Abstract
Physical inactivity, i.e., not reaching the recommended level of physical activity (PA) and sedentary behaviors (SB), i.e., sitting time have been associated with increased risk for common metabolic diseases. Recent epidemiological data suggest that high volumes of SB are detrimental for metabolic health, even in the presence of regular exercise, i.e., moderate/vigorous (MVPA). This suggests that the health effects of SB are independent from those of exercise. However, experimentally testing this hypothesis is complicated because of the difficulty in disassociating SB from PA. Bedrest studies, a traditional space science model, can offer new insights. In some bedrest studies, an exercise training protocol has been used to counteract the harmful effects of inactivity. While bedrest induces an inactive and sedentary state, exercise with bedrest represents a unique model of sedentary yet physically active people. Here, we review bedrest studies with and without exercise training. Although exercise training prevents the loss of muscle mass and function, even large volumes of exercise are not sufficient to fully counteract the negative metabolic adaptations triggered by inactivity. This observation supports the existence of independent adverse health effects of SB, but also the potential benefits of non-exercise activity, i.e., daily living light-intensity activities (LPA). We gathered available data to examine the complex relationships between exercise, non-exercise activity, SB and health outcomes. Given the large amount of SB in modern societies, the sole promotion of exercise, i.e., MVPA may be insufficient, and promotion of LPA may be a complimentary approach to improve health.
Keywords: Physical inactivity, moderate to vigorous physical activity, light physical activity, bedrest, exercise, non-exercise activity, metabolism
Graphical Abstract
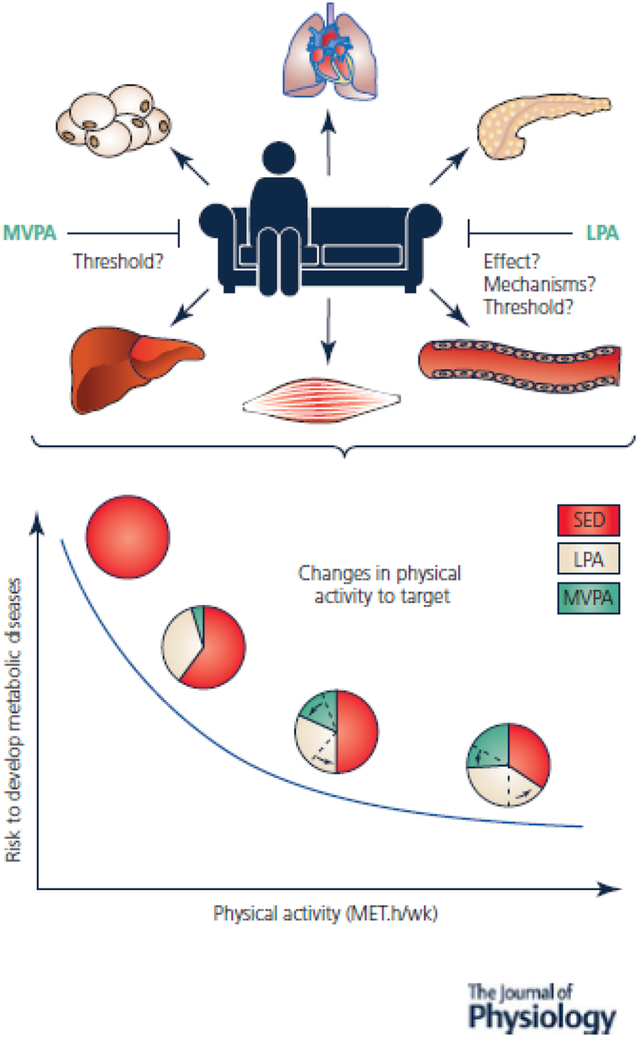
Although the exact physiological mechanisms remain unclear, sedentariness has been shown to negatively affect metabolic health and the main organs involved in its regulation. The 2020 World Health Organization (Bull et al., 2020) guidelines on physical activity recommend practicing a weekly volume of 150–300 min of moderate intensity or 75–150 min of vigorous intensity or an equivalent combination of MVPA to limit the appearance of certain maladaptation. Recently, some large-scale studies have revealed the importance of LPA to prevent the effects of sedentary lifestyles. Since then, limiting periods of sedentary time have become part of the recommendations for the first time, raising the question of how to reduce periods of sedentary living. This review aims to highlight the potential role of LPA in effectively reducing sitting time and preventing its associated harmful health effects. Intervention studies specifically targeting LPA and SB, in addition to MVPA, are necessary to develop specific recommendations and limit the risk of developing metabolic diseases.
Introduction
Insufficient physical activity (PA) is a public health concern and a major risk factor for early mortality and common chronic diseases including obesity, metabolic syndrome, insulin resistance, type 2 diabetes (T2D), certain type of cancers, mental health disorders and others (Lee et al., 2012). Through research efforts to develop strategies to combat physical inactivity, scientists have identified another health risk behavior: sedentary behaviors (SB). SB are distinct from physical inactivity. Although physical inactivity is defined as engaging in less PA necessary to meet the current guidelines (<150 min/week moderate or <75min/week vigorous physical activity – MVPA, with energy expenditure above 3.0 metabolic equivalent or METs), SB correspond to “any waking behavior characterized by an energy expenditure <1.5 METs, while in a sitting or reclining posture” (Tremblay et al., 2017). Although the recommendations encourage reducing periods of SB, no specific strategy has been proposed to combat the effects of sedentary lifestyles (Bull et al., 2020).
SB are found in every domain of modern daily life: transportation, occupational (e.g., desk bound work) and leisure time activities (e.g., video gaming and internet). Adults in Westernized societies spend between 7.7 and 9.7 h/d sitting, which corresponds up to 60% of adult wake time (Ekelund et al., 2019). Several epidemiological studies have reported associations between sedentary time and health outcomes including early mortality, risk of T2D, metabolic syndrome and cardiovascular disease (Dunstan et al., 2012). These associations were observed in both sexes, all ages, ethnicities and independent of adiposity. They were also found in individuals who reach the recommended levels of MVPA, which suggests that SB is a stand-alone factor in the relationship between PA and health. In other words, spending too much time sitting may have different health effects than not exercising enough. While a plethora of epidemiological data is published, experimental evidence supporting the adverse health effects of SB independent of time spent physically active is lacking. This is mainly due to challenges in isolating the effects of SB from those of PA.
The bedrest model can provide unique insights on the independent health effects of SB. Bedrest studies have traditionally been used by the International Space Agencies to understand the physiological effects of microgravity. During these studies, physically active healthy participants free from any predisposition for chronic diseases stay in bed 24h/7d. They are both physically inactive and highly sedentary (figure 1). In some bedrest studies, the efficacy of exercise training protocols to protect the body against the harmful effects of microgravity have also been tested. Participants in these studies perform exercise training (figure 1) while in bedrest. Participants in these studies are both sedentary and physically active, and represent an extreme but unique model of “sedentary exercisers”. Another distinctive characteristic of these bedrest exercisers is that they have very low levels of non-exercise activities of daily living, which correspond to light-intensity physical activities (LPA) with an energy expenditure between 1.6 and 2.9 METs (e.g. walking, taking the stairs, standing, etc.) (figure 1).
Figure 1: Schematic representation of the components of total energy expenditure during bedrest, conducted with or without exercise training.
