Abstract
Objectives
The efficacy of transjugular intrahepatic portosystemic shunt (TIPS) plus extrahepatic collateral embolisation (TIPS+E) in reducing rebleeding and hepatic encephalopathy (HE) post-TIPS was recently reported in a meta-analysis, but further validation is essential. This study aims to confirm the effectiveness of TIPS+E using real-world data.
Methods
The multicentre retrospective cohort included 2077 patients with cirrhosis who underwent TIPS±E (TIPS: 631, TIPS+E: 1446) between January 2010 and December 2022. Regression and propensity score matching (PSM) were used to adjust for baseline characteristic differences. After PSM, clinical outcomes, including rebleeding, HE, survival and further decompensation (FDC), were analysed. Baseline data from all patients contributed to the construction of prognostic models.
Results
After PSM, 1136 matched patients (TIPS+E: TIPS=568:568) were included. TIPS+E demonstrated a significant reduction in rebleeding (HR 0.77; 95% CI 0.59 to 0.99; p=0.04), HE (HR 0.82; 95% CI 0.68 to 0.99; p=0.04) and FDC (HR 0.85; 95% CI 0.73 to 0.99; p=0.04), comparing to TIPS. Significantly, TIPS+E also reduced rebleeding, HE and FDC in subgroup of using 8 mm diameter stents and embolising of gastric varices+spontaneous portosystemic shunts (GV+SPSS). However, there were no differences in overall or subgroup survival analysis. Additionally, the random forest models showed higher accuracy and AUROC comparing to other models. Controlling post-TIPS portal pressure gradient (pPPG) within 7 mm Hg<pPPG<8.5 mm Hg improved prognosis, especially in TIPS+E group.
Conclusion
Our real-world data validation confirms the high efficacy of TIPS+E in reducing rebleeding and HE, particularly when using 8 mm diameter stents, embolising GV+SPSS and maintaining an optimal pPPG.
Keywords: LIVER CIRRHOSIS, PORTAL HYPERTENSION, INTERVENTIONAL RADIOLOGY, GASTROINTESTINAL BLEEDING, HEPATIC ENCEPHALOPATHY
WHAT IS ALREADY KNOWN ON THIS TOPIC
While controversy exists regarding the efficacy of TIPS+E, current guidelines recommend it for variceal bleeding. The clinical outcomes of TIPS+E remain debated, necessitating a comprehensive real-world analysis.
WHAT THIS STUDY ADDS
Our study, based on a large Chinese multicentre cohort, demonstrates that TIPS+E significantly reduces post-TIPS rebleeding, hepatic encephalopathy and further decompensation compared with TIPS alone in patients with cirrhosis. Subgroup analyses highlight benefits with 8 mm diameter stents and gastric varices+spontaneous portosystemic shunts embolisation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings provide robust support for the efficacy of TIPS+E in real-world settings, emphasising potential prognostic advantages with specific procedural considerations. The study is aligned with the principles of precision medicine, incorporating stent diameters and embolisation strategy, informing future guidelines and encouraging personalised interventions in clinical practice.
Introduction
Portal hypertension, a severe complication of liver cirrhosis, could cause a substantial risk of lethal events such as variceal bleeding, ascites and hepatic encephalopathy (HE), significantly impacting life quality and increasing disease burden.1–7 Transjugular intrahepatic portosystemic shunt (TIPS), particularly polytetrafluoroethylene (PTFE)-covered TIPS, is a well-established therapeutic intervention for portal hypertension and its associated complications.1–7 While current guidelines recommend TIPS plus extrahepatic collateral embolisation (TIPS+E) for controlling variceal bleeding and reducing rebleeding,1 2 5 7–10 the clinical outcomes of TIPS+E remain controversial.
In our recent meta-analysis11 including 15 studies and 1408 patients with cirrhosis with variceal bleeding, TIPS+E exhibited superior efficacy in reducing both post-TIPS rebleeding and HE, while no significant difference in risk of shunt dysfunction, new-onset ascites after TIPS, hepatocellular carcinoma, mortality or adverse events was found between the two groups. Similarly, earlier meta-analysis12 and recent studies have indicated the potential of TIPS+E in reducing variceal rebleeding,13–18 although no significant reduction in HE was observed in their analyses. Nevertheless, conflicting reports exist, with several studies reporting that TIPS+E has no significant efficacy on rebleeding or HE,19–21 exemplified by a recent randomised controlled trial (RCT).20 In this RCT, TIPS+E failed to significantly improve clinical outcomes in patients with liver cirrhosis, including rebleeding, HE or overall survival.
To comprehensively assess the efficacy of TIPS+E, this study aims to retrospectively analyse data from a large-sized real-world multicentre cohort in China and construct prognostic models for post-TIPS clinical outcomes.
Materials and methods
Patients and inclusion criteria
The retrospective study enrolled patients with cirrhosis with gastro-oesophageal varices (GOV) who underwent TIPS±E at six academic university affiliated hospitals between January 2010 and December 2022 (online supplemental figure 1). Eligible patients met the following inclusion criteria: (1) age between 18 and 75 years; (2) diagnosed with liver cirrhosis based on clinical features, imaging examinations or liver histology; (3) met TIPS indications according to recent guidelines1 2 4; (4) underwent TIPS±E for variceal bleeding; (5) received covered stents of 6 mm, 8 mm or 10 mm diameters (VIATORR, W.L. Gore & Associates, Phoenix, Arizona, USA or Fluency, Bard Peripheral Vascular, Tempe, Arizona, USA) and (6) had complete electronic health records including both medical records and TIPS procedure records. Exclusion criteria were as follows: (1) presence of contraindications on TIPS; (2) a Child-Turcotte-Pugh (CTP) score >13 points or Model for End-stage Liver Disease (MELD) >18 points; (3) received bared-mental stents of TIPS; (4) poor compliance to conventional therapy (such as entecavir or tenofovir for patients with chronic hepatitis B, strict abstinence from alcohol for individuals with alcoholic-related liver cirrhosis, and the use of ursodesoxycholic acid for primary biliary cirrhosis, among other specified therapies) for eliminating or suppressing the aetiologies; (5) prior TIPS, surgical shunt placement or liver transplantation; (6) non-cirrhotic portal hypertension (such as idiopathic non-cirrhotic portal hypertension, hepatic sinusoidal obstruction syndrome or Budd-Chiari syndrome, etc); (7) ≥50% occlusion of vena cava due to portal vein thrombosis or cavernous transformation; (8) advanced malignancy; (9) end-stage renal disease under renal replacement therapy; (10) severe cardiopulmonary disease; (11) pregnancy or lactation or child and (12) insufficient clinical data or had lost to follow-up within 1 year after the procedure. All patients received adequate treatment for eliminating or suppressing the aetiologies, such as entecavir or tenofovir for patients with chronic hepatitis B, etc.
