Abstract
BACKGROUND:
Development of validated biomarkers to detect early Alzheimer disease (AD) neuropathology is needed for therapeutic AD trials. Abnormal concentrations of “core” AD biomarkers, cerebrospinal fluid (CSF) amyloid beta1–42, total tau, and phosphorylated tau correlate well with neuroimaging biomarkers and autopsy findings. Nevertheless, given the limitations of established CSF and neuroimaging biomarkers, accelerated development of blood-based AD biomarkers is underway.
CONTENT:
Here we describe the clinical significance of CSF and plasma AD biomarkers to detect disease pathology throughout the Alzheimer continuum and correlate with imaging biomarkers. Use of the AT(N) classification by CSF and imaging biomarkers provides a more objective biologically based diagnosis of AD than clinical diagnosis alone. Significant progress in measuring CSF AD biomarkers using extensively validated highly automated assay systems has facilitated their transition from research use only to approved in vitro diagnostics tests for clinical use. We summarize development of plasma AD biomarkers as screening tools for enrollment and monitoring participants in therapeutic trials and ultimately in clinical care. Finally, we discuss the challenges for AD biomarkers use in clinical trials and precision medicine, emphasizing the possible ethnocultural differences in the levels of AD biomarkers.
SUMMARY:
CSF AD biomarker measurements using fully automated analytical platforms is possible. Building on this experience, validated blood-based biomarker tests are being implemented on highly automated immunoassay and mass spectrometry platforms. The progress made developing analytically and clinically validated plasma AD biomarkers within the AT(N) classification scheme can accelerate use of AD biomarkers in therapeutic trials and routine clinical practice.
Introduction
Given that definitive diagnosis of Alzheimer disease (AD) is based on the presence of 2 pathologic hallmarks of AD at autopsy, i.e., amyloid plaques and neurofibrillary tangles, we cannot confirm “definitive AD” in living patients. However, validated fluid AD biomarker and amyloid-β (Aβ) positron emission tomography (PET), and tau PET imaging tests provide objective evidence for the respective AD pathologic changes. Accumulated evidence supports that measurements of cerebrospinal fluid (CSF), amyloid-beta(1–42) (Aβ42) concentration or of brain amyloid deposition by Aβ PET in living persons can serve as proxies for Alzheimer neuropathology (1). Indeed, the National Institute on Aging and the Alzheimer’s Association (NIA-AA) recommended incorporation of AD biomarkers into the diagnostic criteria for this disease (2). Although the NIA-AA recommendation for AD diagnosis has not been implemented in routine clinical practice in the US, the use of imaging and CSF biomarkers provides objective evidence for the underlying neuropathology of cognitive impairment to clinicians and researchers. Subsequently, the 2011 NIA-AA guideline was updated and developed into the “research framework” that describes the biological definition of AD (3).
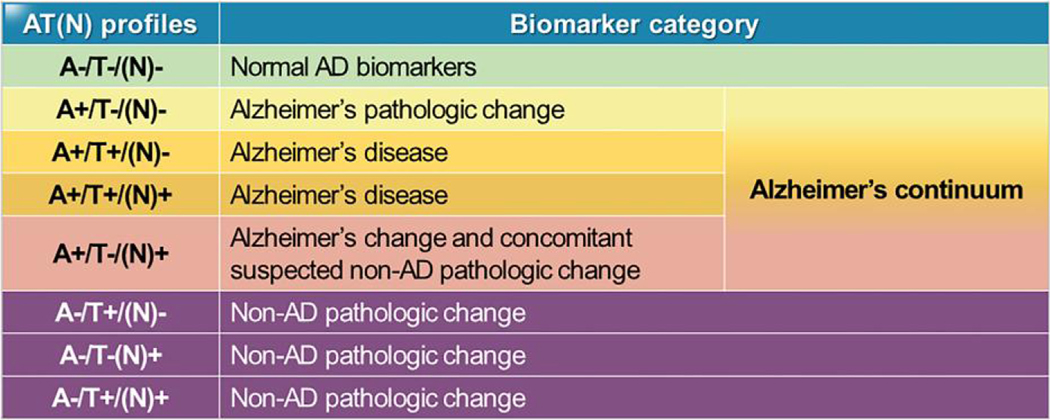
Biomarker-based biological classification of AD by the AT(N) system (Fig. 1 and see next section) in the research framework recommended by the NIA-AA work group creates a scheme to define and stage the disease across the entire disease spectrum, facilitate standardized reporting of research findings, and enable establishment of the longitudinal changes in pathologic criteria in cohort studies. Although the AT(N) system is not “diagnostic criteria or guidelines,” it likely paves the way for the use of these biomarker tests in routine clinical practice.
Fig. 1.
Alzheimer biomarker categories by AT(N) profiles. The 9 possible combinations of each of A, T, and N as binary covariates (above or below the respective cut-point values established for each) are noted in the first column. The second column denotes the biomarker category for each of the 9 combinations. For example, an individual whose A, T, and N test results were all negative, A-T-N-, is denoted as having normal AD biomarkers and is not on an AD trajectory at the time of testing, whereas an individual whose A, T, and N test results were all positive, A + T + N+, has a biomarker category Alzheimer disease at the time of testing. Alzheimer disease is a diagnosis when both A and T are positive with N positive or negative. When A only is positive but T and N negative, the biomarker change is termed Alzheimer pathologic change, which is the very earliest stage for the disease pathologically and progression to a diagnosis of Alzheimer disease may occur many years later.
Together with imaging biomarkers, core CSF AD biomarkers [i.e., Aβ42, total tau (t-tau), and phosphorylated tau (p-tau)] reflect AD brain pathology and neurodegenerative changes. The clinically reliable diagnostic performance of AD biomarkers in antemortem samples has been confirmed in CSF from patients with a “definitive” diagnosis of AD at autopsy (4). In addition, the features of CSF AD biomarkers closely correlate with those measured in imaging modalities including magnetic resonance imaging and PET (5). Despite the established clinical utility for CSF AD biomarkers, there remain several key concerns associated with measurement of CSF AD biomarkers by immunoassays (e.g., invasiveness of lumbar puncture; limited longitudinal studies; limited availability in outpatient settings). These concerns have intensely motivated researchers to study the diagnostic utility of blood-based AD biomarkers and standardize the preanalytical and analytical procedures involved. As a result, several plasma AD biomarkers with high diagnostic sensitivity and specificity were reported in small-scale studies; however, the clinical and analytical performance of these promising blood-based biomarkers require further rigorous validation studies. Importantly, considerable progress in standardization of preanalytical and analytical conditions for CSF biomarker measurements has been achieved (6), and standardization of blood sample collection and handling for developing valid AD biomarkers has progressed (7). In addition, implementation of reference materials and methods are necessary. More recently, analytical variability has been significantly reduced by the emerging fully automated immunoassay platforms for CSF AD biomarkers (8, 9) and these are now in use in plasma AD biomarker development and validation studies (10).
In this narrative review, we critically review published studies documenting progress on clinical implications in CSF AD biomarkers development and emerging blood AD biomarkers. First, we describe the significance of CSF AD biomarkers for disease detection across the Alzheimer continuum. Second, we summarize immunoassay and mass spectrometry (MS) platforms’ contributions toward measuring CSF AD biomarkers and their correlation with neuropathologic diagnosis and imaging data. We also discuss the ethnocultural characteristics of CSF AD biomarkers followed by a discussion of the extensive progress in validating emerging blood-based AD biomarkers. Finally, we describe the significance of plasma AD biomarkers to screen individuals in clinical settings such as treatment trials.
