Abstract
Summary
Approximately 80% of adrenal incidentalomas are benign, and development into adrenal cortical cancer is extremely rare. This is a major reason behind clinical guidelines recommending surveillance of incidentalomas for a relatively short duration of up to 5 years. Surveillance of lesions less than 1 cm is not routinely recommended. A 70-year-old lady was diagnosed with a non-hyperfunctioning 8 mm right adrenal lesion. She underwent annual biochemical and radiological assessment for 5 years before surveillance was extended to 2-yearly intervals. The lesion was stable in size, and radiological characteristics were consistent with a benign adenoma. Seven years after the initial detection of the adrenal lesion, she developed acute abdominal pain. Imaging revealed a 7 cm right adrenal lesion, which was surgically resected and histologically confirmed to be adrenal cortical cancer. She died 1 year later. Clinical guidelines have moved towards a shortened duration of surveillance of incidentalomas. Even though malignant transformation is a rare event, it is possible that this will result in a delayed diagnosis of adrenal cortical cancer, a highly aggressive malignancy with a poor prognosis. To our knowledge, this is the first published case of an adrenal lesion of less than 1 cm developing into adrenal cortical cancer.
Learning points
Adrenal incidentalomas are increasingly common.
Clinical practice guidelines exist to aid in differentiating benign and malignant lesions and assessing functional status.
Transformation of adrenal incidentalomas to adrenal cortical carcinomas is a rare but recognised event.
Patient Demographics: Adult, Female, White, Australia
Clinical Overview: Adrenal, Adrenal
Related Disciplines: Endocrine-related cancer
Publication Details: Unique/unexpected symptoms or presentations of a disease, January, 2024
Background
The diagnosis of adrenal incidentaloma (defined as > 1 cm) is increasingly common, related to the greater frequency of abdominal imaging and to radiological advances. Approximately 80% are benign adenomas, whilst less than 5% are adrenal cortical carcinomas (ACCs), rare malignant lesions that carry a poor prognosis (1, 2). Clinical guidelines assist physicians in the management of incidentalomas and aid in differentiating between benign and malignant lesions and assessing functionality. Most guidelines recommend that small (<4 cm) radiologically benign lesions do not require surveillance for longer than a maximum of 5 years (3, 4). We present a rare case of progression of an apparently benign 8 mm adrenal adenoma to ACC after almost 8years of regular surveillance.
Case presentation
A 70-year-old Hungarian lady regularly reviewed in an Obesity Service complained of flushing. Her medical history was significant for well-controlled type 2 diabetes mellitus, hypertension, osteoarthritis, paroxysmal atrial fibrillation, Gilbert’s syndrome and a resected meningioma. She lived with her husband, and they did not have any children. She was a retired laboratory technician and a lifelong non-smoker. There was a family history of hypertension, and her identical twin sister had benign breast lesions.
Investigation
Investigation of the flushing was performed. Abdominal CT revealed an 8 mm right adrenal nodule and a 1.7 cm hypodense lesion in the liver (no pre-contrast scan performed). A triple-phase CT abdomen deemed the adrenal lesion too small to further characterise, and hormonal testing confirmed a non-hypersecreting lesion. No endocrine cause for flushing was identified, and it spontaneously resolved.
Outcome and follow-up
She underwent 5 years of annual CT imaging (Table 1) and hormonal assessment (Table 2) of her adrenal tumour. The characteristics (density, enhancement pattern, absolute washout and size) remained consistent with a stable, non-functional adrenal adenoma. Surveillance with CT imaging was then extended to 2-yearly intervals. In addition, an MRI scan performed 1 year after initial identification reported that the small size of the lesion limited characterisation, although there appeared to be loss of signal on the out-of-phase T1 sequence, suggestive of a small adenoma.
Table 1.