bmjgast-2023-001310supp001.pdf (1.7MB, pdf)
TIPS and TIPS+E procedure
TIPS procedures in this study were performed according to established protocols as previously described.14 22 23 In brief, TIPS was performed under conditions of haemodynamic stabilisation after accessing the right hepatic vein via the internal jugular vein. An intrahepatic tract was established by crossing the hepatic veins and portal veins, and the tract was validated using portography. A PTFE-covered stent with a diameter of 6 mm, 8 mm or 10 mm was then placed to support the tract, with balloon dilation to suitable diameters. Portal pressure gradient (PPG) was measured before and after TIPS insertion (measured via the main portal vein and the inferior vena cava) (post-TIPS PPG, pPPG). Haemodynamic success was defined as a reduction of absolute pPPG to <12 mm Hg or a relative reduction of PPG by at least 50% from baseline.
For GOV1, GOV2 and IGV1, embolisation would be performed2 for bleeding oesophagogastric varices. If existent of gastric varices (GV) concurrent spontaneous portosystemic shunts (SPSS), embolisation of both GV and SPSS would be performed. In the TIPS+E group, embolisation was performed prior to TIPS stent implantation. Embolisation was performed using cyanoacrylate or other tissue-vessel glues, plus coils as previously described.14 24 Embolisation was performed until the varices or SPSS could no longer be detected on contrast angiography. All interventional procedures were conducted by experienced chief physicians in the six centres.
Data collection and follow-up
Demographic and baseline data were collected from the first inpatient medical records, which included age, gender, aetiology of cirrhosis, platelet levels (PLT), haemoglobin (Hb), albumin (ALB), total bilirubin (TBIL), international normalised ratio (INR), extend of prothrombin time (ePT) and creatinine (Cr). Medical history, such as ascites, variceal classifications, history of variceal bleeding or HE, variceal classifications and timing of TIPS, was also recorded. PPG and pPPG were obtained from TIPS procedure records. CTP and MELD scores were calculated based on the baseline records.
Follow-up was conducted through clinic visits or inpatient visits, and patients were recalled to centres from 1 June 2022 to 31 December 2022. The median follow-up time was 32.5 (19.3, 56.6) months, with a range of 1 week to 140 months. The clinical outcomes were rebleeding, HE, mortality (including death or liver transplantation) and further decompensation (FDC). Rebleeding was defined as haematemesis or melena, or evaluated by endoscopy (Because not all patients received endoscopy examination during follow-up, the outcome of rebleeding was the all-cause rebleeding in practice). HE was defined as grade II or higher on the West Haven criteria (due to lack of scoring systems like number connection test during clinic visits, the outcome of HE was OHE in practice). Mortality was defined as death from any cause. Stent stenosis, also known as shunt dysfunction, was confirmed by imaging examinations conducted at last follow-up. FDC1 was defined as (a) development of a second portal hypertension-driven decompensating event (ascites, variceal haemorrhage or HE) and/or jaundice; (b) development of recurrent variceal bleeding, recurrent ascites (requirement of ≥3 large volume paracenteses within 1 year), recurrent encephalopathy; (c) in patients presenting with bleeding alone, development of ascites, encephalopathy or jaundice after recovery from bleeding but not if these events occur around the time of bleeding (As some of the follow-up assessments were conducted during clinic visits, not all patients underwent a comprehensive re-evaluation of liver function tests and complications, such as spontaneous bacterial peritonitis or hepatorenal syndrome. FDC consideration was limited to patients meeting the specified criteria a–c. All the variables referred to above, such as demographic and baseline data, haemodynamic success, stent stenosis, and embolisation, were included in modelling construction.
Statistical analysis
Continuous data are presented as median (IQR), and categorical data are expressed as percentages. Student’s t-test was applied for continuous variables, while the Mann-Whitney non-parametric test was used for non-normally distributed continuous data. Categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate.
To balance significant differences in baseline characteristics between TIPS+E and TIPS alone, propensity score matching (PSM) was used to mitigate potential confounding factors. Propensity scores were estimated for all patients through a multivariable logistic regression (LR) model, with TIPS+E or TIPS alone as the dependent variable. Covariates included study centres, age, gender, cirrhosis aetiology, CTP score, MELD score, history of ascites, history of HE, variceal classification, timing of TIPS, PPG, post-TIPS PPG (pPPG), stent diameters and follow-up time. A 0.02 calliper was used for 1:1 matching. After PSM, Cox proportional hazards regression model was used for the analysis of cumulative rebleeding rates, HE rates, mortality and FDC rates, with reported HRs and 95% CIs.
Prognostic predictive models were developed using machine learning models, including random forest (RF), artificial neural network (ANN) and support vector machine (SVM), alongside traditional models such as LR and Cox regression. Hyperparameter selection was automated, optimising prediction performance. Model performance was evaluated using receiver operating characteristic (ROC) curves, area under the curve, accuracy (AC), sensitivity (SE) and specificity (Sp). Significance was set at a two-sided p<0.05 for all tests.
