Abstract
Objective:
The MitraClip from Abbott is FDA approved intracardiac implantable device for transcatheter edge-to-edge repair (TEER). Despite a few previously published studies, there is limited safety data for its use in clinical practice, hence, we designed this study using data obtained from a safety nationwide database to demonstrate the safety profile of MitraClip.
Methods:
The first two of the five authors independently queried all reported adverse events from the United State Food and Drug Administration [FDA] Manufacturer and User Facility Device Experience [MAUDE] registry from January 2014 to December 2020. The primary end point was trend in reported fatal events obtained from this database. The secondary end points included the causes of reported nonfatal reports from the MAUDE registry. The trend of reported fatal events was assessed using the Cochran Armitage trend test over the period of the study.
Results:
During the study period, subjects included 3370 patients whose MitraClip-associated adverse events were reported and captured by MAUDE registry. Of these, 211 were fatal and 3159 nonfatal events. Fatal event reports resulted deaths and reported nonfatal events were from injuries and device system malfunction. This study demonstrated an initial upward trend from 2014 to 2015 then a subsequent statistically significant downward trend in reported fatal events from 2015 to 2020 (Cochran-Armitage test P = 0.039). The peak proportion of reported fatal events occurred in 2015, (n = 44; representing 1.25% of reported adverse events) and lowest proportion of reported fatal events took place in 2020 (n = 19; representing 0.56% of reported adverse events). The most reported nonfatal events were from malfunctioning of MitraClip system (n = 1170; representing 37% of reported nonfatal events), new unremarkable repolarization abnormalities on periprocedural EKG (n = 864; representing 27% of reported nonfatal events), leaflet rupture (n = 651; representing 21% of reported nonfatal events), and cardiogenic shock (n = 170; representing 5% of reported nonfatal events).
Conclusions:
This analysis of the MAUDE Registry indicated, especially within the confines of this study’s limitations and poor data quality of information, an apparent downward trend of reported fatal events over the study period. Even though conclusive attributions cannot be made regarding this important finding, perhaps, this points to early evidence of a potential institutional or operator learning curve with this device. However, in view of the inferior quality of the data accrued from the MAUDE Registry, more high-precision studies are needed to better understand these changes, as the utility of MitraClip, becomes more established in clinical practice.
Keywords: MitraClip, Adverse events, Fatal events
1. Introduction
Mitral regurgitation (MR) is the most common valvular heart disease in the United States [1], affecting more than 2 million people [1,2]. Its yearly mortality rate is about 3% for moderate primary mitral regurgitation and about 6% for severe disease, despite guideline-directed medical therapy (GDMT) [2]. This is true for patients aged 65 years and over [2]. In fact, it is the second most common form of Valvular heart disease needing surgery in all of Europe [3]. Historically, surgery is considered the treatment of choice for primary mitral regurgitation, the only modality proven to improve symptoms [4] and prevent heart failure [2], despite inconclusive clinical evidence on mortality benefit [5,6]. There is, however, a subset of patients who were considered prohibitive risk for surgical intervention, and as such, were left to their demise, in the setting of limited therapeutic options. The EVEREST II (Endovascular Valve Edge-to-Edge Repair Study) trial provided one of the initial evidence of transcatheter edge-to-edge repair in the improvement of clinical outcomes for patients with primary mitral regurgitation who were considered high risk for surgery [7]. Furthermore, the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart failure Patients with Functional Mitral Regurgitation) trial also proved that transcatheter edge-to-edge repair using MitraClip resulted in a 32% absolute reduction in hospitalizations and a 16% absolute reduction in all-cause mortality in patients with symptomatic heart failure and severe secondary mitral regurgitation [8]. Clinical practice guidelines now recommend transcatheter edge-to-edge repair with MitraClip for severely symptomatic patients (NYHA class III or IV) with primary severe MR who are high or prohibitive surgical risk with a life expectancy of at least 1 year (class 2A) [9]. Transcatheter Edge-to-Edge Repair (TEER) is also considered reasonable in carefully selected symptomatic patients with chronic severe secondary MR with left ventricular systolic dysfunction (LVEF<50%) despite GDMT and those with appropriate valvular anatomy (class 2A) [9].
