Abstract
Objectives
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease affecting mainly the axial skeleton. Peripheral involvement (arthritis, enthesitis and dactylitis) and extra-musculoskeletal manifestations, including uveitis, psoriasis and bowel inflammation, occur in a relevant proportion of patients. AS is responsible for chronic and severe back pain caused by local inflammation that can lead to osteoproliferation and ultimately spinal fusion. The association of AS with the human leucocyte antigen-B27 gene, together with elevated levels of chemokines, CCL17 and CCL22, in the sera of patients with AS, led us to study the role of CCR4+ T cells in the disease pathogenesis.
Methods
CD8+CCR4+ T cells isolated from the blood of patients with AS (n=76) or healthy donors were analysed by multiparameter flow cytometry, and gene expression was evaluated by RNA sequencing. Patients with AS were stratified according to the therapeutic regimen and current disease score.
Results
CD8+CCR4+ T cells display a distinct effector phenotype and upregulate the inflammatory chemokine receptors CCR1, CCR5, CX3CR1 and L-selectin CD62L, indicating an altered migration ability. CD8+CCR4+ T cells expressing CX3CR1 present an enhanced cytotoxic profile, expressing both perforin and granzyme B. RNA-sequencing pathway analysis revealed that CD8+CCR4+ T cells from patients with active disease significantly upregulate genes promoting osteogenesis, a core process in AS pathogenesis.
Conclusions
Our results shed light on a new molecular mechanism by which T cells may selectively migrate to inflammatory loci, promote new bone formation and contribute to the pathological ossification process observed in AS.
Keywords: Chemokines; Spondylitis, Ankylosing; T-Lymphocyte subsets
WHAT IS ALREADY KNOWN ON THIS TOPIC
Ankylosing spondylitis (AS) is a chronic autoinflammatory condition affecting mainly the axial skeleton, with local new bone formation. The mechanical stress present in the spine contributes to new bone formation by driving local inflammation and the release of several proinflammatory cytokines. Additionally, local inflammation initiates the production of chemokines and the recruitment of immune cells, including CD8+ T cells. However, how the recruited immune cells and the chemokine system contribute to local bone deposition is not fully understood.
WHAT THIS STUDY ADDS
The identification of a subpopulation of circulating high cytotoxic CD8+ T cells upregulating chemokine receptors and genes promoting the ossification process in patients with AS that might contribute to AS pathogenesis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Understanding the role of CD8+CCR4+ T cells and of the relevant chemokines might provide a rationale for the development of additional novel treatments for AS.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease belonging to the group of seronegative spondyloarthritides (SpA), which is defined by radiographic signs of destructive pathologies in the sacroiliac joints and ankyloses.1 2 AS presents a disease continuum including earlier and milder disease forms and affects up to 1% of the population worldwide, with an average onset around 20–30 years of age.3 4 The aetiology of AS is poorly understood, but genetic factors, such as the human leucocyte antigen (HLA)-B27 gene, and environmental triggers might contribute to the development of the disease. Enthesitis, the inflammation of the entheses, located at the attachment of ligaments and tendons to bone, is a hallmark of AS.5 These sites are prone to mechanical stress and microdamages that, in genetically predisposed subjects, might not be resolved by tissue repair mechanisms but instead represent areas of chronic inflammation and subsequent new bone formation.6 The progressive remodelling at sites of spinal entheses of sacroiliac spinal joints ultimately leads to bone fusion (ankylosis).7 8 Peripheral disease manifestations (arthritis, enthesitis and dactylitis) and extra-musculoskeletal manifestations, such as anterior uveitis, psoriasis and inflammatory bowel disease, are present to different degrees in a significant proportion of patients.9
By driving local inflammation with the release of several proinflammatory cytokines, mechanical stress can contribute to new bone formation. It has been recently demonstrated that exposure to tumour necrosis factor (TNF), interleukin (IL)-1β, IL-17, IL-22 and IL-23 aberrantly upregulates the calcium-sensing receptor in osteoblasts, a phenotype also observed in patients with AS, and promotes osteogenic differentiation both in vitro and in vivo.10 In addition, stimulation with low doses of TNF promotes the expression of the osteoinductive molecule Wnt in preosteoblasts.11 IL-1β and TNF have also been reported to upregulate other osteogenic molecules, such as bone morphogenic proteins (BMPs), in mesenchymal cells.12 13 Currently available treatments, ranging from non-steroidal anti-inflammatories to TNF, IL-17 and Janus Kinase inhibitors, target the ongoing inflammation and aim to minimise symptoms.4 14–16
Animal models of SpA, achieved by the dysregulated expression of TNF or IL-22 and IL-23, displayed new bone formation at the enthesis together with extra-articular manifestations.17 18 These models first proved the presence of T cells in regions thought to be populated solely by stromal cells. Studies in patients with AS demonstrated that the enthesis is populated by osteogenic precursors, chondrocytes and osteoblasts together with immune cells, while resident T cells, able to produce TNF and IL-17A, have also been reported in normal enthesis.19–21 Chemokines, such as CXCL12, regulate the migration of both immune cells and bone cells to this site, suggesting that the chemokine system is also involved in AS pathogenesis.19
Alterations in chemokine and chemokine receptor expression, including CCL17, CCL22, CXCL10, CX3CL1, CCR4 and CX3CR1, have been reported in this context, even though their relevance in triggering the pathology has not been fully understood.22–27 Indeed, elevated plasma levels of the chemokines CCL17 and CCL22, known to be involved in different inflammatory conditions,28 29 and of circulating CD4+ T cells expressing the cognate receptor, CCR4, have been described in patients with AS, indicating altered immune cell trafficking via this axis.22–25 We therefore hypothesised that given the importance of HLA-B27 and the elevated levels of CCL17 and CCL22, CD8+ T cells expressing CCR4 might be involved in AS pathogenesis, with the potential of trafficking between different inflammatory sites.
In the present study, we show that CD8+CCR4+ T cells isolated from patients in an active state of the disease display an altered phenotype with a distinct cytotoxic and transcriptomic profile, upregulating genes promoting ossification. Altogether, these results point to new molecular mechanisms by which T cells may promote new bone formation and sustain the pathological ossification process characteristic of AS.
Methods
Ethical approval and patient recruitment
The study was approved by the Ethical Committees of the Canton Ticino (CE-3065) and of the Canton Zurich (EK515). Informed consent from each subject was obtained before enrollment in the study, and all samples were coded. A total of 76 patients with AS undergoing routine disease assessment at the University Hospitals of Zurich or Bern (CH) were enrolled in the study. Blood and sera from each patient were collected at the time of enrolment, and clinicians provided clinical and demographic information. All patients with AS fulfilled the modified New York 1984 criteria,2 and disease activity was assessed by the Ankylosing Spondylitis Disease Activity Score (ASDAS).30 Demographic and clinical characteristics of patients with AS enrolled in the study are shown in table 1. Blood samples from healthy donors (HD) were received from the Central Laboratory of the Swiss Red Cross (Basel, CH), the Centro Trasfusionale Lugano or from spontaneous donations, and usage was approved by the Ethical Committee of the Canton Ticino (CE-3428).
Table 1.