New Diagnostic Approach Based on AT(N) Biomarker Profiles
AD biomarkers, decreased below cut-point concentration values (i.e., fixed concentration or values determined by ROC analysis) for CSF Aβ42 or Aβ42/ Aβ40 ratio and increased, above cut-point values, p-tau or t-tau in CSF, or increased (above cut-point values) accumulated amyloid detected by Aβ PET, have been validated in large-scale prospective clinical studies and are widely used as proxies for AD neuropathologic changes (1). Since a considerable proportion (approximately 30%–50%) of patients who are clinically diagnosed as AD-type dementia showed mixed pathology or even the absence of AD pathology at autopsy (11), clinically defined AD is not regarded as either highly sensitive or specific for AD neuropathologic changes. Therefore, development of well-validated AD biomarkers provides a biological basis for diagnosis that shifts the AD diagnosis paradigm. More importantly, the biologic approach for AD neuropathologic changes made it possible to define “preclinical AD” in persons without overt clinical symptoms who are likely to progress, compared to biologically normal persons (3, 12). To improve diagnostic accuracy, a unified binary AD biomarker classification was proposed in 2018 building on the 2011 NIA-AA recommendation and the growing evidence of the AD pathology continuum [i.e., normal cognition, mild cognitive impairment (MCI), and dementia] aiming to shift diagnosis in living individuals from a syndromal to a biological construct, paralleling neuropathological diagnosis (3, 12). Three components of biomarkers for AD neuropathologic changes are: [A] Aβ deposition, [T] tau pathology, and [(N)] neurodegeneration or injury. Using binary categorization (e.g., [A+] vs [A−]), AT(N) biomarker profiles are represented by CSF and imaging biomarkers, i.e., [A]: CSF Aβ42 or amyloid PET, [T]: CSF p-tau or tau PET, [(N)]: fluorodeoxyglucose PET, or structural magnetic resonance imaging. Through the combination of these 3 different biomarkers, a living person is classified into normal, Alzheimer continuum, or non-AD pathologic change (Fig. 1), independent of clinical symptoms (3). Compared to [A+] or [T+], neurodegeneration [N+] is less specific for AD (13).
Progress in Analytical Platform Development for Measuring Core CSF AD Biomarkers
To measure concentrations of CSF core biomarkers for AD diagnosis, singleplex or multiplex immunoassay platforms were developed. By measuring CSF AD biomarkers using these manual immunoassay platforms, their high interlaboratory variability (%CV for Aβ42 up to 30%) hampered determination of universal cut- points for AD diagnosis (14). Consensus reviews have minimized preanalytical variables (6), but defining and addressing analytical sources of variability remains complicated and can cause significant bias through multiple variables (14). To resolve these shortcomings, development of reference materials and methods is required. To this end, for Aβ42, measurements of absolute CSF levels using LC-MS/MS without immunoaffinity-based sample preparation were developed as reference methods (15, 16). Using LC-MS/MS, Certified Reference Materials (CRM) for Aβ42 were developed (17, 18). Because artificial matrices were not appropriate in a first commutability study across 8 immunoassays and selected reaction monitoring LC-MS (19), human neat CSF collected from normal pressure hydrocephalus patients by lumbar drainage was the CRM starting material. The introduction of CRMs using untreated CSF can lead to harmonization of cut-off values for different CSF Aβ42 methods by minimizing CSF Aβ42 calibrator bias across different immunoassay formats and across kit lots. With the aim to achieve analytical and clinical validity, a novel, fully automated electrochemiluminescence immunoassay system for quantitation of CSF Aβ42 was developed (8). The Elecsys Aβ42 immunoassay developed by Roche Diagnostics demonstrated good analytical sensitivity and specificity, excellent precision, no high-concentration hook effect, no cross-reactivity for Aβ1–38 or Aβ40, and no interfering effects by endogenous substances or medications at therapeutic concentrations. More importantly, the Elecsys immunoassay platform for Aβ42 showed excellent interlaboratory consistency (%CV < 4%) and lot-to-lot comparability (%CV = 0.6%–2.3%) in aqueous control and CSF pools in the results from the Alzheimer’s Association QC program (rounds 16–17) and in a study following Clinical & Laboratory Standards Institute guidelines (8). The Elecsys fully automated immunoassay platform to measure CSF Aβ42, t-tau, and p-tau showed high concordance with amyloid PET classification (90% overall agreement for CSF t-tau/Aβ42 and p-tau/Aβ42 ratios), and optimized cut-points were also highly concordant with PET results in 2 different cohorts (20). Another fully-automated chemiluminescence immunoassay platform (Lumipulse, Fujirebio) for CSF Aβ42 predicted amyloid PET positivity [area under the curve (AUC) = 0.86] that increased to 0.94 to 0.95 when combined with Aβ40 (Aβ42/Aβ40), t-tau (Aβ42/t-tau), or p-tau181 (Aβ42/pt-tau181) (9). Lumipulse assays have excellent analytical performance (inter-assay %CV 0.66% to 3.25%; intra-assay %CV 0.79%–5.5% across CSF Aβ42, Aβ40, t-tau, and p-tau181 concentrations) and strong correlation with the INNOTEST enzyme-linked immunosorbent assay (ELISA) method (21). An open-access automated system integrating Euroimmune ELISA kits for Aβ42, t-tau, and p-tau181 measurements is also available (22). These developments of fully automated immunoassay platforms represent a fundamental advance for clinical application of robust cut-points for AD diagnosis in clinical practice and in clinical trials.
Correlation of Biomarkers with Neuropathological Changes
CSF AD biomarkers or PET imaging provide valid surrogates for detection of AD neuropathologic changes without an autopsy, the gold standard for Alzheimer pathology. However, studies estimating diagnostic cut- offs for CSF AD biomarkers based on autopsy- confirmed diagnosis are limited in number. Even if the detailed nature of CSF AD biomarker abnormalities and amyloid or tau PET are not directly comparable, the abnormalities are closely correlated (e.g., nonlinear inverse association between CSF Aβ42 level and amyloid PET intensity) and have a reasonable degree of concordance and comparable diagnostic utilities (9, 20, 23, 24). Notably, amyloid PET had greater specificity than CSF Aβ42 whereas CSF has greater sensitivity (25). However, CSF Aβ42 and p-tau181 reflect the pathologic state of brain associated with amyloid plaque and paired-helical filament formation, respectively, while amyloid or tau PET reflect cumulative pathologic deposits (3), which may lead to discordance between CSF and imaging measurements depending on measurement timing. Several amyloid PET tracers [e.g., Florbetapir (trade name Amyvid); Flutemetamol (trade name Vizamyl); Florbetaben (trade name NeuroCeq)] have received Food and Drug Administration and European Medicines Agency approval for clinical use, and Aβ burden measured by amyloid PET correlates closely with that measured in autopsied brain tissue (26). For tau PET imaging, one tracer, Flortaucipir, is Food and Drug Administration-approved while others are still in the early stages of study, thus correlation between tau-PET imaging and fluid AD biomarkers is maturing (27).
