Abstract
A wide variety of biological activities including the major metabolic actions of insulin is regulated by phosphatidylinositol (PI) 3-kinase. However, the downstream effectors of the various signaling pathways that emanate from PI 3-kinase remain unclear. Akt (protein kinase B), a serine-threonine kinase with a pleckstrin homology domain, is thought to be one such downstream effector. A mutant Akt (Akt-AA) in which the phosphorylation sites (Thr308 and Ser473) targeted by growth factors are replaced by alanine has now been shown to lack protein kinase activity and, when overexpressed in CHO cells or 3T3-L1 adipocytes with the use of an adenovirus vector, to inhibit insulin-induced activation of endogenous Akt. Akt-AA thus acts in a dominant negative manner in intact cells. Insulin-stimulated protein synthesis, which is sensitive to wortmannin, a pharmacological inhibitor of PI 3-kinase, was abolished by overexpression of Akt-AA without an effect on amino acid transport into the cells, suggesting that Akt is required for insulin-stimulated protein synthesis. Insulin activation of p70 S6 kinase was inhibited by ∼75% in CHO cells and ∼30% in 3T3-L1 adipocytes, whereas insulin-induced activation of endogenous Akt was inhibited by 80 to 95%, by expression of Akt-AA. Thus, Akt activity appears to be required, at least in part, for insulin stimulation of p70 S6 kinase. However, insulin-stimulated glucose uptake in both CHO cells and 3T3-L1 adipocytes was not affected by overexpression of Akt-AA, suggesting that Akt is not required for this effect of insulin. These data indicate that Akt acts as a downstream effector in some, but not all, of the signaling pathways downstream of PI 3-kinase.
Akt is a pleckstrin homology (PH) domain-containing protein serine-threonine kinase whose kinase domain shares structural similarity with protein kinase C (PKC) isozymes and cyclic AMP-dependent protein kinase (PKA) (3). Thus, Akt has also been termed RAC-PK (protein kinase related to A and C kinases) (19) and PKB (protein kinase B) (7). Insulin and various other growth factors activate Akt, and this activation is inhibited by pharmacological blockers of phosphatidylinositol (PI) 3-kinase or by a dominant negative mutant of PI 3-kinase (4, 14, 25). Furthermore, Akt is activated by overexpression of a constitutively active mutant of PI 3-kinase in quiescent cells (11, 23). These observations indicate that Akt is a downstream effector of PI 3-kinase.
PI 3-kinase, which consists of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit (5), is implicated in various metabolic effects of insulin (18, 59). A dominant negative mutant of PI 3-kinase as well as various pharmacological inhibitors, such as wortmannin and LY294002, have been used to block specific signaling pathways that include this enzyme (6, 16, 31, 39, 61). The metabolic actions of insulin that are sensitive to either a dominant negative mutant or pharmacological inhibitors of PI 3-kinase include stimulation of glucose uptake, antilipolysis, activation of fatty acid synthase and glycogen synthase, and stimulation of amino acid transport and protein synthesis (6, 16, 34, 37, 47, 48, 54, 55). Moreover, regulation of the amounts of specific protein participants in metabolic pathways by insulin is mediated by this lipid kinase (52). Although these observations indicate that PI 3-kinase is a major regulator of the metabolic effects of insulin, the roles of the various downstream effectors of PI 3-kinase in each of these actions remain unclear.
The kinase activity of Akt fused with a viral Gag protein or tagged with a myristoylation signal sequence is higher than that of wild-type Akt (Akt-WT) (4, 26). Overexpression of these mutant Akt proteins induced activation of p70 S6 kinase (4, 26), which is also activated by insulin in a wortmannin-sensitive manner (42, 43). Expression of these active Akt mutants also promoted glucose uptake and translocation of GLUT4 glucose transporters in quiescent adipocytes (27, 53). These observations have suggested that Akt is a downstream effector of PI 3-kinase that mediates insulin-induced activation of p70 S6 kinase and glucose uptake. This proposal could be tested further by investigating the effects of specific inhibition of Akt activity on these actions of insulin. However, a mutant Akt that exerts dominant negative effects has not previously been described.
The mechanism by which Akt is activated in response to growth factor stimulation is not fully understood. PI 3,4-bisphosphate, one of the products of PI 3-kinase action, stimulates Akt activity in vitro (15, 24). Furthermore, Akt mutants with substitutions in or lacking the PH domain were not activated by this phospholipid (15, 24), suggesting that Akt is activated as a result of direct interaction of its PH domain with the lipid. On the other hand, other studies have suggested the importance of phosphorylation of Akt on serine and threonine residues in regulation of its activity. Akt is phosphorylated in vivo in response to various growth factors that stimulate Akt activity (4, 14, 26), and dephosphorylation of in vivo-activated Akt by a serine-threonine phosphatase abolished its enzymatic activity (26). Akt is phosphorylated on Thr308 and Ser473 in response to insulin in vivo, and Akt mutants in which either Thr308 or Ser473 was substituted were not activated (1). Moreover, a protein kinase which phosphorylates and activates Akt has been cloned and characterized (2, 44, 50, 51). These data have suggested that Akt is primarily activated as a result of its phosphorylation on serine and threonine residues by an upstream kinase.
We have now shown that when overexpressed in CHO cells or 3T3-L1 adipocytes, a mutant Akt in which growth factor-targeted serine and threonine phosphorylation sites are replaced with alanine exerted a dominant negative effect on endogenous Akt activity stimulated by insulin. With the use of this dominant negative Akt, we have investigated the roles of Akt in insulin-stimulated protein synthesis, p70 S6 kinase activation, and glucose transport in these cells.
MATERIALS AND METHODS
Cells, plasmids, and antibodies.
CHO cells were routinely maintained and 3T3-L1 preadipocytes were maintained and induced to differentiate into adipocytes as described previously (48). To establish CHO cells that stably express FLAG epitope-tagged Akt (CHO-Akt cells), we transfected CHO cells with pSV40-hgh, which confers resistance to hygromycin, and a PECE vector encoding FLAG epitope-tagged rat Akt1 (RAC-PKα) (30). Transfected cells were selected and cloned as described previously (22). PECE vectors encoding FLAG epitope-tagged rat Akt2 (RAC-PKβ) (30) and RAC-PKγ (29) were as described previously. Monoclonal antibodies to the hemagglutinin (HA) epitope tag (12CA5) or to the FLAG epitope tag were obtained from Boehringer Mannheim and Kodak Scientific Imaging Systems, respectively. Polyclonal antibodies to Akt were generated against a glutathione S-transferase fusion protein containing amino acids 428 to 480 of rat Akt1. Polyclonal antibodies to mitogen-activated protein (MAP) kinase (αC92) were as described previously (48). Polyclonal antibodies to p70 S6 kinase were generated against a synthetic peptide corresponding to amino acids 2 to 23 of the rat enzyme (33).
Construction of adenovirus vectors.
Adenovirus vectors encoding a dominant negative PI 3-kinase (AxCAΔp85) or a dominant negative SOS (AxCAΔSOS) were as described previously (48). Rat Akt1 was tagged with the HA epitope by PCR with a sense primer (5′-ACT AAG CTT GCC ATG TAC CCA TAC GAT GTT CCG GAT TAC GCT AAC GAC GTA GCC ATT GTG AAG G), an antisense primer (5′-GAT GAA TTC ACT GGG TGA ACC TGA CCG G), and a full-length rat Akt1 cDNA as template. Both Thr308 and Ser473 of HA-tagged rat Akt1 were replaced by alanine with the use of a Quick Change site-directed mutagenesis kit (Stratagene). Lys179 of HA-tagged rat Akt1 was replaced with asparate by the use of PCR. These mutants were termed Akt-AA and AktK179D, respectively. DNA encoding the HA-tagged wild-type and mutant (Akt-AA or AktK179D) Akt proteins was subcloned into pAxCAwt (36), and adenovirus vectors containing these cDNAs were generated by transfecting 293 cells with the corresponding pAxCAwt plasmid together with a DNA-terminal protein complex (36), as described previously (48). The resulting vectors were termed AxCAAkt-WT, AxCAAkt-AA, and AxCAAkt-K179D, respectively. CHO cells or 3T3-L1 adipocytes were infected with adenovirus vectors at the indicated multiplicity of infection (MOI) as described previously (48, 57). The cells were subjected to experiments 48 h after infection.
