Abstract
Telehealth utilization increased during the COVID-19 pandemic, yet few studies have documented associations of telehealth use with subsequent medical costs and health care utilization. We examined associations of telehealth use during the early COVID-19 public health emergency (March–June 2020) with subsequent total medical costs and health care utilization among people with heart disease (HD). We created a longitudinal cohort of individuals with HD using MarketScan Commercial Claims data (2018–2022). We used difference-in-differences methodology adjusting for patients’ characteristics, comorbidities, COVID-19 infection status, and number of in-person visits. We found that using telehealth during the stay-at-home order period was associated with a reduction in total medical costs (by −$1814 per person), number of emergency department visits (by −88.6 per 1000 persons), and number of inpatient admissions (by −32.4 per 1000 persons). Telehealth use increased per-person per-year pharmacy prescription claims (by 0.514) and average number of days’ drug supply (by 0.773 days). These associated benefits of telehealth use can inform decision makers, insurance companies, and health care professionals, especially in the context of disrupted health care access.
Keywords: telehealth, heart disease, emergency department, inpatient admissions, total medical costs, difference-in-differences, reduction in medical costs
Introduction
Heart disease (HD) is a leading cause of morbidity and mortality in the United States. Over 20.5 million people have been diagnosed with chronic HD in 2017–2020,1 accounting for 21% of total US deaths in 2020.2 Although most HD risk factors are preventable, HD results in millions of emergency department (ED) visits and inpatient admissions, costing the nation $4.6 billion in ED visits and $56.9 billion in hospital inpatient stays in 2018–2019.1 Strengthening HD prevention, managing HD risk factors, and curtailing costs of inpatient and ED visits is a public health priority that can be supported by addressing modifiable risk factors and preserving care continuity.3
Telehealth, the delivery of health-related services via remote technologies,4 is widely considered an effective strategy to prevent and manage HD.5-7 The Centers for Disease Control and Prevention (CDC) Division for Heart Disease and Stroke Prevention refers to telehealth as a best practice,8 and the Community Preventive Services Task Force has found sufficient evidence to support a range of telehealth and complementary telehealth and in-person interventions.5-7 Telehealth, when complementing in-person care, can leverage other evidence-based practices (eg, remote patient monitoring, implementing multidisciplinary teams), while reducing barriers to care (eg, transportation, scheduling, costs).9 By improving health outcomes, enhancing quality of care,9,10 and reducing disruptions in care,11,12 telehealth can reduce the use of emergency resources, especially during public health emergencies (PHEs) such as the COVID-19 pandemic.
Because COVID-19 prompted stay-at-home orders in March 2020,13 “nonessential” care—including preventive screenings and care and chronic disease management and treatment14—was delayed early in the pandemic. To mitigate care disruptions while following physical distancing guidelines, the Centers for Medicare and Medicaid Services relaxed telehealth restrictions in March 2020 and encouraged private health insurers to follow suit.15 Subsequently, telehealth use rapidly increased,16-19 representing approximately one-third of outpatient and primary care visits during the early COVID-19 PHE, March–June 2020.20 Several studies examined associations of telehealth with access to care, quality of care, health outcomes, and costs; however, its association with cost savings has been inconclusive, especially among individuals with HD.9,10,21
Some studies demonstrate cost savings with telehealth vs conventional in-person methods,22-24 while others demonstrate no significant difference, or higher costs vs usual care.25-27 However, most studies are based on targeted programs or small patient cohorts before the COVID-19 pandemic.28,29 Few studies examined whether telehealth reduced medical costs and health care utilization following its widespread adoption in 2020.28,30,31
The American Heart Association found that stroke telemedicine, or telestroke, expands access to acute stroke care and improves the accuracy of diagnosing acute ischemic stroke while being cost-effective.32 In the management of cardiac disease through patient monitoring and engagement, telehealth demonstrated favorable clinical outcomes.32 Furthermore, telehealth for peripheral artery disease management reduced costs and travel time while enhancing patient satisfaction.32 A meta-analysis and systematic review on the efficacy of telehealth for cardiovascular disease (CVD) management found that remote monitoring and consultation for patients with heart failure reduced inpatient admissions, increased medication adherence, and lowered the risk of mortality in the short term.29
While this literature supports the potential of telehealth to enhance the prevention and management of CVD, it lacks examination on how these clinical outcomes translate into impacts on health care utilization and costs, particularly among individuals diagnosed with HD. Therefore, we examined associations of telehealth use during the early COVID-19 PHE when telehealth use increased most19 and almost all states implemented stay-at-home orders13 with subsequent medical costs and health care utilization among patients with HD.
Data and methods
Data
We used the Merative MarketScan Commercial Database from March 1, 2016, to June 30, 2022.33 The MarketScan database relies upon administrative medical claims of a large subsample of employer-sponsored health insurance plans for employees aged younger than 65 years and their dependents. The data were provided by more than 300 large employers, 30 health plans, and more than 500 hospitals in the United States, with 27.6 million enrollees in 2016 and 13.9 million in 2022. We accessed the data through Treatment Pathways, a MarketScan cloud-based tool allowing researchers to extract data using an online query system. This study used secondary data analysis using deidentified information. Therefore, a review by CDC's Institutional Review Board was unnecessary.
Analytic cohort
The analytic cohort included adults aged 18–64 years as of June 30, 2022 (day of last follow-up), with diagnosed HD during March 1, 2016–February 28, 2018, enrolled in a commercial health insurance plan (Appendix Figure S1). Individuals with diagnosed HD were identified as having 1 or more inpatient admission or 2 or more outpatient visits (≥30 days apart) with an International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), diagnosis code (ICD-10-CM = I00–I09, I11, I13, I20–I51) (Appendix Table S1). The HD conditions considered in this study encompass various conditions, including coronary heart disease, acute myocardial infarction (AMI), cardiac dysrhythmia, atrial fibrillation, and heart failure. Persons were excluded if they were not continuously enrolled in a commercial health plan from March 1, 2016, to June 30, 2022; had a pregnancy diagnosis (Appendix Table S1)34; or had a capitated insurance plan (because medical costs are not fully captured in capitated plans) during March 1, 2016–June 30, 2022.
Telehealth use
A telehealth visit was identified as an outpatient encounter with a telehealth-related place of service or procedure-modifier code (Appendix Table S2).33 A non–telehealth visit was defined as an in-person outpatient encounter. Heart disease–related telehealth visits were defined as telehealth visits with an HD diagnosis code (Appendix Table S1). We assessed telehealth use and in-person outpatient encounters during the stay-at-home order period, when telehealth use increased the most.16-19 We defined the stay-at-home order period as March 1–June 30, 2020, when many state, county, and local jurisdictions issued stay-at-home orders.13
Outcome variables
For medical costs, we examined all-cause total medical costs and HD-related total medical costs, which are the sum of costs paid to providers and individuals’ out-of-pocket costs. For health care utilization, we examined numbers of ED visits and inpatient admissions (containing an HD diagnosis code; Appendix Table S1), number of pharmacy prescriptions, and average number of days of prescription supply. We further examined costs associated with ED visits, inpatient admissions, outpatient visits, and pharmacy prescriptions. All of the costs were adjusted to the 2022 Personal Consumption Expenditures Price Index for health from the Bureau of Economic Analysis.35 We measured the outcome variables during the pre–stay-at-home order period, which was the 2 years before (March 2018–February 2020), as well as the post–stay-at-home order period, which was the 2 years after (July 2020–June 2022) the stay-at-home order period.
