Abstract
Background and aims
Metformin has been reported to inhibit the occurrence and development of colorectal cancer (CRC) by mediating changes in intestinal flora. Studies have also indicated that the occurence of familial adenomatous polyposis (FAP) may also be associated with changes in the intestinal flora. Therefore, we investigated the efficacy and safety of metformin in treating FAP and the association with intestinal flora.
Results
Compared with the baseline, the mean number and load of polyps in the areas of nanocarbon labeling and postoperative residuals in the test group were lower than those in the placebo group, while the diversity of intestinal flora species was increased. At the genus level, the relative abundance of g_Ruminococcus in the test group was lower than that at baseline, whereas the relative abundance of g_Lactobacillus was higher. These changes were statistically significant (P < 0.05).
Conclusion
One-year metformin therapy for FAP is safe and effective, potentially mediated by modulating the intestinal flora. This study provides new insights and strategies for preventing adenomatous polyp carcinogenesis in FAP and explores possible preventive action.
Keywords: Familial adenomatous polyposis, Chemoprevention, Metformin, Intestinal flora
Introduction
Familial adenomatous polyposis (FAP) is mainly caused by germline mutations in the adenomatous polyposis gene on chromosome 5 [1], and is characterized by the growth of large numbers of adenomatous polyps in the colorectum during adolescence, with a risk of progression to colorectal cancer (CRC) of close to 100% without timely intervention. Somatic mutations in the adenomatous polyposis coli (APC) gene occur in 80% of sporadic CRC cases, and germline mutations in the APC gene are the main cause of FAP; thus, it is hypothesized that the mechanism of tumorigenesis in FAP may be similar to that of the vast majority of patients with sporadic CRC [2].
Accumulating evidence supports the correlation between intestinal flora and CRC development, so further research on the relationship between intestinal flora and FAP is warranted. In patients with FAP, Dejea et al. [3] discovered a relationship between the polyposis and intestinal flora, which was mainly composed of polyketide synthase-positive Escherichia coli (pks + E. coli) and enterotoxin-producing Bacteroides fragilis (ETBF) in colonic biofilms. Attard et al. [4] were the first to initiate a study of the intestinal flora of pediatric patients with FAP at the mucosal level. The results suggested that, in pediatric patients with FAP, the key flora components on polyp mucosa have pro-cancer properties.
Although resection of the primary site is currently the best treatment for FAP, surgery is not a complete cure. Post-surgical patients remain at risk of developing extracolonic disease. For this reason, endoscopic treatment combined with chemoprophylaxis is slowly gaining acceptance [5]. Existing chemotherapy includes nonsteroidal anti-inflammatory drugs (NSAIDS) [6–8], cyclooxygenase inhibitor combination therapy [9, 10], mammalian target of rapamycin (mTOR) inhibitors [11–13], and traditional Chinese medicine [14]. As a result of adverse effects found in some trials, the use of most of these drugs has been limited due to concerns that long-term use could lead to gastrointestinal damage or cardiovascular events [15–17].
Numerous studies have indicated that metformin has the potential to chemoprevent CRC. In 2010, a study in Japan first reported preliminary evidence that metformin inhibits the occurrence of CRC. The results indicated that metformin inhibited the proliferation of human colonic epithelium as well as the formation of rectal abnormal crypt foci (ACF) [18]. In 2016, a clinical trial suggested that low-dose metformin not only reduces the incidence, but also the number of heterochronic adenomas after polypectomy, and also demonstrated that it is safe for non-diabetic patients to take low-dose metformin for 1 year [19]. In addition, our previous study confirmed that metformin exerted a chemopreventive effect in mice, inhibiting the occurrence and development of ACF, polyps and CRC [20, 21]. In the clinic, metformin combined with endoscopy was confirmed to be superior to endoscopy alone in the treatment of FAP [22].
The effects of metformin on the intestinal flora have been reported, with some results indicating that metformin increased the abundance of short-chain fatty acid-producing and mucin-degrading bacteria [23, 24]. Metformin also promoted the enrichment of beneficial bacteria [25].
In this study, we investigated the ability of metformin to inhibit the development and progression of FAP adenomatous polyps by regulating the intestinal flora.
Methods
Subjects and study design
This prospective randomized controlled double-blind trial was conducted at the endoscopy center of the 900th Hospital of Joint Logistic Support Force. Twenty-six patients who attended from January 2022 to July 2022 and met the diagnostic criteria for FAP were included. The clinical diagnostic criteria for FAP required more than 100 adenomas in the general population or more than 20 in patients with a genetic predisposition. The study protocol adhered to the Declaration of Helsinki, and all participants provided written informed consent. The study was approved by the Ethics Committee of the 900th Hospital of the Joint Security Force and registered in the China Clinical Trial Registry (ChiCTR2300071081). All authors had access to the study data and reviewed and approved the final manuscript.
Inclusion criteria were as follows: 1. Patients aged 18 years or older with a clinical diagnosis or genetic test results confirming FAP. 2. Either of the following conditions sufficed: (a) Patients with an intact colon with moderate adenomatous loads (100–1000 polyps) who were being considered for prophylactic surgery. (b) Patients who had undergone surgical procedures, primarily consisting of subtotal colectomy with ileo-rectal anastomosis (IRA) and ileal-pouch-anal-anastomosis (IPAA).
Exclusion criteria included: (1) Coexisting diseases affecting the intestinal microecology, such as inflammatory bowel disease (IBD). (2) Use of immunosuppressants and glucocorticoids within 3 months, antibiotics, probiotics within 1 month, or proton-pump inhibitors, bismuth-containing drugs within 2 weeks prior to study inclusion. (3) Diagnosis of malignant diseases such as CRC. (4) Use of NSAIDs more than 3 times per week in the 6 months before the test. (5) Diagnosis of diabetes mellitus, pregnancy, or breastfeeding. (6) Significant liver and renal function test abnormalities. (7) Men preparing for conception. Other factors deemed inappropriate for enrollment by the investigator.
