Abstract
Hypothyroidism is a relatively common finding during pregnancy. This may be due either to the presence of existing thyroid disease and/or to the increased demands that pregnancy places the thyroid gland to provide thyroid hormones for the mother and the developing fetus. There is no doubt that overt hypothyroidism is associated strongly with adverse pregnancy outcomes, including miscarriage. Meta-analyses show that thyroid hormone replacement with levothyroxine (LT4) reduces the risk of adverse pregnancy outcomes in the setting of overt hypothyroidism. Accordingly, management guidelines in this area are unanimous in recommending intervention with to control the level of thyrotropin (TSH) to below 2.5 μIU/mL. The evidence for an adverse impact of subclinical hypothyroidism (SCH) on pregnancy outcomes is less clear, although meta-analyses suggest that SCH reduces the chance of a successful pregnancy outcome. Guidelines also support intervention for some patients with SCH, particularly where TSH is high (>10 μIU/mL), or where TSH is above its trimester-specific reference range in a woman with thyroid autoimmunity (giving LT4 to euthyroid women with thyroid autoimmunity is not supported). Real-world evidence suggests that hypothyroidism in pregnancy is often overlooked or that LT4 is not given appropriately to gain tight control of TSH. More research is needed to identify the barriers to optimal thyroid care with LT4 at this crucial time.
Keywords: levothyroxine, hypothyroidism, pregnancy, miscarriage
Diagnosis of hypothyroidism during pregnancy
Evolution of normal thyroid function during pregnancy
Circulating levels of human chorionic gonadotrophin (hCG) increase from about 11 days post conception, reach a peak around the end of the first trimester and decrease thereafter (1). This hormone supports the progress of the new pregnancy in a number of ways, including ensuring an adequate supply of thyroid hormones (TH) for the developing fetus, which does not generate an adequate supply of its own until well into the second trimester (1). In addition, type 3 deiodinase in the placenta degrades a proportion of the maternal supply of triiodothyronine (T3, the principal TH), which also increases the need for additional production of TH at this time. The increase in TH occurs due to a mild activation of the TSH receptor by hCG (which bears structural similarities to TSH), resulting in increased levels of T3 and T4, and a consequent modest fall in TSH (2, 3, 4). Increasing levels of oestrogen also increase the level of thyroid binding protein, and induce a shift the circulating version of this protein towards a form with a longer half-life, which contributes to increased levels of total T3 and T4 (3, 5).
Diagnostic criteria for hypothyroidism during pregnancy
The changes in circulating TH (especially) during the first half of pregnancy suggest that the reference ranges used to diagnose thyroid disease routinely in non-pregnant subjects (6) are likely to be inappropriate during pregnancy. In fact, the criteria for diagnosing hypothyroidism during pregnancy have evolved over time. The US guideline from 2011, based on a relatively limited sample of about 5500 pregnant women, recommended upper limits for the TSH reference range of 2.5 μIU/mL for the first trimester, and 3.0 μIU/mL for each of the second and third trimesters (7). Considerably more data on TSH ranges in pregnant women without thyroid disease had appeared by the time the US guideline was updated in 2017 (8). This later guidance noted that subjects’ ethnicity, location and adiposity, in particular, affected the upper limits of ‘normal’ thyroid function, as expressed by the reference range. As a result, the US guidelines advocate the use where possible of locally derived trimester-specific reference ranges that are of direct relevance to the population being tested. Where this is not available, an upper limit of about 4 μIU/mL for the first trimester can be used, as this reflects the average reduction of about 0.5 μIU/mL in TSH that occurs early in pregnancy (see above). The upper limit can then move closer to the value used for non-pregnant subjects as the pregnancy progresses.
A very recent (2023) draft ‘Green Top Guideline’ from the UK Royal College of Gynaecology (RCOG) proposes an upper limit of 4 μIU/mL for the TSH reference range throughout pregnancy (9), which is similar to the latest thinking from the USA. This guidance also notes that this threshold value has been used widely in epidemiology studies that have associated hypothyroidism during pregnancy with adverse maternal and neonatal outcomes (see below). A 2021 guideline for the management of thyroid disorders in women undergoing assisted reproduction also proposes 4.0 μIU/mL as an upper limit to the TSH reference range, unless a locally derived reference range has a higher value (10). As in other settings, the diagnosis of subclinical hypothyroidism (SCH) is made when TSH is above the upper limit of its pregnancy-specific reference range, when free T4 is within its reference range.
Epidemiology of hypothyroidism during pregnancy
The prevalence of overt hypothyroidism during pregnancy has been described as about 1–3%, with a higher prevalence of SCH of 3–15% (11, 12, 13), although the clinical studies reported below (using ATA trimester-specific diagnostic criteria/reference ranges for TSH (see above), unless specified differently) demonstrate a very high level of variation in individual estimates from different regions. Routine screening of 2509 pregnant women for thyroid disorders in Spain revealed overt hypothyroidism in 1.9% and SCH in 3.6% with no effect of maternal age (14). A retrospective analysis in Turkey revealed a low prevalence of overt hypothyroidism (0.4%) and a moderate prevalence of SCH (8.9%) in the third trimester of 573 pregnancies in women without any history of thyroid problems (15). About 1 in 6 (17.1%) of pregnant women in Lebanon had hypothyroidism (overt hypothyroidism and SCH were not reported separately) (16).
