Abstract
Background:
The present study aims to determine the possible low dose-dependent adverse effects of 2.45 GHz microwave exposure and Wi-Fi frequency on the cochlea.
Methods:
Twelve pregnant female rats (n = 12) and their male newborns were exposed to Wi-Fi frequencies with varying electric field values of 0.6, 1.9, 5, 10 V/m, and 15 V/m during the 21-day gestation period and 45 days after birth, except for the control group. Auditory brainstem response testing was performed before exposure and sacrification. After removal of the cochlea, histopathological examination was conducted by immunohistochemistry methods using caspase (cysteine-aspartic proteases, cysteine aspartates, or cysteine-dependent aspartate-directed proteases)-3, -9, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Kruskal–Wallis and Wilcoxon tests and multivariate analysis of variance were used.
Results:
Auditory brainstem response thresholds in postexposure tests increased statistically significantly at 5 V/m and above doses. When the number of apoptotic cells was compared in immunohistochemistry examination, significant differences were found at 10 V/m and 15 V/m doses (F (5,15) = 23.203, P = .001; Pillai’s trace = 1.912, η 2 = 0.637). As the magnitude of the electric field increased, all histopathological indicators of apoptosis increased. The most significant effect was noted on caspase-9 staining (η 2c9 = 0.996), followed by caspase-3 (η 2c3 = 0.991), and TUNEL staining (η 2t = 0.801). Caspase-3, caspase-9, and TUNEL-stained cell densities increased directly by increasing the electric field and power values.
Conclusion:
Apoptosis and immune activity in the cochlea depend on the electric field and power value. Even at low doses, the electromagnetic field in Wi-Fi frequency damages the inner ear and causes apoptosis.
Keywords: Electromagnetic field, cochlea, wireless technology, cochlear disease, apoptosis
Main Points
This is the first research to evaluate the effects of different doses of MW radiation on cochlea.
Apoptosis in the inner ear increases with a rise in electromagnetic field. Wi-Fi has ototoxic effect which is dose dependent.
In the Corti organ, 2.45 GHz microwave radiation causes an increase in the expression of apoptotic markers.
ABR thresholds showed no change at low doses, but at dosages of 5 V/m and above, the thresholds were significantly increased.
Introduction
Radiofrequency (RF) and microwave (MW) radiation used in wireless communication can harm biological systems.1 Wireless local area networks (such as Wi-Fi) operate at a frequency of 2.45 GHz MW is an electromagnetic wave that operates between 30 MHz and 300 GHz frequency and generates electromagnetic field (EMF) in near region.2-4 Modern electronic equipment, such as Bluetooth headphones, laptop computers, mobile phones, radars, and receivers, all communicate using MW. Besides, a cochlear implant system consists of an internal implant and an external speech processor that uses different frequency emitters of 900 MHz, 1.8 GHz, and 2.45 GHz to provide hearing for people with profound sensorineural hearing loss.5 The effect of these devices, which are implanted directly into the hearing organ, on residual hearing is also an issue that will be raised. This is also well established, as MW has been found to damage cellular and molecular processes in living organisms.3 Due to its susceptibility to heating, paying particular attention to specific absorbed rates (SARs) in certain body organs such as the brain, ears, eyes, salivary glands, and skin is recommended.5 The SAR value indicates the amount of energy that causes the thermal response on the tissue caused by RF/MW exposure. Microwaves can generate heat in tissues. It may also affect cell proliferation, cell cycle progression, and DNA replication via unknown nonthermal mechanisms.6 It is stated that there are ear region warmings in power in theoretical cochlear SAR estimates using numerical modeling on a close implant system in a human model.7 Accordingly, it has been discovered that a biological impact known as the MW auditory effect occurs between 915 and 2450 MHz in the auditory system’s auditory pathway.8 Histological studies are one of the most reliable ways for determining the cochlea’s adverse effects of MW. Caspases (cysteine-aspartic proteases, cysteine aspartates, or cysteine-dependent aspartate-directed proteases) are critical regulators of programmed cell death (apoptosis). Previously, it was demonstrated that MW radiation triggered apoptosis in brain cells via the classical mitochondria-dependent caspase-3.9 Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) is used to identify DNA breaks generated during the last stage of apoptosis when DNA fragmentation occurs.10 In our study, we used these biomarkers to assess apoptosis in the inner ear of exposed rats.
Due to the way mobile phones and Bluetooth headphones are used, they are frequently positioned close to the ear for several hours daily. With the widespread usage of communication devices today, there is compelling evidence that the adverse effects of MW radiation released on the hearing organ should be investigated. The ear can be the leading site of damage from these devices because of its delicate structure. The organ of Corti was investigated following experimental MW exposure due to its possible vulnerability to MW radiation. Pregnant women, neonates, and children all have a high water content during their growth cycles, making them especially susceptible to MW radiation.10
We investigated the dose-dependent effects on rats exposed to Wi-Fi frequency at low electric field doses, beginning on the first day of pregnancy (intrauterine 21 days and postnatal 45 days). Therefore, current research aims to demonstrate possible effects of Wi-Fi frequency at low doses on the inner ear in young rats during their developmental period by audiological and histopathological evaluation using auditory brainstem response (ABR) testing, immunohistochemistry, and TUNEL method, respectively.
Material and Methods
Animal Care and Groups
Twelve pregnant Wistar rats and male rats born of them in the pubertal period from the Ondokuz Mayıs University Animal Research Centre [the Institutional Ethical Board for Animal Experiments (Approval No: OMUHADYEK 2019/23)] were used for the experimental study. At the experimental animal facility, the rats were kept in a 12-hour night/day cycle by the circadian rhythm in a room with 24-25°C temperature and a humidity of 40%-50%. All of the study group rats had free access to conventional food and tap water. Before mating, 12 adult female rats were randomly separated into 6 groups of 2. Twelve adult female rats with vaginal plugs were accepted as 0 days pregnant after mating day. In the control group, pregnant rats were not subjected to any electrical field. Pregnant rats in the other 5 groups were kept at different electric field values during the 21-day gestation period. At the end of 21 days, control group rats and 5 exposed groups of pregnant rats, about 1-3 day, gave birth to newborn rats. The newborn males in exposed groups were subjected to 2.45 GHz MW radiation for 1 hour a day for 45 days in their electric field values of their groups after birth. The tympanic membranes and external auditory ear canals of included pubertal term male rats were examined by a microscope before sacrification. Animals with tympanic membrane perforation and acute or chronic otitis externa/media were excluded. The histological study rats were grouped as follows:
Control group rats (2 pregnant and 7 male rats born of them).