CT characteristics of the right adrenal lesion.
| Year of surveillance | Density | Enhancement pattern | Absolute washout | Size | Reported conclusion |
|---|---|---|---|---|---|
| 0 | 8 mm | Most likely an adenoma. | |||
| 0 | Stable small right adrenal nodule, too small to characterise. | ||||
| 1 | Unchanged | Right adrenal is unchanged with only slight bulkiness. | |||
| 2 | 3 HU (non-contrast) | 10 × 6 mm, unchanged | Right adrenal nodule is unchanged, likely an adenoma. | ||
| 3 | 30 HU pre-contrast, 73 HU arterial phase, 102 HU portal venous phase and 42 HU delayed phase | 72% | Unchanged | Stable small right adrenal lesion. The stability and absolute washout are in keeping with an adenoma. | |
| 4 | Consistent with adenoma | Consistent with adenoma | Unchanged | Small right adrenal lesion, corresponding to an adenoma. Unchanged in size. | |
| 5 | Consistent with adenoma | Consistent with adenoma | Unchanged | Stable right adrenal adenoma. | |
| 7 | 32 HU pre-contrast, 60 HU in arterial/portal venous/delayed phases | 0% | 66 × 49 mm | Significant increase in size of the right adrenal mass since previous scan. |
CT, computed tomography; HU, Hounsfield units.
Table 2.
Functional studies at baseline and at diagnosis of ACC.
| 2009 | 2017 | |||||
|---|---|---|---|---|---|---|
| Ur vol (L) | Value | RR | Ur vol (L) | Value | RR | |
| 24-h urine studies | ||||||
| Cortisol (nmol/day) | 2.53 | 104 | 70–430 | 1.69 | 237 | 54–319 |
| Metanephrines (µmol/day) | 3.01 | 0.6 | < 2.1 | 1.23 | 0.4 | < 0.4 |
| Normetanephrines (µmol/day) | 3.01 | 2.5 | < 5.1 | 1.23 | 2.4 | < 2.3 |
| Plasma studies | ||||||
| Aldosterone (pmol/L) | – | 489 | 100–950 | |||
| Renin (mU/L) | – | 206 | 3.3–41 | |||
| ARR | – | 2 | < 70 | |||
Baseline aldosterone and renin were not measured as patient was on medications that interfered with testing.
ACC, adrenal cortical carcinoma; ARR, aldosterone-to-renin ratio; RR, reference range; Ur vol, urine volume.
Almost 8 years after the initial diagnosis of the incidentaloma, but only 21 months since her last CT scan, she presented to her general practitioner with new-onset epigastric/right upper quadrant abdominal pain. An abdominal ultrasound showed a 7.3 cm right adrenal lesion abutting the right lobe of the liver, and a triple-phase CT abdomen confirmed that the adrenal lesion had increased in size to 66 × 49 mm (Fig. 1). There was homogeneous enhancement but a consistent increase from 32 HU pre contrast to 60 HU in the arterial, portal venous and delayed phase sequences. There was no internal calcification or necrosis. The segment 4 liver lesions remained unchanged. A CT chest did not demonstrate any suspicious masses. Hormonal testing was unremarkable.
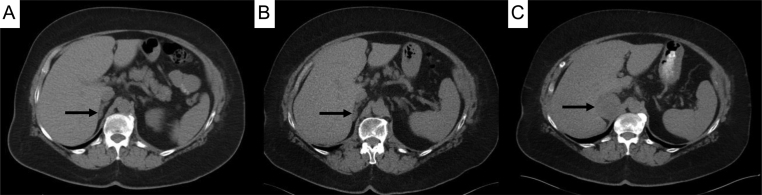
Figure 1.
Pre-contrast triple-phase abdominal CT images of right adrenal lesion (black arrows). A. Initial triple-phase CT scan showing a small right adrenal lesion. B. After 5 years of annual imaging, the lesion remained stable in size and characteristics. C. CT scan performed 21 months after the scan B showing a marked increase in size of the right adrenal lesion.