A study in women of European/Caucasian or African heritage highlighted the importance of choosing the most appropriate TSH cut-off for diagnosing SCH. The prevalence of SCH was comparable when a cut-off value of 2.5 μIU/mL was used to diagnose SCH, but the use of 4 μIU/mL as a cut-off value (cf. the ATA and RCOG guidance, as per above) resulted in a prevalence of SCH of 5.4% for women of Caucasian heritage and 2.1% for women of North African heritage (P = 0.008). The use of a cut-off value derived at the authors’ institution (3.71 μIU/mL) resulted in similar findings (SCH prevalence of 7.1% vs 3.3%, P = 0.013). The use of cut-off values specific for ethnicity reduced the prevalence of SCH by more than 70%, relative to the use of 2.5 μIU/mL as the cut-off value, with no change relative to the institutional cut-off value (17). Thus, rigid adherence to low cut-off values for TSH in diagnosing SCH may be inappropriate, with risk of missing cases, under appreciation of other determinants of thyroid function, and subsequent overtreatment (18).
Hypothyroidism and pregnancy outcomes
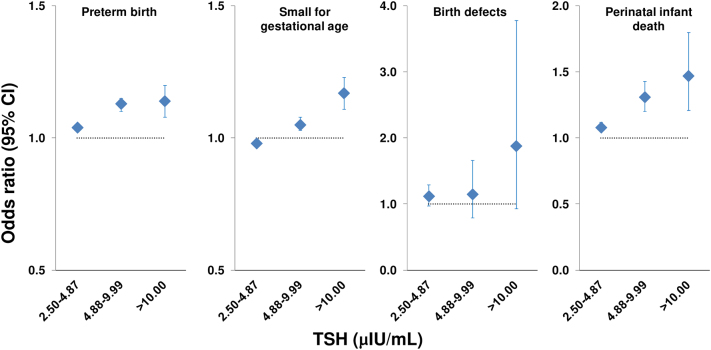
Overt hypothyroidism has been associated with adverse pregnancy outcomes in multiple studies (reviewed elsewhere (19, 20, 21, 22)). Maternal overt hypothyroidism was associated with increased risk in offspring of attention deficit hyperactivity disorder (OR 1.14 (95% CI 1.03 to 1.26)), autism spectrum disorder (OR 1.41 (95% CI 1.05 to 1.90)) and epilepsy (OR 1.21 (95% CI 1.06 to 1.39)) in a meta-analysis of 29 studies (23). Figure 1 summarises data on the relationship between maternal TSH and pregnancy outcomes from a survey of 5,840,894 women who received a prenatal check-up in China. The risk of preterm birth, small for gestational age and perinatal infant death increased in line with increasing category of TSH, and there was a non-significant trend (P = 0.08) to increased risk of birth defects at the highest TSH category (>10 μIU/mL) (24). A similar analysis from the same overall cohort, based on study of 4,739,421 pregnancies, found that elevated TSH was associated with an increased risk of miscarriage (ORs 1.33 (95% CI 1.28 to 1.38) for TSH 4.88–9.99 μIU/mL and 1.25 (95% CI, 1.14 to 1.36) for TSH ≥10.00 μIU/mL) (25). While these studies added compelling evidence to our understanding of the danger of hypothyroidism per se during pregnancy, it should be noted that they did not differentiate between the effects of overt hypothyroidism and SCH.
Figure 1.
Relationships between levels of thyrotropin (TSH) consistent with hypothyroidism and neonatal pregnancy outcomes from a study of 5,840,894 women who underwent prenatal care in China. The reference group (odds ratio = 1, shown by the dotted line in each panel), contained subjects with TSH 0.37–2.49 μIU/mL. Odds ratios were adjusted for pre-pregnancy maternal age, maternal education, location, pre-pregnancy body mass index, alcohol, passive smoking, history of thyroid disease, hypertension, diabetes, history of adverse pregnancy outcomes. Drawn from data presented in reference 24.
The influence of SCH on pregnancy outcomes has also been studied. A meta-analysis that included 18 cohort studies at low risk of bias found that the presence of SCH (defined as elevated TSH with normal serum total or free T4 (where available), or as TSH 2.5–5 μIU/mL) during pregnancy was associated with approximately 1.5- to 2.5-fold increases in the risk of pregnancy loss, placental abruption, neonatal death, and premature rupture of membranes, compared with euthyroid pregnant women (26). Another meta-analysis of 22 studies found that pregnant women with SCH were about 50% more likely to develop hypertensive disorder of pregnancy when an upper diagnostic cut-off of 4.5 μIU/mL was used (OR 1.54 (95% CI 1.21 to 1.96)) than euthyroid women; the effect disappeared if the cut-off was reduced to 3 μIU/mL (27). Pooled data from 19 cohorts (1234 women with SCH among 47,045 pregnancies) reported a significant association between SCH and preterm birth (OR 1.29 (95% CI 1.01 to 1.64)) (28). Another meta-analysis found a significant association of SCH, with pre-eclampsia (3.6% vs 2.1%; OR, 1.53 (95% CI, 1.09 to 2.15)) (29). A large retrospective analysis of 14,744 pregnancies using national databases in Denmark reported that the risk of spontaneous abortion or stillbirth increased sharply in women with TSH >10 μIU/mL (30); however, it should be noted that FT4 levels were not measured in this study, so it was not possible to distinguish the effects of SCH and overt hypothyroidism on pregnancy outcomes.