Characteristics of the patients with AS
| Patient characteristics | Total | Active AS | Inactive AS |
| Number of patients | 76 | 54 | 22 |
| Gender (M/F) | M (54) / F (22) | M (37) / F (17) | M (17) / F (5) |
| Age (mean±SD) | 42.9±12.1 | 45.3±12.0 | 37.4±10.2 |
| Human leucocyte antigen-B27+ (%) | 89.47% | 88.9% | 90.9% |
| Inflammatory bowel disease (%) | 6.6% | 7.4% | 4.5% |
| Psoriasis (%) | 3.9% | 5.5% | 0% |
| Uveitis (%) | 27.6% | 22.2% | 36.4% |
| Tumour necrosis factor inhibitor (%) | 53.9% | 42.6% | 81.8% |
| ASDAS (mean±SD) | 1.92±1.00 | 2.35±0.87 | 0.86±0.23 |
| ASDAS range | 0.2–4.1 | 1.3–4.1 | 0.2–1.2 |
AS, ankylosing spondylitis; ASDAS, Ankylosing Spondylitis Disease Activity Score.
Blood collection and cell isolation
Sixteen millilitres of peripheral blood from each patient were collected in BD Vacutainer CPT cell preparation tubes (362782, BD Biosciences). Blood from HD was provided as buffy coats or was withdrawn and collected in BD Vacutainer CPT tubes.
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque density centrifugation within 24 hours of blood withdrawal. CD8+ T cell enrichment from total PBMCs was performed either using positive immunoselection (130-045-201, Miltenyi Biotec), according to the manufacturer’s instructions, or through cell sorting using FACSAria III (BD Biosciences).
Flow cytometric analysis
Phenotyping
Freshly isolated PBMCs were used for surface staining and incubated for 30 min at 4°C with fluorochrome-conjugated antibodies (online supplemental table 1) in phosphate-buffered saline (PBS; D8537, Sigma-Aldrich) with 1% fetal bovine serum (10270106, Gibco, Life Technologies). Cells were then washed with PBS and immediately acquired or fixed in 1% (w/v) paraformaldehyde (158127, Sigma-Aldrich) in PBS for subsequent acquisition.
rmdopen-2023-003926supp001.pdf (295.9KB, pdf)
Cytokine and cytotoxic molecule production
To assess cytokines, perforin (PRF) and granzyme B (GZMB) production, CD8+CCR4+ T cells were sorted and left overnight in T cell medium (Roswell Park Memorial Institute-1640 medium (11875093) supplemented with 1% (v/v) glutaMAX-I (35050061), 1% (v/v) non-essential amino acids (11140068), 1 mM sodium pyruvate (11360088), 50 μM β-mercaptoethanol (31350010), penicillin (50 U/mL), streptomycin (50 µg/mL) (15070063), 1% (v/v) kanamycin (15160047) (all from Gibco, Life Technologies) and 5% (v/v) human serum (Swiss Blood Centre, Basel)). Cells were then stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (P1585, Sigma-Aldrich) and 1 μg/mL ionomycin (I0634, Sigma-Adrich), resuspended in T cell medium, for 5 hours at 37°C in a 5% carbon dioxide-humidified atmosphere. After 2.5 hours, 10 μg/mL brefeldin A (B5936, Sigma-Aldrich) and 2 mM monensin (M5273, Sigma-Aldrich) were supplemented. Where indicated, directly conjugated anti-CX3CR1 antibody (online supplemental table 1) was added to the cells 30 min prior to fixation. Intracellular staining of cytokines, PRF and GZMB, was performed using fluorochrome-conjugated antibodies (online supplemental table 1) and Cytofix/Cytoperm kit (554714, BD Biosciences), according to the manufacturer’s instructions. All samples were acquired on BD LSRFortessa (BD Biosciences), and the results were analysed with FlowJo software V.10.7.1 (Tree Star).
RNA sequencing
RNA isolation and purification
After sorting, CD8+CCR4+ T cells from six patients with AS and five HDs were stored in TRIzol Reagent (15596026, Life Technologies) at −80°C until RNA extraction was performed. Total RNA was extracted with Zymo-Spin IC Columns (C1004-50, Zymo Research) and the Direct-zol RNA MiniPrep Kit (R2050, Zymo Research) according to the manufacturer’s instructions. RNA sequencing was performed using NEBNext Ultra Directional RNA Library Prep for Illumina (New England BioLabs) according to the manufacturer’s instructions. The libraries were sequenced using NextSeq 500 (Illumina) or Novaseq 6000 (Illumina). Detailed data analysis information is provided in online supplemental file 1.
Statistical analysis
Data were analysed using Prism V.9.0 software (GraphPad) and presented as mean±SEM. The statistical significance between HD and AS groups was determined using the Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Simple linear regression analysis was performed to correlate the percentages of CD8+CCR4+ TEM and the ASDAS for active patients with AS under TNFi treatment. Values were considered statistically significant when probability (p) values were equal or below 0.05 (*), 0.01 (**), 0.001 (***) and 0.0001 (****).
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Altered phenotype of CCR4+ T cells in active AS
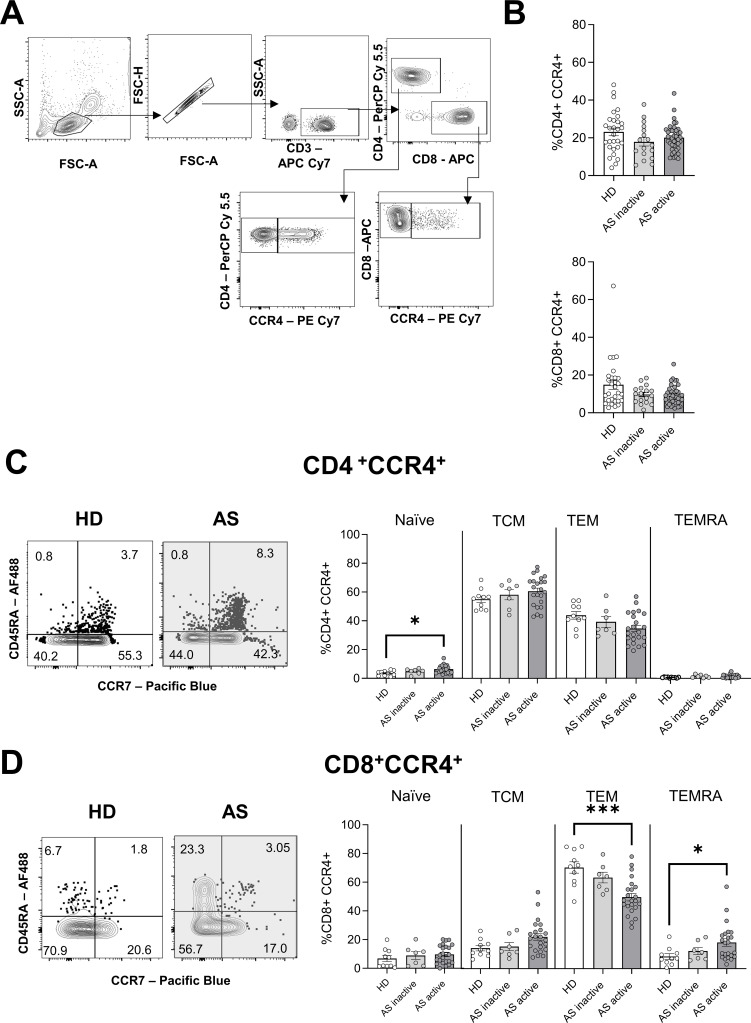
Previous reports showing elevated levels of the ligands of CCR4 in the sera of patients with AS22 23 prompted us to study the role of CCR4-expressing T cells in disease development and progression. Patients with AS (table 1) (n=76) were classified according to their disease activity, measured as ASDAS,30 at the time of inclusion in the study. Patients with an ASDAS below 1.3 were considered in the inactive phase of the disease (n=22, inactive AS), while patients with an ASDAS above or equal to 1.3 were assigned to the active status group (n=54, active AS). Blood samples from HDs (n=42) were used as a control. Flow cytometric analysis revealed no difference in the frequency of circulating CD4+CCR4+ and CD8+CCR4+ T cells in patients with AS, regardless of disease activity (figure 1A,B). In humans, the combined expression of CD45RA and CCR7 allows the distinction of naive T cells (CD45RA+ CCR7+) from antigen-experienced T cells, comprising central memory (TCM, CD45RA- CCR7+), effector memory (TEM, CD45RA- CCR7-) and effector memory re-expressing CD45RA (TEMRA, CD45RA+ CCR7-).31–33 CD4+CCR4+ T cells displayed a TCM and TEM phenotype, and a small but significant increase in the frequencies of naive cells was observed in patients with active disease (figure 1C). As no major differences in frequencies or phenotypes were found in CD4+CCR4+ T cells, we did not explore this population any further.