0.6 V/m (2 pregnant and 6 male rats born of them).
1.9 V/m (2 pregnant and 5 male rats born of them).
5 V/m (2 pregnant and 6 male rats born of them).
10 V/m (2 pregnant and 6 male rats born of them).
15 V/m (2 pregnant and 6 male rats born of them).
Exposure System and Design
In this study, a 2004X-RF Wi-Fi system generator with a monopole antenna and a 0-1 Watt max out (Everest Global System, Turkey) was used to generate different electric field doses at Wi-Fi frequency, 2.45 GHz MW radiation. Before starting the study, reflections and electric field values in the environment were determined with an RF Explorer device. The environment’s electric and magnetic field values range from 0.001 to 0.18 V/m and from 0.0011 to 0.095 A/m, respectively. Pregnant rats of exposed groups and male rats born of pregnant rats were exposed to 2.45 GHz MW radiation in a 6-section circular pie-cage restrainer with a monopole antenna in the middle. Electric field measurements during exposure were taken daily by the Department of Biophysics, and changes in the electric field were prevented. During electric field exposure, electric field measurements for 21 days were taken from the head and back parts of each pregnant rat for 6 minutes, according to the Information Technologies Authority and International Commission on Non-Ionizing Radiation Protection regulations. Newborn rats were exposed to 2.45 GHz MW radiation for 45 days with their mothers for 15 days and individually after 15 days.
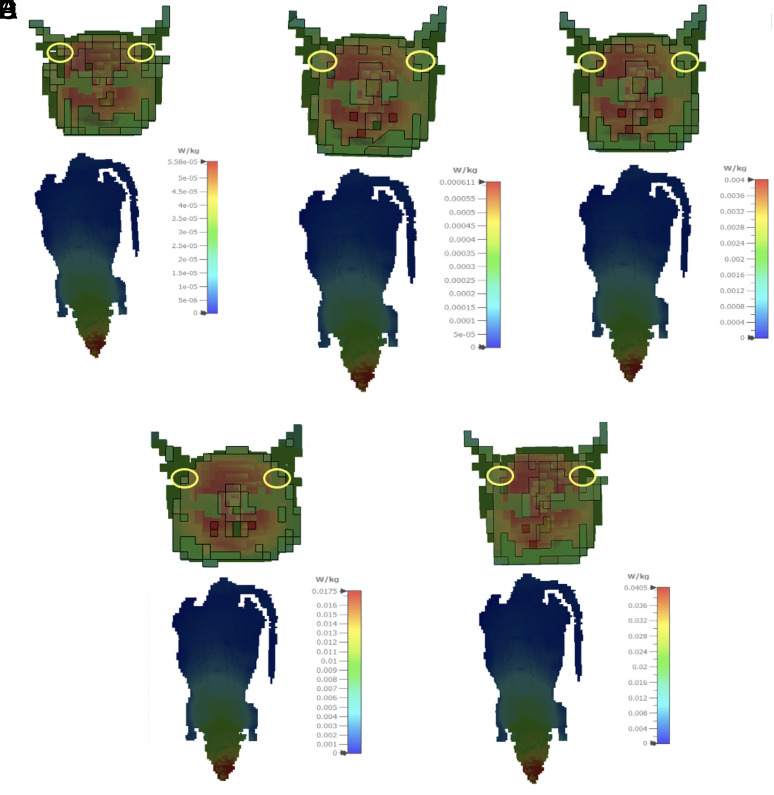
The EMF values of exposed groups were created using the electromagnetic simulation software Computer Simulation Technology (CST) STUDIO SUITE for the specific absorbed rate (SAR) calculation of exposed groups. Simulations in this EMF solver are based on the finite integration technique. Each rat tissue dielectric properties were altered according to the Cole-Cole model in the 2.45 GHz computations, and the SAR (10 g) values were produced. The SAR (10 g) simulation results are given in W/kg for electric field values of 0.6, 1.9, 5, 10, and 15 V/m (Figure 1).
Figure 1.
Posterior coronal section of the cochlea and whole body specific absorption rate distributions (10 g average) in 0.6 V/m EF (A), 1.9 V/m EF (B), 5 V/m EF (C), 10 V/m EF (D), and 15 V/m EF (E).
In the simulations, gamma/4 monople antenna with 2.45 GHz frequency was designed to be used as a signal generator. The specified electric field values were obtained at power values of 0.0845 mW, 0.9245 mW, 6.05 mW, 26.45 mW, and 61.25 mW using the antenna designed in the CST program, respectively. It is ensured that equal radio power is radiated in all directions due to the omnidirectional feature of the monopole antenna, which is positioned at the center point of the circular pie-cage restrainer as in the laboratory environment. For exposed groups, the average SAR values of local cochlear recovery obtained using the standard Institute of Electrical and Electronics Engineers/International Electrotechnical Commission 62704-1 were 0.325 mW/kg, 0.4 mW/kg, 2.3 mW/kg, 12 mW/kg, and 32 mW/kg, respectively.
Audiological Evaluation
Before the rats were exposed to MW radiation at different electric field values, ear examinations and audiologic tests were applied. The Neuro-Audio (Neurosoft, Ivanovo, Russia) was used to assess ABR in a silent room with a noise level of no more than 50 dB (ambient noise). Hearing screening probes for infant rats were used. To record the ABR, the subcutaneous needle electrodes were put in the vertex, the ipsilateral, and the contralateral mastoid regions. The click stimulus was delivered via headphones. The filter constituted of 100-3000 Hz. The repetition rate and the time window was set to 21.1/s and 15 ms. After hearing thresholds were measured starting at 80 dB SPL and decreasing by 10 dB, signal averaging of a total of 2000 samples was taken. When the threshold was close, 5 dB modifications were used to find the exact threshold. The ABR thresholds were determined by defining the lowest intensity at which wave II was obtained.
Histopathological Examination
At the end of 45 days after birth, all-male rats were euthanized with intraperitoneal administration of 5 mg/kg xylazine and 40 mg/kg ketamine hydrochloride. Then the rats were decapitated, and the ears and bullas were removed and transferred to 4% paraformaldehyde. The removed tissues were fixed for 24 hours in neutral formalin; after that, they were immersed to facilitate osseous tissue decalcification with Ethylenediamine Tetraacetic Acid (Sigma-Aldrich, Burlington, Massachusetts, USA) solution, 0.1 mol/L, for 3 weeks. This process was followed by washing with flowing water over night. After dehydrating in a graded ethanol series and clearing in xylene, cleaned tissues were processed by embedding in paraffin wax according to established standardized methods, and the cochlea basal turn was examined. In total, 72 ears of 36 rats were analyzed histopathologically.