Statistical analyses and figures were conducted using SPSS (V.26.0, IBM), GraphPad Prism (V.8.3.1 for Windows; GraphPad Software, www.graphpad.com) and Pycharm (JetBrains s.r.o., https://www.jetbrains.com.cn/en-us/pycharm/; based on Python V.3.10.0, Python Software Foundation; https://www.python.org/downloads/release/python-3100).
Results
Demographic and baseline characteristics
A total of 2077 patients enrolled in the study, with 631 undergoing TIPS alone and 1446 opting for TIPS+E. Key demographic and clinical features are outlined in online supplemental table 1. The median age was 52.0 years (44.0, 60.0), and the majority were male (64.3%). Viral-related cirrhosis constituted the primary aetiology (67.4%), with median CTP and MELD scores of 7.0 (6.0, 8.0) and 11.0 (9.0, 13.0), respectively. At baseline, 67.4% (1339) presented with ascites, while only 1.0% (21, 14 in TIPS+E and 7 in TIPS) reoccurred (Due to the low recurrence frequency, it was not analysed as a separate outcome). According to Sarin’s criteria for variceal classification, 32.8% had oesophageal varices, 33.9% had GOV1, 11.7% had GOV type 2 (GOV2) and 21.5% had isolated gastric varices type 1 (IGV1). Concerning the timing of TIPS, 85.1% (1767) received it as secondary prevention for variceal rebleeding, while 14.9% (310) received it for acute variceal bleeding. The majority (90.8%) underwent TIPS with 8 mm diameter stents, with 6 mm and 10 mm diameters chosen by 4.4% and 4.8% of patients, respectively. Haemodynamic success was achieved in 88.7% (1843) of patients. The median PPG before the procedure was 24.0 mm Hg (20.0, 27.3), decreasing to 8.8 mm Hg (6.0, 11.0). The median follow-up time was 32.5 months (19.3, 56.6).
Prior to PSM, several variables showed significant differences between TIPS+E and TIPS, including age, gender, variceal classifications, PPG, pPPG, the proportion of PPG decline, stent diameters and follow-up time. Following PSM (details in online supplemental table 2), 568 pairs of matched patients were selected, and no significant differences in baseline characteristics were observed between the two groups (online supplemental table 1).
PSM analysis: reduction in rebleeding, HE and FDC with TIPS+E
Following PSM of 1312 patients, TIPS+E demonstrated a substantial decrease in rebleeding, HE and FDC compared with TIPS alone.
Rebleeding
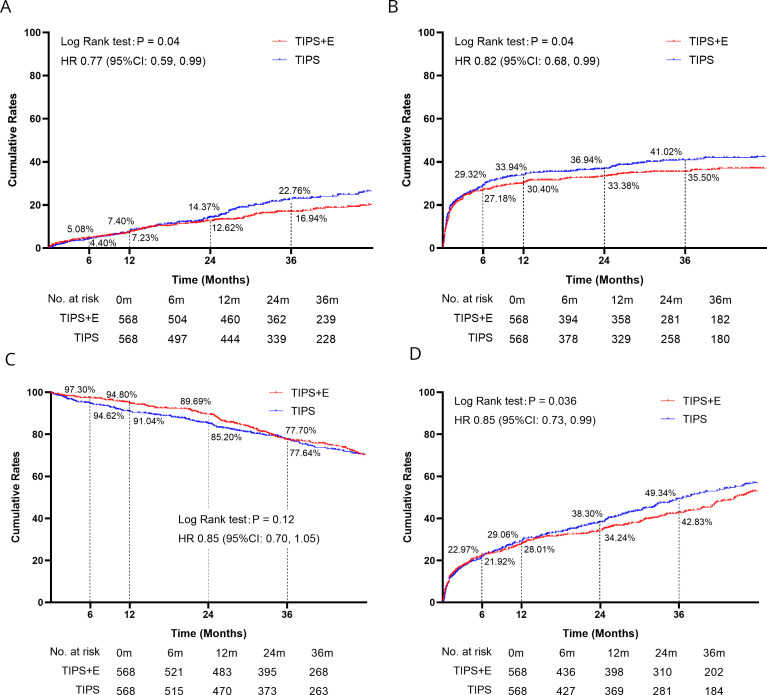
Among the matched patients, 232 (20.4%) experienced variceal rebleeding, with 132 (23.2%) in the TIPS group and 100 (17.6%) in the TIPS+E group. The TIPS+E group exhibited a significantly lower rebleeding rate compared with the TIPS group (HR 0.77; 95% CI 0.59 to 0.99; p=0.04) (figure 1A).
Figure 1.
The clinical outcomes of TIPS and TIPS+E. (A) Cumulative rebleeding rates; (B) cumulative hepatic encephalopathy rates; (C) cumulative survival rates; (D) cumulative further decompensation rates. TIPS, transjugular intrahepatic portosystemic shunt; TIPS+E, TIPS plus extrahepatic collateral embolisation.
Hepatic encephalopathy
In terms of HE, 433 (38.1%) patients experienced it after the TIPS±E procedure, with 235 (41.4%) in the TIPS group and 198 (34.9%) in the TIPS+E group. Only four patients who had experienced HE at baseline encountered HE again (three in TIPS, one in TIPS+E). TIPS+E presented a significantly lower risk of HE compared with TIPS alone (HR 0.82; 95% CI 0.68 to 0.99; p=0.04) (figure 1B).
Survival
Regarding survival, 375 (33.0%) patients died during the follow-up, with 198 (34.9%) in the TIPS group and 177 (31.2%) in the TIPS+E group (figure 1C). No significant difference in cumulative survival rates was observed between the two groups (HR 0.85; 95% CI 0.70 to 1.05; p=0.12) (figure 1C).
