Abstract
Objectives
To evaluate the safety and effectiveness of computed tomography (CT)-guided radioactive 125I seeds brachytherapy (RISB) for lung oligometastases (LO) from colorectal cancer (CRC).
Methods
Data for 144 LOs from 70 CRC patients who underwent CT-guided RISB were retrospectively analyzed. The primary endpoints were progression-free survival (PFS) and overall survival (OS), and the secondary endpoints were technical success, local control rate (LCR), and complications. Kaplan–Meier method was used for survival analysis. Cox model was used to identify the independent predictors of poor prognosis.
Results
The RISB procedures were successfully performed in all patients, and the success rate was 100%. The median follow-up was 27.8 months. The median PFS was 10.0 months (95% CI: 8.9–11.1) and the 1- and 2-year PFS rates were 32.9% and 5.9%, respectively. On multivariate analysis, serum carcinoembryonic antigen (CEA) ≤ 15 ng/ml (P = 0.048), middle-high differentiated pathological classification (P = 0.015), primary TNM stages I-III (P = 0.001), LO number ≤ 2 (P < 0.001) and cumulative gross tumor volume (GTV) ≤ 40 cm3 (P < 0.001) showed superior PFS. The median OS was 30.8 months (95% CI: 27.1–34.4) and the 1-, 2-, and 3-year OS rates were 95.7%, 67.4%, and 42.5%, respectively. On multivariate analysis, serum CEA ≤ 15 ng/ml (P = 0.004), middle-high differentiated pathological classification (P < 0.001), primary TNM stages I-III (P < 0.001), LO number ≤ 2 (P < 0.001), cumulative GTV ≤ 40 cm3 (P < 0.001) and system treatments combined with chemotherapy and target therapy (P < 0.001) showed superior OS. The LCR for 3, 6, and 12 months was 97.9%, 91.0%, and 83.6%, respectively. There were 4 cases of pneumothorax at 5.7% that required drainage.
Conclusions
RISB for LO from CRC is safe and effective, and serum CEA, TNM stage, LO number, cumulative GTV, and system treatments should be emphasized for long OS.
Keywords: Lung oligometastases, Colorectal cancer, 125I brachytherapy, Clinical study
Key points
• CT-guided RISB is a new treatment for lung oligometastases from CRC.
• RISB can be an alternative therapy for patients who are unsuitable for surgery, ablation, or SBRT.
• Serum CEA, TNM stage, LO number, cumulative GTV, and system treatments are prognostic factors.
• RISB is a safe method with a curative effect.
Introduction
Colorectal cancer (CRC) ranks fourth in the incidence rate of malignant tumors in China and has become one of the most important killers threatening people’s life [1]. About 50–60% of CRC patients have metastasis when diagnosed, and liver and lung are the most common sites of metastasis [2]. There is an oligometastatic state (no more than 3 metastatic organs and 5 metastatic lesions) between local tumor progression and extensive metastasis, which concept was expressed by Hellman and Weichsel Baum in 1995 [3], who emphasized the role of local treatments such as surgery, percutaneous ablation, and stereotactic body radiotherapy (SBRT) on long-term survival benefits.
Surgery is the standard treatment for resectable oligometastases from CRC, and 5-year overall survival (OS) can reach 30–60% after surgery resection of liver oligometastases, and it can reach 25–35% for patients with lung oligometastases (LOs) in previous studies [4, 5]. However, it may not be suitable for patients with contraindications such as general anesthesia problems, low performance status, serious concomitant diseases, and subjective refusal. Non-surgical local treatment strategies, represented by SBRT and ablation, are gradually becoming the preferred choice for these patients. Ablation is safe, effective, repeatable, and low in cost, which provides good curative results on peripheral LO. However, it is challenging for LO closed to the main bronchus, large vessels, or pericardium and may result in thermal damage. SBRT is characterized by the irradiation of a relatively small target with a high single dose and few fractions to achieve an equivalent biological dose to the tumor, which is similar to or even higher than conventional segmentation irradiation and has a steep dose fallout on surrounding healthy tissues. A recent meta-analysis based on 943 patients with 1290 oligometastases from 21 clinical studies concluded that 1-year progression-free survival (PFS) and OS were 51.4% (95% CI: 42.7–60.1%) and 85.4% (95% CI: 77.1–92.0%), respectively [6]. But SBRT also has its own limitations, such as the high potential for radiation-induced pneumonitis and pulmonary fibrosis that cannot be repeated in the short term [7], the equipment shortage at most Chinese hospitals because of the high price, the insufficient number of professionals because of the high requirement for tumor localization and implementation, and the difficulty paying for patients from low-income families.
Radioactive 125I seeds brachytherapy (RISB) was a new minimally invasive method in which the 125I seeds were implanted into the target lesion under the guidance of medical imaging [such as Ultrasound, Computer Tomography (CT)] and brachytherapy treatment planning system (BT-TPS). 125I seeds, a brachytherapy nuclide with low energy and a moderate half-life (59.6 days), can continuously emit a low dose of X and γ-rays and gradually accumulate in the tumor tissue. This highly conformal brachytherapy can ensure accurate attack on the tumor while simultaneously protecting the surrounding normal tissues and organs at risk (OAR) to the greatest extent. RISB has been widely used in the local treatment of prostate cancer [8], lung cancer [9], pancreatic cancer [10], esophageal cancer [11], biliary cancer [12] and so on. What’s more, RISB for prostate cancer and brachytherapy stent loaded with 125I seeds for malignant esophageal obstruction is recommended by some guidelines from the European Endoscopic Society and the Chinese Society for Esophageal Cancer Radiotherapy [13, 14]. Compared with SBRT, both can achieve high dose radiation to tumors and minimize radiation injury to the surrounding normal tissue, but RISB has its own advantages [15, 16]: (1) RISB can be widely applied under conventional CT without expensive SBRT equipment additionally; (2) all patients can complete RISB with warm home-care without frequent hospital visits; (3) RISB is cheaper for patients to reduce economic burden; (4) continuous low dose radiation is in accordance with radiotherapy “4R theory” such as repair of radiation damage, redistribution within cell cycle, reoxygenation of tumors, and repopulation of cells in tissue; (5) the local control rate (LCR) of lung tumors after RISB can be as high as 80%–96% in recent Meta analysis [17], causing relatively slighter radiation-induced lung injury that can be repeated in the short term [7], which gradually attracted the attention of radiotherapists, interventionists, oncologists and nuclide specialists. So can RISB achieve comparable efficacy to SBRT in LO from CRC? There were no related reports in the previous clinical study, as far as we know. This retrospective study preliminarily evaluated the clinical efficacy of RISB for LO from CRC, and possible influencing factors on PFS and OS were also analyzed.