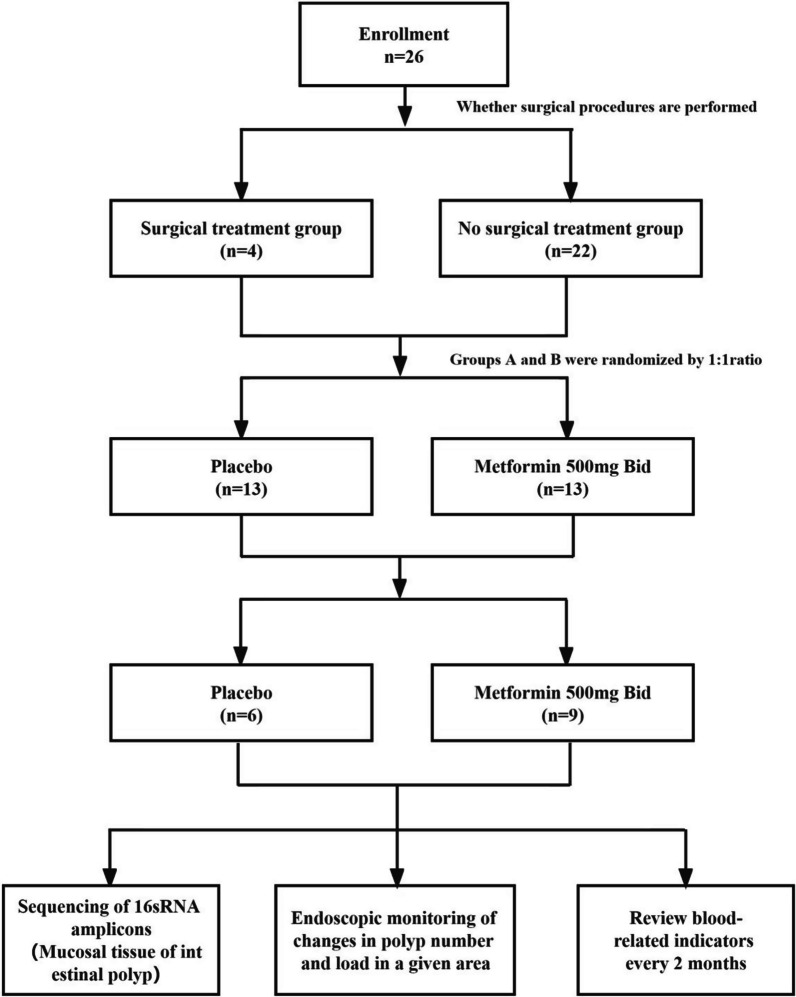
The study subjects were initially divided into a surgical treatment group and a non-surgical treatment group based on whether they underwent surgery. Each group was then randomly assigned in a 1:1 ratio to either a placebo group or a test group according to the order of enrollment, using a random number method, for a study period of 1 year. During the follow-up, the placebo group received a placebo matching metformin in size, appearance, and odor, primarily composed of starch. In contrast, the test group received 500 mg of metformin (produced by Tianfang Pharmaceutical Company) twice daily. Randomized grouping and medication distribution were conducted blindly by a nurse, with grouping and medication information concealed from both the managing physician and the participants until the study's conclusion. A schematic diagram of the study protocol is shown in Fig. 1.
Fig. 1.
A schematic diagram of the study protocol
During the baseline period, patients who had not undergo surgery received upper gastrointestinal endoscopy and colonoscopy. A 10 cm section in the middle part of the descending colon was selected for nanocarbon labeling. For patients who underwent IRA or IPAA, the remaining the rectal or ileal storage pouch area was identified for observation, depending on the surgery type. Endoscopic resection targeted all polyps more than 1 cm in diameter in the gastrointestinal tract, with immediate surgical treatment recommendation if pathology suggested a high-grade atypical hyperplasia or adenocarcinoma. After 1 year, the designated areas (including the descending duodenum, the nanocarbon-labeled area, and the postoperative residual area) were reassessed, focusing on changes in polyps numbers and load.
The endoscopy was video-recorded, with the operator and two other experienced endoscopists on-site to perform the first count of polyps in a designated area, while images were taken on-site in freeze-frame. Images were selected by two independent and experienced endoscopists to compare polyp changes before and after treatment of the designated areas for a second data review. The video served as a reference to resolve disagreements and assist in confirming the counts of polyp. The operator assessed the polyp size by closing and opening the biopsy forceps, only polyps with a diameter of ≥ 2 mm were included during the count. We categorized polyp diameters into the following five categories: 2–4 mm, 5–6 mm, 7–8 mm, 9–10 mm, and > 10 mm, and multiplied the number of polyps in each category by the median number of polyp diameters corresponding to that category. Finally, all the polyp diameters were summed to obtain the polyp load. Polyp mucosal tissue was also obtained from areas that were not counted.
Tissue processing and bioinformatics analysis
Intestinal polyp mucosal tissues were collected from both groups at baseline and at endoscopy after 1 year, processed with liquid nitrogen and immediately frozen and stored in an ultra-low-temperature refrigerator at – 80 °C. Primers (515F and 806R) targeting the V4 region of the bacterial 16S rRNA gene were used. Paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. The valid data were then rigorously processed to obtain the final amplicon sequence variants (ASVs) using the DADA2 module in the QIIME2 software (Version QIIME2-202006) for noise reduction, while ɑ and β diversity were calculated using QIIME2.
Statistical analysis
All statistical analysis was performed using SPSS 25.0 software. Normally distributed continuous variables are presented as mean ± standard deviation (SD), and comparisons between the two groups were conducted using either the two independent samples t-test or the paired samples t-test. Non-normally distributed continuous variables were expressed as median (quartiles), and comparisons between the two groups were performed using the Mann–Whitney rank sum test. The level of significance was set at α = 0.05, and the test was statistically significant (P < 0.05).