Further decompensation
According to the definition of FDC, 637 (56.0%) patients experienced it, with 337 (59.3%) in the TIPS group and 300 (26.4%) in the TIPS+E group (figure 1D). TIPS+E demonstrated a significantly lower risk of FDC compared with TIPS alone (HR 0.85; 95% CI 0.73 to 0.99; p=0.04) (figure 1D).
Subgroup analysis
Stents of 8 mm diameter: significant reduction in rebleeding, HE and FDC with TIPS+E
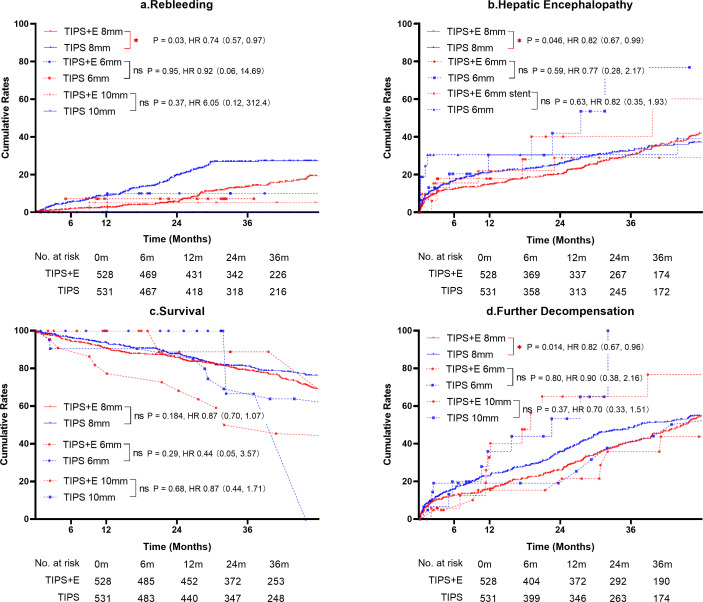
In our previous study,25 compared with TIPS alone, TIPS+E using stents of 8–10 mm in diameter reduced both rebleeding and HE, whereas using 8 mm decreased HE. Notably, 90.8% of patients used 8 mm stents in our present study. In the PSM cohort, 1059 (93.2%) used 8 mm stents, with 531 (93.5%) in TIPS alone and 528 (93.0%) in TIPS+E; 34 (3.0%) used 6 mm stents, with 18 (3.2%) in TIPS and 16 (2.8%) in TIPS+E; 43 (3.8%) used 10 mm stents, with 22 (3.9%) in TIPS and 21 (3.7%) in TIPS+E.
In patients using 8 mm diameter stents, TIPS+E showed significantly lower rates of rebleeding (HR 0.74; 95% CI 0.57 to 0.97; p=0.03), HE (HR 0.82; 95% CI 0.67 to 0.99; p=0.046) and FDC (HR 0.82; 95% CI 0.67 to 0.96; p=0.01) compared with TIPS (figure 2A,B,D). However, no significant difference in survival was observed (figure 2C). Conversely, in patients using 6 mm or 10 mm diameter stents, there were no significant differences in outcomes between TIPS+E and TIPS.
Figure 2.
Subgroups: clinical outcomes of TIPS and TIPS+E in stents of 6 mm, 8 mm and 10 mm on diameters. (A) Cumulative rebleeding rates; (B) cumulative hepatic encephalopathy rates; (C) cumulative survival rates; (D) cumulative further decompensation rates. TIPS, transjugular intrahepatic portosystemic shunt; TIPS+E, TIPS plus extrahepatic collateral embolisation.* means p<0.05; ** means p<0.01;*** means p<0.001; **** means p<0.0001.
Embolisation of GV and SPSS: significant reduction in rebleeding, HE and FDC compared with GV embolisation only
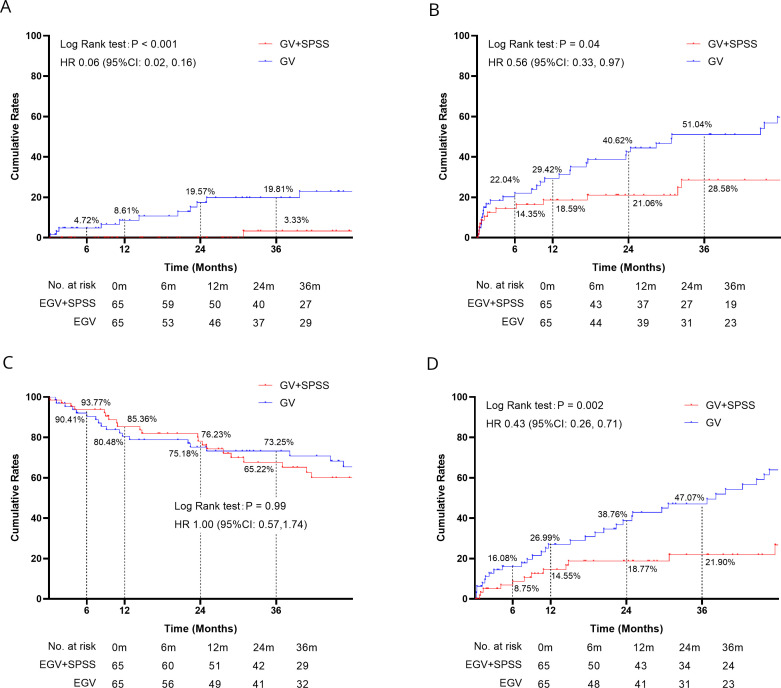
Among the 1447 patients receiving TIPS+E, 1190 underwent GV+SPSS embolisation, while 257 had GV embolisation only. PSM was performed to balance sample sizes, resulting in 65 matched pairs for subgroup analysis (online supplemental table 3).