Since the approval of MitraClip by the United State Food and Drug Administration [FDA], there have been limited safety data published outside the setting of clinical trials in the US. To the best of our knowledge, two previously published MAUDE reports were from Benjamin et al. and Mahabir et al. in 2018 and 2020 respectively. In the Benjamin et al. report, they concluded that the natural language processing technology that sorted through raw MAUDE data, allowed the identification of most common Trans catheter aortic valve replacement (TAVR) and MitraClip associated events. However, when the reported event rates from the MAUDE database were compared with rates from Transcatheter Valve Therapy (TVT) registry, they were not statistically different [12]. Furthermore, Mahabir et al. showed that while injuries reported to the FDA steadily increased over the first 4 year after approval of MitraClip, device- or procedure- related death reported accrued more slowly, corroborating a potential learning curve with the device [13]. Thus, we analyzed the Manufacturer and User Facility Device Experience [MAUDE] registry to report our findings of the safety profile of TEER using MitraClip over a longer period of seven year post FDA approval.
2. Material and methods
The MAUDE is a registry administered under the auspices of the FDA, consisting of mandatory reports of adverse events from manufacturers and dealers of medical devices in the United States. It also allows for discretionary reporting of these events by healthcare providers, patients, and users of these devices [10]. These adverse events include device-associated injuries, malfunctions, and death. Data from the MAUDA registry is freely available to general public. The first two of five authors independently queried the MAUDE registry for all fatal and nonfatal MitraClip- associated adverse events from January 2014 to December 2020 using following search filters: product code “NKM,” (FDA assigned code for MitraClip) and product class “Mitral Valve Repair Devices”. The primary end point was trend in reported fatal adverse event. The secondary end points included the causes of reported nonfatal reports. Cochran-Armitage trend analysis was applied to number of fatal adverse events to assess a statistically significant trend over the period of study.
3. Results
During the study period between January 2014 to December 2020, 3370 unique adverse events from FDA approved transcatheter implantable device, MitraClip were obtained. Reports of All cause adverse events were 482(14.3% of the reported adverse events over the study period) in 2014, which apparently decrease to 417(12.37% of the reported adverse events over the study period) in 2015. Thereafter, reports of All cause adverse events appeared to increase to 518(15.37% of the reported adverse events over the study period) in 2018. They subsequently trended downwards to 465(13.80% of the reported adverse events over the study period) in 2020 (Fig. 1). Trend analysis using Cochran-Armitage testing was applied, however, this showed that the apparent trend seen was not statistically significant over the seven years of the study (P = 0.507). An analysis of reported All-cause adverse events, revealed a total of 211 reported fatal events, representing 6.26% of reported All-cause reports during the study period. A trend depiction of the fatal events using the Cochran-Armitage trend testing showed a downward trend of deaths from a peak 1.31% of reported All-cause adverse events (n = 44 deaths) in 2015 to a trough of 0.56% of reported All-cause adverse events (n = 19 deaths) in 2020, P = 0.039. (Fig. 2). Non-fatal events were from device-associated injury reports and MitraClip device malfunctions. A total of 3159 reported non-fatal events were recorded which represented 93.74% of reported All cause adverse events and consisted of 1989(62.96% of reported non-fata events) device-associated injuries and 1170(37.04% of reported non-fata events) MitraClip device malfunctions. The Most commonly reported nonfatal events were caused by malfunctioning of MitraClip system representing 37% of reported nonfatal events (n = 1170), new unremarkable repolarization abnormalities on periprocedural EKG, 27% of reported nonfatal events (n = 864), leaflet rupture 21% of reported nonfatal events (n = 651), and cardiogenic shock 5% of reported nonfatal events (n = 170). (Fig. 3).
Fig. 1.
