Abstract
Recent experimental studies suggest that wet–dry cycles and coexisting phases can each strongly alter chemical processes. The mechanisms of why and to what degree chemical processes are altered when subjected to evaporation and condensation are unclear. To close this gap, we developed a theoretical framework for nondilute chemical reactions subject to nonequilibrium conditions of evaporation and condensation. We find that such conditions can change the half-time of the product’s yield by more than an order of magnitude, depending on the substrate–solvent interaction. We show that the cycle frequency strongly affects the chemical turnover when the system is maintained out of equilibrium by wet–dry cycles. There exists a resonance behavior in the cycle frequency where the turnover is maximal. This resonance behavior enables wet–dry cycles to select specific chemical reactions, suggesting a potential mechanism for chemical evolution in prebiotic soups at early Earth.
1. Introduction
The presence of catalysts severely alters the speed of the chemical reactions. In contrast to reactants, they are not converted during the turnover of a substrate to a product. Catalysts affect the activation energy ΔE without changing the thermodynamic free energies of the substrate and product state.1 The use of catalysts ensures the economic efficiency of most applications in chemical and biomolecular engineering.2−4 In living systems, the catalytic activity is mediated by enzymes. Enzymes are specialized proteins involved in almost all metabolic processes in the cell.5−7 Enzymes do not solely maximize the speed of chemical reactions; they are optimal concerning reaction speed and specificity;8,9 i.e., they catalyze only specific reactions in a mixture of extremely many reacting components. However, this optimal catalytic property for life had to develop during evolution.10−12 In other words, there were likely no enzymes at the molecular origin of life. It remains one of the mysteries of the molecular origin of life how functional products such as self-replicating RNA strands could have formed despite activation barriers significantly larger than kBT.13,14
An alternative perspective on how to speed up chemical reactions is through nonequilibrium conditions.15−21 In fact, nonequilibrium applies to many processes in chemical engineering, almost all processes in living cells and prebiotic soup at early Earth. The reason is that these are all open systems that can exchange matter and entropy with their environment, termed dissipate systems.22−24 Continuous dissipation is often achieved by cycles of the system’s control parameters, such as temperature or chemical potentials. Recently studied cases are wet–dry cycles.25−30
The ability of wet–dry cycles to affect the synthesis of compounds that are also relevant for the molecular origin of life is progressively backed by experiments.27,31,32 Wet–dry cycles have been applied to catalyze the formation of polyacids,33 esters,31,32 oligopeptides,34−36 nucleosides,27 polynucleotides,37,38 poly(ether sulfone),39 phosphorylation,25 and lipid structures.37 Up to now, the mechanisms of how wet–dry cycles affect chemical processes, particularly in comparison to catalysts, remain elusive.
Unraveling such mechanisms is complex since reducing the solvent amount through evaporation generally leads to nondilute conditions. As a result, nonlinear thermodynamic contributions to chemical activities emerge in addition to altered kinetic activation barriers. Additionally, nondilute systems can induce phase transitions, such as liquid–liquid phase separation,40−42 gel formation,43−46 and solid precipitation.47,48 Recent experiments show that coacervate formation can be triggered by wet–dry cycles.26 To understand how wet–dry cycles affect the synthesis of chemical buildings and to decipher the underlying physiochemical mechanisms, a theoretical framework is lacking.
This work proposes a theoretical framework based on thermodynamic principles for a nondilute chemically reacting mixture coupled to a cyclic wet–dry reservoir that drives the system out of equilibrium; see Figure 1b,c. We show to what extent and under which conditions both evaporation and condensation of solvent can speed up or slow down rates of chemical reactions. In contrast to catalysts that solely affect activation barriers, we find that solvent evaporation and condensation can significantly alter the chemical potentials of substrate and product once the system is driven toward nondilute conditions, illustrated in Figure 1a. We also study how wet–dry cycles allow for continuous chemical activity by keeping the system out of equilibrium. For this case, a resonance cycling frequency exists for each evaporation/condensation flux, at which the chemical turnover is maximal. This resonance behavior provides a selection pressure for specific chemical reaction networks suggesting wet–dry cycles as potential catalyst supplements to kick-start the molecular evolution of life.49−51
Figure 1.

Wet–dry cycles in chemically reacting systems: (a) Wet–dry cycles can enhance chemical reaction rates by increasing the chemical potential of the substrate μA and decreasing the chemical potential μB of the product. (b) Wet–dry cycles lead to nondilute conditions, which affect chemical reactions and may induce liquid–liquid phase separation. (c) Cyclic behavior is included in the theory through an oscillating solvent reservoir with periods of evaporation and condensation.
2. Theory for Wet–Dry Cycles with Chemical Reactions
We consider a mixture of volume V composed of
(M + 1) components, each of particle number Ni (i = 0,
1, ..., M) in the Gibbs (T–P–Ni) ensemble with the Gibbs free energy G(T, P, Ni). Evaporation and condensation of molecule i is governed by the difference between the bulk chemical potential 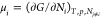 , and the reservoir chemical
potential μir. In all
of our studies, the
chemical potential of the reservoir μir is a control parameter that can be constant or
vary in time. The evaporation and condensation flux hi describes the number of molecules passing
through the surface area A per unit time40
, and the reservoir chemical
potential μir. In all
of our studies, the
chemical potential of the reservoir μir is a control parameter that can be constant or
vary in time. The evaporation and condensation flux hi describes the number of molecules passing
through the surface area A per unit time40
| 1 |
where kBT denotes the thermal energy. Here, ke,i is the kinetic evaporation/condensation coefficient for component i. Equilibrium is reached when the chemical potentials between the reservoir and the bulk are equal, μi = μir, implying no exchange between the bulk and the reservoir, hi = 0. Note that eq 1 considers a symmetric form, hi = hicond – hievap, of the condensation flux, hicondA = ke,iexp{μir/kBT}, and evaporation flux, hievapA = ke,iexp{μi/kBT}, without the loss of generality. The key property is that detailed balance of the rates, hicond/hievap = exp{(μir – μi)/kBT}, is satisfied.
To calculate the chemical potential, we consider the mean field Gibbs free energy G per volume V(52)
| 2 |
where χij is the interaction parameter characterizing the interaction between components i and j. Moreover, ωi and νi denote the internal energy and molecular volume of component i, respectively. The chemical potential of component i, thus becomes
| 3 |
where Λ is given by
| 4 |
The volume fraction of a component is defined by the volume it occupies relative to the total system volume, ϕi = νiNi/V, i ∈ {0, 1, 2, ..., M}. The solvent volume fraction ϕM is set by all other volume fractions
| 5 |
The total volume change due to evaporation and condensation is equal to the volume of molecules removed from or added to the system per unit of time
| 6 |
Moreover,
the volume change alters the volume fractions of all
components, even components where hi = 0, as 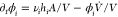 , and thus affect their chemical
potentials
and chemical reactions. A reaction α is written as
, and thus affect their chemical
potentials
and chemical reactions. A reaction α is written as
| 7 |
where Ci denotes a chemical component and σ⇌iα are the stoichiometric matrix elements.53 For the forward and backward chemical reaction rates r⇌α,40 we choose a symmetric form
| 8 |
From chemical potential (3), we see that the forward and backward reaction rates are proportional to the product of their volume fractions ϕi. This implies that once a component gets depleted, reactions slow down and arrest when reaching zero volume fractions. The ratio between the forward and backward reaction rates satisfies detailed balance of the rates54
| 9 |
where we have defined σiα ≡ σ↽iα – σ⇀iα. The difference between the forward and backward reaction pathways, for R different reactions, drives the net reaction rate
| 10 |
If the interaction strengths cross a certain threshold value, the system phase-separates into two phases (denoted I and II) with different composition ϕiI/II but the same chemical potential
| 11 |
The time evolution of the nonsolvent volume fraction of a phase-separated system follows18,40,53,55
| 12 |
where jiI/II gives the diffusive flux between the phases. The volume change of each phase for volume-conserving chemical reactions is given by
| 13 |
To determine the diffusive flux ji in the phase-separated system, we use the (M + 1) phase equlibrium constraints (eq 11). See Appendix C for more details. When the system is homogeneous, the governing equations are retrieved by setting jiI/II = 0, and dropping the superscript specifying the phase. Please note that when accounting for transition states in the theory leads to the same kinetic equations as long as the transition state is short-lived; see Appendix D for a detailed discussion.