Primary and secondary end-points
Primary end-points: Changes in the number of adenomatous polyps in the designated area recorded before and after the trial were compared first, by comparing the two groups and second, by comparing the changes in the load of adenomatous polyps.
Secondary end-points: Changes in the composition of the intestinal flora and the relative abundance of species in the two groups recorded before and after the trial.
Assessment of drug safety
Patient adherence and monitoring of adverse events were assessed by conducting monthly telephone follow-up, micro-telephone video, or in-person conversations. To evaluate the safety of metformin, a range of blood tests were conducted every 2 months. These tests included fasting blood glucose (FBG), fasting insulin (FI), glycosylated hemoglobin (HbA1c), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), blood creatinine, total cholesterol (TC), and low-density lipoprotein (LDL). Adverse events were systematically graded using the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4.0).
Results
Baseline characteristics of the study population
Of the 26 FAP patients who met the enrollment criteria, seven dropped out of the placebo group and four dropped out of the test group. Of the remaining 15 patients, eight participated in genetic testing and had results suggestive of mutations in the APC gene. F1 and F2 were sons of F10, F3 was a nephew of F10, F4 was a son of F12, F5 and F7 were sisters, and F6 was a daughter of F7. Four patients underwent surgery, three patients retained their smoking habit to date, and two patients presented with extracolonic manifestations of which one patient presented with congenital hypertrophy of the retinal pigment epithelium (CHRPE) and desmoid tumor (DT), and the other patient presented with DT. Baseline demographic data characteristics are shown in Table 1.
Table 1.
Baseline demographic data characteristics of patients
| NO | Sex | Age | Family history | APC mutation | Operation | EIM | Current smoker |
|---|---|---|---|---|---|---|---|
| 1 | M | 32 | Mother | Exon16 (codon 1062) | NO | NO | Yes |
| 2 | M | 30 | Mother | Exon16 (codon 1062)) | NO | NO | Yes |
| 3 | M | 21 | Father | Exon16 (codon 1062) | NO | NO | NO |
| 4 | M | 18 | Father | N/A | NO | NO | NO |
| 5 | F | 66 | Father | Exon15 (codon 716) | Yes | CHRPE、DT | NO |
| 6 | F | 24 | Mother | Exon16 (codon 690) | NO | NO | NO |
| 7 | F | 59 | Father | Exon16 (codon 690) | NO | NO | NO |
| 8 | M | 18 | Father | Exon17 (codon 1114) | NO | NO | NO |
| 9 | M | 65 | unknown | N/A | NO | NO | NO |
| 10 | F | 51 | Mother | Exon16 (codon 1062) | Yes | NO | NO |
| 11 | M | 71 | Father | N/A | NO | NO | Yes |
| 12 | M | 44 | Mother | N/A | Yes | DT | NO |
| 13 | M | 19 | Father | N/A | NO | NO | NO |
| 14 | M | 57 | Mother | N/A | Yes | NO | NO |
| 15 | F | 32 | Mother | N/A | NO | NO | NO |
N/A, not available; EIM, extraintestinal manifestation; CHRPE, congenital hypertrophy of the retinal pigment epithelium; DT, Desmoid tumor
Primary end-point results
At the baseline level, there was no significant difference between the placebo group and the test group in the mean number and load of polyps in the designated areas (P > 0.05). After 1 year, there was still no significant difference between the two groups in the mean number and load of polyps in the descending part of the duodenum (P > 0.05). In contrast, the mean number and load of polyps in the area of nanocarbon-labeling and residual area after the operation were reduced in the test group compared with those in the placebo group (P < 0.05).
After 1 year, the mean number of polyps in the designated area of the placebo group had increased from the number detected at the baseline (P < 0.05), whereas the mean number in the test group had decreased (P < 0.05). The difference in the change in the mean number of polyps in the designated areas between the two groups was statistically significant compared with the baseline after 1 year (P < 0.001) (Fig. 2 and Table 2). After 1 year, the mean polyp load in the placebo group had increased from that detected at the baseline in the descending duodenum (P = 0.013) and in the nanocarbon-labeled and post-surgical residual regions (P = 0.066). In contrast, the mean polyp load in the test group had decreased significantly from that detected at the baseline in the indicated regions (P < 0.001). After 1 year, the difference in the change in the mean polyp load of the two groups in the designated region was statistically significant when compared to that detected at baseline (P < 0.001) (Fig. 3 and Table 3).
Fig. 2.
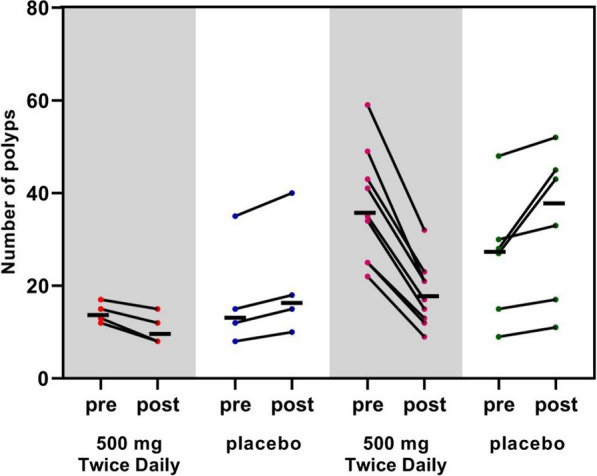
The number of polyps changes in Schematic diagram. Mean number of polyps (indicated by horizontal lines). The two groups on the left indicate changes in the number of polyps in the descending duodenal region; the two groups on the right indicate changes in the number of polyps in the nanocarbon-labeled and postoperative residual regions
Table 2.
