Abstract
Introduction
Osteoarthritis (OA) is a common inflammatory joint disease characterised by progressive cartilage destruction. Management of this condition remains a significant challenge, and new therapies are required. We investigated the protective effects of miR-106a mimics in a murine model of OA.
Material and methods
This study was performed using both in vitro and in vivo OA models. Primary chondrocytes were isolated from female rats, with inflammation induced via treatment with lipopolysaccharide (LPS). Then the effects of a miR-106a mimic were examined based on the level of inflammatory cytokine production and apoptotic signalling following LPS stimulation. An in vivo rat model of OA was generated by injecting LPS into the anterior cruciate ligament, followed by treatment with miR-106a mimics. Then, inflammatory and apoptotic protein expression was assessed in the cartilage tissue.
Results
Treatment with miR-106a mimic reduced the levels of inflammatory cytokines and apoptotic proteins in cartilage tissues following LPS-induced inflammation. Furthermore, the mimic ameliorated the expression of DR-6 mRNA and DR6, IκBα, and p65 proteins in chondrocytes. Similar effects were seen in the in vivo model, with the mimic attenuating expression of NF-κB, p65, IκBα, and DR6 proteins and improving histopathological outcomes in the chondrocytes of OA rats.
Conclusions
Treatment with miR-106a mimic ameliorates inflammation in cartilage tissues of OA subjects by activating death receptor 6 via the NF-κB signalling pathway.
Keywords: osteoarthritis, miR-106a, chondrocytes, cytokines, nuclear factor-κB, death receptor
Introduction
Osteoarthritis (OA) is a common inflammatory joint disease characterised by progressive cartilage destruction [1]. As a long-term, progressive condition, OA is commonly observed in elderly patients, resulting in a significant economic burden worldwide. OA pathology is thought to arise as a result of changes in chondrocyte homeostasis, resulting in an imbalance between degeneration and repair of cartilage [2]. Further progression of OA is driven by chronic joint inflammation, which is responsible for much of the pathology seen in this disease [3]. Therefore, identifying mechanisms capable of targeting joint inflammation and apoptosis of chondrocytes is a promising strategy for the management of OA pathology.
miRNAs regulate a wide range of cellular functions including proliferation, differentiation, and apoptosis [4]. Recent studies have identified numerous miRNAs that are dysregulated in many disorders, including OA [5], which makes them an attractive target for the management of OA. Moreover, several pathogenic mechanisms associated with OA have been linked to miRNAs, including regulation of bone and extracellular matrix remodelling and production of inflammatory mediators, further supporting their importance in disease pathology [6]. miRNA-106a exhibits nephroprotective, anticancer, and antiproliferative activities [7–9], and contributes to the development of skeletal muscle. Moreover, inhibition of miRNA-106a ameliorates sepsis-induced renal injury by regulating various inflammatory pathways [10]. Among the more specific targets, miRNA-106a has been shown to regulate various death receptors (DRs, a family of tumour necrosis factor receptors present on the cell surface), including DR6 [11]. Among these receptors, DR6 signalling triggers apoptosis via activation of the nuclear factor κB (NF-κB) signalling pathway [12]. Here, we evaluated the effects of an miRNA-106a mimic in the context of OA.
Material and methods
Animals
Female Sprague Dawley rats (200–220 g) were housed under a 12 h light/dark cycle in standard conditions (i.e., 24 ±3°C at 60 ±5% humidity). All protocols were approved by the institutional animal ethical committee of The First Hospital of Shan Xi Medical University, China (IAEC/FH-SXMU/2017/09).
Chemicals
DR6 siRNA, control siRNA, negative mimic, and miR-106a mimic were purchased from Genepharma, Shanghai, China. All antibodies were purchased from Thermo Fisher Scientific, USA. ELISA kits were procured from Thermo Fisher Scientific.
Isolation and culture of chondrocytes
Isolation of primary chondrocytes was performed as described previously [13]. Tibial plateau and femoral condyles were used for cartilage slices. Further cartilage tissues were cultured for 1 day at 37°C in Dulbecco’s modified Eagle medium (DMEM) supplemented with penicillin (100 U/ml), glutamine (2 mmol/l), streptomycin (100 µg/ml), and FSC (10%). Then cartilage tissue was removed, and chondrocytes were separated by centrifugation for 5 min at 2000 RPM, followed by resuspension in fresh medium.
LPS (100 ng/ml) was used to induce immune activation, and the role of NF-κB was estimated in the context of LPS-induced inflammation. Cells were pre-treated for 60 min with an NF-κB inhibitor (pyrrolidine dithiocarbamate), followed by LPS for 4 h, after which cytokine levels were assessed in the chondrocyte supernatant.