For a phase-separated system, the chemical reactions concomitantly occur in both phases, while the fluxes related to evaporation/condensation are considered to occur at a surface area enclosing the dilute phase. As the two phases are at phase equilibrium, the choice of which phase is in contact with the reservoir becomes irrelevant. Furthermore, we will assume that the reaction rate coefficient does not depend on the phase kIc,α = kIIc,α. We consider that evaporation/condensation of macromolecules is negligible compared to the solvent,56 such that ke,i = keδiC. When only the solvent is evaporating/condensing, and no chemical reactions occur, the ratio of each nonsolvent volume fraction, ϕi/ϕj, is conserved. To reduce dimensionality and the number of free parameters, we will consider a single monomolecular reaction A ⇌ B, such that σ⇀iα = δiAδα1, σ↽iα = δiBδα1, and kc,α = kcδα,1. The kinetic algorithm to evolve the system according to eq 12 builds on a method derived by Bauermann and Laha;53 see Appendix C for how the theory accounts for the phase equilibrium constraint, and Appendix A for the properties a system should have for phase equilibrium to hold well at each time during the kinetics.
3. Results
3.1. Thermodynamics of Evaporation/Condensation Mixtures without Chemical Reactions
To understand chemical kinetics in systems that can phase separate and undergo wet–dry cycles, it is instrumental first to understand equilibrium states in the absence of chemical reactions. Equilibrium states composed of coexisting phases form through evaporation and condensation if the equilibria are compatible. Compatible equilibria refer to the case where the phase equilibrium condition (11) is concomitantly satisfied together with one or more other equilibrium conditions.53 In the absence of chemical processes, this other condition corresponds to evaporation–condensation equilibrium. Graphically, such compatible equilibria correspond to the intersections in Figure 2a between the binodal and the mononodal line that is set by the reservoir chemical potential μrC (orange dashed line). During evaporation and condensation, ϕB/ϕA is conserved. Thus, the domain of compatible equilibria is cone-shaped and is spanned by two distinct conservation lines of constant ϕB/ϕA (orange domains in Figure 2b). Any initial state will move during its evaporation/condensation kinetics along a conservations line and settle in a compatible equilibrium with coexisting phases. The number and the area of the domain with compatible equilibria depend on the interactions among the molecules. The area of compatible domains increases when A and B interact more differently with the solvent (case i in contrast to cases ii and iii), or when A and B phase-separate independently of the solvent (case iv).
Figure 2.
Evaporation/condensation phase diagrams: (a) Evaporation/condensation equilibrium for different reservoirs are displayed together with the constraint line of constant ϕB/ϕA. The binodal encloses the blue-shaded area of phase coexistence, where the system phase-separates into two phases connected by straight black tie lines. (b) Four qualitatively different phase diagrams corresponding to four sets of interaction strengths (i–iv). The orange-shaded region displays the initial states leading to the coexistence of two phases. (c) The composition of each phase depends differently on the reservoir value μrC for each phase diagram type.
The equilibrium states are homogeneous when evaporation–condensation and phase equilibrium are incompatible. Such incompatible states graphically correspond to the complement of the orange shaded domains in Figure 2b, i.e., the values of ϕB/ϕA that lie outside of the binodal. Incompatible equilibria are favored when the A and B interact similarly with the solvent, and when neither the A or B phase separates with the solvent, as discussed for compatible equilibria. For some values of the reservoir chemical potential all equilibria are incompatible (green and yellow lines in Figure 2a).
The phase behavior of the evaporating/condensing mixtures (i–iv) without chemical reactions can be summarized by the thermodynamic phase diagram spanned by the reservoir chemical potential μrC and the conserved variable ϕB/ϕA. The different interactions (i–iv) lead to very different shapes where two phases can coexist with very different values of the conserved variable ϕB/ϕA, as shown in Figure 2c. For case iv, where the coexisting phases have a similar solvent volume fraction, the nonsolvent components phase separate independently of the solvent. As a result, each phase has a large difference in ϕB/ϕA, creating a significant phase-separated domain.
3.2. Thermodynamics of Evaporation/Condensation Mixtures with Chemical Reactions
In the presence of chemical reactions, ϕB/ϕA is not conserved, and compatible equilibria are no longer achievable. Thermodynamic equilibrium, therefore, always corresponds to a homogeneous state where the conditions of chemical equilibrium (μA = μB) and evaporation–condensation equilibrium (μC = μrC) are fulfilled; see the intersection between the red and green line in Figure 3a. Any initial state (ϕA, ϕB) will evolve following a flow field in the phase diagram toward the fixed point of thermodynamic equilibrium; see Figure 3b. Each point in this flow diagram has two independent directions that characterize the rates of change in the average volume fractions, which correspond to the chemical turnover flux of constant ϕA + ϕB and evaporation–condensation flux of constant ϕB/ϕA; see vectors shown in Figure 3a. Such fluxes of strength visualized by the length of each vector are set as ke and kc. Gibbs’ phase rule (discussed in Appendix E) makes the domain of coexisting states inaccessible at thermodynamic equilibrium, such that small changes in reservoir chemical potential μrC close to the binodal line pronounce significant changes in the equilibrium compositions. This behavior is shown in Figure 3c, where solvent-rich and solvent-poor equilibrium states are separated by the phase coexistence line ending at the critical point (red cross) in Figure 3c.
Figure 3.
Evaporation/condensation and chemically reacting phase diagrams: (a) The intersection of the equilibrium lines of evaporation/condensation and the chemical reaction corresponds to thermodynamic equilibrium. All initial states evolve toward this point in the phase diagram. An example of the rate of change in volume fraction is displayed as a black arrow, which is composed of contributions related to chemical reactions (red) and evaporation/condensation (green). (b) The rate of changes at each point in the phase diagram give rise to a flow field in the phase diagram, where each trajectory evolves toward the thermodynamic equilibrium point, displayed for ke/kc = 4. (c) Phase-separated thermodynamic equilibrium states are achievable for only a single reservoir value for each interaction strength. The phase coexistence line separates solvent-poor and solvent-rich states until the critical point (red cross).
3.3. Speed-Up and Slow-Down of Chemical Reactions through Evaporation and Condensation
Evaporation and condensation can speed up chemical reactions by driving the system along different phase diagram trajectories. For unidirectional chemical reactions, where the internal free energies ωproduct ≪ ωsubstrate, these trajectories are characterized by solvent-poor or solvent-rich conditions during the relaxation to thermodynamic equilibrium (Figure 4a,d). Such different solvent conditions have a dramatic impact on the time (τ1/2) it takes to turn over 50% of A to B, seen as the half-time of the yield ϕB/(ϕA + ϕB) in Figure 4b,e. For the depicted molecular interactions, i.e., A favors the solvent while B does not, a quick removal of the solvent (large ke/kc) causes an increase in the chemical potential of A. This increase in the substrate’s chemical potential, visualized in Figure 1a, speeds up the turnover from A to B.
Figure 4.
Chemical speed-up through evaporation/condensation: The pathway through the phase diagram during evaporation (a–c) and condensation (d–f) toward the same equilibrium (red dot) for different ke/kc are shown in (a) and (d), respectively. The yield of product B, ϕB/(ϕA + ϕB), is displayed as a function of time in (b) and (e) showing a significant speed-up through the different pathways. How this speed-up depends on the interaction strengths for ke/kc = 10 is displayed in (c) and (f). The top row (a–c) shows the results for evaporation, and the bottom row for condensation (d–f).
The threshold between slow-down and speed-up of chemical reactions corresponds to the case of no interactions between the components (χij = 0), as seen in Figure 4c,f. Thus, solely up-concentrating the components does not affect the speed-up, whose origin is a pure consequence of nondilute conditions and the interactions among reacting components. The substrate–solvent interaction enters exponentially as χACϕC in the reaction rate in eq B3. The sign of χ determines whether evaporation or condensation aids the chemical turnover, while the magnitude determines the speed-up. In other words, reactions of solvophilic substrates are sped up through evaporation and solvophobic substrates under condensation. Though other interactions (χAB, χBS) also affect the speed-up, they are less relevant than the substrate–solvent interaction (χAS), as seen in Figure 4c,f. All statements about interaction strengths are qualitatively reversed once we switch from evaporation (Figure 4a–c) to condensation (Figure 4d–f).