Effect of metformin on the number of polyps at different segments
| Segment | Number of polyps at baseline | Number of polyps at 1 year | p value |
|---|---|---|---|
| Descending duodenum | |||
| Test group | 14.25 ± 2.22 | 10.75 ± 3.40 | 0.012 |
| Placebo | 17.5 ± 12.01 | 20.75 ± 13.25 | 0.014 |
| p value | 0.077 | 0.109 | |
| Carbon nanoparticles tattooing and Post-surgical residual areas | |||
| Test group | 37.00 ± 12.28 | 18.11 ± 6.99 | < 0.001 |
| Placebo | 26.17 ± 13.53 | 30.67 ± 14.71 | 0.012 |
| p value | 0.132 | 0.043 |
Fig. 3.
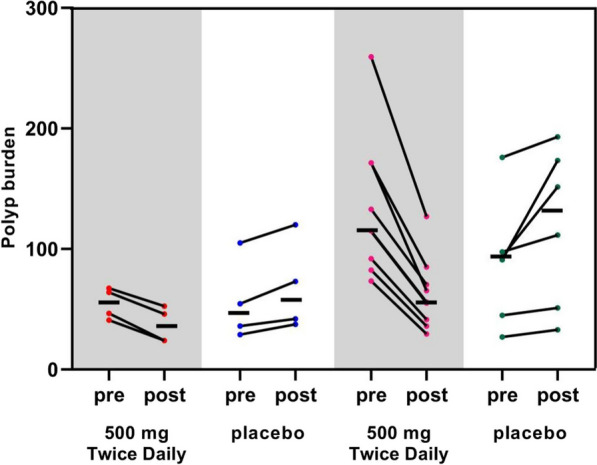
Polyps burden changes in Schematic diagram. Mean polyp load (indicated by horizontal line) in millimeters (mm). The two groups on the left indicate changes in polyp load in the descending duodenal region; the two groups on the right indicate changes in polyp load in the nanocarbon-labeled and postoperative residual regions
Table 3.
Effect of metformin on polyp loading at different segments
| Segment | Number of polyps at baseline | Number of polyps at 1 year | p value | |
|---|---|---|---|---|
| Descending duodenum | Test group | 54.75 ± 12.98 | 36.63 ± 14.82 | < 0.001 |
| Placebo | 56.13 ± 34.30 | 68.88 ± 37.35 | 0.013 | |
| p value | 0.943 | 0.160 | ||
| Carbon nanoparticles tattooing and Post-surgical residual areas | Test group | 134.72 ± 58.46 | 62.89 ± 29.73 | < 0.001 |
| Placebo | 88.33 ± 51.85 | 118.92 ± 65.69 | 0.066 | |
| p value | 0.140 | 0.041 |
Secondary end-point results
Cluster analysis of ASVs in the intestinal flora
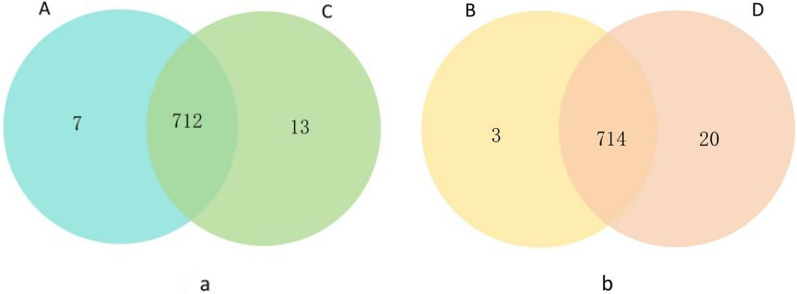
At the baseline level, 719 ASVs were identified in the placebo group (A) and 725 in test group (C). Of these ASVs, 712 were shared by the two groups, while seven were unique to the placebo group and 13 were unique to the test group (Fig. 4a). After 1 year, 717 ASVs were identified in the placebo group (B) and 734 in the test group (D). Of these ASVs, 714 were shared by the two groups, while three were unique to the placebo group and 20 were unique to the test group (Fig. 4b). These data revealed that metformin intervention did not significantly increase the number of ASVs.
Fig. 4.