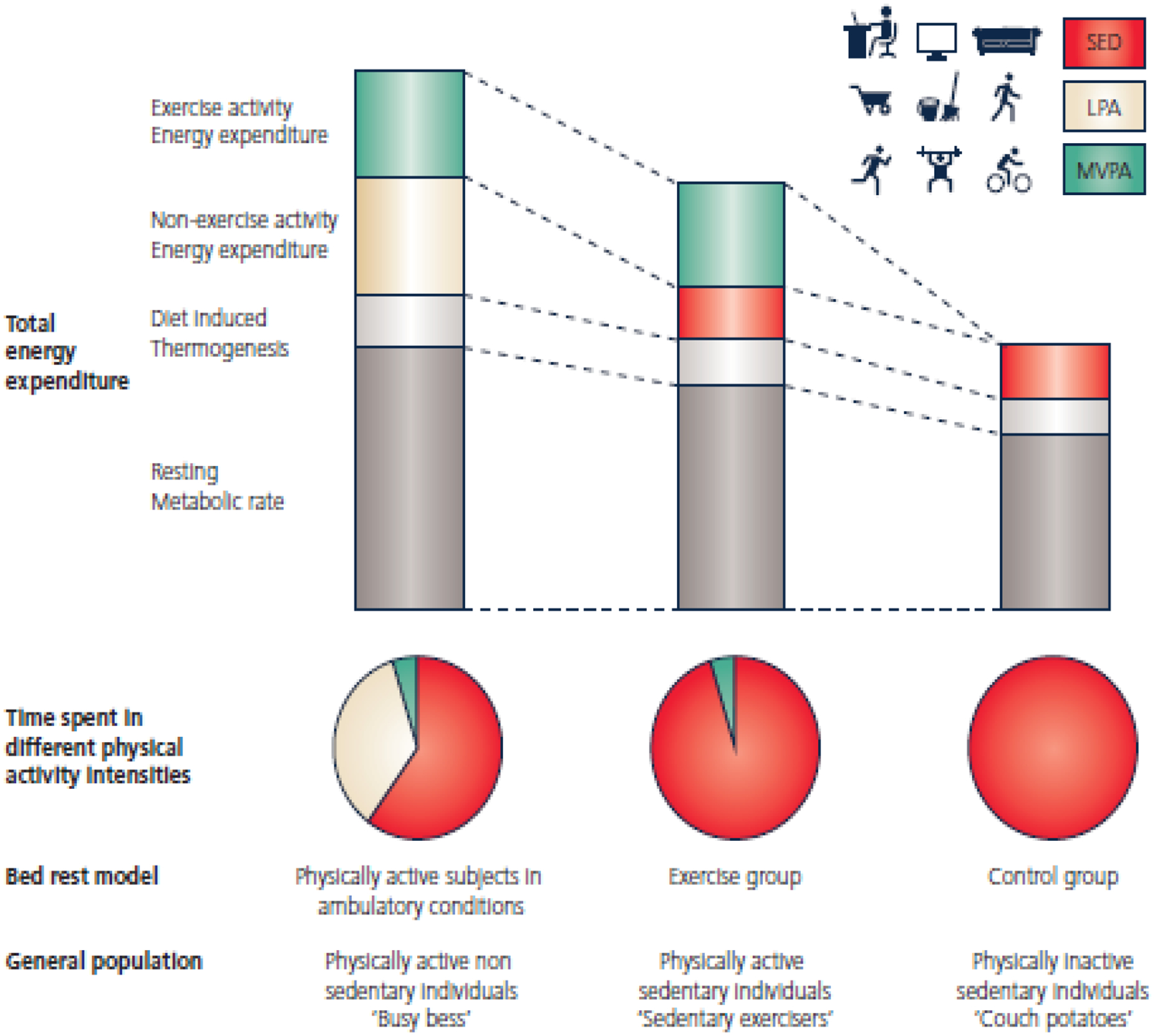
Based on total energy expenditure, participants enrolled in bedrest protocols can be compared to the general population. Strict bedrest suppresses both components of physical activity energy expenditure: exercise activity energy expenditure and non-exercise activity energy expenditure. Exercise activity energy expenditure refers to the energy spent in MVPA and/or structured exercise. Non-exercise activity energy expenditure corresponds to any activity of daily life, which are essentially LPA. Participants who are subjected to moderate to vigorous exercise training along with bedrest maintain high exercise activity energy expenditure mainly due to MVPA. However, they are sedentary with very low levels of non-exercise activity energy expenditure and are lacking LPA. These individuals represent an extreme but unique model of “sedentary exercisers”, i.e. physically active yet sedentary people. Strict bedrest leads to a decrease of both MVPA and LPA while increasing SB. These bedrest individuals represent a model of the modern physically inactive sedentary individuals. SED: sedentary activities; LPA: light-intensity physical activity; MVPA: moderate-to-vigorous physical activity. Adapted from Bergouignan et al 2010.
The objective of this review is to present experimental evidence from bedrest studies with or without concomitant exercise training to provide new information on the metabolic health effects of SB independent from those of MVPA/exercise. To better understand the complex relationship between SB, LPA, MVPA and metabolic health outcomes, we will also review briefly the health benefits of LPA.
The physiology of physical inactivity: insights from strict bedrest investigations
To understand the respective effects of SB and PA, it is important to first briefly summarize the physiological effects of combined sedentariness and physical inactivity induced by strict bedrest (figure 2). Bedrest induces hypokinesia (loss of body movements) and hypodynamia (loss of strength and power), which leads to modifications in all physiological systems (Bergouignan et al., 2011). Among other changes, bedrest reduces muscle function and mass as demonstrated by muscle atrophy and a shift from slow oxidative to fast glycolytic muscle fibers (Trappe et al., 2007a; Salanova et al., 2008; Trappe et al., 2008), reduced mitochondrial volume and oxidative capacity (Kenny et al., 2017), and an impaired expression of genes involved in mitochondrial function (Alibegovic et al., 2010b). Bedrest also rapidly decreases insulin sensitivity in muscle (Alibegovic et al., 2009) in association with lower content and activity of key proteins involved in glucose transport, phosphorylation and storage (Biensø et al., 2012). This results in hyperinsulinemia to maintain normal glucose disposal. This development of glucose intolerance seems to be preceded by a metabolically inflexible state, i.e. an inability of the body to adjust substrate use to changes in substrate availability (Rudwill et al., 2018). Gene expression and activity of enzymes coupled with oxidative metabolism are decreased (Bergouignan et al., 2009; Alibegovic et al., 2010b; Fernandez-Gonzalo et al., 2020) in association with a reduction in lipid oxidation in favor of carbohydrate oxidation (Bergouignan et al., 2006; Bergouignan et al., 2009). These changes are particularly relevant following meal ingestion since they lead to decreased clearance of dietary lipids, which contributes to hyperlipidemia. Despite reduced adipose tissue lipolysis (Alibegovic et al., 2009; Alibegovic et al., 2010a), excess of plasma lipids enhances fat accumulation in the visceral adipose depot (Belavý et al., 2014) and ectopic fat storage in muscle, liver and bone (Bergouignan et al., 2009; Trudel et al., 2009; Trudel et al., 2012; Rudwill et al., 2015). This in turn exacerbates the development of insulin resistance. Fat accumulation in liver likely stimulates de novo lipogenesis and an increased synthesis of atherogenic lipid particles (VLDL), as suggested by a recent study in free-living individuals who reduced their PA levels (Damiot et al., 2019). This increased secretion of VLDL further facilitates hyperlipidemia and ectopic fat storage. A decrease in high-density lipoprotein (HDL) cholesterol, known to be associated with a reduction in cardiometabolic risk, has also been observed (Alibegovic et al., 2009). Concomitantly, the liver is less able to suppress hepatic glucose production, which results in increased gluconeogenesis, thus worsening hyperinsulinemia. These changes are finally associated with the development of low-grade inflammation as indicated by an increase in plasma pro-inflammatory markers (Rudwill et al., 2013; Mutin-Carnino et al., 2014).
Figure 2: Preventive effect of exercise (resistance exercise or resistance plus aerobic exercise) against the bedrest-induced physiological and metabolic alterations.