In patients underwent TIPS+E, GV+SPSS embolisation significantly reduced rates of rebleeding (HR 0.06; 95% CI 0.02 to 0.16; p<0.001), HE (HR 0.56; 95% CI 0.33 to 0.97; p=0.04) and FDC (HR 0.43; 95% CI 0.26 to 0.71; p=0.002) compared with GV embolisation only (figure 3A,B,D). Similar survival rates were observed in both groups (figure 3C).
Figure 3.
Subgroups: clinical outcomes of TIPS+E in embolisation of GV or GV+SPSS. (A) Cumulative rebleeding rates; (B) cumulative hepatic encephalopathy rates; (C) cumulative survival rates; (D) cumulative further decompensation rates. GV+SPSS, gastric varices+spontaneous portosystemic shunts; TIPS+E, transjugular intrahepatic portosystemic shunt plus extrahepatic collateral embolisation.
RF models outperform other methods in predictive value
We used machine learning methods, including RF, ANN and SVM, alongside traditional methods such as LR and Cox regression, to establish prognostic models for rebleeding, HE, survival and FDC. Principal component analysis with an explained variance threshold of 0.95 was used for feature extraction, yielding 37 principal components. The data were split into training and testing sets with a 0.3 ratio. The RF model, with 126 classifiers and a maximum depth of 9, demonstrated superior performance. Parameters were optimised for sensitivity, resulting in the best-performing parameters. Similar methods were applied to ANN and SVM. In LR and Cox regression, variables with p<0.1 in univariate analysis were included in a backward stepwise regression.
Models using the RF method had higher accuracy (online supplemental table 4) in rebleeding (0.85, 95% CI 0.80 to 0.90), HE (0.80, 95% CI 0.76 to 0.84), survival (0.83, 95% CI 0.79 to 0.87) and FDC (0.80, 95% CI 0.76 to 0.84) compared with other methods, with SVM and ANN performing better than LR and Cox.
The area under the receiver operating characteristic (AUROC) of RF models for rebleeding was 0.86 (0.84, 0.89), for HE was 0.71 (0.69, 0.74), for survival was 0.78 (0.75, 0.80) and for FDC was 0.90 (0.87, 0.92). All AUROC values of RF models were higher than other methods, with SVM and ANN outperforming LR and Cox, as well as in sensitivity and specificity (online supplemental table 4).
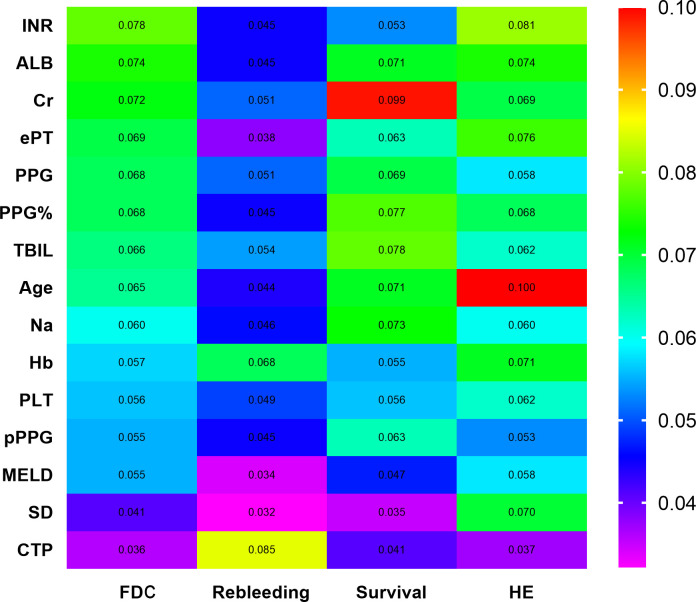
Subgroup analysis based on RF prognostic models variables
In RF models, the top 15 variables, ranked by features importance (figure 4), were INR, ALB, Cr, ePT, PPG, PPG%, TBIL, age, Na, Hb, PLT, pPPG, MELD, CTP and shunt dysfunction. Further analysis revealed that a pPPG<8.5 mm Hg cut-off was associated with significantly lower rebleeding rates (HR 0.77, 95% CI 0.60 to 0.98; p=0.04) (online supplemental figure 2a), while pPPG≤7 mm Hg was associated with increased mortality (HR 1.25, 95% CI 1.00 to 1.57; p=0.048) (online supplemental figure 2b).
Figure 4.
The top 15 variables ranked by features importance by random forest model. Alb, albumin; Cr, creatinine; CTP, Child-Turcotte-Pugh; ePT, extend of prothrombin time; Hb, haemoglobin; HE, hepatic encephalopathy; INR, international normalised ratio; MELD, model for end-stage liver disease; Na, serum natrium; PLT, platelet; pPPG, post-TIPS portal pressure gradient; SD, shunt dysfunction; TBIL, total bilirubin.
To validate our findings, we compared pPPG thresholds in the TIPS+E group. Surprisingly, in the TIPS+E group, patients with pPPG≥8.5 mm Hg had the highest rebleeding rates among all groups (HR 0.63, 95% CI 0.47 to 0.86; p=0.004) (online supplemental figure 2c). Additionally, patients with pPPG≤7 mm Hg had significantly higher mortality rates compared with all other groups (HR 1.40, 95% CI 1.06 to 1.85; p=0.01) (online supplemental figure 2d).
