Abstract
Background
The majority of congenital heart diseases (CHDs) are thought to result from the interactions of genetics and the environment factors. This study aimed to assess the association of maternal non-occupational phthalates exposure, metabolic gene polymorphisms and their interactions with risk of CHDs in offspring.
Methods
A multicenter case-control study of 245 mothers with CHDs infants and 268 control mothers of health infant was conducted from six hospitals. Maternal urinary concentrations of eight phthalate metabolites were measured by ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS). Twenty single nucleotide polymorphisms (SNPs) in cytochrome P450 family 2 subfamily C member 9 (CYP2C9) and 19 (CYP2C19), uridine diphosphate (UDP) glucuronosyl transferase family 1 member A7 (UGT1A7), family 2 member B7 (UGT2B7) and B15(UGT2B15) genes were genotyped. The multivariate logistic regressions were used to estimate the association between maternal phthalates exposure or gene polymorphisms and risk of CHDs. Generalized multifactor dimensionality reduction (GMDR) was used to analyze the gene–gene and gene–phthalates exposure interactions.
Results
There was no significant difference in phthalate metabolites concentrations between the cases and controls. No significant positive associations were observed between maternal exposure to phthalates and CHDs. The SNPs of UGT1A7 gene at rs4124874 (under three models, log-additive: aOR = 1.74, 95% CI:1.28–2.37; dominant: aOR = 1.86, 95% CI:1.25–2.78; recessive: aOR = 2.50, 95% CI: 1.26–4.94) and rs887829 (under the recessive model: aOR = 13.66, 95% CI: 1.54–121) were significantly associated with an increased risk of CHDs. Furthermore, the associations between rs4124874 (under log-additive and dominant models) of UGT1A7 were statistically significant after the false discovery rate correction. No significant gene-gene or gene-phthalate metabolites interactions were observed.
Conclusions
The polymorphisms of maternal UGT1A7 gene at rs4124874 and rs887829 were significantly associated with an increased risk of CHDs. More large-scale studies or prospective study designs are needed to confirm or refute our findings in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06343-z.
Keywords: Congenital heart diseases, Phthalates, Uridine diphosphate (UDP) glucuronosyl transferase family 1 member A7 (UGT1A7), Interaction
Introduction
Congenital heart diseases (CHDs), typically defined as structural and functional abnormalities of the heart and great vessels during embryonic development, are the most common type of birth defects. The reported birth prevalence of CHDs varies widely across countries and continents. A recent meta-analysis has estimate that the worldwide prevalence of CHDs in live births increased to 9.41/‰ in the period 2010–2017 [1]. In China, total CHDs birth prevalence increased continuously, from 0.201‰ in 1980–1984 to 4.905‰ in 2015–2019 [2]. CHDs are the leading cause of infant morbidity and mortality, and account for approximately 40% of prenatal deaths and 20% of mortality in the first year of life [3]. Therefore, CHDs place a heavy burden on the healthcare system, and have become a sizable public health concern. The etiology of CHDs is complex and multifactorial. To date,  20–30% of the CHDs cases could be identified with clear environmental or genetic factors, most CHDs cases are considered to be caused by the interaction of genetic and environmental factors [4–6].
20–30% of the CHDs cases could be identified with clear environmental or genetic factors, most CHDs cases are considered to be caused by the interaction of genetic and environmental factors [4–6].
Phthalates, are a family of synthetic chemicals widely used as plasticizers in many consumer products. Low-molecular-weight phthalates, such as di-n-butyl phthalate (DBP) and di-isobutyl phthalate (DiBP), are present in fragrances, pharmaceuticals, cosmetics, personal-care products, and packaging materials, whereas high-molecular-weight phthalates, such as di-2-ethylhexyl phthalate (DEHP) and benzyl-n-butyl ortho-phthalate (BBP), are used to soften plastics, particularly PVC building materials [7, 8]. Phthalates are not covalently bound to other substances and can easily permeate and migrate into environmental media, as result, the general population is ubiquitously exposed to phthalates via oral, dermal, inhalation, and intravenous routes [9, 10]. Phthalate metabolites have been consistently detected in urine from the general population including pregnant women worldwide [7–9, 11]. Between 2000 and 2017, indoor phthalate exposure in China has led to 3.32 million disability-adjusted life years (DALYs) per year, accounting for 0.90% of total DALYs across China, with DBP, DiBP and DEHP being the most abundant phthalates in indoor environments of residences, offices, and schools [12].
Phthalates can easily cross the placental barrier and impact the developing fetus in utero [7]. Growing literatures have reported that prenatal exposure to phthalates were associated with the risk of multiple adverse pregnancy outcomes, including preterm birth [13, 14], spontaneous pregnancy loss [15, 16], low birth weight [17], male newborn genital anomalies [18], and fetal retarded growth [19]. Two animal experiment have observed that BBP or DBP exposure could cause abnormalities in zebrafish embryo morphology, including cardiac structure deformities [20, 21]. To date, population epidemiological studies on the association between maternal occupational phthalates exposure and CHDs are limited, and the results are inconsistent. For example, two case-control studies have shown that maternal occupational phthalates exposures are associated with increased risk of total CHDs [22] or some CHDs subtypes [23]. However, three case-control studies have not found significant association between maternal occupational phthalates exposure to and CHDs [24–26]. Besides, phthalates exposure is assessed through subjective reporting, which may result in recall bias and not fully objectively reflect the exact level in vivo. To our knowledge, there have no studies on the association between maternal non-occupational phthalates exposure, identified by the biomarker phthalates, and the risk of CHDs.
The metabolism of environmental exposure is the first step to affect the occurrence of disease, and the individual metabolism level can significantly affect the risk of disease occurrence. The cytochrome P450 enzymes (CYPs), such as CYP2C9, and CYP2C19, and uridine diphosphate (UDP) -glucuronyl transferases (UGTs), such as UGT1A7, UGT2B7 and UGT2B15, have been proposed to play important roles in the phase I and phase II biotransformation of phthalates, respectively [7, 27, 28]. Common single nucleotide polymorphisms (SNPs) genetic polymorphisms in these genes could affect individual susceptibility to adverse effects of exposure to phthalates. One study suggested that the SNPs of UGT, such as rs7439366 of UGT2B7, rs1902023 of UGT2B15, were associated with the clearance of bisphenol A and phthalates in in patients with polycystic ovary syndrome [29]. One study observed that rs1799853 and rs1057910 of CYP2C9 could reduce DEHP biotransformation, and rs1799853 and rs1057910 of CYP2C9, rs12248560 of CYP2C19, and rs11692021of UGT1A7 might represent biomarkers of susceptibility or resilience in phthalates exposure [28]. However, few studies have investigated maternal genetic susceptibility to CHDs related to phthalates. Additionally, few studies have explored possible gene-environment interactions.
In the present study, we first evaluated the association between maternal exposure to phthalates by measuring urinary levels of phthalate metabolites during the second pregnancy and the risk of fetal CHDs. Then, we investigated the association between maternal genetic polymorphisms and the risk of fetal CHDs. Finally, we explored the potential interaction between maternal genetic variants and exposure to phthalates on the risk of CHDs.
Methods
Study population
This study was based on the project of a multicenter hospital-based case-control study which was performed from February 2010 to July 2015. The subjects were recruited from six tertiary maternal and child health hospitals with the qualification of performing prenatal diagnoses, located in Fuzhou, Nanning, Chengdu, Zhengzhou, Wuhan and Shenzhen city, respectively.
The cases group were pregnant women whose fetuses were diagnosed with CHDs by echocardiography and no any extracardiac abnormalities and with gestational age more than 12 weeks. CHDs were further confirmed by humanitarian examination of the pathological anatomy for aborted fetuses or ultrasound examination performed within 30 postnatal days and telephone follow-up performed within 60 days for liveborn fetuses. The exclusion criteria were as follows: (1) fetuses with syndromic diseases and chromosomal aberrations, (2) pregnant women with multiple pregnancies, (3) a mother with a family history of CHDs.
The control group were pregnant women with a singleton pregnancy without major congenital malformations diagnosed by echocardiography in the same hospital and with gestational age more than 12 weeks. A further ultrasound examination was performed within 30 postnatal days, and a telephone follow-up was performed within 60 days.