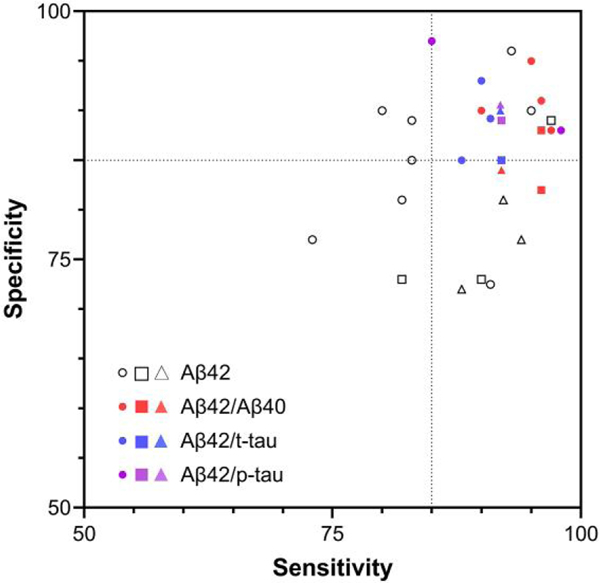
The correlation of CSF AD biomarkers with autopsy-confirmed amyloid or tau pathology in mixed pathology in AD, non-AD, and pure AD is significant. In an autopsy-confirmed cohort study, CSF AD biomarkers have added diagnostic value in differential diagnosis when clinical dementia diagnosis is ambiguous (28). Moreover, subjects at normal or prodromal stages of cognitive decline, who progressed to autopsy-confirmed AD, showed AD-like CSF biomarker profiles at baseline (29–31). It can be expected that implementation of the AT(N) framework for characterization of AD status in individuals permits more refined assessment of progression likelihood. As CSF AD biomarkers have a higher benefit to cost ratio compared to PET imaging, CSF biomarkers can substitute for PET-based AD diagnosis under certain circumstances. Because of disease instability and considerable confounding effects of mixed pathology in the brain of AD patients in the clinical diagnosis of AD (32), determination of diagnostic cut-points of AD biomarkers based on clinical diagnosis has inherent limitations. Rather, application of predefined antemortem cut-off values based on amyloid PET positivity (standardized uptake value ratio or visual), surrogates for autopsy-based cut-points, have provided reliable diagnostic and predictive performance (Fig. 2 and Supplemental Table 1). Autopsy-based predefined diagnostic cut-points of CSF AD biomarkers are considered the gold standard. However, this approach is not as widely available in all countries without establishing nationwide brain banks. Instead, cut-point values determined based on amyloid PET positivity are more widely applicable. However, it should be noted that CSF Aβ42 reflects cerebral amyloid burden of both soluble Aβ42 and plaque, while amyloid PET positivity is generally more reflective of brain neuritic amyloid plaque burden. Therefore, there is expected to be limited discordance between abnormalities of CSF Aβ42 and amyloid PET, although it is a small percentage (23). Based on the observed high concordance rate of amyloid PET positivity and neuropathologic examination, amyloid PET-based determination of CSF Aβ42 cut-points was found to be more accurate and constant across centers, compared to clinically based determination (33). Nevertheless, subgroups of PET and CSF Aβ mismatch and mixed pathology were observed, suggesting that additional nonamyloid AD biomarkers development is warranted (see Supplemental Table 2).
Fig. 2.
Sensitivity and specificity of CSF biomarkers to predict amyloid positivity using cut-off values determined by amyloid-PET (circle, flutemetamol; square, Pittsburgh compound B; triangle, florbetapir) as the reference methodology. Dotted lines indicate 85% sensitivity and specificity. Each symbol indicates an individual cohort in previous studies with sufficient number (n > 100) of subjects (see details in the Supplemental Table 1 and Supplemental References).
Consideration of Ethnocultural Characteristics in AD Biomarker Development
There is increasing evidence of AD disparities including prevalence, neuropathological features, survival rate, clinical features, comorbidity, and biological and medical risk factors (34–36). Age-adjusted dementia incidence rates are highest in African American (26.6 per 1000 person-years) followed by American Indian and Alaskan Native (22.2), Pacific Islander (19.6), Latino (19.6), White (19.4), and Asian American (15.2) patients (34). The levels of t-tau and p-tau in CSF of African American individuals were lower than in White individuals for unknown reasons (36, 37). These racial differences were not explained by disease stage or neurodegeneration status; therefore, race is an important factor when interpreting CSF AD biomarkers for prodromal AD diagnosis (37). Racial disparities in Aβ42 and p-tau in both CSF and plasma may be influenced by differences in the prevalence of medical comorbidities associated with increasing AD risk, social determinants of health, frequency of brain amyloidosis, and genetic differences (38). A study noted genetic variants with different frequencies for the CSF microglial biomarker, soluble triggering receptor expressed in myeloid cells 2, suggesting racial disparities in AD risk (39). Since studies of racial disparities in AD biomarkers are limited, it is crucial to ensure that AD biomarker assays are accurate and consistent across racial groups. Large-scale multicenter studies are necessary to address this issue, as current patient-level datasets generated in 9 major clinical cohort studies are heavily biased toward White individuals (79.3%) compared to African American (11.5%), Latin/Hispanic (5.6%), Asian (2.7%), and other (1.3%) individuals (40). The Alzheimer’s Disease Neuroimaging Initiative-4 study has a major focus on inclusion of a much more representative spectrum of underrepresented populations to better reflect the nation’s population and to provide highly standardized measurements of AD biomarkers in plasma of these individuals (41). These planned studies will need to take into account the differences in comorbidities and include objective amyloid and tau pathologic biomarkers (amyloid and tau PET, CSF Aβ42/40, p-tau, and, where possible, autopsy-based AD diagnosis) rather than clinical diagnosis alone. The latter has been used as reference standard in most of the studies comparing African American to White AD biomarkers for underrepresented populations and makes it challenging to establish cut-points across individuals with different ethnic and socioeconomic backgrounds.
Blood Biomarkers for AD
Both CSF and PET imaging AD biomarkers have shortcomings; i.e., the modest invasiveness of lumbar puncture for CSF collection and the expense and lower accessibility of PET imaging limit cohort enrollment and retention in clinical studies, particularly in minority groups, and are not practical for serial monitoring. Therefore, broad-scale clinical implementation of these established AD biomarkers is challenging. These disadvantages have led researchers to develop plasma AD biomarkers as a more practical approach. Given that the concentration of brain-derived proteins in blood is extremely low compared with other plasma proteins (e.g., albumin), technological developments were made that allow accurate analysis of molecules with very low concentrations in a complicated matrix. Systematic studies of preanalytical factors such as anticoagulant used in the blood collection procedure, temperature maintained during blood sample preparation and centrifugation, aliquot storage temperature, and number of freeze-thaw cycles, have been conducted (7). This evaluation supports use of K2 EDTA blood collection tubes, centrifugation at room temperature within 3 h of collection, tube filling of 250 to 1000 L in polypropylene storage tubes, sample stability at −80°C through 2 freeze-thaw cycles, and long-term storage stability assessment at −80°C is ongoing. Although further evaluations of analytical performance of assay platforms and sources of preanalytical variability remain to be fully completed, measurement of plasma AD biomarkers following clinical evaluation in individuals with cognitive complaints has the potential to screen for suspected Alzheimer pathology before more advanced diagnostic assessments such as CSF analysis and neuroimaging are utilized. In addition, development of validated plasma AD biomarkers for early diagnosis that can replace or complement analysis of CSF or PET imaging will likely save time and cost for therapeutic trials. For example, in one evaluation, the use of plasma p-tau screening to recruit asymptomatic individuals for therapeutic AD trials resulted in a cost savings of approximately 60% compared with Aβ PET-only screening in addition to savings in time and logistics (43, 44).