Kinase assays.
CHO cells or 3T3-L1 adipocytes were deprived of serum for 16 to 20 h, incubated in the absence or presence of insulin, and then immediately frozen with liquid nitrogen. The MAP kinase assay was performed with immunoprecipitates prepared with antibodies to MAP kinase as described previously (47, 48).
For p70 S6 kinase assays, the frozen cells were lysed in a solution containing 50 mM Tris-HCl (pH 8.0), 120 mM NaCl, 20 mM NaF, 1 mM benzamidine, 1 mM EDTA, 6 mM EGTA, 15 mM sodium pyrophosphate, 1% Nonidet P-40, 30 mM p-nitrophenyl phosphate, 0.5 mM dithiothreitol, and 0.1 mM phenylmethylsulfonyl fluoride. The lysate was centrifuged (at 15,000 × g for 20 min), and the resulting supernatant was subjected to immunoprecipitation with polyclonal antibodies to p70 S6 kinase. After being washed three times with HEPES-buffered saline (pH 7.5) containing 0.1% Triton X-100, the immunoprecipitates were incubated for 30 min at 25°C in a reaction mixture (30 μl) containing 50 mM morpholine propanesulfonic acid (pH 7.2), 5 mM MgCl2, 1 mM dithiothreitol, 10 mM p-nitrophenyl phosphate, 100 μM unlabeled ATP, 3.0 μCi of [γ-32P]ATP, and 100 μM S6 synthetic peptide (KRRRLSSLRASTSKSESSQK) as the substrate. The reaction mixture was then centrifuged (at 15,000 × g for 3 min), and the resulting supernatant was transferred to P81 (Whatman) filter paper. After extensive washing of the filters with 0.5% phosphoric acid, 32P incorporation into the peptide was determined by liquid scintillation spectroscopy.
To assay the activities of FLAG- or HA-tagged Akt kinase, we lysed the cells in a solution containing 50 mM HEPES-NaOH (pH 7.6), 150 mM NaCl, 1% Triton X-100, bacitracin (1 mg/ml), 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 mM sodium orthovanadate, 10 mM NaF, and 30 mM sodium pyrophosphate. The lysates were subjected to immunoprecipitation with antibodies to HA or to FLAG. After being washed three times with HEPES-buffered saline (pH 7.5) containing 0.1% Triton X-100, the immunoprecipitates were incubated for 30 min at 30°C with 3.0 μCi of [γ-32P]ATP in a reaction mixture (30 μl) containing 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 25 μM unlabeled ATP, 1 μM protein kinase inhibitor, and histone 2B (0.2 mg/ml) as the substrate. The reaction was terminated by the addition of sodium dodecyl sulfate (SDS) sample buffer, and the samples were then fractionated by SDS-polyacrylamide gel electrophoresis on a 15% gel. The radioactivity incorporated into histone 2B was determined with a Fuji BAS 2000 image analyzer.
For assay of endogenous Akt activity in CHO cells or 3T3-L1 adipocytes that had been infected with AxCAAkt-AA, the cells were lysed as described for determination of the activity of epitope-tagged Akt. The lysates were subjected to three sequential immunoprecipitations for 90 min at 4°C (in a final volume of 400 μl) with 20 μl of protein G-Sepharose (Pharmacia) that had been coupled with 20 μg of antibodies to HA. The final supernatant was then subjected to immunoprecipitation with polyclonal antibodies to Akt, and Akt kinase assays were performed with the resulting immunoprecipitates as described above.
Glucose uptake and translocation of GLUT4.
Glucose uptake was assayed as described previously (16, 48). In brief, CHO cells and 3T3-L1 adipocytes cultured in six-well plates were incubated for 16 h in Ham’s F12 and Dulbecco’s modified Eagle’s media, respectively, containing 5.6 mM glucose and 0.5% fetal bovine serum. The cells were washed twice with DB buffer (140 mM NaCl, 2.7 mM KCl, 1 mM CaCl2, 1.5 mM KH2PO4, 8 mM Na2HPO4 [pH 7.4], 0.5 mM MgCl2) and incubated with 100 nM insulin for 15 min, after which 1 ml of DB buffer containing bovine serum albumin (BSA; 1 mg/ml) and 0.1 mM 2-deoxy-d-[1,2-3H]glucose (1 μCi) was added to each well. After 5 min, the cells were washed and then solubilized with 0.1% SDS. The radioactivity incorporated into the cells was measured by liquid scintillation spectroscopy.
Translocation of GLUT4 to the plasma membrane was measured by the plasma membrane lawn assay as previously described (48).
Protein synthesis and amino acid transport.
Insulin-stimulated protein synthesis was assayed essentially as described previously (34), with the following modifications. CHO cells or 3T3-L1 adipocytes were deprived of serum for 24 h and then incubated for 1 h either with a mixture of Ham’s F12 and DB buffer (1:100 [vol/vol]) or with methionine- and cysteine-free Dulbecco’s modified Eagle’s medium (Sigma), respectively. After addition of Tran35S-label (16 μCi/ml; ICN) and 100 nM insulin, the cells were incubated for an additional 1 h and the medium was then aspirated. The cells were washed three times with phosphate-buffered saline containing 10 mM methionine and then lysed in a solution containing 30 mM Tris-HCl (pH 7.5), 140 mM NaCl, and 0.5% Nonidet P-40. Proteins were precipitated by the addition of ice-cold trichloroacetic acid (final concentration, 10% [wt/vol]) containing 10 mM methionine. The protein precipitates were washed three times with ice-cold phosphate-buffered saline containing 10% trichloroacetic acid and 10 mM methionine and then solubilized in 1 M NaOH at 37°C for 10 min. The protein-associated radioactivity was assayed by liquid scintillation spectroscopy.
Amino acid uptake was assayed as described previously (54), with the following modifications. CHO cells cultured in six-well plates were deprived of serum for 16 h, washed twice with DB buffer, and incubated for 20 min with 2 ml of DB buffer containing BSA (1 mg/ml) and 10 mM unlabeled α-methylaminoisobutyrate (MeAIB). One milliliter of DB buffer containing BSA (1 mg/ml), 10 mM unlabeled MeAIB, 10 μM [14C]MeAIB (1 μCi/ml), and 100 nM insulin was then added to each well; after 30 min, the cells were washed three times with ice-cold DB buffer containing BSA (1 mg/ml) and solubilized with 0.1% SDS. The amount of radioactivity incorporated into the cells was measured by liquid scintillation spectroscopy.
Apoptosis assay.
Apoptosis was assayed by measuring characteristic DNA laddering. DNA laddering was analyzed essentially as described previously (20), with the following modifications. CHO cells cultured in 6-cm-diameter plates were infected with AxCAAkt-AA or AxCAAkt-WT at the indicated MOI (PFU/cell). After 32 h, the infected cells were deprived of serum for 16 h, washed once in phosphate-buffered saline, and lysed in 0.1 ml of a buffer containing 0.5% Triton X-100, 10 mM Tris-HCl (pH 7.5), and 10 mM EDTA. The lysates were incubated for 20 min at 4°C and then centrifuged at 15,000 × g for 20 min. The DNA-containing soluble fraction was extracted from the resultant supernatants with phenol-chloroform, ethanol precipitated, resuspended in a buffer containing 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, and 20 μg of RNase A per ml, and then incubated at 37°C for 1 h. DNA was loaded onto a 1.5% agarose gel; after electrophoresis, the gel was stained with ethidium bromide and photographed.