Patient characteristics
Patient characteristics included age group (18–34 [reference], 35–44, 45–54, and 55– 64 years), sex (male [reference], female), patient's geographic location (urban [reference], rural, per US Census Metropolitan Statistical Area [MSA] designation33,36: urban = MSA, rural = non-MSA), patient's census region (Northeast [reference], Midwest, South, West), number of in-person outpatient visits during the stay-at-home order periods, telehealth use (only had telehealth or had both telehealth and in-person outpatient visits during the stay-at-home order period) or no telehealth use (only had in-person outpatient visits or had no outpatient encounters or telehealth or in-person visits during the stay-at-home order period), COVID-19 diagnosis (persons with ICD-10-CM of U07.1, identified before, during, and after the stay-at-home order periods), and Quan-Charlson comorbidity conditions37 (identified before and after the stay-at-home order period to account for potential time-varying confounders).38 Comorbidity conditions were identified as 1 or more inpatient admission or 2 or more outpatient encounters (≥30 days apart) with the ICD-10-CM codes documented in Quan et al.37 Acute myocardial infarction and congestive heart failure (CHF) were excluded as comorbidities because they were included in our HD definition (Appendix Table S1).37 However, we presented the results after including the 2 conditions as a sensitivity analysis.
Statistical analysis
The Wilcoxon nonparametric rank-sum test was used to compare differences in means for continuous variables, and Pearson's chi-square test was used to compare differences in proportions for categorical variables by telehealth use status during the stay-at-home order period.
We calculated difference-in-differences (DID) estimates to compare the difference in medical costs and health care utilization before and after the stay-at-home order period among those who used telehealth (telehealth users) and those who did not use telehealth (telehealth nonusers). The DID methodology is widely used in public health to minimize potential confounding factors that potentially affect both the exposure (telehealth use) and outcome variables.39 We adjusted for patient characteristics, comorbidities, and COVID-19 diagnosis. We also controlled for the number of in-person outpatient visits during the stay-at-home order period. Therefore, the DID estimates compare pre-post changes in outcomes between telehealth users and telehealth nonusers for patients with either zero or the same number of in-person visits. The overall DID estimate is a weighted average of differences in pre-post difference in outcomes for several comparison group pairs, which can be broadly categorized into patients who only used telehealth (compared with patients who had no outpatient encounters) and patients who had both telehealth and in-person visits (compared with patients who had the same number of in-person visits only).
We graphically examined unadjusted trends in the average values of the outcomes in the pre– and post–stay-at-home order periods by telehealth use status to test the parallel trend assumption for the DID method.
For sensitivity analysis, we repeated the DID analysis using a generalized linear model (GLM) with a gamma distribution and a log-link function for cost-related outcomes and a zero-inflated negative binomial (ZINB) model for health care utilization.
Our primary models incorporated a control for the number of in-person visits to accommodate variations in the use of in-person care and no care during the early PHE. This ensures that the interpretation of telehealth estimates is framed within the context of being complementary to in-person visits. To present a comprehensive view of the overall impacts of telehealth use, we conducted a sensitivity analysis without controlling for the number of in-person visits. These alternative estimates capture both complementary and substitution effects of telehealth use.
We also conducted additional sensitivity analyses to assess whether our results were influenced by the regression to the mean (RTM) hypothesis.40 Utilizing the Stata package “rtmci” (StataCorp LLC, College Station, TX),41 we estimated the impacts of RTM using the mean values of the outcome variables during the pre-period as the cutoff value. We focused on total medical costs, number of ED visits, inpatient admissions, and other cost outcomes, including ED, inpatient admissions, outpatient, and pharmacy costs. These were chosen as certain patterns in these outcomes appeared to differ between telehealth users and nonusers before and during the COVID-19 pandemic. Bootstrap resampling with 1000 replications was used to derive SEs and 95% CIs.
Last, we provided estimates from GLM (for cost-related outcomes) and negative binomial models (for health care utilization) without using the DID study design. This comparison allowed us to analyze the association of telehealth use with the same subsequent outcomes as used in the DID models. Comparing these models and the DID models sheds light on the significance of endogeneity issues—highlighting differences between individuals self-selecting whether or not to use telehealth.
The average marginal effects with 95% CIs were reported for the DID estimates from the linear, GLM, and ZINB models. Both adjusted and unadjusted DID estimates were reported. All estimates are per patient per year. The coefficients of the DID models reflect relative changes (not absolute changes from the pre-period) for the telehealth user group when compared with the telehealth nonuser group.
P values less than .05 were used to indicate statistical significance for 2-sided testing. All analyses were conducted using Stata/MP statistical software (version 17, StataCorp LLC) for December 2021–March 2023.
Results
A total of 70 041 commercially insured individuals aged 18–64 years had a diagnosis of HD and received health care services in an inpatient or outpatient setting between March 1, 2016, and February 28, 2018 (Appendix Figure S1). Of these, 21 598 (30.8%) had 1 or more telehealth visits and 5858 (8.4%) had 1 or more HD-related telehealth visits during the stay-at-home order period. Telehealth users (mean age, 54.8 years) were similar in age to non–telehealth users (mean, 54.9 years; P = .28) but were more likely to be female (46.0% vs 37.8%; P < .001), live in an urban area (88.2% vs 83.4%; P < .001), and have a COVID-19 diagnosis during this period (1.6% vs 0.5%; P < .001) (Table 1). The average number of in-person outpatient visits during this period was higher for individuals who used telehealth than for those who did not (8.0 vs 5.4; P < .001). Those who used telehealth had a higher percentage of comorbidities (for most conditions assessed) vs those who did not. Appendix Table S3 outlines the frequency distributions of in-person visits categorized by telehealth utilization status. The analysis revealed a notably higher number of in-person visits among individuals engaging with telehealth.
Table 1.