Materials and methods
Patient selection
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Ethical Review Number: 2021-KY-400), and all data were collected from the hospital electronic information system. The inclusion criteria were as follows: (1) age range 18–75 years old; (2) radical surgical resection of primary CRC; (3) metastasis involved only lung and liver; (6) total metastases number ≤ 5; (4) limited liver metastases (metastases number ≤ 3 and max. Diameter ≤ 5 cm) are completely controlled by surgery or local ablation; (7) max. Diameter of singel LO ≤ 5 cm; (8) no lymph node metastasis previous RISB; and (9) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1. The exclusion criteria were as follows: (1) metastasis not limited to liver and lungs before RISB; (2) total metastases number > 5; (3) diameter of LO > 5 cm; (4) previous history of radiotherapy for thoracic tumor; (5) insufficient cardiovascular, hepatic, and renal function; (6) complicated with severe coagulation dysfunction (platelet count < 50 × 109/L and prothrombin time > 21 s); (7) life expectancy ≤ 6 months; and (8) incomplete data. The final diagnosis of LO depended on biopsy pathology (n = 27) or medical image evidence (n = 43), and the selection process of 70 patients is presented in Fig. 1.
Fig. 1.
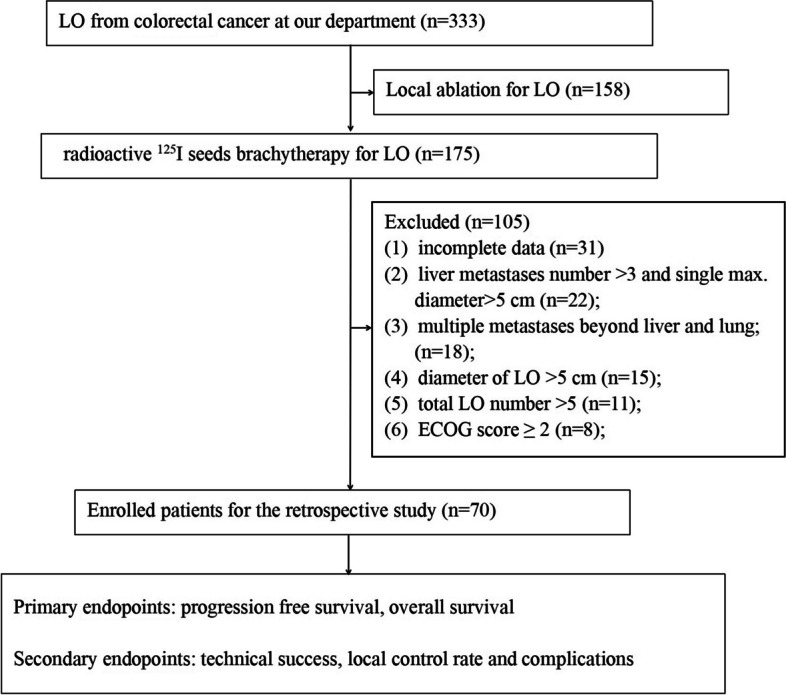
The workflow for 70 patients
125I seeds and TPS
The size of 125I seed was (Type-6711, cylindrical, Tianjin Saide Biopharmaceutical Co., Ltd., China) 0.8 mm in diameter and 4.5 mm in length with titanium capsules, and seed radioactivity was 0.6–0.8 mCi (22.2–29.6 MBq) with a half-life of 59.6 days, which mainly emits low-dose γ-rays (35.5 keV) and soft X-rays (28.6 keV). The valid anticancer radius was 1.7 cm, and more than 90% of the barchytherapy dose was gradually released within ten months. All patients underwent pretreatment TPS (Beijing Atom & High Technical Industries Inc., China) by a clinician and physiologist (Zhou X, who had 8 years of experience) according to enhanced chest CT (lung width: 1000 HU, lung level: − 650 HU, and 5 mm slice thickness) data. With reference to International Commission on Radiation Units and Measurements (ICRU) report (No.58), to acquire the clinical target volume (CTV), the gross tumor volume (GTV) should expand by 5 mm in all directions; however, unless there is external invasion, the CTV should not exceed the anatomical limit. The prescription dose (PD) is recommended to cover the CTV with 120 Gy according to American Brachytherapy Society recommendations for prostate cancer [18] and our prior clinical experience [19]. Organs at risk (OAR) are defined as important or irradiation-sensitive organs such as spinal cord, heart or major blood vessels.
RISB procedure
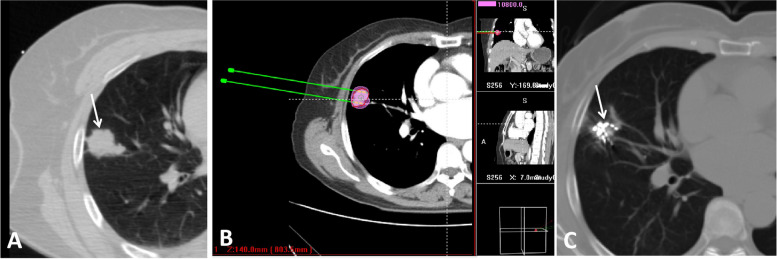
Two experienced doctors (DC Jiao and XW Han, with 15 and 22 years of experience, respectively) performed all RISB procedures. The patient’s position was determined according to the LO location to facilitate puncture (The puncture approaches should avoid the lung vessels as much as possible). After local disinfection of the puncture point and towel laying, 2% lidocaine (5 ml) was used to achieve satisfactory local anesthesia. An 18 G puncture needle (15 cm in length, Plastic hub, Hakko Co. LTD, Japan) was inserted into the farthest end of the LO under CT guidance. Then the seed implatation gun (Tianjin Saide Biopharmaceutical Co. Ltd. China) was attached to the inserted seed needles, and the seeds were implanted from the distal to the proximal with an interval distance of 0.5–0.8 mm. Multiple needles were used at equal intervals in parallel to achieve uniform 125I seeds distribution when the transverse diameter of the tumor was larger than 1.5 cm according to pretreatment TPS. Finally, another CT was performed to exclude puncture-related complications, and dose verification was performed again on TPS to calculate the D90 (cumulative dose absorbed by 90% GTV) and OAR doses (Fig. 2).