MEASUREMENT OF AΒ IN PLASMA
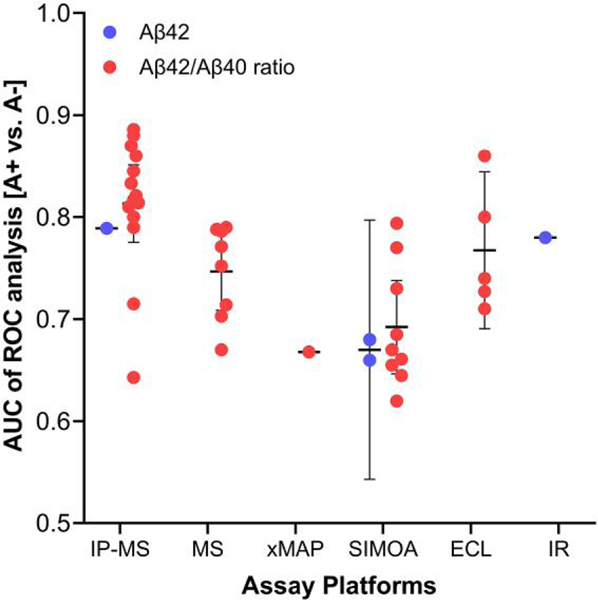
Early reports from 2003 to 2011 of Aβ species measurement using a conventional ELISA platform produced conflicting results (45). One of the reasons for inconsistent results was the analytical performance of the immunoassay platform to detect free plasma Aβ, in addition to other possible reasons including interference by high abundance proteins such as albumin, endogenous immunoglobulins, autoantibodies, and heterophilic antibodies. An analytical platform developed more recently, single molecule array (SIMOA, Quanterix), which detects proteins at the single molecule level utilizing digital counting technology, was used to analyze Aβ42 and tau in plasma (46, 47). For this review, we reexamined previous studies (n > 100 individuals) using various analytical platforms [i.e., SIMOA, antibody-free MS, immunoprecipitation with liquid chromatography- tandem mass spectrometry (IP-MS), Luminex platform, ELISA, electrochemiluminescence immunoassay, and immune-infrared detection technology] to measure plasma Aβ concentration, demonstrating AUC values to predict brain amyloid pathology (Fig. 3 and Supplemental Table 3; and CLIA immunoassay Supplemental Table 3). Among them, the reported AUC values for plasma Aβ42 measured by SIMOA assay were 0.66 and 0.68 to discriminate [A+] from [A−] in individuals with a clinical diagnosis of subjective cognitive decline. The range of AUC values for Aβ42/Aβ40 ratio in cognitively unimpaired normal (CU), MCI, or AD patients to discriminate [A+] from [A−] was 0.645 to 0.794 (mean = 0.70, 95% CI, 0.65–0.75). The levels of plasma Aβ42 and Aβ42/Aβ40 measured by the SIMOA platform were lower in AD than those in MCI or controls and weakly but significantly correlated with corresponding CSF concentration and amyloid deposition measured using flutemetamol PET (46). Weak correlations between plasma Aβ42 concentration measured by the Luminex-xMAP platform and CSF Aβ42, t-tau, and p-tau level by Luminex (48) or ELISA (49) were also observed. Since early studies using ELISA- or multiplex-based assay for plasma Aβ levels to discriminate AD patients were not consistent, Ovod et al. measured plasma Aβ42 and Aβ40 concentration by IP-MS and showed decreased ratios of Aβ42/Aβ40 by 14.3% on average in the presence of amyloidosis relative to amyloid- negative patients and close correlation with CSF Aβ42/ Aβ40 ratio (50). Nakamura et al. independently measured plasma Aβ species using IP-MS and found increased concentration ratios of APP(699–711)/Aβ42 and Aβ40/Aβ42 were useful to predict [A+] (PET positivity) in test and validation cohorts (51). The mean ROC AUC value for plasma Aβ42/Aβ40 ratio determined by IP-MS analysis in previous studies (7 reports, n > 100) to discriminate [A+] from [A−] determined by amyloid PET in CU, MCI, and AD patients is 0.81 (95% CI, 0.77–0.85) (Fig. 3). Measurement of Aβ42/Aβ40 using immunoprecipitation-free MS also showed fair discriminating ability for amyloid pathology (mean of AUC = 0.75, 95% CI, 0.71–0.78) (52) (Fig. 3). Overall, more recent studies in which the plasma Aβ species were measured by immunoassay platforms or MS-based assay have consistently shown that lower concentrations of Aβ42 or Aβ42/Aβ40, but not Aβ40, in plasma can predict Aβ-PET status, despite the relatively narrow range between [A+] and [A−] (Fig. 3, Supplemental Table 3). In fact, analytical and clinical performance of ELISA (Euroimmune, ABtest) and SIMOA platforms were comparable in detecting brain amyloidosis even in the asymptomatic stage (53). Further, it should be noted that the combination of plasma Aβ40 and Aβ42 as a ratio for discriminating brain amyloidosis outperforms Aβ42 alone, as observed in many CSF studies (Fig. 2) and subsequently in plasma (Fig. 3) (50, 51, 54) likely due to normalization by Aβ40, which is much more abundant and less affected by natural differences in Aβ production (55, 56). Therefore, development of new detection technologies for Aβ species are expected to grow. Although the fold- changes of plasma Aβ42/Aβ40 between [A+] and [A−] individuals is substantially lower in comparison to that observed using CSF, likely due to peripheral sources of Aβ, use of plasma Aβ measurement as a screening test in primary care and clinical trials can significantly reduce the required number of amyloid PET scans or CSF testing (44, 57).
Fig. 3.
AUC values of plasma Aβ biomarkers measured by different assay platforms for discriminating brain amyloidosis determined by Aβ PET or CSF Aβ. Detailed information for each cohort (n > 100), diagnostic groups, and methodology determining brain amyloidosis is presented in Supplemental Table 3 and Supplemental References. Horizontal and vertical bars indicate mean and 95% CI, respectively. Abbreviations: ECL, Elecsys electrochemiluminescent fully automated immunoassay; IR, immuno- infrared assay.