3.4. Chemical Reactions Subject to Wet–Dry Cycles
Now we discuss the effects on chemical reactions when the system is subject to cycles of the reservoir chemical potential
| 14 |
Here, Ω denotes the cycling frequency, ⟨μrC⟩ the average reservoir value, and μampC,r the oscillation amplitude. We now discuss two cases of cycles: (i) the chemical path toward the orbit and (ii) the continuous movement among this chemical orbit. For case (i), we are interested in the chemical turnover for a unidirectional chemical reaction, as before, and how it is affected by cycles. After the initial phase, the trajectory becomes periodic. In case (ii), we are interested in the chemical turnover rate during such an orbit.
(i) We find that wet–dry cycles can speed up chemical turnover relative to no cycling with a resonance peak at a specific cycling frequency. Different cycling frequencies Ω result in the different pathways displayed in Figure 5a, with different conversion half-times. Oscillating reservoirs decrease the conversion half-time relative to no oscillations, τ1/2(Ω)/τ1/2(0), by up to a factor of 5 for the parameters used in Figure 5b. The resonance cycling frequency Ωres is stable, changing less than a decade when varying ke/kc over four decades, as displayed in Figure 5c. Slow chemical reactions have the largest speed-up due to wet–dry cycles.
Figure 5.
Resonance behavior in chemical reactions with an oscillating reservoir: the top row (a–c) shows the conversion from A to B on the path toward an orbit, while the bottom row (d–f) shows equivalent quantities during the orbits. The change in the phase-diagram pathway with cycling frequency Ω is displayed in (a) and (d) for ke/kc = 101.5 and 102.75, respectively. Both cases exhibit a resonance behavior between the cycling frequency of the reservoir and the chemical turnover, quantified by the conversion half-time (b, c) and periodic chemical turnover (eq 15) (e, f). Both values increase with ke/kc at the resonance frequency.
(ii) With repeated cycling, the system will eventually enter an orientation, where the path in the phase diagram is a closed loop (Figure 5d). To quantify the chemical activity per period, we define the relative chemical turnover per cycle
| 15 |
This quantity is at its minimal 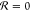 if no chemical reactions take place during
the cycle, and maximal
if no chemical reactions take place during
the cycle, and maximal  if all molecules undergo both the forward
and backward reactions per cycle.
if all molecules undergo both the forward
and backward reactions per cycle.
Different cycling frequencies
Ω/kc produce different orbits in
the phase diagram, as shown in Figure 5d. Small Ω/kc values
allow the system to remain close to
chemical equilibrium, while large values do not allow the system to
respond to the change in the reservoir. In between, there exists a
frequency where the system has time to react to the reservoir and
be driven away from the chemical equilibrium line, maximizing 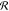 . For larger
values of ke/kc, the ability of the system
to respond to the reservoir increases, making
. For larger
values of ke/kc, the ability of the system
to respond to the reservoir increases, making 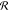 larger as
well. With increasing ke/kc, the system
is able to follow the reservoir equilibrium, saturating
larger as
well. With increasing ke/kc, the system
is able to follow the reservoir equilibrium, saturating 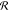 . Without interactions
(χij = 0), the chemical equilibrium
(μA = μB) is set by ϕB/ϕA = constant. As the equilibrium condition
is identical to
the evaporation/condensation constraint, reservoir oscillations cannot
drive the system away from chemical equilibrium. Thus, again, changes
in the solvent composition can only affect chemical reactions when
nondilute interactions and interactions among reacting components
are considered.
. Without interactions
(χij = 0), the chemical equilibrium
(μA = μB) is set by ϕB/ϕA = constant. As the equilibrium condition
is identical to
the evaporation/condensation constraint, reservoir oscillations cannot
drive the system away from chemical equilibrium. Thus, again, changes
in the solvent composition can only affect chemical reactions when
nondilute interactions and interactions among reacting components
are considered.
The resonance behavior provides a selection mechanism for the specific chemical reactions. This selection is achieved by the speeding up of specific chemical reactions, while other reactions do not benefit from the wet–dry cycles. The selection conditions depend on the value of the evaporation flux coefficient ke, which, however, does not change much for water for typical temperature and pressure oscillation on Earth.57−59 A specific resonance frequency, and thus the selection of specific chemical reactions, can however be realized by varying the system’s geometry, i.e., surface-to-volume ratio A/V0 of the system (Figure 6). Increasing the surface area-to-volume ratio from a puddle to a thin film increases the resonance frequency, allowing for faster chemical processes.
Figure 6.

Resonance behavior determined by system’s geometry: The resonance frequency of the periodic chemical turnover in Figure 5f can be reached by varying the geometry of the mixture through its surface-to-volume ratio. Different reaction timescales lead to different resonance frequencies, making the resonance behavior act as a selection mechanism. For this figure, the evaporation speed is 5 cm/day.
4. Conclusions
In this work, we present a thermodynamic framework for evaporation and condensation of nondilute, reacting mixtures and focus on the effects on chemical reactions. We find that the kinetics of evaporation and condensation can significantly speed up chemical reactions. This speed-up stems from evaporation and condensation driving the system away from equilibrium. For wet–dry cycles, the turnover from the substrate to the product can be sped up even further. We found that the cycling frequency strongly affects the rate of the chemical reactions per cycle. A key finding of our work is the existence of a resonance cycling frequency where turnover is maximal. This resonance behavior can act as a selection mechanism to speed up only specific reactions.
The mechanism for how evaporation and condensation for constant and cycling reservoirs affect chemical reactions relies on effectively lowering the free energy barrier for a reaction to occur (eqs D5 and D6). By reducing the amount of solvent through evaporation, a solvophilic component will interact less with the solvent and more with the other components, making it less energetically favorable to be solvophilic. Likewise, solvophobic substrates interacting more with the solvent makes it less energetically favorable to be solvophobic. In both cases, the substrate chemical potential μA increases, thereby reducing the free energy barrier ΔE. For realistic thermodynamic and kinetic parameters of chemical compounds in aqueous solutions,57,59−63 speed-ups of chemical reactions by more than a factor 10 are possible through wet–dry cycles. Importantly, if dilute chemistry is assumed (χij = 0), no speed-up or resonance would appear; the effect of wet–dry cycles results from dense chemistry interactions. This pronounced effect on chemical reactions suggests a strong contender as a naturally occurring process analogous to enzymes. However, while enzymes speed up reactions by lowering the kinetic contributions of the energetic barrier between the substrate and the product, evaporation and condensation can increase the chemical potential of the substrate μA and lower the chemical potential of the product. As a result, the speed of reactions can be enhanced (Figure 1a).
Most chemical reactions in biology or chemical engineering involve enzymes or catalysts to enhance the speed of reactions.2−7 Subjecting such catalyzed reactions to wet–dry cycles could speed up the reactions even further. In particular, active sites of enzymes are typically solvent-depleted64−67 implying that enzymatic activity is enhanced in the dry state of the cycle. Pronounced enhancement is expected when changing the time-dependent reservoir chemical potential from symmetric wet–dry cycles (eq 14) to strongly asymmetric cycles with long dry and short wet periods.
In our work, the change in chemical reactions stems from the nondilute conditions and the interactions among reacting components. Interestingly, phase separation did not qualitatively alter the speed-up or resonance phenomena observed. This might arise from our choice of equal kinetic coefficients in each phase. By including phase-dependent reaction coefficients or explicitly accounting for reactions at the interface between the two phases, we expect that phase separation can alter the resonance behavior.
In our work, we have focused on how chemical reactions are affected by wet–dry cycles in ternary mixtures. However, the theoretical framework provided can be applied to an arbitrary number of components with higher-order chemical reactions or reaction networks, as outlined in the theory section. For such complex reaction schemes, we expect that the speed up of chemical reactions gets even more pronounced as the reaction rates become proportional to a product of all of the substrate volume fractions (eq 8). This allows wet–dry cycles to affect chemical reaction rates even when assuming dilute chemistry.30 Moreover, the resonance behavior should persist, as it arises from a generic coupling between reservoir driving and reaction rates. Exploiting this resonance behavior in reacting mixtures with many components poses challenges at the interface between theory and experiments, which include proper characterization of interaction parameters and kinetic rates.