Each participant participated in face-to-face interview. The structured questionnaire was composed of parental social demographics, living environment and habits, working environment, maternal reproductive history, maternal illness and drug use history, maternal diet and nutrition, and maternal life events and mental state.
Four millilitres of blood was collected in EDTA from each participant during the second trimester by venepuncture and stored at − 70℃until genotyping. Ten millilitres single-spot urine samples of each participant during the second trimester was collected in a nonsterile clean polypropylene container, then divided into aliquots and stored at − 80 °C until further analysis.
Measurement of urinary concentrations of phthalate metabolites
Phthalate metabolites concentration was measured at the West China School of Public Health, Sichuan University by ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS). Eight phthalate metabolites from four parent compounds were quantified, including mono-n-butyl phthalate (MnBP, metabolite of di-n-butyl phthalate (DnBP)); mono-isobutyl phthalate (MiBP, metabolite of diisobutyl phthalate (DiBP)); mono-benzyl phthalate (MBzP, metabolite of butylbenzyl phthalate (BBzP)); five metabolites of di (2-ethylhexyl) phthalate (DEHP):[mono(2-ethyl-hexyl) phthalate (MEHP),mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP),mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono (2-ethyl-5-carboxypentyl)phthalate (MECCP), and mono-2- carboxymethyl hexyl phthalate (MCMHP)].
The analysis was performed using an ultra-high performance liquid chromatography ACQUITY UPLC I-Class coupled to an Xevo TQ-XS triple stage quadrupole mass spectrometer (Waters, USA). The details regarding the preparation and analysis of the samples are available in Supplementary Appendix A.
To adjust the dilution of urine, creatinine-adjusted concentrations of urinary phthalate metabolites were calculated. The concentrations below the limit of detection (LOD) were replaced by LOD/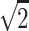 .
.
DNA extraction and genotyping
Genomic DNA was extracted with magnetic bead method (BioTeke, Wuxi, China) according to the recommended protocol. Twenty SNPs in the UGT1A7, UGT2B7, UGT2B15, CYP2C19, CYP2C9 were selected based on the following principal criteria: (1) an association with diseases in previous studies or the metabolic level of phthalates [28–44], (2) a minor allele frequency > 0.05 in Han Chinese. In total, 20 SNPs were selected. The genotypes of these SNPs were detected using multiple-polymerase chain reaction amplification and next generation sequencing (iGeneTech Bioscience Co., Ltd, Beijing, China). More detailed information about the studied genetic variants and genotyping is presented in Supplementary Appendix, Table S2.
For quality-control assessment, genotyping was repeated in 10% of samples, and the consistency rate was 100%.
Statistical analyses
The composition ratio of baseline characteristics between case and control groups was compared by χ2 test using Statistical Package for Social Sciences (SPSS) version 16.0 software (SPSS Inc., IBM, Chicago, USA).These characteristics included maternal age (at the time of the last menstrual period), maternal ethnicity, maternal education level, parental smoking or environmental tobacco smoke (ETS) exposure, maternal alcohol consumption, parity, maternal pre-pregnancy body mass index (ppBMI) (BMI = weight/(height × height) (kg/m2) before known pregnancy), maternal folic acid supplement.
As the distributions of phthalate metabolites concentrations did not meet the normality assumption, they were described as median (interquartile range) and compared with Mann–Whitney U test. The concentrations of phthalate metabolites were performed natural log10 transformation and further divided into three categories according to the tertile which they fell into. Logistic regression analysis was used to estimate the associations of the levels of phthalate metabolites with CHDs using SPSS version 16.0 software (SPSS Inc., IBM, Chicago, USA).
Hardy–Weinberg equilibrium was assessed in the controls using Plink software (http://zzz.bwh.harvard.edu/plink/ld.shtml). Logistic regression analysis was performed to investigate the association between individual genetic polymorphisms and CHDs using Plink software.
The effects of the gene–gene and gene-phthalates exposure interactions on CHDs occurrence were evaluated by logistic models using generalized multifactor dimensionality reduction (GMDR, version 0.7, University of Virginia, Charlottesville, VA).
All analyses were adjusted for covariates or potential confounders. False discovery rate (FDR) correction of multiple hypothesis testing was performed. Two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of the study participants
In this study, a total of 513 subjects were analyzed, including 245 cases with CHDs fetuses and 268 controls. The baseline characteristics of participants were listed in Table 1.
Table 1.
Descriptive characteristics of the participants
| Variable/Characteristic | Controls (n = 268) | CHDs cases (n = 245) | χ2 | P-values |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Maternal age (years) | 1.067 | 0.302 | ||
| < 35 | 234(87.31%) | 221(90.2%) | ||
| ≥ 35 | 34(12.69%) | 24(9.8%) | ||
| Maternal ethnicity | 43.979 | < 0.001 | ||
| Han | 259(96.64%) | 189(77.14%) | ||
| Others | 9(3.36%) | 56(22.86%) | ||
| Maternal education level | 63.325 | < 0.001 | ||
| Junior school or lower | 37(13.81%) | 101(41.22%) | ||
| High school | 42(15.67%) | 52(21.22%) | ||
| College or higher | 189(70.52%) | 92(37.55%) | ||
| Parental smoking or ETS exposurea | 6.524 | 0.011 | ||
| Yes | 124(46.27%) | 141(57.55%) | ||
| No | 144(53.73%) | 104(42.45%) | ||
| Maternal alcohol consumptiona | 0.321 | 0.571 | ||
| Yes | 41(15.3%) | 42(17.14%) | ||
| No | 227(84.7%) | 203(82.86%) | ||
| Parity | 15.727 | < 0.001 | ||
| Nullipara | 143(53.36%) | 88(35.92%) | ||
| Multipara | 125(46.64%) | 157(64.08%) | ||
| Pre-pregnancy BMI (kg/m2) | 0.283 | 0.868 | ||
| ≤ 18.5 | 60(22.39%) | 56(22.86%) | ||
| 18.5–24 | 183(68.28%) | 163(66.53%) | ||
| ≥ 24 | 25(9.33%) | 26(10.61%) | ||
| Folic acid supplementsa | 1.360 | 0.244 | ||
| Yes | 226(84.33%) | 197(80.41%) | ||
| No | 42(15.67%) | 48(19.59%) |
a The exposure was defined from the 3 months before pregnancy to the second trimester
There were significant differences in the distributions of maternal ethnicity (96.64% Han in controls and 77.14% Han in cases, P < 0.001), maternal education level (70.52% controls vs. 37.55% cases had College or higher education, P < 0.001), parental smoking or ETS exposure (53.73% controls vs. 42.45% cases, P = 0.011).
Compared with control mothers, CHDs case mothers were also more likely to be multipara (46.64 controls vs.64.08% cases, P < 0.001). Maternal age, maternal alcohol consumption, pre-pregnancy BMI and folic acid supplements did not show statistical differences between the two groups (Pall>0.05).
Distribution of phthalate metabolite concentrations in maternal urine
The LODs, detection rates, and distributions of phthalate metabolites were summarized in Table 2. The LOD for MiBP, MnBP, MBzP, MEHP, MEHHP, MEOHP, MECPP and MCMHP was 0.50 ng/mL, 0.50 ng/mL, 0.05 ng/mL, 0.50 ng/mL, 0.05 ng/mL, 0.05 ng/mL, 0.10 ng/mL and 0.20 ng/mL, respectively. In addition to relatively low detection rate of 84.60% for MBzP, the detection rates of other seven metabolites were nearly or equal to 100%.
Table 2.