Figure 1.
Phenotype of CCR4+ T cells from patients with AS and HD. (A) Gating strategy for the analysis of CCR4+ T cells. (B) Mean frequency±SEM of CD4+ (top) and CD8+ (bottom) T cells expressing CCR4 in HD and patients with active or inactive AS, according to Ankylosing Spondylitis Disease Activity Score. (C and D) Representative plot from the flow cytometric analysis showing the expression of CD45RA and CCR7 by CD4+CCR4+ (C) and CD8+CCR4+ (D) T cells. The numbers indicate the proportion of the population in each quadrant. Mean frequency±SEM of naive (CD45RA+CCR7+), TCM (CD45RA-CCR7+), TEM (CD45RA-CCR7-) and TEMRA (CD45RA+CCR7-) are represented for HD and patients with AS. Each symbol represents an individual. Kruskal-Wallis was used for the analysis, and asterisks indicate significant differences between the groups (*p<0.05, ***p<0.001). AS, ankylosing spondylitis; HD, healthy donor; TCM, T cell central memory; TEM, T cell effector memory; TEMRA, T cell effector memory re-expressing CD45RA.
On the other hand, CD8+CCR4+ T cells from patients with active disease showed a significant reduction in TEM (active AS vs HD, p=0.001) phenotype and a concomitant increase in TEMRA (active AS vs HD, p=0.036) (figure 1D). To investigate whether these observed differences in TEM and TEMRA populations reflected an altered differentiation state, we assessed their CD27 and CD28 expression. During differentiation, CD27+CD28+ CD8+ T cells progressively lose both CD27 and CD28, upregulating cytotoxic molecules.34 35 Highly differentiated TEMRA, lacking the expression of both CD27 and CD28, was predominant in both HD and patients with AS, with no differences between the groups (online supplemental figure 1A). CD8+CCR4+ TEM were less differentiated, and the majority of cells expressed both receptors. Overall, these results indicate that the altered phenotype present in CCR4-expressing CD8+ T cells is not accompanied by differences in CD27 and CD28 expression.
The use of TNFi is widely adopted as a therapeutic strategy for the treatment of AS. To study the impact of TNFi therapy on the phenotype and function of CCR4+ T cells, patients with active AS were divided according to their therapeutic regimen at the time of enrolment in the study (table 1). CD8+CCR4+ T cells were reduced in patients treated with TNFi and presented a higher proportion of TCM and a subsequent lower level of TEM (online supplemental figure 1B). The reduction in TEM levels was also present in the patients who did not receive TNFi (TNFi−), suggesting that this is a unique feature of active AS (online supplemental figure 1B). The significant reduction of CD8+CCR4+ TEM in patients with active AS compared with controls follows the trend present in total CD8+ T cells (HD vs TNFi−: 46.4% vs 27.5%, p=0.0099; HD vs TNFi+: 46.4% vs 20.1%, p=0.0002). Of note, in patients with active AS under TNFi treatment, the number of circulating CD8+CCR4+ TEM correlates with the ASDAS (r=0.859, p=0.006), while no correlation was observed in the TNFi− group (online supplemental figure 1C). The higher proportion of TEMRA in CD8+CCR4+ T cells observed in patients with active AS was not dependent on TNFi treatment (online supplemental figure 1C).
These results showed that CD8+CCR4+ T cell frequencies are not altered in the blood of patients with AS; however, they exhibit a distinct phenotype. Moreover, the reduction in frequencies of TEM appears to be independent of TNFi treatment.
CD8+CCR4+ T cells in AS exhibit distinct homing features
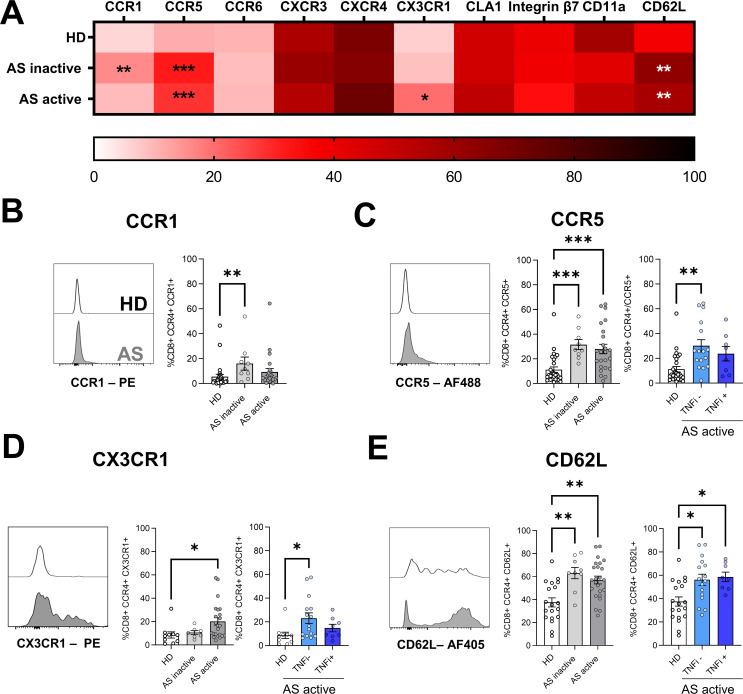
To further characterise the trafficking potential of CD8+CCR4+ T cells to inflamed tissues in AS, we investigated the expression of additional chemokine receptors that guide leucocytes to specific tissues under homeostatic (CCR6 and CXCR4) or inflammatory (CCR1, CCR5, CXCR3 and CX3CR1) conditions. Increased expression of CCR1 was found in patients with inactive AS (figure 2A,B). Significantly higher levels of CCR5 were present in cells isolated from patients with AS, regardless of the disease activity (figure 2A,C). In patients with active AS, TNFi treatment was associated with decreased CCR5 expression to levels similar to the control group (figure 2C). In addition, CX3CR1 was upregulated in active AS and restored to normal levels in patients receiving TNFi treatment (figure 2A,D). By contrast, no differences between the groups were observed for CCR6, CXCR3 and CXCR4 (figure 2A).