Patient characteristics and number of telehealth/in-person visits during the stay-at-home order period among individuals with heart disease.
| All (n = 70 041) (100%) | Telehealth = no (n = 48 443) (69.2%) | Telehealth = yes (n = 21 598) (30.8%) | P a | |
|---|---|---|---|---|
| Age, mean (SD), y | 54.8 (9.1) | 54.9 (9.2) | 54.8 (8.9) | .28 |
| Age groups, n (%) | ||||
| 18–34 y | 2942 (4.20%) | 2083 (4.30%) | 859 (3.98%) | .049 |
| 35–44 y | 4579 (6.54%) | 3089 (6.38%) | 1490 (6.90%) | .010 |
| 45–54 y | 17 579 (25.10%) | 11 967 (24.70%) | 5612 (25.98%) | <.001 |
| 55–64 y | 44 941 (64.16%) | 31 304 (64.62%) | 13 637 (63.14%) | <.001 |
| Female, n (%) | 28 237 (40.31%) | 18 302 (37.78%) | 9935 (46.00%) | <.001 |
| Urban residency, n (%) | 59 428 (84.85%) | 40 380 (83.36%) | 19 048 (88.19%) | <.001 |
| Census region, n (%) | ||||
| Northeast | 9846 (14.06%) | 5818 (12.01%) | 4028 (18.65%) | <.001 |
| Midwest | 13 629 (19.46%) | 9896 (20.43%) | 3733 (17.28%) | <.001 |
| South | 35 597 (50.82%) | 25 388 (52.41%) | 10 209 (47.27%) | <.001 |
| West | 6694 (9.56%) | 4448 (9.18%) | 2246 (10.40%) | <.001 |
| COVID-19 diagnosis, n (%) | ||||
| Stay-at-home order period: March 1, 2020–June 30, 2020 | 601 (0.86%) | 262 (0.54%) | 339 (1.57%) | <.001 |
| July 1, 2020–June 30, 2021 | 6412 (9.15%) | 4214 (8.70%) | 2198 (10.18%) | <.001 |
| July 1, 2021–June 30, 2022 | 10 114 (14.44%) | 6563 (13.55%) | 3551 (16.44%) | <.001 |
| Number of telehealth/in-person visits during stay-at-home order, mean (SD) | ||||
| Telehealth visits | 0.6 (1.6) | 0.0 (0.0) | 2.0 (2.4) | <.001 |
| In-person visits | 4.1 (6.4) | 3.4 (5.4) | 5.7 (8.0) | <.001 |
| HD-related telehealth visits | 0.1 (0.4) | 0.0 (0.0) | 0.3 (0.6) | <.001 |
| HD-related in-person visits | 0.5 (1.6) | 0.5 (1.5) | 0.7 (1.8) | <.001 |
| Charlson comorbidities,b n (%) | ||||
| Peripheral vascular disease | 3173 (4.53%) | 1894 (3.91%) | 1279 (5.92%) | <.001 |
| Cerebrovascular disease | 589 (0.84%) | 318 (0.66%) | 271 (1.25%) | <.001 |
| Dementia | 31 (0.04%) | 23 (0.05%) | 8 (0.04%) | .54 |
| Chronic pulmonary disease | 5176 (7.39%) | 2835 (5.85%) | 2341 (10.84%) | <.001 |
| Rheumatic disease | 1498 (2.14%) | 630 (1.30%) | 868 (4.02%) | <.001 |
| Peptic ulcer disease | 206 (0.29%) | 106 (0.22%) | 100 (0.46%) | <.001 |
| Mild liver disease | 1338 (1.91%) | 709 (1.46%) | 629 (2.91%) | <.001 |
| Diabetes without chronic complication | 12 651 (18.06%) | 7774 (16.05%) | 4877 (22.58%) | <.001 |
| Diabetes with chronic complication | 3934 (5.62%) | 2162 (4.46%) | 1772 (8.20%) | <.001 |
| Hemiplegia or paraplegia | 273 (0.39%) | 117 (0.24%) | 156 (0.72%) | <.001 |
| Renal disease | 2669 (3.81%) | 1373 (2.83%) | 1296 (6.00%) | <.001 |
| Any malignancyc | 3027 (4.32%) | 1722 (3.55%) | 1305 (6.04%) | <.001 |
| Moderate or severe liver disease | 157 (0.22%) | 69 (0.14%) | 88 (0.41%) | <.001 |
| Metastatic solid tumor | 346 (0.49%) | 182 (0.38%) | 164 (0.76%) | <.001 |
| AIDS/HIV | 158 (0.23%) | 83 (0.17%) | 75 (0.35%) | <.001 |
Abbreviation: HD, heart disease.
aThe Wilcoxon nonparametric rank-sum test was used to compare differences in means for continuous variables, and the Pearson's chi-square test was used to compare differences in proportions for categorical variables by telehealth use status during the stay-at-home order period.
bComorbidities were identified from March 1, 2019, to February 28, 2020. Myocardial infarction and congestive heart failure were excluded as comorbidities because they are included in our definition for HD.
cIncludes lymphoma and leukemia, except for malignant neoplasm of skin.
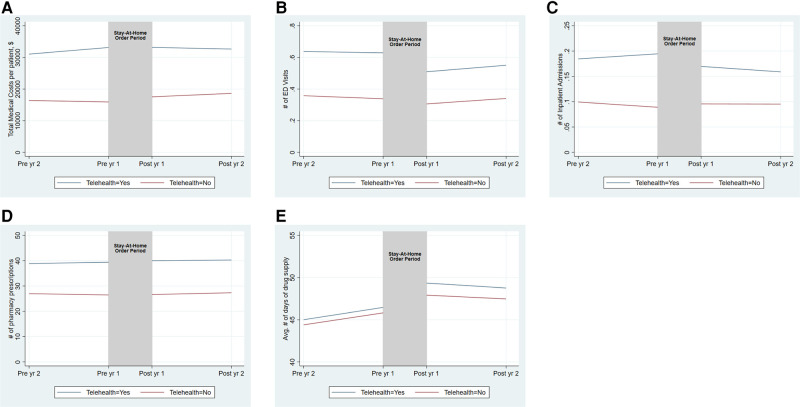
Pre–stay-at-home order trends were parallel for those who used telehealth during the stay-at-home order period and those who did not for the outcomes of numbers of ED visits and pharmacy prescriptions (Figure 1 and Appendix Table S4). Conversely, pre–stay-at-home order trends were not parallel for those who used telehealth and those who did not for the outcomes of total medical costs, number of inpatient admissions, and HD-related outcomes. The pre–stay-at-home order trends were upward (ie, increased costs/utilization) for those who used telehealth but downward (ie, decreased costs/utilization) for those who did not (Figure 1, Appendix Figure S2, and Appendix Tables S4 and S5). The post–stay-at-home order trends were downward for individuals who used telehealth and upward for those who did not.
Figure 1.
Trends in medical costs and health care utilization among telehealth users and nonusers before and after the stay-at-home order period (unadjusted). (A) Total medical costs. (B) Number of ED visits. (C) Number of inpatient admissions. (D) Number of pharmacy prescriptions. (E) Average number of days of drug supply. The vertical shaded area indicates telehealth use during the stay-at-home order periods (March–June 2020). We provided pre– and post–stay-at-home order trends of the outcomes among individuals who used and did not use telehealth during the stay-at-home order periods. Pre and Post indicate pre– and post–stay-at-home order periods. All estimates are per patient per year. The corresponding estimates are available in Appendix Table S4. Abbreviations: Avg, average; ED, emergency department; yr, year.
