Abstract
Chemoresistance is the major cause of cancer recurrence, relapse and eventual death. Doxorubicin resistance is one such challenge in breast cancer. The use of quercetin, an antioxidant, in combination with doxorubicin has been investigated for offering protection to normal cells from the toxic side effects of doxorubicin in addition to modulation of its resistance. The present study aimed to investigate the effects of quercetin in prevention of a doxorubicin-chemoresistant phenotype in both doxorubicin-sensitive and -resistant human MCF-7 breast cancer cell lines. A doxorubicin-resistant MCF-7 cell line was established. The development of resistant cells was closely monitored for changes in morphological features. Sensitivity to doxorubicin and the doxorubicin/quercetin combination was assessed using the tetrazolium assay. To determine the mechanism by which quercetin sensitizes the doxorubicin MCF-7-resistant cell line to doxorubicin, gene expression alterations in breast cancer-related genes were examined using the reverse transcription-quantitative PCR (RT-qPCR) array technology. Resistant MCF cells were successfully developed and the inhibitory concentration (IC50) value of doxorubicin increased from 0.133 to 4 µM (wild-type to resistant). The effects of the quercetin/doxorubicin combination exhibited different effects on wild-type vs. resistant cells. The IC50 of doxorubicin was reduced in wild cells, whereas resistant cells showed an increase in cell viability at lower concentrations and a potentiation of the effects of doxorubicin only at higher concentrations. Annexin V/propidium iodide staining demonstrated that quercetin drives cells into late apoptosis and necrosis, but in resistant cells, necrosis predominates. RT-qPCR results revealed that quercetin led to a reversal in doxorubicin effects via up- and downregulation of important genes such as SNAI2, PLAU and CSF1 genes. Downregulation of cell migration genes, SNAI2 (-31.23-fold) and plasminogen activator, urokinase (PLAU; -30.62-fold), and the apoptotic pathway gene, colony stimulating factor 1 (CSF1; -17.25-fold) were the most important querticin-associated events. Other gene alterations were also observed involving cell cycle arrest and DNA repair pathways. The results of the present study indicated that quercetin could lead to a reversal of doxorubicin resistance in breast cancer cells via downregulation of the expression of important genes, such as SNAI2, PLAU and CSF1. Such findings may represent a potential strategy for reversing breast cancer cell-related chemoresistance.
Keywords: cancer resistance, chemoresistance, doxorubicin, quercetin, breast cancer, MCF7
Introduction
Chemotherapy usually leads to an improvement in tumor cell death and produces a decreases in tumor mass; however, in some patients this therapy fails due to recurrence or even death (1). The response to chemotherapy varies greatly from individual to individual. Some patients can achieve a complete response with just one or two cycles, while others require eight or more cycles (2,3).
Although progress in the treatment of breast cancer has been made, chemoresistance still remains an obstacle to the effective management of all breast cancer types (4). In general, two types of drug resistance exist: i) Intrinsic, which exists before treatment; and ii) acquired, which is generated after chemotherapy. Chemoresistance is the major cause of breast cancer recurrence, relapse and mortality (5). It is caused by different mechanisms, such as drug efflux pumps, dysregulation of apoptosis and cancer stem cells (6) and genetic mutations and/or epigenetic changes (5). The two main mechanisms of chemoresistance are drug efflux and DNA repair, both of which epigenetically regulated (7).
Doxorubicin is one of the most effective chemotherapeutic drugs used for treatment of breast cancer (7-9). It is a naturally occurring anthracycline antibiotic (10). One major cause of treatment failure with doxorubicin is the development of drug resistance and even tumor growth (11) that leads to poor prognosis and survival (12-14). Doxorubicin resistance is still an unresolved issue in treatment of patients with breast cancer despite investigations into several doxorubicin resistance mechanisms (10). Unfortunately, doxorubicin has acute adverse effects that occur within 2 to 3 days of administration and include nausea, vomiting, neutropenia, alopecia and/or arrhythmias (15). Moreover, doxorubicin leads to an increase in the risk of clinical cardiotoxicity by 5.43-fold and the risk of heart attack by 4.94-fold when compared with other non-anthracycline regiments (16). The emergence of drug resistance and life-threatening cardiac injury after doxorubicin treatment has led to limitations for successful cancer treatment (17). Overcoming doxorubicin resistance and minimizing its toxic cardiac and kidney effects would represent a major improvement in the effective management of breast cancer (18).
Quercetin is a polyphenolic compound that is found in abundance in a number of fruits, vegetables and plants and exerts various biological effects, such as antioxidant, antiviral, anticancer, cell cycle modulatory and anti-angiogenesis effects (19). It has been reported that quercetin is effective in inhibiting cancer cell growth via the induction of apoptosis (20,21). In addition, quercetin has been reported to upregulate the expression of estrogen receptors α and β.
The overall outcome on cell fate is reflected by inhibition of cell proliferation, cell cycle arrest in the G1 phase and reduction in cell migratory potential due to actin cytoskeleton disorganization (22). To produce an improvement in antitumor efficacy and a reduction in chemotherapeutic chemoresistance, the natural flavonoid quercetin has been found to have antitumor potential and synergistic effects when combined with doxorubicin (23). The safety of quercetin makes it an attractive candidate for producing a reduction in cardiotoxicity attributed to anticancer drugs, such as doxorubicin (24). The effects of addition of quercetin to doxorubicin has been previously reported, and multiple mechanisms accounting for such effects have been suggested (24-26). Its use in combination with doxorubicin in resistant cell lines and the exploration of possible mechanisms of enhancing doxorubicin toxicity toward cancer cells has yet to be explored.
The aim of the present study was to investigate the effects of quercetin treatment in prevention of a doxorubicin-chemoresistant phenotype in both doxorubicin-sensitive and -resistant human MCF-7 breast cancer cell lines.
Materials and methods
Cell culture growth conditions
A wild-type MCF-7 cell line. MCF-7 breast cancer cell line was originally obtained from the American Cell Culture Collection (cat. no. HTB-22). The starting passage numbers was five. Cells were grown as an attached monolayer culture in the commercially defined RPMI 1640 medium (Euroclone SpA), supplemented with 10-20% (v/v) heat-inactivated fetal bovine serum (FBS; Euroclone SpA), 1% 2 mM L-glutamine (Euroclone SpA), 1% 100X penicillin-streptomycin concentration (Euroclone SpA) and 0.2% 10 mg/ml gentamicin concentration (Euroclone SpA). The cells were grown in 75 or 25 cm2 filter-cap culture flasks (SPL life Sciences). The cells were then incubated at 37˚C in a 90% humidified atmosphere of 5% CO2 and maintained in a tissue culture incubator (BINDER GmbH).
Establishment of doxorubicin resistant sub-line
Doxorubicin 2 mg/ml (Fresenius SE & Co.) was used to induce resistance. A freshly prepared stock solution consisted of 100 µM and was stored at 4˚C. The drug was further diluted with cell culture medium to a concentration of 6.65 nM and added to the cell media.
First, 6.65 nM of doxorubicin was added to the MCF-7 cells, and when the cells reached appropriate confluency at a certain concentration, they were passaged and doxorubicin concentration was increased by 0.05 nM over the previous one. This process took ~3 months as growth of the cells usually has a lag period to allow them to adapt to the higher concentration. To exclude the effects associated with long term culture of MCF-7 wild type cells, the wild-type cells were cultured under identical conditions and maintained in culture for the same period as the resistant cells but without doxorubicin addition.
Cell viability and proliferation assays for the treatment of MCF-7 with doxorubicin and quercetin
For wild-type cell lines, 5,000 MCF-7 cells from the appropriate cell line were seeded per well in a 96-well plate. After 24 h, six different concentrations of doxorubicin (100, 10, 1.0, 0.1, 0.01 or 0.001 µM) were added to the final volume of 200 µl per well of culture RPMI 1640 medium. For the resistant cell lines, 7,000 MCF-7 cells from the appropriate cell line were seeded per well in a 96-well plate. After 24 h, complete growth media was used for doxorubicin dilution, and using a serial dilution technique, 16 different concentrations of doxorubicin were prepared starting at 500 and decreasing each subsequent concentration by 50% until a concentration of 0.015 µM was reached and added to the final volume of 200 µl per well of culture media. A stock solution of quercetin (Sigma-Aldrich; Merck KGaA) was prepared [50 nM in dimethylsulfoxide (DMSO); Euroclone SpA]. Complete growth media was used for quercetin dilution and nine different concentrations of quercetin were prepared (100, 50, 25, 12.5, 6.25, 3.125, 1.652, 0.826 and 0.413 µM). For the combination (both for wild and resistant cell lines), the same concentrations of doxorubicin were mixed with 20 µM of quercetin for MCF-7 cell line.
