchers and clinicians alike to recognize this movement. Although the exact effect these compounds have on various conditions remains to be elucidated, their use should be recognized and documented in detail. It is no longer sufficient to only screen for recreational marijuana use. There is now a growing body of literature that demonstrates these legal over the counter and prescription compounds have active local and systemic effects that may help, or interfere with, conditions and treatments experienced by patients. Thus, further efforts must be made to accurately document the type and amount of cannabinoids a patient is utilizing.
When discussing new medical treatments, side effects must be considered. The negative systemic effects of cannabinoids are well known and include exacerbations of various psychiatric conditions and both acute and long‐term cognitive deficits. They have been shown to affect the cardiovascular system and may be associated with myocardial infarction, cardiomyopathy, and sudden cardiac death. When smoked, they can have deleterious effects on the respiratory system. Additionally, they can negatively affect the reproductive system. 53 Topical cannabinoid treatment will likely prevent many of the systemic side effects by directing the treatment directly to the tissue of interest. However, topical medications have a risk of acute skin reactions such as contact dermatitis or urticaria and this would also be a concern for topical cannabinoids. Interestingly, the endocannabinoid system may attenuate contact dermatitis and play a protective in these reactions. 54 A recent study evaluated the dermatological side effects of topical cannabinoids, including CBD and hemp seed oil. Overall, they found no significant reactions via patch testing and only saw a mild phototoxicity at 48 h when treated with hemp seed oil. The findings suggest that these products appear to be safe. However, given the various formulations that are in the market and the difficulty in regulating topical cannabinoids, these results are likely not generalizable to all cannabinoid products. 55 Further research is needed to understand the full spectrum of side effects of topical cannabinoids.
5. CONCLUSION
Cannabinoids appear to improve cutaneous wound healing through a variety of mechanisms, most notably through the CB2 receptor. Additional, more robust, in vivo and human studies are needed to better define these mechanisms as well as examine cannabinoid's role in human cutaneous wound healing. Finally, while the risks of systemic cannabinoids are well described, the risks of topical cannabinoids are not well known. Although their side effects do appear to be relatively mild, further studies are needed to understand the adverse effects of topical cannabinoids.
All authors discussed the results, reviewed the manuscript, and approved the final submitted version. Each author contributed important intellectual content during manuscript drafting or revision, agrees to be personally accountable for the author's own contributions, and to ensure that questions pertaining to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
AUTHOR CONTRIBUTIONS
Aniruddha C. Parikh: Conceptualization; data curation; investigation; methodology; writing—original draft; writing—review and editing. Christopher S. Jeffery: Conceptualization; data curation; investigation; methodology; writing—original draft; writing—review and editing. Zainab Sandhu: Investigation; writing—original draft; writing—review and editing. Benjamin P. Brownlee: Investigation; writing—original draft; writing—review and editing. Lurdes Queimado: Conceptualization; investigation; methodology; supervision; writing—review and editing. Mark M. Mims: Conceptualization; investigation; methodology; supervision; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Mark M. Mims affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
This research was supported by the National Cancer Institute (NCI) of the National Institutes of Health (R01CA242168). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Parikh AC, Jeffery CS, Sandhu Z, Brownlee BP, Queimado L, Mims MM. The effect of cannabinoids on wound healing: a review. Health Sci Rep. 2024;7:e1908. 10.1002/hsr2.1908
Part of this data was presented at the Combined Otolaryngology Spring Meeting, Dallas, TX, April 27–May 1, 2022.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new datasets were generated or analyzed during the current study.
REFERENCES
- 1. Russo EB, Jiang HE, Li X, et al. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J Exp Bot. 2008;59(15):4171‐4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skoglund G, Nockert M, Holst B. Viking and early middle ages Northern Scandinavian textiles proven to be made with Hemp. Sci Rep. 2013;3(1):2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodiversity. 2007;4(8):1614‐1648. [DOI] [PubMed] [Google Scholar]
- 4. Adrian M. What the history of drugs can teach us about the current cannabis legalization process: unfinished business. Subst Use Misuse. 2015;50(8‐9):990‐1004. [DOI] [PubMed] [Google Scholar]
- 5. Cerdá M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120(1‐3):22‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saad L Gallup. 2022 . Americans Not Convinced Marijuana Benefits Society.