MEASUREMENT OF P-TAU IN BLOOD
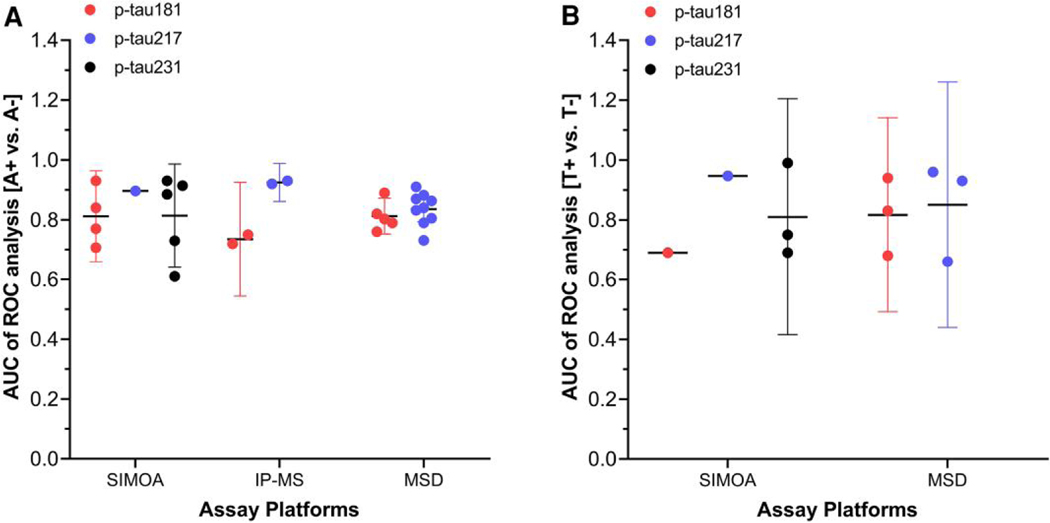
In addition to plasma Aβ reflecting brain amyloid pathology (A), the measurement of tau pathology (T) using plasma samples is emerging. In CSF, phosphorylated tau species are the most AD-specific “T” biomarkers, inspiring studies that evaluate concentrations of various p-tau species in plasma. Several p-tau protein forms in plasma (e.g., p-tau181, p-tau217, or p-tau231) measured by immunoassay or by IP-MS have been studied as AD biomarkers. The lower concentrations of p-tau in blood, compared to that in CSF, limits detection by conventional immunoassays and led to development of novel plasma p-tau detection methods, such as SIMOA technology. Since tau phosphorylated on the N-terminus or mid-region epitopes are more abundant in the soluble protein fraction of brain than C-terminal p-tau forms (58) that are prone to aggregate, development efforts for plasma p-tau assays have focused on N-terminal peptide fragments of p-tau. MS-based analysis of blood enables the simultaneous detection of multiple p-tau proteoforms. Among the p-tau forms, p-tau181 and p-tau217 showed reliable diagnostic utility in several cohorts for early diagnosis and significant correlation with brain amyloidosis and/or tau pathology [see review in (42, 59)]. Using the SIMOA or Meso Scale Diagnostics (MSD) immunoassay platform, measurement of p-tau181 or p-tau217 levels in plasma can discriminate [A+] or [T+] from [A−] or [T−] determined by amyloid/tau PET imaging or CSF analysis (Fig. 4). For p-tau181 and p-tau217 measured by MSD, mean AUC values to discriminate [A+] from [A −] in CU, MCI, and/or AD subjects were 0.81 (95% CI, 0.75–0.87) and 0.84 (95% CI, 0.79–0.88) from 5 and 9 studies, respectively. SIMOA-based assay of p-tau231 afforded a mean AUC value of 0.81 (95% CI, 0.64–0.99) from 5 studies to discriminate [A+] from [A−]. Recent head-to-head comparisons of p-tau assays (p-tau181, p-tau217, and p-tau231 measured by MS, MSD, or SIMOA) have reported variable performance in discriminating [A+] abnormality determined by CSF AD biomarkers (60–62). These studies suggest that optimizing the analytical and clinical performance of plasma p-tau assay requires using different antibodies in combination with different immunoassay platforms to detect different p-tau isoforms, warranting further head-to-head comparison studies for AD diagnosis and prediction of disease progression.
Fig. 4.
(A, B), AUC values of plasma p-tau proteoforms p-tau181, p-tau217, and p-tau231, measured by different assay platforms for discriminating brain amyloidosis (A) and tau brain deposition (B) determined by using autopsy, PET, or CSF. Brain amyloidosis (A) or tau positivity (B) was determined by autopsy with neuropathologic diagnosis, amyloid PET(A), tau PET(T), or CSF (A or T) analysis (see details in Supplemental Table 4 and Supplemental References). Horizontal and vertical bars indicate mean and 95% CI, respectively.
For discrimination of [T+] from [T−], although studies with sufficient numbers of subjects (n > 100) are limited, measurement of p-tau species in plasma by SIMOA or MSD assay fairly discriminated tau pathology in the brain. In Supplemental Table 4, we summarize clinical utility details for clinical diagnosis of AD and correlation with brain neuropathologic findings of p-tau forms. Recent studies strongly suggest that analysis of plasma p-tau would differentiate AD patients from cognitively normal persons, as well as determine if an individual is in the Alzheimer continuum. However, there are still unsolved issues, including the elucidation of possible interethnic differences (63), head-to-head comparison of clinical utility of diverse p-tau forms (64, 65), optimization of the analytical sensitivity required for reliable detection of p-tau in plasma, development of fully validated clinical-grade assays together with CRMs, and establishment of universal cut-points for early diagnosis.
MEASUREMENT OF NEURODEGENERATION AND INFLAMMATION BIOMARKERS IN BLOOD
The SIMOA platform, widely used for measurement of various plasma AD biomarkers, has also been used to measure neurofilament light (NfL) in plasma, demonstrating good analytical performance (intra- and inter- assay %CV of approximately 8.8%–11%, respectively) and excellent analytical sensitivity (lower limit of quantitation of 2.2 ng/L and upper limit of quantitation of 1620 ng/L) (66, 67). Regarding clinical diagnosis, the ability (AUC) of plasma NfL to differentiate CU from AD cases was 0.85 to 0.87 in case-control studies (67, 68). In other longitudinal studies, serum NfL measured (SIMOA platform) could fairly differentiate AD from CU (69) and predict both rate of cortical thinning and cognitive decline (70). In addition, a study showed the association between longitudinal change of plasma NfL concentration (faster increase in NfL) and faster increase in CSF neuronal injury biomarkers and faster rates of atrophy hypometabolism or worsening in global cognition in MCI (71). For t-tau measurement, Mattsson et al. reported that higher plasma concentration was associated with clinical diagnosis of AD, higher CSF tau, and lower CSF Aβ42 in 2 independent cohorts (n = 1284); however, the association was weak and not consistent between cohorts. Higher plasma t-tau was associated with worse disease progression in later disease stages (47). In another longitudinal study, the elevated plasma t-tau levels were associated with cognitive decline in participants with MCI but not in CU and independent of elevated brain amyloid deposition (72). Although the subject number was limited, when tau-PET was used as a reference, plasma levels of t-tau (AUC = 0.80) and t-tau/Aβ42 ratio (AUC = 0.89) were highly predictive of brain tau deposition. In addition, longitudinal changes over 2 years in amyloid deposition, brain glucose metabolism, and hippocampal volume were significantly associated with plasma t-tau/Aβ42 ratio (73). CSF or imaging neurodegeneration biomarkers are not specific to AD, which is likely replicated in blood. In addition, the association of plasma t-tau level with clinical progression was not consistent and likely dependent on disease stage (74). Thus, clinical utilities of plasma neurodegeneration biomarkers in clinical practice and therapeutic trials requires further study. In AD disease progression, glial cells respond to amyloid deposition, contributing to neuroinflammation. Glial fibrillary acidic protein (GFAP) in CSF increases in neurodegenerative disease and is elevated in AD, which is characterized by reactive astrogliosis (75). Interestingly, plasma GFAP more accurately detects amyloid accumulaton and predicts cognitive decline than CSF GFAP (76), highlighting the importance of including plasma GFAP in the cascade of pathological changes occurring in AD and evaluation of anti-Aβ or anti- inflammatory drug effects in clinical trials. Neurogranin (NGRN), a postsynaptic protein known as a marker for synaptic integrity, and NfL, an axonal protein reflecting axonal damage, are emerging nonamyloid biomarkers. Although plasma NGRN levels do not differ between controls and AD patients, combining CSF t-tau, NGRN, and NfL improves diagnostic accuracy. Plasma NfL is elevated not only in AD but also in other dementia types. Supplemental Table 2 summarizes emerging nonamyloid biomarkers.
Implication of AD Biomarkers for Clinical Trials
Since genetic, clinical, and pathological evidence strongly supports the primary role of Aβ in AD pathogenesis, several strategies for reducing brain Aβ accumulation have been applied to develop a “disease-modifying therapy” against AD. In addition, correlations between cognitive dysfunction and tau pathology that are more robust than Aβ load alone has led to a parallel strategy to develop tau-focused therapies. Based on lessons from the failure of previous treatment trials in probable AD patients, including initiation of therapy before onset of clinical symptoms and AD pathologies detection using biologically based AT(N) classification rather than clinical diagnosis, the impact of Aβ load reduction on clinical decline delay may be enhanced in AD trials. Biomarker utility in clinical trials depends on the mechanism of action of the new drug, goal of the trial, questions to be solved, and trial stage. Connected to this, AD biomarkers can be used as surrogate endpoints, tools to enrich study subjects and subgroup selection in phase 2 and 3 trials (particularly using plasma AD biomarkers cost-effectively), proof of mechanisms of action (phase 0 study), and trial-dependent diagnostic measures. Using a structured 5-phase biomarker development framework (77), advances in AD biomarkers using unified protocols to minimize preanalytical variability, CRMs and reference methods, and the fully automated assay platforms for CSF and plasma AD biomarkers have enabled completing phases 1 to 3. To achieve phases 4 to 5, the availability of disease-modifying therapy for AD is necessary as is making generally available diagnostic tools key to initiate treatment (77).