Venn diagram. Note: Blue circles represent baseline levels in the placebo group, green circles represent baseline levels in the test group, yellow circles represent after 1 year in the placebo group, and orange circles represent after 1 year in the test group. Overlapping portions of different circles indicate ASVs sequences common to both groups. The numbers labeled in the figure represent the number of ASVs sequences in different sections
Relative abundance of species in the intestinal flora
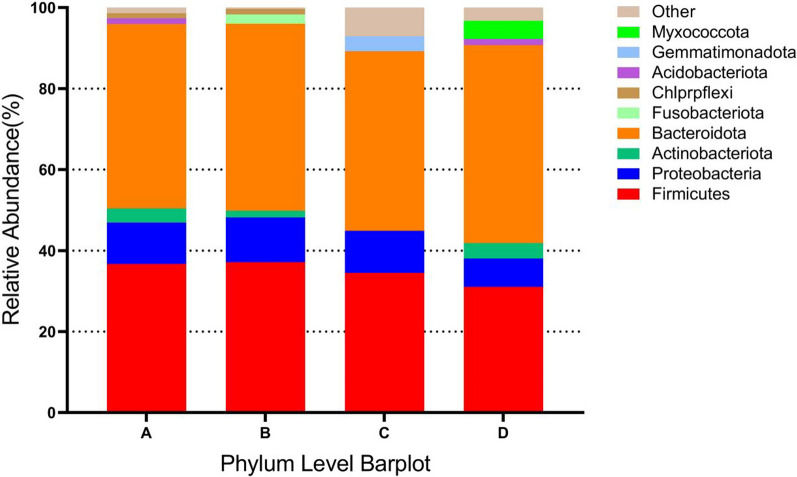
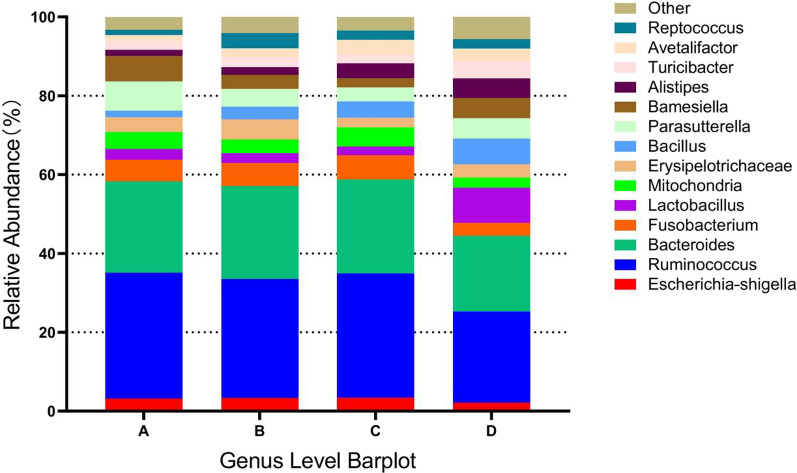
Based on the results of species annotation, the top 10 species with highest abundance at the phylum level for each subgroup were selected to generate the bar chart of species relative abundance shown in Fig. 5. Bacteroidetes, Firmicutes, and Proteobacteria accounted for the highest percentages of species in the two groups. Futhermore, the top 15 species with highest abundance at the genus level for each subgroup were selected to generate the bar chart of species relative abundance shown in Fig. 6. After 1 year of metformin administration, the relative abundance of g_Ruminococcus in the test group was lower than that at the baseline (23.14% vs. 31.46%, P < 0.05), whereas the relative abundance of g_Lactobacillus was higher than that at the baseline (8.91% vs. 2.26%, P < 0.05). Additionally, the relative abundances of g_Escherichia-shigella, g_Bacteroides, and g_Fusobacterium in the test group were lower than those at the baseline level (P > 0.05).
Fig. 5.
Relative abundance of species at the phylum level. Panel A represents the baseline level of the placebo group, panel B represents the placebo group after 1 year, panel C represents the baseline level of the test group, and panel D represents the test group after 1 year
Fig. 6.
Relative abundance of species at the genus level. Panel A represents the baseline level of the placebo group, panel B represents the placebo group after 1 year, panel C represents the baseline level of the test group, and panel D represents the test group after 1 year
Alpha diversity analysis of the intestinal flora
Analysis of the differences in the gut flora ɑ diversity index between the two groups (Table 4) suggested that the abundance as well as the diversity of gut flora species in the test group increased after 1 year of metformin administration compared to the baseline. This was evidenced by significantly higher values of Shannon’s index (P = 0.025) and Simpson’s index (P = 0.009) than those at the baseline.
Table 4.
Alpha diversity analysis of the gut microbiota
| Observed species | Chao1 | Shannon | Simpson | |
|---|---|---|---|---|
| At baseline | ||||
| Placebo | 822 | 405.3 | 6.614 | 0.902 |
| Test group | 810 | 420.7 | 6.098 | 0.908 |
| p value | 0.596 | 0.906 | 0.456 | 0.480 |
| At 1 year | ||||
| Placebo | 806 | 411 | 6.516 | 0.888 |
| Test group | 863 | 443 | 7.392 | 0.989 |
| p value | 0.289 | 0.814 | 0.025 | 0.009 |
Beta diversity analysis of the intestinal flora
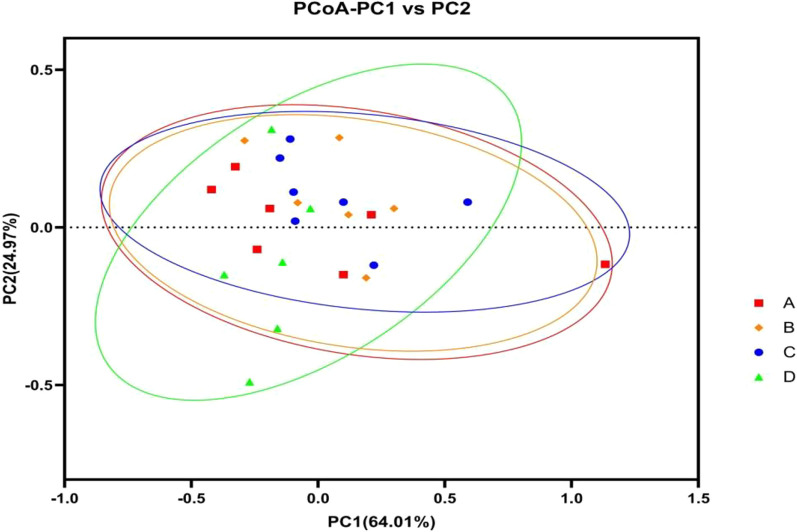
Weighted Unifrac Principal Coordinates Analysis (PCoA) analysis was performed to compare the species composition of the intestinal flora in FAP patients in both groups at baseline and after 1 year. The analysis revealed no significant trend of separation along PC1 or PC2, indicating a similarity in species composition. The horizontal coordinate (PC1) explained 64.01% of the differences, while the vertical coordinate (PC2) accounted for 24.97%. According to the analysis of similarities (ANOSIM), there was no significant difference in the species composition of the intestinal flora between the two groups (P > 0.05) (Fig. 7).
Fig. 7.
