Abstract
Ras mutants with the ability to interact with different effectors have played a critical role in the identification of Ras-dependent signaling pathways. We used two mutants, RasS35 and RasG37, which differ in their ability to bind Raf-1, to examine Ras-dependent signaling in thyroid epithelial cells. Wistar rat thyroid cells are dependent upon thyrotropin (TSH) for growth. Although TSH-stimulated mitogenesis requires Ras, TSH activates protein kinase A (PKA) and downregulates signaling through Raf and the mitogen-activated protein kinase (MAPK) cascade. Cells expressing RasS35, a mutant which binds Raf, or RasG37, a mutant which binds RalGDS, exhibited TSH-independent proliferation. RasS35 stimulated morphological transformation and anchorage-independent growth. RasG37 stimulated proliferation but not transformation as measured by these indices. TSH exerted markedly different effects on the Ras mutants and transiently repressed MAPK phosphorylation in RasS35-expressing cells. In contrast, TSH stimulated MAPK phosphorylation and growth in cells expressing RasG37. The Ras mutants, in turn, exerted differential effects on TSH signaling. RasS35 abolished TSH-stimulated changes in cell morphology and thyroglobulin expression, while RasG37 had no effect on these activities. Together, the data indicate that cross talk between Ras and PKA discriminates between distinct Ras effector pathways.
Ras proteins are important signaling intermediates that convey signals initiated at the cell surface to effector pathways in the cytoplasm. Ras exerts effects on cell transformation and proliferation, the actin cytoskeleton, differentiation, and apoptosis. It is likely that these effects are mediated by multiple effectors, including Raf-1, RalGDS (2), phosphatidylinositol 3-kinase (PI3K) (48), and other Ras-binding proteins (reviewed in reference 38), as well as members of the Rho family (26, 44, 45).
The elucidation of multiple Ras effector pathways was greatly facilitated by the isolation of Ras mutants which interact with single downstream effectors (60). RasS35 binds preferentially to Raf, while RasG37 binds to RalGDS (49, 53, 60). RasS35 stimulates mitogen-activated protein kinase (MAPK) activity and serum response element-dependent gene expression, while RasG37 fails to do so. Individually, neither RasS35 nor RasG37 stimulated focus formation as effectively as RasV12; however, coexpression of the mutants restored this activity (60). Similarly, microinjection of a RasS35 expression vector into REF52 cells failed to stimulate DNA synthesis when injected alone, although coinjection with a second mutant, RasC40, stimulated DNA synthesis (23). These findings, along with others, indicate that Ras mediates its effects through multiple pathways.
While the differential effects of RasS35 and RasG37 on Raf and MAPK activity are consistently observed (23, 25, 27, 46, 49, 60), other effects of these mutants vary. Both RasS35 and RasG37 transform some NIH 3T3 strains, inducing growth in medium with low serum levels, anchorage-independent proliferation, and tumor formation in nude mice (27). The activity of RasG37 in these assays suggests that Ras stimulates some of the same biological effects through Raf-independent pathways and that the effector pathways used by Ras vary in different cell types. Consistently, RasV12 stimulates transformation in most cells, while activated forms of Raf transform fibroblasts but not epithelial cells (41). If Ras signals through multiple effectors, the selection of a particular effector pathway should be regulated. One potential way to achieve such regulation is through cross talk between Ras and other signaling pathways.
One of the best-studied examples of cross talk is that between Ras and protein kinase A (PKA). In many cells, cyclic AMP (cAMP) inhibits Ras signaling through Raf and the MAPK cascade (reviewed in reference 6). cAMP activates PKA, which phosphorylates Raf-1 at multiple serine residues. PKA-mediated phosphorylation reduces the affinity of Raf for Ras (17, 63) and decreases Raf kinase activity (10, 17, 57), effects which may inhibit Ras-mediated proliferation. Ras and cAMP also collaborate to produce similar effects. In PC12 cells, Ras and cAMP induce neurite extension (13, 54) and promote cell survival (64). Ras and cAMP stimulate proliferation in thyroid cells (7, 36, 39), and Ras activity is required for the mitogenic effects of thyrotropin (TSH) (32). Despite the requirement for Ras, TSH downregulates signaling through Raf and the MAPK cascade (1), suggesting that Ras mediates its effects through alternate effectors in these cells. Microinjection of a dominant negative RalA protein, RalAN28 (22), reduced TSH- and Ras-stimulated DNA synthesis, supporting a role for RalGDS in this signaling pathway (40).
We set out to determine if cross talk between Ras and PKA affects Ras-mediated signaling. We isolated thyroid cells stably expressing RasV12 and GTPase-deficient forms of RasS35 and RasG37. RasV12 and the mutants conferred TSH-independent proliferation through distinct pathways. TSH increased the growth rate of RasG37-expressing cells, but not that of RasS35-expressing cells and slowed the growth of RasV12 cells. TSH also stimulated MAPK activity in RasG37 cells and repressed it in RasS35 cells. In turn, RasV12 and RasS35 inhibited TSH effects on thyroglobulin (Tg) expression and the actin cytoskeleton, while RasG37 did not. These results demonstrate that cross talk between Ras and PKA is an important determinant of signaling pathways activated by Ras and that interplay between Ras and PKA exerts profound effects on thyroid cell biology.
MATERIALS AND METHODS
Cell culture and transformation assays.
Wistar rat thyroid (WRT) cells (5) were maintained in Coon’s modified Ham’s F-12 medium with crude bovine TSH (10 mU/ml), insulin (10 μg/ml), transferrin (5 μg/ml), and 5% calf serum (3H medium). 2H medium, used in some studies, lacks TSH. Ras-expressing cells were isolated following Lipofectin-mediated gene transfer with pDCR plasmids encoding RasV12, RasV12G37, or RasV12S35 (abbreviated as RasG37 and RasS35). Transfected cells were maintained in 3H medium containing 150 μg of G418 (GIBCO) per ml.
For colony formation assays, vectors were transfected into early-passage subconfluent cells. Following recovery for 24 h in 3H medium, G418 (300 μg/ml) was added in the presence or absence of TSH for 72 h. The G418 concentration was reduced to 150 μg/ml, and 21 days later the cells were fixed and stained.