Figure 2.
Expression of chemokine receptors and adhesion molecules in CD8+CCR4+ T cells. (A) Heatmap summarising mean frequencies of expression of the reported chemokine receptor and adhesion molecules in CD8+CCR4+ T cells. (B-E) Expression of significantly different receptors is represented as overlaid histograms (left) and mean frequencies±SEM (right) of CD8+CCR4+ T cells. Each symbol represents an individual, and Kruskal-Wallis test was used to compare the markers among the groups; asterisks indicate significant differences between the groups (*p<0.05, **p<0.01 ***p<0.001).
The increased expression of inflammatory receptors prompted us to study the activation and exhaustion states of CD8+CCR4+ T cells. Recently activated T cells express both CD38 and HLA-DR and upregulate PD-1. On average, about 20% of CD8+CCR4+ T cells co-expressed both CD38 and HLA-DR molecules, but no significant differences were observed between healthy individuals and patients with AS (online supplemental figure 2A). Similar levels of PD-1 were present in CD8+CCR4+ T cells in both HD and patients with AS (online supplemental figure 2B). CCR4-expressing cells were also evaluated for their proliferation status based on the expression of Ki67, but no differences were found (online supplemental figure 2C).
To further characterise the migratory capacity of this T cell subpopulation, we assessed the expression of adhesion molecules promoting infiltration at the site of inflammation, selecting those involved in migration to lymphoid organs (CD62L) as well as to sites of AS extramusculoskeletal manifestations: cutaneous lymphocyte-associated antigen 1 (CLA1), CD11a and integrin β7. In both inactive and active AS, a significant increase in CD62L expression was found in CD8+CCR4+ T cells (figure 2A,E). TNFi treatment did not appear to affect the high expression of this selectin. No significant differences in CLA1, CD11a or integrin β7 expression were found (figure 2A).
Together, these results suggest a unique trafficking capability of CD8+CCR4+ T cells in patients with AS mediated by the upregulation of CCR1, CCR5, CX3CR1 and CD62L.
CD8+CCR4+ T cells in AS have increased cytotoxic potential
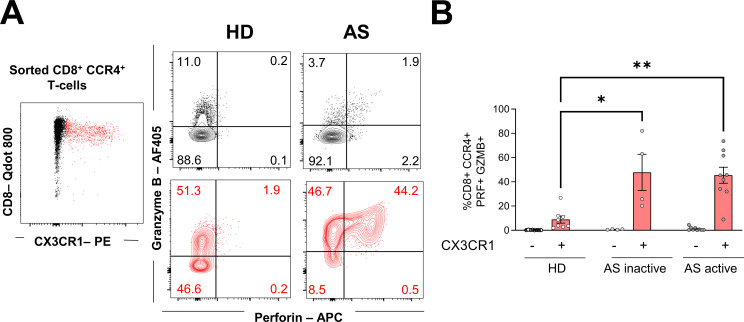
CX3CR1 expression on memory CD8 T cells has been associated with cytotoxic effector functions.36 37 Therefore, we investigated the production of PRF and GZMB upon ex vivo stimulation by sorting CD8+CCR4+ T cells based on their expression of CX3CR1. Consistent with previous studies, higher expression of GZMB was present in CX3CR1+ T cells (figure 3A,B). CD8+CCR4+CX3CR1+ T cells from patients with inactive or active AS produced significantly higher levels of PRF and GZMB than those from HD. On the other hand, CX3CR1- cells only marginally produce PRF.
Figure 3.
PRF and GZMB secretion by CD8+CCR4+ T cells. Sorted CD8+CCR4+ T cells were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 5 hours and expression of CX3CR1, PRF and GZMB was assessed. (A) Representative plot showing CX3CR1- (black) and CX3CR1+ (red) cells expressing PRF and GZMB. Numbers in each quadrant report percentages relative to the parental population. (B) Mean frequencies±SEM of PRF+GZMB+ cells relative to CD8+CCR4+ CX3CR1- (black) or CX3CR1+ (red) cells. Each symbol represents an individual, and Kruskal-Wallis was used to compare PRF and GZMB production between groups within the CX3CR1+ or the CX3CR1- populations. Asterisks indicate significant differences between the groups (*p<0.05, **p<0.01). GZMB, granzyme B; PRF, perforin.
In addition, several cytokines, both with proinflammatory and anti-inflammatory properties, are elevated in sera of patients with AS, among others, TNF, IL-17A and IL-23.38 Overall, ex vivo stimulated CD8+CCR4+ T cells, isolated from patients with active AS, produced fewer cytokines, with a significanly lower proportion of interferon (IFN)-γ, TNF and IL-2 compared with HD (online supplemental figure 3). IL-4 and IL-17 were also reduced, while all groups equally secreted IL-22 and a minimal level of IL-23 (online supplemental figure 3). Together these data indicate that, among the CD8+CCR4+ T cells, generally presenting a low cytokine production, those expressing CX3CR1+ show an enhanced cytotoxicity.