Beta diversity analysis of intestinal flora
Drug safety assessment of metformin
The differences in blood creatinine, BUN, TC, LDL, AST, ALT, HbA1c, body mass index (BMI), and homeostatic model assessment of insulin resistance (HOMA-IR) between the two groups were analyzed at baseline and after 1 year (Table 5). In the test group, there were no significant changes in these indexes after 1 year of metformin treatment compared to the baseline measurements (P > 0.05), with the HOMA-IR remaining constant during this period. Adverse events occurred in three patients (23.1%), all of which were classified as NCI-CTCAE grade 1, indicating mild severity (Table 6). These included abdominal pain, diarrhea, and rash. Notably, One patient withdrew from the study due to an adverse event (diarrhea).
Table 5.
Drug safety assessment of metformin
| Test group | Placebo | p value | |
|---|---|---|---|
| Creatinine (mg/dL) | |||
| At baseline | 72.92 | 67.73 | 0.126 |
| At 1 year | 72.5 | 69.22 | 0.352 |
| p value (baseline vs. 1 year) | 0.913 | 0.378 | |
| Blood urea nitrogen (mg/dL) | |||
| At baseline | 3.39 | 4.92 | 0.02 |
| At 1 year | 3.73 | 5.42 | 0.006 |
| p value (baseline vs. 1 year) | 0.229 | 0.575 | |
| Total cholesterol (mg/dL) | |||
| At baseline | 3.75 | 2.93 | 0.112 |
| At 1 year | 3.74 | 2.62 | 0.023 |
| p value (baseline vs. 1 year) | 0.978 | 0.66 | |
| LDL cholesterol (mg/dL) | |||
| At baseline | 2.06 | 2.26 | 0.768 |
| At 1 year | 2.21 | 2.34 | 0.988 |
| p value (baseline vs1 year) | 0.757 | 0.735 | |
| Aspartate aminotransferase (AST) | |||
| At baseline | 19.6 | 14.85 | 0.045 |
| At 1 year | 17.4 | 15.22 | 0.061 |
| p value (baseline vs. 1 year) | 0.377 | 0.886 | |
| Alanine aminotransferase (ALT) | |||
| At baseline | 33.19 | 15.02 | 0.089 |
| At 1 year | 18.29 | 16.47 | 0.336 |
| p value (baseline vs. 1 year) | 0.008 | 0.395 | |
| HbA1c (%) | |||
| At baseline | 5.53 | 5.45 | 0.494 |
| At 1 year | 5.49 | 5.55 | 0.558 |
| p value (baseline vs. 1 year) | 0.665 | 0.412 | |
| HOMA-IR | |||
| At baseline | 1.18 | 0.97 | 0.528 |
| At 1 year | 1.16 | 0.93 | 0.335 |
| p value (baseline vs. 1 year) | 0.968 | 0.826 | |
| BMI (Kg/m2) | |||
| At baseline | 23.10 | 21.7 | 0.491 |
| At 1 year | 22.16 | 21.57 | 0.745 |
| p value (baseline vs. 1 year) | 0.543 | 0.956 |
Data are mean (SD). HOMA = fasting blood × glucose fasting insulin/22.5
Table 6.
Adverse events
| Test group (n = 13) | Placebo (n = 13) | |
|---|---|---|
| Abdominal pain | 1 (7.7%) | 0 |
| Diarrhea | 1 (7.7%) | 0 |
| Rash | 1 (7.7%) | 0 |
Discussion
Many clinical trials have shown that chemopreventive drugs can inhibit the growth of adenomatous polyps in patients with FAP. However, their long-term use is often limited by side effects. Metformin, a well-known first-line antidiabetic drug, has also been recognized for its antitumor effects [26–28].
Previous studies have indicated that certain bacteria may promote the occurrence and development of CRC. Metformin is thought to exert its preventive and therapeutic effects on CRC by affecting the intestinal flora [29–31]. As research progresses, the significance of changes in the intestinal flora of FAP patients is becoming increasingly acknowledged. Dejea et al. reported that co-colonization by pks + E. coli and ETBF promoted the development and progression of cancer in FAP patients [3]. Futhermore, Kim et al. [32] observed that FAP patients had a significantly lower diversity of intestinal flora compared to healthy individuals, and also a higher ratio of thick-walled phyla to anaplasmatoid phyla, as well as greater relative abundance of metaplasmatoid phyla. Based on these finding, our hypothesis is that metformin could potentially inhibit the occurrence and development of adenomatous polyps in FAP by regulating the intestinal flora.
In this study, we found that after 1 year of metformin treatment, the mean number of polyps and the mean polyp load in the designated areas were significantly reduced in the test group compared with baseline (P < 0.05). Futhermore, both the mean number of polyps and the mean polyp load in the nanocarbon-labeled and postoperative residual areas were much lower in the test group than in the placebo group (P < 0.05). These results suggest that metformin may inhibit the incidence and development of adenomatous polyps in FAP patients. Our findings align with those of Higurashi et al., who reported that low-dose metformin administered over 1 year suppressed the development of heterochronic adenomas after polypectomy [19]. Conversely, a Korean study in randomized, double-blind clinical trial found no significant reduction in the mean number and size of colorectal or duodenal adenomas in FAP patients after 7 months of metformin treatment at two different doses [33]. The shorter duration of this trial could be a key reason for the observed, lack of adenoma regression in FAP patients, who may need a longer treatment period to see the effects similar to those in most cases of sporadic adenomas. Additionally, the small sample size of this trial may explain the limited clinical impact of the modest reductions in polyps number and size observed in FAP patients.
The flora assays conducted in this study indicated an increased diversity of the intestinal flora following metformin treatment compared to the baseline. Specifically, after 1 year of metformin administration, there was an increase in the relative abundance of Bacteroidetes, and a decrease in Firmicutes and Proteobacteria. In animal experiments, Liu et al. [34] found that the relative abundance of Bacteroidetes in the feces of the metformin-treated group increased compared with that of the normal control group, whereas the relative abundance of Firmicutes decreased. In a clinical trial, Yuan et al. [35] found that the relative abundance of Proteobacteria and Bacteroidetes in the feces of metformin-treated group increased, while the relative abundance of Firmicutes decreased in the metformin-treated group. These patterns mirror the changes in Firmicutes and Bacteroidetes seen in our study.