Anchorage-independent growth was evaluated by plating 5 × 103 cells in 0.33% agar in 2H medium. Cells were refed 0.5 ml of 2H medium once a week for 21 days. Colonies were stained in 2H medium containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (0.5 mg/ml; Sigma).
Microinjection and immunostaining.
For DNA synthesis measurements, cells were starved in basal medium (Coon’s modified Ham’s F-12 medium, 0.3% bovine serum albumin, 0.5 μg of insulin per ml) for 48 h. Glutathione S-transferase fusion constructs encoding RasV12, RasV12G37, and RasV12S35 were expressed and the proteins were purified as described previously (40). Purified proteins were injected into the cytoplasm with an Eppendorf semiautomated injector, the cells were labeled with bromodeoxyuridine (BrdU) for 48 h, and DNA synthesis was monitored by immunostaining (34). Rabbit immunoglobulin G (IgG) (7 mg/ml) was included in the injection solutions to identify injected cells. Ras proteins were injected at concentrations ranging from 0.075 to 0.50 mg/ml. Based on an injection volume of 2 × 10−14 liter, injection of Ras at 0.5 mg/ml delivers approximately 290,000 molecules/cell, or an approximately 5- to 15-fold-larger amount than does cellular Ras (50), since approximately 30% of the purified Ras proteins are biologically active (12, 51).
Actin stress fibers were visualized by staining with rhodamine-labeled phalloidin as described by Joneson et al. (23).
Tg expression was assessed by immunostaining with an antibody raised to human Tg as described previously (33).
Western blot analysis.
Ras expression was detected by Western blotting with anti-pan-Ras (1:100; Oncogene Science) and antihemagglutinin antibodies (1:300; kindly provided by J. Field) followed by alkaline phosphatase-conjugated anti-mouse immunoglobulin antibodies (1:1,000; New England Biolabs) and detection with CDP-Star (New England Biolabs).
MAPK phosphorylation was assessed by Western blotting with a phosphospecific MAPK antibody (1 μg/ml; Promega).
RESULTS
Multiple Ras effector pathways stimulate thyroid cell proliferation.
To determine if PKA influences Ras effectors in cells where Ras and PKA promote growth, we assessed the ability of Ras mutants which differ in their ability to bind Raf to stimulate colony formation in thyroid cells. Vector-transfected cells formed small colonies which failed to proliferate in the absence of TSH (Fig. 1). Transfection with constructs encoding GTPase-deficient (V12) forms of Ras, RasG37, and RasS35, as well as RasG12, stimulated the number and size of colonies formed compared to the number and size of colonies formed by vector-transfected cells (Fig. 1 and Table 1). TSH exerted differential effects on colony formation stimulated by RasG12 and the mutants. Colonies formed following transfection with RasS35 were larger in the absence of TSH than in its presence. In contrast, TSH increased the size of colonies formed following transfection with RasG37 or RasG12. The morphology of these cells was indistinguishable from that of parental cells. Cells transfected with RasV12 and RasS35 were morphologically transformed, although in these assays the effects of RasS35 were less pronounced than those of RasV12. These results demonstrate that both Raf-dependent (i.e., RasS35) and -independent (i.e., RasG37) pathways promote thyroid cell proliferation and suggest that these pathways may be regulated differently by PKA.
FIG. 1.
Ras effector mutants stimulate colony formation in WRT cells. Cells were transfected with pDCR or pDCR encoding RasG12, RasV12, or GTPase-deficient (V12) forms of RasG37 and RasS35. Twenty-four hours after transfection, TSH-supplemented (+TSH) or -deficient (−TSH) medium containing G418 was added to replicate plates, and the cells were fixed and stained 21 days later. Colony size could not be evaluated in RasV12-transfected cells since these cells were morphologically transformed and did not form compact colonies. Four experiments were performed with similar results.
TABLE 1.
Colony formation in Ras-transfected WRT cellsa
| Transfected construct | No. of colonies formed
|
|
|---|---|---|
| With TSH | Without TSH | |
| pDCR | 405 | 290 |
| RasG12 | 746 | 476 |
| RasG37 | 760 | 671 |
| RasS35 | 458 | 507 |
Only colonies larger than 0.5 mm in diameter were scored. Results shown are from the experiment depicted in Fig. 1. Three independent experiments yielded similar results.
cAMP exerts differential effects on RasG37- and RasS35-mediated proliferation.
To explore potential cross talk between Ras and PKA, cells stably expressing GTPase-deficient forms of Ras, RasG37, and RasS35 were isolated. Western blotting revealed that all G418-resistant cells expressed abundant levels of transfected Ras (Fig. 2). RasG37 and RasS35 were expressed at similar levels, while RasV12 was expressed at levels 7.5- to 10-fold lower than those of the effector mutants. Compared to cellular Ras, RasV12 was overexpressed approximately fourfold and the effector mutants were overexpressed 30- to 40-fold (Fig. 2B). One mass population and two clonal populations of RasS35-expressing cells and two independent mass populations of RasG37-expressing cells were analyzed in our initial studies. Cells expressing either mutant exhibited TSH-independent DNA synthesis (Fig. 3), supporting the stimulatory effects on proliferation seen in the colony formation assays. To discriminate between direct effects of the mutants on proliferation and their ability to establish and/or maintain an autocrine loop, acute microinjection experiments were performed. Microinjection of purified RasS35 or RasG37 proteins stimulated DNA synthesis in quiescent cells (Fig. 4) (40). The effector mutants appeared less active than RasV12 in these assays (Fig. 4, inset), although it is possible that differences in the activity of these proteins contribute to these results. However, the effector domain mutants are consistently reported to be less active than RasV12 following transfection of expression plasmids for these constructs (27, 49, 60). Our results demonstrate that the acute effects of RasG37 and RasS35 can include the stimulation of DNA synthesis in some cells.