The antiproliferative effects of doxorubicin on MCF7/WT and MCF7/D53.2 cells were analyzed using the tetrazolium MTT dye (Sigma-Aldrich; Merck KGaA). This test is based on the reduction of MTT, a yellow tetrazole, to a purple formazan, a process that occurs in the mitochondria of viable cells. A total of 10 ml of MTT solution were prepared by weighing 50 mg of MTT dye dissolved in 10 ml of phosphate-buffered saline (PBS) 1X (Euroclone SpA).
After 72 h of incubation, media were aspirated from the cells containing the drugs and replaced with fresh media (100 µl/well) and incubated for 30 min after which 10-13 µl of the MTT dye solution was added to each well. The plates were returned to the incubator at 37˚C for 4 h after which 100 µl of DMSO (Euroclone SpA) was added to each well. Optical density (OD) at 570 nm wavelength was recorded using a 96-well plate reader (Biotek instruments, Inc.) after 20 min of shaking on the plate shaker (cat. no. 130,000; Boekel Scientific). The percentage of living cells (% living) was calculated by dividing the absorbance of viable cells per well over the mean absorbance of the viable cells in control wells. The mean percentage per concentration was then calculated as mean ± standard deviation (SD). Measurements were performed in triplicate.
Results of the MTT cell proliferation assay were evaluated using GraphPad Prism 8 software (GraphPad Software, Inc.). The inhibitory concentration (IC50), which is the drug concentration at which 50% of cells are viable, was calculated from the logarithmic trend line of the cytotoxicity graphs for each drug alone and in combination. The degree of resistance was estimated in terms of resistance index (R), which is calculated according to the equation: R=IC50 resistant cells/IC50 sensitive cells.
Phase contrast microscopy
Flasks for control wild-type MCF-7 cells and for each concentration was reached for resistant MCF-7 cells were observed under EVOS XL core imaging system (Thermo Fisher Scientific, Inc.) at magnifications of 4, 10 and 20X and images were captured.
RNA isolation
Next, 75 cm2 flasks were seeded with 7x106 MCF-7 cells. Treatment was started with 5 µM doxorubicin or the combination of 5 µM doxorubicin with 20 µM quercetin. One flask was allotted as control. RNA was extracted from each individual cell pellet using RNeasy® Plus Mini kit (Qiagen GmbH) following manufacturer's instructions.
Then RNA concentration and purity were measured using a nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). Furthermore, the quality of RNA was visualized under ultraviolet light after performing gel electrophoresis.
cDNA synthesis
The first strand of cDNA was prepared using the RT2 Profiler PCR Array kit (Qiagen GmbH). According to the manufacturer's instructions, aliquots containing 1 µg of total RNA were used from each sample.
Gene expression profiling
Pathway-focused gene expression profiling was performed using a 96-well human cancer drug resistance and metabolism PCR array, RT2 Profiler PCR array (PAHS-131ZD-12), Human Cancer Drug Resistance & Metabolism PCR Array (Qiagen, Inc.). In this array, 84 wells contained all components required for the PCR reaction in addition to a primer for a single gene in each well. A total of five housekeeping genes (ACTB, B2M, GAPDH, HPRT1 and RPLP0) were used as a control for normalization. Notably, Qiagen provide the array with their design primers and they do not provide the sequence of such primers. These particular genes are involved in tumor classification, signal transduction, DNA repair and other commonly affected pathways such as the cell cycle, angiogenesis, apoptosis adhesion and proteolysis. A diluted cDNA, equivalent to 1 µg RNA for each plate, was mixed with the RT2 SYBR® green master mix (Qiagen, Inc.) according to the manufacturer's instructions, and loaded into the 96-well array. Reverse transcription-quantitative (RT-qPCR) reaction was performed using the iCycler (Bio-Rad Laboratories, Inc.) machine by heating the plate to 95˚C for 10 min followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min.
The cycle threshold (Cq) values for each sample were given by the iCycler. The threshold value was manually set on 0.01 as recommended by the PCR array user manual. The analysis was performed automatically according to the SA Biosciences company (Qiagen, Inc.) web portal (https://geneglobe.qiagen.com/jo/analyze), and expression levels were expressed as a fold-increase or decrease (27). The data were normalized across the plates to the following housekeeping genes were used: i) Actin β; ii) β-2-microglobulin, glyceraldehyde-3-phosphate dehydrogenase; iii) hypoxanthine phosphoribosyltransferase 1; and iv) ribosomal protein large, P0.
Gene functional annotation
The Gene Ontology (GO; https://david.ncifcrf.gov/tools.jsp) and pathway database for genes whose expression levels changed by 2-fold was investigated using the web-based application DAVID (http://david.abcc.ncifcrf.gov), which provides for ontology and pathway mapping, annotation, and visualization of results. Results appear in tables that describe the most affected function and the pathway based on genes changes.
Apoptosis analysis
Apoptosis events induced by anticancer drug treatment in MCF-7 wild-type and resistant cell lines treated with doxorubicin alone or in combination with quercetin in addition to no treatment were investigated. The mechanism of cell death was determined by Annexin V/propidium iodide (PI) staining using flow cytometry. MCF-7 wild-type and resistant cells were seeded into six-well plates and incubated overnight at 37˚C. Wild-type MCF-7 cells were exposed to 1.3 µM doxorubicin alone or to a combination of 1.3 µM doxorubicin combined with 20 µM of quercetin. Resistant cells were exposed to 4 µM doxorubicin or to a combination of 4 µM doxorubicin combined with 20 µM of quercetin. For both cell types, control cells remained untreated. After incubation for 72 h, media were removed, and cell cultures were washed two times with PBS. Cells were collected into flow tubes using Accutase solution (800 µl on each well at room temperature for 10 min). After this step, an Annexin V/PI apoptosis kit was used to stain the cell pellets following the kit's instructions. The apoptosis assay was carried out using the annexin v assay (Molecular Probes; Thermo Fisher Scientific, Inc.), and cell death (either apoptosis or necrosis) was analyzed by FACS Diva7, a fluorescein-activated sorter (FACS) Canto II version 3 (BD Biosciences). Flow experiments was performed using single color staining.
The results from the apoptosis assay groups were tested for significance using a two-way analysis of variance (ANOVA) followed by a Šidák multiple comparison test to identify the statistical significance using GraphPad Prism. Data was presented as mean ± standard deviation and the experiments were repeated three times. P<0.05 was considered to indicate a statistically significant difference. Fig. 1 presents the study flowchart.
Figure 1.
Study flowchart.
Results
Determination of IC50 value of doxorubicin alone and combination in wild-type and resistant MCF-7 breast cancer cell lines
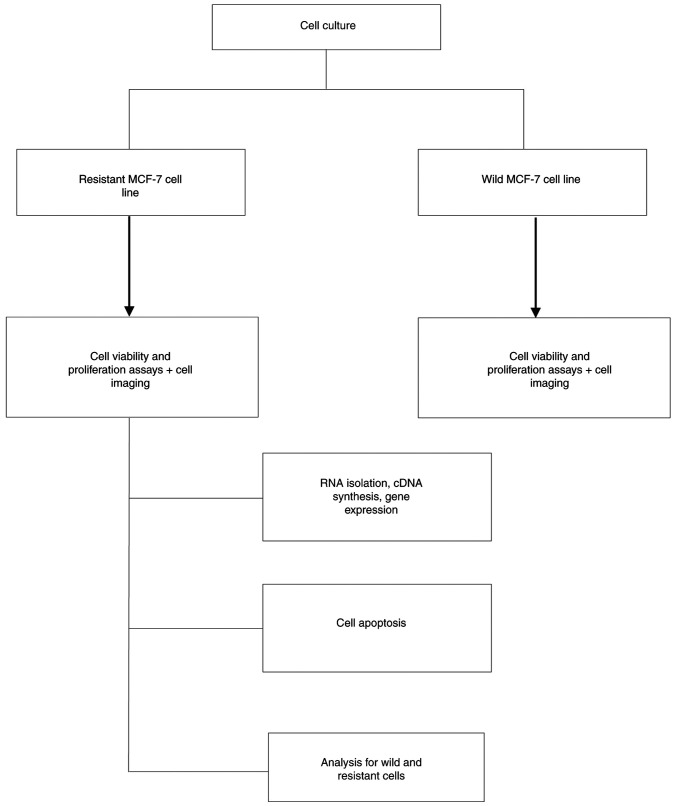
The wild MCF-7 cell viability was determined using the MTT assay as is presented in Fig. 2. The IC50 values for doxorubicin only and doxorubicin with 10 µM quercetin combination were 0.133 and 0.114 µM, respectively. Treatment with doxorubicin with 10 µM quercetin at 0.01, 0.001 and 0.0001 µM only yielded significant inhibition of the growth of MCF-7 cells when compared with the growth of cells treated with higher doxorubicin concentrations. Overall, these findings indicated that quercetin had a potentiation effect toward doxorubicin against the MCF7 breast cancer cells.