The use of AD biomarkers—fluid or imaging—can provide proof of target engagement in treatment trials. In fact, several clinical trials targeting amyloid or tau pathology include CSF AD biomarkers, which provide information on pharmacodynamic efficacy through the change of CSF AD biomarker levels [see review in (78)]. More importantly, based on the necessity to recruit patients for clinical testing at earlier stages of the AD continuum, fluid or imaging AD biomarkers are commonly used in prevention trials in subjects with prodromal and preclinical disease stages. However, it should be noted that more studies using fluid AD biomarkers are needed to determine if they can serve as surrogates for clinical endpoints, such as the Alzheimer’s Disease Assessment Scale-Cognitive, or for determining the degree of clinical improvement.
As evidence for their clinical utility, plasma AD biomarkers will provide the most cost-efficient screening tool modality to reduce sample size and cost for trials. For example, a simulation study using Alzheimer’s Disease Neuroimaging Initiative cohort data indicated that, compared with amyloid PET-only screening, plasma p-tau measurement to screen amyloid PET-positive asymptomatic participants resulted in approximately 60% cost savings (42, 43). The first therapeutic trial setting that used plasma p-tau as an outcome is in the TRAILBLAZER-ALZ study that demonstrated decreased plasma p-tau217 concentrations paralleling amyloid and tau PET signals associated with donanemab treatment (79). More recently, in the Clarity AD trial studying lecanemab, CSF (Aβ42, t-tau, p-tau181, NGRN, and NfL) and plasma (Aβ42/Aβ40, p-tau181, GFAP, NfL) biomarkers were included as outcome measures. Lecanemab resulted in moderately but significantly less cognitive and functional decline, which was largely matched by similar changes in fluid biomarkers (except NfL) and consistent with the change in amyloid PET burden (80). In other studies, the use of plasma p-tau or Aβ42/Aβ40 as a screening tool to predict longitudinal tau or amyloid PET abnormality changes, compared to PET-only testing, could reduce the required sample size by 43% to 68% or the required number of amyloid PET scans by 62%, respectively (81, 82). Overall, these studies support the idea that blood-based AD biomarkers may be useful to screen individuals with brain amyloidosis prior to more expensive confirmatory PET or invasive CSF AD biomarker evaluations. Recent advances in analytical performance (10), studies of preanalytical variability (6, 7), diagnostic accuracy noninferior to CSF and PET biomarkers, and introduction of reference methodology (e.g., IP-MS) in blood-based AD biomarker assays supports their use in the clinic, therapeutic trials, and research cohort studies. Although greater study cohort breadth, increased numbers of expert laboratories performing plasma biomarker tests, and head-to-head comparison studies of analytical and clinical performance are necessary, the acquired experience from CSF AD biomarker development will ensure rapid validation of relevant blood-based AD biomarkers in several contexts of use.
Conclusion
During the past 20-plus years, development of optimal immunoassays and MS analyses for AD biomarker measurements in human CSF and imaging technologies has clear clinical implications. First, studies of CSF and imaging AD biomarkers characterized their temporal changes over the entire pathological cascade of the disease, providing evidence for AT(N) classification of AD, and have made possible greater accuracy than clinical diagnosis in the definition of the antemortem AD pathologic continuum and discrimination of AD from non-AD dementia. Second, considering barriers to applying universal cut-point values of CSF AD biomarkers for early AD diagnosis, current technical advances provided by fully automated analytical platforms and implementation of reference material and methods have made routine clinical use of CSF biomarkers possible. Third, plasma AD biomarkers are considered to be the next-generation biomarkers for AD, although further validation and replication are necessary, with several advantages compared to CSF biomarkers including ease of access and allowing for repeated testing by circumventing the invasiveness barrier of lumbar punctures. Furthermore, as a screening tool, blood AD biomarkers can reduce several burdens in clinical trials, including cost reduction and enrichment of populations with likely underlying AD pathology. Current efforts for blood AD biomarker development include the promise of fully automated immunoassays, preanalytical standardization, and implementation of reference methodology by head-to-head comparisons with IP-LC-MS/MS assays and, importantly, inclusion of reference materials for calibration standardization and harmonization across methods. Therefore, plasma AD biomarkers are likely to replace CSF AD biomarkers in the future. Finally, further development of new biomarker tests is warranted, including those reflecting AD copathologies (vascular pathology, Lewy bodies, TAR DNA-binding protein 43 pathology, and hippocampal sclerosis). Continued improvements in analytical performance of the plasma tests for amyloid and tau pathology will not only facilitate clinical trials but also optimize drug-developing strategies by integration of biomarkers into clinical trial design, dependent on the goals of trials, as well as use for AD diagnosis in clinical practice. This viewpoint is made more realistic by the great deal of expertise and government support for evaluating test performance in diverse populations that better mirror the population of the world.
Supplementary Material
Research Funding:
J.-H. Kang is supported by grants from the National Research Foundation funded by the Korea government (Ministry of Science, ICT and Future Planning) Medical Research Center program (NRF-2021RIA5A2031612). L.M. Shaw is supported by grants from the NIA (U19 AG024904; P30 AG072979) and The Michael J. Fox Foundation for Parkinson’s Disease Research (Grant 13637.01). T.F. Tropea, NIH-National Institute of Neurological Disorders and Stroke (K23NS114167), Parkinson Foundation-PDGENE Clinical Trial, Michael J. Fox Foundation-Fox BioNet Clinical Trial. E.B. Lee, NIH (U19 AG024904; P30 AG072979). A.A. Chen-Plotkin, Chan Zuckerberg Initiative Neurodegeneration Challenge, NIH-NIA (P30 AG 072979 and U19 AG 062418), AHA/Allen Institute Brain Health Initiative, NIH-National Institute of Neurological Disorders and Stroke (RO1 NS 115139 and RO1 NS 082265). D.J. Irwin is supported by NIH grants to institution. D. Wolk, NIH and Biogen grants to institution. K.A.Q. Cousins, University of Pennsylvania’s Alzheimer’s Disease Research Center Project Development Grant (P30 AG072979) and Alzheimer’s Association (AARF-D-619473, AARF-D-619473-RAPID).
Other Remuneration:
L.M. Shaw has received support for attending meetings and/or travel from Biogen, Fujirebio, Roche and in- kind support for ADNI3 CSF AD biomarkers immunoassay reagents and instrumentation from Roche. T.F. Tropea has received support for attending meetings and/or travel from the Parkinson Study Group. E.B. Lee received support for attending meetings and/or travel from Alzheimer’s Association, Alzheimer’s Association International Conference, and Packard Center Meeting, Johns Hopkins University. A.A. Chen-Plotkin receives royalties from licensing IP surrounding gene targeting for progranulin deficiency from Prevail Therapeutics. D.J. Irwin has received travel support from invited talks for the International Society for Frontotemporal Dementias and the Association for Frontotemporal Degeneration caregiver meeting. D. Wolk, royalties or licenses from UptoDate.