She underwent an open right adrenalectomy with removal of part of the right posterior section of the liver and diaphragm fibres. Histopathology was consistent with ACC (AJCC 8th Edition, pT4Nx). The macroscopic specimen (Fig. 2A) showed a 90 × 70 × 52 mm tumour, and microscopically there were features consistent with an invasive and aggressive tumour (Fig. 2B, C and D). The Weiss score was 5/9 (high Furhman nuclear grade, mitotic grade >5 per 10 mm2, necrosis, capsular invasion and venous/vascular invasion). The Ki-67 score was 20%. She was referred to a sub-specialist in the management of ACC for ongoing care but was intolerant of multiple medical therapies. There was rapid local recurrence and metastasis, and she passed away 1 year after the diagnosis of ACC. Prior to her death, she had recommended that her identical twin sister undergoes screening; her abdominal imaging also revealed an adrenal lesion. Further information about the results and outcome for her twin sister is not known.
Figure 2.
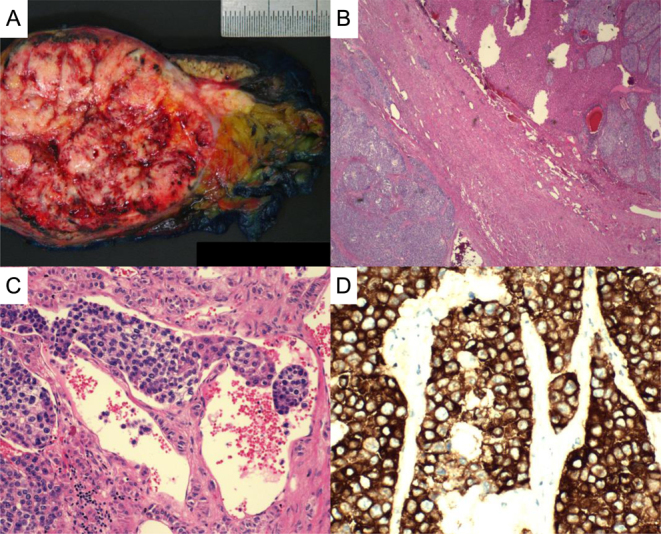
Histopathology of resected adrenal cortical carcinoma. A. Macroscopic specimen of 90 × 70 × 52 mm tumour. B. Haematoxylin and eosin staining showing adrenal tumour surrounded by a fibrous capsule with invasion into the adjacent liver. C. Presence of lympho-vascular invasion. D. Positive synaptophysin staining consistent with ACC.
Discussion
Current guidelines aim to distinguish between adrenal lesions that can be safely monitored versus those that need surgical intervention. However, there is little consensus between guidelines, which are generally based on low-level evidence and relatively short follow-up periods of up to 5 years.
The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Adrenal Incidentaloma Guidelines (3), published in 2009, recommend that patients with a non-functioning adrenal incidentaloma <4 cm in size with benign radiological features (homogeneous, regular borders, HU <10 on non-contrast CT) should have repeat imaging after 3–6 months, and then annually for 1–2 years, along with hormonal assessment annually for 5 years. The guidelines do not offer recommendations for what surveillance is required beyond 5 years for a stable lesion, in part because the transformation of a benign adenoma/hyperplasia into ACC is thought to be extremely rare (5). However, if a lesion was indeterminate on the initial CT scan, then these guidelines advocate for adrenalectomy.
In contrast, the 2016 European Society of Endocrinology (ESE) Clinical Practice Guideline for management of adrenal incidentalomas suggests against any further imaging of adrenal masses <4 cm with clearly benign radiological features and functionally inactive (6). Although this weak recommendation was based on ‘very low’ quality of evidence, an update to these guidelines published in 2023 increased the strength of the recommendation because of the publication of higher quality literature (4). In patients whose initial imaging is indeterminate and who opt against surgical resection, then repeat imaging with non-contrast CT or MRI in 6–12 months is recommended, with re-stratification of the lesion.