- 7. Small E, Cronquist A. A practical and natural taxonomy for cannabis. Taxon. 1976;25(4):405‐435. [Google Scholar]
- 8. Schultes RE, Klein WM, Plowman T, Lockwood TE. Cannabis: an example of taxonomic neglect. Bot Mus Lealf Harv Univ. 1974;23(9):337‐367. [Google Scholar]
- 9. El‐Alfy AT, Ivey K, Robinson K, et al. Antidepressant‐like effect of Δ9‐tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95(4):434‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahrens J, Demir R, Leuwer M, et al. The nonpsychotropic cannabinoid cannabidiol modulates and directly activates Alpha‐1 and Alpha‐1‐Beta glycine receptor function. Pharmacology. 2009;83(4):217‐222. [DOI] [PubMed] [Google Scholar]
- 11. Cohen K, Weinstein A. The effects of cannabinoids on executive functions: evidence from cannabis and synthetic Cannabinoids—a systematic review. Brain Sci. 2018;8(3):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calabria B, Degenhardt L, Hall W, Lynskey M. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29(3):318‐330. [DOI] [PubMed] [Google Scholar]
- 13. Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and chronic effects of cannabinoids on human Cognition—a systematic review. Biol Psychiatry. 2016;79(7):557‐567. [DOI] [PubMed] [Google Scholar]
- 14. Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95(2):191‐202. [DOI] [PubMed] [Google Scholar]
- 15. Atalay S, Jarocka‐Karpowicz I, Skrzydlewska E. Antioxidative and anti‐inflammatory properties of cannabidiol. Antioxidants (Basel, Switzerland). 2019;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1‐2):81‐94. [DOI] [PubMed] [Google Scholar]
- 17. Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(s1):10‐14. [DOI] [PubMed] [Google Scholar]
- 18. Correia‐Sá I, Paiva A, Carvalho CM, Vieira‐Coelho MA. Cutaneous endocannabinoid system: does it have a role on skin wound healing bearing fibrosis? Pharmacol Res. 2020;159:104862. [DOI] [PubMed] [Google Scholar]
- 19. Ogawa LM, Burford NT, Liao YH, et al. Discovery of selective cannabinoid CB2 receptor agonists by high‐throughput screening. SLAS Discovery. 2018;23(4):375‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soethoudt M, Grether U, Fingerle J, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off‐target activity. Nat Commun. 2017;8(1):13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramot Y, Sugawara K, Zákány N, Tóth BI, Bíró T, Paus R. A novel control of human keratin expression: cannabinoid receptor 1‐mediated signaling down‐regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ. 2013;1(1):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Styrczewska M, Kostyn A, Kulma A, et al. Flax fiber hydrophobic extract inhibits human skin cells inflammation and causes remodeling of extracellular matrix and wound closure activation. BioMed Res Int. 2015;2015:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bort A, Alvarado‐Vazquez PA, Moracho‐Vilrriales C, et al. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol Pain. 2017;13:174480691668822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruhl T, Kim BS, Beier JP. Cannabidiol restores differentiation capacity of LPS exposed adipose tissue mesenchymal stromal cells. Exp Cell Res. 2018;370(2):653‐662. [DOI] [PubMed] [Google Scholar]
- 25. Sangiovanni E, Fumagalli M, Pacchetti B, et al. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother Res. 2019;33(8):2083‐2093. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ptr.6400 [DOI] [PubMed] [Google Scholar]
- 26. Ruhl T, Karthaus N, Kim BS, Beier JP. The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs. Exp Cell Res. 2020;389(1):111881. [DOI] [PubMed] [Google Scholar]
- 27. Correia‐Sá IB, Carvalho CM, Serrão PV, et al. A new role for anandamide: defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci Rep. 2020;10(1):11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruhl T, Corsten C, Beier JP, Kim BS. The immunosuppressive effect of the endocannabinoid system on the inflammatory phenotypes of macrophages and mesenchymal stromal cells: a comparative study. Pharmacological Reports. 2021;73(1):143‐153. [DOI] [PubMed] [Google Scholar]
- 29. Correia‐Sá IB, Carvalho CM, Serrão PV, et al. AM251, a cannabinoid receptor 1 antagonist, prevents human fibroblasts differentiation and collagen deposition induced by TGF‐β—an in vitro study. Eur J Pharmacol. 2021;892:173738. [DOI] [PubMed] [Google Scholar]
- 30. Atalay S, Gęgotek A, Domingues P, Skrzydlewska E. Protective effects of cannabidiol on the membrane proteins of skin keratinocytes exposed to hydrogen peroxide via participation in the proteostasis network. Redox Biol. 2021;46:102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller H, De Leo N, Badach J, et al. Role of marijuana components on the regenerative ability of stem cells. Cell Biochem Funct. 2021;39(3):432‐441. [DOI] [PubMed] [Google Scholar]