Transfection
siRNA or mimic (0.4 nmol) was used to transfect chondrocytes by mixing it with Geneporter 2 Transfection Reagent (15 µl). Cells were cultured for 2 days and the supernatant medium was replaced every 6 h with fresh medium.
Estimation of inflammatory cytokines
Levels of inflammatory cytokines in chondrocyte supernatants were estimated using a commercial ELISA kit according to the manufacturer’s recommendations.
Estimation of cellular apoptosis
Cellular apoptosis was assessed by flow cytometry using an Annexin V-FITC Apoptosis Detection Kit according to the manufacturer’s instructions. Briefly, cells were washed with PBS twice and incubated at room temperature for 10 min with Annexin V-FITC. Then they were treated with 1 mg/l propidium iodide solution. A FACSCalibur system was used to analyse the number of stained cells.
RT-PCR
Trizol reagent was used to extract total RNA from Iry chondrocytes and articular cartilage, followed by reverse transcriptase to generate cDNA. DR6 and β-actin expression in chondrocytes was examined using a SYBR Green gene expression assay. Analyses of miR-106a were performed using a TaqMan microRNA assay kit. Analyses were performed using the following primers: DR6 (forward: 5′-ACAGAAGGCCTCGAATCTCA-3′; reverse: 5′-TGCATTCTCGGTCAGTCAAG-3′), β-actin (forward: 5′-CAACTTGATGTATGAAGGCTTTGGT-3′; reverse: 5′-AC-TTTTATTGGTCTCAAGTCAGTGTACAG-3′), and miR-106a (forward: 5′-AAAAGUGCUUACAGUGCAGGU-AG-3′; reverse: 5′-ACCUGCACUGUAAGCACUUUUU-U-3′).
Western blot
M-PER protein extraction reagent supplemented with a protease inhibitor was used for chondrocyte lysis and protein extraction. A Bradford assay kit was used to determine the concentration of total cellular protein. Following quantification, proteins were separated by 10% SDS-PAGE and the reaction was blocked by treating the cells with 5% skim milk. Then the proteins were transferred to a nitrocellulose membrane and incubated overnight in the presence of antibodies against IκBα (1 : 200 dilution), p65 (1 : 100 dilution), DR6 (1 : 200 dilution), and β-actin (1 : 100 dilution). Next, the membranes were rinsed with PBS and incubated with peroxidase-conjugated secondary antibodies for 60 min. An ECL kit was used for the detection of chemiluminescence and images were estimated using a Bio-Rad ChemiDoc MP imaging system.
MTT assay
An MTT assay was used to determine the effects of miR-106a on the viability of chondrocytes. Cells (2 × 105 cells/cm2) were seeded into 96-well plates and then incubated with 0.5 mg/ml MTT reagent at 37°C for 4 h. Absorbance of supernatant solution was determined using a Bio-Rad microplate reader.
Development of an in vivo model of osteoarthritis
All animals were randomly allocated to OA, miR-106a mimic-treated, and sham surgery control groups (n = 8 animals per group). OA was induced in animals as described previously by injecting anterior cruciate ligament transection (ACLT) into the right knees. All animals were anesthetized by injecting them with 60 mg/kg ketamine hydrochloride and 7 mg/kg xylazine. An incision was made on the medial side of the patellar tendon, the patella was dislocated from the leg, and a surgical blade was used to transect the anterior cruciate ligament. Skin was closed after repairing the medial retinaculum. In the miR-106a mimic-treated group, rats were treated with miR-106a mimic applied directly into the articular cavity.
Estimation of protein expression and cytokine levels
All animals were sacrificed via decapitation, and articular cartilage tissues were isolated from the medial tibial plateau of each animal. Expression of IκBα, p65, DR6, and miR-106a in the tissue homogenate was assessed by Western blotting. SF was isolated and assessed for cytokine abundance by ELISA.
Histopathological analyses
Knee joints were isolated and fixed in 10% formalin solution for 3 days. Following fixation, the joints were seeded into molten paraffin and cut into sections 6 µm thick using a microtome. Safranin-O was used to stain the tissue section and cartilage degeneration was scored as described previously.
Statistical analysis
All data are expressed as the mean ± SEM (n = 8). Statistical analyses were performed using a one-way ANOVA. Post hoc comparison of means was carried out using Dunnett’s post hoc test (Version 4.1, GraphPad Software, Inc., San Diego, CA, USA). The level of statistical significance was set at p < 0.05.
Results
miR-106a mimic ameliorates inflammatory cytokine expression
Figure 1 illustrates the effects of the miR-106a mimic on cytokine levels in LPS-induced chondrocytes. Clear increases in cytokine levels following LPS treatment were observed in the OA group compared to the sham group. miR-106a mimic treatment significantly reduced cytokine levels in LPS-induced chondrocytes relative to the OA group.