Our findings suggest that wet–dry cycles could have played a vital role in speeding up prebiotic chemistry on the early Earth, where biological enzymes were absent. During early Earth settings, systems were likely exposed to wet–dry cycles. The reservoir oscillations can originate from weather changes such as temperature, humidity, or pressure,68,69 or salt deliquescence,70 or freeze–thaw cycles.71−73 Alternatively, oscillations in the solvent can be found in foams,28 or porous rock containing trapped gas-bubbles.19,20
An important implication of our work is that the resonance behavior in the frequency of wet–dry cycles provides a selection mechanism for chemical reactions. Since the system’s geometry determines the resonance frequency, different chemical reactions are selected in a puddle versus a mesoscopic aqueous droplet. This suggests that pores of different sizes subjected to wet–dry cycles can provide a setting for chemical selection and evolution at the molecular origin of life.
Acknowledgments
The authors thank Evan Spruijt and Iris Smokers for discussions on the experimentally observed effects of wet–dry cycles on chemical reactions and acknowledge their feedback on the manuscript. The authors also thank Sudarshana Laha for stimulating discussions and Hidde Vuijk, Samuel Gomez, and Gaetano Granatelli for feedback on the manuscript. Figure 1 was created using BioRender.74 C. Weber acknowledges the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Fuelled Life, grant number 949021) for financial support.
Appendix A
Validity of Phase Equilibrium and Parameter Choices
Our work is valid for systems for which phase equilibrium approximately holds at each time during the kinetics. Note that at phase equilibrium, the volume fractions in each phase are spatially homogeneous. For phase equilibrium to hold in the presence of other kinetic processes such as evaporation/condensation with a rate coefficient ke and chemical reactions with a rate coefficient kc, such processes have to be slow compared to diffusion
| A1 |
where l is the characteristic system length, and Dmin is the smallest diffusion coefficient out of all the components. Assuming phase equilibrium at each time of the kinetics also implies that the nucleation of coexisting phases is not a rate-limiting step, and the effects of having a single droplet or an emulsion of the same total condensed volume are negligible. Indeed, nucleation for many phase-separating polymeric systems shows for fast (often seconds or less) formation of mesoscopically sized condensed phases75 and the effect of droplet number on composition is typically only relevant for droplets around the critical nucleation radius.40
To see whether the condition in eq can be fulfilled for chemical systems we consider typical physicochemical values for diffusion coefficients and chemical reaction rates. In water, small reacting molecules often have a diffusion coefficient of around 100 μm2/s. When considering chemical reactions occurring with rates around min–1, the condition for phase equilibrium (AA1) is satisfied for system sizes of 0.1 mm or smaller. For larger system sizes, gradients in the system should be taken into account by using a sharp interface model to calculate diffusive fluxes driven spatial inhomogeneities.76
We have considered parameters consistent with the validity of the phase equilibrium condition (A1) during the wet–dry cycles and the reaction kinetics. The parameters used are listed in Table 1. In Figure 3, we span different interaction parameters to understand what class of interactions results in a kinetic speed-up. Our finding that turnover of solvophilic substrates is enhanced during drying agrees with the interactions of substrates used in phosphorylation reactions,16,25 where speed-up through wet–dry cycles also have been observed. The parameters in interactions used for Figures 2–4 are motivated by systems with similar phase behavior.25,26,77,78 Moreover, also purified proteins show similar interactions.79,80
Table 1. Interaction Strengths χij, Internal Energies ωi, and Reservoir Chemical Potential Values μrCa.
| Figure | χAC/kBT | χBC/kBT | χAB/kBT | ωA/kBT | ωB/kBT | μrC/kBT |
|---|---|---|---|---|---|---|
| 2a | 3.0 | 0.6 | –0.6 | –0.2 | 0.1 | (−0.2, −0.5, −0.9) |
| 2b(i) | 3.0 | 0.6 | –0.6 | –0.2 | 0.1 | –0.2 |
| 2b(ii) | 2.84 | 2.04 | –0.6 | –0.2 | 0.1 | –0.15 |
| 2b(iii) | 1.72 | 1.72 | –2.19 | –0.1 | 0.4 | –0.2 |
| 2b(iv) | –0.60 | 0.60 | 3.0 | –0.2 | 0.1 | –1.40 |
| 3a,b | 3.0 | 0.6 | –0.6 | –0.2 | 0.1 | –0.5 |
| 4a,b | 3.0 | –3.0 | 2.0 | –10 | 0.0 | –10 |
| 4c | 0.0 | –6.0 | Δ2 | |||
| 4d,e | 2.0 | 3.5 | –2.0 | –10 | 0.0 | 2.77 |
| 4f | –6.0 | 0.0 | –Δ5 | |||
| 5a,b,c | 3.0 | –0.6 | 0.6 | 0.0 | –6.0 | ⟨−0.3⟩ |
| 5d,e,f | 3.0 | –0.6 | 0.6 | 0.4 | 1.8 | ⟨−0.538⟩ |
These are used for all figures. Additionally, the Δ in the reservoir chemical potential means change relative to the initial chemical potential. For all figures in this work, we have chosen νi = 1, kc = 1, V(t = 0) = 1, and ωC = 0.
We note that evaporating a major fraction of the solvent can strongly decrease the diffusion coefficient Dmin, potentially violating the condition (A1) as reacting compounds can become more crowded. However, removing the solvent also decreases the system’s size l, potentially maintaining the validity of the condition above throughout the evaporation process.
Appendix B
Unidirectional Chemical Reactions and Analytic Scaling Relation
Unidirectional chemical reactions are achieved by choosing a large difference in the internal energies between the product and substrate.
| B1 |
In turn, the chemical reaction becomes dominated by the direction from substrate to product, where the backward pathway is exponentially damped by the factor (ωproduct – ωsubstrate)/kBT. The chemical reaction in eq 10 can, under these conditions, be approximately written as
| B2 |
when A is acting as the substrate and B as
the product. We have defined 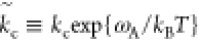 , and
, and 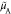 represents the nonentropic contribution
to the chemical potential of component A, defined as
represents the nonentropic contribution
to the chemical potential of component A, defined as
| B3 |
To speed up the chemical reaction, this quantity should be maximized. During evaporation, we reduce ϕC, increasing ϕA and ϕB. A negative value of χAC increases the substrate’s internal energy. For condensation, ϕC is increased relative to ϕA, such that the chemical potential is increased for positive values of χAC. Though the interaction between χAB might appear as important as χAC, the volume fraction of the product will generally vary less during evaporation/condensation. However, it will become important toward the later part of the turnover process. Importantly, Λ, defined in eq 4, also depends on the volume fractions and will vary during evaporation/condensation. The changes in Λ through evaporation/condensation will still contribute less to the overall speed-up as its interaction terms contribute only as second order in the ϕ′s.
The reaction coefficients kI/IIc,α can generally depend on the volume fractions and differ in each phase. However, for simplicity, we treat it as constant and equal in the two phases.
Appendix C
Phase Equilibrium Constraint during Reaction and Wet–Dry Cycle Kinetics
In our work, we consider the kinetics of chemical reactions and wet–dry cycles, where we focus on spatially homogeneous chemical potential. Either the system is spatially homogeneous in terms of composition, or phase-separated with phase equilibrium between phases I and II. Below we review how the constraint of phase equilibrium is accounted for in the numerical solutions; more details can be found in ref (53).
To evolve the phase equilibrium constraint, μIi = μIIi, and thereby calculate the volume fractions in the two phases (eq 12), we use
| C1 |
Using the product rule, this can be rewritten to
| C2 |
Inserting the time-derivative of ϕiI/II from eq 12, eq C1 becomes
 |
C3 |
where rjI/II, jjI/II, and hjI/II are the reaction, diffusive, and evaporation/condensation fluxes, respectively. The conservation of particle number through the interphase implies
| C4 |
and can be plugged into eq C3. Thus, our (M + 1) unknown jiI can be calculated by solving the (M + 1) coupled algebraic equation in eq C3.