Increased thyroid antibody levels (TAB), usually reflected by positive anti-thyroid peroxidase antibodies (TPOAb+), are a common finding during pregnancy and have been detected in as many as one woman in six at this time (31). A retrospective study found TAB in a 75% of population of pregnant women with hypothyroidism diagnosed before or during pregnancy (32). Even if the mother is clinically euthyroid, thyroiditis caused by these antibodies may limit the mother’s ability to produce the additional TH needed to support a pregnancy, as described above (33, 34). The presence vs absence of TAB has been associated with an increased risk of subfertility, miscarriage or other pregnancy complications, or of later maternal thyroid disease, irrespective of whether or the mothers were biochemically euthyroid (35, 36, 37, 38, 39). A meta-analysis found that 28/31 relevant studies found a significant association between TAB and increased risk of miscarriage, with OR of 3.90 (95% CI 2.48 to 6.12; P < 0.001) and for preterm birth of 2.07 (95% CI 1.17 to 3.68; P = 0.01) (39). The US guidelines recommend measuring TSH in women with TAB at the time of confirmation of pregnancy and monthly during mid-pregnancy (8).
Effects of levothyroxine during pregnancy
A large database study of 1013 pregnancies in the UK, where LT4 was started at least 6 months before conception, reported that women with TSH >2.5 μIU/mL in the first trimester were at a higher risk of miscarriage, compared with women within the first-trimester guideline goal of 0.2–2.5 µIU/mL (40). The magnitude of the risk increased with increasing strata of TSH (Fig. 2). Thus, the quality of control of thyroid function during a hypothyroid pregnancy is important for optimising pregnancy outcomes, rather than prescription of LT4 per se.
Figure 2.
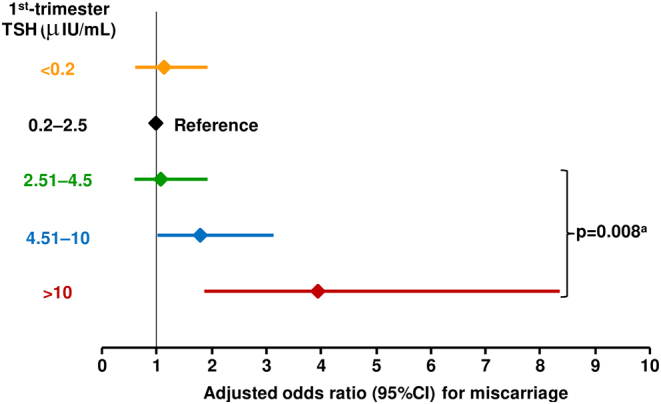
Risk of miscarriage associated with the level of TSH in pregnant women who received levothyroxine for at least 6 months before conception. aThe P-value compares data from subjects with TSH >2.5 μIU/mL with the reference range of 0.2–2.5 μIU/mL. Data were adjusted for age, year of pregnancy, diabetes during or before pregnancy, and social class. Drawn from data presented in reference 40.
A systematic review and meta-analysis of seven randomised trials and 6 observational studies (n = 7342 pregnancies) found that treatment of SCH (TSH 2.5–10.0 μIU/mL) during pregnancy with LT4 reduced the risks of miscarriage (RR 0.79 (95% CI: 0.67 to 0.93)) and neonatal death (RR 0.35 (95% CI: 0.17 to 0.72)) (41). LT4 treatment did not influence the risk of adverse perinatal outcomes (placental abruption, postpartum haemorrhage, premature rupture of membranes, preterm delivery, or preterm labour) or neonatal outcomes (low Apgar score or anthropometric indices) in this analysis. Another meta-analysis (six studies, n = 7955 pregnancies), including only women with TSH levels >4.0 μIU/mL also found a reduced risk of miscarriage, preterm birth and gestational hypertension in women with vs without LT4 treatment (42). The findings persisted after correction for TPOAb.
Finally, LT4 treatment did not appear to influence pregnancy outcomes in euthyroid women with TAB, according to systematic reviews and meta-analyses (43, 44). Also, two recent randomised trials in euthyroid TPOAb+ women with recurrent pregnancy loss found no difference in the live birth rate between LT4 and placebo groups (45, 46). With regard to observational data, a large (n = 984 pregnancies), prospective study found a high rate of miscarriage in euthyroid TPOAb+ women who did not receive LT4, compared with women who did receive LT4 (relative risk (RR) 1.72 (95% CI 1.13 to 2.25)) (33). The risk of miscarriage was also higher for the non-LT4 TPOAb+ women compared with a control group without TPOAb (RR 4.95 (95% CI 2.59 to 9.48)). Similar data were presented for the risk of premature birth. Although these women were described as ‘euthyroid’, mean TSH increased in the non-LT4 treated TPOAb+ group from 1.7 μIU/mL at 10 weeks’ gestation to 3.5 μIU/mL at delivery; mean TSH in the TPOAb+ group who received LT4 remained within the range 1.6–1.9 μIU/mL throughout the pregnancy. A prospective study evaluated the efficacy of screening-led management of thyroid disease with LT4 in 537 pregnant women, 96 of whom were TPOAb+, with a policy of treating TPOAb+ women with TSH >1 μIU/mL with 50 μg/day of LT4. There were no miscarriages among 49 women treated with LT4, compared with 16% in 47 TPOAb+ untreated women (P = 0.02), and 8% in TPOAb− women (P = 0.17) (47). A systematic review found that universal screening for thyroid dysfunction during pregnancy would facilitate intervention with LT4 to reduce the frequency of adverse pregnancy outcomes and would be cost-effective (48).