Discussion
Our present study based on large real-world data in China, demonstrated that, after PSM, TIPS+E could significantly reduce post-TIPS rebleeding, HE and FDC comparing to TIPS alone in patients with cirrhotic portal hypertension. Notably, our subgroup analysis suggested additional benefits in prognosis when using 8 mm diameter stents and performing embolisation of GV+SPSS. These real-world data provide robust support and supplementation to our recent meta-analysis.25
Indeed, TIPS+E in controlling variceal rebleeding has been in controversy. It has been reported that TIPS+E has been routinely practised in 24%–48% of patients20 (in our data for 69.6%). We reviewed for studies reported for the clinical outcomes of TIPS±E, and 15 studies13–15 17–20 26–33 were found. All the 15 studies13–15 17–20 26–33 that reported on variceal rebleeding of TIPS±E, 4 studies13–15 32 showed a statistically significant reduction in variceal rebleeding with TIPS+E compared with TIPS alone, while 11 studies17–20 26–31 33 showed no significant difference between the two groups. Of the nine studies14 15 17–20 26 28 33 that reported on HE, four studies14 26 28 34 showed a statistically significant reduction in HE with TIPS+E compared with TIPS alone, while six studies15 17–20 33 showed no significant difference between the two groups. Of the 10 studies13–15 17 20 26 28 30 32 33 that reported on mortality, none of the studies showed a statistically significant reduction in mortality with TIPS+E compared with TIPS alone.
Notably, the meta-analysis,25 consolidating findings from 15 prior studies, revealed a significant reduction in rebleeding rates (OR 0.57; 95% CI 0.42 to 0.76; p<0.001; I2=29%) and HE (OR 0.65; 95% CI 0.48 to 0.88; p=0.005; I2=1%) with the use of TIPS+E compared with TIPS alone. No significant difference was observed in mortality (OR 0.84, 95% CI 0.63 to 1.12; p=0.24; I2=0%). After PSM to balance confounding factors, our real-world retrospective data validated these results. Notably, our subgroup analysis reinforced these findings, highlighting that the use of 8 mm stents significantly improved prognosis rather than 8–10 mm range. Recent RCTs reported varied results for TIPS+E, specifically focusing on embolisation positions, such as GV and GV+SPSS. Our own data, validated through PSM, indicated a significant improvement in prognosis with GV+SPSS embolisation.
We extended our exploration to prognostic predictive models, revealing that RF is most suited for our dataset. The predictive performance of the RF-based model surpassed that of other machine learning methods and traditional regression algorithms. The top 15 variables, prominently featuring the MELD score and its components, provided crucial insights. A particularly notable observation pertained to MELD>20, showcasing high incidences of FDC, survival, HE and rebleeding (88.2%, 64.7%, 76.5% and 45.2%, respectively), though in a small sample size (17 patients). Another intriguing finding focused on pPPG, indicating that maintaining pPPG within the 7–8.5 mm Hg threshold ensures a balanced survival rate without excessive bleeding, especially beneficial for the TIPS+E group. These results suggest that TIPS+E, as a modified technology, aligns with expectations for an ideal TIPS. Alternative approaches, such as TIPS with small-diameter stents or ‘controlled-expansion’ stents,35 36 may also prove effective and safe when combined with collateral embolisation. Although a pPPG<12 mm Hg or a reduction of more than 50% remains the standard for haemodynamic success,37 38 the definition of haemodynamic success, as indicated by pPPG, may evolve for TIPS+E.39
In considering the materials used for embolisation, existing studies suggest that using vascular plugs, coils or tissue glue showed similar outcomes in terms of rebleeding and mortality, while the combination of coils and tissue glue may enhance survival at the cost of increased hospitalisation expenses.40–42 An interesting nuance arises from variations between Western and Eastern practices in the sequencing of TIPS and embolisation.25 This discrepancy may influence PPG and subsequently impact rebleeding and HE. However, our study’s adherence to standardised procedures, materials and techniques precludes direct comparisons among these diverse subgroups.
A surprising observation emerges from our data: although TIPS+E is associated with reduced rebleeding and HE rates, this improvement does not translate into an improvement on overall survival. We speculate that this discrepancy may be attributed to a potential deterioration in liver function following TIPS+E compared with TIPS alone, thereby nullifying any survival benefit. Intriguingly, a previous study43 compared preprocedural and postprocedural liver function between TIPS+E and TIPS alone. The results indicated that 1 year after the procedure, liver function in the TIPS+E group was superior to TIPS, as assessed by the MELD score. Additionally, there was an improvement in liver function levels 1 year post-TIPS+E, evaluated by the CTP levels, while there was no change in the TIPS group. This nuanced insight prompts further exploration into the complex interplay between procedural interventions, liver function dynamics and overall patient outcomes.
In our study, there were some limitations. First, given its retrospective study, limitations in the completeness and reliability of clinical data collection were inevitable, introducing potential information and recall biases. Second, the low occurrence of outcomes might underestimate the actual risk due to the possibility of missed outcomes during clinic follow-ups and the unavailability of post-TIPS clinical examinations. Third, the lack of data on embolisation range, varied sequences of TIPS and embolisation, diverse combinations of materials (vascular coils, plugs and liquid embolic materials like cyanoacrylate glue), postembolisation syndrome and post-TIPS clinical examinations restricted our ability to explore the beneficial population and identify genuine risk factors for TIPS+E.
In conclusion, our findings suggested the significant benefits of TIPS+E in reducing post-TIPS rebleeding, HE and FDC, compared with TIPS alone in patients with cirrhotic portal hypertension. Notably, our subgroup analysis highlights additional prognostic advantages associated with the use of 8 mm diameter stents and the inclusion of embolisation for GV+SPSS. Furthermore, meticulous control of pPPG emerges as a potential avenue for improving prognosis, particularly in TIPS+E. However, these findings warrant further validation through large-scale RCTs in the future.
