Abstract
Objective
Severe preeclampsia complicates roughly 1% of all pregnancies. One defining feature of severe preeclampsia is new onset visual disturbance. The accessibility of the choroid to high-resolution, noninvasive imaging makes it a reasonable target of investigation for disease prediction, stratification, or monitoring in preeclampsia. This study aimed to compare subfoveal choroidal thickness between women with severe preeclampsia and those with normotensive pregnancies, and to investigate associations between such findings and other indicators of disease severity, including gestational age and serum angiogenic factors.
Study Design
We designed a case-control study comprised of 36 women diagnosed with severe preeclampsia (cases) matched to 37 normotensive women (controls) by race/ethnicity and parity, all diagnosed in the postpartum period. All patients underwent enhanced depth imaging spectral-domain optical coherence tomography and serum analysis.
Results
Cases showed no difference in subfoveal choroidal thickness compared with controls (p = 0.65). Amongst cases, subfoveal choroidal thickness and gestational age at delivery were inversely related (r = 0.86, p <.001). There was a positive association of placental growth factor with subfoveal choroidal thickness amongst cases (r = 0.54, p = 0.002).
Conclusion
This study suggests a relationship between the degree of disease severity and the magnitude of choroidal thickening. We also show an association between this index and placental growth factor level in the postpartum period. Preeclampsia is a multisystem disease of pregnancy characterized by new onset hypertension with proteinuria or end-organ dysfunction after 20 weeks of gestation.1 It occurs in approximately 3.4% of pregnancies in the United states.2 Because the only known cure is delivery of the placenta, preeclampsia is a leading cause of preterm delivery and associated morbidities in the newborn. Severe preeclampsia, defined by the presence of severely elevated blood pressure or other systemic features, complicates roughly 1% of all pregnancies.3 One defining feature of preeclampsia with severe features (sPE) is new onset visual disturbance, which can include obscuration, photopsia, scotoma, or visual loss.4,5
Keywords: EDI SD-OCT, placental growth factor, preeclampsia with severe features, soluble endoglin, soluble fms-like tyrosine kinase, subfoveal choroidal thickness
Condensation
Subfoveal choroidal thickness is increased in patients with preeclampsia diagnosed at earlier GAs as compared with later GAs and those patients without hypertensive disorders of pregnancy.
There are several case reports and series in the literature describing ophthalmic findings in patients with preeclampsia, including macular edema, subretinal fluid, and serous retinal detachments.6–9 Angiography in patients with retinal findings in the acute phase of the disease has revealed choroidal nonfilling, retinal vascular leakage, and defects in the retinal pigment epithelium (RPE).8,10 Dysregulation of angiogenesis, systemic intravascular inflammation, and endothelial dysfunction, particularly of the fenestrated renal vasculature, are central features of preeclampsia pathophysiology.11 One well-established correlate of these processes is that levels of the circulating factors soluble endoglin (sEng), soluble fms-like tyrosine kinase-1 (sFlt-1), and placental growth factor (PlGF), are demonstrably altered in preeclampsia.12–14
Given that the choroid is (1) a microvascular structure with fenestrated endothelium similar to the renal vasculature, and (2) responsive to sEng, placental growth factor (PlGF) and vascular endothelial growth factor (VEGF), it has been a logical target of investigation for derangements during preeclampsia.15,16 Given the unique accessibility of the choroid to high- resolution, noninvasive imaging, several studies have sought to identify changes in this structure’s thickness. Any significant quantitative changes to the structure or function of this potentially representative microvascular tissue in the setting of preeclampsia could be clinically useful in disease prediction, stratification, and/or monitoring.
Studies have been inconclusive on whether the choroid thickens in women with preeclampsia as compared with normotensive pregnant controls or nonpregnant women. A review of available evidence suggests that while the choroid thickens during a normal pregnancy, it is unclear whether it thickens excessively during preeclampsia; except perhaps in severe cases demonstrating frank macular edema.17,18 We explored multiple factors potentially affecting subfoveal choroidal thickness (SFCT) in preeclampsia. Specifically, we hypothesized that in pregnancies affected by preeclampsia, elevations in sFlt-1 and sEng, as well as decreases in PlGF, would correlate with changes in choroidal thickness (CT).
Materials and Methods
Study Population
The study population for this case-control study consisted of postpartum women diagnosed with severe PE who were matched by ethnicity and parity to a group of postpartum normotensive patients (controls). Inclusion criteria for cases included a diagnosis of severe PE or eclampsia as defined by American College of Obstetricians and Gynecologists guidelines.1 Controls included women who were normotensive before, during, and after delivery. Women with diagnoses of chronic or gestational hypertension, as well as pregestational or gestational diabetes, were excluded from both groups to avoid the potentially confounding effects these processes have on choroidal anatomy and function. Patients meeting inclusion criteria in either group, and lacking any aforementioned exclusion criteria were identified and recruited in the postpartum period (median postpartum day 3, range: 1–14 days), and underwent an ophthalmic examination and blood draw as described below. Features defining the severity of diagnosis were recorded, including maximum systolic and diastolic blood pressure during delivery admission, gestational age (GA) at delivery, and subjective visual disturbances.
Patients were recruited between December 2012 and June 2016. This study received approval through the ethics review committee of The Columbia University Medical Center’s (CUMC) Institutional Review Board (IRB-AAAK4352). Written informed consent was obtained from all patients.