Morever, after 1 year of metformin administration, the relative abundances of g_Escherichia-shigella, g_Ruminococcus, g_Bacteroides, and g_Fusobacterium in the test group decreased from the baseline, whereas the relative abundance of g_Lactobacillus increased. Liu et al. [36] also found that metformin restored the biodiversity of the intestinal flora in IBD mice, leading to a decreased abundance of g_Escherichia-shigella and increased abundances of g_Lactobacillus, and g_Akkermansia. Additionally, it was reported that metformin significantly reduced the relative abundance of g_Bacteroides in the feces of mice and inhibited CRC development [31].
Futhermore, several studies have indicated that Fusobacterium nucleatum (F. nucleatum) could promote the occurrence and development of CRC [37–40], while metformin has been shown to alleviate symptoms and inhibit the formation of intestinal tumors in F. nucleatum-administered APCMin/ + mice [28]. Additionally, g_Ruminococcus has been increasingly recognized for its connection to metabolic diseases [41]. This evidence collectively suggests that metformin may contribute to restoring species diversity in the intestinal flora and inhibits the development and progression of adenomatous polyps in patients with FAP by altering the relative abundance of specific genera.
However, this study has several limitations. Firstly, the limited sample size, comprising a small number of families, may not fully capture the chemopreventive effect of metformin in different groups. Secondly, regarding the design of the study endpoints, we focused only on the changes induced by metformin on polyp number and load. For FAP chemoprevention, more critical endpoints might include delaying major surgery, endoscopic resection of advanced adenomas, the emergence of highly atypical hyperplasia in rectal or ileal reservoirs, and duodenal disease progression [42]. The relatively brief duration of our study also limits insights into the long-term impacts of metformin on FAP patients. Lastly, the absence of fecal collection in our study precluded a comparative analysis of metformin's effects on mucosal and fecal flora. Future studies, incorporating a larger cohort and extended trial periods, are essential to more definitively ascertain metformin's chemopreventive efficacy in FAP patients.
Conclusion
We conclude that 1-year metformin therapy for FAP is both safe and effective, potentially exerting its effects by modulating the intestinal flora. This study not only offers novel insights and approaches for preventing adenomatous polyp carcinogenesis in FAP, but also sheds light on the possible mechanisms underlying such preventive actions.
Acknowledgements
Not applicable.
Abbreviations
- FAP
Familial adenomatous polyposis
- CRC
Colorectal cancer
- APC
Adenomatous polyposis coli
- mTOR
Mammalian target of rapamycin
- pks + E. coli
Polyketide synthase-positive Escherichia coli
- ETBF
Enterotoxin-producing Bacteroides fragilis
- ACF
Rectal abnormal crypt foci
- NSAIDS
Nonsteroidal anti-inflammatory drugs
- IBD
Inflammatory bowel disease
- IRA
Ileo-rectal anastomosis
- IPAA
Ileal-pouch-anal-anastomosis
- ASVs
Amplicon sequence variants
- SD
Standard deviation
- FBG
Fasting blood glucose
- FI
Fasting insulin
- HbA1c
Glycosylated hemoglobin
- BMI
Body mass index
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- BUN
Blood urea nitrogen
- TC
Total cholesterol
- LDL
Low-density lipoprotein
- HOMA-IR
Homeostatic model assessment of insulin resistance
- CHRPE
Congenital hypertrophy of the retinal pigment epithelium
- DT
Desmoid tumor
- PCoA
Rincipal coordinates analysis
- ANOSIM
Analysis of similarities
- F. nucleatum
Fusobacterium nucleatum
Author contributions
LXZ and LFZ designed and directed the study, and were also involved in writing and editing the manuscript. WW and DL contributed substantial intellectual discussion and critical reading of the manuscript. LFZ, BX, and ZY performed endoscopy, collected intestinal mucosa samples, and gathered data. LXZ prepared all figures, provided biological expertise, and played a key role in writing and editing the manuscript. All authors have approved the final version of the manuscript.
Funding
This project was supported in part by the Foreign Cooperation Project of the Department of Science and Technology of Fujian Province (2022I0033), Natural Science Foundation of Fujian Province (2023J011358), and Intra-hospital Project at the 900th Hospital of Joint Logistic Support Force (2020L07).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved the Ethics Committee of the 900th Hospital of Joint Logistic Support Force (Lundquist No. 2023-025). All patients provided informed consent before endoscopic examination or intestinal mucosa sampling.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Linxin Zhou and Linfu Zheng contributed equally to this project and should be regarded as co-first authors.
Contributor Information
Dazhou Li, Email: LDZ7302999@sina.com.
Wen Wang, Email: wangwenfj@163.com.