Table 2 shows the adjusted DID estimates for the associations between telehealth use during the stay-at-home order period and subsequent medical costs and health care utilization (see Appendix Table S6 for the unadjusted DID estimates and Appendix Table S7 for all coefficients). Compared with individuals with HD who did not use telehealth, individuals with HD who used telehealth had greater reductions in total medical costs by $1814 per patient per year (95% CI, −$2732 to −$895.8; P < .001), ED visit costs by $208.8 (95% CI, −$272 to −$147), inpatient admission costs by $1574 (95% CI, −$2062 to −$1085), and outpatient costs by $816 (95% CI, −$1448 to −$183) (Table 2). Individuals with HD who are telehealth users had greater reduction in numbers of ED visits (−0.0886; 95% CI, −0.110 to −0.0675; P < .001) and inpatient admissions (−0.0324; 95% CI, −0.0396 to −0.0252; P < .001) but an increase in pharmacy prescriptions (0.514; 95% CI, 0.126–0.903; P < .01), average days of prescription supply (0.773; 95% CI, 0.406–1.139; P < .001), and total pharmacy prescription costs ($838.1; 95% CI, $520.3–$1156) (Table 2).
Table 2.
Relative changes in medical costs and health care utilization among individuals with HD among telehealth users and telehealth nonusers (adjusted).a
| Variables | Total costs, $ | No. of ED visits | No. of inpatient admissions | No. of pharmacy prescriptions | Average no. of days of drug supply | ED visit costs, $ | Inpatient admission costs, $ | Outpatient costs, $ | Pharmacy costs, $ |
|---|---|---|---|---|---|---|---|---|---|
| Diff-in-diff | −1814*** (−2732 to −895.8) | −0.0886*** (−0.110 to −0.0675) | −0.0324*** (−0.0396 to −0.0252) | 0.514** (0.126-0.903) | 0.773*** (0.406-1.139) | −208.8*** (−272.0 to −145.6) | −1574*** (−2062 to −1085) | −815.8* (−1448 to −183.3) | 838.1*** (520.3 to 1156) |
| Mean telehealth nonusers prea | $10 976 | 0.392 | 0.101 | 11.34 | 24.96 | $724.4 | $3939 | $3928 | $1865 |
| Mean telehealth users preb | $17 224 | 0.548 | 0.130 | 19.50 | 26.31 | $1148 | $5493 | $5621 | $4243 |
| Diff—prec | $6248 | 0.157 | 0.0293 | 8.165 | 1.350 | $423.8 | $1554 | $1693 | $2379 |
| Mean telehealth nonusers posta | $11 301 | 0.324 | 0.0836 | 10.82 | 27.74 | $583.8 | $3534 | $4253 | $2360 |
| Mean telehealth users postb | $15 735 | 0.392 | 0.0804 | 19.50 | 29.87 | $798.8 | $3514 | $5130 | $5577 |
| Diff—postc | $4434 | 0.0680 | −0.00314d | 8.680 | 2.123 | $215 | −$19.98 | $877 | $3217 |
Abbreviations: diff-in-diff, difference-in-differences estimates; ED, emergency department; HD, heart disease.
Of the 70 041 individuals with HD (Appendix Figure S1), 21 598 (30.8%) individuals had any telehealth during the stay-at-home order periods. All estimates came from difference-in-differences models, adjusted for age group, sex, urban/rural, census region of individuals’ place of residence, COVID-19 infection status during the stay-at-home order and subsequent periods, number of in-person outpatient visits during the stay-at-home order period, and comorbidities. The difference-in-differences estimates are the differences in means between telehealth users and telehealth nonusers in the pre– and post–stay-at-home order periods. 95% CIs are in parentheses. All estimates are per patient per year. All of the original coefficients from the difference-in-difference models are shown in Appendix Table S7. ***P < .001; **P < .01; *P < .05.
aMean telehealth nonusers pre/post indicates the average of outcomes in the pre– and post–stay-at-home order period (March–June 2020) among telehealth nonusers.
bMean telehealth users pre/post indicates the average of outcomes in the pre– and post–stay-at-home order period among telehealth users.
cDiff—pre/post indicates the differences in the average of outcomes between telehealth users and telehealth nonusers in pre– and post–telehealth use during the stay-at-home order periods.
dNegative average values are adjusted averages of the outcomes after controlling for the list of control variables. Unadjusted estimates are all positive and available in Appendix Table S5.
Table 3 presents the adjusted DID estimates for the associations of HD-related telehealth use with subsequent HD-related outcomes. Individuals with HD who used HD-related telehealth had a $2329 (95% CI, −$3159 to −$1499; P < .001) greater reduction in HD-related total medical costs, HD-related ED visits (−0.0516; 95% CI, −0.0696 to −0.0337; P < .001), and inpatient admissions (−0.0324; 95% CI, −0.0416 to −0.0232; P < .001).
Table 3.
Relative changes in HD-related medical costs and health care utilization among individuals with HD among HD-related telehealth users and telehealth nonusers (adjusted).
| Variables | Total medical costs, $ | No. of ED visits | No. of inpatient admissions | ED visit costs, $ | Inpatient admission costs, $ | Outpatient costs, $ |
|---|---|---|---|---|---|---|
| Diff-in-diff | −2329*** (−3159 to −1499) | −0.0516*** (−0.0696 to −0.0337) | −0.0324*** (−0.0696 to −0.0337) | −125.0*** (−190.3 to −59.81) | −1705*** (−2452 to −958.7) | −487.9*** (−775.2 to −200.6) |
| Mean telehealth nonusers prea | $2230 | −0.00189d | 0.00855 | −$23.78d | $1487 | $1072 |
| Mean telehealth users preb | $8143 | 0.116 | 0.0777 | $301.9 | $4530 | $3527 |
| Diff—prec | $5913 | 0.118 | 0.0692 | $325.7 | $3044 | $2454 |
| Mean telehealth nonusers posta | $1841 | −0.0225 | 0.000154 | −$77.51 | $1172 | $1054 |
| Mean telehealth users postb | $5425 | 0.0441 | 0.0369 | $123.1 | $2511 | $3021 |
| Diff—postc | $3584 | 0.0666 | 0.0368 | $200.7 | $1338 | $1966 |
Abbreviations: diff-in-diff, difference-in-differences estimates; ED, emergency department; HD, heart disease.
Of the 70 041 individuals with HD (Appendix Figure S1), 5858 (8.4%) had HD-related telehealth during the stay-at-home order period. All estimates came from difference-in-differences models, adjusted for age group, sex, urban/rural, census region of individuals’ place of residence, COVID-19 infection status during the stay-at-home order and subsequent periods, number of in-person outpatient visits during the stay-at-home order period, and comorbidities. The difference-in-differences estimates are the differences in means between telehealth users and telehealth nonusers in the pre– and post–stay-at-home order periods. 95% CIs are shown in parentheses. All estimates are per patient per year. ***P < .001.
aMean telehealth nonusers pre/post indicates the average of outcomes in the pre– and post–stay-at-home order period (March–June 2020) among telehealth nonusers. All the original coefficients from the difference-in-difference models are shown in Appendix Table S7.
bMean telehealth users pre/post indicates the average of outcomes in the pre– and post–stay-at-home order period among telehealth users.
cDiff—pre/post indicates the differences in the average of outcomes between telehealth users and telehealth nonusers in pre– and post–telehealth use during the stay-at-home order periods.
dNegative average values are adjusted averages of the outcomes after controlling for the list of control variables. Unadjusted estimates are all positive and available in Appendix Table S5.