Figure 2.
Antiproliferative activity of DOX and DOX/quercetin combination against wild MCF-7. The data represent the viable percentile at each concentration of doxorubicin and DOX/quercetin. (A) DOX and 10 µM quercetin combination; (B) DOX/quercetin combination. Results present the mean ± standard deviation of at least 3 replicates. **P<0.01 and ****P<0.0001. DOX, doxorubicin.
Development and characterization of the MCF-7 resistant cells. The doxorubicin-resistant MCF-7 cell line was established and examined at two different doxorubicin concentrations: i) A final concentration of 26.6 nM; and ii) a final concentration of 53.2 nM.
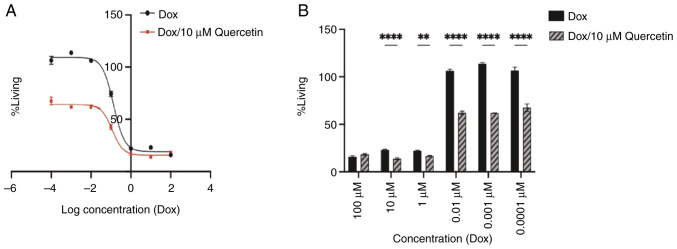
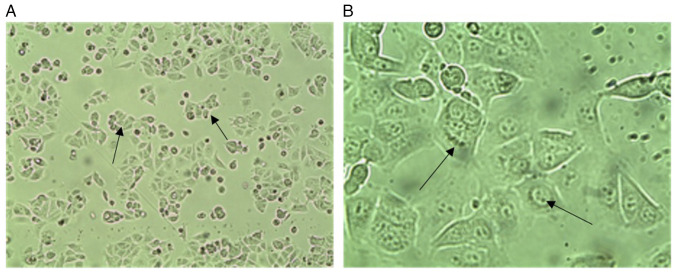
Morphological features. Fig. 3 presents phase contrast images of wild MCF-7 cells, which present epithelial-like morphology of small, spindle-shaped cells with a single nucleus. Cells grow in colonies, tightly packed and uniform in size. While confocal microscopy images of DAPI-staining are presented in Fig. 4 for wild untreated and 1.3 µM doxorubicin treated cells. It represent how the doxorubicin accumulates within the nuclease of the cells in the treated cells.
Figure 3.
Phase-contrast images of the wild-type MCF-7 cell line. The arrows point to cells that have an epithelial-like morphology; small, spindle-shaped cells with single nucleus (A) at a magnification of 10X (B) and at a magnification of 20X.
Figure 4.
Confocal images of wild-type MCF-7 cells. (A) Control wild-type MCF-7 cell line. (B) 1.3 µM DOX treated wild-type MCF-7 cell line, cell nuclei (blue) stained by DAPI. free DOX diffuses through the cytoplasm. Scale bar, 10 µm. DOX, doxorubicin.
Sensitivity to doxorubicin
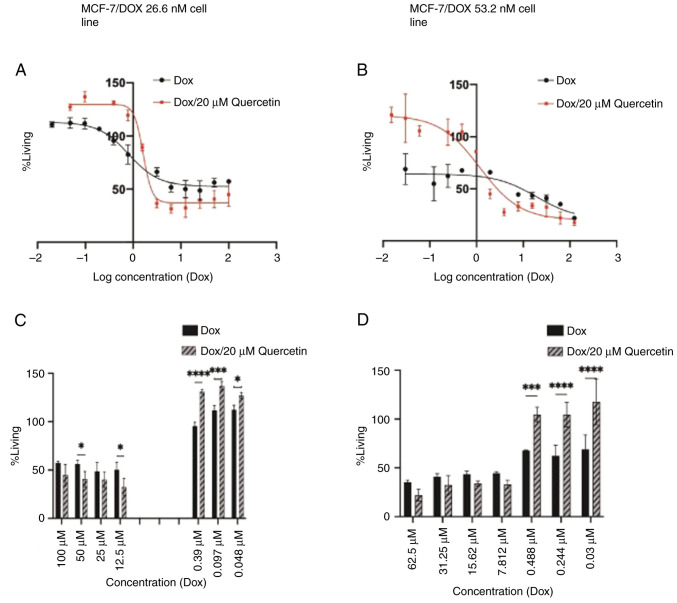
As presented in Fig. 5 the IC50 values of doxorubicin for resistant MCF-7 cell line in addition to the resistance index were calculated and are shown in Table I.
Figure 5.
Antiproliferative activity of doxorubicin and Dox/quercetin combination against resistant MCF-7 cells. (A and B) Doxorubicin and 20 µM quercetin combination. (C and D) Doxorubicin/quercetin combination and differences. Results present the mean ± standard deviation of at least 3 replicates. *P<0.05, ***P<0.001, ****P<0.0001. Dox, doxorubicin.
Table I.
IC50 and R for wild and resistant MCF-7 cell lines.
| Cell line | DOX only, µM | R | DOX/20 µM quercetin, µM |
|---|---|---|---|
| MCF-7 wild | 0.133 | 0.114 | |
| MCF-7/DOX 26.6 nM | 0.800 | 6.01 | 1.620 |
| MCF-7/DOX 53.2 nM | 4.000 | 30.0 | 1.290 |
DOX, Doxorubicin; R, resistance index.
Although the IC50 of the doxorubicin and 20 µM quercetin combination was higher compared with doxorubicin alone in the MCF-7/DOX 26.6 nM group, it was observed that quercetin enhanced anticancer activity when added to higher doxorubicin concentrations as is presented in Fig. 5. The IC50 value of the MCF-7/DOX 53.2 nM group was 4.0, µM, which is 30-fold higher compared with the sensitive parent cell line. Treatment with quercetin in the MCF-7/DOX 53.2 nM group led to a significant decrease in the doxorubicin IC50 value and resistance fold value from 30- to 9-fold. These findings revealed that quercetin had significant doxorubicin re-sensitizing effects.
Reversal of doxorubicin resistance with verapamil was performed to further investigate the inhibition of P-glycoprotein (P-gp) by verapamil as a standard drug after which proliferation of the cells was compared to the resistant MCF-7 cells with MCF-7 cells in combination (doxorubicin with 10 µM verapamil) as presented in Fig. 6. This figure demonstrates how the verapamil forces doxorubicin outside the cells and therefore reduces the concentration that can reach the nucleus.
Figure 6.
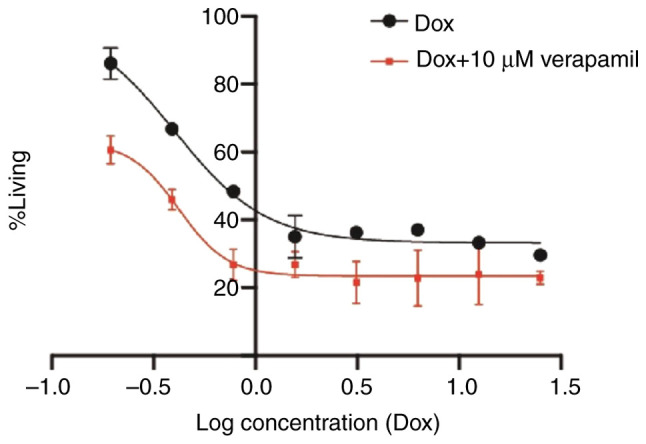
Logarithmic curves for the response of resistant MCF-7 cells to resistant MCF-7 in combination of doxorubicin/10 µM verapamil. The effects of inhibition of P-glycoprotein and partial reversal of doxorubicin resistance. Results present the mean ± standard deviation of at least three wells and the experiment was repeated using 2 different passages. Dox, doxorubicin.