Footnotes
Contributors: Conceptualisation: LZ, GW and CZ. Data curation: LZ, JX, FZ, BW, PL, CC, QW and YX. Formal analysis: LZ and ZL. Funding acquisition: CZ and GW. Investigation: All authors. Methodology: LZ, XS, YX and QW. Project administration: All authors. Resources: JT, YZ, HW, HX and CZ. Software: LZ, QW and ZL. Supervision: GW. Validation: QW and XS. Visualisation: LZ. Writing-original draft: LZ. Writing-review and editing: LZ, QW, GW and CZ. Study materials will not be made available to other researchers. All authors approved the final version of the manuscript. The corresponding author CZ will accept full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81970533), the National Natural Science Foundation of China (82000566) and the Provincial Natural Science Foundation of Shandong, China (ZR2022MH010).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but the study was approved by the ethics committee of Provincial Hospital afflicted to Shandong First Medical University (approval number: SWYX: No. 2021-503). This study was conducted in accordance with the principles of the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1.de Franchis R, Bosch J, Garcia-Tsao G, et al. Renewing consensus in portal hypertension. J Hepatol 2022;76:959–74. 10.1016/j.jhep.2021.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boike JR, Thornburg BG, Asrani SK, et al. North American practice-based recommendations for transjugular intrahepatic portosystemic shunts in portal hypertension. Clin Gastroenterol Hepatol 2022;20:1636–62. 10.1016/j.cgh.2021.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tripathi D, Stanley AJ, Hayes PC, et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut 2020;69:1173–92. 10.1136/gutjnl-2019-320221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allaire M, Walter A, Sutter O, et al. TIPS for management of portal-hypertension-related complications in patients with cirrhosis. Clin Res Hepatol Gastroenterol 2020;44:249–63. 10.1016/j.clinre.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver . Electronic address EEE, European Association for the study of the L. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu . EASL clinical practice guidelines: vascular diseases of the liver. J Hepatol 2016;64:179–202. 10.1016/j.jhep.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310–35. 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 8.Interventionalists CCo . CCI clinical practice guidelines: management of TIPS for portal hypertension (2019 edition. Zhonghua Gan Zang Bing Za Zhi 2019;27:582–93. [DOI] [PubMed] [Google Scholar]
- 9.Intervention Group CSoR, Chinese Medical Association . Expert consensus on transjugular intrahepatic portosystemic shunt. J Clin Hepatol 2017;33:1218–28. [Google Scholar]
- 10.Fagiuoli S, Bruno R, Debernardi Venon W, et al. Consensus conference on TIPS management: techniques, indications, contraindications. Dig Liver Dis 2017;49:121–37. 10.1016/j.dld.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Wu Q, Li Q, et al. TIPSS plus extrahepatic collateral Embolisation may decrease Variceal Rebleeding and post-TIPSS hepatic encephalopathy. Gut 2023. 10.1136/gutjnl-2023-330255 [DOI] [PubMed] [Google Scholar]
- 12.Qi X, Liu L, Bai M, et al. Transjugular intrahepatic portosystemic shunt in combination with or without variceal embolization for the prevention of variceal rebleeding: a meta-analysis. J Gastroenterol Hepatol 2014;29:688–96. 10.1111/jgh.12391 [DOI] [PubMed] [Google Scholar]
- 13.Tesdal IK, Filser T, Weiss C, et al. Transjugular intrahepatic portosystemic shunts: adjunctive embolotherapy of gastroesophageal collateral vessels in the prevention of variceal rebleeding. Radiology 2005;236:360–7. 10.1148/radiol.2361040530 [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Tian X, Hu J, et al. Efficacy of transjugular intrahepatic portosystemic shunt with adjunctive embolotherapy with cyanoacrylate for esophageal variceal bleeding. Dig Dis Sci 2014;59:2325–32. 10.1007/s10620-014-3150-2 [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Wang X, Jiang M, et al. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone and combined with embolisation for the management of cardiofundal varices: a retrospective study. Eur Radiol 2019;29:699–706. 10.1007/s00330-018-5645-2 [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Yang C, Huang S, et al. The combination of balloon-assisted antegrade transvenous obliteration and transjugular intrahepatic portosystemic shunt for the management of cardiofundal varices hemorrhage. European Journal of Gastroenterology & Hepatology 2020;32:656–62. 10.1097/MEG.0000000000001705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Li X, Wei B, et al. Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology 2013;268:900–6. 10.1148/radiol.13120800 [DOI] [PubMed] [Google Scholar]
- 18.Shah KY, Ren A, Simpson RO, et al. Combined transjugular intrahepatic portosystemic shunt plus variceal obliteration versus transjugular intrahepatic portosystemic shunt alone for the management of gastric varices: comparative single-center clinical outcomes. J Vasc Interv Radiol 2021;32:282–91. 10.1016/j.jvir.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Xue H, Yuan J, Chao-Li Y, et al. Follow-up study of transjugular intrahepatic portosystemic shunt in the treatment of portal hypertension. Dig Dis Sci 2011;56:3350–6. 10.1007/s10620-011-1744-5 [DOI] [PubMed] [Google Scholar]
- 20.Lv Y, Chen H, Luo B, et al. Transjugular intrahepatic portosystemic shunt with or without gastro-oesophageal variceal embolisation for the prevention of variceal rebleeding: a randomised controlled trial. The Lancet Gastroenterology & Hepatology 2022;7:736–46. 10.1016/S2468-1253(22)00087-5 [DOI] [PubMed] [Google Scholar]
- 21.Leng X, Zhang F, Zhang M, et al. Comparison of transjugular intrahepatic portosystemic shunt for treatment of variceal bleeding in patients with cirrhosis with or without spontaneous portosystemic shunt. European Journal of Gastroenterology & Hepatology 2019;31:853–8. 10.1097/MEG.0000000000001349 [DOI] [PubMed] [Google Scholar]