- 32. Antezana PE, Municoy S, Pérez CJ, Desimone MF. Collagen hydrogels loaded with silver nanoparticles and Cannabis sativa oil. Antibiotics. 2021;10(11):1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng JL, Yu TS, Li XN, et al. Cannabinoid receptor type 2 is time‐dependently expressed during skin wound healing in mice. Int J Legal Med. 2012;126(5):807‐814. [DOI] [PubMed] [Google Scholar]
- 34. Li SS, Wang LL, Liu M, et al. Cannabinoid CB2 receptors are involved in the regulation of fibrogenesis during skin wound repair in mice. Mol Med Rep. 2016;13(4):3441‐3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang LL, Zhao R, Li JY, et al. Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re‐epithelialization during skin wound healing. Eur J Pharmacol. 2016;786:128‐136. [DOI] [PubMed] [Google Scholar]
- 36. Wohlman IM, Composto GM, Heck DE, et al. Mustard vesicants alter expression of the endocannabinoid system in mouse skin. Toxicol Appl Pharmacol. 2016;303:30‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. del Río C, Navarrete C, Collado JA, et al. The cannabinoid quinol VCE‐004.8 alleviates bleomycin‐induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator‐activated receptor‐γ and CB2 pathways. Sci Rep. 2016;6(1):21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mehrabani M, Seyyedkazemi SM, Nematollahi MH, et al. Accelerated burn wound closure in mice with a new formula based on traditional Medicine. Iran Red Crescent Med J. 2016;18(11):e26613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Du Y, Ren P, Wang Q, et al. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J Inflamm (Lond). 2018;15(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klein M, de Quadros De Bortolli J, Guimarães FS, Salum FG, Cherubini K, de Figueiredo MAZ. Effects of cannabidiol, a Cannabis sativa constituent, on oral wound healing process in rats: clinical and histological evaluation. Phytother Res. 2018;32(11):2275‐2281. [DOI] [PubMed] [Google Scholar]
- 41. Koyama S, Purk A, Kaur M, et al. Beta‐caryophyllene enhances wound healing through multiple routes. PloS one. 2019;14(12):e0216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Casares L, García V, Garrido‐Rodríguez M, et al. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020;28:101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McIver V, Tsang A, Symonds N, et al. Effects of topical treatment of cannabidiol extract in a unique manuka factor 5 manuka honey carrier on second intention wound healing on equine distal limb wounds: a preliminary study. Aust Vet J. 2020;98(6):250‐255. [DOI] [PubMed] [Google Scholar]
- 44. Zhao C, Dong Y, Liu J, et al. An enzyme‐responsive Gp1a‐hydrogel for skin wound healing. J Biomater Appl. 2021;36(4):714‐721. [DOI] [PubMed] [Google Scholar]
- 45. Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self‐initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol. 2018;35(4):e224‐e227. [DOI] [PubMed] [Google Scholar]
- 46. Palmieri B, Laurino C, Vadalà M. A therapeutic effect of cbd‐enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170(2):e93‐e99. [DOI] [PubMed] [Google Scholar]
- 47. Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis‐based medicines—a novel paradigm and treatment for non‐uremic calciphylaxis leg ulcers: an open label trial. Int Wound J. 2020;17(5):1508‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis‐based medicines—a novel adjuvant treatment for venous leg ulcers: an open‐label trial. Exp Dermatol. 2021;30(9):1258‐1267. [DOI] [PubMed] [Google Scholar]
- 49. Diaz P, Katz T, Langleben A, Rabinovitch B, Lewis E. Healing of a chronic pressure injury in a patient treated with medical cannabis for pain and sleep improvement: a case report. Wound Manag Prev. 2021;67(10):42‐47. [PubMed] [Google Scholar]
- 50. Schräder NHB, Gorell ES, Stewart RE, et al. Cannabinoid use and effects in patients with epidermolysis bullosa: an international cross‐sectional survey study. Orphanet J Rare Dis. 2021;16(1):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Correia‐Sá I, Carvalho C, A. Machado V, et al. Targeting cannabinoid receptor 2 (CB2) limits collagen production—an in vitro study in a primary culture of human fibroblasts. Fundam Clin Pharmacol. 2022;36(1):89‐99. [DOI] [PubMed] [Google Scholar]
- 52. Ruhl T, Lippold EF, Christer T, Schaefer B, Kim BS, Beier JP. Genetic deletion of the cannabinoid receptors CB1 and CB2 enhances inflammation with diverging effects on skin wound healing in mice. Life Sci. 2021;285:120018. [DOI] [PubMed] [Google Scholar]
- 53. Cohen K, Weizman A, Weinstein A. Positive and negative effects of cannabis and cannabinoids on health. Clin Pharm Ther. 2019;105(5):1139‐1147. [DOI] [PubMed] [Google Scholar]
- 54. Karsak M, Gaffal E, Date R, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316(5830):1494‐1497. [DOI] [PubMed] [Google Scholar]
- 55. Maghfour J, Rietcheck H, Szeto MD, et al. Tolerability profile of topical cannabidiol and palmitoylethanolamide: a compilation of single‐centre randomized evaluator‐blinded clinical and in vitro studies in normal skin. Clin Exp Dermatol. 2021;46(8):1518‐1529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new datasets were generated or analyzed during the current study.