Tables 2 and 3 present the DID estimates for reduced costs associated with telehealth use for ED visits and inpatient admissions. Telehealth users had a greater reduction in costs associated with ED visits ($208.8; 95% CI, −$272.0 to −$145.6; P < .001) and inpatient admissions ($1574; 95% CI, −$2062 to −$1085; P < .001) per patient per year compared with telehealth nonusers. Individuals who used HD-related telehealth had a greater reduction in costs associated with HD-related ED visits ($125.0; 95% CI, −$190.3 to −$59.81; P < .001) and inpatient admissions ($1705; 95% CI, −$2452 to −$958.7; P < .001) compared with those who did not use telehealth.
Appendix Table S8 presents the average marginal effects for the DID estimates from our sensitivity analyses, extending to the GLM and ZINB models for total medical costs and health care utilization (overall and HD-related). All findings were consistent with our main results.
Appendix Table S9 shows the results without controlling for the number of in-person visits. Overall, the results closely mirrored our primary findings that controlled the number of in-person visits. For instance, individuals using telehealth had a $1914 relative reduction in total medical costs without controlling for the number of in-person visits, whereas after accounting for these visits, the reduction was $1814 in total medical costs (Table 2). Similarly, other health care utilization and HD-related results remained highly consistent.
Appendix Table S10 showed the results after controlling for AMI and CHF, both being sub-diseases of the broader HD category. Notably, the results were quite similar and did not alter the findings of our main results that did not control AMI and CHF.
Appendix Table S11 presents the RTM results testing whether the outcomes were influenced by the RTM hypothesis. These findings indicate no evidence of RTM effects. The observed coefficients above and below the cutoff points differed, and their 95% CIs overlapped for all outcomes, except for the number of inpatient visits, where the inpatient admission costs showed no evidence of RTM. These results affirm that our findings were not influenced by the RTM phenomenon.
Last, Appendix Tables S12 and S13 present additional regression analyses examining the association between telehealth use and subsequent outcomes without implementing the DID study design. In Appendix Table S12 panel A, the results showed that individuals utilizing telehealth had an increase in total medical costs by $14 017 (95% CI, $12 498–$15 536), the number of ED visits by 0.095 (95% CI, 0.084–0.107), and the number of inpatient admissions by 0.024 (95% CI, 0.018–0.031) compared with non–telehealth users. Panel B of Appendix Table S12 shows that individuals using HD-related telehealth had an increase in HD-related total medical costs by $5318 (95% CI, $4243–$6392), the number of ED visits by 0.065 (95% CI, 0.055–0.075), and the number of inpatient admissions by 0.034 (95% CI, 0.032–0.047). These results notably differ from the results from the DID models, emphasizing the significance of addressing endogeneity issues.
Discussion
This study was one of the first to examine the association of widespread COVID-19–related telehealth adoption with subsequent medical costs and health care utilization among individuals diagnosed with HD. We found that telehealth was associated with a relative reduction in ED visits, inpatient admissions, and medical costs. Telehealth users had a greater reduction in total medical costs (−$1814 per patient per year), ED visits (88.6 fewer per 1000 persons per year), and inpatient admissions (32.4 fewer per 1000 people per year) compared with telehealth nonusers. There was a corresponding reduction in costs (−$209 for ED visits and −$1574 for inpatient admissions per patient per year). Telehealth users had a greater increase in the number of prescription fills and a slightly higher average number of days of drug supply. The results were similar with and without controlling for patient characteristics, in the sensitivity analyses, and when extended to the associations between HD-related telehealth use and HD-related outcomes.
While pre–stay-at-home order trends of numbers of ED visits, pharmacy prescriptions, and average number of days of drug supply were parallel among telehealth users and nonusers, pre–stay-at-home order trends for total medical costs, number of inpatient admissions, and HD-related outcomes were not parallel—the outcomes were increasing for telehealth users and were slightly decreasing for telehealth nonusers (ie, the differences were increasing in pre–stay-at-home order trends). These trends reversed after telehealth use during the stay-at-home period (ie, the differences were decreasing in post–stay-at-home order trends). This reversal may imply that our findings on the costs and utilization associated with telehealth were conservative, compared with if we had extrapolated the differences in pre–stay-at-home order trends.42 If the differences in pre–stay-at-home order trends persisted, the estimated associations of telehealth with medical costs and health care utilization may have been much higher than our estimates.42,43
During the COVID-19 pandemic, hospital visits, including ED visits and inpatient admissions, notably declined.44-46 Patients hesitated to seek in-person care due to concerns about COVID-19 exposure, resulting in altered health care–seeking patterns.47 This decrease in hospital encounters was consistently observed in ED visits among both telehealth users and nonusers. Compared with pre–COVID-19 periods, there was a reduction in the number of ED visits for telehealth users (a decrease of 120 visits per 1000 individuals) and for telehealth nonusers (a decrease of 30 visits per 1000 individuals) after the widespread adoption of telehealth during the early COVID-19 pandemic. Understanding these changes in hospital utilization during the pandemic is crucial for interpreting the evolving health care–seeking behaviors relevant to this study's post-period analysis.
Our findings highlight that telehealth utilization during the early PHE was associated with a relative reduction in total medical costs and health care utilization, alongside an increase in pharmacy prescriptions. All results were derived after controlling for the number of in-person outpatient visits, reflecting a narrative of telehealth as a “complementary” aspect. The debate over whether telehealth acts as a complement or substitute to in-person visits remains varied.48-50 Notably, our supplementary analysis, which omitted control for in-person visits, yielded outcomes consistent with our primary findings. For instance, telehealth utilization showed a relative reduction in total medical costs by $1814 when controlling for the number of in-person outpatient visits. Conversely, without controlling for these visits, the reduction was slightly higher at $1914. These findings may suggest that the dominant effects of telehealth use are complementary rather than substitutionary.
There is mixed evidence regarding the impacts of telehealth use on subsequent outcomes. Some studies have documented an increase in total medical costs51,52 and health care utilization after telehealth use,51,53 while others have shown a reduction in costs30 and health care utilization.29,54 Several factors may explain these differences. First, self-selection into telehealth use presents a significant endogeneity issue. Individuals who self-select into telehealth use during the early PHE might represent a sicker and systematically different group compared with those who did not use telehealth. Our estimates compared those who used telehealth with those who did not during both pre– and post–stay-at-home order periods (ie, DID settings), which aimed to consider some of the time-invariant characteristics that could impact this decision. Additionally, we controlled for time-varying individual comorbidities that might affect the decision to use telehealth and subsequent outcomes. Our supplementary results from non-DID models indicated an increase in total medical costs and health care utilization. This underscores the significance of self-selection and endogeneity issues within the model. Second, our study concentrated on individuals with HD enrolled in a commercial insurance plan, and the advantages of telehealth might vary among different population groups and individuals with specific conditions. For instance, while a systematic review28 highlighted the benefits of telehealth, these advantages may not uniformly apply to individuals with other medical conditions.