Role of Sponsor:
The funding organizations played no role in the design of study, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Nonstandard Abbreviations:
- AD
Alzheimer disease
- Aβ
amyloid-β
- PET
positron emission tomography
- CSF
cerebrospinal fluid
- Aβ42
amyloid-beta(1–42)
- NIA-AA
National Institute on Aging and the Alzheimer’s Association
- t-tau
total tau
- p-tau
phosphorylated tau
- MS
mass spectrometry
- MCI
mild cognitive impairment
- CRM
Certified Reference Material
- AUC
area under the curve
- ELISA
enzyme-linked immunosorbent assay
- SIMOA
single molecule array
- IP-MS
immunoprecipitation-coupled mass spectrometry
- CU
cognitive unimpaired
- MSD
Meso Scale Diagnostics
- NfL
neurofilament light
- GFAP
glial fibrillary acidic protein
- NGRN
neurogranin
Footnotes
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: T.F. Tropea, Parkinson Study Group. E.B. Lee, Executive Council, American Association of Neuropathologists.
Consultant or Advisory Role: L.M. Shaw, Biogen. T.F. Tropea, participation on a Data Safety Monitoring Board or Advisory Board for Bial Pharmaceuticals. D.J. Irwin, participation on a Data Safety Monitoring Board or Advisory Board for Denali Therapeutics. D. Wolk, Eli Lilly, Qynapse, GE Healthcare, and Neuronix; participation on a Data Safety Monitoring Board or Advisory Board for Functional Neuromodulation.
Stock Ownership: None declared.
Honoraria: L.M. Shaw, Biogen. T.F. Tropea, Parkinson Study Group. E.B. Lee, honoraria for lectures from University of Toronto, University of Michigan, Columbia University; for grant review from the Alzheimer’s Drug Discovery Foundation, Department of Defense, National Institutes of Health (NIH), and Duke University; from The University of Texas at San Antonio for participating in an external advisory board meeting. A.A. Chen-Plotkin, honoraria for publication of papers in special issues from the Journal of Parkinson’s Disease and for lectures given at Mount Sinai (New York, NY) and the Movement Disorders Society International Meeting. D. Wolk, honoraria for Harvard Dementia CME. K.A.Q. Cousins, Weston Foundation for grant review.
Expert Testimony: None declared.
Patents: None declared.
References
- 1.Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010;9:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009;65:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 2013;9:e111–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson O, Mikulskis A, Fagan AM, Teunissen C, Zetterberg H, Vanderstichele H, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: A review. Alzheimers Dement 2018;14:1313–33. [DOI] [PubMed] [Google Scholar]
- 7.Verberk IMW, Misdorp EO, Koelewijn J, Ball AJ, Blennow K, Dage JL, et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer’s disease-related blood-based biomarkers: results from the Standardization of Alzheimer’s Blood Biomarkers (SABB) working group. Alzheimers Dement 2022; 18:1484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement 2016;12: 517–26. [DOI] [PubMed] [Google Scholar]
- 9.Janelidze S, Pannee J, Mikulskis A, Chiao P, Zetterberg H, Blennow K, Hansson O. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol 2017;74:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmqvist S, Stomrud E, Cullen N, Janelidze S, Manuilova E, Jethwa A, et al. An accurate fully automated panel of plasma biomarkers for Alzheimer’s disease. Alzheimers Dement 2023;19:1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuropathology Group. Medical Research Council Cognitive Function and Aging Study. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology group of the medical research council cognitive function and ageing study (MRC CFAS). Lancet 2001;357:169–75. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallin AK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O. CSF Biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 2010;74:1531–7. [DOI] [PubMed] [Google Scholar]
- 14.Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF Biomarker variability in the Alzheimer’s association quality control program. Alzheimers Dement 2013;9: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leinenbach A, Pannee J, Dulffer T, Huber A, Bittner T, Andreasson U, et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin Chem 2014;60:987–94. [DOI] [PubMed] [Google Scholar]
- 16.Pannee J, Gobom J, Shaw LM, Korecka M, Chambers EE, Lame M, et al. Round robin test on quantification of amyloid-beta 1–42 in cerebrospinal fluid by mass spectrometry. Alzheimers Dement 2016;12:55–9. [DOI] [PubMed] [Google Scholar]
- 17.Kuhlmann J, Andreasson U, Pannee J, Bjerke M, Portelius E, Leinenbach A, et al. CSF Abeta1–42—an excellent but complicated Alzheimer’s biomarker—a route to standardisation. Clin Chim Acta 2017;467: 27–33. [DOI] [PubMed] [Google Scholar]
- 18.Boulo S, Kuhlmann J, Andreasson U, Brix B, Venkataraman I, Herbst V, et al. First amyloid beta1–42 certified reference material for re-calibrating commercial immunoassays. Alzheimers Dement 2020;16:1493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjerke M, Andreasson U, Kuhlmann J, Portelius E, Pannee J, Lewczuk P, et al. Assessing the commutability of reference material formats for the harmonization of amyloid-beta measurements. Clin Chem Lab Med 2016;54:1177–91. [DOI] [PubMed] [Google Scholar]
- 20.Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. CSF Biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14:1470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leitao MJ, Silva-Spinola A, Santana I, Olmedo V, Nadal A, Le Bastard N, Baldeiras I. Clinical validation of the Lumipulse G cerebrospinal fluid assays for routine diagnosis of Alzheimer’s disease. Alzheimers Res Ther 2019;11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med 2018;284:643–63. [DOI] [PubMed] [Google Scholar]
- 23.Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease. Brain 2015;138(Pt 3): 772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmqvist S, Zetterberg H, Mattsson N, Johansson P; Alzheimer’s Disease Neuroimaging Initiative; Minthon L, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 2015;85: 1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattsson N, Insel PS, Landau S, Jagust W, Donohue M, Shaw LM, et al. Diagnostic accuracy of CSF Ab42 and florbetapir PET for Alzheimer’s disease. Ann Clin Transl Neurol 2014;1:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 2012;11:669–78. [DOI] [PubMed] [Google Scholar]
- 27.Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol 2022;21:726–34. [DOI] [PubMed] [Google Scholar]
- 28.Niemantsverdriet E, Feyen BFE, Le Bastard N, Martin JJ, Goeman J, De Deyn PP, et al. Added diagnostic value of cerebrospinal fluid biomarkers for differential dementia diagnosis in an autopsy-confirmed cohort. J Alzheimers Dis 2018;63:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeburger JL, Holder DJ, Combrinck M, Joachim C, Laterza O, Tanen M, et al. Cerebrospinal fluid biomarkers distinguish postmortem-confirmed Alzheimer’s disease from other dementias and healthy controls in the OPTIMA cohort. J Alzheimers Dis 2015;44:525–39. [DOI] [PubMed] [Google Scholar]
- 30.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow- up study. Lancet Neurol 2006;5:228–34. [DOI] [PubMed] [Google Scholar]
- 31.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of beta-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 2012;69:98–106. [DOI] [PubMed] [Google Scholar]
- 32.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005– 2010. J Neuropathol Exp Neurol 2012;71: 266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol 2014;71:1282–9. [DOI] [PubMed] [Google Scholar]