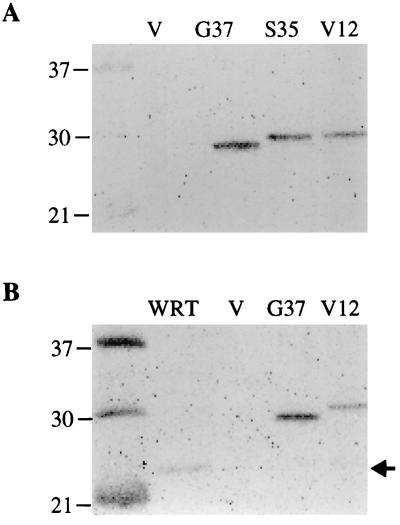
FIG. 2.
Ras expression in stably transfected WRT cells. Total cell lysates were prepared and protein determinations were made. (A) Western blot probed with antihemagglutinin antibody to detect transfected Ras. Samples from left to right are vector-transfected (V) cells, RasG37 (pool 1), RasS35 (pool 10b) (2 μg each), and RasV12 (clone 3, 10 μg). The levels of Ras expression were similar in multiple pools and single-cell clones of cells transfected with RasS35 and RasG37. RasV12 was expressed at levels approximately 7.5- to 10-fold lower than those of the effector mutants. (B) Western blot probed with pan-Ras antibody to detect cellular Ras. Samples from left to right are WRT (100 μg), vector-transfected (V) cells, RasG37 (5 μg), and RasV12 (50 μg). Densitometric analysis revealed that RasV12 was overexpressed 4-fold and RasG37 was overexpressed 40-fold relative to cellular Ras. Cellular Ras expression was not altered by transfection of RasV12 or the mutants.
FIG. 3.
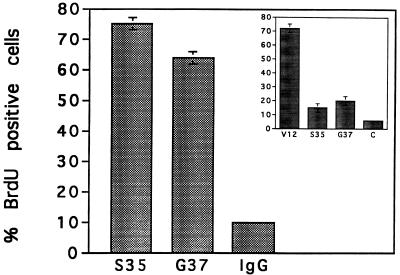
Ras-expressing cells exhibit TSH-independent DNA synthesis. Ras-expressing cells were incubated in TSH-deficient medium for 48 h and then labeled with BrdU for 48 h. The results shown are for RasV12 (clone 3), RasS35 (pool 10b), RasG37 (pool 1), and vector-transfected cells (C). More than 200 cells were scored on each coverslip. Error bars indicate 95% confidence intervals based on the standard error of proportion. The absence of an error bar indicates a standard error of proportion of 1 or less. Three experiments performed in duplicate gave similar results. Three independent RasS35 isolates and two independent RasG37 isolates were analyzed with similar results.
FIG. 4.
Microinjection of RasV12, RasS35, and RasG37 proteins stimulates DNA synthesis. Quiescent cells were injected with RasS35 or RasG37 (0.5 mg/ml, the minimal active concentration in these cells) and labeled with BrdU for 48 h. BrdU incorporation in IgG-injected cells is shown as a negative control. (Inset) BrdU incorporation following injection of RasV12, RasS35, and RasG37 at similar concentrations (0.075, 0.1, and 0.1 mg/ml, respectively). C, IgG-injected cells. Error bars indicate 95% confidence intervals based on the standard error of proportion. The absence of an error bar indicates a standard error of proportion of 1 or less. More than four independent preparations of the Ras proteins were tested with similar results.
To evaluate effects of RasG37 and RasS35 on proliferation, growth rates were determined in cells expressing these mutants. Expression of either mutant conferred TSH-independent proliferation (Table 2) on cells, as did expression of RasV12. Because the proliferation of WRT cells is TSH dependent, their growth rate is very low in its absence. Cells expressing RasV12 or either mutant exhibited much shorter doubling times in the absence of TSH than did parental cells. In the presence of TSH, the growth rate of RasG37 cells was markedly increased (Table 2 and Fig. 5A). RasG37 cells grew more rapidly than did cells expressing either RasS35 or RasV12 in TSH-containing medium. Similar effects were not observed in RasV12 or RasS35 cells, which grew more slowly, at least transiently, following TSH addition (Fig. 5B). Consistent with these results, TSH increased DNA synthesis in RasG37 cells (Fig. 5C) and decreased it in RasS35 cells (Fig. 5D). These results demonstrate that TSH exerts differential effects on signaling pathways stimulated by RasG37 and RasS35.
TABLE 2.
Growth rates of Ras-expressing cellsa
| Cell line | Doubling time (h) in medium
|
|
|---|---|---|
| 3H (+TSH) | 2H (−TSH) | |
| WRT | 34.2 ± 2.4 | 62.7 ± 3.6 |
| RasV12 | 36.7 ± 1.9 | 26.6 ± 2.7 |
| RasS35 | 37.2 ± 4.1 | 32.0 ± 6.2 |
| RasG37 | 27.5 ± 2.3 | 35.7 ± 3.3 |
The P value for the RasG37 trials was <0.025, while the P value for the RasV12 trials was <0.005. The effects of TSH on growth rate in RasS35 cells were not statistically significant although they were consistently observed. The results shown are representative of four independent experiments performed with similar results.
FIG. 5.
TSH exerts differential effects on RasS35 and RasG37-mediated proliferation. Ni, cell number at day N; No, number of cells plated on day 1. (A) Cells were plated in 3H medium overnight and transferred to 2H medium, and cell number was assessed at days 2 and 4 (open symbols). On day 4, replicate plates were fed 3H (filled symbols) or 2H (open symbols) medium, and the cell number was determined 2 days later. Results shown are the means ± standard deviations from three experiments performed in duplicate with similar results. The absence of an error bar indicates a standard deviation of 1 or less. Two independent isolates of RasG37-expressing cells were analyzed with similar results. (B) Experimental conditions were the same as for panel A with RasS35 cells. Three experiments with three independent RasS35 isolates were performed in duplicate with similar results. (C) RasG37 cells were incubated in 2H medium (−TSH) or 3H medium (+TSH), and BrdU incorporation was measured over 24 h. WRT cells were included for comparison. Error bars indicate 95% confidence intervals. Three experiments performed in duplicate gave similar results. (D) BrdU incorporation in RasS35 cells with or without TSH. Cells were incubated in basal medium for 48 h and subsequently stimulated with the same medium (−TSH) or TSH-supplemented medium (+TSH) containing BrdU for 24 h. Error bars indicate 95% confidence intervals. Four experiments yielded similar results.