Fig. 2.
A 62 female patient who had a right lung oligometastase (white arrow, diameter: 3.1 cm) after radical surgical resection of primary rectal carcinoma and local ablation for one liver metastase. B Treatment planning system was done before radioacitve seed implantation with 120Gy prescription dose; C Local tumor disapeared after two months125I brachytherapy (white arrow)
Definition and follow-up
Technical success was defined as the successful completion of RISB. Cumulative GTV was calculated by summing individual GTV for patients with multiple lesions. Progression disease (PD) was defined as any tumor recurrence or metastasis after RISB, including modality. Local failure was defined as an increase in tumor size during follow-up or the development of a new lesion in the 125I seed brachytherapy field. Regional failure was defined as a new lesion’s development outside the brachythrapy field in the same organ, and distant metastasis was defined as a new lesion beyond the organ. PFS was defined as the time from the start of RISB to the first documented date of PD or to the last follow-up visit. OS was defined as the time from the start of RISB to the date of death or the last follow-up visit. Local control rate (LCR) will be conducted according to the evaluation standard for solid tumor efficacy (RECIST 1.1) [20]. LCR = [complete response (CR) + partial response (PR) + stable disease (SD)]/total number of lesions × 100%. Adverse reactions were evaluated according to the criteria for common adverse events (CTCAE 4.03) of the National Institutes of Health (NIH) [21]. All patients were followed up every month during the first three months and then every 2–3 months thereafter. The contents of follow-up included routine blood tests, liver and kidney function, serum CEA, and enhanced CT.
Statistical analysis
SPSS 19.0 software (SPSS, Inc., Chicago, IL) was used to perform all statistical analyses. All continuous data are expressed as the mean ± standard deviation (SD). PFS and OS were calculated and compared by the Kaplan–Meier method and log-rank test, and all possible variables were finally analyzed by multivariate analysis (Cox hazards model) to identify independent prognostic factors. P < 0.05 was considered statistically significant.
Results
Study population
From October 2012 to January 2019, 70 patients [mean age: (57.5 ± 7.6) years old, (range: 41–71); male/female = 37:33, 144 LO with mean GTV (31.2 ± 18.1) cm3] with CRC met the inclusion criteria, whose data were collected by electronic information system. Of all 70 patients, the primary CRCs (colon cancer = 34 and rectum = 36) were completely controlled by surgery, and 44 patients had limited liver metastases (metastases number ≤ 3 and max. Diameter ≤ 5 cm) that were completely controlled by surgery (n = 14) or local ablation (n = 30). There were 1, 2, 3, 4, and 5 LO in 26 (37.1%), 21 (30.0%), 17 (24.3%), 5 (7.1%) and 1 (1.4%) patients, respectively. Primary TNM stage I-III and IV were in 47 (67.1%) and 23 (32.9%) patients, and low and middle-high differentiated pathological classification were in 28 (40.0%) and 42 (60.0%) patients, respectively. The serum CEA was (28.7 ± 21.8) ng/ml (range 3.60–108.20). The total implanted 125I seeds were 3089, and the implanted 125I number per patient was (44.1 ± 16.1) (range 20.0–95.0). According to postoperative TPS evaluation, the D90 was (116.4 ± 11.8) Gy (range 92.5–144.6). As for systemic treatments, 43 (61.4%) and 27 (38.6%) accepted chemotherapy combined target therapy and chemotherapy alone, respectively, and other detailed information is presented in Table 1, and the chemothrapy regimen was capecitabine plus oxaliplatin.
Table 1.
Basic characteristics
| Characteristics | Value (%) |
|---|---|
| Total patients/LO number | 70/144 |
| Sex(male/female) | 37(52.9%)/33(47.1%) |
| Age (mean ± SD, years old) | 57.5 ± 7.6 |
| BMI (mean ± SD, mg/m2) | 24.6 ± 2.9 |
| Serum CEA (mean ± SD, ng/ml) | 28.7 ± 21.8 |
| Serum CEA stratification (≤ 15 ng/ml/ > 15 ng/ml) | 32(45.7%)/38(54.3%) |
| Primary tumor site (colon/rectum) | 34(48.6%)/36(51.4%) |
| Pathological classification (low/middle- high differentiated) | 28(40.0%)/42(60.0%) |
| TNM stage (stage I-III/stage IV) | 47(67.1%)/23(32.9%) |
| ECOG score (0/1) | 35(50.0%)/35(50.0%) |
| Number of LO per person(1/2/3/4/5 LO) | 26(37.1%)/21(30.0%)/17(24.3%)/5(7.1%)1(1.4%) |
| Mean number of LO per person ( mean ± SD) | 2.1 ± 1.0 |
| Number of LO stratification (≤ 2 / > 2 nodules) | 47(67.1%)/23(32.9%) |
| LO location (left/right lung) | 79(54.9%)/65(45.1%) |
| Mean GTV per LO ( mean ± SD, cm3) | 31.2 ± 18.1 |
| Cumulative GTV per person ( cm3) | 53.0 ± 22.4 |
| Cumulative GTV stratification (≤ 40 / > 40 cm3) | 31(44.3%)/39(55.7%) |
| D90 per LO (mean ± SD, Gy) | 116.4 ± 11.8 |
| Systemic treatments (C + TT/C alone) | 43(61.4%)/27(38.6%) |
| Progression disease (Yes/no) | 64(91.4%)/6(8.6%) |
| Disease-free interval (months) | 11.0 ± 6.9 |
| Disease-free interval stratification (≤ 12 / > 12 months) | 47(67.1%)/23(32.9%) |
| Disease progression type (local/regional/distance failure) | 9/22/61 |
| PFS (median, 95% CI) (months) | 10.0(95% CI: 8.9–11.1) |
| 6-, 12-, 24-month PFS rates (%) | 72.9%/32.9%/5.9% |
| OS (median, 95% CI) (months) | 30.8(95% CI: 27.1–34.4) |
| 1-, 2-, 3-year OS rates (%) | 95.7%/67.4%/42.5% |
LO Lung oligometastases, ECOG Eastern Cooperative Oncology Group, PFS progression free survival, OS overall survival, CEA Carcinoembryonic antigen, C Chemotherapy (capecitabine plus oxaliplatin), TT target therapy, GTV gross tumor volume
Local control and time to progression
During the median follow-up of 27.8 months (range: 10.0–61.0), the median PFS was 10.0 months (95% CI: 8.9–11.1), and the median OS was 30.8 months (95% CI: 27.1–34.4). Technical success was achieved in 105 procedures performed to treat 144 LO, corresponding to a technical success rate of 100%. 64 (91.4%) patients experienced PD because of local failure (n = 9), regional failure (n = 22), distant metastasis (n = 61) (Fig. 3). The 3-, 6-, and 12-month LCR were 97.9%, 91.0% and 83.6%, respectively (Table 2). All further treatments were decided by a multidisciplinary panel composed of thoracic surgeons, respiratory physicians, oncologists, radiation physicians, pathologists, interventional radiologists and radiologists. There were 50 (time to PD ≤ 12 months) and 14 (time to PD > 12 months) patients who experienced disease progression, respectively (Fig. 4). There were 39 (55.7%) patients who experienced death. The reasons were extensive metastases (n = 29), cachexia (n = 8), acute myocardial infarction (n = 1), cerebral hemorrhage (n = 1).