Image Acquisition and Analysis
The Heidelberg Spectralis (Heidelberg Spectralis HRA.OCT version 1.7.0.0; Heidelberg Engineering, Heidelberg, Germany) was used to perform enhanced depth imaging spectral-domain optical coherence tomography (EDI SD- OCT) imaging. Horizontal foveal line scans were used for measurements of the subfoveal choroidal thickness. SD-OCT images were viewed with Heidelberg software (Spectralis Viewing Module 5.4.6.0; Heidelberg Engineering, Heidelberg, Germany). SFCT was measured at the fovea and defined as the distance between the outer portion of the RPE to the inner surface of the sclera, measured in micrometers with the Heidelberg Eye Explorer interactive software’s manual calipers tool.19 Axial length measurements were obtained with IOLMaster (Carl Zeiss Meditec). As SFCT decreases with myopia, elongated axial length was adjusted according to measurements observed by Flores-Moreno et al; adjustments in axial length were made for all patients with an axial length greater than 23.7 mm, with 25.9 μm added to the measured SFCT for every 1-mm increase above 23.7 mm.20
Serum Analysis
All patients underwent a 10 mL blood draw by standard venipuncture performed prior to image acquisition by a qualified research assistant. After clotting at room temperature, serum was centrifugally isolated within 3 hours of collection, then stored on site in a −20°C freezer. All serum was ultimately batch analyzed for PlGF (limit of quantitation (LOQ): 15.6 pg/mL, intra-assay coefficient of variation (CV):5.4%, interassay CV: 11.2%), sEng (LOQ: 0.156 ng/mL, intra-assay CV: 3%, inter-assay CV: 6.5%), and sFlt-1 (LOQ: 31.3pg/ml, intra-assay CV: 2.6%, interassay CV: 6.36%), by the Irving Institute’s Biomarker Core Laboratory. Serum was not available for six of the cases and one of the control patients.
Statistical Analysis
Statistical analyses were performed by creating generalized linear regression models based on the method of generalizing estimating equations to examine the SFCT in relation to the cases and controls. As assessments and data were available from both eyes for each patient, models were corrected for intracluster correlation. Failure to correct for clustering between two eyes within a woman would lead to imprecise variance estimates, and consequently incorrect statistical inferences. As not all cases had a match, residual imbalance was accounted for by adjusting for the confounding effects of race/ethnicity and parity, in addition to maternal age, body mass index. A sample size of 68 (34 subjects and 34 controls) was required to detect a 15% difference in SFCT between cases and controls with a power of 0.8 (β = 0.2) and a two-tailed α = 0.05 based on an expected distribution of SFCT in pregnant women.21
The following measures were independently compared between case and control groups using a nonparametric two-tailed Mann–Whitney U-test: sFlt-1, sEng, PlGF, and sFlt-1:PlGF ratio levels. To investigate the relationship between SFCT and these biomarkers of preeclampsia, we determined a Pearson’s correlation coefficient (r) for each comparison and determined the significance of these correlations with t-tests.