CD8+CCR4+ T cells in AS upregulate gene promoting osteogenesis
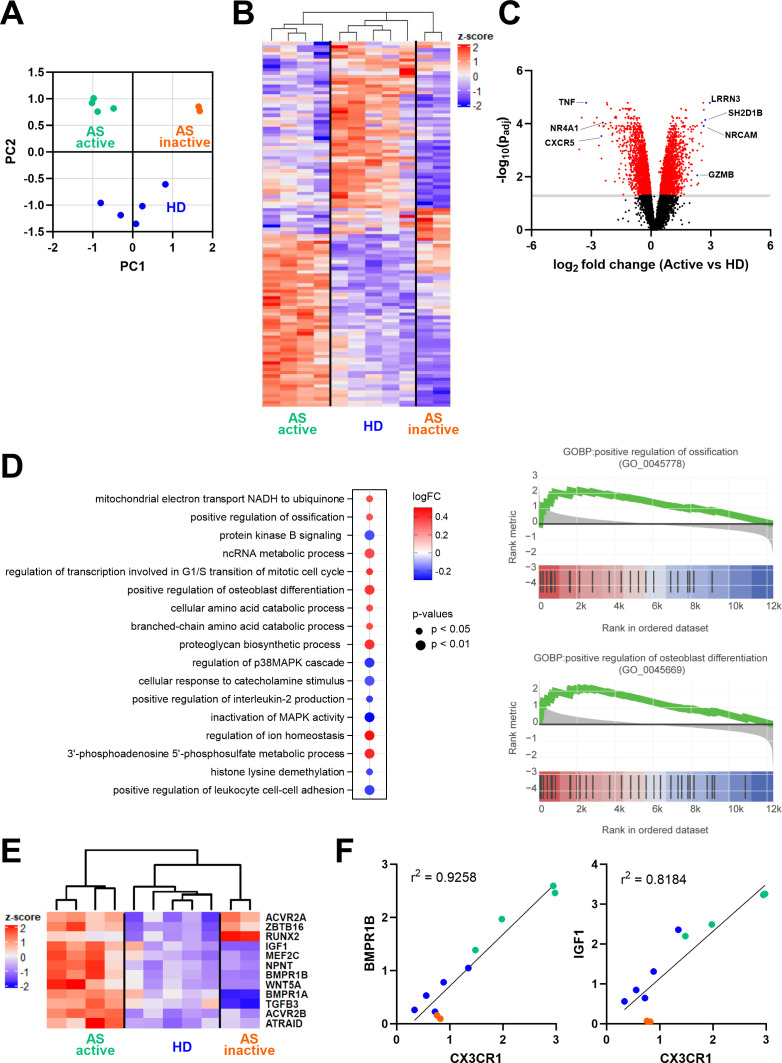
Whole-transcriptome analysis was performed on sorted CD8+CCR4+ T cells from patients with active or inactive AS and from HD. The principal component analysis of the expressed genes highlighted that cells from patients with AS clustered according to disease activity and were distinct from those derived from HD (figure 4A). Of all 12 334 genes detected, 777 genes were significantly differently expressed (false discovery rate ≤0.05 and the absolute value of log2 fold change ≥1) between active AS and HD. Genes involved in the cytolytic process (SH2D1B, NRCAM and GZMB) and expressed in non-senescent cells (IRRN3) were upregulated in active AS, while, among others, TNF, the nuclear receptor NR4A1, implicated in early TCR signalling,39 and the chemokine receptor CXCR5 were downregulated (figure 4B,C). On the other hand, 2161 genes were expressed differently in the active versus inactive AS and 1670 genes between inactive AS and HD. Gene set enrichment analysis of active AS versus HD identified 153 pathways differentially expressed. Interestingly, among the top 10 upregulated pathways in active AS, two were involved in the ossification process: positive regulation of ossification (Gene Ontology Biological Process (GOBP): 0045778, adjusted p value (q)=0.013) and positive regulation of osteoblast differentiation (GOBP: 0045669, q=0.002) (figure 4D). Among these pathways, BMP receptors (BMPR1A, BMPR1B, ACVR2A and ACVR2B), insulin growth factor 1 (IGF1), Wnt proteins (WNT5) and nephronectin (NPNT) genes were significantly upregulated in active AS compared with HD (figure 4E). Both pathways were not significantly altered in inactive AS compared with HD; however, some genes, including the transcription factor RUNX2 and the BMP receptor ACVR2A remained upregulated (figure 4E). Interestingly, the expression of BMPR1B and IGF1 positively correlated with the expression of the fractalkine receptor gene CX3CR1 (figure 4F).
Figure 4.
Gene expression analysis of sorted CD8+CCR4+ T cells. (A) Principal component analysis and sample clustering based on the whole transcriptome. (B) Heatmap showing normalised expression of the significantly differentially expressed genes between active AS and HD. (C) Volcano plot displaying differentially expressed genes between active AS and HD; in red are highlighted the significantly different genes (−log10(p value adjusted) > 1). (D) The graph on the left shows the top 10 upregulated and the seven downregulated pathways identified in the gene set enrichment analysis between active AS and HD. Pathways were ordered based on the number of identified genes in the dataset. The graphs on the right show gene set enrichment analysis of the Gene Ontology Biological Process (GOBP) related to positive regulation of ossification and osteoblast differentiation in patients with active AS. The y-axis reports the fold change expression compared with control samples, while in the x-axis, black bars indicate the rank of genes in the gene set list. The green curve corresponds to the running statistics of the enrichment score. (E) The heatmap reports significantly upregulated genes promoting ossification in active AS. (F) Correlation of CX3CR1 and BMPR1B or IGF1 gene expression detected in the RNA sequencing analysis. AS, ankylosing spondylitis; HD, healthy donor.
Taken together, our results show that CD8+CCR4+ T cells from patients with active AS upregulate genes promoting ossification, which correlates with CX3CR1 expression.
Discussion
The genetic predisposition associated with HLA-B27, discovered 50 years ago,36 40 leads to the hypothesis that T cells may play an important role in AS pathogenesis via TCR-HLA-B27 engagement. Nevertheless, the precise mechanism by which T cells influence new bone formation remains poorly understood. In this study, we provide for the first time the characterisation of a unique subset of CD8+ T cells with osteoregulatory potential. These cells have the potential to travel to extra-musculoskeletal sites of inflammation, where they might cause damage thanks to enhanced production of GZMB and PRF. We have focused on the radiographic state (AS) of a disease continuum now known as axial SpA to also include earlier and milder disease forms.41 Spinal radiographic progression is minimal during the non-radiographic disease state,42 and we targeted a population with demonstrated potential for further structural damage.
Contrasting data on the frequencies of circulating CCR4+ T cells and their correlation with disease activity have been reported.24 25 Our study expands the first characterisations of an altered CCR4+ T cells presence in the circulation of patients with AS and shows, in a larger cohort, that CCR4-expressing CD4+ and CD8+ T cells are not altered in frequencies, regardless of disease activity. Interestingly, we have found that patients with active disease under TNFi treatment displayed lower frequencies of CD8+CCR4+ T cells compared with HD, suggesting a potential egress of these cells from the circulation to the inflamed sites (online supplemental figure 1B). These data are corroborated by initial findings on the presence of a CCR4+ T cell subpopulation in the synovial fluid of patients with AS.43
We have found high expression of CCR1 and CCR5 on CD8+CCR4+ T cells from patients with AS (figure 2A), and their ligands, CCL3, CCL4 and CCL5, have been reported to guide effector T cell migration during inflammation.44 45 These chemokines are also expressed in psoriatic skin46 and in a mouse model of uveitis.47 This suggests that, in AS, CD8+CCR4+ T cells have the potential to travel to sites of extra-musculoskeletal manifestations, including eyes, skin and gut, thanks to the upregulation of inflammatory chemokine receptors and the expression of CLA1 and integrin β7.
Chemokine–chemokine receptor interaction, in addition to its homing regulation, can also influence other T cell activities, such as proliferation, survival and activation.48 CX3CR1 expression on antigen-experienced CD8+ T cells is associated with their ability to produce GZMB, irrespective of CD62L or CCR7 expression.36 49 50 Patients with active AS presented a higher proportion of CD8+CCR4+ T cells expressing CX3CR1.