Effects on MAPK phosphorylation.
RasS35 and RasG37 differ in their ability to bind Raf and stimulate MAPK activity (23, 25, 27, 49, 60). These mutants’ properties of binding to proteins in whole-cell lysates from thyroid cells were identical to those described in previous reports. RasS35 interacted with Raf-1 but not RalGDS. Conversely, RasG37 bound RalGDS but not Raf-1 (47).
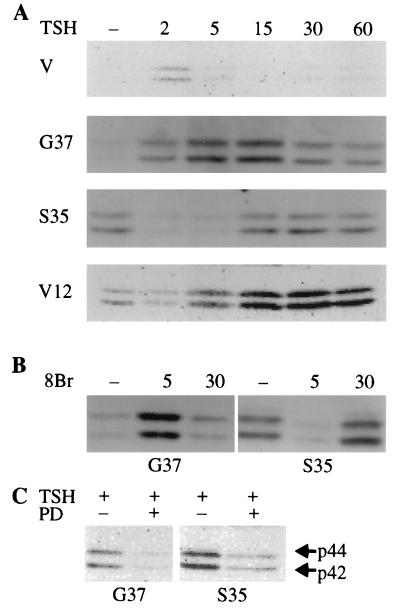
To examine the signaling pathways activated by these mutants, we examined their effects on MAPK phosphorylation. In the absence of TSH, cells expressing RasS35 or RasV12 exhibited basally elevated levels of phosphorylated MAPK (Fig. 6A). MAPK phosphorylation was not elevated in cells expressing RasG37 or vector-transfected cells under these conditions. TSH rapidly and transiently decreased MAPK phosphorylation in both RasV12 and RasS35 cells (Fig. 6A), an effect that was mimicked by cAMP-elevating agents (Fig. 6B). Maximal repression was observed 2 to 5 min after TSH addition, after which MAPK phosphorylation returned to levels observed in untreated cells. In RasS35 cells, both basal levels of MAPK phosphorylation (data not shown) and the return to these levels following TSH stimulation were MEK dependent (Fig. 6C).
FIG. 6.
MAPK phosphorylation in Ras-expressing cells is differentially regulated by cAMP. (A) Cells maintained in basal medium for 24 h were stimulated with TSH (1 mU/ml) for various times (given in minutes), and MAPK phosphorylation was assessed. TSH diminished MAPK phosphorylation in RasS35 (pool 10b) and RasV12 (clone 3) cells. TSH increased MAPK phosphorylation in RasG37 (pool 1) cells. TSH stimulated a small increase in MAPK phosphorylation in vector (V)-transfected cells, as it did in parental cells (data not shown). The results shown are from one of two experiments performed with these clones which yielded similar findings. A third experiment with independent isolates of RasG37 (pool 2) and RasS35 (pool C) gave similar results. (B) RasG37 (pool 1) and RasS35 (pool 10b) cells were treated as for panel A except that they were stimulated with 8BrcAMP (8Br) (1 mM) for various times (given in minutes). (C) Pretreatment with PD98059 (25 μM) for 24 h abolished MAPK phosphorylation 30 min after TSH treatment in RasS35 cells and TSH-stimulated phosphorylation (at 30 min) in RasG37 cells. Two experiments were performed with similar results.
In contrast to its effects in RasS35 cells, TSH stimulated a marked increase in MAPK phosphorylation in RasG37 cells (Fig. 6A). TSH increased MAPK phosphorylation within 2 to 5 min, and this increase was sustained for at least 60 min. The effects of TSH on MAPK phosphorylation were mimicked by cAMP-elevating agents, demonstrating that they were PKA mediated (Fig. 6B). Interestingly, in RasG37 cells, pretreatment with the MEK inhibitor PD98059 abolished the effects of TSH on MAPK phosphorylation, supporting a role for MEK1 in TSH-mediated regulation of MAPK (Fig. 6C). The differential effects of cAMP on MAPK phosphorylation provide additional evidence that RasS35 and RasG37 activate distinct signaling pathways, although these pathways may converge at MEK or MAPK.
RasS35, but not RasG37, inhibits thyroglobulin expression.
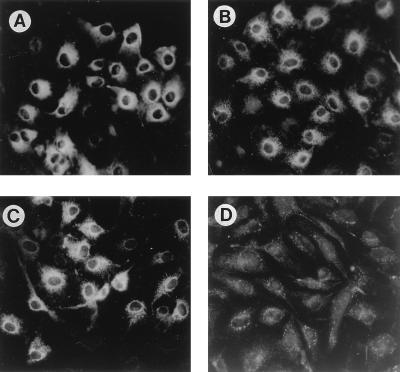
Acute or chronic expression of RasV12 decreased the expression of Tg, a marker of the differentiated phenotype, in WRT cells (33). To explore the pathways which mediate this effect, the effects of RasG37 and RasS35 on Tg expression were examined. Because Tg expression is stimulated by TSH, cells were incubated in TSH-deficient medium for 6 days to reduce Tg expression. Basal levels of Tg expression, as measured by immunostaining with a Tg-specific antibody, were undetectable under these conditions (data not shown). TSH stimulated Tg expression in RasG37-expressing cells to levels similar to those achieved in parental and vector-transfected cells (Fig. 7). In contrast, TSH failed to stimulate Tg expression in cells expressing RasS35, similarly to the effects previously reported for cells expressing RasV12 (33). Because dedifferentiation frequently accompanies transformation of thyroid cells, the effects of RasS35 and RasG37 on morphological transformation and anchorage-independent growth were examined.
FIG. 7.
RasS35, but not RasG37, inhibits Tg expression. WRT cells (A), pDCR-transfected controls (B), and RasG37 (pool 1) (C) and RasS35 (pool 10b) (D) cells were stimulated with TSH (1 mU/ml) and stained for Tg expression 48 h later. Photomicrographs were taken at identical exposure times. Three independent experiments with these isolates yielded similar results. Three independent isolates of RasS35-expressing cells and two independent isolates of RasG37-expressing cells were analyzed.
RasS35, but not RasG37, stimulates morphological transformation and anchorage-independent growth.