Urinary concentrations of unadjusted (ng/mL) and adjusted PAEs (µg/g Cr)
| Diether phthalate | Phthalate metabolites (µg/g creatinine) |
LOD (ng/mL) |
Concentration≥ LOD, n (%) |
Median (IQR) | P-Valuea | ||
|---|---|---|---|---|---|---|---|
| Total participants (n = 529) |
Controls (n = 268) |
CHDs Cases (n = 245) |
|||||
| DiBP | MiBP | 0.50 | 511(99.61%) | 10.23(5.94,17.43) | 10.43(6.09,18.77) | 10.01(5.53,16.12) | 0.452 |
| DnBP | MnBP | 0.50 | 513(100%) | 36.9(17.58,71.91) | 34.08(16.6,68.19) | 41.11(19.02,76.23) | 0.106 |
| BBzP | MBzP | 0.05 | 434(84.60%) | 0.08(0.04,0.23) | 0.09(0.04,0.19) | 0.08(0.04,0.28) | 0.407 |
| DEHP | MEHP | 0.50 | 492(95.90%) | 2.53(1.30,4.58) | 2.71(1.41,4.95) | 2.37(1.19,4.04) | 0.052 |
| MEHHP | 0.05 | 513(100%) | 2.45(1.39,4.11) | 2.35(1.38,3.85) | 2.51(1.41,4.84) | 0.137 | |
| MEOHP | 0.05 | 513(100%) | 1.44(0.86,2.45) | 1.40(0.83,2.3) | 1.48(0.88,2.81) | 0.176 | |
| MECPP | 0.10 | 513(100%) | 5.48(3.29,9.10) | 5.43(3.25,8.25) | 5.49(3.38,10.36) | 0.219 | |
| MCMHP | 0.20 | 513(100%) | 1.59(1.12,2.58) | 1.60(1.16,2.31) | 1.55(1.10,2.89) | 0.489 | |
aP values for the Mann‒Whitney U test between case and control group
The median concentrations of MnBP, MEHHP, MEOHP and MECPP were higher in CHDs than in controls (41.11 vs. 34.08, 2.51 vs. 2.35, 1.48 vs. 1.40, 5.49 vs. 5.43 µg/g creatinine, respectively), whereas the median concentrations of MiBP, MBzP, MEHP and MCMHP were lower in CHDs than in controls (10.01 vs. 10.43, 0.08 vs. 0.09, 2.37 vs. 2.71, 1.55 vs. 1.60 µg/g creatinine, respectively). However, these eight phthalate metabolites concentrations did not show significant differences between two groups (Pall>0.05).
Associations between maternal phthalates exposure and CHDs risk
The relation between maternal phthalates exposure and the risk of CHDs was displayed in Table 3. Among the eight metabolites, including MiBP, MnBP, MBzP, MEHP, MEHHP, MEOHP, MECPP, MCMHP, the first-tertile log10-transformed concentration of each was used as a reference, the second- and third-tertile concentrations were not associated with the risks of CHDs. Stratified analysis in the Han Chinese maternal population also did not observe significant positive associations between maternal exposure to phthalates and CHDs (Supplementary Appendix Table S3).
Table 3.
Logistic regression analyses of the association between phthalate metabolites in maternal urinary samples and the risk of CHDs
| Elements | Concentration levels a | Controls | CHDs Cases | cOR (95% CI) | aOR (95% CI)b |
|---|---|---|---|---|---|
| No. (%) | No. (%) | ||||
| MiBP | First-tertile | 90(33.58%) | 82(33.47%) | Reference | Reference |
| Second-tertile | 87(32.46%) | 85(34.69%) | 1.07(0.70–1.64) | 1.04(0.65–1.67) | |
| Third-tertile | 91(33.96%) | 78(31.84%) | 0.94(0.62–1.44) | 0.87(0.54–1.41) | |
| MnBP | First-tertile | 96(35.82%) | 76(31.02%) | Reference | Reference |
| Second-tertile | 94(35.07%) | 76(31.02%) | 1.02(0.67–1.57) | 1.14(0.69–1.79) | |
| Third-tertile | 78(29.10%) | 93(37.96%) | 1.51(0.98–2.31) | 1.48(0.92–2.38) | |
| MBzP | First-tertile | 81(30.22%) | 85(34.69%) | Reference | Reference |
| Second-tertile | 98(36.57%) | 78(31.84%) | 0.76(0.50–1.16) | 0.75(0.46–1.21) | |
| Third-tertile | 89(33.21%) | 82(33.47%) | 0.88(0.57–1.35) | 0.93(0.58–1.51) | |
| MEHP | First-tertile | 81(30.22%) | 90(36.73%) | Reference | Reference |
| Second-tertile | 88(32.84%) | 83(33.88%) | 0.85(0.56–1.30) | 0.67(0.41–1.08) | |
| Third-tertile | 99(36.94%) | 72(29.39%) | 0.66(0.43-1.00) | 0.63(0.39–1.02) | |
| MEHHP | First-tertile | 92(34.33%) | 79(32.24%) | Reference | Reference |
| Second-tertile | 94(35.07%) | 75(30.61%) | 0.93(0.61–1.42) | 0.76(0.47–1.23) | |
| Third-tertile | 82(30.60%) | 91(37.14%) | 1.29(0.85–1.97) | 1.25(0.77–2.01) | |
| MEOHP | First-tertile | 93(34.7%) | 77(31.43%) | Reference | Reference |
| Second-tertile | 90(33.58%) | 85(34.69%) | 1.14(0.75–1.74) | 0.90(0.56–1.45) | |
| Third-tertile | 85(31.72%) | 83(33.88%) | 1.18(0.77–1.81) | 1.09(0.68–1.77) | |
| MECPP | First-tertile | 94(35.07%) | 76(31.02%) | Reference | Reference |
| Second-tertile | 86(32.09%) | 85(34.69%) | 1.22(0.80–1.87) | 0.98(0.61–1.59) | |
| Third-tertile | 88(32.84%) | 84(34.29%) | 1.18(0.77–1.81) | 1.01(0.62–1.64) | |
| MCMHP | First-tertile | 85(31.72%) | 82(33.47%) | Reference | Reference |
| Second-tertile | 100(37.31%) | 74(30.2%) | 0.77(0.50–1.18) | 0.70(0.43–1.15) | |
| Third-tertile | 83(30.97%) | 89(36.33%) | 1.11(0.73–1.70) | 1.12(0.69–1.81) |
aData were divided by overall maternal urine tertiles log10-transformed phthalate metabolites concentrations
baOR, adjusted odds ratio. Logistic regression was used to calculate odds ratios and 95% CIs; all models were adjusted for maternal age (continuous), maternal ethnicity, maternal education level, parental smoking or ETS exposure, maternal alcohol consumption, gravidity, pre-pregnancy BMI (continuous), folic acid supplements
Association between maternal gene polymorphisms and CHDs risk
The genotype distributions for polymorphisms of UGT1A7, UGT2B15, UGT2B7, CYP2C19 and CYP2C9 in the controls were consistent with Hardy-Weinberg equilibrium (see Supplementary Appendix, Table S4).
The association between single gene loci polymorphisms and the risk of CHDs when assuming various genetic models was shown in Table 4. In the UGT1A7 gene, the SNP rs4124874 was associated with an increased risk of CHDs (under the log-additive model: aOR = 1.74, 95% CI:1.28–2.37; under the dominant model: aOR = 1.86, 95% CI:1.25–2.78; under the recessive model: aOR = 2.50, 95% CI: 1.26–4.94), and the SNP rs887829 was associated with an increased risk of CHDs under the recessive model (aOR = 13.66, 95% CI: 1.54–121). However, for the recessive model, the associations were not statistically significant after the false discovery rate (FDR) correction. No significant association was found between any of the remaining 18 selected loci and the risk of CHDs.
Table 4.