- 34.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babulal GM, Quiroz YT, Albensi BC, Arenaza-Urquijo E, Astell AJ, Babiloni C, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement 2019;15: 292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JC, Schindler SE, McCue LM, Moulder KL, Benzinger TLS, Cruchaga C, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol 2019;76:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrett SL, McDaniel D, Obideen M, Trammell AR, Shaw LM, Goldstein FC, Hajjar I. Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Netw Open 2019;2:e1917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajjar I, Yang Z, Okafor M, Liu C, Waligorska T, Goldstein FC, Shaw LM. Association of plasma and cerebrospinal fluid Alzheimer disease biomarkers with race and the role of genetic ancestry, vascular comorbidities, and neighborhood factors. JAMA Netw Open 2022;5: e2235068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindler SE, Cruchaga C, Joseph A, McCue L, Farias FHG, Wilkins CH, et al. African Americans have differences in CSF soluble TREM2 and associated genetic variants. Neurol Genet 2021;7:e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birkenbihl C, Salimi Y, Domingo-Fernandez D, Lovestone S; AddNeuroMed consortium; Fröhlich H, et al. Evaluating the Alzheimer’s disease data landscape. Alzheimers Dement (N Y) 2020;6:e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner MW, Veitch DP, Miller MJ, Aisen PS, Albala B, Beckett LA, et al. Increasing participant diversity in AD research: plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement 2023;19: 307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karikari TK, Ashton NJ, Brinkmalm G, Brum WS, Benedet AL, Montoliu-Gaya L, et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol 2022;18:400–18. [DOI] [PubMed] [Google Scholar]
- 43.Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suarez-Calvet M, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry 2021;26:429–42. [DOI] [PubMed] [Google Scholar]
- 44.Keshavan A, Pannee J, Karikari TK, Rodriguez JL, Ashton NJ, Nicholas JM, et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain 2021; 144:434–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid- beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 2012;69: 824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janelidze S, Stomrud E, Palmqvist S, Zetterberg H, van Westen D, Jeromin A, et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci Rep 2016;6:26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figurski MJ, Waligorska T, Toledo J, Vanderstichele H, Korecka M, Lee VM, et al. Improved protocol for measurement of plasma beta-amyloid in longitudinal evaluation of Alzheimer’s Disease Neuroimaging Initiative study patients. Alzheimers Dement 2012;8:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanon O, Vidal JS, Lehmann S, Bombois S, Allinquant B, Treluyer JM, et al. Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement 2018;14: 858–68. [DOI] [PubMed] [Google Scholar]
- 50.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017;13:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018; 554:249–54. [DOI] [PubMed] [Google Scholar]
- 52.Janelidze S, Palmqvist S, Leuzy A, Stomrud E, Verberk IMW, Zetterberg H, et al. Detecting amyloid positivity in early Alzheimer’s disease using combinations of plasma Abeta42/Abeta40 and p-tau. Alzheimers Dement 2022;18:283–93. [DOI] [PubMed] [Google Scholar]
- 53.De Meyer S, Schaeverbeke JM, Verberk IMW, Gille B, De Schaepdryver M, Luckett ES, et al. Comparison of ELISA- and SIMOA-based quantification of plasma Abeta ratios for early detection of cerebral amyloidosis. Alzheimers Res Ther 2020;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doecke JD, Perez-Grijalba V, Fandos N, Fowler C, Villemagne VL, Masters CL, et al. Total Abeta(42)/Abeta(40) ratio in plasma predicts amyloid-PET status, independent of clinical AD diagnosis. Neurology 2020;94:e1580–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheimers Dis 2015;43:183–91. [DOI] [PubMed] [Google Scholar]
- 56.Wiltfang J, Esselmann H, Bibl M, Hull M, Hampel H, Kessler H, et al. Amyloid beta peptide ratio 42/40 but not A beta 42 correlates with phospho-Tau in patients with low- and high-CSF A beta 40 load. J Neurochem 2007;101:1053–9. [DOI] [PubMed] [Google Scholar]
- 57.Verberk IMW, Slot RE, Verfaillie SCJ, Heijst H, Prins ND, van Berckel BNM, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018;84:648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017;547: 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Telser J, Risch L, Saely CH, Grossmann K, Werner P. P-tau217 in Alzheimer’s disease. Clin Chim Acta 2022;531:100–11. [DOI] [PubMed] [Google Scholar]
- 60.Ashton NJ, Puig-Pijoan A, Mila-Aloma M, Fernandez-Lebrero A, Garcia-Escobar G, Gonzalez-Ortiz F, et al. Plasma and CSF biomarkers in a memory clinic: head-to-head comparison of phosphorylated tau immunoassays. Alzheimers Dement 2022;19:1913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayoumy S, Verberk IMW, den Dulk B, Hussainali Z, Zwan M, van der Flier WM, et al. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther 2021;13:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janelidze S, Bali D, Ashton NJ, Barthelemy NR, Vanbrabant J, Stoops E, et al. Head-to- head comparison of 10 plasma phospho- tau assays in prodromal Alzheimer’s disease. Brain 2023;146:1592–601: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schindler SE, Karikari TK, Ashton NJ, Henson RL, Yarasheski KE, West T, et al. Effect of race on prediction of brain amyloidosis by plasma Abeta42/Abeta40, phosphorylated tau, and neurofilament light. Neurology 2022;99:e245–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barthelemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med 2020;217:e20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karikari TK, Emersic A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimers Dement 2021;17:755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016;87:1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mattsson N, Andreasson U, Zetterberg H, Blennow K; Alzheimer’s Disease Neuroimaging Initiative. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther 2018;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinacker P, Anderl-Straub S, Diehl-Schmid J, Semler E, Uttner I, von Arnim CAF, et al. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 2018;91:e1390–e401. [DOI] [PubMed] [Google Scholar]
- 70.Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019;25:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2019;76:791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the mayo clinic study on aging. JAMA Neurol 2017;74: 1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park JC, Han SH, Yi D, Byun MS, Lee JH, Jang S, et al. Plasma tau/amyloid-beta1– 42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 2019;142:771–86. [DOI] [PubMed] [Google Scholar]
- 74.Chen YH, Lin RR, Huang HF, Xue YY, Tao QQ. Microglial activation, tau pathology, and neurodegeneration biomarkers predict longitudinal cognitive decline in Alzheimer’s disease continuum. Front Aging Neurosci 2022;14:848180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellaver B, Ferrari-Souza JP, Uglione da Ros L, Carter SF, Rodriguez-Vieitez E, Nordberg A, et al. Astrocyte biomarkers in Alzheimer disease: A systematic review and meta-analysis. Neurology 2021;96: e2944–55. [DOI] [PubMed] [Google Scholar]
- 76.Pereira JB, Janelidze S, Smith R, Mattsson-Carlgren N, Palmqvist S, Teunissen CE, et al. Plasma GFAP is an early marker of amyloid-beta but not tau pathology in Alzheimer’s disease. Brain 2021;144:3505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leuzy A, Ashton NJ, Mattsson-Carlgren N, Dodich A, Boccardi M, Corre J, et al. 2020 Update on the clinical validity of cerebrospinal fluid amyloid, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Eur J Nucl Med Mol Imaging 2021;48:2121–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zetterberg H, Bendlin BB. Biomarkers for Alzheimer’s disease-preparing for a new era of disease-modifying therapies. Mol Psychiatry 2021;26:296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. N Engl J Med 2021;384:1691–704. [DOI] [PubMed] [Google Scholar]
- 80.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med 2023;388:9–21. [DOI] [PubMed] [Google Scholar]
- 81.Leuzy A, Smith R, Cullen NC, Strandberg O, Vogel JW, Binette AP, et al. Biomarker-based prediction of longitudinal tau positron emission tomography in Alzheimer disease. JAMA Neurol 2022;79: 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High-precision plasma beta-amyloid 42/ 40 predicts current and future brain amyloidosis. Neurology 2019;93:e1647–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.