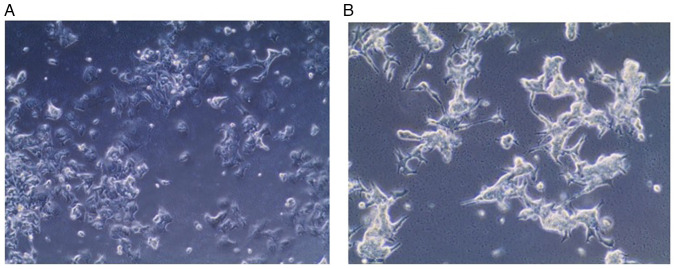
Morphological features. In comparison with parent wild type cells, the resistant MCF-7 cells appeared to be larger, irregular and had a satellite-like shape with multiple nuclei and multiple vesicles and stronger adhesion to the underlying surface (Fig. 7).
Figure 7.
Phase-contrast photography of resistant MCF-7 cell line. Cells appear large, irregular, rounded and have multiple vesicles, and images are presented (A) at a magnification of 10X and (B) at a magnification of 20X.
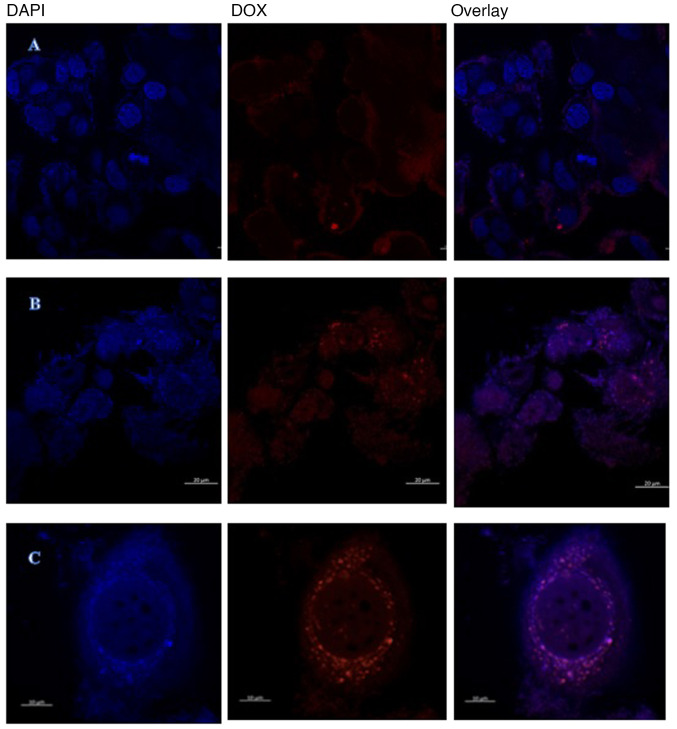
Confocal imaging in Fig. 8 was used to investigate intracellular doxorubicin accumulation in resistant MCF-7/DOX 53.2 nM cells. The cells were incubated with 400 µM of doxorubicin for 4 h, it was also viewed at 0 h as a control. This demonstrated that control resistant MCF-7/DOX 26.6 nM cells show a low level of fluorescence with a narrow doxorubicin intracellular distribution, while in 400 µM treated doxorubicin group the free doxorubicin surrounded the nucleus. These figures collectively indicated that the main resistant mechanism exemplified in effluxing doxorubicin toward the cytoplasm and to engulf them in special type of vesicles.
Figure 8.
Confocal images of resistant MCF-7. (A) Confocal imaging of control resistant MCF-7/DOX 26.6 nM cell line. (B) Confocal imaging of treated resistant MCF-7 cell line, the free DOX surrounding the nucleus and also presented at (C) a higher magnification of image B. Magnification, 20X. DOX, doxorubicin.
Apoptosis analysis
To determine whether the effects of quercetin on wild-type and resistant MCF-7 cancer cell proliferation were related to apoptosis, flow cytometry using Annexin V/PI staining was performed. Wild-type MCF-7 cells were treated with 1.3 µM doxorubicin only or a combination of 1.3 µM doxorubicin and 20 µM quercetin, and resistant MCF-7 were treated with 4 µM doxorubicin or a combination of 4 µM doxorubicin and 20 µM quercetin for 72 h.
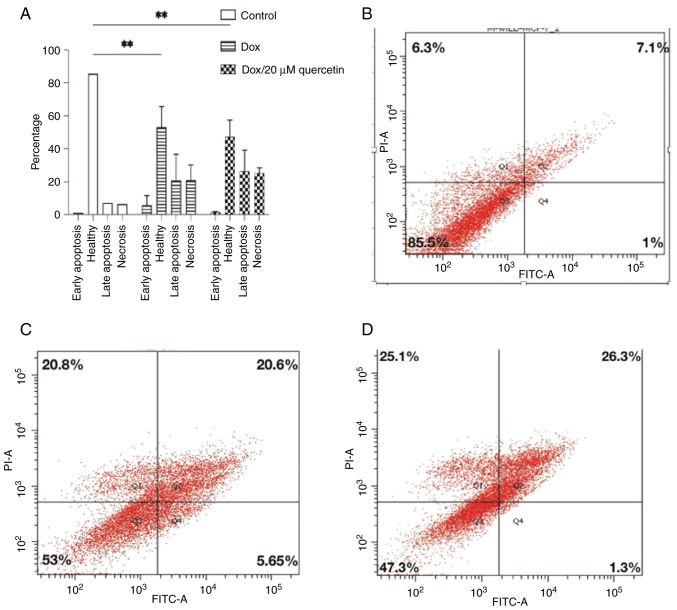
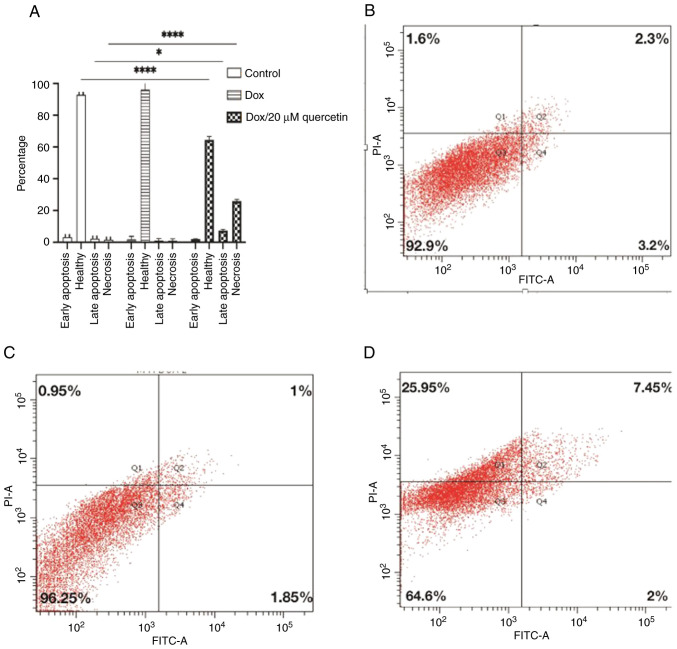
The cells were sorted according to annexin V and PI status into early apoptotic Q4, late apoptotic Q2, necrotic Q1 and viable cells Q3, and results are presented in Figs. 9 and 10. In wild-type MCF-7 cells as shown in Fig. 9, the late apoptosis rate increased from 7.1% in control cells to 20.6 and 26.3% after treatment corresponding to doxorubicin or the doxorubicin and quercetin combination, respectively. Wild-type MCF-7 cells treated with 1.3/20 µM Dox/quercetin combination had the highest apoptosis rates. Similarly, the percentage of necrotic cells increased from 6.3% in control cells to 20.8 and 25.1% after treatment corresponding to doxorubicin only or the doxorubicin and quercetin combination.
Figure 9.
Annexin V-FITC PI binding assay for evaluation of apoptosis for wild MCF-7. (A) The bar chart represents the percentages of the apoptosis assays. (B-D) Cellular death modality by flow cytometry of (B) control wild MCF-7 cells, (C) wild-type MCF-7 cells treated with 1.3 µM Dox and (D) wild-type MCF-7 cells treated with 1.3/20 µM Dox/quercetin combination that had the highest apoptosis rates. Results present the mean ± standard deviation of at least 2 replicates. **P<0.01. PI, propidium iodide; Dox, doxorubicin.
Figure 10.
Annexin V-FITC PI binding assay for the evaluation of apoptosis for resistant MCF-7 cells. (A) Percentages of apoptosis assays. Cellular death modality by flow cytometry of (B) control resistant MCF-7 cells, (C) resistant MCF-7 cells treated with Dox and (D) resistant MCF-7 cells treated with Dox/quercetin combination. Results present the mean ± standard deviation of at least 2 replicates. *P<0.05, ****P<0.0001. PI, propidium iodide; Dox, doxorubicin.