Association between maternal genotypes and the risk of CHDs
| Gene | dbSNP_ID | Model | Genotype | Controls | Cases | aORa | P-Value | FDR-BH P-Value |
|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | |||||||
| UGT1A7 | rs11692021 | Log-additive | - | - | - | 0.79(0.56–1.11) | 0.1754 | 0.7015 |
| Dominant | T/T | 154(57.46) | 159(64.9) | 1 | ||||
| T/C-C/C | 114(42.54) | 86(35.1) | 0.75(0.49–1.13) | 0.1632 | 0.6298 | |||
| Recessive | T/T-T/C | 252(94.03) | 234(95.51) | 1 | ||||
| C/C | 16(5.97) | 11(4.49) | 0.78(0.31–1.93) | 0.5863 | 0.7818 | |||
| UGT1A7 | rs4124874 | Log-additive | - | - | - | 1.74(1.28–2.37) | 0.0004 | 0.009* |
| Dominant | T/T | 142(52.99) | 96(39.18) | 1 | ||||
| T/G-G/G | 126(47.01) | 149(60.82) | 1.86(1.25–2.78) | 0.0023 | 0.0469* | |||
| Recessive | T/T-T/G | 250(93.28) | 208(84.9) | 1 | ||||
| G/G | 18(6.72) | 37(15.1) | 2.50(1.26–4.94) | 0.0085 | 0.1253 | |||
| UGT1A7 | rs10929302 | Log-additive | - | - | - | 1.23(0.80–1.90) | 0.3456 | 0.7325 |
| Dominant | G/G | 215(80.22) | 194(79.18) | 1 | ||||
| G/A-A/A | 53(19.78) | 51(20.82) | 1.04(0.64–1.70) | 0.8754 | 0.9423 | |||
| Recessive | G/G-G/A | 267(99.63) | 236(96.33) | 1 | ||||
| A/A | 1(0.37) | 9(3.67) | 13.66(1.54–121) | 0.0188 | 0.1253 | |||
| UGT1A7 | rs887829 | Log-additive | - | - | - | 1.22 (0.79–1.88) | 0.3663 | 0.7325 |
| Dominant | C/C | 214(79.85) | 194(79.18) | 1 | ||||
| C/T-T/T | 54(20.15) | 51(20.82) | 1.03(0.63–1.68) | 0.9094 | 0.9423 | |||
| Recessive | C/C-C/T | 267(99.63) | 236(96.33) | 1 | ||||
| T/T | 1(0.37) | 9(3.67) | 13.66(1.54–121) | 0.0188 | 0.1253 | |||
| UGT1A7 | rs4148323 | Log-additive | - | - | - | 0.69 (0.47–1.01) | 0.0577 | 0.5055 |
| Dominant | G/G | 174(64.93) | 181(73.88) | 1 | ||||
| G/A-A/A | 94(35.07) | 64(26.12) | 0.70(0.45–1.08) | 0.1095 | 0.6298 | |||
| Recessive | G/G-G/A | 255(95.15) | 240(97.96) | 1 | ||||
| A/A | 13(4.85) | 5(2.04) | 0.38 (0.11–1.29) | 0.1199 | 0.5994 | |||
| UGT2B15 | rs3100 | Log-additive | - | - | - | 1.38 (0.95–2.01) | 0.0888 | 0.5055 |
| Dominant | G/G | 193(72.01) | 160(65.31) | 1 | ||||
| G/A-A/A | 75(27.99) | 85(34.69) | 1.41 (0.92–2.16) | 0.1113 | 0.6298 | |||
| Recessive | G/G-G/A | 262(97.76) | 238(97.14) | 1 | ||||
| A/A | 6(2.24) | 7(2.86) | 1.83(0.55–6.10) | 0.3281 | 0.7149 | |||
| UGT2B15 | rs4148269 | Log-additive | - | - | - | 1.37(0.94–1.99) | 0.1011 | 0.5055 |
| Dominant | T/T | 192(71.64) | 160(65.31) | 1 | ||||
| T/G-G/G | 76(28.36) | 85(34.69) | 1.39(0.91–2.13) | 0.1284 | 0.6298 | |||
| Recessive | T/T-T/G | 262(97.76) | 238(97.14) | 1 | ||||
| G/G | 6(2.24) | 7(2.86) | 1.83(0.55–6.10) | 0.3281 | 0.7149 | |||
| UGT2B15 | rs2045100 | Log-additive | - | - | - | 0.93(0.68–1.26) | 0.6269 | 0.7973 |
| Dominant | T/T | 125(46.64) | 119(48.57) | 1 | ||||
| T/A-A/A | 143(53.36) | 126(51.43) | 0.89(0.60–1.33) | 0.5612 | 0.9423 | |||
| Recessive | T/T-T/A | 244(91.04) | 223(91.02) | 1 | ||||
| A/A | 24(8.96) | 22(8.98) | 0.97(0.50–1.88) | 0.9232 | 1 | |||
| UGT2B15 | rs1902023 | Log-additive | - | - | - | 0.93 (0.71–1.23) | 0.6104 | 0.7973 |
| Dominant | C/C | 84(31.34) | 85(34.69) | 1 | ||||
| C/A-A/A | 184(68.66) | 160(65.31) | 0.97(0.64–1.48) | 0.8849 | 0.9423 | |||
| Recessive | C/C-C/A | 208(77.61) | 200(81.63) | 1 | ||||
| A/A | 60(22.39) | 45(18.37) | 0.83(0.51–1.36) | 0.4569 | 0.7149 | |||
| UGT2B15 | rs9994887 | Log-additive | - | - | - | 0.96(0.73–1.26) | 0.7578 | 0.7973 |
| Dominant | G/G | 85(31.72) | 83(33.88) | 1 | ||||
| G/A-A/A | 183(68.28) | 162(66.12) | 1.02 (0.67–1.56) | 0.916 | 0.9423 | |||
| Recessive | G/G-G/A | 208(77.61) | 199(81.22) | 1 | ||||
| A/A | 60(22.39) | 46(18.78) | 0.84(0.52–1.38) | 0.5004 | 0.7149 | |||
| UGT2B15 | rs13112099 | Log-additive | - | - | - | 0.96(0.73–1.26) | 0.7578 | 0.7973 |
| Dominant | G/G | 85(31.72) | 83(33.88) | 1 | ||||
| G/T-T/T | 183(68.28) | 162(66.12) | 1.02(0.67–1.56) | 0.916 | 0.9423 | |||
| Recessive | G/G-G/T | 208(77.61) | 199(81.22) | 1 | ||||
| T/T | 60(22.39) | 46(18.78) | 0.84(0.52–1.38) | 0.5004 | 0.7149 | |||
| UGT2B15 | rs7686914 | Log-additive | - | - | - | 1.04(0.79–1.37) | 0.7578 | 0.7973 |
| Dominant | T/T | 60(22.39) | 46(18.78) | 1 | ||||
| T/C-C/C | 208(77.61) | 199(81.22) | 1.19(0.72–1.94) | 0.916 | 0.9423 | |||
| Recessive | T/T-T/C | 183(68.28) | 162(66.12) | 1 | ||||
| C/C | 85(31.72) | 83(33.88) | 0.98(0.64–1.49) | 0.5004 | 0.7149 | |||
| UGT2B15 | rs7696472 | Log-additive | - | - | - | 1.05(0.80–1.38) | 0.7415 | 0.7973 |
| Dominant | G/G | 60(22.39) | 46(18.78) | 1 | ||||
| G/A-A/A | 208(77.61) | 199(81.22) | 1.19(0.72–1.94) | 0.9423 | 0.9423 | |||
| Recessive | G/G-G/A | 183(68.28) | 161(65.71) | 1 | ||||
| A/A | 85(31.72) | 84(34.29) | 0.98(0.65–1.50) | 0.5004 | 0.7149 | |||
| UGT2B7 | rs4587017 | Log-additive | - | - | - | 0.83(0.61–1.13) | 0.228 | 0.7325 |
| Dominant | T/T | 19(7.09) | 22(8.98) | 1 | ||||
| T/G-G/G | 249(92.91) | 223(91.02) | 0.71(0.35–1.45) | 0.2988 | 0.8538 | |||
| Recessive | T/T-T/G | 121(45.15) | 115(46.94) | 1 | ||||
| G/G | 147(54.85) | 130(53.06) | 0.81(0.54–1.21) | 0.3473 | 0.7149 | |||
| UGT2B7 | rs7662029 | Log-additive | - | - | - | 0.85(0.62–1.16) | 0.2996 | 0.7325 |
| Dominant | A/A | 19(7.09) | 22(8.98) | 1 | ||||
| A/G-G/G | 249(92.91) | 223(91.02) | 0.71(0.35–1.45) | 0.4125 | 0.9423 | |||
| Recessive | A/A-A/G | 125(46.64) | 116(47.35) | 1 | ||||
| G/G | 143(53.36) | 129(52.65) | 0.85(0.57–1.26) | 0.3473 | 0.7149 | |||
| UGT2B7 | rs12233719 | Log-additive | - | - | - | 0.94(0.65–1.36) | 0.7391 | 0.7973 |
| Dominant | G/G | 184(68.66) | 177(72.24) | 1 | ||||
| G/T-T/T | 84(31.34) | 68(27.76) | 0.95 (0.62–1.46) | 0.8184 | 0.9423 | |||
| Recessive | G/G-G/T | 259(96.64) | 238(97.14) | 1 | ||||
| T/T | 9(3.36) | 7(2.86) | 0.79(0.25–2.45) | 0.6793 | 0.8491 | |||
| UGT2B7 | rs10028494 | Log-additive | - | - | - | 1.14(0.80–1.62) | 0.4861 | 0.7973 |
| Dominant | A/A | 166(61.94) | 152(62.04) | 1 | ||||
| A/C-C/C | 102(38.06) | 93(37.96) | 1.10(0.73–1.66) | 0.658 | 0.9423 | |||
| Recessive | A/A-A/C | 260(97.01) | 236(96.33) | 1 | ||||
| C/C | 8(2.99) | 9(3.67) | 1.69(0.58–4.94) | 0.3395 | 0.7149 | |||
| CYP2C19 | rs12248560 | Log-additive | - | - | - | 0.82(0.22–3.12) | 0.7719 | 0.7973 |
| Dominant | C/C | 262(97.76) | 239(97.55) | 1 | ||||
| C/T-T/T | 6(2.24) | 6(2.45) | 0.82(0.22–3.12) | 0.7719 | 0.9423 | |||