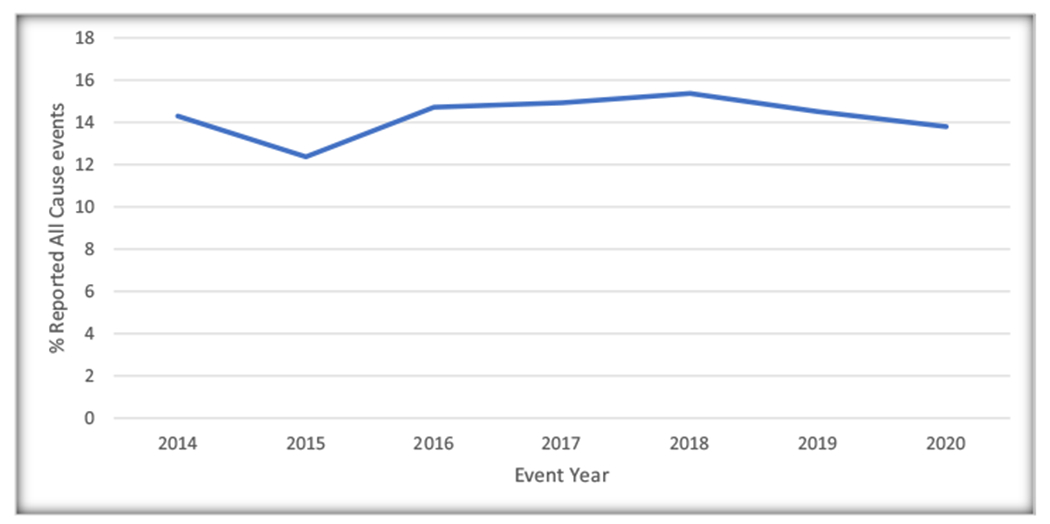
Yearly percentage of total All-Cause Adverse Events reported to the United States Food and Drug Administration [FDA] Manufacturer and User Facility Device Experience [MAUDE] database for MitraClip, 2014–2020.
Fig. 2.
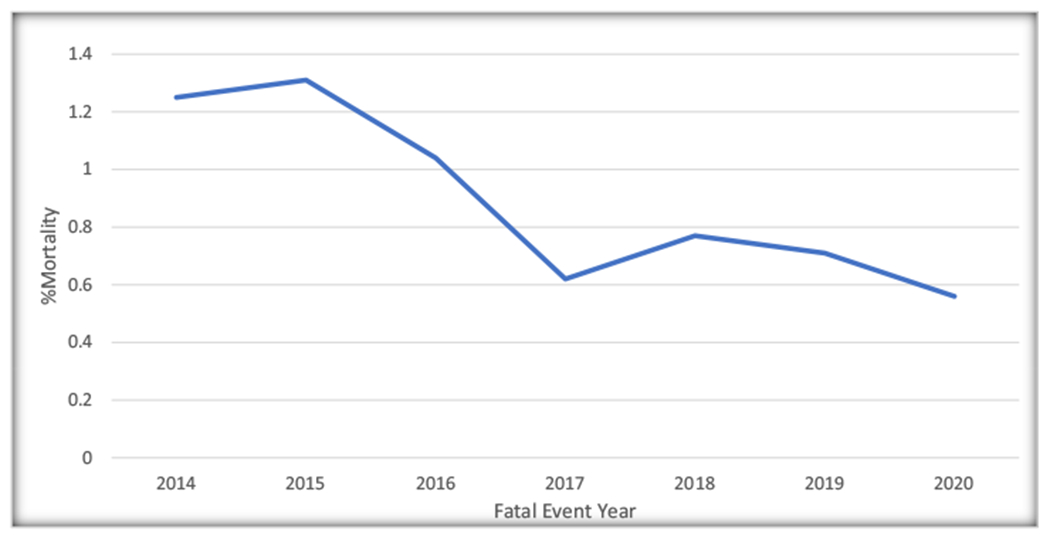
Trend of Reported MitraClip associated fatal events (expressed as a percentage of all reported events) reported to the United State Food and Drug Administration [FDA] Manufacturer and User Facility Device Experience [MAUDE] database for MitraClip, 2014–2020.
Fig. 3.