In resistant MCF-7 cells (Fig. 10), the effects of quercetin on the induction of cell apoptosis in breast cancer cells was also measured. It was found that the late apoptosis rate changed from 2.3% in control cells to 1% or 7.25% after treatment corresponding to doxorubicin only or to the doxorubicin and quercetin combination, respectively. By contrast, the percentage of necrotic cells changed from 1.6% in control cells to 0.95% or 25.95% after treatment corresponding to doxorubicin only or the doxorubicin and quercetin combination, respectively. Collectively, the apoptosis assay findings indicated that the mechanism of killing for doxorubicin differs in the presence of quercetin, especially in the resistant MCF-7 cells.
Gene expression changes in the resistant MCF-7 cell line
A gene expression profile analysis was performed using the PCR array and a standard 2-fold change in expression was used as the cut-off. The effects of 5 µM of doxorubicin on the expression of the breast cancer genes were tested against MCF/DOX, such treatment was called Track (1). While the combination of 5 µM dox and 20 µM quercetin were named Track (2). Analysis of track (1) revealed that 52 genes out of the 84 genes were modulated, 41 of these were upregulated, and 11 genes were downregulated (Tables SI and SII).
In the Track 2 analysis, it was revealed that in 46 genes out of the total 84 genes that were examined, 35 were upregulated and 11 genes were downregulated (Tables SIII and SIV). The difference between the fold regulation between Tracks (1) and (2) was calculated as shown in Tables SV and SVI using a standard of 5-fold change as the cut-off.
Gene ontology and pathways analysis
Gene Ontology (GO) categories for both up- and downregulated genes for Track (1) are shown in Tables II and III, respectively. The data indicated that the selection for doxorubicin resistance led to changes in gene expression, mainly in upregulated genes, and consisted of ‘protein binding’, ‘negative regulation of cell proliferation’, ‘positive regulation of transcription from RNA polymerase II promoter’, ‘negative regulation of apoptotic process’ and ‘positive regulation of cell migration’. In downregulated genes, it was found that ‘positive regulation of transcription from RNA polymerase II promoter’, ‘positive regulation of transcription, DNA-templated’ and ‘positive regulation of apoptotic process’ were affected. The most important pathways in which the up- and the downregulated genes participated are presented in Tables IV and V, respectively. In the downregulated side, ‘pathways in cancer’, ‘microRNAs in cancer’, ‘p53 signaling pathway’, ‘cell cycle’ and ‘signaling pathway’ are the main pathways and ‘pathways in cancer’ is the only pathway in the downregulated side.
Table II.
Gene Ontology groups for upregulated genes (≥2-fold) in (MCF-7/DOX 53.2 nM).
| Category | Count | Genes |
|---|---|---|
| Protein binding | 37 | CDKN1C, CDKN1A, NOTCH1, CSF1, GSTP1, SERPINE1, PTEN, TWIST1, NR3C1, GLI1, PTGS2, MAPK8, CCND2, PLAU, CDH1, ADAM23, SFN, SLIT2, JUN, TGFB1, CDKN2A, MMP2, KRT5, IGF1, HIC1, ESR2, NME1, VEGFA, CCNA1, SFRP1, IL6, APC, CCNE1, BCL2, CTNNB1, SNAI2, ABCG2 |
| Negative regulation of cell proliferation | 14 | CDKN1A, JUN, TGFB1, NOTCH1, CDKN2A, PTEN, PTGS2, NME1, IL6, SFRP1, APC, CDH13, CTNNB1, RARB |
| Positive regulation of transcription from RNA polymerase II promoter | 14 | JUN, TGFB1, NOTCH1, CDKN2A, SERPINE1, TWIST1, IGF1, GLI1, NR3C1, VEGFA, IL6, CDH13, CTNNB1, RARB |
| Positive regulation of transcription, DNA- templated | 13 | CDKN1C, JUN, TGFB1, NOTCH1, CDKN2A, IGF1, GLI1, ESR2, IL6, SFRP1, CCNE1, CDH1, CTNNB1 |
| Negative regulation of apoptotic process | 12 | IL6, CDKN1A, SFRP1, MAPK8, CCND2, GSTP1, PTEN, BCL2, RARB, TWIST1, IGF1, VEGFA |
| Positive regulation of cell proliferation | 12 | IL6, SFRP1, TGFB1, NOTCH1, CCND2, CSF1, PTEN, BCL2, RARB, IGF1, GLI1, VEGFA |
| Positive regulation of cell migration | 10 | TGFB1, NOTCH1, APC, CSF1, PLAU, CDH13, SNAI2, IGF1, GLI1, VEGFA |
| Negative regulation of transcription from RNA polymerase II promoter | 10 | CDKN1C, TGFB1, NOTCH1, CTNNB1, RARB, TWIST1, SNAI2, HIC1, ESR2, VEGFA |
DOX, doxorubicin.
Table III.
Gene ontology groups for downregulated genes (≥2-fold) in (MCF-7/DOX 53.2 nM).
| Category | Count | Genes |
|---|---|---|
| Positive regulation of transcription from RNA polymerase II promoter | 7 | RB1, AR, XBP1, PGR, BRCA1, ESR1, MAPK3 |
| Transcription, DNA-templated | 7 | RB1, AR, BIRC5, PGR, BRCA1, ESR1, MAPK3 |
| Positive regulation of transcription, DNA-templated | 6 | RB1, AR, BRCA1, BRCA2, ESR1, MAPK3 |
| Regulation of apoptotic process | 3 | BIRC5, BRCA1, ESR1 |
DOX, doxorubicin.
Table IV.
Pathway analysis for upregulated genes in (MCF-7/DOX 53.2 nM).
| Category | Count | Genes |
|---|---|---|
| Pathways in cancer | 19 | CDKN1A, JUN, TGFB1, CDKN2A, GSTP1, MMP2, PTEN, IGF1, GLI1, PTGS2, VEGFA, IL6, MAPK8, APC, CCNE1, CDH1, BCL2, CTNNB1, RARB |
| MicroRNAs in cancer | 11 | CDKN1A, NOTCH1, CCND2, APC, PLAU, CCNE1, CDKN2A, PTEN, BCL2, PTGS2, VEGFA |
| p53 signaling pathway | 8 | CDKN1A, CCND2, CCNE1, CDKN2A, SERPINE1, PTEN, SFN, IGF1 |
| Cell cycle | 8 | CDKN1C, CCNA1, CDKN1A, TGFB1, CCND2, CCNE1, CDKN2A, SFN |
| Signaling pathway | 7 | IL6, CDKN1A, MAPK8, TGFB1, CCND2, PTEN, IGF1 |
DOX, doxorubicin.
Table V.
Pathway analysis for downregulated genes in (MCF-7/DOX 53.2 nM).
| Category | Count | Genes |
|---|---|---|
| Pathways in cancer | 5 | RB1, AR, BIRC5, BRCA2, MAPK3 |
DOX, doxorubicin.
In Track (2) the genes that were upregulated 2-fold were functionally clustered into main categories consisting of ‘negative regulation of cell proliferation’, ‘negative regulation of apoptotic process’, ‘cell cycle arrest’ and ‘regulation of cell cycle’, as shown in Table VI. In downregulated genes, it was found that ‘positive regulation of cell migration’, ‘positive regulation of apoptotic process’, ‘negative regulation of transcription from RNA polymerase II promoter’ and negative regulation of cell proliferation’ were affected, as shown in Table VII. The main pathways in up- and the downregulated genes are presented in Tables VIII and IX, respectively. In the upregulated side, those pathways are ‘pathways in cancer, ‘microRNAs in cancer’, ‘p53 signaling pathway’ and ‘cell cycle’. In the downregulated side, they are ‘microRNAs in cancer’ and ‘pathways in cancer’.
Table VI.
Gene Ontology groups for upregulated genes (≥2-fold) in (MCF-7/DOX 53.2 nM) [Track (2)].
| Category | Count | Genes |
|---|---|---|
| Negative regulation of cell proliferation | 11 | IL6, CDKN1A, SFRP1, JUN, APC, CDKN2A, PTEN, CDH13, RARB, PTGS2, NME1 |
| Negative regulation of apoptotic process | 10 | IL6, CDKN1A, SFRP1, MAPK8, CCND2, GSTP1, PTEN, BCL2, RARB, IGF1 |
| Positive regulation of apoptotic process | 9 | IL6, SFRP1, MAPK8, APC, CDKN2A, RARB, ATM, SLIT2, PTGS2 |
| Positive regulation of cell proliferation | 9 | IL6, SFRP1, CCND2, CSF1, PTEN, BCL2, RARB, IGF1, GLI1 |
| Cell cycle arrest | 5 | CDKN1C, CDKN1A, APC, CDKN2A, ATM |
| Regulation of cell cycle | 4 | JUN, CCNE1, PTEN, ATM |
| DNA damage response, signal transduction by p53 class mediator resulting in cell cycle arrest | 3 | CDKN1A, ATM, SFN |
DOX, doxorubicin.