Despite the differences in prior studies’ findings, our findings are consistent with those from other studies—prior to COVID-19, in small patient cohorts—investigating the associations of telehealth among patients diagnosed with chronic and cardiovascular conditions other than HD.28,30,31 When leveraged as a complementary modality to in-person care, telehealth has the potential to support the triple aim of better care, improved population health, and reduced health care costs.9,21,55,56 Telehealth can improve care experiences by supporting continuity of care and engaging patients regularly to help manage chronic conditions. The rise of telehealth mitigated the initial drop in outpatient visit volume during the stay-at-home period19,20 and may have facilitated timely access to care.10,12 This, in turn, may have prevented the exacerbation of chronic conditions, and improved health outcomes.10,29,57,58 Furthermore, telehealth can increase health provider efficiency, can reduce burden on EDs,10,57 and has been shown to reduce hospitalization.9,10,28,29,31,59
Telehealth users had increased pharmacy prescriptions and days of drug supply—variables used in the proportion of days covered and medication possession ratio, 2 common measures of medication adherence60,61—and might imply that telehealth improved chronic disease management through increased medication intensification, medication access, and/or medication adherence. Studies before and after the stay-at-home order found that telehealth interventions successfully improved medication use and adherence among individuals diagnosed with hypertension, heart failure, and CVD.5-7,62 Increased health care utilization and reduced medical costs for telehealth users may have been driven by increased patient engagement, enabling providers to address barriers to medication adherence.63 Improved medication adherence can address the modifiable risk factors for chronic conditions and reduce health care utilization, related costs, and morbidity and mortality.63
Although this study did not evaluate the associations of telehealth with health disparities, results showed that people living in urban areas were more likely to use telehealth, aligning with concerns over growing disparities in telehealth access, also known as the digital divide.20,55,64 Our analysis highlighted that telehealth users were also more likely to use in-person care and more likely to have comorbidities. A potential explanation could be the effectiveness of telehealth interventions for the prevention, assessment,8,28,29 and access to specialty health care services between in-person appointments, thereby meeting the more intensive treatment needs of those with chronic conditions and comorbidities.
During the COVID-19 pandemic, state and federal governments implemented various measures to promote the rapid adoption of telehealth. Some of these telehealth flexibilities have become permanent. Others are temporary, suspended with the lifting of the PHE.65 Among the primary obstacles to ongoing policy flexibility is the assumption by policymakers and providers that expanding telehealth access will result in increased utilization and higher costs66 through supply-induced demand.67 Our key findings—including reductions in total medical costs, inpatient admissions, and ED utilization—challenge some of these assumptions, and point toward telehealth as a potentially effective and financially viable alternative to in-person care in a post-pandemic setting.
The Consolidated Appropriations Act, which extended telehealth flexibilities for Medicare enrollees post-PHE, became law on March 15, 2022. This law was designed to prevent a “telehealth cliff” in Medicare post-PHE and to enable Congress to review future telehealth-related data. These changes may include restrictions related to provider and patient geography, Health Insurance Portability and Accountability Act compliance, and reimbursements for both video and audio-only telehealth.65 Amid this changing landscape, our findings contribute evidence that could inform decisions regarding the continuation of reimbursement parity post-PHE.68,69
Future research can investigate optimal workflows of telehealth and in-person care delivery to meet quality-of-care standards, address concerns about disparities in accessing telehealth among patients with HD, and identify circumstances in which telehealth is an appropriate modality.64 More research on the association between telehealth and medication adherence could contribute to evidence regarding risk factor management for HD. Additionally, future analysis of total medical costs may consider other reimbursement mechanisms beyond noncapitated insurance.59 In addition, costs related to transportation, logistics, and scheduling can be considered when assessing total medical costs and costs incurred by patients. Also, this study primarily focused on evaluating the effects of both general and HD-related telehealth visits on subsequent outcomes. Given the varied purposes of telehealth, including tele-mental health and other behavioral telehealth visits, future studies might benefit from further analysis by disaggregating the results based on different telehealth modalities. Finally, our study focused on individuals with HD, who may have been sicker than individuals in the general population enrolled in a commercial plan. The benefits of telehealth use might vary depending on the population groups, and future studies may consider examining outcomes among all commercially insured enrollees, publicly insured individuals, and those without health insurance.
Limitations
Our analysis had several limitations. First, our study did not examine programmatic and other costs associated with setting up telehealth technology and did not account for other types of nonmedical cost reductions often associated with telehealth use, such as reduced transportation time and costs.70 Second, although we controlled for time-varying COVID-19 diagnoses and individuals’ comorbidities to adjust for potential observable confounders, if time-varying unobservable covariates (such as patients’ income, wealth, and willingness to access preventive care independent of their underlying health status) differed between those who used telehealth and those who did not, the DID telehealth estimates might be underestimated.38 Third, our results are not generalizable to those without continuous enrollment, with public insurance (eg, Medicare, Medicaid), or with no insurance.70 Telehealth use during the stay-at-home order period might differ among individuals with public health insurance or without insurance. There might also be differences between those without and those with continuous commercial insurance enrollment during the stay-at-home order period. Fourth, our findings are based on telehealth use during March–June 2020, when telehealth use increased most19 and most states implemented stay-at-home orders.13 Our findings may not be generalizable to telehealth use in other time frames. Fifth, because the DID estimate is a weighted average, the analysis cannot infer whether and by how much each comparison group's contribution to the overall relative reduction in outcomes can be attributed to telehealth use. Sixth, because the DID analysis is applied to a pre-post (and not a quasi-experimental) study design, the findings are only interpreted as associative, and no causal inferences are drawn. Seventh, recording errors in the MarketScan database may exist due to the imperfect nature of claims data. Our results might be an underestimate if health care providers were unfamiliar with telehealth-related claims during the stay-at-home order period.
Conclusion
Our analysis of commercial insurance claims reflecting telehealth use during the stay-at-home order period (March–June 2020) indicated that telehealth use was associated with (1) a significant decrease in medical costs and numbers of ED visits and inpatient admissions and (2) an increase in pharmacy prescriptions and length of drug supply. As the pandemic strained hospital and ED resources, telehealth use is associated with reduced ED and inpatient admissions among populations at the highest risk of contracting a serious COVID-19 infection. This may be due to telehealth's ability to promote communication between patients and providers, improve access to continuous care, and manage chronic conditions more effectively. Our findings align with evidence of telehealth's effectiveness in the prevention and management of chronic disease. As medical costs and health care utilization grow, our findings suggest the value of telehealth in reducing medical costs and health care utilization in ED and inpatient settings.
Supplementary Material
Acknowledgments
The authors are genuinely grateful to Michael Schooley, Kakoli Roy, Fatima Coronado, and Adam Vaughan (Centers for Disease Control and Prevention) for their guidance, suggestions, and article review. They sincerely appreciate Moira Urich (Centers for Disease Control and Prevention) for her edits to the manuscript.