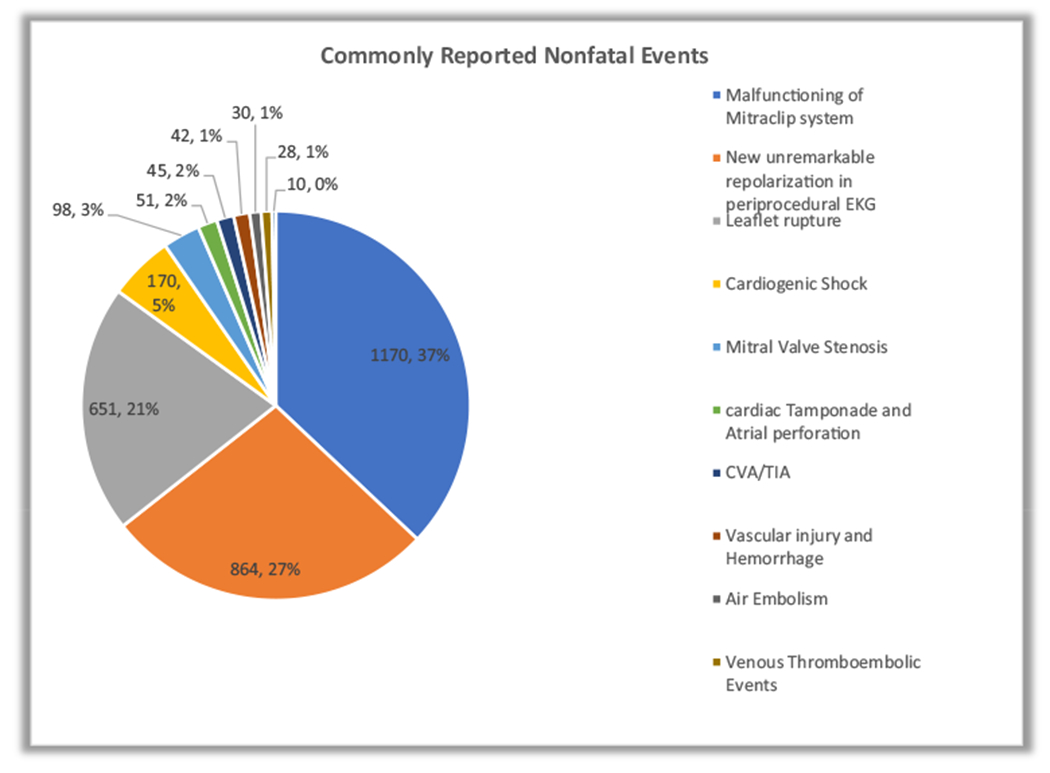
Nonfatal Events (expressed as a percentage of all reported nonfatal events) reported to the United States Food and Drug Administration [FDA] Manufacturer and User Facility Device Experience [MAUDE] database for MitraClip, 2014–2020.
4. Discussion
In this study of a national Database of reported adverse everts following MitraClip procedure, the apparent trend of reported fatal events showed a progressive decrease in reported MitraClip-related deaths from peak number of 44 to 19 during the study period (P = 0.039, using Cochran-Armitage test). This represented more than 50% decrease in these reported events over the last 5 years from 2015 to 2020. This apparent downward trend of reports of fatal events may likely point to, perhaps, a potential operator and institutional learning curve which may, perhaps, be due to improved proficiency in the placement of these devices especially, in the later years compared to the earlier years of its approval. Mahabir and colleagues also reported this potential institutional or operator learning curve with MitraClip as they showed device- or procedure- related death reports accrued more slowly during the first 4 years of its FDA approval in light of the incomplete and poor data quality obtained from MAUDE [13]. This trend is likely to continue into the foreseeable future.
The apparent downward trend of reported All-cause events from the high reported number of events in the early years following FDA approval of the device, compared with the latter is difficult to interpret in the context of decreasing trends in procedural deaths. This may perhaps be attributed to flaws in device engineering likely to be improved in the latest iteration of the device. This must be interpreted in its proper context as the trend analysis using Cochran-Armitage testing showed no statistically significant trend over the period of the study. Furthermore, Benjamin et al. also showed that Natural language processing technology was able to identify the most common reported MitraClip-related and TAVR-related events using MAUDE registries. However, there was no statistical difference in event rate when MAUDE obtained results were compared to those of TVT database [12].
In the EVEREST II trial, the two most reported adverse events in the MitraClip group were new onset Atrial fibrillation and Acute Kidney Injury, when compared to the surgical arm [7,11]. These were all patient related injuries, however, in this study, the topmost causes of non-fatal events were attributed to the malfunction of the mitraClip device. Most of these malfunctions, though, not fatal, were because of incomplete coaptation of the mitral valve leaflets, failure of the delivery mechanism of the device, and difficulty with positioning of the device. This may indicate engineering flaws. These engineering flaws may like to be corrected by more refined iterations of the old version of earlier MitraClip device. Overall, the future outlook of the MitraClip looks very favorable, especially in the setting of decreasing trends of reported device-related death and its application in clinical practice is likely to be more commonplace.
4.1. Study limitations
The limitation of the study stems largely from the nature of the data gleaned from the MAUDE Registry. Since the generation of these reports partly relies on voluntary reporting from patients and customers, some important safety events are likely to be omitted and not reported. Hence skewing any result obtained from studies with data obtained from the MAUDE Registry. Additionally, some of the adverse events reported are likely to be reported multiple times, especially, when manufacturers, providers and patients all report the same event. Furthermore, some long-term adverse events are likely to be omitted, or under reported in this passive self-reporting database. Results from this study should be interpreted in its proper contexts. Since the MAUDE registry does not provide records of all MitraClip device utilization in the country, prevalence, rates, and incidence of any entity cannot be extrapolated from the reported events in the MAUDE database. Finally, the veracity of the data obtained are not authenticated by any independent bodies, as the FDA only collects the reports of adverse events reported but does not verify their validity.