References
- 1.Kinzler KW, Nilbert MC, Vogelstein B, et al. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251(4999):1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 2.Liang S, Mao Y, Liao M, Xu Y, Chen Y, Huang X, Wei C, Wu C, Wang Q, Pan X, Tang W. Gut microbiome associated with APC gene mutation in patients with intestinal adenomatous polyps. Int J Biol Sci. 2020;16:135–146. doi: 10.7150/ijbs.37399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359:592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attard TM, Septer S, Lawson CE, Attard MI, Lee STM, Umar S. Microbiome insights into pediatric familial adenomatous polyposis. Orphanet J Rare Dis. 2022;17(1):416. doi: 10.1186/s13023-022-02569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) Gut. 2020;69(3):411–444. doi: 10.1136/gutjnl-2019-319915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 7.Burke CA, Phillips R, Berger MF, et al. Children’s international polyposis (CHIP) study: a randomized, double-blind, placebo-controlled study of celecoxib in children with familial adenomatous polyposis. Clin Exp Gastroenterol. 2017;10:177–185. doi: 10.2147/CEG.S121841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burn J, Bishop DT, Chapman PD, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011;4(5):655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giardiello FM, Hamilton SR, Hylind LM, et al. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57(2):199–201. [PubMed] [Google Scholar]
- 10.Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1(1):32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rad E, Murray JT, Tee AR. Oncogenic signalling through mechanistic target of rapamycin (mTOR): a driver ofmetabolic transformation and cancer progression. Cancers (Basel) 2018;10(1):5. doi: 10.3390/cancers10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faller WJ, Jackson TJ, Knight JR, et al. mTORC1-mediated translational elongation limits intestinal tumourinitiation and growth. Nature. 2015;517(7535):497–500. doi: 10.1038/nature13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuksekkaya H, Yucel A, Gumus M, et al. Familial adenomatous polyposis; succesful use of sirolimus. AmJ Gastroenterol. 2016;111(7):1040–1041. doi: 10.1038/ajg.2016.159. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa H, Wakabayashi K, Suzuki S, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med. 2013;2(1):50–56. doi: 10.1002/cam4.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 17.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 18.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3(9):1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 19.Higurashi T, Hosono K, Takahashi H, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–483. doi: 10.1016/S1470-2045(15)00565-3. [DOI] [PubMed] [Google Scholar]
- 20.Dazhou LI. Chemopreventive effect of metformin on colorectal cancer. Naval Medical University; 2016. [Google Scholar]
- 21.Gui-lin XU, Dazhou LI, Zhou YE, et al. The effect of metformin combined with endoscopy in the treatment of familial adenomatous polyposis. Chin J Clin Gastroenterol. 2020;32(02):84–89. [Google Scholar]
- 22.Dazhou LI, Wen WANG, Binbin XU, et al. Chemopreventive mechanism of metformin or celecoxib on chemically induced colorectal aberrant crypt foci in mice. J Fujian Med Univ. 2016;50(03):163–166. [Google Scholar]
- 23.Zhang X, Zhao Y, Xu J, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 26.Fujimori T, Kato K, Fujihara S, et al. Antitumor effect of metformin on cholangiocarcinoma: in vitro and in vivo studies. Oncol Rep. 2015;34(6):2987–2996. doi: 10.3892/or.2015.4284. [DOI] [PubMed] [Google Scholar]
- 27.Gagnaire A, Nadel B, Raoult D, Neefjes J, Gorvel JP. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat Rev Microbiol. 2017;15(2):109–128. doi: 10.1038/nrmicro.2016.171. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Hong X, Wang J, et al. Metformin elicits antitumour effect by modulation of the gut microbiota and rescues Fusobacterium nucleatum-induced colorectal tumourigenesis. EBioMedicine. 2020;61:103037. doi: 10.1016/j.ebiom.2020.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan L, Zeng X, Xu G. Metformin regulates gut microbiota abundance to suppress M2 skewing of macrophages and colorectal tumorigenesis in mice. J Microbiol. 2023;61(1):109–120. doi: 10.1007/s12275-022-00010-8. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Kim YJ, Oh GM, Jung W, Park SJ. How is gut microbiome of patients with familial adenomatous polyposis different from healthy people? Medicine (Baltimore) 2022;101(49):e32194. doi: 10.1097/MD.0000000000032194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato K, Gong J, Iwama H, et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11(3):549–560. doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 32.Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99(11):2136–2141. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JJ, Kim BC, Hong SP, et al. The effect of metformin in treatment of adenomas in patients with familial adenomatous polyposis. Cancer Prev Res (Phila) 2021;14(5):563–572. doi: 10.1158/1940-6207.CAPR-20-0580. [DOI] [PubMed] [Google Scholar]
- 34.Yuzhao LIU, Yingshan SUN, Yangang WANG, et al. Effects of metformin on serum homocysteine level and intestinal flora in diabetes rats. Shandong Med J. 2023;63(07):50–54. [Google Scholar]
- 35.Fengyi Y, Zhun S, Fangming Y, et al. Impacts of Metformin treatment on the gut microbiota in newly⁃diagnosed type 2 diabetes mellitus patients. Chin J Diabetes. 2022;30(09):672–677. [Google Scholar]
- 36.Liu Z, Liao W, Zhang Z, et al. Metformin affects gut microbiota composition and diversity associated with amelioration of dextran sulfate sodium-induced colitis in mice. Front Pharmacol. 2021;12:640347. doi: 10.3389/fphar.2021.640347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ternes D, Tsenkova M, Pozdeev VI, et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metab. 2022;4(4):458–475. doi: 10.1038/s42255-022-00558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu NN, Jiao N, Tan JC, et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat Microbiol. 2022;7(2):238–250. doi: 10.1038/s41564-021-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu X, Yin F, Pei M, et al. Modulation of intratumoral fusobacterium nucleatum to enhance sonodynamic therapy for colorectal cancer with reduced phototoxic skin injury. ACS Nano. 2023;17(12):11466–11480. doi: 10.1021/acsnano.3c01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duggan WP, Salvucci M, Kisakol B, et al. Increased Fusobacterium tumoural abundance affects immunogenicity in mucinous colorectal cancer and may be associated with improved clinical outcome. J Mol Med (Berl) 2023;101(7):829–841. doi: 10.1007/s00109-023-02324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grahnemo L, Nethander M, Coward E, et al. Cross-sectional associations between the gut microbe Ruminococcus gnavus and features of the metabolic syndrome [published correction appears in Lancet Diabetes Endocrinol. 2022 Sep;10(9):e9]. Lancet Diabetes Endocrinol. 2022;10(7):481–483. [DOI] [PubMed]
- 42.Burke CA, Dekker E, Lynch P, et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med. 2020;383(11):1028–1039. doi: 10.1056/NEJMoa1916063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].