The importance of avoiding repetitive radiological evaluation, as proposed by the ESE guidelines (4), is supported by the high financial costs of imaging, high false-positive rates and significant radiation exposure (2). The amount of radiation required to follow an adrenal incidentaloma for 3 years confers a risk of developing a fatal cancer that is comparable to the risk of malignant transformation of the adrenal lesion (2). Indeed, it may be possible in the presented case that repeated exposure to radiation contributed to the development of the ACC, although this would be difficult to prove.
The recent surge in artificial intelligence has raised questions about how it can be best utilised in healthcare to improve patient outcomes. The use of radiomics with CT texture analysis has been proposed to discriminate benign from malignant adrenal lesions (7). Such technology, which operates via an unsupervised machine learning approach, could be useful in early detection of suspicious adrenal lesions and thus limit repeated radiation exposure. Alternatively, this may prevent unnecessary surgical resection of otherwise benign lesions.
The question remains what the natural history of a benign adrenal adenoma is, and what the optimal length of follow-up is required to detect transformation to a malignant lesion. A systematic review of over 1400 people with incidentalomas followed for a mean of 2 years identified only two cases of malignancy, neither of which were a primary adrenal cancer (2). But in 2017, the first-ever case report of an incidentaloma developing into ACC was published (8). A 71-year-old male developed ACC 14 years after the first detection of an adrenal lesion, with the initial non-contrast CT revealing a 17 mm right adrenal lesion with a density of 7.9 HU. He died within months of the diagnosis of metastatic ACC. A small number of other cases have since been reported, but unlike the case presented here, the adrenal lesions were always greater than 1 cm, and they did not have benign radiological features at the initial assessment.
It is impossible to tell in any of these cases whether the ACC transformed directly from the benign adrenal adenoma, or whether it was a de novo event. Adrenal cortical cancers are frequently aggressive and invasive, and histopathology often shows complete infiltration of the adrenal gland with no, or very little (such as in our case), normal tissue left. On extremely rare occasions, there can be the co-existence of two adjacent but histologically distinct tumours of the adrenal gland, termed adrenal collision tumours (9). These collision tumours might support the hypothesis of two independent events; however, there is also evidence supporting multistep tumourigenesis of adrenal lesions (10). The latter mechanism is responsible for the pathogenesis of colorectal carcinoma from benign polyps.
The diagnosis of an adrenal lesion in this patient’s identical twin sister raises the possibility of an underlying genetic mutation associated with the development of ACC. Unfortunately, we do not have access to any further results or outcomes for her. Approximately 5–10% of ACCs are caused by a germline mutation, and there are several familial cancer syndromes that are associated with ACC (11). Li–Fraumeni syndrome (predominantly childhood ACC), Lynch syndrome and multiple endocrine neoplasia type 1 have a prevalence of ACC in adulthood of between 1% and 7%. There are also case reports in the literature of ACC in familial adenomatous polyposis, Beckwith–Wiedemann, neurofibromatosis type 1, Carney complex and Birt–Hogg–Dube syndromes (11). There are currently no formal guidelines for genetic testing of patients with ACC and their families, but in the future, this may aid in developing a better understanding of the pathogenesis of ACC as well as targeted treatment for patients and/or prevention strategies for affected family members.
A recent large series of 512 patients with ACC reported that ACC first detected as an incidentaloma accounted for almost 40% of cases, with a frequency that increased with age (12). Patients who had an incidental finding of ACC were more likely to have a better outcome compared with patients who presented with symptomatic disease (12). Whilst these prognostic presentation features are less relevant to the case described here, the study also identified tumour factors such as Ki67%, Weiss score, ENSAT stage III and cortisol secretion as markers associated with increased risk of disease recurrence and mortality in ACC (12).