| Recessive | C/C-C/T | 268(100) | 245(100) | 1 | ||||
| T/T | 0(0) | 0(0) | NA | NA | NA | |||
| CYP2C19 | rs4244285 | Log-additive | - | - | - | 0.96(0.72–1.29) | 0.7973 | 0.7973 |
| Dominant | G/G | 118(44.03) | 108(44.08) | 1 | ||||
| G/A-A/A | 150(55.97) | 137(55.92) | 0.92(0.62–1.37) | 0.6817 | 0.9423 | |||
| Recessive | G/G-G/A | 239(89.18) | 213(86.94) | 1 | ||||
| A/A | 29(10.82) | 32(13.06) | 1.03(0.56–1.90) | 0.9237 | 1 | |||
| CYP2C9 | rs1057910 | Log-additive | - | - | - | 1.58(0.72–3.47) | 0.2585 | 0.7325 |
| Dominant | A/A | 256(95.52) | 225(91.84) | 1 | ||||
| A/C-C/C | 12(4.48) | 20(8.16) | 1.76(0.76–4.08) | 0.189 | 0.6298 | |||
| Recessive | A/A-A/C | 267(99.63) | 245(100) | 1 | ||||
| C/C | 1(0.37) | 0(0) | 0(0-inf) | 0.9993 | 1 |
aaOR, adjusted odds ratio. Logistic regression was used to calculate odds ratios and 95% CIs; all models were adjusted for maternal age (continuous), maternal ethnicity, maternal education level, parental smoking or ETS exposure, maternal alcohol consumption, gravidity, pre-pregnancy BMI (continuous), folic acid supplements
GMDR analyses for gene–gene and gene–environment interactions on CHDs
The gene-gene and gene-environment interaction model by GMDR was presented in Table 5. The P value was determined using the permutation test with 1000 replications. For gene-gene interaction, rs4124874 was a susceptibility locus for CHDs risk. Two-locus to five-locus interaction models were observed, but there were no statistical differences. In addition, for gene-phthalate metabolites interaction, five interaction combinations with no statistical significance were observed.
Table 5.
GMDR analysis for gene–gene and gene–phthalates exposure interaction models in CHDs
| Model | Training Bal. Acc | Testing Bal. Acc | Sign Test (P-Value) |
CV Consistency |
|---|---|---|---|---|
| Gene-gene interaction | ||||
| rs4124874 | 0.5690 | 0.5691 | 10(0.0010) | 10/10 |
| rs1057910 rs4124874 | 0.5933 | 0.5397 | 8(0.0547) | 5/10 |
| rs4124874 rs3100 rs2045100 rs12233719 | 0.6565 | 0.5292 | 7(0.1719) | 5/10 |
| rs4244285 rs11692021 rs4124874 rs1902023 rs4587017 | 0.7091 | 0.4999 | 4(0.8281) | 3/10 |
| Gene-PAEs phthalates interaction | ||||
| rs11692021 rs1902023 MEHP | 0.6161 | 0.5186 | 7(0.1719) | 3/10 |
| rs4244285 rs11692021 rs4124874 rs1902023 rs12233719 MEHP | 0.7740 | 0.4988 | 5(0.6230) | 2/10 |
| rs4244285 rs11692021 rs4124874 rs9994887 rs7662029 MiBP MEHP | 0.8376 | 0.4832 | 5(0.6230) | 3/10 |
| rs4244285 rs11692021 rs4124874 rs9994887 rs7662029 rs12233719 MnBP MBzP | 0.8912 | 0.5023 | 4(0.8281) | 5/10 |
| rs4244285 rs11692021 rs4124874 rs2045100 rs9994887 rs7662029 MiBP MEHP MCMHP | 0.9361 | 0.4287 | 1(0.9990) | 2/10 |
Notes Training Bal. Acc: training balanced accuracy; Testing Bal. Acc: testing balanced accuracy; CV Consistency: cross validation consistency
Discussion
In this case-control study, we observed no difference in the concentration of maternal phthalate metabolites between the case and control groups, and did not find significant association between the concentrations of maternal phthalate metabolites and the risk of CHDs, while observed that the SNPs rs4124874 and rs887829 of UGT1A7 gene were associated with an increased risk of CHDs, but did not find significant gene-gene or gene-phthalate metabolite interactions on CHDs.
Our findings suggested that maternal phthalates exposure was not associated with the risk of CHDs, which was similar to the results of two Netherlands studies showing no association of maternal exposure to phthalates with risk of CHDs [24, 26]. Another case-control study in Hungary also reported that maternal job-exposure matrix (JEM)-assessed and self-reported exposures to phthalates were not associated with the risk of CHDs or subtypes [25]. Moreover, our analysis in the Han Chinese maternal population found no significant positive associations between phthalates exposure to and CHDs. The results of stratified analysis were inconsistent with one previous studies reported in China, which found that maternal occupational exposure to phthalates was associated with a higher incidence of total congenital heart defects, with aOR 1.6 (95% CI: 1.0-2.6) [22]. Similarly, a later Chinese study confirmed that maternal occupation exposure to phthalates was associated with CHDs subtypes, including ventricular septal defect (VSD), atrial septal defect (ASD), patent ductus arteriosus (PDA), pulmonary valve stenosis (PVS) [23]. However, due to the small sample size, we did not perform the association analysis between phthalates exposure with CHDs subtypes. In addition to CHDs, maternal occupational exposure to phthalate obtained by a job exposure matrix was associated with increased risks of hypospadias (OR = 3.12; 95% CI, 1.04–11.46) [45]. First trimester urinary DEHP metabolite concentrations were associated with increased odds of neonatal genital anomaly (OR = 2.54, 95% CI, 1.09–5.92) [18]. One study found that the detection ratio of positive BBP and its metabolites in maternal urine was obviously higher in neural tube defects population than that in normal controls [46]. Overall, there are only a few limited reports on phthalate exposure during pregnancy and birth defects.
It is important to note that studies on the association between maternal phthalates exposure and the risk of CHDs in the Chinese population have shown inconsistent results, which may be due to differences in the way exposure was evaluated. Phthalates exposure is ubiquitous. Two previous China studies focused occupational exposures, which only considered a single source of exposure at work, ignoring comprehensive exposure in the living environment. Moreover, it was difficult to estimate accurately the amount of exposure, the time and the frequency due to recall bias. In addition, the discrepancies across studies may be due to the heterogeneity of populations. For example, two European studies found that maternal occupational phthalates exposure was not associated with CHDs, however, the inverse associations were observed between maternal occupational phthalates exposure and the risk of CHDs in China studies. The conflicting results can likely be explained by differences in sensitivity to the biochemical and toxic effects of phthalates due to genetic polymorphisms.