Results
Patient Demographics
This study included 73 patients, 36 severe PE cases and 37 normotensive controls. ►Table 1 summarizes demographic characteristics of these two groups. Cases were examined on postpartum day 3 (range: 1–14 days), and controls were examined on postpartum day 2 (range: 1–3 days). Seventeen of the 36 severe PE cases (47%) and two of 37 controls (5%) reported having at least one subjective visual disturbance during the pregnancy or early postpartum period.
Table 1.
Demographic comparison across the two groups: women with severe preeclampsia and normotensive postpartum controls
| Maternal characteristics | Severe preeclampsia cases (n = 36) | Normotensive controls (n = 37) | p-Value |
|---|---|---|---|
| Maternal age (mean, SD; years) | 33.2 (6.1) | 31.8 (4.9) | 0.298 |
| Race/ethnicity (%) | |||
| African American | 20 | 12 | 0.100 |
| Asian | 8 | 12 | |
| Caucasian | 22 | 30 | |
| Hispanic | 50 | 46 | |
| Primiparity (%) | 65 | 54 | 0.770 |
| Gestational age at delivery (wk) | 33.1 ± 4.5 | 39 ± 1.7 | <0.001 |
| Maximum MAP (mean, SD; mm Hg) | 128.5 ± 13.6 | 105.5 ± 13 | <0.001 |
| Visual symptoms, % | 47 | 5 | <0.001 |
Abbreviatons: MAP, mean arterial pressure; SD, standard deviation.
Features Noted on EDI SD-OCT
After adjusting for myopia, the mean subfoveal choroidal thickness of cases and controls was 351 ± 84 and 351 ± 108 μm, respectively. After adjusting for confounding variables and intracluster correlation between eyes, there was no difference between groups (p = 0.652). Results are summarized in ►Fig. 1, with a representative EDI SD-OCT image and choroidal thickness measurement shown in ►Fig. 2.
Fig. 1.
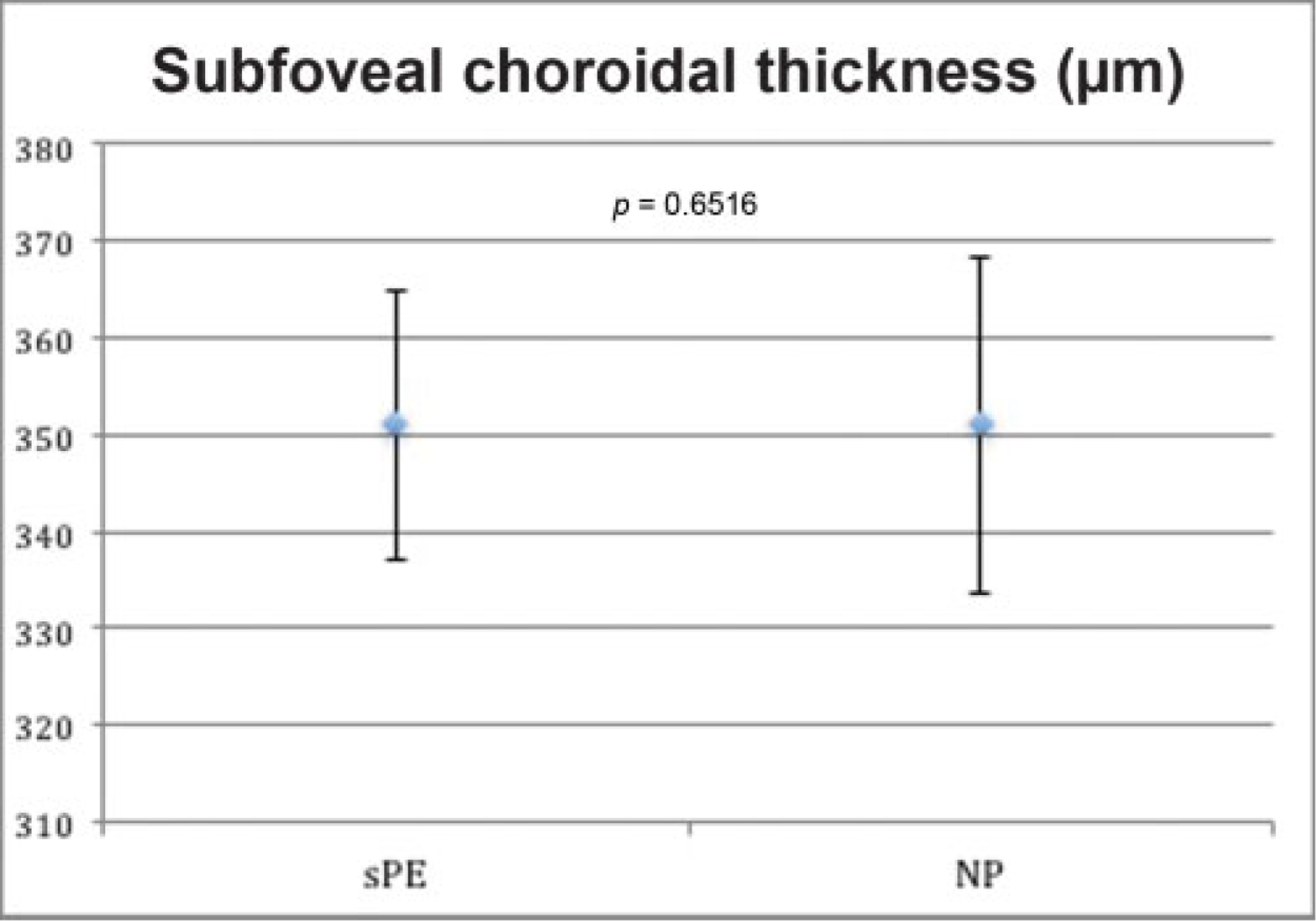
Mean choroidal thickness in severe preeclampsia and normotensive postpartum controls with standard error bars. After adjusting for confounders and intracluster correlation between eyes, there was no significant difference between the groups (p = 0.652). NP, normotensive postpartum; sPE, severe preeclampsia.
Fig. 2.
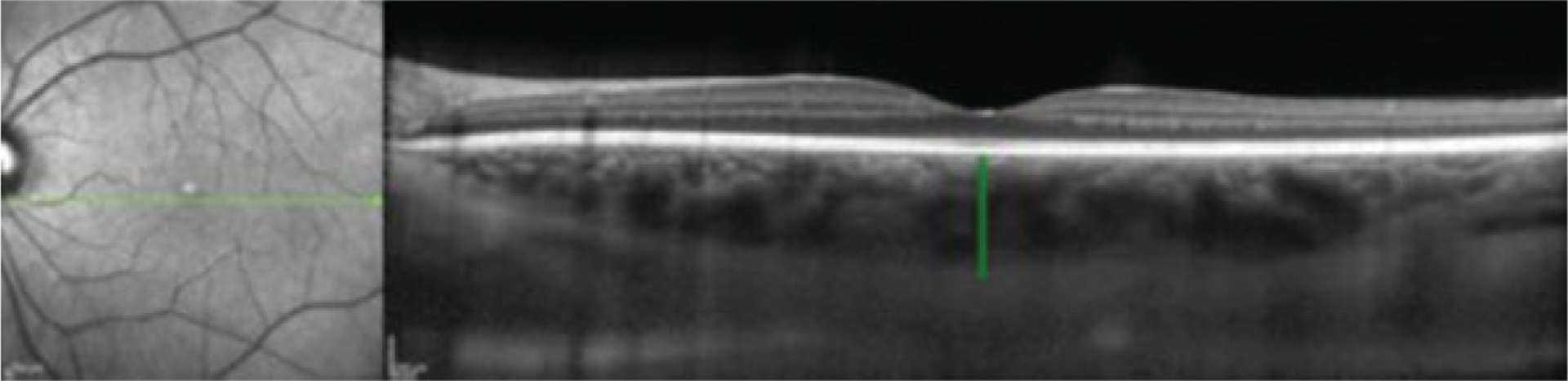
Subfoveal choroidal thickness measurement. EDI SD-OCT image from a patient with severe preeclampsia, demonstrating caliper measurement of subfoveal choroidal thickness. EDI-SD-OCT, enhanced depth imaging spectral-domain optical coherence tomography.
Comparison of SFCT and Disease Severity
Amongst cases, SFCT and GA at delivery were inversely related (r = − 0.71, p < 0.001). Conversely, no relationship was found between choroidal thickness and GA in controls (r = 0.53, p = 0.05). No relationship was found between choroidal thickness and maximum peripartum mean arterial pressure (MAP) amongst cases or controls (r = 0.47, p = 0.09; r = 0.53, p = 0.05). Similarly, no difference was found in CT when comparing those patients who had any reported visual disturbances and those who hadn’t, amongst both cases and controls (p = 0.7). These results are illustrated in ►Figs. 3 and 4. A subanalysis of ophthalmic studies disclosing subretinal fluid revealed an SFCT of 512 ± 74 μm, different from the overall severe PE group (p = 0.003).
Fig. 3.