Immunohistochemistry
After tissue paraffin blocks were sliced as 5 µm sections, they were stained with the caspase-3 and the caspase-9 primary antibodies (1 : 100, LabVision, Bucharest, Romania) for evaluation by using immunohistochemical techniques. 3-amino-9-ethylcarbazole (AEC) was also used to label the samples, and Mayer’s hematoxylin was used to stain the background which was then coated with a mounting medium. The slices examined via a light microscope (Olympus Model: CX41, Tokyo, Japan) were achieved from the cochlea basal turn. The control samples were processed the same way as the test samples. But the incubation time with the primary antibody was omitted. The immunolabeling scores were determined independently by 2 observers blinded to the data. The staining intensity on the slices with their immunohistochemical protocol was scored semi-quantitatively. The Histochemical Scoring (HSCORE) was evaluated by the equation HSCORE = Σ Pi (I + 1). In equation of the HSCORE, Pi = the percentage of staining cells for each severity, and I = 1 (weak), 2 (moderate), and 3 (strong) is the staining intensity.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling method
According to the manufacturer’s instructions, rehydrated and deparaffinized sections were stained with a commercial kit (Apoptag, S7101, Chemicon, Calif, USA). A CX41 bright-field microscope was used to examine the TUNEL-stained slices (Olympus, Model: CX41, Tokyo, Japan). The TUNEL scores were measured independently by 2 researchers blinded to the experimental information. The positive reactive cell number in all TUNEL-stained slices was accounted and was analyzed from apical to basal switch. The TUNEL reactive cells were accounted in randomly selected fields per case to calculate the average apoptotic cell number. In each case, 100 cells were determined to be TUNEL positive/negative, and the percentage of TUNEL reactive cell number was assigned. All histopathological specimens were assessed by 2 expert pathologists. In evaluation, cells in regions with necrosis, those placed in borders, or poor morphology were excluded.
Statistical Analysis
The data obtained in this study were analyzed using R Studio software version 1.2.5019 (RStudio, PBC, Boston, MA, USA). Whether the data were normally distributed was analyzed by the Shapiro–Wilk test. The analysis results were presented as mean, SD, or median (minimum–maximum). If the variables were normally distributed, parametric tests were used; otherwise, nonparametric tests were used. The Kruskal–Wallis test was used to compare groups, and the Dunn test was used for post hoc pairwise comparisons. The Wilcoxon signed rank tests were applied to evaluate the differences in ABR thresholds before and after EMF exposure. Multivariate analysis of variance (MANOVA) was used to compare groups in a single analysis and to reduce type-1 error in histopathological data. The Levene’s test and the Box test were employed to determine variance homogeneity. The Gamess–Howell test was used for post hoc pairwise comparisons. P-values of <.05 were regarded as statistically significant.
Results
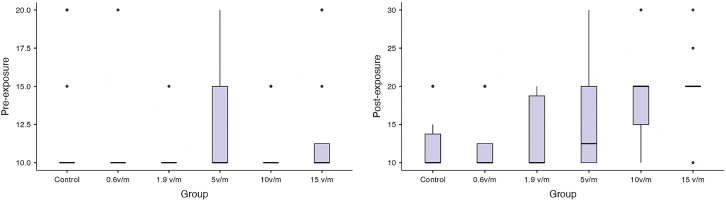
There were no significant differences between preexposure and postexposure measurements for control, 0.6 V/m, 1.9 V/m, and 5 V/m groups (P-values > .05). There was a significant increase in postexposure thresholds in the 10 V/m and 15 V/m groups (Wilcoxon test; P = .005 and P = .007, respectively). In preexposure measurements, no statically significant difference was obtained between groups, indicating that ABR thresholds were homogeneous. When postexposure ABR latencies were examined within groups, no statistically significant difference was found between 0.6 V/m, 1.9 V/m, and 5 V/m groups. However, ABR thresholds were elevated statistically significantly at doses of 5 V/m and above (Kruskal–Wallis test; P < .001) (Figure 2). Table 1 summarizes the intra- and intergroup comparisons of preexposure and postexposure measurements.
Figure 2.
Comparison of preexposure and postexposure auditory brainstem response thresholds between groups. Boxes indicate the first and third quartiles, and median observations are denoted by a line in each box.
Table 1.
Comparison of Preexposure and Postexposure Auditory Brainstem Response Threshold Measurements
| Group | Preexposure ABR Threshold |
Postexposure ABR Threshold |
P 2 | |
|---|---|---|---|---|
| Control | Mean + SD | 11.8 ± 3.7 | 12.1 ± 3.8 | .752 |
| Median (minimum–maximum) | 10 (10-20) | 10 (10-20)a | ||
| 0.6 V/m | Mean + SD | 10.8 ± 2.9 | 12.5 ± 4.5 | .346 |
| Median (minimum–maximum) | 10 (10-20) | 10 (10-20)a | ||
| 1.9 V/m | Mean + SD | 10.5 ± 1.6 | 13.5 ± 4.7 | .129 |
| Median (minimum–maximum) | 10 (10-15) | 10 (10-20)a | ||
| 5 V/m | Mean + SD | 12.5 ± 3.9 | 15.0 ± 6.4 | .193 |
| Median (minimum–maximum) | 10 (10-20) | 12.5 (10-30)ab | ||
| 10 V/m | Mean + SD | 10.8 ± 1.9 | 18.3 ± 5.4 | .005 |
| Median (minimum–maximum) | 10 (10-15) | 20 (10-30)b | ||
| 15 V/m | Mean + SD | 12.1 ± 3.9 | 20.4 ± 4.5 | .007 |
| Median (minimum–maximum) | 10 (10-20) | 20 (10-30)b | ||
| P 1 | .559 | <.001 |
Bold values in P-value row indicate statistically significant difference.
ABR, auditory brainstem response; V/m, volt/meter.
1Comparison of preexposure measurements and postexposure measurements between groups (Kruskal–Wallis test with Dunn test).
2Comparison of preexposure and postexposure thresholds in each group (Wilcoxon signed rank test).
a-b There is no statistically significant difference in the comparison of post-exposure thresholds between groups with the same letter.