Fig. 3.
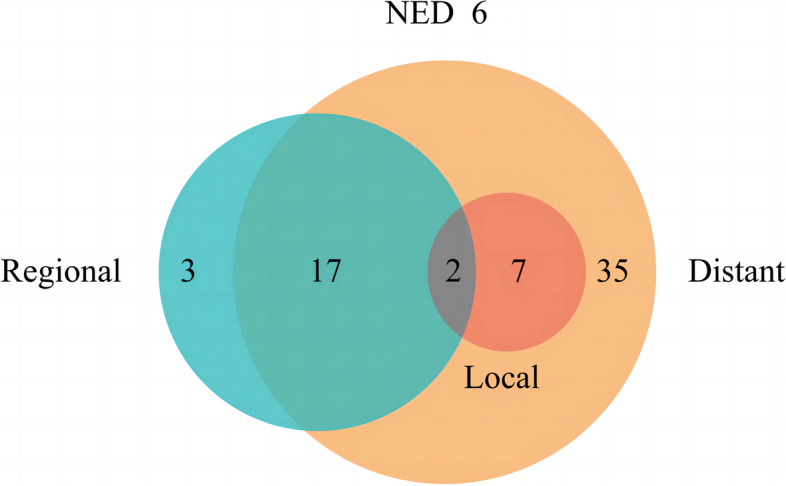
The patterns of progression disease (PD) in 64 patients: local failure (n = 9), regional failure (n = 22), distant metastasis (n = 61). NED: no evidence of disease progression
Table 2.
Local response of all 144 lesions
| Months | Total LO evaluated | CR | PR | SD | PD | LCR |
|---|---|---|---|---|---|---|
| 3 month | 144 | 89(57.6%) | 46(31.9%) | 12(8.3%) | 3 (%) | 97.9% |
| 6 month | 144 | 81(56.3%) | 38(26.4%) | 12(8.3%) | 13(9.0%) | 91.0% |
| 12 month | 134 | 72 (50.0%) | 30 (20.8%) | 10 (6.9%) | 22(15.3%) | 83.6% |
LCR local control rate, CR complete response, PR partial response, SD stable disease, LCR (CR + PR + SD)/total LO evaluated
Fig. 4.
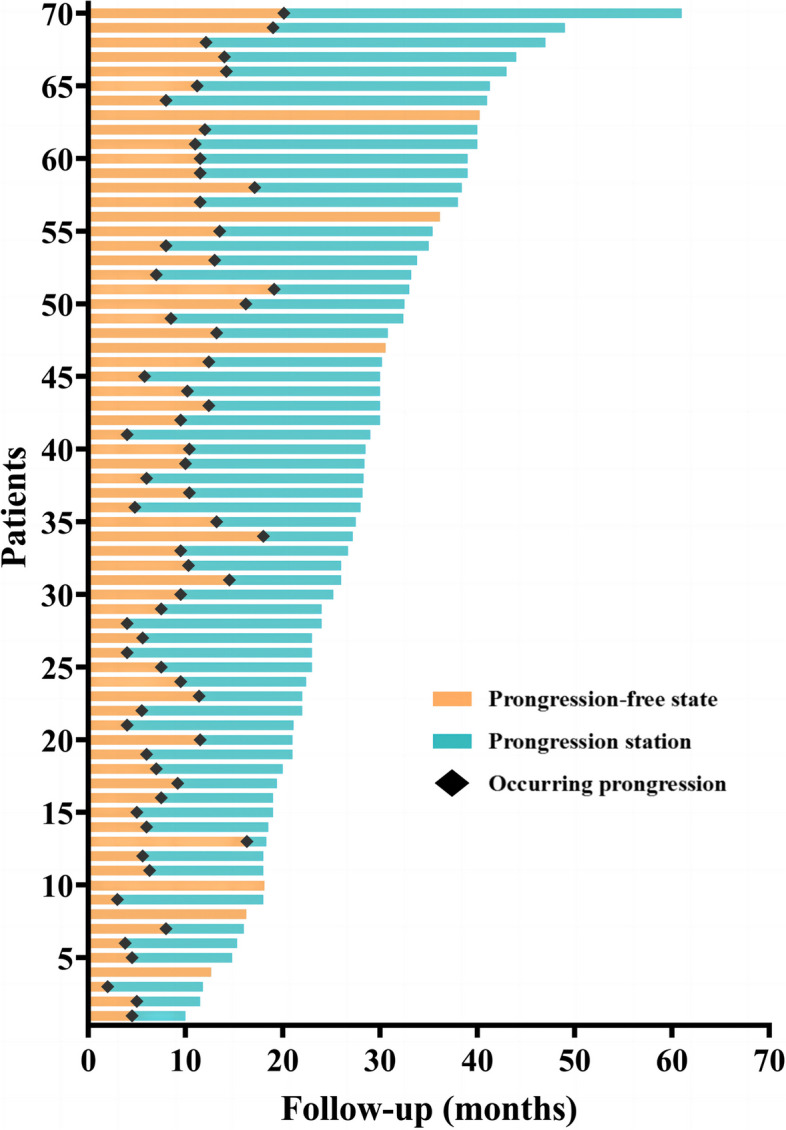
The progression free and disease progression state of all 70 patients
PFS and prognostic factors
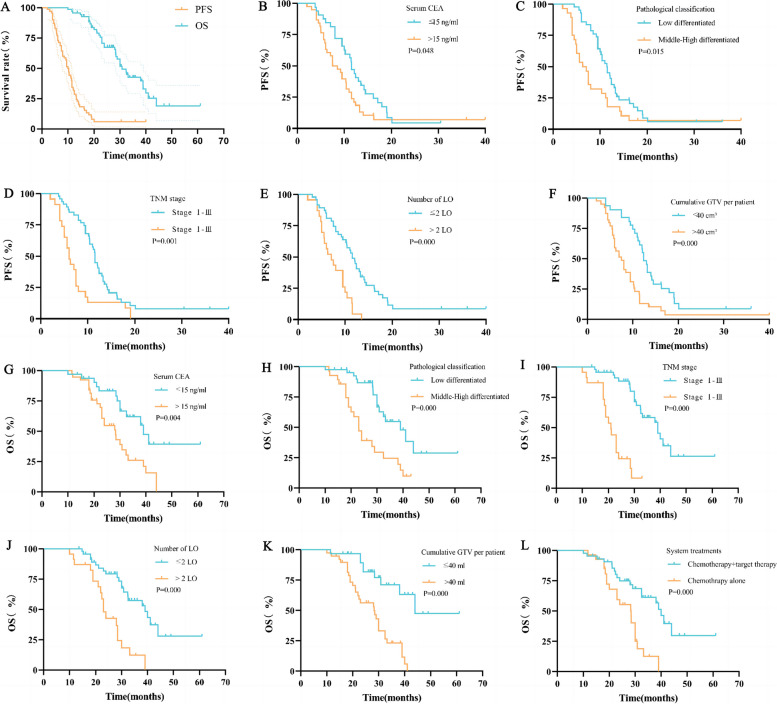
The median PFS was 10.0 months (95% CI: 8.9–11.1), and the 1- and 2-year PFS rates were 32.9% and 5.9%, respectively. Patients with the following characteristics had superior PFS: serum CEA ≤ 15 ng/ml [11.50 months (95% CI: 10.26–12.75) vs 7.50 months (95% CI: 4.84–10.16), P = 0.048], middle-high differentiated pathological classification [11.40 months (95% CI: 9.77–13.03) vs 6.30 months (95% CI: 4.45–8.15), P = 0.015], primary TNM stage I-III [11.50 months (95% CI: 11.10–11.90) vs 6.00 months (95% CI: 4.90–7.10), P = 0.001], LO number ≤ 2 [11.50 months (95% CI: 10.02–12.98) vs 7.00 months (95% CI: 4.65–9.35), P < 0.001], cumulative GTV ≤ 40 cm3 [12.40 months (95% CI: 11.31–13.49) vs 7.50 months (95% CI: 5.77–9.23), P < 0.001] (Fig. 5). The above five factors were included in the multivariate analyses using the Cox hazards model, and serum CEA ≤ 15 ng/ml (HR: 1.906, P = 0.020), primary TNM stage I-III (HR: 2.749, P = 0.003), LO number ≤ 2 (HR: 2.150, P = 0.025), and cumulative GTV ≤ 40 cm3 (HR: 2.012, P = 0.033) were independent factors for superior PFS. The following characteristics were not significantly different in the univariate analysis: sex (P = 0.168), age (P = 0.790), Body Mass Index (BMI) (P = 0.564), smoke abuse (P = 0.751), primary tumor site (P = 0.151), ECOG score (P = 0.671) and system treatments (P = 0.356) (Tables 3 and 4).
Fig. 5.
Kaplan–Meier univariate analyses of PFS and OS. A PFS and OS of all patients; B PFS of serum CEA; C PFS of pathological classification; D PFS of TNM stage; E PFS of LO number; F PFS of cumulative GTV per patient; G OS of serum CEA; H OS of pathological classification; I OS of TNM stage; J OS of LO number; K OS of cumulative GTV per patient; L OS of system treatments