Therapeutic use of LT4 during pregnancy
Management guidelines
Overt hypothyroidism
There is unanimous support for intervention with LT4 in the setting of overt hypothyroidism during pregnancy, given the clear association between this condition and adverse pregnancy outcomes, as described above (8, 9, 49). The 2017 US guideline notes that while this statement is not supported by evidence from a randomised, controlled trial, the strength of the evidence available suggests that withholding of LT4 treatment from a control group would be unethical (8).
Women with hypothyroidism should have TSH maintained above the lower limit of the reference range and below 2.5 μIU/mL (8, 9). When a woman becomes pregnant when taking LT4, she should increase her LT4 dose by 20–30% (e.g. with an additional two of their usual tablets per week (50)) and immediately seek evaluation of their thyroid status, with monthly ongoing monitoring, from their healthcare team (8, 9).
The use of other thyroid preparations, including formulations of T3 or those derived from desiccated thyroid tissue, is not supported during pregnancy (this applies also to SCH, described separately below) (8, 49).
Subclinical hypothyroidism
The European (ETA) guideline from 2014 recommends treatment with LT4 of SCH that develops before or during a pregnancy (49). The 2017 US guideline recommends intervention with LT4 during pregnancy for TPOAb+ women with SCH and TSH above their trimester-specific reference range, or for TPOAb-negative women with SCH and TSH > 10 μIU/mL (8). According to this guidance, LT4 may be considered during pregnancy for TPOAb+ women with TSH >2.5 μIU/mL but below the upper limit of their reference range, or for TPOAb-negative women with TSH between the upper limit of the reference range and 10 μIU/mL (8). The guidance from the RCOG is consistent, and advocates use of LT4 for ‘severe’ SCH (TSH >10 μIU/mL), with a target for TSH of <2.5 μIU/mL (9). Women with SCH and TSH between the upper limit of the reference range, but <10 μIU/mL, may be considered for LT4 therapy, according to the RCOG; if LT4 is not given, regular monitoring should take place in case severe SCH or overt hypothyroidism develops during the pregnancy (9).
There is no general recommendation for LT4 treatment of infertile women with SCH (TSH >4.0 μIU/mL) who are seeking to become pregnant via natural conception, although the use of a low dose of LT4 (25–50 μg) is generally safe and may be useful to prevent progression to overt hypothyroidism during the pregnancy (8). Women with SCH undergoing ART should receive LT4 to control TSH to <2.5 μIU/mL (8, 10).
Euthyroid subjects
The 2017 US guideline concluded that there was insufficient evidence to propose routine treatment of euthyroid women with TAB, although conceded that this may be considered for women with a history of pregnancy loss given the proven safety of LT4 treatment in this setting. The recent guidance from the RCOG included consideration of one of the recent randomised trials in euthyroid, TPOAb+ women (45) and recommended that LT4 should not be given in this setting (9). This position will become the dominant one, taking into account the recent negative study in women with recurrent miscarriage and TPOAb+ (46).
Real world practice
An analysis from a primary care database in the UK showed that TSH was higher than the first-trimester guideline goal of 2.5 μIU/mL for 46% of 1,013 pregnancies in women who had been taking LT4 for at least 6 months before conception (40). Importantly, 22% of these women had TSH >4.5 μIU/mL, which was the threshold for increased risk of pregnancy outcome in this study (see above). Retrospective data from the USA showed that the use of LT4 in pregnancies complicated by SCH (untreated TSH 2.5–10 μIU/mL) increased from 12–19% during the period 2010–2014 (51). An observational study from Italy also suggested that while use of LT4 before pregnancy decreased between 1990 and 2019, the use of LT4 once a pregnancy was established tended to increase during this period (as did the accuracy of LT4 dosing, according to measured levels of TSH) (52). The use of LT4 during pregnancy appears to be increasing in Finland, based on data from all pregnancies that occurred between 2004 and 2016, in that country’s national Birth Register (n = 736,873) (53). The proportion of women who received thyroxine increased year by year, from 1.1% on 2004 to 6.2% in 2016, a difference of more than five-fold. About four-fifths started LT4 shortly before or after discovery of the pregnancy. Overall, 3.4% of pregnant women received LT4 during this period, which is consistent with the prevalence rates for pregnancy complicated by hypothyroidism discussed above. However, a formal diagnosis of hypothyroidism was recorded for 1.2% in 2004 and in 3.1% on 2015, implying an increasing tendency to treat with LT4 with or without a diagnosis. Additionally, women receiving LT4 during pregnancy in 2016 tended to be older and heavier than their peers in 2004.
Should we screen for thyroid dysfunction during pregnancy?
This has long been a matter for debate, with many articles appearing in peer-reviewed journals (see below) and in the lay press (54). Approaches to this issue have varied widely across the world. For example surveys in Latin America in 2014 (55) and in Europe in 2012 (56) found that almost half (42%) of healthcare professionals who manage pregnant patients with thyroid dysfunction favoured a universal screening approach. In the same year, about one-fifth (21%) of institutions in Asia reported screening all pregnant women for thyroid dysfunction (57).