RESULTS
Dominant negative effects of a mutant Akt with alanine substitutions at Thr308 and Ser473.
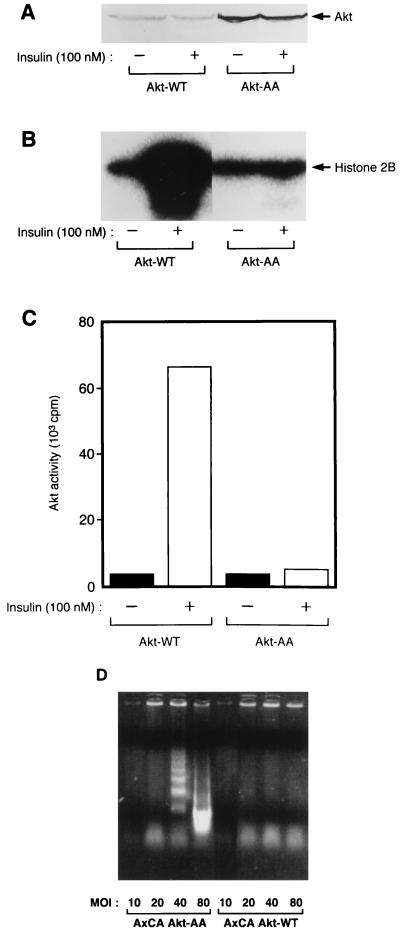
A rat Akt1 (RAC-PKα) (28) mutant (termed Akt-AA) in which Thr308 and Ser473 were replaced by alanine and rat Akt-WT were tagged with the HA epitope at their NH2 termini and expressed in CHO cells with the use of adenovirus vectors (AxCAAkt-WT or AxCAAkt-AA) containing the corresponding cDNA. The infected cells were then incubated in the absence or presence of insulin, after which recombinant Akt was immunoprecipitated with antibodies to HA and assayed for Akt kinase activity with histone 2B as the substrate. Consistent with previous observations (1), insulin increased the activity of Akt1-WT about 20-fold but had no effect on the activity of Akt-AA (Fig. 1A to C).
FIG. 1.
(A to C) Effects of insulin on the kinase activity of Akt-WT and Akt-AA in CHO cells. CHO cells were infected with either AxCAAkt-WT or AxCAAkt-AA at an MOI of 5 PFU/cell. The cells were incubated for 10 min in the absence or presence of 100 nM insulin, lysed, and subjected either to immunoblot analysis with antibodies to HA (A) or to immunoprecipitation with antibodies to HA (B and C). The immunoprecipitates were assayed for Akt kinase activity, and the radioactivity incorporated into histone 2B was visualized (B) or quantitated (C) with an image analyzer as described in Materials and Methods. Data are representative of three independent experiments. (D) Effects of Akt-AA and Akt-WT on apoptosis in CHO cells. CHO cells were infected with AxCAAkt-AA (left) or AxCAAkt-WT (right) at the indicated MOI (PFU/cell). The cells were deprived of serum for 16 h, and DNA laddering was examined as described in Materials and Methods. Data are representative of two independent experiments.
Because Akt has been shown to be involved in cell survival signals (13, 20), we first examined the effects of Akt-AA on apoptosis. CHO cells were infected with AxCAAkt-AA or AxCAAkt-WT at the indicated MOI, and then DNA laddering was examined. Infection of CHO cells with AxCAAkt-AA led to DNA laddering in an MOI-dependent manner whereas AxCAAkt-WT did not exhibit such an effect (Fig. 1D), suggesting that the induction of apoptosis was due not to a nonspecific effect of viral infection but to the effect of Akt-AA. However, DNA laddering was not evident with the cells infected with AxCAAkt-AA at an MOI of 20. We thus examined the effects of this mutant on various insulin-induced biological activities in CHO cells infected with AxCAAkt-AA at an MOI of 20 or less.
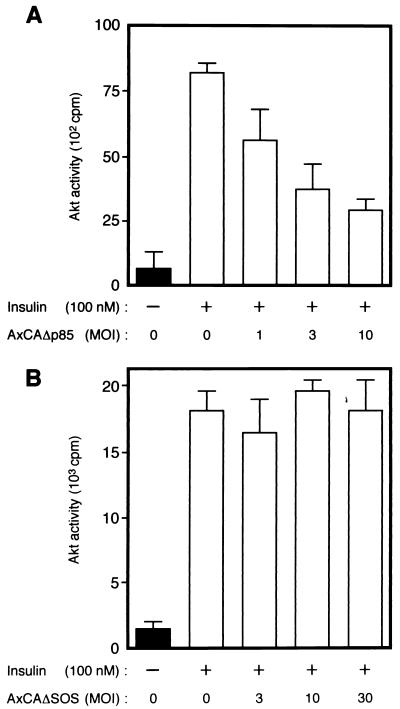
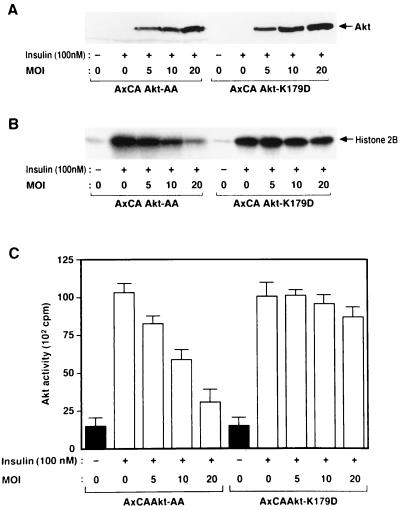
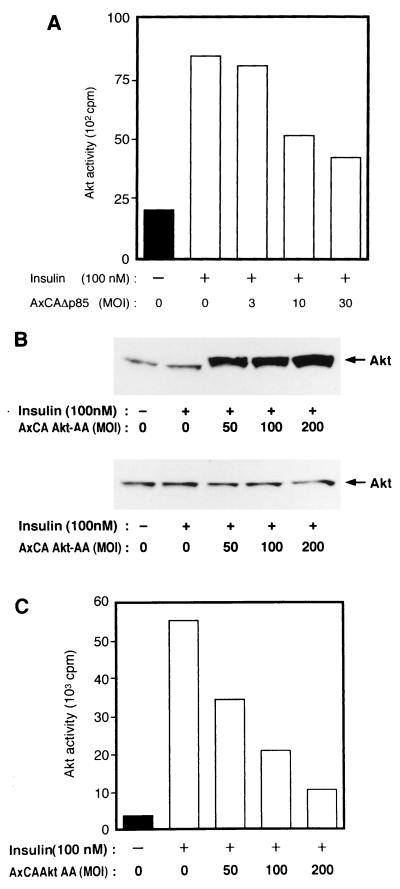
We also established CHO-Akt cells, which stably express FLAG epitope-tagged rat Akt1. The extent of expression of Akt protein in these cells is approximately seven times that in parental CHO cells (data not shown). CHO-Akt cells were infected with various adenovirus vectors, incubated in the absence or presence of insulin, lysed, and subjected to immunoprecipitation with antibodies to FLAG. Assay of the resulting immunoprecipitates for Akt activity revealed that insulin induced a 6- to 10-fold increase in the activity of FLAG-tagged Akt (Fig. 2 and 3). Infection of the cells with AxCAΔp85 (48), an adenovirus encoding a dominant negative mutant of PI 3-kinase, inhibited insulin-induced activation of Akt (Fig. 2A). In contrast, infection of CHO-Akt cells with an adenovirus encoding a dominant negative mutant of SOS (AxCAΔSOS) (48, 57), even at a virus concentration sufficient for almost complete inhibition of insulin-induced activation of MAP kinase activity in CHO cells (data not shown), had no effect on Akt activity (Fig. 2B). Infection of the cells with AxCAAkt-AA resulted in a dose-dependent inhibition of Akt activity precipitated with antibodies to FLAG (Fig. 3B and C); the extent of inhibition paralleled the expression of Akt-AA protein (Fig. 3A), with ∼75% inhibition apparent at an MOI of 20 PFU/cell. We also constructed an adenovirus vector that encodes a mutant Akt in which Lys179 in the kinase domain was replaced by asparate (Akt-K179D). This mutant Akt did not exhibit kinase activity (data not shown). Infection of the cells with AxCAAkt-K179D had little effect on Akt activity precipitated with antibodies to FLAG (Fig. 3B and C), whereas the extent of expression of Akt-K179D protein assessed by immunoblot analysis with antibodies to HA was similar to that of Akt-AA protein in the cells infected with AxCAAkt-AA (Fig. 3A).