Table 3.
Univariate analysis of PFS and OS
| Factors | PFS (months) | OS (months) | ||
|---|---|---|---|---|
| Median, (95% CI) | P | Median, (95% CI) | P | |
| Sex | 0.168 | 0.250 | ||
| Male | 8.00, (6.01–9.99) | 30.00, (24.44–35.56) | ||
| Female | 11.50, (10.16–12.84) | 38.00, (22.88–53.13) | ||
| Age | 0.790 | 0.530 | ||
| ≤ 60 years old | 10.40, (1.20–8.04) | 30.00, (26.35–33.65) | ||
| > 60 years old | 9.50, (6.96–12.04) | 32.50, (25.86–39.14) | ||
| Body mass index | 0.564 | 0.345 | ||
| ≤ 25 kg/m2 | 8.50, (5.79–11.21) | 30.00, (26.87–33.14) | ||
| > 25 kg/m2 | 10.40, (9.28–11.52) | 38.00, (25.067–50.93) | ||
| Smoke abuse | 0.751 | 0.133 | ||
| Yes | 9.50, (6.87–12.13) | 30.00, (27.61–32.38) | ||
| No | 10.40, (8.64–12.16) | 39.00, (27.19–50.81) | ||
| Serum CEA | 0.048 | 0.004 | ||
| ≤ 15 ng/ml | 11.50, (10.26–12.75) | 39.00, (30.30–47.69) | ||
| > 15 ng/ml | 7.50, (4.84–10.16) | 28.30, (22.85–33.75) | ||
| Primary tumor site | 0.151 | 0.201 | ||
| Colon | 11.00, (9.29–12.71) | 32.50, (29.50–35.49) | ||
| Rectum | 8.00, (5.65–10.35) | 30.00, (27.90–32.10) | ||
| Pathological classification | 0.015 | 0.000 | ||
| Low differentiated | 6.30, (4.45–8.15) | 23.00, (20.17–25.84) | ||
| Middle-High differentiated | 11.40, (9.77–13.03) | 39.00, (30.54–47.46) | ||
| TNM stage | 0.001 | 0.000 | ||
| Stage I-III | 11.50, (11.10–11.90) | 39.00, (31.95–46.05) | ||
| Stage IV | 6.00, (4.90–7.10) | 21.00, (17.94–24.06) | ||
| ECOG score | 0.671 | 0.504 | ||
| PS 0 | 10.40, (8.95–11.85) | 32.50, (27.62–37.38) | ||
| PS 1 | 9.50, (8.35–10.65) | 29.00, (20.18–37.82) | ||
| Number of LO | 0.000 | 0.000 | ||
| ≤ 2 LO | 11.50, (10.02–12.98) | 39.00, (29.33–48.67) | ||
| > 2 LO | 7.00, (4.65–9.35) | 23.00, (20.82–25.18) | ||
| Cumulative GTV per patient | 0.000 | 0.000 | ||
| ≤ 40 cm3 | 12.40, (11.31–13.49) | 45.95(38.25–53.66) | ||
| > 40 cm3 | 7.50, (5.77–9.23) | 28.30, (20.74–35.86) | ||
| System treatments | 0.356 | 0.000 | ||
| C + TT | 11.20, (10.10–12.30) | 40.00, (36.57–43.43) | ||
| C alone | 7.50, (4.32–10.68) | 28.40, (22.26–34.54) | ||
AFP alpha fetoprotein, BCLC Barcelona Clinic Liver Cancer, CI Confidence interval, ECOG Eastern Cooperative Oncology Group, OS overall survival, PFS progression free survival, PO Pulmonary oligometastases, C Chemotherapy, TT target therapy
Table 4.