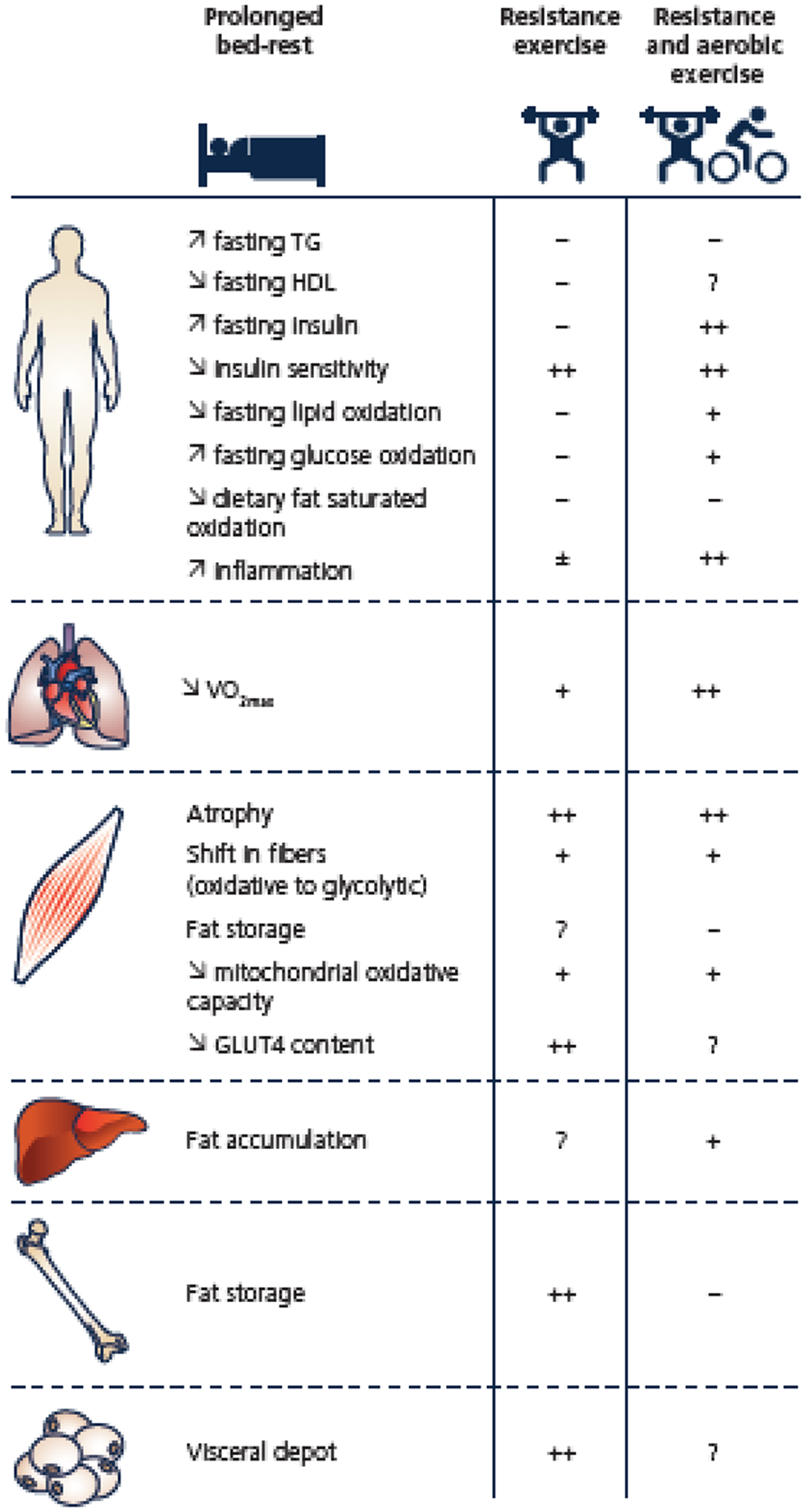
− : no effect; + : partially protected; ++ : fully protected; ?: no data available; ± : no consensus.
All these metabolic features are commonly observed in individuals with obesity, T2D or metabolic syndrome. These observations therefore support a key role of physical inactivity in the onset and progression of metabolic diseases. Although the health enhancing effects of exercise (or MVPA) on these metabolic outcomes are well established, it is unclear whether they are sufficient to reverse the adverse health effects of sedentariness.
Can MVPA reverse the adverse health effects of physical inactivity and SB?: Insights from bedrest studies with concomitant exercise training
To the best of our knowledge, 10 bedrest studies have tested the protective effects of an exercise training program against the metabolic alterations induced by bedrest. These studies span from 20 to 90 days and the exercise prescriptions varied in the type (resistance or aerobic exercise), duration, frequency and intensity (Table 1). Some training protocols were below the current recommendations for PA while others were above. Results have been reported in 26 published articles and are summarized in figure 2.
Table 1:
Summary of the exercise training protocols tested during bedrest studies
| Publication | Study name Duration of BR Sample size | Exercise modalities | Estimated duration of MVPA | Estimated energy cost |
|---|---|---|---|---|
| Resistance exercise | ||||
|
Bergouignan A. et al. 2006
Fernandez-Gonzalo R. et al. 2020 Irimia J. et al. 2017 Rudwill F. et al. 2013 Trappe S. et al. 2004 |
LTBR 2001–2002 90 d HDT-BR n=18 ♂ |
35 min every 3 d during BR on flywheel ergometer. Progressive warm-up + 4×7 max concentric/eccentric squat + 4×14 in calf press. 2 min rest between sets and 5 min between EX. | 82 min MVPA/wk 12 min MVPA/d |
8 MET.h/wk 1.2 MET.h/d |
| Brooks N. et al. 2014 | 28 d HDT-BR n=31 ♂ |
1 h/d, 6 d/wk. Target intensity: 70–80% of 1RM as estimated by the OMNI rating of perceived EX 10 category scale. 7 to 8 REX targeting major muscle groups during each session. Lower body (squats, single leg squats, diagonal jump, calf raise, single-leg hip extension, leg curl, single-leg hip abduction) and upper body (pull-ups, pull-over, triceps press, chest fly, shoulder press, biceps curl, upright row, lateral arm raise) EX were performed on alternating days. |
360 min MVPA/wk 51 min MVPA/d |
36 MET.h/wk 5.2 MET.h/d |
| Ferrando A. et al. 1997 | 14 d HDT-BR n=6 ♂ |
Squat on horizontal leg-training device every 2 d. 3×12 squats, training volume progressively increased to reach 5×8 squat at session 3 till the end of BR. | ? | ? |
|
Guinet P. et al. 2020
Kenny H. et al. 2017 Kenny H. et al. 2020 |
MNX 21 d HDT-BR n=12 ♂ |
5 sessions of EX, on leg press machine with a vibration platform (8 mm peak-to-peak, 25 Hz): bilateral squats (10 rep, 75% 1-RM, 8 s/rep), single heel raises (×1.3 body weight, contractions performed as fast as possible until fatigue) and bilateral heel raises (×1.8 body weight, contractions performed as fast as possible until fatigue). A 5% load adjustment was made based on the ability of volunteers to complete the set of EX. | 5–15 min MVPA/wk 0.7–2.1 min MVPA/d |
0.5–1.5 MET.h/wk 0.01–0.2 MET.h/d |
| Moriggi M. et al. 2010 | BBR1 55 d HDT-BR n=12 ♂ |
2 bouts/d of EX (6min each) of RVE at preset frequencies ranging from 19 at the beginning to 25Hz. Total of 89 sessions. 1. Squatting EX: Knees were extended from 90° to almost full extension in cycles of 6s for each squat (knee extensors). 2. Heel raises: With knees almost extended, heels were raised to fatigue. Only then, brief rests (< 5s) were allowed with the entire foot on the vibration platform in order to recover, and subjects started to raise their heels again (foot plantar flexors). 3. Toe raises: Similar to 2, but toes were raised instead of heels (foot dorsi-flexors). 4. “Kicks”: With the same loading as in 1–3, knees were extended as quickly and forcefully as possible. The platform was struck with the balls of the feet, and legs rested on the Galileo Space framework in between the kicks. This was done 10 times with 10s of rest inserted. |
36 min MVPA/wk 5.1 min MVPA/d |
3.6 MET.h/wk 0.5 MET.h/d |
| Belavy D. et al 2014 Trudel G. et al. 2012 |
BBR2–2 60 d HDT-BR n=24 ♂ |
3 d/wk: 1. Bilateral leg press (~75–80% of pre-bed-rest max voluntary contraction); 2. Dingle-leg heel raises (~1.3 times body weight); 3. Double leg heel raises (~1.8 times body weight); 4. back and forefoot raise (performing hip and lumbar spine extension against gravity with ankle dorsiflexion, but with ~1.5 times body weight applied at the shoulders). The RVE group performed the same exercises as the REX group, except that whole body vibration was applied. The corresponding vibration parameters were as follows: 1. frequency 24 Hz, amplitude 3.5–4 mm, and peak acceleration ~8.7 g, where g ~ 9.81 ms−2; 2. frequency 26 Hz, amplitude 3.5–4 mm, and peak acceleration ~10.2 g; 3. frequency 26 Hz, amplitude 3.5–4 mm, and peak acceleration ~10.2 g; 4. frequency 16 Hz, amplitude 3.5–4 mm, and acceleration ~3.9 g |
15.8 min MVPA/wk 2.3 min MVPA/d |
1.6 MET.h/wk 0.2 MET.h/d |
| Combined resistance and aerobic exercise | ||||
|
Bergouignan A. et al., 2009 Bergouignan A. et al., 2010 Lee SM. et al., 2014 Mutin-Carnino M. et al., 2013 Rudwill F. et al., 2015 Salanova M. et al., 2008 Trappe T.A. et al., 2007 Trappe S. et al., 2007 Trappe S. et al., 2008 Trudel G. et al., 2009 |
WISE 60 d HDT-BR n=16 ♀ |