FIG. 2.
Effects of Δp85 (A) and ΔSOS (B) on insulin-induced activation of Akt in CHO-Akt cells. CHO-Akt cells were infected with adenoviruses encoding Δp85 (AxCAΔp85) (A) or ΔSOS (AxCAΔSOS) (B) at the indicated MOI (PFU/cell). The cells were incubated for 10 min in the absence or presence of 100 nM insulin, after which lysates from individual 6-cm-diameter plates were subjected to immunoprecipitation with antibodies to FLAG and the precipitates were assayed for Akt kinase activity. Data are means ± standard errors from three experiments.
FIG. 3.
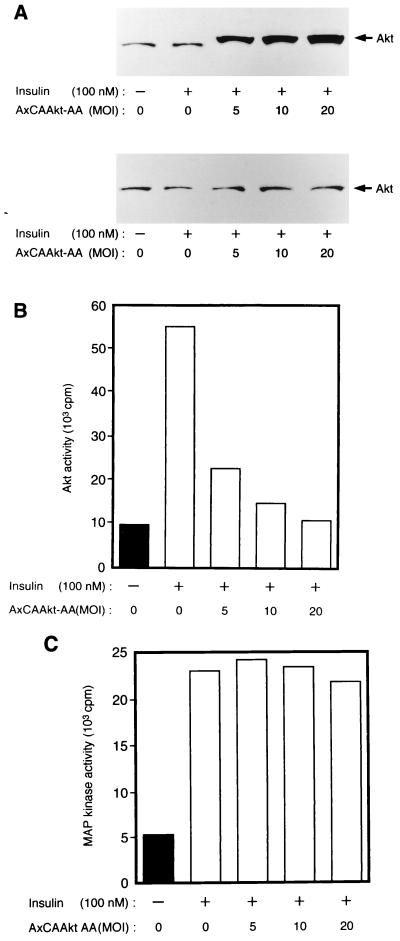
Effects of Akt-AA and Akt-K179D on insulin-induced activation of Akt in CHO-Akt cells. CHO-Akt cells were infected with an adenovirus encoding Akt-AA (AxCAAkt-AA) or Akt-K179D (AxCAAkt-K179D) at the indicated MOI (PFU/cell), incubated for 10 min in the absence or presence of 100 nM insulin, lysed, and subjected either to immunoblot analysis with antibodies to HA (A) or to immunoprecipitation with antibodies to FLAG (B and C). The immunoprecipitates were assayed for Akt kinase activity, and radioactivity incorporated into histone 2B was visualized (B) or quantitated (C) with an image analyzer. Data in panel C are means ± standard errors from three experiments.
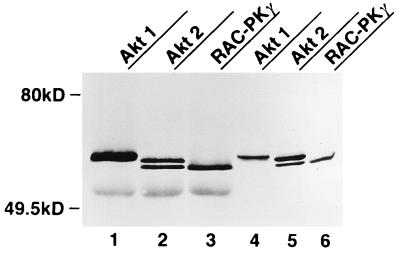
We next investigated the effect of Akt-AA on endogenous Akt activity in CHO cells by precipitating the endogenous protein with polyclonal antibodies to Akt. The polyclonal antibodies recognize all three known rat Akt isoforms, Akt1 (RAC-PKα), Akt2 (RAC-PKβ), and RAC-PKγ, which were transiently expressed in COS cells (Fig. 4). Because these antibodies recognize both endogenous and recombinant Akt proteins, Akt-AA was immunodepleted from cell lysates with antibodies to HA before endogenous Akt was immunoprecipitated with the polyclonal antibodies and assayed for kinase activity toward histone 2B. Infection of CHO cells with AxCAAkt-AA resulted in the expression of Akt-AA, the electrophoretic mobility of which was slightly less than that of endogenous Akt because of the presence of the epitope tag, in an MOI-dependent manner (Fig. 5A). At an MOI of 20 PFU/cell, the abundance of Akt-AA was ∼30 to 50 times that of endogenous Akt (data not shown). After three sequential immunoprecipitations with antibodies to HA, the amounts of Akt protein remaining in the supernatant were similar in infected and noninfected cells (Fig. 5A), indicating that Akt-AA was removed by this procedure. The insulin-induced activation of endogenous Akt was found to be inhibited by AxCAAkt-AA in an MOI-dependent manner, with 95% inhibition apparent at an MOI of 20 (Fig. 5B). In contrast, insulin-induced activation of MAP kinase was not affected by the expression of Akt-AA (Fig. 5C). Furthermore, infection of the cells with a control virus containing the lacZ gene (AxCALacZ) at an MOI of 20 had no effect on insulin-stimulated Akt activity (data not shown), suggesting that the inhibition of insulin-induced Akt activation by AxCAAkt-AA was not due to a nonspecific effect of viral infection.
FIG. 4.
Polyclonal antibodies to Akt recognize all three known isoforms of Akt. COS-7 cells cultured in 6-cm-diameter plates were transiently transfected with 3 μg of plasmid-encoded FLAG-tagged Akt1, Akt2, or RAC-PKγ. Lysates prepared from COS-7 cells expressing each isoform of Akt were subjected to immunoprecipitation with polyclonal antibodies to Akt. The immunoprecipitates (lanes 1 to 3) or the total cell lysates (lanes 4 to 6) were subjected to immunoblot analysis with antibodies to FLAG.
FIG. 5.
Effects of Akt-AA on insulin-induced activation of endogenous Akt and MAP kinase in CHO cells. CHO cells cultured in 6-cm-diameter plates were infected with AxCAAkt-AA at the indicated MOI (PFU/cell), incubated for 10 min in the absence or presence of 100 nM insulin, and lysed. (A) Cell lysates were subjected either to immunoblot analysis with polyclonal antibodies to Akt (top) or to three sequential rounds of immunoprecipitation with antibodies to HA in order to deplete Akt-AA, after which the final supernatant was subjected to immunoblot analysis with polyclonal antibodies to Akt (bottom). (B and C) The Akt-AA-depleted final supernatant from panel A was subjected to immunoprecipitation with polyclonal antibodies to Akt (B) or with antibodies to MAP kinase (C), and the precipitates were assayed for Akt kinase or MAP kinase activity, respectively. Data are representative of three independent experiments, with bars representing means of duplicate determinations.
We confirmed the dominant negative effect of Akt-AA with 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes were infected with AxCAAkt-AA or AxCAΔp85 and incubated in the absence or presence of insulin. The activity of endogenous Akt was then assayed after immunodepletion of Akt-AA. As with CHO cells, three sequential immunoprecipitations with antibodies to HA removed Akt-AA (Fig. 6B). Infection of the adipocytes with AxCAΔp85 inhibited insulin-induced activation of endogenous Akt in an MOI-dependent manner, with ∼65% inhibition apparent at an MOI of 30 (Fig. 6A). Infection of the cells with AxCAAkt-AA also inhibited insulin-induced activation of endogenous Akt, with ∼80% inhibition apparent at an MOI of 200 (Fig. 6C). DNA laddering was not evident with 3T3-L1 adipocytes infected with AxCAAkt-AA at an MOI of 200 (data not shown). Infection of 3T3-L1 adipocytes with AxCAAkt-AA did not affect either the abundance of GLUT4 protein or morphological characteristics of the adipocytes (data not shown), suggesting that Akt-AA did not cause a change in phenotype of the adipocytes during the time course of the experiments. Thus, Akt-AA exerts a dominant negative effect in both CHO cells and 3T3-L1 adipocytes.