When the apoptotic cell number was compared by caspase-3, -9, and TUNEL methods (MANOVA model [F (5,15) = 23.203, P < .001; Pillai’s trace = 1.912, η 2 = 0.637)], a significant difference was found in comparison between the groups. Table 2 demonstrates the descriptive statistics and intergroup comparisons as MANOVA results are summarized in Table 3. All histopathological indices of apoptosis increased as the magnitude of the electric field increased. The greatest impact was determined on the caspase-9 staining (η 2c9 = 0 .996), followed by caspase-3 (η 2c3 = 0.991), and TUNEL staining (η 2t = 0 .801).
Table 2.
Caspase-3, Caspase-9, and TUNEL-Stained Apoptotic Cells Between Groups
| Group | Caspase-3 Mean (SD) |
Caspase-9 Mean (SD) |
TUNEL Mean (SD) |
|---|---|---|---|
| Control (n = 14) | 33.4 (3.95)a | 28.5 (3.06)a | 2.7 (1.27)a |
| 0.6 V/m EF (n = 12) | 46.6 (3.55)b | 36.3 (3.10)b | 4.3 (1.60)ab |
| 1.9 V/m EF (n = 10) | 61.0 (5.54)c | 42.1 (3.06)c | 5.8 (2.04)b |
| 5 V/m EF (n = 12) | 103.1 (4.91)d | 56.7 (3.08)d | 8.2 (2.21)bc |
| 10 V/m EF (n = 12) | 123.8 (6.70)e | 79.8 (3.22)e | 10.6 (2.16)c |
| 15 V/m EF (n = 12) | 199.2 (8.85)f | 160.4 (3.23)f | 16.8 (4.39)d |
EF, electrical field; n, number; V/m, volt/meter.
a-fEach different superscript letter denotes a statistically significant difference in intergroup comparisons (post hoc Games–Howell test).
Table 3.
Multiple Analysis of Variance Results
| Stain | Type III Sum of Squares | F | P | η 2 |
|---|---|---|---|---|
| Caspase-3 | 233741.605 | 1378.402 | <.001 | 0.991 |
| Caspase-9 | 147816.993 | 3036.829 | <.001 | 0.996 |
| TUNEL | 1612.654 | 53.046 | <.001 | 0.801 |
F (5,15) = 23.203, Pillai’s trace = 1.912, P < .001.
η 2, partial eta square; F, test statistics; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Increased staining of caspase-3 and caspase-9 from the control group to 15 V/m was identified. In other words, while the weakest staining was in the control group, the most significant staining was in the 15 V/m groups.
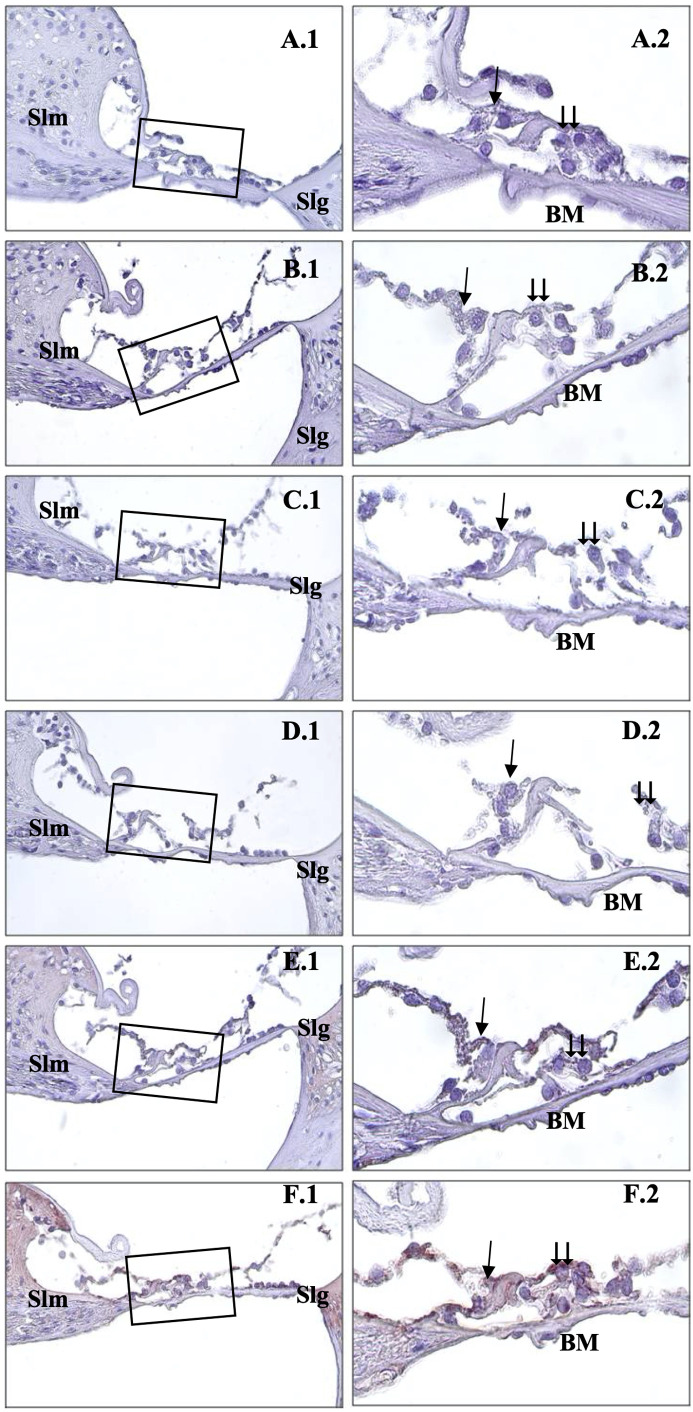
As the electric field value increased, the density of caspase-3-stained cells increased in direct proportion (Figure 3). For caspase-3 immunostaining, weak expression was detected in the control group’s organ of Corti (Figure 3A). In the 0.6 V/m, the immune reaction was weak but more intense than in the control group (Figure 3B). Caspase-3 reactions were more substantial in the 1.9 V/m group than in the 0.6 V/m group (Figure 3C). In 5 V/m and 10 V/m groups, the immune reaction was strong in the outer hair cells (Figure 3D, and 3E). It was noted that the strongest caspase-3 staining was observed in all organ of Corti cells of the 15 V/m group (Figure 3F).
Figure 3.
. Immunohistochemical staining of cochlea with caspase-3. →: inner hair cells, ⇉: outer hair cells, Slm: spiral limbus, Slg: spiral ligament, BM: basillary membrane, Control (A), 0.6 V/m EF (B), 1.9 V/m EF (C), 5 V/m EF (D), 10 V/m EF (E), 15 V/m EF (F). Organ of Corti ×40 (1) and organ of Corti ×100 (2). Mayers hematoxylin base staining.