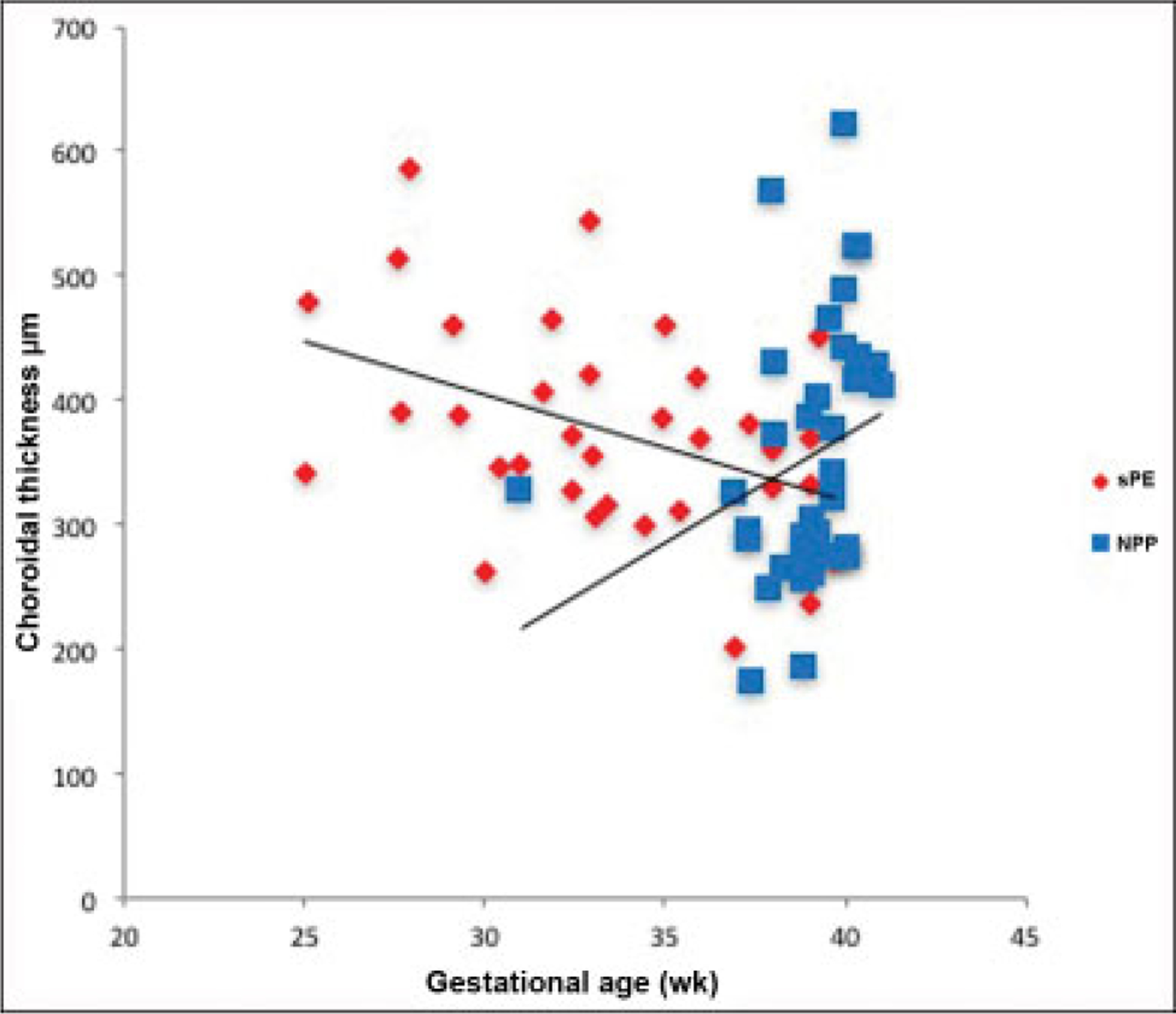
Choroidal thickness in μm versus gestational age at delivery in weeks. For patients with severe preeclampsia, there was an inverse relationship (r = −0.71, p < 0.001). No relationship was seen in normotensive postpartum controls (r = 0.53, p = 0.05). NP, normotensive postpartum; sPE, severe preeclampsia.
Fig. 4.
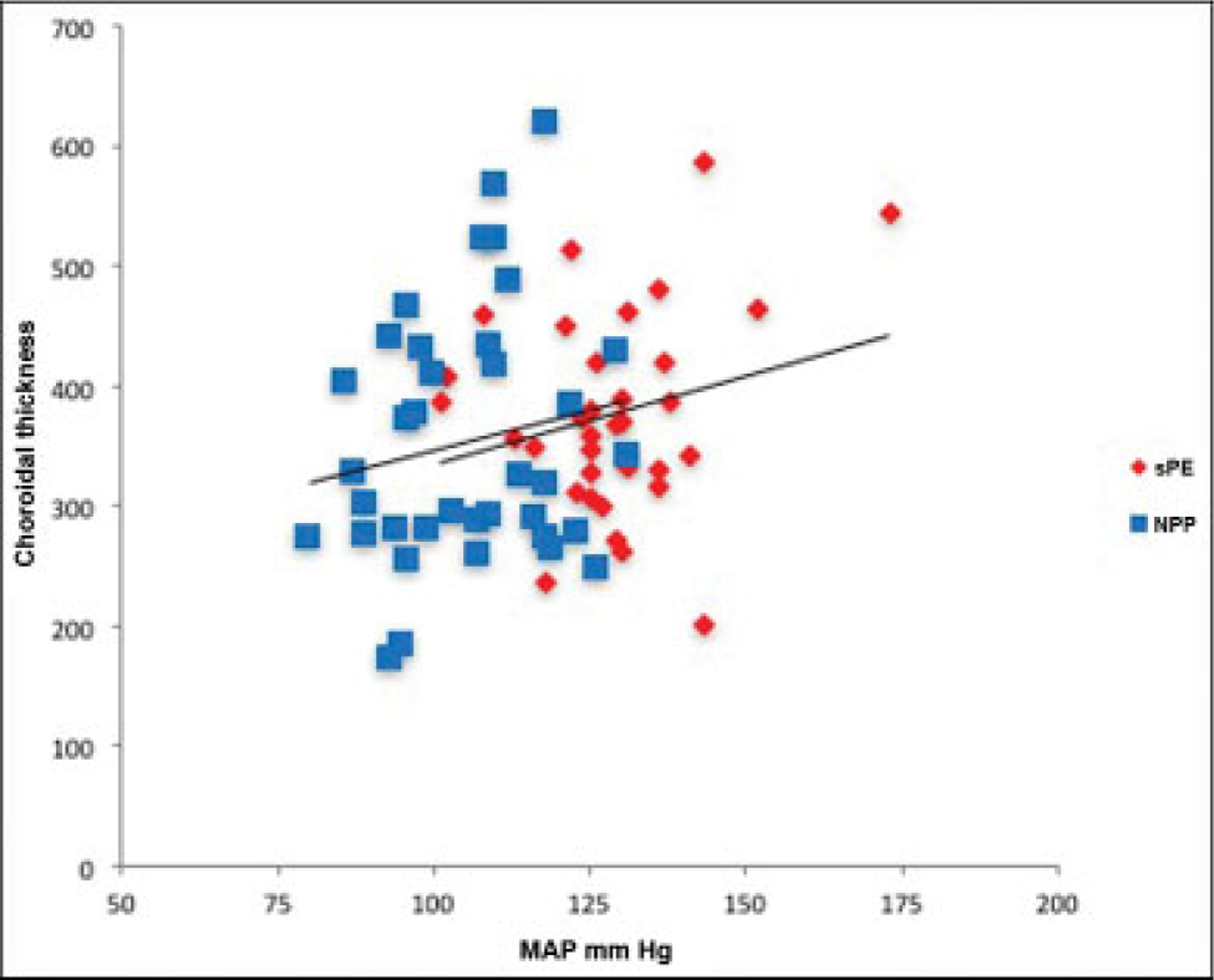
Choroidal thickness in μm versus maximum peripartum MAP in mmHg. No relationship was seen in cases or controls (r = 0.47, p = 0.09; r = 0.53, p = 0.05). MAP, mean arterial pressure; NP, normotensive postpartum; sPE, severe preeclampsia.