FIG. 6.
Effects of Δp85 (A) and Akt-AA (B and C) on insulin-induced activation of endogenous Akt in 3T3-L1 adipocytes. 3T3-L1 adipocytes were infected with AxCAΔp85 (A) or AxCAAkt-AA (B and C) at the indicated MOI (PFU/cell), incubated for 10 min in the absence or presence of 100 nM insulin, and lysed. Cell lysates infected with AxCAAkt-AA were subjected either to immunoblot analysis with polyclonal antibodies to Akt (B, top) or to three sequential rounds of immunoprecipitation with antibodies to HA in order to deplete Akt-AA, after which the final supernatant was subjected to immunoblot analysis with polyclonal antibodies to Akt (B, bottom). Cell lysates infected with AxCAΔp85 or the Akt-AA-depleted final supernatant from panel B were subjected to immunoprecipitation with polyclonal antibodies to Akt; then these immunoprecipitates were assayed for Akt kinase activity. Data are representative of three independent experiments, with bars representing means of duplicate determinations.
Effects of Akt-AA on insulin-stimulated protein synthesis in CHO cells and 3T3-L1 adipocytes.
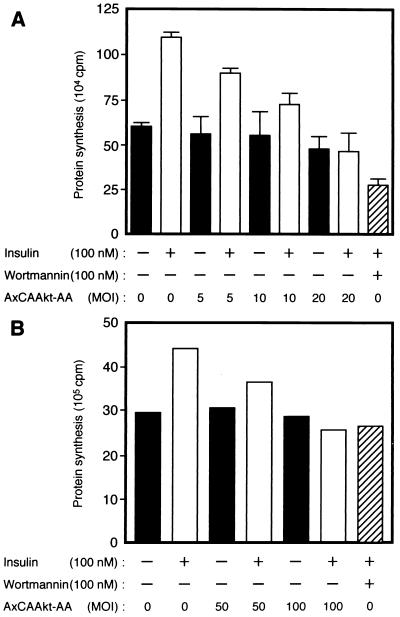
We next investigated the effect of Akt-AA on insulin-stimulated bulk protein synthesis, which has previously been shown to be regulated by PI 3-kinase (10, 34). Insulin stimulated an ∼1.8-fold increase in protein synthesis in CHO cells within 1 h (Fig. 7A). Cells that had been exposed to wortmannin before treatment with insulin showed a level of protein synthesis that was less than the basal value. Infection of cells with AxCAAkt-AA inhibited insulin-stimulated protein synthesis in an MOI-dependent manner, without affecting the basal level; insulin-stimulated protein synthesis was completely abolished at an MOI of 20 PFU/cell.
FIG. 7.
Effects of Akt-AA on insulin-stimulated protein synthesis in CHO cells (A) and 3T3-L1 adipocytes (B). CHO cells or 3T3-L1 adipocytes were infected with AxCAAkt-AA at the indicated MOI (PFU/cell). The cells were incubated in the absence or presence of 100 nM wortmannin for 30 min, after which insulin-stimulated protein synthesis was assayed as described in Materials and Methods. Data are means ± standard errors from three experiments (A) or means of two experiments (B).
Because wortmannin inhibits insulin-stimulated amino acid transport (54), we examined the effect of Akt-AA on this action of insulin. Insulin induced an ∼1.2-fold increase in amino acid transport in CHO cells (Table 1), and treatment of cells with wortmannin before incubation with insulin reduced the extent of amino acid transport to below the basal value. Infection of the cells with AxCAAkt-AA at an MOI of 20 PFU/cell had no effect on insulin-stimulated amino acid transport, indicating that the inhibition of insulin-stimulated protein synthesis by Akt-AA was not attributable to an effect on amino acid transport.
TABLE 1.
Effect of Akt-AA on insulin-stimulated amino acid uptake in CHO cellsa
| Condition | MeAIB uptake (cpm) at AxCAAkt-AA MOI of:
|
|
|---|---|---|
| 0 | 20 | |
| Basal | 1,154 ± 109 | 1,120 ± 60 |
| Insulin (100 nM) | 1,387 ± 20 | 1,401 ± 41 |
| Wortmannin (100 nM) + insulin (100 nM) | 867 ± 19 | |
CHO cells cultured in six-well plates were infected or not with AxCAAkt-AA at an MOI of 20 PFU/cell and incubated in the absence or presence of 100 nM wortmannin for 20 min. Insulin-stimulated MeAIB transport into the cells was then assayed as described in Materials and Methods. Data are means ± standard errors from three experiments.
In 3T3-L1 adipocytes, insulin induced an ∼1.5-fold increase in protein synthesis within 1 h (Fig. 7B). Pretreatment of the cells with wortmannin abolished insulin stimulation of protein synthesis in these cells. As with CHO cells, infection of 3T3-L1 adipocytes with AxCAAkt-AA resulted in an MOI-dependent inhibition of insulin-stimulated protein synthesis. When CHO cells or 3T3-L1 adipocytes were infected with a control virus containing the lacZ gene at an MOI of 20 or 100, respectively, insulin-stimulated protein synthesis in these cells was not affected (data not shown). Furthermore, infection of AxCAΔSOS at an MOI sufficient for almost complete inhibition of insulin-induced activation of MAP kinase activity in the adipocytes had no effect on insulin-induced protein synthesis (data not shown).
Effects of Akt-AA on insulin-stimulated p70 S6 kinase activity.
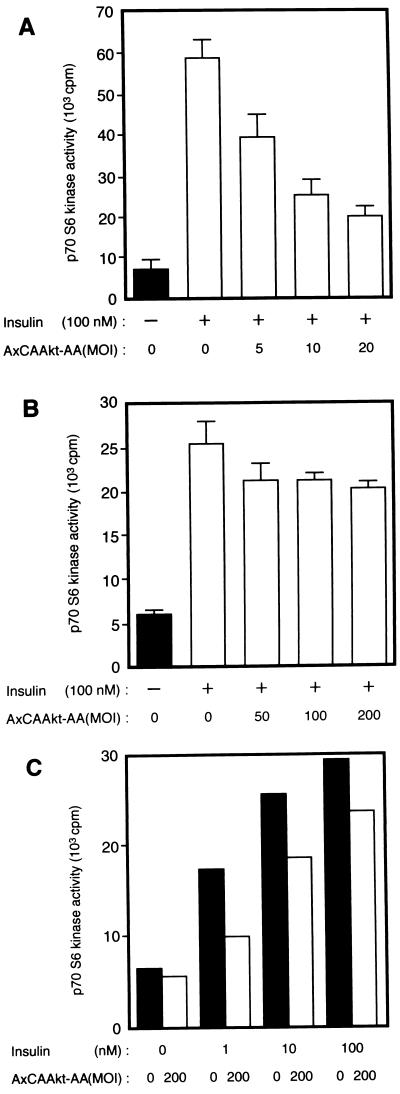
We next investigated the effects of Akt-AA on insulin-induced activation of p70 S6 kinase, the activity of which has previously been shown to be increased as a result of overexpression of a membrane-targeted mutant Akt or a Gag-Akt fusion protein (4, 26). Insulin induced an approximately sixfold increase in p70 S6 kinase activity in CHO cells (Fig. 8A). Infection of the cells with AxCAAkt-AA inhibited insulin-stimulated activation of p70 S6 kinase in an MOI-dependent manner, with ∼75% inhibition apparent at an MOI of 20 PFU/cell.