Interestingly, CD8+CCR4+CX3CR1+ T cells isolated from patients with AS, regardless of disease activity, produced both PRF and GZMB, in contrast to the exclusive production of GZMB registered in HD (figure 3B). This enhanced cytotoxic profile may reflect the enrichment of TEMRA, a subset of CD8+ T cells known for their low proliferative rate, high cytotoxicity, sensitivity to apoptosis and association with chronic inflammation.51 52 The overexpression of cytotoxic molecules is in line with previous work highlighting the upregulation of GZMH, GZMB and NKG7 on circulating CD8+ TEM cells isolated from patients with AS.53
Of note, elevated levels of CX3CL1 are present in the gut, bone marrow and synovium of patients with AS, but not in sera samples.26 53 CX3CR1 and CX3CL1 overexpression has been reported in other autoimmune diseases, and in rheumatoid arthritis (RA), this axis has been targeted by an antibody-based therapeutic intervention.54 Synovial fibroblasts and synoviocytes, expressing CX3CL1, are able to promote CD4+ T cell adhesion in vitro, adhesion that is impaired by the addition of soluble chemokine.55 Thanks to the presence of CX3CL1 on mature osteoblasts,56 cells specialised in bone matrix deposition, we envision a similar interaction between CD8+CCR4+ T cells expressing CX3CR1 and osteoblasts in AS. Indeed, it has been shown that CD8+ T cells, as well as osteoblasts, are present in the enthesis and in the perientheseal bone.19 21
Importantly, CX3CR1 expression levels in CD8+CCR4+ T cells correlated with many of the ossification-promoting genes upregulated in patients with active AS (figure 4F). While the activity of these genes has been extensively studied on osteoblasts,57 little is known about their role in leucocyte biology. IGF1 promotes the differentiation and acquisition of a proinflammatory and cytotoxic profile in both T and natural killer (NK) cells58 59 and induces chondrocyte and osteoblast proliferation and the deposition of extracellular matrix.60 NPNT protein contains epidermal growth factor-like motifs, responsible for the regulation of osteoblast differentiation and angiogenesis in the bone,61 while its function on T cells and immune cell development has not yet been identified. BMPs induce regulatory T cell differentiation and promote an M2 phenotype in macrophages, while NK cells, activated by BMPs, secrete more IFNγ.62 The upregulation of BMPR1A, B and ACVR2 in CD8+CCR4+ T cells from patients with active AS and the fact that their ligand, BMP-2, is found at high levels in the ossifying enthesis,63 point to a role of this axis in AS pathogenesis. Of note, ACVR2A and RUNX2, but not BMPR1A and BMPR1B, all involved in BMPs/transforming growth factor-β signalling, were also upregulated in CD8+CCR4+ T cells from patients with inactive AS. Functional experiments will need to clarify if this difference reflects a ‘quiescent’ state of this pathway in patients with inactive AS that may be ‘reactivated’ by inflammatory cues, present during the active disease.
This report highlights, for the first time, a new pathway by which T cells could directly regulate new bone formation through the expression of ossification-related molecules. Other mechanisms by which T cells regulate bone growth and resorption have been previously proposed. Ahuja and colleagues reported the expression of CD40 and major histocompatibility complex-I on murine osteocytes and osteoblasts, hypothesising that CD8+ T cells, expressing CD40L, could provide these cells with a survival signal.64 CD40–CD40L interaction has also been shown to indirectly induce bone loss in a model of hormone-driven osteoporosis.65 A second mechanism, demonstrated in vitro, relies on cytokine production by bystander activated T cells and the subsequent release of BMP-2 by osteoblast precursors and their matrix mineralization.66 67 Our study shows that CD8+CCR4+ T cells from patients with AS produced low levels of IFNγ, TNF and IL-17 (online supplemental figure 3), suggesting that these cells do not rely on cytokine production to promote bone mineralisation. These data were further corroborated by the low level of TNF gene expression found by RNA sequencing (figure 4C). Other studies pointed at a minimal contribution of CD8+ T cells in AS for the production of major inflammatory cytokines, with decreased levels of IFNγ+ and TNF+ CD8+ T cells both in circulation and in inflamed sites.68 69 On the other hand, excessive IL-17 production was demonstrated to be crucial in the pathogenesis of AS.15 Nevertheless, in line with our data, increasing evidence points to mucosal-associated invariant T cells and γδ cells as major producers of IL-17 in AS, with limited production by CD8+ T cells.70 71 CD8+IL-17+ T cells are found in the synovial fluid of patients suffering from other inflammatory joint diseases, such as psoriatic arthritis, but are absent in RA.72 We cannot exclude that in the inflamed tissues of patients with AS, CD8+CCR4+ T cells might acquire a different cytokine expression profile.
Our study reports a CD8+ T cell subset in AS displaying both osteoregulatory and cytotoxic functions. These cells, due to their chemokine receptor expression pattern, have the potential to traffic to the bone, promoting ossification, and to sites of extra-musculoskeletal manifestations, where they could contribute to inflammation via tissue damage.
A better understanding of the crosstalk between immune cells and the bone in AS will be invaluable for establishing novel therapeutic approaches. Current treatments successfully target inflammation but only partially influence new bone formation. The present data suggest that targeting the chemokine system might represent an additional approach to effectively address this unmet medical need.
Acknowledgments
The authors are grateful to all patients enrolled in the study and to Kristina Bürki (Department of Rheumatology, University Hospital Zurich) for her constant support. We also want to acknowledge the Functional Genomics Center Zurich for the support in the RNA sequencing.
Footnotes
VM and YS contributed equally.
Contributors: VC and MU designed the study and were responsible for the general organisation. VC and MU accept full responsability for the work, had access to the data, and controlled the decision to publish. YS, VM, VC and GD performed most of the experiments. AC and BM provided patient samples and clinical information. AR performed sequencing analysis. VM, IK and MF designed and performed bioinformatics analysis. DJ performed flow cytometry cell sorting. VM, VC, YS, AC and MU discussed the project, experiments and results. VM, VC and MU wrote the manuscript. All authors revised the paper critically for important intellectual content and gave their final approval for submission.
Funding: This work was supported by the Ceschina Foundation, the Swiss National Science Foundation (3100A0-143718/1 and 141773-RM3) and the European Union’s Programs for research technological development and demonstration (ADITEC-280873 (FP7) and TIMER-281608 (FP7)).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. RNA sequencing data were deposited into the GEO repository under accession number GSE243689.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Ethical Committees of the Canton Ticino (CE 3065) and of the Canton Zurich (EK515). Participants gave informed consent to participate in the study before taking part.