Reviewing relatively recent literature on this topic (2015 onwards), those in favour of universal screening for thyroid dysfunction in pregnancy point to the relatively high prevalence of thyroid disorders, the existence of low-cost tests (TSH, T4) and treatment (LT4), the potential of screening to avoid the potential devastating consequences of undiagnosed thyroid disease at this time, its cost-effectiveness, and a better prospect of finding most or all cases, compared with a targeted screening approach focussed on individuals with risk factors for thyroid disease (58, 59, 60, 61). In one study, targeted screening focussed on women with at least one risk factor for hypothyroidism found that this approach was more effective for detecting overt hypothyroidism than SCH (except for obese Caucasian women, where both were detected) (62). Those against argue that, while the dangers of (especially overt) hypothyroidism in pregnancy are acknowledged universally, we do not have enough data on improved outcomes resulting from universal screening approaches to make evidence-based recommendations in favour of this approach (63, 64, 65). Opponents also point out that most cases of thyroid dysfunction during pregnancy represent relatively mild, borderline cases, where the benefits of intervention are less certain than for overt hypothyroidism (12, 59).
Current guidelines tend towards opposition to universal screening for thyroid disease during pregnancy. The 2017 ATA guideline considered that there was insufficient evidence to make any recommendation on whether or not to screen before or early during pregnancy (other than asking them about any history of thyroid disease and reviewing their treatment history for thyroid medications) (8). A weak recommendation against screening for low T4 in pregnancy was based on a ‘weak recommendation’ and ‘moderate-quality evidence’. Rather, target screening should be carried out where women present with one or more of a list of risk factors for thyroid disease. The 2014 ETA guideline for the management of SCH takes a similar approach, citing the lack of an evidence base to support universal screening, while acknowledging that targeted screening would miss a substantial number of cases (49). The 2023 draft guideline from the RCOG also favours targeted (risk-based) screening (9). This approach is not taken universally across Europe, however: for example, a guideline from Poland recommends routine measurement of TSH in women planning pregnancy, and measurement of thyroid function in women with risk factors for thyroid disease, or who have received interventions or drugs that affect thyroid function, before conception (66). An exception in guidelines from either side of the Atlantic relates to a recommendation to screen women who have TAB or who are planning ART (8, 9, 49). A separate 2021 guideline from the ETA also recommends measurement of TSH and TAB in women of subfertile couples who are planning ART (10).
Conclusions
Management guidelines are explicit on the need to give thyroid hormone replacement therapy with LT4 (not T3 or thyroid extracts) for pregnant women with overt hypothyroidism. There is a consensus that intervention with LT4 is likely justified in people with SCH where TSH is >10 μIU/mL, although the use of LT4 according to lower TSH cut-offs (e.g. >2.5 μIU/mL or >5 μIU/mL) requires further investigation. Real world data suggest that the use of LT4 in the setting of pregnancy is variable, however, suggesting that sub-optimal control of thyroid function is common at this time and associated with an increased risk of adverse pregnancy outcomes. More studies are needed to identify the barriers to optimal thyroid care with LT4 at this crucial time.
Declaration of interest
Merck Healthcare KGaA, Darmstadt, Germany, funded fast track review and open access publication of this article, and editorial assistance (see below). No other funding applied. BU is a full time employee of Merck Healthcare KGaA, Darmstadt, Germany. KGP received lecture fees from the IBSA Institut Biochimique SA, Berlin-Chemie AG and the Merck company between 2016 and 2022. From September 2023 on, he has been the secretary of the European Thyroid Association (ETA).
Funding
Merck Healthcare KGaA, Darmstadt, Germany, funded fast track review and open access publication of this article, and editorial assistance (see below).
Artificial intelligence
No AI-related technology was used in the preparation of this article.
Acknowledgement
Dr Mike Gwilt (GT Communications) provided editorial assistance.
References
- 1.Gridelet V Perrier d'Hauterive S Polese B Foidart JM Nisolle M & Geenen V. Human chorionic gonadotrophin: new pleiotropic functions for an "old" hormone during pregnancy. Frontiers in Immunology 202011343. ( 10.3389/fimmu.2020.00343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medici M Korevaar TI Visser WE Visser TJ & Peeters RP. Thyroid function in pregnancy: what is normal? Clinical Chemistry 201561704–713. ( 10.1373/clinchem.2014.236646) [DOI] [PubMed] [Google Scholar]
- 3.Yap YW Onyekwelu E & Alam U. Thyroid disease in pregnancy. Clinical Medicine 202323125–128. ( 10.7861/clinmed.2023-0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng L & Lam L. Thyroid function during the fetal and neonatal periods. NeoReviews 202021e30–e36. ( 10.1542/neo.21-1-e30) [DOI] [PubMed] [Google Scholar]