FIG. 8.
Effects of Akt-AA on insulin-induced activation of p70 S6 kinase in CHO cells (A) and 3T3-L1 adipocytes (B and C). Cells were infected with AxCAAkt-AA at the indicated MOI (PFU/cell), incubated in the absence or presence of the indicated concentrations of insulin for 10 min, lysed, and subjected to immunoprecipitation with antibodies to p70 S6 kinase. The resulting immunoprecipitates were assayed for p70 S6 kinase activity as described in Materials and Methods. Data are means ± standard errors from three experiments (A and B) or means of two experiments (C).
We also examined the effect of Akt-AA on insulin-stimulated p70 S6 kinase activity in 3T3-L1 adipocytes. Infection of the adipocytes with AxCAAkt-AA at an MOI of 200 PFU/cell, a dose that inhibited insulin-induced activation of endogenous Akt by ∼80% (Fig. 6C), reduced the p70 S6 kinase activity promoted by 100 nM insulin by ∼30% (Fig. 8B). More than 70% inhibition was apparent in the cells stimulated with 1 nM insulin, and ∼40% inhibition was observed in those stimulated with 10 nM insulin (Fig. 8C).
Effects of Akt-AA on insulin-stimulated glucose uptake and GLUT4 translocation.
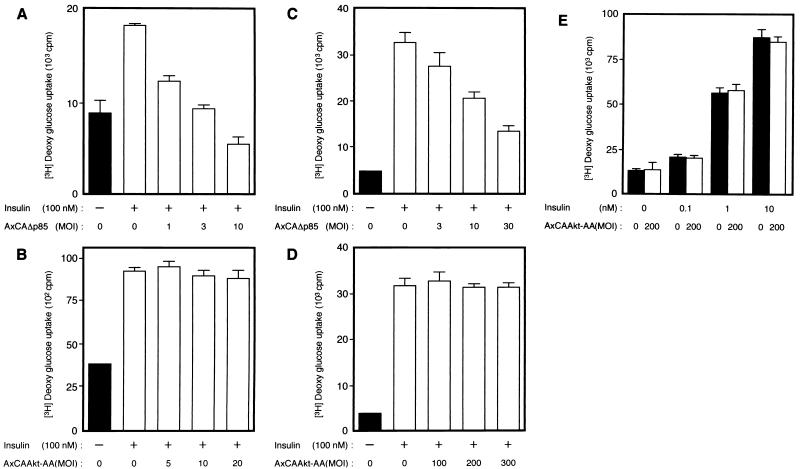
Finally, we examined the effects of Akt-AA on insulin-stimulated glucose uptake. Insulin induced an approximately twofold increase in glucose uptake in CHO cells, an effect that was inhibited by infection of the cells with AxCAΔp85 (Fig. 9A), consistent with our previous data (16). However, infection of CHO cells with AxCAAkt-AA had no effect on insulin-stimulated glucose uptake (Fig. 9B), even at a virus concentration (MOI of 20 PFU/cell) that inhibited insulin-induced activation of endogenous Akt by ∼95% (Fig. 5B). Wortmannin abolished insulin stimulation of glucose uptake in cells that had been infected with AxCAAkt-AA (data not shown).
FIG. 9.
Effects of Δp85 or Akt-AA on insulin-stimulated glucose uptake in CHO cells (A and B) and 3T3-L1 adipocytes (C to E). Cells were infected with AxCAΔp85 (A and C) or AxCAAkt-AA (B, D, and E) at the indicated MOI (PFU/cell), and insulin-stimulated glucose uptake was assayed as described in Materials and Methods. Data are means ± standard errors from three experiments.
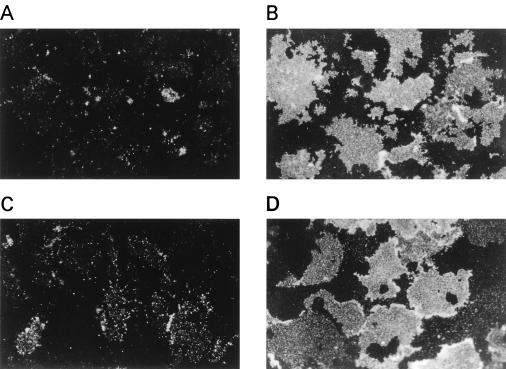
Constitutively active Akt mutant proteins have been shown to stimulate glucose uptake and translocation of GLUT4 in adipocytes (27, 53). We therefore examined the effects of Akt-AA on glucose uptake and GLUT4 translocation in 3T3-L1 adipocytes. Insulin induced an approximately eightfold increase in glucose uptake in these cells. Consistent with our previous results (48), AxCAΔp85 inhibited insulin-stimulated glucose uptake in an MOI-dependent manner (Fig. 9C). In contrast, AxCAAkt-AA had no effect on glucose uptake (Fig. 9D) even at an MOI of 200. Furthermore, a dose-response curve of insulin-stimulated glucose uptake in the cells infected with AxCAAkt-AA at an MOI of 200 was similar to that of noninfected cells (Fig. 9E). We examined the effect of Akt-AA on insulin-induced translocation of GLUT4 by the plasma membrane lawn assay. Whereas plasma membrane lawns prepared from quiescent adipocytes showed little GLUT4 immunoreactivity, insulin induced a marked increase in the amount of the glucose transporter in the plasma membrane (Fig. 10A and B). Infection of the cells with AxCAΔp85 inhibited the insulin-induced increase in GLUT4 immunoreactivity in the membrane (Fig. 10C), whereas AxCAAkt-AA had no effect on this action of insulin (Fig. 10D).
FIG. 10.
Effects of Δp85 and Akt-AA on insulin-induced translocation of GLUT4 to the plasma membrane of 3T3-L1 adipocytes. Cells were not infected (A and B) or infected with AxCAΔp85 (C) or AxCAAkt-AA (D) at an MOI of 30 or 200 PFU/cell, respectively. The cells were incubated in the absence (A) or presence (B to D) of 100 nM insulin for 10 min, after which plasma membrane fragments were prepared and subjected to immunofluorescence microscopy with antibodies to GLUT4 and tetramethyl rhodamine isothiocyanate-labeled secondary antibodies. Data are representative of three independent experiments. Magnification, ×400.
DISCUSSION
We have investigated the roles of Akt in cells by specifically inhibiting the activity of the endogenous enzyme. Such inhibition was achieved by expression of a mutant Akt (Akt-AA) in which the sites of ligand-induced phosphorylation are mutated to alanine and that acts in a dominant negative manner. Akt-AA was introduced into both CHO cells and 3T3-L1 adipocytes with the use of an adenovirus expression system, which has previously been used to express a variety of genes with high efficiency (36). The maximal abundance of Akt protein in CHO cells or 3T3-L1 adipocytes infected with AxCAAkt-AA was 30 to 50 times that of endogenous Akt.
We tested several mutants of Akt for dominant negative effects, including a protein in which Lys179 in the kinase domain was replaced by asparate (Akt-K179D). Although Akt-K179D did not exhibit kinase activity, it was less effective than Akt-AA in inhibiting the activity of endogenous Akt when overexpressed by the adenovirus system. Activity-deficient mutants of protein kinases in which phosphorylation sites targeted by extracellular stimuli have been replaced by neutral amino acids have previously been shown to act in a dominant negative manner. For example, mutants of MAP kinase in which either Thr192 or Tyr194 is replaced by alanine (40) act in a dominant negative manner. Furthermore, a MEK protein in which extracellular stimulus-dependent phosphorylation sites were changed to alanine also behaved as a dominant negative mutant (8). Such a mutant MEK showed increased binding to Raf, a kinase upstream of MEK, compared with wild-type MEK (60). These data, together with our observation that Akt-K179D is phosphorylated in vivo in response to insulin (unpublished data) and by a putative upstream kinase in vitro (51), suggest that Akt-AA may interact with its upstream kinase with higher affinity than does either wild-type Akt or Akt-K179D and that this higher-affinity interaction underlies its dominant negative effects.