REX: 35min every 3 d, 4×7 max concentric/eccentric squat + 4×14 in calf press. AEX: 50min every 2 d, 50min in lower body negative pressure vertical treadmill at 40–80% pre-bedrest VO2max |
247 min MVPA/wk 35.3 min MVPA/d |
33.1 MET.h/wk 4.7 MET.h/d |
| Krainski F. et al., 2014 | 35 d HDT-BR n=27 ♂/♀ |
REX: 25–30min 2 d/wk. 2×8–12 of lower body exercises (leg press, plantar flexion, knee flexion, hip flexion, and hip abduction) and 1×8–12 of upper body EX (shoulder press, elbow flexion and extension, chest press, pullovers, and abdominal crunches) were performed in the supine position, loads were adjusted weekly to reach muscle fatigue during each set of EX. After 5 wk of BR, 2×20 plantar flexion exercises on each leg 2/d (6–8min) against an elastic band were added for all remaining subjects in EX group. AEX: 6 d/wk. During each week of BR, subjects completed 1 recovery (low intensity, typically <70% max HR), 2 base (moderate intensity, between 70–80% max HR), 1 MSS (vigorous intensity, 80–90% maximal HR), and 2 interval sessions (high intensity, 90–95% max HR or above), each lasting a total of 30 – 46 min and separate warm-up/cool-down phases lasting 5 min each. Intervals consisted of 6 cycles of 3 min at 90–95% of max HR, followed by 3 min at recovery pace. |
381 min MVPA/wk 54.4 min MVPA/d |
49.5 MET.h/wk 7.1 MET.h/d |
| Ploutz-Snyder L. et al. 2018 | 70 d HDT-BR n=26 ♂ |
REX: 3 d/wk. 3×4 supine lifts (squat, leg press, unilateral leg curl, and heel raise); squats and leg press were each performed using a standard shoulder-width stance, single-leg stance, or wide-leg stance on a rotating basis. Training followed a nonlinear periodized model in which load and repetitions were varied on a daily basis to optimize adaptations. AEX: 6 d/wk. Alternating days of continuous cycle EX for 30 min at 75% of VO2peak (3 d/w) with interval treadmill sessions of 30s, 2min, or 4min intervals (3 d/wk) at nearly max intensity. |
314.5 min MVPA/wk 45 min MVPA/d |
40 MET.h/wk 5.7 MET.h/d |
| Ward K. et al. 2020 (In press) | RSL 60 d HDT-BR n=23 ♂ |
48 sessions including 4 types of training sessions based on varying CMJ and repetitive hops between 80–90% of BW during 1.5–3min preceded by a warm-up and 3 max CMJ at 80% of BW. | 17.5 min MVPA/wk 2.5 min MVPA/d |
2.6 MET.h/wk 0.4 MET.h/d |
HDT-BR: 6° head-down tilt bedrest; d: days; wk: week; max: maximal; BW: body weight; CMJ: countermovement jump; EX: exercise; REX: resistive exercise; AEX: aerobic exercise
Effect of resistance exercise alone:
Resistance exercise has been shown to mitigate the decrease in cardiorespiratory fitness (Guinet et al., 2020; Kenny et al., 2020) and prevent the loss of muscle function and mass including the reduction in fiber diameter during bedrest (Trappe et al., 2004; Moriggi et al., 2010). However, the mechanisms underlying the protective effects of resistance exercise against unloading-muscle atrophy are not fully clear. Resistance exercise was shown to prevent the bedrest-induced decrease in muscle protein synthesis (Ferrando et al., 1997) and downregulate the gene expression of myostatin (Irimia et al., 2017), a myokine known to contribute to muscle wasting. The effect of resistance exercise on muscle protein balance, i.e. muscle protein synthesis and breakdown, during bedrest is however still unknown. Despite these positive effects on skeletal muscle, resistance exercise only partially prevents the whole-body metabolic alterations induced by bedrest. It protects against the rise in visfatin (Rudwill et al., 2013), an adipocytokine that mimics the effects of insulin, but does not prevent the increase in IL-6 and C-reactive protein, two pro-inflammatory markers, or the decrease in adiponectin (Brooks et al., 2014), a change associated with inflammation, lipid abnormalities, and insulin resistance. Even when performed at high intensity, resistance exercise does not mitigate the decrease in HDL (Brooks et al., 2014; Guinet et al., 2020), the development of insulin resistance, hyperlipidemia, or the shift towards the use of carbohydrate as fuel (Bergouignan et al., 2006). This later observation is surprising knowing that resistance exercise prevents against the shift of muscle fibers from oxidative to glycolytic types (Trappe et al., 2004), offsets the transcriptomic alterations in muscle related to aerobic energy metabolism (e.g. electron transport chain, fatty acid beta-oxidation and tricarboxylic cycle pathways) (Fernandez-Gonzalo et al., 2020), and partially maintains the activity and gene expression of enzymes controlling oxidative metabolism (e.g. citrate synthase, succinate dehydrogenase) at the mitochondrial level (Irimia et al., 2017). Although resistance exercise alone does not restore the levels of fatty acid oxidation to baseline values (Bergouignan et al., 2006), no accumulation of fat in bone (Trudel et al., 2012) or visceral depots (Belavý et al., 2014) was reported. Furthermore, no data exists on the effects of resistance exercise on ectopic fat storage in liver or muscle during bedrest. In all these studies, the resistance exercise session was performed as a single continuous bout; however novel data suggest that spreading activity throughout the day as multiple short active bouts may have more potent health-enhancing effects (Loh et al., 2019). Nevertheless, when exercise was performed as intermittent, frequent jumping squats spread throughout the day muscle mass loss was prevented, but not the reduction in whole-body and peripheral insulin sensitivity (Ward et al., 2020). Taken together, the low energy expenditure associated with resistance exercise (Table 1) may be responsible for partial or limited protective effects on metabolic health.