5. Conclusion
In the Era of post- COAPT trial, the utility and clinical application of TEER is likely to expand to include other subsets of Mitral regurgitation patients. This analysis of the MAUDE Registry indicated, especially within the confines of this study’s limitations and the inferior quality of MAUDE-derived data, an apparent downward trend of reported fatal events over the study period. Even though conclusive attributions cannot be made regarding this important finding, perhaps, this points to early evidence of a potential institutional or operator learning curve with this device. As more interventionalists and institutions continue to perform these procedures, they are likely to obtain valuable experience with the implantation of the MitraClip in a safe and effective manner leading to improved outcomes. Manufacturers are also likely to improve the engineering of the MitraClip further improving the safety profile of the device. However, in view of the incomplete and poor quality of data accrued from the MAUDE Registry, more high precision studies are needed to better understand these changes, as the utility of MitraClip, become more established in clinical practice.
Footnotes
CRediT authorship contribution statement
Sheriff N. Dodoo: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization, Investigation, Writing – review & editing. Alexis K. Okoh: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization, Investigation, Writing – review & editing. Abdullahi Oseni: Writing – review & editing. Oghenerukevwe Odiete: Writing – review & editing. Henry E. Okafor: Supervision.
Declaration of competing interest
This is to state that the Author of this Manuscript titled “Adverse Events Following Transcatheter Mitral Valve Repair using MitraClip: Lessons learned from the Manufacturer and User Facility Device Experience (MAUDE) Registry” have no conflicts of interest to declare.
References
- [1].Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- [2].Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–94. 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- [3].Lung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: the euro heart survey on valvular heart disease. Eur Heart J. 2003;24:1231–43. 10.1016/s0195-668x(03)00201-x12831818. [DOI] [PubMed] [Google Scholar]
- [4].Phillips HR, Levine FH, Carter JE, Boucher CA, Osbakken MD, Okada RD, et al. Mitral valve replacement for isolated mitral regurgitation: analysis of clinical course and late postoperative left ventricular ejection fraction. Am J Cardiol. 1981;48:647–54. 10.1016/0002-9149(81)90142-97282546. [DOI] [PubMed] [Google Scholar]
- [5].Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al. Joint task force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome). 2013;14:167–214. 10.1714/1234.13659. [DOI] [PubMed] [Google Scholar]
- [6].Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, Butter C, et al. Percutaneous mitral valve interventions in the real world: early and 1- year results from the ACCESS-EU, a prospective, multicenter, non-randomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62:1052–61. 10.1016/j.jacc.2013.02.09423747789. [DOI] [PubMed] [Google Scholar]
- [7].Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: results of the EVEREST II study. J Am Coll Cardiol. 2014;64:172–81. 10.1016/j.jacc.2013.12.062. [DOI] [PubMed] [Google Scholar]
- [8].Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–18. 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- [9].Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–227. 10.1161/CIR.0000000000000923 Epub 2020. Erratum in: Circulation. 2021 Feb 2;143: e229. [DOI] [PubMed] [Google Scholar]
- [10].MAUDE. Manufacturer and user facility device experience. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. [Accessed 15 July 2021].
- [11].Kelley C, Lazkani M, Farah J, Pershad A. Percutaneous mitral valve repair: a new treatment for mitral regurgitation. Indian Heart J. 2016;68:399–404. 10.1916/j.ihj.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benjamin ZG, David EB, Gregory L, Englander LL. Comparison of adverse event and device problem rates for transcatheter aortic valve replacement and MitraClip procedures as reported by the transcatheter valve therapy registry and the Food and Drug Administration postmarket surveillance data. Am Heart J. 2018;198:64–74. 10.1016/j.ahj.2017.10.013. [DOI] [PubMed] [Google Scholar]
- [13].Mahabir CA, DeFilippis EM, Aggarwal S, Bath A, Qamar A, Patel NK, et al. The first 4 years of postmarketing safety surveillance related to the MitraClip device: a United States Food and Drug Administration MAUDE experience. J Invasive Cardiol. 2020;32:E130–2. [DOI] [PubMed] [Google Scholar]