Overexpression of a membrane-targeted mutant Akt or a Gag-Akt fusion protein, the kinase activity of both of which is greater than that of Akt-WT, was previously shown to increase glucose uptake or translocation of GLUT4 in quiescent adipocytes (27, 53). However, we have now shown that inhibition of endogenous Akt activity by Akt-AA had no effect on glucose transport. The simplest explanation for these observations would be that Akt is not necessary for insulin-stimulated glucose uptake, although activated Akt is sufficient to increase glucose uptake under certain conditions. A constitutively active mutant of Ras also increases glucose uptake and translocation of GLUT4 (32, 45), whereas Ras activation and its downstream signaling are not required for insulin stimulation of glucose uptake (12, 17, 45, 48).
It is possible that the inhibition of endogenous Akt activity by overexpression of Akt-AA is not sufficient to affect insulin-stimulated glucose uptake. However, the same extent of inhibition of insulin-stimulated Akt activity achieved by a dominant negative mutant of PI 3-kinase (Δp85) was sufficient to inhibit insulin-induced glucose transport. The polyclonal antibodies used to examine endogenous Akt activity are capable of precipitating all three known isoforms of Akt (Akt1 [RAC-PKα], Akt2 [RAC-PKβ], and RAC-PKγ). Thus, our data indicate that the activities of all three Akt isoforms are inhibited by Akt-AA. However, we cannot exclude the possibility that an unidentified isoform of Akt that is resistant to Akt-AA is present in the cells studied and responsible for insulin-stimulated glucose uptake.
We have shown that Akt-AA inhibited insulin-stimulated p70 S6 kinase activity, suggesting that Akt is required for activation of p70 S6 kinase. It is not clear why insulin-induced activation of p70 S6 kinase in CHO cells was inhibited by only ∼75% whereas insulin activation of endogenous Akt was almost completely abolished in these cells. However, previous evidence has suggested that growth factor stimulation of p70 S6 kinase is mediated by redundant signaling pathways (43, 58). Thus, interruption of only one pathway (the Akt pathway) may be insufficient for complete inhibition of p70 S6 kinase activation. The relatively small inhibitory effect of Akt-AA on insulin activation of p70 S6 kinase in 3T3-L1 adipocytes may reflect a minor contribution of Akt to this action of insulin in these cells.
Wortmannin inhibits insulin-stimulated bulk protein synthesis (10, 34). Furthermore, we have recently shown that a dominant negative mutant of PI 3-kinase inhibited insulin-stimulated protein synthesis in CHO cells (52a). These data suggest that PI 3-kinase is important for insulin-stimulated bulk protein synthesis. However, it has not been known at which of the multiple steps of protein synthesis (21) PI 3-kinase exerts its effect. Tsakiridis et al. (54) showed that wortmannin inhibits insulin-stimulated amino acid transport in L6 myoblast cells. We have now shown that Akt-AA inhibited protein synthesis but not amino acid transport in CHO cells. These data demonstrate that the Akt pathway affects protein synthesis at a step distinct from amino acid transport. However, we cannot exclude the possibility that upstream kinases of Akt have physiological substrates other than Akt and that the dominant negative effects of Akt-AA are, at least in part, due to the inhibition of phosphorylation of these substrates but not of Akt.
p70 S6 kinase is thought to contributes to the regulation of protein synthesis by phosphorylating ribosomal protein S6 (43). Phosphorylation of S6 in intact cells correlates with increased protein synthesis (21). However, the inhibitory effect of Akt-AA on bulk protein synthesis is probably not due to the inhibition of p70 S6 kinase because rapamycin, which prevents insulin activation of p70 S6 kinase (41, 43), has only a small effect on insulin-stimulated protein synthesis (10, 34). We recently showed that insulin-induced activation of guanine nucleotide exchange activity toward translation initiation factor eIF-2 in total lysates of CHO-IR cells was completely inhibited by overexpression of a dominant negative PI 3-kinase (57). The eIF-2B factor is thought to mediate this guanine nucleotide exchange activity and subsequently to regulate recruitment of the initiator Met-tRNA to the 40S ribosomal subunit (46), an essential step for initiation of translation. Glycogen synthase kinase-3β (GSK-3β), a putative downstream effector of Akt (9), has been suggested to participate in the regulation of eIF-2B (56). Although the effects of Akt-AA on regulation of GSK-3β and eIF-2B remain to be elucidated, Akt-AA may affect bulk protein synthesis by inhibiting the GSK-3β–eIF-2B pathway.
In summary, we have identified a dominant negative mutant of Akt and, with the use of an adenovirus encoding this protein, shown that Akt mediates some, but not all, signaling pathways downstream of PI 3-kinase. Because PI 3-kinase contributes not only to the metabolic actions of insulin but to a variety of biological effects, it will be important to determine which other signaling pathways downstream of PI 3-kinase are mediated through Akt. An atypical PKC isozyme, PKCζ, is a putative downstream effector of PI 3-kinase (38) and has recently been shown to be required for insulin-stimulated bulk protein synthesis (35). It is not clear which steps of protein synthesis are regulated by PKCζ, and so it remains to be determined whether Akt and PKCζ participate in the same signaling pathway or whether each controls protein synthesis by regulating different steps.
ACKNOWLEDGMENTS
We thank I. Saito for pAxCAwt, AxCALacZ, and technical advice on preparation of adenovirus vectors.