This paper was presented at the American Society of Health Economists' 12th Annual Conference as a poster presentation on June 11, 2023, and at the Atlanta/Athens Health Economists Research Conference as an oral presentation on September 29, 2023. Additionally, it was presented at the American Economic Association Annual Meeting as a poster on January 5, 2024.
Contributor Information
Jun Soo Lee, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA 30341, United States.
Ami Bhatt, ASRT, Inc, Atlanta, GA 30346, United States.
Lisa M Pollack, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA 30341, United States.
Sandra L Jackson, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA 30341, United States.
Ji Eun Chang, Department of Public Health Policy and Management, School of Global Public Health, New York University, New York, NY 10003, United States.
Xin Tong, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA 30341, United States.
Feijun Luo, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta, GA 30341, United States.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services.
Supplementary material
Supplementary material is available at Health Affairs Scholar online.
Notes
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93–e621. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelen GD, Wolfe R, D’Onofrio G, et al. Emergency department crowding: the canary in the health care system. NEJM Catalyst. 2021;8. [Google Scholar]
- 4.NEJM Catalyst. What is telehealth? 2018;4(1). 10.1056/CAT.18.0268 [DOI]
- 5.Guide to Community Preventive Services. Heart disease and stroke prevention: mobile health (mHealth) interventions for treatment adherence among newly diagnosed patients. Accessed February 10, 2023. https://www.thecommunityguide.org/pages/tffrs-heart-disease-stroke-prevention-mobile-health-interventions-treatment-adherence-newly-diagnosed-patients.html
- 6.Guide to Community Preventive Services. Health information technology: text messaging interventions for medication adherence among patients with chronic diseases. Accessed February 10, 2023. https://www.thecommunityguide.org/findings/health-information-technology-text-messaging-medication-adherence-chronic-disease.html
- 7.Guide to Community Preventive Services. Heart disease and stroke prevention: interactive digital interventions for blood pressure self-management. Accessed February 10, 2023. https://www.thecommunityguide.org/findings/heart-disease-stroke-prevention-interactive-digital-interventions-blood-pressure-self-management.html
- 8.Centers for Disease Control and Prevention. Best practices for heart disease and stroke: a guide to effective approaches and strategies. 2022. Accessed February 10, 2023. https://stacks.cdc.gov/view/cdc/122290
- 9.Schwamm LH, Chumbler N, Brown E, et al. Recommendations for the implementation of telehealth in cardiovascular and stroke care: a policy statement from the American Heart Association. Circulation. 2017;135(7):e24–e44. 10.1161/CIR.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 10.Butzner M, Cuffee Y. Telehealth interventions and outcomes across rural communities in the United States: narrative review. J Med Internet Res. 2021;23(8):e29575. 10.2196/29575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baum A, Barnett ML, Wisnivesky J, Schwartz MD. Association between a temporary reduction in access to health care and long-term changes in hypertension control among veterans after a natural disaster. JAMA Netw Open. 2019;2(11):e1915111. 10.1001/jamanetworkopen.2019.15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieneck C, Weaver E, Maryon T. Outpatient telehealth implementation in the United States during the COVID-19 global pandemic: a systematic review. Medicina (Kaunas). 2021;57(5):462. 10.3390/medicina57050462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreland A, Herlihy C, Tynan MA, et al. Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement—United States, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1198–1203. 10.15585/mmwr.mm6935a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czeisler MÉ, Marynak K, Clarke KE, et al. Delay or avoidance of medical care because of COVID-19–related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. Current emergencies. Accessed February 10, 2023. https://www.cms.gov/about-cms/what-we-do/emergency-response/current-emergencies
- 16.Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January–March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao GF, Li KY, Zhu Z, et al. Use of telehealth by surgical specialties during the COVID-19 pandemic. JAMA Surg. 2021;156(7):620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demeke HB, Merali S, Marks S, et al. Trends in use of telehealth among health centers during the COVID-19 pandemic—United States, June 26–November 6, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(7):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Lowe Beasley K, Schooley MW, Luo F. Trends and costs of US telehealth use among patients with cardiovascular disease before and during the COVID-19 pandemic. J Am Heart Assoc. 2023;12(4):e028713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States. Health Aff (Millwood). 2021;40(2):349–358. 10.1377/hlthaff.2020.01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blok S, van der Linden EL, Somsen GA, Tulevski II, Winter MM, van den Born BH. Success factors in high-effect, low-cost eHealth programs for patients with hypertension: a systematic review and meta-analysis. Eur J Prev Cardiol. 2021;28(14):1579–1587. 10.1177/2047487320957170 [DOI] [PubMed] [Google Scholar]
- 22.Cichosz SL, Ehlers LH, Hejlesen O. Health effectiveness and cost-effectiveness of telehealthcare for heart failure: study protocol for a randomized controlled trial. Trials. 2016;17(1):590. 10.1186/s13063-016-1722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaambwa B, Bryan S, Jowett S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a cost-effectiveness analysis. Eur J Prev Cardiol. 2014;21(12):1517–1530. 10.1177/2047487313501886 [DOI] [PubMed] [Google Scholar]
- 24.Perednia DA, Allen A. Telemedicine technology and clinical applications. JAMA. 1995;273(6):483–488. [PubMed] [Google Scholar]
- 25.Henderson C, Knapp M, Fernández JL, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator Telehealth Questionnaire Study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ. 2013;346:f1035. 10.1136/bmj.f1035 [DOI] [PubMed] [Google Scholar]
- 26.Stoddart A, Hanley J, Wild S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomised controlled trial. BMJ Open. 2013;3(5):e002681. 10.1136/bmjopen-2013-002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen LB, Christiansen T, Kirkegaard P, Pedersen EB. Economic evaluation of home blood pressure telemonitoring: a randomized controlled trial. Blood Press. 2011;20(2):117–125. 10.3109/08037051.2010.532306 [DOI] [PubMed] [Google Scholar]
- 28.Battineni G, Sagaro GG, Chintalapudi N, Amenta F. The benefits of telemedicine in personalized prevention of cardiovascular diseases (CVD): a systematic review. J Pers Med. 2021; 11(7):658. 10.3390/jpm11070658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuan PX, Chan WK, Fern Ying DK, et al. Efficacy of telemedicine for the management of cardiovascular disease: a systematic review and meta-analysis. Lancet Digit Health. 2022;4(9):e676–e691. 10.1016/S2589-7500(22)00124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nord G, Rising KL, Band RA, Carr BG, Hollander JE. On-demand synchronous audio video telemedicine visits are cost effective. Am J Emerg Med. 2019;37(5):890–894. 10.1016/j.ajem.2018.08.017 [DOI] [PubMed] [Google Scholar]
- 31.Farabi H, Rezapour A, Jahangiri R, Jafari A, Rashki Kemmak A, Nikjoo S. Economic evaluation of the utilization of telemedicine for patients with cardiovascular disease: a systematic review. Heart Fail Rev. 2020;25(6):1063–1075. 10.1007/s10741-019-09864-4 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi EA, Schwamm LH, Adeoye OM, et al. An overview of telehealth in the management of cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2022;146(25):e558–e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merative MarketScan® Research Databases. Accessed December 20, 2022. https://marketscan.truvenhealth.com/marketscanportal
- 34.Kuklina E, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42(9):2564–2570. 10.1161/STROKEAHA.110.610592) [DOI] [PubMed] [Google Scholar]
- 35.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53(1):175–196. 10.1111/1475-6773.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014.