- 5.Cignini P Cafà EV Giorlandino C Capriglione S Spata A & Dugo N. Thyroid physiology and common diseases in pregnancy: review of literature. Journal of Prenatal Medicine 2012664–71. [PMC free article] [PubMed] [Google Scholar]
- 6.Razvi S Bhana S & Mrabeti S. Challenges in interpreting thyroid stimulating hormone results in the diagnosis of thyroid dysfunction. Journal of Thyroid Research 201920194106816. ( 10.1155/2019/4106816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, et al.Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011211081–1125. ( 10.1089/thy.2011.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, et al.2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 201727315–389. ( 10.1089/thy.2016.0457) [DOI] [PubMed] [Google Scholar]
- 9.Chan S Marsh M Boelaert K Evans C Gilbert J & Dhillon-Smith R. RCOG Green-top Guideline (New), May-June 2023 – Peer Review Draft 2023. London, UK: Royal College of Obstetricians and Gynaecologists. (available at: https://www.rcog.org.uk/media/4v3hsepc/thyroiddisordersinpregnancyvpeerreviewfinal.pdf) [Google Scholar]
- 10.Poppe K Bisschop P Fugazzola L Minziori G Unuane D & Weghofer A. 2021 European Thyroid Association guideline on thyroid disorders prior to and during assisted reproduction. European Thyroid Journal 20219281–295. ( 10.1159/000512790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus JH. Epidemiology and prevention of thyroid disease in pregnancy. Thyroid 200212861–865. ( 10.1089/105072502761016485) [DOI] [PubMed] [Google Scholar]
- 12.Negro R & Stagnaro-Green A. Clinical aspects of hyperthyroidism, hypothyroidism, and thyroid screening in pregnancy. Endocrine Practice 201420597–607. ( 10.4158/EP13350.RA) [DOI] [PubMed] [Google Scholar]
- 13.Dong AC & Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: a systematic review and meta-analysis. Thyroid 201929278–289. ( 10.1089/thy.2018.0475) [DOI] [PubMed] [Google Scholar]
- 14.Diéguez M Herrero A Avello N Suárez P Delgado E & Menéndez E. Prevalence of thyroid dysfunction in women in early pregnancy: does it increase with maternal age? Clinical Endocrinology 201684121–126. ( 10.1111/cen.12693) [DOI] [PubMed] [Google Scholar]
- 15.Dulek H Vural F Aka N & Zengin S. The prevalence of thyroid dysfunction and its relationship with perinatal outcomes in pregnant women in the third trimester. Northern Clinics of Istanbul 20196267–272. ( 10.14744/nci.2018.51422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzeddine D Ezzeddine D Hamadi C Abbas HA Nassar A Abiad M & Ghazeeri G. Prevalence and correlation of hypothyroidism with pregnancy outcomes among Lebanese women. Journal of the Endocrine Society 20171415–422. ( 10.1210/js.2017-00014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veltri F Belhomme J Kleynen P Grabczan L Rozenberg S Pepersack T & Poppe K. Maternal thyroid parameters in pregnant women with different ethnic backgrounds: do ethnicity-specific reference ranges improve the diagnosis of subclinical hypothyroidism? Clinical Endocrinology 201786830–836. ( 10.1111/cen.13340) [DOI] [PubMed] [Google Scholar]
- 18.Korevaar TIM Medici M Visser TJ & Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nature Reviews. Endocrinology 201713610–622. ( 10.1038/nrendo.2017.93) [DOI] [PubMed] [Google Scholar]
- 19.American College of Obstetricians and Gynecologists. Thyroid disease in pregnancy. ACOG Practice Bulletin, number 223. Obstetrics & Gynecology 2020135e261–e274. ( 10.1097/aog.0000000000003893) [DOI] [PubMed] [Google Scholar]
- 20.Lee SY & Pearce EN. Testing, monitoring, and treatment of thyroid dysfunction in pregnancy. Journal of Clinical Endocrinology and Metabolism 2021106883–892. ( 10.1210/clinem/dgaa945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li SW & Chan SY. Management of overt hypothyroidism during pregnancy. Best Practice and Research Clinical Endocrinology and Metabolism 202034101439. ( 10.1016/j.beem.2020.101439) [DOI] [PubMed] [Google Scholar]
- 22.Taylor PN & Lazarus JH. Hypothyroidism in pregnancy. Endocrinology and Metabolism Clinics of North America 201948547–556. ( 10.1016/j.ecl.2019.05.010) [DOI] [PubMed] [Google Scholar]
- 23.Ge GM Leung MTY Man KKC Leung WC Ip P Li GHY Wong ICK Kung AWC & Cheung CL. Maternal thyroid dysfunction during pregnancy and the risk of adverse outcomes in the offspring: a systematic review and meta-analysis. Journal of Clinical Endocrinology and Metabolism 2020105dgaa555. ( 10.1210/clinem/dgaa555) [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Guo T, Fu J, Kuang J, Wang Y, Zhang Y, Zhang H, He Y, Peng Z, Wang Q, et al.Preconception thyrotropin levels and risk of adverse pregnancy outcomes in Chinese women aged 20 to 49 years. JAMA Network Open 20214e215723. ( 10.1001/jamanetworkopen.2021.5723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Guo T, Fu J, Zhao J, Wang Y, He Y, Peng Z, Zhang Y, Zhang H, Zhang Y, et al.Association of preconception thyrotropin levels with fecundability and risk of spontaneous abortion in China. JAMA Network Open 20225e2228892. ( 10.1001/jamanetworkopen.2022.28892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maraka S Ospina NM O'Keeffe DT Espinosa De Ycaza AE Gionfriddo MR Erwin PJ Coddington CC Stan MN Murad MH & Montori VM. Subclinical hypothyroidism in pregnancy a systematic review and meta-analysis. Thyroid 201626580–590. ( 10.1089/thy.2015.