Table VII.
Gene Ontology groups for downregulated genes (≥2-fold) in (MCF-7/DOX 53.2 nM) [Track(2)].
| Category | Count | Genes |
|---|---|---|
| Positive regulation of cell migration | 5 | APC, CSF1, PLAU, CDH13, SNAI2 |
| Positive regulation of apoptotic process | 4 | APC, RARB, SLIT2, PTGS2 |
| Negative regulation of cell proliferation | 4 | APC, CDH13, RARB, PTGS2 |
| Negative regulation of transcription from RNA polymerase II promoter | 4 | RARB, SNAI2, HIC1, ESR2 |
| Transcription, DNA-templated | 4 | RARB, SNAI2, HIC1, ESR2 |
DOX, doxorubicin.
Table VIII.
Pathway analysis for upregulated genes in (MCF-7/DOX 53.2 nM) [Track(2)].
| Category | Count | Genes |
|---|---|---|
| Pathways in cancer | 16 | CDKN1A, JUN, CDKN2A, GSTP1, MMP2, PTEN, IGF1, PTGS2, GLI1, IL6, MAPK8, APC, CCNE1, CDH1, BCL2, RARB |
| MicroRNAs in cancer | 10 | CDKN1A, CCND2, APC, PLAU, CCNE1, CDKN2A, PTEN, BCL2, ATM, PTGS2 |
| p53 signaling pathway | 9 | CDKN1A, CCND2, CCNE1, CDKN2A, SERPINE1, PTEN, ATM, SFN, IGF1 |
| Cell cycle | 8 | CDKN1C, CCNA1, CDKN1A, CCND2, CCNE1, CDKN2A, ATM, SFN |
DOX, doxorubicin.
Table IX.
Pathway analysis for downregulated genes in (MCF-7/DOX 53.2 nM) [Track(2)].
| Category | Count | Genes |
|---|---|---|
| MicroRNAs in cancer | 3 | APC, PLAU, PTGS2 |
| Pathways in cancer | 3 | APC, RARB, PTGS2 |
DOX, doxorubicin.
In terms of the difference between Tracks (1) and (2) genes that were upregulated ≥5-fold, the main functional groups included those involved in ‘negative regulation of apoptotic process’, ‘positive regulation of cell proliferation’, ‘positive regulation of transcription, DNA-templated’, ‘cellular response to tumor necrosis factor’ and ‘DNA replication’ (Table X). In downregulated genes, these groups included ‘positive regulation of cell migration’, ‘positive regulation of apoptotic process’, ‘negative regulation of cell proliferation’ and ‘transcription, DNA-templated’ (Table XI). The main pathways in up- and downregulated genes are presented in Tables XII and XIII, respectively. ‘Pathways in cancer’, ‘p53 signaling pathway’, ‘signaling pathway’ and ‘cell cycle’ are the main pathway on the upregulated side and ‘microRNAs in cancer’ and ‘pathways in cancer’ on the downregulated side.
Table X.
Gene Ontology groups for upregulated genes (≥5-fold) in the difference between Track (1) and Track (2).
| Category | Count | Genes |
|---|---|---|
| Negative regulation of apoptotic process | 6 | IL6, SFRP1, CCND2, GSTP1, BIRC5, IGF1 |
| Positive regulation of cell proliferation | 5 | IL6, SFRP1, CCND2, BIRC5, IGF1 |
| Positive regulation of transcription, DNA-templated | 5 | IL6, SFRP1, CDKN2A, BRCA1, IGF1 |
| Positive regulation of apoptotic process | 4 | IL6, SFRP1, CDKN2A, ATM |
| Cellular response to tumor necrosis factor | 3 | IL6, SFRP1, BRCA1 |
| DNA replication | 3 | ATM, BRCA1, IGF1 |
| Replicative senescence | 2 | CDKN2A, ATM |
Table XI.
Gene Ontology groups for downregulated genes (≥5-fold) in the difference between Track (1) and Track (2).
| Category | Count | Genes |
|---|---|---|
| Positive regulation of cell migration | 5 | APC, CSF1, PLAU, CDH13, SNAI2 |
| Positive regulation of apoptotic process | 4 | APC, RARB, SLIT2, PTGS2 |
| Negative regulation of cell proliferation | 4 | APC, CDH13, RARB, PTGS2 |
| Transcription, DNA-templated | 4 | RARB, SNAI2, HIC1, ESR2 |
Table XII.
| Category | Count | Genes |
|---|---|---|
| Pathways in cancer | 6 | IL6, CDKN2A, GSTP1, MMP2, BIRC5, IGF1 |
| p53 signaling pathway | 5 | CCND2, CDKN2A, SERPINE1, ATM, IGF1 |
| Signaling pathway | 4 | IL6, CCND2, ATM, IGF1 |
| Cell cycle | 3 | CCND2, CDKN2A, ATM |
Table XIII.
| Category | Count | Genes |
|---|---|---|
| MicroRNAs in cancer | 3 | APC, PLAU, PTGS2 |
| Pathways in cancer | 3 | APC, RARB, PTGS2 |
Notably, after quercetin treatment the colony stimulating factor 1 (CSF1) gene, an apoptotic gene, decreased from 35.26- to 17.58-fold. In addition, as shown in Table II, another alteration in the apoptotic pathway that occurred in the present study after quercetin addition was upregulation of the ATM gene by 4.02-fold as shown in Table VI.
In the DNA repair pathway, BRCA1 and BRCA2 gene expression levels were downregulated in (MCF-7/DOX 53.2 nM) cells by -9.45- and -3.94-fold compared with -2.65 and -4.04-fold in cells treated with quercetin (Table III).
Another valuable observations in the present study was the upregulation of PLAU expression gene in (MCF-7/DOX 53.2 nM) cells by 44.32-fold and downregulation by 13.7-fold after combination treatment as shown in Table II. Moreover, SNAI2 expression decreased by 30.06-fold to -1.17-fold after combination treatment as shown in Table II.
Discussion
The present study was designed to investigate the effects of quercetin in reversing the doxorubicin-chemoresistant phenotype in a MCF-7 breast cancer cell line. First, the doxorubicin-resistant MCF-7 cell line was established using a series of stages of increasing doxorubicin concentrations. The development of resistant cells was closely monitored. Sensitivity to doxorubicin and the doxorubicin with quercetin combination was assessed using the MTT assay. The concentration ranges of quercetin were proven not to be cytotoxic for the cells under investigation. Annexin V/PI staining demonstrated that the presence of quercetin drives cells into late apoptosis and necrosis in agreement with the present findings.
It has been suggested that the reversal property of quercetin is due to its anti-oxidant effects and its capability to disrupt mitochondrial membrane potential, leading to the release of cytochrome C, which is a pro-caspase protein (16). Li et al (28) demonstrated that quercetin has little effect on cell proliferation at concentrations <0.7 µM; however, when combined with doxorubicin, the combination leads to significant inhibition of cell proliferation and invasion and suppression of the expression of HIF-1α and P-gp. In 2012, Wang et al (29) also found that co-treatment with quercetin (20 mM) and doxorubicin (1 mM) leads to significant potentiation of the antitumor effects of doxorubicin in human liver cancer cells. Shu et al (30) reported that the combination treatment of doxorubicin and quercetin leads to a significant promotion of doxorubicin-induced apoptosis in resistant prostate cancer cells. Another study by Huang et al (31) showed that quercetin can inhibit oral cancer cell proliferation through induction of apoptosis and death in tongue squamous cell carcinoma cells.
Doxorubicin accumulation patterns or accumulation defects have been described earlier by AbuHammad and Zihlif as reflecting resistance to doxorubicin (11). It is assumed that doxorubicin resistance in several P-glycoprotein-positive non-small cell lung cancer and breast cancer multidrug resistant cell lines can be explained by a summation of accumulation defect and alterations in the efficacy of the drug once present in the cell (32). Furthermore, in our previous study that describes the gene expression changes in MCF-7 cells, a significant overexpression of p-gp gene was reported (11). The accumulation of doxorubicin in the vesicles outside the nucleus has been reported by our group previously (11). Resistant MCF-7 cells were treated with a combination of doxorubicin and verapamil. Verapamil is a calcium channel inhibitor, and it has been shown to efficiently suppress the function of P-gp, leading to the reverse of drug resistance and eventual increase in drug accumulation and enhanced DNA damage (33-35). Notably, the impact of quercetin is more obvious in term of reversing the resistance. Such impact is clear at both the necrosis and apoptosis levels. Verapamil antiproliferation has not been performed on wild-type MCF7 cells as it does not have an effect on these cells by itself nor have an impact on the Doxorubicin IC50 when there is no doxorubicin resistance (11). Verapamil impact can only be seen in the resistant MCF7 cells that increased the P-gp expression (36).