- 37.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 38.Zeldow B, Hatfield LA. Confounding and regression adjustment in difference-in-differences studies. Health Serv Res. 2021;56(5):932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wing C, Simon K, Bello-Gomez RA. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health. 2018;39(1):453–469. [DOI] [PubMed] [Google Scholar]
- 40.Barnett AG, Van Der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34(1):215–220. [DOI] [PubMed] [Google Scholar]
- 41.Linden A. rtmci: Stata module for estimating regression to the mean effects with confidence intervals. 2013. Accessed February 10, 2023. http://www.lindenconsulting.org
- 42.Rambachan A, Roth J. A more credible approach to parallel trends. Rev Econ Stud. 2023;90(5):2555–2591. [Google Scholar]
- 43.Hernandez-Cortes D, Meng KC. Do environmental markets cause environmental injustice? Evidence from California's carbon market. J Public Econ. 2023;217:104786. [Google Scholar]
- 44.Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smulowitz PB, O’Malley AJ, Khidir H, Zaborski L, McWilliams JM, Landon BE. National trends in ED visits, hospital admissions, and mortality for Medicare patients during the COVID-19 pandemic: study examines trends in emergency department visits, hospital admissions, and mortality for Medicare patients during the COVID-19 pandemic. Health Aff. 2021;40(9):1457–1464. [DOI] [PubMed] [Google Scholar]
- 46.Hartnett KP, Kite-Powell A, DeVies J, et al. Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore R, Purvis RS, Hallgren E, et al. “I am hesitant to visit the doctor unless absolutely necessary”: a qualitative study of delayed care, avoidance of care, and telehealth experiences during the COVID-19 pandemic. Medicine (Baltimore). 2022;101(32):e29439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson TN, Sreedhara M, Bostic M, et al. Telehealth use to address cardiovascular disease and hypertension in the United States: a systematic review and meta-analysis, 2011–2021. Telemed Rep. 2023;4(1):67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewinski AA, Walsh C, Rushton S, et al. Telehealth for the longitudinal management of chronic conditions: systematic review. J Med Internet Res. 2022;24(8):e37100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Y, Chen D, Smith M. Use telehealth as needed: telehealth substitutes in-person primary care and associates with the changes in unplanned events and follow-up visits. BMC Health Serv Res. 2023;23(1):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485–491. [DOI] [PubMed] [Google Scholar]
- 52.The Medicare Payment Advisory Commission (MedPAC). June 2023 Report to the Congress: Medicare and the Health Care Delivery System. Accessed December 12, 2023. https://www.medpac.gov/wp-content/uploads/2023/06/Jun23_Ch7_MedPAC_Report_To_Congress_SEC.pdf
- 53.Chen K, Zhang C, Gurley A, Akkem S, Jackson H. Primary care utilization among telehealth users and non-users at a large urban public healthcare system. PLoS One. 2022;17(8):e0272605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayabakan S, Bardhan IR, Zheng Z. Impact of telehealth and process virtualization on healthcare utilization. Inform Syst Res. 2023. 10.1287/isre.2023.1220 [DOI] [Google Scholar]
- 55.Haynes N, Ezekwesili A, Nunes K, Gumbs E, Haynes M, Swain J. Can you see my screen?” Addressing racial and ethnic disparities in telehealth. Curr Cardiovasc Risk Rep. 2021;15(12):23. 10.1007/s12170-021-00685-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah VV, Villaflores CW, Chuong LH, et al. Association between in-person vs telehealth follow-up and rates of repeated hospital visits among patients seen in the emergency department. JAMA Netw Open. 2022;5(10):e2237783. 10.1001/jamanetworkopen.2022.37783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel P, Dhindsa D, Eapen DJ, et al. Optimizing the potential for telehealth in cardiovascular care (in the era of COVID-19): time will tell. Am J Med. 2021;134(8):945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bitar H, Alismail S. The role of eHealth, telehealth, and telemedicine for chronic disease patients during COVID-19 pandemic: a rapid systematic review. Digit Health. 2021;7:20552076211009396. 10.1177/20552076211009396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chin MH. Uncomfortable truths—what COVID-19 has revealed about chronic-disease care in America. N Engl J Med. 2021;385(18):1633–1636. [DOI] [PubMed] [Google Scholar]
- 60.Loucks J, Zuckerman AD, Berni A, Saulles A, Thomas G, Alonzo A. Proportion of days covered as a measure of medication adherence. Am J Health Syst Pharm. 2021;79(6):492–496. 10.1093/ajhp/zxab392 [DOI] [PubMed] [Google Scholar]
- 61.Canfield SL, Zuckerman A, Anguiano RH, et al. Navigating the Wild West of medication adherence reporting in specialty pharmacy. J Manag Care Spec Pharm. 2019;25(10):1073–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molloy GJ, O'Carroll RE, Witham MD, McMurdo ME. Interventions to enhance adherence to medications in patients with heart failure: a systematic review. Circ Heart Fail. 2012;5(1):126–133. 10.1161/circheartfailure.111.964569 [DOI] [PubMed] [Google Scholar]
- 63.Neiman AB, Ruppar T, Ho M, et al. CDC grand rounds: improving medication adherence for chronic disease management—innovations and opportunities. MMWR Morb Mortal Wkly Rep. 2017;66(45):1248–1251. 10.15585/mmwr.mm6645a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nana-Sinkam P, Kraschnewski J, Sacco R, et al. Health disparities and equity in the era of COVID-19. J Clin Transl Sci. 2021;5(1):e99. 10.1017/cts.2021.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.US Department of Health and Human Services . Telehealth policy changes after the COVID-19 public health emergency. 2023. Accessed February 10, 2023. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency/
- 66.Antall P, Berry K, Benjamin R, et al. Taskforce on telehealth policy (TTP) findings and recommendations. 2020. Accessed February 10, 2023. https://www.ncqa.org/wp-content/uploads/2020/09/20200914_Taskforce_on_Telehealth_Policy_Final_Report.pdf
- 67.Volk J, Palanker D, O’Brien M, Goe C. States’ actions to expand telemedicine access during COVID-19 and future policy considerations. Commonwealth Fund; June 2021. 10.26099/r95z-bs17 [DOI]
- 68.American Medical Association . Future of health. Closing the digital health disconnect: a blueprint for optimizing digitally enabled care. 2022. Accessed February 10, 2023. https://www.ama-assn.org/system/files/ama-future-health-report.pdf
- 69.American Medical Association . Equity in telehealth: taking key steps forward. 2022. Accessed February 10, 2023. https://www.ama-assn.org/system/files/issue-brief-equity-in-telehealth.pdf
- 70.Russo JE, McCool RR, Davies L. VA telemedicine: an analysis of cost and time savings. Telemed e-Health. 2016;22(3):209–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.