Accumulating evidence have suggested that inter-individual differences in the ability of the xenobiotic metabolism due to high variability in certain metabolic enzyme activities can influence the effects of environmental exposure on birth defects (e.g., oral clefts, neural tube defects, CHDs) [47–50]. The uridine diphosphate (UDP)-glucuronosyl transferase (UGTs), UGT1A7, UGT2B7 and UGT2B15, catalyze the glucuronidation of multiple substrates. As for the UGT1A7 gene, rs11692021 polymorphism was related to higher risk of chronic pancreatitis (aOR = 1.76, 95% CI: 1.26–2.46) [30]. In addition, rs4124874 and rs4148323 polymorphism were associated with in increased risk of hyperbilirubinemia [31, 32]. However, rs887829 genotype was related to a decreased risk of hyperbilirubinemia (aOR = 0.55, 95% CI: 0.34–0.89) [32]. As for the UGT2B7 gene, rs7662029 polymorphism was associated with the withdrawal symptoms in methadone maintenance patients [33]. SNP rs4587017 polymorphism might influence the analgesic effects of fentanyl in the cold pressor-induced pain test [34]. SNP rs12233719 polymorphism was associated with never-smoking female lung cancer risk [35]. As for the UGT2B15 gene, rs2045100 locus, six SNPs (rs3100, rs4148269, rs9994887, rs13112099, rs7686914 and rs7696472), and rs1902023 polymorphism, showed significant associations with increased risk for prostate cancer [36–38]. In the present study, among 17 SNPs in UGT1A7, UGT2B7 and UGT2B15, the polymorphisms of maternal UGT1A7 gene at rs4124874 (under additive, dominant and recessive models), and rs887829 (under recessive model) were associated with increased CHDs risk. The molecular mechanism remains unclear. It is possible that these two intronic loci affect the alternative spicing of the gene products, or they might be in linkage disequilibrium with other causal loci or genes, thereby affecting the metabolism of phthalates.
Two members of the CYP family, CYP2C9 and CYP2C19 are the main Phase I metabolizing enzymes mediating the toxicity of phthalates, their polymorphisms are associated with the risk of many types of diseases. It has been reported that rs1057910 genotype of CYP2C9 was related to increased risk of adenoma recurrence (aRR = 1.47, 95% CI: 1.19–1.83) [39], or developing sporadic colorectal carcinoma (aOR = 2.77, 95% CI: 1.1653–4.643) [40]. In addition, for the two common polymorphic loci rs12248560 and rs4244285 of CYP2C19, the genotype of rs12248560 was associated with decreased breast cancer risk (aOR = 0.77, 95% CI: 0.65–0.93) [41], while rs4244285 polymorphism was related to higher risk of epilepsy (aOR = 4.24, 95% CI: 2.52–7.15) [42], long-term ischemic stroke events (hazard ratio: 1.64, 95% CI: 1.06–2.53) [43], hypertension (aOR = 2.433, 95% CI: 1.797–3.293) [44]. Meanwhile, several studies have observed that CYP2C9 rs1057910 polymorphism was associated with increased risk for adenoma recurrence (aRR = 1.47, 95% CI 1.19–1.83) [39], or developing sporadic colorectal carcinoma (aOR = 2.589, 95% CI: 1.549–4.330) [40]. In our study, no significant associations between CHDs risk and genotype were seen for the rs12248560 and rs4244285 of CYP2C19, or rs1057910 of CYP2C9 polymorphisms.
It is now widely believed that most structural birth defects including CHDs are caused by a complex combination of genetic and environmental factors that interact to interfere with morphogenetic processes. More and more studies have reported significant interaction effects between gene-environment interactions for the development of CHDs. One study found maternal dietary factors and cystathionine beta synthase (CBS) gene variants (rs2851391, rs234714) interactions were significantly associated with risk of CHDs [51]. One study observed the interaction between maternal tobacco exposure and polymorphisms of the MTHFD1 gene including rs1950902, rs2236222, rs1256142, rs11849530 and rs2236225, was significantly associated with the risk of CHDs in offspring [52]. One study reported a significantly positive interaction between maternal folic acid supplementation and genetic variation at rs828858 of MTHFD2 for the risk of CHDs [53]. In our previous study, we found that polymorphisms of maternal GST genes (GSTM1, GSTT1, GSTP1) might modify the association of maternal smoke exposure with CHDs [54]. In addition, we also observed that the polymorphisms of maternal AHR rs2158041 and rs7811989, or UGT1A1 rs4148323 might modify the association of PAHs exposure with CHDs, CYP1A2 rs4646425 or CYP2E1 rs915908 polymorphisms significantly interacted with PAHs exposure on CHDs [55, 56]. In the present study, no significantly positive interaction of gene-gene or gene-phthalate metabolites for the risk of CHDs was observed, more large-scale studies or prospective study designs are needed to explore the interactions in the future.
This study has several strengths. First, to the best of our knowledge, this is the first study to evaluate the effect of the interaction between maternal phthalates exposure and maternal gene polymorphism on the risk of CHDs. Second, compared with previous phthalates exposure assessment that relied solely on expert industrial hygienist consensus or the self-reported questionnaire, we used a urinary bio-markers based approach to evaluate the association between maternal phthalates exposure and the risk of CHDs, offering an objective measure of exposure. Finally, the subjects of our study were non-occupational, low-dose phthalates exposed pregnant women, thus, the results can be generalized to all women because the environmental factors can be assessed at the individual level.
However, our study still had several limitations. First, small sample size limited the statistical power; besides, due to a small number of cases, our study could not perform the analysis for type-specific CHDs; future studies with larger sample sizes are warranted to confirm or refute our findings. Second, we only measured phthalate metabolites from a single spot urine sample taken at the second trimester which could not precisely estimate the mother’s long-term exposure level. Thus, future studies are needed to collect multiple urine samples. Third, only maternal phthalates exposure and genetic susceptibilities were considered; future studies are needed to investigate the effects of fetal exposure, fetal genotypes, and the interaction between them on the risk of CHDs.
In conclusion, our analysis results indicated that maternal phthalates exposure was not associated with the risk of CHDs. The polymorphisms of maternal UGT1A7 gene at rs4124874 and rs887829 were significantly associated with an increased risk of CHDs. No significant gene-gene or gene-phthalate metabolites interactions on CHDs was observed. However, due to the complex pathogenesis of CHDs and the limitation of small sample size, more large-scale studies or prospective study designs are needed to confirm or refute our findings in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are indebted to the pediatric cardiologists, geneticists, and epidemiologists who collaborated in this program and made the study possible. We thank the obstetricians, pediatricians, pathologists, experimental technician and other participants involved in the project for recruiting the participants and collecting the data. We thank all participating families for their cooperation and for providing personal information. We also thank the reviewers for their helpful comments.
Abbreviations
- CHDs
congenital heart diseases
- aOR
adjusted odds ratio
- 95% CI
95% confidence interval
- UHPLC-MS/MS
ultra-high performance liquid chromatography coupled with tandem mass spectrometry
- SNPs
single nucleotide polymorphisms
- CYP2C9
cytochrome P450 family 2 subfamily C member 9
- CYP2C9
cytochrome P450 family 2 subfamily C member 19
- UGT1A7
uridine diphosphate (UDP) glucuronosyl transferase family 1 member A7
- UGT2B7
uridine diphosphate (UDP) glucuronosyl transferase family 2 member B7
- UGT2B15
uridine diphosphate (UDP) glucuronosyl transferase family 2 member B15
- GMDR
generalized multifactor dimensionality reduction
- FDR
false discovery rate
- MnBP
mono-n-butyl phthalate
- DnBP
di-n-butyl phthalate
- MiBP
mono-isobutyl phthalate
- DiBP
diisobutyl phthalate
- MBzP
mono-benzyl phthalate
- BBzP
butylbenzyl phthalate
- DEHP
di (2-ethylhexyl) phthalate
- MEHP
mono(2-ethyl-hexyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MECCP
mono (2-ethyl-5-carboxypentyl)phthalate
- MCMHP
mono-2- carboxymethyl hexyl phthalate
- LOD
limit of detection
- ETS
environmental tobacco smoke
Author contributions
P. Y. and N. L. developed the study design and drafted the manuscript. H. K. and J. T. assisted in conducting the experiment and analyzing the data. L. L., M. W., Y. L. and W. X. assisted in preparing samples and extracting DNA. L. Z., D. Y., X. L., Y. W. and J. Z. participated in reviewing, editing, and revising the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (grant numbers 2022YFC2703300, 2022YFC2703302, 2022YFC2703700, 2022YFC2703702); Sichuan Science and Technology Program (grant numbers 2022NSFSC0661, 2022NSFSC0656).
Data availability
The variant data for this study have been deposited in the European Variation Archive (EVA) at EMBL-EBI under accession number PRJEB70402.
Declarations
Ethics approval and consent to participate
All the participants signed an informed consent form. This research was approved by the Ethics Committee of Sichuan University (No. 2010004) and followed the tenets of the Declaration of Helsinki.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nana Li and Hong Kang contributed equally to this work.
Contributor Information
Jing Tao, Email: taotao20081988@126.com.
Ping Yu, Email: yup@scu.edu.cn.