Appendix D
Role of Transition States
In this
section, we discuss
the role of transition states in the kinetics of chemical reactions
when subject to wet–dry cycles. To this end, we introduce the
transition state A*, which is associated with energetic barriers,
typically referred to as activation energies, relative to the states
A and B, 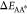 and
and  , respectively (Figure 7a). For the sake of illustration, we restrict
ourselves to homogeneous mixtures but allow the system to be nondilute.
Due to the change in composition during wet–dry cycles, the
activation energies, in general, vary during the chemical kinetics.
Here, we show that the effects of transition state A* on the kinetics
are negligible if the activation energies heights are in the order
of a few kBT or larger.
Such large energetic barriers will lead to a dilution of the transition
state corresponding to low volume fractions ϕA*.
, respectively (Figure 7a). For the sake of illustration, we restrict
ourselves to homogeneous mixtures but allow the system to be nondilute.
Due to the change in composition during wet–dry cycles, the
activation energies, in general, vary during the chemical kinetics.
Here, we show that the effects of transition state A* on the kinetics
are negligible if the activation energies heights are in the order
of a few kBT or larger.
Such large energetic barriers will lead to a dilution of the transition
state corresponding to low volume fractions ϕA*.
Figure 7.
Chemical reactions with a transition state: The transition state
A* between the reacting components A and B gives rise to an energetic
barrier for the reaction A ⇌ B. (a) The energy landscape is
set by the chemical potential 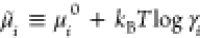 , where μi0 is the reference chemical potentials
and γi is the activity coefficient
that includes
the interactions among the nondilute components. The activation energy
for the reaction A ⇌ A* to occur is ΔEAA* = μ0A* – μ0A + kBTlogγA*/γA, and ΔEA*B = μ0A* – μ0B + kBTlog(γA*/γB) for the reaction A* ⇌ B. For large energetic barriers,
the transition state will be both dilute and short-lived, yielding
a separation of time scales. (b) The chemical potentials μi change in time and become equal at chemical
equilibrium. Moreover, for a quasi-static transition state (∂tϕA* ≃ 0), the chemical
potential of A* corresponds to a weighted average between μA and μB at each time of the kinetics (eq D10).
, where μi0 is the reference chemical potentials
and γi is the activity coefficient
that includes
the interactions among the nondilute components. The activation energy
for the reaction A ⇌ A* to occur is ΔEAA* = μ0A* – μ0A + kBTlogγA*/γA, and ΔEA*B = μ0A* – μ0B + kBTlog(γA*/γB) for the reaction A* ⇌ B. For large energetic barriers,
the transition state will be both dilute and short-lived, yielding
a separation of time scales. (b) The chemical potentials μi change in time and become equal at chemical
equilibrium. Moreover, for a quasi-static transition state (∂tϕA* ≃ 0), the chemical
potential of A* corresponds to a weighted average between μA and μB at each time of the kinetics (eq D10).
The chemical reaction rates for a chemical system with the substrate A, transition state A* (Figure 7), and a product B are given as
| D1 |
| D2 |
In a homogeneous system, they govern the rate of change of the volume fraction of the three components
 |
D3 |
Solving these equations, we find that
for large enough barriers, 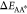 and
and 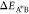 , the transition state quickly relaxes to
quasi-static conditions (∂tϕA* ≃ 0). To understand this analytically, we consider
an initial state ϕA(0) = ϕA0, ϕA*(0) = 0, and ϕB(0)
= 0, and consider that ϕA(t) ≃
ϕA(0) is at excess at early times. Thus, one can
write the time-derivative of A* (1D–D3) as
, the transition state quickly relaxes to
quasi-static conditions (∂tϕA* ≃ 0). To understand this analytically, we consider
an initial state ϕA(0) = ϕA0, ϕA*(0) = 0, and ϕB(0)
= 0, and consider that ϕA(t) ≃
ϕA(0) is at excess at early times. Thus, one can
write the time-derivative of A* (1D–D3) as
| D4 |
To achieve these expressions, we have rewritten the chemical potentials, μi = μ0i(T,p) + kBTlog(ϕiγi), where
| D5 |
| D6 |
Here, γi are the activity coefficients, including the effects of interactions, and μ0i are the reference chemical potentials. The chemical potential (μ0i + log γi) describes the energy landscape that a molecule experiences while reacting between A and B. It allows us to define activation energies for nondilute systems, ΔEAA* = μ0A* – μ0A + kBTlog γA*/γA and ΔEA*B = μ0A* – μB0 + kBTlog(γA*/γB) (Figure 7a). Note that such activation energies, in general, depend on composition and are only constant for dilute mixtures.
The solution to eq D4 at early times reads
| D7 |
with the characteristic relaxation time
| D8 |
and the early time plateau value of the transition state
| D9 |
where 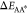 is the activation energy between A and
A* as depicted in Figure 7.
is the activation energy between A and
A* as depicted in Figure 7.
From the expression, we observe that the occupation
of the transition
state decreases exponentially with a characteristic time-scale τ.
This time scale has an exponential dependence on the activation energy  . Thus, for larger barriers, the relaxation
of the transition state occurs on a time scale much faster
. Thus, for larger barriers, the relaxation
of the transition state occurs on a time scale much faster 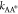 and
and 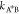 . After this relaxation, the kinetics
of
the transition state is quasi-statically slaved to the slow changes
of A and B. Thus, the transition state satisfies ∂tϕA* ≃ 0. On such time scales,
i.e.,
. After this relaxation, the kinetics
of
the transition state is quasi-statically slaved to the slow changes
of A and B. Thus, the transition state satisfies ∂tϕA* ≃ 0. On such time scales,
i.e., 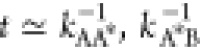 , ΦA* and ϕA change concomitantly
in time leading to the population of the B
state.
, ΦA* and ϕA change concomitantly
in time leading to the population of the B
state.
We note that large activation energies 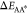 and
and 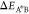 also imply that the transition state A*
gets diluted, which is reflected in the exponential dependence of
ΦA* on the barrier height (eq D9). In other words, transition states with
large energetic barriers lead to a separation of time scales and a
dilution of the transition evolving accordingly to a quasi-static
kinetics.81,82 This statement is confirmed by the excellent
agreement between the approximate analytic expression (D7) and the full numerical solution of the equation (Figure 8). At later time
scales when A is converted to B, the plateau value of ΦA* is slowly changing and thus deviates from the analytic value eq D9. However, solving the
equation using a quasi-static approximation (∂tϕA* ≃ 0) agrees extremely
well with the full numerical solution of eq at later times.
also imply that the transition state A*
gets diluted, which is reflected in the exponential dependence of
ΦA* on the barrier height (eq D9). In other words, transition states with
large energetic barriers lead to a separation of time scales and a
dilution of the transition evolving accordingly to a quasi-static
kinetics.81,82 This statement is confirmed by the excellent
agreement between the approximate analytic expression (D7) and the full numerical solution of the equation (Figure 8). At later time
scales when A is converted to B, the plateau value of ΦA* is slowly changing and thus deviates from the analytic value eq D9. However, solving the
equation using a quasi-static approximation (∂tϕA* ≃ 0) agrees extremely
well with the full numerical solution of eq at later times.
Figure 8.
Analytic equilibration of the transition state is fast: the full numerical solution is seen to agree with the approximate analytic solution in eq D7. The transition state’s relaxation time scale is faster than for A and B and remains dilute throughout.
We conclude that for large barriers and large times t > τ, the quasi-static condition is well satisfied. Using eq D3, we find that the chemical potential of the transition state corresponds to a weighted average of the chemical potentials of A and B
| D10 |
This equation can be solved for ϕA* at any time point of the later-time kinetics. By substituting eq D10 into eq D1, we find
| D11 |
and 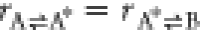 . Comparing to eqs 10 and 8, we see that
the expression is equivalent to a system with a single monomolecular
chemical reaction σ⇀iα = δiAδα1, σiα↽ = δiBδα1, and kc,α = kcδα,1. Thus, we introduce an effective
modified chemical reaction rate coefficient.