The gene expression investigation in the present study revealed that quercetin produced a reversal in the doxorubicin resistance through up- and downregulation of important genes. Those genes are involved in a number of important cellular processes, such as cell cycle, apoptotic pathway, DNA-repair and/or cell migration.
CDKN2A (p16) and CDKN1A are upregulated by 5.21- and 6.11-fold, respectively. The p16 protein binds to two other proteins, CDK4 and CDK6, which assist in cell cycle regulation. CDK4 and CDK6 normally stimulate the cell to continue through the cell cycle and divide; however, binding with p16 blocks cell cycle progression (37). According to AbuHammad and Zihlif (11), p16 regulates the G1/S cell cycle transition and has the effect of causing cell arrest in the G1-phase. In the present study, after treatment with quercetin, it was found that CDKN2A (p16) was upregulated by 13.7-fold, which could be due to the effect of quercetin in reversing doxorubicin resistance. Other studies have showed that quercetin causes S phase arrest via decreases in protein expression levels of CDK2 and cyclins A and B while producing an increase in p53 and p57 protein levels (38-40).
In the present study, the CSF1 gene decreased from 35.26- to 17.58-fold after quercetin treatment, thereby leading to a reduction in the expression of anti-apoptotic proteins and rendering cells more sensitive to doxorubicin. In 2016, Zhang et al (41) revealed that the CSF1 gene, which encodes M-CSF protein, induced significant doxorubicin resistance of MCF-7 cell via inhibition of apoptosis through activation of the PI3K/Akt/Survivin pathway.
Another alteration in the apoptotic pathway that occurred in the present study after quercetin addition was upregulation of the ATM gene by 4.02-fold, which further stimulated the p53 pathway. ATM acts as a binary switch by regulating the capability of p53 to induce cell death after chemotherapy. AbuHammad and Zihlif (11) revealed that ATM selectively activates p53, providing a mechanism for controlling the cell cycle and apoptotic responses. Another study showed that treatment with quercetin resulted in p53 upregulation, a finding that was consistent with an earlier report (38).
Since DNA repair mechanisms play an important role in regulating cellular sensitivity to DNA damaging agents, DNA repair plays an important role in the development of resistance to chemotherapy (42).
Breast cancer types 1 (BRCA) and 2 (BRCA2) genes produce proteins that help repair damaged DNA (43). In the present study, BRCA1 and BRCA2 gene expression levels were downregulated in (MCF-7/DOX 53.2 nM) cells by -9.45- and -3.94-fold compared with -2.65 and -4.04-fold in cells treated with quercetin.
Variations in the studies on the role of BRCA1 and BRCA2 proteins in doxorubicin resistance exists. One study found that BRCA1-defective breast cancer cells are significantly less sensitive to doxorubicin when compared with MCF-7 and MDA-MB231(44), but another study found that reduced BRCA1 protein expression in ovarian cancer cells leads to an improvement in survival (45). As a result, downregulation of BRCA1 and BRCA2 gene expression levels seen in resistant MCF-7 cells may be one of the main changes seen in the resistant phenotype. The present results showed that quercetin led to an increase in the expression of BRCA1 gene by 6.8-fold; this result is consistent with those from a study by Kundur et al in 2019(46), who reported that quercetin and curcumin lead to a dose-dependent enhancement in BRCA1 expression.
In the present study, SNAI2 expression decreased by 30.06-fold to -1.17-fold after combination treatment. This gene encodes a member of the Snail family as described by Alves et al (47). The encoded protein represents a transcriptional repressor to E-cadherin transcription in breast carcinoma. It is also involved in epithelial-mesenchymal transitions and has antiapoptotic activity. Another study by Côme et al (48) reported that SNAI2 has been found to be upregulated in all endocrine resistant cells when compared with parental cell lines. Reduction of SNAI2 expression levels lead to a disabling of cell migration and an increase in E-cadherin levels in two fulvestrant-resistant breast cancer cell models, proving the role of SNAI2 in controlling cell motility and maintenance of a mesenchymal phenotype in resistant cells (47). Another valuable observation in the present study was the upregulation of PLAU expression gene in (MCF-7/DOX 53.2 nM) cells by 44.32-fold and downregulated to 13.7-fold after combination treatment.
Lin et al (49) showed that downregulation of PLAU expression can suppresses colorectal cancer development via inhibition of colorectal cancer cell growth, cell migration and angiogenesis. Another study by Ai et al in 2020(50) revealed that inhibition of PLAU expression can repress the migratory and invasive capability of cervical cancer cells through downregulation of MMP2. This finding may provide a clue that quercetin can cause reduction in the metastatic phenotype of MCF-7 resistant cells as published by Hoca et al (51) who demonstrated that quercetin can lead to inhibition of EMT in cancer cell lines, including oral cancer cells, breast cancer stem cells and prostate cancer cells.
In conclusion, the results of the present study indicated that quercetin could lead to reversal of breast cancer cell doxorubicin resistance via downregulation of the expression of important genes, such as SNAI2, PLAU and CSF1. Such findings may represent a potential strategy for reversing the chemoresistance of breast cancer. Notably, the present study needs a further protein level confirmation for the obtained gene expression changes.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: This project received funding from the Deanship of Scientific Research at the University of Jordan.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MZ and BAA confirm the authenticity of all the raw data. MZ, RA-D and HZ conceptualized the present study. MZ, BAA and RA-D designed the methodology. All the authors worked on the validation. MZ, BAA and RA-D worked on the formal analysis of data. MZ, BAA and RA-D performed the experiments. All authors worked together on resources, writing-original draft preparation, writing-review and editing. MZ, RA-D and HZ supervised the study. MZ, RA-D and HZ were project administrators. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Authors' information
Ms. Hiba Zalloum (ORCID: 0000-0002-5296-2776).