References
- 1.Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455–63. doi: 10.1093/ije/dyz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L, Chen L, Yang T, Wang T, Zhang S, Ye Z, Luo L, Qin J. Birth prevalence of congenital heart disease in China, 1980–2019: a systematic review and meta-analysis of 617 studies. Eur J Epidemiol. 2020;35(7):631–42. doi: 10.1007/s10654-020-00653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagy O, Barath S, Ujfalusi A. The role of microRNAs in congenital heart disease. EJIFCC. 2019;30(2):165–78. [PMC free article] [PubMed] [Google Scholar]
- 4.Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, Mital S, Priest JR, Pu WT, Roberts A, et al. Genetic basis for congenital Heart Disease: Revisited: A Scientific Statement from the American Heart Association. Circulation. 2018;138(21):e653–e711. doi: 10.1161/CIR.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauss RS, Hong M. Gene-environment interactions and the etiology of birth defects. Curr Top Dev Biol. 2016;116:569–80. doi: 10.1016/bs.ctdb.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Moreau JLM, Kesteven S, Martin E, Lau KS, Yam MX, O’Reilly VC, Del Monte-Nieto G, Baldini A, Feneley MP, Moon AM et al. Gene-environment interaction impacts on heart development and embryo survival. Development 2019, 146(4). [DOI] [PubMed]
- 7.Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. Phthalates impact human health: epidemiological evidences and plausible mechanism of action. J Hazard Mater. 2017;340:360–83. doi: 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhu H, Kannan K. A review of Biomonitoring of Phthalate exposures. Toxics 2019, 7(2). [DOI] [PMC free article] [PubMed]
- 9.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015;85:27–39. doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Ma J, Wang T, Qin C, Hu X, Mosa A, Ling W. Environmental health risks induced by interaction between phthalic acid esters (PAEs) and biological macromolecules: a review. Chemosphere. 2023;328:138578. doi: 10.1016/j.chemosphere.2023.138578. [DOI] [PubMed] [Google Scholar]
- 11.Gao H, Zhu YD, Xu YY, Zhang YW, Yao HY, Sheng J, Jin ZX, Ren LL, Huang K, Hao JH, et al. Season-dependent concentrations of urinary phthalate metabolites among Chinese pregnant women: repeated measures analysis. Environ Int. 2017;104:110–7. doi: 10.1016/j.envint.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Sun Y, Liu N, Hou J, Huo X, Zhao Y, Zhang Y, Deng F, Kan H, Zhao Z, et al. Indoor exposure to phthalates and its burden of disease in China. Indoor Air. 2022;32(4):e13030. doi: 10.1111/ina.13030. [DOI] [PubMed] [Google Scholar]
- 13.Gao H, Wang YF, Huang K, Han Y, Zhu YD, Zhang QF, Xiang HY, Qi J, Feng LL, Zhu P, et al. Prenatal phthalate exposure in relation to gestational age and preterm birth in a prospective cohort study. Environ Res. 2019;176:108530. doi: 10.1016/j.envres.2019.108530. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Mustieles V, Yland J, Braun JM, Williams PL, Attaman JA, Ford JB, Calafat AM, Hauser R, Messerlian C. Association of Parental Preconception Exposure to phthalates and phthalate substitutes with Preterm Birth. JAMA Netw Open. 2020;3(4):e202159. doi: 10.1001/jamanetworkopen.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Gao F, Ben Y, Su Y. Association between phthalate exposure and risk of spontaneous pregnancy loss: a systematic review and meta-analysis. Environ Pollut. 2020;267:115446. doi: 10.1016/j.envpol.2020.115446. [DOI] [PubMed] [Google Scholar]
- 16.Ji H, Wu Z, Chen D, Miao M, Chen H, Shuai W, Liang H, Yuan W. Individual and joint effects of phthalates exposure on the risk of early miscarriage. J Expo Sci Environ Epidemiol 2023. [DOI] [PubMed]
- 17.Jin S, Cui S, Xu J, Zhang X. Associations between prenatal exposure to phthalates and birth weight: a meta-analysis study. Ecotoxicol Environ Saf. 2023;262:115207. doi: 10.1016/j.ecoenv.2023.115207. [DOI] [PubMed] [Google Scholar]
- 18.Sathyanarayana S, Grady R, Barrett ES, Redmon B, Nguyen RHN, Barthold JS, Bush NR, Swan SH. First trimester phthalate exposure and male newborn genital anomalies. Environ Res. 2016;151:777–82. doi: 10.1016/j.envres.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernandez MF, Garcia-Esteban R, Iniguez C, Martinez D, Murcia M, Monfort N, et al. Exposure to Bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell cohort. Environ Health Perspect. 2016;124(4):521–8. doi: 10.1289/ehp.1409190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun G, Liu K. Developmental toxicity and cardiac effects of butyl benzyl phthalate in zebrafish embryos. Aquat Toxicol. 2017;192:165–70. doi: 10.1016/j.aquatox.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Li Y. Exposure to DBP induces the toxicity in early development and adverse effects on cardiac development in zebrafish (Danio rerio) Chemosphere. 2019;218:76–82. doi: 10.1016/j.chemosphere.2018.11.095. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Xie L, Zhou K, Zhan Y, Li Y, Li H, Qiao L, Wang F, Hua Y. Increased risk for congenital heart defects in children carrying the ABCB1 gene C3435T polymorphism and maternal periconceptional toxicants exposure. PLoS ONE. 2013;8(7):e68807. doi: 10.1371/journal.pone.0068807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Zhan Y, Wang F, Li H, Xie L, Liu B, Li Y, Mu D, Zheng H, Zhou K, et al. Parental occupational exposures to endocrine disruptors and the risk of simple isolated congenital heart defects. Pediatr Cardiol. 2015;36(5):1024–37. doi: 10.1007/s00246-015-1116-6. [DOI] [PubMed] [Google Scholar]
- 24.Snijder CA, Vlot IJ, Burdorf A, Obermann-Borst SA, Helbing WA, Wildhagen MF, Steegers EA, Steegers-Theunissen RP. Congenital heart defects and parental occupational exposure to chemicals. Hum Reprod. 2012;27(5):1510–7. doi: 10.1093/humrep/des043. [DOI] [PubMed] [Google Scholar]
- 25.Fazekas-Pongor V, Fekete M, Csaky-Szunyogh M, Cseh K, Penzes M. Parental occupational exposure and congenital heart diseases in a Hungarian case-control study. Int Arch Occup Environ Health 2021. [DOI] [PMC free article] [PubMed]
- 26.Wijnands KP, Zeilmaker GA, Meijer WM, Helbing WA, Steegers-Theunissen RP. Periconceptional parental conditions and perimembranous ventricular septal defects in the offspring. Birth Defects Res Clin Mol Teratol. 2014;100(12):944–50. doi: 10.1002/bdra.23265. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez-Romero E, Scheringer M. A review of phthalate pharmacokinetics in human and rat: what factors drive phthalate distribution and partitioning? Drug Metab Rev. 2019;51(3):314–29. doi: 10.1080/03602532.2019.1620762. [DOI] [PubMed] [Google Scholar]
- 28.Stajnko A, Runkel AA, Kosjek T, Snoj Tratnik J, Mazej D, Falnoga I, Horvat M. Assessment of susceptibility to phthalate and DINCH exposure through CYP and UGT single nucleotide polymorphisms. Environ Int. 2022;159:107046. doi: 10.1016/j.envint.2021.107046. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Nie Y, Tang L, Xu CC, Xu L. The correlation between UDP-glucuronosyltransferase polymorphisms and environmental endocrine disruptors levels in polycystic ovary syndrome patients. Med (Baltim) 2020;99(11):e19444. doi: 10.1097/MD.0000000000019444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ockenga J, Vogel A, Teich N, Keim V, Manns MP, Strassburg CP. UDP glucuronosyltransferase (UGT1A7) gene polymorphisms increase the risk of chronic pancreatitis and pancreatic cancer. Gastroenterology. 2003;124(7):1802–8. doi: 10.1016/s0016-5085(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Song L, Hao L. The role of UGT1A1 (c.-3279 T > G) gene polymorphisms in neonatal hyperbilirubinemia susceptibility. BMC Med Genet. 2020;21(1):218. doi: 10.1186/s12881-020-01155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Wang SN, Li H, Zha W, Wang X, Liu Y, Sun J, Peng Q, Li S, Chen Y, et al. Association of UGT1A1 variants and hyperbilirubinemia in breast-fed full-term Chinese infants. PLoS ONE. 2014;9(8):e104251. doi: 10.1371/journal.pone.0104251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian JN, Ho IK, Tsou HH, Fang CP, Hsiao CF, Chen CH, Tan HK, Lin L, Wu CS, Su LW, et al. UGT2B7 genetic polymorphisms are associated with the withdrawal symptoms in methadone maintenance patients. Pharmacogenomics. 2012;13(8):879–88. doi: 10.2217/pgs.12.69. [DOI] [PubMed] [Google Scholar]