References
- 1.Rudwaleit M, van der Heijde D, Landewé R, et al. The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31. 10.1136/ard.2010.133645 [DOI] [PubMed] [Google Scholar]
- 2.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 3.de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016;18:196. 10.1186/s13075-016-1093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauro D, Thomas R, Guggino G, et al. Ankylosing spondylitis: an autoimmune or autoinflammatory disease Nat Rev Rheumatol 2021;17:387–404. 10.1038/s41584-021-00625-y [DOI] [PubMed] [Google Scholar]
- 5.McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet 1998;352:1137–40. 10.1016/S0140-6736(97)12004-9 [DOI] [PubMed] [Google Scholar]
- 6.Gracey E, Burssens A, Cambré I, et al. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat Rev Rheumatol 2020;16:193–207. 10.1038/s41584-019-0364-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sieper J, Poddubnyy D. Axial Spondyloarthritis. Lancet 2017;390:73–84. 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 8.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. 10.1056/NEJMra1406182 [DOI] [PubMed] [Google Scholar]
- 9.Stolwijk C, van Tubergen A, Castillo-Ortiz JD, et al. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:65–73. 10.1136/annrheumdis-2013-203582 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Chen S, Hu Z, et al. Aberrant upregulation of Casr promotes pathological new bone formation in ankylosing spondylitis. EMBO Mol Med 2020;12:e12109. 10.15252/emmm.202012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Wang J, Zhan Z, et al. Inflammation intensity-dependent expression of osteoinductive WNT proteins is critical for ectopic new bone formation in ankylosing spondylitis. Arthritis Rheumatol 2018;70:1056–70. 10.1002/art.40468 [DOI] [PubMed] [Google Scholar]
- 12.Fukui N, Ikeda Y, Ohnuki T, et al. Pro-inflammatory cytokine tumor necrosis factor-alpha induces bone morphogenetic protein-2 in chondrocytes via mRNA stabilization and transcriptional up-regulation. J Biol Chem 2006;281:27229–41. 10.1074/jbc.M603385200 [DOI] [PubMed] [Google Scholar]
- 13.Lories RJU, Derese I, Ceuppens JL, et al. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum 2003;48:2807–18. 10.1002/art.11389 [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, He X, Cheng K, et al. Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res 2019;7:22. 10.1038/s41413-019-0057-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGonagle DG, McInnes IB, Kirkham BW, et al. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis 2019;78:1167–78. 10.1136/annrheumdis-2019-215356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toussirot E. The use of Janus kinase inhibitors in axial spondyloarthritis: current insights. Pharmaceuticals (Basel) 2022;15:270. 10.3390/ph15030270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacques P, Lambrecht S, Verheugen E, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis 2014;73:437–45. 10.1136/annrheumdis-2013-203643 [DOI] [PubMed] [Google Scholar]
- 18.Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR-Gammat+ Cd3+Cd4-Cd8- entheseal resident T cells. Nat Med 2012;18:1069–76. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- 19.Cui H, Li Z, Chen S, et al. Cxcl12/Cxcr4-Rac1-mediated migration of osteogenic precursor cells contributes to pathological new bone formation in ankylosing spondylitis. Sci Adv 2022;8:14. 10.1126/sciadv.abl8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appel H, Sieper J. Spondyloarthritis at the crossroads of imaging, pathology, and structural damage in the era of Biologics. Curr Rheumatol Rep 2008;10:356–63. 10.1007/s11926-008-0058-x [DOI] [PubMed] [Google Scholar]
- 21.Watad A, Rowe H, Russell T, et al. Normal human enthesis harbours conventional Cd4+ and Cd8+ T cells with regulatory features and inducible IL-17A and TNF expression. Ann Rheum Dis 2020;79:1044–54. 10.1136/annrheumdis-2020-217309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zhao Q, Wang G, et al. Circulating levels of Th1 and Th2 chemokines in patients with ankylosing spondylitis. Cytokine 2016;81:10–4. 10.1016/j.cyto.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 23.Shi H, Chen L, Ridley A, et al. GM-CSF primes proinflammatory monocyte responses in ankylosing spondylitis. Front Immunol 2020;11:1520. 10.3389/fimmu.2020.01520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang PT, Kasai H, Zhao LJ, et al. Increased Ccr4 expression on circulating Cd4(+) T cells in ankylosing spondylitis, rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol 2004;138:342–7. 10.1111/j.1365-2249.2004.02617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duftner C, Dejaco C, Kullich W, et al. Preferential type 1 chemokine receptors and cytokine production of Cd28- T cells in ankylosing spondylitis. Ann Rheum Dis 2006;65:647–53. 10.1136/ard.2005.042085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciccia F, Guggino G, Zeng M, et al. Proinflammatory Cx3Cr1+Cd59+Tumor necrosis factor-like molecule 1A+Interleukin-23+ monocytes are expanded in patients with ankylosing spondylitis and modulate innate lymphoid cell 3 immune functions. Arthritis Rheumatol 2018;70:2003–13. 10.1002/art.40582 [DOI] [PubMed] [Google Scholar]
- 27.Proost P, Struyf S, Loos T, et al. Coexpression and interaction of Cxcl10 and Cd26 in mesenchymal cells by synergising inflammatory cytokines: Cxcl8 and Cxcl10 are discriminative markers for autoimmune arthropathies. Arthritis Res Ther 2006;8:R107. 10.1186/ar1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupancu TJ, Eivazitork M, Hamilton JA, et al. Ccl17/TARC in autoimmunity and inflammation-not just a T-cell chemokine. Immunol Cell Biol 2023;101:600–9. 10.1111/imcb.12644 [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto S, Nakamura K, Oyama N, et al. Macrophage-derived Chemokine (MDC)/Ccl22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J Dermatol Sci 2006;44:93–9. 10.1016/j.jdermsci.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 30.Machado P, Landewé R, Lie E, et al. Ankylosing spondylitis disease activity score (ASDAS): defining cut-off values for disease activity States and improvement scores. Ann Rheum Dis 2011;70:47–53. 10.1136/ard.2010.138594 [DOI] [PubMed] [Google Scholar]
- 31.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and Effector human Cd8+ T cells. J Exp Med 1997;186:1407–18. 10.1084/jem.186.9.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahnke YD, Brodie TM, Sallusto F, et al. The who’s who of T-cell differentiation: human memory T-cell Subsets. Eur J Immunol 2013;43:2797–809. 10.1002/eji.201343751 [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–12. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 34.Romero P, Zippelius A, Kurth I, et al. Four functionally distinct populations of human effector-memory Cd8+ T lymphocytes. J Immunol 2007;178:4112–9. 10.4049/jimmunol.178.7.4112 [DOI] [PubMed] [Google Scholar]
- 35.Rufer N, Zippelius A, Batard P, et al. Ex vivo characterization of human Cd8+ T subsets with distinct replicative history and partial effector functions. Blood 2003;102:1779–87. 10.1182/blood-2003-02-0420 [DOI] [PubMed] [Google Scholar]
- 36.Böttcher JP, Beyer M, Meissner F, et al. Functional classification of memory Cd8(+) T cells by Cx3Cr1 expression. Nat Commun 2015;6:8306. 10.1038/ncomms9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlach C, Moseman EA, Loughhead SM, et al. The chemokine receptor Cx3Cr1 defines three antigen-experienced Cd8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 2016;45:1270–84. 10.1016/j.immuni.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xueyi L, Lina C, Zhenbiao W, et al. Levels of circulating Th17 cells and regulatory T cells in Ankylosing Spondylitis patients with an inadequate response to anti-TNF-alpha therapy. J Clin Immunol 2013;33:151–61. 10.1007/s10875-012-9774-0 [DOI] [PubMed] [Google Scholar]