Effect of combined resistance and aerobic exercise:
The majority of bedrest studies has combined aerobic exercise with resistance exercise, which likely induced a greater energy expenditure compared to resistance exercise alone. This training approach preserves or at least attenuates cardiorespiratory fitness, muscle structure and function, leg muscle size and power, muscle strength and endurance, muscle fiber composition and diameter and mitochondrial content and oxidative capacity (Trappe et al., 2007a; Trappe et al., 2007b; Salanova et al., 2008; Trappe et al., 2008; Bergouignan et al., 2009; Krainski et al., 2014; Lee et al., 2014; Ploutz-Snyder et al., 2018). Although muscle alterations were prevented by all the tested resistance and aerobic exercise protocols regardless of the type, duration, intensity and frequency of the training, the protective effects on metabolic outcomes were variable. Combined resistance and aerobic exercise training prevents the development of a pro-inflammatory state (Mutin-Carnino et al., 2014), insulin resistance and the shift in substrate use from total fat oxidation to carbohydrate oxidation (Bergouignan et al., 2009). However, it does not counteract the increase in fasting triglycerides, the reduced oxidative rate of dietary fatty acids likely due to an impaired transport of fatty acids into the myocyte, and fat accumulation in skeletal muscle (Bergouignan et al., 2009) and bone (Trudel et al., 2009). Hepatic fat accumulation is however likely offset (Rudwill et al., 2015). Surprisingly, these alterations were observed despite levels of MVPA mostly above recommended levels (Table 1), and total daily energy expenditure maintained to pre-bedrest levels in the exercising participants (Bergouignan et al., 2010).
Taken together these studies show that exercise (or MVPA) protects skeletal muscle mass and function, and cardiorespiratory function against large volumes of SB induced by bedrest. However, even if a dose-response relationship exists (Figure 2 and Table 1), very high levels of exercise do not fully prevent the manifestation of metabolic dysfunction, i.e. whole-body insulin resistance, glucose intolerance, alterations of lipid metabolism, and systemic inflammation. These observations support the role of organs other than muscle in the health-enhancing effects of physical activity (Thyfault & Bergouignan, 2020), and the existence of health effects of SB independent of those from MVPA. It further highlights the importance of non-exercise activity (i.e. LPA), which mainly corresponds to daily living activities performed throughout the day.
Health benefits of daily living activities
Evidence from epidemiological studies indicate that LPA has a potential role in reducing the risk of early mortality. In cross-sectional studies, LPA is favorably associated with waist circumference, body mass index (BMI), plasma triglyceride, insulin, HDL-cholesterol concentrations (Amagasa et al., 2018) and 2h plasma glucose (Healy et al., 2007), independent of MVPA. Iso-temporal substitution modelling suggests that replacing 30 min of SB per day with 30 min of LPA (and not MVPA) is associated with lower waist circumference and BMI (Healy et al., 2015). A growing number of experimental studies have also examined the effects of LPA prescribed as short bouts spread throughout the day on metabolic health (table 2). As previously reviewed (Dempsey et al., 2016a), LPA bouts (15–40 min) acutely decrease postprandial glycemia and insulinemia. Even brief intermittent bouts (≤5 min) of walking spread throughout the day reduce glucose and insulin concentrations following meal ingestion, with more potent effects observed in adults with overweight to obesity and T2D (Chastin et al., 2019), and those with lower cardiorespiratory fitness compared to healthy lean individuals (McCarthy et al., 2017). Importantly, acute exposure to bouts of LPA elicits similar responses to those observed with short, frequent bouts of MVPA. With regards to standing, although some studies did not show a reduction in postprandial glycemic response (Bailey & Locke, 2015; Pulsford et al., 2017), others did (Benatti et al., 2017). Benatti and colleagues even reported that intermittent standing, but not a single continuous bout of MVPA, lowers postprandial glycemia in healthy adults. The difference in the observed effects may be explained by the duration of the standing bouts (2 min vs 15 min) and the total active duration (30 min MVPA vs 15 min standing every 30 min for 8.5 hours). Although these acute studies suggest that LPA of higher energy expenditures or longer duration decrease glycemia and insulinemia in a dose-dependent manner, none of these studies controlled for energy expenditure across the interventions. In an elegant series of studies, Duvivier and colleagues compared the metabolic effects of replacing SB with LPA walking and standing to those of 1h/d of MVPA. Both interventions lasted four days and were matched for energy expenditure. Replacing SB with high volumes of LPA without any increase in MVPA, decreased postprandial insulin, fasting triglycerides and non-HDL cholesterol in healthy adults (Duvivier et al., 2013). In adults with T2D, increasing time spent standing and walking improved glucose control and insulin sensitivity. It further reduced diastolic blood pressure, blood triglycerides and non-HDL cholesterol while increasing HDL cholesterol (Duvivier et al., 2017a; Duvivier et al., 2017b). The MVPA intervention tended to improve these metabolic parameters, but the effects were less pronounced. These studies show that when energy expenditure is matched, replacing SB with high volumes of LPA (i.e. non-exercise activity) is more beneficial than performing MVPA as a single continuous bout (i.e. structured exercise), at least for glucose control, insulin sensitivity and circulating lipids. On the contrary, if frequent LPA bouts (>2 min) improves endothelial function (Thosar et al., 2015; Dempsey et al., 2016b), a single bout of MVPA may be more beneficial for microvascular function than increasing LPA throughout the day (Duvivier et al., 2018). These findings suggest that MVPA (i.e., exercise) and LPA (i.e., non-exercise activity) might elicit differential cardiometabolic effects. Future studies will need to further compare the effects of LPA versus MVPA on cardiometabolic health outcomes, including maximal aerobic capacity, muscle strength, substrate metabolism, glucose control, and insulin sensitivity in different populations and investigate the mechanistic underpinnings.