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Y Wang J Wang X Ouyang L & Li Y. Relationship between subclinical hypothyroidism in pregnancy and hypertensive disorder of pregnancy: a systematic review and meta-analysis. Frontiers in Endocrinology 202213823710. ( 10.3389/fendo.2022.823710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth, Korevaar TIM, Derakhshan A, Taylor PN, Meima M, Chen L, Bliddal S, Carty DM, Meems M, Vaidya B, et al.Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth. JAMA 2019322632–641. ( 10.1001/jama.2019.10931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toloza FJK, Derakhshan A, Männistö T, Bliddal S, Popova PV, Carty DM, Chen L, Taylor P, Mosso L, Oken E, et al.Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes and Endocrinology 202210243–252. ( 10.1016/S2213-8587(2200007-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knøsgaard L Andersen S Hansen AB Vestergaard P & Andersen SL. Maternal hypothyroidism and adverse outcomes of pregnancy. Clinical Endocrinology 202398719–729. ( 10.1111/cen.14853) [DOI] [PubMed] [Google Scholar]
- 31.De Leo S & Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet. Diabetes and Endocrinology 20186575–586. ( 10.1016/S2213-8587(1730402-3) [DOI] [PubMed] [Google Scholar]
- 32.Kiran Z Sheikh A & Islam N. Association of thyroid antibodies status on the outcomes of pregnant women with hypothyroidism (maternal hypothyroidism on pregnancy outcomes, MHPO-4). BMC Pregnancy and Childbirth 202121136. ( 10.1186/s12884-021-03594-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negro R Formoso G Mangieri T Pezzarossa A Dazzi D & Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Journal of Clinical Endocrinology and Metabolism 2006912587–2591. ( 10.1210/jc.2005-1603) [DOI] [PubMed] [Google Scholar]
- 34.Glinoer D Riahi M Grün JP & Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. Journal of Clinical Endocrinology and Metabolism 199479197–204. ( 10.1210/jcem.79.1.8027226) [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya R Mukherjee K Das A Biswas MR Basunia SR & Mukherjee A. Anti-thyroid peroxidase antibody positivity during early pregnancy is associated with pregnancy complications and maternal morbidity in later life. Journal of Natural Science, Biology, and Medicine 20156402–405. ( 10.4103/0976-9668.160021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meena M Chopra S Jain V & Aggarwal N. The effect of anti-thyroid peroxidase antibodies on pregnancy outcomes in euthyroid women. Journal of Clinical and Diagnostic Research 201610QC04–QC07. ( 10.7860/JCDR/2016/19009.8403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan S. The relationship between thyroid autoantibody positivity and abnormal pregnancy outcomes and miscarriage in euthyroid patients. Journal of Obstetrics and Gynecological Investigations 2020317–22. ( 10.5114/jogi.2020.100977) [DOI] [Google Scholar]
- 38.Lata K Dutta P Sridhar S Rohilla M Srinivasan A Prashad GR Shah VN & Bhansali A. Thyroid autoimmunity and obstetric outcomes in women with recurrent miscarriage: a case-control study. Endocrine Connections 20132118–124. ( 10.1530/EC-13-0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thangaratinam S Tan A Knox E Kilby MD Franklyn J & Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011342d2616. ( 10.1136/bmj.d2616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor PN, Minassian C, Rehman A, Iqbal A, Draman MS, Hamilton W, Dunlop D, Robinson A, Vaidya B, Lazarus JH, et al.TSH levels and risk of miscarriage in women on long-term levothyroxine: a community-based study. Journal of Clinical Endocrinology and Metabolism 2014993895–3902. ( 10.1210/jc.2014-1954) [DOI] [PubMed] [Google Scholar]
- 41.Bein M Yu OHY Grandi SM Frati FYE Kandil I & Filion KB. Levothyroxine and the risk of adverse pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. BMC Endocrine Disorders 20212134. ( 10.1186/s12902-021-00699-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding Z Liu Y Maraka S Abdelouahab N Huang HF Fraser WD & Fan J. Pregnancy and neonatal outcomes with levothyroxine treatment in women with subclinical hypothyroidism based on new diagnostic criteria: a systematic review and meta-analysis. Frontiers in Endocrinology 202112797423. ( 10.3389/fendo.2021.797423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau L Benham JL Lemieux P Yamamoto J & Donovan LE. Impact of levothyroxine in women with positive thyroid antibodies on pregnancy outcomes: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 202111e043751. ( 10.1136/bmjopen-2020-043751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X Hou N Wang H Ma L Sun J & Liu Y. A meta-analysis of pregnancy outcomes with levothyroxine treatment in euthyroid women with thyroid autoimmunity. Journal of Clinical Endocrinology and Metabolism 2020105dgz217. ( 10.1210/clinem/dgz217) [DOI] [PubMed] [Google Scholar]
- 45.Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, Bender-Atik R, Agrawal R, Bhatia K, Edi-Osagie E, et al.Levothyroxine in women with thyroid peroxidase antibodies before conception. New England Journal of Medicine 20193801316–1325. ( 10.1056/NEJMoa1812537) [DOI] [PubMed] [Google Scholar]
- 46.van Dijk MM, Vissenberg R, Fliers E, van der Post JAM, van der Hoorn MP, de Weerd S, Kuchenbecker WK, Hoek A, Sikkema JM, Verhoeve HR, et al.Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes and Endocrinology 202210322–329. ( 10.1016/S2213-8587(2200045-6) [DOI] [PubMed] [Google Scholar]
- 47.Lepoutre T Debiève F Gruson D & Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecologic and Obstetric Investigation 201274265–273. ( 10.1159/000343759) [DOI] [PubMed] [Google Scholar]