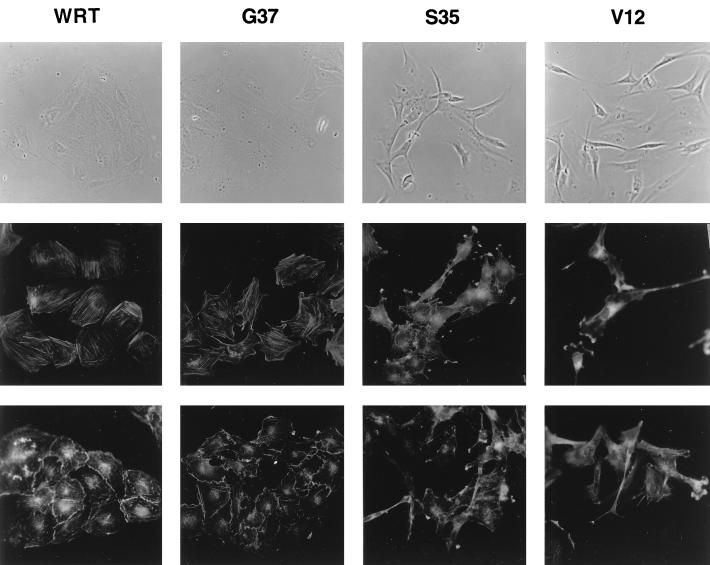
RasV12 and RasS35 cells, but not those expressing RasG37, were morphologically transformed (Fig. 8, top). RasG37 cells exhibited an epithelial morphology indistinguishable from that of parental cells and proliferated as small, well-defined colonies. Cells expressing RasV12 or RasS35 exhibited a fibroblast-like morphology characterized by highly refractile, elongated, spindle-shaped cells which were scattered across the culture dishes. Ras-mediated alterations in cell morphology were accompanied by profound changes in the actin cytoskeleton. In the absence of TSH, RasG37-expressing cells, like parental cells, displayed abundant longitudinal actin stress fibers (Fig. 8, middle). RasV12 and RasS35 cells exhibited a marked decrease in stress fibers. The most striking feature of these cells was the presence of abundant large membrane extensions with prominent ruffles.
FIG. 8.
Morphological changes in cells expressing RasV12 and RasS35. (Top) Phase-contrast photomicrographs of WRT, RasG37 (pool 1), RasS35 (pool 10b), and RasV12 (clone 3) cells. (Middle) Actin staining in the same cells maintained in basal medium (i.e., without TSH) for 48 h. (Bottom) Actin staining in cells acutely stimulated with TSH (1 mU/ml) for 30 min. Results shown are representative of three independent experiments with these isolates. Two independent pools of cells expressing RasG37 and RasS35 were examined.
TSH and cAMP analogs exerted dramatic effects on the cytoskeleton, including cytoplasmic retraction, the dissolution of actin stress fibers, increased peripheral actin staining, and extensive membrane ruffling at sites of cell-cell contact (Fig. 8, bottom). These effects were preserved in cells expressing RasG37 but lost in RasV12 and RasS35 cells, which retained membrane extensions in the presence of TSH.
Because actin rearrangements are often associated with changes in cell-substrate attachment, the ability of these cells to proliferate in soft agar was examined. Both RasV12 and RasS35 cells exhibited anchorage-independent proliferation in the absence of TSH, while parental cells and vector-transfected cells (data not shown) were unable to form colonies under these conditions (Fig. 9). RasG37 cells occasionally formed small colonies in either the presence or absence of TSH; however, these results were inconsistent. Together, these results demonstrate that RasV12 and RasS35 stimulate several markers of transformation: dedifferentiation, cytoskeletal alterations, and anchorage-independent growth. In contrast, RasG37 stimulates proliferation but not transformation.
FIG. 9.
RasV12 and RasS35 stimulate anchorage-independent proliferation. Cells were plated in agar and 2H medium as described in Materials and Methods. Three independent experiments were performed in duplicate with similar results.
DISCUSSION
Two Ras effector mutants which differ in their ability to bind Raf (60) conferred hormone-independent proliferation on thyroid cells through signaling pathways differentially regulated by PKA. cAMP is a mitogen in WRT cells, which are TSH dependent for growth. TSH stimulates proliferation through PKA and Ras (32), but not through Raf-1 (1). RasG37, a mutant that binds RalGDS (61), increased the growth rate of cells in the presence of TSH. Interestingly, this effect was not observed in cells expressing RasV12 or RasS35, proteins competent to bind Raf. These cells grew more slowly than cells expressing RasG37 in the presence of TSH. These results suggest that growth rates in TSH may reflect the affinities of these proteins for Raf-1, an interaction which is inhibited by PKA (reviewed in reference 6). In support of this idea, the rank order activity of the Ras proteins was reversed when the cells were grown in the absence of TSH. Under these conditions, cells expressing RasV12 and RasS35 grew more rapidly than did cells expressing RasG37. The apparent reduction in growth rate observed in RasV12 and RasS35 cells in the presence of TSH could be a consequence of increased apoptosis (25). Because TSH inhibits Ras-Raf signaling, expression of Ras proteins which bind Raf may constitute an inappropriate signal for proliferation in the presence of TSH-stimulated acute cAMP elevation. We have not directly examined apoptosis in these cells; however, cells injected with these Ras proteins are recovered in the expected numbers in the presence of TSH. This indicates that if apoptosis occurs, it does so in a small fraction of cells or with such asynchrony that it is not detected in these assays.
Our results provide the first demonstration that the acute effects of RasS35 or RasG37 include the stimulation of DNA synthesis. These results differ from those of Joneson et al., who reported that microinjection of a vector encoding RasS35 failed to stimulate DNA synthesis in REF52 cells (23). Whether these differences are technical or a consequence of different cellular environments is unclear. Consistent with our findings, RasS35 and RasG37 enhanced the growth rate of NIH 3T3 cells in low serum levels (27).