Angiogenic Factors
The average levels of sEng, sFlt-1, PlGF, and sFlt-1:PlGF ratio for the normotensive postpartum and severe preeclamptic groups were 8.06 ± 3.42, 822 ± 465, 19 ± 6, and 45 ± 25 and 27.82 ± 18.91, 1,551 ± 1,245, 21 ± 8, and 74 ± 46 pg/mL, respectively. Mann–Whitney U-test comparison between the groups for each of these factors showed that sEng, sFlt-1, and the sFlt-1:PlGF ratio was all significantly greater in the preeclamptic group than the normotensive group (p < 0.001, p = 0.002, and p =0.006, respectively), while there was no difference for PlGF (p = 0.249). These findings are illustrated in ►Fig. 5.
Fig. 5.
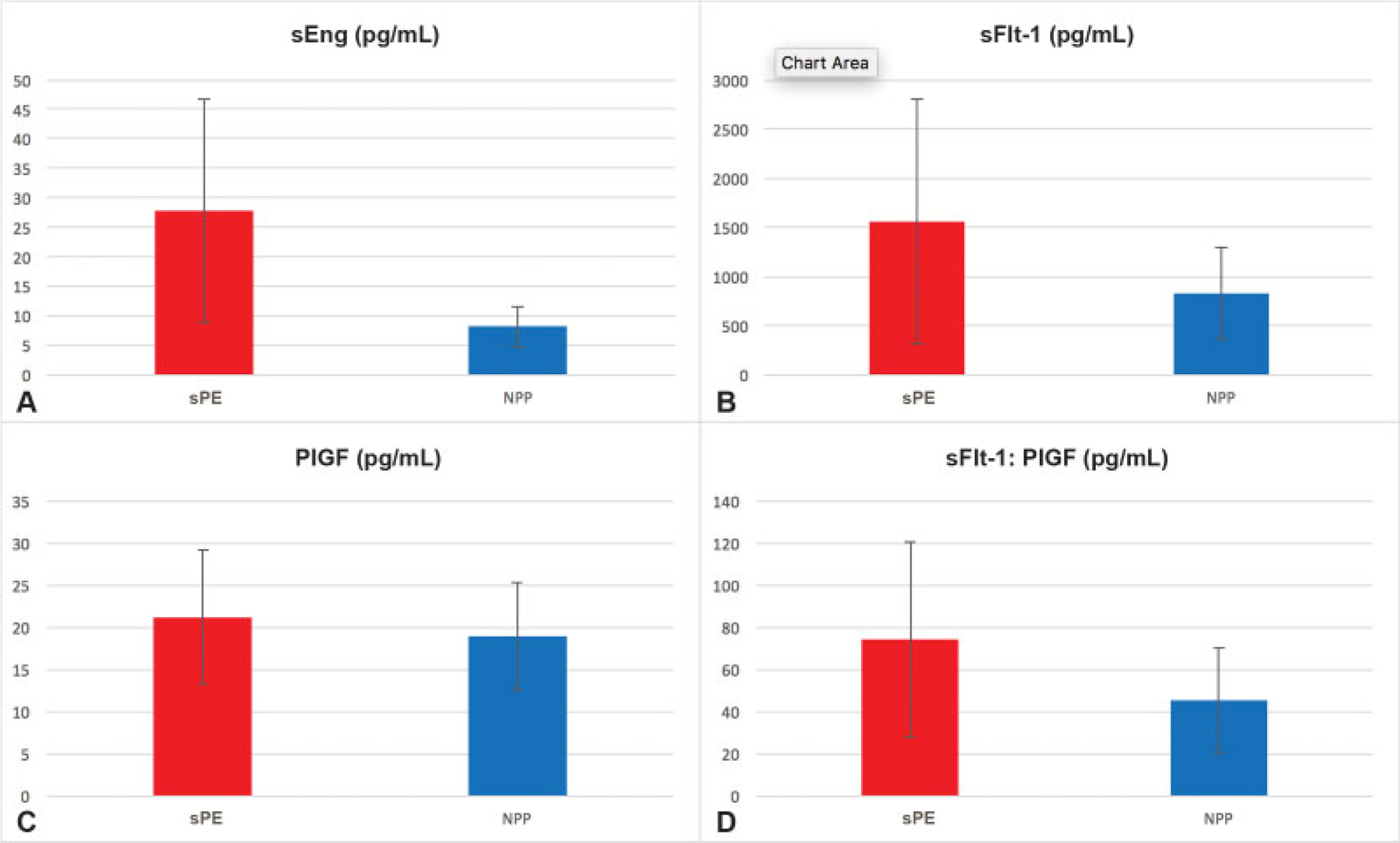
(A–D) Serum angiogenic factor analysis. All serum analysis is in terms of pg/mL and each analyzed factor is displayed with mean and standard deviation in light blue bars for the two separate populations, severe preeclampsia cases and normotensive controls. p-Values performed for each factor are (A) p < 0.001 (B) p = 0.002, (C) p = 0.249, (D) p = 0.006, respectively. NPP, normotensive postpartum; PlGF, placental growth factor; sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase-1; sPE, severe preeclampsia.