. Comparing to eqs 10 and 8, we see that
the expression is equivalent to a system with a single monomolecular
chemical reaction σ⇀iα = δiAδα1, σiα↽ = δiBδα1, and kc,α = kcδα,1. Thus, we introduce an effective
modified chemical reaction rate coefficient.
| D12 |
We conclude that for large barrier heights, the kinetics of A and B separated by a transition state A* can be mapped on a reduced chemical system without a transition state with an effective reaction rate kc (eq D12).
This reduction is also valid when the system is subject to evaporation if the time scales of evaporation/condensation ke–1, reaction kc–1 and Ω–1 are slow compared to the time scale of the transition state relaxation τ (D8)
| D13 |
This statement is supported by Figure 9, where the integrated relative difference between the substrate volume fraction for the kinetics with and without the transition state decreases exponentially with the barrier height.
Figure 9.
Relative error with and without a transition state: the integrated relative difference between the substrate volume fraction with and without a transition state decreases exponentially with the barrier height. By including interactions and/or evaporation/condensation, the required barrier height to achieve a given error increases. For this graph ωA = 0 and ωB = −3kBT.
The elimination of the kinetic equation for the
transition state
A* does not imply that the effective rate kc (eq D12) was independent
of the transition state. Using Kramer’s transition state theory,83 the kinetic rate coefficient 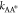 and
and 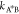 exponentially depends on the respective
activation energies
exponentially depends on the respective
activation energies
| D14 |
| D15 |
The above relationships not only encode the effects of transition state A* for large activation energies but also imply that such effects in general depend on the composition of all components due to the interaction among each other. Yet, as long as the reference chemical potentials dominate the activation energies, i.e., they exceed the contribution related to the activity coefficients
| D16 |
the kinetic rates 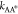 and
and 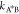 (eq D14) are approximately composition-independent.
In this
case, the effective reaction coefficient (eq D12) is approximately composition-independent,
too.
(eq D14) are approximately composition-independent.
In this
case, the effective reaction coefficient (eq D12) is approximately composition-independent,
too.
Appendix E
Gibbs’ Phase Rule
For a system of 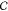 nonsolvent
components,
nonsolvent
components, 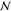 external thermodynamic
parameters, and
external thermodynamic
parameters, and 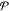 number
of
phases, the number of degrees
of freedom
number
of
phases, the number of degrees
of freedom 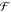 can be calculated
from
can be calculated
from
| E1 |
The number of degrees of freedom gives
the number of independent intensive variables uniquely defining the
thermodynamic equilibrium. The number of components 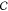 is calculated
as the number of components
needed to specify the composition of all phases (incompressibility
reduces
is calculated
as the number of components
needed to specify the composition of all phases (incompressibility
reduces 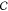 by one) minus
the number of constraints
between the concentrations (reduced by the number of chemical reactions).
Furthermore, the evaporation/condensation equilibrium can be regarded
as an external intensive variable defining the system. Such that the
set {T, p, μrC} or alternatively
{T, μrC}, gives
by one) minus
the number of constraints
between the concentrations (reduced by the number of chemical reactions).
Furthermore, the evaporation/condensation equilibrium can be regarded
as an external intensive variable defining the system. Such that the
set {T, p, μrC} or alternatively
{T, μrC}, gives
| E2 |
Therefore, two-phase coexistence in
a system is defined as a volume
in {T, p, μrC}-space and a
plane in {T, μCr}-space, while the homogeneous system has an additional degree
of freedom. For a fixed reservoir and a single chemical reaction,
the degree of freedom becomes  , such
that two-phase coexistence is only
achievable for a single point in the phase diagram. This is consistent
with the phase diagram in Figure 3c.
, such
that two-phase coexistence is only
achievable for a single point in the phase diagram. This is consistent
with the phase diagram in Figure 3c.
The authors declare no competing financial interest.
References
- Masel R. I.Chemical Kinetics and Catalysis; Wiley, 2001. [Google Scholar]
- Singch S.; Tandon P. Catalysis: a brief review on nano-catalyst. J. Energy Chem. Eng. 2014, 2, 106. [Google Scholar]
- Werner S.; Haumann M.; Wasserscheid P. Ionic Liquids in Chemical Engineering. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 203–230. 10.1146/annurev-chembioeng-073009-100915. [DOI] [PubMed] [Google Scholar]
- Blaser H.-U.; Studer M. The role of catalysis for the clean production of fine chemicals. Appl. Catal., A 1999, 189, 191–204. 10.1016/S0926-860X(99)00276-8. [DOI] [Google Scholar]
- Alberty R. A.Thermodynamics of Biochemical Reactions; Wiley-Interscience: Hoboken, N.J, 2003. [Google Scholar]
- Srere P. A. Complexes of Sequential Metabolic Enzymes. Annu. Rev. Biochem. 1987, 56, 89–124. 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Underkofler L. A.; Barton R. R.; Rennert S. S. Production of Microbial Enzymes and Their Applications. Appl. Microbiol. 1958, 6, 212–221. 10.1128/am.6.3.212-221.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigen M.; Hammes G. G.. Advances in Enzymology - and Related Areas of Molecular Biology; John Wiley & Sons, Ltd, 1963; pp 1–38. [Google Scholar]
- Eigen M.Quarterly Reviews of Biophysics; Cambridge University Press, 1968; Vol. 1, p 3. [DOI] [PubMed] [Google Scholar]
- Kauffman S. A. Approaches to the Origin of Life on Earth. Life 2011, 1, 34–48. 10.3390/life1010034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Molecular Diversity Preservation International
- Oparin A. I. The Origin of Life and the Origin of Enzymes. Adv. Enzymol. Relat. Area Mol. Biol. 1965, 27, 347–380. 10.1002/9780470122723.ch7. [DOI] [PubMed] [Google Scholar]
- Orgel L. E. The Origin of Life on the Earth. Sci. Am. 1994, 271, 76–83. Scientific American, a division of Nature America, Inc 10.1038/scientificamerican1094-76. [DOI] [PubMed] [Google Scholar]
- Hay S.; Johannissen L. O.; Sutcliffe M. J.; Scrutton N. S. Barrier Compression and Its Contribution to Both Classical and Quantum Mechanical Aspects of Enzyme Catalysis. Biophys. J. 2010, 98, 121–128. 10.1016/j.bpj.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeyssens F.; Harvey J. N.; Manby F. R.; Mata R. A.; Mulholland A. J.; Ranaghan K. E.; Schütz M.; Thiel S.; Thiel W.; Werner H.-J. High-Accuracy Computation of Reaction Barriers in Enzymes. Angew. Chem., Int. Ed. 2006, 45, 6856–6859. 10.1002/anie.200602711. [DOI] [PubMed] [Google Scholar]
- Mast C. B.; Schink S.; Gerland U.; Braun D. Escalation of polymerization in a thermal gradient. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 8030–8035. Proceedings of the National Academy of Sciences 10.1073/pnas.1303222110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasch M.; Liu J.; Dirscherl C. F.; Ianeselli A.; Kühnlein A.; Le Vay K.; Schwintek P.; Islam S.; Corpinot M. K.; Scheu B.; et al. Heated gas bubbles enrich, crystallize, dry, phosphorylate and encapsulate prebiotic molecules. Nat. Chem. 2019, 11, 779–788. 10.1038/s41557-019-0299-5. [DOI] [PubMed] [Google Scholar]