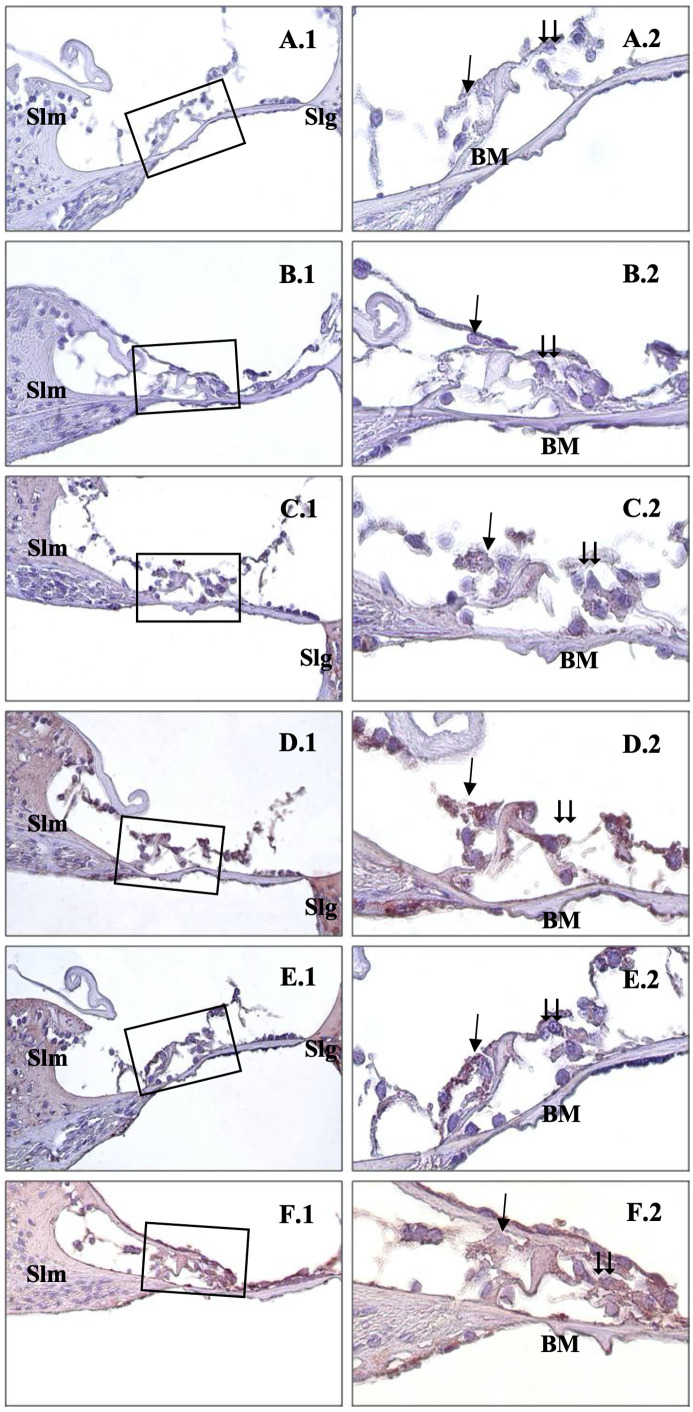
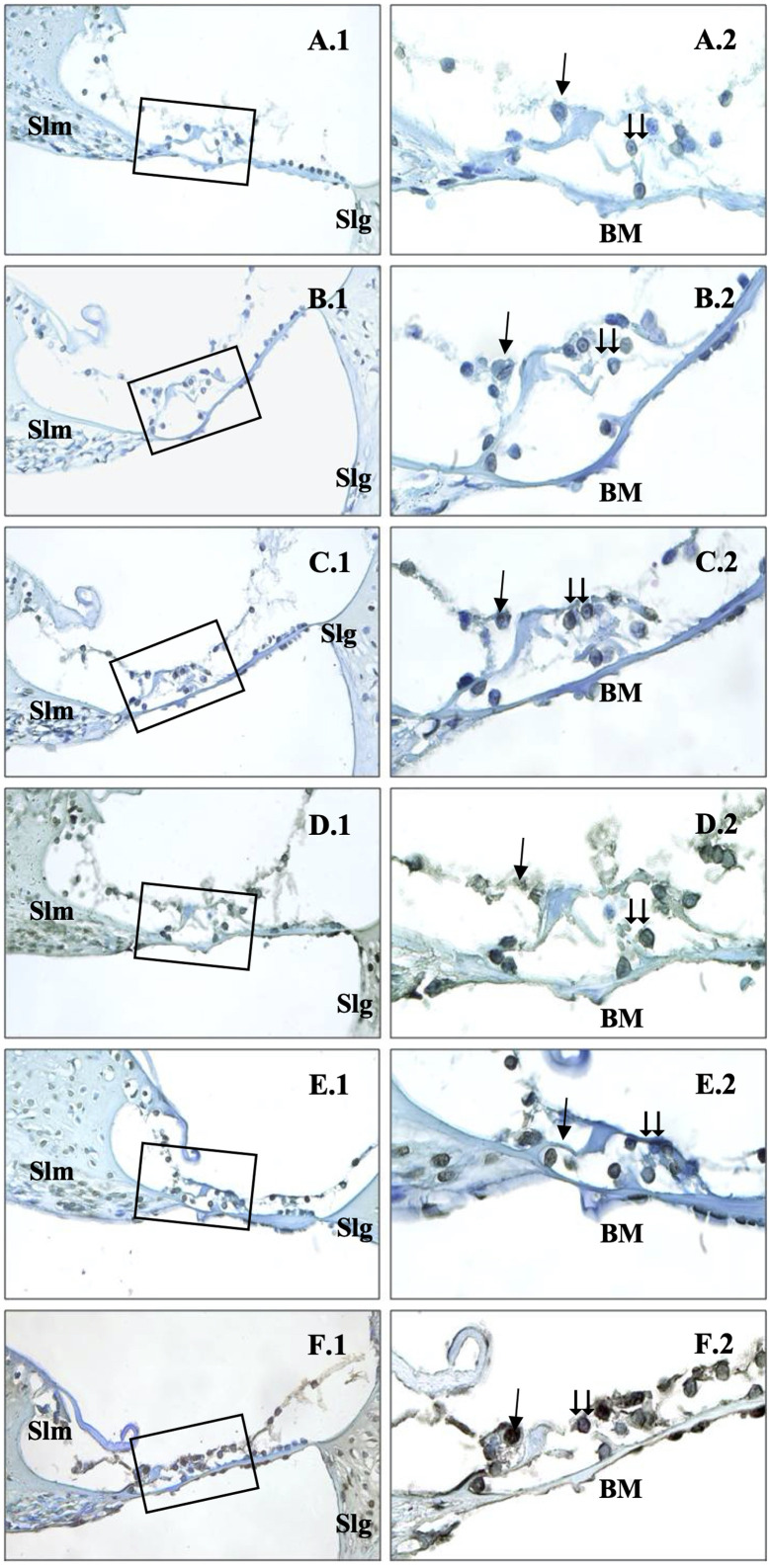
Also, the intensity of caspase-9 staining gradually increased as the electromagnetic wave dose increased. Caspase-9 was weakly expressed in the Corti organs of the control, 0.6 V/m, and 1.9 V/m groups (Figure 4A, 4B, and 3C). Also, mild–moderate staining was observed in the 5 V/m groups (Figure 3D). Moderate–strong reactions were detected, particularly in the outer hair cells of the 10 V/m group (Figure 4E). In the rats exposed to 15 V/m, significantly strong expression was detected in the inner and outer hair cells of organ of Corti (Figure 4F).