- 39.Moran AE, Holzapfel KL, Xing Y, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011;208:1279–89. 10.1084/jem.20110308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewerton DA, Hart FD, Nicholls A, et al. Ankylosing Spondylitis and HL-A 27. Lancet 1973;1:904–7. 10.1016/s0140-6736(73)91360-3 [DOI] [PubMed] [Google Scholar]
- 41.Navarro-Compán V, Sepriano A, El-Zorkany B, et al. Axial spondyloarthritis. Ann Rheum Dis 2021;80:1511–21. 10.1136/annrheumdis-2021-221035 [DOI] [PubMed] [Google Scholar]
- 42.Hebeisen M, Micheroli R, Scherer A, et al. Spinal radiographic progression in axial spondyloarthritis and the impact of classification as nonradiographic versus radiographic disease: data from the swiss clinical quality management cohort. PLoS One 2020;15:e0230268. 10.1371/journal.pone.0230268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simone D, Penkava F, Ridley A, et al. Single cell analysis of spondyloarthritis regulatory T cells identifies distinct synovial gene expression patterns and clonal fates. Commun Biol 2021;4. 10.1038/s42003-021-02931-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castellino F, Huang AY, Altan-Bonnet G, et al. Chemokines enhance immunity by guiding naive Cd8+ T cells to sites of Cd4+ T cell-dendritic cell interaction. Nature 2006;440:890–5. 10.1038/nature04651 [DOI] [PubMed] [Google Scholar]
- 45.Schall TJ, Bacon K, Toy KJ, et al. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990;347:669–71. 10.1038/347669a0 [DOI] [PubMed] [Google Scholar]
- 46.Giustizieri ML, Mascia F, Frezzolini A, et al. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol 2001;107:871–7. 10.1067/mai.2001.114707 [DOI] [PubMed] [Google Scholar]
- 47.Curnow SJ, Murray PI. Inflammatory mediators of uveitis: cytokines and chemokines. Curr Opin Ophthalmol 2006;17:532–7. 10.1097/ICU.0b013e32801094b5 [DOI] [PubMed] [Google Scholar]
- 48.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659–702. 10.1146/annurev-immunol-032713-120145 [DOI] [PubMed] [Google Scholar]
- 49.Gordon CL, Lee LN, Swadling L, et al. Induction and maintenance of Cx3Cr1-intermediate peripheral memory Cd8(+) T cells by persistent viruses and vaccines. Cell Rep 2018;23:768–82. 10.1016/j.celrep.2018.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwijnenburg AJ, Pokharel J, Varnaite R, et al. Graded expression of the chemokine receptor Cx3Cr1 marks differentiation states of human and murine T cells and enables cross-species interpretation. Immunity 2023;56. 10.1016/j.immuni.2023.06.025 [DOI] [PubMed] [Google Scholar]
- 51.Appay V, Dunbar PR, Callan M, et al. Memory Cd8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002;8:379–85. 10.1038/nm0402-379 [DOI] [PubMed] [Google Scholar]
- 52.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human Cd8+ memory T-cell Subsets in response to antigen or homeostatic cytokines. Blood 2003;101:4260–6. 10.1182/blood-2002-11-3577 [DOI] [PubMed] [Google Scholar]
- 53.Alber S, Kumar S, Liu J, et al. Single cell Transcriptome and surface EPITOPE analysis of Ankylosing Spondylitis facilitates disease classification by machine learning. Front Immunol 2022;13:838636. 10.3389/fimmu.2022.838636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka Y, Takeuchi T, Yamanaka H, et al. Long-term safety and efficacy of E6011, an anti-Fractalkine monoclonal antibody, in patients with rheumatoid arthritis inadequately responding to methotrexate. Mod Rheumatol 2023;34:37–44. 10.1093/mr/road004 [DOI] [PubMed] [Google Scholar]
- 55.Sawai H, Park YW, Roberson J, et al. T cell costimulation by fractalkine-expressing synoviocytes in rheumatoid arthritis. Arthritis Rheum 2005;52:1392–401. 10.1002/art.21140 [DOI] [PubMed] [Google Scholar]
- 56.Koizumi K, Saitoh Y, Minami T, et al. Role of Cx3Cl1/Fractalkine in osteoclast differentiation and bone resorption. J Immunol 2009;183:7825–31. 10.4049/jimmunol.0803627 [DOI] [PubMed] [Google Scholar]
- 57.Wu M, Chen G, Li YP. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 2016;4:16009. 10.1038/boneres.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiToro D, Harbour SN, Bando JK, et al. Insulin-like growth factors are key regulators of T helper 17 regulatory T cell balance in autoimmunity. Immunity 2020;52:650–67. 10.1016/j.immuni.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ni F, Sun R, Fu B, et al. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat Commun 2013;4:1479. 10.1038/ncomms2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Shen X, Wan C, et al. Effects of insulin and insulin-like growth factor 1 on Osteoblast proliferation and differentiation: differential signalling via AKT and ERK. Cell Biochem Funct 2012;30:297–302. 10.1002/cbf.2801 [DOI] [PubMed] [Google Scholar]
- 61.Sun Y, Kuek V, Qiu H, et al. The emerging role of NPNT in tissue injury repair and bone homeostasis. J Cell Physiol 2018;233:1887–94. 10.1002/jcp.26013 [DOI] [PubMed] [Google Scholar]
- 62.Sconocchia T, Sconocchia G. Regulation of the immune system in health and disease by members of the bone Morphogenetic protein family. Front Immunol 2021;12:802346. 10.3389/fimmu.2021.802346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng G, Xie Z, Wang P, et al. Enhanced osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis: a study based on a three-dimensional biomimetic environment. Cell Death Dis 2019;10:350. 10.1038/s41419-019-1586-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahuja SS, Zhao S, Bellido T, et al. Cd40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone Osteocyte-Y4. Endocrinology 2003;144:1761–9. 10.1210/en.2002-221136 [DOI] [PubMed] [Google Scholar]
- 65.Gao Y, Wu X, Terauchi M, et al. T cells potentiate PTH-induced cortical bone loss through Cd40L signaling. Cell Metab 2008;8:132–45. 10.1016/j.cmet.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rifas L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J Cell Biochem 2006;98:706–14. 10.1002/jcb.20933 [DOI] [PubMed] [Google Scholar]
- 67.Rifas L, Arackal S, Weitzmann MN. Inflammatory T cells rapidly induce differentiation of human bone marrow Stromal cells into mature Osteoblasts. J Cell Biochem 2003;88:650–9. 10.1002/jcb.10436 [DOI] [PubMed] [Google Scholar]
- 68.Rudwaleit M, Siegert S, Yin Z, et al. Low T cell production of Tnfalpha and Ifngamma in Ankylosing Spondylitis: its relation to HLA-B27 and influence of the TNF-308 gene polymorphism. Ann Rheum Dis 2001;60:36–42. 10.1136/ard.60.1.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Damme N, De Vos M, Baeten D, et al. Flow cytometric analysis of gut mucosal lymphocytes supports an impaired Th1 cytokine profile in spondyloarthropathy. Ann Rheum Dis 2001;60:495–9. 10.1136/ard.60.5.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kenna TJ, Davidson SI, Duan R, et al. Enrichment of circulating Interleukin-17-secreting interleukin-23 receptor-positive gamma/Delta T cells in patients with active Ankylosing Spondylitis. Arthritis Rheum 2012;64:1420–9. 10.1002/art.33507 [DOI] [PubMed] [Google Scholar]
- 71.Rosine N, Rowe H, Koturan S, et al. Characterization of blood Mucosal-associated invariant T cells in patients with axial Spondyloarthritis and of resident Mucosal-associated invariant T cells from the axial Entheses of non-axial Spondyloarthritis control patients. Arthritis Rheumatol 2022;74:1786–95. 10.1002/art.42090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+Cd8+ T cells are enriched in the joints of patients with Psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. 10.1002/art.38376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003926supp001.pdf (295.9KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data are available upon reasonable request. RNA sequencing data were deposited into the GEO repository under accession number GSE243689.