- Baaske P.; Weinert F. M.; Duhr S.; Lemke K. H.; Russell M. J.; Braun D. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 9346–9351. Proceedings of the National Academy of Sciences 10.1073/pnas.0609592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolucci G.; Calaça Serrão A.; Schwintek P.; Kühnlein A.; Rana Y.; Janto P.; Hofer D.; Mast C. B.; Braun D.; Weber C. A. Sequence self-selection by cyclic phase separation. Proc. Natl. Acad. Sci. U.S.A. 2023, 120, e2218876120 10.1073/pnas.2218876120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianeselli A.; Tetiker D.; Stein J.; Kühnlein A.; Mast C. B.; Braun D.; Dora Tang T.-Y. Non-equilibrium conditions inside rock pores drive fission, maintenance and selection of coacervate protocells. Nat. Chem. 2022, 14, 32–39. 10.1038/s41557-021-00830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nature Publishing Group
- Matreux T.; Le Vay K.; Schmid A.; Aikkila P.; Belohlavek L.; Çalışkanoǧlu A. Z.; Salibi E.; Kühnlein A.; Springsklee C.; Scheu B.; et al. Heat flows in rock cracks naturally optimize salt compositions for ribozymes. Nat. Chem. 2021, 13, 1038. 10.1038/s41557-021-00772-5. [DOI] [PubMed] [Google Scholar]; Nature Publishing Group
- Busiello D. M.; Liang S.; Piazza F.; De Los Rios P. Dissipation-driven selection of states in non-equilibrium chemical networks. Commun. Chem. 2021, 4, 16. 10.1038/s42004-021-00454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nature Publishing Group
- Nicolis G. Dissipative systems. Rep. Prog. Phys. 1986, 49, 873–949. 10.1088/0034-4885/49/8/002. [DOI] [Google Scholar]
- England J. L. Dissipative adaptation in driven self-assembly. Nat. Nanotechnol. 2015, 10, 919–923. 10.1038/nnano.2015.250. [DOI] [PubMed] [Google Scholar]; Nature Publishing Group
- Brogliato B.; Lozano R.; Maschke B.; Egeland O.. Dissipative Systems Analysis and Control: Theory and Applications, Communications and Control Engineering; Springer International Publishing: Cham, 2020. [Google Scholar]
- Maguire O. R.; Smokers I. B. A.; Huck W. T. S. A physicochemical orthophosphate cycle via a kinetically stable thermodynamically activated intermediate enables mild prebiotic phosphorylations. Nat. Commun. 2021, 12, 5517. 10.1038/s41467-021-25555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nature Publishing Group
- Fares H. M.; Marras A. E.; Ting J. M.; Tirrell M. V.; Keating C. D. Impact of wet-dry cycling on the phase behavior and compartmentalization properties of complex coacervates. Nat. Commun. 2020, 11, 5423. 10.1038/s41467-020-19184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S.; Schneider C.; Okamura H.; Crisp A.; Amatov T.; Dejmek M.; Carell T. Wet-dry cycles enable the parallel origin of canonical and non-canonical nucleosides by continuous synthesis. Nat. Commun. 2018, 9, 163. 10.1038/s41467-017-02639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin E.; Salditt A.; Schwintek P.; Wunnava S.; Langlais J.; Saenz J.; Tang D.; Schwille P.; Mast C.; Braun D. Prebiotic Foam Environments to Oligomerize and Accumulate RNA. ChemBioChem 2022, 23, e202200423 10.1002/cbic.202200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass A. V.; Wunnava S.; Langlais J.; von der Esch B.; Krusche M.; Ufer L.; Chrisam N.; Dubini R. C. A.; Gartner F.; Angerpointner S.; et al. RNA Oligomerisation without Added Catalyst from 2′,3′-Cyclic Nucleotides by Drying at Air-Water Interfaces**. ChemSystemsChem 2023, 5, e202200026 10.1002/syst.202200026. [DOI] [Google Scholar]
- Higgs P. G. The Effect of Limited Diffusion and Wet–Dry Cycling on Reversible Polymerization Reactions: Implications for Prebiotic Synthesis of Nucleic Acids. Life 2016, 6, 24. 10.3390/life6020024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multidisciplinary Digital Publishing Institute
- Forsythe J. G.; Yu S.-S.; Mamajanov I.; Grover M. A.; Krishnamurthy R.; Fernández F. M.; Hud N. V. Ester-Mediated Amide Bond Formation Driven by Wet–Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem., Int. Ed. 2015, 54, 9871–9875. 10.1002/anie.201503792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamajanov I.; MacDonald P. J.; Ying J.; Duncanson D. M.; Dowdy G. R.; Walker C. A.; Engelhart A. E.; Fernández F. M.; Grover M. A.; Hud V.; Schork F. J.; et al. Ester Formation and Hydrolysis during Wet–Dry Cycles: Generation of Far-from-Equilibrium Polymers in a Model Prebiotic Reaction. Macromolecules 2014, 47, 1334–1343. 10.1021/ma402256d. [DOI] [Google Scholar]
- Weber A. L. Thermal synthesis and hydrolysis of polyglyceric acid. Orig. Life Evol. Biosph. 1989, 19, 7–19. 10.1007/BF01808284. [DOI] [PubMed] [Google Scholar]
- Lahav N.; White D.; Chang S. Peptide Formation in the Prebiotic Era: Thermal Condensation of Glycine in Fluctuating Clay Environments. Science 1978, 201, 67–69. American Association for the Advancement of Science 10.1126/science.663639. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia M.; Surman A. J.; Cooper G. J. T.; Suárez-Marina I.; Hosni Z.; Lee M. P.; Cronin L. Formation of oligopeptides in high yield under simple programmable conditions. Nat. Commun. 2015, 6, 8385. 10.1038/ncomms9385. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nature Publishing Group
- Fox S. W.; Harada K. Thermal Copolymerization of Amino Acids to a Product Resembling Protein. Science 1958, 128, 1214. 10.1126/science.128.3333.1214. [DOI] [PubMed] [Google Scholar]
- Rajamani S.; Vlassov A.; Benner S.; Coombs A.; Olasagasti F.; Deamer D. Lipid-assisted Synthesis of RNA-like Polymers from Mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. 10.1007/s11084-007-9113-2. [DOI] [PubMed] [Google Scholar]
- Da Silva L.; Maurel M.-C.; Deamer D. Salt-Promoted Synthesis of RNA-like Molecules in Simulated Hydrothermal Conditions. J. Mol. Evol. 2015, 80, 86–97. 10.1007/s00239-014-9661-9. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Pham V. A.; Matsuura T.; Santerre J. P.; Narbaitz R. M. Effect of surface-modifying macromolecules and solvent evaporation time on the performance of polyethersulfone membranes for the separation of chloroform/water mixtures by pervaporation. J. Appl. Polym. Sci. 1994, 54, 1937–1943. 10.1002/app.1994.070541216. [DOI] [Google Scholar]
- Weber C. A.; Zwicker D.; Jülicher F.; Lee C. F. Physics of active emulsions. Rep. Prog. Phys. 2019, 82, 064601.IOP Publishing 10.1088/1361-6633/ab052b. [DOI] [PubMed] [Google Scholar]
- Kato S.; Garenne D.; Noireaux V.; Maeda Y. T. Phase Separation and Protein Partitioning in Compartmentalized Cell-Free Expression Reactions. Biomacromolecules 2021, 22, 3451–3459. American Chemical Society 10.1021/acs.biomac.1c00546. [DOI] [PubMed] [Google Scholar]
- Hyman A. A.; Weber C. A.; Jülicher F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Blankschtein D.; Thurston G. M.; Benedek G. B. Theory of phase separation in micellar solutions. Phys. Rev. Lett. 1985, 54, 955. 10.1103/PhysRevLett.54.955. [DOI] [PubMed] [Google Scholar]
- Semenov A. N.; Rubinstein M. Thermoreversible Gelation in Solutions of Associative Polymers. 1. Statics. Macromolecules 1998, 31, 1373–1385. 10.1021/ma970616h. [DOI] [Google Scholar]
- Deviri D.; Safran S. A. Equilibrium size distribution and phase separation of multivalent, molecular assemblies in dilute solution. Soft Matter 2020, 16, 5458–5469. 10.1039/C9SM02408E. [DOI] [PubMed] [Google Scholar]
- Bartolucci G.; Haugerud I. S.; Michaels T. C. T.; Weber C. A. The interplay between molecular assembly and phase separation. bioRxiv 2023, 2023. 10.1101/2023.04.18.537072. [DOI] [Google Scholar]