Cox-regression multivariate analysis of PFS and OS
| Factors | PFS (months) | OS (months) | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| CEA level (≤ 15 / > 15 ng/ml) | 1.906(1.107–3.283) | 0.020 | 2.493(1.146–5.423) | 0.021 |
| Pathological classification (Low/Middle-High differentiated) | 1.298(0.707–2.384) | 0.400 | 1.972(0.793–4.906) | 0.144 |
| TNM stage (stage I-III/IV) | 2.749(1.412–5.351) | 0.003 | 9.215(3.199–26.549) | 0.000 |
| Number of LO (≤ 2 / > 2 LO) | 2.150(1.100–4.200) | 0.025 | 1.678(0.772–3.650) | 0.192 |
| Cumulative GTV (≤ 40 / > 40 cm3) | 2.012(1.060–3.821) | 0.033 | 4.164(1.791–9.679) | 0.001 |
| System treatments(C + TT/C alone) | - | - | 2.388(1.024–5.572) | 0.044 |
CI Confidence interval, HR hazard ratio, OS overall survival, PFS progression-free survival, LO Lung oligometastases, C Chemotherapy, TT target therapy
OS and prognostic factors
The median OS was 30.8 months (95% CI: 27.1–34.4), and the 1-, 2- and 3-year OS rates were 95.7%, 67.4% and 42.5%, respectively. Patients with the following characteristics had superior OS: serum CEA ≤ 15 ng/ml [39.00 months (95% CI: 30.30–47.69) vs 28.30 months (95% CI: 22.85–33.75), P = 0.004], middle-high differentiated pathological classification [39.00 months (95% CI: 30.54–47.46) vs 23.00 months (95% CI: 20.17–25.84), P < 0.001], primary TNM stage I-III [39.00 months (95% CI: 31.95–46.05) vs 21.00 months (95% CI: 17.94–24.06), P < 0.001], LO number ≤ 2 [39.00 months (95% CI: 29.33–48.67) vs 23.00 months (95% CI: 20.82–25.18), P < 0.001] and cumulative GTV ≤ 40 cm3 [45.95 months (95% CI: 38.25–53.66) vs 28.30 months (95% CI: 20.74–35.86), P < 0.001] (Fig. 5). The above six factors were included in the multivariate analyses, and serum CEA ≤ 15 ng/ml (HR: 2.493, P = 0.021), primary TNM stage I-III (HR: 9.215, P < 0.001), cumulative ≤ 40 cm3 (HR: 4.164, P = 0.001) and systemic treatments combined with chemotherapy and target therapy (HR: 2.388, P = 0.044), were independent factors for superior OS. The following characteristics were not significantly different in the univariate analysis:sex (P = 0.250), age (P = 0.530), BMI (P = 0.345), smoke abuse (P = 0.133), primary tumor site (P = 0.201), ECOG score (P = 0.504) (Tables 3 and 4).
Complications
Four patients experienced pneumothorax pulmonary compression of 30%–60% during the procedure, and chest drainage was needed to relieve the symptom. All of them required 2–3 days of hospitalization, and they were evaluated as major complications. Minor complications were intrapulmonary hemorrhage (n = 31), a small amount of pneumothorax not requiring treatment (n = 11), and hemoptysis (≤ 10 ml, n = 3). No RISB-associated massive bleeding, irradiation pneumonitis or pulmonary fibrosis, pulmonary infection, pleural fistula, or death occurred during treatment and follow-up.
Discussion
Approximately half of the patients developed distant organ metastases when CRC was initially diagnosed, and the most common metastatic sites include liver, lung, brain, and bone [22]. Active local treatments such as hepatectomy, ablation, and SBRT for liver metastasis have been accepted by multiple clinical guidelines, and it is believed that this strategy can significantly improve the long-term survival rate [23, 24]. However, as the second most common site of metastasis for CRC after the liver, there are few detailed guidelines focusing on CRC with lung metastases. Previous study showed that only 10% patients were suitable for radical lung metastasectomy among the initial lung metastases, for the remaining 90% of patients, non-surgical local therapy is an alternative option combined with systematic therapy [25].