- 34.Muraoka W, Nishizawa D, Fukuda K, Kasai S, Hasegawa J, Wajima K, Nakagawa T, Ikeda K. Association between UGT2B7 gene polymorphisms and fentanyl sensitivity in patients undergoing painful orthognathic surgery. Mol Pain. 2016;12:1744806916683182. doi: 10.1177/1744806916683182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian Y, Xie L, Li L, Feng T, Zhu T, Wang R, Yang Y, Zhou B, Yu H, Qian B. Association between sex hormones regulation-related SNP rs12233719 and lung cancer risk among never-smoking Chinese women. Cancer Med. 2021;10(5):1880–8. doi: 10.1002/cam4.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun T, Oh WK, Jacobus S, Regan M, Pomerantz M, Freedman ML, Lee GS, Kantoff PW. The impact of common genetic variations in genes of the sex hormone metabolic pathways on steroid hormone levels and prostate cancer aggressiveness. Cancer Prev Res (Phila) 2011;4(12):2044–50. doi: 10.1158/1940-6207.CAPR-11-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal AC, Tucker C, Schildkraut JM, Richardson RM, McPhail M, Freedland SJ, Hoyo C, Grant DJ. Novel associations of UDP-glucuronosyltransferase 2B gene variants with prostate cancer risk in a multiethnic study. BMC Cancer. 2013;13:556. doi: 10.1186/1471-2407-13-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant DJ, Hoyo C, Oliver SD, Gerber L, Shuler K, Calloway E, Gaines AR, McPhail M, Livingston JN, Richardson RM, et al. Association of uridine diphosphate-glucuronosyltransferase 2B gene variants with serum glucuronide levels and prostate cancer risk. Genet Test Mol Biomarkers. 2013;17(1):3–9. doi: 10.1089/gtmb.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barry EL, Poole EM, Baron JA, Makar KW, Mott LA, Sandler RS, Ahnen DJ, Bresalier RS, McKeown-Eyssen GE, Ulrich CM. CYP2C9 variants increase risk of colorectal adenoma recurrence and modify associations with smoking but not aspirin treatment. Cancer Causes Control. 2013;24(1):47–54. doi: 10.1007/s10552-012-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao LH, Zhang H, Lai MP, Lau KW, Lai AK, Zhang JH, Wang Q, Wei W, Chai JH, Lung ML, et al. The association of CYP2C9 gene polymorphisms with colorectal carcinoma in Han Chinese. Clin Chim Acta. 2007;380(1–2):191–6. doi: 10.1016/j.cca.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Justenhoven C, Hamann U, Pierl CB, Baisch C, Harth V, Rabstein S, Spickenheuer A, Pesch B, Bruning T, Winter S, et al. CYP2C19*17 is associated with decreased breast cancer risk. Breast Cancer Res Treat. 2009;115(2):391–6. doi: 10.1007/s10549-008-0076-4. [DOI] [PubMed] [Google Scholar]
- 42.Makowska M, Smolarz B, Brys M, Forma E, Romanowicz H. An association between the rs1799853 and rs1057910 polymorphisms of CYP2C9, the rs4244285 polymorphism of CYP2C19 and the prevalence rates of drug-resistant epilepsy in children. Int J Neurosci. 2021;131(12):1147–54. doi: 10.1080/00207454.2020.1781110. [DOI] [PubMed] [Google Scholar]
- 43.Wu P, Liu Z, Tian Z, Wu B, Shao J, Li Q, Geng Z, Pan Y, Lu K, Wang Q et al. CYP2C19 loss-of-function variants Associated with Long-Term ischemic stroke events during Clopidogrel Treatment in the Chinese Population. Clin Pharmacol Ther 2023. [DOI] [PubMed]
- 44.Cai N, Li C, Gu X, Zeng W, Zhong J, Liu J, Zeng G, Zhu J, Hong H. CYP2C19 loss-of-function is associated with increased risk of hypertension in a Hakka population: a case-control study. BMC Cardiovasc Disord. 2023;23(1):185. doi: 10.1186/s12872-023-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, Elliott P. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect. 2009;117(2):303–7. doi: 10.1289/ehp.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song G, Wang R, Cui Y, Hao CJ, Xia HF, Ma X. Oxidative stress response associates with the teratogenic effects of benzyl butyl phthalate (BBP) Toxicol Res (Camb) 2020;9(3):222–9. doi: 10.1093/toxres/tfaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi M, Christensen K, Weinberg CR, Romitti P, Bathum L, Lozada A, Morris RW, Lovett M, Murray JC. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am J Hum Genet. 2007;80(1):76–90. doi: 10.1086/510518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L, Jin L, Liu J, Zhang Y, Yuan Y, Yi D, Ren A. Maternal genetic polymorphisms of phase II metabolic enzymes and the risk of fetal neural tube defects. Birth Defects Res Clin Mol Teratol. 2014;100(1):13–21. doi: 10.1002/bdra.23196. [DOI] [PubMed] [Google Scholar]
- 49.Padula AM, Yang W, Schultz K, Lurmann F, Hammond SK, Shaw GM. Genetic variation in biotransformation enzymes, air pollution exposures, and risk of spina bifida. Am J Med Genet A. 2018;176(5):1055–90. doi: 10.1002/ajmg.a.38661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, Wang M, Yu P, Li X, Lin Y, Duan Y, Tian Y, Zhu J, Deng Y, Li N. Maternal trichloroethylene exposure and metabolic gene polymorphisms may interact during fetal cardiovascular malformation. Reprod Toxicol. 2021;106:1–8. doi: 10.1016/j.reprotox.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Diao J, Li J, Luo L, Zhao L, Zhang S, Wang T, Chen L, Yang T, Zhu P, et al. Association of maternal dietary intakes and CBS gene polymorphisms with congenital heart disease in offspring. Int J Cardiol. 2021;322:121–8. doi: 10.1016/j.ijcard.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Song X, Li Q, Diao J, Li J, Li Y, Zhang S, Zhao L, Chen L, Wei J, Shu J, et al. Association of MTHFD1 gene polymorphisms and maternal smoking with risk of congenital heart disease: a hospital-based case-control study. BMC Pregnancy Childbirth. 2022;22(1):88. doi: 10.1186/s12884-022-04419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, Ou J, Chen Y, Chen Q, Luo M, Wang T, Qin J. Association of Maternal Folate Intake and offspring MTHFD1 and MTHFD2 genes with congenital heart disease. Nutrients 2023, 15(16). [DOI] [PMC free article] [PubMed]
- 54.Li X, Liu Z, Deng Y, Li S, Mu D, Tian X, Lin Y, Yang J, Li J, Li N, et al. Modification of the association between maternal smoke exposure and congenital heart defects by polymorphisms in glutathione S-transferase genes. Sci Rep. 2015;5:14915. doi: 10.1038/srep14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N, Mu Y, Liu Z, Deng Y, Guo Y, Zhang X, Li X, Yu P, Wang Y, Zhu J. Assessment of interaction between maternal polycyclic aromatic hydrocarbons exposure and genetic polymorphisms on the risk of congenital heart diseases. Sci Rep. 2018;8(1):3075. doi: 10.1038/s41598-018-21380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao J, Li N, Liu Z, Deng Y, Li X, Luo F, Yu P, Zhu J. Polymorphisms in gene UGT1A1 modify the association of prenatal exposure to polycyclic aromatic hydrocarbons with congenital heart diseases risk. J Matern Fetal Neonatal Med. 2023;36(1):2183743. doi: 10.1080/14767058.2023.2183743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The variant data for this study have been deposited in the European Variation Archive (EVA) at EMBL-EBI under accession number PRJEB70402.