Table 2:
Summary of the acute metabolic health effects of light-intensity physical activity from experimental laboratory-controlled studies
| Publication | Sample size and characteristics | Study design Study conditions | Primary results |
|---|---|---|---|
| Studies investigating light-physical activity compared to sitting | |||
| Bailey et al. 2015 | N=10 (3♀/7♂) BMI: 26.5 ± 4.3 kg/m2 Non-insulin resistant |
Cross-over (5h/condition) 1) Uninterrupted Sitting 2) Sit + 2min stand every 20min 3) Sit + 2min LIW every 20min |
Plasma glucose AUC: LIW < sitting and standing; standing vs. sitting: NS Blood pressure: No between-conditions difference Plasma lipids: No between-conditions difference |
| Benatti et al. 2017 | N=14 ♂ BMI kg/m2: 24.9 ± 4.3 kg/m2 Non-insulin resistant |
Cross-over (27h/condition) 1) Uninterrupted Sitting 2) Sit + 15min stand every 30min 3) Sit + 30min MIW 4) Sit + 30min MIW and 15min stand every 30-min |
Postprandial plasma glucose iAUC: standing < sitting; MIW vs sitting: NS |
| Dempsey et al. 2016b | N=24 (10♀/14♂) BMI: 33.0 ± 3.4 kg/m2 Type 2 diabetes |
Cross-over (8h/condition) 1) Uninterrupted sitting 2) Sitting + 3min LIW every 30min 3) Sitting + 3min resistance activities every 30min |
Blood pressure: LIW < sitting Noradrenaline concentration: LIW < sitting |
| Duvivier et al. 2017a | N=24 (11♀/13♂) BMI: 29.0 ± 2.0 kg/m2 Non-insulin resistant |
Cross-over (4 days/condition) 1) Sit 13.5h/d, stand 1.4h/d, LIW 0.7 h/d 2) Sit 7.6h/d, stand 4.0h/d, LIW 4.3h/d |
OGTT insulin AUC: LIW < sitting Insulin sensitivity (Matsuda index): LIW < sitting Fasted lipids: LIW < sitting Fasted lipoproteins: LIW < sitting Diastolic blood pressure: LIW < sitting |
| McCarthy et al. 2017 | N=34 (18♀/16♂) BMI: 23.8 ± 6.1 kg/m2 Non-insulin resistant |
Cross-over (7.5h/condition) 1) Uninterrupted Sitting 2) Sit + 5min LIW every 30min |
Plasma glucose iAUC: LIW < sitting Plasma insulin iAUC: LIW < sitting |
| Pulsford et al. 2017 | N=25 ♂ BMI: 24.9 ± 4.3 kg/m2 Non-insulin resistant |
Cross-over (7h/condition) 1) Uninterrupted sitting 2) Sit + 2min stand every 20min 3) Sit + 2min LIW every 20min |
Postprandial plasma glucose AUC: LIW < sitting Postprandial plasma insulin AUC: LIW < sitting Insulin sensitivity (Matsuda index): LIW < standing and sitting |
| Thosar et al. 2015 | N=12 ♂ BMI: 23.7 ± 3.4 kg/m2 Non-insulin resistant |
Cross-over (3h/condition) 1) Uninterrupted sitting 2) Sit + 5min LIW every hour |
Flow-mediated dilation: LIW > sitting Shear rate: LIW > sitting |
| Studies comparing the health effects of light-intensity and moderate-vigorous physical activity to sitting | |||
| Duvivier et al. 2013 | N=18 (16♀/2♂) BMI: 22.6 ± 2.6 kg/m2 Non-insulin resistant |
Cross-over (4d/condition) 1) Sit 14h/d 2) Sit 13h/d and 1h EX 3) Sit 8h/d, 4h LPA, 2h stand |
OGTT plasma insulin AUC: LPA < sitting and exercise Fasting lipids: LPA < sitting; LPA vs exercise: NS Fasting lipoproteins: LPA < sitting; LPA vs exercise: NS |
| Duvivier et al. 2017b | N=19 (6♀/13♂) BMI: 30 ± 2.0 kg/m2 Type 2 diabetes |
Cross-over (4d/condition) 1) Sit 14h/d with 4415 steps/d 2) Sit + 1.1h/d EX with 4823 steps/d 3) Sit + stand 2.5h/d and LIW 2.2h/d with 17,502 steps/d |
Glycemia (CGMs): LIW < exercise Insulin sensitivity index (HOMA2-IR): LIW < exercise |
| Duvivier et al. 2018 | N= 61 (33♀/28♂) BMI: 27.8 ± 4.3 kg/m2 Non-insulin resistant and type 2 diabetics |
Pooled analysis Cross-over (4d/condition) (1) Sit 14h/d (2) Sit + 1h/d MIW (3) Sit + 5–6h/d LIW and standing |
Endothelial function: LIW < MIW Insulin sensitivity index (HOMA2-IR): LIW < MIW Plasma lipids: LIW < MIW |
BMI: body mass index (kg/m2); h: hours; d: day; EX: exercise; body mass index; AUC: area under the curve; iAUC: incremental area under the curve; LIW: light-intensity walking; MIW: moderate-intensity walking; OGTT: oral glucose tolerance test; LPA: light-intensity physical activity; CGMs: continuous glucose monitoring system.
Where do we go from here?
Although MVPA produces a myriad of health benefits, it does not reduce time spent sedentary. Indeed, physically active people, even those who exceed the current guidelines, can be as sedentary as their inactive counterparts (Rantalainen et al., 2018). Increasing MVPA can even trigger spontaneous behavioral compensations in sedentary adults leading to a decrease in non-exercise activities (i.e. LPA) in favor of sedentary time (Lefai et al., 2017). Furthermore, MVPA does not fully offset the adverse health effects of large volumes of SB, as shown by the bedrest studies with concomitant exercise training. The remaining question is how much MVPA is needed to offset the effects of a certain amount of SB. It was shown that independent of physical activity, every hour spent sitting increases the risk of mortality by 5.9%, of T2D by 22% and of obesity by 23% (Hu et al., 2003; Wilmot et al., 2012; Chau et al., 2013). A meta-analysis including more than 1 million individuals further showed that 60–75 min/d of MVPA are needed to prevent the risk of premature death associated with 9h/d or more of sitting time (Ekelund et al., 2016); 9h/d being close to the average sitting time observed in modern societies. When most of the population does not reach the recommended guidelines (i.e. 30 min/d of MVPA, 5d/wk.), adding 30–45 more minutes per day of MVPA is unrealistic. Therefore, other pragmatic and efficient strategies are needed.
Activities of daily living (i.e. LPA) are inversely associated with SB; increases in LPA are associated with reductions in sedentary time (Pate et al., 2008). In addition, large volumes of LPA, here considered as any body movements associated with activities of daily living, have been shown to confer health benefits. Knowing that lack of time is a major barrier to the practice of exercise/MVPA, reintroducing LPA into daily life could be an effective strategy to reduce sedentary time and prevent its effects on metabolic health. In this line, the latest guidelines from the World Health Organization (Bull et al., 2020) promote the practice of physical activity of any intensity to reduce sedentary activities. In other words, moving is better than sitting. Future mechanistic studies will need to establish the physiology of SB and LPA to better understand the respective negative and positive health effects, and thus better define the dose-response relationship between the components of PA behavior and key health outcomes. Experimental research examining these relationships will foster the development of more specific and pragmatic public health guidelines.
Funding
This publication was made possible by funding to ELR from the CNRS GDR Sport & Activité Physique, by funding to NDJ from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institute for Health (NIH) (F31DK125061), by funding to DHB from the Colorado Nutrition Obesity Research Center (NORC P30DK048520), by funding to CS and SB from the French Space Agency, by funding to AB from the French Space Agency (CNES), the NIH/NIDDK (R00DK100465), the NORC (P30DK048520).
Biography
The authors are a group of researchers from the University of Colorado in the USA, the French National Centre for Scientific Research (CNRS) and the University of Lyon who are experts in metabolism, exercise and physical inactivity physiology. Elisa Le Roux and Nathan DeJong are graduate students working under the mentorship of Audrey Bergouignan. Stéphane Blanc, PhD, is studying the role of environment on the regulation of energy balance. Chantal Simon, MD, PhD, is professor of Nutrition and expert in metabolism, physical activity and sedentary behaviors. Daniel Bessesen, MD, is an endocrinologist who studies the regulation of body weight at the University of Colorado. Audrey Bergouignan, PhD, is an expert in metabolism, sedentary behaviors and physical inactivity physiology. The group, led by Dr Bergouignan, is building an international lab including members from the two institutions to address the role of sedentary behaviors in the onset and progression of metabolic diseases.

Elisa Le Roux

Nathan DeJong
Footnotes
Conflict of interest
No conflict of interest to declare.