Non-surgical local treatment strategies, represented by SBRT and ablation, are a valid option for patients with oligometastatic disease. SBRT is a non-invasive treatment and is able to deliver ablative radiation doses to target lesions, short of equipment limit its application at China [7, 26]. Therefore, we hypothesized that RISB may be ideal for the treatment of lung oligometastases from CRC, hoping it has a similar effect compared to conventional local therapy and can be an alternative choice when SRBT and ablation are unsuitable for specific patients. RISB has been increasingly practiced in the clinical treatment of NSCLC since the 1980s [27]. A recent meta-analysis including 15 studies and 1188 cases has systematically reported that RISB combined with chemotherapy has a higher overall response rate (RR = 1.84, 95% CI: 1.65–2.05), better OS (HR = 0.66, 95% CI: 0.50–0.86) as compared to chemotherapy alone for the treatment of NSCLC [17]. However, compared to liver oligometastases, the studies of local therapy, especially RISB, that focus on LOs from CRC are much fewer, and there are no authoritative guides that describe the therapy in detail. Its efficacy and safety remain unclear.
Kinj et al. [17] treated 53 oligometastatic patients with 87 lung lesions from CRC and reported that 1-year LCR of SBRT was 79.8%, which is also similar to the present study (1-year LCR of 83.6% at present study). Agolli et al. [28] previously reported the SBRT treatment of 44 patients and 69 lesions. The study showed 2-year OS was 67.7%, which is similar to the present study (2-year OS rate of 67.4%). A prospective study about RISB conducted by Wang et al. [29] reveals a 1-year LCR of 33.3% and a mOS of 18.8 months in 33 patients with 126 bilateral lung recurrence lesions from CRC, which is much lower than our study (79.8% and 30.8 months). This difference may be due to the fact that the inclusion criteria for their study were limited to patients with bilateral lung oligometastases and failure of standard chemotherapy in the majority of patients. The 2-year PFS reported in most of the SBRT studies were 16.2%–27% [17, 28, 30–32], which is significantly higher than that of our study ( 5.9% at present study). The reasons for the analysis are as follows: (1) RISB has high puncture skills requirement for the operator, and when the puncture cannot obtain a satisfactory 125I seed distribution, it means that the dose distribution is uneven (the D90 range in this study was 92.5–144.6 Gy), while SBRT has a standard operating process, controllable and repeatable dose, so the efficacy can be replicated in different centers; (2) The difference in inclusion criteria for selected cases is also an important operational aspect; (3) These studies come from different countries, and there may be differences in the efficacy of RISB and SBRT among different ethnic groups.
Numerous studies also investigated the potential variables that affect effectiveness when focusing on local therapies for lung metastases. Agolli et al. [28] reported that multiple lung metastases were significantly associated with worse PFS. Kinj et al. [17] demonstrated that there was a significant OS detriment in patients with ≥ 3 metastases and patients with a larger GTV). In a prospective study, it was reported that the degree of tumor differentiation, growth rate, and tumor size could affect local efficacy. It was also reported that serum CEA level was associated with the OS [33]. In our study, we similarly included the potential factors in survial analysis, and the results show that lower serum CEA levels (≤ 15 ng/ml), middle-high pathological differentiation, lower primary TNM stages (I-III), fewer LO numbers (≤ 2) and smaller cumulative GTV (≤ 40 cm3) were significantly associated with better PFS. In regards to OS, in addition to the factors mentioned above, systemic treatment combined with chemotherapy and target therapy is also a contributing factor to the superior prognosis. These results were similar to the data from the literature mentioned above and suggest which patients are more likely to benefit from RISB. Therefore, these factors should be taken into account when designing treatment strategies for patients during our clinical practice.
As for complications including hemoptysis, pneumothorax, and pneumorrhagia occurred in a significant portion of patients who underwent RISB, which is mainly due to punctures. These complications can be reduced by minimizing the number of punctures and ensuring that they are as far as possible from large vessels and trachea. In addition, it is suggested that both lungs should not be treated at the same time because of life threatening bilateral pneumothorax. No irradiation-related pneumonitis or pulmonary fibrosis occurred during the therapy period. The results proved that it has an equal effect but milder lung tissue damage compared to SBRT, which is also demonstrated at clincial study from Li et al. [26].
Our study has some limitations. This study was a retrospective, small-sample, single-center study with a median follow-up of 27.8 months. The conclusion needs to be confirmed by prospective multiple center large sample study with longer follow-up in the future. In conclusion, our study suggests that RISB for LO from CRC is safe and effective, and serum CEA, TNM stage, LO number, cumulative GTV, and system treatments should be emphasized for long OS.
Acknowledgements
We thank the patients enrolled in this study.
Abbreviations
- RISB
Radioactive 125I seeds brachytherapy
- LO
Lung oligometastases
- CRC
Colorectal cancer
- SBRT
Stereotactic body radiotherapy
- BT-TPS
Brachytherapy treatment planning system
- OAR
Organs at risk
- CT
Computer tomography
- CEA
Carcinoembryonic antigen
- GTV
Gross tumor volume
- PFS
Progression-free survival
- OS
Overall survival
- LCR
Local control rate
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- PD
Progression disease
- ECOG
Eastern Cooperative Oncology Group
- NIH
National Institutes of Health
- ICRU
International Commission on Radiation Units and Measurements
Authors’ contributions
RH, MS, YL, KD, CZ, KX and XH collected or interpreted the patient data. MY and DJ were responsible for the conception and design of the article. All authors participated in the manuscript writing and substantively revised it. All authors read and approved the final manuscript.
Funding
This study has received fundings by Major Science and Technology Projects of Henan Province in 2022 (fund No:221100310100) and Henan Province Science and Technology Research Project in 2023 (fund No:232102311132).
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committees of the First Affiliated Hospital of Zhengzhou University (Ethical Review Number: 2021-KY-400). The requirement for informed consent was waived by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mengyao Song and Xueliang Zhou contributed equally to the work.