Figure 1.
miR-106a mimic ameliorates inflammatory cytokine production in the supernatant of LPS-induced chondrocytes
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
miR-106a mimic ameliorates cellular apoptosis
Effects of the miR-106a mimic on cell viability and apoptosis in LPS-induced chondrocytes were assessed using an MTT assay and flow cytometry, respectively (Figure 2). Cell viability was reduced by up to 49.6% in LPS-induced chondrocytes, relative to controls. miRNA-106a mimic treatment significantly increased the percentage of viable cells (62.7%), relative to the OA group. Similarly, the apoptosis rate increased to 46.8% in the OA group vs. only 6.2% in the sham control group. Treatment with the miR-106a mimic significantly reduced the rate of apoptosis in LPS-induced chondrocytes, relative to the OA group.
Figure 2.
miR-106a mimic attenuates LPS-induced apoptosis in chondrocytes. A – Assessment of cell viability by MTT assay, B – assessment of apoptosis of chondrocytes by flow cytometry
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
miR-106a mimic ameliorates expression of DR-6
Expression of DR6 was assessed in miR-106a mimic- and LPS-treated chondrocytes using RT-PCR and Western blotting (Figure 3). Clear increases in the expression of both DR6 mRNA and protein were observed in the sham control group relative to the OA group. Treatment with the miR-106a mimic reduced the expression of DR6 mRNA and protein in LPS-induced chondrocytes.
Figure 3.
miR-106a mimic attenuates DR6 expression. A – DR6 mRNA expression, B – DR6 protein expression
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
miR-106a mimic ameliorates expression of IκBα and p65 protein
Figure 4 shows the effects of the miR-106a mimic and an NF-κB inhibitor on the expression of IκBα and p65 proteins in LPS-treated chondrocytes. Expression of IκBα and p65 protein was significantly increased in the chondrocytes of the OA group relative to the sham control group. By contrast, treatment with either the miR-106a mimic or NF-κB inhibitor significantly attenuated the expression of IκBα and p65 proteins following LPS treatment.
Figure 4.
miR-106a mimic ameliorates relative expression of IkBα and p65 protein via inhibition of the NF-κB pathway
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
Effect of miR-106a mimic on expression of inflammatory mediators and miR-106a
Figure 5 shows the effects of the miR-106a mimic on the expression of inflammatory mediators, as well as cell-derived miR-106a in the cartilage of OA rats. Expression of miR-106a was significantly reduced in the cartilage of OA rats relative to the sham control group. Treatment with the miR-106a mimic enhanced the expression of miR-106a in cartilage tissues. Expression of inflammatory mediators was also significantly enhanced (p < 0.01) in the cartilage homogenate of the OA group relative to the sham control group. Overall concentrations of inflammatory mediators were significantly reduced in the cartilage of the group treated with miR-106a mimic relative to the OA group.
Figure 5.
miR-106a mimic suppresses miR-106a and inflammatory gene expression in OA rats
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
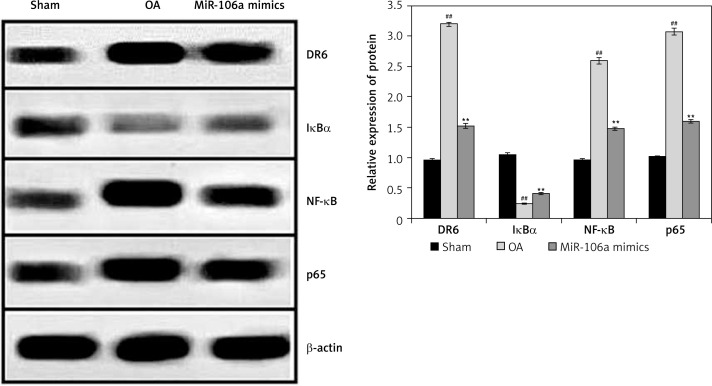
Effect of miR-106a mimic on protein expression of NF-κB, p65, IκBα, and DR
Expression of NF-κB, p65, IκBα, and DR6 proteins was examined in the cartilage of OA rats (Figure 6). Compared to controls, the OA group had higher expression levels of DR6, NF-κB, and p65 proteins, and lower levels of Iκ-Bα protein. Treatment with the miR-106a mimic attenuated these changes in all four proteins relative to the OA group.