Following the analysis of distributions, we calculated correlations for each factor with SFCT in the severe preeclampsia group, bivariate linear fits are demonstrated in ►Fig. 6. The sole significant correlation was a positive one between PlGF and SFCT (r = 0.55, p = 0.002). No relationship was found between sEng, sFlt-1, or the SFlt-1:PlGF ratio, and SFCT (r = 0.26, p = 0.738; r = 0.36, p = 0.497; r < 0.001, p = 0.973).
Fig. 6.
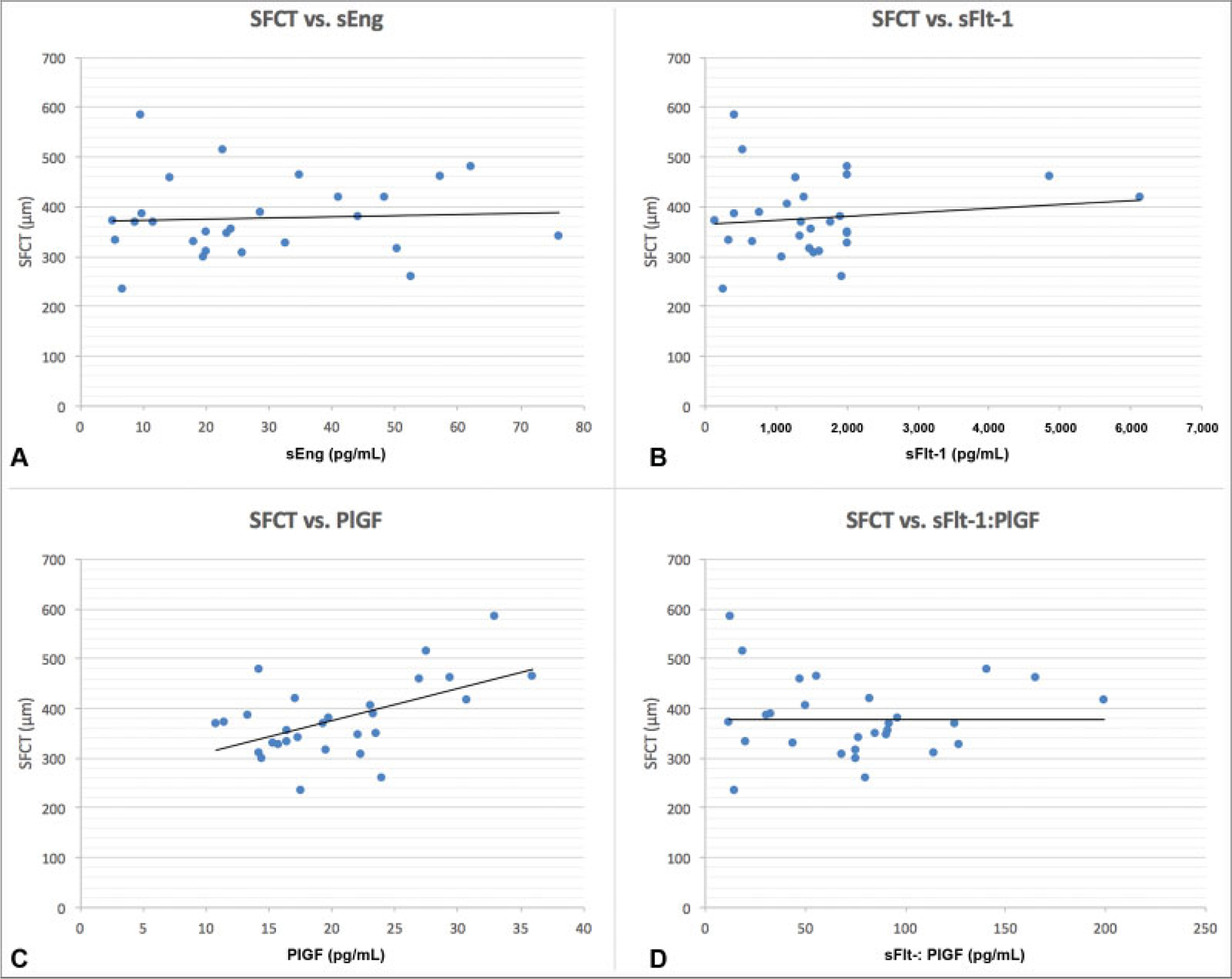
(A–D) Subfoveal choroidal thickness correlations with angiogenic factors. A bivariate distribution of each of three serum factor levels and the sFlt-1:PlGF ratio with SFCT in the severe preeclampsia group is displayed with a red linear fit line. Correlation values are (A) r = 0.26, p = 0.738; (B) r = 0.36, p = 0.497 (C) r = 0.55, p = 0.002, and (D) r < 0.001, p = 0.973. NPP, normotensive postpartum; PlGF, placental growth factor; sEng, soluble endoglin; SFCT, subfoveal choroidal thickness; sFlt-1, soluble fms-like tyrosine kinase-1; sPE, severe preeclampsia.
Comment
Principal Findings
Our primary result is that there is no significant difference in SFCT in the early postpartum period between severe preeclamptics and pregnancies unaffected by hypertensive disorders. However, we show that despite a direct relationship between GA and choroidal thickness in normal pregnancy, there exists an inverse relationship between these measures within patients with severe preeclampsia. We also show an association between this ophthalmic imaging index and placental growth factor level in the postpartum period.
Clinical Interpretation
Many case reports and series in the literature have described qualitative ophthalmic findings in patients with preeclampsia, including macular edema, subretinal fluid, serous retinal detachments, retinal vascular leakage, and RPE defects.6–10 There have been fewer quantitative studies, and of these, results are mixed. To our knowledge, this is the largest quantitative study of choroidal changes in severe preeclampsia and the first to associate changes in choroidal thickness to other more firmly established factors.