References
- Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Alsted TJ, Kiens B, Stallknecht B, Dela F & Vaag A. (2010a). Increased rate of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. American Journal of Physiology Endocrinology and Metabolism 298, E555–564. [DOI] [PubMed] [Google Scholar]
- Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Stallknecht B, Dela F & Vaag A. (2009). Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 58, 2749–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibegovic AC, Sonne MP, Højbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F & Vaag A. (2010b). Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. American Journal of Physiology Endocrinology and Metabolism 299, E752–763. [DOI] [PubMed] [Google Scholar]
- Amagasa S, Machida M, Fukushima N, Kikuchi H, Takamiya T, Odagiri Y & Inoue S. (2018). Is objectively measured light-intensity physical activity associated with health outcomes after adjustment for moderate-to-vigorous physical activity in adults? A systematic review. The International Journal of Behavioral Nutrition and Physical Activity 15, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DP & Locke CD. (2015). Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. Journal of science and medicine in sport 18, 294–298. [DOI] [PubMed] [Google Scholar]
- Belavý DL, Möhlig M, Pfeiffer AF, Felsenberg D & Armbrecht G. (2014). Preferential deposition of visceral adipose tissue occurs due to physical inactivity. Int J Obes (Lond) 38, 1478–1480. [DOI] [PubMed] [Google Scholar]
- Benatti FB, Larsen SA, Kofoed K, Nielsen ST, Harder-Lauridsen NM, Lyngbæk MP, Eriksen D, Karstoft K, Krogh-Madsen R, Pedersen BK & Ried-Larsen M. (2017). Intermittent Standing but not a Moderate Exercise Bout Reduces Postprandial Glycemia. Med Sci Sports Exerc 49, 2305–2314. [DOI] [PubMed] [Google Scholar]
- Bergouignan A, Momken I, Schoeller DA, Normand S, Zahariev A, Lescure B, Simon C & Blanc S. (2010). Regulation of energy balance during long-term physical inactivity induced by bed rest with and without exercise training. The Journal of Clinical Endocrinology and Metabolism 95, 1045–1053. [DOI] [PubMed] [Google Scholar]
- Bergouignan A, Rudwill F, Simon C & Blanc S. (2011). Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol (1985) 111, 1201–1210. [DOI] [PubMed] [Google Scholar]
- Bergouignan A, Schoeller DA, Normand S, Gauquelin-Koch G, Laville M, Shriver T, Desage M, Le Maho Y, Ohshima H, Gharib C & Blanc S. (2006). Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary Fatty acids: results of a randomized trial. PLoS clinical trials 1, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergouignan A, Trudel G, Simon C, Chopard A, Schoeller DA, Momken I, Votruba SB, Desage M, Burdge GC, Gauquelin-Koch G, Normand S & Blanc S. (2009). Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes 58, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biensø RS, Ringholm S, Kiilerich K, Aachmann-Andersen N-J, Krogh-Madsen R, Guerra B, Plomgaard P, van Hall G, Treebak JT, Saltin B, Lundby C, Calbet JAL, Pilegaard H & Wojtaszewski JFP. (2012). GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes 61, 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks NE, Cadena SM, Cloutier G, Vega-López S, Roubenoff R & Castaneda-Sceppa C. (2014). Influence of Exercise on the Metabolic Profile Caused by 28 days of Bed Rest with Energy Deficit and Amino Acid Supplementation in Healthy Men. International Journal of Medical Sciences 11, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput J-P, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V & Willumsen JF. (2020). World Health Organization 2020 guidelines on physical activity and sedentary behaviour. British Journal of Sports Medicine 54, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastin SFM, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M & Stamatakis E. (2019). How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. British journal of sports medicine 53, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, Bauman AE & van der Ploeg HP. (2013). Daily sitting time and all-cause mortality: a meta-analysis. PloS one 8, e80000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiot A, Demangel R, Noone J, Chery I, Zahariev A, Normand S, Brioche T, Crampes F, de Glisezinski I, Lefai E, Bareille MP, Chopard A, Drai J, Collin-Chavagnac D, Heer M, Gauquelin-Koch G, Prost M, Simon P, Py G, Blanc S, Simon C, Bergouignan A & O’Gorman DJ. (2019). A nutrient cocktail prevents lipid metabolism alterations induced by 20 days of daily steps reduction and fructose overfeeding: result from a randomized study. Journal of Applied Physiology (Bethesda, Md: 1985) 126, 88–101. [DOI] [PubMed] [Google Scholar]
- Dempsey PC, Owen N, Yates TE, Kingwell BA & Dunstan DW. (2016a). Sitting Less and Moving More: Improved Glycaemic Control for Type 2 Diabetes Prevention and Management. Curr Diab Rep 16, 114. [DOI] [PubMed] [Google Scholar]
- Dempsey PC, Sacre JW, Larsen RN, Straznicky NE, Sethi P, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA & Dunstan DW. (2016b). Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens 34, 2376–2382. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Howard B, Healy GN & Owen N. (2012). Too much sitting--a health hazard. Diabetes Res Clin Pract 97, 368–376. [DOI] [PubMed] [Google Scholar]
- Duvivier B, Schaper NC, Koster A, van Kan L, Peters HPF, Adam JJ, Giesbrecht T, Kornips E, Hulsbosch M, Willems P, Hesselink MKC, Schrauwen P & Savelberg H. (2017a). Benefits of Substituting Sitting with Standing and Walking in Free-Living Conditions for Cardiometabolic Risk Markers, Cognition and Mood in Overweight Adults. Front Physiol 8, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvivier BM, Schaper NC, Bremers MA, van Crombrugge G, Menheere PP, Kars M & Savelberg HH. (2013). Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PloS one 8, e55542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvivier BM, Schaper NC, Hesselink MK, van Kan L, Stienen N, Winkens B, Koster A & Savelberg HH. (2017b). Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 60, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvivier BMFM, Bolijn JE, Koster A, Schalkwijk CG, Savelberg HHCM & Schaper NC. (2018). Reducing sitting time versus adding exercise: differential effects on biomarkers of endothelial dysfunction and metabolic risk. Sci Rep 8, 8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Steene-Johannessen J, Brown WJ, Fagerland MW, Owen N, Powell KE, Bauman A & Lee IM. (2016). Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. The Lancet 388, 1302–1310. [DOI] [PubMed] [Google Scholar]
- Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn IM, Hagstromer M, Edwardson C, Yates T, Shiroma E, Anderssen SA & Lee IM. (2019). Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 366, l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalo R, Tesch PA, Lundberg TR, Alkner BA, Rullman E & Gustafsson T. (2020). Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Tipton KD, Bamman MM & Wolfe RR. (1997). Resistance exercise maintains skeletal muscle protein synthesis during bed rest. Journal of Applied Physiology (Bethesda, Md: 1985) 82, 807–810. [DOI] [PubMed] [Google Scholar]
- Guinet P, MacNamara JP, Berry M, Larcher F, Bareille MP, Custaud MA, Pavy-Le Traon A, Levine BD & Navasiolava N. (2020). MNX (Medium Duration Nutrition and Resistance-Vibration Exercise) Bed-Rest: Effect of Resistance Vibration Exercise Alone or Combined With Whey Protein Supplementation on Cardiovascular System in 21-Day Head-Down Bed Rest. Front Physiol 11, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ & Owen N. (2007). Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 30, 1384–1389. [DOI] [PubMed] [Google Scholar]