Figure 6.
miR-106a mimic reduces NF-κB, p65, IkBα and DR6 protein expression in OA rats
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
Effects of miR-106a mimic on histopathology of cartilage tissue
Histopathological analyses of cartilage tissues were performed using safranin-O stain in miR-106a mimic-treated OA rats (Figure 7). The TS of cartilage in the OA group exhibited clear increases in erosion of the cartilage surface along with reductions in the staining of the superficial layer. Treatment with the miR-106a mimic ameliorated these changes. Histological scores were significantly higher in the cartilage of the OA group relative to sham-operated controls. Treatment with the miR-106a mimic significantly improved these scores, relative to the OA group.
Figure 7.
miR-106a mimic rescues the histopathology of cartilage tissue in OA rats by safranin-O staining
Mean ± SEM (n = 8), ##p < 0.01 vs. sham operated group; **p < 0.01 vs. OA group.
Discussion
OA is an important joint disease characterised by widespread inflammation and cartilage destruction, with few good management and treatment options [14]. Therefore, there is a significant need for the development of new therapies for the treatment of OA. In the present investigation, we evaluated the protective effects of miR-106a mimics in OA subjects using a combination of both in vitro and in vivo models. Primary chondrocytes were isolated from female rats and inflammation was induced by treating them with LPS. The effects of miR-106a mimic treatment on the level of inflammatory cytokines and apoptosis in LPS-induced inflammation were assessed. Moreover, RT-PCR and Western blotting analyses were performed to estimate mRNA and protein expression in chondrocytes. An in vivo model of OA in which ACLT was injected directly into the knees of OA rats, with or without miR-the effects better and helps to recruit others.
LPS-induced inflammation is regulated by miR-106a via the TLR4/NF-κB signalling pathway [15]. miRNA-106a has also been shown to regulate the activation of macrophages. Levels of inflammatory cytokines are strongly enhanced in the blood and cartilage tissues of OA patients [16]. In this study, we found that miRNA-106a significantly reduced inflammatory cytokine levels in affected cartilage, while simultaneously reducing the rate of apoptosis in cartilage tissues. NF-κB is a nuclear transcription factor that plays an important role in the inflammatory response. Activation of NF-κB can be inhibited by IκBα, as well as by reducing the expression of p65 [17]. The data presented here show that treatment with miR-106a mimics attenuates the expression of NF-κB pathway proteins.
DR6 plays an important role in the activation of NF-κB, and in turn contributes to apoptosis [18]. Activation of DR6 stimulates apoptosis, an important factor contributing to the development of OA [19]. The data presented here show that treatment with miR-106a attenuates the activation of DR6, leading to a reduction in apoptosis.
In conclusion, treatment with miR-106a mimics ameliorates inflammation in the cartilage tissue of OA subjects by activating DR6 via the NF-κB signalling pathway.
Acknowledgments
Luping Cui and Yongbin Han contribute to this work equally.
The authors of this manuscript would like to thank the First Hospital of Shan Xi Medical University, China, for providing the necessary facilities to conduct the work.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012; 64: 1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiao YQ, Jiang PF, Gao YZ. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch Med Sci 2018; 14: 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013; 5: 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumourigenesis. Br J Cancer 2006; 94: 776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papanagnou P, Stivarou T, Tsironi M. The role of miRNAs in common inflammatory arthropathies: osteoarthritis and gouty arthritis. Biomolecules 2016; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malemud CJ. MicroRNAs and osteoarthritis. Cells 2018; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Yue D, Zhou Y, et al. The role of microRNAs in Abeta deposition and tau phosphorylation in Alzheimer’s disease. Front Neurol 2017; 8: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Q, Ma Q. MicroRNA-106a inhibits cell proliferation and induces apoptosis in colorectal cancer cells. Oncol Lett 2018; 15: 8941–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoey C, Ray J, Jeon J, et al. miRNA-106a and prostate cancer radioresistance: a novel role for LITAF in ATM regulation. Mol Oncol 2018; 12: 1324–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Y, Yu J, Jing Y, Zhang J. MiR-106a aggravates sepsis-induced acute kidney injury by targeting THBS2 in mice model. Acta Cir Bras 2019; 34: e201900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Z, Jiang J, Kokkinaki M, et al. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells 2013; 31: 2205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 2001; 21: 5299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gartland A, Mechler J, Mason-Savas A, et al. In vitro chondrocyte differentiation using costochondral chondrocytes as a source of primary rat chondrocyte cultures: an improved isolation and cryopreservation method. Bone 2005; 37: 530–44. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 2017; 5: 16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arenas-Padilla M, Mata-Haro V. Regulation of TLR signalling pathways by microRNAs: implications in inflammatory diseases. Cent Eur J Immunol 2018; 43: 482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014; 2014: 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giridharan S, Srinivasan M. Mechanisms of NF-kappaB p65 and strategies for therapeutic manipulation. J Inflamm Res 2018; 11: 407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1: a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlier E, Relic B, Deroyer C, et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci 2016; 17: 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]