References
- 1.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021 doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Li N, Ren J, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019 doi: 10.1136/gutjnl-2018-317124. [DOI] [PubMed] [Google Scholar]
- 3.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995 doi: 10.1200/JCO.1995. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Wang G, Sun J, et al. The prognosis of radiofrequency ablation versus hepatic resection for patients with colorectal liver metastases: a systematic review and meta-analysis based on 22 studies. Int J Surg. 2021 doi: 10.1016/j.ijsu.2021.105896. [DOI] [PubMed] [Google Scholar]
- 5.Salah S, Watanabe K, Park JS, et al. Repeated resection of colorectal cancer pulmonary oligometastases: pooled analysis and prognostic assessment. Ann Surg Oncol. 2013 doi: 10.1245/s10434-012-2860-y. [DOI] [PubMed] [Google Scholar]
- 6.Lehrer EJ, Singh R, Wang M, et al. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: a systematic review and meta-analysis. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2020.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owen D, Olivier KR, Mayo CS, et al. Outcomes of stereotactic body radiotherapy (SBRT) treatment of multiple synchronous and recurrent lung nodules. Radiat Oncol. 2015 doi: 10.1186/s13014-015-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding XF, Huang TB, Gao Y, et al. Permanent 125I prostate brachytherapy for castration-resistant prostate cancer. Int J Urol. 2019 doi: 10.1111/iju.13866. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z, Yao W, Zhang T, et al. Computed tomography-guided implantation of 125I radioactive seeds in patients with malignant airway compression induced by advanced lung cancer: effectiveness and safety in 40 patients. J Contemp Brachytherapy. 2020 doi: 10.5114/jcb.2020.98113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan T, Zhou JY. Computed tomography-guided 125I radioactive seed implantation therapy for pancreatic cancer pain. J Coll Physicians Surg Pak. 2020 doi: 10.29271/jcpsp.2020.04.364. [DOI] [PubMed] [Google Scholar]
- 11.Zhang QZ, Li GL, Shang JB, Ren YM, Xie JL. Clinical study of a 125I particle-integrated esophageal covered stent and hyperbaric oxygen in the treatment of advanced esophageal cancer. Rev Esp Enferm Dig. 2021 doi: 10.17235/reed.2020.7377/2020. [DOI] [PubMed] [Google Scholar]
- 12.Jiao D, Xu K, Mukhiya G, et al. Brachytherapy drainage catheter and chemotherapy for unresectable pancreatic carcinoma combined with obstructive jaundice. Front Oncol. 2022 doi: 10.3389/fonc.2022.941336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Radiation Oncology Branch of the Chinese Medical Association, the Radiation Oncology Branch of the Chinese Medical Association, and the Cancer Radiation Therapy Professional Committee of the Chinese Anti Cancer Association. Chinese guidelines for radiotherapy of esophageal cancer (2021 Edition). Int J Oncol. 2022. 10.3760/cma.j.cn371439-20211021-00002.
- 14.Spaander MC, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2016 doi: 10.1055/s-0042-114210. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Zhao Z, Lu J, et al. A comparison of the biological effects of 125I seeds continuous low-dose-rate radiation and 60Co high-dose-rate gamma radiation on non-small cell lung cancer cells. PLoS One. 2015 doi: 10.1371/journal.pone.0133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Zhang W, Dou M, et al. 125I seeds inhibit proliferation and promote apoptosis in cholangiocarcinoma cells by regulating the AGR2-mediated p38 MAPK pathway. Cancer Lett. 2022 doi: 10.1016/j.canlet.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Li J, Li R, Zhang Y, Han M, Ma W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy. 2018 doi: 10.1016/j.brachy.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Nag S. Brachytherapy for prostate cancer: summary of American Brachytherapy Society recommendations. Semin Urol Oncol. 2000;18(2):133–136. [PubMed] [Google Scholar]
- 19.Liu Y, Zhang C, Xu K, Wu K, Han X, Jiao D. 125I brachytherapy: a useful treatment to control painful osteoblastic bone metastases. Clin Transl Oncol. 2023 doi: 10.1007/s12094-022-03025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min SJ, Jang HJ, Kim JH. Comparison of the RECIST and PERCIST criteria in solid tumors: a pooled analysis and review. Oncotarget. 2016 doi: 10.18632/oncotarget.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Chen Q, Wang Q. The use of and adherence to CTCAE v3.0 in cancer clinical trial publications. Oncotarget. 2016. 10.18632/oncotarget.11576. [DOI] [PMC free article] [PubMed]
- 22.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 23.Morris VK, Kennedy EB, Baxter NN, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol. 2023 doi: 10.1200/JCO.22.01690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016 doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 25.Tampellini M, Ottone A, Bellini E, et al. The role of lung metastasis resection in improving outcome of colorectal cancer patients: results from a large retrospective study. Oncologist. 2012 doi: 10.1634/theoncologist.2012-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Zhang L, Xie Q, Wang W, Hua Y, Sun Z. Comparison of clinical efficacy and complications of 125I seed brachytherapy and stereotactic body radiation therapy for recurrent pulmonary metastases from colorectal carcinoma. J Contemp Brachytherapy. 2018 doi: 10.5114/jcb.2018.77956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heelan RT, Hilaris BS, Anderson LL, et al. Lung tumors: percutaneous implantation of I-125 sources with CT treatment planning. Radiology. 1987 doi: 10.1148/radiology.164.3.3615870. [DOI] [PubMed] [Google Scholar]
- 28.Agolli L, Bracci S, Nicosia L, Valeriani M, De Sanctis V, Osti MF. Lung metastases treated with stereotactic ablative radiation therapy in oligometastatic colorectal cancer patients: outcomes and prognostic factors after long-term follow-up. Clin Colorectal Cancer. 2017 doi: 10.1016/j.clcc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Zamdborg L, Ye H, Grills IS, Yan D. A matched-pair analysis of stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer versus early stage non-small cell lung cancer. BMC Cancer. 2018 doi: 10.1186/s12885-018-4865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvajal C, Navarro-Martin A, Cacicedo J, Ramos R, Guedea F. Stereotactic body radiotherapy for colorectal lung oligometastases: preliminary single-institution results. J BUON. 2015;20(1):158–165. [PubMed] [Google Scholar]
- 31.Qiu H, Katz AW, Chowdhry AK, et al. Stereotactic body radiotherapy for lung metastases from colorectal cancer: prognostic factors for disease control and survival. Am J Clin Oncol. 2018 doi: 10.1097/COC.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 32.Filippi AR, Badellino S, Ceccarelli M, et al. Stereotactic ablative radiation therapy as first local therapy for lung oligometastases from colorectal cancer: a single-institution cohort study. Int J Radiat Oncol Biol Phys. 2015 doi: 10.1016/j.ijrobp.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Zhang F, Yang B, et al. Feasibility and clinical value of CT-guided (125)I brachytherapy for bilateral lung recurrences from colorectal carcinoma. Radiology. 2016 doi: 10.1148/radiol.2015150641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.