- Healy GN, Winkler EAH, Brakenridge CL, Reeves MM & Eakin EG. (2015). Accelerometer-derived sedentary and physical activity time in overweight/obese adults with type 2 diabetes: cross-sectional associations with cardiometabolic biomarkers. PloS One 10, e0119140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Li TY, Colditz GA, Willett WC & Manson JE. (2003). Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. Jama 289, 1785–1791. [DOI] [PubMed] [Google Scholar]
- Irimia JM, Guerrero M, Rodriguez-Miguelez P, Cadefau JA, Tesch PA, Cussó R & Fernandez-Gonzalo R. (2017). Metabolic adaptations in skeletal muscle after 84 days of bed rest with and without concurrent flywheel resistance exercise. Journal of Applied Physiology (Bethesda, Md: 1985) 122, 96–103. [DOI] [PubMed] [Google Scholar]
- Kenny HC, Rudwill F, Breen L, Salanova M, Blottner D, Heise T, Heer M, Blanc S & O’Gorman DJ. (2017). Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia 60, 1491–1501. [DOI] [PubMed] [Google Scholar]
- Kenny HC, Tascher G, Ziemianin A, Rudwill F, Zahariev A, Chery I, Gauquelin-Koch G, Barielle M-P, Heer M, Blanc S, O’Gorman DJ & Bertile F. (2020). Effectiveness of Resistive Vibration Exercise and Whey Protein Supplementation Plus Alkaline Salt on the Skeletal Muscle Proteome Following 21 Days of Bed Rest in Healthy Males. Journal of Proteome Research. [DOI] [PubMed] [Google Scholar]
- Krainski F, Hastings JL, Heinicke K, Romain N, Pacini EL, Snell PG, Wyrick P, Palmer MD, Haller RG & Levine BD. (2014). The effect of rowing ergometry and resistive exercise on skeletal muscle structure and function during bed rest. Journal of Applied Physiology (Bethesda, Md: 1985) 116, 1569–1581. [DOI] [PubMed] [Google Scholar]
- Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT & Group LPASW. (2012). Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (London, England) 380, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SMC, Schneider SM, Feiveson AH, Macias BR, Smith SM, Watenpaugh DE & Hargens AR. (2014). WISE-2005: Countermeasures to prevent muscle deconditioning during bed rest in women. Journal of Applied Physiology (Bethesda, Md: 1985) 116, 654–667. [DOI] [PubMed] [Google Scholar]
- Lefai E, Blanc S, Momken I, Antoun E, Chery I, Zahariev A, Gabert L, Bergouignan A & Simon C. (2017). Exercise training improves fat metabolism independent of total energy expenditure in sedentary overweight men, but does not restore lean metabolic phenotype. International Journal Of Obesity 41, 1728. [DOI] [PubMed] [Google Scholar]
- Loh R, Stamatakis E, Folkerts D, Allgrove JE & Moir HJ. (2019). Effects of Interrupting Prolonged Sitting with Physical Activity Breaks on Blood Glucose, Insulin and Triacylglycerol Measures: A Systematic Review and Meta-analysis. Sports Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M, Edwardson CL, Davies MJ, Henson J, Bodicoat DH, Khunti K, Dunstan DW, King JA & Yates T. (2017). Fitness Moderates Glycemic Responses to Sitting and Light Activity Breaks. Med Sci Sports Exerc 49, 2216–2222. [DOI] [PubMed] [Google Scholar]
- Moriggi M, Vasso M, Fania C, Capitanio D, Bonifacio G, Salanova M, Blottner D, Rittweger J, Felsenberg D, Cerretelli P & Gelfi C. (2010). Long term bed rest with and without vibration exercise countermeasures: effects on human muscle protein dysregulation. Proteomics 10, 3756–3774. [DOI] [PubMed] [Google Scholar]
- Mutin-Carnino M, Carnino A, Roffino S & Chopard A. (2014). Effect of muscle unloading, reloading and exercise on inflammation during a head-down bed rest. Int J Sports Med 35, 28–34. [DOI] [PubMed] [Google Scholar]
- Pate RR, O’Neill JR & Lobelo F. (2008). The evolving definition of “sedentary”. Exerc Sport Sci Rev 36, 173–178. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Downs M, Goetchius E, Crowell B, English KL, Ploutz-Snyder R, Ryder JW, Dillon EL, Sheffield-Moore M & Scott JM. (2018). Exercise Training Mitigates Multisystem Deconditioning during Bed Rest. Medicine and Science in Sports and Exercise 50, 1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsford RM, Blackwell J, Hillsdon M & Kos K. (2017). Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: A randomised crossover study in inactive middle-aged men. Journal of science and medicine in sport 20, 278–283. [DOI] [PubMed] [Google Scholar]
- Rantalainen T, Pesola AJ, Quittner M, Ridgers ND & Belavy DL. (2018). Are habitual runners physically inactive? J Sports Sci 36, 1793–1800. [DOI] [PubMed] [Google Scholar]
- Rudwill F, Bergouignan A, Gastebois C, Gauquelin-Koch G, Lefai E, Blanc S & Simon C. (2015). Effect of enforced physical inactivity induced by 60-day of bed rest on hepatic markers of NAFLD in healthy normal-weight women. Liver International: Official Journal of the International Association for the Study of the Liver 35, 1700–1706. [DOI] [PubMed] [Google Scholar]
- Rudwill F, Blanc S, Gauquelin-Koch G, Choukèr A, Heer M, Simon C & Bergouignan A. (2013). Effects of different levels of physical inactivity on plasma visfatin in healthy normal-weight men. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquee, Nutrition Et Metabolisme 38, 689–693. [DOI] [PubMed] [Google Scholar]
- Rudwill F, O’Gorman D, Lefai E, Chery I, Zahariev A, Normand S, Pagano AF, Chopard A, Damiot A, Laurens C, Hodson L, Canet-Soulas E, Heer M, Meuthen PF, Buehlmeier J, Baecker N, Meiller L, Gauquelin-Koch G, Blanc S, Simon C & Bergouignan A. (2018). Metabolic Inflexibility Is an Early Marker of Bed-Rest-Induced Glucose Intolerance Even When Fat Mass Is Stable. The Journal of Clinical Endocrinology and Metabolism 103, 1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova M, Schiffl G, Püttmann B, Schoser BG & Blottner D. (2008). Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. Journal of Anatomy 212, 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thosar SS, Bielko SL, Mather KJ, Johnston JD & Wallace JP. (2015). Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47, 843–849. [DOI] [PubMed] [Google Scholar]
- Thyfault JP & Bergouignan A. (2020). Exercise and metabolic health: beyond skeletal muscle. Diabetologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N & Trappe T. (2008). Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. American Journal of Physiology Regulatory, Integrative and Comparative Physiology 294, R939–947. [DOI] [PubMed] [Google Scholar]
- Trappe S, Creer A, Slivka D, Minchev K & Trappe T. (2007a). Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. Journal of Applied Physiology (Bethesda, Md: 1985) 103, 1242–1250. [DOI] [PubMed] [Google Scholar]
- Trappe S, Trappe T, Gallagher P, Harber M, Alkner B & Tesch P. (2004). Human single muscle fibre function with 84 day bed-rest and resistance exercise. The Journal of Physiology 557, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe TA, Burd NA, Louis ES, Lee GA & Trappe SW. (2007b). Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiologica (Oxford, England) 191, 147–159. [DOI] [PubMed] [Google Scholar]
- Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM & Participants STCP. (2017). Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. The International Journal of Behavioral Nutrition and Physical Activity 14, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel G, Coletta E, Cameron I, Belavy DL, Lecompte M, Armbrecht G, Felsenberg D & Uhthoff HK. (2012). Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. Journal of Applied Physiology (Bethesda, Md: 1985) 112, 1824–1831. [DOI] [PubMed] [Google Scholar]
- Trudel G, Payne M, Mädler B, Ramachandran N, Lecompte M, Wade C, Biolo G, Blanc S, Hughson R, Bear L & Uhthoff HK. (2009). Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. Journal of Applied Physiology (Bethesda, Md: 1985) 107, 540–548. [DOI] [PubMed] [Google Scholar]
- Ward K, Mulder E, Frings-Meuthen P, O’Gorman DJ & Cooper D. (2020). Fetuin-A as a Potential Biomarker of Metabolic Variability Following 60 Days of Bed Rest. Frontiers in Physiology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T & Biddle SJ. (2012). Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 55, 2895–2905. [DOI] [PubMed] [Google Scholar]