- Lewis A. E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. 10.1016/j.hydromet.2010.06.010. [DOI] [Google Scholar]
- Agarwal V.; Peters B. Solute Precipitate Nucleation: A Review of Theory and Simulation Advances. Adv. Chem. Phys. 2014, 155, 97–160. 10.1002/9781118755815.ch03. [DOI] [Google Scholar]
- Carrea G.; Colonna S.; Kelly D. R.; Lazcano A.; Ottolina G.; Roberts S. M. Polyamino acids as synthetic enzymes: mechanism, applications and relevance to prebiotic catalysis. Trends Biotechnol. 2005, 23, 507–513. 10.1016/j.tibtech.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Eijsink V. G. H.; Gåseidnes S.; Borchert T. V.; van den Burg B. Directed evolution of enzyme stability. Biomol. Eng. 2005, 22, 21–30. 10.1016/j.bioeng.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Schulz L.; Guo Z.; Zarzycki J.; Steinchen W.; Schuller J. M.; Heimerl T.; Prinz S.; Mueller-Cajar O.; Erb T. J.; Hochberg G. K. A. Evolution of increased complexity and specificity at the dawn of form I Rubiscos. Science 2022, 378, 155–160. American Association for the Advancement of Science 10.1126/science.abq1416. [DOI] [PubMed] [Google Scholar]
- Adame-Arana O.; Weber C. A.; Zaburdaev V.; Prost J.; Jülicher F. Liquid Phase Separation Controlled by pH. Biophys. J. 2020, 119, 1590–1605. 10.1016/j.bpj.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermann J.; Laha S.; McCall P. M.; Jülicher F.; Weber C. A. Chemical Kinetics and Mass Action in Coexisting Phases. J. Am. Chem. Soc. 2022, 144, 19294–19304. American Chemical Society 10.1021/jacs.2c06265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülicher F.; Ajdari A.; Prost J. Modeling molecular motors. Rev. Mod. Phys. 1997, 69, 1269–1282. American Physical Society 10.1103/revmodphys.69.1269. [DOI] [Google Scholar]
- Bartolucci G.; Adame-Arana O.; Zhao X.; Weber C. A. Controlling composition of coexisting phases via molecular transitions. Biophys. J. 2021, 120, 4682–4697. 10.1016/j.bpj.2021.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loche P.; Bonthuis D. J.; Netz R. R. Molecular dynamics simulations of the evaporation of hydrated ions from aqueous solution. Commun. Chem. 2022, 5, 55. 10.1038/s42004-022-00669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nature Publishing Group
- Hisatake K.; Tanaka S.; Aizawa Y. Evaporation rate of water in a vessel. J. Appl. Phys. 1993, 73, 7395–7401. 10.1063/1.354031. [DOI] [Google Scholar]
- Heinen M.; Vrabec J. Evaporation sampled by stationary molecular dynamics simulation. J. Chem. Phys. 2019, 151, 044704. 10.1063/1.5111759. [DOI] [PubMed] [Google Scholar]
- Xiong T. Y.; Yuen M. C. Evaporation of a liquid droplet on a hot plate. Int. J. Heat Mass Transfer 1991, 34, 1881–1894. 10.1016/0017-9310(91)90162-8. [DOI] [Google Scholar]
- Clary D. C. Fast Chemical Reactions: Theory Challenges Experiment. Annu. Rev. Phys. Chem. 1990, 41, 61–90. 10.1146/annurev.pc.41.100190.000425. [DOI] [Google Scholar]
- Kok C. M.; Rudin A. Prediction of Flory–Huggins interaction parameters from intrinsic viscosities. J. Appl. Polym. Sci. 1982, 27, 353–362. 10.1002/app.1982.070270203. [DOI] [Google Scholar]
- Russell T. H.; Edwards B. J.; Khomami B.. EPL; EDP Sciences, IOP Publishing and Società Italiana di Fisica, 2015; Vol. 108, p 66003. [Google Scholar]
- Zwicker D.; Seyboldt R.; Weber C. A.; Hyman A. A.; Jülicher F. Growth and division of active droplets provides a model for protocells. Nat. Phys. 2017, 13, 408–413. Nature Publishing Group 10.1038/nphys3984. [DOI] [Google Scholar]
- Zaks A.; Klibanov A. M. The effect of water on enzyme action in organic media. J. Biol. Chem. 1988, 263, 8017–8021. 10.1016/S0021-9258(18)68435-2. [DOI] [PubMed] [Google Scholar]
- Brogan A. P. S.; Sharma K. P.; Perriman A. W.; Mann S. Enzyme activity in liquid lipase melts as a step towards solvent-free biology at 150 °C. Nat. Commun. 2014, 5, 5058.Nature Publishing Group 10.1038/ncomms6058. [DOI] [PubMed] [Google Scholar]
- Helms V.; Wade R. C. Hydration energy landscape of the active site cavity in cytochrome P450cam. Proteins: Struct., Funct., Bioinf. 1998, 32, 381–396. . [DOI] [PubMed] [Google Scholar]
- Klibanov A. M. Trends Biotechnol. 1997, 15, 97–101. 10.1016/S0167-7799(97)01013-5. [DOI] [PubMed] [Google Scholar]
- Mulkidjanian A. Y.; Bychkov A. Y.; Dibrova D. V.; Galperin M. Y.; Koonin E. V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, E821 10.1073/pnas.1117774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damer B.; Deamer D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. 10.3390/life5010872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T. D.; Febrian R.; McCarthy J. T.; Kleinschmidt H. E.; Forsythe J. G.; Bracher P. J. Prebiotic condensation through wet–dry cycling regulated by deliquescence. Nat. Commun. 2019, 10, 4508. 10.1038/s41467-019-11834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov A. V.; Johnston B. H.; Landweber L. F.; Kazakov S. A. Ligation activity of fragmented ribozymes in frozen solution: implications for the RNA world. Nucleic Acids Res. 2004, 32, 2966–2974. 10.1093/nar/gkh601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vay K.; Song E. Y.; Ghosh B.; Tang T.-Y. D.; Mutschler H. Enhanced Ribozyme-Catalyzed Recombination and Oligonucleotide Assembly in Peptide-RNA Condensates. Angew. Chem., Int. Ed. 2021, 60, 26096–26104. 10.1002/anie.202109267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler H.; Wochner A.; Holliger P. Freeze–thaw cycles as drivers of complex ribozyme assembly. Nat. Chem. 2015, 7, 502–508. Nature Publishing Group 10.1038/nchem.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scientific Image and Illustration Software | BioRender. 2023, Oct 10, 2023, https://www.biorender.com/.
- Bergmann A. M.; Donau C.; Späth F.; Jahnke K.; Göpfrich K.; Boekhoven J. Evolution and Single-Droplet Analysis of Fuel-Driven Compartments by Droplet-Based Microfluidics. Angew. Chem. 2022, 134, e202203928 10.1002/ange.202203928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauermann J.; Weber C. A.; Jülicher F. Energy and Matter Supply for Active Droplets. Ann. Phys. 2022, 534, 2200132. 10.1002/andp.202200132. [DOI] [Google Scholar]
- Fritsch A. W.; Diaz-Delgadillo A. F.; Adame-Arana O.; Hoege C.; Mittasch M.; Kreysing M.; Leaver M.; Hyman A. A.; Jülicher F.; Weber C. A. Local thermodynamics govern formation and dissolution ofCaenorhabditiselegans P granule condensates. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2102772118Proceedings of the National Academy of Sciences 10.1073/pnas.2102772118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A. M.; Bauermann J.; Bartolucci G.; Donau C.; Stasi M.; Holtmannspötter A.-L.; Jülicher F.; Weber C. A.; Boekhoven J. Liquid spherical shells are a non-equilibrium steady state. Nat. Commun. 2023, 14, 6552. 10.1038/s41467-023-42344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S.; Saha S.; Woodruff J. B.; Franzmann T. M.; Wang J.; Hyman A. A. A User’s Guide for Phase Separation Assays with Purified Proteins. J. Mol. Biol. Phase Separ. Biol. Dis. 2018, 430, 4806–4820. 10.1016/j.jmb.2018.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T.; Linke D. Phase separation in the isolation and purification of membrane proteins. BioTechniques 2007, 43, 427–440. Future Science 10.2144/000112566. [DOI] [PubMed] [Google Scholar]
- Van Kampen N. G. Elimination of fast variables. Phys. Rep. 1985, 124, 69–160. 10.1016/0370-1573(85)90002-X. [DOI] [Google Scholar]
- Janssen J. A. M. The elimination of fast variables in complex chemical reactions. II. Mesoscopic level (reducible case). J. Stat. Phys. 1989, 57, 171–185. 10.1007/BF01023639. [DOI] [Google Scholar]
- Gardiner C. W.Handbook of Stochastic Method; Springer Berlin, 1985; Vol. 3. [Google Scholar]