The effects of TSH on Ras-stimulated proliferation were reflected in effects on MAPK activity. In the absence of TSH, basal levels of MAPK phosphorylation were elevated in RasV12 and RasS35 cells, but not in RasG37 cells. These results are consistent with previous reports that RasS35, but not RasG37, stimulates MAPK activity (23, 25, 27, 49, 60). TSH transiently repressed MAPK phosphorylation in RasV12 and RasS35 cells. This effect most likely reflects the inhibitory action of PKA on Ras-Raf signaling, since it was mimicked by cell-permeative cAMP analogs. The transient nature of this inhibition may reflect downregulation of PKA activity or the stimulation of other pathways that contribute to MAPK activation, since 8-bromo-cAMP-stimulated DNA synthesis is Ras dependent in these cells (32). In RasV12-expressing cells, MAPK inhibition by TSH was accompanied by a decrease in growth rate. The effects of TSH on RasS35-mediated growth were less pronounced, possibly as a consequence of the lower affinity of RasS35 than of RasV12 for Raf, or perhaps due to the higher levels of overexpression of RasS35 than of RasV12. While basal levels of MAPK phosphorylation were low in RasG37 cells, TSH stimulated a dramatic increase in MAPK phosphorylation. This was unexpected, since RasG37 has not been reported to stimulate MAPK activity either alone or in cooperation with Raf-1 (61), and TSH decreased serum- as well as insulin-like growth factor 1-stimulated MAPK activity in parental WRT cells (40). TSH was also reported not to stimulate MAPK activity in primary canine thyroid cells (35). Nonetheless, WRT cells expressing RasG37 grew faster than cells expressing RasV12 or RasS35 in the presence of TSH, suggesting that RasG37 collaborates with pathways activated by TSH to stimulate growth.
Several mechanisms can be envisioned for the effects of TSH on MAPK activity in RasG37 cells. RasG37 binds RalGDS or a related molecule in thyroid cell extracts (47). Microinjection of RalAN28 (22), a mutant protein which binds RalGDS, decreases Ras- and TSH-stimulated DNA synthesis, supporting a role for RalGDS in growth stimulation (40). Consistent with such a role, RalGDS is phosphorylated by PKA in vitro, although phosphorylation reportedly had no effect on exchange factor activity (29). It is conceivable that PKA relocalizes RalGDS to a membrane compartment where it binds Ras, is activated, and stimulates pathways leading to MAPK phosphorylation. We failed to detect any consistent effects of stable expression of RalGDS on cell proliferation; however, we were unable to drive RalGDS expression in thyroid cells to high levels. In addition to binding Ras (20, 28, 52, 60), RalGDS binds Rap1A (18, 21, 43, 56, 62), originally identified as K-rev1 (30). PKA activates Rap1B (3), and microinjection of Rap1B stimulates DNA synthesis in cAMP-responsive Swiss 3T3 cells (65), suggesting a role for Rap in cAMP-mediated proliferation. cAMP activates MAPK through Rap1 and B-Raf in PC12 cells (59), providing one potential mechanism through which TSH could stimulate MAPK activity in RasG37 cells. However, in PC12 cells, the effects of cAMP on MAPK were Ras independent. In Swiss 3T3 cells, prolonged platelet-derived growth factor-stimulated MAPK activation requires protein kinase C and PI3K activity (16). The mitogenic activity of TSH requires PI3K and p70S6k activity (9a). It is possible that PI3K contributes to the effects of TSH on MAPK activity observed in RasG37 cells in a manner that does not invoke PI3K binding to RasG37. Rho family members cooperate with Raf to activate MAPK (14), as does p21-activated kinase (55). Whether TSH signals through Rho family members is not yet clear, although it is interesting that Ral binding proteins with cdc42 GTPase-activating protein-like domains have been identified (8, 24, 42).
Cross talk between Ras and PKA is bidirectional. While TSH differentially affects Ras-mediated growth, Ras mutants exert distinct effects on TSH signaling. RasV12 and RasS35 stimulate morphological transformation, anchorage-independent growth, and dedifferentiation. In contrast with earlier reports (23, 49), thyroid cells expressing RasS35 exhibited dramatic membrane ruffles, an effect that was mimicked in cells expressing RasV12 but not RasG37. Whether this is a direct effect of RasS35 or is secondary to autocrine secretion is not yet clear. As reported previously, NIH 3T3 cells expressing RasV12 or RasS35 but not RasG37 also exhibited a decrease in the number of actin stress fibers (27). In WRT cells, TSH stimulates rapid morphological changes including cytoplasmic retraction and extensive ruffling at sites of cell-cell contact. These effects of TSH were abolished in RasV12 and RasS35 cells but retained in RasG37 cells. This provides another molecular marker of differential cross talk between PKA and Ras effectors. In this case, the effects of Ras predominate over those of TSH.
Morphological transformation elicited by RasV12 and RasS35 was accompanied by a new ability of these cells to form colonies in soft agar in the absence of TSH. WRT cells form small colonies in the presence, but not in the absence, of TSH. Expression of RasV12 or RasS35 conferred TSH-independent growth in agar, while expression of RasG37 did not. These effects differ somewhat from those reported for NIH 3T3 cells, where both mutants promoted anchorage-independent growth.
TSH is required for maintenance of the differentiated phenotype (reviewed in references 11 and 58) by affecting the expression of thyroid-specific gene products, including the TSH receptor (31), the Na-I symporter (reviewed in reference 9), thyroid peroxidase (37), and Tg (19). Ras decreases the expression of some thyroid-specific genes (4, 15). In WRT cells, acute or chronic expression of RasV12 abolished TSH-stimulated Tg expression (33). This effect appears to be Raf dependent, since RasS35 exhibited similar effects while RasG37 did not. As for the actin cytoskeleton, the effects of RasV12 and RasS35 on differentiation were dominant over the effects of TSH. In 10T1/2 cells, RasV12 and RasS35 also interfered with a marker of differentiation, MyoD-induced myogenesis (46).
Substantial progress in understanding the mechanisms through which Ras exerts its biological effects has been made. There are multiple effector pathways activated by Ras, and those pathways can vary in different cells. The challenge is to understand how Ras-mediated signals are directed at distinct effector pathways. Our results indicate that cross talk between signaling pathways, i.e., Ras and PKA, influences the effectors through which Ras conveys its signals. In turn, discrete Ras effector pathways differentially affect the cAMP/PKA pathway. Interplay between these signaling pathways may allow cells to respond appropriately to changing cues in their cellular environment.