In preeclamptic patients, Sayin et al22 found that SFCT values were similar to those of nonpregnant women, and less than those of healthy pregnant women. This is in contrast to other studies showing an increased SFCT in preeclampsia. Ataş et al23 found that both healthy pregnant women and those with preeclampsia had increased SFCT compared with nonpregnant women, but that the increase in preeclampsia was less than that of healthy pregnant controls, and suggested that peripheral vasospasm might be contributing to this difference. Kim et al18 showed an increased SFCT in women with preeclampsia compared with normotensive controls in the third trimester and postpartum period, hypothesizing that the difference results from increased hydrostatic pressure of the tissue. Previous research from our ongoing study by Garg et al17 showed increased SFCT in patients with severe preeclampsia as well and found that there was no difference between these values in normotensive controls and a nonpregnant reference group.
The present study found that the mean adjusted SFCT was not increased in women with severe preeclampsia cases compared with normotensive postpartum controls. These findings differ from our earlier results. It is more difficult to obtain accurate measurements of the choroid, as it becomes more edematous, which biases the results toward the null. It is possible that the results presented here are skewed toward showing no association because of this phenomenon.
Dissimilar conclusions from previous studies measuring this outcome can be attributed to several factors. The first relates to the small sample size that each study was able to attain, and a bias toward recruiting less-severely-ill patients who are more clinically stable and therefore more likely to volunteer their time for a potentially lengthy eye examination. Variation in the severity of disease is another potential source of bias between studies. Given the heterogeneous nature of preeclampsia and its pathophysiology, including early-onset and late-onset disease in a single cohort might bias toward the null hypothesis by including patients with a less severe form of disease with a more limited duration and degree of damage to choroidal microvasculature. Finally, the stage of pregnancy at which each study group was imaged would impact SFCT, as evidenced by studies showing an upward trend of choroidal thickness in the first and second, followed by a decline in the third trimester.24–26
We also investigated the correlation between SFCT and alternative indicators of disease severity, including maximum blood pressure and earlier GAs of delivery. We hypothesized that there would be a linear relationship between these alternative measures of preeclampsia severity and presence of ophthalmic findings. We showed that amongst cases, CT and GA at delivery were indeed inversely related as anticipated. However, no relationship was found between choroidal thickness and maximum MAP. These findings support the notion that more severe disease, as indicated by the need to deliver at an earlier GA, leads to more severe ophthalmic findings. The fact that a similar correlation was not seen between imaging findings and MAP supports the idea that the ophthalmic findings in severe PE are distinct from hypertensive retinal changes.10,17 The increase in CT seen at earlier GA might again be biased by the reported physiologic rise and fall of SFCT during the second and third trimesters, rather than as a consequence of disease severity. As we did not collect imaging data throughout pregnancy, we did not have the ability to make this direct comparison. Other measures of disease severity, such as degree of renal or hepatic injury, were not collected in this study, thereby limiting any conclusion we could make regarding the independent effects this end-organ damage may have on choroidal thickness.
This study also reported on the state of several angiogenic factors measured in the postpartum period. We found, consistent with previously reported literature that there is an increase in sFlt-1, sEng, and the sFlt-1:PlGF ratio in cases versus controls.13,27,28 The fact that our study group expressed severe features of the disease made this observation more likely, with a linear relationship reported between severity and factor derangement, even in the postpartum period.35 Several studies show that normalization of sFlt1 and sEng takes 1 to 7 days, and so our postpartum findings confirm the literature.29,30
Serum PlGF normalizes by the first day postpartum, potentially explaining the lack of difference between our groups for this factor.31 While there was no difference between serum levels, we did find a positive association of PlGF with SFCT in severe preeclampsia. This might be understood in the context of PlGF’s role as a proangiogenic agent in both retinal and choroidal vasculature.16,32 PlGF is thought to contribute to angiogenesis in pathological states of repair, after ischemic damage has occurred. In the case of preeclampsia, where PlGF is reduced compared with normotensive controls, an antiangiogenic balance might reduce the ability of the choroid to maintain its thickness following an inflammatory or ischemic insult.14 In this way, we anticipate that more severe or early-onset disease might produce a thinner choroid. The lack of association between sFlt-1and sEng and SFCT might be explained by the insensitivity of these measures to choroidal thickness. In their roles, as soluble receptors for transforming growth factor-B1 and vascular endothelial growth factor family proteins, sFlt-1 and sEng act to reduce endothelial function and ability to vasodilate but not directly on vessel density or volume.32 In this way, choroid thickness may be maintained despite the increase in these anti-angiogenic factors and subsequent damage to endothelial cell function.33
The finding of a correlation of PlGF with SFCT in preeclampsia might be interpreted in multiple ways. Intravascular inflammation and vasospasm induced by preeclampsia may impact choroidal thickness in a thresholded manner, whereby only an acute or prolonged ischemic event of the vasculature would lead to either increased permeability of the tissue or a compensatory increase in upstream blood flow that force-dilates the microcirculation. This theory is supported by our finding that the SFCT was increased in those patients who presented with subretinal fluid, that is, a break-through event. Alternatively, there may be competing effects to the SFCT by the two major components of the choroid. Hypothetically, if the stromal area of choroid was increasing with transudative edema, as the disease progressed, the luminal area of the choroid might be contracting with vasospasm or reducing in density in response to the antiangiogenic balance generated by a diseased placenta, thereby suppressing any global increases in thickness.
While the role of SFCT as a predictive or risk-stratifying factor for preeclampsia isn’t supported by our work, it is clear that the choroid does respond differentially in some ways in preeclampsia. Our findings may encourage the pursuit of a more granular characterization of the choroid, such as the choroidal vascular index (CVI) or caliber, branching, and flow indices of choroidal vasculature provided by OCT angiography.34 Future studies might also report on similar outcomes as this one, but in a strictly gestational-age matched series throughout the third trimester, to control for the dynamic physiological changes to choroid that are likely occurring during this period. Finally, improved ultrasound resolution of choroidal blood flow characteristics using plane-wave technology might help to clarify the relationship between choroidal thickness and its immediate perfusion by ciliary arteries in both physiologic and pathologic states.
Funding
Support was received from The New York Community Trust-Frederick J and Theresa Dow Wallace Fund, Columbia University (S.B.).