This work was supported by grants (to M.K.) from the Ministry of Education, Science, Sports, and Culture of Japan and from the Uehara Memorial Foundation.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. A retroviral oncogene, AKT, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:244–247. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 4.Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter L C, Cantley L C. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 6.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffer P J, Woodgett J R. Molecular cloning and characterization of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase-C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 8.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 9.Cross D A E, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 10.Dardevet D, Sornet C, Vary T, Grizard J. Phosphatidylinositol 3-kinase and p70 S6 kinase participate in the regulation of protein turnover in skeletal muscle by insulin and insulin-like growth factor I. Endocrinology. 1996;137:4087–4094. doi: 10.1210/endo.137.10.8828461. [DOI] [PubMed] [Google Scholar]
- 11.Didchenko S A, Tlton B, Hemmings B A, Ballmer-Hofer K, Thelen M. Constitutive activation of protein kinase B and phosphorylation of p47phox by a membrane-targeted phosphoinositide 3-kinase. Curr Biol. 1996;6:1271–1278. doi: 10.1016/s0960-9822(02)70713-6. [DOI] [PubMed] [Google Scholar]
- 12.Dorrestijn J, Ouwens D M, Van den Berghe N, Bos J L, Maassen J A. Expression of a dominant negative Ras mutant does not affect stimulation of glucose uptake and glycogen synthesis by insulin. Diabetologia. 1996;39:558–563. doi: 10.1007/BF00403302. [DOI] [PubMed] [Google Scholar]
- 13.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 14.Franke T F, Yang S I, Chan T O, Datta K, Kazluskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the AKT protooncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 15.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct binding of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–667. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 16.Hara K, Yonezawa K, Sakaue H, Ando A, Kotani K, Kitamura T, Kitamura Y, Ueda H, Stephens L, Jackson T R, Hawkins P T, Dhand R, Clark A E, Holman G D, Waterfield M D, Kasuga M. 1-Phosphatidylinositol 3-kinase activity is required for insulin stimulated glucose transport but not for activation in CHO cells. Proc Natl Acad Sci USA. 1994;91:7415–7419. doi: 10.1073/pnas.91.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausdroff S F, Frangioni J V, Birnbaum M J. Role of p21ras in insulin-stimulated glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1994;269:21391–21394. [PubMed] [Google Scholar]
- 18.Holman G H, Kasuga M. From receptor to transporter: insulin signaling to glucose transport. Diabetologia. 1997;40:991–1003. doi: 10.1007/s001250050780. [DOI] [PubMed] [Google Scholar]
- 19.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimball S R, Vary T C, Jefferson L S. Regulation of protein synthesis by insulin. Annu Rev Physiol. 1994;56:321–348. doi: 10.1146/annurev.ph.56.030194.001541. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura Y, Kitamura T, Sakaue H, Maeda T, Ueno H, Nishio S, Ohno S, Osada S-I, Sakaue M, Ogawa W, Kasuga M. Interaction of Nck-associated protein 1 with activated GTP-binding protein Rac. Biochem J. 1997;322:873–878. doi: 10.1042/bj3220873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klippel A, Reinhard C, Kavanaugh M, Apell G, Escobedo M A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohn A D, Kovacina K S, Roth R A. Insulin stimulates the kinase-activity of RAC-PK, a pleckstrin homology domain-containing Ser/Thr kinase. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohn A D, Takeuchi F, Roth R A. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 27.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 28.Konishi H, Shinomura T, Kuroda S-I, Ono Y, Kikkawa U. Molecular cloning of rat RAC protein kinase α and β and their association with protein kinase C ζ. Biochem Biophys Res Commun. 1994;205:817–825. doi: 10.1006/bbrc.1994.2738. [DOI] [PubMed] [Google Scholar]
- 29.Konishi H, Kuroda S, Tanaka M, Matsuzaki H, Ono Y, Kameyama K, Haga T, Kikkawa U. Molecular cloning and characterization of a new member of the RAC protein kinase family: association of the pleckstrin homology domain of three types of RAC protein kinase with protein kinase C subspecies and beta gamma subunits of G proteins. Biochem Biophys Res Commun. 1995;216:526–534. doi: 10.1006/bbrc.1995.2654. [DOI] [PubMed] [Google Scholar]
- 30.Konishi H, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S, Kikkawa U. Activation of RAC-protein kinase by heat shock and hyperosmolarity stress through a pathway independent of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, Nishiyama M, Waterfield M D, Kasuga M. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozma L, Baltensperger K, Klarlund J, Porras A, Santos E, Czech M P. The ras signaling pathway mimics insulin action on glucose transporter translocation. Proc Natl Acad Sci USA. 1993;90:4460–4464. doi: 10.1073/pnas.90.10.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozma S C, Ferrari S, Bassand P, Siegmann M, Totty N, Thomas G. Cloning of the mitogen-activated S6 kinase from rat liver reveals an enzyme of the second messenger subfamily. Proc Natl Acad Sci USA. 1990;87:7365–7369. doi: 10.1073/pnas.87.19.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez R, Myers M G, White M F, Rhoads R E. Stimulation of protein synthesis, eukaryotic translation initiation factor 4E phosphorylation, and PHAS-I phosphorylation by insulin requires insulin receptor substrate 1 and phosphatidylinositol 3-kinase. Mol Cell Biol. 1996;16:2857–2864. doi: 10.1128/mcb.16.6.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendez R, Kollmorgen G, White M F, Rhoads R E. Requirement of protein kinase Cζ for stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA terminal protein complex and a cosmid bearing the full length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moule S K, Edgell N J, Welsh G I, Diggle T A, Foulstone E J, Heesom K J, Proud C G, Denton R M. Multiple signalling pathways involved in the stimulation of fatty acid and glycogen synthesis by insulin in rat epididymal fat cells. Biochem J. 1995;311:595–601. doi: 10.1042/bj3110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakanishi H, Brewer K A, Exton J H. Activation of the ζ isoenzyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 39.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 40.Pagés G, Lenormand P, L’Allemain G, Chambard J-C, Meloche S, Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price D J, Grove R, Calvo V, Avruch J, Bierer B E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- 42.Proud C G. p70 S6 kinase: an enigma with variations. Trends Biochem Sci. 1996;21:181–185. [PubMed] [Google Scholar]
- 43.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 44.Pullen N, Dennis P B, Andjelkovic M, Dafner A, Kozma S C, Hemmings B A, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 45.Quon M J, Chen H, Ing B R, Liu M-L, Zarnowski M J, Yonezawa K, Kasuga M, Cushman S W, Taylor S I. Roles of 1-phosphatidylinositol 3-kinase and Ras in regulating translocation of GLUT4 in transfected rat adipose cells. Mol Cell Biol. 1995;15:5403–5411. doi: 10.1128/mcb.15.10.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redpath N T, Proud C G. Molecular mechanisms in the control of translation by hormones and growth factors. Biochim Biophys Acta. 1994;1220:147–162. doi: 10.1016/0167-4889(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 47.Sakaue H, Hara K, Noguchi T, Matozaki T, Kotani K, Ogawa W, Yonezawa K, Waterfield M D, Kasuga M. Ras-independent and wortmannin-sensitive activation of glycogen synthase by insulin in Chinese hamster ovary cells. J Biol Chem. 1995;270:11304–11309. doi: 10.1074/jbc.270.19.11304. [DOI] [PubMed] [Google Scholar]
- 48.Sakaue H, Ogawa W, Takata M, Kuroda S, Kotani K, Matsumoto M, Sakaue M, Nishio S, Ueno H, Kasuga M. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol Endocrinol. 1997;11:1552–1562. doi: 10.1210/mend.11.10.9986. [DOI] [PubMed] [Google Scholar]
- 49.Sakaue M, Bowtell D, Kasuga M. A dominant-negative mutant of mSOS1 inhibits insulin-induced Ras activation and reveals Ras-dependent and -independent insulin signaling pathways. Mol Cell Biol. 1995;15:379–388. doi: 10.1128/mcb.15.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens L, Anderson K, Stokes D, Erdjumrnt-Bromega H, Painter G F, Holmes A B, Gaffney P R J, Reese C B, McCormick F, Tempst P, Coadwell J, Hawkins P T. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-triphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 51.Stokes D, Stephens L R, Copeland T, Gaffney P J, Reese C, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 52.Sutherland C, O’Brien R M, Granner D K. Phosphatidylinositol 3-kinase, but not p70/p85 ribosomal S6 protein kinase, is required for the regulation of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by insulin. Dissociation of signaling pathways for insulin and phorbol ester regulation of PEPCK gene expression. J Biol Chem. 1995;270:15501–15506. doi: 10.1074/jbc.270.26.15501. [DOI] [PubMed] [Google Scholar]
- 52a.Takata, M., W. Ogawa, and M. Kasuga. Unpublished data.
- 53.Tanti J F, Grillo S, Gremeaux T, Coffer P J, Van-Obberghen E, Le-Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 54.Tsakiridis T, McDowell H E, Walker T, Downes C P, Hundal H S, Vranic M, Klip A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315–4322. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- 55.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1994;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 56.Welsh G I, Proud C G. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welsh G I, Stokes C M, Wang X, Sakaue H, Ogawa W, Kasuga M, Proud C G. Activation of translation initiation factor eIF2B by insulin requires phosphatidylinositol 3-kinase. FEBS Lett. 1997;410:418–422. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]
- 58.Weng Q-P, Andrabi K, Kozlowski M T, Grove J R, Avruch J. Multiple independent inputs are required for activation of the p70 S6 kinase. Mol Cell Biol. 1995;15:2333–2340. doi: 10.1128/mcb.15.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White M F, Kahn C R. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 60.Wu X, Noh S J, Zhou G, Dixon J E, Guan K-L. Selective activation of MEK1 but not MEK2 by A-Raf from epidermal growth factor-stimulated Hela cells. J Biol Chem. 1996;271:3265–3271. doi: 10.1074/jbc.271.6.3265. [DOI] [PubMed] [Google Scholar]
- 61.Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]