ACKNOWLEDGMENTS
We thank Lisa Cass for helpful discussions and Jonathan Nissanov for technical advice.
This work was supported by PHS grants DK45696 and DK 02494 awarded to J.M.
REFERENCES
- 1.Al-Alawi N, Rose D W, Buckmaster C, Ahn N, Rapp U, Meinkoth J, Feramisco J R. Thyrotropin-induced mitogenesis is Ras dependent but appears to bypass the Raf-dependent cytoplasmic kinase cascade. Mol Cell Biol. 1995;15:1162–1168. doi: 10.1128/mcb.15.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright C F, Giddings B W, Liu J, Vito M, Weinberg R A. Characterization of a guanine nucleotide dissociation stimulator for a ras-related GTPase. EMBO J. 1993;12:339–347. doi: 10.1002/j.1460-2075.1993.tb05662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschuler D L, Peterson S N, Ostrowski M C, Lapetina E G. Cyclic AMP-dependent activation of Rap1b. J Biol Chem. 1995;270:10373–10376. doi: 10.1074/jbc.270.18.10373. [DOI] [PubMed] [Google Scholar]
- 4.Avvedimento V E, Musti A, Fusco A, Bonapace M J, Lauro R D. Neoplastic transformation inactivates specific trans-acting factor(s) required for the expression of the thyroglobulin gene. Proc Natl Acad Sci USA. 1988;85:1744–1748. doi: 10.1073/pnas.85.6.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandi M L, Rotella C M, Mavilia C, Franceschelli F, Tanini A, Toccafondi R. Insulin stimulates cell growth of a new strain of differentiated rat thyroid cells. Mol Cell Endocrinol. 1987;54:91–103. doi: 10.1016/0303-7207(87)90142-0. [DOI] [PubMed] [Google Scholar]
- 6.Burgering B M T, Bos J L. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- 7.Burns J S, Blaydes J P, Wright P A, Lemoine L, Bond J A, Williams E D, Wynford-Thomas D. Stepwise transformation of primary thyroid epithelial cells by a mutant Ha-ras oncogene: an in vitro model of tumor progression. Mol Carcinog. 1992;6:129–139. doi: 10.1002/mc.2940060208. [DOI] [PubMed] [Google Scholar]
- 8.Cantor S B, Urano T, Feig L A. Identification and characterization of Ral-binding protein 1, a potential downstream target of Ral GTPases. Mol Cell Biol. 1995;15:4578–4584. doi: 10.1128/mcb.15.8.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 9a.Cass L A, Meinkoth J L. Differential effects of cyclic adenosine 3′, 5′-monophosphate or p70 ribosomal S6 kinase. Endocrinology. 1998;139:1991–1998. doi: 10.1210/endo.139.4.5880. [DOI] [PubMed] [Google Scholar]
- 10.Cook S J, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1059–1062. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 11.Dumont J E, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. 1992;72:667–697. doi: 10.1152/physrev.1992.72.3.667. [DOI] [PubMed] [Google Scholar]
- 12.Feramisco J R, Gross M, Kamata T, Rosenberg M, Sweet R W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984;38:109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- 13.Frodin M, Peraldi P, Obberghen E V. Cyclic AMP activates the mitogen-activated protein kinase cascade in PC12 cells. J Biol Chem. 1994;269:6207–6214. [PubMed] [Google Scholar]
- 14.Frost J A, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P E, Cobb M H. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusco A, Berlingieri M T, Di Fiore P P, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grammer T C, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- 17.Häfner S, Adler H S, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994;14:6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann C, Horn G, Spaargaren M, Wittinghofer A. Differential interaction of the Ras family GTP-binding proteins H-Ras, Rap1A, and R-Ras with the putative effector molecules Raf kinase and Ral-guanine nucleotide exchange factor. J Biol Chem. 1996;271:6794–6800. doi: 10.1074/jbc.271.12.6794. [DOI] [PubMed] [Google Scholar]
- 19.Heuverswyn B V, Steydio C, Brocas H, Refetoff S, Dumont J, Vassart G. Thyrotropin controls transcription of the thyroglobulin gene. Proc Natl Acad Sci USA. 1984;81:5941–5945. doi: 10.1073/pnas.81.19.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofer F, Fields S, Schneider C, Martin G S. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci USA. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda M, Koyama S, Okazaki M, Dohi K, Kikuchi A. rap1 p21 regulates the interaction of ras p21 with RGL, a new effector protein of ras p21. FEBS Lett. 1995;375:37–40. doi: 10.1016/0014-5793(95)01169-f. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Luo J-Q, Urano T, Frankel P, Lu Z, Foster D A, Feig L A. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995;378:409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- 23.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 24.Julien-Flores V, Dorseuil O, Romero F, Letourneur F, Saragosti S, Berger R, Tavitian A, Gacon G, Camonis J H. Bridging Ral GTPase to Rho pathways. J Biol Chem. 1995;270:22473–22477. doi: 10.1074/jbc.270.38.22473. [DOI] [PubMed] [Google Scholar]
- 25.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulruch E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 26.Khosravi-Far R, Solski P A, Clark C J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Aelst L V, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi A, Demo S D, Ye Z-H, Chen Y-W, Williams L T. ralGDS family members interact with the effector loop of ras p21. Mol Cell Biol. 1994;14:7483–7491. doi: 10.1128/mcb.14.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi A, Williams L T. Regulation of interaction of rasp21 with RalGDS and Raf-1 by cyclic AMP-dependent protein kinase. J Biol Chem. 1996;271:588–594. doi: 10.1074/jbc.271.1.588. [DOI] [PubMed] [Google Scholar]
- 30.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 31.Kohn L D, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287–385. doi: 10.1016/s0083-6729(08)60658-5. [DOI] [PubMed] [Google Scholar]
- 32.Kupperman E, Wen W, Meinkoth J L. Inhibition of thyrotropin-stimulated DNA synthesis by microinjection of inhibitors of cellular Ras and cyclic AMP-dependent protein kinase. Mol Cell Biol. 1993;13:4477–4484. doi: 10.1128/mcb.13.8.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kupperman E, Wofford D, Wen W, Meinkoth J L. Ras inhibits thyroglobulin expression but not cyclic adenosine monophosphate-mediated signaling in Wistar rat thyrocytes. Endocrinology. 1996;137:96–104. doi: 10.1210/endo.137.1.8536648. [DOI] [PubMed] [Google Scholar]