Figure 4.
Immunohistochemical staining of cochlea with caspase-9. →: inner hair cells, ⇉: outer hair cells, Slm: spiral limbus,Slg: spiral ligament, BM: basillary membrane, Control (A), 0.6 V/m EF (B), 1.9 V/m EF (C), 5 V/m EF (D), 10 V/m EF (E), 15 V/m EF (F). Organ of Corti ×40 (1) and organ of Corti ×100 (2). Mayers hematoxylin base staining.
The TUNEL-positive reactive cell number in the organ of Corti was almost nonexistent in the control group (Figure 5A). The 0.6 V/m group had a slightly nonsignificantly higher TUNEL-positive reactive cell number in outer hair cells than the control group (Figure 5B). The TUNEL-positive reactive cell number was slightly but nonsignificantly stronger in the 1.9 V/m group when compared to 0.6 V/m (Figure 5C). Staining density was similar and robust in the 5 V/m and 10 V/m groups, especially in the outer hair cells (Figure 5D and 5E). In the 15 V/m groups, it was found that especially the inner and outer hair cells, as well as the other support cells in the organ of Corti, showed the strongest TUNEL staining (P < .001) (Figure 5F).
Figure 5.
Immunohistochemical staining of cochlea with TUNEL. →: inner hair cells, ⇉: outer hair cells, Slm: spiral limbus, Slg: spiral ligament, BM: Basillary membrane, Control (A), 0.6 V/m EF (B), 1.9 V/m EF (C), 5 V/m EF (D), 10 V/m EF (E), 15 V/m EF (F). Organ of Corti ×40 (1) and organ of Corti ×100, methyl green base staining (2).
Discussion
In this study, the low dose-dependent adverse effects of 2.45 GHz MW radiation transmitted by wireless devices were investigated, which might cause histopathological changes in the inner ear tissues of rats. According to our findings, there is an apparent ototoxic effect of 2.45 GHz MW radiation on rat cochlea. The dose-dependent effects of 2.45 GHz MW radiation on cochlear cells are uncertain. Previous experiments on cochlear damage were conducted at the GSM (Global System for Mobile communication) frequency, and apoptosis in cochlear cells was identified.11,12,13
Current wireless devices (such as Wi-Fi-activated devices or wireless internet access devices) use higher frequency ranges (2.4-2.5 GHz) than mobile phones and typically have a prolonged exposure time and a broader exposure area.14 The effects of 2.45 GHz electromagnetic radiation on various body parts, such as the nervous system, tissue morphology and histology, serum biochemical parameters, metabolism, immune response, and reproductive organs, have already been established.14-17
The cochlea is the hearing unit where the beginning of electrical hearing pathways and inner and outer hair cells exist. The cochlear cells are susceptible to external factors such as acoustic trauma, metabolic diseases, drugs, trauma, and radiation.17 Although various adverse biological effects have been observed following MW exposure, the possible ototoxic effect of wireless devices is still not well established. According to recent studies, MW emitted by Wi-Fi devices and cell phones can induce oxidative stress due to the overproduction of oxygen-derived free radicals.18 Also, it is well known that oxidative stress induces apoptosis in the organ of Corti. In a previous review, Balbani et al18 reported no scientific proof that cell phone RF/MW radiation cause thermal damage to consumers. According to this review, acute exposure to MW radiation emitted by mobile phones does not affect cochlear outer hair cells or cochlear nerve function.10
In an earlier in vivo study, dose-dependent cochlear cell apoptosis of radiation was demonstrated in an experimental model.19 According to Tuhanioglu et al,17 pulsed magnetic fields (1.5 mT, 40 Hz) can cause auditory dysfunction and cochlea histopathological damage. They showed a significant increase of apoptosis, especially in outer hair cells, by using caspase-3, -9, and TUNEL methods as in our study.
Caspases (cysteine-aspartic proteases, cysteine aspartates, or cysteine-dependent aspartate-directed proteases) are a protease enzyme family that plays critical roles in the induction of apoptosis. Caspase-3 is an active death protease that catalyzes the selective cleavage of several essential cellular proteins.20 Caspase-9 is an enzyme encoded in humans by the CASP9 gene. It is a promoter caspase essential for the apoptotic cascade in many tissues. Overactivation of specific caspases, such as caspase-3, can result in exaggerated programmed cell death.21 Caspase-3 is actively expressed in sensory and nonsensory cells in the organ of Corti, and caspase-dependent apoptosis in the cochlea was reported in previous reports.13 Caspase inhibitors assist in hearing restoration by preserving the cochlea from acute mitochondrial dysfunction.15 In the intrinsic mitochondrial apoptosis pathway, caspase-9 is the initiator, and caspase-3 is the terminator caspase. The initiator caspase-9 then cleaves and activates the executioner caspases-3, -6, and -7, resulting in cell apoptosis.22,23 We chose caspase-3 and caspase-9 in our study to show the exact start and end pathways of apoptosis. In order to clearly diagnose apoptosis, it is necessary to determine that at least these caspase-9 and caspase-3 molecules from the caspase molecule family react. Thus, we prefer to use these apoptosis indices to examine the potentially detrimental effect of 2.45 GHz MW radiation in the inner ear. We observed that 2.45 GHz MW radiation increased the expression of caspase-3 and caspase-9 enzymes in the organ of Corti.