Footnotes
Conflict of Interest
S.B. reports grants from The New York Community Trust–Frederick J and Theresa Dow Wallace Fund, grants from National Center for Advancing Translational Sciences, National Institutes of Health, during the conduct of the study.
References
- 1.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol 2013; 122(05):1122–1131 [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Keyes KM, Wapner RJ. Preeclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013; 347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003;22(02):203–212 [DOI] [PubMed] [Google Scholar]
- 4.Sheth BP, Mieler WF. Ocular complications of pregnancy. Curr Opin Ophthalmol 2001;12(06):455–463 [DOI] [PubMed] [Google Scholar]
- 5.Roos NM, Wiegman MJ, Jansonius NM, Zeeman GG. Visual disturbances in (pre)eclampsia. Obstet Gynecol Surv 2012;67(04): 242–250 [DOI] [PubMed] [Google Scholar]
- 6.Somfai GM, Miháltz K, Tulassay E, Rigó J Jr. Diagnosis of serous neuroretinal detachments of the macula in severe preeclamptic patients with optical coherence tomography. Hypertens Pregnancy 2006;25(01):11–20 [DOI] [PubMed] [Google Scholar]
- 7.Pastore MR, De Benedetto U, Gagliardi M, Pierro L. Characteristic SD-OCT findings in preeclampsia. Ophthalmic Surg Lasers Imaging 2012;43(6, Suppl):S139–S141 [DOI] [PubMed] [Google Scholar]
- 8.Lin P, Hahn P, Fekrat S. Peripheral retinal vascular leakage demonstrated by ultra-widefield fluorescein angiography in preeclampsia with HELLP syndrome. Retina 2012;32(08): 1689–1690 [DOI] [PubMed] [Google Scholar]
- 9.Neudorfer M, Spierer O, Goder M, et al. The prevalence of retinal and optical coherence tomography findings in preeclamptic women. Retina 2014;34(07):1376–1383 [DOI] [PubMed] [Google Scholar]
- 10.Fastenberg DM, Fetkenhour CL, Choromokos E, Shoch DE. Choroidal vascular changes in toxemia of pregnancy. Am J Ophthalmol 1980;89(03):362–368 [DOI] [PubMed] [Google Scholar]
- 11.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Preeclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014;10(08):466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matias DS, Costa RF, Matias BS, Gordiano L, Correia LC. Predictive value of ophthalmic artery Doppler velocimetry in relation to development of preeclampsia. Ultrasound Obstet Gynecol 2014; 44(04):419–426 [DOI] [PubMed] [Google Scholar]
- 13.Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, et al. ; EBM CONNECT Collaboration. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of preeclampsia: a systematic review and meta-analysis. BJOG 2012; 119(07):778–787 [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor- 1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med 2008;21(01):9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grisanti S, Canbek S, Kaiserling E, et al. Expression of endoglin in choroidal neovascularization. Exp Eye Res 2004;78(02):207–213 [DOI] [PubMed] [Google Scholar]
- 16.Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 2003;44(07):3186–3193 [DOI] [PubMed] [Google Scholar]
- 17.Garg A, Wapner RJ, Ananth CV, et al. Choroidal and retinal thickening in severe preeclampsia. Invest Ophthalmol Vis Sci 2014;55(09):5723–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JW, Park MH, Kim YJ, Kim YT. Comparison of subfoveal choroidal thickness in healthy pregnancy and preeclampsia. Eye (Lond) 2016;30(03):349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol 2009; 147(05):801–810 [DOI] [PubMed] [Google Scholar]
- 20.Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol 2013;155(02):314–319 [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Kuang GP, Luo DX, Lu XH. Choroidal thickness in pregnant women: a cross-sectional study. Int J Ophthalmol 2016;9(08): 1200–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayin N, Kara N, Pirhan D, et al. Subfoveal choroidal thickness in preeclampsia: comparison with normal pregnant and nonpregnant women. Semin Ophthalmol 2014;29(01):11–17 [DOI] [PubMed] [Google Scholar]
- 23.Ataş M, Açmaz G, Aksoy H, et al. Evaluation of the macula, retinal nerve fiber layer and choroid in preeclampsia, healthy pregnant and healthy non-pregnant women using spectral-domain optical coherence tomography. Hypertens Pregnancy 2014;33(03): 299–310 [DOI] [PubMed] [Google Scholar]
- 24.Goktas S, Basaran A, Sakarya Y, et al. Measurement of choroid thickness in pregnant women using enhanced depth imaging optical coherence tomography. Arq Bras Oftalmol 2014;77(03): 148–151 [DOI] [PubMed] [Google Scholar]
- 25.Ulusoy DM, Duru N, Ataş M, Altınkaynak H, Duru Z, Açmaz G. Measurement of choroidal thickness and macular thickness during and after pregnancy. Int J Ophthalmol 2015;8(02):321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dadaci Z, Alptekin H, Oncel acir N, Borazan M. Changes in choroidal thickness during pregnancy detected by enhanced depth imaging optical coherence tomography. Br J Ophthalmol 2015;99(09):1255–1259 [DOI] [PubMed] [Google Scholar]
- 27.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350(07):672–683 [DOI] [PubMed] [Google Scholar]
- 28.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 2010;122(05):478–487 [DOI] [PubMed] [Google Scholar]
- 29.Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth 2015;15:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrozella L, Mahendroo M, Timmons B, Roberts S, McIntire D, Alexander JM. Endothelial microparticles and the antiangiogenic state in preeclampsia and the postpartum period. Am J Obstet Gynecol 2012;207(02):140.e20–140.e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wikström AK, Larsson A, Eriksson UJ, Nash P, Olovsson M. Early postpartum changes in circulating pro- and anti-angiogenic factors in early-onset and late-onset preeclampsia. Acta Obstet Gynecol Scand 2008;87(02):146–153 [DOI] [PubMed] [Google Scholar]
- 32.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011; 123(24):2856–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannan NJ, Brownfoot FC, Cannon P, et al. Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction - implications as a preeclampsia treatment. Sci Rep 2017;7(01):1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep 2016;6:21090. [DOI] [PMC free article] [PubMed] [Google Scholar]