References
- 1.Gucalp A, Gupta GP, Pilewskie ML, Sutton EJ, Norton L. Advances in managing breast cancer: A clinical update. F1000Prime Rep. 2014;6(66) doi: 10.12703/P6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson TR, Longley DB, Johnston PG. Chemoresistance in solid tumours. Ann Oncol. 2006;17 (Suppl 10):x315–x324. doi: 10.1093/annonc/mdl280. [DOI] [PubMed] [Google Scholar]
- 3.Prihantono , Faruk M. Breast cancer resistance to chemotherapy: When should we suspect it and how can we prevent it? Ann Med Surg (Lond) 2021;70(102793) doi: 10.1016/j.amsu.2021.102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquette C, Nabell L. Chemotherapy-resistant metastatic breast cancer. Curr Treat Options Oncol. 2012;13:263–275. doi: 10.1007/s11864-012-0184-6. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moulder S. Intrinsic resistance to chemotherapy in breast cancer. Womens Health (Lond) 2010;6:821–830. doi: 10.2217/whe.10.60. [DOI] [PubMed] [Google Scholar]
- 7.Crea F, Nobili S, Paolicchi E, Perrone G, Napoli C, Landini I, Danesi R, Mini E. Epigenetics and chemoresistance in colorectal cancer: An opportunity for treatment tailoring and novel therapeutic strategies. Drug Resist Updat. 2011;14:280–296. doi: 10.1016/j.drup.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N, Palmieri C, Plummer CJ, Stanley A, Verrill MW. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol. 2009;20:816–827. doi: 10.1093/annonc/mdn728. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Bieerkehazhi S, Ma H. Next-generation proteasome inhibitor oprozomib enhances sensitivity to doxorubicin in triple-negative breast cancer cells. Int J Clin Exp Pathol. 2018;11:2347–2355. [PMC free article] [PubMed] [Google Scholar]
- 10.Smith L, Watson MB, O'Kane SL, Drew PJ, Lind MJ, Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol Cancer Ther. 2006;5:2115–2120. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
- 11.AbuHammad S, Zihlif M. Gene expression alterations in doxorubicin resistant MCF7 breast cancer cell line. Genomics. 2013;101:213–220. doi: 10.1016/j.ygeno.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Lu Y, Liang K, Liu B, Fan Z. Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res. 2005;7:R589–R597. doi: 10.1186/bcr1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, Choi HY, Jeong MY, Cho SG. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta. 2006;1763:958–968. doi: 10.1016/j.bbamcr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, Carbone M, Testa JR, Mossman BT. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer. 2010;9(314) doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Moon M, Dawood S, McManus B, Liu PP. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36:296–305. doi: 10.1007/s00059-011-3470-3. [DOI] [PubMed] [Google Scholar]
- 16.Elmadany N, Khalil E, Vaccari L, Birarda G, Yousef I, Abu-Dahab R. Antiproliferative activity of the combination of doxorubicin/quercetin on MCF7 breast cancer cell line: A combined study using colorimetric assay and synchrotron infrared microspectroscopy. Infrared Phys Technol. 2018;95:141–147. [Google Scholar]
- 17.Yang F, Teves SS, Kemp CJ, Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014;1845:84–89. doi: 10.1016/j.bbcan.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbeau D, Persoons R, Marques M, Hervé C, Laffitte-Rigaud G, Maitre A. Relevance of urinary 3-hydroxybenzo(a)pyrene and 1-hydroxypyrene to assess exposure to carcinogenic polycyclic aromatic hydrocarbon mixtures in metallurgy workers. Ann Occup Hyg. 2014;58:579–590. doi: 10.1093/annhyg/meu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang ZP, Liu XJ, Zou BX, Wang LG, Zhou T. The complete recanalization of PICC-related venous thrombosis in cancer patients: A series of case reports. Exp Ther Med. 2013;6:411–412. doi: 10.3892/etm.2013.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G, et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep. 2017;38:819–828. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slighoua M, Amrati FE, Chebaibi M, Mahdi I, Al Kamaly O, El Ouahdani K, Drioiche A, Saleh A, Bousta D. Quercetin and ferulic acid elicit estrogenic activities in vivo and in silico. Molecules. 2023;28(5112) doi: 10.3390/molecules28135112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notas G, Nifli AP, Kampa M, Pelekanou V, Alexaki VI, Theodoropoulos P, Vercauteren J, Castanas E. Quercetin accumulates in nuclear structures and triggers specific gene expression in epithelial cells. J Nutr Biochem. 2012;23:656–666. doi: 10.1016/j.jnutbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Liu C, Tang C, Yin C. Dual stimulus-responsive chitosan-based nanoparticles co-delivering doxorubicin and quercetin for cancer therapy. Mater Lett. 2021;305(130826) [Google Scholar]
- 24.Henidi HA, Al-Abbasi FA, El-Moselhy MA, El-Bassossy HM, Al-Abd AM. Despite blocking doxorubicin-induced vascular damage, quercetin ameliorates its antibreast cancer activity. Oxid Med Cell Longev. 2020;2020(8157640) doi: 10.1155/2020/8157640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JY, Hu RY, Chou HC. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J Biomed Sci. 2013;20(95) doi: 10.1186/1423-0127-20-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Yuan S, Zhao Q, Wang B, Wang X, Li K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed Pharmacother. 2018;100:441–447. doi: 10.1016/j.biopha.2018.02.055. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Li SZ, Li K, Zhang JH, Dong Z. The effect of quercetin on doxorubicin cytotoxicity in human breast cancer cells. Anticancer Agents Med Chem. 2013;13:352–355. doi: 10.2174/1871520611313020020. [DOI] [PubMed] [Google Scholar]
- 29.Wang G, Zhang J, Liu L, Sharma S, Dong Q. Quercetin potentiates doxorubicin mediated antitumor effects against liver cancer through p53/Bcl-xl. PLoS One. 2012;7(e51764) doi: 10.1371/journal.pone.0051764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu Y, Xie B, Liang Z, Chen J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol Lett. 2018;15:2252–2258. doi: 10.3892/ol.2017.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CF, Liu SH, Ho TJ, Lee KI, Fang KM, Lo WC, Liu JM, Wu CC, Su CC. Quercetin induces tongue squamous cell carcinoma cell apoptosis via the JNK activation-regulated ERK/GSK-3α/β-mediated mitochondria-dependent apoptotic signaling pathway. Oncol Lett. 2022;23(78) doi: 10.3892/ol.2022.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuurhuis GJ, Van Heijningen TH, Cervantes A, Pinedo HM, de Lange JH, Keizer HG, Broxterman HJ, Baak JP, Lankelma J. Changes in subcellular doxorubicin distribution and cellular accumulation alone can largely account for doxorubicin resistance in SW-1573 lung cancer and MCF-7 breast cancer multidrug resistant tumour cells. Br J Cancer. 1993;68:898–908. doi: 10.1038/bjc.1993.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Li Y, Fan GF, Zhang TY, Sun B, Fan PS. Effect of verapamil in the reversal of doxorubicin chemotherapy resistance in advanced gastric cancer. Eur Rev Med Pharmacol Sci. 2020;24:7753–7763. doi: 10.26355/eurrev_202007_22278. [DOI] [PubMed] [Google Scholar]
- 34.Rogan AM, Hamilton TC, Young RC, Klecker RW Jr, Ozols RF. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984;224:994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- 35.Bellamy WT, Dalton WS, Kailey JM, Gleason MC, McCloskey TM, Dorr RT, Alberts DS. Verapamil reversal of doxorubicin resistance in multidrug-resistant human myeloma cells and association with drug accumulation and DNA damage. Cancer Res. 1988;48:6365–6370. [PubMed] [Google Scholar]
- 36.AlQudah DA, Zihlif MA, Taha MO. Ligand-based modeling of diverse aryalkylamines yields new potent P-glycoprotein inhibitors. Eur J Med Chem. 2016;110:204–223. doi: 10.1016/j.ejmech.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Poi MJ, Tsai MD. Regulatory mechanisms of tumor suppressor P16(INK4A) and their relevance to cancer. Biochemistry. 2011;50:5566–5582. doi: 10.1021/bi200642e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6(24049) doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh DK, Lee EJ, Kim HC, Kim JH. Induction of G(1)/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch Pharm Res. 2010;33:781–785. doi: 10.1007/s12272-010-0519-4. [DOI] [PubMed] [Google Scholar]
- 40.Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837–844. doi: 10.3892/ijo.19.4.837. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M, Zhang H, Tang F, Wang Y, Mo Z, Lei X, Tang S. Doxorubicin resistance mediated by cytoplasmic macrophage colony-stimulating factor is associated with switch from apoptosis to autophagic cell death in MCF-7 breast cancer cells. Exp Biol Med (Maywood) 2016;241:2086–2093. doi: 10.1177/1535370216660399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torgovnick A, Schumacher B. DNA repair mechanisms in cancer development and therapy. Front Genet. 2015;6(157) doi: 10.3389/fgene.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, Goel A, Barbieri V, Costanzo F, Boland CR, Venuta S. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88:1285–1291. doi: 10.1038/sj.bjc.6600859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GK, Mullan PB, Johnston PG, Wilson RH, Harkin DP. BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res. 2007;13:7413–7420. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- 46.Kundur S, Prayag A, Selvakumar P, Nguyen H, McKee L, Cruz C, Srinivasan A, Shoyele S, Lakshmikuttyamma A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J Cell Physiol. 2019;234:11103–11118. doi: 10.1002/jcp.27761. [DOI] [PubMed] [Google Scholar]
- 47.Alves CL, Elias D, Lyng MB, Bak M, Ditzel HJ. SNAI2 upregulation is associated with an aggressive phenotype in fulvestrant-resistant breast cancer cells and is an indicator of poor response to endocrine therapy in estrogen receptor-positive metastatic breast cancer. Breast Cancer Res. 2018;20(60) doi: 10.1186/s13058-018-0988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Côme C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 49.Lin M, Zhang Z, Gao M, Yu H, Sheng H, Huang J. MicroRNA-193a-3p suppresses the colorectal cancer cell proliferation and progression through downregulating the PLAU expression. Cancer Manag Res. 2019;11:5353–5363. doi: 10.2147/CMAR.S208233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ai C, Zhang J, Lian S, Ma J, Győrffy B, Qian Z, Han Y, Feng Q. FOXM1 functions collaboratively with PLAU to promote gastric cancer progression. J Cancer. 2020;11:788–794. doi: 10.7150/jca.37323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoca M, Becer E, Kabadayı H, Yücecan S, Vatansever HS. The effect of resveratrol and quercetin on epithelial-mesenchymal transition in pancreatic cancer stem cell. Nutr Cancer. 2020;72:1231–1242. doi: 10.1080/01635581.2019.1670853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.