The TUNEL is an immunohistochemical technique commonly used to classify and measure apoptotic cells. It detects apoptotic DNA fragmentation and severe DNA deformation in different tissues. It might also label cells whose DNA has been damaged in ways other than apoptosis. According to our findings, as the electrical field increases, DNA damage and apoptosis increase.
Tuhanioğlu et al17 studied otoacoustic emission and caspase in 40 Hz pulsed magnetic field for 1 h/day for 30 days. According to their findings, a shallow dosage magnetic field, which has lately been investigated for therapeutic applications, can produce auditory function problems and histopathologic damage in cochlea cells. They did not, however, investigate the dose-dependent effect or the auditory pathway’s integrity with ABR. According to our findings, 2.45 GHz MW radiation significantly affects both electrophysiologically and histopathologically, especially at doses of 10 V/m and higher. Çelikler et al13 investigated the effects of a 2100 MHz GSM-like EMF generated by a field generator on the auditory system of rats. They discovered an increased apoptotic index histopathologically but no change in ABR latencies between the exposed and nonexposed groups. They could have seen electrophysiologic alterations if they had analyzed exposure at different intensities as we performed. In a study in which pregnant rats were exposed to 900 MHz and 1800 MHz RF radiation, there was no difference in the distortion product otoacoustic emission test. However, electron microscopic evaluation revealed significant differences in the groups’ number of regular, apoptotic, and necrotic cells. In this study, Distortion Product Otoacoustic Emission (DPOAE) was studied up to 8000 Hz, and the higher frequencies to which the rat ear is sensitive were not tested.24
Since the ears are often closer to mobile phones rather than other parts of the body, the possible adverse effect of Wi-Fi frequency on the cochlea may be more significant than the other organs. Similar studies are required for better understanding Wi-Fi-related cochlear damage. Zuo et al9 used a very similar design to our study and demonstrated the effect of cellular phone-induced electromagnetic radiation on spiral ganglion neurons in neonatal rats.
Electromagnetic waves, like sound waves, have an oscillating frequency but differ profoundly from sound waves. While sound waves are mechanical vibrations that spread through matter and carry energy, electromagnetism does not involve a medium. The ear detects pressure waves in the audible frequency between 0 and 20 kHz. Microwave pulse listening (30-300 Hz) differs from air or bone-conduction hearing.25 Since electromagnetic waves (for example, light) can be seen but not heard, the report on the auditory detection of MW pulses was both surprising and fascinating. As reported by Lin and Wang26 in their 2007 review article, the MW auditory effect is not caused by an association of MW pulses with nerves along the auditory neurophysiological pathways. Instead, upon absorption by soft tissues in the brain, the MW pulse generates a thermoelastic wave of acoustic energy that travels to the inner ear through bone conduction. It then stimulates the cochlear receptors in the same way as natural hearing does.26 Since cell phones commonly use the Wi-Fi frequency alongside the GSM frequency, the impact of Wi-Fi on the ear has become a question worth investigating. According to our findings, apoptosis increases in the inner ear as the EMF increases. In other words, Wi-Fi shows an ototoxic effect. This effect may be due to thermal or acoustic trauma due to MW heating. Our research has demonstrated MW ototoxicity, and we hope it will serve as a baseline for future publications.
As far as we know, this is the first study that assesses the impact of MW radiation on cochlea in various doses. The most important finding in our study was the dose-dependent rate of apoptosis and immune activity. Organ of Corti inflammation and apoptosis are direct causes of hearing impairment. Although the clinical importance of our findings in humans is not evident at this time, further studies should evaluate the effect of MW on humans audiologically. From an ordinary individual’s point of view, devices that use MW frequencies, like cochlear implants, wireless headphones, Bluetooth devices, wireless technologies, and cell phones, work by sending out electromagnetic waves with frequencies between hundreds of MHz and tens of GHz. When using Bluetooth with a cell phone, it should be used where the connection strength is good. If not, these gadgets would have to send out additional radiation to communicate.
The main limitation of this study is the lack of electron microscopical imaging at the cochlear level. The cochlea is a crucial element of the auditory pathway that is very vulnerable to external risk factors. To assess the whole auditory pathway, histopathological examination of the cochlear nerve would also be beneficial. Future studies should investigate the effect of MW radiation on the vestibular system in a molecular level. In conclusion, our study demonstrated that 2.45 GHz MW radiation has a dose-dependent detrimental effect on the inner ear. Apoptosis and DNA damage in the cochlea also increase as the electric field value increases.
In conclusion, 1.245 mW radiation exposed to newborn rats, both histopathological damage and physiological damage in the inner ear increase as the dose of the electric field increases. Further studies are required to detail the negative effects of wifi technology, which has become a part of life in today’s technology, on the hearing system.
Footnotes
Research data for this article: The data of the study can be shared on demand and it was uploaded to Mendeley data storage as; Tahir, Emel (2021), “radiation rat,” Mendeley Data, V1, doi: 10.17632/bsx7j7bfx3.1
Ethics Committee Approval: This study was approved by Ethics Committee of Ondokuz Mayıs University (Approval No: OMUHADYEK 2019/23, Date: 2019).
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.A.K., E.T.; Design – A.A.K., E.T., S.G.G., B.K.E.; Supervision – A.A.K.; Resources – A.A.K., B.K.E., E.T., A.T.; Materials – A.K.E.T., A.T., S.G.G.; Data Collection and/or Processing – E.T., S.G.G.; Analysis and/or Interpretation – E.T.; Literature Search – E.T., A.A.K.; Writing Manuscript – E.T., A.A.K., B.K.E., S.G.G., A.T.; Critical Review – A.A.K.