Conclusion
We present a case of the diagnosis of ACC more than 7 years after the commencement of surveillance of a benign adrenal adenoma, well beyond the time frame for surveillance recommended in all major clinical guidelines. To our knowledge, it is the first published case to describe an adrenal lesion that measured less than 1 cm at the commencement of surveillance, and therefore, was not large enough to even be defined as an incidentaloma.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the case study reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Prior to her passing, the patient verbally consented to her adrenal cancer diagnosis being presented in meetings and wider forum as a teaching point. Every effort was made to contact the next of kin of the deceased patient to obtain their consent, but was unsuccessful.
Author contribution statement
SNP wrote the first draft manuscript. NSL was the physician responsible for the patient and reviewed and edited the manuscript.
References
- 1.Barzon L Sonino N Fallo F Palu G & Boscaro M. Prevalence and natural history of adrenal incidentalomas. European Journal of Endocrinology 2003149273–285. ( 10.1530/eje.0.1490273) [DOI] [PubMed] [Google Scholar]
- 2.Cawood TJ Hunt PJ O'Shea D Cole D & Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? European Journal of Endocrinology 2009161513–527. ( 10.1530/EJE-09-0234) [DOI] [PubMed] [Google Scholar]
- 3.Zeiger MA, Thompson GB, Duh Q-Y, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J, Zeiger MA, Thompson GB, et al.American Association of Clinical Endocrinologists and American Association of endocrine surgeons medical guidelines for the management of adrenal incidentalomas. Endocrine Practice 200915(Supplement 1) 1–20. ( 10.4158/EP.15.S1.1) [DOI] [PubMed] [Google Scholar]
- 4.Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, Pelsma I, Marina L, Lorenz K, Bancos I, et al.European Society of endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the european network for the study of adrenal tumors. European Journal of Endocrinology 2023189G1–G42. ( 10.1093/ejendo/lvad066) [DOI] [PubMed] [Google Scholar]
- 5.Barzon L Scaroni C Sonino N Fallo F Paoletta A & Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. Journal of Clinical Endocrinology and Metabolism 199984520–526. ( 10.1210/jcem.84.2.5444) [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, et al.Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology 2016174G1–G34. ( 10.1530/EJE-16-0467) [DOI] [PubMed] [Google Scholar]
- 7.Torresan F, Crimì F, Ceccato F, Zavan F, Barbot M, Lacognata C, Motta R, Armellin C, Scaroni C, Quaia E, et al.Radiomics: a new tool to differentiate adrenocortical adenoma from carcinoma. BJS Open 20215. ( 10.1093/bjsopen/zraa061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmihoub I Silvera S Sibony M Dousset B Legmann P Bertagna X Bertherat J & Assié G. From benign adrenal incidentaloma to adrenocortical carcinoma: an exceptional random event. European Journal of Endocrinology 2017176K15–K19. ( 10.1530/EJE-17-0037) [DOI] [PubMed] [Google Scholar]
- 9.Katabathina VS Flaherty E Kaza R Ojili V Chintapalli KN & Prasad SR. Adrenal collision tumors and their mimics: multimodality imaging findings. Cancer Imaging 201313602–610. ( 10.1102/1470-7330.2013.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard MH Sidhu S Berger N Peix JL Marsh DJ Robinson BG Gaston V Le Bouc Y & Gicquel C. A case report in favor of a multistep adrenocortical tumorigenesis. Journal of Clinical Endocrinology and Metabolism 200388998–1001. ( 10.1210/jc.2002-021117) [DOI] [PubMed] [Google Scholar]
- 11.Petr EJ & Else T. Adrenocortical carcinoma (ACC): when and why should we consider germline testing? Presse Médicale 201847e119–e125. ( 10.1016/j.lpm.2018.07.004) [DOI] [PubMed] [Google Scholar]
- 12.Puglisi S, Calabrese A, Ferraù F, Violi MA, Laganà M, Grisanti S, Ceccato F, Scaroni C, Di Dalmazi G, Stigliano A, et al.New findings on presentation and outcome of patients with adrenocortical cancer: results from a national cohort study. Journal of Clinical Endocrinology and Metabolism 20231082517–2525. ( 10.1210/clinem/dgad199) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a