- 48.Jouyandeh Z Hasani-Ranjbar S Qorbani M & Larijani B. Universal screening versus selective case-based screening for thyroid disorders in pregnancy. Endocrine 201548116–123. ( 10.1007/s12020-014-0385-9) [DOI] [PubMed] [Google Scholar]
- 49.Lazarus J Brown RS Daumerie C Hubalewska-Dydejczyk A Negro R & Vaidya B. European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. European Thyroid Journal 2014376–94. ( 10.1159/000362597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yassa L Marqusee E Fawcett R & Alexander EK. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. Journal of Clinical Endocrinology and Metabolism 2010953234–3241. ( 10.1210/jc.2010-0013) [DOI] [PubMed] [Google Scholar]
- 51.Maraka S, Mwangi R, Yao X, Sangaralingham LR, Singh Ospina NM, O'Keeffe DT, Rodriguez-Gutierrez R, Stan MN, Brito JP, Montori VM, et al.Variation in treatment practices for subclinical hypothyroidism in pregnancy: US National Assessment. Journal of Clinical Endocrinology and Metabolism 20191043893–3901. ( 10.1210/jc.2019-00057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giusti M. Management of thyroid hypofunction during pregnancy: a real-world experience in a secondary endocrine centre in Liguria. Gynecological and Reproductive Endocrinology and Metabolism 20212168–177. [Google Scholar]
- 53.Turunen S Vääräsmäki M Leinonen M Gissler M Männistö T & Suvanto E. The increased trend of medical treatment for thyroid diseases during pregnancy: a 13-year national study. European Thyroid Journal 202110230–236. ( 10.1159/000515125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen I. Prenatal testing of thyroid is debated. New York Times, 13 April 2009. (available at: https://www.nytimes.com/2009/04/14/health/14thyr.html) [Google Scholar]
- 55.Medeiros MF, Cerqueira TL, Silva Junior JC, Amaral MT, Vaidya B, Poppe KG, Carvalho GA, Gutierrez S, Alcaraz G, Abalovich M, et al.An international survey of screening and management of hypothyroidism during pregnancy in Latin America. Arquivos Brasileiros de Endocrinologia e Metabologia 201458906–911. ( 10.1590/0004-2730000003382) [DOI] [PubMed] [Google Scholar]
- 56.Vaidya B Hubalewska-Dydejczyk A Laurberg P Negro R Vermiglio F & Poppe K. Treatment and screening of hypothyroidism in pregnancy: results of a European survey. European Journal of Endocrinology 201216649–54. ( 10.1530/EJE-11-0729) [DOI] [PubMed] [Google Scholar]
- 57.Azizi F Amouzegar A Mehran L Alamdari S Subekti I Vaidya B Poppe K San Luis T & Akamizu T. Screening and management of hypothyroidism in pregnancy: results of an Asian survey. Endocrine Journal 201461697–704. ( 10.1507/endocrj.ej14-0083) [DOI] [PubMed] [Google Scholar]
- 58.Stagnaro-Green A Dong A & Stephenson MD. Universal screening for thyroid disease during pregnancy should be performed. Best Practice and Research Clinical Endocrinology and Metabolism 202034101320. ( 10.1016/j.beem.2019.101320) [DOI] [PubMed] [Google Scholar]
- 59.Taylor PN Zouras S Min T Nagarahaj K Lazarus JH & Okosieme O. Thyroid screening in early pregnancy pros and cons. Frontiers in Endocrinology 20189626. ( 10.3389/fendo.2018.00626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira JL Gomes M & Príncipe RM. Controversial screening for thyroid dysfunction in preconception and pregnancy: an evidence-based review. Journal of Family and Reproductive Health 202014209–220. ( 10.18502/jfrh.v14i4.5204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor PN Okosieme OE Premawardhana L & Lazarus JH. Should all women be screened for thyroid dysfunction in pregnancy? Women’s Health 201511295–307. ( 10.2217/whe.15.7) [DOI] [PubMed] [Google Scholar]
- 62.Sitoris G Veltri F Kleynen P Belhomme J Rozenberg S & Poppe K. Screening for thyroid dysfunction in pregnancy with targeted high-risk case finding: can it be improved? Journal of Clinical Endocrinology and Metabolism 20191042346–2354. ( 10.1210/jc.2018-02303) [DOI] [PubMed] [Google Scholar]
- 63.van der Spek AH & Bisschop PH. Universal screening for thyroid disease SHOULD NOT be recommended before and during pregnancy. Best Practice and Research Clinical Endocrinology and Metabolism 202034101429. ( 10.1016/j.beem.2020.101429) [DOI] [PubMed] [Google Scholar]
- 64.Spencer L Bubner T Bain E & Middleton P. Screening and subsequent management for thyroid dysfunction pre-pregnancy and during pregnancy for improving maternal and infant health. Cochrane Database of Systematic Reviews 20152015CD011263. ( 10.1002/14651858.CD011263.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amiri M Nazarpour S Ramezani Tehrani F Sheidaei A & Azizi F. The targeted high-risk case-finding approach versus universal screening for thyroid dysfunction during pregnancy: thyroid-stimulating hormone (TSH) and/or thyroid peroxidase antibody (TPOAb) test? Journal of Endocrinological Investigation 2022451641–1651. ( 10.1007/s40618-021-01738-7) [DOI] [PubMed] [Google Scholar]
- 66.Hubalewska-Dydejczyk A, Trofimiuk-Müldner M, Ruchala M, Lewiński A, Bednarczuk T, Zgliczyński W, Syrenicz A, Kos-Kudla B, Jarząb B, Gietka-Czernel M, et al.Thyroid diseases in pregnancy: guidelines of the Polish Society of Endocrinology [Choroby tarczycy w ciąży: zalecenia postępowania Polskiego Towarzystwa Endokrynologicznego]. Endokrynologia Polska 202172425–488. ( 10.5603/EP.a2021.0089) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a