Acknowledgments: The authors would like thank to staff of Laboratory Animals Research Center for their assistance during experiment.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Yakymenko I, Burlaka A, Tsybulin I, et al. Oxidative and mutagenic effects of low intensity GSM 1800 MHz microwave radiation. Exp Oncol. 2018;40(4):282 287. ( 10.31768/2312-8852.2018.40(4):282-287) [DOI] [PubMed] [Google Scholar]
- 2. Yu Y, Yao K. Non-thermal cellular effects of lowpower microwave radiation on the lens and lens epithelial cells. J Int Med Res. 2010;38(3):729 736. ( 10.1177/147323001003800301) [DOI] [PubMed] [Google Scholar]
- 3. Wongkasem N. Electromagnetic pollution alert: microwave radiation and absorption in human organs and tissues. Electromagn Biol Med. 2021;40(2):236 253. ( 10.1080/15368378.2021.1874976) [DOI] [PubMed] [Google Scholar]
- 4. Talebnejad MR, Sadeghi-Sarvestani A, Nowroozzadeh MH, Mortazavi SMJ, Alighanbari A, Khalili MR. The effects of microwave radiation on rabbit’s retina. J Curr Ophthalmol. 2018;30(1):74 79. ( 10.1016/j.joco.2017.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai H, Blake Levitt B. The roles of intensity, exposure duration, and modulation on the biological effects of radiofrequency radiation and exposure guidelines. Electromagnetic Biology Med. 2022;41:230 255. [DOI] [PubMed] [Google Scholar]
- 6. Alkis ME, Akdag MZ, Dasdag S. Effects of low-intensity microwave radiation on oxidant-antioxidant parameters and DNA damage in the liver of rats. Bioelectromagnetics. 2021;42(1):76 85. ( 10.1002/bem.22315) [DOI] [PubMed] [Google Scholar]
- 7. McIntosh RL, Iskra S, McKenzie RJ, Chambers J, Metzenthen B, Anderson V. Assessment of SAR and thermal changes near a cochlear implant system for mobile phone type exposures. Bioelectromagnetics. 2008;29(1):71 80. ( 10.1002/bem.20364) [DOI] [PubMed] [Google Scholar]
- 8. International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020;118(5):483 524. ( 10.1097/HP.0000000000001210) [DOI] [PubMed] [Google Scholar]
- 9. Zuo H, Lin T, Wang D, et al. Neural cell apoptosis induced by microwave exposure through mitochondria-dependent caspase-3 pathway. Int J Med Sci. 2014;11(5):426 435. ( 10.7150/ijms.6540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lozano GM, Bejarano I, Espino J, et al. Relationship between caspase activity and apoptotic markers in human sperm in response to hydrogen peroxide and progesterone. J Reprod Dev. 2009;55(6):615 621. ( 10.1262/jrd.20250) [DOI] [PubMed] [Google Scholar]
- 11. Panda NK, Jain R, Bakshi J, Munjal S. Audiologic disturbances in long-term mobile phone users. J Otolaryngol Head Neck Surg. 2010;39(1):5 11. [PubMed] [Google Scholar]
- 12. Kizilay A, Ozturan O, Erdem T, Kalcioglu MT, Miman MC. Effects of chronic exposure of electromagnetic fields from mobile phones on hearing in rats. Auris Nasus Larynx. 2003;30(3):239 245. ( 10.1016/s0385-8146(03)00054-3) [DOI] [PubMed] [Google Scholar]
- 13. Çeliker M, Özgür A, Tümkaya L, et al. Effects of exposure to 2100MHz GSM-like radiofrequency electromagnetic field on auditory system of rats. Braz J Otorhinolaryngol. 2017;83(6):691 696. ( 10.1016/j.bjorl.2016.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aweda MA, Gbenebitse S, Meidinyo RO. Effects of 2.45 GHz microwave exposures on the peroxidation status in Wistar rats. Niger Postgrad Med J. 2003;10(4):243 246. ( 10.4103/1117-1936.174222) [DOI] [PubMed] [Google Scholar]
- 15. Kumar S, Kesari KK, Behari J. Influence of microwave exposure on fertility of male rats. Fertil Steril. 2011;95(4):1500 1502. ( 10.1016/j.fertnstert.2010.04.078) [DOI] [PubMed] [Google Scholar]
- 16. Oksay T, Naziroğlu M, Doğan S, Güzel A, Gümral N, Koşar PA. Protective effects of melatonin against oxidative injury in rat testis induced by wireless (2.45 GHz) devices. Andrologia. 2014;46(1):65 72. ( 10.1111/and.12044) [DOI] [PubMed] [Google Scholar]
- 17. Tuhanioğlu B, Erkan SO, Gürgen SG, et al. The effect of very low dose pulsed magnetic waves on cochlea. Braz J Otorhinolaryngol. 2019;85(3):282 289. ( 10.1016/j.bjorl.2018.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balbani AP, Montovani JC. Mobile phones: influence on auditory and vestibular systems. Braz J Otorhinolaryngol. 2008;74(1):125 131. ( 10.1016/s1808-8694(15)30762-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Low WK, Tan MG, Sun L, Chua AW, Goh LK, Wang DY. Dose-dependant radiation-induced apoptosis in a cochlear cell-line. Apoptosis. 2006;11(12):2127 2136. ( 10.1007/s10495-006-0285-4) [DOI] [PubMed] [Google Scholar]
- 20. Porter AG, Jänicke RU. Emerging roles of caspase -3 in apoptosis. Cell Death Differ. 1999;6(2):99 104. ( 10.1038/sj.cdd.4400476); Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22(4): 526 539. () [DOI] [Google Scholar]
- 21. Van De Water TR, Lallemend F, Eshraghi AA, et al. Caspases, the enemy within, and their role in oxidative stress-induced apoptosis of inner ear sensory cells. Otol Neurotol. 2004;25(4):627 632. ( 10.1097/00129492-200407000-00035) [DOI] [PubMed] [Google Scholar]
- 22. Rowinsky EK. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23(36):9394 9407. ( 10.1200/JCO.2005.02.2889) [DOI] [PubMed] [Google Scholar]
- 23. Loreto C, Almeida LE, Trevilatto P, Leonardi R. Apoptosis in displaced temporomandibular joint disc with and without reduction: an immunohistochemical study. J Oral Pathol Med. 2011;40(1):103 110. ( 10.1111/j.1600-0714.2010.00920.x) [DOI] [PubMed] [Google Scholar]
- 24. Seckin E, Suren Basar F, Atmaca S, et al. The effect of radiofrequency radiation generated by a Global System for Mobile Communications source on cochlear development in a rat model. J Laryngol Otol. 2014;128(5):400 405. ( 10.1017/S0022215114000723) [DOI] [PubMed] [Google Scholar]
- 25. Lubner RJ, Kondamuri NS, Knoll RM, et al. Review of audiovestibular symptoms following exposure to acoustic and electromagnetic energy outside conventional human hearing. Front Neurol. 2020;11:234. ( 10.3389/fneur.2020.00234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin JC, Wang Z. Hearing of microwave pulses by humans and animals: effects, mechanism, and thresholds. Health Phys. 2007;92(6):621 628. ( 10.1097/01.HP.0000250644.84530.e2) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a