- 22.Lv Y, Zuo L, Zhu X, et al. Identifying optimal candidates for early TIPS among patients with cirrhosis and acute variceal bleeding: a multicentre observational study. Gut 2019;68:1297–310. 10.1136/gutjnl-2018-317057 [DOI] [PubMed] [Google Scholar]
- 23.Lv Y, Wang Z, Li K, et al. Risk stratification based on chronic liver failure consortium acute decompensation score in patients with child-pugh B cirrhosis and acute variceal bleeding. Hepatology 2021;73:1478–93. 10.1002/hep.31478 [DOI] [PubMed] [Google Scholar]
- 24.Ni JB, Xiang XX, Wu W, et al. Transjugular intrahepatic portosystemic shunt in patients treated with a balloon tamponade for variceal hemorrhage without response to high doses of vasoactive drugs: a real-world multicenter retrospective study. J Dig Dis 2021;22:236–45. 10.1111/1751-2980.12978 [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Wu Q, Li Q, et al. TIPSS plus extrahepatic collateral embolisation may decrease variceal rebleeding and post-TIPSS hepatic encephalopathy. Gut 2023:gutjnl–2023. 10.1136/gutjnl-2023-330255 [DOI] [PubMed] [Google Scholar]
- 26.Lv Y, Chen H, Luo B, et al. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: a randomized controlled trial. Hepatology 2022;76:676–88. 10.1002/hep.32453 [DOI] [PubMed] [Google Scholar]
- 27.Taussig M, Ronald J, Seyferth E, et al. Use of covered stent-graft for transjugular intrahepatic portosystemic shunt placement reduces variceal rebleeding rate with or without variceal embolization. Journal of Vascular and Interventional Radiology 2020;31:S148. 10.1016/j.jvir.2019.12.381 [DOI] [Google Scholar]
- 28.He C, Lv Y, Wang Z, et al. Association between non-variceal spontaneous portosystemic shunt and outcomes after TIPS in cirrhosis. Digestive and Liver Disease 2018;50:1315–23. 10.1016/j.dld.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 29.Lakhoo J, Bui JT, Lokken RP, et al. Transjugular intrahepatic portosystemic shunt creation and variceal coil or plug embolization ineffectively attain gastric variceal decompression or occlusion: results of a 26-patient retrospective study. J Vasc Interv Radiol 2016;27:1001–11. 10.1016/j.jvir.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 30.Weber CN, Ge BH, Clark T, et al. Clinical outcomes of selective variceal coil embolization during PTFE-covered transjugular intrahepatic portosystemic shunt (TIPS) placement for variceal hemorrhage. Journal of Vascular and Interventional Radiology 2015;26:S92–3. 10.1016/j.jvir.2014.12.252 [DOI] [Google Scholar]
- 31.Jaju P, Amin S, Sze DY, et al. Effect of embolization or sclerosis on recurrence of variceal hemorrhage after transjugular intrahepatic portosystemic shunt placement. Journal of Vascular and Interventional Radiology 2014;25:S83. 10.1016/j.jvir.2013.12.235 [DOI] [Google Scholar]
- 32.Gaba RC, Omene BO, Podczerwinski ES, et al. TIPS for treatment of Variceal hemorrhage: clinical outcomes in 128 patients at a single institution over a 12-year period. J Vasc Interv Radiol 2012;23:227–35. 10.1016/j.jvir.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 33.Xiao T, Chen L, Chen W, et al. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol 2011;45:643–50. 10.1097/MCG.0b013e318203dfb3 [DOI] [PubMed] [Google Scholar]
- 34.Tripathi D, Lui HF, Helmy A, et al. Randomised controlled trial of long term portographic follow up versus variceal band ligation following transjugular intrahepatic portosystemic stent shunt for preventing oesophageal variceal rebleeding. Gut 2004;53:431–7. 10.1136/gut.2003.013532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosch J. Small diameter shunts should lead to safe expansion of the use of TIPS. J Hepatol 2021;74:230–4. 10.1016/j.jhep.2020.09.018 [DOI] [PubMed] [Google Scholar]
- 36.Tripathi D, Bureau C. Prophylactic embolization of large spontaneous portosystemic shunts with transjugular intrahepatic portosystemic shunt (TIPS): a panacea for post-TIPS hepatic encephalopathy Hepatology 2022;76:551–3. 10.1002/hep.32525 [DOI] [PubMed] [Google Scholar]
- 37.Silva-Junior G, Turon F, Baiges A, et al. Timing affects measurement of portal pressure gradient after placement of transjugular intrahepatic portosystemic shunts in patients with portal hypertension. Gastroenterology 2017;152:1358–65. 10.1053/j.gastro.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 38.Casado M, Bosch J, García-Pagán JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology 1998;114:1296–303. 10.1016/S0016-5085(98)70436-6 [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Luo X, Yang L. Achieving an effective pressure reduction after TIPS: the need for a N Ew target. Journal of Hepatology 2021;75:246–8. 10.1016/j.jhep.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 40.Sarwar A, Esparaz AM, Tapper EB, et al. Comparison of vascular plugs and pushable coils for variceal embolization after TIPS. AJR Am J Roentgenol 2017;208:650–5. 10.2214/AJR.16.16012 [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Zhong B, Du H, et al. Comparison of embolic agents for varices during transjugular intrahepatic portosystemic shunt for variceal bleeding: tissue GEL or coil J Interv Med 2020;3:195–200. 10.1016/j.jimed.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolter K, Praktiknjo M, Boie J, et al. Glue Embolization of gastroesophageal Varices during transjugular intrahepatic portosystemic shunt (TIPS) improves survival compared to coil-only Embolization—A single-center retrospective study. Cardiovasc Intervent Radiol 2021;44:1240–50. 10.1007/s00270-021-02852-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huan H, Tong H, Wei B, et al. The influence of transjugular intrahepatic portosystemic shunt with concurrent left gastric vein embolization on the liver function of patients with liver cirrhosis. Gastroenterology 2017;152:S1135–6. 10.1016/S0016-5085(17)33819-2 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2023-001310supp001.pdf (1.7MB, pdf)
Data Availability Statement
Data are available on reasonable request.