- 34.LaMorte V, Goldsmith A, Spiegel J, Meinkoth J, Feramisco J. Inhibition of DNA synthesis in living cells by microinjection of Gi2 antibodies. J Biol Chem. 1992;267:691–694. [PubMed] [Google Scholar]
- 35.Lamy F, Wilkin F, Baptist M, Posada J, Roger P P, Dumont J E. Phosphorylation of mitogen-activated protein kinases is involved in the epidermal growth factor and phorbol ester, but not in the thyrotropin/cAMP, thyroid mitogenic pathway. J Biol Chem. 1993;268:8398–8401. [PubMed] [Google Scholar]
- 36.Lemoine N R, Staddon S, Bond J, Wyllie F S, Shaw J J, Wynford-Thomas D. Partial transformation of human thyroid epithelial cells by mutant Ha-ras oncogene. Oncogene. 1990;5:1833–1837. [PubMed] [Google Scholar]
- 37.Magnusson R P, Rapoport B. Modulation of differentiated function in cultured thyroid cells: thyrotropin control of thyroid peroxidase activity. Endocrinology. 1985;116:1493–1500. doi: 10.1210/endo-116-4-1493. [DOI] [PubMed] [Google Scholar]
- 38.Marshall M S. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–1318. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 39.Meinkoth J L, Cruz J D, Burrow G N. TSH, IGF-1 and activated ras protein induce DNA synthesis in cultured thyroid cells. Thyroidology. 1991;3:97–102. [PubMed] [Google Scholar]
- 40.Miller M J, Prigent S, Kupperman E, Rioux L, Park S-H, Feramisco J R, White M A, Rutkowski J L, Meinkoth J L. RalGDS functions in Ras- and cAMP-mediated growth stimulation. J Biol Chem. 1997;272:5600–5605. doi: 10.1074/jbc.272.9.5600. [DOI] [PubMed] [Google Scholar]
- 41.Oldham S M, Clark G J, Gangarosa L M, Coffey J R., Jr Activation of the Raf-1/MAP kinase cascade is not sufficient for Ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA. 1996;93:6924–6928. doi: 10.1073/pnas.93.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park S-H, Weinberg R A. A putative effector of Ral has homology to Rho/Rac GTPase activating proteins. Oncogene. 1995;11:2349–2355. [PubMed] [Google Scholar]
- 43.Peterson S N, Trabalzini L, Brtva T R, Fischer T, Altschuler D L, Martelli P, Lapetina E G, Der C J, White G C., II Identification of a novel RalGDS-related protein as a candidate effector for Ras and Rap1. J Biol Chem. 1996;271:29903–29908. doi: 10.1074/jbc.271.47.29903. [DOI] [PubMed] [Google Scholar]
- 44.Prendergast G C, Khosravi-Far R, Solski P A, Kurzawa H, Lebowitz P F, Der C J. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- 45.Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 46.Ramocki M B, Johnson S E, White M A, Ashendel C L, Konieczny S F, Taparowsky E J. Signaling through mitogen-activated protein kinase and Rac/Rho does not duplicate the effects of activated Ras on skeletal myogenesis. Mol Cell Biol. 1997;17:3547–3555. doi: 10.1128/mcb.17.7.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rioux, L. Unpublished data.
- 48.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–530. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 50.Scheele J S, Rhee J M, Boss G R. Determination of the absolute amounts of GDP and GTP bound to Ras in mammalian cells: comparison of parental and Ras-overproducing NIH3T3 fibroblasts. Proc Natl Acad Sci USA. 1995;92:1097–1100. doi: 10.1073/pnas.92.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Self A J, Hall A. Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 1995;256:3–10. doi: 10.1016/0076-6879(95)56003-3. [DOI] [PubMed] [Google Scholar]
- 52.Spaargaren M, Bischoff J R. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, and Rap. Proc Natl Acad Sci USA. 1994;91:12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stang S, Bottorff D, Stone J C. Interaction of activated Ras with Raf-1 alone may be sufficient for transformation of rat2 cells. Mol Cell Biol. 1997;17:3047–3055. doi: 10.1128/mcb.17.6.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szeberenyi J, Erhardt P, Cai H, Cooper G M. Role of Ras in signal transduction from the nerve growth factor receptor: relationship to protein kinase C, calcium and cyclic AMP. Oncogene. 1992;7:2105–2113. [PubMed] [Google Scholar]
- 55.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs J B, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997;17:4454–4464. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 57.Vaillancourt R R, Gardner A M, Johnson G L. B-Raf-dependent regulation of the MEK-1/mitogen-activated protein kinase pathway in PC12 cells and regulation by cyclic AMP. Mol Cell Biol. 1994;14:6522–6530. doi: 10.1128/mcb.14.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vassart G, Dumont J E. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 59.Vossler M R, Yao H, York R D, Pan M-G, Rim C S, Stork P J. cAMP activates MAP kinase and elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 60.White M A, Nicolette C, Minden A, Polverino A, Aelst L V, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 61.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for RalGDS in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 62.Wolthuis R M W, Bauer B, van’t Veer L J, de Vries-Smits A M M, Cool R H, Spaargaren M, Wittinghofer A, Burgering B M, Bos J L. RalGDS-like factor (Rlf) is a novel Ras and Rap1A-associating protein. Oncogene. 1996;13:353–362. [PubMed] [Google Scholar]
- 63.Wu J, Dent P, Jelinek T, Wolfman A, Weber M J, Sturgill T A. Inhibition of EGF-activated MAP kinase signaling pathway by adenosine 3′5′-monophosphate. Science. 1993;262:1065–1068. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 64.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida Y, Kawata M, Miura Y, Musha T, Sasaki T, Kikuchi A, Takai Y. Microinjection of smg/rap1/Krev-1 p21 into Swiss 3T3 cells induces DNA synthesis and morphological changes. Mol Cell Biol. 1992;12:3407–3414. doi: 10.1128/mcb.12.8.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]